Abstract
Microfluidic lab-on-a-chip devices are usually fabricated using replica molding, with poly(dimethylsiloxane) (PDMS) casting on a mold. Most common techniques used to fabricate microfluidic molds, such as photolithography and soft lithography, require costly facilities such as a cleanroom, and complicated steps, especially for the fabrication of three-dimensional (3D) features. For example, an often-desired 3D microchannel feature consists of intersecting channels with depth variations. This type of 3D flow focusing geometry has applications in flow cytometry and droplet generation. Various manufacturing techniques have recently been developed for the rapid fabrication of such 3D microfluidic features. In this paper, we describe a new method of mold fabrication that utilizes water jet cutting technology to fabricate free-standing structures on mild steel sheets to make a mold for PDMS casting. As a proof-of-concept, we use this fabrication technique to make a PDMS chip that has a 3D flow focusing junction, an inlet for the sample fluid, two inlets for the sheath fluid, and an outlet. The flow focusing junction is patterned into the PDMS slab with an abrupt, nearly stepwise change to the depth of the microchannel junction. We use confocal microscopy to visualize the 3D flow focusing of a sample flow using this geometry, and we also use the same geometry to generate water-in-oil droplets. This alternative approach to create microfluidic molds is versatile and may find utility in reducing the cost and complexity involved in fabricating 3D features in microfluidic devices.
I. INTRODUCTION
Among the various materials that are used for microfluidics devices, polymers have found more use than silicon and glass because they can be molded or hot-embossed rather than direct etching, making them suitable for high-volume manufacturing processes.1,2 Among many polymers, cross-linkable or castable materials are of the most interest in lab on a chip (LOC) devices3 and the most commonly used is poly(dimethylsiloxane) (PDMS). PDMS is very commonly used because of its biocompatibility and nontoxicity,2,4 high hydrophobic nature,5 relatively high tensile strength,6 and high fidelity in transferring mold contours.7 An ubiquitous and efficient method to make microfluidic devices is PDMS casting with a mold, which has generally been fabricated using photolithography8 and soft lithography.9 These fabrication techniques, however, require costly facilities such as a cleanroom. Perhaps, more importantly, photolithography and soft lithography methods, which rely on selective exposure of light to pattern substrates, inherently complicate efforts to create structures that have 3D features. Indeed, the steps needed to fabricate 3D microfluidic features using these classical approaches are laborious and often require the careful alignment of multiple layers of two-dimensionally patterned PDMS.10
Low-cost micro-fabrication techniques that do not require clean rooms, and can create high aspect ratio features, for example, aspect ratios greater than 20, are highly desirable.11,12 Thus, recent developments in the micro-fabrication of such devices have been mainly focused on developing simpler, less expensive, and more robust fabrication techniques.13 Wong et al. developed a new protocol for the fabrication of microfluidic molds in a nickel alloy using a combination of photolithography for channel patterns on silicon, followed by deep reactive-ion etching (DRIE) and deposition of a seeding layer, and finally electroplating on a nickel mold.14 The manufacturing process was aimed at creating microfluidic chips in poly(methyl methacrylate) (PMMA) with channel depths of ∼100 μm.14 Joanni et al. developed a laser polymer swelling technique for creating molds in PMMA and found that by a proper control of the process parameters features having an aspect ratio of ∼4 could be cast in PDMS.15 Another technique, Xurography, uses a cutting plotter machine and adhesive vinyl films to generate master molds with dimensions of ∼50 μm.16 In another study, laser micro-machining was used to make molds in order to fabricate PMMA chips using a hot embossing technique.17 Direct CO2 laser ablation has also been used to make microfluidic molds and to fabricate channels on wax-coated glass substrates.18,19 Finally, advanced micromachining techniques such as x-ray lithography20 and CNC (Computer Numerical Control) micro-milling1 have also been developed. Koesdjojo et al.21 successfully demonstrated the use of micro-milling aluminum molds to emboss a polyetherimide (PEI) substrate for the fabrication of microfluidic devices. Wilson et al.22 utilized micro-milling to fabricate microfluidic channels with complex cross-sectional geometries including 3D features. For this purpose, they used a combination of micro-scale milling tools and soft lithography to fabricate semi-circular patterns on planar metallic (brass) surfaces to create a master mold and then cast PDMS to make chips. However, one of the main disadvantages of CNC milling is that it requires complex tool alignment and tool breakage may occur.11 Furthermore, as found by Guckenberger et al.,1 for applications in which optical clarity is critical, such as for phase contrast cell microscopy and imaging, the roughness created from the milling process may not be acceptable.
Recently, rapid prototyping (RP) techniques such as print-and-peel,23 aluminum foil replica,24 and PZT (lead zirconate titanate) drop-on-demand droplet generators for wax25 have been developed to fabricate molds for microfluidic devices. To overcome challenges with high cost and low prototyping speed, Lin et al.26 utilized 3D printing as an alternative for fabricating molds from a stainless steel 420 powder mixture.27 In order to overcome the limitations of 3D printing of polymers, including transparency problems28 and a need to cover the surface of the mold by a PDMS-compatible material,29 Lynh and Pin-Chuan30 used solvent bonding PMMA and poly (lactic acid) thermoplastic materials for the creation of hybrid microfluidic chips. Micro-injection is another mold fabrication technique that has been used for microfluidic devices,31 although it may introduce some residual stress and subsequently decrease replication fidelity.32
Despite these recent advances in microfluidics manufacturing, none of these approaches appear to be displacing the classical soft lithography method for making microfluidic devices. This lack of uptake by the microfluidics community suggests that there is great interest in a better manufacturing approach—one that does not require using a clean room, is of low cost, has good resolution, and is capable of creating complex 3D features without any limitations on achievable channel heights.33,34
A common and important feature shared by many flow systems for biomedicine is flow confinement, which is usually achieved by 3D flow focusing in microfluidic devices.35,36 One method for 3D flow focusing involves introducing a shallow sample channel into an intersection of deep main and sheath flow channels.37 This 3D microfluidic geometry is difficult to fabricate using conventional planar mold manufacturing techniques.38,39 It is also time-consuming to make such molds using soft lithography and two-step molding,40,41 and newer methods such as electroplating nickel molds.42 Therefore, the fabrication of 3D flow focusing geometries can be made much simpler by avoiding strategies that rely on layer-by-layer photolithography.43
Powder blasting or abrasive jet micro-machining (AJM) is used to machine micro-grooves for microfluidic application44,45 and in the industry by, e.g., Micron-it technology.46 It is, however, not well suited for use in the fabrication of 3D multi-level features with abrupt changes in depth. When using AJM on glass, thermoplastic, or thermoset materials, it is almost impossible to create steps at a 90°,47 because the erosive potential or “erosive efficacy” changes gradually across the jet diameter. This means that changes in channel depth must occur with transition regions having slopes of 60°,48 at most. Moreover, when fabricating intersecting channels, a “blast lag phenomenon”49 occurs whereby undesirable erosion occurs within the channel intersection. Finally, a limitation of using AJM techniques for mold manufacturing and even direct machining of chips is the relatively high roughness and waviness of the fabricated mold surfaces,50 which makes it difficult to bond and seal cast chips. For example, in glass, the achievable roughness using AJM is around ∼0.4–0.6 μm.47
Recent advances in the miniaturization of abrasive water jet technology have made possible functioning abrasive water jet micro-machining (AWJM) nozzles as small as 250 μm.51 AWJM allows the cutting or milling of virtually any material using a flow of high-pressure water mixed with abrasive particles, which is forced through a micro-nozzle.52 Azarsa et al. have very recently proposed a technique using masked AWJM and abrasive slurry jet machining (ASJM) to directly mill, using stainless steel masks, flat pockets and pockets with raised features in Al6061 and SS316 to be used as molds for PDMS casting.53 They demonstrated that these substrates could be milled to create intersecting protruding ridges of uniform height as narrow as ∼300 μm, representing molds for casting intersecting microfluidic channels. They found that direct surface machining of molds resulted in surfaces with significant waviness, which were difficult to bond using plasma bonding, and the heights of the fabricated structures were limited by the durability of the mask to less than 500 μm. More importantly, when attempting to change the height of a portion of the ridges in order to create a 3D chip, the gradient of erosive efficacy across the abrasive jet footprint unavoidably created a gradual transition in height, limiting the effective slope in the transition region to a maximum of 50°.53 This made the proposed technique unsuitable for, e.g., the 3D flow focusing devices described above, which require very abrupt changes in channel depth.
Here, we describe a new AWJM microfluidic mold fabrication technique that allows the rapid prototyping of master molds with 3D features having abruptly changing heights. We first describe the new mold manufacturing method and discuss its advantages and limitations. Then, we use the method to create a proof-of-concept 3D flow focusing microchannel geometry and demonstrate applications in 3D flow focusing sample streams and in generating water-in-oil droplets. To the best of our knowledge, this is the first report of using AWJM technology to micro-machine microfluidic master molds. We anticipate that this low cost and simple fabrication method will find utility in democratizing microfluidics for laboratories that do not have access to soft lithography facilities.
II. EXPERIMENTAL
A. Machining setup
AWJM is conducted using an OMAX 2626 Jet Machining Centre (OMAX Corp., Kent, WA, USA). The nozzle movement is computer controlled by the OMAX Make program with a maximum traverse speed of 4572 mm/min and a positional accuracy of 76 μm over 30 cm. We use angular garnet particles (220 UT Barton International, Glens Falls, NY, USA) with an average spherical diameter of 75 μm as the abrasive. We adopt the setup used by Azarsa et al.,54 which includes a special micro-nozzle assembly with an orifice and a mixing tube having diameters of 127 μm and 254 μm, respectively. We perform all machining experiments with both the nozzle and the workpiece submerged under water since Haghbin et al.55 showed that this results in an effectively narrower cutting jet.
B. Mold fabrication technique
The technique uses AWJM to cut thin structures from sheets of steel that, when placed against a flat substrate, will form a mold for casting PDMS. We use an electromagnet to firmly secure the structures against the backing substrate during the casting process, without the contamination and spew filets that would result from using an adhesive. Using a magnet to hold the ferromagnetic (steel) mold features allows a choice of virtually any substrate from a wide variety of readily available ultra-smooth and flat materials. Thus, sufficiently smooth and uniform surface finishes can be chosen to allow the fabricated chips to be bonded to cover plates using direct bonding techniques such as plasma bonding17,42 or with intermediate layers such as adhesive bonding by spin coating.56,57 This provides an advantage over the majority of previously developed mold manufacturing techniques13 that are limited to the surface finish resulting from the microfabrication technique.
Abrupt transitions in the height for the fabrication of 3D molds are made possible by machining stacked sheets of different thicknesses. The range and number of different heights of the structures is virtually unlimited since steel sheets are readily available off-the-shelf in thicknesses between 50 μm and several mm or more. More gradual changes in height can be made by abrasive jet milling the structures after they have been cut or by milling the sheets themselves before they are cut. The structure width is mostly limited by the stiffness and strength of the sheets, although this may be partially overcome in the future by using a different (ferromagnetic) sheet material. As we show below, for the mild steel sheets used in this study, minimum widths of 100–260 μm are easily achievable. Thinner structures are likely possible using a stiffer and stronger material.
In Secs. II C–II E, we demonstrate the technique for a particular 3D microfluidic geometry that is difficult to achieve using traditional soft lithography-based techniques.
C. Fabrication of mold for 3D flow focusing device
The proof-of-concept chip design used to demonstrate our mold fabrication technique is based on the 3D flow focusing design of Chiu et al.37 Figure 1 shows a schematic of the steps required. We used AWJM to cut stacked low carbon steel sheets of two different thicknesses (760 and 100 μm) into thin intersecting micro-features that, when cast, represent an intersecting channel network with the main inlet channel at a shallower depth that meets two sheath flow inlet channels and the outlet channel at the junction. The top sheet has a cut out window to allow access for machining the lower sheet, and the two sheets are bonded together using a thin layer of spray glue (3M Canada Corp., London, Ontario, Canada) as shown in Fig. 1(a). We cut the sheets using AWJM to make the desired intersecting micro-features, as shown in Fig. 1(b). We will discuss the choice of optimal process parameters in this step in Sec. III A.
FIG. 1.
Schematic of mold fabrication of 3D device process using AWJM. (a) Spray glue bonding of a windowed thicker steel sheet to a thinner one, (b) AWJ cutting to create features representing deep channels and intersection (thick sheet) and shallower channels (thin sheet), (c) attachment of silicon backing plate using electromagnet, (d) pour PDMS onto mold, (e) peel PDMS from mold after curing, (f) punch inlets and trim it into a rectangle, (g) plasma bond PDMS to glass substrate, and (h) seal channel ends using PDMS.
As shown in Fig. 1(c), the resulting cut features are held firmly against a 100 μm thick smooth silicon backing plate (University Water Inc., Boston, MA, USA) using a surface-contact DC-powered electromagnet (24 V DC 5.6 W, Magnetech Corp. Novi, MI, USA) with a DC power supply (Model LPD 422A-FM, Lambda Electronics Corp., New York, NY, USA).
In the next stages of mold fabrication, shown in Fig. 1(d), we pour PDMS onto the mold and cure it, peel off the fabricated PDMS chip from the mold and trim it into a rectangle [Fig. 1(e)], punch inlet and outlet holes [Fig. 1(f)], and bond the PDMS to a glass slide following the methods that will be explained in Sec. II D [Fig. 1(g)]. In the final stage of the mold fabrication, we seal the open-ended channel ends by pouring PDMS over the back of the chip as shown in Fig. 1(h).
There are advantages that this mold fabrication technique provides over other methods,40,41 but the sealing step is an extra step not present in most fabrication techniques. However, the sealing step does not hamper the utilization of the device. After we pour PDMS on the channel ends, the PDMS will be cured and irreversibly bond into PDMS and glass slabs as shown in Fig. 1(h) and therefore seal the channel openings.
D. Device fabrication
We make microfluidic devices by pouring a 10:1 ratio of degassed mixture of PDMS 365 resin and curing agent (Sylgard 184, Dow-Corning, Midland, MI, USA) into the mold and curing at ambient temperature for 24 h. The flow focusing device is comprised of three inlets to introduce dispersed and continuous phases and an outlet. The PDMS device replicates the geometry of the mold with a step of 100 μm and a total height of 860 μm. After curing, we remove the PDMS slab from the mold, and we make inlet and outlet holes using a biopsy puncher (Integra Miltex, Inc., Rietheim-Weilheim, Germany). We use oxygen–plasma treatment (Harrick Plasma, Ithaca, NY, USA) to bond the PDMS slab to the glass slide.
E. Measurements
1. Mold and PDMS measurements
We measure the 3D profiles of the structures in the molds using a non-contact optical profilometer (model ST 400, Nanovea, Irvine, CA, USA) having a lateral and vertical resolution of 0.1 μm. We also use an optical microscope system (LEICA DM 2500M based Clemex DM Vision PE system, Clemex Technologies Inc., Quebec, Canada) to analyze the samples and to measure the width of the structures. We use scanning electron microscopy (SEM) (JSM-6380, JEOL, Tokyo, Japan) to characterize the fabricated PDMS chips.
2. Chip performance
We test the fabricated microfluidic devices to study their ability to focus the sample fluid, as will be explained in Sec. III C. We use syringe pumps (Harvard Apparatus Pump 11 Elite, Holliston, MA, USA) to infuse the sample fluid. A Nikon A1 inverted confocal laser scanning microscope (Nikon Instruments, Melville, NY, USA) is used to acquire 3D fluorescent images of the sample flow in the microchannel. After data acquisition from the confocal microscope, we use digital software (ImageJ software, http://rsb.info.nih.gov/ij/) to analyze the confocal tests results, to measure the centroid of the sample flow in the microchannel, and determine the degree of flow focusing. For each measurement, we sample the degree of flow focusing at five different locations downstream of the flow focusing junction.
F. Chemicals
In the flow focusing experiments, we use de-ionized (DI) water as a sheath fluid and the sample fluid is DI water with 1% (w/v) fluorescein (Sigma-Aldrich, St. Louis, MI, USA). The sample solution is passed through a syringe filter with 0.45 μm pore size (Corning Inc., NY, USA) to remove particulates and impurities prior to use.
In droplet formation experiments, in order to generate aqueous droplets inside the microfluidic device, we prepare two liquid phases: olive oil as the continuous phase and DI water as the dispersed phase. Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, St. Louis, MI, USA) is premixed in DI water with a concentration at ∼8mM to stabilize the droplets.
III. RESULTS AND DISCUSSION
A. Choice of machining parameters
The depth of the structures can be varied by changing the thickness of the machined sheets. As mentioned in Sec. II B, since steel sheets are available off-the-shelf in a wide range of heights, fabricating molds with a wide variety of differences in heights (channel depths) is possible. For the mold in Sec. II C, we use a step ratio—height of the tall structures on the mold divided by the height of the shorter structure—of ∼8. The ability to fabricate molds with large step ratios is another advantage of our technique, because such ratios are difficult to achieve using conventional photolithographic approaches developed for microfluidic fabrication.11,13 Although the availability of thick-layer photo-resist sheets makes it possible to fabricate deep channels for microfluidic chips made from SU-8,58 or as molds for PDMS casting,59 as mentioned in Sec. I, the fabrication of a stepped channel for 3D applications remains challenging because of the need for optical alignment during the patterning of the different thickness photo-resists.
The width of the cut structures depends on the choice of the AWJM machining parameters used to cut out the structures in the steel sheets [Fig. 1(b)]. Figure 2 shows a schematic of the AWJM nozzle movement path used to study the effect of nozzle traverse speed and offset values (distance between two adjacent AWJM nozzle passes) on the resulting structure width. Although these tests are conducted on a single plate (760 μm), which is used as the thicker plate for the device in Sec. II C, the trends are similar for thinner plates or for the stacked plates used to fabricate 3D structures. The top of the structure is generally narrower than the bottom (Fig. 3), consistent with many studies,54,55 and is attributed to both the conical jet shape and the loss of jet energy as it cuts through the thickness. In order to minimize this effect and achieve the highest possible material removal rate during cutting according to the findings of Azarsa et al.,53 we use a water jet pressure of 207 MPa and a relatively high abrasive mass flow rate of 75 g/min in our experiments.
FIG. 2.
Schematic of abrasive water jet machining for cutting thick mild steel sheet.
FIG. 3.
Top and bottom width of the structures in the thick mild steel sheet made using three different offsets at two different nozzle traverse speeds. Scatter bars are the standard deviation of 15 measurements from five different locations on three different molds made under identical conditions.
Figure 3 shows the effect of the offset size on the widths of the features at two different nozzle traverse speeds. We observe that the minimum possible feature top (channel bottom) and bottom (channel top) widths are ∼100 and 260 μm, respectively. Narrower features are also possible, but the features may be so fragile that they cannot be used as mold structures. It would be difficult to peel PDMS off such fragile mold structures. As will be explained in Sec. III C, the difficulty in fabricating narrow structures is one of the limitations of our novel technique.
At higher nozzle traverse speeds, the structures are wider for all offset sizes because of the reduced erosion associated with the lower delivered abrasive dose. Figure 3 is useful for choosing the correct offset size in order to achieve a desired structure width on the metal sheets. For the mold in Sec. II C, we choose an offset of 0.8 mm with 50 mm/min traverse speed from Fig. 3 in order to achieve channel widths of about 480 and 370 μm for the channel bottom and top, respectively.
B. Mold and resulting PDMS chip
Figure 4 shows a profilometer scan and a photograph of the mold of Sec. II C at the 3D flow focusing junction. Importantly, there is an ∼90° step from the shallow to deep sections of the microchannel. Images of the 3D PDMS chip can be seen in Fig. 4 with shallow and deep channel heights of 100 and 860 μm, respectively. Figure 5 also shows SEM images of the molded PDMS slab. Upstream of the flow focusing junction, the channel width is 380 μm, and downstream from the junction, the deeper channels are 480 μm wide. The difference in the widths occurs because the erosive footprint of the approximately conical jet changes with the standoff (distance from the nozzle to the machined sheets), which, in turn, depends on the sheet thickness.54,55 For applications requiring all channels to be of the same width, the standoff used while machining one side of the mold can be appropriately adjusted by the difference in sheet thicknesses.
FIG. 4.
(a) Three-dimensional profilometer scan and (b) image of the AWJ machined microfluidic mold. Notably, there is an ∼90° step from the shallower to the deeper channel.
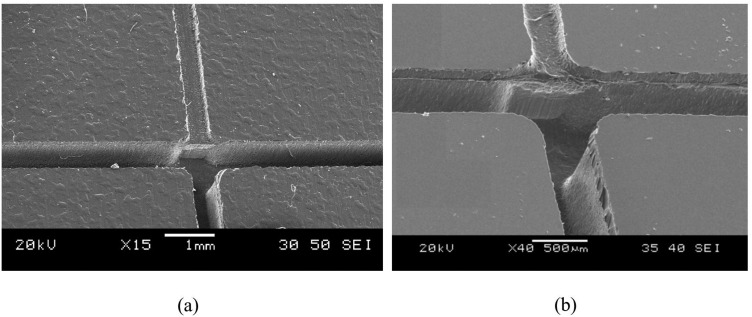
FIG. 5.
(a) SEM and (b) close-up SEM images of the molded PDMS slab, showing the step from the shallow upstream channel, to the deep downstream channels. The shallow and deep channels have depths of 100 and 860 μm, respectively.
C. Comparison with other LOC mold fabrication techniques
As discussed in Sec. I, there are numerous direct machining and mold fabrication techniques for microfluidic device fabrication, each with their advantages and disadvantages. Thus far, we have focused on comparison with lithography-based techniques as our benchmark cases because these approaches are the most commonly used.8,9 Our technique allows the fabrication of micro-molds suitable for mass production of 3D microfluidic devices. Other technologies that have been used in the past with some success for these purposes include laser machining, 3D printing, and micro-milling.
For indirect fabrication of molds using laser ablation techniques, laser energy is used to create raised structures on a substrate, which can be later used as molds for device fabrication. This makes it possible to create complex in-plane shapes on the mold such as helical or serpentine channels.36 This capability overcomes one limitation of our AWJM technique, where intersecting 3D stepped channels are possible, but cannot be curved. However, the laser machining technique has its own limitations. For example, laser ablation can be used to fabricate features with a maximum height of about 100–150 μm60,61 and features as narrow as ∼100 μm. Our AWJM technique can be used to create much taller features simply by using a thicker cut substrate, but AWJM and laser ablation have similar limitations in width. Molds made using indirect laser ablation have also been shown to have a relatively rough surface finish and create chips, which can suffer from edge swelling, debris, width fluctuation, bubbles, and micro-cracks.15 For the limited geometry tested in the present work, there were no such problems with AWJM.
3D printing for rapid prototyping (RP)62 is another promising technique for mold manufacture of LOC devices. Commercially available 3D printing techniques are divided mainly into five categories:62 (1) extrusion-based such as fused deposition modeling (FDM), (2) photocuring such as stereolithography apparatus (SLA), (3) photo-melting including SLS (selective laser sintering) or SLM (selective laser melting) techniques, (4) inkjet-based, and (5) paper cutting. These techniques make it possible to fabricate structures with a wide range of heights.8 Although 3D printing is most often used as a direct manufacturing technique, the first three techniques are also promising for the fabrication of microfluidic molds.63 For example, Macdonald et al.64 compared the three most common 3D printing technologies including FDM, Polyjet, and digital light processing stereolithography (DLP-SLA) in terms of their ability to fabricate molds for microfluidic devices. They reported that the minimum achievable widths using FDM, Polyjet, and DLP-SLA were 500, 200, and 100 μm, respectively. Using our technique, it is possible to create a minimum channel width of ∼100 μm, about the same as for DLP-SLA. Although the authors did not characterize how the roughness was measured, they found values of 10, 2, and 1 μm for FDM, Polyjet, and DLP-SLA, respectively, on the flat portions of the chips. In our experience, these relatively high roughness values can make it very difficult to bond a cover plate to close the chip using, e.g., oxygen–plasma bonding.
In general, extrusion-based printers such as FDM are very inexpensive compared to AWJM apparatuses, but the fabricated chips or molds have usually very low surface quality with high roughness values.28 As mentioned in Sec. I, for methods such as SLA, there are some limitations in the 3D printing of polymers including transparency problems,28 and only a limited range of resins can be used with this technique. The SLM technique can be used to make molds out of metals to fabricate PDMS chips for mass production, but it also may result in poor surface properties with high roughness and waviness, which, as mentioned above, can make it difficult to use oxygen–plasma bonding to close the fabricated chips, or may cause leakage problems.62 Furthermore, the capital cost is about as high as it is with AWJM. A major advantage of our technique is that, as mentioned in Sec. II C, it avoids any machining of the flat surfaces or tops of the raised mold features allowing for highly polished substrates to be used for the surfaces that need to be bonded. The only machined part in a microfluidic device manufactured by our presented technique is the channel sidewalls, cut with AWJM with roughness value of about 2 μm.
As discussed in Sec. I, it has been demonstrated that CNC micro-milling technologies make it possible to fabricate aluminum master molds for microfluidic devices with 3D curved channel geometries.22 Fabrication of curved channels is not possible using our technique; however, as discussed in Sec. I, the main disadvantages of CNC milling are tool alignment, tool breakage,11 and reduced surface quality compared with lithography-based techniques.1
The ranges of tested flow rates for 3D printed molded chips in the study of Eluru et al. are up to 2 ml/h,65 and our PDMS chips were tested up to about 200 ml/h. Because the geometry of our device is different than the flow focusing device of others such as Eluru et al.65 and Chiu et al.,37 we are not able to directly compare the microfluidic flow focusing performance. In general, the flow focusing performance will depend on the dimensions (discussed above) and roughness of the channels.4,5 As discussed above, using our technique, the flat surfaces and tops of the raised mold features are not machined; therefore, we can use highly polished substrates to define the replicated PDMS channel bottom roughness and the PDMS surface that will be bonded to the glass cover plate. Because published studies do not generally report channel sidewall roughness, we can only compare our channel wall roughness of ∼2 μm to the channel bottom roughness for the other techniques. This is done in Table I, which shows that the obtained roughnesses are comparable to other technologies.
TABLE I.
Comparison of roughness for various microfabrication technologies.
| Manufacturing technique | Surface roughness (μm) | Channel roughnessa (μm) |
|---|---|---|
| Present technique | Defined by substrate that can be highly polished | 2 |
| 3D printing of stainless steel molds26 | 3.85 | 3.85 |
| 3D printing (FDM, Polyjet, DLP-SLA) by Macdonald et al.64 | 1–10 | 1–10 |
| 3D printing omni phobic-lubricant-infused molds (OLIMs) | 0.2 | N/A |
| 3D printing of molds from photopolymers for PDMS casting28 | 1.9–3.6 | 1.9–3.6 |
| Micro-milling of PMMA molds66 | 2.5 | 2.5 |
| Laser ablation of PDMS molds18 | 1 | 5 |
| Paraffin based polymer molding27 | 1 | 1 |
| Xurography: rapid prototyping using a cutting plotter16 | 2 | N/A |
Channel roughness refers to channel bottom for all techniques except present which refers to the channel sidewall. N/A indicates that quantity was not measured in study.
In summary, although there are other microfabrication technologies that show promise for the creation of microfluidic molds with 3D features, they almost always involve surface material removal or addition, which can cause deleterious changes in surface quality. Our AWJM technique is designed such that the backing plate is not machined, thus avoiding these problems. Moreover, AWJM can be used to easily create sharply stepped geometries allowing for 3D microfluidic features. Our technique, however, presently has some limitations including a nonstandard step required for sealing the channel ends, a maximum feature width of ∼100 μm, and difficulty in fabricating curved or more complex channel geometries. In general, when compared with the high costs associated with cleanroom facilities, for relatively simple in-plane channel geometries that, however, require abrupt changes in depth, the technique is a very promising potential alternative to conventional soft lithography and other competing technologies.
D. Flow focusing device test
As explained in Sec. I, fabrication of PDMS devices for 3D flow focusing applications relying on conventional techniques such as multilayer soft lithography67 or photolithography,40,41 is difficult since different channel heights are required. While 2D flow focusing is quite ubiquitous in microfluidics,68,69 3D flow focusing applications often require sophisticated manufacturing procedures. For example, Gnyawali et al. used a suspended needle manually aligned with a gap from the bottom of the channel, and guided through the sample inlet to the cross-junction of PDMS chip (cast from a metal mold), to create a 3D flow focusing device.70–73
Flow focusing microfluidic devices can be used to fabricate liposomes, which have a wide range of delivery applications, such as enhancing the performance of enzymes in cosmetics, pharmaceuticals, target-specific drug release, and chemical reaction chambers.74 In addition to flow cytometry,70 focused flows are also useful in applications such as drug discovery and delivery, deoxyribonucleic acid (DNA)-stretching, reagent mixing in microfluidics,71 and the formation of protein microfibers.75
As explained in Sec. II C and shown in Fig. 1, we exploit AWJM to make PDMS-based microfluidic devices with a 3D flow focusing junction in a fast and inexpensive way. This technique enables the fabrication of micro-scaled 3D features on metal sheets that allows molds for PDMS-based features such as PDMS-based microfluidic chips. As explained in Sec. II C, it is possible to achieve any desired surface finish and step ratios without using complex fabrication and manufacturing techniques, or expensive facilities such as a cleanroom. Our approach also does not need any post-processing or alignment. In the rest of this section, we test the proof-of-concept 3D flow focusing device by introducing a fluorescently labeled aqueous solution through the sample fluid channel and DI water through the side sheath fluid channels, and we quantify the degree of 3D flow focusing by confocal microscopy.
Figure 6 represents a schematic of the flow focusing experiment. When the two phases reach the junction, the sheath fluid pushes the sample fluid to the lateral center of the channel, and to the vertical bottom of the channel, toward the glass slide (bottom channel), as shown in Fig. 6(b). This happens because the PDMS chip possesses an approximately right-angle sharp step at the junction, and the depth variation in the channels enables the sheath fluid to push the sample fluid to the bottom of the channel while the sheath fluid stays on the top. Similar to conventional 2D flow focusing systems, here, the sheath flow also pushes the sample flow to the lateral center of the microchannel.
FIG. 6.
Schematic image of confocal microscopy experiments: (a) Isometric view of the chip and (b) section view of the deep channel in xz plane representing vertically and laterally focused flow downstream of the deep channel.
As explained in Sec. II E, we use confocal microscopy to quantify the degree of flow focusing of the sample fluid by the sheath fluid. We characterize the degree of vertical focusing of the sample fluid by measuring the centroid of the fluorescently labeled sample fluid using ImageJ software. The sample centroid and the bottom of the channel are separated by a distance D1. To quantify the degree of flow focus, we use a tightness measure (TM),65
| (1) |
where Z is the distance between the channel centroid and the bottom of the channel shown in Fig. 6.
Figures 7(a)–7(c) show confocal cross-sectional view images of the experiment performed at a sample fluid flow rate of 2 ml/h, and sheath flow rates of 3, 18, and 36 ml/h, respectively. It shows that an increase in the sheath flow rate from 3 to 36 ml/h causes the sample fluid to focus and push toward the bottom of the channel. Figure 8 indicates that due to this increase in the sheath flow rate, the tightness measure percentage decreased from 35% to 10%. A decrease in this measure is a clear indication of the increase in the ability of the chip to focus the sample fluid in the vertical direction, similar to previously developed chips for cytometry applications.65,71 At higher sample fluid flow rates, such as 6 ml/h, a higher sheath flow rate is required to reach to the same TM%, as can be seen in Fig. 8. This indicates that a tighter focusing or a lower TM% along the depth direction can be obtained by choosing either a higher sheath fluid flow rate or lower sample fluid flow rate. Further increases in the sheath flow rate at a sample flow rate of 6 ml/h can result in the sample flow splitting, as in Figs. 7(d) and 7(e). The view from the bottom of the channel at these experimental parameters can be observed in Fig. 7(f), showing that the sample flow is divided into two separate streams. The same effect of increasing the sheath flow on splitting the sample core flow is also observed for a core flow rate of 18 ml/h. The same phenomenon was also reported by Azarmanesh et al.,76 who attributed it to the high shear stress at the junction at higher sheath flow rates. This high shear stress creates a stagnation point that splits the core flow to two jet streams each of which are surrounded by the second phase (sheath flow).
FIG. 7.
(a)–(e) show cross-sectional images of confocal microscopy using the sample and sheath flows of, respectively, (a) 2 and 3 ml/h, (b) 2 and 18 ml/h, (c) 2 and 36 ml/h, (d) 6 and 72 ml/h, and (e) 6 and 108 ml/h. (f) Bottom view of experiment shown in (e). Scale bars represent 200 μm.
FIG. 8.
Tightness measurements at three different sample flow rates at various sheath flow rates. Scatter bars are standard deviation of five measurements for different locations along downstream of the main channel.
E. Droplet formation
Droplet microfluidics is an emerging technology with a wide range of applications in diagnostics, proteomics, pharmaceutical and drug discovery, and synthetic biology.77 Water-in-oil and oil-in-water two-phase systems have been extensively used to produce discrete monodisperse droplets.78 These systems typically employ hydrodynamically controlled flow focusing geometries to generate droplets by exploiting the Rayleigh–Plateau instability of a central liquid jet within a continuous outer phase fluid.79
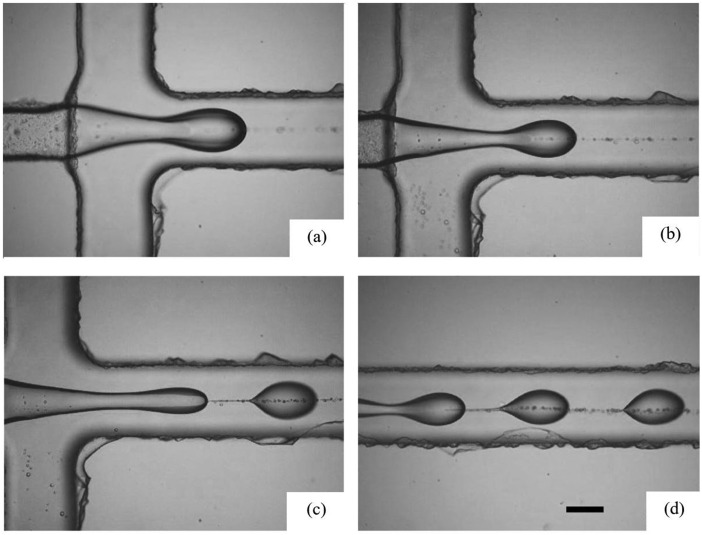
As a further demonstration of the utility of the microfluidic geometry fabricated using our abrasive water jet machined mold, we conduct a series of experiments to generate microdroplets. For these experiments, we introduce the oil phase as the continuous phase with flow rates of 8, 14, 16, and 24 ml/h, and the aqueous phase as the dispersed phase with flow rates of 1, 2, 3, and 4 ml/h. The geometry of our flow focusing device facilitates the fabrication of 300–400 μm water-in-oil droplets, as shown in Fig. 9 (Multimedia view).
FIG. 9.
IV. CONCLUSIONS
In this study, we present a new technique for mold fabrication that can be used to make PDMS microfluidic devices. The technique utilizes water jet machining technology to fabricate free-standing structures on readily available off-the-shelf steel sheets. The sheets are held together and against a backing substrate using an electromagnet to make a mold for PDMS casting. Since substrates are available at low-cost in a wide variety of surface finishes, a variety of cover plate bonding techniques can be used. This provides an advantage over most previously developed mold manufacturing techniques that are limited to the surface finish resulting from the particular microfabrication technique that was used. We show that this technique makes it possible to fabricate 3D PDMS chips with features having a wide range of depths, from 50 μm up to several millimeters and widths as small as ∼200 μm.
To demonstrate the technique, we fabricate a mold for a 3D flow focusing microchannel geometry and demonstrate its capability in two applications: 3D sample flow focusing and the generation of water-in-oil droplets. These applications are made possible by the patterned PDMS slab, which features an abrupt, nearly stepwise, change in the depth of the microchannel junction.
Our experiments and quantitative measurements by confocal microscopy clearly show that the fabricated chips can perform both 3D sample flow focusing and generate water-in-oil droplets. We also show that a tighter focusing, or a lower TM%, along the depth direction can be obtained by choosing either a higher sheath fluid flow rate or higher sample fluid flow rate.
Although this alternative approach to create microfluidic molds has limitations in feature width and cannot be used to create curved channels, it is nonetheless quite versatile and may find utility in reducing the cost and complexity involved in fabricating 3D features in microfluidic devices. With these limitations in mind, we anticipate that this low cost and simple fabrication method will be useful for many laboratories to create microfluidic systems, without the need for complex soft lithography facilities.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support of the Natural Sciences and Engineering Research Council of Canada (NSERC) (No. RGPIN-2019-04633) and Ontario Graduate Scholarships.
DATA AVAILABILITY
The data that support the findings of this study are available within the article or from the corresponding author upon the reasonable request.
REFERENCES
- 1.Guckenberger D. J., de Groot T. E., Wan A. M. D., Beebe D. J., and Young E. W. K., Lab Chip 15, 2364 (2015). 10.1039/C5LC00234F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald J. C., Duffy D. C., Anderson J. R., Chiu D. T., Wu H., Schueller O. J. A., and Whitesides G. M., Electrophoresis 21, 27 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Fujii T., Microelectron. Eng. 61–62, 907 (2002). 10.1016/S0167-9317(02)00494-X [DOI] [Google Scholar]
- 4.Becker H. and Locascio L. E., Talanta 56, 267 (2002). 10.1016/S0039-9140(01)00594-X [DOI] [PubMed] [Google Scholar]
- 5.Zhou J., Ellis A. V., and Voelcker N. H., Electrophoresis 31, 2 (2010). 10.1002/elps.200900475 [DOI] [PubMed] [Google Scholar]
- 6.Mata A., Fleischman A. J., and Roy S., Biomed. Microdevices 7, 281 (2005). 10.1007/s10544-005-6070-2 [DOI] [PubMed] [Google Scholar]
- 7.McDonald J. C. and Whitesides G. M., Acc. Chem. Res. 35, 491 (2002). 10.1021/ar010110q [DOI] [PubMed] [Google Scholar]
- 8.Kamei K., Mashimo Y., Koyama Y., Fockenberg C., Nakashima M., Nakajima M., Li J., and Chen Y., Biomed. Microdevices 17, 36 (2015). 10.1007/s10544-015-9928-y [DOI] [PubMed] [Google Scholar]
- 9.Kim P., Kwon K. W., Park M. C., Lee S. H., Kim S. M., and Suh K. Y., Biochip J. 2, 1–11 (2008). [Google Scholar]
- 10.Jo B.-H. and Beebe D. J., Proc. SPIE 3877 (1999). [Google Scholar]
- 11.Faustino V., Catarino S. O., Lima R., and Minas G., J. Biomech. 49, 2280 (2016). 10.1016/j.jbiomech.2015.11.031 [DOI] [PubMed] [Google Scholar]
- 12.Bukatin A. S., Mukhin I. S., Malyshev E. I., Kukhtevich I. V., Evstrapov A. A., and Dubina M. V., Tech. Phys. 61, 1566 (2016). 10.1134/S106378421610008X [DOI] [Google Scholar]
- 13.Gale B. K., Jafek A. R., Lambert C. J., Goenner B. L., Moghimifam H., Nze U. C., and Kamarapu S. K., Inventions 3, 60 (2018). 10.3390/inventions3030060 [DOI] [Google Scholar]
- 14.Wong T. I., Limantoro J., Fong K. P., Tan C. Y. L., Quan C., Sun L. L., and Zhou X., J. Micromech. Microeng. 26, 065016 (2016). 10.1088/0960-1317/26/6/065016 [DOI] [Google Scholar]
- 15.Joanni E., Peressinotto J., Domingues P. S., Setti G. d. O., and de Jesus D. P., RSC Adv. 5, 25089 (2015). 10.1039/C5RA03122B [DOI] [Google Scholar]
- 16.Bartholomeusz D. A., Boutte R. W., and Andrade J. D., J. Microelectromech. Syst. 14, 1364 (2005). 10.1109/JMEMS.2005.859087 [DOI] [Google Scholar]
- 17.Shiu P. P., Knopf G. K., Ostojic M., and Nikumb S., J. Micromech. Microeng. 18, 025012 (2008). 10.1088/0960-1317/18/2/025012 [DOI] [Google Scholar]
- 18.Isiksacan Z., Guler M. T., Aydogdu B., Bilican I., and Elbuken C., J. Micromech. Microeng. 26, 035008 (2016). 10.1088/0960-1317/26/3/035008 [DOI] [Google Scholar]
- 19.da Costa E. T., Santos M. F. S., Jiao H., do Lago C. L., Gutz I. G. R., and Garcia C. D., Electrophoresis 37, 1691 (2016). 10.1002/elps.201600065 [DOI] [PubMed] [Google Scholar]
- 20.Saez J., Basabe-Desmonts L., and Benito-Lopez F., Microfluid. Nanofluid. 20, 116 (2016). 10.1007/s10404-016-1781-7 [DOI] [Google Scholar]
- 21.Koesdjojo M. T., Tennico Y. H., Rundel J. T., and Remcho V. T., Sens. Actuators B Chem. 131, 692 (2008). 10.1016/j.snb.2008.01.008 [DOI] [Google Scholar]
- 22.Wilson M. E., Kota N., Kim Y., Wang Y., Stolz D. B., LeDuc P. R., and Ozdoganlar O. B., Lab Chip 11, 1550 (2011). 10.1039/c0lc00561d [DOI] [PubMed] [Google Scholar]
- 23.Morbioli G. G., Speller N. C., Cato M. E., Cantrell T. P., and Stockton A. M., Sens. Actuators B Chem. 284, 650 (2019). 10.1016/j.snb.2018.12.053 [DOI] [Google Scholar]
- 24.Micheal I. J., Vidyasagar A. J., Bokara K. K., Mekala N. K., Asthana A., and Rao C. M., Lab Chip 14, 3695 (2014). 10.1039/C4LC00659C [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Hou L., Zhang W., and Zhu L., Anal. Methods 6, 4716 (2014). 10.1039/C4AY00798K [DOI] [Google Scholar]
- 26.Lin T.-Y., Do T., Kwon P., and Lillehoj P. B., Lab Chip 17, 241 (2017). 10.1039/C6LC01430E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogi K., Sakata K., Hashimoto Y., and Yamamoto T., Materials 9, 621 (2016). 10.3390/ma9080621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang Y., Paydar O. H., and Candler R. N., Sens. Actuators A Phys. 226, 137 (2015). 10.1016/j.sna.2015.02.028 [DOI] [Google Scholar]
- 29.Comina G., Suska A., and Filippini D., Lab Chip 14, 424 (2013). 10.1039/C3LC50956G [DOI] [PubMed] [Google Scholar]
- 30.Lynh H. D. and Pin-Chuan C., Sens. Actuators A Phys. 280, 350 (2018). 10.1016/j.sna.2018.08.002 [DOI] [Google Scholar]
- 31.Attia U. M., Marson S., and Alcock J. R., Microfluid. Nanofluid. 7, 1 (2009). 10.1007/s10404-009-0421-x [DOI] [Google Scholar]
- 32.Jena R. K., Dev K., Yue C. Y., and Asundi A., RSC Adv. 2, 5717 (2012). 10.1039/c2ra20159c [DOI] [Google Scholar]
- 33.Wang L., Liu W., Li S., Liu T., Yan X., Shi Y., Cheng Z., and Chen C., Microsyst. Technol. 22, 677 (2016). 10.1007/s00542-015-2465-z [DOI] [Google Scholar]
- 34.Hoyland J., Kunstmann-Olsen C., and Rubahn H.-G., Microelectron. Eng. 98, 689 (2012). 10.1016/j.mee.2012.05.053 [DOI] [Google Scholar]
- 35.Lee M. G., Choi S., and Park J.-K., Lab Chip 9, 3155 (2009). 10.1039/b910712f [DOI] [PubMed] [Google Scholar]
- 36.Lee C.-Y., Chang C.-L., Wang Y.-N., and Fu L.-M., Int. J. Mol. Sci. 12, 3263 (2011). 10.3390/ijms12053263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu Y.-J., Hwan Cho S., Mei Z., Lien V., Wu T.-F., and Lo Y.-H., Lab Chip 13, 1803 (2013). 10.1039/c3lc41202d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang R., Feeback D. L., and Wang W., Sens. Actuators A Phys. 118, 259 (2005). 10.1016/j.sna.2004.09.001 [DOI] [Google Scholar]
- 39.Sundararajan N., Pio M. S., Lee L. P., and Berlin A. A., J. Microelectromech. Syst. 13, 559 (2004). 10.1109/JMEMS.2004.832196 [DOI] [Google Scholar]
- 40.Hongbin Y., Guangya Z., Siong C. F., Shouhua W., and Feiwen L., Sens. Actuators B Chem. 137, 754 (2009). 10.1016/j.snb.2008.11.035 [DOI] [Google Scholar]
- 41.Wang C.-K., Liao W.-H., Wu H.-M., Lo Y.-H., Lin T.-R., and Tung Y.-C., J. Micromech. Microeng. 27, 115003 (2017). 10.1088/1361-6439/aa874d [DOI] [Google Scholar]
- 42.Novak R., Ranu N., and Mathies R. A., Lab Chip 13, 1468 (2013). 10.1039/c3lc41362d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan H. N., Chen Y., Shu Y., Chen Y., Tian Q., and Wu H., Microfluid. Nanofluid. 19, 9 (2015). 10.1007/s10404-014-1542-4 [DOI] [Google Scholar]
- 44.Yun D. J., Seo T. I., and Park D. S., Sensors 8, 1308 (2008). 10.3390/s8021308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park D.-S., Cho M.-W., Lee H., and Cho W.-S., J. Mater. Process. Technol. 146, 234 (2004). 10.1016/j.jmatprotec.2003.11.013 [DOI] [Google Scholar]
- 46.See https://www.micronit.com/technologies/capabilities/etching.html for DRIE etching in technologies.
- 47.Ghobeity A., Crabtree H. J., Papini M., and Spelt J. K., J. Micromech. Microeng. 22, 025014 (2012). 10.1088/0960-1317/22/2/025014 [DOI] [Google Scholar]
- 48.Ghobeity A., Spelt J. K., and Papini M., J. Micromech. Microeng. 18, 055014 (2008). 10.1088/0960-1317/18/5/055014 [DOI] [Google Scholar]
- 49.Shafagh S. and Papini M., Precis. Eng. 62, 162 (2020). 10.1016/j.precisioneng.2019.12.001 [DOI] [Google Scholar]
- 50.Wensink H., Schlautmann S., Goedbloed M. H., and Elwenspoek M. C., J. Micromech. Microeng. 12, 616 (2002). 10.1088/0960-1317/12/5/316 [DOI] [Google Scholar]
- 51.Liu P., J. Manuf. Mater. Process. 1(1), 1 (2017). [Google Scholar]
- 52.Kartal F., Int. J. Adv. Manuf. Technol. 88, 495–505 (2017). 10.1007/s00170-016-8777-z [DOI] [Google Scholar]
- 53.Azarsa E., Ibrahim A., and Papini M., Precis. Eng. 65, 197–215 (2020). 10.1016/j.precisioneng.2020.05.009 [DOI] [Google Scholar]
- 54.Azarsa E., Cinco L., and Papini M., J. Mater. Process. Technol. 275, 116318 (2020). 10.1016/j.jmatprotec.2019.116318 [DOI] [Google Scholar]
- 55.Haghbin N., Spelt J. K., and Papini M., Int. J. Mach. Tools Manuf. 88, 108 (2015). 10.1016/j.ijmachtools.2014.09.012 [DOI] [Google Scholar]
- 56.Satyanarayana S., Karnik R. N., and Majumdar A., J. Microelectromech. Syst. 14, 392 (2005). 10.1109/JMEMS.2004.839334 [DOI] [Google Scholar]
- 57.Eddings M. A., Johnson M. A., and Gale B. K., J. Micromech. Microeng. 18, 067001 (2008). 10.1088/0960-1317/18/6/067001 [DOI] [Google Scholar]
- 58.Lin C.-H., Lee G.-B., Chang B.-W., and Chang G.-L., J. Micromech. Microeng. 12, 590 (2002). 10.1088/0960-1317/12/5/312 [DOI] [Google Scholar]
- 59.Oh S. R., J. Micromech. Microeng. 18, 115025 (2008). 10.1088/0960-1317/18/11/115025 [DOI] [Google Scholar]
- 60.Suriano R., Kuznetsov A., Eaton S. M., Kiyan R., Cerullo G., Osellame R., Chichkov B. N., Levi M., and Turri S., Appl. Surf. Sci. 257, 6243 (2011). 10.1016/j.apsusc.2011.02.053 [DOI] [Google Scholar]
- 61.Antończak A. J., Stępak B. D., and Abramski K. M., J. Micromech. Microeng. 25(10), 107001 (2015). 10.1088/0960-1317/25/10/107001 [DOI] [Google Scholar]
- 62.He Y., Wu Y., Fu J., Gao Q., and Qiu J., Electroanalysis 28, 1658 (2016). 10.1002/elan.201600043 [DOI] [Google Scholar]
- 63.Bhattacharjee N., Urrios A., Kang S., and Folch A., Lab Chip 16, 1720 (2016). 10.1039/C6LC00163G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macdonald N. P., Cabot J. M., Smejkal P., Guijt R. M., Paull B., and Breadmore M. C., Anal. Chem. 89, 3858 (2017). 10.1021/acs.analchem.7b00136 [DOI] [PubMed] [Google Scholar]
- 65.Eluru G., Julius L. A. N., and Gorthi S. S., Lab Chip 16, 4133 (2016). 10.1039/C6LC00935B [DOI] [PubMed] [Google Scholar]
- 66.Zhou Z., Chen D., Wang X., and Jiang J., Micromachines 8, 287 (2017). 10.3390/mi8100287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim J.-M., Bertrand N., Valencia P. M., Rhee M., Langer R., Jon S., Farokhzad O. C., and Karnik R., Nanomed. Nanotechnol. Biol. Med. 10, 401 (2014). 10.1016/j.nano.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng L., Yang M., Guo S., Liu W., and Zhao X., Biomed. Microdevices 13, 559 (2011). 10.1007/s10544-011-9526-6 [DOI] [PubMed] [Google Scholar]
- 69.Anna S. L. and Mayer H. C., Phys. Fluids 18, 121512 (2006). 10.1063/1.2397023 [DOI] [Google Scholar]
- 70.Strohm E. M., Gnyawali V., Sebastian J. A., Ngunjiri R., Moore M. J., Tsai S. S. H., and Kolios M. C., Sci. Rep. 9, 4775 (2019). 10.1038/s41598-019-40895-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gnyawali V., Saremi M., Kolios M. C., and Tsai S. S. H., Biomicrofluidics 11, 034104 (2017). 10.1063/1.4983147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gnyawali V., Strohm E. M., Wang J.-Z., Tsai S. S. H., and Kolios M. C., Sci. Rep. 9, 1585 (2019). 10.1038/s41598-018-37771-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeyhani M., Gnyawali V., Abbasi N., Hwang D. K., and Tsai S. S. H., J. Colloid Interface Sci. 553, 382 (2019). 10.1016/j.jcis.2019.05.100 [DOI] [PubMed] [Google Scholar]
- 74.Davies R. T., Kim D., and Park J., J. Micromech. Microeng. 22, 055003 (2012). 10.1088/0960-1317/22/5/055003 [DOI] [Google Scholar]
- 75.Kamada A., Mittal N., Söderberg L. D., Ingverud T., Ohm W., Roth S. V., Lundell F., and Lendel C., Proc. Natl. Acad. Sci. U.S.A. 114, 1232 (2017). 10.1073/pnas.1617260114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azarmanesh M., Bawazeer S., Mohamad A. A., and Sanati-Nezhad A., Sci. Rep. 9, 1 (2019). 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schaerli Y. and Hollfelder F., Mol. BioSyst. 5, 1392 (2009). 10.1039/b907578j [DOI] [PubMed] [Google Scholar]
- 78.Teh S.-Y., Lin R., Hung L.-H., and Lee A. P., Lab Chip 8, 198 (2008). 10.1039/b715524g [DOI] [PubMed] [Google Scholar]
- 79.Jeyhani M., Thevakumaran R., Abbasi N., Hwang D. K., and Tsai S. S. H., Small 16, 1906565 (2020). 10.1002/smll.201906565 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available within the article or from the corresponding author upon the reasonable request.