Abstract
Objective
Coronavirus disease 2019 (COVID-19) has raised concern around the world as an epidemic or pandemic. As data on COVID-19 has grown, it has become clear that older adults have a disproportionately high rate of death from COVID-19. This study describes the early clinical characteristics of COVID-19 in patients with more than 80 years of age.
Methods
Epidemiological, clinical, laboratory, radiological, and treatment data from 17 patients diagnosed with COVID-19 between January 20 and February 20, 2020 were collected and analyzed retrospectively. Treatment outcomes among subgroups of patients with non-severe and severe symptoms of COVID-19 were compared.
Results
Of the 17 hospitalized patients with COVID-19, the median age was 88.0 years (interquartile range, 86.6–90.0 years; range, 80.0–100.0 years) and 12 (70.6%) were men. The age distribution of patients was not significantly different between non-severe group and severe group. All patients had chronic pre-existing conditions. Hypertension and cardiovascular diseases were the most common chronic conditions in both subgroups. The most common symptoms at the onset of COVID-19 were fever (n = 13; 76.5%), fatigue (n = 11; 64.7%), and cough (n = 5; 29.4%). Lymphopenia was observed in all patients, and lymphopenia was significantly more severe in the severe group than that in non-severe group (0.4 × 109/L vs 1.2 × 109/L, P = 0.014). The level of serum creatinine was higher in the severe group than in the non-severe group (99.0 μmol/L vs 62.5 μmol/L, P = 0.038). The most common features of chest computed tomography images were nodular foci in 10 (58.8%) patients and pleural thickening in 7 (41.2%) patients. All patients received antiviral therapy, while some patients also received intravenous antibiotics therapy (76.5%), Chinese medicinal preparation therapy (Lianhuaqingwen capsule, 64.7%), corticosteroids (35.3%) or immunoglobin (29.4%). Eight patients (47.1%) were transferred to the intensive care unit because of complications. Ten patients (58.8%) received intranasal oxygen, while 3 (17.6%) received non-invasive mechanical ventilation, and 4 (23.5%) received high-flow oxygen. As of June 20, 7 (41.2%) patients had been discharged and 10 (58.8% of this cohort, 77.8% of severe patients) had died.
Conclusion
The mortality of patients aged 80 years and older with severe COVID-19 symptoms was high. Lymphopenia was a characteristic laboratory result in these patients, and the severity of lymphopenia was indicative of the severity of COVID-19. However, the majority of patients with COVID-19 in this age cohort had atypical symptoms, and early diagnosis depends on prompt use of a viral nucleic acid test.
Keywords: Coronavirus, 2019-nCoV, SARS-CoV-2, COVID-19, Older patients
1. Introduction
Coronavirus disease 2019 (COVID-19) has raised the concern of a global epidemic [1], [2], [3] and the World Health Organization declared COVID-19 to be a global pandemic in March of 2020. As of June 20, 2020, 8,569,962 confirmed cases and 457,388 deaths had been reported worldwide [4]. In recent months, numerous studies have looked at how COVID-19 has affected specific populations, such as psychiatric patients [5], healthcare workers [3], [6], and the general working population [7]. Other studies have looked in more detail at epidemiological findings [8], clinical presentations, laboratory parameters, imaging features and clinical outcomes [9] of patients with COVID-19. The median age of patients included in several studies was 47–57 years [1], [10], [11], [12], and it has been reported clearly and consistently that greater patient age was associated with more severe cases [1], [10], [11], [12], [13]. Yang et al. [14] analyzed data from 710 patients with COVID-19, including 52 critically ill adult patient with a mean age of 59.7 years. Patients aged greater than 65 years with comorbidities and acute respiratory distress syndrome were at an increased risk of death [14]. In fact, the proportion of patients aged ≥ 65 years in population is increasing, and this age comprises about 12% of the Chinese population [15]. Aging is associated with a decline in immune function, which is generally referred to as immunosenescence. A consequence of immunosenescence is that many viral infections are more severe in older individuals [16], [17]. However, the clinical characteristics of patients aged > 80 years remains unknown. Therefore, we conducted a retrospective observational study to describe the early clinical characteristics of this age group with COVID-19.
2. Methods
2.1. Study design and participants
The retrospective study was performed at the Department of Geriatrics, Renmin Hospital of Wuhan University, Wuhan, China. This case series was approved by the institutional ethics board of Renmin Hospital of Wuhan University (No. WDRY2020-K089). We retrospectively analyzed records of patients in hospital and newly admitted to hospital who had been diagnosed with COVID-19 between January 20 and February 20, 2020. Diagnostic criteria for COVID-19 and the severity of COVID-19 were determined according to the guidance provided by the National Health Commission of the People’s Republic of China and the National Administration of Traditional Chinese Medicine [18]. Those who met the following criteria were defined as having severe-type infection: (1) respiratory distress with a respiratory rate over 30 breaths per minute, (2) oxygen saturation ≤ 93% in the resting state, and (3) arterial blood oxygen partial pressure (PaO2)/fraction of inspiration oxygen (FiO2) ≤ 300 mmHg. Once diagnosis was confirmed, patients were transferred to the designated department. Patients less than 80 years old were excluded. Patients with severe acute cardiovascular and cerebrovascular diseases before the onset of COVID-19 symptoms were also excluded from the study.
2.2. Data collection
The medical records of patients were analyzed by a trained team of physicians. Relevant data were extracted from electronic medical records of patients, including demographic data, medical history, epidemiological history, underlying diseases (chronic cardiac disease, chronic pulmonary disease, cerebrovascular disease, chronic neurological disorder, diabetes, and malignancy), clinical symptoms and signs, laboratory and radiological characteristics, treatments and outcomes. Two physicians independently reviewed the data collection forms to double check the accuracy. If data were not available from electronic medical records, the researchers directly communicated with patients and/or health-care providers and/or their families to ascertain epidemiological and symptom data.
2.3. Procedures
Throat swabs and/or nasal-pharyngeal swabs were used to collect nucleic acid samples from patients suspected of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, i.e., COVID-19. These samples were tested for the presence of viral nucleic acids and testing was confirmed by repeating the swab and analysis on a daily interval, at least once. The detailed procedure for the real-time reverse-transcriptase polymerase chain reaction assay has been described by Xu et al. [9]. Other lab data included a complete blood count and serum biochemical test for liver function, serum amyloid A, and C-reactive protein, which were detected on the day or the next day after the confirmation of a COVID-19 diagnosis. Patients were subjected to chest computed tomography (CT) scans within three days after the onset of COVID-19. Radiological diagnosis was reviewed by two experienced chest radiologists.
2.4. Statistical analysis
Continuous variables were described using mean and standard deviations, or median and interquartile range (IQR) values. Categorical variables were described as percentages and compared using the χ2 test, although the Fisher exact test was used when data were limited. Continuous variables were compared using independent-group t test when the data were normally distributed; otherwise, the Mann-Whitney test was used. All statistical analyses were conducted using GraphPad Prism 5.0 software (USA). A two-sided P of less than 0.05 was considered statistically significant.
3. Results
3.1. Demographics and clinical characteristics
A total of 17 patients with COVID-19 were included in this study. All cases were confirmed by virus nucleic acid test within 3 days after a SARS-CoV-2 infection was suspected. Sixteen (94.1%) patients received additional treatment to manage their chronic conditions, including hypertension, diabetes mellitus, cardiovascular disease, neurodegenerative diseases, chronic obstructive airway disease, chronic kidney disease and malignancy, and retained full-time nursing aids to administer this care. All patients were residents of Wuhan. None of them had a history of contact with individuals suspected or confirmed to have COVID-19.
The median age was 88.0 years (IQR, 86.6–90.0 years; range, 80.0–100.0 years). The ratio of male to female cases was 2.4:1. Fever (76.5%) and fatigue (64.7%) were the most common symptoms at the onset of COVID-19, followed by cough (29.4%), abdominal distention (29.4%), and sore throat (17.6%). According to clinical manifestation, 8 (47.1%) patients and 9 (52.9%) patients were diagnosed as non-severe and severe subgroups, respectively. The age was not significantly different between the two subgroups (P = 0.669). Hypertension and cardiovascular diseases were the most common chronic conditions in both two subgroups.
The demographic characteristics of participants are shown in Table 1 .
Table 1.
Demographic and epidemiologic characteristics of 17 patients aged > 80 years with COVID-19.
| Characteristics | All patients (N = 17) |
Disease severity |
P value | |
|---|---|---|---|---|
| Non-severe (n = 8) | Severe (n = 9) | |||
| Age (years, median [IQR]) | 88 (86.6–90) | 88 (80–90) | 88 (86–90) | 0.6534 |
| Sex (n [%]) | ||||
| Male | 12 (70.6) | 6 (75) | 6 (66.7) | 0.8759 |
| Female | 5 (29.4) | 2 (25) | 3 (33.3) | 0.7805 |
| Chronic conditions (n [%]) | ||||
| Hypertension | 9 (52.9) | 4 (50) | 5 (55.6) | 0.8988 |
| Cardiovascular disease | 8 (47.1) | 4 (50) | 4 (44.4) | 0.8908 |
| Chronic kidney disease | 6 (35.3) | 1 (12.5) | 5 (55.6) | 0.1897 |
| Diabetes mellitus | 5 (29.4) | 2 (25) | 3 (33.3) | 0.7805 |
| Neurodegenerative diseases | 5 (29.4) | 3 (37.5) | 2 (22.2) | 0.6109 |
| Chronic obstructive airway disease | 3 (17.6) | 1 (12.5 | 2 (22.2) | 0.6595 |
| Malignancy | 2 (11.8) | 2 (25) | 0 (0) | 0.1561 |
| Clinical symptom (n [%]) | ||||
| Fever | 13 (76.5) | 6 (75) | 7 (77.8) | 0.9607 |
| Fatigue | 11 (64.7) | 6 (75) | 5 (55.6) | 0.6988 |
| Cough | 5 (29.4) | 2 (25) | 3 (33.3) | 0.7805 |
| Abdominal distention | 5 (29.4) | 3 (37.5) | 2 (22.2) | 0.6109 |
| Sore throat | 3 (17.6) | 2 (25) | 1 (11.1) | 0.5312 |
COVID-19: coronavirus disease 2019; IQR: interquartile range. P values indicate differences between non-severe and severe patients.
3.2. Laboratory findings and radiologic features
The laboratory findings and radiologic features from data collected on the day that patients were first tested for COVID-19 are showed in Table 2 and Fig. 1 . All patients had normal total leukocyte counts and lymphopenia. The lymphopenia was significantly worse in the severe group than that in the non-severe group (0.4 × 109/L vs 1.2 × 109/L, P = 0.014). All patients demonstrated mild anemia, and normal levels of alanine aminotransferase and aspartate aminotransferase. The level of serum creatinine was higher in the severe group than that in the non-severe group (99.0 μmol/L vs 62.5 μmol/L, P = 0.038). All patients exhibited elevated levels of C-reactive protein and serum amyloid A, but there was no significant difference between the two subgroups. Chest CT images showed nodular foci in 10 (58.8%) patients, pleural thickening in 7 (41.2%) patients, fibrous cord in 5 (29.4%) patients, typical ground-glass shadow in 2 (11.8%) patients, and pleural effusion in 2 (11.8%) patients.
Table 2.
Laboratory parameters and radiologic features of 17 patients aged > 80 years with COVID-19.
| Characteristics | All patients (N = 17) |
Disease severity |
P value | |
|---|---|---|---|---|
| Non-severe (n = 8) | Severe (n = 9) | |||
| Laboratory examinations (median [IQR]) | ||||
| White blood cells (×109/L) | 7.0 (4.9–8.7) | 8.3 (7.3–9.5) | 5.0 (3.6–6.6) | 0.0206 |
| Lymphocytes (×109/L) | 1.0 (0.4–1.2) | 1.2 (0.9–1.4) | 0.4 (0.3–1.1) | 0.0141 |
| Hemoglobin level (g/L) | 113.0 (92.5–119.5) | 113.5 (97.3–124.3) | 113.0 (91.5–117.5) | 0.4946 |
| Platelet count (×109/L) | 157.0 (133.0–331.0) | 182.5 (145.5–241.8) | 152.0 (92.5–200.5) | 0.2460 |
| Alanine aminotransferase (U/L) | 13.0 (12.5–19.0) | 14.0 (11.5–25.3) | 13.0 (12.5–18.0) | 0.7571 |
| Aspartate aminotransferase (U/L) | 25.0 (18.5–31.5) | 21.5 (16.5–40.0) | 25.0 (22.0–30.0) | 0.7251 |
| Creatinine (μmol/L) | 76.0 (60–115) | 62.5 (52.8–85) | 99.0 (74.5–212.5) | 0.0377 |
| C-reaction protein (mg/L) | 14.3 (7.5–68.5) | 11.2 (5.9–74.8) | 28.3 (12.0–68.5) | 0.4234 |
| Serum amyloid A (mg/L) | 85.2 (42.1–210.7) | 51.7 (27.0–134.3) | 153.1 (73.55–227.7) | 0.0927 |
| Radiologic features (n [%]) | ||||
| Nodular foci | 10 (58.8) | 5 (62.5) | 5 (55.6) | 0.8826 |
| Pleural thickening | 7 (41.2) | 5 (62.5) | 2 (22.2) | 0.2761 |
| Fibrous cord | 5 (29.4) | 2 (25) | 3 (33.3) | 0.7805 |
| Ground-glass opacity | 2 (11.8) | 1 (12.5) | 1 (11.1) | 0.9372 |
| Pleural effusion | 2 (11.8) | 1 (12.5) | 1 (11.1) | 0.9372 |
COVID-19: coronavirus disease 2019; IQR: interquartile range. P values indicate differences between patients with severe and non-severe cases.
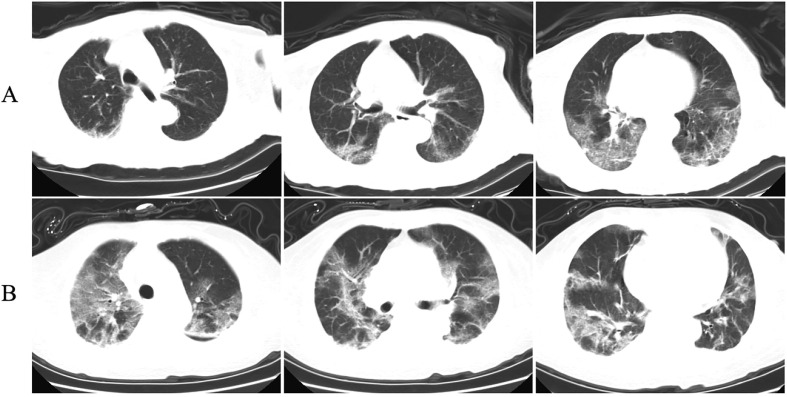
Fig. 1.
Chest computed tomography (CT) images of two patients with COVID-19. A: Chest CT images of a 90-year-old female patient show ground-glass opacity in both lungs 3 days after symptom onset. B: Chest CT images of an 87-year-old female patient show ground-glass opacity in both lungs 2 days after symptom onset.
3.3. Treatments and clinical outcomes
The treatments and clinical outcomes are shown in Table 3 . All patients received antiviral therapy, and 13 (76.5%) patients received intravenous antibiotics therapy. At the same time, 11 (64.7% of severe and 87.5% of non-severe patients) patients were given Lianhuaqingwen capsules, a traditional Chinese medicinal preparation. In addition, 35.3% of the patients received corticosteroid therapy and 29.4% received treatment with intravenous immunoglobin. Ten (58.8%) patients received intranasal oxygen, 3 (17.6%) received non-invasive mechanical ventilation, and 4 (23.5%) received high-flow oxygen. Eight (47.1%) patients were referred to the intensive care unit. Due to underlying disease, great age, and refusal by patients and their families, no patient received invasive treatments such as continuous renal replacement therapy, invasive mechanical ventilation and extracorporeal membrane oxygenation. As of June 20, 2020, 7 (41.2%) patients had been discharged and 10 (58.8% of this cohort, 77.8% of severe patients) had died.
Table 3.
Treatments and clinical outcomes of 17 patients aged > 80 years with COVID-19.
| Characteristics | All patients (N = 17) |
Disease severity |
P value | |
|---|---|---|---|---|
| Non-severe (n = 8) | Severe (n = 9) | |||
| Treatments (n [%]) | ||||
| Arbidol | 17 (100) | 8 (100) | 9 (100) | > 0.9999 |
| Intravenous antibiotics | 13 (76.5) | 6 (75) | 7 (77.8) | 0.9607 |
| Lianhuaqingwen capsule | 11 (64.7) | 7 (87.5) | 4 (44.4) | 0.3903 |
| Corticosteroids | 6 (35.3) | 2 (25) | 4 (44.4) | 0.5598 |
| Immunoglobin | 5 (29.4) | 2 (25) | 3 (33.3) | 0.7805 |
| Intranasal oxygen | 10 (58.8) | 8 (100) | 2 (22.2) | 0.0925 |
| High-flow oxygen | 4 (23.5) | 0 (0) | 4 (44.4) | 0.0812 |
| Non-invasive mechanical ventilation | 3 (17.6) | 0 (0) | 3 (33.3) | 0.1250 |
| Intensive care unit admission | 8 (47.1) | 0 (0) | 8 (88.9) | 0.0186 |
| Clinical outcomes (n [%]) | ||||
| Death | 10 (58.8) | 3 (37.5) | 7 (77.8) | 0.3837 |
| Discharge from hospital | 7 (41.2) | 5 (62.5) | 2 (22.2) | 0.2761 |
COVID-19: coronavirus disease 2019.
4. Discussion
In this study, we report on the cases of 17 patients aged 80 and older with laboratory-confirmed COVID-19. Most patients had hypertension and cardiovascular disease. Severe illness occurred in 52.9% of patients. The most common symptoms at onset were fever (76.5%) and fatigue (64.7%). Lymphopenia and renal dysfunction were significantly worse in the severe group than those in the non-severe group. Only two patients showed typical ground-glass shadows on chest CT scans, while others showed nonspecific lesions such as nodular foci, pleural thickening, and fibrous cord, which may be related to age. Overall, 47.1% of patients required intensive care, 41.2% of patients were discharged, and 77.8% of patients with severe symptoms were died.
In agreement with recent publications [1], [13], we found that fever and cough were the dominant symptoms of COVID-19. This differs from physical symptoms reported by the general public who were concerned about COVID-19 (coryza [16.9%], cough [13.9%], sore throat [11.5%], and fever [0.5%]) but did not have COVID-19 [8]. We reported the most common symptoms at onset were fever (76.5%) and fatigue (64.7%), which suggested older patients had atypical symptoms in the early days of infection. Consistent with previous studies [1], [13], lymphopenia was common in patients included in our study. Further, lymphopenia was significantly worse in the severe group than that in the non-severe group. It has been established that lymphopenia is the result of apoptosis of lymphocytes [19], which suggested that the severity of lymphopenia reflects the severity of COVID-19. We also found the level of serum creatinine was higher in the severe group than that in the non-severe group. In the severe group, 5 of 9 patients had underlying chronic kidney disease, which could explain the severity of renal dysfunction in the severe group. However, future studies would need to determine whether renal dysfunction is linked to the severity of COVID-19.
Up to now, there has been no specialized treatment for COVID-19. The mainstay of treatment is symptomatic supportive therapy, and severe cases have a significantly worse prognosis. A total of 77.8% of severe patients died in this study. This mortality rate is higher than that in a recent study reporting that 61.5% of critically ill patients had died [14]. Due to the underlying diseases of patients included in this study, some invasive treatments, including continuous renal replacement therapy and extracorporeal membrane oxygenation, were difficult to implement in the clinic, which directly affected the mortality rate. Thus, it is important to identify treatment methods that may be effective, safe and practical for the elderly patients. At present, COVID-19 is considered to be related to an immune inflammatory response, and immunomodulatory therapy has been recommended for the treatment of COVID-19 [20]. Meanwhile, patients with COVID-19 often have secondary bacterial infections; 76.5% of the patients in this study were treated with intravenous antibiotics. Lianhuaqingwen capsule is a compounded Chinese herbal medicine that is composed of Maxing Shigan decoction and Yinqiao powders, which has anti-inflammatory, antiviral and immunomodulatory effects [21]. Lianhuaqingwen capsule has been shown to reduce the symptoms and improve the prognosis of influenza, Middle East respiratory syndrome (MERS) and COVID-19 [22], [23], [24]; further, it has no obvious side effects that pose a threat to elderly patients. In our study, most non-severe patients with COVID-19 were treated with Lianhuaqingwen capsule, but the efficacy of Lianhuaqingwen capsule on COVID-19 cannot be determined from our data, as this is a retrospective study with a small number of patients. However, in view of the advantages of multi-target treatments and fewer side effects of Chinese herbal medicine, we should actively explore the efficacy of Chinese herbal medicine in the treatment of COVID-19.
In the present study the median age of patients was 88.0 years. To our knowledge, this is the first study to focus on older patients with COVID-19. Previous studies have shown that the median age of patients admitted for COVID-19 treatment was 47–57 years, and indicated that greater age was associated with more severe cases [1], [10], [11], [12], [13]. It has also been reported that the mean age of critically ill patients was 59.7 years and that the mean age of non-survivors was older (64.6 years) than that of survivors (51.9 years) [14]. These studies suggest that older patients are at increased risk of death. The high mortality rate in older patients is not understood. Possible explanations may be hinted in the published data from other outbreaks of coronavirus infection, such as MERS coronavirus (MERS-CoV) and SARS coronavirus (SARS-CoV). It has been established that old age and the existence of underlying comorbidities significantly increased mortality from MERS-CoV and SARS-CoV [25], [26], which may also be a characteristic of coronavirus infection. It is well-known that aging is associated with a decline in immune function, referred to as immunosenescence. A consequence of immunosenescence is that many viral infections are more severe in older patients [17]. We observed that lymphopenia was typical of COVID-19 in older patients, which may be related to immunosenescence and contribute to the high mortality rate. The exact relationship among lymphopenia, immunosenescence and high mortality rate needs to be explored further.
It should be noted that psychiatric disorders are also associated with increased mortality rates [27]. It has already been reported that depression in later life reflects depressive symptoms caused by underlying aging-related processes, such as inflammation-based sickness behavior, frailty, and mild cognitive impairment, which have all been associated with increased mortality [28]. Most patients in this study employed full-time nurses and did not receive day care from family members, which may have affected their psychological and mental status. Thus, psychological and mental status are potential factors that contribute to the high mortality rate in older patients and deserve further attention as COVID-19 research is analyzed.
Our study has several limitations. First, all patients had underlying diseases. It is no doubt that SARS-CoV-2 infection is a trigger for disease progression. However, we have no data for the role of the SARS-CoV-2 infection in the development of underlying diseases. Second, this study had a small sample size, with only 17 patients aged 80 years and over included in the dataset. Third, this is a retrospective study. We only analyzed available medical information collected within 3 days after patients suspected infection of SARS-CoV-2, and were not able to gather dynamic observations on the functional state of organs and mental status. We also did not estimate immune function. Finally, we did not evaluate viral load and thus failed to evaluate whether a patient’s viral load was associated with clinical severity of COVID-19 symptoms.
In conclusion, the mortality rate of patients aged 80 years and older with COVID-19 is high. The majority of old patients with COVID-19 have atypical symptoms. Early diagnosis is depended on virus nucleic acid test, and lymphopenia appears to be a characteristic laboratory result that correlates with the severity of COVID-19.
Author contributions
JZD designed the hypotheses and the experiments, and GYZ performed the experiments and their analysis. YJY were responsible for data collection. JZD and FZ participated in data analysis. All authors participated in data interpretation and manuscript review and writing. JZD and FZ were responsible for preparation of the tables and figures. All authors contributed to the scientific discussion of the data and the manuscript.
Funding
This work was supported by grant from the National Nature Sciences Foundation of China (No. 81500639).
Conflicts of interest
The authors declare that they have no actual or potential conflict of interest.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Pan R., Wan X., Tan Y., Xu L., McIntyre R.S. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. 2020;87:40–48. doi: 10.1016/j.bbi.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chew N.W.S., Lee G.K.H., Tan B.Y.Q., Jing M., Goh Y., Ngiam N.J.H. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–565. doi: 10.1016/j.bbi.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Center for Systems Science and Engineering at Johns Hopkins University. The COVID-19 Global Map. (2020-06) [2020-06-20]. https://coronavirus.jhu.edu/map.html.
- 5.Hao F., Tan W., Jiang L., Zhang L., Zhao X., Zou Y. Do psychiatric patients experience more psychiatric symptoms during COVID-19 pandemic and lockdown? A case-control study with service and research implications for immunopsychiatry. Brain Behav Immun. 2020;87:100–106. doi: 10.1016/j.bbi.2020.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan B.Y.Q., Chew N.W.S., Lee G.K.H., Jing M., Goh Y., Yeo L.L.L. Psychological impact of the COVID-19 pandemic on health care workers in Singapore. Ann Intern Med. 2020;173(4):317–320. doi: 10.7326/M20-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan W., Hao F., McIntyre R.S., Jiang L., Jiang X., Zhang L. Is returning to work during the COVID-19 pandemic stressful? A study on immediate mental health status and psychoneuroimmunity prevention measures of Chinese workforce. Brain Behav Immun. 2020;87:84–92. doi: 10.1016/j.bbi.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 13.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X. Steady growth of population and improvement of urbanization process. (2019-01-23) [2019-01-23]. http://www.stats.gov.cn/tjsj/sjjd/201901/t20190123_1646380.html.
- 16.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsolamy S. Middle East respiratory syndrome: knowledge to date. Crit Care Med. 2015;43(6):1283–1290. doi: 10.1097/CCM.0000000000000966. [DOI] [PubMed] [Google Scholar]
- 18.National Health Commission of the People’s Republic of China, National Administration of Traditional Chinese Medicine. Guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection (Trial version 7). Quan Ke Yi Xue Lin Chuang Yu Jiao Yu 2020; 18(2):100–5 [Chinese].
- 19.Chu H., Zhou J., Wong B.H., Li C., Chan J.F., Cheng Z.S. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213(6):904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Xie B., Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia W., Wang C., Wang Y., Pan G., Jiang M., Li Z. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen Capsule by UPLC-DAD-QTOF-MS. ScientificWorldJournal. 2015;2015 doi: 10.1155/2015/731765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan Z.P., Jia Z.H., Zhang J., Liu S., Chen Y., Liang L.C. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chin Med J (Engl) 2011;124(18):2925–2933. [PubMed] [Google Scholar]
- 23.National Health Commission of the People’s Republic of China. Guideline on diagnosis and treatmemt of Middle East respiratory syndrome (2015 version). Zhongguo Bing Du Bing Za Zhi 2015; 5(5): 352–4 [Chinese].
- 24.Hu K., Guan W.J., Bi Y., Zhang W., Li L.J., Zhang B. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alqahtani F.Y., Aleanizy F.S., El Hadi A., Mohamed R., Alanazi M.S., Mohamed N. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 2018;147:1–5. doi: 10.1017/S0950268818002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan T.Y., Miu K.Y., Tsui C.K., Yee K.S., Chan M.H. A comparative study of clinical features and outcomes in young and older adults with severe acute respiratory syndrome. J Am Geriatr Soc. 2004;52(8):1321–1325. doi: 10.1111/j.1532-5415.2004.52362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker E.R., McGee R.E., Druss B.G. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatr. 2015;72(4):334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Berg KS, Wiersema C, Hegeman JM, van den Brink RHS, Rhebergen D, Marijnissen RM, et al. Clinical characteristics of late-life depression predicting mortality. Aging Ment Health 2019; 10.1080/13607863.2019.1699900. [DOI] [PubMed]