Lower respiratory tract infection (LRTI) is a leading cause of death in the United States and the most common infection identified in patients admitted to the intensive care unit (ICU).1,2 This burden will only increase as the population ages.3
The diagnosis and treatment of LRTIs including community-acquired pneumonia (CAP) has focused traditionally on bacterial pathogens.4 Enthusiasm for the study of respiratory viral pathogens in severe respiratory illness has been tempered in the past by cumbersome diagnostic techniques and limited pharmacologic therapies. However, as pneumonia epidemiology and diagnostic platforms evolve, this focus has begun to change. The success of childhood vaccination programs and the aging of the US population have altered the landscape of severe respiratory infection.
Invasive pneumococcal disease has declined dramatically and viral pathogens that particularly impact the elderly are now recognized as common causal pathogens in severe disease.5,6 Concurrently, the widespread use of nucleic acid amplification testing has markedly improved the detection of viral pathogens.7
This review focuses on the importance of respiratory viral pathogens in the pathogenesis of severe respiratory infections with a particular emphasis on community-acquired infections. Given widespread knowledge of influenza’s important role in severe respiratory infections, we will focus on the noninfluenza viruses rhinovirus, human adenovirus (HAdV), respiratory syncytial virus (RSV), and human metapneumovirus (hMPV; Table 1).8
Table 1.
Characteristics of common noninfluenza respiratory viral pathogens
| Virus | Structure | Peak Infectivity | Notable Groups at Risk | Notable Features | Preferred Diagnostic Test | Investigational Therapies |
|---|---|---|---|---|---|---|
| Rhinovirus | Single-stranded negative-sense RNA virus | Late spring and early fall | • Immunocompromised patients • Patients with COPD |
• Common cause of asthma exacerbations in children • Most common pathogen isolated in CDC EPIC study |
RT-PCR | Pegylated interferon-α2A + ribavirin |
| Human adenovirus | Nonenveloped double-stranded DNA virus | No seasonal peak | • Immunocompromised patients • Adults in crowded living environments including military barracks and long-term care facilities |
• HAdV-14 linked to outbreaks of severe respiratory infection in the US • HAdV-55 an important cause of CAP in China |
PCR | Cidofivir |
| Respiratory syncytial virus | Enveloped negative-sense single-stranded RNA virus | December to February | • Immunocompromised patients • Elderly patients • Patients with COPD |
• Most common cause of LRTIs in children • Commonly presents with wheezing |
RT-PCR | • Ribavirin ± IVIG • Viral replication inhibitor ALS-008176 |
| Human metapneumovirus | Single-stranded negative-sense RNA virus | Winter to spring | • Immunocompromised patients • Residents of long-term care facilities • Patients with COPD |
• Commonly presents with wheezing | RT-PCR | Ribavirin + IVIG |
Abbreviations: CAP, community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; EPIC, Etiology of Pneumonia in the Community; IVIG, intravenous immunoglobulin; LRTI, lower respiratory tract infection; PCR, polymerase chain reaction; RT-PCR, reverse-transcriptase polymerase chain reaction.
THE EVOLVING EPIDEMIOLOGY OF SEVERE RESPIRATORY INFECTIONS
As the US population ages, the number of homebound elderly, patients discharged to long-term care facilities, and adults with chronic medical conditions has increased.3,9,10 It is, therefore, not surprising that the number of elderly patients admitted to the hospital with pneumonia is increasing. In 1 study, hospitalizations for pneumonia in patients 65 years of age or older increased by 20% over a 15-year period with an 11% increase in the number of patients with chronic cardiac or pulmonary disease.3 Elderly and functionally limited adults are particularly prone to severe viral infection.11 The incidence of rhinovirus infection in patients 65 years of age or older is 10 times higher than in younger adults; likewise, the majority of deaths attributable to RSV infection occur in patients older than 65 years of age.12,13 Outbreaks of severe viral infections at long-term care facilities are common for numerous respiratory viral pathogens.14–16
As the number of adults susceptible to severe viral infections has increased, the incidence of invasive bacterial pneumonia has decreased owing to widespread pneumococcal vaccination, increased awareness of the importance of early antimicrobial therapy, and decreased rates of cigarette smoking. In 1 study, the incidence of invasive pneumococcal disease decreased by almost 30% over a 5-year period in adults greater than 50 years of age.17 This shift in CAP pathogenesis may in part explain why the percentage of pneumonia hospitalizations with no reported pathogen increased by almost 20% from 1993 to 2011 despite improvements in diagnostic testing.18
Concurrently, our ability to diagnose viral infections rapidly and accurately has improved. Conventional diagnostic tests for respiratory viral pathogens include viral culture, acute and convalescent phase viral serologies, and direct fluorescence antibody staining. These methods are limited by slow turnaround time and limited sensitivity.19 Nucleic acid amplification testing with the use of polymerase chain reaction (PCR) platforms has greatly improved the diagnosis of respiratory viral infections. The sensitivity of PCR testing is up to 5 times higher than conventional diagnostic methods, which may be particularly important in elderly patients who shed lower titers of virus.20–23 PCR can also aid with viral subtyping and quantification of viral burden. Multiplex assays are now available, which allow for the testing of up to 19 viruses simultaneously.19 Numerous clinical samples can be used for PCR testing including nasopharyngeal swabs, tracheal aspirates, bronchoalveolar lavage fluid, and pleural fluid.
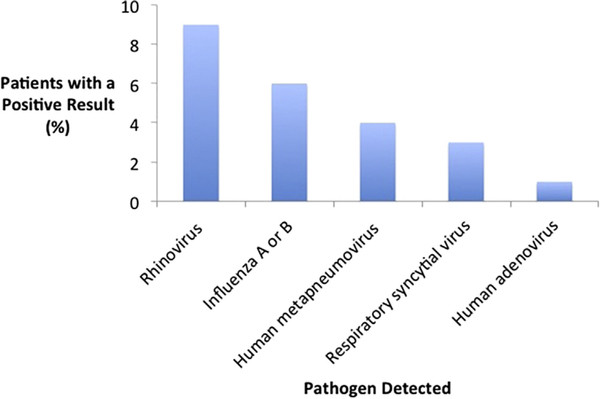
The widespread use of PCR-based testing has allowed for a more accurate assessment of the role respiratory viral pathogens play in severe disease. In studies of hospitalized patients with CAP, between 15% and 35% have evidence of a viral infection.21,24–27 This was best illustrated in the recent Centers for Disease Control and Prevention (CDC) EPIC study (Etiology of Pneumonia in the Community), a multicenter population-based surveillance study conducted in the United States, which used rigorous microbiologic testing in 2259 hospitalized adults with CAP.12 Viruses were the most common type of pathogen isolated, found in 23% of patients compared with just 11% of patients with bacterial pathogens (Fig. 1).
Fig. 1.
Percentage of all adults in the Centers for Disease Control and Prevention EPIC (Etiology of Pneumonia in the Community) study in whom specific respiratory viral pathogens were detected. (Data from Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373(5):415–27.)
Viral pathogens are also frequently isolated in patients with severe CAP requiring ICU admission. In a single-site study from Korea, viral pathogens were isolated by reverse transcription PCR (RT-PCR) from nasopharyngeal swabs or lavage fluid in 72 of 198 (36%) patients with severe CAP or health care-associated pneumonia.28 Viral detection rates in similar studies of ICU patients have ranged from 16% to 41%.29–31
Studies have also found respiratory viral pathogens present in over 20% of patients with hospital-acquired pneumonia (HAP)32,33 and between 14% and 29% of patients undergoing bronchoalveolar lavage for suspected infection.22,34
As our understanding of the importance of respiratory viral pathogens in the pathogenesis of severe respiratory infection continues to evolve, it is important for clinicians to be familiar with the unique characteristics of the most commonly identified pathogens.
RHINOVIRUS
Rhinoviruses are singe-stranded negative-sense RNA viruses that are divided into 3 species (rhinovirus-A, -B, -C) and more than 160 distinct serotypes.35 Rhinovirus infections occur throughout the year with increased prevalence noted in the late spring and early fall.36 Transmission occurs most commonly through autoinoculation after contact with contaminated objects, although aerosolization also contributes to viral spread.37 Nosocomial outbreaks of rhinovirus have been reported and highlight the importance of infection control protocols when caring for infected patients.38
The clinical importance of rhinovirus is well described in children where it may be responsible for more than 70% of asthma exacerbations in children greater than 2 years of age.39 Infection with rhinovirus early in childhood has been linked to asthma pathogenesis, particularly in children with a genetic predisposition to the disease.40,41 Rhinovirus is also recognized as an important cause of pediatric CAP.42
Rhinovirus Infection in Adults
In immunocompetent adults, rhinovirus most commonly causes a self-limited upper respiratory tract infection (URI) and may be responsible for more than 80% of common colds during the fall and spring.43 The frequent association with benign URIs has led many clinicians to question its relevance to pneumonia. However, rather than simply a precursor to more serious infections, rhinovirus can itself be an important pathogen. In the clearest example, immunocompromised patients are particularly prone to severe rhinovirus infection. Infection after lung transplantation is common and may contribute to graft dysfunction.44 Rhinovirus is also a common cause of severe LRTIs in adults with hematologic malignancies, commonly in association with bacterial coinfection.45–47
In patients with chronic obstructive pulmonary disease (COPD), rhinovirus is an important cause of exacerbations. In a study of 77 patients with COPD and frequent exacerbations, rhinovirus prevalence and viral load, measured in sputum by quantitative RT-PCR, were significantly higher in patients during acute exacerbations. Of patients with rhinoviral infection, 73% were found to have bacteria in their sputum by day 14.48 This association between rhinovirus and bacterial coinfection may be due in part to changes in the host microbiome. A recent study of rhinovirus infection in patients with COPD and healthy controls found that rhinovirus altered the microbiome of COPD patients, allowing for an increase in pathogenic bacterial species such as Haemophilus influenzae.49 Rhinovirus may also degrade antimicrobial peptides in the lung, predisposing susceptible patients to bacterial coinfection.50
Rhinovirus is isolated frequently in adult patients with CAP. In the CDC EPIC study, rhinovirus was the most common pathogen identified and was found in 9% of all patients.12 Importantly, rhinovirus was rarely isolated in the study’s healthy controls. In a single-center prospective study of 304 hospitalized patients with CAP in New Zealand, rhinovirus was also the most frequently identified pathogen and was isolated in 10% of patients.26 The incidence of rhinovirus in other studies of CAP both in the United States and around the world have ranged from 1% to 4%.24,25,27
Several studies have focused specifically on patients with severe CAP requiring admission to the ICU. In a prospective multicenter study from Kentucky, rhinovirus was identified from nasopharyngeal swab in 33 of 393 patients (36%) with severe CAP.31 In a study of 49 patients with CAP requiring mechanical ventilation in Finland, 15 (31%) were found to be infected with rhinovirus.51 Similarly, rhinovirus was identified in 4 of 64 patients (6%) with severe CAP in Korea.28
Rhinovirus also plays an important role in HAP. Rhinovirus was identified in 15 of 262 patients (6%) with HAP requiring admission to an ICU in Korea.28 Similarly, a retrospective single-center study found rhinovirus in 19 (11%) of 174 patients with non-ventilated HAP.33
Clinical Presentation and Diagnosis
Sore throat and rhinorrhea are typical early symptoms of rhinovirus infection.52 Common presenting symptoms in patients with CAP secondary to rhinovirus are not well-described. In 1 study of 304 hospitalized patients with CAP, the most common symptoms in 31 patients with documented rhinovirus infection were cough (94%), lethargy (87%), anorexia (77%), sputum production (74%), and pleuritic pain (58%).26
RT-PCR is the preferred diagnostic test for severely ill patients with rhinovirus owing to improved sensitivity and more rapid turnaround time than conventional culturebased diagnostic methods.53 In the future, identifying specific host transcriptional changes may help to differentiate between true infection and asymptomatic carriage.54
Treatment
Treatment of even severe rhinoviral infection is supportive. Case reports have described the use of pegylated interferon-α2A and ribavirin in immunosuppressed patients with evidence of persistent infection, but this strategy has not been tested in randomized trials.55
HUMAN ADENOVIRUSES
HAdVs are nonenveloped double-stranded DNA viruses that have long been recognized as an important cause of respiratory tract infections in children.56 HAdVs are divided into seven species (HAdV-A through HAdV-G) with species B, C, and E most commonly associated with respiratory infections.57 Based on serotypes and genomic analysis, 67 subtypes of adenovirus have been identified.58
Unlike other respiratory viruses, HAdV infections do not demonstrate clear seasonal variation.58 Transmission can occur via inhalation of aerosolized droplets, direct conjunctival inoculation, fecal–oral spread, and contact with infected environmental surfaces.59 HAdVs are resistant to many common disinfectants, so rigorous infection control policies, including the use of 95% ethanol for decontamination, are essential to prevent nosocomial spread of infection.59,60
Human Adenovirus Infection in Adults
Severe HAdV infection is most commonly encountered in immunocompromised hosts, where disease can range from asymptomatic viremia to invasive multiorgan disease. Patients with human immunodeficiency virus and those who have undergone solid organ transplantation or allogeneic stem cell transplantation are particularly at risk.61 Common disease manifestations in the immunocompromised patient include pneumonia, colitis, hemorrhagic cystitis, hepatitis, and graft dysfunction.58
By adulthood, almost all immunocompetent individuals have evidence of prior HAdV exposure and exhibit HAdV-specific T cells.57 As a result, HAdV infection is usually mild and self-limited. However, outbreaks of severe respiratory infection are well-described and it is important for clinicians to be aware of recent trends in HAdV epidemiology.
Crowded living environments are a risk factor for outbreaks of severe HAdV in otherwise healthy individuals. The best documented example is US military recruits who for decades have been found to be at high risk for severe HAdV infection.61 Recognition of this association led to routine vaccination of military trainees against HAdV-4 and HAdV-7, which produced a dramatic decrease in HAdV disease.62 However, a recent epidemic of HAdV pneumonia at a US Air Force base in Texas was found to be caused by HAdV-14, an uncommon subtype not usually associated with severe disease.63 Of 66 hospitalized trainees, 23 (35%) were found to have HAdV-14 infection, including 4 (17%) who required ICU admission. HAdV infection has been responsible for outbreaks of febrile respiratory infections at military training facilities outside of the United States.64–67 and infections requiring hospitalization at mental health facilities,68 job training sites,69 and boarding schools.70
Recent community outbreaks of HAdV-14 in the United States emphasize the increasing importance of this particular subtype even outside of communal living environments. In Oregon, 28 cases of HAdV-14 pneumonia were identified including 18 (47%) who required admission to the ICU and 7 (18%) who died.71 Similarly, 46 cases of HAdV-14 respiratory illness were recently documented in an Alaskan community, including 11 patients who required hospitalization.72 In both of these outbreaks, elderly patients with underlying lung disease and other chronic health problems were at particular risk.
Outside of the United States, HAdV has emerged as an increasingly important cause of CAP. A recent multicenter surveillance study in China documented HAdV as a causative pathogen in 5% of all cases of CAP and found that infection with serotype HAdV-55 was associated with a particularly high pneumonia severity index score.73 A retrospective analysis of all cases of CAP caused by HAdV-55 at 2 hospitals in northern China noted a 27% mortality rate.74 Interestingly, 2 cases of severe CAP secondary to HAdV-55 were also recently described in France, perhaps signaling the importance of this serotype outside of Asia.75
Clinical Presentation and Diagnosis
Patients with pneumonia owing to HAdV present with symptoms indistinguishable from other types of pneumonia, including fever, cough, and shortness of breath.71,72 No clinical factors reliably discriminate between pneumonia caused by HAdV and pneumonia caused by other pathogens. In a study of infected military personnel, those with HAdV infection were more likely to have cytopenias than those without HAdV infection.76 This association between HAdV infection and cytopenias has been documented in other studies, but not with enough consistency to impact clinical practice.68,77 Although chest imaging is usually abnormal, findings are nonspecific and can include focal areas of consolidation or interstitial abnormalities.71,78
Numerous methods are available to diagnose HAdV infection, although PCR is the most practical choice for acutely ill patients. Viral culture was previously considered the “gold standard” although the time needed to observe the characteristic cytopathic effect in human epithelial cells makes it impractical for use in critically ill patients.61 Shell vial cultures have improved turnaround time although may have lower sensitivity.58 HAdV-specific antigens can be identified by enzyme-specific immunoassays, although this method is not recommended in immunocompromised patients owing to poor sensitivity.79 Although tissue sampling is rarely pursued in immunocompetent patients, HAdV can be diagnosed readily on histopathology by visualizing characteristic intranuclear viral inclusions. In recent years, PCR has become the test of choice owing to rapid turnaround time and high sensitivity and specificity.80 Molecular typing, although helpful for epidemiologic studies, is not recommended for individual patients.
Treatment
The mainstay of therapy for immunocompetent patients with HAdV infection is supportive care. No high-quality randomized trials inform the decision to use pharmacologic therapy in any patient population. Of available antiviral agents, cidofovir, the nucleoside analogue of cytidine monophosphate, has the most supporting data and several case reports have described the safe and successful use of cidofovir in the treatment of severe HAdV infection in immunocompromised patients.81,82 However, routine use is limited by significant side effects, including nephrotoxicity and neutropenia.83,84
RESPIRATORY SYNCYTIAL VIRUS
RSV is an enveloped, negative-sense, single-stranded RNA virus first identified more than 50 years ago.85 The 2 serotypes, RSV-A and RSV-B, are discriminated by reactivity to monoclonal antibodies. RSV has a worldwide circulation and peak infectivity in temperate climates between December and February.86 Exposure to the virus by 2 years of age is nearly universal.85 RSV is highly infectious and can spread via aerosolized droplets or contact with infected secretions.87 Outbreaks of RSV infections in hospitalized patients are well-described and strict infection control protocols are essential when caring for infected patients.88
The clinical and economic burden of RSV infection in children is substantial. Globally, RSV is the most common cause of LRTIs in children, with more than 3 million hospitalizations and up to 200,000 deaths in children less than 5 years of age per year.89 Annual direct medical costs in the United States are estimated at more than $650 million.90 Respiratory bronchiolitis, characterized by inflammation and obstruction of the small airways, is one of the most common manifestations of RSV infection and is a significant cause of pediatric morbidity and mortality in the United States.91 Children with Down syndrome seem to be at particular risk of severe infection.92 RSV infection early in life has also been associated with the development of asthma.93 RSV can cause numerous extrapulmonary diseases in children, including myocarditis, hepatitis, and seizures.94
Respiratory Syncytial Virus Infection in Adults
As with other respiratory viruses, immunocompromised patients are at particular risk of severe RSV infection. Severe LRTIs have been described in multiple patient populations, including after hematopoietic stem cell transplantation, patients with hematologic malignancies, and after solid organ transplantation, where infection may predispose to graft dysfunction.95–98 Outbreaks of severe RSV infections in bone marrow transplantation wards highlight the susceptibility of this patient population to infection.99
In otherwise healthy adults, RSV infection typically produces a URI characterized by a productive cough, nasal congestion, and sinus involvement.100 In elderly patients and those with underlying cardiac and pulmonary disease, RSV is an important cause of LRTIs and pneumonia (Fig. 2). Studies using national mortality and viral surveillance data have found that more than 75% of deaths attributable to RSV infection occur in patients older than 65 years of age.101 In this age group, RSV is responsible for an estimated 62,000 hospitalizations per year and 9% of all hospitalizations for pneumonia.102 The numerous reports of RSV outbreaks at long-term care facilities highlight the susceptibility of elderly patients to severe RSV infection.15,103
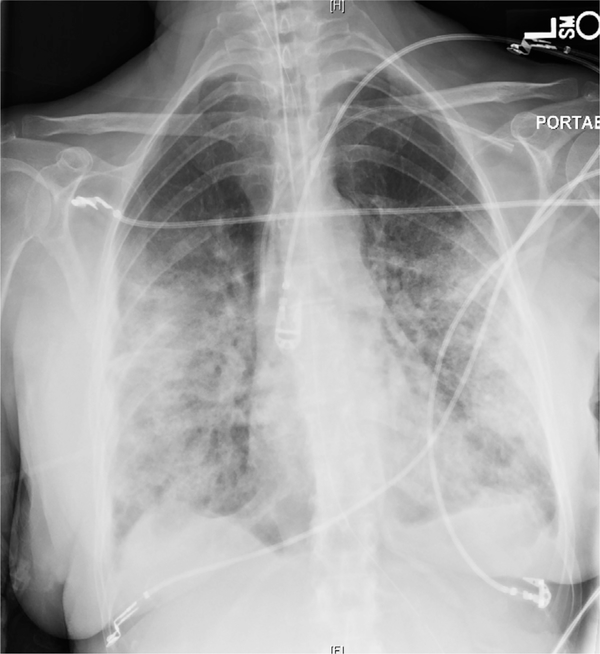
Fig. 2.
Posteroanterior chest radiograph in an elderly woman with acute hypoxemic respiratory failure secondary to respiratory syncytial virus pneumonia demonstrating dense bilateral airspace disease.
In one of the most rigorous studies to date, Falsey and colleagues13 prospectively evaluated the impact of RSV infection over 4 consecutive winters in 3 patient cohorts: healthy adults 65 years of age or older, elderly adults with chronic cardiac or pulmonary disease, and adult patients hospitalized with acute respiratory symptoms. Importantly, in addition to viral culture and serologies, RT-PCR was used to aid the diagnosis of RSV infection. The annual rate of RSV infection was 3% to 7% in healthy elderly patients and 4% to 10% in high-risk adults. Of 56 high-risk patients with RSV infection, 25 (45%) were unable to perform activities of daily living owing to their acute illness, 9 (16%) required hospitalization, and 2 (4%) died. In the cohort of hospitalized patients with confirmed RSV infection, 20 (15%) required ICU admission, 17 (13%) required mechanical ventilation, and 10 (8%) died. During the study period, RSV accounted for 11% of hospitalizations for pneumonia, 11% for COPD, 5% for congestive heart failure, and 7% for asthma.
In studies of CAP, RSV has been found to be an important pathogen. In the CDC EPIC study, RSV was detected in 3% of adults hospitalized with CAP with detection rates varying significantly by season.12 A similar detection rate has been found in other studies.25,104 In patients with severe CAP requiring admission to the ICU, RSV may be responsible for up to 10% of cases.28,29,31
Patients with COPD seem to be at particular risk of RSV infection. Although persistent RSV infection in stable COPD seems to be uncommon,105,106 RSV is a common trigger for COPD exacerbations.107 COPD is frequently identified as a risk factor for severe RSV infection108 and mortality rates in infected COPD patients may eclipse those of infected patients after stem cell transplantation.109
Clinical Presentation and Diagnosis
Among adults presenting to the hospital with confirmed RSV infection, wheezing is encountered more frequently than with other viral infections, including influenza.104,110,111 Cough, shortness of breath, and fever are other common presenting symptoms.13,104 Chest radiography is frequently normal, although radiographic evidence of pneumonia may be found more frequently than in patients with influenza.13,104 On chest computed tomography scans, tree-in-bud opacities and abnormalities in a bronchocentric distribution are more common in RSV infection than with other respiratory viruses.112,113
As with other respiratory viruses, nucleic acid amplification, specifically with RT-PCR, has become the test of choice for suspected RSV infection in adults.114 Culture techniques including shell vial culture are challenging given the unstable nature of the RSV virus and lack sensitivity.85 Rapid antigen detection tests, which are used commonly in children, perform less well in adults likely owing to lower viral titers present in the secretions of elderly patients.115 Detection of acute and convalescent phase serologies is useful for epidemiologic study and may increase the yield of RT-PCR, but is not widely used in clinical practice.85
Treatment
The mainstay of therapy for immunocompetent adults with severe RSV infection is supportive care. In immunocompromised patients and other select high-risk adult groups, additional therapy may be considered. The guanosine analogue ribavirin has been used with some success in patients with RSV infection after hematopoietic stem cell transplantation. In a recent single-center study of 280 patients after allogeneic stem cell transplantation, early use of aerosolized ribavirin was associated with a reduction in progression to LRTI and improved mortality.116 Similar results were found in a recent review of published case series.117 Concerns regarding cost, teratogenicity, and adverse effects including hemolytic anemia have limited routine use in adults.118 Immunotherapy with intravenous immunoglobulin in combination with ribavirin has been described in case reports, but has not been studied in randomized trials.119
In children, passive immunoprophylaxis with palivizumab, a monoclonal antibody directed against the RSV F glycoprotein, has been used with success and is recommended by the American Academy of Pediatrics for use in infants with hemodynamically significant heart disease or chronic lung disease of prematurity.120 Unfortunately, results with the use of palivizumab in at-risk adult patients have been disappointing.117,121 A novel oral viral replication inhibitor ALS-008176 was recently used with encouraging results in a small RSV challenge study in healthy adults, but further trials are required before it can be recommended for routine use.122
The substantial morbidity and mortality associated with RSV infection in the elderly has heightened calls for the development of an RSV vaccine.13 Although progress has been made, no vaccines are currently available.123
HUMAN METAPNEUMOVIRUS
hMPV is a single-stranded, negative-sense RNA virus first isolated in 2001 from children with respiratory tract infections in the Netherlands.124 The virus is present worldwide and exhibits clear seasonality with peak circulation in temperate climates between winter and spring.125 Exposure to the virus by 5 years of age is nearly universal.126 Modes of transmission are not well-described, but outbreaks of hMPV at long-term care facilities and hospital wards highlight the importance of infection control protocols when caring for infected patients.14,16,127
The clinical importance of hMPV is well-documented in children. In a recent prospective study in the United States, hMPV was identified in 6% of all children hospitalized with an acute respiratory illness and associated with increased ICU duration of stay.128 hMPV may be responsible for more than 10% of all LRTIs in children in the United States and 5% to 7% of all pediatric respiratory tract infections worldwide.129,130 Disease manifestations in children range from croup and bronchiolitis to exacerbations of asthma and severe pneumonia requiring mechanical ventilation.129
Human Metapneumovirus Infection in Adults
hMPV is recognized as an important respiratory pathogen in immunocompromised adults. Studies using RT-PCR have identified hMPV as the cause of severe pneumonia in hematopoietic stem cell transplant recipients,131 patients with hematologic malignancies,132 and solid organ transplant recipients where infection may increase the risk of graft dysfunction.133–135 A recent systematic review found a 26% mortality in immunocompromised patients with hMPV LRTI.135
In immunocompetent adults with suspected viral infection, the incidence of hMPV ranges from 2% to 9%.136–138 In the recent CDC EPIC study, hMPV was identified in 4% of hospitalized adults with CAP.12 Although severe hMPV infection in immunocompetent adults is uncommon, several at-risk patient populations deserve mention. Outbreaks of hMPV are common at long-term acute care facilities. In California, 26 residents and staff were infected with hMPV including 8 (31%) who developed radiographically confirmed pneumonia and 2 (5%) who required hospitalization.14 Similarly, during an outbreak of severe respiratory infection at a long-term care facility in Quebec, hMPV was identified in 6 of 96 infected patients, with a 50% mortality.16 Outbreaks have also been described at long-term care facilities in Oregon,139 the Netherlands,140 and Japan.141
Limited data suggest that hMPV may be an important cause of hospitalizations in patients with COPD. In a single-center, observational study of 50 adults hospitalized for a COPD exacerbation, RT-PCR of nasopharyngeal specimens identified 6 patients (12%) with hMPV infection.142 Documented hMPV infection rates in other studies of patients with COPD exacerbations have ranged from 2.3% to 5.5%.143,144
Clinical Presentation and Diagnosis
Patients hospitalized with hMPV present with nonspecific symptoms. In 1 study of 91 hospitalized adults with hMPV, the most common symptoms were dyspnea (98%), cough (94%), wheezing (79%), and sputum production (74%).145 High rates of wheezing have been noted in other studies and are similar to the incidence of bronchospasm found with RSV infection.138,146 Chest imaging is similarly nonspecific and may be normal in more than one-third of hospitalized patients.145 Reports of chest computed tomography findings in hPMV infection are limited. In 1 study of high-resolution computed tomography findings in 4 patients with hMPV, groundglass opacities, consolidation, and parenchymal bands were present in all patients.147
Although uncommon, hMPV infection can lead to severe disease. In a single study from Korea of 198 patients with severe pneumonia requiring admission to the ICU, hMPV infection was identified by RT-PCR in 13 patients including 5 (8%) with CAP.28 Similarly, in a recent review of all admissions to a single ICU over 4 years, 40 cases of hMPV infection were identified, of which 55% required mechanical ventilation, 23% developed shock, and 48% met criteria for acute respiratory distress syndrome.148 Importantly, 6 of these 40 patients (15%) had only minor comorbidities. Finally, in a study of 91 patients hospitalized with hMPV, 12 (13%) required admission to the ICU, 11 (12%) required mechanical ventilation, and 6 (7%) died.145
hMPV replicates slowly and is difficult to isolate with typical cell culture techniques, making viral culture impractical for routine use in the ICU.149 RT-PCR is the preferred diagnostic test for hMPV and is now available as part of a multiplex PCR panel for simultaneous testing with other viruses.138
Treatment
Treatment of severe hMPV infection is supportive and no pharmacologic therapies are currently approved for use. Ribavirin has shown promising activity in murine models of infection150 and several case reports describe the drug’s potential efficacy in humans when used in conjunction with intravenous immunoglobulins.151,152 However, concerns regarding the cost of ribaviran, teratogenicity and reports showing underwhelming clinical results have tempered enthusiasm for more routine use.153
CHALLENGES AND FUTURE DIRECTIONS
With the improved sensitivity of PCR-based testing, a major challenge in the diagnosis and treatment of viral pneumonia is distinguishing true infection from asymptomatic carriage or isolated URI.6 This is especially true for samples obtained from the upper respiratory tract in patients with suspected LRTI. The specificity of PCR testing likely depends on both the age of the patient and the pathogen identified and further studies are needed to refine test interpretation.154 The results of the CDC EPIC study, where only 2% of 238 asymptomatic control subjects had a pathogen identified, suggest that the majority of identified respiratory viral pathogens play a causal role in disease pathogenesis.12
Measuring convalescent phase serum antibodies may help to improve the diagnostic yield and specificity of PCR-based testing although further studies are needed to validate this approach.155 Transcriptional profiling of the host response to infection may also aid the diagnosis of viral pneumonia. Recently, an 11-gene influenza virusspecific host response signature was identified in human blood that accurately diagnosed influenza infection, identified bacterial coinfection, and predicted outcomes in patients with influenza pneumonia.156 Similarly, a host transcriptional signature defined largely by the overexpression of interferon-related genes was found to discriminate between viral and bacterial pneumonia.157
Perhaps the greatest challenge facing both clinicians and researchers is the large number of patients with a clinical diagnosis of pneumonia in whom a causative pathogen is never identified. Of the more than 2000 patients in the CDC EPIC study, 62% had no identifiable pathogen despite a degree of microbiologic testing that exceeded usual clinical practice.12 Over the past 2 decades, the percentage of patients hospitalized with pneumonia who had no reported pathogen increased by almost 20% in the United States.18 Research that better characterizes this large group of patients has the potential to profoundly impact health care costs and antimicrobial stewardship.158 Our evolving understanding of the link between the respiratory microbiome and pneumonia pathogenesis may prove an important engine of innovation in the coming years.159
KEY POINTS.
The epidemiology of severe lower respiratory tract infection is changing due in part to the aging of the US population and the success of childhood vaccination programs.
Diagnostic advances including nucleic acid amplification platforms have greatly improved the detection of respiratory viral pathogens.
Respiratory viral pathogens are now recognized as an important cause of severe respiratory infection in both immunocompetent and immunocompromised adults.
Despite advances in diagnostic testing, a large number of patients with severe community-acquired respiratory infections do not have a causative pathogen identified.
Better characterizing this group of patients remains an ongoing challenge.
Footnotes
Disclosure Statement: Dr J.M. Walter has nothing to disclose. Dr R.G. Wunderink has consulted for Genmark and Accelerate Diagnostics.
REFERENCES
- 1.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302(21):2323–9. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Natl Vital Stat Rep 2016;64(2):1–119. [PubMed] [Google Scholar]
- 3.Fry AM, Shay DK, Holman RC, et al. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA 2005;294(21):2712–9. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med 1995; 333(24):1618–24. [DOI] [PubMed] [Google Scholar]
- 5.Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013;369(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruuskanen O, Lahti E, Jennings LC, et al. Viral pneumonia. Lancet 2011; 377(9773):1264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somerville LK, Ratnamohan VM, Dwyer DE, et al. Molecular diagnosis of respiratory viruses. Pathology 2015;47(3):243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009;361(7):674–9. [DOI] [PubMed] [Google Scholar]
- 9.Ornstein KA, Leff B, Covinsky KE, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med 2015;175(7):1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn JM, Benson NM, Appleby D, et al. Long-term acute care hospital utilization after critical illness. JAMA 2010;303(22):2253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falsey AR, Walsh EE. Viral pneumonia in older adults. Clin Infect Dis 2006;42(4): 518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia Requiring hospitalization among U.S. adults. N Engl J Med 2015;373(5):415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005;352(17):1749–59. [DOI] [PubMed] [Google Scholar]
- 14.Louie JK, Schnurr DP, Pan CY, et al. A summer outbreak of human metapneumovirus infection in a long-term-care facility. J Infect Dis 2007;196(5):705–8. [DOI] [PubMed] [Google Scholar]
- 15.Sorvillo FJ, Huie SF, Strassburg MA, et al. An outbreak of respiratory syncytial virus pneumonia in a nursing home for the elderly. J Infect 1984;9(3):252–6. [DOI] [PubMed] [Google Scholar]
- 16.Boivin G, De Serres G, Hamelin ME, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis 2007;44(9):1152–8. [DOI] [PubMed] [Google Scholar]
- 17.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005;294(16):2043–51. [DOI] [PubMed] [Google Scholar]
- 18.Smith SB, Ruhnke GW, Weiss CH, et al. Trends in pathogens among patients hospitalized for pneumonia from 1993 to 2011. JAMA Intern Med 2014;174(11):1837–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahony JB, Petrich A, Smieja M. Molecular diagnosis of respiratory virus infections. Crit Rev Clin Lab Sci 2011;48(5–6):217–49. [DOI] [PubMed] [Google Scholar]
- 20.Diederen BM, Van Der Eerden MM, Vlaspolder F, et al. Detection of respiratory viruses and Legionella spp. by real-time polymerase chain reaction in patients with community acquired pneumonia. Scand J Infect Dis 2009;41(1):45–50. [DOI] [PubMed] [Google Scholar]
- 21.Templeton KE, Scheltinga SA, van den Eeden WC, et al. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis 2005;41(3):345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garbino J, Gerbase MW, Wunderli W, et al. Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med 2004;170(11):1197–203. [DOI] [PubMed] [Google Scholar]
- 23.She RC, Polage CR, Caram LB, et al. Performance of diagnostic tests to detect respiratory viruses in older adults. Diagn Microbiol Infect Dis 2010;67(3): 246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu JX, Gu L, Pu ZH, et al. Viral etiology of community-acquired pneumonia among adolescents and adults with mild or moderate severity and its relation to age and severity. BMC Infect Dis 2015;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnstone J, Majumdar SR, Fox JD, et al. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest 2008;134(6):1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax 2008;63(1):42–8. [DOI] [PubMed] [Google Scholar]
- 27.Zhan Y, Yang Z, Chen R, et al. Respiratory virus is a real pathogen in immunocompetent community-acquired pneumonia: comparing to influenza like illness and volunteer controls. BMC Pulm Med 2014;14:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SH, Hong SB, Ko GB, et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med 2012;186(4):325–32. [DOI] [PubMed] [Google Scholar]
- 29.Ostby AC, Gubbels S, Baake G, et al. Respiratory virology and microbiology in intensive care units: a prospective cohort study. APMIS 2013;121(11):1097–108.23682902 [Google Scholar]
- 30.Cilloniz C, Ewig S, Ferrer M, et al. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit Care 2011; 15(5):R209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiemken T, Peyrani P, Bryant K, et al. Incidence of respiratory viruses in patients with community-acquired pneumonia admitted to the intensive care unit: results from the severe influenza pneumonia surveillance (SIPS) project. Eur J Clin Microbiol Infect Dis 2013;32(5):705–10. [DOI] [PubMed] [Google Scholar]
- 32.Hong HL, Hong SB, Ko GB, et al. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS One 2014;9(4):e95865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shorr AF, Zilberberg MD, Micek ST, et al. Viruses are prevalent in non-ventilated hospital-acquired pneumonia. Respir Med 2017;122:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garbino J, Soccal PM, Aubert JD, et al. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax 2009;64(5):399–404. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg SB. Update on Human Rhinovirus and Coronavirus Infections. Semin Respir Crit Care Med 2016;37(4):555–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner RB. Rhinovirus: more than just a common cold virus. J Infect Dis 2007; 195(6):765–6. [DOI] [PubMed] [Google Scholar]
- 37.Musher DM. How contagious are common respiratory tract infections? N Engl J Med 2003;348(13):1256–66. [DOI] [PubMed] [Google Scholar]
- 38.Reese SM, Thompson M, Price CS, et al. Evidence of nosocomial transmission of human rhinovirus in a neonatal intensive care unit. Am J Infect Control 2016; 44(3):355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med 1999;159(3):785–90. [DOI] [PubMed] [Google Scholar]
- 40.Caliskan M, Bochkov YA, Kreiner-Moller E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med 2013;368(15):1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camargo CA Jr. Human rhinovirus, wheezing illness, and the primary prevention of childhood asthma. Am J Respir Crit Care Med 2013;188(11):1281–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372(9):835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arruda E, Pitkaranta A, Witek TJ Jr, et al. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol 1997;35(11):2864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser L, Aubert JD, Pache JC, et al. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med 2006;174(12):1392–9. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs SE, Lamson DM, Soave R, et al. Clinical and molecular epidemiology of human rhinovirus infections in patients with hematologic malignancy. J Clin Virol 2015;71:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobs SE, Soave R, Shore TB, et al. Human rhinovirus infections of the lower respiratory tract in hematopoietic stem cell transplant recipients. Transpl Infect Dis 2013;15(5):474–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malcolm E, Arruda E, Hayden FG, et al. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J Clin Virol 2001;21(1):9–16. [DOI] [PubMed] [Google Scholar]
- 48.George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J 2014;44(1):87–96. [DOI] [PubMed] [Google Scholar]
- 49.Molyneaux PL, Mallia P, Cox MJ, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188(10):1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mallia P, Footitt J, Sotero R, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186(11):1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karhu J, Ala-Kokko TI, Vuorinen T, et al. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis 2014;59(1):62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris JM 2nd, Gwaltney JM Jr. Incubation periods of experimental rhinovirus infection and illness. Clin Infect Dis 1996;23(6):1287–90. [DOI] [PubMed] [Google Scholar]
- 53.Hammond SP, Gagne LS, Stock SR, et al. Respiratory virus detection in immunocompromised patients with FilmArray respiratory panel compared to conventional methods. J Clin Microbiol 2012;50(10):3216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinonen S, Jartti T, Garcia C, et al. Rhinovirus detection in symptomatic and asymptomatic children: value of host transcriptome analysis. Am J Respir Crit Care Med 2016;193(7):772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruuskanen O, Waris M, Kainulainen L. Treatment of persistent rhinovirus infection with pegylated interferon alpha2a and ribavirin in patients with hypogammaglobulinemia. Clin Infect Dis 2014;58(12):1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardner PS. Virus infections and respiratory disease of childhood. Arch Dis Child 1968;43(232):629–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lion T Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 2014;27(3):441–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ison MG, Hayden RT. Adenovirus. Microbiol Spectr 2016;4(4). [DOI] [PubMed] [Google Scholar]
- 59.Sandrock C, Stollenwerk N. Acute febrile respiratory illness in the ICU: reducing disease transmission. Chest 2008;133(5):1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cassir N, Hraiech S, Nougairede A, et al. Outbreak of adenovirus type 1 severe pneumonia in a French intensive care unit, September-October 2012. Euro Surveill 2014;19(39) [pii:20914]. [DOI] [PubMed] [Google Scholar]
- 61.Lynch JP 3rd, Kajon AE. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med 2016;37(4):586–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potter RN, Cantrell JA, Mallak CT, et al. Adenovirus-associated deaths in US military during postvaccination period, 1999–2010. Emerg Infect Dis 2012;18(3): 507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tate JE, Bunning ML, Lott L, et al. Outbreak of severe respiratory disease associated with emergent human adenovirus serotype 14 at a US air force training facility in 2007. J Infect Dis 2009;199(10):1419–26. [DOI] [PubMed] [Google Scholar]
- 64.Kurian PV, Lal R, Pandit V. Adenovirus infections in Indian army personnel. Indian J Med Res 1966;54(9):812–8. [PubMed] [Google Scholar]
- 65.Pavilanis V, Davignon L, Podoski MO. Incidence of Adenovirus Infections in a Camp of Canadian Recruits. Rev Can Biol 1964;23:291–8 [in French]. [PubMed] [Google Scholar]
- 66.Hierholzer JC, Pumarola A, Rodriguez-Torres A, et al. Occurrence of respiratory illness due to an atypical strain of adenovirus type 11 during a large outbreak in Spanish military recruits. Am J Epidemiol 1974;99(6):434–42. [DOI] [PubMed] [Google Scholar]
- 67.Kajon AE, Dickson LM, Metzgar D, et al. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J Clin Microbiol 2010;48(4):1438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klinger JR, Sanchez MP, Curtin LA, et al. Multiple cases of life-threatening adenovirus pneumonia in a mental health care center. Am J Respir Crit Care Med 1998;157(2):645–9. [DOI] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention (CDC). Civilian outbreak of adenovirus acute respiratory disease–South Dakota, 1997. MMWR Morb Mortal Wkly Rep 1998;47(27):567–70. [PubMed] [Google Scholar]
- 70.Zhu Z, Zhang Y, Xu S, et al. Outbreak of acute respiratory disease in China caused by B2 species of adenovirus type 11. J Clin Microbiol 2009;47(3): 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis PF, Schmidt MA, Lu X, et al. A community-based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J Infect Dis 2009; 199(10):1427–34. [DOI] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention (CDC). Outbreak of adenovirus 14 respiratory illness–Prince of Wales Island, Alaska, 2008. MMWR Morb Mortal Wkly Rep 2010;59(1):6–10. [PubMed] [Google Scholar]
- 73.Cao B, Huang GH, Pu ZH, et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest 2014;145(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan D, Zhu H, Fu Y, et al. Severe community-acquired pneumonia caused by human adenovirus in immunocompetent adults: a multicenter case series. PLoS One 2016;11(3):e0151199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lafolie J, Mirand A, Salmona M, et al. Severe pneumonia associated with adenovirus type 55 infection, France, 2014. Emerg Infect Dis 2016;22(11):2012–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vento TJ, Prakash V, Murray CK, et al. Pneumonia in military trainees: a comparison study based on adenovirus serotype 14 infection. J Infect Dis 2011; 203(10):1388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon H, Jhun BW, Kim SJ, et al. Clinical characteristics and factors predicting respiratory failure in adenovirus pneumonia. Respirology 2016;21(7):1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan D, Fu Y, Xu J, et al. Severe adenovirus community-acquired pneumonia in immunocompetent adults: chest radiographic and CT findings. J Thorac Dis 2016;8(5):848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis 2006; 43(3):331–9. [DOI] [PubMed] [Google Scholar]
- 80.Damen M, Minnaar R, Glasius P, et al. Real-time PCR with an internal control for detection of all known human adenovirus serotypes. J Clin Microbiol 2008; 46(12):3997–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SJ, Kim K, Park SB, et al. Outcomes of early administration of cidofovir in non-immunocompromised patients with severe adenovirus pneumonia. PLoS One 2015;10(4):e0122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee M, Kim S, Kwon OJ, et al. Treatment of adenoviral acute respiratory distress syndrome using cidofovir with extracorporeal membrane oxygenation: case series and literature review. J Intensive Care Med 2016;32:231–8. [DOI] [PubMed] [Google Scholar]
- 83.Ison MG, Green M. Practice ASTIDCo. Adenovirus in solid organ transplant recipients. Am J Transplant 2009;9(Suppl 4):S161–5. [DOI] [PubMed] [Google Scholar]
- 84.Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the infectious diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2003;31(6):481–6. [DOI] [PubMed] [Google Scholar]
- 85.Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev 2017; 30(1):277–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bont L, Checchia PA, Fauroux B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther 2016;5(3):271–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drysdale SB, Green CA, Sande CJ. Best practice in the prevention and management of paediatric respiratory syncytial virus infection. Ther Adv Infect Dis 2016;3(2):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bont L Nosocomial RSV infection control and outbreak management. Paediatr Respir Rev 2009;10(Suppl 1):16–7. [DOI] [PubMed] [Google Scholar]
- 89.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375(9725):1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paramore LC, Ciuryla V, Ciesla G, et al. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22(5):275–84. [DOI] [PubMed] [Google Scholar]
- 91.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360(6):588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stagliano DR, Nylund CM, Eide MB, et al. Children with down syndrome are high-risk for severe respiratory syncytial virus disease. J Pediatr 2015;166(3): 703–9.e2. [DOI] [PubMed] [Google Scholar]
- 93.Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65(12):1045–52. [DOI] [PubMed] [Google Scholar]
- 94.Bohmwald K, Espinoza JA, Rey-Jurado E, et al. Human respiratory syncytial virus: infection and pathology. Semin Respir Crit Care Med 2016;37(4):522–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Englund JA, Sullivan CJ, Jordan MC, et al. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med 1988;109(3):203–8. [DOI] [PubMed] [Google Scholar]
- 96.Hertz MI, Englund JA, Snover D, et al. Respiratory syncytial virus-induced acute lung injury in adult patients with bone marrow transplants: a clinical approach and review of the literature. Medicine (Baltimore) 1989;68(5):269–81. [DOI] [PubMed] [Google Scholar]
- 97.Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis 2008;46(3):402–12. [DOI] [PubMed] [Google Scholar]
- 98.Gottlieb J, Zamora MR, Hodges T, et al. ALN-RSV01 for prevention of bronchiolitis obliterans syndrome after respiratory syncytial virus infection in lung transplant recipients. J Heart Lung Transplant 2016;35(2):213–21. [DOI] [PubMed] [Google Scholar]
- 99.Kelly SG, Metzger K, Bolon MK, et al. Respiratory syncytial virus outbreak on an adult stem cell transplant unit. Am J Infect Control 2016;44(9):1022–6. [DOI] [PubMed] [Google Scholar]
- 100.Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis 2001;33(6):792–6. [DOI] [PubMed] [Google Scholar]
- 101.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289(2):179–86. [DOI] [PubMed] [Google Scholar]
- 102.Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis 1999; 179(1):25–30. [DOI] [PubMed] [Google Scholar]
- 103.Hart RJ. An outbreak of respiratory syncytial virus infection in an old people’s home. J Infect 1984;8(3):259–61. [DOI] [PubMed] [Google Scholar]
- 104.Dowell SF, Anderson LJ, Gary HE Jr, et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis 1996;174(3):456–62. [DOI] [PubMed] [Google Scholar]
- 105.Falsey AR, Formica MA, Hennessey PA, et al. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173(6):639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giannakaki S, Politi L, Antonogiannaki EM, et al. Absence of human rhinovirus and respiratory syncytial virus from bronchoalveolar lavage and bronchial biopsies of selected patients with stable chronic obstructive pulmonary disease. Respir Res 2016;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramaswamy M, Groskreutz DJ, Look DC. Recognizing the importance of respiratory syncytial virus in chronic obstructive pulmonary disease. COPD 2009; 6(1):64–75. [DOI] [PubMed] [Google Scholar]
- 108.Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004;189(2):233–8. [DOI] [PubMed] [Google Scholar]
- 109.Anderson NW, Binnicker MJ, Harris DM, et al. Morbidity and mortality among patients with respiratory syncytial virus infection: a 2-year retrospective review. Diagn Microbiol Infect Dis 2016;85(3):367–71. [DOI] [PubMed] [Google Scholar]
- 110.O’Shea MK, Ryan MA, Hawksworth AW, et al. Symptomatic respiratory syncytial virus infection in previously healthy young adults living in a crowded military environment. Clin Infect Dis 2005;41(3):311–7. [DOI] [PubMed] [Google Scholar]
- 111.Wald TG, Miller BA, Shult P, et al. Can respiratory syncytial virus and influenza a be distinguished clinically in institutionalized older persons? J Am Geriatr Soc 1995;43(2):170–4. [DOI] [PubMed] [Google Scholar]
- 112.Mayer JL, Lehners N, Egerer G, et al. CT-morphological characterization of respiratory syncytial virus (RSV) pneumonia in immune-compromised adults. Rofo 2014;186(7):686–92. [DOI] [PubMed] [Google Scholar]
- 113.Miller WT Jr, Mickus TJ, Barbosa E Jr, et al. CT of viral lower respiratory tract infections in adults: comparison among viral organisms and between viral and bacterial infections. AJR Am J Roentgenol 2011;197(5):1088–95. [DOI] [PubMed] [Google Scholar]
- 114.Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol 2002;40(3):817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chartrand C, Tremblay N, Renaud C, et al. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol 2015;53(12):3738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 2013;68(8):1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011;117(10):2755–63. [DOI] [PubMed] [Google Scholar]
- 118.Chemaly RF, Aitken SL, Wolfe CR, et al. Aerosolized ribavirin: the most expensive drug for pneumonia. Transpl Infect Dis 2016;18(4):634–6. [DOI] [PubMed] [Google Scholar]
- 119.Ghosh S, Champlin RE, Englund J, et al. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant 2000;25(7):751–5. [DOI] [PubMed] [Google Scholar]
- 120.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134(5):e1474–502. [DOI] [PubMed] [Google Scholar]
- 121.de Fontbrune FS, Robin M, Porcher R, et al. Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2007;45(8):1019–24. [DOI] [PubMed] [Google Scholar]
- 122.DeVincenzo JP, McClure MW, Symons JA, et al. Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med 2015;373(21): 2048–58. [DOI] [PubMed] [Google Scholar]
- 123.Chiu C Novel immunological insights in accelerating RSV vaccine development. Vaccine 2017;35(3):459–60. [DOI] [PubMed] [Google Scholar]
- 124.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 2001;7(6):719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haynes AK, Fowlkes AL, Schneider E, et al. Human Metapneumovirus Circulation in the United States, 2008 to 2014. Pediatrics 2016;137(5) [pii:e20152927]. [DOI] [PubMed] [Google Scholar]
- 126.Falsey AR. Human metapneumovirus infection in adults. Pediatr Infect Dis J 2008;27(10 Suppl):S80–3. [DOI] [PubMed] [Google Scholar]
- 127.Kim S, Sung H, Im HJ, et al. Molecular epidemiological investigation of a nosocomial outbreak of human metapneumovirus infection in a pediatric hematooncology patient population. J Clin Microbiol 2009;47(4):1221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. N Engl J Med 2013;368(7):633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004;350(5):443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J 2004;23(1 Suppl):S25–32. [DOI] [PubMed] [Google Scholar]
- 131.Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006;144(5):344–9. [DOI] [PubMed] [Google Scholar]
- 132.Godet C, Le Goff J, Beby-Defaux A, et al. Human metapneumovirus pneumonia in patients with hematological malignancies. J Clin Virol 2014;61(4):593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hopkins P, McNeil K, Kermeen F, et al. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med 2008;178(8):876–81. [DOI] [PubMed] [Google Scholar]
- 134.Larcher C, Geltner C, Fischer H, et al. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant 2005;24(11):1891–901. [DOI] [PubMed] [Google Scholar]
- 135.Shah DP, Shah PK, Azzi JM, et al. Human metapneumovirus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett 2016;379(1):100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gray GC, Capuano AW, Setterquist SF, et al. Multi-year study of human metapneumovirus infection at a large US midwestern medical referral center. J Clin Virol 2006;37(4):269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stockton J, Stephenson I, Fleming D, et al. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis 2002;8(9):897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Falsey AR, Erdman D, Anderson LJ, et al. Human metapneumovirus infections in young and elderly adults. J Infect Dis 2003;187(5):785–90. [DOI] [PubMed] [Google Scholar]
- 139.Liao RS, Appelgate DM, Pelz RK. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility for the elderly in Oregon. J Clin Virol 2012;53(2):171–3. [DOI] [PubMed] [Google Scholar]
- 140.Te Wierik MJ, Nguyen DT, Beersma MF, et al. An outbreak of severe respiratory tract infection caused by human metapneumovirus in a residential care facility for elderly in Utrecht, the Netherlands, January to March 2010. Euro Surveill 2012;17(13) [pii:20132]. [PubMed] [Google Scholar]
- 141.Honda H, Iwahashi J, Kashiwagi T, et al. Outbreak of human metapneumovirus infection in elderly inpatients in Japan. J Am Geriatr Soc 2006;54(1):177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martinello RA, Esper F, Weibel C, et al. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect 2006;53(4):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rohde G, Borg I, Arinir U, et al. Relevance of human metapneumovirus in exacerbations of COPD. Respir Res 2005;6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vicente D, Montes M, Cilla G, et al. Human metapneumovirus and chronic obstructive pulmonary disease. Emerg Infect Dis 2004;10(7):1338–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med 2008;168(22):2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boivin G, Abed Y, Pelletier G, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis 2002; 186(9):1330–4. [DOI] [PubMed] [Google Scholar]
- 147.Wong CK, Lai V, Wong YC. Comparison of initial high resolution computed tomography features in viral pneumonia between metapneumovirus infection and severe acute respiratory syndrome. Eur J Radiol 2012;81(5):1083–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hasvold J, Sjoding M, Pohl K, et al. The role of human metapneumovirus in the critically ill adult patient. J Crit Care 2016;31(1):233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wen SC, Williams JV. New approaches for immunization and therapy against human metapneumovirus. Clin Vaccine Immunol 2015;22(8):858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hamelin ME, Prince GA, Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother 2006;50(2):774–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bonney D, Razali H, Turner A, et al. Successful treatment of human metapneumovirus pneumonia using combination therapy with intravenous ribavirin and immune globulin. Br J Haematol 2009;145(5):667–9. [DOI] [PubMed] [Google Scholar]
- 152.Kitanovski L, Kopriva S, Pokorn M, et al. Treatment of severe human metapneumovirus (hMPV) pneumonia in an immunocompromised child with oral ribavirin and IVIG. J Pediatr Hematol Oncol 2013;35(7):e311–3. [DOI] [PubMed] [Google Scholar]
- 153.Renaud C, Xie H, Seo S, et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant 2013;19(8):1220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Self WH, Williams DJ, Zhu Y, et al. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2016;213(4):584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y, Sakthivel SK, Bramley A, et al. Serology enhances molecular diagnosis of respiratory virus infections other than influenza in children and adults hospitalized with community-acquired pneumonia. J Clin Microbiol 2017;55(1):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Andres-Terre M, McGuire HM, Pouliot Y, et al. Integrated, multi-cohort analysis identifies conserved transcriptional signatures across multiple respiratory viruses. Immunity 2015;43(6):1199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suarez NM, Bunsow E, Falsey AR, et al. Superiority of transcriptional profiling over procalcitonin for distinguishing bacterial from viral lower respiratory tract infections in hospitalized adults. J Infect Dis 2015;212(2):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nathan C, Cars O. Antibiotic resistance–problems, progress, and prospects. N Engl J Med 2014;371(19):1761–3. [DOI] [PubMed] [Google Scholar]
- 159.Yan Q, Cui S, Chen C, et al. Metagenomic analysis of sputum microbiome as a tool toward culture-independent pathogen detection of patients with ventilatorassociated pneumonia. Am J Respir Crit Care Med 2016;194(5):636–9. [DOI] [PubMed] [Google Scholar]