Abstract
It is clear that existing cardiovascular disease is a major risk factor for COVID-19 and related adverse outcomes. In addition to acute respiratory syndrome, a large cohort also develop myocardial or vascular dysfunction, in part from inflammation and renin angiotensin system activation with increased sympathetic outflow, cardiac arrhythmias, ischemia, heart failure, and thromboembolic complications that portend poor outcomes related to COVID-19.
We summarize recent information for hospitalists and internists on the front line of this pandemic regarding its cardiovascular impacts and management and the need for cardiovascular consultation.
Keywords: Cardiovascular consult, Cardiovascular disease, COVID-19, Multidisciplinary care, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Clinical Significance.
-
•
Current prognostic trends of cardiac involvement in COVID-19 infection are reviewed.
-
•
A practical algorithm is provided for the internist or hospitalist on efficient cardiac workup in patients with COVID-19.
-
•
Cardiac arrhythmia considerations with associated treatment options for patients with COVID-19 are reviewed.
Alt-text: Unlabelled box
Introduction
The COVID-19 pandemic focuses on respiratory manifestations, but evidence has emerged relative to major cardiovascular implications. Preexisting cardiovascular conditions increase risk for COVID-19 and also for cardiovascular manifestations sometimes leading to death. Understanding this aspect is imperative for the internal medicine community.
Cardiovascular manifestations range from arrhythmias to acute cardiomyopathies, myocarditis, thromboembolic disorders, and shock. Conversely in presentations appearing to be cardiovascular, COVID-19 infection should also be considered.1 Given the extent and variability of cardiovascular manifestations, care of these patients is challenging and includes direct virus effects and possible iatrogenic effects from treatments.
Cardiologists should be prepared to assist other specialties in managing these cardiac complications and protocols for triaging, diagnosing, and managing patients with COVID-19 with cardiovascular complications should be refined as data become available. Caution should be exercised because classical myocardial infarction symptoms may be obscured leading to underdiagnosis of cardiac injury or overdiagnosis and inappropriate diagnostic testing or imaging during shortages in personal protective equipment and staffing.
This review provides recommendations for a systematic approach to the cardiovascular evaluation of patients with COVID-19 for the internist or hospitalist. Pressing cardiovascular queries related to COVID-19 faced by frontline physicians, from baseline cardiac evaluation needed, to appropriate cardiac imaging, and timing of cardiology input, are addressed.
Electrocardiogram and Biomarker Testing
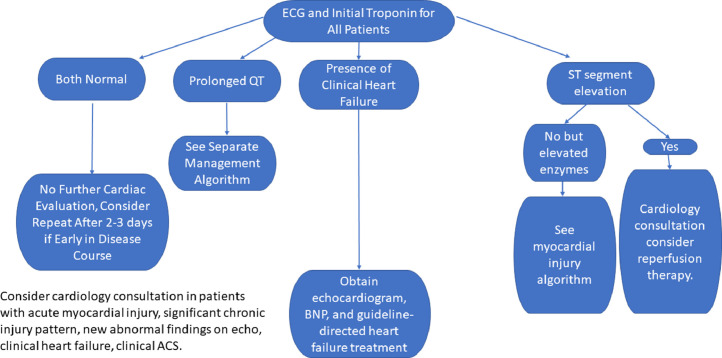
Clinical data (eg, symptoms, electrocardiogram [ECG], and echocardiography) and cardiac troponin (cTn) are key to evaluate myocardial injury in COVID-19. High-sensitivity (hs) cTn increases our ability to detect cardiomyocyte injury resulting from a variety of processes (eg, ischemia, hypoxia, microvascular dysfunction, microvascular thrombosis, cytokine storm, myocarditis, underlying heart failure, etc.). Among patients with COVID-19 with cTn >99th percentile mortality may approach ~50% compared with ~5% without cTn elevation.2 , 3 Rapidly obtainable, cTn is useful for identifying increased mortality risk. Interpretation using the fourth-universal definition of myocardial infarction4 maintains standardized language regarding cTn patterns and facilitates appropriate cardiovascular consultation because >20% of patients hospitalized with COVID-19 may have elevated cTn.2 , 3 , 5 An initial negative cTn does not necessarily require repeat testing unless indicated clinically to exclude acute coronary syndrome (Figure 1 ). An initial elevated cTn should be trended to clarify the injury pattern as acute or chronic/stable and to quantify the injury. Testing frequency should be based on clinical and laboratory findings to minimize extra testing and staff exposure.
Figure 1.
Suggested algorithm for cardiac assessment of patients with COVID-19. BNP = brain natriuretic peptide; ECG = electrocardiogram.
Among patients with COVID-19 with elevated cTn, we recommend echocardiography as a baseline and to identify preclinical left-ventricular dysfunction that may portend poor prognosis. Patients with low levels of cTn (chronic injury pattern) without clinical heart failure or echocardiographic abnormalities may be managed conservatively with close monitoring. Acute myocardial injury may be further delineated with brain natriuretic peptide, clinical findings, repeat ECGs, and echocardiography to guide care. These patients have a broad differential diagnosis because multiple factors may affect cTn (eg, renal disease, coronary artery disease, etc.), so setting a threshold level to trigger consultation is challenging. Concern for acute coronary syndrome or heart failure signal important opportunities for consultation.
Anticoagulation
Patients with COVID-19 are at elevated risk for venous thromboembolism or microvascular thrombosis. Prophylactic anticoagulation with low-molecular-weight heparin is recommended for patients without contraindications.6 , 7 Some attempt to risk-stratify by trending D-dimer levels. We suggest maintaining anticoagulation in patients with another indication for anticoagulation (eg, atrial fibrillation, mechanical valve, known venous thromboembolism, etc.). Of note, apixaban and rivaroxaban levels may be increased in patients treated with sarilumab or tocilizumab, and alternative agents should be considered in this setting. Warfarin may need to be increased with closer monitoring. When to initiate anticoagulation in patients without thromboembolic events is unknown but may be considered in patients with significantly elevated D-dimer levels as part of a clinical trial.
Approach to Acute Myocardial Injury in the COVID-19 Era
Internists are skilled at initial assessment and therapeutic decision making for suspected acute coronary syndromes. Multiple and varied myocardial effects of COVID-19 are apparent, and patients with preexisting cardiovascular disease are at increased risk for adverse outcomes.2 COVID-19 complications may render decision making more difficult in patients with presumed acute coronary syndrome. Patients presenting with myocardial injury in the setting of COVID-19 may have a Type-1 myocardial infarction with atherothrombosis and coronary occlusion as a result of acute plaque rupture or erosion. These patients may also present with viral myocarditis, stress-related cardiomyopathy, another form of nonischemic cardiomyopathy, coronary vasospasm, or nonspecific injury without Type-1 or -2 myocardial infarction.8 Among patients in New York City, 3%-7% had a cardiac complication.9 In 18 patients with ST elevation, more than half had it at presentation at the emergency department, and the remainder developed ST elevation later. Only one-third of patients with ST elevation had chest pain and the majority were men. The pattern of ST elevation was diffuse in 22%. Of 9 patients who underwent coronary angiography, 6 had disease and only 5 underwent percutaneous coronary intervention (PCI).10 Importantly, 72% of these patients died. Although this is a small series, it illustrates the varied presentations for patients with COVID-19 and management challenges.
COVID-19 has prompted reevaluation of established algorithms for patient care for ST-segment elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI). A balance is needed between invasive therapy in patients with acute coronary syndrome in the pandemic environment and contamination protection of health care workers and facilities. The number of STEMI activations has declined,11 suggesting that patients in need of acute care are not seeking it because of fear of going to the hospital. Another concern is the possibility of misdiagnosis or “missed” diagnosis when the focus is solely on the respiratory issues.
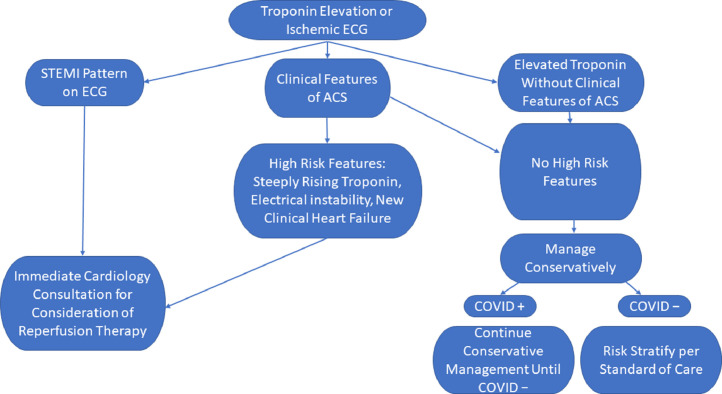
The following strategies will hopefully assist the internist or hospitalist in management of patients with COVID-19 presenting with evidence for acute myocardial injury (Figure 2 ).
Figure 2.
Suggested algorithm for management of myocardial injury in patients in the COVID-19 era. ACS = acute coronary syndrome; ECG = electrocardiogram; STEMI = ST-segment elevation myocardial infarction.
ST-Segment Elevation Myocardial Injury
The approach to patients with chest pain with STEMI should not differ from before this pandemic. Evaluation should include clinical assessment and an ECG. Primary PCI is recommended within 90 minutes of presentation, and centers without access to primary PCI within 90 minutes should consider thrombolytic therapy. In the current era, patients with STEMI should be considered positive for COVID-19.12 If severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing is rapid enough to impact decision making, it may be used to streamline care. Cardiology consultation may be helpful in distinguishing Type-1 STEMI from virus-associated Type-2 STEMI mimics such as myopericarditis. Delays in reperfusion may occur if studies such as echocardiography or computed tomography are required when ECGs or symptoms are atypical. The goal remains timely primary PCI whenever possible. Although thrombolytic therapy may be considered at centers without PCI capabilities, patients with ST elevation from 1 of the Type-2 causes of myocardial infarction seen with COVID-19 may be exposed to bleeding risks without benefit. Currently, patients with COVID-19 requiring intubation have a high mortality, so those who develop a STEMI may be considered for palliative care after discussions with family or caregivers.13
Unstable Angina or NSTEMI Myocardial Injury
Without ST elevation, biomarker elevation in patients with COVID-19 is difficult to interpret. The patient may have Type-1 myocardial infarction with atherothrombosis, or other causes of myocardial injury may be present. The challenge is deciding when to use the catheterization laboratory. Stable patients can be managed conservatively until SARS-CoV2 status is known. Before COVID-19, patients with NSTEMI or unstable angina were often treated conservatively before invasive procedures. Exceptions include hemodynamic or electrical instability, a high Global Registry of Acute Coronary Events (GRACE) score, or other high-risk feature. These patients should be offered an invasive evaluation and intervention if indicated. Patients without high-risk features can be managed conservatively until they either test negative for SARS-CoV2 or until the infection has resolved. All patients should receive guideline-directed medical therapy.12
Cardiogenic Shock or Out-of-Hospital Cardiac Arrest
Patients with cardiogenic shock or out-of-hospital cardiac arrest remain at high risk for death. Before COVID-19 there was lack of consensus about taking these patients to cardiac catheterization. In the current environment it is recommended that patients who have been resuscitated after out-of-hospital cardiac arrest be considered for invasive therapy when presenting with STEMI. Those who are hemodynamically unstable or in cardiogenic shock may also be considered for invasive cardiac evaluation despite their ECG findings. Mechanical circulatory support may be considered after consultation with an advanced heart failure team.
Heart Failure Considerations in COVID-19 Management
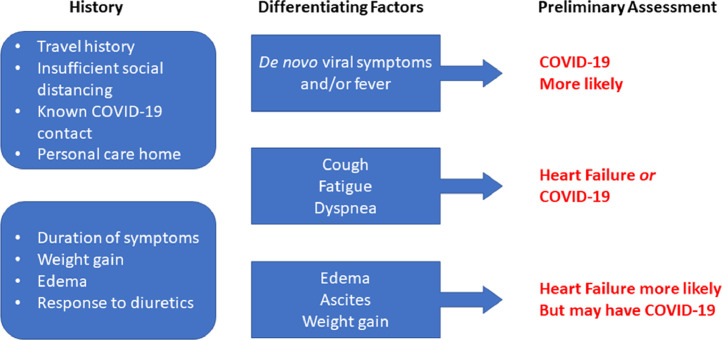
An unexpected challenge of COVID-19 is the management of heart failure beginning before hospital arrival because undifferentiated symptoms may be mischaracterized, causing delay or confusion in management, and potential inappropriate treatment. Furthermore, if these patients require in-person assessment, symptom-based virtual assessment is needed to preserve health care and personal protective resources, while limiting exposure of vulnerable patients. Virtual assessment should focus on features that increase the probability of COVID-19 compared with only heart failure exacerbation.14 Features suggesting increased COVID-19 probability include de novo viral syndrome symptoms, inadequate social distancing, lack of typical heart failure features (eg, weight gain or prior heart failure exacerbations), or lack of improvement with diuretics. Results can then be used to guide further assessment and intervention, and should in-person assessment be required, it will inform the best setting for such an assessment (Figure 3 ).
Figure 3.
Heart failure or COVID-19 virtual assessment guide. Adapted from Canadian Cardiovascular Society.14
An important question is continuation of neurohormonal blockade. Therapies under investigation to mitigate COVID-19 complications include immune-modulating medications such as chloroquine and hydroxychloroquine. Although these medications have myocardial toxicities (eg, cardiomyopathy or worsening heart failure), these often take months to develop, and short therapy duration may confer lower risk.15 , 16 Of concern is the inhibitory effect of chloroquine on CYP2D6. Because CYP2D6 inhibition increases beta-blocker concentrations, it may cause greater decreases in blood pressure and heart rate.17 Decisions to continue beta-blockade should consider respiratory and hemodynamic status; we recommend continuation of these agents in patients with chronic systolic heart failure with a decreased dose if receiving an antimalarial drug.
Of interest is the complex, incompletely understood interaction of renin-angiotensin-aldosterone system (RAAS) inhibition with SARS-CoV2 because the angiotensin-converting enzyme 2 (ACE2) receptor is used for host cell entry. Theoretically, ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) may upregulate ACE2 and increase SARS-CoV2 susceptibility.17 , 18 This, however, contrasts with experimental data that suggest that ACEIs may protect against lung injury, and data from the SARS-CoV 2002-2003 epidemic where ARBs were proposed as potential severe acute respiratory syndrome (SARS) therapeutics.19 , 20 Given incomplete and conflicting data, it has been recommended to continue RAAS inhibition in COVID-19. A multicenter retrospective study (>1000 patients with hypertension) with COVID-19 suggested improved survival with an ACEI or ARB.21 We continue these medications in patients with hypertension or chronic systolic heart failure in the absence of shock or renal disease.
Most important in the management of patients with heart failure and COVID-19 is maintaining euvolemia and rapidly correcting underlying hypoxemia to prevent more severe complications. In this context, a cardiology consultation can assist the patient with COVID-19 who is not in the intensive care unit (ICU).
Cardiac Arrhythmia Considerations
Drug-Induced QT Interval Prolongation and Torsades de Pointes
Chloroquine, hydroxychloroquine with or without azithromycin, and other antiviral medications are purported to benefit patients with COVID-19. These medications are relatively weak inhibitors of cardiac potassium channels (mainly Ikr) responsible for repolarization; when used alone in patients who are healthy, they result in only minor QT prolongation. Chloroquine has been used for malaria prophylaxis worldwide, and drug-induced torsades de pointes and life-threatening ventricular tachyarrhythmias are rare in these patients, even with prolonged use.22 , 23 In contrast, anticipated duration of exposure to these medications for COVID-19 is relatively short, 5-10 days.
However, when 2 or more QT-interval prolonging drugs are used together, or used in patients who are older and critically ill with multiple risk factors for arrhythmias related to QT prolongation (see below), prolongation of repolarization may be sufficient to result in life-threatening proarrhythmia, polymorphic ventricular tachycardia or torsades de pointes.24 Although only a small proportion of patients with QT prolongation develop torsades, it is most likely to occur when the QTc is ≥500 ms. The half-life of hydroxychloroquine is 40-60 days,25 raising the possibility that drug-induced QTc prolongation could remain for a substantially longer period than actual drug administration, and if a QT-prolonging drug were added, risk of torsades may increase. QT prolongation may not be the only cause of ventricular arrhythmias in patients with COVID-19 receiving hydroxychloroquine or other antivirals; viral myocarditis or other organ damage that may alter drug metabolism may increase arrhythmia susceptibility.
Preliminary data indicate that most patients with COVID-19 showed minor increases in QTc duration on combination hydroxychloroquine and azithromycin, yet 12% had QTc prolongation >60 ms from baseline and 11% developed QTc intervals >500 ms.26 Another study found QTc prolongation >500 ms in 19% of patients given higher doses of chloroquine.27 Most of these patients were also receiving oseltamivir, which also may prolong the QTc. Patients with COVID-19 pneumonia in the ICU may be particularly prone to excessive QT prolongation, especially when hydroxychloroquine is combined with azithromycin.28 , 29
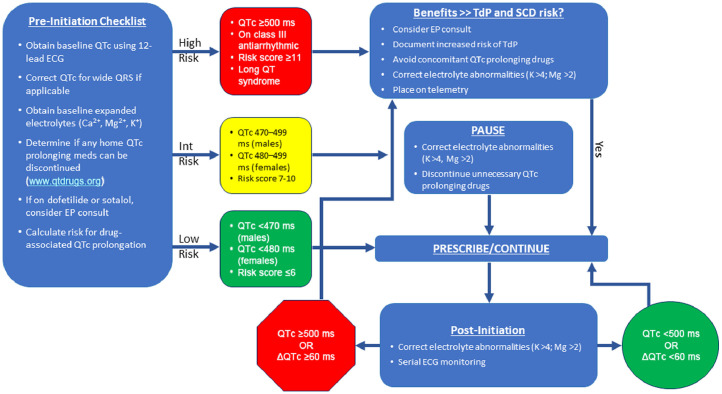
Prior to COVID-19, a risk score for predicting QT prolongation in patients in the ICU was developed.30 Risk factors were totaled: 1 point included age ≥68 years, female sex, and loop diuretic; 2 points, serum potassium ≤3.5, admission QTc ≥450 ms, and acute myocardial infarction; 3 points, 1 QTc-prolonging drug, 2 or more QTc-prolonging drugs, sepsis, and heart failure. The maximum risk score was 21. Patients at low risk for QT prolongation or torsades de pointes were defined as those with a score ≤6 points, moderate risk 7-10 points, and high risk ≥11 points. Importantly, loop diuretic, low potassium, or concomitant QT-prolonging drugs are modifiable. This scoring system was incorporated into an algorithm by others31 (Figure 4 ). A baseline QTc is obtained using 12-lead ECG. If the QRS is wide (eg, bundle branch block or ventricular pacing), a correction can be estimated by the following formula:
Figure 4.
University of Florida Health algorithm for initiation of QTc prolonging drugs* in COVID-19 pathway. Modified from Giudicessi et al.31
*Hydroxychloroquine, chloroquine, azithromycin, lopinavir/ritonavir.
ECG = electrocardiogram; EP = electrophysiology; SCD = sudden cardiac death; TdP = torsades de pointes.
Using the baseline QTc and risk score,30 patients are categorized as low, intermediate, or high risk, and any modifiable risk factors for QT prolongation should be addressed. Serum potassium should be maintained >4 mEq/L and magnesium >2 mEq/L. If the patient is receiving concomitant drugs that prolong the QT-interval, including dofetilide or sotalol, consider discontinuing them if possible. A list of offending drugs is at www.qtdrugs.org. If the patient is low or intermediate risk for QT prolongation and reversible factors have been addressed, QT-prolonging COVID-19 drugs can be initiated. The high-risk category is not an absolute contraindication to hydroxychloroquine with or without azithromycin, but potential benefit versus risk must be addressed with the patient.
The intensity of QTc follow-up for patients receiving these drugs depends on the patient's risk category. Due to proarrhythmia and difficulty with QT-interval follow-up, the Food and Drug Administration (FDA) recommends chloroquine, hydroxychloroquine, or azithromycin use in patients with COVID-19 be limited to clinical trials or for certain patients who are hospitalized.32 Even in the inpatient setting, patients with COVID-19 present unique challenges in obtaining serial ECGs because of isolation issues. If the patient is low risk, it may be reasonable to forego serial ECG monitoring or obtain QTc measurements on Days 2 and 4 of therapy if feasible. For patients at intermediate risk, telemetry should be maintained and QTc measurement on Days 2 and 4 of therapy. For high-risk patients, QTc measurements should be obtained 2-4 hours after the first dose and daily thereafter if feasible, and the patient should be monitored via telemetry.31 Single-lead telemetry or smartphone tracings are not sufficiently accurate for QTc monitoring, but many telemetry systems have the ability to obtain multiple simultaneous leads, and the longest QT- interval measured from those leads is a reasonable estimate of the QTc. The Food and Drug Administration has approved smartphone applications providing simultaneous 6-lead ECGs for QTc monitoring. The smartphone can either be a placed in a sterile bag and the recording apparatus sanitized after every use, or the patient can use their own smartphone.31 , 33 , 34
Arrhythmia Management in COVID-19
In patients with COVID-19, cardiovascular complications may be associated with serious arrhythmias: arrhythmias occurred in 17% of hospitalized patients and 44% of those admitted to the ICU, but details were lacking.35 COVID-19 is associated with myocarditis and myocardial ischemia; both may lead to ventricular tachyarrhythmias, atrial tachyarrhythmias, or bradycardia/atrioventricular conduction block. Associated hypoxia, shock, and electrolyte disturbances also contribute to arrhythmias. Arrhythmia management is per usual arrhythmia guidelines. Bradyarrhythmias may be transient, and if pacing is required, one can consider temporary rather than permanent pacing.
Special considerations may apply in patients with preexisting arrhythmias, especially in those already receiving antiarrhythmic drugs that prolong the QT interval (eg, sotalol and dofetilide). Doses may need to be adjusted or discontinued if hydroxychloroquine or other QT-prolonging COVID-19 therapies are employed. Amiodarone also prolongs the QT interval but usually does not cause torsades de pointes. Patients with preexisting long QT syndromes should be given QT-prolonging drugs only after considering risk versus benefit and with close QT monitoring. High body temperature commonly unmasks serious ventricular arrhythmias in patients with Brugada syndrome.36
Cardiac Implantable Electronic Devices
Remote cardiac implantable electronic device (CIED) interrogations and device management via telemonitoring should be performed.37 , 38 We recommend initiation of remote monitoring at device implantation; patients not enrolled should be strongly encouraged to do so. Patients should transmit a device interrogation the day before a scheduled telemedicine visit, so the physician can review and discuss device function with the patient at the visit. Postoperative wound checks can be performed using video telemedicine. Office personnel should closely monitor CIED remote transmissions for significant arrhythmias or device malfunctions requiring early intervention.
Despite COVID-19, situations to consider in-person device interrogation37 , 38 include abnormalities on remote monitoring that may require device reprogramming, defibrillator shocks, presyncope/syncope symptoms concerning for arrhythmia that may require reprogramming, symptoms or monitor evidence of device/lead malfunction, suspected device infection, incessant arrhythmias (especially with multiple shocks), or need for emergent or urgent magnetic resonance imaging. In a patient dependent on a pacemaker undergoing surgery, a doughnut magnet can be placed over the pacemaker to ensure pacing during electrocautery. During surgery in a patient with an implantable defibrillator, a magnet can be placed over the pulse generator to suspend tachyarrhythmia detection and shocks.
Conclusions
The scale and speed of the COVID-19 pandemic is unprecedented; data change rapidly as this disease outpaces usual information-sharing and consensus processes. We are a collaborative team of physicians, respiratory therapists, pharmacists, and nurses from multiple disciplines. Although emphasis is on the lung, many important cardiovascular implications are emerging. Making these recommendations available to internists and hospitalists should help everyone to receive the best care possible.
Footnotes
Funding: None.
Conflicts of Interest: Authors do not have conflicts or funding specific to this manuscript.
Authorship: All authors had access to the data and a role in writing this manuscript.
References
- 1.Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China [e-pub ahead of print] JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. Available at: Accessed April 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection [e-pub ahead of print]. J Am Coll Cardiol Available at: https://doi:10.1016/j.jacc.2020.06.007. Accessed June 8, 2020. [DOI] [PMC free article] [PubMed]
- 4.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 5.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) [e-pub ahead of print] JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Available at: Accessed April 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt B, Retter A, McClintock C. ISTH Academy. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. Available at: https://academy.isth.org/isth/2020/covid-19/290533/. Accessed April 28, 2020.
- 8.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with Covid-19 - A case series. N Engl J Med. 2020;382(25) doi: 10.1056/NEJMc2009020. 2478-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmud E, Dauerman HL, Welt FG, et al. Management of acute myocardial infarction during the COVID-19 pandemic [e-pub ahead of print]. J Am Coll Cardiol Available at: 10.1016/j.jacc.2020.04.039. Accessed April 28, 2020. [DOI] [PMC free article] [PubMed]
- 13.Bennett CE, Anavekar NS, Gulati R, et al. ST-segment elevation, myocardial injury, and suspected or confirmed COVID-19 patients: Diagnostic and treatment uncertainties. Mayo Clin Proc. 2020;95(6):1107–1111. doi: 10.1016/j.mayocp.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canadian Cardiovascular Society COVID-19 Rapid Response Team. Is it COVID-19 or is it heart failure? Management of ambulatory heart failure patients. Available at: http://www.ccs.ca/images/Images_2020/COVID_or_HF_RRT_doc_01Apr.pdf. Accessed April 29, 2020.
- 15.Page RL, 2nd, O'Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134 doi: 10.1161/CIR.0000000000000426. e32-69. [DOI] [PubMed] [Google Scholar]
- 16.Tonnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy - a review of the literature. Immunopharmacol Immunotoxicol. 2013;35:434–442. doi: 10.3109/08923973.2013.780078. [DOI] [PubMed] [Google Scholar]
- 17.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurwitz D.Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics [e-pub ahead of print]. Drug Dev Res. Available at: 10.1002/ddr.21656. Accessed April 28, 2020. [DOI] [PMC free article] [PubMed]
- 20.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaria Policy Advisory Committee, World Health Organization. The cardiotoxicity of antimalarials. Executive Summary, WHO Evidence Review Group Meeting, 13-14 October 2016, Geneva, Switzerland. Available at: https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2.pdf. Accessed April 29, 2020.
- 23.Haeusler IL, Chan XHS, Guerin PJ, White NJ. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16:200. doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 (Coronavirus Disease 2019) treatment. Circulation. 2020;141(24):e906–e907. doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 25.Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 26.Chorin E, Dai M, Shulman E, et al. The QT interval in patients with SARS-CoV-2 infection treated with hydroxychloroquine/azithromycin [e-pub ahead of print]. Available at: 10.1101/2020.04.02.20047050. Accessed April 28, 2020. [DOI]
- 27.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bessiere F, Roccia H, Deliniere A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [e-pub ahead of print]. JAMA Cardiol. Available at: 10.1001/jamacardio.2020.1787. Accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 29.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) [e-pub ahead of print]. JAMA Cardiol Available at: 10.1001/jamacardio.2020.1834. Accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 30.Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giudicessi JR, Noseworthy PA, Friedman PA, Acherman MJ. Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for COVID-19. Mayo Clin Proc. 2020;95(6):1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FDA Drug Safety Communication. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems.Available at:https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. Accessed April 29, 2020.
- 33.Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992–2993. doi: 10.1016/j.jacc.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung CC, Davies B, Gibbs K, Laksman ZW, Krahn AD. Multi-lead QT screening is necessary for QT measurement: implications for management of patients in the COVID-19 era [e-pub ahead of print]. JACC: Clin Electrophysiol Available at: 10.1016/j.jacep.2020.04.001. Accessed April 28, 2020. [DOI] [PMC free article] [PubMed]
- 35.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CI, Postema PG, Arbelo E, et al. SARS-CoV-2, COVID-19 and inherited arrhythmia syndromes [e-pub ahead of print]. Heart Rhythm. Available at: 10.1016/j.hrthm.2020.03.024. Accessed April 28, 2020. [DOI] [PMC free article] [PubMed]
- 37.HRS COVID-19 Task Force Update: April 15, 2020. Cardiac implantable electronic device (CIED) management. Available at:https://www.hrsonline.org/COVID19-Challenges-Solutions/hrs-covid-19-task-force-update-april-15-2020. Accessed April 29, 2020.
- 38.Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020;141:e823–e831. doi: 10.1161/CIRCULATIONAHA.120.047063. [DOI] [PMC free article] [PubMed] [Google Scholar]