SUMMARY
Fluorescent RNA aptamers have been used in cells as biosensor reporters and tags for tracking transcripts. Recently, combined SELEX and microfluidic fluorescence sorting yielded three aptamers that activate fluorescence of TO1-Biotin: Mango-II, Mango-III, and Mango-IV. Of these, Mango-IV was best at imaging RNAs in both fixed and live mammalian cells. To understand how Mango-IV achieves activity in cells, we determined its crystal structure complexed with TO1-Biotin. The structure reveals a domain-swapped homodimer with two independent G-quadruplex fluorophore binding pockets. Structure-based analyses indicate that the Mango-IV core has relaxed fluorophore specificity, and a tendency to reorganize binding pocket residues. These molecular properties may endow it with robustness in the cellular milieu. Based on the domain-swapped structure, heterodimers between Mango-IV and the fluorescent aptamer iSpinach, joined by Watson-Crick base-pairing, were constructed. These exhibited FRET between their respective aptamer-activated fluorophores, advancing fluorescent aptamer technology towards multi-color, RNA-based imaging of RNA coexpression and colocalization.
Graphical Abstract
IN BRIEF
Mango-IV is a fluorogenic RNA aptamer found to be active in both live and fixed cells. Trachman et al. describe the X-ray crystal structure of Mango-IV, revealing a domain swapped homodimer. The structure of Mango-IV is used to engineer a heterodimeric FRET pair with the fluorescent iSpinach aptamer.
INTRODUCTION
Fluorescence turn-on aptamers (Strack and Jaffrey, 2013; Trachman and Ferré-D'Amaré, 2019; Trachman et al., 2017a) are in vitro selected RNAs that bind and activate conditionally fluorescent small molecules. They have been employed as genetically encoded tags for visualizing and tracking RNA in live cells, and reporter modules for biosensors (Kellenberger et al. 2013; Paige et al. 2012). One of the first fluorescent aptamers, Mango-I (Dolgosheina et al., 2014), was selected (Ellington and Szostak, 1990; Tuerk and Gold, 1990) to bind the fluorophore TO1-Biotin (Figure 1A) with high affinity so as to enable imaging of low copy number RNAs. The selection yielded an aptamer of exceptional affinity (Kd ~ 3 nM); however, fluorescence was limited by a quantum yield (Φ) of 0.14. The X-ray crystal structure of Mango-I in complex with TO1-Biotin revealed that the benzothiazole (BzT) and methylquinoline (MQ) heterocycles of the fluorophore were twisted by 52° about the methyne linker. Yet, a coplanar conformation should maximize Φ (Trachman et al., 2017b).
Figure 1.
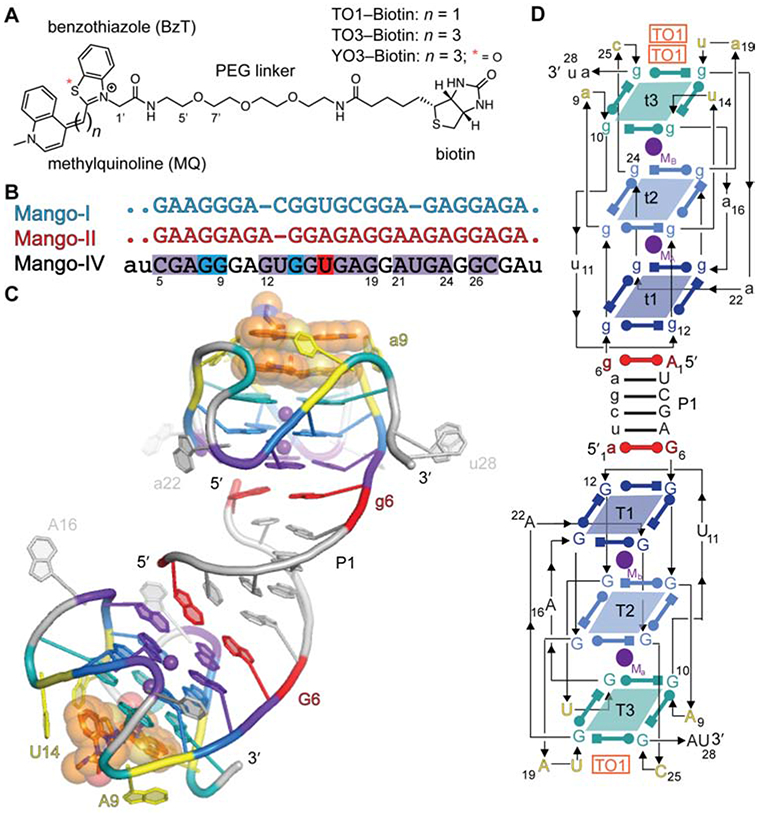
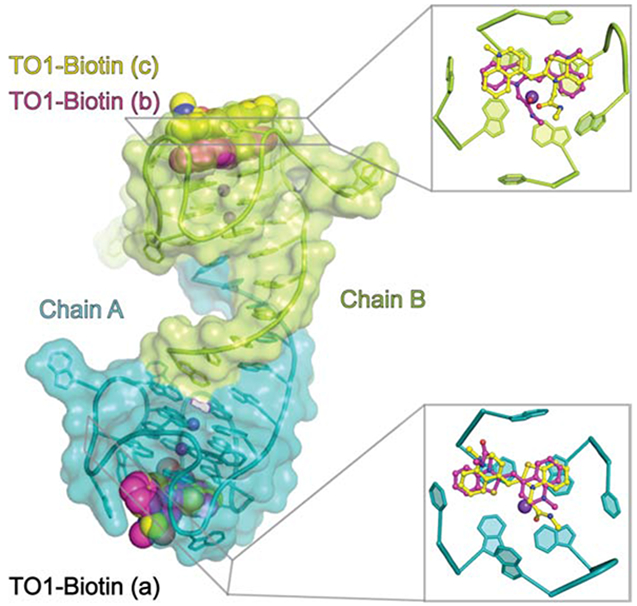
Overall structure of the Mango-IV-TO1-Biotin complex. (A) Chemical structures of TO1-Biotin, TO3-Biotin, and YO3-Biotin. (B) Sequence alignment of Mango-I, Mango-II, and Mango-IV. Residues identical between Mango-IV and Mango-I, Mango-II, or both are highlighted (blue, red and purple respectively). Capital letters denote conserved regions while period (.) and lowercase letters denote variable nucleotides for Mango variants, and Mango-IV, respectively. (C) Cartoon representation of the three-dimensional structure. Nucleotides of protomers A and B are labeled in upper- and lower-case, respectively. Translucent spheres depict Van der Waals surfaces of the bound fluorophores. (D) Secondary structure. Thin lines with arrowheads denote connectivity. Base pairs are represented with Leontis-Westhof symbols (Leontis and Westhof 2001). Approximate locations of metal ions (Mx) and fluorophores (TO1) are indicated.
To search for brighter variant aptamers, a functional selection using fluorescence-activated microfluidic droplet sorting was performed on the final pool of the Mango-I selection. This resulted in three new fluorescent aptamers, Mango-II, Mango-III, and Mango-IV (Autour, et al. 2018). In addition to maintaining high affinity for TO1-Biotin (1 nM < Kd < 11 nM), the new Mango aptamers demonstrated larger Φ. Mango-II has the highest affinity for TO1-Biotin (Kd ≤ 1 nM) while Mango-III is the brightest (Φ = 0.56). Despite having the lowest affinity for TO1-Biotin (Kd = 11 nM) and the second brightest fluorescence (Φ = 0.41), Mango-IV exhibited the greatest brightness in live mammalian cells, being resolved as distinct foci when transcribed as either a 5S or U6 RNA fusion. Statistical analysis of photobleaching indicated that as few as four Mango-IV molecules could yield an observable fluorescent focus, thus nearing single-fluorophore imaging of tagged RNAs in cells (Autour et al., 2018).
Why Mango-IV exhibits superior activity in live cells is not apparent from sequence comparison with Mango-I and Mango-II, with which it is 70-80% identical in the conserved core (Figure 1B; Mango-III is more divergent). To provide a structural framework for understanding its robust fluorescence activity in cells, and as a starting point for structure-guided engineering, we have now determined the co-crystal structure of Mango-IV in complex with TO1-Biotin. Two molecular properties of Mango-IV uncovered in our studies, an open fluorophore binding site and a propensity to adopt multiple folds, may account for the usefulness of this aptamer in cellular studies. Unexpectedly, but consistent with the results of hydrodynamic analyses, Mango-IV forms a domain-swapped homodimer. The domain-swapped structure of Mango-IV is thus far unique among fluorescence turn-on aptamers, and allowed us to engineer heterodimerizartion with the iSpinach fluorescent aptamer (Autour et al., 2016; Fernandez-Millan et al., 2017). The heterodimer exhibits substantial Förster resonance energy transfer (FRET) between its two bound, aptamer-activated fluorophores, and opens the way to designer multi-color imaging tools for tracking higher-order RNA interactions.
RESULTS
Overall Structure of Mango-IV
An RNA construct comprising the conserved 25-nt Mango-IV sequence (Figure 1B) flanked by three nucleotides (two on 5′ end, one on 3′ end) presumed to form two base pairs extending a terminal helix was co-crystalized with TO1-Biotin (Figure 1A). Co-crystals were also obtained with RNAs in which U2 and U11 were replaced with 5-bromouracil (5BrU). The structure was solved at 2.8 Å resolution by the single anomalous dispersion (SAD) method. Strong anomalous features were observed for 5BrU2 (Supplementary Figure 1).; the nucleobase of U11 is mostly disordered with an anomalous signal located adjacent to C5′ of U11. We were unable to model this feature, which may reflect radiation damage or multiple conformations that are not resolved at the current resolution. Refinement against a native dataset yielded the current 2.4 Å resolution model (Materials and Methods; Supplementary Table 1).
The crystallographic asymmetric unit contains two Mango-IV aptamers forming a domain-swapped homodimer that is linked through the intermolecular P1 helix (Figure 1C, D). P1 is formed by the first six nucleotides of the sequence. Residues 2 through 5 make canonical Watson-Crick pairs, while residues 1 and 6 form two A•G pairs, one at each end of P1. The core residues of each Mango-IV fold into a three-tiered G-quadruplex, coordinated with two axial K+ ions. The G-quadruplexes stack coaxially on the terminal A•G pairs of P1. The guanine residues of the four guanine tracts of the two duplex-proximal G-quartets are consecutive; therefore, these two tiers (T1 and T2) are parallel. T3 is anti-parallel to T1 and T2, because its four guanines are each in a propeller loop connecting consecutive guanine tracts, after the RNA chain has reversed direction (Figure 1C, D). Five unpaired propeller loop nucleobases surround the fluorophore-binding face of the G-quadruplex.
Fluorophore Binding Sites of Mango-IV
Unbiased residual electron density corresponding to the benzothiazole (BzT), methylquinoline (MQ), and a short segment of the polyethylene glycol (PEG) linker was observed for three distinct TO1-Biotin fluorophores in the Mango-IV homodimer (Figure 2A). RNA chains A and B bind to one and two fluorophore molecules, respectively. The TO1-Biotin bound to chain A (TO1A) is observed in two distinct, mutually exclusive, conformations. In the first conformation, MQ and BzT stack on G26 and G10, respectively, while in the second, the heterocycle stacking order is reversed (Figure 2B,C). In both poses, the fluorophore headgroup is modestly twisted about the methyne linker (by 30 and 25 degrees, respectively), and thus slightly out of the mean nucleobase plane of T3.
Figure 2.
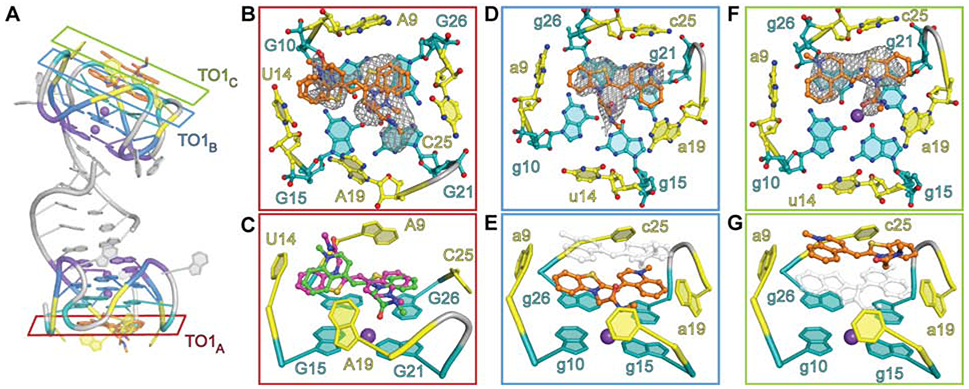
TO1-Biotin recognition by Mango-IV. (A) Location of the three bound fluorophores in the homodimer crystal structure. (B) Close-up of TO1A cross section from panel (A) outlined in red. Ligand binding pocket is shown as ball-stick representation. (C) 75° rotation of panel B in cartoon representation. Two conformers are modeled purple and green. (D) Close up of TO1B cross section from panel (A) outlined in blue. Ligand binding pocket is shown as ball-stick representation. (E) 75° rotation of panel (D) in cartoon representation. (F) Close up of TO1C cross section from panel (A) outlined in green. Ligand binding pocket is shown as ball-stick representation. (G) 75° rotation of panel (F) in cartoon representation. Panels depict ligand binding pocket superimposed on the ∣Fo∣-∣FC∣ electron density map, contoured at 1.5 σ, prior to placement of the fluorophore in the model.
The two fluorophores bound to chain B of the Mango-IV dimer (TO1B and TO1C) are each in a unique conformation (Figure 2A, D-G). The BzT and MQ groups of TO1B stack on g26 and g21 (chain B nucleotides are denoted by lower-case letters) of T3. TO1C stacks on TO1B such that its BzT overlies the MQ of TO1B and vice versa. TO1C makes van der Waals interactions with TO1B and the nucleobases of propeller loop residues a9, c25, and a19 (Figure 2F, G). Electron density was not observed within 5.3 Å of TO1C in the native dataset (Supplementary Figure 2A), although we cannot exclude contacts made by crystallographically disordered moieties. The lower resolution anomalous dataset did reveal a weak positive feature along a symmetry axis in both 2∣Fo∣-∣Fc∣ and ∣Fo∣-∣Fc∣ difference maps (peak heights of 2 and 3 σ, respectively) Noise is exacerbated at special positions, and efforts to model these features were unsuccessful. Job plot analysis shows that, in solution, the TO1-Biotin:Mango-IV stoichiometry is 1:1.4 (Supplementary Figure 2B), suggesting a substantial binding-inactive aptamer population. Given that fluorophore stacking has the potential to quench fluorescence, stacked dimers likely do not exist in solution.
Oligomeric State of Mango-IV
The sequence-variable P1 helix is minimized in our crystallization construct. It is possible that the dimerization and domain swapping observed crystallographically are caused by the shortened P1. We examined by size-exclusion chromatography (SEC) four Mango-IV constructs with 5′ and 3′ complementary sequences of 3, 6, and 8 nucleotides (hereafter Mango-IV3, Mango-IV6 and Mango-IV8, respectively), as well as a construct consisting only of the Mango-IV core sequence, residues 3-27 (Mango-IVcore). When loaded at an RNA concentration of 35 μM after thermal annealing (STAR Methods), Mango-IV6 and Mango-IV8 exhibit a major species with an elution volume (Ve) presumed to be, and consistent with, that of a dimer. Smaller chromatographic peaks corresponding to monomer and unfolded species are also present (Figure 3A). The non-conserved region of Mango-IV8 has low self-complementarity (only 4 consecutive canonical base pairs; STAR Methods KEY RESOURCES TABLE), suggesting that dimerization can occur through mechanisms other than the formation of P1 by reciprocal Watson-Crick base pairing. We isolated the presumed monomer and dimer Mango-IV8 peaks by SEC and incubated the fractionated RNAs at room temperature for 15 minutes. When re-analyzed by SEC, the monomer fraction had formed an additional higher molecular weight species (Ve = 10 ml), while the dimer fraction exhibited a dominant species eluting at 10.9 ml, comparable to its initial Ve (Supplementary Figure 3). It should be noted that these two elution volumes and the populations of species are not identical to the chromatograms after thermal annealing and suggests that the population is not yet at equilibrium after a 15 minute incubation at room temperature. SEC analysis shows that Mango-IV6 has a larger fraction of dimeric species than Mango-IV8 (Figure 3A). Under the same conditions, Mango-IVcore elutes as a mixture of monomer (Ve = 14.8 ml) and dimer (Ve = 12.6 ml), with a preponderance of the former (Figure 3A). Mango-IV3 exhibited primarily a monomeric species (Figure 3B). Overall, the results of SEC analysis suggest that dimerization is at least in part a result of intermolecular interactions between conserved core nucleotides of Mango-IV, and can be modulated by the sequence and complementarity of P1.
Figure 3.
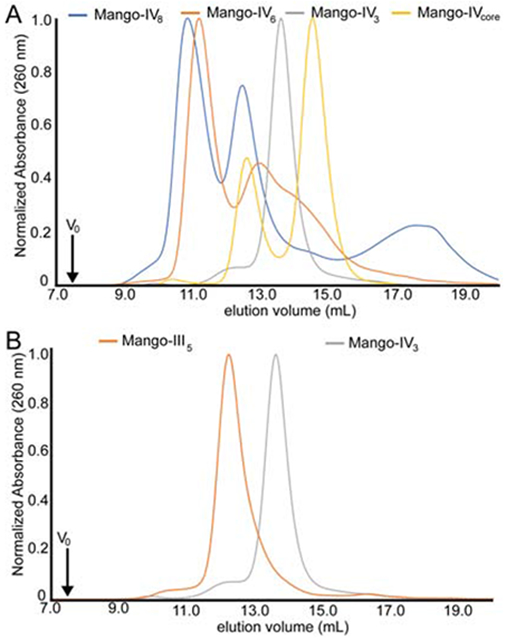
Size-exclusion chromatography (SEC) of Mango-IV variants. (A) SEC of Mango-IV constructs that can form P1 helices with 0 (core), 3, 6 and 8 base pairs (V0, void volume). (B) SEC of the Mango-IV3 and the monomeric Mango-III aptamer.
Mango-IV6 and Mango-IV8 were further examined by constant velocity analytical ultracentrifugation (AUC). Sedimentation coefficients could be determined for multiple species at concentrations ranging from 0.1 μM – 1.0 μM (detected by absorbance at 260 nm), and from 2.5 μM - 7.5 μM (absorbance at 295 nm; Figure 4; Supplementary Figure 4). This analysis shows that both Mango-IV6 and Mango-IV8 exhibit concentration-dependent dimerization, even at these relatively low concentrations (Figure 4A; Supplementary Table 2). Dimerization was not affected by the presence of TO1-Biotin.
Figure 4.
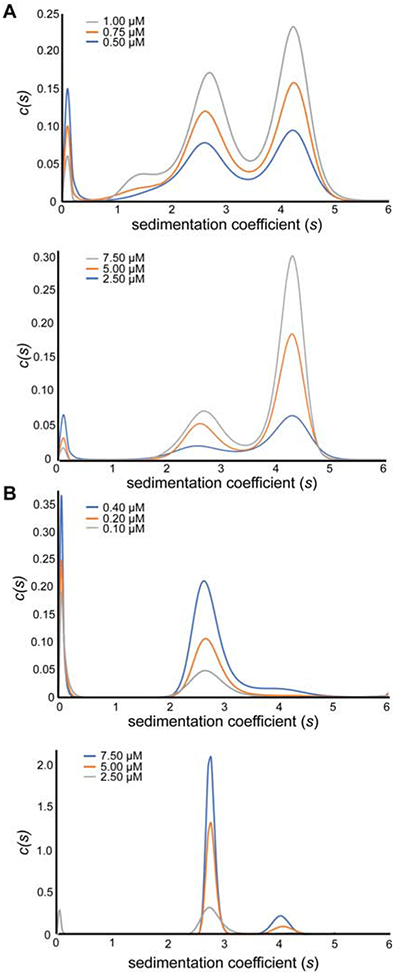
Sedimentation velocity analytical ultracentrifugation (AUC) of Mango-IV6. (A) Non-normalized c(s) distributions of the Mango-IV6 aptamer determined at 260 nm absorbance (top) or 295 nm absorbance (bottom) at three concentrations each, in the presence of TO1-Biotin. (B) Non-normalized c(s) distributions of the Mango-IV6 Δ27 aptamer determined at 260 nm absorbance (top) or 295 nm absorbance (bottom) at three concentrations each, in the presence of TO1-Biotin.
To test how dimerization is affected by the conserved nucleotides that connect the Mango-IV G-quadruplex core to the non-conserved 5' and 3' P1-forming sequences, RNAs bearing mutations to nucleotides 3, 4, and 27 were analyzed by AUC. The corresponding residues in the sequence alignment (Figure 1B) with the monomeric fluorescent aptamers Mango-I (Trachman et al., 2017b) and Mango-II (Trachman et al., 2018) fold into a split GAA^A tetraloop-junction (^ denotes the insertion point of the Mango-I and II G-quadruplexes). Although Mango-IV residues 3, 4, 5 and 27 might fold into a CGA^A tetraloop junction, this sequence does not conform to either the GNRA or UNCG (N = any residue, R= purine) tetraloop consensus. To examine if Mango-IV has a propensity to form a tetraloop junction, we determined the oligomerization state of its C3G, G4A double mutant (Mango-IVGAAA) by velocity AUC (Supplementary Table 2). Normalized c(S) traces reveal that Mango-IVGAAA forms both monomer and dimer populations, suggesting that the monomeric fold of Mango-I and Mango-II are not exclusively a result of the split GAA^A tetraloop-junction. To further assess the importance of the connecting sequences for oligomerization, A27 was deleted, and this mutant (Mango-IVΔ27) analyzed by AUC (Figure 4B). Mango-IVΔ27 exists almost exclusively as a monomer, with only a small dimer population present at concentrations as high as 7.5 μM. Thus, Mango-IV can be turned into a monomer by mutating a residue immediately 3' to its G-quadruplex core. Overall, these results are consistent with dimerization being an intrinsic property of the core sequences of Mango-IV and the immediately adjacent residues, independent of (but modulated by) P1.
A Guanine-rich Segment Results in Frustrated Folding
The five-nucleotide segment that forms the first guanine tract of the three-tiered Mango-IV G-quadruplex (residues 6-10) contains four guanines. This could give rise to alternative folds, either forming the quadruplex with the consecutive G6, G7 and G8, or by shifting register, with G7, G8, and G10. The consecutive and shifted registers are analogous to the G-quadruplex structures of Mango-I (Figure 5A, left) and Mango-II (Figure 5A, right), respectively. The structure of this segment of Mango-IV more closely resembles that of Mango-II than Mango-I, despite having higher core sequence identity to the latter (70% and 80%, respectively). The Mango-II-like register in the crystal structure is not consistent with the results of Mango-IV dimethylsulfate (DMS) probing (Autour et al., 2018), in which G10 (crystal structure numbering scheme) was reactive, and judged to be unpaired. Given that multiple conformations and oligomeric states are observed for Mango-IV in solution (Figure 3, 4), it is possible that the crystal structure and the chemical probing are reporting on subpopulations with different folds.
Figure 5.
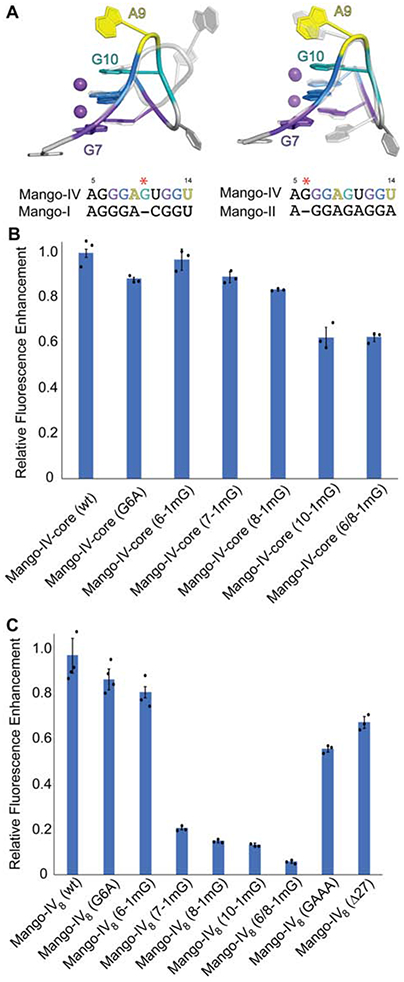
Structural comparison and mutational analysis of the first guanine tract of Mango-IV. (A) Overlay of first guanine tract of Mango-IV (colored) with Mango-I (grey transparent, left) and Mango-II (grey, right). Sequence alignments of first guanine tract region are shown below each overlay. Red asterisk indicates location of guanine insertion for the Mango-IV sequence. (B) Fluorescence enhancement of the Mango-IVcore sequence and mutants to the first guanine tract. Residue numbering is consistent with nucleotide positions of crystalizing construct depicted in panel A. (C) Fluorescence enhancement of the Mango-IV8 aptamer and mutants to the first guanine tract and helical linker. Residue numbering is consistent with nucleotide positions of crystalizing construct depicted in panel A. Data are represented as the mean ± SEM.
To test the contribution of residues 6, 7, 8 and 10 to function, we site-specifically replaced these guanines with 1-methylguanine (1mG) in the Mango-IVcore and Mango-IV8 constructs. For Mango-IVcore, 1mG incorporation was most detrimental at position 10, while it was least disruptive at position 6. Incorporation at positions 7 and 8 had intermediate effects (Figure 5B). Incorporation of 1mG into Mango-IV8 positions 7, 8 and 10 resulted in more pronounced effects, while the effect was again muted at position 6 (Figure 5C). This gradation of severity of G-quartet disruption from T3 to T1 is consistent with the Mango-IV crystal structure, in which G10 stacks directly under the MQ of TO1-Biotin (Figure 2B). Taken together, the results of 1mG incorporation indicate that, in the absence of a register-imposing P1 helix, mutations of single nucleotides in the first G-tract of Mango-IV can be compensated for by refolding of the core into the alternative register.
Mango-IV Accommodates Fluorophores with Extended Conjugation
Like Mango-II, Mango-IV has an open binding pocket in which 'flap' nucleobases do not stack on top of the fluorophore heterocycles (on the face opposite to the G-quadruplex). This contrasts with Mango-I and Mango-III, in which propeller loop nucleotides form either unpaired flaps, or a tertiary base pair, respectively, that stack on and restrain the MQ and BzT of TO1-Biotin. To compare the fluorophore selectivity and fluorescence enhancement of the structurally diverse Mango aptamer binding pockets, their ability to promote fluorescence of several variant fluorophores was examined (Figure 6). This shows that the fluorophores with propenate (three-carbon) conjugated linkers (TO3-acetate, TO3-Biotin, and YO3-Biotin) are better accommodated by Mango-II and Mango-IV, the RNAs with open binding pockets. Conversely, Mango-III(A10U) is remarkably adept at enhancing fluorescence of TO1-Biotin and derivatives with methyne (one carbon) conjugated linkers. However, its tertiary U•U base pair, which stacks on the MQ and BzT groups of TO1-Biotin, is less adept at restraining and turning on the larger fluorophores of TO3 and YO3. Among the known Mango aptamers, Mango-IV achieves the greatest fluorescence turn-on of TO3-Biotin and YO3-Biotin.
Figure 6.
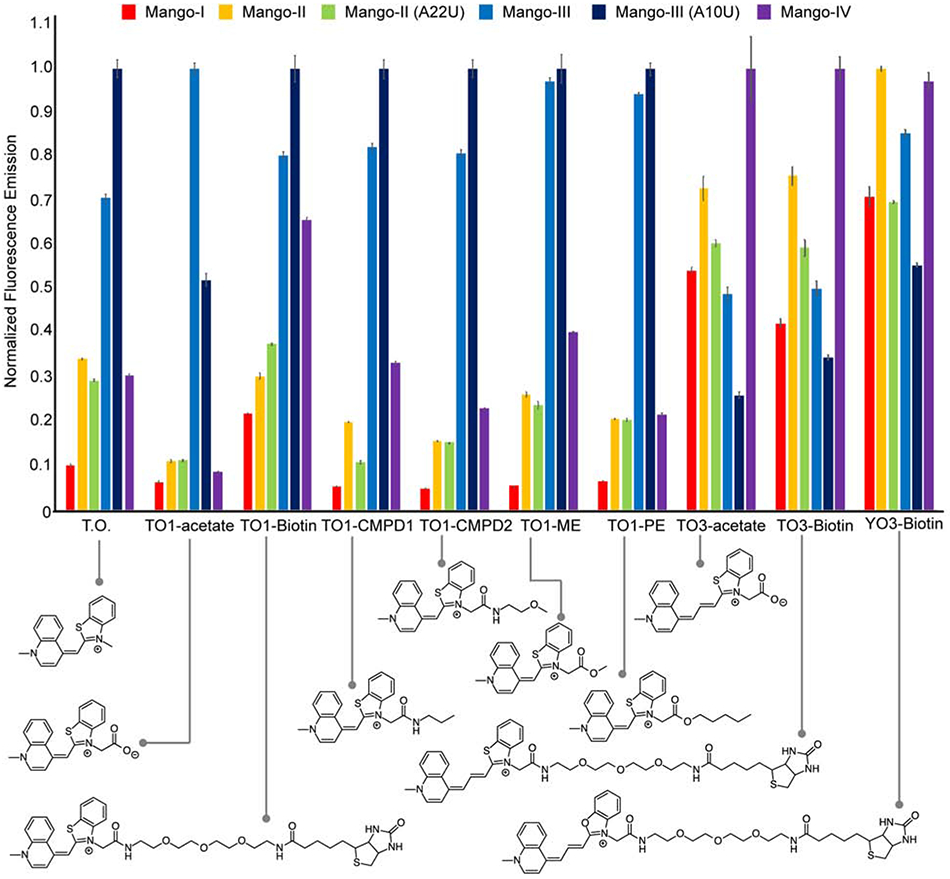
Normalized fluorescence emission of cyanine dye derivatives by Mango aptamers. Fluorescence emission is normalized to the brightest RNA-dye complex for each fluorophore. Fluorescence emission is shown for Mango-I (red), Mango-II (yellow), Mango-II-A22U (green), Mango-III (blue), Mango-III-A10U (navy), Mango-IV (purple). Chemical structures of fluorophores are shown below the graph. Data are represented as the mean ± SEM.
An Aptamer FRET Pair Based on a Mango-IV Heterodimer
Previously, a fluorescence turn-on aptamer-based FRET system was reported in which Spinach and Mango-I were held together, intramolecularly, using an RNA origami scaffold (Jepsen et al., 2018). The domain-swapped homodimeric architecture of Mango-IV suggested that this aptamer could form the basis of an intermolecular FRET pair that associates through sequence-specific base pairing in P1. Such a FRET aptamer would be able to report, for instance, on the co-localization of two RNAs bearing complementary single-stranded RNA segments that hybridize to form P1.
The emission spectrum of DFHBI-1T (Song, et al. 2014), a small-molecule analog of the intrinsic fluorophore of green fluorescent protein (GFP), bound to the optimized aptamer iSpinach (Autour et al., 2016; Fernandez-Millan et al., 2017) overlaps substantially with the absorption spectrum of the oxazole-containing fluorophore YO3-Biotin bound to Mango-IV (Figure 7A). We truncated the 3' terminus of iSpinach and made its resulting 5' single-stranded sequence complementary to a 5' unpaired sequence on Mango-IV (also with a truncated 3' terminus), reasoning that annealing of the two RNAs would result in an intermolecular aptamer FRET pair (Figure 7B). Heterodimeric FRET pairs were designed with P1 helices with 10, 11 and 12 base pairs.
Figure 7.
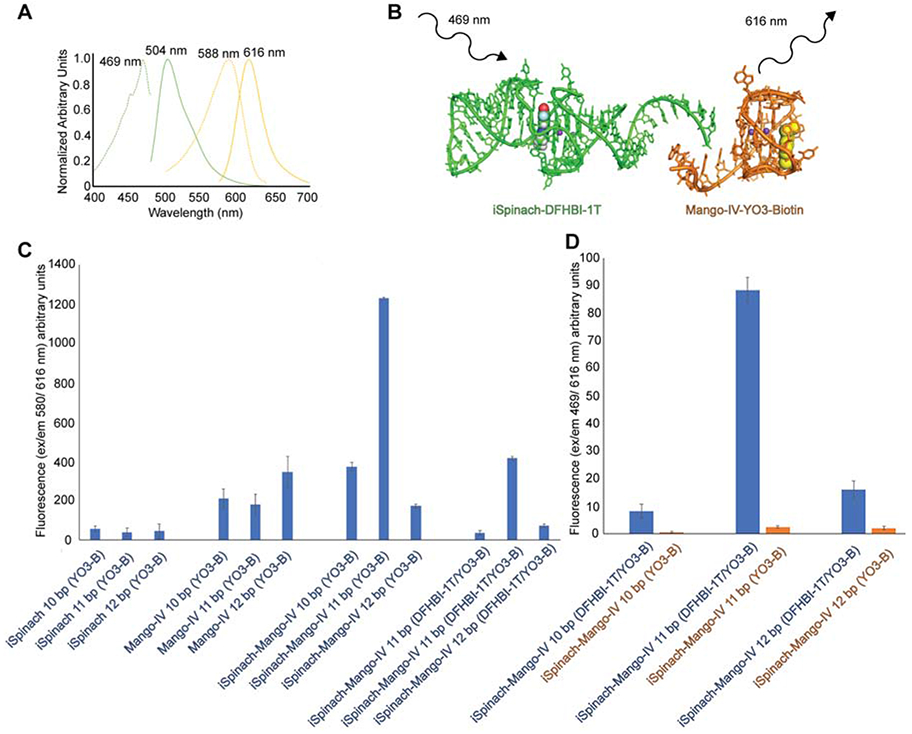
Design of an aptamer FRET reporter using Mango-IV. (A) Excitation (dashed lines) and emission (solid lines) of the iSpinach-DFHBI-1T (green) and Mango-IV-YO3-Biotin complexes (yellow). (B) iSpinach and Mango-IV are joined through Watson-Crick base pairing of complementary, single-stranded 5' extensions. The iSpinach-bound DFHBI-1T is excited, donating energy to the Mango-IV-bound YO3-Biotin. (C) Fluorescence of YO3-Biotin (excitation: 580 nm; emission: 616 nm) in the presence of iSpinach, Mango-IV, and iSpinach-Mango-IV chimeras, with 10-12 complementary base pairs. Fluorophore(s) present in solution are in parentheses. (D) Fluorescence emission (excitation: 469 nm; emission: 616 nm) of iSpinach-Mango-IV complexes in the presence of DFHBI-1T and YO3-Biotin (blue) or just YO3-Biotin (orange). Data are represented as the mean ± SEM.
To determine whether the 3'-truncated Mango-IV constructs retain YO3-Biotin activation, and to examine cross-activation of this fluorophore by our iSpinach constructs, YO3-Biotin fluorescence was measured for iSpinach, Mango-IV, and the iSpinach-Mango-IV chimeras (Figure 7C). Consistent with previous reports of limited selectivity of Spinach (Jeng et al., 2016), the truncated iSpinach constructs activate YO3-Biotin, but more weakly than the truncated Mango-IV constructs. The 10 and 11 base-pair iSpinach-Mango-IV chimeras activate YO3-Biotin more than either of their constituents alone, suggesting that formation of P1 stabilizes the fluorescent cores of the aptamers. Consistent with this interpretation, measurement of YO3-Biotin activation by the chimeric RNAs in the presence of DFHBI-1T (which presumably outcompetes YO3-Biotin from iSpinach) shows reduction in emission, compared to YO3-Biotin alone. When we determined FRET between the two fluorophores in the presence of the chimeras by illuminating at the excitation peak for DFHBI-1T, the 11-bp variant exhibited over 20% of the fluorescence emission measured by directly exciting at the DFHBI-1T absorption maximum (Figure 7D), demonstrating activation through intramolecular FRET in the heterodimeric aptamer complex. In the presence of both fluorophores, the 10 and 12 bp chimeras have minimal YO3-Biotin fluorescence when illuminating at DFHBI-1T peak. The rather modest FRET signal observed for the 11 bp chimera is likely due to a mixture of heterodimers and homodimers. The minimal FRET signal reported by the 10 bp and 12 bp chimeras are likely a result of misfolding and or misalignment of dipole moments, which can reduce FRET upon a single nucleotide addition (Jepsen, et al. 2018). However, the FRET signal observed for all chimeras is dependent on the presence of DFHBI-1T, and therefore derived from active heterodimers (Figure 7D).
DISCUSSION
When compared to the other Mango aptamers, three structural features of Mango-IV are noteworthy. First is the connectivity of its G-quadruplex, which results in an open fluorophore binding pocket. Mango-I, Mango-II and Mango-IV all have 3-tiered G-quadruplex cores, but the fluorophore-proximal G-quartets (Tier 3) of the latter two aptamers are fully anti-parallel to their two other G-quartets (Tiers 1 and 2). Tier 3 of Mango-I is partially parallel, yielding an asymmetric, partially constrained ligand binding site (Trachman et al., 2017 b). Consistent with the open pockets of Mango-II and Mango-IV, their bound fluorophores exhibit multiple poses in their respective crystal structures. In contrast, Mango-I binds to one conformation of TO1-Biotin. (Mango-III has a two-tiered G-quadruplex (Trachman et al., 2019a), a fluorophore binding site overlaid by a trans Watson-Crick base pair, and is not directly comparable). Second, Mango-IV lacks a junction connecting its P1 duplex and G-quadruplex cores. Mango-I and Mango-II both link P1 and their G-quadruplex using a GAA^A tetraloop-like junction. Both Mango-III and iMango-III (Trachman et al., 2019b) use base triples to coaxially stack P1 helices and G-quadruplexes. Mango-IV has no analogous structural element between P1 and the G-quadruplex, except a Watson-Crick A•G pair, that at least in our crystal structure, results from domain swapping. Third, Mango-IV is a domain-swapped dimer, whereas all three other Mango aptamers are monomeric.
While most structurally characterized fluorescence turn-on aptamers are monomeric, Corn (Song et al. 2014), which was selected to bind DFHO (a small molecule analog of the red fluorescent protein fluorophore) was found to be a homodimer (Warner et al., 2017). Corn binds one molecule of its cognate fluorophore at its dimer interface, which lacks any inter-protomer base-pairing. DFHO makes contact with both protomers of the Corn homodimer, each of which contributes a G-quadruplex to forming the ligand-binding pocket. The Mango-IV homodimer, in contrast, assembles through a Watson-Crick base-paired A-form duplex, and has two independent fluorophore binding sites. Our hydrodynamic analyses show that unlike the extremely stable Corn homodimer (Kd < 1 nM), Mango-IV partitions into monomer and dimer at micromolar concentration, and associates reversibly in a concentration-dependent manner. Whereas the Corn residues that form its interfacial fluorophore binding site also participate in DFHO binding, and therefore their mutation affects both, dimerization and fluorescence turn-on (Warner et al., 2017; Sjekloća and Ferré-D'Amaré, 2019), the interprotomer interface of the Mango-IV is distant from its TO1-Biotin binding sites, and therefore can be mutated without directly affecting fluorophore activation (although we found that mutations in P1 can destabilize the RNA, possibly through formation of an altered G-quadruplex).
Two molecular properties of the Mango-IV aptamer uncovered by our studies may explain its effectiveness for imaging RNAs in cells. First, its open fluorophore binding pocket, while exhibiting limited selectivity, is likely less sensitive to its precise cellular context and the manner in which the aptamer is tagged onto the RNA that is being visualized, as perturbation to the nucleotides surrounding the binding pocket is less likely to affect fluorophore activation, contrary to what would result if those nucleotides interacted intimately with the fluorophore (as in Mango-III). Second, the propensity of the Mango-IV core to adopt alternative folds, possibly due to frustrated folding, while a liability in biophysical studies, may be advantageous for an in vivo RNA tag. This plasticity may allow the aptamer to sample multiple, fluorescently active, conformations and folding schemes in the cellular milieu, even if it is placed in an RNA context where a substantial fraction of the Mango-IV tag initially misfolds. Overall, the effectiveness of Mango-IV as an imaging tag in cells despite its apparently suboptimal molecular properties points to the importance of selecting molecular tags for their intended cellular context, and not simply for optimal performance in vitro.
The ability to observe simultaneously the location and interactions of different RNAs is important for elucidating higher-order RNA assembly in cells. Numerous biological processes such as splicing (Crick, 1979), translational initiation and ribosome assembly (Hinnebusch and Lorsch, 2012), and stress granule formation (Van Treeck and Parker, 2018) rely upon co-localization and complex formation between multiple RNA species. Split fluorescent aptamers (Alam et al. 2017; Rogers et al., 2015; Warner et al., 2014), which upon strand hybridization form a single fluorophore binding pocket to induce fluorescence, have been engineered to probe RNA colocalization. However, split aptamers require that long sequences of unstructured RNA (up to 45 nucleotides) be genetically fused to the RNA of interest. These long, unstructured segments may induce misfolding of the native RNA component and have the potential to artificially over stabilize RNA complexes. The heterodimeric iSpinach-Mango-IV FRET pair has the benefit of requiring as few as eleven nucleotides flanking the 5′ aptamer cores, thus reducing the potential to misfold and providing a simple platform to engineer dimerization through complementary strand annealing. Since fluorescent aptamer heterodimers form two distinct binding pockets, they provide the added benefit of substituting fluorophore components in a “plug and play” manner to optimize FRET. By combining the minimal fluorescence background of a GFP derived fluorophore (DFHBI-1T) with the bright, red-shifted emission of a cyanine dye derivative (YO3-Biotin), a unique fluorescence signal can be generated at a desirable emission wavelength. Further optimization of this intramolecular FRET pair may yield improvements in folding behavior, as well as additional fluorophore combinations.
STAR♦ METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact: Adrian R. Ferré-D’Amaré.
Materials Availability
This study did not generate new unique reagents
Data and Code Availability
Structure coordinates generated during this study are available at the Protein Data Bank under accession codes 6V9B and 6V9D.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
No experimental models and subjects were used in this study
METHOD DETAILS
RNA preparation
RNA constructs used in this study are listed in Key Resources Table. RNAs 1-2, 5-7, and 10-21 were chemically synthesized (Dharmacon), deprotected according the manufacturer's instructions, and purified by electrophoresis on 14% polyacrylamide (19:1 acrylamide/bisacrylamide), 1xTBE, 8M Urea gels; electroeluted from gel slices; washed once with 1M KCl; desalted and exchanged into 20 mM MOPS-KOH pH 7.0, 150 mM KCl and 10 μM EDTA through centrifugal ultrafiltration (3,000-Da cutoff, Millipore), filtered (0.1 μm cutoff, Amicon Ultrafree-MC, Millipore); and stored at 4°C. RNAs 3-4, 8-9, 22-33 were in vitro transcribed as described (Trachman et al., 2017 b); purified by electrophoresis on 14% polyacrylamide (19:1 acrylamide/bisacrylamide), 1xTBE, 8M Urea gels; electroeluted from gel slices; washed once with 1M KCl; desalted by ultrafiltration, filtered (0.1 μm cutoff, Amicon Ultrafree-MC, Millipore) and stored at 4°C. In vitro transcription was performed using recombinantly expressed and purified T7 RNA polymerase in 70 mM Tris•HCl pH 7.5, 50 mM MgCl2, 2 mM DTT, 0.01 % Triton, 2.5 mM each trinucleotide (ATP, GTP, CTP, and UTP).
Fluorophores
TO1-Biotin, TO3-Biotin, TO1-Acetate, TO1-CMPD1, TO1-CMPD2, and TO3-Acetate were synthesized at a purity of >95% using synthetic methods as described previously (Dolgosheina et al., 2014). Briefly, the acetate forms of TO1 and TO3 were synthesized from the metylquinolinium and benzotiazole groups. For TO1-Biotin and TO3-Biotin, PEG-Biotin was covalently attached to the fluorophore by nucleophilic attack of a primary amine. TO1-CMPD1 and TO1-CMPD2 were synthesized from TO1-Acetate by nucleophylic attack of a primary amine on propyl and ethyl esther substituents, respectively. TO1-ME and TO1-PE were synthesized and purified by HPLC as described previously (Trachman et al., 2018). Briefly, these compounds were synthesized from the TO1-Acetate starting material using a Williamson reaction. Alkylation was performed from 1-Iodo reagents. All chemicals were purified by reverse-phase HPLC using a C18 column at a flow rate of 1ml/min in an aqueous buffer of 50 mM ammonium acetate. A linear gradient of 0% to 100% acetonitrile was run for 60 minutes.
Crystallization and diffraction data collection
Mango-IV-TO1-Biotin (RNA 1) in 20 mM MOPS-KOH pH 7.0, 150 mM KCl, 10 μM EDTA was heated to 95°C for 3 minutes, incubated at RT for 10 minutes, mixed with equimolar TO1-Biotin, then incubated at RT for 30 minutes. For crystallization, 0.2 μl of RNA solution (300 μM) and 0.2 μl reservoir solution (0.1 M Tris-HCl pH 8.5, 0.01 M CoCl2, 1.65-1.75 M NH4SO4, 0.01-0.015 M Phenol) were mixed and equilibrated at 294 K by sitting drop vapor diffusion. Hexagonal plate crystals grew in 1-3 days to maximum dimensions of 75 x 75 x 20 μm3. Cryoprotection was performed by supplementing the reservoir solution with 150 mM KCl and 10% glycerol prior to mounting the crystal in a nylon loop and vitrifying by plunging into liquid nitrogen. Data were collected at 100 K at APS 24-ID-C with 1.495 Å X-radiation. Bromine derivatized RNA (RNA 2) for phasing was crystalized under the same conditions as RNA 1. Crystals were soaked in cryoprotectant conditions above supplemented with 10 mM Iridium hexamine for 90 minutes and then vitrifying by plunging into liquid nitrogen. Data sets of iridium-soaked crystals were collected at 100 K at ALS BCSB 5.0.2 using 0.917 Å X-radiation. An anomalous signal corresponding to iridium hexamine was not observed. Data were reduced in XDS (Kabsch, 2010 a; Kabsch, 2010 b) and DIALS (Parkhurst et al. 2016) with 10% of reflections flagged for RFree calculation. Data collection statistics are summarized in Supplementary Table 1.
Structure determination and refinement
Data sets reporting a significant anomalous signal by DIALS were further analyzed for phasing. SHELXC (Sheldrick, 2010) reported significant anomalous signal extending to 2.7 Å from a single crystal. Two heavy atom sites were located by AutoSol (Terwilliger et al., 2009; Terwilliger, 2000) yielding a mean overall figure of merit of 0.304. Density modification using Resolve (Terwilliger, 2000) resulted in an electron density map into which 50 nucleotides could be built manually using Coot. This model was then refined using Phenix.refine (Afonine et al., 2012) and molecular replaced into the native data set (2.4 Å resolution) collected at APS 24-ID-C using the program Phaser (McCoy et al., 2007). The top solution had a TFZ score of 24.1. Manual building and refinement was performed in Coot and Phenix.refine (Afonine et al., 2012), respectively. HL coefficients generated from the Bromine dataset were used as a target during refinement. Electron density for terminal residue U28 was not observed in chain A despite being observed in chain B, thus resulting in a difference in perceived sequence length. Refinement statistics are summarized in Supplementary Table 1. Structure factors and coordinates have been uploaded to the Protein Data Bank under accession codes 6V9B and 6V9D.
Size Exclusion Chromatography
To monitor oligomerization state, RNA samples (RNA 3-6) were refolded in 20 mM MOPS pH 7.0, 150 mM KCl, 10 μM EDTA at 35 μM concentration by heating to 95°C for 3 minutes following incubation at RT for 30 minutes. Samples were run on an ÄKTApurifier at 1 ml/minute over a Superdex 75 Increase size exclusion column (GE Life Sciences) at room temperature. Absorbance was monitored at 260 nm, 280 nm, and 295 nm.
To monitor exchange between oligomerization states, RNA samples (RNA 3-4) were refolded in 20 mM MOPS pH 7.0, 150 mM KCl, 10 μM EDTA at 10 μM concentration by heating to 95°C for 3 minutes following incubation at RT for 30 minutes. Samples were run on an ÄKTApurifier at 1 ml/minute over a Superdex 75 Increase size exclusion column (GE Life Sciences) at room temperature. Absorbance was monitored at 260 nm, 280 nm, and 295 nm. Fractions corresponding to the peak of interest where separated and incubated at room temperature for 15 minutes prior to reanalyzing by ÄKTApurifier using the same method above.
Analytical ultracentrifugation
Analytical Ultracentrifugation was performed on RNAs 3,6,8, and 9 in the presence and absence of TO1-Biotin. RNAs were thermally annealed as previously described and incubated at room temperature for a minimum of 30 minutes prior to analysis. Cells contained Mango-IV RNAs at a concentration range of 0.25-7.5 μM in addition to 20 mM MOPS-KOH, pH 7.0, 150 mM KCl, 10 μM EDTA. Samples were prepared as above. 500 scans were collected and averaged on a Beckman XLI analytical ultracentrifuge. Radial scans were collected on a Beckman XLI analytical ultracentrifuge at a run speed 60,000 r.p.m. and 20°C, using either 260 nm or 295 nm absorbance detection. The viscosity and density of the buffer were estimated as 0.01015 P and 1.0068 g ml−1, respectively, with the Sednterp server (http://rasmb.org/sednterp). c(S) distributions were calculated using SEDFIT assuming a partial specific volume of 0.53 cm3 g−1 (Ramesh et al., 2011).
Fluorescence enhancement of thiazole orange derivatives
Fluorescence measurements were recorded on a Varian Cary Eclipse Spectrofluorimeter set to measure at excitation wavelength of 510 nm (for TO1 dyes), 637 nm (for TO3 dyes), and 595 nm for YO3-Biotin, with emission wavelengths of 535 nm (TO1 dyes), 658 nm (TO3 dyes), and 620 nm (YO3-Biotin). RNA aptamers (M1, M2, M2 A22U, M3, M3 A10U, M4) at 500 nM final concentration were incubated at room temperature for 1 hr with dyes (Thiazole Orange, TO1-Ac, TO1-Biotin, TO1-CMPD1, TO1-CMPD2, TO1-ME, TO1-PE, TO3-Ac, TO3-Biotin) at 10 nM final concentration in Mango selection buffer (140 mM KCl, 1 mM MgCl2, 10 mM NaH2PO4 pH 7.2, 0.05% (v/v) Tween-20).
Fluorescence enhancement of Mango-IV mutants
Fluorescence was measured on a Photon Technologies International/820 Photomultiplier Detection System with excitation and emission tuned to 510 nm and 535 nm, respectively. Initial readings at 0 nm and 500 nM TO1-Biotin were performed in a 0.1 mm cuvette. Prefolded RNA was added to the same cuvette to a final concentration of 2 μM, and allowed to incubate at RT for approximately 5 minutes. The fluorescence signals were acquired and averaged over 60s. RNA samples (RNA 3,6, and 9-21) in 20 mM MOPS pH 7.0, 150 mM KCl, 10 μM EDTA at 20 μM concentration, and equimolar concentration of DFHBI-1T, YO3-Biotin, or both, were heated to 95°C for 3 minutes and incubated at RT for 20 minutes. Samples were run on an ÄKTApurifier at 1 ml/minute over a Superdex 200 Increase size exclusion column (GE Life Sciences) at room temperature. The running buffer was the same as the RNA folding buffer supplemented with 400 nM DFHBI-1T, 20 nM YO3-Biotin, or both. Fractions corresponding to the complex of interest were collected, and concentrations were immediately determined on a NanoDrop 2000 (Thermo Scientific). Fluorescence was measured on a Photon Technologies International/820 Photomultiplier Detection System. Measurements were performed at an excitation of either 469 nm or 580 nm, and an emission of 616 nm.
QUANTIFICATION AND STATISTICAL ANALYSIS
Structure modeling and refinement were validated by both Rwork and Rfree as described in “Method Detials”. Ten percent of reflections were randomly selected by XDS and the model validated against these data using Phenix.refine. Fluorescence enhancement is reported as the mean of at least three measurements with error reported by standard error measurement (SEM).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| TO1-Biotin | Ref Dolgosheina 2014 | N/A |
| Thiazole orange | Sigma-Aldrich | 17237 |
| TO1-Acetate | Ref Dolgosheina 2014 | N/A |
| TO1-CMPD1 | Ref Dolgosheina 2014 | N/A |
| TO1-CMPD2 | Ref Dolgosheina 2014 | N/A |
| TO1-ME | Ref Trachman 2018 | N/A |
| TO1-PE | Ref Trachman 2018 | N/A |
| TO3-Biotin | Ref Dolgosheina 2014 | N/A |
| YO3-Biotin | Jepsen et al. 2018 | N/A |
| Deposited Data | ||
| Mango-IV-TO1-Biotin (native) | This study | 6V9D |
| Mango-IV-TO1-Biotin (Br derivative) | This study | 6V9B |
| Oligonucleotides | ||
| See Supplementary Table 3 | ||
| Software and Algorithms | ||
| HKL2000 | Otwinowski and Minor, 1997 | http://www.hkl-xray.com/ |
| XDS | Kabsch, 2010 b | http://xds.mpimf-heidelberg.mpg.de/ |
| DIALS | Winter et al., 2018 | http://dials.github.io/ |
| Phenix | Adams et al., 2010 | https://www.phenix-online.org/ |
| Coot | Emsley and Cowtan, 2004 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Pymol | DeLano, 2002 | https://pymol.org/2/ |
| SednterpPymol | Laue, et al., 1992 DeLano, 2002 | http://www.jphilo.mailway.com/download.htmhttps://pymol.org/2/ |
| SEDFIT | Peter Schuck, 2019 | https://sedfitsedphat.nibib.nih.gov/software/default.aspx |
SIGNIFICANCE.
The Mango-IV aptamer activates conditionally fluorescent ligands such as TO1-Biotin, and is particularly effective for imaging RNA transcripts in both fixed and live cells. Its crystal structure reveals an unexpected domain-swapped homodimer, and structure-guided analyses indicate that the RNA has a tendency to sample alternative folds. While reducing the binding-competent fraction of molecules in vitro, this may endow Mango-IV with robustness when deployed for imaging in cells, as the RNA may be able more effectively to refold, even if expressed in contexts where it initially misfolds. The biophysical and imaging properties of Mango-IV underscore the importance of functionally selecting imaging probes in their intended cellular context. The domain-swapped homodimer of Mango-IV is linked by a simple base-paired helix. This feature was exploited to design heterodimers of Mango-IV and a second fluorescence turn-on aptamer, iSpinach. These dimers, whose interaction depends on a short segment of sequence complementarity that links them through A-form helix formation, exhibit intermolecular FRET between the respective aptamer bound fluorophores. Further engineering of the fluorophore-binding sites of the two aptamers, as well as the conditional fluorophores, may yield improvements in orthogonality and FRET efficiency.
HIGHLIGHTS.
The Mango-IV fluorescence turn-on aptamer RNA is a domain-swapped homodimer
The Mango-IV ligand-binding site is open, and activates red-shifted fluorophores
The aptamer samples multiple folds, endowing it with robustness for in vivo imaging
Mango-IV was engineered to assemble into a heterodimeric FRET pair with iSpinach
ACKNOWLEDGMENTS
We thank the staff of beamlines 5.0.1 and 5.0.2 of the Advanced Light Source, Lawrence Berkeley National Laboratory (ALS), and 24-ID-C of the Advanced Photon Source, Argonne National Laboratory (APS) for crystallographic data collection; D.-Y. Lee of the Biochemistry Core of NHLBI and R. Levine for high-performance liquid chromatography; and members of the Ferré-D’Amaré, Ryckelynck and Unrau laboratories for discussions. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Pilatus 6M detector on 24-ID-C beam line is funded by a NIH-ORIP HEI grant (S10 RR029205). This work was partially supported by an NSERC (Canada) operating grant (P.J.U.), the LabEx NetRNA (ANR-10-LABX-0036) (M.R.), ‘Agence Nationale pour la Recherche’ (ANR-16-CE11-0010-01) (M.R.), and the Intramural Program of the NHLBI, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
The authors declare no competing financial interests.
References
- Afonine P, Grosse-Kunstleve R, Echols N, Headd J, Moriarty N, Mustyakimov M, Terwilliger T, Urzhumtsev A, Zwart P, and Adams P (2012). Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica Section D-Biological Crystallography 68, 352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam KK, Tawiah KD, Lichte MF, Porciani D, and Burke DH (2017). A fluorescent split aptamer for visualizing RNA-RNA assembly in vivo. ACS Synth Biol 6, 1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autour A, C Y Jeng S, D Cawte A, Abdolahzadeh A, Galli A, Panchapakesan SSS, Rueda D, Ryckelynck M, and Unrau PJ (2018). Fluorogenic RNA Mango aptamers for imaging small non-coding RNAs in mammalian cells. Nat Commun 9, 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autour A, Westhof E, and Ryckelynck M (2016). iSpinach: a fluorogenic RNA aptamer optimized for in vitro applications. Nucleic Acids Res 44, 2491–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F (1979). Split genes and RNA splicing. Science 204, 264–271. [DOI] [PubMed] [Google Scholar]
- Dolgosheina EV, Jeng SCY, Panchapakesan SSS, Cojocaru R, Chen PSK, Wilson PD, Hawkins N, Wiggins PA, and Unrau PJ (2014). RNA Mango Aptamer-Fluorophore: A bright, high-affinity complex for RNA labeling and tracking. ACS Chem. Biol. 9, 2412–2420. [DOI] [PubMed] [Google Scholar]
- Ellington AD, and Szostak JW (1990). In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822. [DOI] [PubMed] [Google Scholar]
- Fernandez-Millan P, Autour A, Ennifar E, Westhof E, and Ryckelynck M (2017). Crystal structure and fluorescence properties of the iSpinach aptamer in complex with DFHBI. RNA 23, 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG, and Lorsch JR (2012). The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng SCY, Chan HHY, Booy EP, McKenna SA, and Unrau PJ (2016). Fluorophore ligand binding and complex stabilization of the RNA Mango and RNA Spinach aptamers. RNA 22, 1884–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen MDE, Sparvath SM, Nielsen TB, Langvad AH, Grossi G, Gothelf KV, and Andersen ES (2018). Development of a genetically encodable FRET system using fluorescent RNA aptamers. Nat Commun 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W (2010 a). Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr D Biol Crystallogr 66, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W (2010 b). XDS. Acta Crystallogr D Biol Crystallogr 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger CA, Wilson SC, Sales-Lee J, and Hammond MC (2013). RNA-based fluorescent biosensors for live cell imaging of second messengers cyclic di-GMP and cyclic AMP-GMP. J. Am. Chem. Soc 135, 4906–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontis NB, and Westhof E (2001). Geometric nomenclature and classification of RNA base pairs. RNA 7, 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, and Read RJ (2007). Phaser crystallographic software. J Appl Crystallogr 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige JS, Nguyen-Duc T, Song WJ, and Jaffrey SR (2012). Fluorescence imaging of cellular metabolites with RNA. Science 335, 1194–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst JM, Winter G, Waterman DG, Fuentes-Montero L, Gildea RJ, Murshudov GN, and Evans G (2016). Robust background modelling in. J Appl Crystallogr 49, 1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A, Wakeman CA, and Winkler WC (2011). Insights into metalloregulation by M-box riboswitch RNAs via structural analysis of manganese-bound complexes. J. Mol. Biol 407, 556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TA, Andrews GE, Jaeger L, and Grabow WW (2015). Fluorescent monitoring of RNA assembly and processing using the split-spinach aptamer. ACS Synth Biol 4, 162–166. [DOI] [PubMed] [Google Scholar]
- Sheldrick GM (2010). Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr D Biol Crystallogr 66, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Strack RL, Svensen N, and Jaffrey SR (2014). Plug-and-Play fluorophores extend the spectral properties of Spinach. J. Am. Chem. Soc 136, 1198–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack RL, and Jaffrey SR (2013). New approaches for sensing metabolites and proteins in live cells using RNA. Current Opinion in Chemical Biology 17, 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger T, Adams P, Read R, Mccoy A, Moriarty N, Grosse-Kunstleve R, Afonine P, Zwart P, and Hung L (2009). Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallographica Section D-Biological Crystallography 65, 582–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC (2000). Maximum-likelihood density modification. Acta Crystallogr D Biol Crystallogr 56, 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, Truong L, and Ferré-D'Amaré AR (2017. a). Structural principles of fluorescent RNA aptamers. Trends in Pharmacological Sciences 38, 928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, Demeshkina NA, Lau MWL, Panchapakesan SSS, Jeng SCY, Unrau PJ, and Ferré-D'Amaré AR (2017. b). Structural basis for high-affinity fluorophore binding and activation by RNA Mango. Nat. Chem. Biol 13, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, Abdolahzadeh A, Andreoni A, Cojocaru R, Knutson JR, Ryckelynck M, Unrau PJ, and Ferré-D'Amaré AR (2018). Crystal structures of the Mango-II RNA aptamer reveal heterogeneous fluorophore binding and guide engineering of variants with improved selectivity and brightness. Biochemistry 57, 3544–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, and Ferré-D'Amaré AR (2019). Tracking RNA with light: selection, structure, and design of fluorescence turn-on RNA aptamers. Q Rev Biophys 52, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, Autour A, Jeng SCY, Abdolahzadeh A, Andreoni A, Cojocaru R, Garipov R, Dolgosheina EV, Knutson JR, Ryckelynck M, et al. (2019 a). Structure and functional reselection of the Mango-III fluorogenic RNA aptamer. Nat. Chem. Biol 15, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachman RJ, Stagno JR, Conrad C, Jones CP, Fischer P, Meents A, Wang YX, and Ferré-D'Amaré AR (2019 b). Co-crystal structure of the iMango-III fluorescent RNA aptamer using an X-ray free-electron laser. Acta Crystallogr F Struct Biol Commun 75, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C, and Gold L (1990). Systematic evolution of ligands by exponential enrichment - RNA ligands to bacteriophage-T4 DNA-polymerase. Science 249, 505–510. [DOI] [PubMed] [Google Scholar]
- Van Treeck B, and Parker R (2018). Emerging Roles for Intermolecular RNA-RNA Interactions in RNP Assemblies. Cell 174, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KD, Chen MC, Song WJ, Strack RL, Thorn A, Jaffrey SR, and Ferré-D'Amaré AR (2014). Structural basis for activity of highly efficient RNA mimics of green fluorescent protein. Nat. Struct. Mol. Biol 21, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KD, Sjekloca L, Song WJ, Filonov GS, Jaffrey SR, and Ferré-D'Amaré AR (2017). A homodimer interface without base pairs in an RNA mimic of red fluorescent protein. Nat. Chem. Biol 13, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Structure coordinates generated during this study are available at the Protein Data Bank under accession codes 6V9B and 6V9D.


