Abstract
To date, only two pigments have been identified in avian eggshells: rusty-brown protoporphyrin IX and blue-green biliverdin IXα. Most avian eggshell colours can be produced by a mixture of these two tetrapyrrolic pigments. However, tinamou (Tinamidae) eggshells display colours not easily rationalised by combination of these two pigments alone, suggesting the presence of other pigments. Here, through extraction, derivatization, spectroscopy, chromatography, and mass spectrometry, we identify two novel eggshell pigments: yellow–brown tetrapyrrolic bilirubin from the guacamole-green eggshells of Eudromia elegans, and red–orange tripyrrolic uroerythrin from the purplish-brown eggshells of Nothura maculosa. Both pigments are known porphyrin catabolites and are found in the eggshells in conjunction with biliverdin IXα. A colour mixing model using the new pigments and biliverdin reproduces the respective eggshell colours. These discoveries expand our understanding of how eggshell colour diversity is achieved. We suggest that the ability of these pigments to photo-degrade may have an adaptive value for the tinamous.
Subject terms: Biosynthesis, Animal physiology, Chemical modification, Structure elucidation
Introduction
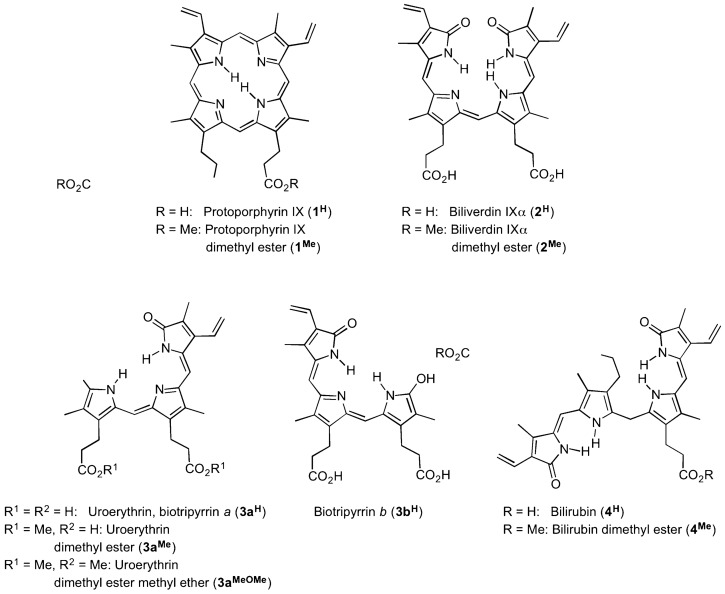
Birds’ eggs are found in an expansive variety of shapes, sizes, and colourings1. The diverse array of appearances found across Aves is achieved—in large part—through a combination of structural features, solid or patterned colorations, the use of two different dyes, and differential pigment deposition. Eggshell pigments are embedded within the white calcium carbonate matrix of the egg and within a thin outer proteinaceous layer called the cuticle2–4. These pigments are believed to play a key role in crypsis5,6, although other, possibly dynamic7,8, roles in inter- and intra-species signalling5,9–12 are also possible. In addition, these pigments may provide a range of structural, thermoregulatory, UV-protective, and photo-dependent antimicrobial benefits5,13–18. Despite the diversity of observable colours, there is a universal consensus that all of these colors are generated by only two pigments1,4,19–22: the tetrapyrrolic compounds protoporphyrin IX (referred to here as 1H, rusty-brown) and biliverdin IXα (2H, blue-green) (Fig. 1).
Figure 1.
Molecular structures of the two eggshell pigments (1H and 2H) identified to date, and the two new pigments (3aH/3bH and 4H) reported here, isolated as their dimethyl esters (3Me and 4Me, respectively); structure of the literature-known uroerythrin dimethyl ester methyl ether 3aMeOMe is also included.
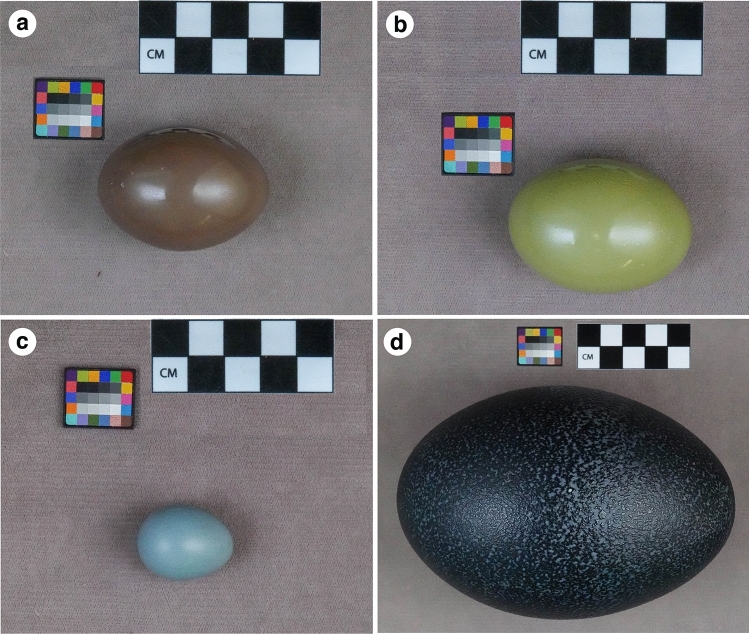
Tinamous (Tinamidae) are ground-dwelling, chicken-like, flying paleognathes native to Central and South America23. Tinamou eggs exhibit a diversity of bright colours, ranging from blues and greens to exotic greys and deep purplish-browns. Their eggshell surfaces have a distinctive glossy, porcelain-like appearance generated by nanostructured surface calcite and calcium phosphate crystals3. Interestingly, only the blue-green pigment biliverdin has been previously detected in the purplish-brown eggshells of the Spotted Nothura (Nothura maculosa; Fig. 2a) and the guacamole-green eggshell of the Elegant Crested Tinamou (Eudromia elegans; Fig. 2b)20,24. However, these tinamou eggshells differ strikingly in colour from other bird eggshells, including the biliverdin-only blue-green eggshells of the American robin (Turdus migratorius; Fig. 2c) and the dark-green eggshells of the emu (Dromaius novaehollandiae; Fig. 2d)1. We thus hypothesized that the purplish and green hues of the N. maculosa and E. elegans eggshells, respectively, are generated by mixing biliverdin with other, yet unknown, pigments. Therefore, we re-examined these tinamou eggshells, specifically extracting and identifying their pigments, and analysed their contributions to the observed eggshell coloration.
Figure 2.
Photographs of eggshells of (a) Nothura maculosa, (b) Eudromia elegans, (c) Turdus migratorius, and (d) Dromaius novaehollandiae. Image credits: Richard O. Prum.
Results
Nothura maculosa eggshell extraction
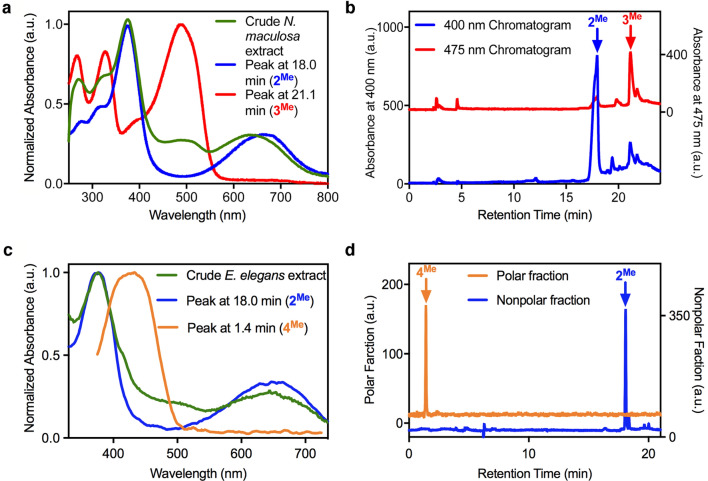
We applied a variation of a classic methanolic sulfuric acid-based eggshell pigment extraction protocol that esterifies the carboxylic acids of the pigments, yielding efficient partitioning of the pigments into an organic phase25–28. The UV–Vis absorption spectrum of the raw organic extract from the purplish-brown N. maculosa eggshell exhibits characteristic features of a bilin-type spectrum (i.e. two broad bands near 380 nm and 650 nm)29,30, as well as prominent bands centred at 325 and ~ 490 nm (Fig. 3a, green). The absence of any sharp feature in the UV–Vis absorption spectrum near 400 nm arising from the diagnostic Soret band of porphyrins suggests none are present.
Figure 3.
(a) Normalized UV–Vis spectra of the raw N. maculosa eggshell extract (green trace, EtOAc), extracted biliverdin 2Me (blue trace, MeOH), and extracted uroerythrin 3Me (red trace, MeOH). (b) HPLC traces of the raw extract from purple N. maculosa eggshells at two different wavelengths of detection. (c) Normalized UV–Vis spectra of the raw E. elegans eggshell extract (green trace, EtOAc), and of the two major components: biliverdin 2Me (blue trace, in MeOH) and bilirubin 4Me (orange trace, in MeOH). (d) NP-HPLC traces (at 400 nm detection wavelength) of the polar and nonpolar fractions from the E. elegans extract.
Analysis of the crude extract using normal-phase high-performance liquid chromatography (NP-HPLC) revealed the presence of two major chromophores and some minor components (Fig. 3b). The first component, eluting at 18.0 min and absorbing strongly at 400 nm (Fig. 3b, blue), displayed a typical biliverdin UV–Vis spectrum29,30 (Fig. 3a, blue). The compound proved to be identical in retention time, composition (C35H39N4O6 for [M + H]+ as per electrospray high-resolution mass spectrometry in the positive ion mode (ESI + HR-MS)), and molecular ion fragmentation pattern to biliverdin dimethyl ester 2Me extracted from emu (Dromaius novaehollandiae) eggshells28 and of commercial samples (Supplementary Figs. S1–4).
Inspecting the 475 nm detection chromatogram (Fig. 3b, red), we observe that the biliverdin peak (at 18.0 min) is, as expected, diminished; however, a second major peak, eluting at 21.1 min, is prominent. This peak is associated with a pigment that appears red–orange to the human eye and that possesses a three-band UV–Vis spectrum with features centred at 269, 325, and 495 nm (Fig. 3a, red). When compared to bilins, such blue-shifted spectra correlate with those of conjugated tripyrrolic compounds30,31. The pigment’s composition was determined to be C27H31N3O6, (for [M + H]+ as per ESI + HR-MS; Supplementary Fig. S5), and tandem HR-MS2 experiments supported the presence of a tripyrrolic pigment (Supplementary Fig. S6). Taken together, the data identify the orange pigment as the dimethyl ester 3Me of uroerythrin 3aH32 (for a discussion of the presence of its isomer 3bH, see below). The UV–Vis absorption spectrum of the crude extract allowed us to estimate the relative molar ratio of the two pigments biliverdin 2Me and uroerythrin 3Me to be about 3.5:1, with biliverdin 2Me being present in the range of 20–40 nmol g−1 eggshell. The colour mixing model described below provides an independently verified match of the pigment ratios.
We experimentally corroborated with independently sourced protoporphyrin 1H and biliverdin 2H that our pigment extraction protocol did not lead to their degradation, in general, or to the production of uroerythrin 3Me, in particular. Inversely, we found that purified uroerythrin extracts degraded in solution or as a film within days at ambient conditions (air, room temperature, light), but also—albeit slower—at − 20 °C in the dark. This speaks of the general lability of the pigment—an aspect of possible biological function, see below—and the need for rapid analysis after extraction for accurate quantitation.
Extractions of N. maculosa eggshells using EDTA, followed by ESI + MS–MS analysis of the extracts separated by reverse-phase HPLC (RP-HPLC), confirmed that the pigments were, as expected, present in the eggshells in their diacid forms 2H and 3H (Supplementary Figs. S7–9, S12). Furthermore, this analysis also shed more light on the nature of the minor pigments present, one of which could be identified as a uroerythrin isomer33 (Supplementary Fig. S7) and one to be likely a formyldipyrrinone34 (Supplementary Fig. S13). Of note, trace amounts of bilirubin 4H were detectable in the EDTA extracts through ESI + HR-MS, also (Supplementary Figs. S10–11). However, the absence of a 4H chromophore in the 400 nm RP-HPLC chromatogram (Supplementary Fig. S7), coupled with our colour blending model discussed below (cf. Figure 4), suggests that bilirubin 4H does not play any noticeable role in colouring N. maculosa eggshells.
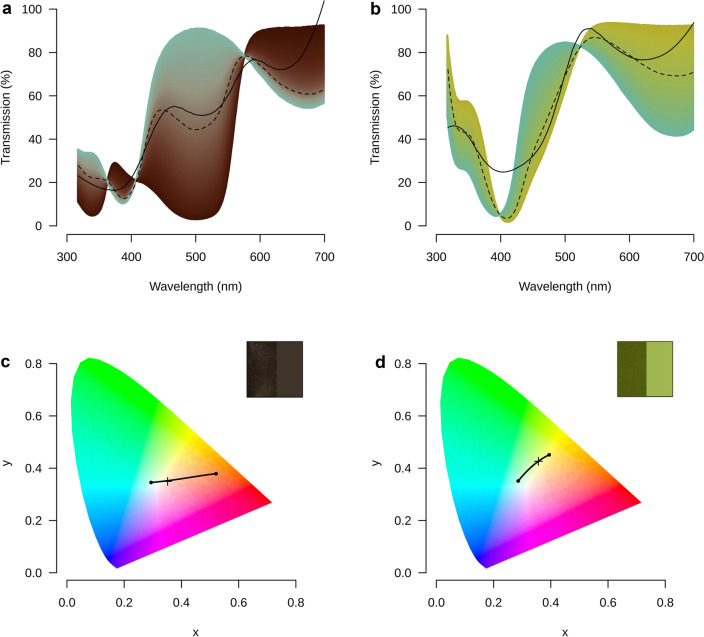
Figure 4.
Transmission spectra for the variable subtractive mixtures of (a) biliverdin and uroerythrin from N. maculosa and (c) biliverdin and bilirubin from E. elegans eggshells, respectively plotted in their modelled colours. Measured spectral reflectance of those eggshells (solid) is compared to the best predicted spectrum (dashed). All spectra are depicted from 318 to 700 nm. The colour of each spectrum is calculated from its spectrum, and the brightness of those colours was modified to approximate the reflectance of whole eggshells (solid black lines). Intermediate spectra for (b) biliverdin and uroerythrin and (d) biliverdin and bilirubin were plotted within the CIE coordinate space (small black dots) varying from entirely biliverdin (left most large dot) to entirely the novel pigment (rightmost large dot). For comparison, the reflectance values of whole eggshells are plotted (+ symbol) within each coordinate space. Insets (b) and (d): Colour swatch for our best prediction (left) compared against a close-up photograph of the surface colour (right).
Eudromia elegans eggshell extraction
The guacamole-green eggshells of E. elegans were also extracted and analysed using NP-HPLC, UV–Vis spectroscopy, and ESI + HR-MS spectrometry as described above. The UV–Vis spectrum of the raw extract is overall bilin-like (i.e. broad bands near 380 nm and 650 nm), with the exception of the presence of a small shoulder at ~ 420 nm and a stronger than expected absorption in the range between ~ 450 and 550 nm (Fig. 3c, green). The lack of a sharp Soret band near 400 nm, again, indicates that porphyrins are not present. The crude sample was fractionated into a non-polar (eluent ethyl acetate), blue-green fraction from a more polar (eluent 4:1 ethyl acetate:methanol) yellow–brown-coloured fraction.
HPLC analysis showed that the non-polar fraction contains a single major chromophore (Fig. 3d, blue), identified as biliverdin dimethyl ester 2Me (Supplementary Fig. S16). Likewise, the polar fraction also contains only one major chromophore (Fig. 3d, orange). The composition of this pigment (C35H40N4O6Na as per ESI + HR-MS for its sodium adduct [M + Na]+) and diagnostic UV–Vis spectrum (single band absorption spectrum centred at ~ 440 nm; Fig. 3c, orange) characterize it as bilirubin dimethyl ester 4Me29. Accordingly, it also proved to be identical in retention time, UV–Vis absorbance profile, and gas phase behaviour under ESI + MS conditions to independently sourced bilirubin dimethyl ester (Supplementary Figs. S15 and S17–18). The presence of the diacid forms of 2H and 4H in the eggshells was also confirmed using EDTA eggshell extractions, followed by RP-HPLC and ESI + MS–MS analysis of the extracts (Supplementary Figs. S19, S21 and S23).
An analysis of the UV–Vis spectrum of the crude yellow extract approximates the molar ratio of 4Me:2Me to be roughly 0.2:1, with biliverdin 2Me present in the range of 5–10 nmol g−1 eggshell. Bilirubin 4Me proved to be quite unstable under the extraction conditions and in the crude extract solutions; handling of bilirubin samples for even short periods under ambient conditions (including light exposure) resulted in decomposition and formation of, inter alia, the oxidation product, biliverdin, resulting in an underestimation of the bilirubin contents. Accordingly, the eggshell colour mixing model described below also suggests that the molar ratio of bilirubin:biliverdin in the native eggshells is significantly higher than we estimated based on the optical data of the crude extract.
Colour mixing models
Employing colour mixing models35, we find that combinations of biliverdin 2Me with uroerythrin 3Me or bilirubin 4Me could generate approximate colour matches (within 0.01 and 0.1 just noticeable difference, respectively) to the reflectance spectra of the surfaces of the N. maculosa and E. elegans eggshells, respectively. The predicted reflectance spectra closely approximated the measured reflectance spectra (Fig. 4a, b), and the modelled colours were very similar in appearance (Fig. 4c, d). These findings support the conclusion that the eggshell colours of both species are generated by the previously identified biliverdin pigment in combination with the previously unknown colorants: uroerythrin for N. maculosa and bilirubin for E. elegans. Notably, the purplish-brown colour of the N. maculosa eggshells did not require the presence of a brown porphyrin.
Additionally, these models predicted a ratio of biliverdin to uroerythrin of 3.74:1, which closely (within 7%) approximated the relative molar ratio of these pigments estimated by UV–Vis spectroscopy of the crude extract (see above). By contrast, the colour mixing model predicted a bilirubin to biliverdin molar ratio of 1.77:1, which is much larger than the spectroscopically estimated ratio of 0.2:1 (see above). This discrepancy is rationalized by the decomposition (oxidation) of the bilirubin and conversion to biliverdin under the extraction conditions. Additionally, the shape of the spectral reflectance curves for both eggshell surfaces (Fig. 4a, b, solid line) were closely approximated by those predicted by these unique admixtures of biliverdin and novel colourants (Fig. 4a, b, dashed line).
Discussion
Contrary to previous reports that found only biliverdin in N. maculosa and E. elegans eggshells20,24, this study also discovered the orange pigment uroerythrin 3H and the yellow–brown pigment bilirubin 4H, respectively. We can confidently conclude that both of these newly found pigments are genuine eggshell pigments as we experimentally verified that they are not artefacts generated in the extraction process. Additionally, these results are supported by colour mixing models which found that unique combinations of these pigments would generate the unusual surface colours of these eggshells. Furthermore, the excellent colour matches generated by our colour mixing models suggest that the presence of other minor pigments, such as the light-yellow dipyrrolic degradation products seen in the extract of the N. maculosa eggshells, do not contribute to the colour of the eggshells.
The orange-red tripyrrolic pigment uroerythrin 3H is a member of the urochromes, pigments arising from haem catabolism and present in, for example, human urine32. Uroerythrin is excreted in particularly high levels in individuals exhibiting metabolic pathologies and is associated with increased stress levels32. The biosynthesis of uroerythrin is not well understood; however, its origin as an oxidative degradation product of biliverdin 2H is assumed32. Model studies also hint at its chemical sensitivity36. Biliverdin, as well as bilirubin, were shown to be able to photosensitize oxygen leading to their own photodegradation33,37.
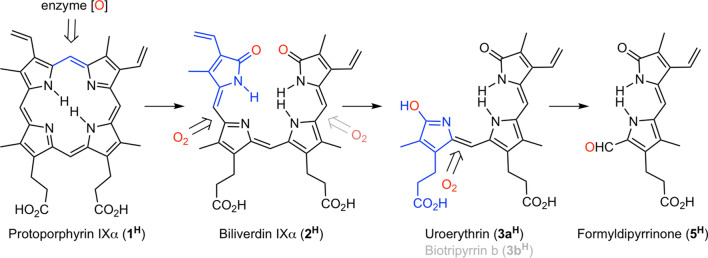
In the HPLC trace of the crude EDTA extract of the N. maculosa eggshells (Supplementary Fig. S7) as well as in the ESI + extracted ion chromatograms corresponding to the molecular mass of 3H (m/z = 466.2 Da; Supplementary Fig. S24), we find evidence for the presence of two isomeric compounds. Indeed, the oxidative loss of any one of the terminal pyrrolic moieties in the tetrapyrrolic pigment biliverdin will produce one of the isomers, 3aH or 3bH, also known as biotripyrrins a and b33. The occurrence of either isomer might point to their non-enzymatic origin. Furthermore, the N. maculosa EDTA extract also contained a small fraction of a dipyrrolic pigment with the composition C17H19N2O4 (for MH+). Characterized by its UV–Vis spectrum (Supplementary Fig. S7) and particularly through its well-resolved tandem MS spectrum (Supplementary Fig. S13), we assign it the formyldipyrrinone34 structure 5H. It is formally derived by continued oxidative degradation of uroerythrin (Fig. 5). Whether the first biliverdin oxidation step actually takes place at the bond indicated or at the adjacent double bond, followed by the loss of the meso-carbon, cannot be determined, but direct and circumstantial evidence exist that at least singlet oxygen reacts specifically with the double bond33,38.
Figure 5.
Degradation chain of protoporphyrin IX via biliverdin 2H to form uroerythrin 3aH (biotripyrrin b) and formydipyrrinone 5H. The pyrrolic fragments lost in the subsequent products are indicated in blue, the oxygens introduced in the oxidation step in red.
Bilirubin 4H is a well-studied catabolite of biliverdin39, generated through a reductive enzymatic process40. The adventitious formation of bilirubin from biliverdin under the oxic conditions of the pigment isolation and derivatization process is highly unlikely. This suggests that bilirubin 4H is a genuine eggshell pigment in Eudromia elegans. Since bilirubin degrades rapidly under the typical extraction conditions (converting, in part, to biliverdin, but also generating smaller (dipyrrolic), unidentified fragments, Supplementary Figs. S19–20, S22), it is understandable how bilirubin in E. elegans eggshells has eluded detection in the past20, 24. In addition, because of their structural similarity, the Raman spectra of biliverdin and bilirubin do not permit facile differentiation41.
Birds generally produce little bilirubin as most either lack, or have significantly reduced levels of, biliverdin reductase42,43. We are unaware if E. elegans possesses biliverdin reductase; however, work analysing the complete mitochondrial genome (GeneBank Accession no. NC_027260) of the closely related white-throated tinamou (Tinamus guttatus) reveals that T. guttatus contains genes corresponding to both biliverdin reductases A and B44. Moreover, the mitogenome order of T. guttatus is identical to that of the published mitogenome of T. major (GeneBank Accession no. NC_002781.3)45. We therefore deem it reasonable to assume that E. elegans can also generate bilirubin by enzymatic reduction of biliverdin.
Our colour mixing model accurately modelled the natural colours of the purple and green N. maculosa and E. elegans eggshells, respectively, by mixing the colours of the two pigments found in each eggshell. As such, this model provides supporting evidence that the extracted compounds are genuine eggshell pigments contributing toward the unique eggshell colours. Thus, tinamous have access to an expanded eggshell pigment colour palette. Furthermore, since this two-pigment model, which assumes an even mixing of the pigments throughout the pigmented layer, achieved a close match to the experimental eggshell colours, it also indicates that none of the additional mechanisms discovered to modulate eggshell colours—such as uneven distribution of the pigments throughout the cuticle and eggshell46–48, colour modulation by the cuticle and eggshell structure2,3, pigment aggregation49, or the strongly solvatochromic properties and conformational plasticity of linear tetrapyrroles30,50–52 that might express different hues in variable protein matrices—play a prominent role here. Likewise, the much less deeply yellow-coloured dipyrrins present also do not play any major role in the coloration of the eggshells.
Both novel pigments are more (photo)-labile than biliverdin. This suggests that the surface coloration for both eggs should vary substantially over time when exposed to light. Interestingly, such colour changes have been observed in tinamou eggshells and may have an adaptive function in communally nesting polyandrous birds8. In both species, females will lay their eggs in a male’s nest and the colour change could be useful for communally nesting females to discriminate freshly laid clutches from older clutches, thus serving as a useful cue for deciding where and when to lay8. Conspicuous eggshell colours may also serve as an attractive signal to other females, encouraging them to add to the clutch, which can lower predation by initiating male incubation53. This said, it is also plausible that these colour changes are simply non-adaptive, non-costly by-products of the underlying pigments. Further research is necessary to determine whether the (photo)-lability of these dyes has any ecological significance. The use of ephemeral, tetrapyrrole-based photo-degradable coloration to convey biological information has been proposed previously54.
The finding that oligopyrrolic pigments are exclusively used as eggshell pigments attests to the strong colouring ability of extended π-systems. The open-chain pigments can be derived from the degradation of haem within the shell gland of the laying bird55 and represent an essentially linear catabolic sequence already present elsewhere in the avian body56; it is a further testament that the eggshell pigments are derived by the repurposing of existing metabolic pathways56. The parent pigment, protoporphyrin, may itself also be derived in deviation from regular haem metabolism pathways from haem in the oviduct57. Future research is required to determine if the shell gland is also the synthesis site for bilirubin and uroerythrin. Interestingly, the only other study we are aware of which reports a non-porphyrin/non-biliverdin pigment (albeit without providing details or an independent verification) found another biliverdin metabolite, the tetrapyrrolic purple pigment mesobiliviolin, in the brown markings on the greenish eggshells of Lissotis melanogaster19. The finding that the eggshell pigments identified to date are oligopyrrolic pigments suggests that birds are limited to utilizing metabolites from the levulinic acid route to dye their eggs. This is in contrast to the mevalonic acid and tyrosine metabolic routes taken by the birds for the majority of the dyes to colour their plumage58.
Conclusion
In conclusion, the investigation of the unusually coloured eggshells of two tinamou species revealed, next to the well-known eggshell pigment biliverdin 2H, the presence of two hitherto unrecognized oligopyrrolic eggshell pigments: the orange tripyrrolic uroerythrin 3H (in eggshells of N. maculosa) and the brown-yellow tetrapyrrolic bilirubin 4H (in eggshells of E. elegans), both isolated and identified as their diacids and their dimethyl esters. A colour mixing model supports the conclusion that the eggshell colours of both species are generated by the presence of the previously identified pigment biliverdin in combination with the two previously unknown colourants. Notably, the chocolate-brown coloration of the E. elegans eggshells can be achieved without any contribution of the traditional brown pigment, protoporphyrin IX. The yellow–brown bilirubin and orange uroerythrin thus expand the pallete of the known eggshell pigments. Furthermore, we suggest that the layering of biliverdin with these pigments possessing different abilities to photo-degrade may have an adaptive value for the tinamou species investigated.
Methods
Materials
The N. maculosa eggshell from captive-bred birds (Chile) were sourced from The Eggery Place (https://theeggeryplace.com). The E. elegans eggshells came from the Peabody Museum collection. All solvents used were HPLC or spectroscopy grade and used as provided by commercial suppliers. Protoporphyrin IX 1H, its dimethyl ester 1Me , biliverdin IXα 2H, its dimethyl ester 2Me, bilirubin 4H, its dimethyl ester 4Me28 were either provided by Porphyrin Products, Logan, UT, or were extracted from hen (1Me)27 or emu eggshells (2Me)28, or chemical reduction of biliverdin 2Me, respectively (4Me)28.
Acid-based eggshell extraction
A slightly modified version of the classic eggshell pigments extraction protocol using methanolic H2SO4 solution was used25–28. A detailed protocol is provided in the ESI.
EDTA-based eggshell extraction
The EDTA pigment extractions were performed as described in Gorchein et al.21. A detailed protocol is provided in the ESI.
Instrumentation
UV–Vis spectroscopy
We either used a Cary 50 spectrometer or the Agilent 1,100 series HPLC UV-detector to record the UV–Vis spectra of the fractions and mixtures in the solvents indicated.
NP-HPLC
A portion of the N. maculosa eggshell extracts and the blue-green, non-polar band of the E. elegans eggshells were dissolved in ethyl acetate (~ 1 mL) and analysed using an Agilent 1,100 series HPLC (equipped with a Grace analytical normal-phase Apollo silica column, 4.6 × 250 mm, 5 μm and autosampler). The mobile phase employed a gradient delivery of hexanes and EtOAc: linear gradient of pure hexanes to 70:30 v/v hexanes:EtOAc over 6 min, then isocratic delivery of 70:30 v/v hexanes:EtOAc over 7 min, followed by linear gradient to pure ethyl acetate over 2 min, all with a flow rate of 1.5 mL/min. The detection wavelengths of the UV–Vis detector were set to 400 nm and 475 nm. The polar, green band of the E. elegans eggshell extracts were dissolved in MeOH (~ 1 mL) and analysed using the setup described above but using an isocratic delivery of 100% MeOH. The detection wavelength of the UV–Vis detector was set to 400 nm.
HR-MS
High-resolution mass spectra were recorded on a Thermo Scientific Q Exactive Quadrupole-Orbitrap mass spectrometer in the ESI + mode using 100% acetonitrile.
RP–HPLC–MS/MS
The dried EDTA eggshells extracts from the EDTA extraction were dissolved in MeOH (100 µL), centrifuged at 13,000 rpm, and transferred to LC–MS vials. RP-HPLC-ESI+-MS was performed using a SCIEX ExionLC (using a Kinetex C18 Reversed-Phase Column, 100 × 2.1 mm) coupled to a SciEX X500B QTof mass spectrometer. The samples were separated employing a 30 min gradient delivery of 5:95 to 98:2 ACN (+ 0.01% TFA) to water (+ 0.01% TFA). The measurements were contrasted against those using biliverdin 2H and bilirubin 4H standards.
Colour mixing model
Details to the colour mixing model used are provided in the ESI.
Supplementary information
Acknowledgements
Funding for this work was provided by the U.S. National Science Foundation (NSF) through grants CHE-1800361 (to C.B.), and a fellowship to R.H. through Grant HRD-1400382. We thank Blandy Experimental Farm and Arboretum for research support to collect gray catbird spectra (United States Fish and Wildlife Service (USFWS): MB81216C-1, Virginia Department of Game and Inland Fisheries (VADGIF): 062147, Long Island University Institutional Animal Care and Use Committee (IACUC): 18-01).
Author contributions
The manuscript was written through contributions of all authors. R.H. and C.B. performed the pigment extraction and identification, D.H. the colour modelling, R.P. provided the images for Fig. 2. All authors reviewed and have given approval to the final version of the manuscript.
Data availability
All data is available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-68070-7.
References
- 1.Hauber ME. The Book of Eggs. Chicago: Chicago University Press; 2014. [Google Scholar]
- 2.Fecheyr-Lippens DC, et al. The cuticle modulates ultraviolet reflectance of avian eggshells. Biol. Open. 2015;4:753–759. doi: 10.1242/bio.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Igic B, et al. A nanostructural basis for gloss of avian eggshells. J. R. Soc. Interface. 2015;12:20141210. doi: 10.1098/rsif.2014.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanley D, Grim T, Cassey P, Hauber Mark E. Not so colourful after all: Eggshell pigments constrain avian eggshell colour space. Biol. Lett. 2015;11:20150087. doi: 10.1098/rsbl.2015.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilner RM. The evolution of egg colour and patterning in birds. Biol. Rev. 2006;81:383–406. doi: 10.1017/S1464793106007044. [DOI] [PubMed] [Google Scholar]
- 6.Caswell Stoddard M, Marshall KLA, Kilner RM. Imperfectly camouflaged avian eggs: artefact or adaptation? Avian Biol. Res. 2011;4:196–213. [Google Scholar]
- 7.Hanley D, et al. Dynamic egg color mimicry. Ecol. Evol. 2016;6:4192–4202. doi: 10.1002/ece3.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanley D, Stoddard MC, Cassey P, Brennan PLR. Eggshell conspicuousness in ground nesting birds: Do conspicuous eggshells signal nest location to conspecifics? Avian Biol. Res. 2019;6:147–156. [Google Scholar]
- 9.Moreno J, Osorno JL. Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol. Lett. 2006;6:803–806. [Google Scholar]
- 10.Moreno J, et al. Experimental evidence that egg color indicates female condition at laying in a songbird. Behav. Ecol. 2006;17:651–655. [Google Scholar]
- 11.Stoddard Mary C, Stevens M. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Proc. R. Soc. B: Biol. Sci. 2010;277:1387–1393. doi: 10.1098/rspb.2009.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherry MI, Gosler AG. Avian eggshell coloration: New perspectives on adaptive explanations. Biol. J. Linn. Soc. 2010;100:753–762. [Google Scholar]
- 13.Gosler AG, Higham JP, Reynolds SJ. Why are birds’ eggs speckled? Ecol. Lett. 2005;8:1105–1113. [Google Scholar]
- 14.Magige F, Moe B, Røskaft E. The white colour of the Ostrich (Struthio camelus) egg is a trade-off between predation and overheating. J. Ornithol. 2008;149:323–328. [Google Scholar]
- 15.Ishikawa S-I, et al. Photodynamic antimicrobial activity of avian eggshell pigments. FEBS Lett. 2010;584:770–774. doi: 10.1016/j.febslet.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 16.Lahti DC, Ardia DR. Shedding light on bird egg color: Pigment as parasol and the dark car effect. Am. Nat. 2016;187:547–563. doi: 10.1086/685780. [DOI] [PubMed] [Google Scholar]
- 17.Dearborn DC, Page SM, Dainson M, Hauber ME, Hanley D. Eggshells as hosts of bacterial communities: An experimental test of the antimicrobial egg coloration hypothesis. Ecol. Evol. 2017;7:9711–9719. doi: 10.1002/ece3.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisocki PA, et al. The global distribution of avian eggshell colours suggest a thermoregulatory benefit of darker pigmentation. Nat. Ecol. Evol. 2019;4:148–155. doi: 10.1038/s41559-019-1003-2. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy GY, Vevers HG. A survey of eggshell pigments. Comp. Biochem. Physiol. 1976;55B:117–123. doi: 10.1016/0305-0491(76)90183-8. [DOI] [PubMed] [Google Scholar]
- 20.Verdes A, et al. Nature’s Palette: Characterization of shared pigments in colorful avian and mollusk shells. PLoS ONE. 2015;10:e0143545. doi: 10.1371/journal.pone.0143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorchein A, Lima CK, Cassey P. Extraction and analysis of colourful eggshell pigments using HPLC and HPLC/electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2008;23:602–606. doi: 10.1002/bmc.1158. [DOI] [PubMed] [Google Scholar]
- 22.Sorby HC. On the colouring-matters of the shells of birds’ eggs. Proc. Zool. Soc. Lond. 1875;23:351–365. [Google Scholar]
- 23.Davies SJJF. Ratites and Tinamous. Oxford: Oxford University Press; 2002. [Google Scholar]
- 24.Thomas DB, et al. Analysing avian eggshell pigments with Raman spectroscopy. J. Exp. Biol. 2015;218:2670. doi: 10.1242/jeb.124917. [DOI] [PubMed] [Google Scholar]
- 25.Tixier R. Contribution a l'étude de l'ester méthylique de la biliverdine des coquille d'oeufs d'Emeu. Bull. Soc. Chim. Biol. 1945;27:627–631. [PubMed] [Google Scholar]
- 26.Kennedy GY, Vevers HG. Eggshell pigments of the Araucano fowl. Comp. Biochem. Physiol. 1973;44B:11–25. doi: 10.1016/0305-0491(73)90336-2. [DOI] [PubMed] [Google Scholar]
- 27.Dean ML, Miller TA, Brückner C. Egg-citing! Isolation of protoporphyrin IX from brown eggshells and its detection by optical spectroscopy and chemiluminescence. J. Chem. Educ. 2011;88:788–792. [Google Scholar]
- 28.Halepas S, Hamchand R, Lindeyer SED, Brückner C. Isolation of biliverdin IXα, as its dimethyl ester, from emu eggshells. J. Chem. Educ. 2017;94:1533–1537. [Google Scholar]
- 29.Taniguchi M, Lindsey JS. Database of absorption and fluorescence spectra of >300 common compounds for use in PhotochemCAD. Photochem. Photobiol. 2018;94:290–327. doi: 10.1111/php.12860. [DOI] [PubMed] [Google Scholar]
- 30.Falk H. The Chemistry of Linear Oligopyrroles and Bile Pigments. Berlin: Springer; 1989. [Google Scholar]
- 31.Roth SDST, Lightner DA. Intermolecularly hydrogen-bonded dimeric helices: Tripyrrindiones. Tetrahedron. 2007;63:11030–11039. doi: 10.1016/j.tet.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berüter J, Colombo J-P, Schlunegger UP. Isolation and identification of the urinary pigment uroerythrin. Eur. J. Biochem. 1975;56:239–244. doi: 10.1111/j.1432-1033.1975.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi T, Shioji I, Sugimoto A, Komoda Y, Nakajima H. Chemical structure of a new family of bile pigments from human urine. J. Biochem. 1994;116:298–303. doi: 10.1093/oxfordjournals.jbchem.a124523. [DOI] [PubMed] [Google Scholar]
- 34.Boiadjiev SE, Lightner DA. Converting 9-methyldipyrrinones to 9-H and 9-CHO dipyrrinones. Tetrahedron. 2007;63:8962–8976. doi: 10.1016/j.tet.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonot L, Hébert M. Between additive and subtractive color mixings: Intermediate mixing models. J. Opt. Soc. Am. A. 2014;31:58–66. doi: 10.1364/JOSAA.31.000058. [DOI] [PubMed] [Google Scholar]
- 36.Bahnmüller S, et al. Hexaethyltripyrrindione (H3Et6tpd): A non-innocent ligand forming stable radical complexes with divalent transition-metal ions. Eur. J. Inorg. Chem. 2016;2016:4761–4768. [Google Scholar]
- 37.Lightner DA. Products of bilirubin photodegradation. In: Berk PD, Berlin NI, editors. Chemistry and Physiology of Bile Pigments. Washington: US Department of Health, Education, and Welfare; 1977. pp. 93–102. [Google Scholar]
- 38.Dorazio SJ, et al. Singlet oxygen oxidation products of biliverdin IXα dimethyl ester. Bioorg. Med. Chem. 2015;23:7671–7675. doi: 10.1016/j.bmc.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Heirwegh KPM, Brown SB, editors. Bilirubin—Metabolism. Boca Raton: CRC Press; 2018. [Google Scholar]
- 40.McDonagh AF. Turning green to gold. Nat. Struct. Biol. 2001;8:198–200. doi: 10.1038/84915. [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Morris MD, Xie M, Lightner DA. Resonance Raman spectroscopy of bilirubins: Band assignments and application to bilirubin/lipid complexation. Biochemistry. 1991;30:688–694. doi: 10.1021/bi00217a015. [DOI] [PubMed] [Google Scholar]
- 42.Lin GL, Himes JA, Cornelius CE. Bilirubin and biliverdin excretion by the chicken. Am. J. Physiol. 1974;226:881–885. doi: 10.1152/ajplegacy.1974.226.4.881. [DOI] [PubMed] [Google Scholar]
- 43.Lumeij JT, Westerhof I. Blood chemistry for the diagnosis of hepatobiliary disease in birds. A review. Vet. Q. 1987;9:255–261. doi: 10.1080/01652176.1987.9694110. [DOI] [PubMed] [Google Scholar]
- 44.An M, Zhang Z, Li X, Yang S. The complete mitochondrial genome of the White-throated Tinamou, Tinamus guttatus (Tinamiformes, Tinamidae) Mitochondrial DNA A. 2016;27:2800–2801. doi: 10.3109/19401736.2015.1053073. [DOI] [PubMed] [Google Scholar]
- 45.Haddrath O, Baker AJ. Complete mitochondrial DNA genome sequences of extinct birds: Ratite phylogenetics and the vicariance biogeography hypothesis. Proc. R. Soc. B: Biol. Sci. 2001;268:939–945. doi: 10.1098/rspb.2001.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu HC, Hsiao MC, Hu YH, Lee SR, Cheng WTK. Eggshell pigmentation study in blue-shelled and white-shelled ducks. Asian-Australas J. Anim. Sci. 2010;23:162–168. [Google Scholar]
- 47.Butler MW, Waite HS. Eggshell biliverdin concentration does not sufficiently predict eggshell coloration. J. Avian Biol. 2016;47:491–499. [Google Scholar]
- 48.Cassey P, et al. Avian eggshell pigments are not consistently correlated with colour measurements or egg constituents in two Turdus thrushes. J. Avian Biol. 2012;43:503–512. [Google Scholar]
- 49.Ostertag E, Scholz M, Klein J, Rebner K, Oelkrug D. Pigmentation of white, brown, and green chicken eggshells analyzed by reflectance, transmittance, and fluorescence spectroscopy. ChemistryOpen. 2019;8:1084–1093. doi: 10.1002/open.201900154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagniere G, Blauer G. Calculations of optical properties of biliverdin in various conformations. J. Am. Chem. Soc. 1976;98:7806–7810. doi: 10.1021/ja00440a056. [DOI] [PubMed] [Google Scholar]
- 51.Braslavsky SE, et al. Phytochrome models. IV. Conformational heterogeneity and photochemical changes in biliverdin dimethyl esters in solution. Isr. J. Chem. 1980;20:196–202. [Google Scholar]
- 52.Braslavsky SE, Holzwarth AR, Schaffner K. Solution conformations, photophysics, and photochemistry of bile pigments; bilirubin and biliverdin, dimethyl esters and related linear tetrapyrroles. Angew. Chem. Int. Ed. Engl. 1983;22:656–674. [Google Scholar]
- 53.Brennan PLR. Clutch predation in great tinamous Tinamus major and implications for the evolution of egg color. J. Avian Biol. 2010;41:419–426. [Google Scholar]
- 54.Galván I, Camarero PR, Mateo R, Negro JJ. Porphyrins produce uniquely ephemeral animal colouration: a possible signal of virginity. Sci. Rep. 2016;6:39210. doi: 10.1038/srep39210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao R, Xu G-Y, Liu Z-Z, Li J-Y, Yang N. A study on eggshell pigmentation: Biliverdin in blue-shelled chickens. Poult. Sci. 2006;85:546–549. doi: 10.1093/ps/85.3.546. [DOI] [PubMed] [Google Scholar]
- 56.Warren M, Smith A, editors. Tetrapyrroles: Birth, Life and Death. New York: Springer; 2009. [Google Scholar]
- 57.Wang XT, et al. Comparison of the total amount of eggshell pigments in Dongxiang brown-shelled eggs and Dongxiang blue-shelled eggs. Poult. Sci. 2009;88:1735–1739. doi: 10.3382/ps.2008-00434. [DOI] [PubMed] [Google Scholar]
- 58.Hill GE, McGraw KJ, editors. Bird Colorationa: Mechanisms and Measurements. Cambridge: Harvard University Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available upon request.