Abstract
Objective
The aims of this study is to investigate the matrix metalloproteinase-2 (MMP-2) levels and the toxicity evaluation to determine the safety of carbonate apatite material that will be used as an endodontic sealer.
Methods
Carbonate apatite as endodontic sealer material is fabricated with a size of 2 × 2 × 1 mm, sterilized, and then implanted in the subcutaneous area on the murine back. Total of 28 rats was divided into 2 groups: the implantation group and the control group (positive and negative). We observed behavior, macroscopic and microscopic images (through hematoxylin-eosin/HE staining) and MMP-2 levels in the serum (through ELISA examination) to determine the reaction of implant material in experimental animals.
Results
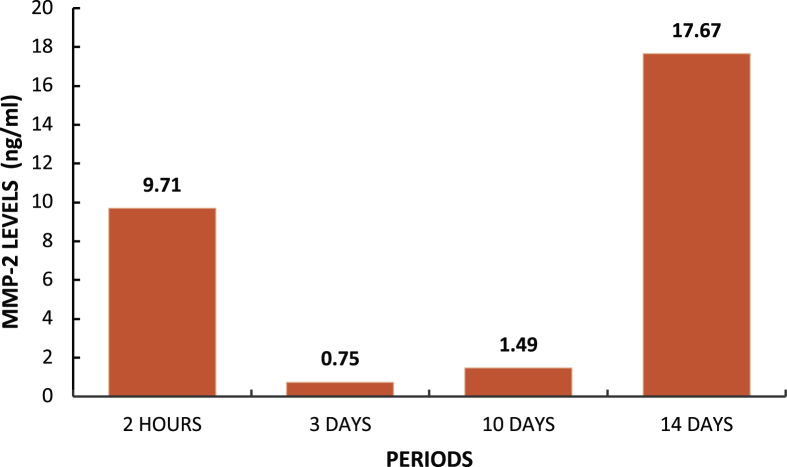
Behavioral test shows impaired motor function within 2–24 h in all rats, autonomic behavioral test does not show any disturbance, macroscopic images show appearance of tumor and rubor within 2–72 h in all rats, microscopic images shows an increase in the mean neutrophils number with the highest point located in the 24 h (3.92×103/μL) and lymphocytes with the highest point in the 14 days (15.31×103/μL). The average value of MMP-2 levels of the negative control group was 1.33 ng/ml, 1.29 ng/ml of the positive control group, and 7.32 ng/ml of the implantation group. Level of MMP-2 in rats with visible implants increased on day 3 (0.75 ng/ml), day 10 (1.49 ng/ml) and day 14 (17.67 ng/ml).
Conclusion
The implantation of carbonate apatite material did not cause behavioral disorders or abnormalities in the tissues surrounding of the implant site and did not show signs of toxicity or death.
Keywords: Matrix metalloproteinase-2 (MMP-2), Behavioral alteration, Tissue reaction, Carbonate apatite, Endodontic sealer material, Tissue engineering, Biomedical materials, Medical implant, Biomedical engineering, Histology, Dental materials, Toxicology
Matrix metalloproteinase-2 (MMP-2); Behavioral alteration; Tissue reaction; Carbonate apatite; Endodontic sealer material; Tissue engineering; Biomedical materials; Medical implant; Biomedical engineering; Histology; Dental materials; Toxicology
1. Introduction
An acute toxicity evaluation is a test to detect toxic effects that appear in a short time after administration of a test material given in a single dose or repeated doses given within 24 h. In this study, an acute toxicity evaluation for carbonate apatite-based implants was carried out. Although, this test does not fully describe the real safety in humans, the result of the toxicity evaluation can be a reference related to the safety of a product. The assessment of acute toxicity is determined by the death of the test animal as the final parameter. Animals that died during the experiment and lived until the end of the experiment underwent an autopsy to evaluate the presence of toxicity. In the present study, carbonate apatite implants were implanted subcutaneously below the back skin.
Materials with good biocompatibility, able to stimulate hard tissue regeneration, and bind well with mineralized structures can be an alternative material for the application of various clinical needs in the field of dentistry. One of the materials that have been used for quite a long time in healthcare, especially orthopedics, is bioceramic of carbonate apatite as bone graft material [1, 2, 3, 4, 5]. Carbonate apatite has the ability to bind to bone structure and can stimulate hard tissue formation [5, 6, 7, 8, 9, 10, 11]. Besides, when carbonate apatite granules implanted in the cranial bone of rats, after 24 weeks almost all carbonate apatite granules were changed to new bone [12]. Other using apatite cement with CO2 treatment to modify the surface layer of the sample until turn into carbonate apatite, proved after 6-months of time implantation in tibia rat, the carbonate apatite layer was resorbed gradually and form new bone [5].
Judging by the basic ingredients of bone which are almost the same as dental tissue, and the domestic ease of production, it becomes almost imperative to examine its function more deeply as an endodontic filler so it can be applied in dentistry [1, 13, 14, 15, 16]. Materials used in healthcare must be biocompatible so it does not cause side effects or bad outcome because every foreign object that enters the body will receive an inflammatory response by the body. One of such materials requiring high biocompatibility is endodontic filling agents placed close to the soft tissue around the apex of the teeth and capillaries. This material is able to form hydroxyapatite layers that resemble the bone mineral phase and produce a good adaptation between apatite cement and bone tissue. Carbonate apatite can adapt to the physiological environment and can be resorbed by tissue that will replace damaged tissue [1, 13, 17]. Inflammation which is the body's response to foreign matter can be interpreted as a series of secondary changes in tissue that are traumatized, whether physical, thermal, chemical or caused by micro-organisms [18].
Inflammation is characterized by vasodilation of local blood vessels which causes an increase in local blood flow, increased capillary permeability which allows extravasation into the interstitial cavity, and common fluid clotting in the interstitial cavity caused by fibrinogen and excessive blood protein, migration of granulocytes and monocytes in large numbers into the network, and swelling of tissue cells [6, 19]. In clinical examination symptoms can be found in the form of increased temperature (calor), pain (dolor), reddened tissue (rubor), enlargement (tumor) and often also lose or decrease of tissue function (functio laesa) [7, 8, 18, 20].
Acute dermal toxicity evaluation for carbonate apatite-based endodontic sealer is carried out by implantation of the material in the subcutaneous layer which will be observed macroscopically on gross tissue anatomy and microscopically under blood smear, histologically, and by measuring serum matrix metalloproteinase (MMP-2) levels because these animals have relative tissue response similar to human tissue. The response can be visualized as a response to human tissue [2, 17].
The behavior of animals is be observed after implantation because physiological changes or pathological conditions can change the behavior of living things. Preliminary test results obtained motor behavioral change in rats within 2–24 h, macroscopic tissue reactions (rubor and tumor) in rats within 2–72 h, the highest number of neutrophil inflammatory cells before 14 days and the highest lymphocyte increase at 14 days. This study aims to investigate the changes induced in rats post carbonate apatite material implantation whether macroscopically, microscopically, behaviorally and by MMP-2 serum levels change.
2. Materials and methods
This was an experimental study, using a randomized controlled design with a pre-test post-test control group design pattern. The study population was male white rats (Rattus norvegicus) aged 8–10 weeks old, weighing 200–350 g, at adult age (approximately 10 weeks), with normal behavior, no anatomical abnormalities, no visible dullness/hair loss/baldness. The research sample based on the Federer formula obtained 28 samples that divided into a control group (positive/negative) and an implant group. This study used tools and materials in the form of a behavioral form, microscope, experimental animals, carbonate apatite-based endodontic materials, Giemsa and HE stain, SEA100Ra 96 Enzyme-Linked Immunosorbent Assay (ELISA) for MMP-2 (Cloud-Clone Corp., Houston, USA). The carbonate apatite-based endodontic materials were prepared as our previous study which also consists of the evaluation of the basic properties [15, 16]. Additionally, the carbonate apatite as endodontic sealer material used in this study was fabricated from cement with a size of 2 × 2 × 1 mm and incubated at 37 °C in 100% relative humidity for 72 h, after the cement was set then sterilized. The carbonate apatite-based endodontic materials forming method was based on dissolution and precipitation reaction. When powder particle was mixed with the solution, the powder was dissolved to supply Ca2+, PO43- and CO32- ions. When the supersaturation phase was reached to carbonate apatite, the carbonate apatite crystals were precipitated and form the set cement [3].
The protocols study was approved by The Research Ethics Committee of Padjadjaran University number 1328/UN6.KEP/EC/2018. The experimental animals were put into a cage and let to adapt for one week. In the experimental animals, carbonate apatite-based endodontic material was implanted in the rat's subcutaneous back region by surgery. We observed motoric behavior (platform and hanging test) and autonomous sensory function (pineal, corneal, respiratory test), microscopic test (blood smear and hematoxylin-eosin), macroscopic test, as well as measured the level of MMP-2 serum ELISA using Multiscan FC Thermo Scientific (Thermo Scientific, Vantaa, Finland) in all samples at day 1, 3, 7, and 14. Data analysis is descriptive non-parametric which obtained using the mean formula.
3. Results
3.1. Observation of behavioral test, Sensoric dan motoric response
Behavioral test observation showed a decrease in the above platform activity 24 h after carbonate apatite material implantation. However, similar results were observed in the control group so the decrease was not caused by the presence of implants in the rat's body. Although, above platform activity decreased, observations on motor activity showed normal results in both groups.
The 24th hours and 14th days observation post-installation of carbonate apatite did not find Straub, piloerection, ptosis, lacrimation, catalysis, salivation, tremor, convulsions or writhing. All test animals showed normal responses to pineal reflex, corneal reflex, and respiration. Grooming, urination, and defecation activity in the test group is comparable to the normal control group.
Table 1 shows the results of implant and control rat control testing. Motoric nerve behavior test in experimental rat produce “abnormal” in 2 h period (100%), results vary in 24 h period consisting (64%) “normal”in control rat with (62%) in controls, and all rats in periods of 0 h, 3 days, 7 days, 10 and 14 days give “normal” results (100%).
Table 1.
Motoric behavior test on carbonate apatite based endodontic sealer material implanted animals and control animals.
| Animal Type (Σ) | Effects | Period(s) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 H | 2 H | 24 H | D 3 | D 7 | D 10 | D 14 | ||||
| NC (2) | Pl | N | % | 100 | - | - | - | - | - | - |
| AN | % | 0 | - | - | - | - | - | - | ||
| Ba | N | % | 100 | - | - | - | - | - | - | |
| AN | % | 0 | - | - | - | - | - | - | ||
| C (11) | Pl | N | % | 100 | 0 | 64 | 100 | 100 | 100 | 100 |
| AN | % | 0 | 100 | 36 | 0 | 0 | 0 | 0 | ||
| Ba | N | % | 100 | 0 | 64 | 100 | 100 | 100 | 100 | |
| AN | % | 0 | 100 | 36 | 0 | 0 | 0 | 0 | ||
| I (15) | Pl | N | % | 100 | 0 | 62 | 100 | 100 | 100 | 100 |
| AN | % | 0 | 100 | 38 | 0 | 0 | 0 | 0 | ||
| Ba | N | % | 100 | 0 | 62 | 100 | 100 | 100 | 100 | |
| AN | % | 0 | 100 | 38 | 0 | 0 | 0 | 0 | ||
Noted: NC = Negative Controls, C = Control, I = Implant, Pl = Platform, Ba = Bar hanging, N = Normal, AN = Abnormal.
Table 2 shows the “normal” results in all rats and all periods in the autonomic behavior test. The next table will explain the results of macroscopic tests on experimental animals implanted with carbonate apatite-based endodontic sealers.
Table 2.
Autonomous behavior tests in control animals and experiments implanted in carbonate apatite-based endodontic sealers.
| Animal Type (Σ) | Effects | Period(s) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 H | D 2 | D 24 | D 3 | D 7 | D 10 | D 14 | ||||
| NC (2) | Pi | N | % | 100 | - | - | - | - | - | - |
| AN | % | 0 | - | - | - | - | - | - | ||
| Co | N | % | 100 | - | - | - | - | - | - | |
| AN | % | 0 | - | - | - | - | - | - | ||
| Re | N | % | 100 | - | - | - | - | - | - | |
| TN | % | 0 | - | - | - | - | - | - | ||
| C (11) | Pi | N | % | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| AN | % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Co | N | % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| AN | % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Re | N | % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| AN | % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| I (15) | Pi | N | % | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| AN | % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Co | N | % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| AN | % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Re | N | % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| AN | % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
NC = Negative Controls, C = Control, I = Implant, Pi = Pineal, Co = Corneal, Re = Respiratory, N = Normal, AN = Abnormal.
3.2. Macroscopic observation
Table 3 shows the results of macroscopic observation of implanted and control rats. Rubor and tumor signify “present” test results in all rats within 2 and 24 h. Macroscopic observation found present rubor and tumor (36%) in control rats within 3 days, and (45%) in implanted rats within 3 days, whereas in all rats within 0 h, 7 days, 10 days and 14 days macroscopic observation yielded “none” or “absent” (100%).
Table 3.
Macroscopic test results in control and test animals implanted with carbonate apatite-based endodontic sealers.
| Animal Type (Σ) | Effects | Period(s) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 H | 2 H | 24 H | D 3 | D 7 | D 10 | D 14 | ||||
| NC (2) | Ru | NP | % | 100 | - | - | - | - | - | - |
| P | % | 0 | - | - | - | - | - | - | ||
| Tu | NP | % | 100 | - | - | - | - | - | - | |
| P | % | 0 | - | - | - | - | - | - | ||
| Fu | NP | % | 100 | - | - | - | - | - | - | |
| P | % | 0 | - | - | - | - | - | - | ||
| C (11) | Ru | NP | % | 100 | 0 | 0 | 64 | 100 | 100 | 100 |
| P | % | 0 | 100 | 100 | 36 | 0 | 0 | 0 | ||
| Tu | NP | % | 100 | 0 | 0 | 64 | 100 | 100 | 100 | |
| P | % | 0 | 100 | 100 | 36 | 0 | 0 | 0 | ||
| Fu | NP | % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| P | % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| I (15) | Ru | NP | % | 100 | 0 | 0 | 55 | 100 | 100 | 100 |
| P | % | 0 | 100 | 100 | 45 | 0 | 0 | 0 | ||
| Tu | NP | % | 100 | 0 | 0 | 55 | 100 | 100 | 100 | |
| P | % | 0 | 100 | 100 | 45 | 0 | 0 | 0 | ||
| Fu | NP | % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| P | % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
NC = Negative Controls, C = Control, I = Implant, Ru = Rubor, Tu = Tumor, Fu = Functio laesa, P = Presented, NP = Not Presented.
3.3. Organs observation
Bodyweight was observed for 14 days and showed an increase in weight. However, the increase was greater in control rats than in implanted rats. There was no death of implanted rats. No deaths were found after 14 days of carbonate apatite material installation. Body weight profiles for 14 days were similar in the control and test group. No organ abnormalities were found in all test animals on the 14th day after administration of the test preparation (Figure 1).
Figure 1.
Observation of control group organs (A), and implant groups (B).
3.4. Microscopic observation
Microscopic observation was carried out by examination of blood smears and examination of tissue slides through HE staining. The optical equipment was using biological microscopes (Olympus CX21, Tokyo, Japan).
3.4.1. Microscopic observation of blood film
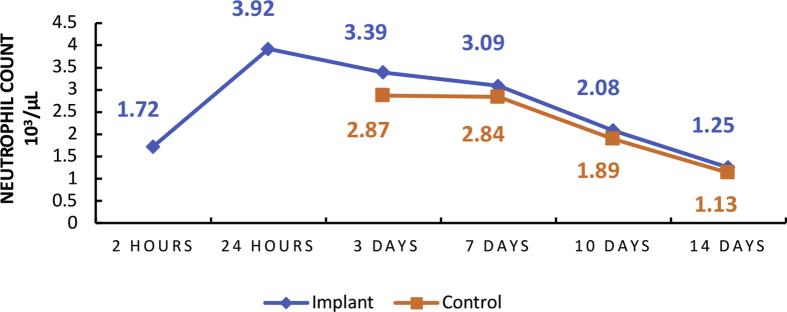
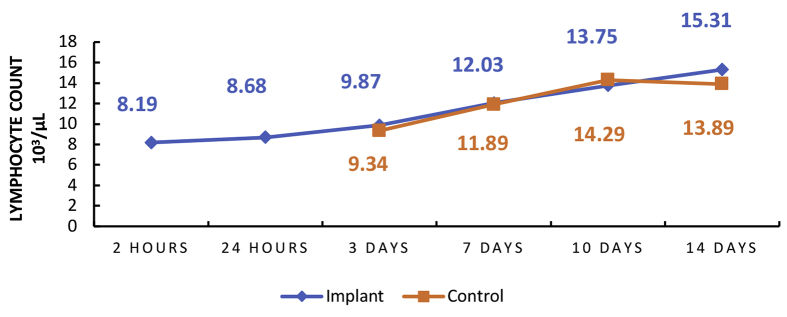
Graphs 1 and 2 above show the highest point in the mean number of neutrophils in implant rats within 24 h with a mean of 3.92×103/μL. The highest lymphocyte count was found in implant rats within 14-days with a mean of 15.31×103/μL. There was an increase in the mean neutrophil level within 2–24 h and then a decrease within 14 days. An increase in mean lymphocytes level within 2 h–14 days.
Graph 1.
Mean number of neutrophil inflammatory cells.
Graph 2.
Mean number of lymphocyte inflammatory cells.
3.4.2. Microscopic observation with HE staining
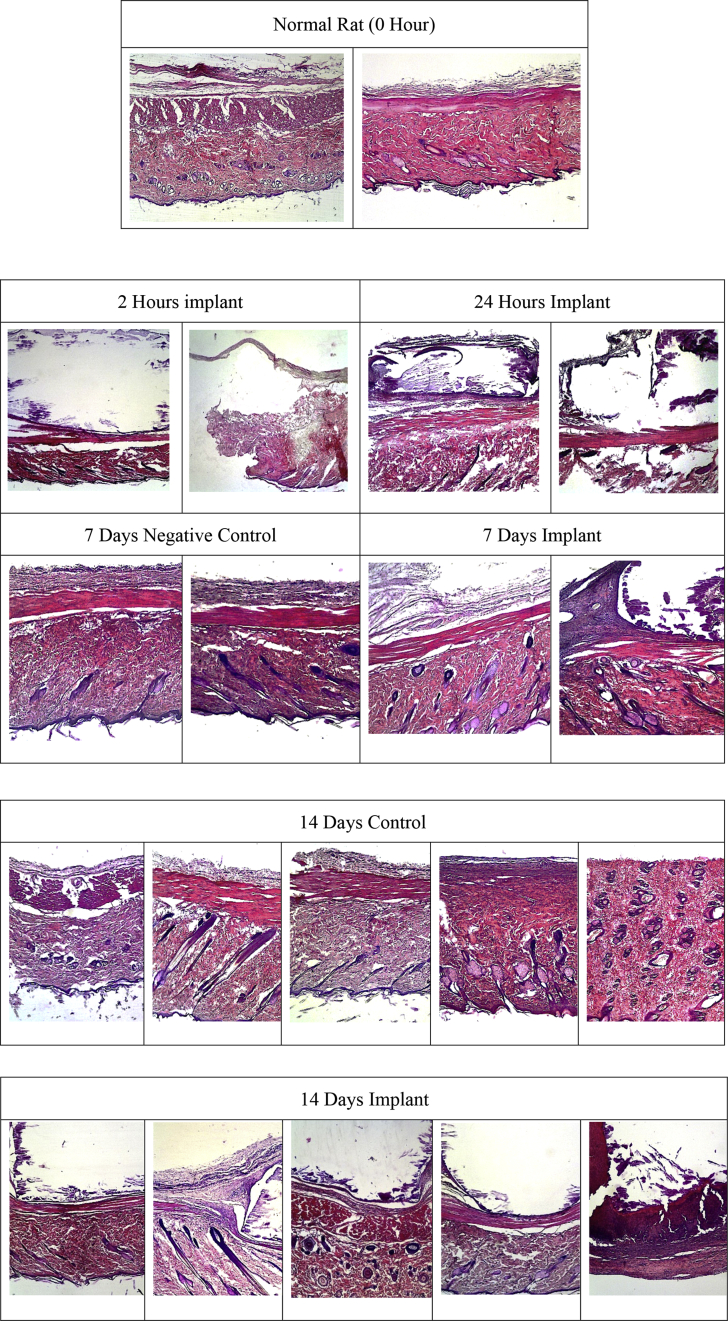
Observations of negative, positive controls and implants with hematoxylin-eosin (HE) staining showed no sign of inflammation around the area exposed to carbonate apatite implants (Figure 2). Subcutaneous tissue observation in the test group was similar to the normal.
Figure 2.
Rat subcutaneous tissue with HE staining (40x magnification) for 0, 2 and 24 h(s), 7 days and 14 days.
3.4.3. Serum MMP-2 level observation
Serum MMP-2 level within murine blood increased by the day as shown in the Graph 3. The observation showed high MMP-2 level within the 2nd hours and 14th days, in accordance with its role in extracellular matrix remodeling.
Graph 3.
MMP-2 serum level.
4. Discussion
Inflammation is the body's mechanism for reacting to wounds, foreign bodies or substances that enter the body. This response can be used as an indicator of the level of the body's acceptance of a material; the more difficult a material is received by the body, the worse the inflammation and the longer the duration. Marker of the emergence of inflammation is the cardinal signs of inflammation which can be visually observed in experimental animals: rubor, tumor, and functio laesa. The severity of the inflammatory response can be characterized by disruption of the wound healing process and the level of leukocytosis or increase in the number of white blood cells that occur systemically. The duration of the inflammatory response can be measured by how long the above signs appear and persist in experimental animals. If inflammation is extensive and ongoing, it can affect the organ tissue function of an organism which can ultimately change the organism's behavior [7, 9, 18, 21, 22].
Table 1 shows the results of the “abnormal" motor behavior test in the control and implant rats within 2 h. The motor behavior test gives “normal” and “abnormal” results within 24 h “Abnormal” results between 2 and 24 h occur due to dolor caused by compressive stimulation of nerve endings by edema resulting from vasodilation and opening of nerve endings due to injuries, anesthetic effects, as well as fatigue and discomfort due to energy depletion and body nutrition for healing processes [8, 23, 24, 25, 26, 27].
Results vary in implant and control rats within 24 h between “normal” and “abnormal”. This variation is caused by the inflammatory and wound healing process which happens at different rates. The rate of inflammatory processes that occur is influenced by the regeneration factor of each individual hence some samples regenerate faster [28, 29, 30].
Table 2 explains the results of the autonomous behavior test. The test showed “normal” results in all rats in every temporal setting. These results are consistent with the theory conveyed by Williams and Maier (1997) that local inflammation can localize trauma or stimulus, remove damaged tissue, destroy bacteria, and rebuild damaged tissue, so the inflammation will end; however, in general inflammation, trauma can extend to the surrounding tissue and can cause functio laesa in the surrounding tissue. Because trauma has been localized successfully, it does not cause widespread tissue damage so disruption of the autonomic nervous system does not occur [8, 31].
Table 3 shows the results of macroscopic observation of inflammatory signs in the form of tumor, rubor, and functio laesa. Signs of tumor and rubor inflammation that appear within 2 and 24 h are normal according to Nguyen (2009) that tumors and rubor occur are the results of vasodilation intended to enable platelets and blood clotting factors to exudate out of the blood vessels in order maintain homeostasis in the wound area [28]. This is also followed by A. Gonzalez et al., (2016) who maintains that inflammation can occur for 24 h to 4 days, so during this period vasodilation and exudation of fluids can cause redness and swelling [29]. Signs of inflammation that appear in some implant and control rats within 3 days is normal because the inflammatory period or wound healing of each individual can be different. S. Guo (2010) proposed several factors affecting wound healing: oxygenation, infections, foreign substances, adequacy of local blood vessels, age, sex, hormones, stress, systemic diseases, body weight, drugs, immunological conditions, and nutrition [30]. Within these factors, we can observe bodyweight and infection. Every rat has a different weight so the ability to heal wounds also yields different results. Microorganisms on the skin, dirt and urine can come into contact with the wound so contamination can occur which will cause slow wound healing [30].
The ultimate function of foreign body response (FBR) that occurs under normal physiological conditions is to protect the body from the foreign object. Usually, the work of FBR based on nonspecific protein adsorption, immune, and inflammatory cells. To avoid health complications of the patient, the effects of the implant on the host tissue and the host on the implantable device must be clearly understood. When the level of the homeostatic mechanisms is concerned, the pathophysiological conditions formed, then analysis of the inflammatory response should be considered as a reaction of the host, which ultimately resolves the relative compatibility of the materials. Whereas it is beneficial to split homeostatic mechanisms into tissue–material interactions, it is unique that many of the mechanism associated with homeostasis is a part of the same physiologic processes. A tissue/material interface is directly formed after intramuscular and sub cutan implantation. Nonspecific blood adsorption and tissue fluid proteins are also usually affected by the implant surface. The FBR severity depends on composition, length of contact, percentage of degradation, morphology, porosity, roughness, form, dimension, sterility, and surface chemistry of the material. FBR is characterized by an inflammatory response that involves a primary acute phase and following by the chronic phase. The wound healing process triggered by material implantation and associated tissue injury. The acute phase is marked by exudation as well as a neutrophilic reaction, and it lasts from hours to days. It is responsible for matrix formation and wound site cleaning. The vessels expand and blood will move into the injury area. The FBR reaction can release such as cytokines and growth factors from blood and tissue protein. After that, leukocytes adhere to the blood vessels endothelium and penetrate the injury area. Monocytes (part of leukocytes) then charged into the site and differentiate into macrophages. The continual presence of biomaterial as inflammatory stimuli can cause chronic inflammation [32].
Graphs 1 and 2 show the results of microscopic observations. The highest serum neutrophil level was found in the 24 h implant rat with a mean of 3.92×103/μL. The highest serum lymphocyte level was found in the 14 days implant rat with a mean of 15.31×103/μL. The control variable for Graphs 1 and 2 began to be observed on the third day (3 days) because the acute phase lasts from hours to days and is marked by fluid and protein exudation as well as a neutrophilic reaction and the numbers of neutrophil increase rapidly and reach a peak after 24–48 h [26, 27, 28, 29]. According to Martin, C. W. (1990), peak serum lymphocytes will be found within 8–14 days, after which it will decrease [33, 34]. The discussion above gives the impression that the material in this study has fairly good biocompatibility in the acute stage.
In Graph 3, we found elevated serum MMP-2 level in implanted rats with a longer period of implantation, showing that gelatinase has extra fibronectin domain located within the catalytic domain, which allows binding and processing of denatured collagen or gelatin. This shows that this enzyme also plays a key role in extracellular matrix remodeling. MMP gene expression is mainly regulated at the level of transcription, which usually results in a low basal level in normal physiology. Most members of the MMP family share a common cis-element in their promoter sequence, which allows strict control of cell-specific expression. As a result, MMP is often expressed or repressed in response to multiple stimuli, including inflammatory cytokines, growth factors, glucocorticoids or retinoids. The response at the transcription level occurs a few hours after exposure to the stimulus, indicating that the MMP promoter is a downstream target in the initial response gene signal pathway, which is induced shortly after cellular stimulation and in the absence of new protein synthesis. This initial response gene encodes a signaling protein that phosphorylates different transcription factors, which can then bind to the MMP gene promoter. Matrix metalloproteinases (MMPs) form a family of enzymes that mediate various functions in tissue destruction, remodeling, and immune responses by hydrolyzing components of the extracellular matrix under physiological and pathological conditions. The relationship between the MMP expression and inflammatory lesions was also examined [35, 36]. Carbonate apatite is a biocompatible implantation material. Behavioral, microscopic, macroscopic, and serum MMP-2 level observation did not show signs of toxicity.
5. Conclusion
The implant of subcutaneous carbonate-based endodontic sealer material in experimental animals using behavioral, microscopic, macroscopic, and serum MMP-2 level observation did not show signs of toxicity or death. The carbonate-based endodontic sealer material has an excellent result and promising to be use as an endodontic sealer material for endodontic dental treatment.
Declarations
Author contribution statement
A. Cahyanto and S.S. Widayaputra: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. Tjahajawati: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Zulhazmi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
M.S. Mariam: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
N. Djustiana, D. Aripin and K. Usri: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
The authors are grateful to Padjadjaran University under Academic Leaderships Grant (ALG) Program No. 2957/UN6.F/LT/2019 for funding this research and to Faculty of Dentistry and Oral Biomaterials Study Centre, Padjadjaran University for support and kind help in this study.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge the Laboratory of Veterinary, School of Pharmacy, ITB and the Integrated Laboratory, Faculty of Dentistry, Padjadjaran University where study was conducted.
References
- 1.Hing K.A. Bioceramic bone graft substitutes: influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005;2(3):184–199. [Google Scholar]
- 2.Ayukawa Y., Suzuki Y., Tsuru K., Koyano K., Ishikawa K. Histological comparison in rats between carbonate apatite fabricated from gypsum and sintered hydroxyapatite on bone remodeling. BioMed Res. Int. 2015;2015(1):1–8. doi: 10.1155/2015/579541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahyanto A., Maruta M., Tsuru K., Matsuya S., Ishikawa K. Fabrication of bone cement that fully-transform to carbonate apatite. Dent. Mater. J. 2015;34(3):394–401. doi: 10.4012/dmj.2014-328. [DOI] [PubMed] [Google Scholar]
- 4.Djustiana N., Amaranila M., Greviana A., Zakaria M.N., Sunarso Cahyanto A. Hardness evaluation of carbonate apatite cement based on various ratio of precursor. Key Eng. Mater. 2017;758:52–55. [Google Scholar]
- 5.Cahyanto A., Tsuru K., Ishikawa K. Effect of setting atmosphere on apatite cement resorption: an in vitro and in vivo study. J. Mech. Behav. Biomed. Mater. 2018;88:463–469. doi: 10.1016/j.jmbbm.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 7.Punchard N.A., Whelan C.J., Adcock I. The journal of inflammation. J Inflamm. 2004;1(1):1–4. doi: 10.1186/1476-9255-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zetoune F.S., Serhan C.N., Ward P.A. Inflammatory disorders. https://www.sciencedirect.com/science/article/pii/B9780128012383050960 Ref Modul Biomed Sci [Internet]. 2014 Jan 1 [cited 2019 Aug 21]; Available from:
- 9.Zakaria M.N., Pauziah N.F.N., Sabirin I.P., Cahyanto A. Evaluation of carbonate apatite cement in inducing formation of reparative dentin in exposed dental pulp. Key Eng. Mater. 2017;758:250–254. [Google Scholar]
- 10.Zakaria M.N., Cahyanto A., El Ghannam A. Calcium release and physical properties of modified carbonate apatite cement as pulp capping agent in dental application. Biomater. Res. 2018;22:35. doi: 10.1186/s40824-018-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahyanto A., Rezano A., Zakaria M.N., El-Ghannam A. Synthesis and characterization of a novel SCPC-CO3Ap cement for pulp capping application in dentistry. Key Eng. Mater. 2017;758:29–33. [Google Scholar]
- 12.Ishikawa K. Bone substitute fabrication based on dissolution-precipitation reactions. Materials. 2010;3:1138–1155. [Google Scholar]
- 13.Oryan A., Alidadi S., Moshiri A., Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014;9(1):1–27. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce A.I., Richards R.G., Milz S., Schneider E., Pearce S.G. Animal models for implant biomaterial research in bone: a review. Eur. Cell. Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 15.Cahyanto A., Megasari E., Zakaria M.N., Djustiana N., Sunarso, Usri K., Aripin D., Tjahajawati S., Mariam A.S., Widyaputra S.S. Fabrication of carbonate apatite cement as endodontic sealer. Key Eng. Mater. 2017;758:61–65. [Google Scholar]
- 16.Megasari E., Dharsono H.D.A., Fadil R., Zakaria M.N., Widyaputra S.S., Cahyanto A. The evaluation of setting time and ftir spectroscopy of carbonate apatite cement as endodontic sealer. Key Eng. Mater. 2018;782:32–37. [Google Scholar]
- 17.Seok J., Warren H.S., Cuenca A.G., Mindrinos M.N., Baker H.V., Xu W. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciaccia L. Fundamentals of inflammation. Yale J. Biol. Med. 2011;81(1):64–65. [Google Scholar]
- 19.Guyton A.C., Hall J.E. Guyton and Hall textbook of medical physiology. In: Hall J.E., editor. Guyton and Hall Textbook of Medical Physiology. twelfth ed. Elsevier; Jackson, Mississippi: 2011. p. 966. [Google Scholar]
- 20.Newman M.G., Takei H.H., Klokkevold P.R. In: eleventh ed. Carranza F.A., editor. Vol. 14. Elsevier; 2018. pp. 25–33. (Carranza's Clinical Periodontology). [Google Scholar]
- 21.Dąbrowska A.M., Słotwiński R. The immune response to surgery and infection. Cent. Eur. J. Immunol. 2014;39(4):532–537. doi: 10.5114/ceji.2014.47741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan G., Majno G. Acute inflammation. A review. Am. J. Pathol. 1977;86(1):185–274. [PMC free article] [PubMed] [Google Scholar]
- 23.Hammer G.D., McPhee S.J. eighth ed. 2018. Pathophysiology of Disease: an Introduction to Clinical Medicine; p. 1875. Ann Arbor. [Google Scholar]
- 24.McCance K.L., Huether S.E. eighth ed. Evolve Elsevier; Salt Lake City: 2019. Pathophysiology: the Biologic Basis for Disease in Adults and Children; p. 5816. [Google Scholar]
- 25.Hovens I.B., Schoemaker R.G., van der Zee E.A., Heineman E., Nyakas C., van Leeuwen B.L. Surgery-induced behavioral changes in aged rats. Exp. Gerontol. 2013;48(11):1204–1211. doi: 10.1016/j.exger.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Anusavice K.J., Shen C., Rawls H.R. twelfth ed. Elsevier Saunders; St. Louis, Mo: 2013. Phillip's Science of Dental Materials; pp. 111–147. [Google Scholar]
- 27.Zargar-Shoshtari K., Hill A. Postoperative fatigue: a review. World J. Surg. 2009;33:738–745. doi: 10.1007/s00268-008-9906-0. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen D.T., Orgill D.P., Murphy G.F. The pathophysiologic basis for wound healing and cutaneous regeneration. Biomater. Treat Ski Loss. 2009:25–57. [Google Scholar]
- 29.Gonzalez A.C.D.O., Costa T.F., Andrade Z.D.A., Medrado A.R.A.P. Wound healing - a literature review. An. Bras. Dermatol. 2016;91(5):614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo S., Dipietro L.A. Factors affecting wound healing. J. Dent. Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams J.G., Maier R.V. The inflammatory response. J. Intesive Care Med. 1992;7(2):53–66. [Google Scholar]
- 32.Morais J.M., Papadimitrakopoulos F., Burgess D.J. Biomaterials/Tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12(2):188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin C.W., Muir I.F.K. The role of lymphocytes in wound healing. Br. J. Plast. Surg. 1990;43(6):655–662. doi: 10.1016/0007-1226(90)90185-3. [DOI] [PubMed] [Google Scholar]
- 34.Boyce D.E., Jones W.D., Ruge F., Harding K.G., Moore K. The role of lymphocytes in human dermal wound healing. Br. J. Dermatol. 2000;143(1):59–65. doi: 10.1046/j.1365-2133.2000.03591.x. [DOI] [PubMed] [Google Scholar]
- 35.Fanjul-Fernandez M., Folgueras A.R., Cabrera S., Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta. 2010;1803(1):3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Klein T., Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41(2):271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]