Abstract
The review focuses on the role of vitamin A (retinol) in the control of energy homeostasis, and on the manner in which certain retinoids subvert this process, leading potentially to disease. In eukaryotic cells, the pyruvate dehydrogenase complex (PDHC) is negatively regulated by four pyruvate dehydrogenase kinases (PDKs) and two antagonistically acting pyruvate dehydrogenase phosphatases (PDPs). The second isoform, PDK2, is regulated by an autonomous mitochondrial signal cascade that is anchored on protein kinase Cδ (PKCδ), where retinoids play an indispensible co-factor role. Along with its companion proteins p66Shc, cytochrome c, and vitamin A, the PKCδ /retinol complex is located in the intermembrane space of mitochondria. At this site, and in contrast to cytosolic locations, PKCδ is activated by the site-specific oxidation of its cysteine-rich activation domain (CRD) that is configured into a complex RING-finger. Oxidation involves the transfer of electrons from cysteine moieties to oxidized cytochrome c, a step catalyzed by vitamin A. The PKCδ/retinol signalosome monitors the internal cytochrome c redox state that reflects the workload of the respiratory chain. Upon sensing demands for energy PKCδ signals the PDHC to increase glucose-derived fuel flux entering the KREBS cycle. Conversely, if excessive fuel flux surpasses the capacity of the respiratory chain, threatening the release of damaging reactive oxygen species (ROS), the polarity of the cytochrome c redox system is reversed, resulting in the chemical reduction of the PKCδ CRD, restoration of the RING-finger, refolding of PKCδ into the inactive, globular form, and curtailment of PDHC output, thereby constraining the respiratory capacity within safe margins. Several retinoids, notably anhydroretinol and fenretinide, capable of displacing retinol from binding sites on PKCδ, can co-activate PKCδ signaling but, owing to their extended system of conjugated double bonds, are unable to silence PKCδ in a timely manner. Left in the ON position, PKCδ causes chronic overload of the respiratory chain leading to mitochondrial dysfunction. This review explores how defects in the PKCδ signal machinery potentially contribute to metabolic and degenerative diseases.
Keywords: Vitamin A, Protein kinase C, Mitochondria, Energy homeostasis
When eukaryotic cells evolved 2.5 billion years ago they gained independence from fixed energy sources, allowing them to spread into vast new environmental niches. Those adopting mitochondrial endosymbionts eventually switched to oxidative metabolism, enabled by the co-evolution of chloroplast-carrying plant cells that created an oxygen-rich atmosphere. With the availability of a portable, oxygen-based energy source multicellular organisms arose. However, reliance on as corrosive a chemical as molecular oxygen for energy metabolism had drawbacks, necessitating powerful anti-oxidant systems. Despite these, various oxygen byproducts, collectively known as reactive oxygen species (ROS), inevitably escape from mitochondria, especially during phases of high energy demands. In animal cells, some of these ROS species are coopted to perform normal functions, including signaling, but when in excess multiple pathologies can ensue. To forestall such harmful ROS production, mitochondria evolved internal quality control mechanisms. One example among these is a signaling system based on protein kinase A which regulates the efficiency of electron transfer by coordinating Krebs cycle metabolism with cytochrome c oxidase capacity [1]. A second, feed-forward/feed-back loop, anchored on protein kinase Cδ (PKCδ), is the subject of this review [2, 3]. This signal system operates upstream of the pyruvate dehydrogenase complex and is designed to coordinate the fuel flux entering the Krebs cycle with the workload of the electron-transfer chain (ETC), ostensibly to speed up electron flux during high energy demands but, importantly, to also maintain energy generation within safe margins. The PKCδ signaling systems is entirely confined to mitochondria, and while the import and assembly of the biochemical machinery, the uptake of a variety of fuels, as well as the export of numerous products, are under cytoplasmic control, monitoring the performance of the ETC by the PKCδ signaling system occurs in real time and quasi-autonomously. Such uniquely independent status seems necessary because cells harbor in their cytoplasm hundreds to thousands of mitochondria, with no readily discernible central coordination of these widely dispersed organelles.
Brief history of the discovery of the regulation of oxidative phosphorylation by the PKCδ / vitamin A complex.
Vitamin A came into existence as “fat-soluble substance A” based on its requirement by chicks for normal growth [4, 5]. Support of growth promotion was also implied by findings in the 1920’s that the development of lymphoid organs (among other organs) was stunted in vitamin A deficient mice. While, for instance, the thymus Anlage was present, the lymphocytes normally populating this organ were missing [6], which in retrospect we now know proliferate rapidly during the immune response, demanding peak energy levels. That vitamin A deprivation correlated with insufficient cell proliferation foretold an important regulatory role of this vitamin in energy homeostasis. Yet in the following decades vitamin A physiology took a back-seat, upstaged by the groundbreaking discoveries of the roles of vitamin A metabolites in vision [7] and gene transcription [8, 9]. When the growth-promoting properties of vitamin A were rediscovered by us in the early 90’s [10–12], and when only vitamin alcohol, but not retinoic acid, rescued vitamin A-deprived cultures of lymphocytes it became clear that vitamin A has its own biological purpose and is not a mere precursor for activated retinoids.
A search for cellular vitamin A receptors by affinity chromatography netted protein kinase Cα, and subsequent studies revealed that several other PKC isoforms, along with all three Raf serine/threonine kinase isoforms, harbored vitamin A binding sites as well [13]. All members of the family of serine/threonine kinases share in common a conserved, cysteine-rich, circa 50-amino acid long domain within their regulatory C1 domain [14]. An unusual structural feature of the cysteine-rich domains is the coordination of six cysteines, together with 2 histidines by 2 Zn2+ ions into dual zinc-finger-like structures, with the motif HX12CX2CXnCX2CX4HX2CX7C, called a RING finger. These structures constitute the activation domains, where diacyl-glycerol (DAG), the classic second messenger of PKCδ, binds [15], or where RAS, an activator of Raf family members, attaches [16]. The RING-finger domain is also where vitamin A binds, although at a site separate from the classical second-messenger binding site [17]. In contrast to DAG and RAS, vitamin A does not activate serine-threonine kinases per se. However, the alternate activation mode by oxidizing agents such as H2O2, discovered by Nishizuka three decades ago [18, 19], was found by us to require vitamin A as essential co-factor [20].
All mammalian PKC and Raf isoforms carry at least one RING-finger with an associated vitamin A binding site. These structures are evolutionarily conserved from Mammals to Drosophila, implying functional relevance. Among the kinase isoforms studied in detail all registered positive in the vitamin A-catalyzed redox activation assay [21]. It is therefore likely that the emerging paradigm, ie the redox activation of RING-finger bearing kinases with participation of vitamin A cofactor, finds broad usage in redox signal networks. In the following we review the principles of this paradigm, as it applies to a unique PKCδ signaling complex in mitochondria, the PKCδ signalosome, distinct of PKCδ signals elsewhere in the cell.
Composition and redox-activation of the murine PKCδ signalosome.
A large pool of inactive PKCδ exists in the cytosol from where a portion translocates to the intermembrane space of mitochondria. Here PKCδ associates with p66Shc, a versatile signal adaptor [22], using the SH2 domain of the latter to attach to a phospho-tyrosine residue (Tyr 332 in mice) of PKCδ [23]. Cytochrome c also binds to the p66Shc assembly platform, using a hydrophobic interaction with glutamic acid residues, E132 and E133 [24]. This arrangement juxtaposes cytochrome c towards PKCδ, thus facilitating the site-specific oxidation of the PKCδ RING-finger, leading to the activation of the PKCδ enzyme, independently of the DAG second-messenger. This alternate, oxidation-based activation mechanism entails the transfer of electrons from the PKCδ RING-fingers to cytochrome c. However, despite being tethered to the same platform in close proximity the passage of electrons proceeds too slowly between these two proteins to be physiologically useful. Nature overcame this problem by deploying vitamin A as catalyst. Vitamin A attaches to both PKCδ RING-finger domains via its head group, the β-ionone ring. The tail, comprising a system of conjugated double bonds, is thought to contact the juxtaposed cytochrome c, thereby establishing an electronic bridge [25] that accelerates the passage of electrons by several orders of magnitude (Figure 1).
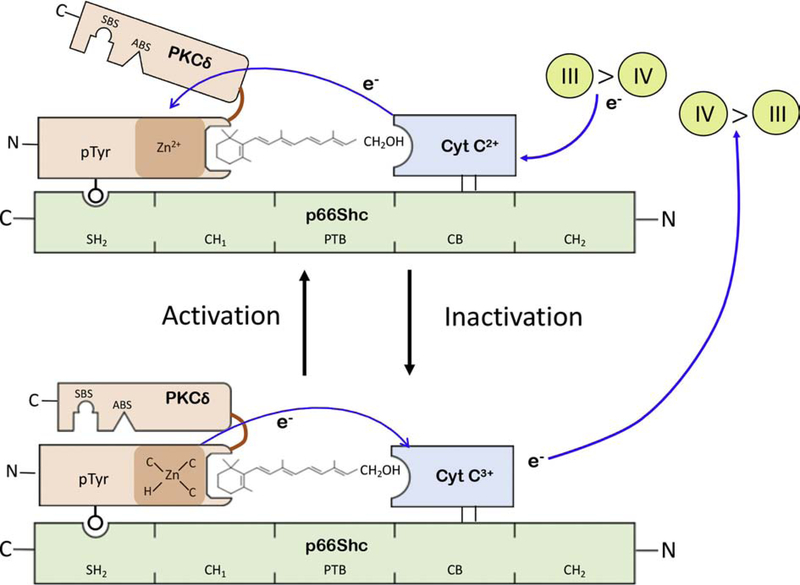
Figure 1: Model of the PKCδ/retinol signalosome in mitochondria.
The PKCδ signalosome is shown as an assemblage of two proteins, PKCδ (in brown) and cytochrome c (in blue), on the p66Shc platform (in green). The phosphotyrosine residue 332 of PKCδ binds the SH2 domain of p66Shc, and cytochrome c (cyt c) connects to the p66Shc CB (cytochrome c binding domain) by hydrophobic interaction. Retinol occupies dedicated binding pockets on PKCδ which coincides with the two cysteine-rich activation domains (CRD) of this kinase, only one them being shown for clarity. The CRD contains two Zn2+ ions, configured into a rigid zinc-finger (also known as RING Finger) by chelation of each Zn cation with 3 cysteinyl-sulfhydryl anions (C), and one histidinyl anion (H). In mitochondria PKCδ is activated by oxidation of RING-finger cysteines, presumably generating one or two cystine residues, depending on whether one or both zinc-coordination centers are oxidized. Oxidation entails the transfer of at least one pair of electrons (e-) from cysteines to cyt c3+. Electron transfer is catalyzed by vitamin A (retinol) which bridges the gap between the PKCδ CRD and cyt c. In consequence to RING-finger oxidation the CRD undergoes a local structural change resulting, when amplified by allosteric means, in the retraction of the regulatory domain from the catalytic domain and providing access to substrate binding (SBS) and ATP binding sites (ABS).
The unfolding process from globular, inactive PKCδ to the active enzyme is postulated to be reversible. This step presumably entails the reduction of cystines (generated during the activation step) by vitamin A-catalyzed transfer of electrons from cyt c2+ that accumulates in the IMS during periods of high respiratory activity. It is presently unknown whether the restoration of the RING-finger, along with the large-scale restoration of the globular protein, occurs spontaneously or, as seems likely, involves auxiliary chaperones. It is noteworthy that the silencing of the bacterial HSP33 protein refoldase is assisted by the DNAK/DNAJ/GrpE auxiliary chaperone machinery. Refolding PKCδ might require orthologous auxiliary chaperones.
That retinol is electronically coupled to the RING-finger domains was demonstrated with recombinant versions of PKC C1 domains. In the presence of retinol these peptides displayed quenching of intrinsic protein (tryptophan) fluorescence and resonance energy transfer of intrinsic protein fluorescence (FRET) [13, 21]. In fact, the quenching of fluorescence elicited by UV irradiation at 280nm of a conserved tryptophan situated near the retinol binding cavity forms the basis of measurements of retinoid binding affinities to recombinant RING-finger proteins of a number PKC isoforms, as well as of cRAF [13]. Binding constants ranged from 20 nM (PKCα C1A, PKCμ C1A, and cRAF C1 domains) to 65nM (PKCδ C1A) or 95nM (PKCδ C1B), to name a few examples [13]. The recorded binding constants represent nominal values since these were measured with recombinant peptides. The true values in native PKC and Raf proteins may differ. However, in operational terms vitamin A binding is essential for PKC and Raf function, since genetic elimination of vitamin A binding sites abolished redox activation [20, 26], while preserving activation by second-messengers, phorbol esters, or RAS, respectively.
Vitamin A receptor sites on PKCδ do not only bind vitamin A alcohol (retinol) but also a number of other retinoids. However, retinol is the most important response modifier for PKCδ. As mentioned above, binding occurs via the β-ionone head group, and hence retinoids sharing the β-ionone ring, which include retinaldehyde, retinoic acid, and certain β-apocarotenoids all can bind and co-activate PKCδ in vitro, throwing this phenomenon into confusion as to its biological specificity. Nature largely resolved this problem by differentiating cytoplasmic, including mitochondrially located, vitamin A alcohol receptors of PKC and Raf families from those of the nuclear retinoic acid receptor families, RARs and RXRs. Thus, retinoid binding affinities for PKC and Raf kinases measure in the ten’s of nanomoles [13], and hence are two orders of magnitude lower than those of retinoic acid for retinoic acid receptors (ranging from 0.1 to 1 nM). Therefore, in order to work as PKC or Raf cofactors local retinol concentrations must reach tens of nanomoles, which is achievable since vitamin A is ubiquitously distributed in micromolar concentrations in the circulation. On the other hand, because retinoic acids are produced locally near their sites of usage at concentrations never exceeding the low-nanomolar range, and because they are moreover sequestered to these sites by the degrading enzyme, Cyp26 [27], they are unlikely to interfere with retinol-mediated signaling events in mitochondria. However, in the event that retinoids, such as retinaldehyde and anhydroretinol (AR) were to accrue in pathological circumstances in concentrations above 10 to 100 nanomoles they may out-compete retinol via the shared the β-ionone ring. As experiments with cultured cells show, several of these retinoids function as competent PKCδ co-activators. However, as described below, the long-term (>30 min) stimulation of cells with retinoids other than retinol itself causes cytotoxicity by an as yet incompletely explained mechanism [28]. To what extent these in vitro inhibitory effects are biologically relevant is still uncertain.
Role of RING-fingers in PKCδ activation.
Substantial biochemical evidence supports the notion that the 2 PKCδ RING-fingers tandemly arranged within the regulatory C1 domain principally function as activation domains. In this regard, the PKCδ RING-finger is reminiscent of the activation domain of the bacterial Hsp33 chaperone which, like PKCs, comprises a zinc-coordinated structure as its key feature [29]. The Hsp33 zinc-finger is simpler in structure, comprising a single zinc ion chelated by four cysteines [30], as compared to the RING-finger which contains 2 zinc centers, each coordinated by three cysteinyl-plus one histidinyl-anion [15]. Nevertheless, the two structures are true functional orthologs. Because these ligands are derived from a single peptide and are arranged in space at the four corners of a perfect tetrahedron with the Zn2+ ion at its center, Zn-chelation gives rise to rigid structures that dominate the overall tertiary structure of PKCs. RING-fingers are stable as long as intracellular reducing conditions prevail. Jakob, Bardwell et al. were the first to point out the dynamic nature of zinc-finger structures in vivo by demonstrating that zinc-coordination centers dissolve under oxidizing conditions, but reform when reducing conditions return [29]. This concept of a reversible redox switch underlies the dynamic regulation of the Hsp33 chaperone [31]. Whether the PKCδ RING-finger structures are subject to a similar, redox-dependent dynamism, including especially full reversibility, has not been established in ultra-structural detail, but it was noted that PKC activation was linked to changes in their zinc-coordination centers.
In the inactive state, PKCs are subject to the common paradigm of auto-inhibition, where the regulatory domain sterically obstructs the kinase domain. In essence, activation entails the retraction of the regulatory domain from the catalytic domain, to uncover substrate recognition surfaces, to gain access to ATP binding sites and to dislodge the pseudo substrate from the catalytic cavity. A stepwise unfolding process was described in exemplary detail for the PKCβII isoform by Hurley and co-workers [32]. In their model Ca++ release triggers the translocation of PKC from cytosol to the plasma membrane where it becomes anchored to membrane lipids, phosphatidyl serine and phosphatidylinositol (4,5)-bisphosphate, by the synergistic action of two C2-domain associated receptors. Next, membrane-associated di-acyl-glycerol (DAG) binds both tandem RING-finger subunits, promoting the further unfolding of the regulatory domain to yield the active enzyme. Although widely accepted, this model does not allow for conformation changes induced within the RING-finger domains in response to DAG binding. To the contrary, our group showed that activated PKCα recovered by immunoprecipitation from phorbol-ester stimulated live cells had lost a substantial portion of its zinc [33], indicating that zinc-coordination centers dissolved during the activation process. Follow-up studies showed that recombinant αClb RING-finger domain structures responded to phorbol-12-myristoyl-13-acetate (PMA) in a dose-dependent manner by shedding zinc ions, but not to phorbol-13-acetate (P13A) [34]. Recombinant αClb also released zinc in response to DAG, and this effect was enhanced by simultaneous stimulation with phosphatidyl-serine. The DAG-nonbinding PKCζ Cl domain was unresponsive to PMA. Full-length recombinant PKCα protein shed 2 equivalents of zinc upon PMA, but not P13A, stimulation, acquiring at the same time full phospho-transferase activity in vitro. A chimeric Hsp33 reporter protein, in which the mammalian PKCε Clb RING-finger replaced the bacterial zinc-finger, yielded a chaperone that underwent dramatic conformation change upon stimulation with PMA, but not P13A, as revealed by NMR [34]. This Hsp33 chimeric chaperone moreover responded to PMA by gaining the ability to refold aggregated test protein into the soluble, native conformation, just as the native Hsp33 would do in response to oxidation. In toto, these findings strongly support our hypothesis that PKCδ activation is associated with the reorganization of RING-finger peptides from their rigid, zinc-coordinated forms to flexible structures. Whether initiated by PMA, DAG, or oxidation, the dissolution of the RING-finger fold may represent the primary event in the series of unfolding steps recorded by Leonard et al [32] that yield the active enzyme.
Mitochondria lack DAG generating enzymes. Hence the activation of PKCδ, residing within mitochondria, is accomplished by the alternate pathway via oxidation of PKCδ RING-fingers. Although oxidation by diffusible oxidants, such as peroxide, is the main activation mechanism in bacteria this is not permissible in intact mammalian cells because random oxidation of sensitive cysteines and/or methionines located in the catalytic domain can be expected to inactivate PKCδ. This was in fact shown for several PKC isoforms [35]. In lieu of soluble oxidants, mammalian cells evolved the use of oxidoreductases, because these enzymes possess intrinsic site-recognition, much as phosphatases, kinases, methylases or demethylases do. The PKCδ signalosome is a cogent example of how pin-point precise oxidation can be achieved, not only with regard to site selectivity (ie the RING-finger) but also to isoform selectivity. As mentioned above, PKCδ was found to assemble in the mitochondrial intermembrane space with cytochrome c on the p66Shc platform, bringing the oxidizing agent (cytochrome c3+) into close proximity with the PKCδ substrate [36]. Moreover, retinol bound to the RING-finger domain, marks this structure as the target of oxidation, at the same time creating a conduit for efficient transfer of electrons from PKCδ to oxidized cytochrome c3+.
Our model is well vetted by biochemical and genetic experiments [2, 26] although ultra-structural analyses are still outstanding. Genetic ablation of PKCδ, p66Shc, or cytochrome c in mouse embryo fibroblasts (MEFs) all rendered the PKCδ signalosome inoperative. Genetic complementation by re-introduction of the respective transgenes normalized signaling. The withdrawal of retinol from cell cultures similarly silenced PKCδ signaling. Replenishment with vitamin A (retinol) reactivated the PKCδ signalosome in a dose-dependent manner, with an optimum dose at 1 μM – close to the physiological concentration of vitamin A in plasma. Phosphotyrosine Y332 of PKCδ binds the SH2 domain of p66Shc [23]. Disruption of this interaction by Y332F mutation attenuated signaling, as did the double mutation E132Q ; E133Q on p66Shc that abolishes the binding of cytochrome c to p66Shc [24]. Thus, the integrity of the fully assembled signal complex is critical for the activation and forward signaling of PKCδ. It is noteworthy that the above listed genetic disruptions (except outright PKCδ deletion), including the removal of vitamin co-factor, are readily overridden by pharmacomimetics, such as PMA [2].
We confirmed that redox activation of PKCδ was mediated by cytochrome c3+. Mitoplasts lose cytochrome c along with other small molecular components from the intermembrane space, but retain their intact respiratory machinery. Replenishing mitoplasts from the exterior space with the combination of cytochrome c3+ and retinol restored respiration, while cytochrome c2+ was ineffective [2]. Control mitoplasts of cells harboring the p66Shc E132Q; E133Q mutations did not normalize respiration in response to external cytochrome c3+, indicating that physical binding of cytochrome c to p66Shc was a prerequisite. Cytochrome c3+ mediated PKCδ activation of mitoplasts correlated with increased PKCδ auto-phosphorylation, along with decreased PDH E1 phosphorylation, signifying coordinately increased PKCδ and PDH activities [2]. The changes in in vivo phosphorylation patterns also correlated with up-regulation of oxygen consumption, and with augmentation of ATP synthesis. Considered in toto, these observations support the notion that in intact mitochondria PKCδ is activated by cytochrome c-dependent redox action.
We postulate that cytochrome c3+-mediated activation of PKCδ is reversible. Specifically, we propose that the oxidized RING-finger associated with the active form of PKCδ is reduced by cytochrome c2+, restoring the zinc-coordination centers and initiating the process that returns the molecule to its inactive conformation. Silencing PKCδ would depend on conditions when the cytochrome c2+ concentration in the IMS would prevail over that of the oxidized cytochrome c species. While these dynamics are unknown in detail, there is evidence that oxidation of cytochrome c2+ by cytochrome c oxidase is the rate-limiting, final step in the conveyance of electrons to oxygen [37, 38]. By implication, low respiration would be associated with a high ratio of cytochrome c3+ : c2+, whereas at high respiratory activity the ratio would shift towards prevalence of cytochrome c2+ > c3+. Linking PKCδ activity to the dynamics of the cytochrome c redox status represents the beginnings of a ratiometric feedback/feedforward loop that ties glycolytic fuel production to the workload of the respiratory chain.
Forward target of PKCδ signaling.
In the above studies on retinoid dependence mitochondria were energized by pyruvate/malate, placing the target of retinoid action upstream of the PDHC. By contrast, use of succinate in lieu of pyruvate rendered mitochondrial function retinol-independent, also suggesting that the likely target of PKCδ signaling was upstream of the Krebs cycle, effectively ruling out components of the ETC, especially complex I, as the direct target of PKCδ/retinoid action, and supporting instead the function as upstream regulator of PDHC [26] (Figure 2).
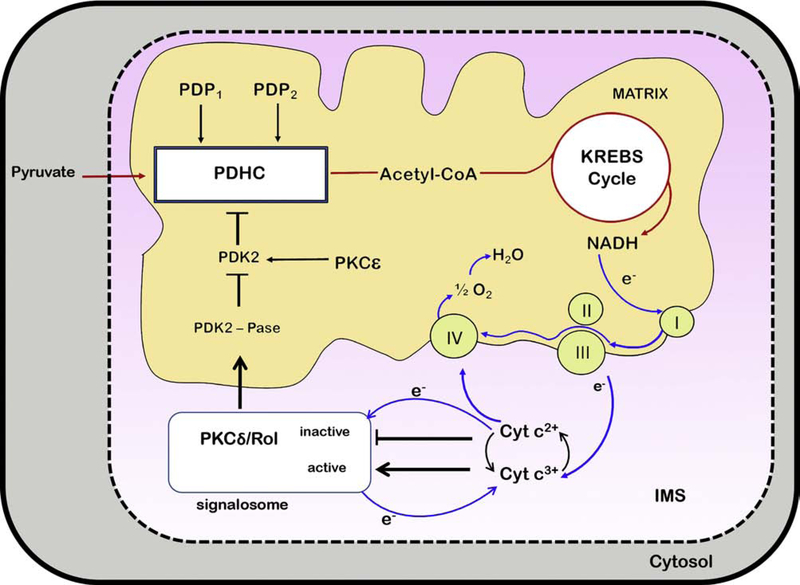
Figure 2: Dynamic model of activation and proposed de-activation of PKCδ in mitochondria.
The PKCδ signalosome comprises PKCδ as the signal actuator, p66Shc as assembly platform, cytochrome c as oxido-reductase, and retinol as electron-bridge catalyzing the transfer of electrons from PKCδ to cyt c3+, initiating enzyme activation, or in reverse from cyt c2+ to PKCδ, silencing PKCδ activity. The PKCδ signal complex is situated in the IMS and signals forward to activate an as yet unidentified matrix-located phosphatase. The latter dephosphorylates the pyruvate dehydrogenase kinase 2 (PDK2), causing its inactivation. As PDK2 acts as negative regulator of PDHC the silencing of this kinase allows the canonical pyruvate dehydrogenase phosphatases, (PDP1 or 2) to dephosphorylate the E1 regulatory subunit of pyruvate dehydrogenase, thereby increasing the enzymatic conversion of pyruvate to acetyl-coenzyme A. Processing of the latter generates NADH which donates the high-energy electrons that pass through the ETC and generate the proton-motive force that drives ATP production (not shown in the cartoon). Electrons are then transferred in complex III to cyt c3+, reducing the latter to cyt c2+, which is transported to complex IV for final disposal by transfer of electrons to molecular oxygen, in the process regenerating cyt c3+.
The model proposes a feedback/feedforward loop that positively and negatively regulates the fuel flux entering the KREBS cycle. The key assumption is that PKCδ monitors the redox state of the cytochrome c pool. PKCδ becomes activated by oxidation of its RING-finger domain when cyt c3+ prevails over cyt c2+, reflecting the demand for ATP generation. It was proposed that cytochrome c oxidase is the rate-limiting step in electron transfer to oxygen, implying dynamic shifts in the ratios of cyt c3+ to 2+. Therefore, at rest most cytochrome c would comprise the oxidized form, whereas at high respiratory load reduced cytochrome c would come to dominate. Hence, when cyt c2+ accumulates in the IMS, the redox polarity in the PKCδ signalosome is reversed, allowing the reduction of the RING-finger cystines, restoring zinc-chelation centers and initiating the de-activation of PKCδ. Consequently PDHC output is reduced, and fuel flux diminished. While incompletely understood the PKCδ signaling system emerges as an important ratiometric regulatory circuit that can accelerate acetyl-CoA production when energy demands are high, but also curtail fuel flux when the danger of ETC overload is imminent, threatening the release of damaging levels of ROS.
If real-time regulation of PDHC by PKCδ can be likened to the cruise control of automobiles, our model also has the makings of a second, more resolute downregulation akin a shift to lower gear. Located in the matrix, the PKCε kinase phosphorylates PDK2, thereby activating this negative regulator of the PDHC, antagonizing PKCδ. Although normally subjugated to PKCδ, during periods of stress added amounts of PKCε are recruited to mitochondria, essentially switching the control circuitry towards suppression of PDHC. The mechanism of PKCε activation, and the nature of potential companion molecules are unknown, as are modes of integration into the overall PKC control circuit.
In further detailed studies to define physiologically meaningful PKCδ targets we employed live cells, isolated mitochondria, and mitoplasts. Focusing on key components, including PDK2, PDHC and PKCδ itself, we determined unbiased phosphorylation patterns by 2D gel electrophoresis, recorded the phosphorylation status of known functional sites, determined, where possible, the intrinsic phosphotransferase capacities, and related measurements with downstream metabolic parameters, including oxygen consumption and ATP synthesis. To further consolidate findings, we applied an array of cell lines with select genetic modifications of pertinent genes, including global gene knock-outs, RNAi-knockdowns, and site-specific function-disrupting mutations. The results were entirely congruent with one another, and supported the model of figure 2 where the PKCδ activation results in PDK2 inactivation, consequently up-regulating PDHC activity. Our results are in conflict with a study which showed that purified PKCδ was capable of phosphorylating recombinant PDK2 protein in a cell-free system [39]. However, such in vitro results are biologically meaningless unless substantiated in intact cells.
The PKCδ signal pathway proposed by us melds seamlessly with the canonical PDHC regulatory system, that involves a set of 4 PDH kinases (PDK1 – 4) and 2 PDH phosphatases (PDP1,2) [40–43]. But it also necessitates a matrix-located PDK2 phosphatase acting upstream of PDK2, the identity of which remains unknown. The mechanism of how PKCδ signals to the PDK2-phosphatase across the inner membrane is also unknown. On the other hand, following the tenet that every phosphatase must be paired with a kinase, and vice versa, we recently identified PKCε as a strong candidate for the PDK2 kinase, as will be described below.
Subversion of the PKCδ signal system by natural vitamin A derivatives and synthetic analogs.
The major metabolic derivatives of retinol are retinoic acid, acting as transcriptional mediator, and retinaldehyde, forming the universal visual pigment in the vertebrate eye, as well as in the compound eye of insects. Additional common derivatives of retinol are two hydroxylated retinoids: 14-hydroxy-retro-retinol (14-HRR) [44] and 13,14-dihydroxy-retinol (13,14-DHR) [45]. A third derivative is AR, generated by enzymatic dehydration of the terminal primary alcohol group of retinol by a de-hydratase [46]. Apocarotenoids, generated by asymmetric enzymatic cleavage of β-carotenoids, further expand the inventory of cellular retinoids [47]. These vitamin A derivatives were identified by analyses of vitamin A metabolism in normal tissues, normal cell lines and cancer cells, but a systematic study of their tissue distribution is still outstanding. Moreover their physiologic purpose has remained unclear. Vitamin A and its natural derivatives 14HRR, 13,14DHR and AR, as well as the synthetic retinoid fenretinide (4HPR) all contain the β-ionone ring that enables binding to the RING-Finger domains of PKCδ, as well as those to other PKC isoforms. As these 5 retinoids have similar binding affinities they can act as competent co-factors during redox activation of PKCδ. However, the consequences of interaction with PKCδ differ dramatically, depending on the chemical structure of the retinoid ligands. Hydroxylated retinoids, retinol, 14-HRR and 13,14-DHR support long-term cell growth, although their inverted U-shape dose-response curves suggest that at supra-optimal doses they exert considerable cytotoxicity, just as retinol does [44, 45]. By contrast, AR and fenretinide are acutely cytotoxic throughout the entire dose range [28, 46, 48]. What accounts for the different outcomes of AR- or fenretinide-mediated versus retinol-mediated PKCδ activation is not fully understood, but contains a potentially important lesson in vitamin A physiology. We propose that the reason for retinoid toxicity is subversion of the PKCδ signaling system, leading to mitochondrial overload.
In our model the high-dose induced retinol cytotoxicity can be attributed to a shift in the equilibrium between apo-PKCδ and holo-PKCδ (ie. the retinol-free, inactive PKCδ species and the retinol-bound, active PKCδ species, respectively). By the law of mass action, cells exposed to supra-optimal retinol concentrations, assuming unobstructed partition to mitochondria, would have increased holo-PKCδ concentrations and hence generate a comparatively stronger signal to PDH, driving fuel flux to levels that elicit chronic mitochondrial overload. As our experimental evidence shows, cultured cells supplemented with retinol concentrations above the physiological optimum display dramatically lower oxygen consumption and ATP synthesis compared to cultures with physiological retinol concentrations [26].
While both high-dose vitamin A- and AR-mediated toxicities can be attributed to mitochondrial overload [49], as defined by increased NADH production in the Krebs cycle, inner membrane hyperpolarization [50], and release of damaging ROS [51, 52] [53] the underlying mechanisms differ. The former can be explained as shifts in the equilibrium from apo- towards holo-PKCδ, increasing PKCδ signal strength, as described above. By contrast, the cytotoxicity mediated by AR and fenretinide results, we propose, from a unique manner in which these 2 retinoids subvert the control of PKCδ. The dynamic model of figure 1 stipulates that retinol catalyzes the forward redox reaction resulting in the conversion of inactive mitochondrial PKCδ to the active enzyme. However retinol also catalyzes the necessary reverse reaction that inactivates this enzyme as soon as demands for substrate are met, but when excess might become a liability. AR on the other hand, while capable of catalyzing the forward reaction as demonstrated by us, might not be able to drive the reverse reaction, thereby leaving the enzyme stranded in its active state. It should be noted that the oxidation of RING-finger cysteines that underlies PKCδ activation likely is an exergonic reaction, whereas the reduction of oxidized cysteines, a step postulated to precede kinase inactivation, is endergonic.
In catalyzing these reactions retinoids can be thought of as devices that shuttle electrons from PKCδ to cytochrome c, as well as in the opposite direction. The concept becomes clearer if one assumes that energy, not electrons, is being transferred. We observed that retinol bound to PKCδ becomes electronically coupled to the RING-finger, potentially enabling resonance energy transfer (RET). A key consideration is that the energy quanta any given retinoid can absorb is determined by the length of its linear polyene. As a rule, the longer the polyene, measured in terms of number of conjugated double bonds, the lower the energy absorption. This is reflected in the redshift of absorption spectra. As a rule of thumb for each C-C=C unit added to the polyene the absorption maximum gains circa 20 nm. Retinol (λmax = 325 nm) possesses 4 conjugated double bonds, compared to 6 in AR λmax = 366 nm) and 5 in fenretinide. Consequently, the quantum of energy retinol can adsorb by RET from PKCδ and transfer to cytochrome c3+ is substantially larger than what AR or fenretinide can absorb. These considerations support our model that AR and fenretinide are capable of co-activating PKCδ, as in fact shown by the experiment [28], but lack the energy to de-activate PKCδ in a timely manner. Under these circumstances mitochondrial overload ensues, generating damaging amounts of ROS, that lead to programmed cell death with the hallmarks of acute mitochondrial membrane hyperpolarization followed by depolarization, PARP-1 dependent ATP deprivation, opening of the transition pores, cytochrome c release, and eventually programmed cell death [49]. It is noteworthy that retinol at physiological concentrations is able to rescue cells when supplied within the first hour after AR supplementation, presumably by competing AR off the PKCδ receptor sites and thereby restoring normal, bi-directional, control of the PDHC [49].
In addition to direct effects on PKCδ fenretinide also exerts an indirect influence on the PKCδ signal cascade. This is mediated by a second mitochondrial kinase, PKCε, localized in the matrix. While the mode of fenretinide-mediated PKCε activation is currently unknown, this kinase was found to act as a negative regulator of the PDHC, thereby antagonizing PKCδ signaling [54]. On preliminary evidence PKCε targets PDK2, which is also the downstream target of PKCδ, with the proviso that the former activates, whereas the latter inactivates PDK2 (Figure 2). The observation that PKCε activates PDK2 raises the possibility that PKCδ represents the long sought PDK2-kinase [28].
Compared to the real-time action of PKCδ on the PDHC, the fenretinide-mediated downregulation of PDHC via PKCε occurs with delayed kinetics. The fenretinide effect is bi-modal, consisting during the first 30 minutes of a phase when PKCδ becomes hyper-activated. This is followed by the second phase when PKCδ action dominates over that of PKCδ. Observations that during the secondary phase substantial amounts of PKCδ are recruited from the cytoplasm to the mitochondrial matrix suggest an explanation of how the balance in the PKCδ/ε Yin-Yang signal system can be shifted towards suppression of the PDHC [28]. That this shift coincides with the intense oxidative stress further implies that cells sensing imminent danger down-regulate oxidative phosphorylation in order to mitigate mitochondrial deterioration. The emerging picture is of a system that, similar to the cruise control of an automobile, monitors the internal performance of the ETC and constantly adjusts the OXPHOS machinery via the PKCε signal system to keep the output within safe margins. However, in the event that run-away fuel flux threatens mitochondrial integrity the PKCδ signal is activated to dampen OXPHOS more resolutely, akin to the chauffeur’s shifting to lower gear.
Association of imbalanced PKCδ signalosome with disease.
PKCδ has been directly linked to many pathophysiologies, including metabolic syndrome, reperfusion injury of the ischemic heart, and cancer. Natural overexpression of PKCδ in C57BL/6J mice correlated with the development of insulin resistance, while experimental deletion of PKCδ in muscle conferred resistance to age-related insulin-resistance [55]. Likewise, mice with liver-specific or global ablation of PKCδ displayed increased hepatic insulin sensitivity, increased glucose tolerance and reduced hepatosteatosis, whereas liver-specific overexpression resulted in the opposite phenotype: insulin resistance, glucose intolerance and obesity [55]. For these pathologies the primary target of PKCδ signaling has not been identified, indeed whether single or multiple targets are involved is uncertain. However, a case can be made that the PKCδ signalosome is the common denominator, in as much as type 2 diabetes is a quintessentially mitochondrial disease, and PKCδ is emerging as a prime regulator of glycolytic fuel processing in mitochondria [26]. Thus, the consequences of the above genetic PKCδ overexpression might relate to abnormally high PDHC activity resulting in excessive ROS production by the chronically overloaded ETC. The converse might also apply: the more subdued pace of glycolytic fuel production by an under-stimulated PDHC might engage increased fatty acid beta-oxidation to maintain energy homeostasis, explaining the lean body mass of PKCδ−/− mice [55, 56]. Noteworthy, global ablation of p66Shc generated a mouse strain that partly phenocopies the PKCδ−/− mouse strain [57]. P66Shc ablation in Ob/Ob mice ameliorated several symptoms of metabolic syndrome, including improved glucose tolerance and protection from premature death. These parameters are typically linked to oxidative stress and therefore ascribed to a propensity of p66Shc to control ROS production. Indeed, on the basis of cell-free studies but never demonstrated in intact cells, it was proposed that p66Shc itself possesses intrinsic capacity to generate ROS by catalyzing the transfer of electrons from cytochrome c2+ to oxygen [24, 58]. On the other hand, ablation of p66Shc disrupts the PKCδ signalosome, thereby curtailing the far more important source of mitochondrially produced ROS. Consistent with our model, we showed that in p66Shc−/− MEFs the PKCδ/PDH signal path was blocked, reducing glycolytic fuel flux. Therefore, the reduced redox stress observed with p66Shc−/− mice [57] might be more aptly attributed to overall diminished intermediate metabolism.
PKCδ was implicated in RBP4-mediated insulin resistance [59]. In this condition elevated RBP4 levels observed in the plasma of type 2 diabetic individuals or mice [60, 61] might indirectly, and at times adversely, influence PKCδ signaling. PKCδ activation requires that the vitamin A (retinol) cofactor be physically bound to a defined pocket in the PKCδ activation domain, as described above. Since the retinol binding affinities of PKCδ and RBP4 are similar in magnitude, holo-PKCδ and holo-RBP4 are in equilibrium. Therefore elevations of holoRBP4, occurring in diabetic conditions, leads by the laws of mass action to increases in the proportion of the fully assembled retinol/PKCδ complexes, i.e. the active form of PKCδ. The resulting increase in signal strength to PDHC would push glycolytic fuel production to higher, and potentially unsafe, levels when the overburdened ETC releases pathogenic amounts of ROS. Like in instances of natural PKCδ overexpression [55], damaging ROS levels would exacerbate diabetic conditions. This hypothesis was tested in mice with experimentally-induced muscle-specific overexpression of RBP4 [59]. These mice had up to threefold elevated levels of retinol in muscle. When fed a high fat diet these mice became prone to develop insulin-resistance and glucose intolerance. This phenotype was PKCδ-dependent since ablation of PKCδ in the RBP4-transgenic mice ameliorated insulin-resistance, along with a number of other parameters of metabolic syndrome.
A tale of misinterpretations and missed opportunities?
The emerging model of intrinsic regulation of intermediary metabolism (see cartoon of Figure 2) contains multiple concatenated Yin/Yang control elements: 1. The PDHC, positively regulated by pyruvate dehydrogenase phosphatases, counterbalanced by pyruvate dehydrogenase kinases; 2. Among these PDK2, negatively controlled by a PKCδ dependent phosphatase, but counterbalanced by PKCδ kinase; 3. PKCδ, regulated positively by cytochrome c3+, but held in check by cytochrome c2+. This hierarchical system of checks and balances, more complex than a Calder sculpture, likely evolved to guarantee rapid PDHC activation, but to also curtail PDHC output when excessive fuel flux might jeopardize the integrity of the electron transfer chain. Since several pathophysiological conditions are attributable to imbalances in the control of PDHC, the PKCδ/PKCε kinase system presents a prime target for therapeutic interventions. For instance, the metabolic syndrome is caused by chronic dependence on glucose-derived fuel at the expense of fats. The latter, being unable to be disposed of, accumulate in adipose tissues. Since our model highlights a profound antagonistic relationship between PKCδ and PKCε, should it not be possible to intervene by either reducing PKCδ signaling, or by strengthening PKCε signaling? Our model predicts that either of such interventions should reduce glucose dependence and increase fat utilization, thus effectively countermanding obesity. As mentioned, PKCδ knock-out mice have lean body mass and resist high-fat diet induced insulin-resistance with aging, whereas, conversely, mice with abnormally high PKCδ expression are prone to become obese, glucose intolerant, and insulin resistant [55]. Thus, methods to shift the Yin/Yang balance towards increased PKCε signaling might be of high clinical interest.
In fact the scientific record shows that this concept has already been proven in principle. Unbeknownst to their authors, the promising outcomes of several therapeutic intervention trials with fenretinide [62] might well be based on adjustments within the PKCδ/PKCε signal system that diminish PDHC output. As described above, sustained fenretinide treatment of cells promotes the recruitment of PKCε into the mitochondrial matrix, resulting in decreased PDHC function via the activation of PKD2, the negative PDHC regulator.
Fenretinide was widely investigated in cancer therapies, based on findings in cell cultures that it preferentially killed malignantly transformed cells, compared to normal cells [63]. Fenretinide was known to interfere with complex I function, but it was only recently found that its true mode of action was upstream of complex I, namely suppression of PDHC function [28]. Clinical trials to control several types of cancer were initially rewarded with favorable outlooks buoyed by good pharmacological parameters, including excellent bioavailability and low toxicity, but resulted in only sporadic clinical improvement. Clinical researchers turned their attention to cancer prevention, specifically asking in one study whether secondary breast cancers in women could be prevented by long-term treatment with fenretinide [64]. Encouragingly, the incidents of secondary breast cancers were reduced, but analyses of the data revealed that weight loss among obese postmenopausal women was the true reason for the lowered cancer incidents. Chronic fenretinide treatment, it transpired, reduced adiposity, a result consistent with our model.
Observations raised by Yang et al in a mouse model of HFD-induced obesity and type 2 diabetes [60] might be relevant here. As this syndrome was known to be driven by elevated serum levels of holoRBP4, chronic treatments with fenretinide were undertaken under the premise that this retinoid would disrupt the TTR/RBP4/retinol transporter leading to efficient renal secretion of RBP4 [65]. Lowered RBP4 levels indeed correlated with improved insulin sensitivity and decreased adiposity. However, as shown with RBP4 knockout mice on HFD, the adiposity-reducing effect of fenretinide was independent of RBP4, and might well result from PKCε-mediated attenuation of PDHC function, as predicted by our model.
Outlook
Mitochondria are the ubiquitous energy-producing organelles. They harbor, so far without exception, the PKCδ signalosome as an important component of an intrinsic energy-regulating circuit. Unsurprisingly mitochondrial dysfunction has been linked to a plethora of pathologies, many of which involve excessive ROS production as a primary etiologic factor. These include the metabolic syndrome, atherosclerosis, neurodegeneration, macula degeneration, cardiac ischemia reperfusion injury, to name a few, but also normal aging. It is unclear how injury to mitochondria that are common to all nucleated cells can result in tissue-specific diseases. In this regard the discovery of the PKCδ/s regulatory system is expected to open new avenues of inquiry. The finding that turning off PKCδ signaling in a timely manner is as important as efficient activation represents a conceptual advance. Currently the mechanism of PKCδ deactivation is unclear, but on thermodynamic grounds auxiliary protein foldases, orthologs to the DNAJ / DNAK / GrpE chaperone machinery of bacteria [66] [67], must exist as additional components of the PKCδ signalosome and should be investigated. When dysfunctional, this postulated refolding machinery might link PKCδ signaling to yet other pathologies, in particular to protein refolding diseases, such as Alzheimer [68] and Parkinson disease [69, 70], for which mitochondrial influences are already apparent. As our understanding of the contributions of PKCδ signaling to mitochondrial integrity and function deepens, opportunities for therapeutic interventions in degenerative diseases are apt to follow.
Highlights.
The mitochondrial PKCδ/retinol signalosome controls PDHC output, hence fuel flux
The PKCδ activation domains comprise RING-fingers that contain retinol-binding sites
Retinol-catalyzed oxidation of RING-fingers with cytochrome c3+ activates PKCδ
Linking PKCδ activity to cytochrome c redox state creates regulatory feedback loop
Reversibility of PKCδ signaling ensures safe operation of the respiratory chain
Footnotes
Declaration of Interest Statement
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- [1].Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G, Cyclic AMP produced inside mitochondria regulates oxidative phopshorylation, Cell Metabolism, 9 (2009) 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Acin-Perez R, Hoyos B, Gong J, Vinogradov V, Fischman DA, Leitges M, Borhan B, Starkov A, Manfredi G, Hammerling U, Regulation of intermediary metabolism by the PKCdelta signalosome in mitochondria, FASEB J, 24 (2010) 5033–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Acin-Perez R, Gatti DL, Bai Y, Manfredi G, Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation, Cell Metab, 13 (2011) 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McCollum E, Davis M, The necessity of certain lipins in the diet during growth, J. Biol. Chem, 15 (1913) 167–175. [Google Scholar]
- [5].Osborne T, LB M, The relation of growth to the chemical constituents of diet, J. Biol. Chem, 15 (1913) 311–326. [Google Scholar]
- [6].Wolbach SB, Howe PR, Tissue changes following deprivation of fat-soluble A vitamin, J. Exp. Med, 42 (1925) 753–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wald G, The molecular basis of visual excitation, Nature, 219 (1968) 800–807. [DOI] [PubMed] [Google Scholar]
- [8].Giguere V, Ong ES, Segui P, Evans RM, Identification of a receptor for the morphogen retinoic acid, Nature, 330 (1987) 624–629. [DOI] [PubMed] [Google Scholar]
- [9].Petkovich M, Brand NJ, Krust A, Chambon P, A human retinoic acid receptor which belongs to the family of nuclear receptors, Nature, 330 (1987) 444–450. [DOI] [PubMed] [Google Scholar]
- [10].Buck J, Ritter G, Dannecker L, Katta V, Cohen SL, Chait BT, Hammerling U, Retinol is essential for growth of activated human B cells, J Exp Med, 171 (1990) 1613–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Garbe A, Buck J, Hammerling U, Retinoids are important cofactors in T cell activation, J Exp Med, 176 (1992) 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].O’Connell M, Chua R, Hoyod B, Buck J, Chen CQ, Derguini F, Hammerling U, Retro-retinoids in regulated cell growth and death, J. Exp. Med, 184 (1996) 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoyos B, Imam A, Chua R, Swenson C, Tong G-X, Levi E, Noy N, Hammerling U, The Cysteine-rich Regions of the Regulatory Domains of Raf and Protein Kinase C as Retinoid Receptors, J. Exp. Med, 192 (2000) 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hommel U, Zurini M, Luyten M, Solution structure of a cysteine rich domain of rat protein kinase C, Nat. Struct. Biol, 1 (1994) 383–388. [DOI] [PubMed] [Google Scholar]
- [15].Zhang G, Kazanietz MG, Blumberg PM, Hurley JH, Crystal Structure of the Cys2 Activator-Binding Domain of Protein Kinase C8 in Complex with Phorbol Ester, Cell, 81 (1995) 917–924. [DOI] [PubMed] [Google Scholar]
- [16].Mott HR, Carpenter JW, Zhong S, Ghosh S, Bell RM, Campbell SL, The solution structure of the Raf-1 cysteine-rich domain: a novel ras and phospholipid binding site, Proc Natl Acad Sci U S A, 93 (1996) 8312–8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hoyos B, Jiang S, Hammerling U, Location and functional significance of retinol binding sites on the serine/threonine kinase, cRaf, J. Biol. Chem, 280 (2005) 6872–6878. [DOI] [PubMed] [Google Scholar]
- [18].Nishizuka Y, Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C, Science, 258 (1992) 607–614. [DOI] [PubMed] [Google Scholar]
- [19].Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y, Activation of protein kinase C by tyrosine phosphorylation in response to H2O2, Proc. Natl. Acad. Sci, 94 (1997) 11233–11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hoyos B, Imam A, Korichneva I, Levi E, Chua R, Hammerling U, Activation of c-Raf Kinase by Ultraviolet Light, J.Biol. Chem, 277 (2002) 23949–23947. [DOI] [PubMed] [Google Scholar]
- [21].Imam A, Hoyos B, Swenson C, Levi E, Chua R, Viriya E, Hammerling U, Retinoids as ligands and coactivators of protein kinase C alpha, FASEB J, 15 (2001) 28–30. [DOI] [PubMed] [Google Scholar]
- [22].Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, Finkel T, The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism, J Biol Chem, 281 (2006) 10555–10560. [DOI] [PubMed] [Google Scholar]
- [23].Morita M, Matsuzaki H, Yamamoto T, Fukami Y, Kikkawa U, Epidermal growth factor receptor phosphorylates protein kinase C δ at Tyr332 to form a trimeric complex with p66Shc in the H2O2-stimulated cells, J Biochem, 143 (2008) 31–38. [DOI] [PubMed] [Google Scholar]
- [24].Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pellicia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG, Electron Transfer between Cytochrome c and p66Shc Generates Reactive Oxygen Species that Trigger Mitochondrial Apoptosis, Cell, 122 (2005) 221–233. [DOI] [PubMed] [Google Scholar]
- [25].Frank HA, Brudvig GW, Redox functions of carotenoids in photosynthesis, Biochemistry, 43 (2004) 8607–8615. [DOI] [PubMed] [Google Scholar]
- [26].Acin-Perez R, Hoyos B, Zhao F, Vinogradov V, Fischman DA, Harris RA, Leitges M, Wongsiriroy N, Blaner WS, Manfredi G, Hammerling U, Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis., FASEB, 24 (2010) 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H, The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo, Genes Dev, 15 (2001) 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gong J, Hoyos B, Acin-Perez R, Vinogradov V, Shabrova E, Zhao F, Leitges M, Fischman D, Manfredi G, Hammerling U, Two protein kinase C isoforms, delta and epsilon, regulate energy homeostasis in mitochondria by transmitting opposing signals to the pyruvate dehydrogenase complex, FASEB J, 26 (2012) 3537–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jakob U, Muse W, Eser M, Bardwell JC, Chaperone activity with a redox switch, Cell, 96 (1999) 341–352. [DOI] [PubMed] [Google Scholar]
- [30].Ilbert M, Graf PCF, Jakob U, Zinc Center as Redox Switch—New Function for an Old Motif, Antioxidants and Redox Signaling, 8 (2006) 835–846. [DOI] [PubMed] [Google Scholar]
- [31].Ilbert M, Horst J, Ahrens S, Winter J, Graf PC, Lilie H, Jakob U, The redox-switch domain of Hsp33 functions as dual stress sensor, Nat Struct Mol Biol, 14 (2007) 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Leonard TA, Rozycki B, Saidi LF, Hummer G, Hurley J, H., Crystal structure and allosteric activation of portein kinase C ßII, Cell, 144 (2011) 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U, Zinc-release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen, J. Biol. Chem, 277 (2002) 44327–44331. [DOI] [PubMed] [Google Scholar]
- [34].Zhao F, Ilbert M, Varadan R, Cremers CM, Hoyos B, Acin-Perez R, Vinogradov V, Cowburn D, Jakob U, Hammerling U, Are zinc-finger domains of protein kinase C dynamic structures that unfold by lipid or redox activation?, Antioxid Redox Signal, 14 (2011) 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gopalakrishna R, Gundimeda U, Schiffman JE, McNeill TH, A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells, J Biol Chem, 283 (2008) 14430–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hoyos B, Acin-Perez R, Fischman DA, Manfredi G, Hammerling U, Hiding in plain sight: uncovering a new function of vitamin A in redox signaling, Biochim Biophys Acta, 1821 (2012) 241–247. [DOI] [PubMed] [Google Scholar]
- [37].Villani G, Greco M, Papa S, Attardi G, Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types., J. Biol. Chem, 273 (1998) 31829–31836. [DOI] [PubMed] [Google Scholar]
- [38].Acin-Perez R, Bayona-Bafaluy MP, Bueno M, Machicado C, Fernandez-Silva P, Perez-Martos A, Montoya J, Lopez-Perez MJ, Sancho J, Enriquez JA, An intragenic suppressor in the cytochrome c oxidase I gene of mouse mitochondrial DNA, Human Molecular Genetics, 12 (2003) 329–339. [DOI] [PubMed] [Google Scholar]
- [39].Churchill EN, Murreil CL, Chen CH, Mochley-Rosen D, Szweda LI, Reperfusion-induced translocation of delta-PKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation Circ. Res, 97 (2005) 78–85. [DOI] [PubMed] [Google Scholar]
- [40].Holness MJ, Sugden MC, Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation, Biochem Soc Trans, 31 (2003) 1143–1151. [DOI] [PubMed] [Google Scholar]
- [41].Harris RA, Huang B, Wu P, Control of pyruvate dehydrogenase kinase gene expression, Adv Enzyme Regul, 41 (2001) 269–288. [DOI] [PubMed] [Google Scholar]
- [42].Kolobova E, Tuganova A, Boulatnikov I, Popov KM, Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites, Biochem J, 358 (2001) 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Patel MS, Korotchkina LG, Regulation of the pyruvate dehydrogenase complex, Biochem Soc Trans, 34 (2006) 217–222. [DOI] [PubMed] [Google Scholar]
- [44].Buck J, Derguini F, Levi E, Nakanishi K, Hammerling U, Intracellular signaling by 14-hydroxy-4,14-retro-retinol, Science, 254 (1991) 1654–1656. [DOI] [PubMed] [Google Scholar]
- [45].Derguini F, Nakanishi K, Hammerling U, Buck J, Intracellular signaling activity of synthetic (14R)-, (14S)-, and (14RS)-14-hydroxy-4,14-retro-retinol, Biochemistry, 33 (1994) 623–628. [DOI] [PubMed] [Google Scholar]
- [46].Buck J, Grun F, Kimura S, Noy N, Derguini F, Hammerling U, Anhydroretinol: A naturally occurring inhibitor of lymphocyte physiology, J. Exp. Med, 178 (1993) 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Harrison EH, Apocarotenoids: Emerging role in mammals, Ann. Rev. Nutr, 38 (2019) 153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen Y, Buck J, Derguini F, Anhydroretinol induces oxidative stress and cell death, Cancer Res., 59 (1999) 3985–3990. [PubMed] [Google Scholar]
- [49].Chiu H-J, Fischman DA, Hammerling U, Vitamin A-depletion causes oxidative stress, mitochondrial dysfunction and PARP-1-dependent energy deprivation, FASEB J, 22 (2008) 3738–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Korichneva I, Waka J, Hammerling U, Regulation of the cardiac mitochondrial membrane potential by retinoids, J Pharmacol Exp Ther, 305 (2003) 426–433. [DOI] [PubMed] [Google Scholar]
- [51].Kwong Y, Henning M, Starkov A, Manfredi G, The mitochondrial respiratory chain is a modulator of apoptosis, Journal of Cell Biology, 179 (2007) 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Starkov AA, Fiskum G, Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state., J. Neurochem, 86 (2003) 1101–1107. [DOI] [PubMed] [Google Scholar]
- [53].Starkov AA, The role of mitochondria in reactive oxygen species mtabolism and signaling, Ann NY Acad Sci., 1147 (2008) 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mayr M, Liem D, Zhang J, Li X, Avliyakulov NK, Yang JI, Young G, Vondriska TM, Ladroue C, Madhu B, Griffiths JR, Gomes A, Xu Q, Ping P, Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts, J Mol Cell Cardiol, 46 (2009) 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bezy O, Tran TT, Pihlajamaki J, Suzuki R, Emanuelli B, Winnay J, Mori MA, Haas J, Biddinger SB, Leitges M, Goldfine AB, Patti ME, King GL, Kahn CR, PKCdelta regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans, J Clin Invest, 121 (2011) 2504–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Leitges M, Gimborn K, Elis W, Kalesnikoff J, Hughes MR, Krystal G, Huber M, Protein kinase C-delta is a negative regulator of antigen-induced mast cell degranulation, Mol Cell Biol, 22 (2002) 3970–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ranieri SC, Fusco S, Panieri E, Labate V, Mele M, Tesori V, Ferrara AM, Maulucci G, De Spirito M, Martorana GE, Galeotti T, Pani G, Mammalian life-span determinant p66shcA mediates obesity-induced insulin resistance, Proc Natl Acad Sci U S A, 107 (2010) 13420–13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Migliaccio E, Giorgio M, Pelicci PG, Apoptosis and aging: role of p66Shc redox protein, Antioxid Redox Signal, 8 (2006) 600–608. [DOI] [PubMed] [Google Scholar]
- [59].Shabrova E, Hoyos B, Vinogradov V, Kim YK, Wassef L, Leitges M, Quadro L, Hammerling U, Retinol as cofactor for PKCδ-mediated impairment of insulin sensitivity in a mouse model of diet-induced obesity, FASEB J, 30 (2016) 1339–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB, Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes, Nature, 436 (2005) 356–362. [DOI] [PubMed] [Google Scholar]
- [61].Broch M, Vendrell J, Ricart W, Richart C, Fernandez-Real JM, Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects, Diabetes Care, 30 (2007) 1802–1806. [DOI] [PubMed] [Google Scholar]
- [62].Johansson H, Gandini S, Guerrieri-Gonzaga A, Iodice S, Ruscica M, Bonanni B, Gulisano M, Magni P, Formelli F, Decensi A, Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer, Cancer Res, 68 (2008) 9512–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hail NJ, Kim HJ, Lotan R, Mechanisms of fenretinide-induced apoptosis, Apoptosis, 11 (2006) 1677–1694. [DOI] [PubMed] [Google Scholar]
- [64].Decensi A, Zanardi S, Argusti A, Bonanni B, Costa A, Veronesi U, Fenretinide and risk reduction of second breast cancer, Nat Clin Pract Oncol, 4 (2007) 64–65. [DOI] [PubMed] [Google Scholar]
- [65].Preitner F, Mody N, Graham TE, Peroni OD, Kahn BB, Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis, Am J Physiol Endocrinol Metab, 297 (2009) E1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Schroeder H, Langer T, Hartl FU, DnaK, DnaJ, and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage., Embo J, 12 (1953) 4137–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Voth W, Jakob U, Stress-activated chaperones: A first line of defense., Trends Biochem Sci, 42 (2017) 899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Park SJ, Shin JH, Jeong JI, Song JH, Jo JK, Kim ES, Lee EH, Hwang JJ, Lee EK, Chung SJ, Koh JY, Jo DG, Cho DH, Down-regulation of mortalin exacerbates Ab-mediated mitochondrial fragmentation and dysfunction., J. Biol. Chem, 289 (2104) 2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Burbulla LF, Schelling C, Kato H, Rapaport D, Woitalla D, Schiesling C, Schulte C, Sharma M, Illig T, Bauer P, Jung S, Nordheim A, Schoels L, Riess O, Krueger R, Dissecting the role of the mitochondrial chaperone mortalin in Parkinson’s disease: functional impact of disease-related variants on mitochondrial homeostasis, Human Molecular Genetics, 19 (2010) 4437–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wadhwa R, Ryu J, Ahn HM, Saxena N, Chaudhary A, Yum CO, Kaul SC, Functional significance of point mutations in stress chaperone mortalin and their relevance to Parkinson Disease., J. Biol. Chem, 290 (2015) 8447–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]