Abstract
Purpose
To evaluate the relationship between microperimetric (MP) sensitivity and retinal thickness measured at co-registered retinal locations in individuals who have mild or no diabetic retinopathy.
Methods
Fifty non-diabetic control subjects and 50 type-2 diabetic subjects participated (25 had no clinically apparent DR [NDR] and 25 had mild nonproliferative DR [MDR]). MP sensitivity was measured at 36 retinal locations that were arranged in three concentric rings centered on the fovea (radii of 3°, 6°, and 12°). Optical coherence tomography (OCT) images were obtained and total retinal thickness (TRT), inner retinal thickness (IRT), and outer retinal thickness (ORT) were quantified from the OCT images at locations that matched the MP measures. Linear quantile mixed models (LQMMs) and linear quantile models (LQMs) were used to compare MP and thickness values for the three subject groups and to quantify structure-function relationships.
Results
The statistical models indicated significant TRT and IRT reductions in the NDR and MDR groups, relative to the controls, that were most apparent in the 3° ring. By contrast, ORT was not reduced significantly for either diabetic group. MP sensitivity was reduced significantly within each ring and for both diabetic groups. Despite reductions in both thickness and sensitivity, the structure-function associations were generally weak with borderline statistical significance. For example, a TRT or IRT reduction of approximately 27 μm was predicted to result in approximately 1 dB of MP sensitivity loss for the MDR group (p = 0.03 and 0.05, respectively).
Conclusions
The results support previous findings of early retinal neurodegeneration in diabetics who have NDR or MDR. Interestingly, the structural and functional deficits appear to be only weakly associated, suggesting that mechanisms in addition to retinal thinning underlie the functional defects in early stage DR.
Keywords: Diabetic retinopathy, microperimetry, optical coherence tomography
Introduction
Diabetic retinopathy (DR) is a progressive retinal complication caused by diabetes mellitus (DM), and is the most common cause of blindness among working-age adults worldwide.1, 2 Although DR is diagnosed and staged according to abnormalities of the retinal vasculature,3 there is accumulating evidence that retinal neurodegeneration may precede the clinically apparent vascular changes. For example, several studies using optical coherence tomography (OCT) have reported thinning of the inner retinal layers of DM subjects who have mild or no DR.4–14 The retinal ganglion cell (RGC) layer, in particular, is typically reported to be more affected than other retinal layers in early-stage DR.9–11, 14 The area of retinal thinning is often reported within the ETDRS pericentral ring, defined as a region with an inner diameter of approximately 1 mm and an outer diameter of approximately 3 mm.9–11, 13
Neurodegeneration in early-stage DR may also lead to functional abnormalities, including psychophysical contrast sensitivity losses that have been assessed using letter contrast sensitivity,15, 16 visual field perimetry,17–19 and microperimetry.20–24 However, only a subset of studies have examined the spatial correlation between abnormalities in retinal structure and contrast sensitivity in early-stage DR.7, 20, 21, 23, 24 As such, the extent to which retinal thinning observed by OCT is associated with functional loss has not been fully established. Of the studies that have examined structure-function associations in early-stage DR, one reported a significant correlation between RCG + inner plexiform layer (RGC+) thickness and microperimetric (MP) sensitivity.21 However, the results of that study indicated that a substantial amount of thinning resulted in only a modest sensitivity abnormality. An important advantage of their approach21 was that structure and function were evaluated at several locations throughout the retina. By contrast, most structure-function studies in DM subjects have compared measures that involve averaging structural and/or perimetry measurements across the retina, which minimizes the ability to evaluate local relationships.7, 20, 23, 24 Taken together, previous work suggests that in the early stages of DR, individuals who do not have diabetic macular edema (DME) can have thinning of the inner-retina and sensitivity loss, but the extent of the structure-function association remains uncertain.
An important consideration in previous structure-function studies in early-stage DR is that the two types of measurements were performed with separate instruments, requiring the spatial locations of the measurements to be aligned in post-acquisition analyses. Factors such as fixation location and instability may introduce errors when attempting to spatially match the structure and function measurements. One approach to overcome this limitation is to perform the measurements with a single instrument through the same optics. As described elsewhere,25 this can be achieved with a combined OCT/scanning laser ophthalmoscope/microperimeter (Optos, Inc., Marlborough, MA) that permits retinal thickness and MP measurements to be performed through the same optics. The MP stimulus is presented in Maxwellian view, which helps to minimize the effects of pupil size differences among subjects. Additionally, this instrument tracks and accounts for eye position during the measurements. These features are expected to improve the spatial structure-function correspondence and may provide new insight into the relationship between retinal thickness and MP sensitivity in diabetes.
In the present study, this instrument was used to 1) evaluate potential MP sensitivity and retinal thickness abnormalities in individuals who have mild or no diabetic retinopathy; 2) quantify the relationship between MP sensitivity and retinal thickness measured at co-registered retinal locations in these individuals. We sought to determine if the expected thinning of the inner-retina is correlated with MP sensitivity loss. In contrast to some previous studies,e.g.21 outer-retina thickness was also measured and compared with MP sensitivity measurements in subjects who have mild or no DR. Thus, we sought to provide a comprehensive analysis of structure-function abnormalities in early-stage DR.
Methods
Subjects
Fifty subjects diagnosed with type-2 DM were recruited from the Retina and General Eye Clinics of the University of Illinois at Chicago, Department of Ophthalmology and Visual Sciences. Medical histories were obtained from their records and each subject was examined by a retina specialist. The examination paid particular attention to the optic nerve, retina, and its vasculature. The stage of NPDR was graded clinically according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) scale3 and the subjects were classified as diabetic with no apparent DR (NDR; N = 25) or diabetic with mild NPDR (MDR; N = 25). Subjects classified as MDR had retinal vascular abnormalities including microaneurysms, hard exudates, cotton-wool spots, and/or mild retinal hemorrhage (equivalent to ETDRS3 level 35 or less). No subject had systemic disease known to affect the retina (other than diabetes) or other ocular disease. Subjects who had sickle cell disease, retinal vascular occlusions, age-related macular degeneration, glaucoma, or high myopia (more than 6 diopters) were not recruited. The lens of each subject was graded by slit lamp examination using a clinical scale that ranged from clear to 4+. Subjects with more than mild (2+) nuclear sclerotic, posterior subcapsular, or cortical lens opacities were excluded. Two MDR subjects had a history of anti-VEGF injection, but neither subject had clinically significant macular edema at the time of enrollment in the present study. All subjects had ETDRS central subfield (1 mm) thickness within the instrument-defined normal range (less than 271 μm). Subject characteristics including age, sex, estimated disease duration, HbA1c percentage, ETDRS central subfield thickness, and mean MP sensitivity are provided in Table 1.
Table 1:
Subject characteristics
| Control (N = 50) | NDR (N = 25) | Mild NPDR (N = 25) | |
|---|---|---|---|
| Age (yr) | 53.4 ± 11.1 | 52.3 ± 8.0 | 55.2 ± 10.3 |
| Sex | 24M 26F | 7M 18F | 9M 16F |
| Disease duration (yr) | 7.4 ± 6.0 | 16.0 ± 9.1 | |
| HbA1c (%) | 8.3 ± 1.8 | 8.1± 1.5 | |
| ETDRS central subfield (μm) | 215 ± 23 | 206 ± 34 | 213 ± 28 |
| MP mean score (dB) | 15.2 ± 1.1 | 14.3 ± 0.9 | 13.8 ± 1.7 |
yr is years; M is male and F is female; HbA1c is glycated hemoglobin; ETDRS is early treatment of diabetic retinopathy study; MP is microperimetry
Fifty visually-normal, non-diabetic control subjects also participated. The exclusion criteria discussed above were applied to the control subjects. A one-way analysis of variance indicated no significant difference in mean age among the control and DM groups (F = 0.50, p = 0.61). The studies conformed to the tenants of the Declaration of Helsinki, institutional review board approval was obtained at the University of Illinois at Chicago, and the experiments were undertaken with the understanding and written consent of each subject.
Apparatus, stimuli, procedure, and analysis
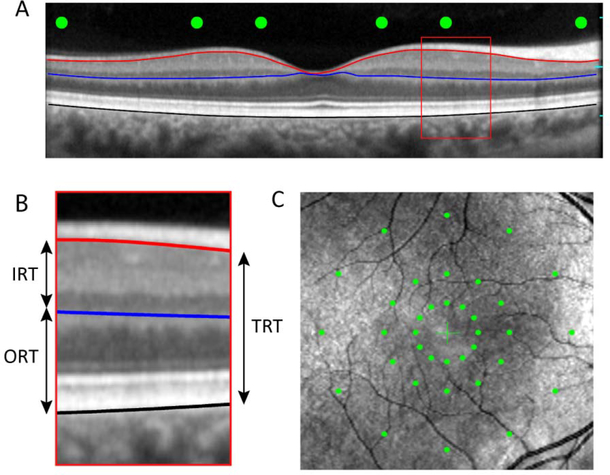
OCT and MP were performed with an Optos OCT/SLO/microperimeter (Optos, Inc., Marlborough, MA) that we have used previously and described elsewhere.25, 26 Figure 1 shows an example OCT image (A), an expanded view of the OCT image that highlights the layers analyzed (B) and an SLO image with the location of the MP measurements indicated (C). For the MP measurements, the stimulus spot size was equivalent to the Goldmann III (0.43°) and the duration was 200 ms. Stimuli were presented against a uniform field with a mean luminance of 10 cd/m2. A 4–2-1 staircase procedure was used to define threshold. As indicated by the green dots in Fig. 1C, stimuli were presented at 36 macular locations within 3 concentric rings that had radii of 3°, 6 °, and 12 °. Each ring included 12 test spots that were positioned according to the clock hours. These locations were selected to sample the parafoveal area that has been reported to be abnormally thin in previous studies of early-stage NPDR,9–11, 13 as well as the perifoveal retina. We note that the 3 o’clock location along the horizontal meridian often falls near the optic nerve head, which can complicate measurements at this location. However, the stimulus did not fall on the optic nerve head for any subject in the present study.
Figure 1:
Example OCT image from a visually-normal control subject (A). The red, blue, and black lines mark the borders of structures that were used to define TRT, IRT, and ORT, as described in the text. The green circles indicate the locations at which MP sensitivity measurements were performed. Panel B shows an expanded view of the OCT image, with the three retinal regions analyzed marked. Panel C is a SLO image that shows the location of the 36 MP sensitivity measurements organized into three concentric rings that have radii of 3°, 6°, 12°.
Six high-resolution (444 × 1268 pixels; scan width of 29.7°) radial SD-OCT b-scans were obtained along the clock hours (spatially matched to the MP measurement locations). Each b-scan that was used for analysis was comprised of an average of approximately 30 individual b-scans scans. To extract the thickness of individual retinal regions, the images were segmented using a standard semi-automated approach27, 28 that was performed in MATLAB using custom-written software. Three anatomical markers were defined, as shown in Fig. 1B: 1) the border between RNFL and retinal ganglion cell layer (RNFL/RGC; red line), 2) the border between the inner nuclear layer and the outer plexiform layer (INL/OPL; blue line), 3) RPE/Bruch’s membrane (RPE/BM; black line). The total retinal thickness (TRT) was defined as the distance between the RNFL/RGC (red line) and the RPE/BM (black line). Note that this definition excludes the RNFL, as this structure is highly asymmetric (thicker in the nasal retina), which complicates associations with retinal function. The inner retinal thickness (IRT) was defined as the distance between the RNFL/RGC (red line) and the INL/OPL (blue line), whereas the outer retinal thickness (ORT) was defined as the distance between the INL/OPL (blue line) and the RPE/BM (black line). The thickness values were averaged over a diameter of 0.43 deg (equivalent to the size of the Goldmann III; 19 A-scans).
Data were analyzed using linear quantile mixed models (LQMMs) 29–31 and linear quantile models (LQMs),32, 33 given the non-normal distributions of the thickness and MP data. For each model, subject group (NDR, MDR versus control) was included as the main predictor. As discussed further in the sections below, LQMMs were first developed to evaluate differences in retinal thickness and sensitivity among the three subject groups at the 36 retinal locations (three rings, twelve locations per ring; Fig.1C). The LQMMs were initially fit to all locations within each ring simultaneously to examine the overall effect of group (control, NDR, MDR) on the thickness and sensitivity measures within each ring (eccentricity). For the LQMMs, three subject groups and 12 retinal locations were included as fixed effects, and a random intercept was added at the subject level to account for subject dependence (i.e. multiple measures performed for each subject). Next, LQMs that only included subject group as the main predictor were developed to examine differences in thickness and sensitivity among the groups point-by-point (12 locations) within each ring, and then within retinal quadrants (superior, inferior, nasal, temporal). All LQMMs and LQMs were adjusted for subject age as a possible confounding variable.
The relationships between retinal thickness and MP sensitivity were evaluated by computing Spearman rank order correlation coefficients for each quadrant within each ring separately, following previous work.34, 35 The association between thickness and sensitivity within the diabetic groups was also evaluated by fitting LQMMs to the structure and function data to predict the amount of MP sensitivity loss for each micron loss of retinal thickness. Other than thickness, this model included eccentricity (ring) and quadrant as fixed effects, and a subject-level random intercept. An alpha-value of 0.05 or less was considered statistically significant and the p-values provided in the Tables below were not corrected for multiple comparisons, as the study was considered exploratory in nature. Statistical analyses were conducted using R (version 3.4.1; R Core Team, Vienna, Austria) and Sigmaplot (version 12; Systat Software, San Jose, CA, USA).
Results
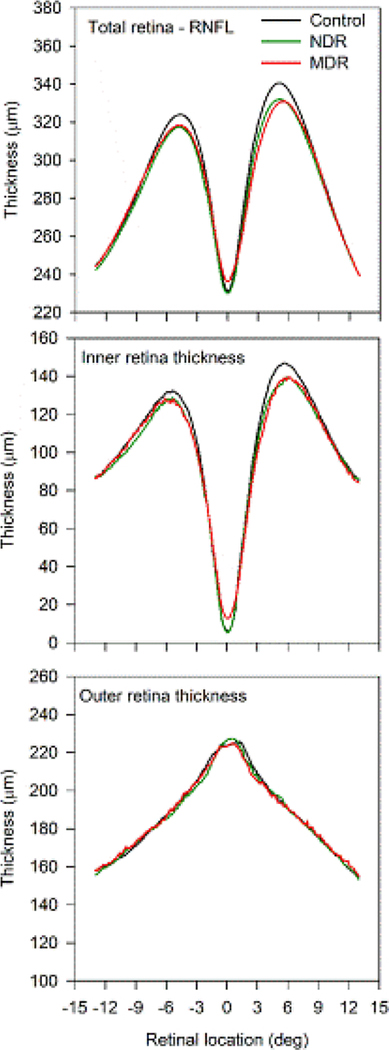
Figure 2 shows the mean thickness profiles for the three subject groups obtained from horizontal scans passing through the fovea. Thickness is plotted as a function of retinal eccentricity, with negative values indicating measurements from the temporal retina and positive values indicating measurements from the nasal retina. The total retinal thickness (top panel) was similar for the three subject groups along much of the horizontal meridian. However, there was a narrow region centered at 5° nasal and temporal to the foveal center that differed in thickness among the groups. In this parafoveal area, the total retinal thickness of both diabetic groups was thinned relative to that of the control group. Of note, the abnormal thinning was more apparent in the nasal retina as compared to the temporal retina. Furthermore, similar TRT reductions that were most apparent 5° nasal and temporal to the foveal center were also observed in the other five scan angles (e.g. vertical, diagonals; data not shown). The middle panel shows that a highly similar pattern was obtained for the IRT measurements: thinning in the diabetic subject groups was apparent in the parafoveal region 5° nasal and temporal to the foveal center. Here too, the IRT of the nasal retina was more affected than that of the temporal retina. The lower panel shows the thickness profiles for the outer retina. There were no apparent differences among the three subject groups in ORT. Indeed, the ORT profiles were nearly identical for the three groups.
Figure 2:
Mean thickness as a function of retinal location along the horizontal meridian. Locations that have negative values correspond to the temporal retina, whereas positive values correspond to the nasal retina. TRT, IRT, and ORT are shown in the top, middle, and bottom panels, respectively. The mean control profile is shown in black, NDR profile in green, and MDR profile in red.
Thinning of the three layers shown in Fig. 2 was examined quantitatively at the same spatial locations at which MP measurements were obtained (illustrated in Fig. 1). Differences in retinal thickness among the three subject groups (control, NDR, MDR) at the 36 retinal locations (three rings, twelve locations per ring) were examined using LQMMs, as described above. For the LQMM that was fit to all locations within each ring simultaneously, there was a reduction in TRT for the MDR group for the 3° ring (median reduction of 10.44 μm, p = 0.014), but not the 6° ring (median reduction of 4.82 μm, p = 0.248) or 12° ring (median increase of 0.52 μm, p = 0.873). In comparison, there were no significant TRT reductions for the NDR group within any ring (all p > 0.110). There was a significant reduction in IRT for the MDR group for the 3° ring (median reduction of 8.05 μm, p =0.010), but not the 6° ring (median reduction of 3.96 μm, p = 0.173) or 12° ring (median increase of 1.2 μm, p = 0.520). There were no significant IRT reductions for the NDR group within any ring (all p > 0.185). There were also no significant ORT reductions for either DM group within any ring (all p > 0.249). Thus, the largest abnormalities were found in the 3° ring for the MDR subject group.
To define the specific locations within the 3° ring that were abnormally thin, LQMs were fit to each of the 12 locations separately. The results of this analysis are presented in Table 2, which shows the median amount of thinning (μm) for each location, relative to the control group and the corresponding p-value (highlighted cells indicate p < 0.05). Overall, for the MDR subject group, thinning of the TRT and IRT were most apparent in the superior, nasal, and inferior quadrants (clock hours: 1 – 7, 11, 12), with relative preservation of the temporal retina (clock hours of 8, 9, 10). For the NDR group, the median TRT and IRT were reduced at each of the 12 locations within the 3° ring, but none reached statistical significance. Likewise, there was no significant ORT reduction at any location for either DR group; consequently, ORT data are not shown in Table 2. Of note, similar analyses were performed for the 6° and 12° rings, but with few exceptions TRT, IRT, and ORT were normal for both DM groups, as expected from the models fit to all locations simultaneously (discussed above). The exceptions included significant thinning in the 6° ring that was restricted to the 3 o’clock location (nasal retina). Specifically, there was a 9.41 μm loss of TRT for the NDR group (p = 0.048), a 8.05 μm loss of IRT for the NDR group (p = 0.022), and a 8.63 μm loss of IRT for the MDR group (p = 0.015).
Table 2:
Location-by-location thickness analysis for the 3° eccentricity
| Quadrant | Location (clock hour) | Layer | NDR | MDR |
|---|---|---|---|---|
| Superior | 11 | TRT | −5.48, p = 0.320 | −11.80, p = 0.011 |
| IRT | −5.29, p = 0.286 | −8.85, p = 0.020 | ||
| 12 | TRT | −7.49, p = 0.133 | −13.93, p = 0.004 | |
| IRT | −6.74, p = 0.113 | −15.62, p < 0.001 | ||
| 1 | TRT | −7.60, p = 0.129 | −14.91, p = 0.007 | |
| IRT | −6.01, p = 0.164 | −9.68, p = 0.003 | ||
| Nasal | 2 | TRT | −7.55, p = 0.159 | −12.89, p = 0.013 |
| IRT | −3.67, p = 0.420 | −9.15, p = 0.029 | ||
| 3 | TRT | −6.17, p = 0.228 | −13.65, p = 0.018 | |
| IRT | −3.58, p = 0.367 | −10.09, p = 0.043 | ||
| 4 | TRT | −5.58, p = 0.280 | −11.80, p = 0.020 | |
| IRT | −0.59, p = 0.885 | −5.65, p = 0.121 | ||
| Inferior | 5 | TRT | −4.59, p = 0.318 | −10.55, p = 0.030 |
| IRT | −2.75, p = 0.530 | −8.60, p = 0.059 | ||
| 6 | TRT | −6.51, p = 0.190 | −7.64, p = 0.083 | |
| IRT | −3.20, p = 0.523 | −4.64, p = 0.247 | ||
| 7 | TRT | −7.82, p = 0.111 | −8.93, p = 0.019 | |
| IRT | −5.02, p = 0.249 | −4.87, p = 0.074 | ||
| Temporal | 8 | TRT | −8.07, p = 0.174 | −6.30, p = 0.100 |
| IRT | −6.32, p = 0.203 | −6.58, p = 0.030 | ||
| 9 | TRT | −8.64, p = 0.156 | −4.21, p = 0.269 | |
| IRT | −6.80, p = 0.158 | −2.91, p = 0.318 | ||
| 10 | TRT | −8.64, p = 0.138 | −9.51, p = 0.092 | |
| IRT | −7.78, p = 0.159 | −10.46, p = 0.039 |
To summarize the location-by-location results: 1) for the inner-most ring (3°) the TRT and IRT had several locations of significant thinning that were generally observed in the superior, inferior, and nasal retina for the MDR group. 2) TRT and IRT were better preserved in the temporal retina. 3) ORT did not differ significantly at any location for either diabetic group. 4) There was generally no significant thinning in the 6° and 12° rings for either group in any layer.
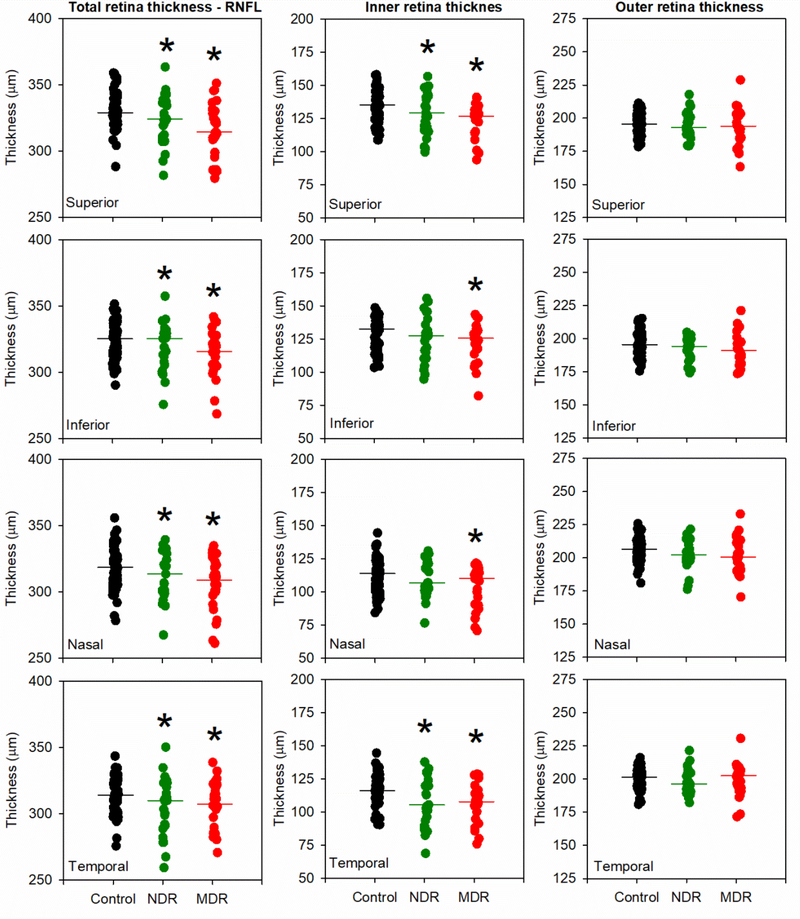
To simplify the extensive data set and examine quadrant-related thinning, the 12 locations were grouped as follows: 11, 12, 1 o’clock were averaged to provide a superior retina value; 2, 3, 4 o’clock were averaged to provide a nasal retina value; 5, 6, 7 o’clock were averaged to provide an inferior retina value; 8, 9, 10 o’clock were averaged to provide a temporal retina value. The thickness value for each subject within each quadrant for the 3° ring is shown in Fig. 3. Data for the 3° ring are shown, as this is the area that was most affected in the location-by-location analysis described above. The first column provides TRT measurements, the second column provides IRT measurements, and the third column provides ORT measurements. The superior, inferior, nasal, and temporal retina locations are shown in rows 1 to 4, respectively. Fig. 3 shows that the median TRT and IRT tended to be reduced in the two diabetic groups, relative to the control group, but there were no apparent differences among the groups in ORT. Furthermore, the TRT and IRT decreases were similar, indicating the TRT reduction can largely be attributed to thinning of the inner-retina. The quadrant data shown in Fig. 3 were analyzed quantitatively using LQMs and the results are shown in Table 3 (significant differences are marked in Fig. 3 by asterisks). There was a decrease in TRT for both the NDR and MDR subjects compared to the controls for all quadrants. A similar pattern was observed for IRT: thickness was reduced significantly for both subject groups in all quadrants, with two exceptions: IRT was normal for the NDR group in the inferior and nasal quadrants. As shown in Table 3, there was also TRT and IRT thinning for both groups in the 6° ring that was generally restricted to the nasal quadrant. No thinning was observed within the 12° ring (data not shown).
Figure 3:
Thickness averaged within each quadrant for the 3° ring is shown for each control (black), NDR (green), and MDR (red) subject. Data are shown for TRT (first column), IRT (second column), and ORT (third column). Each row shows data from a different retinal quadrant (superior in the first row, inferior in the second row, nasal in the third row, temporal in the fourth row). The horizontal lines in each panel represent the group medians and asterisks mark the DM groups that differ significantly from the control.
Table 3:
Retinal quadrant thickness analysis
| Ring | Location | Layer | NDR | MDR |
|---|---|---|---|---|
| 3 | Superior | TRT | −6.86, p = 0.010 | −13.54, p < 0.001 |
| IRT | −6.02, p = 0.026 | −11.38, p < 0.001 | ||
| Inferior | TRT | −6.31, p = 0.023 | −9.04, p < 0.001 | |
| IRT | −3.66, p = 0.215 | −6.04, p = 0.010 | ||
| Nasal | TRT | −6.43, p = 0.028 | −12.78, p < 0.001 | |
| IRT | −2.61, p = 0.312 | −8.29, p < 0.001 | ||
| Temporal | TRT | −8.45, p = 0.008 | −6.68, p = 0.006 | |
| IRT | −6.97, p = 0.037 | −6.64, p = 0.004 | ||
| 6 | Superior | TRT | −0.21, p = 0.926 | −1.57, p = 0.539 |
| IRT | −0.47, p = 0.791 | −1.57, p = 0.533 | ||
| Inferior | TRT | −4.09, p = 0.051 | −4.11, p = 0.081 | |
| IRT | −3.81, p = 0.039 | −3.72, p = 0.082 | ||
| Nasal | TRT | −8.39, p = 0.001 | −9.58, p = 0.002 | |
| IRT | −6.07, p = 0.001 | −7.32, p = 0.001 | ||
| Temporal | TRT | −4.07, p = 0.077 | −3.90, p = 0.070 | |
| IRT | −3.47, p = 0.118 | −3.87, p = 0.059 |
The MP sensitivity data obtained at locations that spatially correspond to the thickness measurements were also analyzed using LQMMs. A LQMM was first fit to all locations within each ring simultaneously to examine the overall effect of group (control, NDR, mild NPDR). There was a significant reduction in MP sensitivity for the MDR group for the 3° ring (median reduction of 1.05 dB, p <0.001), 6° ring (median reduction of 1.11 dB, p = 0.003), and 12° ring (median decrease of 1.13 dB, p = 0.001). Similarly, there was a significant reduction in MP sensitivity for the NDR group for the 3° ring (median reduction of 0.64 dB, p = 0.009), 6° ring (median decrease of 0.72 dB, p = 0.004), and 12° ring (median reduction of 1.22 dB, p < 0.001). Thus, small MP sensitivity reductions were generally found throughout all three rings for both DM groups.
Fig. 4 shows the MP sensitivity values obtained within each quadrant for the three rings (3° first column, 6° second column, 12° third column). In general, MP sensitivity tended to be reduced in both DM groups, relative to the control group, for each quadrant within each ring. These data were also analyzed by fitting LQMs to the data within each quadrant for each ring. Statistically significant reductions in MP sensitivity derived from the LQMs are indicated by asterisks in Fig. 4.
Figure 4:
MP sensitivity averaged within each quadrant is shown for each control (black), NDR (green), and MDR (red) subject. Data are shown for the 3° eccentricity (first column), 6° eccentricity (second column), and 12° eccentricity (third column). All other conventions are as in Fig. 3.
Figs 3 and 4 show significant differences in median retinal thickness and MP sensitivity, respectively, among the three subject groups. However, the ranges of normal thickness and MP sensitivity were relatively large and there was considerable overlap between the control and diabetic groups. Nevertheless, location-by-location analysis of the IRT values within the 3° ring indicated that IRT was abnormally reduced in a total of 59 individual locations, summed over all subjects and locations within the 3° ring (“abnormally reduced” is defined as a value that falls below that of any control subject for the specific location examined). Of these 59 abnormally thin locations, only 9 locations (15%) also had abnormally reduced MP sensitivity. In comparison, there were 24 locations that had abnormally reduced MP sensitivity within the 3° ring, of which 9 locations (38%) also had abnormally reduced IRT. Thus, locations with abnormally low MP sensitivity were often thin (approximately 38% of the locations), whereas inner-retina locations that were abnormally thin were a poor predictor of MP sensitivity loss (approximately 15% of the locations).
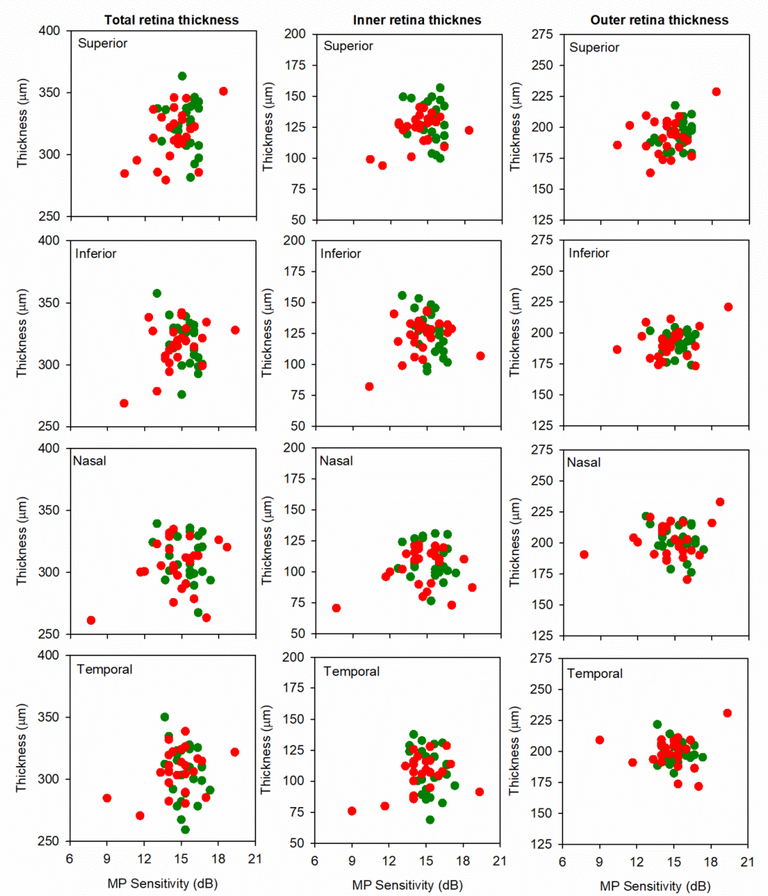
Fig. 5 shows the relationship between retinal thickness (TRT, left column; IRT, middle column; ORT, right column) and MP sensitivity for each quadrant within the 3° ring. Here, retinal thickness in linear units (μm) is plotted as a function of log MP sensitivity (dB), consistent with previous work.34, 35 Overall, trends for associations between the thickness and MP sensitivity measurements were observed for the MDR group. Indeed, it is apparent from the figures that there were generally positive correlations between TRT and MP sensitivity, as well as between IRT and MP sensitivity. The strongest correlation observed was between TRT and MP sensitivity in the inferior quadrant (Spearman’s ρ = 0.41, p = 0.042); this was the only correlation with a p-value less than 0.05 (note that this would not achieve statistical significance if corrected for multiple comparisons).
Figure 5:
Thickness averaged within each quadrant is plotted as a function of MP sensitivity averaged within the corresponding quadrant for each NDR (green) and MDR (red) subject. Data are shown for TRT (first column), IRT (second column), and ORT (third column). Each row shows data from a different retinal quadrant (superior in the first row, inferior in the second row, nasal in the third row, temporal in the fourth row).
As an additional approach to evaluate structure-function associations, LQMMs were used to predict the amount of sensitivity loss (dB) for each micron loss of TRT and IRT. In contrast to the simple correlations discussed above and shown in Fig. 5, the LQMMs included data from all four quadrants and all three rings. The LQMMs indicated that there was a borderline-significant association between TRT and MP sensitivity for the MDR group (p = 0.03), such that a 27 μm TRT reduction is predicted to produce a median sensitivity loss of 1 dB, after adjusting for location. Similarly, the LQMMs indicated a borderline-significant association between IRT and MP sensitivity for the MDR group (p = 0.05), such that a 28 μm IRT reduction is predicted to produce a median sensitivity loss of 1 dB, after adjusting for location. By contrast, TRT and IRT reductions were not significantly associated with MP sensitivity losses for the NDR group, and ORT was not significantly associated with MP sensitivity for either group.
Discussion
This study evaluated abnormalities in MP sensitivity and retinal thickness, as well as the association between these measures, in individuals who have NDR or MDR. In contrast to previous studies, inner retina, outer retina, and total retina thicknesses were measured and correlated with MP sensitivity performed at corresponding retinal locations. Overall, the results showed that MP sensitivity was significantly reduced in the NDR and MDR groups compared to the controls at nearly all retinal locations evaluated. In comparison, TRT and IRT reductions in the DM groups were generally restricted to the inner-most ring that was examined (3° eccentricity); ORT did not differ from control in any ring for either DM group. Although the group differences were statistically significant, relatively few diabetic subjects fell outside of the control range. For example, 24% of the NDR and 28% of the MDR subjects had an IRT value that was below the control range in at least one quadrant in the 3° ring. MP values within the 3° ring were below the control range in at least one quadrant in 24% of the MDR subjects, whereas all NDR subjects had MP values within the control range for the 3° ring. Interestingly, despite the group differences in structure (TRT and IRT) and function, the structure-function associations were relatively weak for the MDR group and non-significant for the NDR group.
The finding that IRT reductions were largely restricted to a parafoveal ring (3° radius, centered on the fovea) is consistent with previous work.9–11, 13 Specifically, these previous studies have shown thinning of the ganglion cell layer in the “pericentral area” imaged by OCT in diabetic subjects who have NDR or MDR. The pericentral area was defined as a ring with an inner radius of 0.5 mm (approximately 1.6°) and an outer radius of 1.5 mm (approximately 5.0°), centered on the fovea. This region closely approximates the region of thinning shown in Fig. 2. These studies9–11 also showed that ORT is generally normal, which was confirmed in our subject sample. The explanation for why TRT and IRT reductions are most apparent in a parafoveal ring in our data is presently uncertain. However, we note that this region of the inner retina, particularly in the nasal region, has the greatest thickness (Fig. 2) and retinal ganglion cell density36, 37 and may therefore have high oxygen demands. However, this is speculative and further work is needed to understand why this region of the inner retina may be more vulnerable to thinning.
We also found MP sensitivity was reduced in the NDR and MDR subjects, but the abnormality was relatively uniform throughout the area measured. Other studies have also reported MP sensitivity loss in early-stage DR. For example, Nittala et al.22 reported significantly reduced MP sensitivity using the Nidek MP-1 microperimeter in diabetic subjects who had mild or no retinopathy, as well as in DM subjects who had more advanced disease. Gella et al.20 and Verma et al.23, 24 also used the Nidek MP-1 to examine MP sensitivity in diabetic subjects. Both studies reported MP sensitivity reductions in diabetics who had no clinically-apparent DR. Taken together, there is now a substantial amount of evidence that MP sensitivity can be affected in early-stage DR, which suggests that this may be a useful approach to measure early functional changes in these individuals.
Despite MP loss and thinning in the same retinal region (3° ring) the structure-function relationships observed in the present study were generally weak. As shown in Fig. 5, there were trends toward relationships between TRT and MP sensitivity and between IRT and MP sensitivity. The LQMMs that defined the relationship between TRT and MP sensitivity, as well as that between IRT and MP sensitivity, indicated that these relationships were statistically significant for the MDR group. Although statistically significant, 27 to 28 μm of thinning was predicted to result in a relatively modest MP sensitivity loss (1 dB). No such relationship was observed between TRT and MP sensitivity for the NDR group. These findings are generally consistent with Montesarno et al.21 who reported a weak, but statistically significant, relationship between MP sensitivity and inner-retina thickness in subjects who had NDR. Their results indicated that a substantial loss of RGC+ thickness was associated with only a small change in MP sensitivity. Specifically, a 40 μm loss of RGC+ (approximately half of the layer) was predicted to produce a 1 dB MP sensitivity loss. Taken together, the results of the present study and of Montesarno et al.21 suggest that sensitivity losses in DR cannot be fully explained by inner-retina thinning, and presumably cell death.
A recent study that examined letter CS, MP sensitivity, and ORT within the central macula (±3° from the fovea) in NDR and MDR subjects showed statistically significant correlations for MDR subjects between ORT and letter CS, as well as between ORT and MP sensitivity.38 Structure-function correlations were observed in that study, despite the generally normal ORT of the MDR subjects. Like the present study, and that of Montesarno et al.21, the correlations were relatively weak, but statistically significant. The present study examined MP sensitivity and ORT at more peripheral locations (±3° to ±12° from the fovea) and found no significant association between MP sensitivity and ORT. Taken together, the results of the present study and that of prior work indicate that structure-function relationships in early-stage DR likely depend on both the retinal layer and retinal region analyzed.
Structure-function comparisons, like those reported by Montesarno et al.,21 and in the present study, have received relatively little attention in the diabetic literature. In contrast, structure-function relationships have been widely studied in glaucoma (reviewed in39, 40). Although there has been substantial progress in understanding structure-function relationships in glaucoma, discordance that is thought to arise from several factors has also been reported in that patient population. Factors that limit structure-function agreement in glaucoma may also be relevant in DM. For example, normal healthy eyes can vary in RGC thickness by a factor of two.41 Thus, a subject who begins life with a relatively high density of RGCs could lose half of his/her RGC layer and remain within the lower limit of normal. Another consideration, as discussed in a recent review,39 is that structural (e.g. OCT) and functional (e.g. MP) measurement variability can differ substantially: abnormalities are expected to be detected by the measure with the least variability first. A final limitation is that the soma of inner-retina neurons are displaced near the central macula. Models have been developed to account for the location of RGC soma relative to the corresponding receptive field.37, 42 However, it is difficult to extend these models to account for the displacement of the inner-retina, given that the inner-retina consists of several sub-structures each of which may have different displacements. Despite these challenges, the accumulating evidence that indicates inner-retina thinning and MP sensitivity loss in DM warrants additional study. The need for additional study of inner-retina thickness is further emphasized in a recent longitudinal study that found that DM subjects lose RGC thickness at a rate of approximately 0.29 μm per year, on average, which is similar to that observed in glaucoma.8
In summary, subjects who have mild or no diabetic retinopathy can have thinning of the inner-retina and MP sensitivity loss. However, the relationship between these measures is generally weak. This suggests that mechanisms in addition to retinal thinning contribute to functional deficits in early-stage DR.
Acknowledgments
This research was supported by National Institutes of Health research grants R01EY026004 (JM), P30EY001792 (core grant), UL1TR000050 (Center for Clinical and Translational Science), an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
Declaration of interests
The authors report no conflicts of interest.
References
- 1.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4): 179–183. [DOI] [PubMed] [Google Scholar]
- 2.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL 3rd, Knatterud GL. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early treatment diabetic retinopathy study report #18. Invest Ophthalmol Vis Sci. 1998;39(2): 233–252. [PubMed] [Google Scholar]
- 4.Chhablani J, Sharma A, Goud A, Peguda HK, Rao HL, Begum VU, Barteselli G. Neurodegeneration in type 2 diabetes: Evidence from spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56(11): 6333–6338. [DOI] [PubMed] [Google Scholar]
- 5.Carpineto P, Toto L, Aloia R, Ciciarelli V, Borrelli E, Vitacolonna E, Di Nicola M, Di Antonio L, Mastropasqua R. Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye (Lond). 2016;30(5): 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gundogan FC, Akay F, Uzun S, Yolcu U, Cagiltay E, Toyran S. Early neurodegeneration of the inner retinal layers in type 1 diabetes mellitus. Ophthalmologica. 2016;235(3): 125–132. [DOI] [PubMed] [Google Scholar]
- 7.Joltikov KA, de Castro VM, Davila JR, Anand R, Khan SM, Farbman N, Jackson GR, Johnson CA, Gardner TW. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58(6): BIO277–BIO290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, Garmager A, Wit F, Kucukevcilioglu M, van Velthoven ME et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113(19): E2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Verbraak FD, Abramoff MD. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50(7): 3404–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51(7): 3660–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dijk HW, Verbraak FD, Kok PH, Stehouwer M, Garvin MK, Sonka M, DeVries JH, Schlingemann RO, Abramoff MD. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012;53(6): 2715–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and muller cells alterations. J Diabetes Res. 2013:905058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biallosterski C, van Velthoven ME, Michels RP, Schlingemann RO, DeVries JH, Verbraak FD. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br J Ophthalmol. 2007;91(9): 1135–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues EB, Urias MG, Penha FM, Badaro E, Novais E, Meirelles R, Farah ME. Diabetes induces changes in neuroretina before retinal vessels: A spectral-domain optical coherence tomography study. Int J Retina Vitreous. 2015;1(4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAnany JJ, Park JC. Reduced contrast sensitivity is associated with elevated equivalent intrinsic noise in type 2 diabetics who have mild or no retinopathy. Invest Ophthalmol Vis Sci. 2018;59(6): 2652–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stavrou EP, Wood JM. Letter contrast sensitivity changes in early diabetic retinopathy. Clin Exp Optom. 2003;86(3): 152–156. [DOI] [PubMed] [Google Scholar]
- 17.Hellgren KJ, Agardh E, Bengtsson B. Progression of early retinal dysfunction in diabetes over time: Results of a long-term prospective clinical study. Diabetes. 2014;63(9): 3104–3111. [DOI] [PubMed] [Google Scholar]
- 18.Lobefalo L, Verrotti A, Mastropasqua L, Chiarelli F, Morgese G, Gallenga PE. Flicker perimetry in diabetic children without retinopathy. Can J Ophthalmol. 1997;32(5): 324–328. [PubMed] [Google Scholar]
- 19.Parravano M, Oddone F, Mineo D, Centofanti M, Borboni P, Lauro R, Tanga L, Manni G. The role of humphrey matrix testing in the early diagnosis of retinopathy in type 1 diabetes. Br J Ophthalmol. 2008;92(12): 1656–1660. [DOI] [PubMed] [Google Scholar]
- 20.Gella L, Raman R, Kulothungan V, Saumya Pal S, Ganesan S, Sharma T. Retinal sensitivity in subjects with type 2 diabetes mellitus: Sankara nethralaya diabetic retinopathy epidemiology and molecular genetics study (sn-dreams ii, report no. 4). Br J Ophthalmol. 2016;100(6): 808–813. [DOI] [PubMed] [Google Scholar]
- 21.Montesano G, Gervasoni A, Ferri P, Allegrini D, Migliavacca L, De Cilla S, Rossetti L. Structure-function relationship in early diabetic retinopathy: A spatial correlation analysis with oct and microperimetry. Eye (Lond). 2017;31(6): 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nittala MG, Gella L, Raman R, Sharma T. Measuring retinal sensitivity with the microperimeter in patients with diabetes. Retina. 2012;32(7): 1302–1309. [DOI] [PubMed] [Google Scholar]
- 23.Verma A, Raman R, Vaitheeswaran K, Pal SS, Laxmi G, Gupta M, Shekar SC, Sharma T. Does neuronal damage precede vascular damage in subjects with type 2 diabetes mellitus and having no clinical diabetic retinopathy? Ophthalmic Res. 2012;47(4): 202–207. [DOI] [PubMed] [Google Scholar]
- 24.Verma A, Rani PK, Raman R, Pal SS, Laxmi G, Gupta M, Sahu C, Vaitheeswaran K, Sharma T. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (sd-oct) study in individuals with diabetes, but no diabetic retinopathy. Eye (Lond). 2009;23(9): 1824–1830. [DOI] [PubMed] [Google Scholar]
- 25.Anastasakis A, McAnany JJ, Fishman GA, Seiple WH. Clinical value, normative retinal sensitivity values, and intrasession repeatability using a combined spectral domain optical coherence tomography/scanning laser ophthalmoscope microperimeter. Eye (Lond). 2011;25(2): 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jivrajka RV, Genead MA, McAnany JJ, Chow CC, Mieler WF. Microperimetric sensitivity in patients on hydroxychloroquine (plaquenil) therapy. Eye (Lond). 2013;27(9): 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hood DC, Cho J, Raza AS, Dale EA, Wang M. Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci. 2011;88(1): 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JC, Collison FT, Fishman GA, Allikmets R, Zernant J, Liu M, McAnany JJ. Objective analysis of hyperreflective outer retinal bands imaged by optical coherence tomography in patients with stargardt disease. Invest Ophthalmol Vis Sci. 2015;56(8): 4662–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geraci M, Bottai M. Quantile regression for longitudinal data using the asymmetric laplace distribution. Biostatistics. 2007;8(1): 140–154. [DOI] [PubMed] [Google Scholar]
- 30.Geraci M Linear quantile mixed models: The lqmm package for laplace quantile regression. J Stat Softw. 2014;57(13): 29. [Google Scholar]
- 31.Geraci M, Bottai M. Linear quantile mixed models. Stat Comput. 2014;24(3): 461–479. [Google Scholar]
- 32.Bottai M, Orsini N, Geraci M. A gradient search maximization algorithm for the asymmetric laplace likelihood. J Stat Comput Simul. 2015;85(10): 1919–1925. [Google Scholar]
- 33.Koenker R, Bassett G. Regression quantiles. Econometrica. 1978;46(1): 33–50. [Google Scholar]
- 34.Hood DC, Anderson SC, Wall M, Kardon RH. Structure versus function in glaucoma: An application of a linear model. Invest Ophthalmol Vis Sci. 2007;48(8): 3662–3668. [DOI] [PubMed] [Google Scholar]
- 35.Raza AS, Cho J, de Moraes CG, Wang M, Zhang X, Kardon RH, Liebmann JM, Ritch R, Hood DC. Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol. 2011;129(12): 1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300(1): 5–25. [DOI] [PubMed] [Google Scholar]
- 37.Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res. 2007;47(22): 2901–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAnany JJ, Park JC, Liu K, Liu M, Chen Y, Chau FY, Lim JI. Contrast sensitivity is associated with outer-retina thickness in early-stage diabetic retinopathy. Acta Ophthalmol. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hood DC. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res. 2017;57:46–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malik R, Swanson WH, Garway-Heath DF. ‘Structure-function relationship’ in glaucoma: Past thinking and current concepts. Clin Exp Ophthalmol. 2012;40(4): 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashimatey BS, Swanson WH. Between-subject variability in healthy eyes as a primary source of structural-functional discordance in patients with glaucoma. Invest Ophthalmol Vis Sci. 2016;57(2): 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turpin A, Chen S, Sepulveda JA, McKendrick AM. Customizing structure-function displacements in the macula for individual differences. Invest Ophthalmol Vis Sci. 2015;56(10): 5984–5989. [DOI] [PubMed] [Google Scholar]