Abstract
Aim:
Breast milk feeding is linked to improved neurodevelopmental outcomes in very low birth weight (VLBW) infants, though the mechanisms are not well understood. This study utilised quantitative magnetic resonance imaging (qMRI) techniques to compare brain growth and white matter development in preterm infants receiving primarily breast milk versus formula feeds.
Methods:
We prospectively enrolled infants born at very low birth weight (<1500 g) and <32 weeks gestational age and performed MRI at term-equivalent age. We utilised volumetric segmentation to calculate regional and total brain volumes and diffusion tensor imaging to evaluate white matter microstructural organisation. Daily nutritional data were extracted from the medical record.
Results:
Nutritional and MRI data were obtained for 68 infants admitted within the first week of life (44 breast milk and 24 formula). Breast milk–fed infants demonstrated significantly larger total brain volumes (P = .04) as well as volumes in the amygdala-hippocampus and cerebellum (P < .01) compared with formula-fed. Infants receiving breast milk also demonstrated greater white matter microstructural organisation in the corpus callosum, posterior limb of internal capsule and cerebellum (P < .01 to .03).
Conclusions:
VLBW infants receiving primarily breast milk versus preterm formula in this small exploratory study demonstrated significantly greater regional brain volumes and white matter microstructural organisation by term-equivalent age.
Keywords: brain, breast milk, MRI, VLBW
1 ∣. INTRODUCTION
Preterm infants born at very low birth weight (VLBW, <1500 g) are at significantly increased risk for neurodevelopmental impairment, and emerging data suggest that breast milk feeding is associated with improved short- and long-term neurodevelopmental outcomes in this vulnerable population. Studies have demonstrated superior behavioural, motor, and cognitive function from infancy through school age and adolescence in preterm neonates receiving maternal breast milk versus formula feeds.1-4 However, longitudinal studies to assess complex, functional outcomes of premature infants later in childhood and adolescence remain fiscally and logistically challenging.
Quantitative magnetic resonance imaging (qMRI) techniques extend beyond standard qualitative assessments and are evolving as promising in vivo biomarkers for these functional outcomes in premature infants, particularly three-dimensional volumetric growth and microstructural assessments.5 Diffusion tensor imaging (DTI) is a qMRI technique that utilises differences in water molecule diffusion to estimate white matter microstructural development. Two common DTI measures used to estimate cellular maturation include fractional anisotropy (FA) and mean diffusivity (MD), which measure the directionality and net diffusion of water molecules, respectively.5
Few studies have utilised qMRI to evaluate the impact of breast milk on microstructural brain development in preterm infants.1,2,6 Long-term follow-up studies in infants born at term suggest that breast milk intake during infancy is associated with greater brain volumes and white matter microstructural organisation, correlating to improved motor and cognitive outcomes including visual and language scores and overall intelligence quotient.7-9 The goal of this exploratory study was to utilise advanced qMRI techniques to assess the impact of early breast milk feeding on microstructural brain development in VLBW premature infants.
2 ∣. PATIENTS AND METHODS
2.1 ∣. Subjects
Preterm infants admitted to our single patient room Level IV Neonatal Intensive Care Unit (NICU) at Children's National Hospital in Washington, DC, USA were enrolled as part of a prospective, observational study of the antecedents and sequelae of prematurity-related brain injury between June 2012 and April 2017. All infants admitted to our NICU were outborn, and gestational age was determined by referral hospital prior to transfer. Infants were eligible for enrolment if they were born at very low birth weight (VLBW < 1500 g) and ≤32 weeks gestational age and admitted within the first week of life. Infants with a known or suspected brain malformation, dysmorphic features or congenital anomalies suggestive of an underlying genetic syndrome, confirmed metabolic disorder, chromosomal abnormality or proven perinatal central nervous system infection were excluded. For our analysis comparing brain development in breast milk versus formula-fed infants, we only included patients with available term-equivalent MRI data and also further excluded infants with Grade III intraventricular haemorrhage, periventricular haemorrhagic infarction or cerebellar haemorrhagic injury. Clinical data were extracted from the medical record. This study was approved by the Children's National Medical Center Institutional Review Board and informed, written consent was obtained from the parents of all participants.
2.2 ∣. Nutritional intake
Daily nutritional intake was retrospectively extracted from the electronic medical record from admission until date of term-equivalent MRI. A standardised feeding protocol for VLBW infants was utilised with specific parameters for initiation, advancement and fortification of enteral feeds based on birth weight and feeding tolerance. Trophic enteral feeds were initiated within 24 hours of life or once the infant was deemed clinically stable by the medical team. Pasteurised donor breast milk was made available in our NICU beginning in April 2015, and was provided when maternal breast milk supply was insufficient and if parents consented until 34 weeks corrected age, at which point infants were transitioned to formula. Maternal (unpasteurised) and donor breast milk were both fortified in the same fashion with a cow's milk-based human milk fortifier. Infants were categorised as having received primarily breast milk or formula feeds (>50% of total enteral feeding volume for duration of NICU admission). No other significant changes were made to the feeding protocol or other NICU policies that could potentially affect maternal-infant bonding, including the provision of Kangaroo Care, during the study time period.
2.3 ∣. MRI acquisition and processing
Non-sedated brain MRI studies were performed at term-equivalent age on a 3 Tesla MRI scanner (Discovery MR750; General Electric Medical, Systems, Waukesha, Wisconsin) with an 8-channel receiver head coil approved for safety in neonates. Infants were immobilised using an InfantVacuum Immobilizer (Newmatic Medical, Caledonia, Michigan) and provided with double ear protection. The MRI acquisition protocol included structural imaging with T2 3D-cube and T1 3D-spoiled gradient recalled images (T2: 84 ms echo time, 2500 ms repetition time, field of view 13 cm, 1 mm slice thickness, 160 × 160 acquisition matrix; T1: 3.8 ms echo time, 6.7 ms repetition time, field of view 13 cm, 1 mm slice thickness, 700 ms inversion time, 160 × 160 acquisition matrix). DTI acquisition consisted of a single-shot, echo-planar sequence with 27 noncollinear direction diffusion gradients with an effective high b-value of 1000 s/mm2 (3 b = 0 s/mm2) 80 ms echo time, 8000 ms repetition time, field of view 200 × 200 mm, 3 mm slice thickness and no gap with a 128 × 128 acquisition matrix.
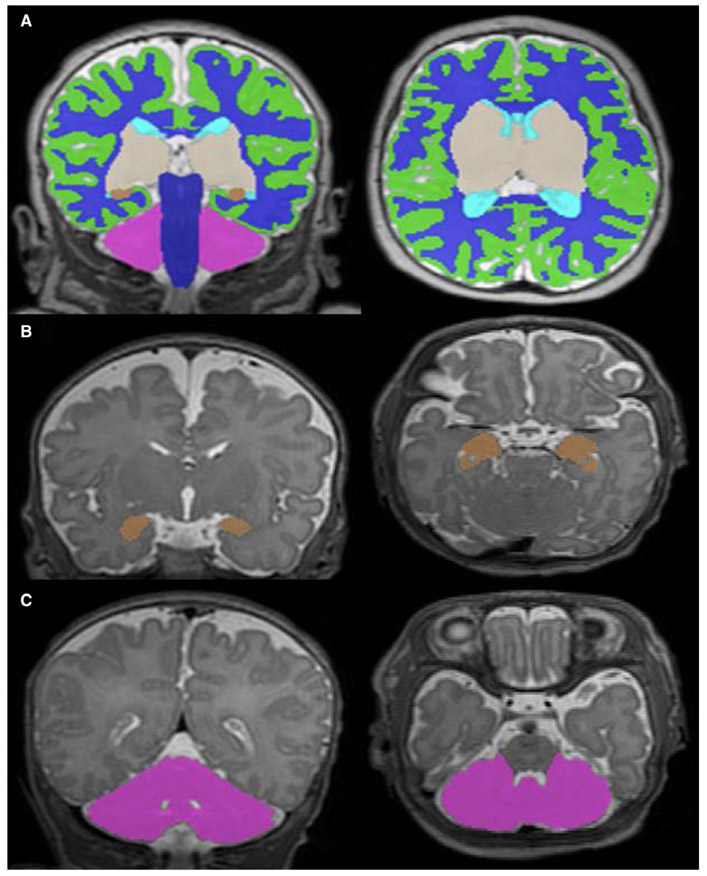
Volumetric segmentation was performed on Coronal T2 Cube 3D images using a validated automated algorithm, with subsequent manual inspection and correction as needed by two investigators blinded to enteral feeding type (KO and KK).10 Regional brain volumes were obtained for the cortical grey matter, white matter, deep grey matter, amygdala-hippocampus, cerebellum, and brainstem and utilised to calculate total brain volume for each infant (Figure 1A). DTI data were preprocessed based on a previously published pipeline.11 Using the B0 image for each patient and its registered FA and MD maps, cubic regions of interest (ROIs, 6.5-49.4 mm3) were manually placed by a single observer (KK) on 2-3 consecutive slices to maximise the coverage of the evaluated structure, using predefined anatomical landmarks to place ROIs in known early areas of white matter development including the corpus callosum, posterior limb of internal capsule (PLIC), cerebellum (middle and superior cerebellar peduncle and anterior vermis), brainstem and pons.11 Mean diffusivity (MD) and fractional anisotropy (FA) values were determined for each ROI. The size of the ROI was same across all the subjects, and the coverage of the ROI on the diffusion map was based on its registered B0 and 3D T2 image.
FIGURE 1.
Volumetric segmentation of T2-weighted MRI images. Coronal and axial views of the (A) total brain, (B) amygdala-hippocampus and (C) cerebellum. Legend: green = cortical grey matter, blue = white matter, grey = deep grey matter, brown = amygdala-hippocampus, pink = cerebellum, navy = brainstem and aqua = ventricles
2.4 ∣. Statistical analysis
Statistical analysis was performed using Statistical Analysis Software (SAS) 9.4. Wilcoxon-Mann-Whitney and chi-square tests were used to compare clinical characteristics and comorbidities between the breast milk and formula groups. Least squares means estimates from generalised linear models adjusted for gestational age at birth and for corrected postmenstrual age at MRI were used to compare quantitative measures from DTI and volumetric segmentation techniques between infants receiving breast milk versus preterm formula. Additional analyses were performed on volumetric data, adjusting generalised linear models for average weight gain and total brain volume. Additional adjustments for significant covariates were subsequently performed. Sub-analyses evaluated differences between maternal breast milk and donor breast milk versus formula.
3 ∣. RESULTS
We studied 68 VLBW infants admitted within the first week of life, 57 (84%) of whom were admitted within the first 48 hours of life. Term-equivalent MRI was obtained at an average age of 40 weeks and 1 day ±1 week and 5 days postmenstrual age (range 36 weeks and 5 days to 45 weeks and 2 days). Forty-four infants received primarily breast milk feeds (30 maternal breast milk, 14 donor breast milk) and 24 received primarily preterm formula. The majority of infants in the breast milk–fed group received exclusive breast milk throughout their NICU admission (without exposure to preterm formula), demonstrated by a mean breast milk intake of 86% of total enteral feeds (median 90%). On sub-group analysis, infants receiving donor breast milk had a greater exposure to formula during their NICU admission with a mean breast milk intake of 69% of enteral feeds (median 64%), likely related to our institutional protocol of changing from donor milk to transitional formula at 34 weeks corrected age. Race/ethnicity was significantly different between breast milk and formula-fed infants, otherwise there were no significant differences in baseline clinical characteristics between feeding groups including proportion of infants born small for gestational age (Table 1 & Supporting Information Table S1). Formula-fed babies were more likely than breast milk–fed babies to have an infection (79% vs. 55%, P = .04); no significant differences were noted with regard to other common comorbidities of prematurity (Table 1).
TABLE 1.
Clinical characteristics of the preterm cohort
| Characteristic | Breast milk (n = 44) | Formula (n = 24) | P-value |
|---|---|---|---|
| Maternal primiparity, n (%) | 8 (18.2) | 6 (25.0) | .54 |
| Race/ethnicity, n (%) | <.01 | ||
| Hispanic | 8 (18.2) | 1 (4.2) | |
| White | 4 (9.1) | 5 (20.8) | |
| Asian | 11 (25.0) | – | |
| Black | 14 (31.8) | 18 (75.0) | |
| Hawaiian/Pacific Islander | 2 (4.6) | – | |
| Other/Unknown | 5 (11.4) | – | |
| Antenatal steroids, n (%) | 19 (43.2) | 10 (41.7) | 1.00 |
| Female gender, n (%) | 28 (63.6) | 13 (54.2) | .45 |
| Birth gestational age, median (IQR) | 26.9 (25.6-29.1) | 28.0 (26.0-30.3) | .11 |
| Birth weight (kg), median (IQR) | 0.90 (0.70-1.20) | 0.96 (0.76-1.25) | .64 |
| Birth HC (cm), median (IQR) | 24.3 (21.8-26.4) | 24.3 (23.0-27.0) | .50 |
| SGA at birth, n (%) | 4 (9.1) | 3 (12.5) | .68 |
| Nutrition days, median (IQR) | 76.5 (49.5-97.5) | 71.0 (55.0-98.5) | .83 |
| Infection, n (%) | 24 (54.5) | 19 (79.1) | .04 |
| Moderate-severe BPD, n (%) | 16 (36.3) | 11 (45.8) | .45 |
| ROP (≥Stage 2), n (%) | 15 (34.1) | 7 (29.2) | .79 |
| Surgical NEC, n (%) | 4 (9.1) | 2 (8.3) | 1.00 |
| Surgery, n (%) | 18 (40.9) | 10 (41.2) | .95 |
| Postnatal steroids, n (%) | 15 (34.1) | 11 (45.8) | .34 |
| Mechanical ventilation (days), median(IQR) | 5.0 (1.5-24.5) | 7.5 (1.5-27.0) | .90 |
Abbreviations: BPD, Bronchopulmonary Dysplasia; HC, Head Circumference; IQR, Interquartile Range; NEC = Necrotising Enterocolitis requiring laparotomy or drain placement; ROP, Retinopathy of Prematurity; SGA, Small for Gestational Age (<10%).
3.1 ∣. Brain volumes
High-quality volumetric data were available for 67 infants (43 primarily breast milk versus 24 primarily formula-fed). Inter-rater reliability measure for the manual correction of volumetric segmentation based on 20 randomly selected subjects was 0.99. Global and regional brain volumes were overall larger in infants receiving breast milk versus formula (Table 2). After adjusting for gestational age at birth, corrected postmenstrual age at MRI, and average weight gain, infants in the breast milk group had significantly larger total brain volume (P = .04) as well as larger regional volumes in the amygdala-hippocampus (P = .0002), cerebellum (P = .002) and brainstem (P = .02) (Figure 1). Following additional adjustment for total brain volume, only the amygdala-hippocampus (P = .03) and cerebellum (0.02) remained significantly larger in breast milk versus formula-fed infants. Sub-group analysis of donor milk–fed infants also revealed significantly larger amygdala-hippocampus (P = .01) volumes when compared to formula-fed infants (Supporting Information Table S2). Additional adjustments for infection, the only significant covariate, had no material effect on these estimates. Sex and race/ethnicity were not significant in any of the models tested, with similar effects noted in analyses that incorporated these variables.
TABLE 2.
Relationship between enteral nutrition type (breast milk versus formula) and brain volume
| Brain volume (cm3) Mean (95% CI) | Breast milk (n = 43) | Formula (n = 24) | Pa |
|---|---|---|---|
| Total brain volume | 322 (312-331) | 305 (293-318) | .04 |
| Cortical grey matter | 126 (122-129) | 119 (114-124) | .06 |
| White matter | 142 (137-147) | 137 (130-143) | .20 |
| Deep grey matter | 24 (24-25) | 23 (22-24) | .05 |
| Amygdala-hippocampus | 2.3 (2.3-2.4) | 2.1 (2.0-2.2) | <.01 |
| Cerebellum | 22 (21-23) | 19 (18-20) | <.01 |
| Brainstem | 5.3 (5.2-5.4) | 5.0 (4.8-5.2) | .02 |
Based on least squares means estimates from generalised linear models adjusted for gestational age at birth, postmenstrual age at MRI and average weight gain.
3.2 ∣. White matter microstructural development
High-quality DTI data were available for 65 infants (42 primarily breast milk versus 23 primarily formula-fed). Intra-rater reliabilities on 10 randomly selected subjects using intraclass correlation coefficients were greater than 0.89 (range 0.89 to 1) for all measured regions. Breast milk compared with formula-fed infants demonstrated significantly lower MD in the corpus callosum, left and right PLIC, and middle cerebellar peduncle, suggestive of more robust white matter maturation in these regions, with lower FA in the cerebellar vermis (Figure 2, Table 3). After adjustment for infection, MD in the corpus callosum and right PLIC were no longer significantly different between groups. In sub-group analysis, donor milk–fed infants exhibited significantly lower MD in the corpus callosum (P = .02), left PLIC (P = .03), and middle cerebellar peduncle (P = .01), with lower FA in the cerebellar vermis (P < .01) compared to formula-fed infants (Supporting Information Tables S3 and S4). Sex and race/ethnicity were not significant in any of the models tested, with similar effects noted in analyses that incorporated these variables.
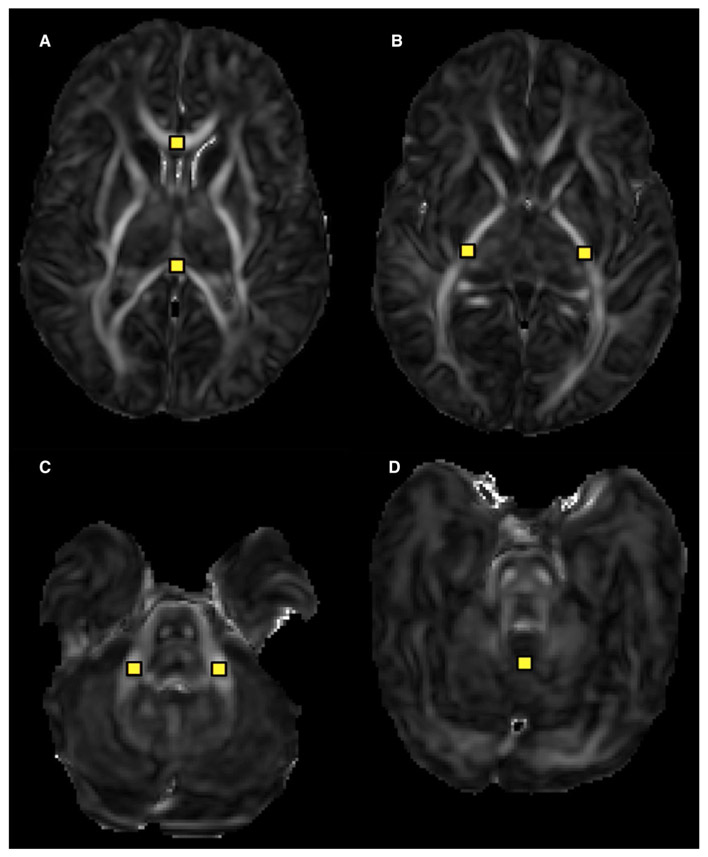
FIGURE 2.
Axial diffusion tensor image (DTI) parametric maps of the significantly different regions of interest (ROI) between breast milk and formula-fed infants. Breast milk–fed infants demonstrated significantly lower MD in the (A) corpus callosum, (B) left and right PLIC, and (C) middle cerebellar peduncle and significantly lower FA in the (D) cerebellar vermis
TABLE 3.
Relationship between enteral nutrition type (breast milk versus formula) and white matter microstructural development
| Fractional Anisotropy |
Mean Diffusivity (× 10−3 mm2/seconds) |
|||||
|---|---|---|---|---|---|---|
| Region of Interest |
Breast Milk (n = 42) Mean (95% CI) |
Formula (n = 23) Mean (95% CI) |
P valuea |
Breast Milk (n = 42) Mean (95% CI) |
Formula (n = 23) Mean (95% CI) |
P valuea |
| CC | 0.55 (0.53-0.58) | 0.54 (0.51-0.57) | .53 | 1.28 (1.23-1.33) | 1.38 (1.31-1.45) | .03 |
| Left PLIC | 0.59 (0.58-0.61) | 0.58 (0.56-0.60) | .21 | 0.92 (0.90-0.95) | 0.99 (0.96-1.02) | <.01 |
| Right PLIC | 0.60 (0.58-0.61) | 0.58 (0.56-0.60) | .26 | 0.94 (0.92-0.96) | 0.98 (0.95-1.02) | .03 |
| MCP | 0.51 (0.47-0.54) | 0.52 (0.48-0.56) | .83 | 0.80 (0.77-0.83) | 0.86 (0.82-0.90) | .02 |
| SCP | 0.37 (0.35-0.38) | 0.36 (0.34-0.38) | .56 | 0.86 (0.84-0.88) | 0.88 (0.86-0.91) | .21 |
| Vermis | 0.19 (0.18-0.20) | 0.23 (0.21-0.24) | <.01 | 0.71 (0.68-0.74) | 0.73 (0.69-0.77) | .56 |
| Pons | 0.39 (0.37-0.40) | 0.39 (0.37-0.42) | .69 | 0.84 (0.81-0.86) | 0.84 (0.81-0.87) | .85 |
Abbreviations: CC, corpus callosum; MCP, middle cerebellar peduncle; PLIC, posterior limb of internal capsule; SCP, superior cerebellar peduncle.
Based on least squares means estimates from generalised linear models adjusted for gestational at birth and postmenstrual age at MRI.
4 ∣. DISCUSSION
Quantitative MRI findings in this small exploratory study revealed improved early brain growth and microstructural development in breast milk compared with formula-fed VLBW infants by term-corrected age, and many of these benefits extended to infants receiving donor breast milk. Breast milk–fed infants demonstrated significantly greater total brain volumes on term-equivalent MRI and in the amygdala-hippocampus and cerebellum, as well as significantly greater white matter microstructural organisation in the corpus callosum, PLIC and cerebellum on DTI.
Our study provides further support for early administration of breast milk to preterm infants in the postnatal period when the brain is undergoing rapid growth and maturation. There are several unique constituents of breast milk that are either absent or present in lower quantities in preterm formula including growth factors, cholesterol and long-chain polyunsaturated fatty acid (LCPUFAs),4 as well as potential factors yet to be identified. In addition to providing important constituents for cell membrane and myelin formation, it has been postulated that breast milk might exert its beneficial effects on the developing brain through a variety of mechanisms including hormonal and anti-inflammatory effects and by conferring immunologic benefits.2,12 This is particularly relevant in white matter development, as the early systemic exposure to infection and inflammation can result in severe injury and death of oligodendrocyte precursors and lead to white matter injury.13
4.1 ∣. Breast milk and brain volumes
Breast milk intake in our study cohort was associated with significantly greater amygdala-hippocampal volumes by term-corrected age. These findings are consistent with Belfort et al, who showed a positive association between average daily breast milk intake in preterm infants and hippocampal volume by term-equivalent age.1 The amygdala and hippocampus are both regions of the brain that are especially vulnerable to perinatal stress, and previous studies in preterm infants have demonstrated that altered development in the amygdala and hippocampus are associated with cognitive and behavioural impairments.14-18 Beauchamp et al found that decreased hippocampal volume at term-equivalent age in very premature infants was associated with working memory deficits at 2 years of age.15 These deleterious effects appear to persist into adolescence, with smaller hippocampal volumes in preterm-born adolescents correlating to deficits in intelligence quotient and every day memory.17,18
We also found greater cerebellar volumes in breast milk compared with formula-fed infants at term-equivalent age. The cerebellum is another particularly vulnerable structure in the developing preterm brain, prone to injury as it undergoes rapid growth during the third trimester.19 In addition to the known role of the cerebellum in motor function, it is increasingly recognised to play a role in cognitive and social-behavioural development.19-21 In particular, cerebellar haemorrhagic injury is associated with neurodevelopmental disabilities including language and cognitive deficits.19 In a long-term follow-up study of infants born very premature, Allin et al found significantly reduced cerebellar volume compared to term controls that persisted through adolescence and was associated with poor cognitive outcome.20
We postulate that the neurocognitive benefits of breast milk for preterm infants may, in part, be mediated by improved development or protective effects on the vulnerable amygdala-hippocampus and cerebellum. Neurodevelopmental follow-up studies are required and are currently underway in this cohort to examine the long-term significance of our findings.
4.2 ∣. Breast milk and white matter microstructure
Using DTI analysis to estimate in vivo white matter development, we found evidence of greater white matter microstructural organisation in the corpus callosum and PLIC in infants receiving primarily breast milk compared with preterm formula. These data augment previous findings of a positive association between the duration of human milk feeding and corpus callosum development.6 The corpus callosum and PLIC have been identified as important markers of early white matter development in preterm infants, and our findings suggest that breast milk intake may somehow lead to enhanced development in these regions. Improved white matter microstructural organisation on DTI studies of preterm infants in the corpus callosum and PLIC have been linked to superior neurodevelopmental outcomes, including cognitive and motor development, through school age.22-25
LCPUFAs present in breast milk may directly contribute to white matter development and myelination; however, preterm infants are born prior to the significant accretion of LCPUFAs that occurs during the third trimester. Breast milk contains a unique composition of LCPUFAs, and studies of LCPUFA supplementation in very preterm infants suggest that the LCPUFAs provided via breast milk may confer neurodevelopmental benefits, with improved behavioural and cognitive outcomes.26,27
We also found significant differences in cerebellar white matter development between infants receiving breast milk versus formula. The lower MD in the middle cerebellar peduncle is consistent with improved white matter tract formation and may reflect mature cerebro-cerebellar connections.5 The lower FA values in the cerebellar vermis of breast milk–fed infants are less intuitive to interpret but are consistent with previous findings by our group. In a study of cerebellar white matter microstructural development in premature infants compared with healthy controls, healthy term infants had lower FA values compared with premature infants.11 While the exact mechanisms are unclear, it may be that maturation of cerebellar tracts in the vermis, including robust dendritic growth, disrupts axonal water molecule movement so that lower FA values reflect greater cerebellar tract maturation.
4.3 ∣. Donor breast milk
Given the documented benefits of human milk for premature infants, donor programmes are increasingly utilised to provide breast milk to infants whose mothers are unable to provide a sufficient milk supply. However, studies have described decreased immunologic and bacteriostatic proteins in stored, pasteurised donor milk when compared to raw maternal milk.28 Additionally, donor breast milk is generally from mothers of term infants, with lower macro- and micronutrient content when compared to expressed milk from mothers who have delivered prematurely.29 Concerns have been raised that these differences may attenuate the neurodevelopmental benefits of breast milk in preterm infants receiving donor breast milk. Indeed, O’Connor et al did not find any differences in Bayley III scores by 18 months of age between premature infants who had received donor breast milk or formula.30 Despite these developmental concerns, our study noted positive MRI findings in infants receiving donor milk compared with formula, although these data should be interpreted with caution as these findings were based on secondary analyses with a small sample size, and should not be utilised to make causal assumptions. Larger prospective studies are required to evaluate the long-term neurodevelopmental effects of donor breast milk compared with raw maternal milk in preterm infants.
There are several strengths of this study, however, our limitations deserve mention. The retrospective nature of our data collection could lead to potential confounding, especially as infants were not randomly assigned to breast milk versus formula groups and we were unable to control parental decision on feeding type. While our preliminary results regarding the potential role of donor breast milk are very intriguing, the number of infants receiving donor breast milk was small, and future studies are needed to confirm the potential benefits. Additionally, our study cohort was limited to a single Level IV NICU with a high incidence of critical illness, and future large, prospective studies in extremely premature infants are warranted to further validate our findings. We also did not have data on maternal intelligence quotient (IQ) and family background that could potentially influence both the decision to breast feed and brain development. Lastly, data on the functional and lasting effects of breast milk on long-term developmental outcomes are also needed, and currently underway in our study cohort. Future studies should consider all possible confounders known to influence breast feeding, including socioeconomic status, lifestyle choices, and racial/ethnic differences, and evaluate both short- and long-term neurodevelopment. Similarly, additional studies to better elucidate mechanistic pathways that could link early nutritional exposures with neurodevelopment are also warranted.
5 ∣. CONCLUSIONS
In this exploratory study, we demonstrate that early breast milk feeding is associated with significantly greater cerebral and cerebellar volumes as well as white matter microstructural organisation in very premature infants by term-equivalent age. These findings may help explain the improved neurodevelopmental outcomes described in breast milk–fed preterm infants. Further research is needed to elucidate which components of breast milk contribute to improved structural brain development in order to optimise nutritional interventions for infants born preterm.
Supplementary Material
Key Notes.
This study evaluated the relationship between breast milk (versus formula) feeds in very low birth weight (VLBW) infants and brain development utilising advanced, quantitative magnetic resonance imaging (qMRI) techniques.
In 68 VLBW infants, breast milk–fed infants demonstrated significantly larger amygdala-hippocampus and cerebellar volumes on term-equivalent qMRI.
Early breast milk nutrition was associated with improved white matter microstructural organisation as measured by diffusion tensor imaging on term-equivalent qMRI.
Acknowledgments
Funding information
This project was supported by Award Numbers UL1TR001876, 1U54HD090257 and R01 HL116585-01 from the NIH National Center for Advancing Translational Sciences, The District of Columbia Intellectual Developmental Disabilities Research Center at Children's National (DCIDDRC), supported through the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) program grant and the National Heart, Lung and Blood Institute (NHLBI)
Abbreviations:
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- LCPUFA
long-chain polyunsaturated fatty acid
- MD
mean diffusivity
- NICU
Neonatal Intensive Care Unit
- PLIC
posterior limb of internal capsule
- qMRI
quantitative magnetic resonance imaging
- VLBW
very low birth weight
Footnotes
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health or the United States Air Force.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Belfort MB, Anderson PJ, Nowak VA, et al. Breast milk feeding, brain development, and neurocognitive outcomes: a 7-year longitudinal study in infants born at less than 30 weeks' gestation. J Pediatr. 2016;177:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gaidan DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and White matter development. Pediatr Res. 2010;67(4):357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7–8 years. Arch Dis Child Fetal Neonatal Ed. 2001;84(1):F23–F27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vohr BR, Poindexter BB, Dusick AM, et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118(1):e115–e123. [DOI] [PubMed] [Google Scholar]
- 5.Hinojosa-Rodriguez M, Harmony T, Carrillo-Prado C, et al. Clinical neuroimaging in the preterm infant: diagnosis and prognosis. Neuroimage Clin. 2017;16:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pogribna U, Yu X, Burson K, et al. Perinatal clinical antecedents of white matter microstructural abnormalities on diffusion tensor imaging in extremely preterm infants. PLoS ONE. 2013;8(8):e72974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deoni SC, Dean DC 3rd, Piryatinsky I, et al. Breastfeeding and early white matter development: a cross-sectional study. Neuroimage. 2013;82:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luby JL Belden AC, Whalen D Harms MP, Barch DM Breastfeeding and childhood IQ: the mediating role of gray matter volume. J Am Acad Child Adolesc Psychiatry. 2016;55(5):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kafouri S, Kramer M, Leonard G, et al. Breastfeeding and brain structure in adolescence. Int J Epidemiol. 2013;42(1):150–159. [DOI] [PubMed] [Google Scholar]
- 10.Makropoulos A, Gousias IS, Ledig C, et al. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans Med Imaging. 2014;33(9):1818–1831. [DOI] [PubMed] [Google Scholar]
- 11.Brossard-Racine M, Poretti A, Murnick J, et al. Cerebellar microstructural organization is altered by complications of premature birth: a case-control study. J Pediatr. 2017;182:28–33. [DOI] [PubMed] [Google Scholar]
- 12.Andreas NJ, Kampmann B, Mehring L-D. Human breast milk: a review on its composition and bioactivity. Early Hum Dev. 2015;91(11):629–635. [DOI] [PubMed] [Google Scholar]
- 13.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F153–F161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson DK, Wood SJ, Doyle LW, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63(5):642–651. [DOI] [PubMed] [Google Scholar]
- 15.Beauchamp MH, Thompson DK, Howard K, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131(Pt 11):2986–2994. [DOI] [PubMed] [Google Scholar]
- 16.Cismaru AL, Gui L, Vasung L, et al. Altered amygdala development and fear processing in prematurely born infants. Front Neuroanat. 2016;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs EB, Lucas A, Chong WK, et al. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res. 2000;47(6):713–720. [DOI] [PubMed] [Google Scholar]
- 18.Abernethy LJ, Palaniappan M, Cooke RW. Quantitative magnetic resonance imaging of the brain in survivors of very low birth weight. Arch Dis Child. 2002;87(4):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limperopoulos C, Bassan H, Gauvreau K, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–593. [DOI] [PubMed] [Google Scholar]
- 20.Allin M, Matsumoto H, Santhouse AM, et al. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 2001;124(Pt 1):60–66. [DOI] [PubMed] [Google Scholar]
- 21.Tavano A, Grasso R, Gagliardi C, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130(Pt 10):2646–2660. [DOI] [PubMed] [Google Scholar]
- 22.Counsell SJ, Edwards AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131(Pt 12):3201–3208. [DOI] [PubMed] [Google Scholar]
- 23.De Bruine FT, Van Wezel-Meijler G, Leijser LM, et al. Tractography of white-matter tracts in very preterm infants: a 2-year follow-up study. Dev Med Child Neurol. 2013;55(5):427–433. [DOI] [PubMed] [Google Scholar]
- 24.Rose J, Cahill-Rowley K, Vassar R, et al. Neonatal brain microstructure correlates of neurodevelopment and gait in preterm children 18–22 mo of age: an MRI and DTI study. Pediatr Res. 2015;78(6):700–708. [DOI] [PubMed] [Google Scholar]
- 25.Thompson DK, Lee KJ, van Bijnen L, et al. Accelerated corpus callosum development in prematurity predicts improved outcome. Hum Brain Mapp. 2015;36(10):3733–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henriksen C, Haugholt K, Lindgren M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121(6):1137–1145. [DOI] [PubMed] [Google Scholar]
- 27.Westerberg AC, Schei R, Henriksen C, et al. Attention among very low birth weight infants following early supplementation with docosahexaenoic and arachidonic acid. Acta Paediatr. 2011;100(1):47–52. [DOI] [PubMed] [Google Scholar]
- 28.Picaud JC, Buffin R. Human milk-treatment and quality of banked human milk. Clin Perinatol. 2017;44(1):95–119. [DOI] [PubMed] [Google Scholar]
- 29.Underwood MA. Human milk for the premature infant. Pediatr Clin North Am. 2013;60(1):189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connor DL, Gibbins S, Kiss A, et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: a randomized clinical trial. JAMA. 2016;316(18):1897–1905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.