Abstract
Background
The high demand for personal protective equipment during the novel coronavirus outbreak has prompted the need to develop strategies to conserve supply. Little is known regarding decontamination interventions to allow for surgical mask reuse.
Aim
To identify and synthesize data from original research evaluating interventions to decontaminate surgical masks for the purpose of reuse.
Methods
MEDLINE, Embase, CENTRAL, Global Health, the WHO COVID-19 database, Google Scholar, DisasterLit, preprint servers, and prominent journals from inception to April 8th, 2020, were searched for prospective original research on decontamination interventions for surgical masks. Citation screening was conducted independently in duplicate. Study characteristics, interventions, and outcomes were extracted from included studies by two independent reviewers. Outcomes of interest included impact of decontamination interventions on surgical mask performance and germicidal effects.
Findings
Seven studies met eligibility criteria: one evaluated the effects of heat and chemical interventions applied after mask use on mask performance, and six evaluated interventions applied prior to mask use to enhance antimicrobial properties and/or mask performance. Mask performance and germicidal effects were evaluated with heterogeneous test conditions. Safety outcomes were infrequently evaluated. Mask performance was best preserved with dry heat decontamination. Good germicidal effects were observed in salt-, N-halamine-, and nanoparticle-coated masks.
Conclusion
There is limited evidence on the safety or efficacy of surgical mask decontamination. Given the heterogeneous methods used in studies to date, we are unable to draw conclusions on the most efficacious and safe intervention for decontaminating surgical masks.
Keywords: COVID-19, SARS-CoV-2, Personal protective equipment, Decontamination, Surgical masks, Systematic review
Introduction
As the global spread of novel coronavirus (SARS-CoV-2) continues to escalate, so has the demand for personal protective equipment (PPE), creating global shortages in the supply of N95 filtering face respirators (FFRs) and surgical masks. N95 FFRs are recommended by the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) for use by healthcare providers (HCPs) caring for coronavirus disease (COVID-19) patients requiring airborne precautions and during aerosol-generating procedures [1,2]. Therefore, N95 FFRs are most commonly needed for HCPs in acute care and inpatient settings.
By contrast, surgical masks are recommended for use by HCPs to protect against the risk of droplet transmission in a broader range of inpatient healthcare settings, as well as outpatient settings (e.g. COVID-19 assessment centres, long-term care facilities, and community care settings) [[2], [3], [4]]. As the supply of N95 FFRs is threatened, HCPs may resort to use of surgical masks for airborne precautions [3,[5], [6], [7]]. Surgical masks are also recommended for use by patients with suspected or confirmed COVID-19 to prevent potential spread in a variety of healthcare settings [2,4]. Several institutions now recommend that everyone entering the hospital setting wear a surgical mask [8].
These practices have created an unprecedented demand for surgical masks; unfortunately, the capacity for surge production of PPE is not sustainable in the long term [[5], [6], [7]]. As most face mask PPE are designed for single use, mask rationing and conservation is now a top priority globally [9]. Mask reuse is now suggested as a crisis capacity strategy to conserve available supplies during a pandemic, and much attention has now turned to decontaminating face masks [2,[9], [10], [11]]. Several strategies have been evaluated, including ultraviolet germicidal irradiation (UVGI), chemical disinfectants, and microwave- and heat-based methods; however, most of this literature has focused on the decontamination of N95 FFRs [[12], [13], [14]].
The evidence on the efficacy and safety of decontamination and reuse of surgical masks is unclear. The objective of this systematic review was to evaluate and synthesize the evidence on decontamination interventions for the purpose of surgical mask PPE reuse.
Methods
This systematic review protocol was designed a priori, registered on PROSPERO (April 15th, 2020; CRD42020178290), and uploaded as a pre-print on Open Science Framework (April 8th, 2020; https://osf.io/8wt37/) [15]. The reporting of this systematic review is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Appendix A) [16].
Eligibility criteria
Studies were eligible for inclusion if the following criteria were met: (1) the study was original research, including systematic reviews; (2) the study evaluated surgical face mask PPE or their components; (3) the study evaluated any intervention(s) to decontaminate, sterilize, or treat surgical masks (applied either before or after their use) for the purposes of reuse as PPE; (4) at least one of the following efficacy or safety outcomes of interest was reported: (a) mask performance (i.e. filtration efficiency and airflow resistance); (b) reduction in pathogen load; (c) in-vivo infection rates following use of decontaminated masks; (d) changes in physical appearance (i.e. mask appearance or physical degradation); (e) adverse effects experienced by the wearer (e.g. skin irritation); or (f) feasibility of the intervention (e.g. time, cost, resource utilization). We excluded editorials, case reports, narrative reviews, study protocols, clinical practice guidelines, grey literature, book chapters, and patents.
Database search and study selection
Two health sciences librarians (L.S., M.S.) searched the following electronic databases from their dates of inception to April 8th, 2020: Medline and Medline in Process via OVID, Embase Classic + Embase via OVID, Cochrane CENTRAL (February 2020 issue) and Global Health via CAB Direct. The search strategy was developed in Medline, and then translated into the other databases, as appropriate (Appendix B). Language was restricted to English or French, with no other publication restrictions.
Three journals were hand-searched, as they were relevant to the review but not indexed in any of the electronic databases: Journal of the International Society for Respiratory Protection, Aerosol Science and Technology, and the Journal of Engineered Fibers and Fabrics. A search of Google Scholar (April 8th, 2020) through Publish or Perish was screened until 50 consecutive apparently irrelevant citations were found. Patents, reports, and books were removed. The WHO database on COVID-19 as of April 7th, 2020, was downloaded and searched within Reference Manager. Disaster Lit: Database for Disaster Medicine and Public Health, medRxiv, and OSF Preprints were searched on April 8th, 2020 and citations pertaining to decontamination were downloaded. Citations were imported into Endnote and duplicates were removed.
Citation screening and data extraction
Citations were uploaded to insightScope (www.insightscope.ca) for title/abstract screening and full text review. Citation screening for title/abstract and full text review stages was conducted independently and in duplicate by a team of 11 reviewers recruited from McMaster University, the University of Ottawa, and the University of Manitoba. Prior to gaining access to the full set of citations, each reviewer read the systematic review protocol and was required to achieve a sensitivity of at least 80% when screening a test set of 50 citations (containing five true positives and 45 true negatives). Reviewers achieving less than 80% sensitivity on the test set were provided with additional training. At both title/abstract and full text review, citations were excluded only if both reviewers agreed to exclude; disagreements were resolved by the study leads (D.Z., K.C.) where necessary. Upon completion of full text review, the study co-lead (D.Z.) reviewed all retained citations to identify potential duplicates and confirm eligibility. The reference lists of all citations included for full text review were searched for potential eligible citations that may have evaded the initial database search.
Data were collected using electronic data extraction forms (Microsoft Excel) modified from previous systematic reviews for this protocol and piloted by two investigators (D.Z., S.G.) on two eligible studies [12,13]. Data was extracted from the full text publication and any related publications, referenced published protocols, or supplementary materials. Data extraction was completed in duplicate by two independent reviewers. Where necessary, graphical data was extracted using SourceForge Plot Digitizer (http://plotdigitizer.sourceforge.net) and checked by the second reviewer for accuracy. Disagreements were resolved by the study leads (D.Z., K.C.), where necessary.
Risk of bias assessment
We planned to use recommended risk of bias tools where appropriate [17]; however, in the absence of a standard risk of bias tool for laboratory studies, we applied objective assessment criteria developed for this purpose [14]. Risk of bias was assessed by two reviewers independently and in duplicate at the study level by outcome in the following domains: study design, methodological consistency, population heterogeneity, sampling bias, outcome evaluation, and selective reporting (Appendix C).
Outcomes
The primary outcome for this study was efficacy and safety of the decontamination intervention, as determined by any of the following: mask performance (filtration efficiency (FE) and airflow resistance); reduction in pathogen load; in-vivo infection rates following use of treated masks; mask appearance or physical degradation; or adverse effects experienced by the wearer. FE refers to the percentage of particles filtered at either a specific particle size or a range of particle sizes depending on the testing agent and standard used [18]. Study results reporting percentage particle penetration were converted to FE units (i.e. FE% = 100 – particle penetration) for comparability [18]. Airflow resistance is measured as the pressure reduction across the mask, quantifying initial resistance to airflow in millimetres of water column height pressure per square centimetre (mmH2O/cm2) [19]. Reduction in pathogen load was reported as a log10 reduction factor from a time zero post inoculation to a subsequently measured time-point. If log10 reductions were not reported by the study, we calculated them using the study data provided (e.g. colony counts), where possible. A log10 reduction factor ≥3 was used as a reference indicating good germicidal effect [20]. The secondary outcome was feasibility of the intervention, such as the time, cost, and resources required to implement the intervention. Where results were presented for multiple experimental conditions, the summary of results conducted at the harshest testing conditions were reported, to allow a conservative interpretation of the outcomes evaluated [18].
Statistical analysis
Primary outcome data was analysed descriptively and presented using absolute values and as a percent change where possible. No three studies evaluated the same intervention, nor applied similar test agents or conditions when evaluating outcomes. This precluded our planned quantitative analysis of outcomes [15]; therefore, selected results from included studies were summarized descriptively.
Results
Study identification
Nine of 11 reviewers achieved the 80% sensitivity threshold on the test set. The two reviewers who did not achieve the 80% threshold were provided additional training regarding the screening protocol prior to citation screening. The review team achieved kappa values of 0.38 and 0.43 for title/abstract and full text screening respectively. Study leads resolved conflicts in 3.0% title/abstract and 12.1% full text screening citations.
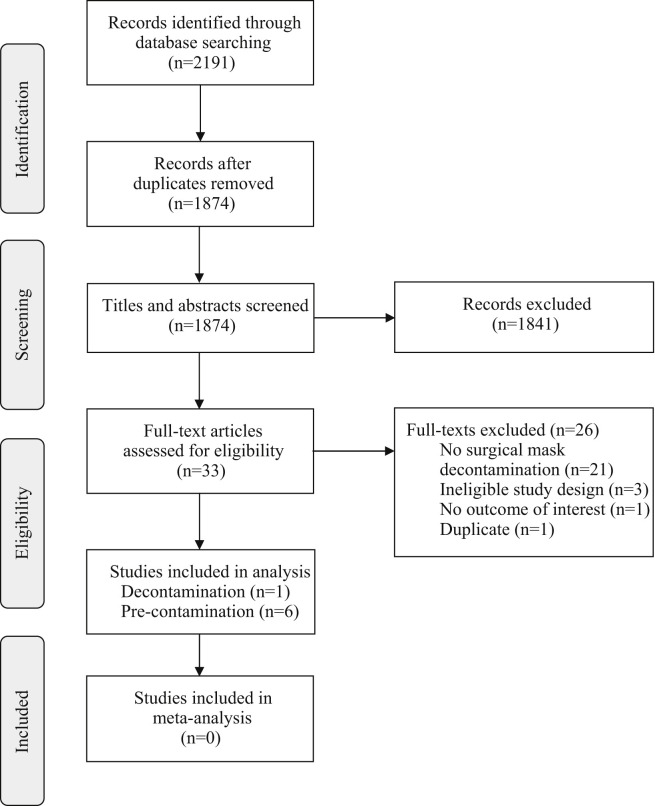
Of 2191 records identified through the initial database search, 1874 unique citations were reviewed and 33 full texts were assessed for eligibility. Twenty-six full texts were excluded for ineligibility, leaving seven unique studies for inclusion in our analysis (PRISMA diagram, Figure 1 ). No additional citations were identified on review of reference lists.
Figure 1.
PRISMA flow diagram of the citation search and screening process.
Study characteristics
Characteristics of included studies are summarized in Table I . Only one of the seven included studies evaluated interventions applied after surgical mask use (i.e. decontamination interventions) [21]. The remaining six studies evaluated interventions applied to masks or mask components prior to use to enhance antimicrobial properties and/or FE for potential reuse or extended use (i.e. pre-contamination interventions) [[22], [23], [24], [25], [26], [27]]. Interventions in these studies were tested on whole masks, pieces of whole masks (referred hereafter as mask pieces) or pieces of individual mask layers (referred hereafter as mask layer pieces). Risk of bias assessments for the included studies are described in Appendix D.
Table I.
Characteristics of included studies
| Interventions | Study | Intervention | Procedure |
Mask/component tested | Testing agent | Control | |||
|---|---|---|---|---|---|---|---|---|---|
| Duration | Cycles | Temperature | Other | ||||||
| Decontamination | Lin et al. [21] | Dry heat (rice cooker) | 3 min | 1 | 149–164°C | – | Mask pieces: 1. Gauze double-layer electret mask 2. Oimo spunlace non-woven masks (models not specified) |
Potassium sodium tartrate tetrahydrate solution (polydispersed droplets) | Untreated mask piece |
| Moist heat (autoclave) | 15 min | 1 | 121°C | Pressure: 1.06 kg/cm2 | |||||
| Ethanol, 70% | 10 min submersion | 1 | Room | Air-dried overnight | |||||
| Isopropanol, 100% | 10 min submersion | 1 | Room | Air-dried overnight | |||||
| Sodium hypochlorite solution, 0.5% | 10 min submersion | 1 | Room | Air-dried overnight | |||||
| Pre-contamination | Quan et al. [25] | Salt (NaCl) coating: 3, 7, 11, 14, and 19 mg NaCl/cm2 |
Solution 1: overnighta Solution 2: 1 daya |
1 | Room | Oven-dried 37°C × 1 day | Salt-coated pieces of polypropylene mask filter (mask model not specified) | Aerosolized virus: H1N1 CA/09 H1N1 PR/34 H5N1 VN/04 |
Uncoated mask filter |
| Tseng et al. [27] | Quaternary ammonium agent (Goldshield 5; GS5), 1% in sterile water | Applied with common spray bottle (4 sprays = 1 mL) |
Room | Air-dried × 24 h | GS5-coated pieces of 3 mask layers and GS5-coated full mask (AERO PRO, Taiwan; model not specified) | Aerosolized bacteria: A. baumannii ATCC17978 E. faecalis ATCC29212 S. aureus ATCC29213 |
Sterile water-coated mask | ||
| Demir et al. [22] | N-halamine (1-chloro-2,2,5,5-tetramethyl-4-imidazolidinone), 1 wt% in ethanol | 10 min submersion. Padded through laboratory wringer |
1 | Room | Air-dried × 24 h | N-halamine-coated pieces of polypropylene mask filter (Hollingsworth & Vose Co., USA; model not specified) | Aerosolized bacteria: S. aureus ATCC6538 E. coli 0157:H7 ATCC43895 |
Uncoated mask filter | |
| Li et al. [24] | Silver nitrate and titanium dioxide nanoparticle emulsion, 0.4 mg/cm2 | Coated to desired concentration with textile-finishing machine (China patent 03142467) |
Full masks with nanoparticle-coated outermost mask layer (Winner Medical Group, China; model not specified) |
Aerosolized pathogen simulant: KCl–fluorescein | Uncoated mask layer | ||||
| Li et al. [23] | Silver nitrate and titanium dioxide nanoparticle emulsion, 0.4 mg/cm2 | Coated to desired concentration with textile-finishing machine (China patent 03142467) | Nanoparticle-coated pieces of outermost mask layer (Winner Medical Group, China; model not specified) | Bacterial suspensions: E. coli ATCC25922 S. aureus ATCC25923 |
Uncoated fabric | ||||
| Shen et al. [26] | Fluorochemical repellent (Zonyl® PPR Protector), 6% and 12% | Unspecified submersion time. Padded through laboratory wringer |
2 | Room | Dried at 176°F × 2 min. Cured at 250°F 2 min |
Three full masks made of nonwoven mask components with repellant applied to cover layer (models not specified) | Aerosolized pathogen simulant: Latex micro-spheres + synthetic blood | Uncoated mask | |
ATCC, American Type Culture Collection; GS5, Goldshield 5 quaternary ammonium agent; H1N1 CA/09, H1N1 influenza virus (A/California/04/2009); H1N1 PR/34, H1N1 influenza virus (A/Puerto Rico/08/2934); H5N1 VN/04, H5N1 influenza virus (A/Vietnam/1203/2004); KCl, potassium chloride; NaCl, sodium chloride.
Incubated in 600 μL of salt-coating solution overnight at room temperature, then immersed and oven-dried for one day in a second salt-coating solution (0, 100, 300, 600, 900, or 1200 μL) to achieve different salt-coating (sodium chloride, in mg) on filter per unit area.
Decontamination interventions for surgical mask reuse
Lin et al. evaluated five decontamination interventions on mask pieces of two surgical mask types commonly used in Taiwanese hospitals (gauze double-layer electret masks and Oimo spunlace non-woven masks; models unspecified): dry heat (via rice cooker), high-pressure moist heat (i.e. autoclave), and three chemical agents (70% ethanol, 100% isopropanol, and 0.5% sodium hypochlorite (i.e. bleach)) [21]. Study methods and findings are summarized in Table II . Mask pieces were assessed for FE, airflow resistance, and physical characteristics following decontamination. FE was presented graphically for a range of particle sizes (0.0146–0.594 μm); the results were summarized for FE at 0.1 μm, a standard particle size for particulate FE testing [18]. At baseline, gauze and spunlace mask pieces had FEs of approximately 87% and 45%, respectively. FE in both mask pieces decreased after each decontamination intervention, but dry heat decontamination of gauze mask pieces demonstrated the smallest change (absolute FE reduction of 1.3%). Moist heat and chemical decontamination interventions all resulted in greater absolute FE reductions (12%–36%). Bleach was the most damaging method, resulting in a 15.3% absolute FE reduction in spunlace mask pieces and destruction of gauze mask pieces.
Table II.
Decontamination methods and summary of results by outcome [21].
| Test agent and conditions | Intervention | Mask/component | Findings | Comments |
|---|---|---|---|---|
| Filtration efficiencya | FE% (absolute difference from control) | |||
| Charge neutralized polydispersed potassium sodium tartrate tetrahydrate droplets (results for 0.1 μm particle diameter). 5.95 L/min flow rate over mask pieces (stated to be equivalent to 85 L/min on full mask) |
Dry heat | Gauze | 85.6 (–1.3) | Dry heat caused the least reduction in FE; P-values not reported for this particle size |
| Spunlace | 40.6 (–4.3) | |||
| Autoclave | Gauze | 74.2 (–12.8) | ||
| Spunlace | 43.7 (–12.1) | |||
| Ethanol | Gauze | 70.5 (–16.5) | ||
| Spunlace | 31.0 (–13.8) | |||
| Isopropanol | Gauze | 50.8 (–36.1) | ||
| Spunlace | Not reported | |||
| Bleach | Gauze | Not assessable (mask destroyed) | ||
| Spunlace | 29.6 (–15.3) | |||
| Airflow resistanceb | Pressure reduction in mmH2O (absolute difference from control) | |||
| Applied: 5.95 L/min flow rate over mask pieces (stated to be equivalent to 85 L/min on full mask) | Dry heat | Gauze | 3.9 (+0.1), NS | No significant changes in airflow resistance following dry heat and ethanol decontamination (gauze mask only) |
| Spunlace | 1.4 (+0.1), P < 0.05 | |||
| Autoclave | Gauze | 3.1 (–0.7), P < 0.05 | ||
| Spunlace | 1.6 (+0.3), P < 0.05 | |||
| Ethanol | Gauze | 3.9 (+0.2), NS | ||
| Spunlace | 1.7 (+0.4), P < 0.05 | |||
| Isopropanol | Gauze | Not reported | ||
| Spunlace | Not reported | |||
| Bleach | Gauze | Not assessable (mask destroyed) | ||
| Spunlace | 1.6 (+0.3), P < 0.05 |
FE, filtration efficiency; NS, not statistically significant.
FE to testing agent used, expressed as a percentage. A higher percentage filtration efficiency indicates better mask performance. Results in study were presented as percentage particle penetration, and converted to filtration efficiency (FE % = 100 – particle penetration) for consistency of reporting in this systematic review.
Airflow resistance assessed the ‘breathability’ of the mask at tidal breathing. A lower airflow resistance means better breathability.
Airflow resistance was assessed at a flow rate of 5.95 L/min [21]. Statistically significant changes in pressure reduction were reported following all decontamination interventions, except for dry heat and ethanol on gauze mask pieces [21]. Airflow resistance results were not reported for bleach on gauze mask pieces (mask destroyed), or for isopropanol for either mask type. Physical characteristics were reported only for gauze mask pieces; the autoclave deformed and caused observable folds in the mask filter, and bleach destroyed the mask. Physical characteristics following decontamination with other interventions, or in spunlace mask pieces, were not reported. Germicidal effects of the five decontamination methods were not assessed.
Pre-contamination interventions
Six studies evaluated five unique pre-contamination methods applied prior to mask use: four were antimicrobial interventions (nanoparticle emulsion, quaternary ammonium agent, monochlorinated N-halamine, sodium chloride (NaCl) salt), and one was a fluorochemical repellent [[23], [24], [25], [26], [27]] (Table I).
These interventions have a variety of proposed mechanisms of action. Quan et al. evaluated multiple concentrations of an NaCl salt coating applied to the polypropylene layer of a three-ply surgical mask, which inactivates viruses by hyperosmotic stress upon the viral envelope [25]. Tseng et al. tested a quaternary ammonium agent (Goldshield 5 [GS5], AP Goldshield LLC, USA), a disinfectant widely used in medical, commercial, and household settings [27]. Demir et al. applied a monochlorinated N-halamine coating on to the polypropylene layer of a surgical mask, which inactivates Gram-negative and -positive bacteria through direct transfer of oxidative halogens to the cell membrane [22]. Li et al. conducted two studies evaluating a silver nitrate and titanium dioxide nanoparticle emulsion coating of the outer layer of a surgical mask [23,24]. Silver inactivates bacteria by interacting with the thiol groups of enzymes, and titanium oxide creates microbicidal hydroxyl radicals [23]. Finally, Shen et al. applied a fluorochemical finish (Zonyl® PPR Protector, Ciba Specialty Chemicals, Basel, Switzerland) to the outer layer of surgical masks to enhance resistance to fluid penetration [26].
Filtration efficiency and airflow resistance following pre-contamination
Five studies evaluated the effects of their intervention on FE, airflow resistance, or both, applying different testing techniques (Table III ). Quan et al. used aerosolized H1N1 influenza virus (2.5–4 μm particle diameter) to evaluate FE on polypropylene mask filter pieces and found that increasing concentrations of the salt coating increased filter FE (54.4%, 69%, and 83.9% for 3, 11, and 19 mg NaCl/cm2, respectively) [25]. Airflow resistance was not assessed. Tseng et al. evaluated FE of GS5-coated mask layer pieces using aerosolized bacteria (0.5–2.1 μm particle diameter) and GS5-coated masks using NaCl (0.075 μm particle diameter), respectively [27]. They found no statistically significant change in FE in GS5-coated masks or mask layer pieces (0.6%–1.8% FE increase in polypropylene filter layer, 1.8% FE reduction in mask; not significant). Airflow resistance was also not significantly affected (pressure decrease of 1.0 mmH2O; not significant). Demir et al. only assessed airflow resistance and found similar results in N-halamine-coated polypropylene filters compared to uncoated filters (26.7 mL/s/cm2; not significant) [22].
Table III.
Pre-contamination methods and summary of results by outcome
| Study | Test agent and conditions | Intervention | Mask/component | Findings | Comments | |||
|---|---|---|---|---|---|---|---|---|
| Filtration efficiencya | FE% (absolute difference from control) | |||||||
| Quan et al. [25] | Unneutralized virus aerosol (H1N1 CA/09; 2.5–4 μm volumetric mean diameter) at 17 kPa vacuum | Salt coating | Polypropylene filter | Uncoated: 0.8 3 mg/cm2: 54.3 (+53.6) 11 mg/cm2: 69.0 (+68.2) 19 mg/cm2: 83.9 (+83.1) |
Increasing FE observed with increasing salt coating concentrations; P-values not reported for comparisons with control. | |||
| Tseng et al. [27] | Unneutralized bacteria aerosol (104 cfu/m3 experiment; 0.5–2.1 μm aerodynamic particle diameter) at 46 L/min flow rate (stated as equivalent to 95 L/min on full mask) | GS5 | A. baumannii | E. faecalis | S. aureus | P-value | No significant increase in FE of mask filter layer with GS5 coating. | |
| Outer layer | 60.7 (+6.3) | 55 (+12.3) | 69.3 (+18.2) | 0.005 | ||||
| Polypropylene filter | 99.3 (+1.8) | 99.8 (+1.1) | 99.9 (+0.6) | NS | ||||
| Interior layer | 65.4 (+8.9) | 59.5 (+10.0) | 62.8 (+6.5) | 0.02 | ||||
| Charge-neutralized NaCl aerosol (0.075 μm median particle diameter) at 85 L/min flow rate | GS5 | Full mask | 77.6 (–1.8), NS | No significant change in FE with GS5 coating. | ||||
| Li et al. [24] | Aerosolized KCl–fluorescein solution sprayed on to full mask worn by exercising human subjects. Flow rate, particle size and charge not reported. | Nanoparticle emulsion | Outer layer | 82.0 (+2.0) | Results presented as percentage of total KCl particles found in each layer, respectively; KCl not reported for subject's face (i.e. cannot determine penetration through mask). P-values not reported for comparisons with control. | |||
| Polypropylene filter | 13.0 (–3.0) | |||||||
| Interior layer | 4.5 (+1.5) | |||||||
| Shen et al. [26] | Aerosolized latex microspheres + synthetic blood (average particle size 1.0 μm). Flow rate and particle charge not reported. Each layer tested three times, in three individual masks. | Repellant | Uncoated (0% repellant) | 6% repellant | 12% repellant | P-value | Results presented as percentage of total particles found on each mask layer respectively in the three masks tested. 0% found in inner layers of all masks, suggesting FE of native mask filter was unchanged by presence of repellent-coated outer layer. Outcome evaluated with laser scanning confocal microscope. | |
| Outer layer | 36.2% | 25.8% | 27.4% | <0.0001 | ||||
| 37.7% | 26.8% | 27.4% | ||||||
| 40.6% | 29.2% | 29.4% | ||||||
| Polypropylene filter | 63.4% | 74.2% | 72.0% | 0.032 | ||||
| 62.2% | 73.2% | 72.0% | 0.043 | |||||
| 55.3% | 56.5% | 57.2% | NS | |||||
| Interior layer | 0.0% | 0.0% | 0.0% | Not reported | ||||
| 0.0% | 0.0% | 0.0% | ||||||
| 0.0% | 0.0% | 0.0% | ||||||
| Airflow resistanceb | Measured variable (absolute difference from control) | |||||||
| Tseng et al. [27] | Applied: flow rate (85 L/min) over full mask | GS5 | Full mask | Pressure reduction: 16.8 (+1.0) mmH2O, NS |
No significant changes in airflow resistance | |||
| Demir et al. [22] | Applied: pressure reduction (12.7 mmH2O) over mask filter piecesc | N-halamine | Polypropylene filter | Flow rate: 26.7 (–0.6) mL/s/cm2, P-values not reported | ||||
| Li et al. [23] | Applied: pressure reduction (10 mmH2O) over full mask | Nanoparticle emulsion | Full mask | Flow rate: 18.4 (–1.4) mL/s/cm2, NS | ||||
| Germicidal effect | Pathogen load (log10 reduction) | |||||||
| Quan et al. [25] | Aerosolized influenza virus (strain not reported) with post-inoculation incubation of 5–60 min. | Salt coating | Polypropylene filter | Log10 reduction at 5, 15, 60 min post exposured,e N0 = viral load on untreated filter at 5 min incubation Uncoated: 0.0 (N0), 1.7, 2.0 3 mg/cm2: 2.7, 2.8, BDL, P < 0.001 11 mg/cm2: 2.9, 3.0, BDL, P < 0.001 19 mg/cm2: 3.0, 3.0, BDL, P < 0.001 |
Good germicidal effect (log10 reduction >3 in pathogen load) after 60 min incubation in all concentrations of salt coating. | |||
| Tseng et al. [27] | Aerosolized bacteria (104 cfu/m3 experiment) with post-inoculation incubation of 0 min. N0 = sterile water-coated mask pathogen load immediately post exposure |
GS5 | Log10 reduction immediately post exposuref | Inadequate germicidal effect (log10 reduction <<3 in pathogen load) on all GS5-coated mask layers. | ||||
| A. baumannii | E. faecalis | S. aureus | P-value | |||||
| Outer layer | 0.8 | 0.9 | 0.8 | Not reported | ||||
| Polypropylene filter | 1.0 | 0.8 | 0.8 | Not reported | ||||
| Interior layer | 0.8 | 0.9 | 0.9 | Not reported | ||||
| Demir et al. [22] | Aerosolized bacteria with post-inoculation incubation of 10 min. N0 = uncoated mask at 10 min post exposure |
N-halamine | Polypropylene filter | Log10 reduction at 10 min post exposured | Good germicidal effect (log10 reduction >3 in pathogen load) by 10 min | |||
| S. aureus | E. coli | p-value | ||||||
| 4.4 | 3.2 | Not reported | ||||||
| Li et al. [23] | Bacterial suspensions (105 cfu/mL) with post-inoculation incubation of 0 and 24 h N0 = uncoated mask at 0 h |
Nanoparticle emulsion | Outer layer | Log10 reduction at 0, 24 h post exposured | Good germicidal effect (log10 reduction >3 in pathogen load) by 24 h | |||
| S. aureus | E. coli | P-value | ||||||
| Uncoated: 0.0 (N0), –0.3 | Uncoated: 0.0 (N0), 0.1 | Not reported | ||||||
| Nanoparticle-coated: –0.2, 5.7 | Nanoparticle-coated: 1.4, 5.9 | |||||||
| In-vivo infection prevention | Measured variable | |||||||
| Quan et al. [25] | Aerosolized virus (H1N1 CA/09, H1N1 PR/34, or H5N1 VN/04) 8-week-old female inbred BALB/c mice N0 = lung viral titer (H1N1 CA/09) following aerosolization through uncoated filter |
Salt coating | Polypropylene filter | 16-day survival (%) | All mice protected by salt-coated filter barriers survived after exposure to lethal dose of aerosolized virus (surrogate mortality endpoint of >25% loss in body weight). Full data across all viruses available only for 11 mg/cm2 salt-coated filter | |||
| H1N1 CA/09 | H1N1 PR/34 | H5N1 VN/04 | ||||||
| Uncoated: 0% 3 mg/cm2: 100% 11 mg/cm2: 100% |
Uncoated: 0% 19 mg/cm2: 100% 11 mg/cm2: 100% |
Uncoated: 0% 11 mg/cm2: 100% |
||||||
| Log10 reduction in lung viral titreg | All surviving mice had reduced, but detectable, lung viral titres. | |||||||
| Uncoated: 0.0 (N0) | ||||||||
| 3 mg/cm2: 0.6, P < 0.005 | ||||||||
| 11 mg/cm2: 1.1, P < 0.005 | ||||||||
| 19 mg/cm2: 1.3, P < 0.005 | ||||||||
BDL, below detection limit; cfu, colony-forming units; FE, filtration efficiency; GS5, Goldshield 5 quaternary ammonium agent; H1N1 CA/09, H1N1 influenza virus (A/California/04/2009); H1N1 PR/34, H1N1 influenza virus (A/Puerto Rico/08/2934); H5N1 VN/04, H5N1 influenza virus (A/Vietnam/1203/2004); KCl, potassium chloride; N0, time zero from which log10 reduction factor was calculated; NaCl, sodium chloride; NS, not statistically significant.
FE to testing agent used, expressed as a percentage. A higher percentage filtration efficiency indicates better mask performance.
Airflow resistance assessed the ‘breathability’ of the mask at tidal breathing. A lower airflow resistance means better breathability.
Study reported pressure reduction and flow rate in inches of water and cubic feet per minute per square foot, respectively. Results converted to SI units.
Colony-forming units or plaque-forming units reported in study, as applicable. Results converted to log10 reduction factors.
Plaque-forming units below detectable limit. Detection limit of assay not reported; log10 reduction factor cannot be calculated.
Colony-forming unit reduction percentages reported in study. Results converted to log10 reduction factors.
Absolute values for lung viral titres reported in study. Results converted to log10 reduction factors.
Li et al. used a potassium fluorescein solution (particle size not reported) to evaluate FE in nanoparticle-coated full masks by: (1) the percentage potassium content of each mask layer relative to the potassium content of the whole mask; and (2) a seven-point scale rating fluorescent stains on mask users' faces [24]. They found that the percentage potassium content of each mask layer was similar compared to that of uncoated masks (+2%, –3% and +1.5% absolute difference from control for outer, middle and interior mask layers, respectively), and similar ratings of fluorescent stains. Airflow resistance was non-significantly increased (+1.4 mL/s/cm2). Shen et al. used an aerosolized pathogen simulant (1.0 μm particle diameter) and quantified FE as the proportion of particle content on each mask layer to that of the whole mask [26]. They reported significant decreases in particle content on the repellant-coated outer mask layer coated with repellant (P < 0.0001), but no changes to particle content on mask layers following the filter layer (suggesting no changes to FE of the mask as a whole). Airflow resistance was not assessed.
Germicidal effects of pre-contamination interventions
Four studies evaluated germicidal effects using a variety of aerosolized pathogens [22,25,27] or bacterial suspensions [22,23], and assessed the pathogen load upon the mask or mask component(s) tested after different incubation periods (Table III). All three tested concentrations of salt-treated polypropylene mask filters exhibited reductions in bacterial colony counts at 5 and 15 min post incubation, and were below detection limit (BDL) by 60 min [25]. Germicidal effect was strongest in the 19 mg NaCl/cm2 salt-coated mask filter (log10 reduction factor ≥3 at 5 and 15 min, and BDL at 60 min). Colony counts demonstrated ≥3 log10 reduction factors in N-halamine-coated mask filters when evaluated 10 min after a 3 h bacterial aerosol test [22], and in the nanoparticle-coated mask outer layer at 24 h post-incubation with bacterial suspensions [23]. GS5-coated mask layers did not appear to achieve adequate reduction in pathogen load (log10 reduction factor ≤1 in all experiments) [27].
In-vivo infection was evaluated by Quan et al., where 16-day survival of mice was assessed following exposure to aerosolized influenza viruses through a salt-coated mask filter barrier compared to an uncoated filter barrier [25]. All mice sheltered by uncoated filter barriers were euthanized after losing more than 25% of their body weight by day 16 (a surrogate outcome for mortality), whereas none of the mice sheltered by any concentration of salt-coated mask filter met this endpoint (i.e. 100% survival rate) [25]. Lung viral titres were also assessed; titres were lower, but still detectable, in lungs of mice sheltered by the salt-coated mask filters compared to those sheltered by uncoated filter barriers.
Physical characteristics and adverse events following pre-contamination
Two studies assessed physical characteristics of nanoparticle-coated masks and/or adverse effects after their use in human subjects [23,24]. A survey of blinded users of nanoparticle-coated and uncoated masks demonstrated no differences in perceived heat, humidity, odour, breathability, itchiness, or user comfort between the two groups (not significant in each survey domain) [24]. Symptom inquiry and physical examination of the facial skin in users of nanoparticle-coated and uncoated masks demonstrated no signs or symptoms of skin irritation [23].
Discussion
This systematic review on decontamination interventions for surgical mask PPE reveals the following key findings. First, there is a paucity of research evaluating decontamination interventions on surgical masks; there is only one study evaluating methods intended for decontaminating masks following use, and six studies evaluating pre-contamination methods to enhance the antimicrobial and/or filtration performance of surgical masks. Second, included studies evaluated different interventions, using heterogeneous and non-standardized test conditions, and assessed a variety of outcomes using inconsistent methods. This limits our ability to compare between interventions or make conclusions regarding their efficacy or safety. No studies provided explicit information on the feasibility of these interventions in institutional or hospital settings, making it challenging to determine whether any of the interventions evaluated could be easily implemented during situations of high PPE demand.
Each decontamination intervention applied in the included studies is founded in evidence-based scientific rationale. Heat is known to inactivate pathogens, including coronaviruses [[28], [29], [30]]; moist heat is a standard intervention for sterilization in healthcare settings [31,32]. The chemical disinfectants evaluated are also widely used in household, commercial, healthcare, and food industry settings [31,33]. Based on the limited evidence in this review, dry heat may alter surgical mask performance less than high-pressure moist heat or chemical interventions; however, the germicidal effect of dry heat in surgical masks is unclear. Bleach is not a safe method of decontaminating surgical masks; mask performance is significantly altered and safety data from N95 FFR studies suggest potential health risks associated with off-gassing [14]. With respect to pre-contamination interventions, salt film, GS5, nanoparticle emulsion, and N-halamine mask coatings were reported not to have detrimental effects on mask performance. N-Halamine and nanoparticle emulsion showed strong germicidal effects in masks (log10 reduction factors ≥3), which is consistent with their application in medical devices [34], and in food and water treatment [35]. Salt films also demonstrated strong germicidal effects, but their application has been experimental to date [25]. An important consideration is that pathogen load was evaluated at different post-inoculation incubation time-points in each study (i.e. 5 min to 24 h); it is well established that viral load reductions can occur by virtue of time [36].
Ideal PPE decontamination methods should not only demonstrate effective reductions in pathogen load, but also preserve mask performance without causing any residual chemical hazard to the wearer [37]. Results of included studies should be interpreted cautiously for the following reasons: (1) some of the mask types used in these experiments appear to have baseline FEs below reference standards, which may have affected the results observed [18]; (2) experiments and test conditions applied to mask pieces or individual layers cannot necessarily be extrapolated to whole masks; and (3) testing methods and outcome assessments were heterogeneous. Unlike N95 FFRs, surgical masks are not certified under standardized National Institute for Occupational Safety and Health regulations. The Food and Drug Administration (FDA) recommends that several standards (ASTM F2101, ASTM 2299, Mil-M369454C, or modified Greene and Vesley method) may be applied to surgical masks, complicating the evaluation of mask performance in this review [18]. There are many test conditions that can impact FE, such as particle size, particle charge (i.e. whether charge-neutralized or not), and face velocity (i.e. flow rate); however, the FDA and ASTM do not have uniform recommended standards [18]. The evidence that we have collated in this systematic review is therefore important and essential.
This systematic review reveals that the body of evidence on decontamination interventions for surgical masks is scant compared to N95 FFRs. Three recent systematic reviews have revealed 22 unique studies evaluating microwave irradiation, heat, chemical disinfectants, and UVGI for decontamination of N95 FFRs [[12], [13], [14]]. UVGI and vaporous hydrogen peroxide showed favourable evidence for germicidal effects without significant changes in mask performance; however, we found no publications evaluating these methods in surgical masks. The lack of research on surgical masks may stem from assumptions that methods effective in N95 FFRs can be extrapolated to surgical masks, and some institutions are already applying the same decontamination methods to both [38]. Considering this systematic review demonstrates that mask types can perform differently after decontamination, and that surgical masks and N95 FFRs perform differently with aerosol challenges [21,39], we cannot conclude that decontamination methods can be effectively or safely applied to all mask types. Furthermore, common components of surgical masks, such as cellulose-based materials, are known to degrade vaporous hydrogen peroxide and reduce the efficacy of sterilization [40]. There is also limited data evaluating the effectiveness of any PPE decontamination intervention against SARS-CoV-2 [38,41], although more studies are underway. Independent research on surgical masks is therefore critical in order to inform clinicians, infection control experts, and public health administrators on how best to advise safe decontamination and reuse practices.
Our systematic review has several important strengths. To our knowledge, this is the first systematic review of decontamination interventions in surgical mask PPE and provides important information describing the nature of interventions and outcomes evaluated to date. Our review highlights the variability in study methods and outcome reporting. As a result, we identified the following core outcomes to consider when conducting research in this field, to encourage consistent methodology and transparent reporting: mask performance (FE, airflow resistance), decontamination effects (germicidal effects, in-vivo infection rates), physical characteristics of decontaminated masks, adverse effects to mask users, and intervention feasibility. We also developed a systematic tool with which to assess risk of bias in this body of literature.
Our review also has limitations. We were unable to conduct any meta-analyses due to the paucity of studies and their heterogeneous methodologies and outcome assessments. Outcomes described in this systematic review required summarizing study results from multiple experiments; we rationalized the selective reporting of results in our methods to encourage conservative interpretation of the findings. Given the rapidly evolving landscape of PPE literature during the SARS-CoV-2 pandemic, we plan to update this systematic review at regular intervals for new relevant evidence as it becomes available (i.e. ‘living systematic review’) [42].
Conclusion
There is inadequate evidence on the safety or efficacy of any decontamination intervention for extended use or reuse of surgical masks in the clinical setting. Further research should therefore be conducted specifically in surgical masks, including decontamination interventions demonstrating promise in N95 FFRs (e.g. UVGI, vaporized hydrogen peroxide). To ensure the safety of HCPs and all end-users, the same rigorous standard of research should be applied to surgical masks as with N95 FFRs, given its much broader applications as PPE. We recommend that future studies consider applying core outcomes and test conditions that are in accordance with acceptable industry standards in their design, to enable transparency of reporting and comparisons of efficacy between interventions.
Acknowledgements
The authors are grateful to the following people for their contributions to citation screening (in alphabetical order): A. Agarwal, K. Agyei, J. Gibson, H. Guerra, R. Gupta, M. Kaur, R. Ng, E. Staykov.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.07.007.
Conflict of interest statement
None declared.
Funding sources
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html [last accessed August 2020].
- 2.World Health Organization . WHO; Geneva: 2020. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages: interim guidance, 6 April 2020. [Google Scholar]
- 3.MacIntyre C.R., Chughtai A.A. Facemasks for the prevention of infection in healthcare and community settings. BMJ. 2015;350:h694. doi: 10.1136/bmj.h694. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . WHO; Geneva: 2020. Advice on the use of masks in the context of COVID-19: interim guidance, 6 April 2020. [Google Scholar]
- 5.Carias C., Rainisch G., Shankar M., Adhikari B., Swerdlow D., Bower W., et al. Potential demand for respirators and surgical masks during a hypothetical influenza pandemic in the United States. Clin Infect Dis. 2015;60(Suppl 1):S42–S51. doi: 10.1093/cid/civ141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel A., D’Alessandro M.M., Ireland K.J., Burel W.G., Wencil E.B., Rasmussen S.A. Personal protective equipment supply chain: lessons learned from recent public health emergency responses. Health Secur. 2017;15:244–252. doi: 10.1089/hs.2016.0129. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan A., Jernign D.B., Liedtke L., Strausbaugh L. Hospital preparedness for severe acute respiratory syndrome in the United States: views from a national survey of infectious diseases consultants. Clin Infect Dis. 2004;39:272–274. doi: 10.1086/421777. [DOI] [PubMed] [Google Scholar]
- 8.Klompas M., Morris C.A., Sinclair J., Pearson M., Shenoy E.S. Universal masking in hospitals in the Covid-19 era. N Eng J Med. 2020 doi: 10.1056/NEJMp2006372. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Strategies for optimizing the supply of facemasks. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html [last accessed August 2020].
- 10.Livingston E., Desai A., Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA. 2020 doi: 10.1001/jama.2020.5317. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html [last accessed August 2020].
- 12.Gertsman S., Agarwal A., O’Hearn K., Webster R.J., Tsampalieros A., Barrowman N., et al. Microwave-and heat-based decontamination of N95 filtering facepiece respirators (FFR): a systematic review. OSF Preprints. 2020 doi: 10.31219/osf.io/4whsx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Hearn K., Gertsman S., Sampson M., Webster R.J., Tsampalieros A., Ng R., et al. Decontaminating N95 masks with ultraviolet germicidal irradiation (UVGI) does not impair mask efficacy and safety: a systematic review. OSF Preprints. 2020 doi: 10.31219/osf.io/29z6u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Hearn K., Gertsman S., Webster R.J., Tsampalieros A., Ng R., Gibson J., et al. Efficacy and safety of disinfectants for decontamination of N95 and SN95 filtering facepiece respirators: a systematic review. OSF Preprints. 2020 doi: 10.31219/osf.io/ct6m8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zorko D.J., Choong K., McNally D., O’Hearn K., Sampson M., Sikora L. Decontamination interventions for the reuse of surgical mask personal protective equipment (PPE): a protocol for a systematic review. OSF Preprints. 2020 doi: 10.31219/osf.io/8wt37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Higgins J.P.T. Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rengasamy S., Shaffer R., Williams B., Smit S. A comparison of facemask and respirator filtration test methods. J Occup Environ Hyg. 2017;14:92–103. doi: 10.1080/15459624.2016.1225157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Society for Testing and Materials (ASTM) F2100-19e1 . ASTM International; West Conshohocken, PA: 2019. Standard specification for performance of materials used in medical face masks. [DOI] [Google Scholar]
- 20.Fisher E.M., Noti J.D., Lindsley W.G., Blachere F.M., Shaffer R.E. Validation and application of models to predict facemask influenza contamination in healthcare settings. Risk Anal. 2014;34:1423–1434. doi: 10.1111/risa.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin T.-H., Chen C.-C., Huang S.-H., Kuo C.-W., Lai C.-Y., Lin W.-Y. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: effects of five decontamination methods. PloS One. 2017;12(10) doi: 10.1371/journal.pone.0186217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demir B., Cerkez I., Worley S., Broughton R., Huang T.-S. N-halamine-modified antimicrobial polypropylene nonwoven fabrics for use against airborne bacteria. ACS Appl Mater Interfaces. 2015;7:1752–1757. doi: 10.1021/am507329m. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Leung P., Yao L., Song Q., Newton E. Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect. 2006;62:58–63. doi: 10.1016/j.jhin.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Wong T., Chung J., Guo T.P., Hu J.Y., Guan Y.T., et al. In vivo protective performance of N95 respirator and surgical facemask. Am J Ind Med. 2006;49:1056–1065. doi: 10.1002/ajim.20395. [DOI] [PubMed] [Google Scholar]
- 25.Quan F.-S., Rubino I., Lee S.-H., Koch B., Choi H.-J. Universal and reusable virus deactivation system for respiratory protection. Sci Rep. 2017;7:1–10. doi: 10.1038/srep39956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen H., Leonas K.K. Study of repellent finish of filtration ability of surgical face masks. Int Nonwovens J. 2005;(4) doi: 10.1177/1558925005os-1400403. [DOI] [Google Scholar]
- 27.Tseng C.-C., Pan Z.-M., Chang C.-H. Application of a quaternary ammonium agent on surgical face masks before use for pre-decontamination of nosocomial infection-related bioaerosols. Aerosol Sci Tech. 2016;50:199–210. doi: 10.1080/02786826.2016.1140895. [DOI] [Google Scholar]
- 28.Leclercq I., Batejat C., Burguière A.M., Manuguerra J.C. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir Viruses. 2014;8:585–586. doi: 10.1111/irv.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabenau H., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin T.H., Tang F.C., Hung P.C., Hua Z.C., Lai C.Y. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018;28:754–762. doi: 10.1111/ina.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutala W.A., Weber D.J. 2008. Guideline for disinfection and sterilization in healthcare facilities.https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html Available at: [last accessed August 2020] [Google Scholar]
- 32.Swenson V.A., Stacy A.D., Gaylor M.O., Ushijima B., Philmus B., Cozy L.M., et al. Assessment and verification of commercially available pressure cookers for laboratory sterilization. PloS One. 2018;13(12) doi: 10.1371/journal.pone.0208769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worley S.D., Williams E., Crawford R.A. Halamine water disinfectants. Crit Rev Environ Sci Technol. 1988;18:133–175. doi: 10.1080/10643388809388345. [DOI] [Google Scholar]
- 34.Prabhu S., Poulose E.K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2:32. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- 35.Kenawy E.-R., Worley S., Broughton R. The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. 2007;8:1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 36.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Health Canada. Important regulatory considerations for the reprocessing of single use N95 respirators during the COVID-19 response: Notice. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/activities/announcements/covid19-notice-reprocessing-n95-respirators.html [last accessed August 2020].
- 38.Kumar A., Kasloff S.B., Leung A., Cutts T., Strong J.E., Hills K., et al. N95 Mask decontamination using standard hospital sterilization technologies. medRxiv. 2020 doi: 10.1101/2020.04.05.20049346. [DOI] [Google Scholar]
- 39.Bałazy A., Toivola M., Adhikari A., Sivasubramani S.K., Reponen T., Grinshpun S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:51–57. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 40.McEvoy B., Rowan N.J. Terminal sterilization of medical devices using vaporized hydrogen peroxide: a review of current methods and emerging opportunities. J Appl Microbiol. 2019;127:1403–1420. doi: 10.1111/jam.14412. [DOI] [PubMed] [Google Scholar]
- 41.Fischer R., Morris D.H., van Doremalen N., Sarchette S., Matson J., Bushmaker T., et al. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.04.11.20062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott J.H., Turner T., Clavisi O., Thomas J., Higgins J.P.T., Mavergames C., et al. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med. 2014;11:e1001603. doi: 10.1371/journal.pmed.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.