Abstract
Among the more than 750 carotenoids identified in nature, only lutein, zeaxanthin, meso-zeaxanthin, and their oxidative metabolites are selectively accumulated in the macula lutea region of the human retina. These retinal carotenoids are collectively referred to as the macular pigment (MP) and are obtained only through dietary sources such as green leafy vegetables and yellow and orange fruits and vegetables. Lutein- and zeaxanthin-specific binding proteins (StARD3 and GSTP1, respectively) mediate the highly selective uptake of MP into the retina. Meso-zeaxanthin is rarely present in the diet, and its unique presence in the human eye results from metabolic conversion from dietary lutein by the RPE65 enzyme. The MP carotenoids filter high-intensity, short-wavelength visible light and are powerful antioxidants in a region vulnerable to light-induced oxidative stress. This review focuses on MP chemistry, absorption, metabolism, transport, and distribution with special emphasis on animal models used for MP study.
Keywords: Age-Related macular degeneration, Carotenoid, Lutein, Macular pigment, Nutrition, Zeaxanthin
1. Introduction
Carotenoids are natural lipophilic pigments with C40H56 core chemical structures that are synthesized by plants, algae, bacteria, yeast, and molds [1]. Xanthophylls are oxygenated carotenoids, and three isomeric xanthophyll carotenoids: lutein, zeaxanthin, and meso- zeaxanthin, share a common molecular formula of C40H56O2 and are collectively referred to as the macular pigment (MP) [2]. They act as anti-oxidants and blue light filters that protect the eye from light-induced oxidative stress [3]. The human eye is comprised of three different layers: the fibrous tunic, the vascular tunic (uvea), and the retina. The fibrous tunic is the outermost layer and consists of the sclera and cornea, while the vascular tunic comprises the choroid, iris, and ciliary body. The innermost layer is the neural retina. Figure 1 shows the anatomy of the human eye en face and in cross-section highlighting the macula, the cone-rich region responsible for high acuity central vision. The retina is further subdivided into 10 layers from outermost to innermost as follows: retinal pigment epithelium (RPE), photoreceptor outer segment (POS) layer, external limiting membrane (ELM), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL), nerve fiber layer (NFL), and internal limiting membrane (ILM). The central region of the retina known as the macula has a small pit at the center of the fovea centralis. This region is commonly referred to as the macula lutea or “yellow spot” due to high accumulation of MP [1]. The macula region appears in primate retina and is absent in other mammals. The macular carotenoids play a significant role in preventing various ocular diseases, which has been described in detail from a clinical perspective in our recent review [4]. This current review has a more biochemical perspective and focuses on MP chemistry, biochemistry, absorption, and transport with particular attention to their roles in animal models and interventional studies.
Figure 1.
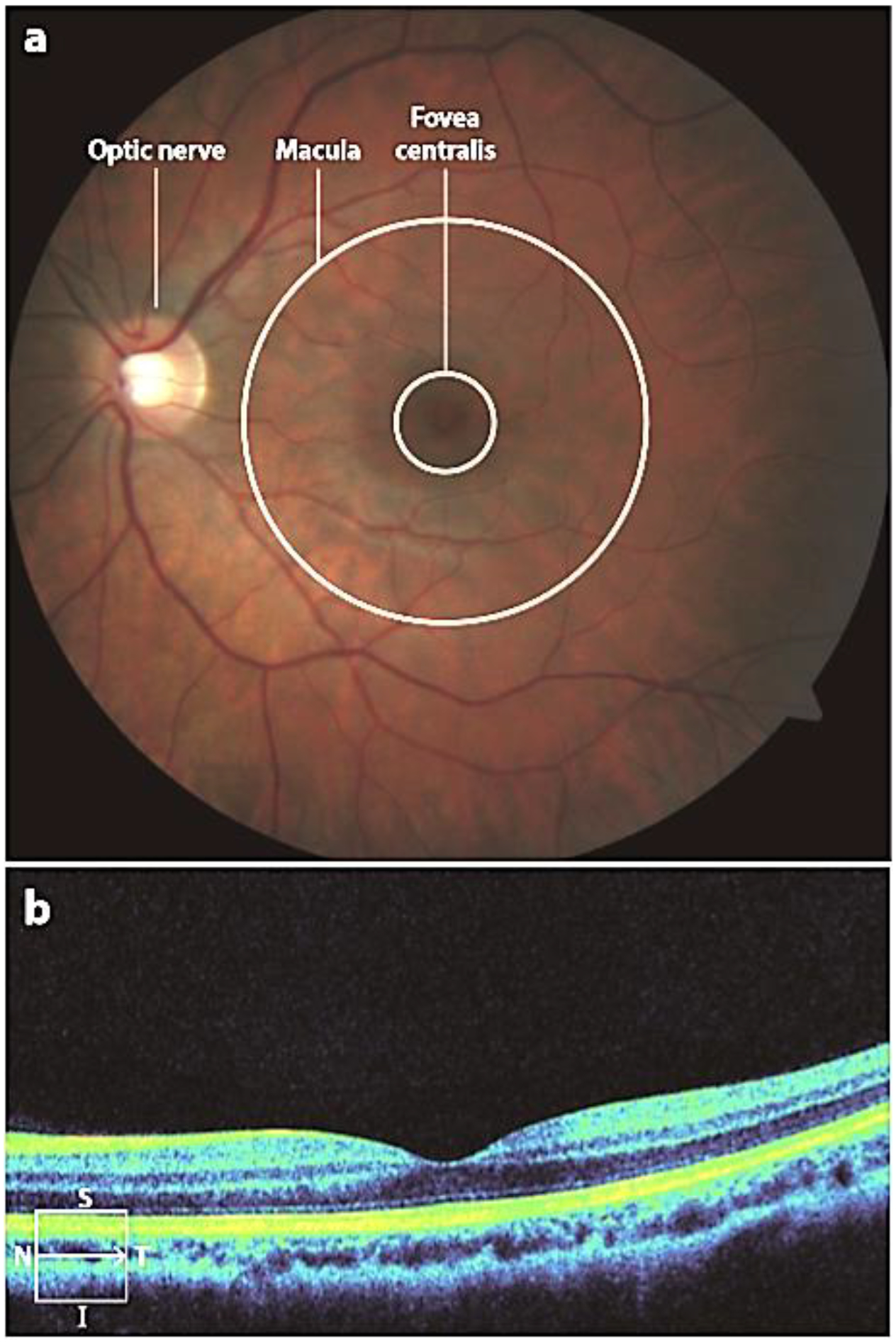
Anatomy of a healthy eye. Images from (a) fundus photography and (b) optical coherence tomography. The line in the lower left corner of panel b indicates the position of the scan within the macula. Abbreviations: I, inferior; N, nasal; S, superior; T, temporal.
2. Macular carotenoid structure and chemistry
The MP carotenoids have a hydroxyl (O-H) functional groups attached at the 3 and 3’ positions of terminal ionone rings connected by a rigid 22-carbon isoprenoid backbone with nine conjugated double bonds as shown in Figure 2. The distinctive structures of individual carotenoids define their physical, chemical, and biological properties [5]. The presence of O-H groups and their total number of conjugated double bonds in MP carotenoids determine their polarity, solubility, light-absorbing, and antioxidant properties. The maximum absorption of lutein is around 445 nm, and zeaxanthin is around 450 nm. The peak absorption of the macular pigment at 460 nm corresponds with the “blue light hazard” wavelength of 450–500 nm (Figure 3). Depending on its concentration, the MP acts as a blue light filter that absorbs 40–90% of incident short-wavelength, high-energy visible blue light [6], protecting the retina from light-induced damage. Due to the presence of many C=C double bonds, numerous cis/trans (E/Z) configurations are possible for a particular molecule, but, in general, the ocular carotenoids are all-trans. Lutein differs from zeaxanthin and meso-zeaxanthin because it has one fewer conjugated double bond. Lutein has eight stereoisomeric forms due to the occurrence of three stereocenters at C-3, C-3ʹand C6ʹ. However, in the retina and most of nature, lutein exists in a single stereoisomeric form, (3R, 3ʹR, 6R)-β, ε-carotene-3,3ʹ-diol. Zeaxanthin has three different stereoisomeric forms: SS-zeaxanthin [(3S, 3ʹS) β, β-carotene-3, 3ʹdiol] (rarely found in nature), RR zeaxanthin [(3R, 3ʹR)-β, β-carotene-3, 3ʹdiol] (dietary zeaxanthin), and meso-zeaxanthin [(3R, 3ʹS)-β, β-carotene-3,3ʹ diol] (unique to the eye in mammals and birds and rarely found elsewhere in nature) [2]. In model lipid membranes, lutein and its isomers can orient both parallel and perpendicular to the plane of the membrane, whereas zeaxanthin prefers a roughly perpendicular orientation. This is in contrast to less polar carotenoids such as β-carotene, which are more disordered in lipid membranes. The MP carotenoids enhance the rigidity of the lipid bilayer and thus act as “molecular rivets,” which may decrease the membrane’s susceptibility to lipid oxidation [7].
Figure 2.
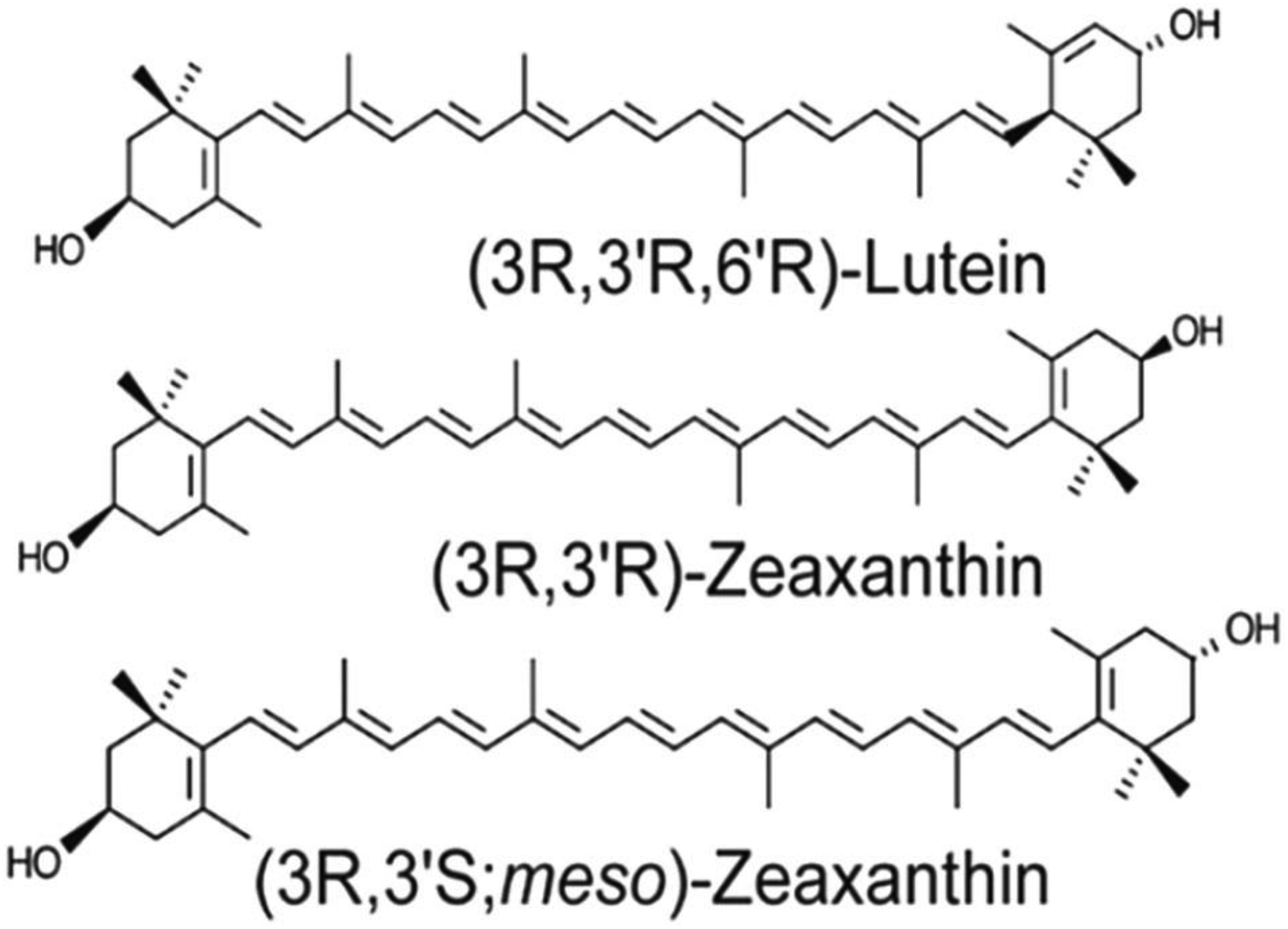
Structures of the macular carotenoids: lutein, zeaxanthin, and meso-zeaxanthin.
Figure 3.
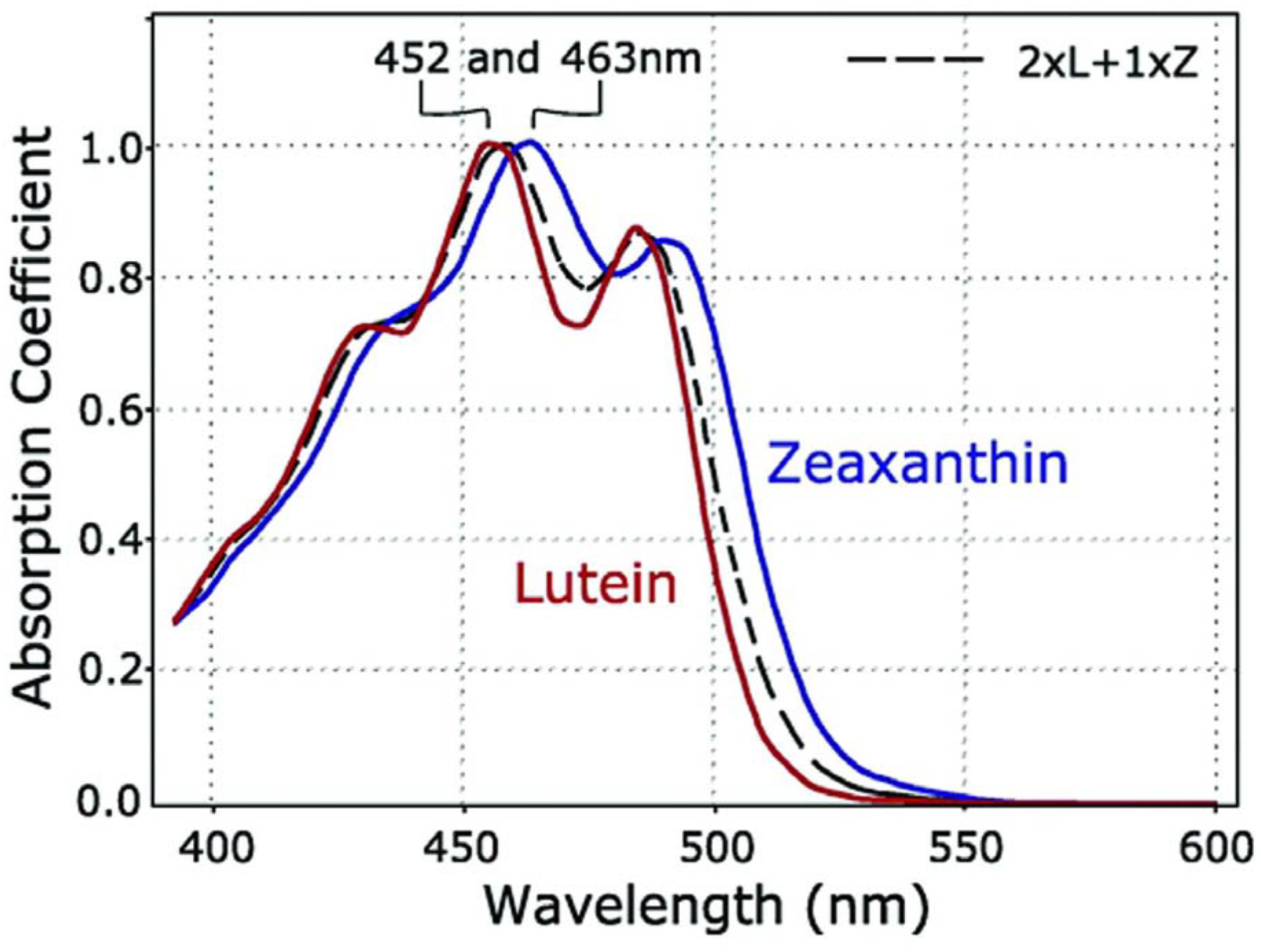
Absorption spectra of lutein (red) and zeaxanthin (blue) in olive oil. A mixture of lutein plus zeaxanthin (dashed black line) closely approximates the absorption spectrum of the macular pigment in the living human eye.
3. Macular carotenoid anatomy and distribution
Among 15 dietary carotenoids detected in human serum, only lutein, zeaxanthin, and their metabolites accumulate at high concentrations in the fovea. Lutein (36%), zeaxanthin (18%), and meso-zeaxanthin (18%) constitute the majority of retinal carotenoid content [1]. Their oxidative metabolites such as oxo-lutein, (3-hydroxy-β,ε-carotene-3`-one), epilutein, and ε, ε -carotene-3,3`-dione, 9 - and 13-Z isomers of both lutein and zeaxanthin constitute approximately 20% of the total carotenoid content of retina [8]. MP has a unique distribution in the retina with a high concentration in the fovea and inner plexiform layer of the retina. The distribution of MP in healthy individuals is shown in Figure 4. In the fovea, the Henle fiber layer and receptor axons have MP concentrations around 0.1 to 1 mM. The ratios and distributions of the macular pigments are not uniform and can vary widely among individuals. The ratio of lutein: zeaxanthin is 1:2.4 in the center versus 2:1 in the periphery of the human retina. The concentration of MP in the peripheral retina is 100-fold lower than the fovea. Unlike lutein and zeaxanthin, meso-zeaxanthin is not detectable in other human tissues or blood unless it is specifically supplemented in the diet [9]. The unique identification of meso-zeaxanthin in the retina implies that isomerization from dietary precursors occurs in ocular tissues [10]. This hypothesis was confirmed by in vivo experiments by Johnson et al. [11] and Bhosale et al. [12] in monkeys and quail, which revealed that lutein is the precursor for meso-zeaxanthin. In the retinal pigment epithelium of vertebrates, the RPE65 isomerase enzyme has been identified as a catalyst for the conversion of lutein to meso-zeaxanthin [13]. The retinas of newborn and fetal human eyes have low ratios of meso-zeaxanthin relative to lutein and zeaxanthin in comparison to the usual adult ratios, possibly due to inefficient isomerization of lutein to meso-zeaxanthin. Although much of the MP is probably stably protein-bound in the retina, the macular carotenoids can also incorporate nonspecifically into membranes [3].
Figure 4.
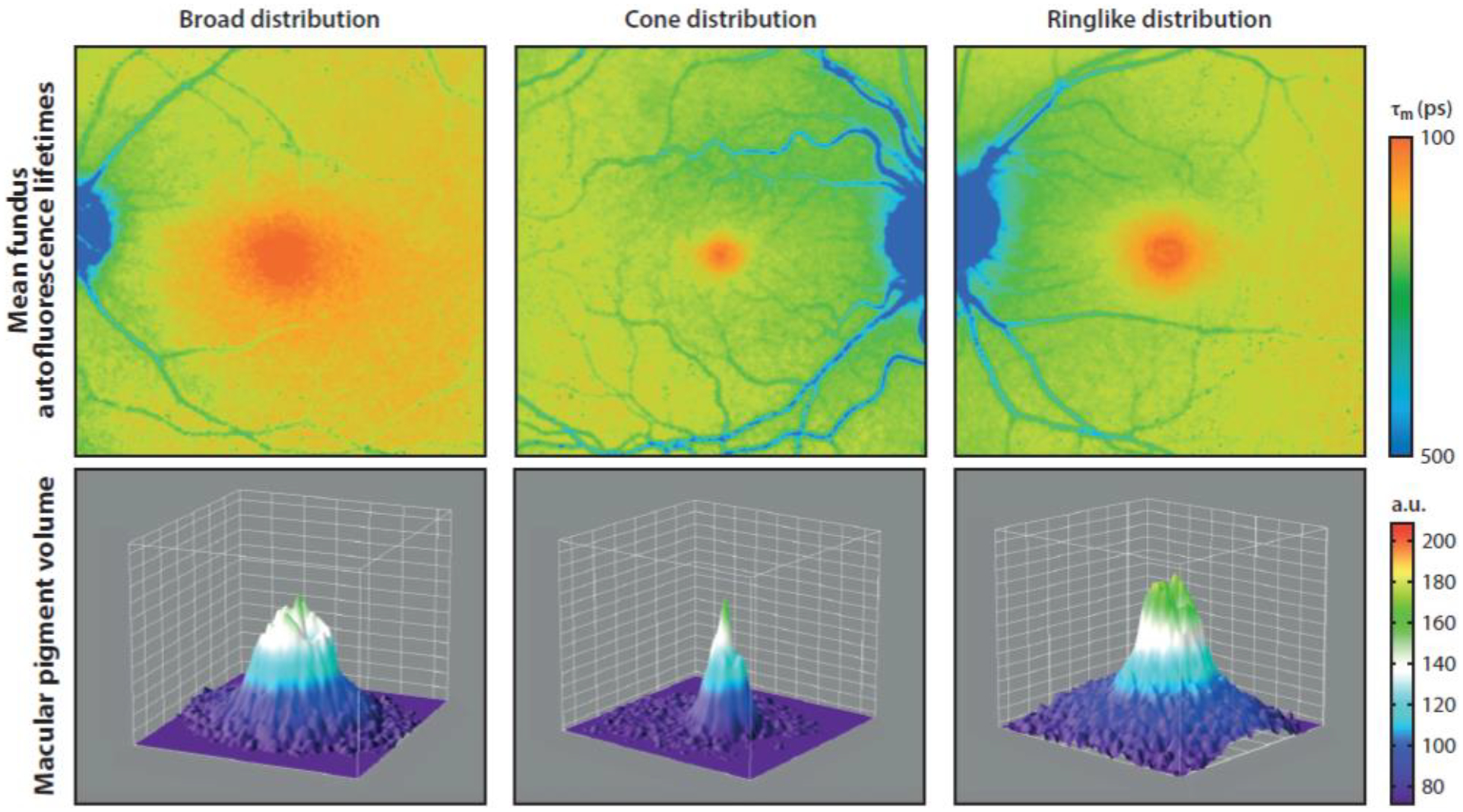
Distribution of macular pigment in healthy individuals. Abbreviations: a.u., arbitrary units; τm, amplitude-weighted mean fluorescence lifetime. Fluorescent life time imaging (FLIO) was used to obtain pigment distribution data and the figure was published in Lydia et al. Investigative Ophthalmology & Visual Science (2018); 59: 3094–3103
4. Clinical significance of the macular carotenoids
The MP’s anatomical location and its spectral properties are vital for protecting the photoreceptors of the fovea from photo-oxidation. [7]. The retina is a potential site for the generation of abundant reactive oxygen species (ROS) due to its high oxygen consumption required for the conversion of captured light photons into electrochemical signals via the transduction cascade and the presence of numerous mitochondria in the inner segment of rod cells. Moreover, the outer segments of retinal photoreceptors are rich in polyunsaturated fatty acids that are susceptible to photo-oxidation [14]. The macular carotenoids efficiently protect the macula from light-induced oxidative damage not only by absorbing incoming blue light but also by physically quenching and chemically scavenging ROS.
The macular carotenoids cannot be synthesized de novo in vertebrates and invertebrates and must be obtained through diet or supplementation. Epidemiological and clinical studies have suggested an essential role of dietary intake and nutritional supplementation with lutein and zeaxanthin in reducing the risk of age-related macular degeneration (AMD). A large case-control study in 1993 detected a plausible role for carotenoids and other serum antioxidants in decreasing the risk of AMD [15]. Subsequent clinical studies have correlated the consumption of carotenoid-rich vegetables using food questionnaires, indicating a need for prospective, randomized supplementation studies to understand the role of carotenoids in a reducing risk of visual loss from AMD [16].
In 1990, the Age-Related Eye Disease Study (AREDS), a randomized, 11-center, double-masked clinical trial, enrolled approximately 3600 AMD patients aged between 55–80 years with drusen, non-central geographic atrophy (GA) in one or both eyes, or advanced AMD (choroidal neovascularization or central GA) in the fellow eye, and followed them for an average of 6.3 years. The active treatment groups received antioxidants (vitamins C and E and zinc) or 15 mg/day β-carotene or a combination of both and achieved a statistically significant decrease in progression to advanced AMD in comparison to the placebo group [17]. It rapidly became standard-of-care to advise persons with intermediate or advanced AMD to take the AREDS formulation; however, subsequent epidemiological studies and small prospective clinical trials suggested that dietary intake of foods rich in lutein and zeaxanthin rather than β-carotene was driving lowered AMD risk [18] [19] and increased macular pigment optical density [20]. Other researchers suggested that β-carotene should be removed from the AREDS formulation because of its increased cancer risk in smokers and lack of correlation with AMD risk [21]. These concerns indicated the need to study next-generation AREDS formulations with the MP carotenoids (10 mg/day lutein and 2 mg/day zeaxanthin) as substitutes for β-carotene.
Like the AREDS study, AREDS2 was a randomized, multi-centered, double-masked, placebo-controlled trial with nearly 4200 subjects with late AMD in one eye or intermediate AMD in both eyes with an age range between 50 to 85 years followed for six years. MP supplementation with lutein and zeaxanthin decreased progression to late AMD by ~10% in comparison to no lutein + zeaxanthin supplementation. Still, this result was not sufficient to achieve the study’s pre-specified primary positive end-point [22] However, pre-planned secondary analyses showed that subgroups receiving lutein + zeaxanthin with no β-carotene had significantly reduced progression to advanced AMD relative to β-carotene subgroups [23]. This positive effect of lutein and zeaxanthin supplementation over β-carotene, coupled with lutein’s and zeaxanthin’s relative safety with regard to cancer risk, led to the rapid adoption of the AREDS2 formulation in the marketplace and clinical practice in preference to the original AREDS formulation.
In addition to its AMD protective effects, lutein + zeaxanthin supplementation has also been reported to improve visual and cognitive function in young, healthy adults [24], in agedadults with and without AMD, and patients with Alzheimer’s disease [25]. However, additional larger randomized, double-blinded, multi-centered trials are necessary to establish the role of lutein and zeaxanthin supplementation in cognition and visual performance definitively. A schematic representation of the protective roles of the macular carotenoids is shown in Figure 5.
Figure 5.
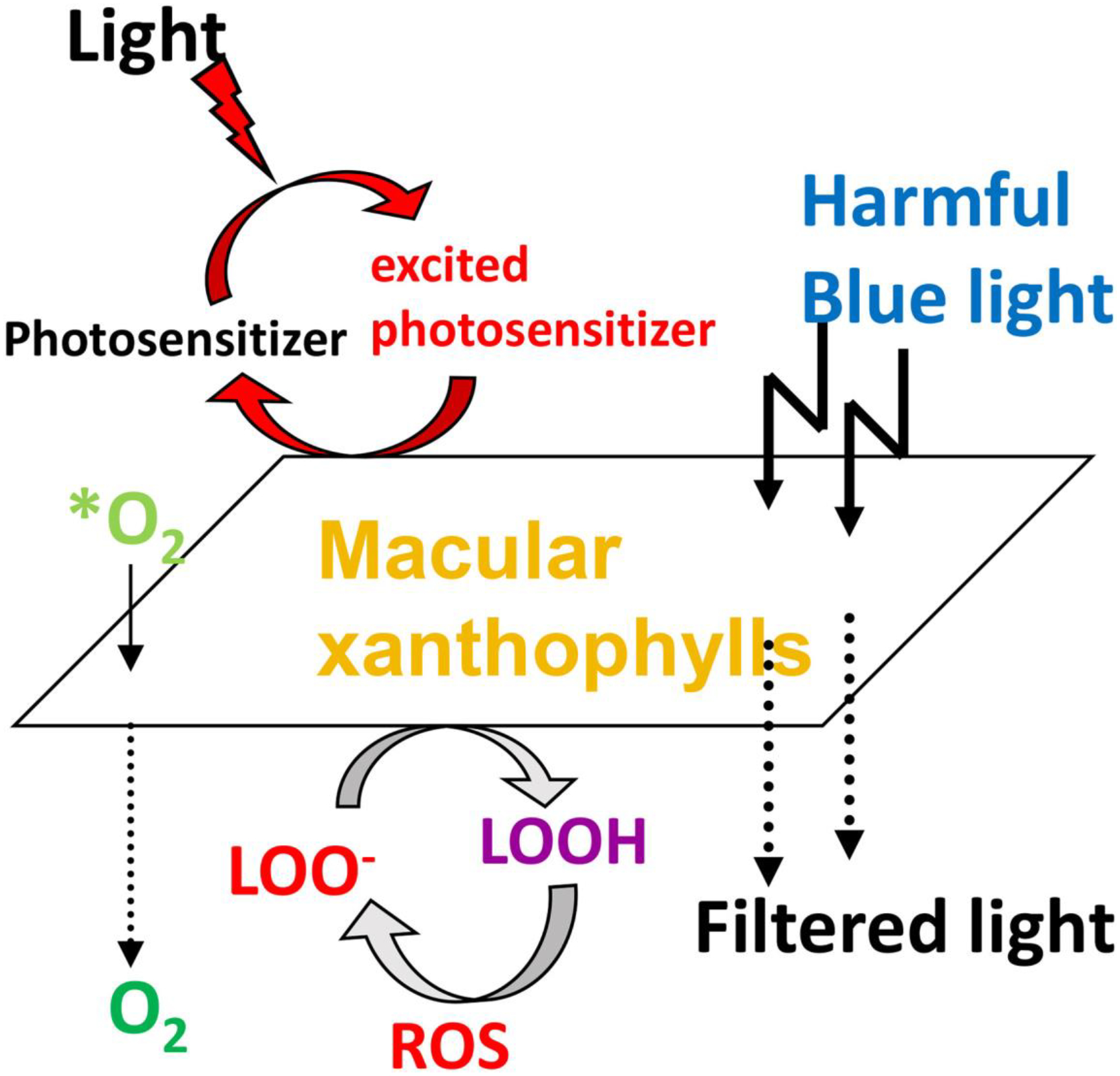
Protective roles of lutein and zeaxanthin as absorbers of harmful blue light and as antioxidants reacting with reactive oxygen species (ROS). *O2, singlet oxygen; LOO−, lipid peroxyl radicals; LOOH, lipid peroxides.
5. Dietary sources of the macular carotenoids
Lutein and zeaxanthin are the common xanthophylls present in green leafy vegetables (spinach, lettuce, kale, peas, broccoli, parsley, collard greens, and zucchini) and yellow-orange fruits (mango, orange, tangerine, papaya) [26]. The other significant sources are maize, einkorn, durum wheat, egg yolk, fish skin, and carapace (shell) of crustaceans [27]. The dietary xanthophylls such as lutein and zeaxanthin are commonly present in foods as di-esters with long-chain fatty acids. The relative amounts of free xanthophylls versus xanthophyll esters differ among various dietary sources. Generally, the carotenoid composition of food varies based on several factors such as species, cultivation, part of the plant, time of harvesting, degree of maturity at the time of harvest, and post-harvest handling methods [28]. To prevent carotenoid loss during processing, it is important to maintain optimal selection and processing parameters. An average western diet contains 1.3–3 mg/day of lutein and zeaxanthin combined. Lutein is typically present at 3–5 times the level of zeaxanthin in a western diet, while meso-zeaxanthin has been identified only in fish skin, shrimp carapace, and sea turtle fat [29]. Even though dietary meso-zeaxanthin is low or nonexistent, it accounts for one-third of the total MP concentration of macula. In the past, it has been challenging to assess the role of individual macular carotenoids in eye health because the macular carotenoid concentration in food was typically quantified as total lutein and zeaxanthin (L+Z), but newer HPLC-based analytical methods allow baseline separation of lutein from zeaxanthin, so these databases are gradually being updated.
Currently, there is no recommended daily dietary allowance for xanthophyll carotenoids. Nonetheless, daily intake of 2 to 12 mg of macular carotenoids appears to help prevent ocular diseases, and macular carotenoid supplements are generally recognized as safe (GRAS) for human consumption at such doses. The no observed-adverse-effect-level (NOAEL) for highest dose tested for lutein and zeaxanthin is 400 mg/kg body weight/day [30] and for meso- zeaxanthin is 300 mg/kg body weight/day [31].
6. Intestinal absorption and transport of macular carotenoids
Carotenoids are lipophilic molecules that follow the same absorption pathways as dietary lipids and other lipophilic molecules. The schematic representation of MP intestinal absorption, transport, and accumulation in the human retina is shown in Figure 6. Carotenoid absorption involves several steps, including the release of carotenoids from the food matrix. The carotenoids are initially released into the aqueous solution in the gut by chemical and mechanical disruption. The released carotenoids are dispersed in lipid emulsions and then transferred to mixed micelles. The degree of carotenoid solubilization and incorporation inside mixed micelles is one of the crucial factors in determining the bioavailability of carotenoids. The composition of mixed micelles consists of oleic acid, cholesterol, lysophosphatidylcholine, fatty acids, and monoglycerol [32]. Solubilized carotenoids in mixed micelles are then taken up by intestinal enterocytes. In the human intestine, the carotenes may be cleaved by β-carotene 15,15’-dioxygenase (BCO1) or β-carotene 9’,10’-monooxygenase (BCO2) enzymes to vitamin A and other metabolites in a regulated manner depending on vitamin A nutritional status [33], while xanthophyll carotenoids are generally absorbed without cleavage. Intact carotenoids or cleaved metabolites are incorporated into lymphatic lipoproteins as chylomicrons. Chylomicrons mediate the transfer of absorbed carotenoids from the gut to the liver via the lymphatic and portal circulations. From the liver, carotenoids are repackaged into plasma lipoproteins and transported to targeted tissues. During different stages of carotenoid absorption, there are several factors that influence carotenoid bioavailability, and it is challenging to assess the effect of each factor on overall carotenoid absorption [33]. The foremost factors that affect carotenoid absorption are Species of carotenoid, molecular Linkage, Amount of carotenoid ingested, carotenoid Matrix, Effectors of absorption and bioconversion, Nutrition status of the host, Genetic and host-related factors, and mathematical Interactions (jointly known by the abbreviation SLAMENGI) [34].
Figure 6.
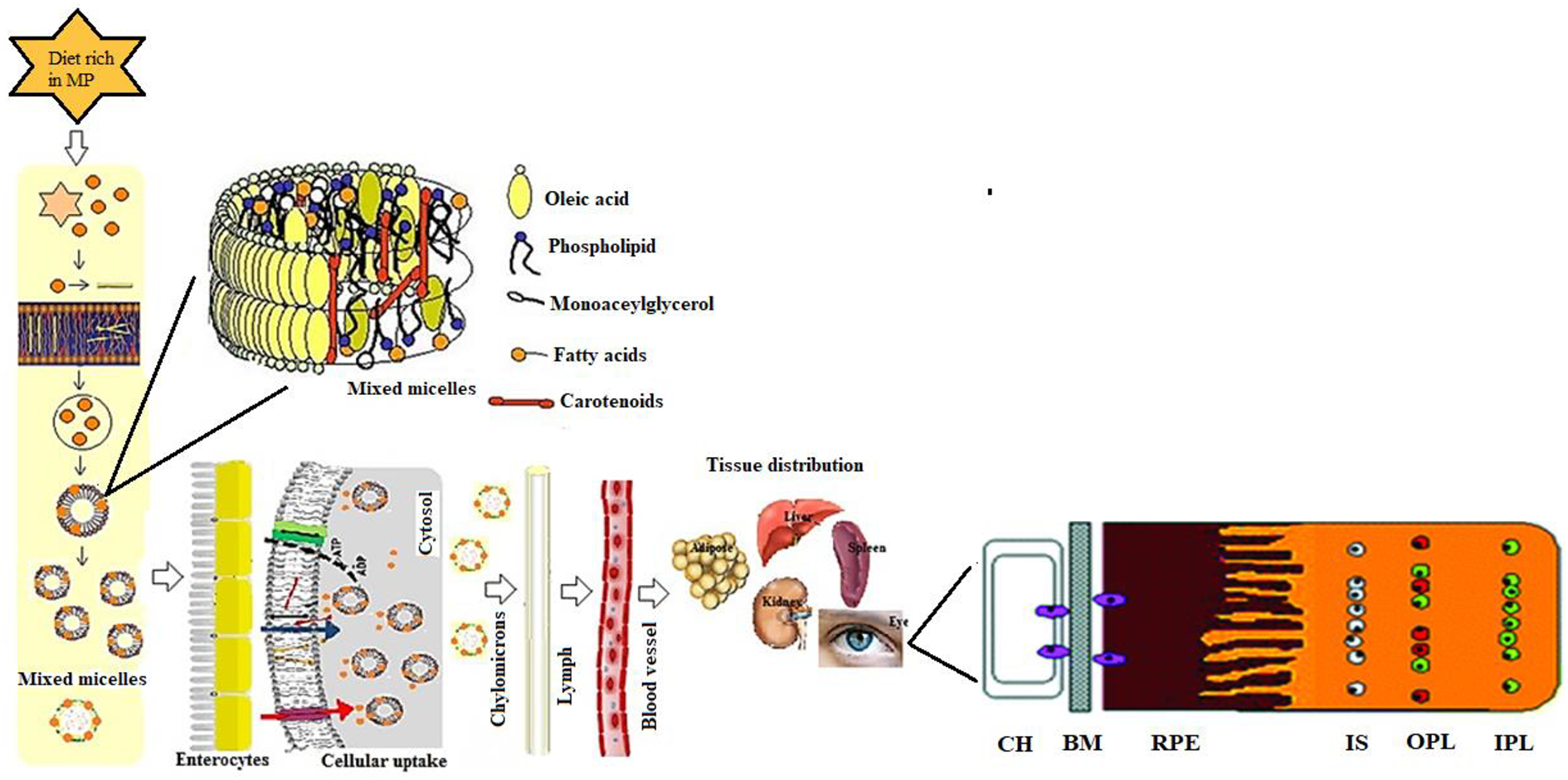
Possible pathways for MP carotenoid uptake, transport, and accumulation in the human retina. Choriocapillaris (CH); Bruch’s membrane (BM); Retinal pigment epithelium (RPE); Inner segments (IS); Outer plexiform layer (OPL); Inner plexiform layer (IPL).
7. Carotenoid transport proteins
Plasma lipoproteins are water-soluble proteins that mediate the transfer of plasma lipids, retinoids, and vitamin E [35]. Lipoprotein constituents include proteins and phospholipids (PL) at the outer shell and neutral lipids in the inner core. Lipoproteins have been classified into six groups: chylomicrons, chylomicron remnants, very-low-density lipoproteins (VLDL), low-density lipoprotein (LDL), and intermediate-density lipoproteins and high-density lipoprotein (HDL) [36]. The proportion of the surface PL to core lipid (cholesterol esters and triglycerides) in HDLs and LDLs are different. In HDLs, the ratio of PL to core lipid is 1.4:1, and in LDLs, it is 0.3:1 [37]. Lutein and zeaxanthin are found predominantly associated with HDL (53%), LDL (31%) and VLDL (16%) respectively [38]. LDL carries about one molecule of carotenoid per LDL particle with a ratio of 1:1, while the HDL carries only one carotenoid molecule in every 20 HDL particles with the ratio of 1:20 [39]. In plasma, lutein: zeaxanthin exists in ratios between 2:1 and 4:1. The mechanisms by which carotenoids are incorporated into LDL or HDL and their exact location in the particles are poorly understood. The difference in PL to core lipids ratio and the polarity of carotenoids seem to account for the affinity of carotenoids for particular lipoproteins, but the specific components responsible for carotenoid binding still need to be identified.
Among lipoprotein groups, HDL is the smallest and densest, and it plays a crucial role in cholesterol metabolism. HDL removes cholesterol from peripheral tissues in a process known as reverse cholesterol transport [40]. Higher HDL levels are positively correlated with cardiovascular health [41]. The normal chicken accumulate macular carotenoids in the retina as oil droplets and the chicken retina lacks macular fovea or the “yellow spot” region. The Wisconsin hypo alpha mutant (WHAM) chicken has a mutation in the ATP binding cassette subfamily A-1 (ABCA1) transporter gene, which causes a 90% reduction in plasma HDL. WHAM chickens fed with a lutein-rich diet have higher lutein levels in liver, heart, plasma, and egg yolk. However, lutein was not detected in the retina, suggesting that HDL plays a crucial role in delivering lutein to the retina [42].
Scavenger receptor class B type-1 (SR-B1) is a multi-ligand, single-chain transmembrane glycoprotein that acts as an HDL cell surface receptor. It plays a prominent role in HDL metabolism in mammals by mediating delivery of cholesterol esters to tissues and discharge of cholesterol from peripheral tissues and macrophages to liver and lipoproteins [40]. It belongs to the CD36 superfamily and is well conserved between species. The molecular structure of scavenger receptor B family members differs from other scavenger receptors by two transmembrane domains, with both the N and C terminals of the proteins residing intracellularly. SR-B1 is expressed at high levels in many mammalian steroidogenic tissues such as adrenals, ovaries, testis, and placenta. It plays a key role in the absorption and accumulation of ocular carotenoids in the human and fly retina. The human SCARB1 gene encoding the 509 amino-acid SR-B1 protein is located on chromosome 12q24 [43]. Due to posttranslational modifications, SR-B1 protein’s molecular weight is detected as 82-kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) instead of its predicted 57-kDa [44]. Abnormal SR-B1 expression is associated with coronary artery disease, prostate, and breast cancer [45], [46].
For many years, researchers thought that intestinal carotenoid uptake occurs by passive diffusion. After learning that the gene ninaD encoded a class B scavenger receptor in Drosophila melanogaster with high sequence homology to the mammalian class B scavenger receptors (SRB1 and CD36), the concept of receptor-mediated carotenoid absorption gathered much attention. The ninaD protein is essential for xanthophyll cellular uptake and distribution in drosophila. Defects in cellular uptake of carotenoids due to a nonsense mutation in the ninaD gene result in impaired vision in drosophila [47]. Further studies on Caco-2 cells demonstrated that SRB1 plays an important role in the cellular uptake of lutein [48]. During et al. reported higher uptake of macular carotenoids over β-carotene via an SR-B1 dependent mechanism by “adult retinal pigment epithelial cells (ARPE-19)”. ARPE 19 is a cell culture model for the retinal pigment epithelium (RPE) that displays some, but not all, of the characteristics of natural human RPE cells. It plays a critical role in the development and maintenance of adjacent photoreceptors in the vertebrate retina. Blocking SR-B1 by its antibody or knocking it down by siRNA leads to a reduction in macular carotenoid uptake. The preferential uptake of macular carotenoids over β-carotene suggests a possible transporter role for SR-B1 [49]. Studies on the selective mechanism of delivery of xanthophyll to ARPE-19 showed HDL-mediated selective uptake of zeaxanthin and meso-zeaxanthin via SR-B1 in contrast to an LDL-mediated uptake of lutein [50].
The intestine specific-homeobox (ISX) transcription factor acts as a retinoic acid-sensitive “gatekeeper” that represses the intestinal expression of SR-B1 [51]. In the intestine, carotenoid absorption is governed by ISX via SR-B1 by negative feedback regulatory mechanisms through its metabolite retinoic acid. In contrast, in the retina, SR-B1 regulatory mechanisms must be different from the intestine because there is no report on the existence of ISX in RPE or retina. A recent study from our laboratory showed that all three human SR-B proteins (SR-B1, SR-B2 (LIMPII), and CD36) are capable of binding to the MP carotenoids [52]. We also demonstrated in vitro that zeaxanthin and meso-zeaxanthin uptake concentrations were increased in the presence of HDL and mediated by all three human SR-B proteins, whereas lutein uptake is mediated by SR-B1 and CD36 and is enhanced in the presence of LDL [52].
The cluster of differentiation (CD-36) protein belongs to the same scavenger receptor family member as SR-B1 and is also known as fatty acid translocase (FAT). CD-36 can bind to long-chain fatty acids and transport them to cells [53]. CD-36 is an integral membrane glycoprotein that is found on the surface of many vertebrate cell types and displays broad specificity. Unlike SR-B1, it is expressed highly in primate neural retina and at the brush border level of the duodenum and the jejunum [54]. CD-36 consists of 472 amino acids with a projected molecular weight of approximately 53-kDa, but due to extensive glycosylation, it is detected at 88-kDa on SDS-PAGE. In humans, its gene is located at chromosome 7 at band 11.2 (7q11.2). Cameo2 is similar to the mammalian CD36 protein and is required for lutein uptake into the silk glands of Bombyx mori [55]. CD-36 is involved in carotenoid uptake in adipocytes and adipose tissue via a facilitated process in 3T3-L1 cells. Inhibition of CD-36 did not impair carotenoid uptake by adipocytes, which strongly suggests that other mechanisms are involved in carotenoid uptake [56]. Recent studies demonstrate that genetic variation of CD-36 is associated with serum lutein levels and macular pigment optical density (MPOD) in AMD patients, suggesting a role for CD-36 in MP uptake [57].
8. Carotenoid cleavage enzymes
Moore et al. first reported the conversion of β-carotene into retinol in the rat small intestine [58]. Karrer elucidated the structure of β-carotene and proposed a central cleavage of the 15,15` C=C double bond of β-carotene to form 2 molecules of retinaldehyde [59]. BCO1 catalyzes the central cleavage at 15, 15` double bond of β-carotene, forming two molecules of all-trans-retinal. The all-trans-retinal can be oxidized to retinoic acid or reduced to retinol. BCO2 participates in eccentric cleavage of β-carotene at the 9,10, and 9`,10` C=C double bonds forming apo-carotenoids, including β-apo-10’-carotenal and β-ionone. BCO1 functions as a prominent enzyme in vitamin A production. Both BCO1 and BCO2 belong to the non-heme iron oxygenase family, which is found in both plants and animals. BCO1 is a cytoplasmic enzyme expressed in the intestinal mucosa which cleaves provitamin A carotenoids with at least one nonsubstituted β-ionone ring. BCO2 is a mitochondrial enzyme that is highly expressed in hepatocytes. The difference in cellular compartments of BCO1 and BCO2 may reflect separate substrate specificities and physiological functions [60].
9. Carotenoid binding proteins
Carotenoid binding proteins have been reported in plants [61], microorganisms [62], and invertebrates [63], but until recently, there was no information available on specific carotenoid binding proteins in vertebrates. Our laboratory initiated a project to understand the biochemical mechanisms underlying the specific binding and accumulation of these macular pigments. Since the macular carotenoids are specifically accumulated in the human fovea, we hypothesized that carotenoid binding proteins would mediate this process. The first vertebrate carotenoid binding protein to be reported was retinal tubulin because when this 55 kDa water-soluble protein was isolated from bovine retinal microtubules, it co-purified with endogenous carotenoids, but with low specificity and affinity [64]. Tubulin is abundant in the receptor axon layer of the fovea, and it can serve as a locus for the accumulation of macular carotenoids. Further studies in our laboratory demonstrated that many carotenoids besides lutein and zeaxanthin could bind to tubulin, indicating that tubulin was unlikely to be the mediator of the specific uptake of the macular carotenoids. Crabtree et al. demonstrated that carotenoids might be bound to the paclitaxel (Taxol) binding site of the β-tubulin subunit of microtubules [65].
9.1. Glutathione S-transferase P1 (GSTP1)
The first specific carotenoid binding protein that we identified in the human retina with a high affinity for zeaxanthin and meso-zeaxanthin was GSTP1. GSTs are better known as enzymes that catalyze the detoxification of cytotoxic agents by reduction and conjugation with glutathione [66]. GSTs are divided into three major families: cytosolic GSTs, mitochondrial GSTs, and microsomal GSTs. In humans, cytosolic GSTs are divided into alpha, zeta, theta, mu, pi, sigma, and omega isoforms [67]. The pi isoform of GST is found abundantly in human epithelial tissue as a dimer of identical 23 kDa subunits, and its gene is located at chromosome locus 11q13 [67]. GSTP1 is a dimeric phase II detoxification enzyme that detoxifies electrophiles by glutathione conjugation.[68]. In the human retina, GSTP1 co-purifies with endogenous zeaxanthin, and immunolocalization of GSTP1 reveals that it is localized to the outer and inner plexiform layers and the inner segment of the photoreceptor ellipsoid region (Figure 7) [69]. Recombinant human GSTP1 exhibits higher affinity and specificity for zeaxanthin with a KD of 0.33 μM for zeaxanthin and 0.52 μM for meso-zeaxanthin, while lutein does not bind to any appreciable extent. Other recombinant GST proteins such as GSTA1 and GSTM1 are close analogs to human GSTP1, but they exhibit low-affinity binding for xanthophylls. GSTP1/zeaxanthin complexes exhibit synergistic antioxidant activities and effectively quench lipid peroxyl radical generators. The GSTP1 protein stabilizes zeaxanthin against degradation induced by peroxyl radicals, thereby enhancing its antioxidant properties [70]. Genetic polymorphisms of GSTP1 are associated with risk of wet AMD and cortical cataracts [71]
Figure 7.
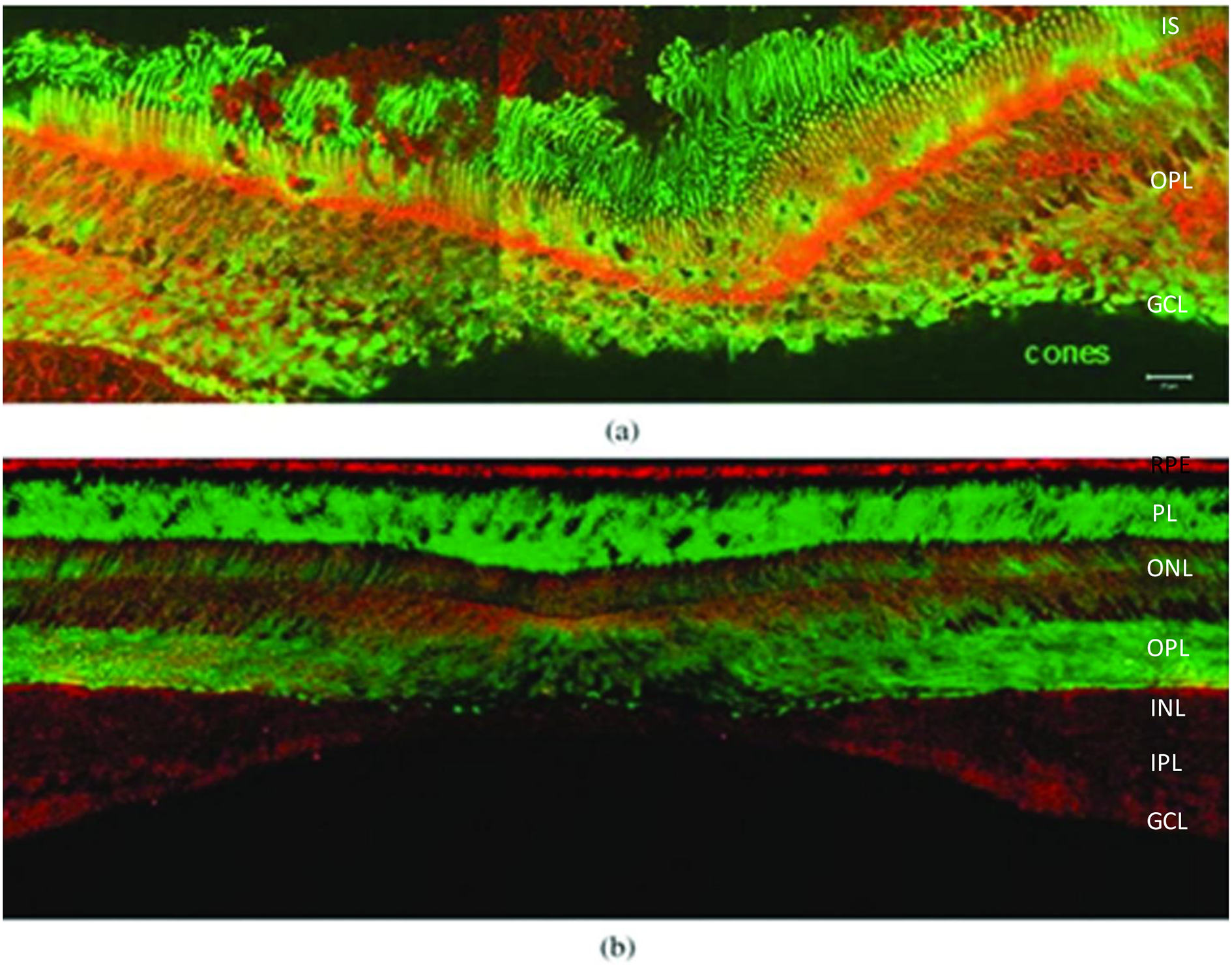
The retinal distribution of macular pigment binding proteins. (a) GSTP1 labeling of foveal cones in the macula of a 3-year-old monkey. This montage shows strongest labeling by antibody against GSTP1 (red) over the myoid and ellipsoid regions of cones identified by monoclonal antibody (7G6, green). (b) A low-magnification view of a near-foveal retina section in which N-62 StAR (red) identifies StARD3, an anti-cone arrestin monoclonal antibody (7G6, green) identifies monkey cones. Images courtesy of Dr. Jeanne M. Frederick and the figure was published in Bernstein et al. 2016 Prog. Retin. Eye Res.; 50:34–36. The layers are labelled as Inner segments of photoreceptors (IS), Outer plexiform layer (OPL), Ganglion cell layer (GCL), Retinal pigment epithelium (RPE), Photoreceptor layer (PL), Outer nuclear layer (ONL), Outer plexiform layer (OPL), Inner nuclear layer (INL), Inner plexiform layer (IPL).
9.2. Steroidogenic acute regulatory domain protein 3 (StARD3)
StAR related lipid transfer domain 3 (StARD3), also known as metastatic lymph node 64 protein (MLN64), was identified by our laboratory as the human retina’s lutein binding protein based on its homology to the silkworm’s lutein binding protein in the gut and silk gland (CBP) and its high expression in the macula. The human StARD3 gene encodes a StARD sub-family protein involved in lipid trafficking that is characterized by an N-terminal metastatic lymph node 64 domain and a C-terminal steroidogenic acute regulatory domain. The gene was first reported in breast and ovarian cancer cells [72], and its chromosomal location is 17q11–q12. It plays a vital role in mitochondrial cholesterol delivery and intercellular sterol transport.
Surface plasma resonance binding assays showed that human StARD3 has high specificity and affinity for lutein (KD =0.45 μM) [73], and lutein/StARD3 complexes may enhance the antioxidant activity of lutein similar to GSTP1/zeaxanthin complexes. Immunolocalization studies in retina show that the protein is present in the outer plexiform (Henle fiber) region (Figure 7). The structure of the lutein-binding domain of StARD3 has been determined to 1.74 Å resolution, and docking studies revealed that the ε-ionone ring of lutein is important for lutein/StARD3 complex formation (Figure 8), suggesting that steric complementarity and ligand asymmetry may play a role in discriminating lutein from the other ocular carotenoids [74]. King et al. reported that StARD3 was also expressed in glia and neurons in specific regions of the human brain [75]. Lutein is the predominant carotenoid in the brain (about 31% of total brain carotenoids), and the selective accumulation of lutein in the brain also appears to be due to StARD3 [76].
Figure 8.
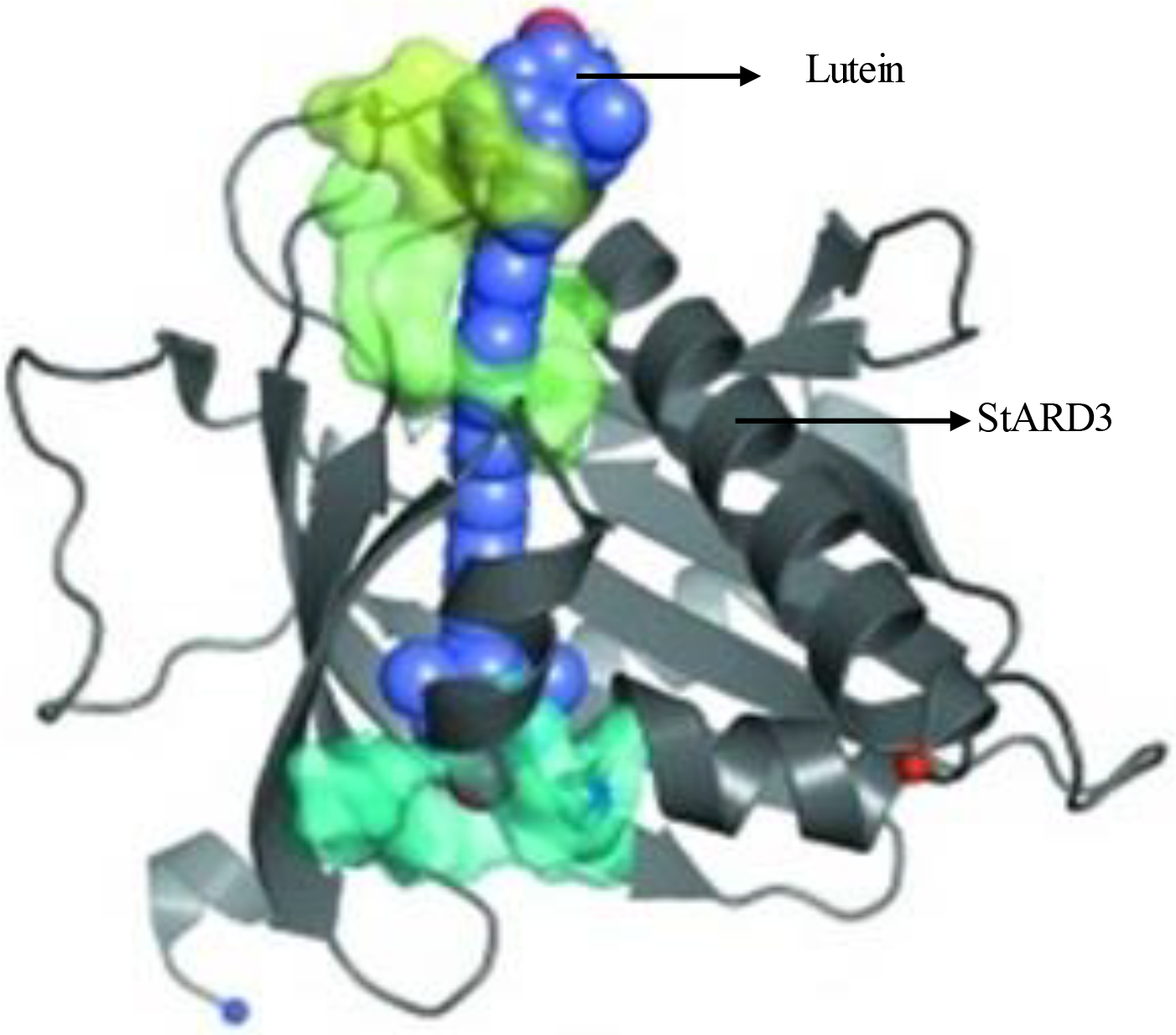
Overview of lutein molecule docked with StARD3 structure shows the best-scoring lutein candidate from the one portal set and the figure was published in Horvath et al. Acta Cryst. (2016); 72:609–618.
10. Animal models in macular carotenoids research
10.1. Avian models
Chickens have been used as a model to study the accumulation of macular carotenoids because their relevant transport and binding proteins for macular carotenoids are similar to those of humans. The chicken retina is comparable in organization to humans and other mammals with some significant differences. The chicken retina lacks a macula, and much of its carotenoid content is esterified in oil droplets. Wang et al. studied selective retention of macular carotenoids in the retina of chickens fed a xanthophyll-free diet for 28 days and found a sharp decline (99%) in carotenoid concentration in all tissues except retina where it was avidly retained [77]. As in humans, HDL is a prominent serum carrier protein for chicken ocular carotenoids. The crucial role of HDL in the transport of macular carotenoids was particularly well demonstrated in WHAM chickens because HDL deficiency in WHAM chickens is associated with a significantly reduced level of lutein and zeaxanthin in the retina [42].
Gorusupudi et al. reported that the RPE/choroid is the primary site for meso-zeaxanthin’s developmentally regulated synthesis [10]. In further studies, the role of RPE65 as the enzyme responsible for the transformation of lutein to meso-zeaxanthin was determined. There was several-fold upregulation of RPE65’s gene expression corresponding to meso-zeaxanthin’s production during retinal development, and pharmacologic inhibition of RPE65’s activity resulted in a significant decrease in meso-zeaxanthin levels. To confirm the RPE activity of meso-zeaxanthin production from lutein, (HEK293T) human embryonic kidney cell lines were used. Cells were transfected with plasmids pCDNA3.1 chicken RPE65 resulted in overexpression of RPE 65 up to 4 days. The cells were treated with purified lutein (4 μM) resulted in production of higher level of meso-zeaxanthin in RPE overexpressing cells compared to control cells where no detectable meso-zeaxanthin was observed [13]. Chickens fed with meso-zeaxanthin for eight weeks had a high accumulation of meso-zeaxanthin in the retina, brain, liver, kidney, heart, demonstrating that meso-zeaxanthin accumulates in non-ocular chicken tissues in a pattern similar to lutein and zeaxanthin [78]. Among the vertebrates, meso-zeaxanthin is present only in the retina due to tissue-specific isomerization of lutein, but these experiments confirmed that dietary supplementation with exogenous meso-zeaxanthin could result in accumulation of mesozeaxanthin in tissues as well.
The Japanese quail, with its cone-rich retina, has been used as a model to study macular carotenoids. Toyoda et al. studied the xanthophyll tissue distribution profile in quails after dietary feeding of zeaxanthin [79]. Dietary supplementation with zeaxanthin increased its concentration in the retina and other tissues several-fold. Quails fed separately with zeaxanthin or β-carotene had a significant increase in lens carotenoid content in the zeaxanthin supplement group, but they observed no detectable lens β-carotene in the β-carotene supplement group. These results suggest that there is selective uptake of xanthophylls by quails, similar to what is seen in primates and humans [80]. In another study, they looked at light-induced retinal damage in zeaxanthin-supplemented quail and found that higher retinal zeaxanthin levels resulted in a reduction of apoptotic rod and cone cells [81]. Our group studied the metabolic transformation of carotenoids in quail ocular tissues [12]. Supplementation with deuterium-labeled zeaxanthin showed that dietary zeaxanthin is the precursor for astaxanthin, adonirubin, 3`-oxolutein, β-apo-2`-carotenol, and galloxanthin, while dietary lutein is the precursor of just meso-zeaxanthin. Furthermore, we reported inhibition of A2E formation in Japanese quail supplemented with macular carotenoids for 16 weeks. These results highlight another protective mechanism of macular carotenoids against macular degeneration [82].
10.2. Non-human primates
The non-human primate’s macula lutea is similar to the human macula lutea making it an appropriate model to study AMD and carotenoid metabolism. Snodderly et al. reported the distribution of individual macular carotenoids in non-human primates and found that zeaxanthin becomes more prominent in the foveal center, while lutein is predominant in peripheral regions [83]. Lutein and zeaxanthin oxidation products have been identified in both human and monkey retinas, and the identified oxidative products in the retina may be interconverted through a series of oxidative-reductive reactions, further supporting the hypothesis that the macular carotenoids act as antioxidants and protect the retina from short-wavelength visible light [8]. Monkeys supplemented with either lutein or zeaxanthin showed an increase in serum macular carotenoids, even after life-long xanthophyll deficiency, and the investigators concluded that monkeys retained their ability to absorb and metabolize macular carotenoids after a long period of xanthophyll deficiency [84]. They also concluded that the macular carotenoids, along with n-3 fatty acids, are essential for healthy development and maintenance of RPE cells [85]. Supplementation with pure lutein or zeaxanthin in monkeys raised on xanthophyll-free diets showed the presence of meso-zeaxanthin in the retina only when lutein was fed to them, which indicates that lutein is the precursor for meso-zeaxanthin [11]. Supplementation with n-3 fatty acids and macular carotenoids alters rod and S-cone density profiles, and they found that photoreceptors are less sensitive than RPE to nutritional manipulation [86]. Fovea’s of the macular carotenoid supplemented group were less sensitive to blue light-induced damage than a control group fed with a xanthophyll-free diet, potentially explaining how the macular carotenoids could protect the fovea from blue light-induced damage [87].
Vishwanathan et al. evaluated the relationship between ocular and brain macular carotenoids levels and reported that macular pigment levels are significantly correlated in the brain and retinal tissues [88]. Studies on the lutein bioaccumulation in the brain and retina show that breastfed infant monkeys have higher total cholesterol, HDL, and apoA-1 and apoB- 100 levels than the supplement fed infant monkeys. This indicates that apoproteins is essential for delivering lutein to retina and brain tissues [89].
10.3. Knockout rodent models
Rats and mice are the most commonly used animal models for carotenoid research. Many research groups have used them to study different aspects of macular carotenoid absorption, physiology, and various biological properties. In most of the studies, researchers used a high level of carotenoids (0.5–100 mg/kg body wt./d (oral administration)), which translates to 30–6000 mg/d in a 60 kg weight adult which is much higher than average human consumption in a typical western diet (2–7 mg/day). Although researchers were able to detect carotenoid uptake into whole eyes [90], mice fail to accumulate carotenoids in their retinas. This is because wild type mice commonly used for macular carotenoid studies have highly active carotenoid cleaving enzymes (BCO1 and BCO2), which cleave carotenoids into their respective metabolites [91]. Thus, to study carotenoid uptake into the retina in mice, it is necessary to genetically knockout these enzymes. To study various biological and physiological properties of macular carotenoids. Amangual et al. reported the biochemical properties of mitochondrial carotenoid oxygenase BCO2 and the effect of BCO2 deficiency in mouse models of accumulation of macular carotenoids in the retina and other tissues [92]. Babino et al. characterized the role of BCO2 in macular carotenoid metabolism and concluded that primate BCO2 expresses as an oxidative stress-induced mitochondrial protein with conserved structural fold and enzymatic function [93]. Our group reported that relative inactivity of human BCO2 underlies the retinal accumulation of macular pigments [91], and we further studied the retinal accumulation of macular pigments in mice deficient in BCO1 and/or BCO2 [94]. BCO2 knockout mice supplemented with lutein or zeaxanthin showed improved visual acuity compared to a control group fed a low carotenoid diet, confirming that BCO2 knockout “macular pigment mice” are a good model for studying carotenoid function in small animal models [95].
10.4. Other animal models
Lobo et al. studied the role of BCO2 as an oxidative stress-regulated protein and found that knock out of BCO2 resulted in anemia during zebrafish larval development. They concluded that BCO2 protects against oxidative stress and plays a vital role in carotenoid scavenging and the apoptotic pathways [96]. Similar to mice, the visual acuity of zebrafish was 14% higher one week after intraocular injection of zeaxanthin [97]. Zhang et al. investigated the impact of lutein on the life span of Drosophila melanogaster. Results revealed that flies fed with a luteinenriched diet showed a significant increase in their life span that was partially accounted for by the up-regulation of antioxidant enzymes and decreases in malondialdehyde (MDA) levels [98]. The beneficial effect of lutein was evaluated on light-induced retinal phototoxicity, and they found that the lutein administered group showed no significant difference in preventing rabbit eyes from photodamage compared to a control group [99]. The neuroprotective effect of lutein in liposome form against cisplatin-induced retinal injury in rabbits was also studied. They concluded that liposomal lutein could prevent the detrimental effects of cisplatin on the retina [100]. Rabbit ocular tissues were used to evaluate the toxicity profile of lutein and zeaxanthin, along with brilliant blue dye. There was no significant difference in electroretinogram, and evaluation of histological sections indicated that lutein and zeaxanthin alone or in combination with dye was safe [101].
11. Conclusion and perspectives
The macular carotenoids have unique chemical structures that define their physical, chemical, and biological properties. Strong evidence from epidemiology, animal studies, and in vitro experiments suggest that the MP carotenoids protect the retina from photo-oxidative damage. The macular carotenoids effectively absorb short-wavelength visible light and quench ROS through their antioxidant properties, and multiple studies suggest that higher levels of macular carotenoids in diet and plasma increase MPOD and inversely correlate with the incidence of AMD.
In the past few decades, our understanding of macular carotenoids and their role in AMD protection has improved dramatically. However, the impact of supplementation with specific combinations and dosages of meso-zeaxanthin, lutein, and zeaxanthin on the progression of AMD remains unclear, and there is considerable controversy among patients and clinicians with regard to preferred formulations; this will require further clinical investigations beyond the current AREDS2 recommendations. Nor do we know whether macular carotenoid supplementation has any value over increased fruit and vegetable consumption for the “worried well” who show no signs of AMD but may have a positive family history that would suggest a future risk for vision loss later in life. Moreover, it is becoming clear that lutein and zeaxanthin play important roles in maintenance of ocular health well before the age when AMD typically manifests. In younger adults, we know that increased MP levels can improve visual performance in subtle but reproducible ways [102], but it is unclear whether lutein and/or zeaxanthin supplementation should be recommended for this population. The MP is detectable at birth and increases steadily through the first seven years of life, which implies that it may have an underappreciated role in foveal and macular development. To address this unmet need, we have initiated a randomized, placebo-controlled trial of lutein and zeaxanthin supplementation in prenatal vitamins (the L-ZIP study, NCT03750968) to assess its effects on combatting maternal carotenoid depletion during pregnancy and promoting enhanced infant MPOD and visual function and development. We hope that when results are available in two years, we will be able to provide evidence-based recommendations for carotenoid supplementation early in life.
While clinical research guides nutritional recommendations and clinical practice, it is still important to expand our basic science knowledge of the chemistry and physiology of lutein and zeaxanthin in the retina. Our laboratory and others have elucidated the pathways of uptake of the xanthophyll carotenoids from the diet and their highly specific deposition in the macula via scavenger receptors, lipoproteins, binding proteins, and eventual metabolism and cleavage by various enzymes. Yet, we still have relatively limited understanding of the biochemical mechanisms and physiological value underlying why the primate eye uniquely deposits only lutein, zeaxanthin, and their metabolites in just a tiny but vital region of the human retina. Further mechanistic studies of the antioxidant and regulatory properties of the macular carotenoids and improvement and implementation of rodent models genetically engineered to have enhanced uptake of lutein and zeaxanthin along with use of smaller and easier to handle non-human primate models will undoubtedly expand our knowledge of the ocular physiology of the macular carotenoids in upcoming years.
Highlights.
Lutein, zeaxanthin, and meso-zeaxanthin are the macular pigment (MP) carotenoids.
MP cannot be synthesized de novo and must be obtained through diet.
Selective accumulation of MP in the retina is mediated by specific binding proteins.
MP filters high intensity visible light and provides retinal antioxidant protection.
Genetically engineered “MP mice” can be used to study MP function and physiology.
ACKNOWLEDGMENTS
This work was funded in part by National Eye Institute grants EY11600 and EY14800 and an unrestricted departmental grant from Research to Prevent Blindness.
Abbreviations:
- A2E
N-retinylidene-N-retinyl-ethanolamine
- ABCA1
ATP binding cassette subfamily A-1
- AMD
Age-related macular degeneration
- AREDS
Age-Related Eye Disease Study
- AREDS2
Age-Related Eye Disease Study 2
- BCO1
β-carotene oxygenase 1 (also known as β-carotene-15, 15’-monoxygenase)
- BCO2
β-carotene oxygenase 2 (also known as β-carotene-9’, 10’-dioxygenase)
- CD 36
Cluster of Differentiation 36
- ELM
External limiting membrane
- GA
Geographic atrophy
- GCL
Ganglion cell layer
- GRAS
Generally recognized as safe
- GSH
Glutathione
- GSTs
Glutathione-S-transferases
- GSTP1
Glutathione S-transferase P1
- HDL
High-density lipoproteins
- HPLC
High pressure/performance liquid chromatography
- ILM
Internal limiting membrane
- INL
Inner nuclear layer
- IPL
Inner plexiform layer
- ISX
Intestine specific homeobox
- LDL
Low-density lipoprotein
- MDA
Malondialdehyde
- MLN64
Metastatic lymph node 64
- MP
Macular pigment
- MPOD
Macular pigment optical density
- NFL
Nerve fiber layer
- NOAEL
No observed-adverse-effect level
- O-H
Hydroxy group
- ONL
Outer nuclear layer
- OPL
Outer plexiform layer
- PL
Phospholipid
- POS
Photoreceptor outer segment
- ROS
Reactive oxygen species
- RPE
Retinal pigment epithelium
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SR-BI
Scavenger receptor class B member 1
- StARD3
Steroidogenic acute regulatory domain protein 3
- VLDL
Very high-density lipoprotein
- WHAM
Wisconsin hypo alpha mutant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].Landrum JT, Bone RA, Lutein, zeaxanthin, and the macular pigment, Arch. Biochem. Biophys 385 (2001) 28–40. 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- [2].Bone RA, Landrum JT, Hime GW, Cains A, Zamor J, Stereochemistry of the human macular carotenoids, Invest. Ophthalmol. Vis. Sci 34 (1993) 2033–2040. [PubMed] [Google Scholar]
- [3].Krinsky NI, Landrum JT, Bone RA, Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye, Annu. Rev. Nutr 23 (2003) 171–201. 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- [4].Sauer L, Li B, Bernstein PS, Ocular Carotenoid Status in Health and Disease, Annual Review of Nutrition. 39 (2019) 95–120. 10.1146/annurev-nutr-082018-124555. [DOI] [PubMed] [Google Scholar]
- [5].Woodall AA, Britton G, Jackson MJ, Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability, Biochimica et Biophysica Acta (BBA) - General Subjects. 1336 (1997) 575–586. 10.1016/S0304-4165(97)00007-X. [DOI] [PubMed] [Google Scholar]
- [6].Arunkumar R, Calvo CM, Conrady CD, Bernstein PS, What do we know about the macular pigment in AMD: the past, the present, and the future, Eye. 32 (2018) 992–1004. 10.1038/s41433-018-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sujak A, Gabrielska J, Grudziński W, Borc R, Mazurek P, Gruszecki WI, Lutein and Zeaxanthin as Protectors of Lipid Membranes against Oxidative Damage: The Structural Aspects, Archives of Biochemistry and Biophysics. 371 (1999) 301–307. 10.1006/abbi.1999.1437. [DOI] [PubMed] [Google Scholar]
- [8].Khachik F, Bernstein PS, Garland DL, Identification of lutein and zeaxanthin oxidation products in human and monkey retinas, Invest. Ophthalmol. Vis. Sci 38 (1997) 1802–1811. [PubMed] [Google Scholar]
- [9].Khachik F, de Moura FF, Zhao D-Y, Aebischer C-P, Bernstein PS, Transformations of Selected Carotenoids in Plasma, Liver, and Ocular Tissues of Humans and in Nonprimate Animal Models, Invest. Ophthalmol. Vis. Sci 43 (2002) 3383–3392. https://iovs.arvojournals.org/article.aspx?articleid=2123268 (accessed September 3, 2019). [PubMed] [Google Scholar]
- [10].Gorusupudi A, Shyam R, Li B, Vachali P, Subhani YK, Nelson K, Bernstein PS, Developmentally Regulated Production of meso-Zeaxanthin in Chicken Retinal Pigment Epithelium/Choroid and Retina, Invest Ophthalmol Vis Sci. 57 (2016) 1853–1861. 10.1167/iovs.16-19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM, Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys, Invest. Ophthalmol. Vis. Sci 46 (2005) 692–702. 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- [12].Bhosale P, Serban B, Zhao DY, Bernstein PS, Identification and Metabolic Transformations of Carotenoids in Ocular Tissues of the Japanese Quail Coturnix japonica, Biochemistry. 46 (2007) 9050–9057. 10.1021/bi700558f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shyam R, Gorusupudi A, Nelson K, Horvath MP, Bernstein PS, RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye, Proc. Natl. Acad. Sci. U.S.A 114 (2017) 10882–10887. 10.1073/pnas.1706332114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cai J, Nelson KC, Wu M, Sternberg P, Jones DP, Oxidative damage and protection of the RPE, Progress in Retinal and Eye Research. 19 (2000) 205–221. 10.1016/S1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- [15].E.D.C.C.S. Group, Antioxidant status and neovascular age-related macular degeneration, Arch Ophthalmol. 111 (1993) 104–109. https://ci.nii.ac.jp/naid/10026077078/ (accessed September 18, 2019). [DOI] [PubMed] [Google Scholar]
- [16].Smith W, Mitchell P, Webb K, Leeder SR, Dietary antioxidants and age-related maculopathy: The blue mountains eye study, Ophthalmology. 106 (1999) 761–767. 10.1016/S0161-6420(99)90164-1. [DOI] [PubMed] [Google Scholar]
- [17].A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss, Arch Ophthalmol. 119 (2001) 1417–1436. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1462955/ (accessed September 18, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W, Plasma Lutein and Zeaxanthin and Other Carotenoids as Modifiable Risk Factors for Age-Related Maculopathy and Cataract: The POLA Study, Invest. Ophthalmol. Vis. Sci 47 (2006) 2329–2335. 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- [19].Robman L, Vu H, Hodge A, Tikellis G, Dimitrov P, McCarty C, Guymer R, Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration, Canadian Journal of Ophthalmology. 42 (2007) 720–726. 10.3129/i07-116. [DOI] [PubMed] [Google Scholar]
- [20].Hammond JB, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM, Dietary modification of human macular pigment density., Invest Ophthalmol Vis Sci. 38 (1997) 1795–1801. http://europepmc.org/abstract/med/9286268 (accessed September 20, 2019). [PubMed] [Google Scholar]
- [21].Tan JSL, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P, Dietary Antioxidants and the Long-term Incidence of Age-Related Macular Degeneration: The Blue Mountains Eye Study, Ophthalmology. 115 (2008) 334–341. 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- [22].Chew EY, Clemons TE, SanGiovanni JP, Danis R, Ferris FL, Elman M, Antoszyk A, Ruby A, Orth D, Bressler S, Fish G, Hubbard B, Klein M, Chandra S, Blodi B, Domalpally A, Friberg T, Wong W, Rosenfeld PJ, Agron E, Toth C, Bernstein P, Sperdut R, Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial, JAMA. 309 (2013) 2005–2015. 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- [23].Chew EY, Clemons TE, SanGiovanni JP, Danis RP, Ferris FL, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agrón E, Toth CA, Bernstein PS, Sperduto RD, Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression: AREDS2 Report No. 3, JAMA Ophthalmol. 132 (2014) 142–149. 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stringham JM, Johnson EJ, Hammond BR, Lutein across the Lifespan: From Childhood Cognitive Performance to the Aging Eye and Brain, Curr Dev Nutr. 3 (2019). 10.1093/cdn/nzz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nolan JM, Mulcahy R, Power R, Moran R, Howard AN, Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined, Journal of Alzheimer’s Disease. 64 (2018) 367–378. 10.3233/JAD-180160. [DOI] [PubMed] [Google Scholar]
- [26].Perry A, Rasmussen H, Johnson EJ, Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products, Journal of Food Composition and Analysis. 22 (2009) 9–15. 10.1016/j.jfca.2008.07.006. [DOI] [Google Scholar]
- [27].Abdel-Aal E-SM, Akhtar H, Zaheer K, Ali R, Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health, Nutrients. 5 (2013) 1169–1185. 10.3390/nu5041169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rodriguez-Amaya DB, Changes in carotenoids during processing and storage of foods, Arch Latinoam Nutr. 49 (1999) 38S–47S. [PubMed] [Google Scholar]
- [29].Maoka T, Arai A, Shimizu M, Matsuno T, The first isolation of enantiomeric and meso-zeaxanthin in nature., Comp Biochem Physiol B. 83 (1986) 121–124. 10.1016/0305-0491(86)90341-x. [DOI] [PubMed] [Google Scholar]
- [30].Ravikrishnan R, Rusia S, Ilamurugan G, Salunkhe U, Deshpande J, Shankaranarayanan J, Shankaranarayana ML, Soni MG, Safety assessment of lutein and zeaxanthin (LutemaxTM 2020): Subchronic toxicity and mutagenicity studies, Food and Chemical Toxicology. 49 (2011) 2841–2848. 10.1016/j.fct.2011.08.011. [DOI] [PubMed] [Google Scholar]
- [31].Thurnham DI, Howard AN, Studies on meso-zeaxanthin for potential toxicity and mutagenicity, Food and Chemical Toxicology. 59 (2013) 455–463. 10.1016/j.fct.2013.06.002. [DOI] [PubMed] [Google Scholar]
- [32].Ranganathan A, Manabe Y, Sugawara T, Hirata T, Shivanna N, Baskaran V, Poly (d, l-lactide-co-glycolide)-phospholipid nanocarrier for efficient delivery of macular pigment lutein: absorption pharmacokinetics in mice and antiproliferative effect in Hep G2 cells, Drug Deliv. and Transl. Res 9 (2019) 178–191. 10.1007/s13346-018-0590-9. [DOI] [PubMed] [Google Scholar]
- [33].Zaripheh S, Erdman JW, Factors That Influence the Bioavailablity of Xanthophylls, The Journal of Nutrition. 132 (2002) 531S–534S. 10.1093/jn/132.3.531S. [DOI] [PubMed] [Google Scholar]
- [34].West CE, Castenmiller JJ, Quantification of the “SLAMENGHI” factors for carotenoid bioavailability and bioconversion, Int J Vitam Nutr Res. 68 (1998) 371–377. [PubMed] [Google Scholar]
- [35].Rigotti A, Miettinen HE, Krieger M, Role of the High-Density Lipoprotein Receptor SR-BI in the Lipid Metabolism of Endocrine and Other Tissues, Endocrine Review. 24 (2003) 357–87. Oxford Academic,. https://academic.oup.com/edrv/article/24/3/357/2424383). [DOI] [PubMed] [Google Scholar]
- [36].Mahley RW, Innerarity TL, Rall SC, Weisgraber KH, Plasma lipoproteins: apolipoprotein structure and function., J. Lipid Res 25 (1984) 1277–1294. http://www.jlr.org/content/25/12/1277 (accessed September 3, 2019). [PubMed] [Google Scholar]
- [37].Borel P, Grolier P, Armand M, Partier A, Lafont H, Lairon D, Azais-Braesco V, Carotenoids in biological emulsions: solubility, surface-to-core distribution, and release from lipid droplets., J. Lipid Res 37 (1996) 250–261. http://www.jlr.org/content/37/2/250 (accessed September 3, 2019). [PubMed] [Google Scholar]
- [38].Parker RS, Absorption, metabolism, and transport of carotenoids, FASEB J. 10 (1996) 542–551. [PubMed] [Google Scholar]
- [39].Harrison EH, Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium, Molecular Nutrition & Food Research. 63 (2019) 1801046 10.1002/mnfr.201801046. [DOI] [PubMed] [Google Scholar]
- [40].Pagler TA, Rhode S, Neuhofer A, Laggner H, Strobl W, Hinterndorfer C, Volf I, Pavelka M, Eckhardt ERM, van der Westhuyzen DR, Schütz GJ, Stangl H, SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux, J. Biol. Chem 281 (2006) 11193–11204. 10.1074/jbc.M510261200. [DOI] [PubMed] [Google Scholar]
- [41].Sirtori CR, Fumagalli R, LDL-cholesterol lowering or HDL-cholesterol raising for cardiovascular prevention: A lesson from cholesterol turnover studies and others, Atherosclerosis. 186 (2006) 1–11. 10.1016/j.atherosclerosis.2005.10.024. [DOI] [PubMed] [Google Scholar]
- [42].Connor WE, Duell PB, Kean R, Wang Y, The Prime Role of HDL to Transport Lutein into the Retina: Evidence from HDL-Deficient WHAM Chicks Having a Mutant ABCA1 Transporter, Invest. Ophthalmol. Vis. Sci 48 (2007) 4226–4231. 10.1167/iovs.06-1275. [DOI] [PubMed] [Google Scholar]
- [43].Rigotti A, Miettinen HE, Krieger M, The Role of the High-Density Lipoprotein Receptor SR-BI in the Lipid Metabolism of Endocrine and Other Tissues, Endocrine Reviews. 24 (2003) 357–387. 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- [44].Acton SL, Scherer PE, Lodish HF, Krieger M, Expression cloning of SR-BI, a CD36-related class B scavenger receptor, J. Biol. Chem 269 (1994) 21003–21009. [PubMed] [Google Scholar]
- [45].McCarthy JJ, Lehner T, Reeves C, Moliterno DJ, Newby LK, Rogers WJ, Topol EJ, Association of genetic variants in the HDL receptor, SR-B1, with abnormal lipids in women with coronary artery disease, Journal of Medical Genetics. 40 (2003) 453–458. 10.1136/jmg.40.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gutierrez-Pajares JL, Ben Hassen C, Chevalier S, Frank PG, SR-BI: Linking Cholesterol and Lipoprotein Metabolism with Breast and Prostate Cancer, Front Pharmacol. 7 (2016). 10.3389/fphar.2016.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kiefer C, Sumser E, Wernet MF, Von Lintig J, A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila, Proc. Natl. Acad. Sci. U.S.A 99 (2002) 10581–10586. 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Reboul E, Abou L, Mikail C, Ghiringhelli O, André M, Portugal H, Jourdheuil-Rahmani D, Amiot M-J, Lairon D, Borel P, Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI), Biochem J. 387 (2005) 455–461. 10.1042/BJ20040554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].During A, Doraiswamy S, Harrison EH, Xanthophylls are preferentially taken up compared with β-carotene by retinal cells via a SRBI-dependent mechanism, J. Lipid Res 49 (2008) 1715–1724. 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Thomas SE and Harrison EH, Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins, J Lip Res. 57(2016) 1865–1878. http://www.jlr.org/content/57/10/1865.short (accessed September 28, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J, ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production, FASEB J. 24 (2010) 1656–1666. 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shyam R, Vachali P, Gorusupudi A, Nelson K, Bernstein PS, All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids, Arch. Biochem. Biophys 634 (2017) 21–28. 10.1016/j.abb.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Silverstein RL, Febbraio M, CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior, Sci Signal. 2 (2009) re3 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez R, Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors, Molecular Vision. (n.d.) 15. [PubMed] [Google Scholar]
- [55].Sakudoh T, Iizuka T, Narukawa J, Sezutsu H, Kobayashi I, Kuwazaki S, Banno Y, Kitamura A, Sugiyama H, Takada N, Fujimoto H, Kadono-Okuda K, Mita K, Tamura T, Yamamoto K, Tsuchida K, A CD36-related transmembrane protein is coordinated with an intracellular lipid-binding protein in selective carotenoid transport for cocoon coloration., J Biol Chem. 285 (2010) 7739–7751. 10.1074/jbc.M109.074435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moussa M, Gouranton E, Gleize B, Yazidi CE, Niot I, Besnard P, Borel P, Landrier J-F, CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures, Mol Nutr Food Res. 55 (2011) 578–584. 10.1002/mnfr.201000399. [DOI] [PubMed] [Google Scholar]
- [57].Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand J-M, Meunier N, Drouault-Holowacz S, Bieuvelet S, Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans, Ann. Med 43 (2011) 47–59. 10.3109/07853890.2010.531757. [DOI] [PubMed] [Google Scholar]
- [58].Moore T, Vitamin A and carotene, Biochem J. 24 (1930) 692–702. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1254511/ (accessed September 3, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Karrer P, Helfenstein A, Wehrli H, Wettstein A, Pflanzenfarbstoffe XXV. Über die Konstitution des Lycopins und Carotins, Helvetica Chimica Acta. 13 (1930) 1084–1099. 10.1002/hlca.19300130532. [DOI] [Google Scholar]
- [60].Palczewski G, Amengual J, Hoppel CL, von Lintig J, Evidence for compartmentalization of mammalian carotenoid metabolism, The FASEB Journal. 28 (2014) 4457–4469. 10.1096/fj.14-252411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ruban AV, Young AJ, Horton P, Dynamic Properties of the Minor Chlorophyll a/b Binding Proteins of Photosystem II, an in Vitro Model for Photoprotective Energy Dissipation in the Photosynthetic Membrane of Green Plants, Biochemistry. 35 (1996) 674–678. 10.1021/bi9524878. [DOI] [PubMed] [Google Scholar]
- [62].Reddy KJ, Masamoto K, Sherman DM, Sherman LA, DNA sequence and regulation of the gene (cbpA) encoding the 42-kilodalton cytoplasmic membrane carotenoprotein of the cyanobacterium Synechococcus sp. strain PCC 7942., Journal of Bacteriology. 171 (1989) 3486–3493. 10.1128/jb.171.6.3486-3493.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zagalsky PF, Mummery RS, Winger LA, Cross-reactivities of polyclonal antibodies to subunits, CRTA and CRTC, of the lobster carapace carotenoprotein, α-crustacyanin, and of monoclonal antibodies to human serum retinol-binding protein against carotenoproteins of different types and from separate invertebrate species, Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 110 (1995) 385–391. 10.1016/0305-0491(94)00160-V. [DOI] [Google Scholar]
- [64].Bernstein PS, Balashov NA, Tsong ED, Rando RR, Retinal tubulin binds macular carotenoids, Invest. Ophthalmol. Vis. Sci 38 (1997) 167–175. [PubMed] [Google Scholar]
- [65].Crabtree DV, Ojima I, Geng X, Adler AJ, Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site, Bioorg. Med. Chem 9 (2001) 1967–1976. 10.1016/s0968-0896(01)00103-1. [DOI] [PubMed] [Google Scholar]
- [66].Townsend DM, Tew KD, Tapiero H, The importance of glutathione in human disease, Biomedicine & Pharmacotherapy. 57 (2003) 145–155. 10.1016/S0753-3322(03)00043-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Oakley A, Glutathione transferases: a structural perspective, Drug Metabolism Reviews. 43 (2011) 138–151. 10.3109/03602532.2011.558093. [DOI] [PubMed] [Google Scholar]
- [68].Strange RC, Spiteri MA, Ramachandran S, Fryer AA, Glutathione-S-transferase family of enzymes, Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 482 (2001) 21–26. 10.1016/S0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- [69].Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS, Identification and Characterization of a Pi Isoform of Glutathione S-Transferase (GSTP1) as a Zeaxanthin-binding Protein in the Macula of the Human Eye, J. Biol. Chem 279 (2004) 49447–49454. 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- [70].Bhosale P, Bernstein PS, Synergistic effects of zeaxanthin and its binding protein in the prevention of lipid membrane oxidation, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1740 (2005) 116–121. 10.1016/j.bbadis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [71].Juronen E, Tasa G, Veromann S, Parts L, Tiidla A, Pulges R, Panov A, Soovere L, Koka K, Mikelsaar A-V, Polymorphic Glutathione S-transferase M1 is a Risk Factor of Primary Open-angle Glaucoma among Estonians, Experimental Eye Research. 71 (2000) 447–452. 10.1006/exer.2000.0899. [DOI] [PubMed] [Google Scholar]
- [72].Strauss JF, Kishida T, Christenson LK, Fujimoto T, Hiroi H, START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells, Molecular and Cellular Endocrinology. 202 (2003) 59–65. 10.1016/S0303-7207(03)00063-7. [DOI] [PubMed] [Google Scholar]
- [73].Li B, Vachali P, Frederick JM, Bernstein PS, Identification of StARD3 as a Lutein-Binding Protein in the Macula of the Primate Retina, Biochemistry. 50 (2011) 2541–2549. 10.1021/bi101906y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Horvath MP, George EW, Tran QT, Baumgardner K, Zharov G, Lee S, Sharifzadeh H, Shihab S, Mattinson T, Li B, Bernstein PS, Structure of the lutein-binding domain of human StARD3 at 1.74 Å resolution and model of a complex with lutein, Acta Crystallogr F Struct Biol Commun. 72 (2016) 609–618. 10.1107/S2053230X16010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ, An Essential Component in Steroid Synthesis, the Steroidogenic Acute Regulatory Protein, Is Expressed in Discrete Regions of the Brain, J. Neurosci 22 (2002) 10613–10620. 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tanprasertsuk J, Li B, Bernstein PS, Vishwanathan R, Johnson MA, Poon L, Johnson EJ, Relationship between Concentrations of Lutein and StARD3 among Pediatric and Geriatric Human Brain Tissue, PLOS ONE. 11 (2016) e0155488 10.1371/journal.pone.0155488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang Y, Connor SL, Wang W, Johnson EJ, Connor WE, The selective retention of lutein, meso-zeaxanthin and zeaxanthin in the retina of chicks fed a xanthophyll-free diet, Experimental Eye Research. 84 (2007) 591–598. 10.1016/j.exer.2006.11.013. [DOI] [PubMed] [Google Scholar]
- [78].Phelan D, Prado-Cabrero A, Nolan JM, Analysis of Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Organs of Carotenoid-Supplemented Chickens, Foods. 7 (2018). 10.3390/foods7020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Toyoda Y, Thomson LR, Langner A, Craft NE, Garnett KM, Nichols CR, Cheng KM, Dorey CK, Effect of dietary zeaxanthin on tissue distribution of zeaxanthin and lutein in quail, Invest. Ophthalmol. Vis. Sci 43 (2002) 1210–1221. [PubMed] [Google Scholar]
- [80].Dorey CK, Granata L, Nichols CR, Cheng KM, Craft NE, Dietary modulation of lens zeaxanthin in quail, Exp. Eye Res. 81 (2005) 464–477. 10.1016/j.exer.2005.03.007. [DOI] [PubMed] [Google Scholar]
- [81].Thomson LR, Toyoda Y, Langner A, Delori FC, Garnett KM, Craft N, Nichols CR, Cheng KM, Dorey CK, Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail, Invest. Ophthalmol. Vis. Sci 43 (2002) 3538–3549. [PubMed] [Google Scholar]
- [82].Bhosale P, Serban B, Bernstein PS, Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium, Arch. Biochem. Biophys 483 (2009) 175–181. 10.1016/j.abb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Snodderly DM, Handelman GJ, Adler AJ, Distribution of individual macular pigment carotenoids in central retina of macaque and squirrel monkeys, Invest. Ophthalmol. Vis. Sci 32 (1991) 268–279. [PubMed] [Google Scholar]
- [84].Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM, Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys, Invest. Ophthalmol. Vis. Sci 45 (2004) 3234–3243. 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- [85].Leung IY-F, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM, Nutritional Manipulation of Primate Retinas, II: Effects of Age, n–3 Fatty Acids, Lutein, and Zeaxanthin on Retinal Pigment Epithelium, Invest. Ophthalmol. Vis. Sci 45 (2004) 3244–3256. 10.1167/iovs.02-1233. [DOI] [PubMed] [Google Scholar]
- [86].Leung IY-F, Sandstrom MM, Zucker CL, Neuringer M, Max Snodderly D, Nutritional manipulation of primate retinas. IV. Effects of n–3 fatty acids, lutein, and zeaxanthin on S-cones and rods in the foveal region, Experimental Eye Research. 81 (2005) 513–529. 10.1016/j.exer.2005.03.009. [DOI] [PubMed] [Google Scholar]
- [87].Barker FM, Snodderly DM, Johnson EJ, Schalch W, Koepcke W, Gerss J, Neuringer M, Nutritional Manipulation of Primate Retinas, V: Effects of Lutein, Zeaxanthin, and n–3 Fatty Acids on Retinal Sensitivity to Blue-Light–Induced Damage, Invest Ophthalmol Vis Sci. 52 (2011) 3934–3942. 10.1167/iovs.10-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ, Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates, Nutr Neurosci. 16 (2013). 10.1179/1476830512Y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jeon S, Neuringer M, Kuchan MJ, Erdman JW, Relationships of carotenoid-related gene expression and serum cholesterol and lipoprotein levels to retina and brain lutein deposition in infant rhesus macaques following 6 months of breastfeeding or formula feeding, Archives of Biochemistry and Biophysics. 654 (2018) 97–104. 10.1016/j.abb.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Xue C, Rosen R, Jordan A, Hu D-N, Management of Ocular Diseases Using Lutein and Zeaxanthin: What Have We Learned from Experimental Animal Studies?, J Ophthalmol. 2015 (2015). 10.1155/2015/523027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Li B, Vachali P, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, and Bernstein PS. Inactivity of human β,β-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment | PNAS, 111 (28) (2014) 10173–10178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J, A mitochondrial enzyme degrades carotenoids and protects against oxidative stress, FASEB J. 25 (2011) 948–959. 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Babino D, Palczewski G, Widjaja-Adhi MAK, Kiser PD, Golczak M, von Lintig J, Characterization of the Role of β-Carotene 9,10-Dioxygenase in Macular Pigment Metabolism, J Biol Chem. 290 (2015) 24844–24857. 10.1074/jbc.M115.668822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li B, Vachali PP, Shen Z, Gorusupudi A, Nelson K, Besch BM, Bartschi A, Longo S, Mattinson T, Shihab S, Polyakov NE, Suntsova LP, Dushkin AV, Bernstein PS, Retinal accumulation of zeaxanthin, lutein, and β-carotene in mice deficient in carotenoid cleavage enzymes, Exp. Eye Res 159 (2017) 123–131. 10.1016/j.exer.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Li B, Rognon GT, Mattinson T, Vachali PP, Gorusupudi A, Chang F-Y, Ranganathan A, Nelson K, George EW, Frederick JM, Bernstein PS, Supplementation with macular carotenoids improves visual performance of transgenic mice, Arch. Biochem. Biophys 649 (2018) 22–28. 10.1016/j.abb.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lobo GP, Isken A, Hoff S, Babino D, von Lintig J, BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway, Development. 139 (2012) 2966–2977. 10.1242/dev.079632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Saidi EA, Davey PG, Camero DJ, The Effect of Zeaxanthin on the Visual Acuity of Zebrafish, PLoS One 12 (2015) e0135211 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0135211 (accessed September 5, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhang Z, Han S, Wang H, Wang T, Lutein extends the lifespan of Drosophila melanogaster, Arch Gerontol Geriatr. 58 (2014) 153–159. 10.1016/j.archger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- [99].Teixeira A, Novais EA, Badaró E, Lima A, Farah ME, Belfort R, Experimental model to evaluate the benefits of lutein to prevent retinal phototoxicity during pars plana vitrectomy surgery using xenon source light illumination in rabbits, International Journal of Retina and Vitreous. 5 (2019) 11 10.1186/s40942-019-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ibrahim AE, Shafaa MW, Khedr MH, Rashed RF, Comparative study between lutein and its liposomal form on cisplatin-induced retinal injury in rabbits, Cutan Ocul Toxicol. 38 (2019) 279–285. 10.1080/15569527.2019.1608227. [DOI] [PubMed] [Google Scholar]
- [101].Furlani BA, Barroso L, Sousa-Martins D, Maia M, Moraes-Filho MN, Badaro E, Portella R, Lima-Filho AA, Rodrigues EB, Belfort R, Lutein and zeaxanthin toxicity with and without brilliant blue in rabbits, J Ocul Pharmacol Ther. 30 (2014) 559–566. 10.1089/jop.2013.0171. [DOI] [PubMed] [Google Scholar]
- [102].Kvansakul J, Rodriguez- Carmona M, Edgar DF, Barker FM, Köpcke W, Schalch W, Barbur JL, Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance, Ophthalmic and Physiological Optics. 26 (2006) 362–371. 10.1111/j.1475-1313.2006.00387.x. [DOI] [PubMed] [Google Scholar]


