Abstract
Neurogenic hypertension is associated with excessive sympathetic nerve activity (SNA) to the kidneys and portions of the cardiovascular system. Here we examine the brain regions that cause heightened SNA in animal models of neurogenic hypertension and we discuss the triggers responsible for the changes in neuronal activity within these regions. We highlight the limitations of the evidence and, whenever possible, we briefly address the pertinence of the findings to human hypertension.
The arterial baroreflex reduces BP variability and contributes to the BP set-point. This set-point can also be elevated by a newly described cerebral blood flow-dependent and astrocyte-mediated sympathetic reflex. Both reflexes converge on the presympathetic neurons of the rostral medulla oblongata (RVLM PSNs) and both are plausible causes of neurogenic hypertension. Sensory afferent dysfunction (reduced baroreceptor activity, increased renal or carotid body afferent) contributes to many forms of neurogenic hypertension. Neurogenic hypertension can also result from activation of brain nuclei or sensory afferents by excess circulating hormones (leptin, insulin, AngII) or sodium. Leptin raises blood vessel SNA by activating the carotid bodies and subsets of arcuate neurons. AngII works in the lamina terminalis and probably throughout the brainstem and hypothalamus. Sodium is sensed primarily in the lamina terminalis. Regardless of its cause, the excess SNA is mediated to some extent by activation of presympathetic neurons (PSNs) located in the rostral ventrolateral medulla (RVLM) or the paraventricular nucleus of the hypothalamus. Increased activity of the orexinergic neurons also contributes to hypertension in selected models.
Neurogenic hypertension refers to a chronic increase in arterial blood pressure (BP) that is associated with and presumably driven by excessive sympathetic nerve activity (SNA) to the kidneys and various parts of the cardiovascular system1–4. We will not discuss here how a global or regional increase in SNA causes or maintains the hypertensive state5–7. Instead, we focus on two specific aspects of neurogenic hypertension research: the brain regions responsible for heightened SNA in animal models and the triggers responsible for the changes in neuronal activity within these regions (e.g. sensory afferent dysfunction, brain hypoperfusion, gliotransmission, excess circulating hormones, hypernatremia, etc.). The topic is vast and the space limited. Despite their importance, oxidative stress and inflammation are not discussed.
The ventrolateral medulla and hypertension
The rostral ventrolateral medulla (RVLM) is a major node of the BP regulating neural network8, 9. Silencing RVLM neurons indiscriminately produces large reductions of BP and SNA in anesthetized or conscious rodents9–11. These effects are attributed to the inhibition of excitatory neurons with monosynaptic projections to sympathetic preganglionic neurons, a.k.a. RVLM presympathetic neurons (PSNs). Many RVLM PSNs (C1 cells) synthesize catecholamines inclusive of epinephrine, the rest do not (non-C1)12–14. Estimates of the ratio between non-C1 and C1 PSNs varies widely (33 to 300%)12, 13, 15, 16. C1 and non-C1 PSNs are glutamatergic and express varying levels of neuropeptides (PACAP, NPY, substance P, enkephalins)9.
The degree to which the C1 and other RVLM neurons contribute to resting BP in an intact unstressed and unanesthetized mammal is unsettled. Extensive lesions of the C1 neurons (>85%) compromise BP stability during hypoxic or hypotensive stresses but cause very little hypotension at rest (<10 mmHg in rats)17, 18 suggesting that the contribution of these neurons to BP is small under resting conditions. Consistent with this view, in freely behaving rats at rest, bilateral optogenetic C1 cell inhibition (with the proton pump archaerhodopsin) also reduced BP very little (~5mmHg)19. Yet, a more substantial BP drop (−27 mmHg) was observed using a pharmacogenetic approach (allatostatin receptor)20. The same type of vector (lentivirus with a Phox2b-enhanced promoter, PRSx8) was used in both studies. This vector does not transduce catecholaminergic neurons with total selectivity. The allatostatin receptor is a G-protein coupled receptor and, as such, presumably needs a much lower level of membrane expression than the proton pump to be an effective actuator, thus increasing the probability of off-target effects21. Conversely, archaerhodopsin requires a high level of expression for efficacy; its use could have led to the underestimation of the contribution of the C1 neurons to resting BP. Finally, the autonomic effects caused by a purely psychological stress may not be mediated via the RVLM22.
SNA to various organs or tissues is differentially regulated (e.g.23, 24). This point is notable because hypertension may be caused by preferential activation of SNA to the heart, resistance vessels or splanchnic capacitance25. The differential control of regional SNA may occur via differential recruitment of subsets of RVLM premotor neurons that control sympathetic efferents to particular vascular beds (e.g. splanchnic vs. muscle or capacitance vs. resistance) and different regions of the kidneys or the myocardium( Figure 1)9, 26–28. However, the RVLM also contains highly collateralized PSNs, e.g. C1 cells, that can activate multiple sympathetic efferent pathways simultaneously29, 30.
Figure 1:
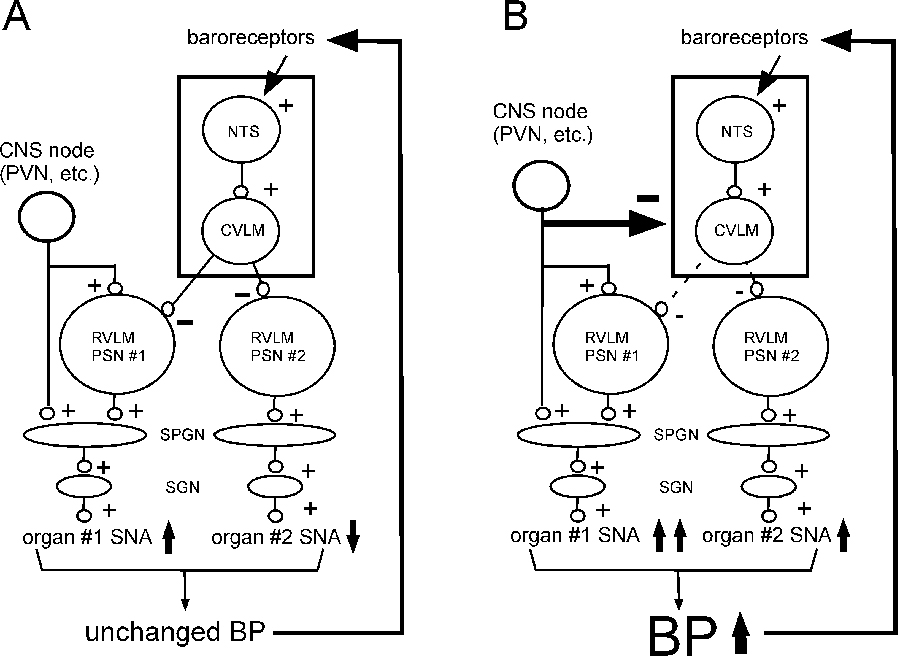
Differential control of regional SNA with or without neurogenic hypertension, a hypothesis. Organ-specific SNA regulation operates via selective recruitment of RVLM presympathetic neurons and/or preganglionic neurons by inputs from a variety of CNS nodes (PVN, midline medulla, DMH, ARC, etc.; only one is represented). A) differential recruitment of regional SNA produces very little BP change if the baroreflex (NTS + CVLM; box) is unaffected. B) differential recruitment of regional SNA is associated with a rise in BP (potentially causing hypertension) if the baroreflex (NTS + CVLM; box) is simultaneously down-regulated.
RVLM PSNs, C1 included, innervate many brain regions implicated in BP control besides the spinal cord including the solitary tract nucleus (NTS), parabrachial region, locus coeruleus, periaqueductal gray matter and raphe pallidus (Figure 2)9, 31. Non-bulbospinal RVLM neurons, including C1 neurons, also target hypothalamic nuclei of prime importance to cardiovascular control, notably orexin neurons and the PVN31, 32. In brief, the notion that RVLM controls SNA exclusively via its spinal projections to preganglionic neurons has not been tested rigorously and seems a priori inconsistent with the available anatomical data.
Figure 2:
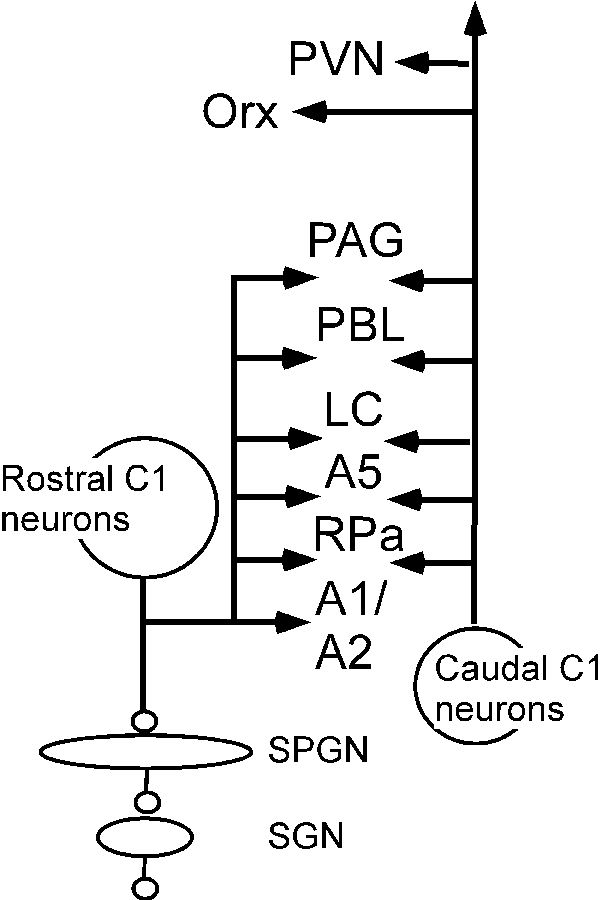
The C1 neurons innervate many nuclei implicated in circulatory control besides the sympathetic preganglionic neurons (see list of abbreviations). The projections of the rostral C1 cells are shown on the left; the projections of the caudal C1 neurons are shown on the right. Note that that both populations target many of the same nuclei.
Many factors (synaptic, intrinsic or paracrine) determine the discharge rate of the PSNs. Conventional excitatory and inhibitory synaptic inputs clearly play a role18. The neurons antecedent to RVLM PSNs reside within the lower brainstem reticular formation, with fewer neurons located in the dorsal vagal complex, dorsolateral pons, midbrain and hypothalamus33, 34. The inhibition of RVLM PSNs elicited by arterial baroreceptor activation is mediated by GABAergic interneurons located in the caudal VLM (CVLM; Figure 1); these interneurons are also strongly respiratory modulated and therefore must contribute to the respiratory modulation of RVLM PSNs and, ultimately, SNA8, 13, 35–38. The CVLM region, in turn, receives extensive input from the dorsal vagal complex and the hypothalamus39.
The activity of C1 and other RVLM PSNs is also partly independent of conventional ionotropic synaptic transmission40, 41. This activity relies on intrinsic properties (auto-depolarization) and local factors such as hypoxia, oxidative stress, circulating hormones and paracrine signals released by the surrounding vasculature or glia (ATP, lactate, PGE2, NO)42–47.
The RVLM contributes to several forms of neurogenic hypertension48–51. Somewhat higher c-Fos expression has been reported in the RVLM of SH rats52. Inhibiting RVLM neurons indiscriminately produces a greater BP drop in hypertensive than normotensive control strains (e.g. SH vs. Wistar-Kyoto) or in a given strain after it has been subjected to a treatment that produces hypertension (salt, overfeeding)48–51. The favored interpretation of such results is that the discharge rate of the PSNs is higher in hypertensive animals but there other possibilities exist. RVLM unit activity could be unchanged or only marginally increased but downstream efferent connections, e.g. SPGNs, could be hyper-responsive to their input; RVLM unit activity and SNA could both be virtually unchanged but vascular reactivity could be enhanced by sympathetic hyper-innervation and arteriolar hypertrophy53, 54 or via sensitized neuroeffector coupling25. Finally, the RVLM could raise BP via projections elsewhere than the spinal cord (e.g. the hypothalamus). These alternative possibilities have been minimally tested50 and never in conscious animals. What is generally underappreciated is that the discharge rate of RVLM PSNs depends to an extreme degree on the resting BP and the degree of impairment of the baroreflex, both of which are highly affected by the preparation and the type and depth of anesthesia. In halothane-anesthetized rats, no difference was found between SH and Wistar-Kyoto except a resetting of the baroreflex55. In neonatal brainstem spinal preparations, RVLM bulbospinal (putative PSNs) neurons discharged at higher rates (e.g.56) and in the arterially perfused SH rat the only difference was an increased respiratory modulation of the PSNs57, 58 which, as discussed later, could be related to excess accumulation of metabolically produced CO2 in preparations with higher vascular resistance. Finally, preferential C1 cell inhibition produced the same BP reduction in control rats as in rats rendered hypertensive by chronic intermittent hypoxia20. This result could mean that the C1 neurons are not hyperactive after CIH and that the postulated heightened SNA might have another cause59.
In sum, the RVLM contributes somehow to the elevated BP present in most hypertensive animal models. PSN hyperactivity is a plausible explanation in need of further evidence.
Cardiorespiratory coupling and hypertension
This topic has been recently reviewed9 and will only be briefly updated. SNA exhibits respiratory-synchronous fluctuations for two reasons. First, cardiopulmonary receptors (including baroreceptors) discharge in phase with chest movements and these periodic fluctuations are transmitted to the network that generates SNA without obligatory relay through the respiratory pattern generator. Secondly the network that generates the SNA, for example CVLM neurons and RVLM PSNs receive inputs from the respiratory pattern generator8, 13, 57. In this section, we discuss whether these inputs are abnormally strong in two animal models of neurogenic hypertension, the SH rat and rats subjected to CIH.
Arterially perfused preparations of SH rats have a higher central respiratory pattern activity than control Wistar rats; this is manifested by narrow, non-ramping, phrenic nerve discharges and by the presence of late expiratory activity in abdominal nerves58. In this model, enhanced respiratory modulation of SNA and RVLM PSNs has been observed in all three phases of the respiratory cycle (inspiration, post-inspiration and late expiration) with qualitative differences between individual RVLM PSNs, between sympathetic nerves and according to the animals’ age57, 58, 60. Similar observations have been made in rats subjected to CIH except that SNA appears enhanced predominantly during the inspiratory or late-expiratory phases, depending on the animals’ sex13, 61. The exaggerated respiratory phasic components of SNA have been attributed to enhanced central respiratory-sympathetic coupling13, 57. However, the enhanced respiratory fluctuations of SNA may be the normal consequence of an increase in central chemoreceptor activity because the abnormal pattern of the hypertensive animals is normalized by lowering perfusate PCO258, 62. This explanation has been rejected by some authors because they could not find any difference in the respiratory chemoreflex of the SH rat60. Yet, hypertensive strains have an elevated cerebrovascular resistance60, 63. If flow is limited, metabolically produced CO2 will inevitably accumulate to a higher level and overstimulate the brain chemoreceptors, ultimately enhancing fictive breathing and the respiratory modulation of SNA64–66.
Unlike in a perfused rodent preparation, breathing at rest is the same in intact unanesthetized SH and WKY rats67 and neither breathing nor the respiratory fluctuations of SNA differ noticeably between normotensive humans and individuals with essential hypertension68. One study did report the presence of active expiration in a few awake rats subjected to CIH but these rats were equally hypertensive during sleep despite the absence of active expiration69. Finally, although daytime SNA is indeed elevated in patients with OSA, resting breathing and SNA respiratory fluctuations seem normal70. That breathing is unaffected in the awake resting state might appear counterintuitive given the well-documented hyperactivity of the carotid bodies (see next section); the powerful countervailing effect of the central chemoreceptors on breathing is one explanation71.
Sensory afferent dysfunction and hypertension
Renal afferents, most of which are unmyelinated, contribute to hypertension and cause a widespread increase in SNA in the DOCA-salt and the 2K-1C (2-kidney-1 clip) models72, 73. Renal inflammation is the suspected trigger. Unmyelinated afferents typically terminate in the dorsal-most laminae of the spinal cord. This region projects in turn to the intermediolateral cell column, the NTS, the parabrachial nucleus and the rostral medulla, all of which could contribute to raise SNA74.
The arterial baroreflex is critically important to BP. Sinoaortic denervation (SAD) causes BP lability. Baroreflex modulation elicited by CNS inputs to the NTS, the CVLM and elsewhere, is likely required for BP to rise or fall appropriately during various behaviors (Figure 1)75. The arterial baroreflex is down-regulated by carotid body afferents, which contributes to various forms of neurogenic hypertension76. Incomplete resetting of the baroreceptor afferents may also cause hypertension even though SAD produces very small BP increases (<10 mmHg)6, 19, 77, 78. Silencing baroreceptors in mice by deleting both Piezo channels from vagal afferents (Phox2bCre+;Piezo1f/fPiezo2f/f) produces hypertension (~15 mmHg) and a modest but significant increase of BP lability79. This genetic approach has limitations (gene knock-out from early developmental stage; gene excision not limited to baroreceptor afferents, etc.) but it suggests that a chronic reduction of the activity of baroreceptor afferents and, by extension incomplete baroreceptor resetting, may indeed cause hypertension.
Why SAD does not cause hypertension remains unexplained. SNA and the activity of the C1 PSNs are greatly elevated immediately after SAD in rats but return to control within a few days19. Accordingly, whatever mechanism restores SNA and BP to control after SAD likely resides within or upstream from the RVLM (NTS, CVLM). This adaptation could result from some form of intrinsic neuron homeostasis80, enhanced synaptic inhibition driven by sensory afferents other than the arterial baroreceptors or an enhancement of the cerebral blood flow-mediated regulation of RVLM neuronal activity81. Interestingly, chronic baroreceptor stimulation produces a reduction in SNA and BP that persists for weeks with very little adaptation6. This remarkable property justifies the use of arterial baroreceptor stimulation to chronically reduce BP in individuals with drug-resistant hypertension82.
Carotid body hyperactivity is another trigger of hypertension. These sensory organs are activated by arterial hypoxia in a pH-dependent manner; they also respond to hypoglycemia, temperature, hormones (angiotensin, leptin) and low blood flow83–86. Carotid body sensory afferents innervate the NTS via the glossopharyngeal nerve and respond primarily to ATP and acetylcholine, transmitters released by the oxygen sensing Type-I glomus cells. Acutely, carotid body stimulation can activate RVLM PSNs via at least four mechanisms whose relative importance to neurogenic hypertension is unsettled: direct excitatory inputs from the NTS to the RVLM, enhanced cardiorespiratory coupling, arterial baroreflex down-regulation and, if the stimulus is intense enough, general arousal87–89. The carotid bodies contribute to the elevated BP of SH rats76, leptin-treated mice90, 2K-1C hypertensive rat91, rats subjected to a hypercaloric diet92 or rodents exposed to chronic intermittent hypoxia (CIH)93, 94. In rodents, the arterial baroreflex is down-regulated when the carotid bodies are activated76. Carotid body denervation in juvenile SH rats also delays the development of hypertension76, 95. In every model, the principal evidence is that bilateral carotid body excision or denervation attenuates the hypertension.
In several of these models (SH rats, CIH) carotid body afferents develop a heightened responsiveness to stimuli (cyanide, hypoxia) and “tonicity”, defined as an increased discharge at rest96–98. In the SH rat, this hyperexcitability is attributed to the upregulation of channels expressed by carotid body afferents such as ASIC3, TASK1 (Kcnk3) or P2X376, 96, 99. In CIH, it is attributed to excessive ROS production by type-I glomus cells100. In mice, hyperleptinemia activates the carotid bodies by activating leptin receptors that are coupled to Trpm7 channels90. Carotid body hyperplasia may also contribute to the outsized influence of these organs on the sympathetic outflow in hypertension85, 101, 102. Finally, the carotid bodies receive a dense sympathetic innervation from the superior cervical ganglion. CIH hypertension is greatly attenuated by both sympathectomy and angiotensin II (AngII) receptor blockade85, 93 suggesting that carotid body hyperactivity could be driven by SNA in a feed-forward loop involving AngII, blood flow restriction via catecholamine-mediated vasoconstriction or a proinflammatory action85.
Unilateral carotid body ablation produced transient BP reductions in a few individuals with essential hypertension103. The potentially deleterious effects of bilateral glomectomy (loss of hypoxic ventilatory reflex, hypoventilation during sleep, breathing difficulties at altitude including in commercial air planes, effects during and after general anesthesia)85 and the possibility of surgical mishaps reduce the translational potential of this intervention but a pharmacological approach based on the use of P2X3 receptor antagonists is promising96.
The hypothalamic paraventricular nucleus (PVN)
Besides the circulation, the parvocellular portion of the PVN controls food intake, appetitive responses to sodium deficiency, gastric, pancreatic and esophageal function, glucose counter-regulation, ventilation, the protection of the cornea and various mucosal tissues and the regulation of cerebral blood flow and possibly body temperature39, 104, 105. Based on their projections, PVN neurons have the potential to elicit cardiovascular stimulation (e.g. via their direct projections to the SPGNs, the caudal pressor area, the C1 neurons), or depression (via sympathoinhibition and parasympathetic bradycardia)39, 106 or the differential regulation of regional SNA107. The PVN undoubtedly regulates SNA and BP but we still do not know whether a subset of PVN neurons is dedicated to regulating the cardiovascular system or whether every PVN neuron influences the cardiovascular system in a unique manner that is best suited to the particular behavior to which this subset of neurons contribute (feeding, drinking, arousal, etc.).
The PVN has been implicated in countless hypertension models and in other conditions associated with increased SNA such as heart failure108. The following section focuses on the SH rat. The contribution of PVN to obesity or salt-dependent hypertension is examined later.
Silencing PVN neurons produces a larger BP drop in the SH rat than in Wistar normotensive controls, anesthetized or conscious109, 110 suggesting that PVN neurons could be “hyperactive”. This interpretation is subject to similar caveats as those evoked for the RVLM. PVN neuron discharge rate was not directly monitored, PVN is a very small nucleus that cannot be selectively manipulated except in mice lines in which Cre recombinase is restricted to this nucleus111 or to subsets of its neurons112. The BP reduction evoked by inhibiting PVN in a hypertensive animal could be larger, not because these neurons are more active, but because of increased vascular reactivity to SNA, increased excitability of the downstream circuits engaged by PVN stimulation, increased or decreased expression of secondary transmitters by PVN neurons, etc. Finally, assuming that PVN activity is indeed elevated, the cause is still speculative e.g. enhanced input from orexin neurons, the VLM, the carotid bodies, direct effects of local inflammation, neuroplasticity and perhaps a difference in gut microbiota32, 108, 113, 114.
The arcuate nucleus (ARC) and obesity-related hypertension
Obesity and a high-fat diet cause a mild hypertension typically associated with elevated muscle SNA in humans and animals115, 116. Hyperleptinemia, hyperinsulinemia, AngII, or even hypoxia caused by hypoventilation are suspected triggers.
The ARC is the primary CNS target of leptin and insulin, blood-borne hormones that control appetite and weight gain (Figure 3)117. ARC neurons elicit behaviors and sensations required for energy homeostasis (appetitive, hedonic, motor), and regulate metabolism and BP via the autonomic nervous system117. Global activation of the ARC can raise or lower BP via changes in SNA and activates brown adipose tissue (BAT) SNA118, 119. ARC lightly innervates the SPGNs and the RVLM120–122 but it probably exerts its effects on SNA predominantly via hypothalamic relays (paraventricular, dorsomedial, and ventromedial nuclei)121, 123, 124.
Figure 3:
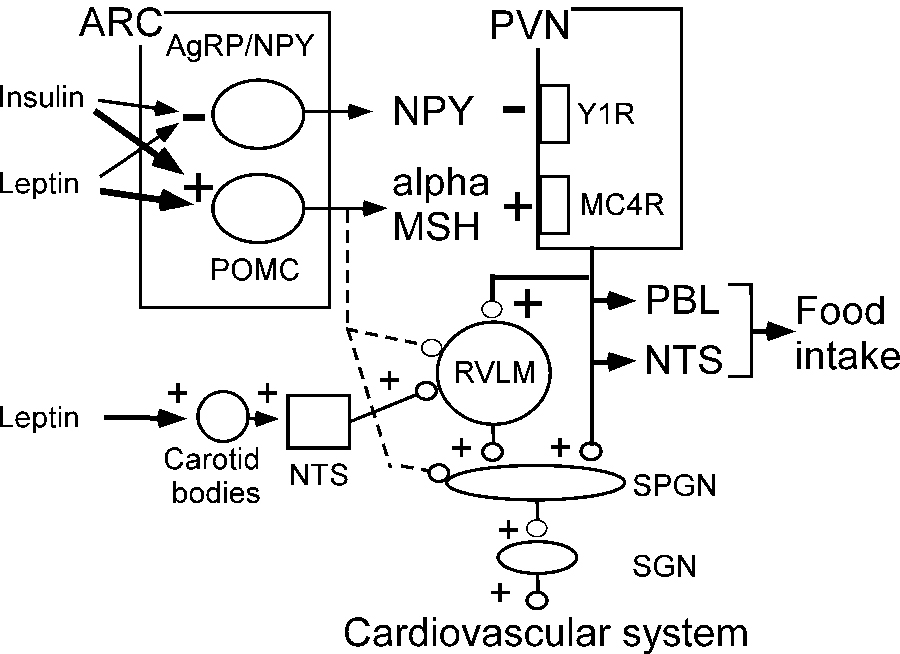
leptin and insulin raise BP by activating POMC neurons and inhibiting AgRP neurons in the arcuate nucleus. POMC neurons activate PVN neurons by releasing α-MSH. AgRP neurons inhibit PVN by releasing NPY. Leptin also raises BP by stimulating the carotid bodies. Dashed lines, minor projections of POMC neurons plausibly involved in SNS control.
Microinjection of leptin into the ARC increases BP and SNA to the kidneys, the lumbar chain, and thermogenic BAT117, 125. Deletion of the leptin receptor from the ARC eliminates the effect of systemic leptin administration on BP but leptin may also act elsewhere in the hypothalamus126. Insulin, on the other hand, probably acts solely in the ARC to increase SNA127, 128. Leptin90, contrary to insulin129, can also increase SNA by activating the carotid bodies (Figure 3). After binding to receptors in the ARC, leptin and insulin regulate SNA via projections from both POMC and AgRP neurons to the PVN (Figure 3). The POMC neurons release α–melanocyte-stimulating hormone (α-MSH) that binds to MC4R and AgRP neurons release neuropeptide Y (NPY) that binds to Y1R. In short, leptin and insulin cause sympathoexcitation by enhancing the excitatory effect of POMC neurons and by reducing the inhibitory effect of NPY neurons in the PVN130.
Hypothalamic sites shown to support basal SNA in diet-induced obese animals include the ARC via leptin126 and insulin131 stimulation, the PVN via α-MSH and glutamatergic stimulation131 and via loss of tonic NPY inhibition132, and the VMH, driven by leptin and α-MSH133. Actions of leptin in the DMH may also support increased BP in mice134 but this point is controversial131, 133.
Leptin has received much attention as the link between increased adiposity and sympathoexcitation and hypertension, yet obese Zucker rats, lacking a functional leptin receptor, also exhibit elevated SNA135. Excessive but leptin-independent activation of MC3/4R may underlie the hypertension136 and CNS mediators other than leptin also contribute to hypertension in this obesity model. Candidates include AngII in the RVLM51 and orexin in the PVN137.
ARC neurons can generate complex patterns of autonomic responses, including a rise in BP, depending on which cell type is recruited. Activation of ARC POMC neurons by leptin and insulin increases BP and SNA to resistance vessels117. This effect is mediated by the release of α-MSH in the PVN and perhaps elsewhere (VMH). One may surmise that in lean and healthy animals, the BP changes elicited by ARC neurons are transient and adapted to periods of food seeking and consumption. Overfeeding may exacerbate and prolong the sympathetic stimulation elicited by ARC under the combined effect of hyperleptinemia, hyperinsulinemia and local tissue inflammation138.
Orexin and hypertension
The orexin neurons reside in the perifornical and lateral hypothalamic region; they are implicated in brain functions as diverse as the control of food intake and energy expenditure, breathing and blood pressure, motivation, circadian rhythm of sleep and wake, memory, cognitive functions, and the cardiovascular, thermoregulatory and respiratory effects of various stresses (including transient hypercapnia)139–143. The orexin neurons are active during waking, when postural muscle tone is high, less active during quiet waking in the absence of movement, and virtually cease firing during sleep144. These neurons are probably further activated by any form of stress including hypercapnia or hypoxia, and they clearly activate breathing and BP143, 145, 146.
The orexin system contributes to hypertension in the SH rat, the Schlager BPH/2J mouse and the obese Zucker rat114, 145, 147. The principal evidence is that systemic administration of a broad-spectrum orexin receptor antagonist, almorexant, reduces BP and plasma catecholamines147–149. This evidence is predicated on two assumptions: the selectivity of almorexant and the belief that orexin does not have pertinent peripheral effects. Also, the brain of the SH rat contains greater numbers of detectably orexin-immunoreactive neurons, increased orexin-A mRNA and denser brainstem orexinergic projections than normotensive controls149–151. In addition, the RVLM seems hyper-responsive to orexin in this strain150. Finally, the orexin system may also contribute to hypertension in the Dahl salt-sensitive (Dahl-S) rat by enhancing vasopressin synthesis in the PVN114, 152.
The SH rat and the BPH/2J mice are also models of behavioral hyperactivity and orexin neuron firing is highly correlated with locomotor activity144. Orexin neurons regulate spontaneous physical activity, non-exercise thermogenesis, the effects of psychological stress and their autonomic correlates (hyperpnea and elevated BP)153. The BP of the SH rat and the BPH/2J mice is partially normalized by amygdala lesions148, 154, 155 implying that a limbic forebrain dysfunction could be driving the orexin system in these rodents. The autonomic effects of increased orexinergic neuron activity are probably relayed through several hypothalamic and brainstem nuclei142, 156. Almorexant reduces CSF noradrenaline dramatically in rats157, possibly because this drug nullifies the excitatory effect of orexin on the locus coeruleus, a major waking-promoting structure158, 159. Orexin also excites the dorsal raphe160. By reducing noradrenaline and serotonin release, orexin receptor antagonists such as almorexant reduce the ability of rodents to wake up from sleep and likely produce sedation161, 162. Accordingly, the sympathoinhibition and hypotension elicited in animal models by orexin receptor antagonists could largely result from a reduction in locomotor activity and, during rest, from interference with wake-promoting systems and circuits (noradrenaline, serotonin)148.
There is no clear indication that the orexin system contributes to hypertension in humans163. Orexin-receptor antagonists could conceivably be effective antihypertensive agents in humans but their current therapeutic use is as sedative-hypnotic drugs164. From past experience, sympatholytic drugs with notable sedative effects (e.g. alpha-2 adrenergic receptor agonists) have fared poorly as anti-hypertensive agents.
The OVLT-PVN connection and hypertension
The lamina terminalis (anterior wall of the 3rd ventricle) includes the median preoptic nucleus (MnPO) and two circumventricular organs, the subfornical organ (SFO) and the organum vasculosum lamina terminalis (OVLT)24, 165, 166. SFO and OVLT detect circulating and brain AngII, [Na+], osmolality and cytokines via receptors expressed by principal neurons, astrocytes and ependymal cells (Figure 4)167–169. The SFO and OVLT have significant reciprocal connections with the MnPO which serves as an integrative hub for signaling from multiple subcortical structures that regulate neuroendocrine and autonomic function and behavior165. The interoceptive function of the SFO and OVLT, like that of the arcuate nucleus170, is regulated by synaptic inputs from regions implicated in drinking and food consumption, thermoregulation, circadian and diurnal patterns, and cardiovascular reflexes166, 171. Sodium sensing by the OVLT and SFO is attributed to several mechanisms. Na(x), a sodium channel encoded by the Scn7a gene and expressed by astrocytes and ependymal cells may be the primary sensing mechanism (Figure 4)167. Other candidates include an N-terminal variant of the transient receptor potential cation channel subfamily V member 1 (TRPV1) and the epithelial sodium channel (ENaC)7.
Figure 4:
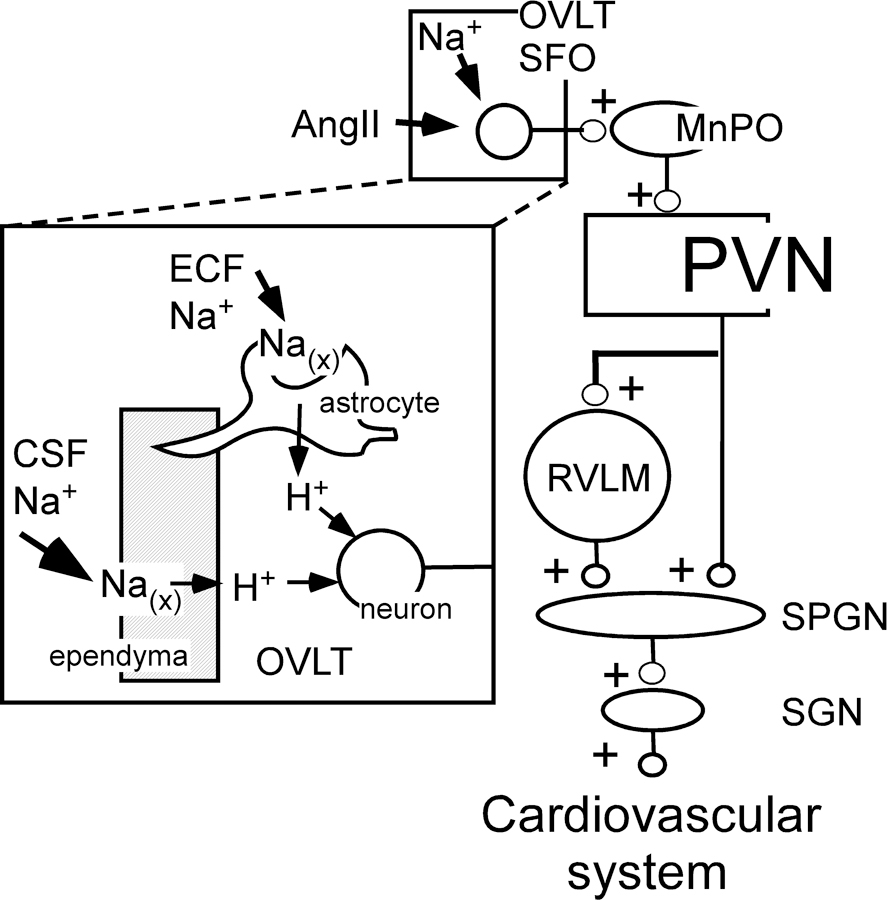
Selected nuclei and pathways that contribute to salt or angiotensin-induced hypertension. Inset: mechanism of action of Na in the OVLT (after167). Sodium present in the CSF and ECF binds to and opens Na(x) channels, elicits the release of protons from astrocytes and ependymal cells. [H+] activates OVLT neurons via acid-sensing ion channel-1a (ASIC). Other types of sodium sensing mechanisms have also been proposed7 . Other abbrs: see list.
Components of the lamina terminalis (SFO or OVLT or MnPO) contribute to BP elevation in several hypertension models: DOCA-salt172, AngII infusion168, chronic intermittent hypoxia59 and high-salt diet7, 167. The sympathetic component of the hypertension probably occurs via an MnPO-PVN connection and PVN projections to SPGNs (direct and indirect via the RVLM; Figure 4)7, 39, 59, 167, 173.
Other brain regions potentially involved in hypertension
SNA is regulated at some level by the entire brain174. Important regions that are little explored from a hypertension perspective include the periaqueductal gray matter, the dorsal colliculi and the cerebellum, dorsomedial hypothalamic nucleus (DMH) and the midline medulla175–178. The DMH relays the cardiovascular response to stress179 and could therefore play a role in stress-induced hypertension. Stimulation of the ventral periaqueductal gray matter may relieve hypertension associated with chronic pain in humans180. The midline medulla oblongata contains the majority of the PSNs identified using the retrograde transport of pseudorabies virus30, 181 and regulates cutaneous flow, the cardiac output and the sympathetic innervation of the brown adipose fat in the context of thermoregulation and body weight homeostasis182, 183.
Hypertension: role of brainstem hypoperfusion and hypoxia
Recent work has reinvigorated the theory that hypertension could be an adaptive mechanism to maintain cerebral blood flow when cerebrovascular resistance increases81, 184–186. A relatively moderate (7–20 mmHg) rise in cerebral pressure increases BP and SNA in unanesthetized sheep and humans186, 187. In anesthetized rats, the BP rise requires the exocytotic release of ATP by RVLM astrocytes81 and ATP may directly activate the PSNs (Figure 5)188. This enticing theory is predicated on the assumption that there is no off-target expression of dnSNARE (in neurons, vessels, etc.) when adeno-associated vectors engineered with an artificial GFAP promoter are used to transduce astrocytes81. Also, whether this glial mechanism can cause hypertension or merely mediates the transient effects of severe brainstem hypoxia or brain hypoperfusion on BP merits additional scrutiny. Finally, BP also rises when blood flow is reduced through the NTS only189, therefore astrocytes may contribute to SNA regulation there too.
Figure 5:
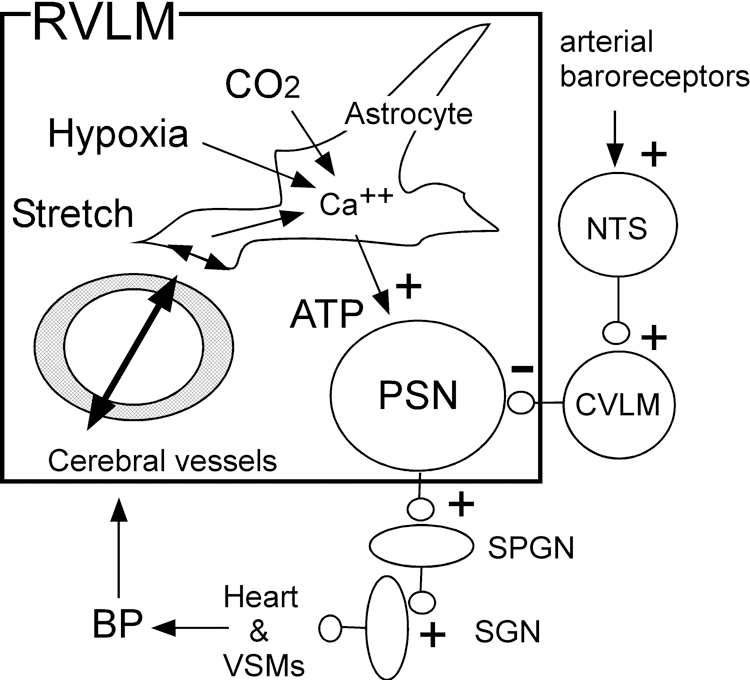
BP regulation via arterial baroreceptors and brainstem perfusion: a hypothesis. A reduction in cerebral blood flow within the RVLM (box) is detected by astrocytes via hypoxia, acidification or end-feet mechanosensitivity elicited by changes in vascular diameter, the latter symbolized by double arrows. The flow-mediated reflex (slow pathway) and the conventional baroreflex (fast pathway, at right of diagram) converge on RVLM PSNs.
The physiological variable detected by astrocytes when cerebral blood flow is limited could be hypoxia, acidification, mechanical stretch of astrocytic end-feet or chemical signals from the vasculature43, 81. In the absence of baroreceptors, this CBF-dependent pathway could conceivably mediate BP homeostasis190.
This astrocyte-mediated homeostatic mechanism evidently does not prevent the substantial hypotension elicited by chronic baroreceptor activation (>25 mmHg; 3 weeks)6. Maybe, baroreceptor activation elicits a countervailing cerebrovascular response that maintains brainstem blood flow despite the hypotension. Alternately, the capacity of astrocytes to depolarize the RVLM PSNs may be limited and may be overridden by the powerful GABA-mediated hyperpolarization elicited by baroreceptor activation (Figure 5). Also unknown is whether excessive flow through the medulla produces the opposite effect, namely sympathoinhibition, and whether this could explain the return to normotension following baroreceptor denervation19.
Conclusions
Myriad brain regions and sensory afferents have now been implicated in various forms of neurogenic hypertension. Progress is constrained by the difficulty to record SNA or brain neurons in conscious animals for long periods and to compare the data between animals. Progress is also limited by our imperfect knowledge of the network that controls SNA and BP. Powerful methods to interrogate the CNS connectome have been recently developed191. They should be more intensively applied to the neural control of BP.
Neurogenic hypertension is typically, and plausibly, attributed to an increase in the activity of RVLM PSNs. However, the supportive evidence has largely consisted of showing that silencing the RVLM (with drugs or vector-delivered actuators), modifying the RVLM redox state or manipulating the RVLM glia produces a larger BP drop in hypertensive rodents. What is actually being measured by these manipulations is the “neurogenic pressor potential” of this brain region, which depends not only on the discharge rate of RVLM neurons but also on a long series of integrative processes that occur between these neurons and vascular smooth muscle and cardiac contraction192. The primacy of the RVLM in BP control is a notion that derives primarily from experiments conducted under anesthesia when the PSNs are disinhibited and sympathetic tone is extremely elevated. SPGNs receive direct input from many sources besides the RVLM e.g. spinal cord, hypothalamus and several brainstem regions193. Most likely, every type of PSN contributes to the differential regulation of regional SNA, albeit in distinct physiological contexts. Brainstem regions such as the NTS and CVLM are clearly as important as the RVLM to BP control and deserve far more attention in the context of hypertension.
Lesions that attenuate hypertension typically enhance the baroreflex (carotid bodies or PVN in SH rats, renal nerves in the 2K-1C model)76, 109, 194 suggesting that baroreflex down-regulation could be a major contributing factor to hypertension. This calls for further investigations of the role of the CVLM and the NTS in hypertension.
The SH rats have been the subjects of most studies. Countless factors are described as playing a “critical” role in the elevated BP of the SH rat (orexin: −33mmHg157, carotid bodies: −17 mmHg95, PVN: −26 mmHg109 or −61 mmHg110; intestinal dysbiosis, −38 mmHg113, amygdala −15 mmHg155, RVLM: −40 mmHg11). This is paradoxical. The homeostasis principle would predict that large and, especially, persistent hypotension should require interfering with multiple pathways simultaneously. Perhaps, at rest, the orexin system, PVN, carotid bodies, amygdala etc. contribute to a roughly comparable extent to the excitatory drive of SPGNs and most of the input summation occurs below action potential threshold in these neurons. If so, the removal of any one of the various inputs could dramatically reduce their firing rate, giving the impression that each is “essential”.
Carotid body hyperactivity contributes to several forms of hypertension but is generally not the predominant factor except when the hypertension results from chronic intermittent hypoxia94. This type of hypertension is also eliminated by adrenal demedullation, sympathetic blockade, AT1 receptor blockade and lesions of the AV3V region59, 93. It is difficult to conceptualize why each of these manipulations would eliminate rather than merely attenuate the effect of carotid body hyperactivity.
SNA elevation and neurogenic hypertension can be caused by excessive levels of circulating hormones (leptin, insulin, AngII). Leptin raises blood vessel SNA by activating the carotid bodies and subsets of ARC neurons but other sites of action may exist195. AngII works in the lamina terminalis and probably throughout the brainstem and hypothalamus via its proinflammatory activity and by reducing the blood brain barrier. Its neurogenic action probably also includes the facilitation of noradrenaline release by sympathetic postganglionic neurons. Sodium works in part via astrocytes and ependymal cells located in the circumventricular organs (OVLT, SFO).
One would also like to know which of the many detected abnormalities have a common origin. Perhaps, all organs with a high metabolic demand (brain, kidneys and the carotid bodies), regulate their blood flow at the expense of systemic BP by eliciting sympathoexcitatory reflexes and the root cause of these neural reflexes is a cerebral or peripheral vascular abnormality. Is hypoxia, oxidative stress or inflammation the trigger in both carotid bodies and brainstem? Are the astrocytes located in the RVLM fundamentally different from those located elsewhere in the brainstem or from type II glomus cells? Finally, the SH rat also highlights the importance of environmental factors (gut microbiome) in hypertension even in this most genetic of models.
Supplementary Material
Sources of Funding:
This work was supported by the National Institutes of Health - R01HL028785 (P.G. Guyenet), RO1HL148004 (S.B.G. Abbott) and RO1HL128181 (V.L. Brooks) and the American Heart Association (#19POST34430205 [G.M.P.R.Souza]).
Abbreviations:
- A1, A2, A5
pontomedullary noradrenergic nuclei
- AgRP
agouti-related peptide
- AngII
angiotensin II
- ARC
arcuate nucleus
- AV3V region
see lamina terminalis
- BP
arterial blood pressure
- C1
RVLM adrenergic/glutamatergic neuron
- CBF
cerebral blood flow
- CIH
chronic intermittent hypoxia
- CSF
cerebrospinal fluid
- CVLM
caudal ventrolateral medulla
- DMH
dorsomedial hypothalamic nucleus
- DOCA
di-hydroxy corticosterone acetate
- ECF
extracellular fluid
- 2K-1C
two-kidney one clip
- LC
locus coeruleus
- MCR
melanocortin receptor
- MnPO
median preoptic nucleus
- αMSH
melanocyte-stimulating hormone
- Na(x)
sodium channel encoded by Scn7a
- NPY
neuropeptide Y
- NTS
solitary tract nucleus
- Orx
orexin
- OSA
obstructive sleep apnea
- OVLT
organum vasculosum lamina terminalis
- PAG
periaqueductal gray matter
- PBL
lateral parabrachial nucleus
- POMC
proopiomelanocortin
- PSN
presympathetic neuron
- PVN
paraventricular nucleus of hypothalamus
- ROS
radical oxygen species
- RPa
raphe pallidus
- RVLM
rostral ventrolateral medulla
- SAD
sinoaortic denervation
- SFO
subfornical organ
- SGN
sympathetic (post) ganglionic neuron
- SH rat
spontaneously hypertensive rat
- SNA
sympathetic nerve activity
- SNS
sympathetic nervous system
- SPGN
sympathetic pre-ganglionic neuron
- VMH
ventromedial hypothalamic nucleus
Footnotes
Disclosures: none.
REFERENCES
- 1.Mann SJ. Neurogenic hypertension: pathophysiology, diagnosis and management. Clin Auton Res 2018;28:363–374. doi: 10.1007/s10286-018-0541-z [DOI] [PubMed] [Google Scholar]
- 2.DiBona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. doi: 10.1161/HYPERTENSIONAHA.111.00633 [DOI] [PubMed] [Google Scholar]
- 3.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stocker SD, Kinsman BJ, Sved AF. Recent Advances in Neurogenic Hypertension: Dietary Salt, Obesity, and Inflammation. Hypertension. 2017;70:474–478. doi: 10.1161/HYPERTENSIONAHA.117.08936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 2010;90:513–557. doi: 10.1152/physrev.00007.2009 [DOI] [PubMed] [Google Scholar]
- 6.Lohmeier TE, Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology (Bethesda). 2015;30:148–158. doi: 10.1152/physiol.00035.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLalio LJ, Sved AF, Stocker SD. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can J Cardiol 2020;36:712–720. doi: 10.1016/j.cjca.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreihofer AM, Sved AF. The ventrolateral medulla and sympathetic regulation of arterial pressure In: Llewellyn-Smith IJ, Verberne AJ, eds. Central regulation of autonomic functions. New-York: Oxford University Press; 2011:78–97. [Google Scholar]
- 9.Guyenet PG, Stornetta RL, Holloway BB, Souza G, Abbott SBG. Rostral Ventrolateral Medulla and Hypertension. Hypertension. 2018;72:559–566. doi: 10.1161/HYPERTENSIONAHA.118.10921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menezes RC, Fontes MA. Cardiovascular effects produced by activation of GABA receptors in the rostral ventrolateral medulla of conscious rats. Neuroscience. 2007;144:336–343. doi: 10.1016/j.neuroscience.2006.08.062 [DOI] [PubMed] [Google Scholar]
- 11.Geraldes V, Goncalves-Rosa N, Liu B, Paton JF, Rocha I. Essential role of RVL medullary neuronal activity in the long term maintenance of hypertension in conscious SHR. Auton Neurosci 2014;186:22–31. doi: 10.1016/j.autneu.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 12.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 1997;387:524–536. doi: [DOI] [PubMed] [Google Scholar]
- 13.Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci 2013;33:19223–19237. doi: 10.1523/JNEUROSCI.3041-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis DJ, Granata AR, Joh TH, Ross CA, Ruggiero DA, Park DH. Brain stem catecholamine mechanisms in tonic and reflex control of blood pressure. Hypertension. 1984;6:II7–I15. doi: 10.1161/01.hyp.6.5_pt_2.ii7 [DOI] [PubMed] [Google Scholar]
- 15.Lipski J, Kanjhan R, Kruszewska B, Smith M. Barosensitive neurons in the rostral ventrolateral medulla of the rat in vivo: Morphological properties and relationship to C1 adrenergic neurons. Neuroscience. 1995;69:601–618. doi: 10.1016/0306-4522(95)92652-z [DOI] [PubMed] [Google Scholar]
- 16.Ross CA, Armstrong DM, Ruggiero DA, Pickel VM, Joh TH, Reis DJ. Adrenaline neurons in the rostral ventrolateral medulla innervate thoracic spinal cord: a combined immunocytochemical and retrograde transport demonstration. Neurosci Lett 1981;25:257–262. doi: 10.1016/0304-3940(81)90401-8 [DOI] [PubMed] [Google Scholar]
- 17.Madden CJ, Stocker SD, Sved AF. Attenuation of homeostatic responses to hypotension and glucoprivation after destruction of catecholaminergic rostral ventrolateral medulla (RVLM) neurons. Am J Physiol Regul Integr Comp Physiol 2006;291:R751–R759. doi: 10.1152/ajpregu.00800.2005 [DOI] [PubMed] [Google Scholar]
- 18.Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep 2003;5:262–268. doi: 10.1007/s11906-003-0030-0 [DOI] [PubMed] [Google Scholar]
- 19.Wenker IC, Abe C, Viar KE, Stornetta DS, Stornetta RL, Guyenet PG. Blood Pressure Regulation by the Rostral Ventrolateral Medulla in Conscious Rats: Effects of Hypoxia, Hypercapnia, Baroreceptor Denervation, and Anesthesia. J Neurosci 2017;37:4565–4583. doi: 10.1523/JNEUROSCI.3922-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moraes DJA, Bonagamba LGH, da Silva MP, Paton JFR, Machado BH. Role of ventral medullary catecholaminergic neurons for respiratory modulation of sympathetic outflow in rats. Sci Rep 2017;7:16883. doi: 10.1038/s41598-017-17113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer KB, Collins HK, Callaway EM. Sources of off-target expression from recombinase-dependent AAV vectors and mitigation with cross-over insensitive ATG-out vectors. Proc Natl Acad Sci U S A 2019. doi: 10.1073/pnas.1915974116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrive P, Gorissen M. Premotor sympathetic neurons of conditioned fear in the rat. EurJ Neurosci 2008;28:428–446. doi: 10.1111/j.1460-9568.2008.06351.x [DOI] [PubMed] [Google Scholar]
- 23.Ramchandra R, Barrett CJ, Guild SJ, Malpas SC. Evidence of differential control of renal and lumbar sympathetic nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 2005. doi: 10.1152/ajpregu.00504.2005 [DOI] [PubMed] [Google Scholar]
- 24.Kinsman BJ, Simmonds SS, Browning KN, Stocker SD. Organum Vasculosum of the Lamina Terminalis Detects NaCl to Elevate Sympathetic Nerve Activity and Blood Pressure. Hypertension. 2017;69:163–170. doi: 10.1161/HYPERTENSIONAHA.116.08372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fink GD. Exaggerated Sympathetic Neurovascular Transduction as a Mechanism of Neurogenic Hypertension: It Is Not All About Activity. Hypertension. 2018;71:64–65. doi: 10.1161/HYPERTENSIONAHA.117.10300 [DOI] [PubMed] [Google Scholar]
- 26.Campos RR, McAllen RM. Cardiac sympathetic premotor neurons. Am J Physiol Regul Integr Comp Physiol 1997;272:R615–R620. doi: 10.1152/ajpregu.1997.272.2.R615 [DOI] [PubMed] [Google Scholar]
- 27.McAllen RM, May CN, Campos RR. The supply of vasomotor drive to individual classes of sympathetic neuron. Clin Exp Hypertens 1997;19:607–618. doi: 10.3109/10641969709083173 [DOI] [PubMed] [Google Scholar]
- 28.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body’s EMTs. Am J Physiol Regul Integr Comp Physiol 2013;305:R187–204. doi: 10.1152/ajpregu.00054.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farmer DGS, Pracejus N, Dempsey B, Turner A, Bokiniec P, Paton JFR, Pickering AE, Burguet J, Andrey P, Goodchild AK, McAllen RM, McMullan S. On the presence and functional significance of sympathetic premotor neurons with collateralized spinal axons in the rat. J Physiol 2019;597:3407–3423. doi: 10.1113/JP277661 [DOI] [PubMed] [Google Scholar]
- 30.Jansen ASP, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system:basis of the fight-or flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644 [DOI] [PubMed] [Google Scholar]
- 31.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol 2006;499:840–859. doi: 10.1002/cne.21140 [DOI] [PubMed] [Google Scholar]
- 32.Abbott SB, DePuy SD, Nguyen T, Coates MB, Stornetta RL, Guyenet PG. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J Neurosci 2013;33:3164–3177. doi: 10.1523/JNEUROSCI.1046-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dempsey B, Le S, Turner A, Bokiniec P, Ramadas R, Bjaalie JG, Menuet C, Neve R, Allen AM, Goodchild AK, McMullan S. Mapping and Analysis of the Connectome of Sympathetic Premotor Neurons in the Rostral Ventrolateral Medulla of the Rat Using a Volumetric Brain Atlas. Front Neural Circuits. 2017;11:9. doi: 10.3389/fncir.2017.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stornetta RL, Inglis MA, Viar KE, Guyenet PG. Afferent and efferent connections of C1 cells with spinal cord or hypothalamic projections in mice. Brain Struct Funct 2016;221:4027–4044. doi: 10.1007/s00429-015-1143-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol 2006;572:881–896. doi: 10.1113/jphysiol.2005.103622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreihofer AM, Guyenet PG. Baro-activated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J Neurophysiol 2003;89:1265–1277. doi: 10.1152/jn.00737.2002 [DOI] [PubMed] [Google Scholar]
- 37.Guyenet PG. Cardiorespiratory integration In: Llewellyn-Smith IJ, Verberne AJ, eds. Central regulation of autonomic functions. New-York: Oxford University Press; 2011:180–201. [Google Scholar]
- 38.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 2014;4:1511–1562. doi: 10.1002/cphy.c140004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 2010;518:1460–1499. doi: 10.1002/cne.22283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Accorsi-Mendonca D, da Silva MP, Souza GM, Lima-Silveira L, Karlen-Amarante M, Amorim MR, Almado CE, Moraes DJ, Machado BH. Pacemaking Property of RVLM Presympathetic Neurons. Front Physiol 2016;7:424. doi: 10.3389/fphys.2016.00424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li YW, Bayliss DA, Guyenet PG. C1 neurons of neonatal rats: intrinsic beating properties and alpha 2-adrenergic receptors. Am J Physiol 1995;269:R1356–1369. doi: 10.1152/ajpregu.1995.269.6.R1356 [DOI] [PubMed] [Google Scholar]
- 42.Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, Teschemacher AG. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun 2014;5:3284. doi: 10.1038/ncomms4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak A, Zwicker J, Teschemacher AG, Ackland GL, Funk GD, Kasparov S, Abramov AY, Gourine AV. Functional Oxygen Sensitivity of Astrocytes. J Neurosci 2015;35:10460–10473. doi: 10.1523/JNEUROSCI.0045-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serrats J, Schiltz JC, Garcia-Bueno B, van RN, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Kempen TA, Dodos M, Woods C, Marques-Lopes J, Justice NJ, Iadecola C, Pickel VM, Glass MJ, Milner TA. Sex differences in NMDA GluN1 plasticity in rostral ventrolateral medulla neurons containing corticotropin-releasing factor type 1 receptor following slow-pressor angiotensin II hypertension. Neuroscience. 2015;307:83–97. doi: 10.1016/j.neuroscience.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reis DJ, Golanov EV, Ruggiero DA, Sun M-K. Sympatho-excitatory neurons of the rostral ventrolateral medulla are oxygen sensors and essential elements in the tonic and reflex control of the systemic and cerebral circulations. J Hypertens. 1994;12 Suppl. 10:S159–S180. [PubMed] [Google Scholar]
- 47.Chen D, Jancovski N, Bassi JK, Nguyen-Huu TP, Choong YT, Palma-Rigo K, Davern PJ, Gurley SB, Thomas WG, Head GA, Allen AM. Angiotensin type 1A receptors in C1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress. J Neurosci 2012;32:2051–2061. doi: 10.1523/JNEUROSCI.5360-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension. 2007;49:640–646. doi: 10.1161/01.HYP.0000254828.71253.dc [DOI] [PubMed] [Google Scholar]
- 49.Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in dahl salt-sensitive rats. Hypertension. 2001;37:687–691. [PubMed] [Google Scholar]
- 50.Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension. 2000;35:413–417. doi: 10.1161/01.hyp.35.1.413 [DOI] [PubMed] [Google Scholar]
- 51.Huber DA, Schreihofer AM. Altered regulation of the rostral ventrolateral medulla in hypertensive obese Zucker rats. Am J Physiol Heart Circ Physiol 2011;301:H230–240. doi: 10.1152/ajpheart.00075.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minson J, Arnolda L, Llewellyn-Smith I, Pilowsky P, Chalmers J. Altered c-fos in rostral medulla and spinal cord of spontaneously hypertensive rats. Hypertension. 1996;27:433–441. doi: 10.1161/01.hyp.27.3.433 [DOI] [PubMed] [Google Scholar]
- 53.Peruzzi D, Hendley ED, Forehand CJ. Hypertrophy of stellate ganglion cells in hypertensive, but not hyperactive, rats. Am J Physiol 1991;261:R979–984. doi: 10.1152/ajpregu.1991.261.4.R979 [DOI] [PubMed] [Google Scholar]
- 54.Wilson C, Zhang X, Buckley C, Heathcote HR, Lee MD, McCarron JG. Increased Vascular Contractility in Hypertension Results From Impaired Endothelial Calcium Signaling. Hypertension. 2019;74:1200–1214. doi: 10.1161/HYPERTENSIONAHA.119.13791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun MK, Guyenet PG. Medullospinal sympathoexcitatory neurons in normotensive and spontaneously hypertensive rats. Am J Physiol 1986;250:R910–R917. doi: 10.1152/ajpregu.1986.250.5.R910 [DOI] [PubMed] [Google Scholar]
- 56.Matsuura T, Kumagai H, Kawai A, Onimaru H, Imai M, Oshima N, Sakata K, Saruta T. Rostral ventrolateral medulla neurons of neonatal Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 2002;40:560–565. doi: 10.1161/01.hyp.0000032043.64223.87 [DOI] [PubMed] [Google Scholar]
- 57.Menuet C, Le S, Dempsey B, Connelly AA, Kamar JL, Jancovski N, Bassi JK, Walters K, Simms AE, Hammond A, Fong AY, Goodchild AK, McMullan S, Allen AM. Excessive Respiratory Modulation of Blood Pressure Triggers Hypertension. Cell Metab 2017;25:739–748. doi: 10.1016/j.cmet.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 58.Moraes DJ, Machado BH, Paton JF. Specific respiratory neuron types have increased excitability that drive presympathetic neurones in neurogenic hypertension. Hypertension. 2014;63:1309–1318. doi: 10.1161/HYPERTENSIONAHA.113.02283 [DOI] [PubMed] [Google Scholar]
- 59.Marciante AB, Wang LA, Little JT, Cunningham JT. Caspase lesions of PVN-projecting MnPO neurons block the sustained component of CIH-induced hypertension in adult male rats. Am J Physiol Heart Circ Physiol 2020;318:H34–H48. doi: 10.1152/ajpheart.00350.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Souza GM, Bonagamba LG, Amorim MR, Moraes DJ, Machado BH. Inspiratory modulation of sympathetic activity is increased in female rats exposed to chronic intermittent hypoxia. Exp Physiol 2016;101:1345–1358. doi: 10.1113/EP085850 [DOI] [PubMed] [Google Scholar]
- 62.Molkov YI, Zoccal DB, Baekey DM, Abdala AP, Machado BH, Dick TE, Paton JF, Rybak IA. Physiological and pathophysiological interactions between the respiratory central pattern generator and the sympathetic nervous system. Prog Brain Res 2014;212:1–23. doi: 10.1016/B978-0-444-63488-7.00001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walas D, Nowicki-Osuch K, Alibhai D, von Linstow Roloff E, Coghill J, Waterfall C, Paton JF. Inflammatory pathways are central to posterior cerebrovascular artery remodelling prior to the onset of congenital hypertension. J Cereb Blood Flow Metab 2018:271678X18769180. doi: 10.1177/0271678X18769180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guyenet PG, Stornetta RL, Souza G, Abbott SBG, Shi Y, Bayliss DA. The Retrotrapezoid Nucleus: Central Chemoreceptor and Regulator of Breathing Automaticity. Trends Neurosci 2019;42:807–824. doi: 10.1016/j.tins.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molkov YI, Shevtsova NA, Park C, Ben-Tal A, Smith JC, Rubin JE, Rybak IA. A closed-loop model of the respiratory system: focus on hypercapnia and active expiration. PLoS One. 2014;9:e109894. doi: 10.1371/journal.pone.0109894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol 2011;105:3080–3091. doi: 10.1152/jn.00070.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li A, Roy SH, Nattie EE. An augmented CO2 chemoreflex and overactive orexin system are linked with hypertension in young and adult spontaneously hypertensive rats. J Physiol 2016;594:4967–4980. doi: 10.1113/JP272199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fatouleh R, Macefield VG. Respiratory modulation of muscle sympathetic nerve activity is not increased in essential hypertension or chronic obstructive pulmonary disease. J Physiol 2011;589:4997–5006. doi: 10.1113/expphysiol.2013.077511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bazilio DS, Bonagamba LGH, Moraes DJA, Machado BH. Cardiovascular and respiratory profiles during the sleep-wake cycle of rats previously submitted to chronic intermittent hypoxia. Exp Physiol 2019;104:1408–1419. doi: 10.1113/EP087784 [DOI] [PubMed] [Google Scholar]
- 70.Fatouleh R, McKenzie DK, Macefield VG. Respiratory modulation of muscle sympathetic nerve activity in obstructive sleep apnoea. Exp Physiol 2014;99:1288–1298. doi: 10.1113/expphysiol.2013.077511 [DOI] [PubMed] [Google Scholar]
- 71.Guyenet PG, Bayliss DA, Stornetta RL, Kanbar R, Shi Y, Holloway BB, Souza G, Basting TM, Abbott SBG, Wenker IC. Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus. J Physiol 2018;596:3029–3042. doi: 10.1113/JP274357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting Afferent Renal Nerve Discharge and Renal Inflammation: Elucidating the Role of Afferent and Efferent Renal Nerves in Deoxycorticosterone Acetate Salt Hypertension. Hypertension. 2016;68:1415–1423. doi: 10.1161/HYPERTENSIONAHA.116.07850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ong J, Kinsman BJ, Sved AF, Rush BM, Tan RJ, Carattino MD, Stocker SD. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol 2019;122:358–367. doi: 10.1152/jn.00173.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 1995;361:225–248. doi: 10.1002/cne.903610204 [DOI] [PubMed] [Google Scholar]
- 75.Dampney RAL. Resetting of the Baroreflex Control of Sympathetic Vasomotor Activity during Natural Behaviors: Description and Conceptual Model of Central Mechanisms. Front Neurosci 2017;11:461. doi: 10.3389/fnins.2017.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun 2013;4:2395. doi: 10.1038/ncomms3395 [DOI] [PubMed] [Google Scholar]
- 77.Cowley AW Jr. Long-term control of arterial blood pressure. Physiol Rev 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231 [DOI] [PubMed] [Google Scholar]
- 78.Dampney RA. Central neural control of the cardiovascular system: current perspectives. Adv Physiol Educ 2016;40:283–296. doi: 10.1152/advan.00027.2016 [DOI] [PubMed] [Google Scholar]
- 79.Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, Patapoutian A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 2018;362:464–467. doi: 10.1126/science.aau6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 2006;7:563–574. doi: 10.1038/nrn1949 [DOI] [PubMed] [Google Scholar]
- 81.Marina N, Christie IN, Korsak A, Doronin M, Brazhe A, Hosford PS, Wells JA, Sheikhbahaei S, Humoud I, Paton JFR, Lythgoe MF, Semyanov A, Kasparov S, Gourine AV. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat Commun 2020;11:131. doi: 10.1038/s41467-019-13956-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lohmeier TE, Hall JE. Device-Based Neuromodulation for Resistant Hypertension Therapy. Circ Res 2019;124:1071–1093. doi: 10.1161/CIRCRESAHA.118.313221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nurse CA. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol 2014;592:3419–3426. doi: 10.1113/jphysiol.2013.269829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol 2013;591:2245–2257. doi: 10.1113/jphysiol.2012.247759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ortega-Saenz P, Lopez-Barneo J. Physiology of the Carotid Body: From Molecules to Disease. Annu Rev Physiol 2019. doi: 10.1146/annurev-physiol-020518-114427 [DOI] [PubMed] [Google Scholar]
- 86.Zera T, Moraes DJA, da Silva MP, Fisher JP, Paton JFR. The Logic of Carotid Body Connectivity to the Brain. Physiology (Bethesda). 2019;34:264–282. doi: 10.1152/physiol.00057.2018 [DOI] [PubMed] [Google Scholar]
- 87.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol 1996;270:R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273 [DOI] [PubMed] [Google Scholar]
- 88.Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res 1993;609:174–184. doi: 10.1016/0006-8993(93)90871-j [DOI] [PubMed] [Google Scholar]
- 89.Marshall JM, Metcalfe JD. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol 1988;407:385–404. doi: 10.1113/jphysiol.1988.sp017422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin MK, Eraso CC, Mu YP, Gu C, Yeung BHY, Kim LJ, Liu XR, Wu ZJ, Paudel O, Pichard LE, Shirahata M, Tang WY, Sham JSK, Polotsky VY. Leptin Induces Hypertension Acting on Transient Receptor Potential Melastatin 7 Channel in the Carotid Body. Circ Res 2019;125:989–1002. doi: 10.1161/CIRCRESAHA.119.315338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pijacka W, McBryde FD, Marvar PJ, Lincevicius GS, Abdala AP, Woodward L, Li D, Paterson DJ, Paton JF. Carotid sinus denervation ameliorates renovascular hypertension in adult Wistar rats. J Physiol 2016;594:6255–6266. doi: 10.1113/JP272708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 2013;62:2905–2916. doi: 10.2337/db12-1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fletcher EC. Invited review: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600 [DOI] [PubMed] [Google Scholar]
- 94.Del Rio R, Andrade DC, Lucero C, Arias P, Iturriaga R. Carotid Body Ablation Abrogates Hypertension and Autonomic Alterations Induced by Intermittent Hypoxia in Rats. Hypertension. 2016;68:436–445. doi: 10.1161/HYPERTENSIONAHA.116.07255 [DOI] [PubMed] [Google Scholar]
- 95.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pijacka W, Moraes DJ, Ratcliffe LE, Nightingale AK, Hart EC, da Silva MP, Machado BH, McBryde FD, Abdala AP, Ford AP, Paton JF. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat Med 2016;22:1151–1159. doi: 10.1038/nm.4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prabhakar NR, Peng YJ, Kumar GK, Pawar A. Altered carotid body function by intermittent hypoxia in neonates and adults: Relevance to recurrent apneas. Respir Physiol Neurobiol 2007;157:148–153. doi: 10.1016/j.resp.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 98.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2012;2:141–219. doi: 10.1002/cphy.c100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nanduri J, Peng YJ, Wang N, Prabhakar NR. Neural Activation of Molecular Circuitry in Intermittent Hypoxia. Curr Opin Physiol 2019;7:9–14. doi: 10.1016/j.cophys.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sobrino V, Gonzalez-Rodriguez P, Annese V, Lopez-Barneo J, Pardal R. Fast neurogenesis from carotid body quiescent neuroblasts accelerates adaptation to hypoxia. EMBO Rep 2018;19 doi: 10.15252/embr.201744598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cramer JA, Wiggins RH, Fudim M, Engelman ZJ, Sobotka PA, Shah LM. Carotid body size on CTA: correlation with comorbidities. Clin Radiol 2014;69:e33–36. doi: 10.1016/j.crad.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 103.Narkiewicz K, Ratcliffe LE, Hart EC, Briant LJ, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, Burchell AE, Durant C, Lobo MD, Sobotka PA, Patel NK, Leiter JC, Engelman ZJ, Nightingale AK, Paton JF. Unilateral Carotid Body Resection in Resistant Hypertension: A Safety and Feasibility Trial. JACC Basic Transl Sci 2016;1:313–324. doi: 10.1016/j.jacbts.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 2011;16:74–104. doi: 10.2741/3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dergacheva O, Yamanaka A, Schwartz AR, Polotsky VY, Mendelowitz D. Direct projections from hypothalamic orexin neurons to brainstem cardiac vagal neurons. Neuroscience. 2016;339:47–53. doi: 10.1016/j.neuroscience.2016.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stocker SD, Lang SM, Simmonds SS, Wenner MM, Farquhar WB. Cerebrospinal Fluid Hypernatremia Elevates Sympathetic Nerve Activity and Blood Pressure via the Rostral Ventrolateral Medulla. Hypertension. 2015;66:1184–1190. doi: 10.1161/HYPERTENSIONAHA.115.05936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dampney RA, Michelini LC, Li DP, Pan HL. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol 2018;315:H1200–H1214. doi: 10.1152/ajpheart.00216.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Geraldes V, Goncalves-Rosa N, Liu B, Paton JF, Rocha I. Chronic depression of hypothalamic paraventricular neuronal activity produces sustained hypotension in hypertensive rats. Exp Physiol 2014;99:89–100. doi: 10.1113/expphysiol.2013.074823 [DOI] [PubMed] [Google Scholar]
- 110.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. [DOI] [PubMed] [Google Scholar]
- 111.Mukerjee S, Gao H, Xu J, Sato R, Zsombok A, Lazartigues E. ACE2 and ADAM17 Interaction Regulates the Activity of Presympathetic Neurons. Hypertension. 2019;74:1181–1191. doi: 10.1161/HYPERTENSIONAHA.119.13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toral M, Robles-Vera I, de la Visitacion N, Romero M, Yang T, Sanchez M, Gomez-Guzman M, Jimenez R, Raizada MK, Duarte J. Critical Role of the Interaction Gut Microbiota - Sympathetic Nervous System in the Regulation of Blood Pressure. Front Physiol 2019;10:231. doi: 10.3389/fphys.2019.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huber MJ, Chen QH, Shan Z. The Orexin System and Hypertension. Cell Mol Neurobiol 2018;38:385–391. doi: 10.1007/s10571-017-0487-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brooks VL, Shi Z, Holwerda SW, Fadel PJ. Obesity-induced increases in sympathetic nerve activity: sex matters. Auton Neurosci 2015;187:18–26. doi: 10.1016/j.autneu.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lambert GW, Schlaich MP, Eikelis N, Lambert EA. Sympathetic activity in obesity: a brief review of methods and supportive data. Ann N Y Acad Sci 2019;1454:56–67. doi: 10.1111/nyas.14140 [DOI] [PubMed] [Google Scholar]
- 117.Rahmouni K Cardiovascular Regulation by the Arcuate Nucleus of the Hypothalamus: Neurocircuitry and Signaling Systems. Hypertension. 2016;67:1064–1071. doi: 10.1161/HYPERTENSIONAHA.115.06425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakamura T, Bhatt S, Sapru HN. Cardiovascular responses to hypothalamic arcuate nucleus stimulation in the rat: role of sympathetic and vagal efferents. Hypertension. 2009;54:1369–1375. doi: 10.1161/HYPERTENSIONAHA.109.140715 [DOI] [PubMed] [Google Scholar]
- 119.Chitravanshi VC, Kawabe K, Sapru HN. Stimulation of the hypothalamic arcuate nucleus increases brown adipose tissue nerve activity via hypothalamic paraventricular and dorsomedial nuclei. Am J Physiol Heart Circ Physiol 2016;311:H433–444. doi: 10.1152/ajpheart.00176.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sim LJ, Joseph SA. Arcuate nucleus projections to brainstem regions which modulate nociception. J Chem Neuroanat 1991;4:97–109. doi: 10.1016/0891-0618(91)90034-a [DOI] [PubMed] [Google Scholar]
- 121.Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, Zhan C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat 2015;9:40. doi: 10.3389/fnana.2015.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x [DOI] [PubMed] [Google Scholar]
- 123.Kawabe T, Kawabe K, Sapru HN. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: role of the hypothalamic paraventricular nucleus. PLoS One. 2012;7:e45180. doi: 10.1371/journal.pone.0045180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi Z, Madden CJ, Brooks VL. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. J Clin Invest 2017;127:2868–2880. doi: 10.1172/JCI92008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2 [DOI] [PubMed] [Google Scholar]
- 126.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 2011;589:1643–1662. doi: 10.1113/jphysiol.2011.205575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol 2013;304:H1538–1546. doi: 10.1152/ajpheart.00081.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Limberg JK, Johnson BD, Mozer MT, Holbein WW, Curry TB, Prabhakar NR, Joyner MJ. Role of the carotid chemoreceptors in insulin-mediated sympathoexcitation in humans. Am J Physiol Regul Integr Comp Physiol 2020;318:R173–R181. doi: 10.1152/ajpregu.00257.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shi Z, Wong J, Brooks VL. Obesity: sex and sympathetics. Biol Sex Differ 2020;11:10. doi: 10.1186/s13293-020-00286-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi Z, Zhao D, Cassaglia PA, Brooks VL. Sites and sources of sympathoexcitation in obese male rats: role of brain insulin. Am J Physiol Regul Integr Comp Physiol 2020. doi: 10.1152/ajpregu.00317.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shi Z, Cassaglia PA, Pelletier NE, Brooks VL. Sex differences in the sympathoexcitatory response to insulin in obese rats: role of neuropeptide Y. J Physiol 2019;597:1757–1775. doi: 10.1113/JP277517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lim K, Barzel B, Burke SL, Armitage JA, Head GA. Origin of Aberrant Blood Pressure and Sympathetic Regulation in Diet-Induced Obesity. Hypertension. 2016;68:491–500. doi: 10.1161/HYPERTENSIONAHA.116.07461 [DOI] [PubMed] [Google Scholar]
- 134.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG Jr., Licinio J, Brown RD, Enriori PJ, O’Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morgan DA, Anderson EA, Mark AL. Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension. 1995;25:834–838. doi: 10.1161/01.hyp.25.4.834 [DOI] [PubMed] [Google Scholar]
- 136.do Carmo JM, da Silva AA, Rushing JS, Hall JE. Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 2012;302:R561–567. doi: 10.1152/ajpregu.00392.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou JJ, Ma HJ, Shao J, Wei Y, Zhang X, Zhang Y, Li DP. Downregulation of Orexin Receptor in Hypothalamic Paraventricular Nucleus Decreases Blood Pressure in Obese Zucker Rats. J Am Heart Assoc 2019;8:e011434. doi: 10.1161/JAHA.118.011434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jais A, Bruning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest 2017;127:24–32. doi: 10.1172/JCI88878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sieminski M, Szypenbejl J, Partinen E. Orexins, Sleep, and Blood Pressure. Curr Hypertens Rep 2018;20:79. doi: 10.1007/s11906-018-0879-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 141.Carrive P, Kuwaki T. Orexin and Central Modulation of Cardiovascular and Respiratory Function. Curr Top Behav Neurosci 2017;33:157–196. doi: 10.1007/7854_2016_46 [DOI] [PubMed] [Google Scholar]
- 142.Kuwaki T Thermoregulation under pressure: a role for orexin neurons. Temperature (Austin) 2015;2:379–391. doi: 10.1080/23328940.2015.1066921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kuwaki T Orexin links emotional stress to autonomic functions. Auton Neurosci 2010;161:20–27. doi: 10.1016/j.autneu.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 144.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li A, Nattie E. Orexin, cardio-respiratory function, and hypertension. Front Neurosci 2014;8:22. doi: 10.3389/fnins.2014.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kuwaki T, Zhang W, Nakamura A, Deng BS. Emotional and state-dependent modification of cardiorespiratory function: role of orexinergic neurons. Auton Neurosci 2008;142:11–16. doi: 10.1016/j.autneu.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 147.Jackson KL, Head GA, Gueguen C, Stevenson ER, Lim K, Marques FZ. Mechanisms Responsible for Genetic Hypertension in Schlager BPH/2 Mice. Front Physiol 2019;10:1311. doi: 10.3389/fphys.2019.01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jackson KL, Dampney BW, Moretti JL, Stevenson ER, Davern PJ, Carrive P, Head GA. Contribution of Orexin to the Neurogenic Hypertension in BPH/2J Mice. Hypertension. 2016;67:959–969. doi: 10.1161/HYPERTENSIONAHA.115.07053 [DOI] [PubMed] [Google Scholar]
- 149.Li AH, Hindmarch CCT, Nattie EE, Paton JFR. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol 2013;591:4237–4248. doi:DOI 10.1113/jphysiol.2013.256271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee YH, Tsai MC, Li TL, Dai YW, Huang SC, Hwang LL. Spontaneously hypertensive rats have more orexin neurons in the hypothalamus and enhanced orexinergic input and orexin 2 receptor-associated nitric oxide signalling in the rostral ventrolateral medulla. Exp Physiol 2015;100:993–1007. doi: 10.1113/EP085016 [DOI] [PubMed] [Google Scholar]
- 151.Clifford L, Dampney BW, Carrive P. Spontaneously hypertensive rats have more orexin neurons in their medial hypothalamus than normotensive rats. Exp Physiol 2015;100:388–398. doi: 10.1113/expphysiol.2014.084137 [DOI] [PubMed] [Google Scholar]
- 152.Huber MJ, Fan Y, Jiang E, Zhu F, Larson RA, Yan J, Li N, Chen QH, Shan Z. Increased activity of the orexin system in the paraventricular nucleus contributes to salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 2017;313:H1075–H1086. doi: 10.1152/ajpheart.00822.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kuwaki T, Zhang W. Orexin neurons as arousal-associated modulators of central cardiorespiratory regulation. Respir Physiol Neurobiol 2010;174:43–54. [DOI] [PubMed] [Google Scholar]
- 154.Jackson KL, Palma-Rigo K, Nguyen-Huu TP, Davern PJ, Head GA. Major contribution of the medial amygdala to hypertension in BPH/2J genetically hypertensive mice. Hypertension. 2014;63:811–818. doi: 10.1161/HYPERTENSIONAHA.113.02020 [DOI] [PubMed] [Google Scholar]
- 155.Folkow B, Hallback-Nordlander M, Martner J, Nordborg C. Influence of amygdala lesions on cardiovascular responses to alerting stimuli, on behaviour and on blood pressure development in spontaneously hypertensive rats. Acta Physiol Scand 1982;116:133–139. doi: 10.1111/j.1748-1716.1982.tb07121.x [DOI] [PubMed] [Google Scholar]
- 156.Llewellyn-Smith IJ, Martin CL, Marcus JN, Yanagisawa M, Minson JB, Scammell TE. Orexin-immunoreactive inputs to rat sympathetic preganglionic neurons. Neurosci Lett 2003;351:115–119. doi: 10.1016/s0304-3940(03)00770-5 [DOI] [PubMed] [Google Scholar]
- 157.Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol 2013;591:4237–4248. doi: 10.1113/jphysiol.2013.256271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev 2012;16:187–197. doi: 10.1016/j.smrv.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031 [DOI] [PubMed] [Google Scholar]
- 160.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Iwakawa S, Kanmura Y, Kuwaki T. Orexin Receptor Blockade-Induced Sleep Preserves the Ability to Wake in the Presence of Threat in Mice. Front Behav Neurosci 2018;12:327. doi: 10.3389/fnbeh.2018.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest 2014;124:604–616. doi: 10.1172/JCI71017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mansukhani MP, Covassin N, Somers VK. Neurological Sleep Disorders and Blood Pressure: Current Evidence. Hypertension. 2019;74:726–732. doi: 10.1161/HYPERTENSIONAHA.119.13456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Herring WJ, Roth T, Krystal AD, Michelson D. Orexin receptor antagonists for the treatment of insomnia and potential treatment of other neuropsychiatric indications. J Sleep Res 2019;28:e12782. doi: 10.1111/jsr.12782 [DOI] [PubMed] [Google Scholar]
- 165.McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 2015;214:8–32. doi: 10.1111/apha.12487 [DOI] [PubMed] [Google Scholar]
- 166.Zimmerman CA, Leib DE, Knight ZA. Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci 2017;18:459–469. doi: 10.1038/nrn.2017.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nomura K, Hiyama TY, Sakuta H, Matsuda T, Lin CH, Kobayashi K, Kobayashi K, Kuwaki T, Takahashi K, Matsui S, Noda M. [Na(+)] Increases in Body Fluids Sensed by Central Nax Induce Sympathetically Mediated Blood Pressure Elevations via H(+)-Dependent Activation of ASIC1a. Neuron 2019;101:60–75 e66. doi: 10.1016/j.neuron.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 168.Young CN, Davisson RL. Angiotensin-II, the Brain, and Hypertension: An Update. Hypertension. 2015;66:920–926. doi: 10.1161/HYPERTENSIONAHA.115.03624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kinsman BJ, Nation HN, Stocker SD. Hypothalamic Signaling in Body Fluid Homeostasis and Hypertension. Curr Hypertens Rep 2017;19:50. doi: 10.1007/s11906-017-0749-7 [DOI] [PubMed] [Google Scholar]
- 170.Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zimmerman CA, Lin YC, Leib DE, Guo L, Huey EL, Daly GE, Chen Y, Knight ZA. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature. 2016. doi: 10.1038/nature18950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Collister JP, Nahey DB, Hartson R, Wiedmeyer CE, Banek CT, Osborn JW. Lesion of the OVLT markedly attenuates chronic DOCA-salt hypertension in rats. Am J Physiol Regul Integr Comp Physiol 2018;315:R568–R575. doi: 10.1152/ajpregu.00433.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kinsman BJ, Browning KN, Stocker SD. NaCl and osmolarity produce different responses in organum vasculosum of the lamina terminalis neurons, sympathetic nerve activity and blood pressure. J Physiol 2017;595:6187–6201. doi: 10.1113/JP274537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blessing WW. Arterial pressure and blood flow to the tissues The lower brainstem and bodily homeostasis. New York: Oxford University Press; 1997:165–268. [Google Scholar]
- 175.Muller-Ribeiro FC, Dampney RA, McMullan S, Fontes MA, Goodchild AK. Disinhibition of the midbrain colliculi unmasks coordinated autonomic, respiratory, and somatomotor responses to auditory and visual stimuli. Am J Physiol Regul Integr Comp Physiol 2014;307:R1025–1035. doi: 10.1152/ajpregu.00165.2014 [DOI] [PubMed] [Google Scholar]
- 176.Dampney RA, Furlong TM, Horiuchi J, Iigaya K. Role of dorsolateral periaqueductal grey in the coordinated regulation of cardiovascular and respiratory function. Auton Neurosci 2013. doi: 10.1016/j.autneu.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 177.Horiuchi J, McDowall LM, Dampney RA. Vasomotor and respiratory responses evoked from the dorsolateral periaqueductal grey are mediated by the dorsomedial hypothalamus. J Physiol 2009;587:5149–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MA, Dampney RA. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol 2004;287:R824–R832. doi: 10.1152/ajpregu.00221.2004 [DOI] [PubMed] [Google Scholar]
- 179.Fontes MA, Xavier CH, de Menezes RC, Dimicco JA. The dorsomedial hypothalamus and the central pathways involved in the cardiovascular response to emotional stress. Neuroscience. 2011;184:64–74. doi: 10.1016/j.neuroscience.2011.03.018 [DOI] [PubMed] [Google Scholar]
- 180.O’Callaghan EL, McBryde FD, Burchell AE, Ratcliffe LE, Nicolae L, Gillbe I, Carr D, Hart EC, Nightingale AK, Patel NK, Paton JF. Deep brain stimulation for the treatment of resistant hypertension. Curr Hypertens Rep 2014;16:493. doi: 10.1007/s11906-014-0493-1 [DOI] [PubMed] [Google Scholar]
- 181.Sams JM, Jansen ASP, Mettenleiter TC, Loewy AD. Pseudorabies virus mutants as transneuronal markers. Brain Res 1995;687:182–190. doi: 10.1016/0006-8993(95)00484-8 [DOI] [PubMed] [Google Scholar]
- 182.Morrison SF, Nakamura K. Central Mechanisms for Thermoregulation. Annu Rev Physiol 2019;81:285–308. doi: 10.1146/annurev-physiol-020518-114546 [DOI] [PubMed] [Google Scholar]
- 183.Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Compr Physiol 2014;4:1677–1713. doi: 10.1002/cphy.c140013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McBryde FD, Malpas SC, Paton JF. Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol (Oxf) 2017;219:274–287. doi: 10.1111/apha.12706 [DOI] [PubMed] [Google Scholar]
- 185.Cushing H Concerning a definitive regulatory mechanism of the vaso-motor centre which controls blood pressure during cerebral compression. B Johns Hopkins Hosp 1901;12:290–292. [Google Scholar]
- 186.Schmidt EA, Despas F, Pavy-Le Traon A, Czosnyka Z, Pickard JD, Rahmouni K, Pathak A, Senard JM. Intracranial Pressure Is a Determinant of Sympathetic Activity. Front Physiol 2018;9:11. doi: 10.3389/fphys.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guild SJ, Saxena UA, McBryde FD, Malpas SC, Ramchandra R. Intracranial pressure influences the level of sympathetic tone. Am J Physiol Regul Integr Comp Physiol 2018;315:R1049–R1053. doi: 10.1152/ajpregu.00183.2018 [DOI] [PubMed] [Google Scholar]
- 188.Sun MK, Wahlestedt C, Reis DJ. Action of externally applied ATP on rat reticulospinal vasomotor neurons. Eur J Pharmacol 1992;224:93–96. doi: 10.1016/0014-2999(92)94824-f [DOI] [PubMed] [Google Scholar]
- 189.Waki H, Bhuiyan ME, Gouraud SS, Takagishi M, Hatada A, Kohsaka A, Paton JF, Maeda M. Acute reductions in blood flow restricted to the dorsomedial medulla induce a pressor response in rats. J Hypertens 2011;29:1536–1545. doi: 10.1097/HJH.0b013e3283484106 [DOI] [PubMed] [Google Scholar]
- 190.Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 2005;288:R846–R855. doi: 10.1152/ajpregu.00474.2004 [DOI] [PubMed] [Google Scholar]
- 191.Saleeba C, Dempsey B, Le S, Goodchild A, McMullan S. A Student’s Guide to Neural Circuit Tracing. Front Neurosci 2019;13:897. doi: 10.3389/fnins.2019.00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shoemaker JK, Badrov MB, Al-Khazraji BK, Jackson DN. Neural Control of Vascular Function in Skeletal Muscle. Compr Physiol 2015;6:303–329. doi: 10.1002/cphy.c150004 [DOI] [PubMed] [Google Scholar]
- 193.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2 [DOI] [PubMed] [Google Scholar]
- 194.Lincevicius GS, Shimoura CG, Nishi EE, Oliveira T, Cespedes JG, Bergamaschi CT, Campos RR. Differential effects of renal denervation on arterial baroreceptor function in Goldblatt hypertension model. Auton Neurosci 2017;208:43–50. doi: 10.1016/j.autneu.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 195.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 2009;514:518–532. doi: 10.1002/cne.22025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


