Abstract
Background and Objective
Unipolar depression is the most common form of depression and demand for treatment, such as psychotherapy, is high. However, waiting times for psychotherapy often considerably exceed their recommended maximum. As a potentially less costly alternative treatment, internet-based cognitive behavior therapy (ICBT) might help reduce waiting times. We therefore analyzed the cost–utility of ICBT compared to face-to-face CBT (FCBT) as an active control treatment, taking differences in waiting time into account.
Methods
We constructed a Markov model to simulate costs and health outcomes measured in quality-adjusted life years (QALYs) for ICBT and FCBT in Germany. We modeled a time horizon of 3 years using six states (remission, depressed, spontaneous remission, undergoing treatment, treatment finished, death). The societal perspective was adopted. We obtained parameters for transition probabilities, depression-specific QoL, and cost data from the literature. Deterministic and probabilistic sensitivity analyses were conducted. Within a scenario analysis, we simulated different time-to-treatment combinations. Half-cycle correction was applied.
Results
In our simulation, ICBT generated 0.260 QALYs and saved €2536 per patient compared to FCBT. Our deterministic sensitivity analysis suggests that the base-case results were largely unaffected by parameter uncertainty and are therefore robust. Our probabilistic sensitivity analysis suggests that ICBT is highly likely to be more effective (91.5%), less costly (76.0%), and the dominant strategy (69.7%) compared to FCBT. The scenario analysis revealed that the base-case results are robust to variations in time-to-treatment differences.
Conclusion
ICBT has a strong potential to balance demand and supply of CBT in unipolar depression by reducing therapist time per patient. It is highly likely to generate more QALYs and reduce health care expenditure. In addition, ICBT may have further positive external effects, such as freeing up capacities for the most severely depressed patients.
Electronic supplementary material
The online version of this article (10.1007/s40258-019-00551-x) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| In the base case internet-based cognitive behavior therapy (ICBT) is simulated to be the dominant strategy generating 0.260 QALYs and saving €2536 per patient compared to face-to-face CBT, which is confirmed by deterministic and probabilistic sensitivity and scenario analyses. |
| Within the context of excess demand for psychotherapy, harmful long waiting times and increasing health care costs, ICBT has the potential to decrease time-to-treatment and to free therapists’ capacity in order to treat more and the most severe patients in a face-to-face setting with a higher intensity. |
Introduction
With an average 1-year prevalence of 6.9%, unipolar depression is the most common type of depression in Europe [1]. However, while the demand for treatment of this disorder is high and has even increased over the past two decades [1–8], the supply of psychotherapists appears to be insufficient. For example, depressed patients in Germany wait an average of 19.9 weeks for psychiatric treatment [9], which considerably exceeds the 4-week maximum waiting time for first appointments recommended by the German Chamber of Psychotherapists [9]. In England, 54% of depressed patients waited longer than 3 months and 12% more than a year for an appointment in 2013 [10]. This led the UK government and English National Health Service (NHS) to publish new access and waiting time standards stipulating that 75% of adults with common mental health conditions referred to the Improved Access to Psychological Therapies program must be treated within 6 weeks of referral [11]. Overall, 26.3% of patients in the European Union (EU) who need psychotherapy must often wait more than 2 months [12].
Although there is strong evidence that face-to-face cognitive behavioral therapy (FCBT) is effective, especially in the treatment of depressive disorders [13–16], alternatives are clearly needed to reduce waiting times and improve access to services more generally. In this regard, internet-based cognitive behavioral therapy (ICBT) may represent a promising approach. With ICBT, patients complete various web-based modules by themselves and contact their therapist to receive support. Several randomized controlled trials (RCTs) and meta-analyses have provided evidence that the effectiveness of ICBT is comparable [17–21] and, in case of guided ICBT, at least equivalent [22, 23] to that of FCBT.
By reducing therapist time per patient, ICBT has the potential to increase efficiency and reduce patient waiting times. Moreover, ICBT may offer advantages to certain subgroups of patients, such as those who fear stigma associated with FCBT or face geographical or other obstacles to travel. Indeed, there is evidence that the more indirect and anonymous form of communication entailed by ICBT reduces barriers to treatment among patients who fear talking about personal problems to strangers, other patients, or even therapists [19, 24]. Furthermore, ICBT is more flexible in terms of time: patients can decide when, where, and how quickly they want to complete the web-based modules, and the content can be reviewed and repeated as required. This can be a particular advantage for patients who work full time or have physical disabilities.
Several systematic reviews, meta-analyses and original studies [25–35] have evaluated the cost-effectiveness of ICBT for use in several psychiatric disorders. However, none of the studies used an established standard treatment such as FCBT as a control and therefore effects are often based on single RCTs with short follow-up periods. To help address this gap in the research and provide payers and decision makers with information that will assist them in making coverage and reimbursement decisions, we conducted the first model-based cost–utility analyses comparing ICBT to an active control comprising standard treatment (FCBT). To do so, we combined different data sources to estimate pooled treatment effects, dropout and mortality rates, costs, and quality-of-life values. In addition, we extrapolated beyond the time horizons reported in the literature and integrated waiting time as a variable in our model.
Methods
Model Structure
Unipolar depression is characterized as a recurrent disease [36]. Phases with mild to severe symptoms (episodes) alternate with phases without, or with only minimal, symptoms (remission) [37, 38]. We employed a cohort-based Markov model to simulate the course of depression as well as treatment by modeling recurring events. According to the included RCTs [39–48], patients experience a relapse after 75.8 weeks on average, which can be understood as a lower bound for the modeled time horizon [49, 50]. Because unipolar depression is a recurrent disease, a longer time horizon is warranted [50]. Based on the available data, we are able to model a time horizon of 3 years, which allows to simulate several depressive episodes and treatment attempts [51, 52]. We do not adopt a life-time horizon because this would be associated with unrealistic assumptions about technological development and the role of treatment alternatives. We chose a cycle length of 1 week (Fig. 1) and defined six discrete Markov states, as follows: remission, depressed, spontaneous remission, undergoing treatment (Weeks 1–11), treatment finished (Week 12), and death (i.e. all-cause mortality) (Fig. 1). We thoroughly defined selection criteria to assure that the data and evidence match the target population. We conducted comprehensive sensitivity and scenario analyses to test the influence of different model specifications on the results and to reveal the impact of both parameter variability and second order parameter uncertainty. In addition, subgroup analyses were carried out to address patient heterogeneity. Structural assumptions reflecting the natural course of the disease and treatment of depression were discussed with medical experts from an affiliated medical center.
Fig. 1.
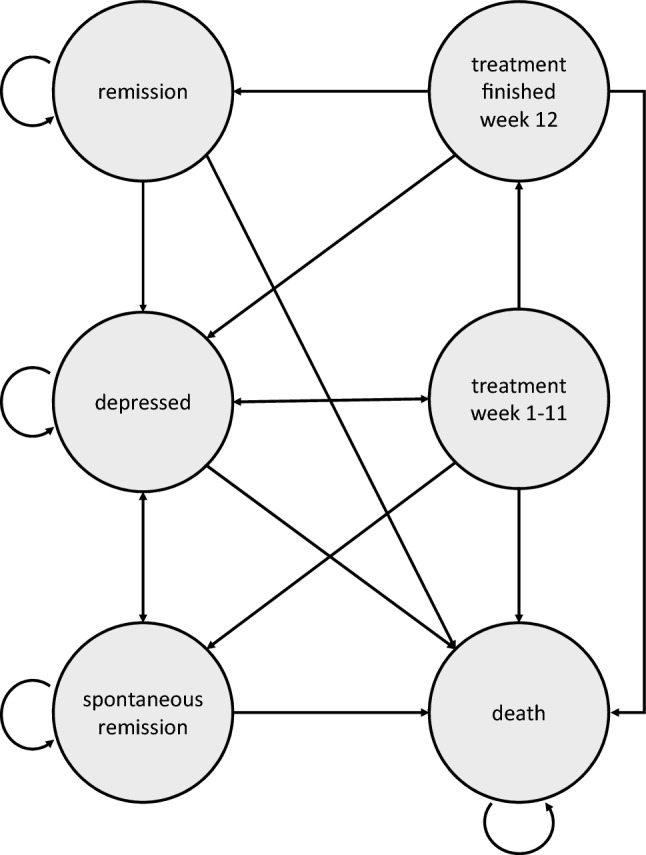
Model structure
The initial population starts in the depressed state. Depressed patients can experience spontaneous remission without any treatment and remain in remission until a relapse occurs, at which point they return to the depressed state. However, after a certain waiting time depressed patients eventually undergo 12 weeks of CBT. At the end of treatment Week 12, patients either enter the remission state, if their treatment has been successful, or return to the depressed state if it has not. Until treatment Week 11 patients may discontinue treatment. After treatment discontinuation, remission is out of reach. Patients either transfer back to the depressed state or remit spontaneously (because treatment discontinuation is often associated with spontaneous or premature remission) [53, 54]. In every state, each individual is at risk of all-cause mortality. We defined costs from the perspective of German society and specified quality-adjusted life years (QALYs) as utility outcomes. Both, costs and QALYs were discounted by 3% per year [49]. Half-cycle correction was applied [55]. We performed all calculations and simulations in R using the packages heemod [56] and metafor [57].
Parameter Assumptions
Transition Probabilities
We defined the effectiveness of CBT as the proportion of patients who remitted after treatment. To identify relevant studies, we used the results of the following two systematic reviews: the first, by Richard and Richardson [58], reviewed the literature on computer- and internet-based psychological treatments for depression and identified 45 publications reporting the results of 40 studies, 19 of which were RCTs and were included in their meta-analysis. The second, by Vittengl et al. [59], reviewed the literature on acute- and continuation-phase FCBT in unipolar depression and identified 28 studies. Of those, 16 studies reported the results of 16 RCTs analyzing acute-phase CBT. By updating both reviews using relevant meta-databases, we identified four additional publications reporting the results of four studies. We excluded studies that did not report remission or dropout rates, analyzed ICBT without therapist support, were restricted to populations with a specific disease (e.g. epilepsy), analyzed CBT in combination with other treatments (e.g. pharmacotherapy), did not clearly report focusing on patients with unipolar depression, or in which CBT was delivered in an inpatient setting.
Seventeen studies remained after we applied these exclusion criteria, ten [39–45, 60–62] and seven [63–69] of which reported remission and dropout rates for FCBT and ICBT, respectively (Table 1). We subsequently pooled these using random effects meta-regression as proposed by DerSimonian and Laird [70]. The online supplement provides full details of our meta-regression. The pooled remission rates after completion of therapy were 0.5174 for ICBT and 0.6090 for FCBT. The pooled spontaneous remission rate in the absence of acute treatment for patients in the depressed state was 0.0123 [42, 63–67, 71, 72]. Relapse rates were 0.0064 after full remission (after treatment completion) [39–47] and 0.0202 after spontaneous remission [48]. The pooled treatment discontinuation rates until Week 11 were 0.0201 for ICBT [63–69] and 0.0145 for FCBT [39–45, 60–62]. We split dropouts equally into the spontaneous remission and depressed states after treatment discontinuation because there were no data in the included studies on this step. We derived data on all-cause mortality from World Health Organization (WHO) mortality tables for the general population in Germany [73]. To reflect disease-specific mortality, we adjusted the 1-week all-cause death rate of 1.890E−05 with relative disease-specific death risks [74], yielding an average 1-week mortality rate of 3.636E−05 for depressed patients.
Table 1.
Included studies
| Study | Year of publication | Design | Treatment | Follow-up (weeks) | N | Proportion of patients | |
|---|---|---|---|---|---|---|---|
| In remission | Discontinuing treatment | ||||||
| Andersson et al. [69] | 2013 | RCT | ICBT | 9 | 33 | 0.394 | 0.061 |
| DeRubeis et al. [61] | 2005 | RCT | FCBT | 16 | 60 | 0.400 | 0.150 |
| Elkin et al. [62] | 1989 | RCT | FCBT | 16 | 56 | 0.649 | 0.339 |
| Evans et al. [44] | 1992 | RCT | FCBT | 12 | 25 | 0.400 | 0.360 |
| Gortner et al. [45] | 1998 | RCT | FCBT | 12 | 50 | 0.500 | 0.120 |
| Hautzinger et al. [42] | 2004 | RCT | FCBT | 12 | 65 | 0.523 | 0.154 |
| Hedman et al. [68] | 2014 | Cohort | ICBT | 12 | 1203 | 0.481 | 0.248 |
| Jarrett et al. [43] | 1998 | Cohort | FCBT | 10 | 60 | 0.617 | 0.183 |
| Jarrett et al. [43] | 1998 | Cohort | FCBT | 10 | 34 | 0.618 | 0.118 |
| Jarrett et al. [39] | 2001 | RCT | FCBT | 13 | 156 | 0.558 | 0.167 |
| Kessler et al. [64] | 2009 | RCT | ICBT | 16 | 149 | 0.289 | 0.242 |
| Murphy et al. [60] | 1984 | RCT | FCBT | 12 | 24 | 0.417 | 0.208 |
| Ruwaard et al. [67] | 2009 | RCT | ICBT | 11 | 36 | 0.444 | 0.083 |
| Shea et al. [40] | 1992 | RCT | FCBT | 16 | 59 | 0.390 | 0.322 |
| Thase et al. [41] | 1992 | Cohort | FCBT | 16 | 76 | 0.658 | 0.158 |
| Titov et al. [65] | 2010 | RCT | ICBT | 8 | 47 | 0.489 | 0.128 |
| Vernmark et al. [66] | 2010 | RCT | ICBT | 8 | 29 | 0.345 | 0.069 |
| Warmerdam et al. [63] | 2008 | RCT | ICBT | 8 | 80 | 0.263 | 0.188 |
FCBT face-to-face cognitive behavior therapy, ICBT internet-based cognitive behavior therapy, N number of study participants, RCT randomized controlled trial
Waiting Time
In Germany, after an initial appointment, a patient’s need for psychotherapy is assessed over two to four evaluation sessions [75]. After this initial assessment phase, patients wait an average of 19.9 weeks to start their psychotherapy [9]. We therefore defined 20 weeks as the average waiting time for our FCBT cohort. Because ICBT has not been used in a regular clinical setting in Germany to date, the potential reduction in waiting times due to ICBT is unknown. Therefore, we used the reported time to treatment from one study on the effectiveness of ICBT for panic disorder within a clinical setting from Sweden, which reported an average waiting time for ICBT of 18 days [76] followed by two days to arrange and commence treatment for those who are assessed to be eligible for it [76]. We therefore defined 3 weeks as the average waiting time for our ICBT cohort. Because it is unknown if a waiting time of 3 weeks can be achieved within the context of the German health care system, we conducted a scenario analysis in which this potential reduction in waiting time is relaxed. Assuming that 50% of the patients who need psychotherapy will start their treatment after the average waiting time, we set the transition probability at 0.5, which yields a 1-week probability of 0.0341 for FCBT and 0.2063 for ICBT.
Quality of Life Measurement
Sapin et al. measured health-related quality of life (QoL) in unipolar depressed patients in France using the EQ-5D-3L [77]. The reported values were broken down according to depression severity and treatment status. Untreated patients who were mildly, moderately, or severely depressed reported mean utility scores of 0.45, 0.33, and 0.15, whereas patients who were undergoing treatment reported substantially higher scores of 0.74, 0.44, and 0.30, respectively. We therefore assigned a weighted average utility score of 0.270 to depressed patients without treatment and 0.417 to patients during treatment in our simulation using weights for the three severity levels according to the distribution in Germany [78]. For patients in remission (valid for remission after treatment and spontaneous remission), Sapin et al. reported a mean utility score of 0.85 [77]. We assigned a score of zero to the state of death.
Measurement of Costs
Krauth et al. [79] reported pharmaceutical, outpatient, inpatient and indirect costs for unipolar depressed patients in Germany based on data from the Primary Care Monitoring for Depressive Patients Trial (PRoMPT) [80]. We inflated these to 2018 prices using data on growth in health care expenditure in Germany [81] (see also Table 2). Doing so yielded average health care costs for untreated depressed patients of €106.25 per week, including pharmaceutical (€6.71), outpatient (€11.75), inpatient (€48.65) and indirect (€39.14) costs.
Table 2.
Model parameters
| Model parameter | Baseline | Deterministic | Probabilistic | Source(s) | |||
|---|---|---|---|---|---|---|---|
| Low | High | Alpha | Beta | ||||
| Costs per cycle and state | |||||||
| Depressed (untreated) | 106.25 | ||||||
| Pharmaceutical | 6.71 | 4.70 | 8.98 | Gamma | 0.51 | 13.26 | [79, 81] |
| Outpatient | 11.75 | 9.76 | 13.92 | Gamma | 1.55 | 7.58 | [79, 81] |
| Inpatient | 48.65 | 18.12 | 83.70 | Gamma | 0.11 | 434.58 | [79, 81] |
| Indirect | 39.14 | 15.28 | 73.87 | Gamma | 0.09 | 425.60 | [79, 81] |
| Depressed (during CBT) | 57.60 | ||||||
| Pharmaceutical | 6.71 | 4.70 | 8.98 | Gamma | 0.51 | 13.26 | [79, 81] |
| Outpatient | 11.75 | 9.76 | 13.92 | Gamma | 1.55 | 7.58 | [79, 81] |
| Indirect | 39.14 | 15.28 | 73.87 | Gamma | 0.09 | 425.60 | [79, 81] |
| Remission | 9.23 | ||||||
| Pharmaceutical | 3.35 | 2.35 | 4.49 | Gamma | 0.51 | 6.63 | [79, 81] |
| Outpatient | 5.88 | 4.88 | 6.96 | Gamma | 1.55 | 3.79 | [79, 81] |
| Treatment costs | |||||||
| FCBT (total) | 1303.22 | ||||||
| ICBT (total) | 736.90 | ||||||
| First appointment (to week 1) | 44.33 | [82] | |||||
| Evaluation sessions (n = 3) | 196.17 | 130.78 | 261.56 | [82] | |||
| Software (ICBT only) | 50.00 | 25.00 | 100.00 | [28, 35] | |||
| FCBT (per week) | 88.56 | [83] | |||||
| ICBT (per week) | 37.20 | 7.08 | 67.31 | Gamma | 1.53 | 24.38 | [68, 83] |
| QoL by state | |||||||
| Remission | 0.85 | 0.72 | 0.98 | Beta | 54,640 | 9642 | [77] |
| Depressed (untreated) | 0.29 | ||||||
| Mild | 0.45 | 0.23 | 0.67 | Beta | 1996 | 2440 | [77] |
| Moderate | 0.33 | 0.09 | 0.57 | Beta | 3804 | 7723 | [77] |
| Severe | 0.15 | 0.00 | 0.36 | Beta | 344 | 1949 | [77] |
| Depressed (during CBT) | 0.47 | ||||||
| Mild | 0.74 | 0.55 | 0.93 | Beta | 4588 | 1612 | [77] |
| Moderate | 0.44 | 0.17 | 0.71 | Beta | 3529 | 4491 | [77] |
| Severe | 0.30 | 0.03 | 0.57 | Beta | 415 | 967 | [77] |
| Death | 0.00 | ||||||
| Discount rate | 0.03 | 0.00 | 0.06 | [49] | |||
| Transition probabilities | |||||||
| FCBT → REM | 0.6090 | 0.5311 | 0.6870 | Beta | 91.13 | 58.50 | [39–45, 60–62] |
| ICBT → REM | 0.5174 | 0.3878 | 0.6470 | Beta | 29.04 | 27.09 | [63–69] |
| FCBT → Drop out | 0.0145 | 0.0054 | 0.0236 | Beta | 9.64 | 655.64 | [39–45, 60–62] |
| ICBT → Drop out | 0.0201 | 0.0132 | 0.0270 | Beta | 31.77 | 1546.96 | [63–69] |
| DPR → sp. REM | 0.0123 | 0.0018 | 0.0227 | Beta | 5.25 | 422.56 | [42, 63–67, 71, 72] |
| REM → DPR | 0.0064 | − 0.0035 | 0.0164 | Beta | 1.59 | 245.61 | [39–47] |
| Sp. REM → DPR | 0.0202 | − 0.0200 | 0.0604 | Beta | 0.93 | 45.07 | [48] |
| All-cause death | 3.29E−05 | [73, 74] | |||||
DPR depressed, Drop-out treatment discontinuation, FCBT face-to-face cognitive behavioral therapy, ICBT internet-based cognitive behavioral therapy, QoL quality of life, REM remission, sp. REM spontaneous remission
Because we modeled CBT as an outpatient treatment, there are no inpatient costs to consider for patients under acute FCBT or ICBT, respectively. Thus, we assigned €57.60 as average weekly health care costs to treatment states. In addition, acute treatment costs for FCBT (€1303.22) and ICBT (€736.90) must be taken into account, including one psychiatric pretreatment consultation (€44.33) [82], three pretreatment evaluation sessions (3 × €65.39) [82] and, for ICBT only, license fees for software (€50.00 according to the literature [28, 35]). These costs are assigned to the first week of treatment. Costs for psychotherapy (FCBT: €88.56 per session; ICBT: €37.20 per session) are assigned weekly for up to 12 treatment weeks. Psychotherapy costs for FCBT have been taken from the German Uniform Value Scheme [83]. Psychotherapy costs for ICBT reflect weekly support by a therapist with an average duration of 21 minutes [68]. Thus, costs per patient and week during treatment sum up to €385.30 for ICBT and €386.66 for FCBT in the first week of treatment and €94.80 for ICBT and €146.16 for FCBT in treatment Weeks 2 to 12.
Although costs for remitted patients are not reported in the literature, some form of maintenance therapy, such as continued pharmacotherapy or outpatient follow-up consultations, is advised [38]. One study from the UK [84] and another from the US [85] estimated the differences in health care utilization between remitted and non-remitted patients in terms of drug and outpatient care costs. They found that health care utilization among remitted patients was between 42 and 70% of that of non-remitted patients. Assuming a share of 50%, we calculated average direct costs for remitted patients of €9.28 per week and assigned these to both remission states.
Sensitivity and Scenario Analyses
To account for uncertainty, we conducted several deterministic and probabilistic sensitivity analyses. For the former, we used reported confidence intervals for the various cost values [79] as lower and upper bounds and varied QoL utility values by one standard deviation around the mean. We set the lower and upper bounds for the one-time software costs of ICBT at €25 and €100 per patient, respectively. As two to four evaluation sessions are compulsory as a pre-treatment in Germany [75], we varied the number of these sessions accordingly. Treatment length in the included studies varied between 8 and 16 weeks, and these two values served as the lower and upper bounds. We varied the time horizon by one to 2 and 4 years. For the discount rate, we set a lower bound of 0% and an upper bound of 6%.
For probabilistic sensitivity analyses, we conducted a Monte Carlo simulation with 10,000 repetitions. We drew the input parameters randomly from gamma (costs and waiting times) or beta (QoL values and transition probabilities) distributions (see Table 2) [86, 87]. In addition, we generated a cost-effectiveness acceptability curve. Waiting times differ across Germany’s 16 states, presumably mostly as a result of varying therapist densities. For example, the average waiting time in the city-state of Berlin is 13.4 weeks, whereas it is 23.7 weeks in the state of Thuringia [9]. We therefore analyzed the impact of six different combinations of waiting times for ICBT / FCBT (20/20, 15/20, 10/20, 5/20, 3/13, 3/24 (in weeks) within a scenario analysis.
Results
In the base-case scenario, with waiting times of 3 weeks for ICBT and 20 weeks for FCBT, ICBT generated 0.260 QALYs and saved €2536 (incremental costs) per patient compared to FCBT. On average, patients in the ICBT cohort spend 55% of their time in remission (FCBT: 35%) and 12% of their time depressed (FCBT: 34%). Moreover, ICBT avoided 0.50 deaths per 1000 patients compared to FCBT. Costs and QALYs per patient and year in the ICBT cohort averaged to €2236 and 0.672 compared to €3081 and 0.586 in the FCBT cohort. The shorter waiting time for ICBT increased the number of patients who completed all 12 weeks of treatment by 70%. Although substantially more patients were treated in the ICBT cohort, total costs were lower than in the cohort of FCBT patients.
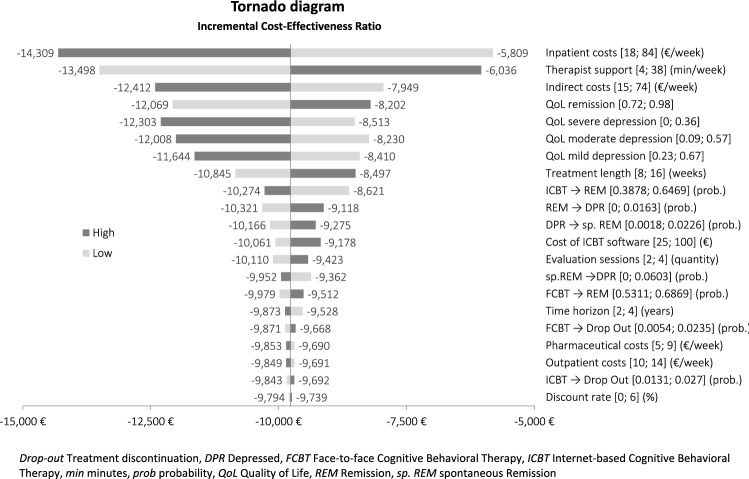
The tornado diagram (Fig. 2) illustrates the results of the deterministic sensitivity analysis. In general, the results appear to be very robust. Health-related inpatient costs and the amount of therapist support are found to have the largest impact on the incremental cost-effectiveness ratio (ICER). For all upper and lower bounds, incremental effects are positive and incremental costs negative, thus presenting ICBT as the dominant strategy.
Fig. 2.
Results of the deterministic sensitivity analysis
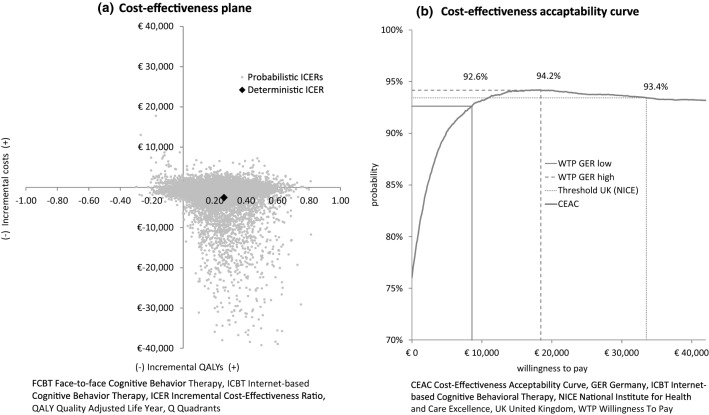
The results of the probabilistic sensitivity analysis are illustrated in Fig. 3. After 10,000 repetitions (Fig. 3a), the incremental effects and costs averaged to 0.209 QALYs and €− 2164 per patient. In 91.5% of simulations, ICBT is more effective and in 76.0% less costly than FCBT. In 69.7% of simulations ICBT is the dominant strategy while it is dominated by FCBT in only 2.2%. In 21.8% of all simulations ICBT is more costly and more effective. The chance of ICBT being cost effective is illustrated by the corresponding cost-effectiveness-acceptability curve in Fig. 3b [86]. For a threshold of zero, ICBT has a chance of 76.0% of being cost effective. Applying other thresholds does not cause substantial variations of this chance.
Fig. 3.
Results of the probabilistic sensitivity analysis
The results of our scenario analysis confirmed the dominance of ICBT over FCBT (Table 3). Only when the waiting times were equal, in scenario A, did ICBT lose its dominance. A reduction in waiting times of 25%, in scenario B, is sufficient to compensate for the lower probability of remission in the ICBT group and turns incremental effects positive.
Table 3.
Results of the scenario analysis
| Scenarios | WT (week) ICBT/FCBT |
Incremental | ICER | |
|---|---|---|---|---|
| Effects | Cost | |||
| Base case | 3/20 | 0.256 | − 1755 | dominant |
| A | 20/20 | − 0.051 | − 530 | 10,434 |
| B | 15/20 | 0.008 | − 761 | dominant |
| C | 10/20 | 0.088 | − 1075 | dominant |
| D | 5/20 | 0.199 | − 1522 | dominant |
| E | 3/13 | 0.165 | − 1528 | dominant |
| F | 3/24 | 0.296 | − 1850 | dominant |
FCBT face-to-face cognitive behavior therapy, ICBT Internet-based cognitive behavior therapy, ICER incremental cost-effectiveness-ratio, QG quality adjusted life year gained, WT waiting time
Discussion
The results of our cost–utility analysis suggest that ICBT is highly likely to be cost effective. By reducing waiting times, ICBT has the potential to improve treatment prospects and reduce costs. A shorter time to treatment reduces the time a patient spends in an untreated depressed state. If only small reductions in waiting times were achieved through ICBT compared to FCBT (scenario B) they would be sufficiently large to compensate for the lower remission rates seen in the ICBT cohort. Overall, the results of our sensitivity and scenario analyses confirmed ICBT as the dominant strategy.
Hedman et al. [25] and Donker et al. [26] reviewed the literature on the cost effectiveness of ICBT and identified four studies that investigated the cost effectiveness of ICBT in depression. One Dutch study [27] reported an ICER of €1817 per clinically improved case compared to waiting list and a chance of 30% for ICBT to be cost effective considering a willingness-to-pay of zero. Another Dutch study [28] evaluated the cost effectiveness of unguided ICBT alone or in combination with treatment as usual compared to treatment as usual alone. The authors did not report an ICER, but rather a 65% chance of ICBT being cost effective compared to treatment as usual at a willingness-to-pay threshold of zero. The third study, from the UK [29], also compared ICBT to treatment as usual and estimated an ICER of £3528 for every clinically improved case and an ICER of £17,173 per QALY gained. This resulted in a 0% chance of ICBT being cost effective at a willingness-to-pay threshold of zero. The fourth study, also from the UK [30] reported no significant difference in the clinical effectiveness, service use, or costs of an unguided ICBT compared to a website providing information on mental health problems. Kolovos et al. [31] conducted a meta-analysis based on patient-level data from five different RCTs that compared ICBT as add-on to care as usual, problem solving therapy or waiting list. The authors report an ICER of €32,706 per QALY gained for a time horizon of 6 months and concluded that ICBT was not cost effective. Paganini et al. [32] in their review also found that ICBT was not cost effective when used either as an adjunct treatment or without support.
One study conducted alongside an RCT in the UK [33] evaluated the cost effectiveness of computerized CBT compared to treatment as usual and reported an 85% chance that the former was cost effective given a societal value of £5000 per QALY gained. An ICER was not reported. Titov et al. [34] evaluated the cost effectiveness of ICBT compared to waiting list for older adults with depression (n = 54) alongside an RCT in Australia. Slightly higher QALYs (+ 0.012) and higher costs ($+ 52.9) in the treatment group resulted in an ICER of $4392 per QALY gained. Duarte et al. [35], in turn, analyzed the cost effectiveness of two computerized CBT programs (“Beating the Blues” and “MoodGym”) within a primary care setting in England. A total of 691 depressed patients were randomized within a clinical RCT to treatment as usual (i.e. by a general practitioner) or to one of the two ICBTs in addition to treatment as usual. With an ICER of £6933 per QALY gained, treatment as usual was cost effective compared to the intervention that combined treatment as usual with ICBT.
However, none of these studies have compared ICBT or computerized CBT to an established standard treatment such as FCBT, making it difficult to establish a more realistic cost effectiveness of ICBT. Moreover, the evidence base is limited to treatment effects from single RCTs and studies with short follow-up periods. In addition, studies have often measured QoL in a unidimensional fashion (e.g. as days with or without depression symptoms) and applied various social values for clinical effects or QALYs gained.
Unlike previous research on this subject, our use of the well-established concept of QALYs and of ICERs to summarize and report our results provides decision makers with information not only on the cost–utility of ICBT compared to an active control treatment (FCBT), but also to compare the cost–utility of ICBT to existing thresholds. In Germany, there is no officially defined threshold for judging the cost effectiveness of medical interventions. However, using information from the only study [88] that has measured a willingness-to-pay threshold for a QALY for Germany, i.e. between €8580 and €18,420, it would seem that ICBT is very likely to be cost effective. The main advantage of ICBT is that it requires less therapist time per patient, allowing therapists to treat more patients overall.
When interpreting these findings, it is important to keep in mind that our study has several limitations. First, the average treatment length in FCBT studies is longer than that in studies that have analyzed ICBT. Although our sensitivity analyses indicate that our overall results were not sensitive to the modeled treatment length, the uncertainty regarding the relationship between treatment length and remission rates of CBT is inherent because treatment lengths vary widely across included studies (8–16 weeks). Using simple weighted average remission rates, and thus disregarding treatment lengths, results in higher remission rates both for FCBT of 0.6446 and for ICBT of 0.5802 but alters the incremental values only slightly. Second, the remission rate of CBT depends on several factors, such as waiting time, depression severity, and the number of previous episodes [38, 89–91]. Although we modeled waiting time explicitly in our analysis, it played no role in the included studies, which were primarily RCTs designed to measure the efficacy of CBT without any consideration of real-world waiting times. Although information on waiting times in these studies was sparse, it is reasonable to assume that participants in the RCTs experienced shorter waiting times than those experienced by patients in everyday care. Therefore, as the simulated waiting times in our model increase, so too does the bias that results from an overestimation of remission rates. In short, at least for our base case, the remission rate for FCBT is overestimated in our model. Conditional remission rates depended on depression severity and the number of previous episodes was not applicable because of missing data. Third, ICBT allows therapists not only to treat more patients, but also to provide those who are severely depressed with higher-intensity treatment in a face-to-face setting. This positive external effect could not be integrated into our analysis, but it is important to note that doing so would have enforced the dominance of ICBT over FCBT. Fourth, our model did not allow for alternative treatment options in non-remitting patients, but rather had them restart CBT. This is most likely not what would happen in everyday practice and ignores the fact that CBT is not the one and only treatment option for every patient. In everyday practice, psychotherapists would probably seek alternative treatment options for non-remitters. If this process were included in our model, it would increase the follow-up costs of acute treatment and decrease incremental costs in favor of FCBT. Fifth, as appropriate data for Germany are not available, health-related QoL values were adopted from a study based on French patients [77]. While we are unable to rule out general differences in health-related QoL values between both countries, we assume that the change in QoL caused by treatment has a similar effect in either population, as long as their socio-economic profile and/or pathology are comparable. Although ranges for QoL values to be covered by deterministic sensitivity analysis (Fig. 2) are relatively large, the impact on the ICER is rather small. Overall, our results are robust to changes in QoL values within a plausible range. Finally, microsimulation may be preferable to cohort simulation for modeling depression [92, 93]. However, the data required to parameterize an individual-level model, i.e. time-dependent relative risks for a range of conditions and patient characteristics, were not available. Since cost data are also available on an aggregated level only (i.e. for discrete states), a Markov model was the obvious choice. We did, however, incorporate patient heterogeneity by distinguishing standard and spontaneous remission as well as mild, moderate and severe depression.
Conclusion
To our knowledge, this is the first model-based study to evaluate the cost–utility of ICBT as a fully substitutive treatment compared to an established active control treatment (FCBT) based on the results of multiple RCTs and cohort studies. Integrating different waiting times into our model revealed the strong potential of internet-based therapies to balance supply and demand of psychotherapy. We provided evidence that ICBT is highly likely to be cost effective or even the dominant strategy. ICBT has the potential to reduce waiting times for patients and free up therapists’ capacity so they can treat more patients and focus on those with more severe depression without increasing overall costs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 5 (TIFF 12727 kb)
Supplementary material 6 (TIFF 12727 kb)
Supplementary material 7 (TIFF 12727 kb)
Supplementary material 8 (TIFF 12727 kb)
Supplementary material 9 (TIFF 12727 kb)
Supplementary material 10 (TIFF 12727 kb)
Acknowledgements
Open Access funding provided by Projekt DEAL.
Author Contributions
MB contributed to the data collection, study design, statistical analysis, interpretation of the results, and synthesis and drafted the first version of the manuscript. TS and SF contributed to the study design and statistical analysis. All authors contributed to interpretation of the results, and the final manuscript. All authors take responsibility for accuracy and integrity of the data analysis.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
Funding
This study has received no external funding.
Conflict of interest
The authors (Mathias Baumann, Simon Frey, Tom Stargardt) hereby declare that no conflicts of interest exist.
Contributor Information
Mathias Baumann, Email: mathias.baumann@uni-hamburg.de.
Tom Stargardt, Email: tom.stargardt@uni-hamburg.de.
Simon Frey, Email: simon.frey@uni-hamburg.de.
References
- 1.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Jacobi F, Hoyer J, Wittchen H-U. Seelische Gesundheit in Ost und West: Analysen auf der Grundlage des Bundesgesundheitssurveys. Z Für Klin Psychol Psychother. 2004;33:251–260. [Google Scholar]
- 3.Jacobi F, Wittchen H-U, Hölting C, Sommer S, Lieb R, Höfler M, et al. Estimating the prevalence of mental and somatic disorders in the community: aims and methods of the German National Health Interview and Examination Survey. Int J Methods Psychiatr Res. 2002;11:1–18. doi: 10.1002/mpr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGee RE, Thompson NJ. Peer reviewed: unemployment and depression among emerging adults in 12 states, behavioral risk factor surveillance system, 2010. Prev Chronic Dis. 2015;12:E38. doi: 10.5888/pcd12.140451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rief W, Nanke A, Klaiberg A, Braehler E. Base rates for panic and depression according to the Brief Patient Health Questionnaire: a population-based study. J Affect Disord. 2004;82:271–276. doi: 10.1016/j.jad.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Wittayanukorn S, Qian J, Hansen RA. Prevalence of depressive symptoms and predictors of treatment among US adults from 2005 to 2010. Gen Hosp Psychiatry. 2014;36:330–336. doi: 10.1016/j.genhosppsych.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Wittchen H-U, Jacobi F, Klose M, Ryl L. Themenheft 51 “Depressive Erkrankungen”; 2019.
- 8.World Health Organization. Global Health Estimates 2015: Disease burden by Cause, Age, Sex, by Country and by Region, 2000–2015 [Internet]; 2016. https://www.who.int/healthinfo/global_burden_disease/GHE2016_DALY_WHOReg_2000_2016_.xls?ua=1. Cited 9 May 2019.
- 9.Bundespsychotherapeutenkammer. Ein Jahr nach der Reform der Psychotherapie-Richtlinie: Wartezeiten 2018; 2018.
- 10.Mind. We still need to talk. A report on access to talking therapies [Internet]; 2013. http://www.mind.org.uk/media/494424/we-still-need-to-talk_report.pdf.
- 11.NHS England. NHS England Guidance to Support the Introduction of Access and Waiting Time Standards for Mental Health Services in 2015/16 [Internet]; 2015. https://www.england.nhs.uk/wp-content/uploads/2015/02/mh-access-wait-time-guid.pdf. Cited 6 May 2019.
- 12.Barbato A, Vallarino M, Rapisarda F, Lora A, Miguel Caldas De Almeida J. Access to mental health care in Europe; 2014.
- 13.Dobson K. A meta-analysis of the efficacy of cognitive therapy for depression. J Consult Clin Psychol. 1989;57:414–419. doi: 10.1037//0022-006x.57.3.414. [DOI] [PubMed] [Google Scholar]
- 14.Gloaguen V, Cottraux J, Cucherat M, Blackburn I-M. A meta-analysis of the effects of cognitive therapy in depressed patients. J Affect Disord. 1998;49:59–72. doi: 10.1016/s0165-0327(97)00199-7. [DOI] [PubMed] [Google Scholar]
- 15.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Cuijpers P, Andersson G, Donker T, Van Straten A. Psychological treatment of depression: Results of a series of meta-analyses; 2011. http://www.academia.edu/download/44812992/Psychological_treatment_of_depression_re20160417-10936-n39y7y.pdf. Cited 5 Jan 2017. [DOI] [PubMed]
- 17.Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther. 2009;38:196–205. doi: 10.1080/16506070903318960. [DOI] [PubMed] [Google Scholar]
- 18.Johansson R, Andersson G. Internet-based psychological treatments for depression. Expert Rev Neurother. 2012;12:861–870. doi: 10.1586/ern.12.63. [DOI] [PubMed] [Google Scholar]
- 19.Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med. 2008;31:169–177. doi: 10.1007/s10865-007-9144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spek V, Cuijpers PIM, Nyklíček I, Riper H, Keyzer J, Pop V. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol Med. 2007;37:319–328. doi: 10.1017/S0033291706008944. [DOI] [PubMed] [Google Scholar]
- 21.Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry. 2013;58:376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- 22.Ahern E, Kinsella S, Semkovska M. Clinical efficacy and economic evaluation of online cognitive behavioral therapy for major depressive disorder: a systematic review and meta-analysis. Expert Rev Pharmacoecon Outcomes Res. 2018;18:25–41. doi: 10.1080/14737167.2018.1407245. [DOI] [PubMed] [Google Scholar]
- 23.Wright JH, Owen JJ, Richards D, Eells TD, Richardson T, Brown GK, et al. Computer-assisted cognitive-behavior therapy for depression: a systematic review and meta-analysis. J Clin Psychiatry. 2019 doi: 10.4088/JCP.18r12188. [DOI] [PubMed] [Google Scholar]
- 24.Marks IM, Kavanagh K, Gega L. Hands-on Help: computer-aided Psychotherapy [Internet]. Maudsley Monographs No. 49. Hove: Psychology Press; 2007. https://ueaeprints.uea.ac.uk/11180/. Cited 5 Sep 2017.
- 25.Hedman E, Ljótsson B, Lindefors N. Cognitive behavior therapy via the Internet: a systematic review of applications, clinical efficacy and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res. 2012;12:745–764. doi: 10.1586/erp.12.67. [DOI] [PubMed] [Google Scholar]
- 26.Donker T, Blankers M, Hedman E, Ljótsson B, Petrie K, Christensen H. Economic evaluations of Internet interventions for mental health: a systematic review. Psychol Med. 2015;45:3357–3376. doi: 10.1017/S0033291715001427. [DOI] [PubMed] [Google Scholar]
- 27.Warmerdam L, Smit F, van Straten A, Riper H, Cuijpers P. Cost–utility and cost-effectiveness of internet-based treatment for adults with depressive symptoms: randomized trial. J Med Internet Res [Internet]; 2010;12. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3057305/. Cited 21 Aug 2017. [DOI] [PMC free article] [PubMed]
- 28.Gerhards SAH, de Graaf LE, Jacobs LE, Severens JL, Huibers MJH, Arntz A, et al. Economic evaluation of online computerised cognitive-behavioural therapy without support for depression in primary care: randomised trial. Br J Psychiatry J Ment Sci. 2010;196:310–318. doi: 10.1192/bjp.bp.109.065748. [DOI] [PubMed] [Google Scholar]
- 29.Hollinghurst S, Peters TJ, Kaur S, Wiles N, Lewis G, Kessler D. Cost-effectiveness of therapist-delivered online cognitive-behavioural therapy for depression: randomised controlled trial. Br J Psychiatry J Ment Sci. 2010;197:297–304. doi: 10.1192/bjp.bp.109.073080. [DOI] [PubMed] [Google Scholar]
- 30.Phillips R, Schneider J, Molosankwe I, Leese M, Foroushani PS, Grime P, et al. Randomized controlled trial of computerized cognitive behavioural therapy for depressive symptoms: effectiveness and costs of a workplace intervention. Psychol Med. 2014;44:741–752. doi: 10.1017/S0033291713001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolovos S, van Dongen JM, Riper H, Buntrock C, Cuijpers P, Ebert DD, et al. Cost effectiveness of guided Internet-based interventions for depression in comparison with control conditions: an individual–participant data meta-analysis. Depress Anxiety. 2018;35:209–219. doi: 10.1002/da.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paganini S, Teigelkötter W, Buntrock C, Baumeister H. Economic evaluations of internet- and mobile-based interventions for the treatment and prevention of depression: a systematic review. J Affect Disord. 2018;225:733–755. doi: 10.1016/j.jad.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 33.McCrone P, Knapp M, Proudfoot J, Ryden C, Cavanagh K, Shapiro DA, et al. Cost-effectiveness of computerised cognitive–behavioural therapy for anxiety and depression in primary care: randomised controlled trial. Br J Psychiatry. 2004;185:55–62. doi: 10.1192/bjp.185.1.55. [DOI] [PubMed] [Google Scholar]
- 34.Titov N, Dear BF, Ali S, Zou JB, Lorian CN, Johnston L, et al. Clinical and cost-effectiveness of therapist-guided internet-delivered cognitive behavior therapy for older adults with symptoms of depression: a randomized controlled trial. Behav Ther. 2015;46:193–205. doi: 10.1016/j.beth.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Duarte A, Walker S, Littlewood E, Brabyn S, Hewitt C, Gilbody S, et al. Cost-effectiveness of computerized cognitive–behavioural therapy for the treatment of depression in primary care: findings from the Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT) trial. Psychol Med. 2017;47:1825–1835. doi: 10.1017/S0033291717000289. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs M, Obrosky S, George C. The course of major depressive disorder from childhood to young adulthood: recovery and recurrence in a longitudinal observational study. J Affect Disord. 2016;203:374–381. doi: 10.1016/j.jad.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beshai S, Dobson KS, Bockting CL, Quigley L. Relapse and recurrence prevention in depression: current research and future prospects. Clin Psychol Rev. 2011;31:1349–1360. doi: 10.1016/j.cpr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 38.DGPPN. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression—Langfassung, 2. Auflage. Version 5—depression-2aufl-vers5-lang.pdf [Internet]; 2015. http://www.leitlinien.de/mdb/downloads/nvl/depression/depression-2aufl-vers5-lang.pdf. Cited 18 July 2017.
- 39.Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: a randomized clinical trial. Arch Gen Psychiatry. 2001;58:381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shea MT, Elkin I, Imber SD, Sotsky SM, Watkins JT, Collins JF, et al. Course of depressive symptoms over follow-up: findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Arch Gen Psychiatry. 1992;49:782–787. doi: 10.1001/archpsyc.1992.01820100026006. [DOI] [PubMed] [Google Scholar]
- 41.Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, et al. Relapse after cognitive behavior therapy of depression: potential implications for longer courses of treatment. Am J Psychiatry. 1992;149:1046. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- 42.Hautzinger M, Welz S. Cognitive behavioral therapy for depressed older outpatients–a controlled, randomized trial. Z Gerontol Geriatr. 2004;37:427–435. doi: 10.1007/s00391-004-0262-x. [DOI] [PubMed] [Google Scholar]
- 43.Jarrett RB, Basco MR, Risser R, Ramanan J, Marwill M, Kraft D, et al. Is there a role for continuation phase cognitive therapy for depressed outpatients? J Consult Clin Psychol. 1998;66:1036. doi: 10.1037//0022-006x.66.6.1036. [DOI] [PubMed] [Google Scholar]
- 44.Evans MD, Hollon SD, DeRubeis RJ, Piasecki JM, Grove WM, Garvey MJ, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49:802–808. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- 45.Gortner ET, Gollan JK, Dobson KS, Jacobson NS. Cognitive–behavioral treatment for depression: relapse prevention. J Consult Clin Psychol. 1998;66:377. doi: 10.1037//0022-006x.66.2.377. [DOI] [PubMed] [Google Scholar]
- 46.Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62:417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 47.Jacobson NS, Fruzzetti AE, Dobson K, Whisman M, Hops H. Couple therapy as a treatment for depression: II. The effects of relationship quality and therapy on depressive relapse. J Consult Clin Psychol. 1993;61:516. doi: 10.1037//0022-006x.61.3.516. [DOI] [PubMed] [Google Scholar]
- 48.Godfrin KA, van Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: a randomized controlled study. Behav Res Ther. 2010;48:738–746. doi: 10.1016/j.brat.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 49.IQWiG. General Methods Version 5.0 [Internet]. Gen. Methods Benefit Assess; 2017. https://www.iqwig.de/en/methods/methods-paper.3020.html.
- 50.National Institute for Health and Clinical Excellence. The guidelines manual [Internet]. London: National Institute for Health and Clinical Excellence; 2012. http://www.nice.org.uk.
- 51.Spijker J, de Graaf R, Bijl RV, Beekman ATF, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Br J Psychiatry. 2002;181:208–213. doi: 10.1192/bjp.181.3.208. [DOI] [PubMed] [Google Scholar]
- 52.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 53.Owen JJ, Adelson J, Budge S, Kopta SM, Reese RJ. Good-enough level and dose-effect models: variation among outcomes and therapists. Psychother Res. 2016;26:22–30. doi: 10.1080/10503307.2014.966346. [DOI] [PubMed] [Google Scholar]
- 54.Kegel AF, Flückiger C. Predicting psychotherapy dropouts: a multilevel approach. Clin Psychol Psychother. 2015;22:377–386. doi: 10.1002/cpp.1899. [DOI] [PubMed] [Google Scholar]
- 55.Naimark DMJ, Bott M, Krahn M. The half-cycle correction explained: two alternative pedagogical approaches. Med Decis Making. 2008;28:706–712. doi: 10.1177/0272989X08315241. [DOI] [PubMed] [Google Scholar]
- 56.Filipović-Pierucci A, Zarca K, Durand-Zaleski I. Markov Models for Health Economic Evaluations: The R Package heemod. ArXiv170203252 Stat [Internet]; 2017. http://arxiv.org/abs/1702.03252.
- 57.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 58.Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:329–342. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: A comparative meta-analysis of cognitive-behavioral therapy’s effects. [Internet]. American Psychological Association; 2007. http://psycnet.apa.org/journals/ccp/75/3/475/. Cited 14 Sep 2017. [DOI] [PMC free article] [PubMed]
- 60.Murphy GE, Simons AD, Wetzel RD, Lustman PJ. Cognitive therapy and pharmacotherapy: singly and together in the treatment of depression. Arch Gen Psychiatry. 1984;41:33–41. doi: 10.1001/archpsyc.1984.01790120037006. [DOI] [PubMed] [Google Scholar]
- 61.DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 62.Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, et al. National Institute of Mental Health treatment of depression collaborative research program: general effectiveness of treatments. Arch Gen Psychiatry. 1989;46:971–982. doi: 10.1001/archpsyc.1989.01810110013002. [DOI] [PubMed] [Google Scholar]
- 63.Warmerdam L, van Straten A, Twisk J, Riper H, Cuijpers P. Internet-based treatment for adults with depressive symptoms: randomized controlled trial. J Med Internet Res [Internet]. 2008;10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2629364/. [DOI] [PMC free article] [PubMed]
- 64.Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S, et al. Therapist-delivered internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet. 2009;374:628–634. doi: 10.1016/S0140-6736(09)61257-5. [DOI] [PubMed] [Google Scholar]
- 65.Titov N, Andrews G, Davies M, McIntyre K, Robinson E, Solley K. Internet treatment for depression: a randomized controlled trial comparing clinician vs. technician assistance. PLoS One. 2010;5:e10939. doi: 10.1371/journal.pone.0010939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vernmark K, Lenndin J, Bjärehed J, Carlsson M, Karlsson J, Öberg J, et al. Internet administered guided self-help versus individualized e-mail therapy: a randomized trial of two versions of CBT for major depression. Behav Res Ther. 2010;48:368–376. doi: 10.1016/j.brat.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Ruwaard J, Schrieken B, Schrijver M, Broeksteeg J, Dekker J, Vermeulen H, et al. Standardized web-based cognitive behavioural therapy of mild to moderate depression: a randomized controlled trial with a long-term follow-up. Cogn Behav Ther. 2009;38:206–221. doi: 10.1080/16506070802408086. [DOI] [PubMed] [Google Scholar]
- 68.Hedman E, Ljótsson B, Kaldo V, Hesser H, El Alaoui S, Kraepelien M, et al. Effectiveness of Internet-based cognitive behaviour therapy for depression in routine psychiatric care. J Affect Disord. 2014;155:49–58. doi: 10.1016/j.jad.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 69.Andersson G, Hesser H, Veilord A, Svedling L, Andersson F, Sleman O, et al. Randomised controlled non-inferiority trial with 3-year follow-up of internet-delivered versus face-to-face group cognitive behavioural therapy for depression. J Affect Disord. 2013;151:986–994. doi: 10.1016/j.jad.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 70.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 71.de Graaf LE, Gerhards SAH, Arntz A, Riper H, Metsemakers JFM, Evers SMA, et al. Clinical effectiveness of online computerised cognitive-behavioural therapy without support for depression in primary care: randomised trial. Br J Psychiatry J Ment Sci. 2009;195:73–80. doi: 10.1192/bjp.bp.108.054429. [DOI] [PubMed] [Google Scholar]
- 72.Farrer L, Christensen H, Griffiths KM, Mackinnon A. Internet-based CBT for depression with and without telephone tracking in a national helpline: randomised controlled trial. PLoS One. 2011;6:e28099. doi: 10.1371/journal.pone.0028099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.WHO Mortality Database [Internet]. http://apps.who.int/healthinfo/statistics/mortality/whodpms/. Cited 30 Aug 2018.
- 74.White J, Zaninotto P, Walters K, Kivimäki M, Demakakos P, Shankar A, et al. Severity of depressive symptoms as a predictor of mortality: the English longitudinal study of ageing. Psychol Med. 2015;45:2771–2779. doi: 10.1017/S0033291715000732. [DOI] [PubMed] [Google Scholar]
- 75.Kassenärtliche Bundesvereinigung (KBV). Strukturreform der Psychotherapeutischen Versorgung [Internet]; 2018. http://www.kbv.de/media/sp/Praxisinformation_Psychotherapie_Reform.pdf. Cited 21 Mar 2018.
- 76.Hedman E, Ljótsson B, Rück C, Bergström J, Andersson G, Kaldo V, et al. Effectiveness of Internet-based cognitive behaviour therapy for panic disorder in routine psychiatric care. Acta Psychiatr Scand. 2013;128:457–467. doi: 10.1111/acps.12079. [DOI] [PubMed] [Google Scholar]
- 77.Sapin C, Fantino B, Nowicki M-L, Kind P. Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual Life Outcomes. 2004;2:20. doi: 10.1186/1477-7525-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wittchen H-U, Pittrow D. Prevalence, recognition and management of depression in primary care in Germany: the Depression 2000 study. Hum Psychopharmacol Clin Exp. 2002;17:S1–S11. doi: 10.1002/hup.398. [DOI] [PubMed] [Google Scholar]
- 79.Krauth C, Stahmeyer JT, Petersen JJ, Freytag A, Gerlach FM, Gensichen J. Resource utilisation and costs of depressive patients in germany: results from the primary care monitoring for depressive patients trial [Internet]. Depress Res Treat; 2014. https://www.hindawi.com/journals/drt/2014/730891/abs/. Cited 18 July 2017. [DOI] [PMC free article] [PubMed]
- 80.Gensichen J, Torge M, Peitz M, Wendt-Hermainski H, Beyer M, Rosemann T, et al. Case management for the treatment of patients with major depression in general practices—rationale, design and conduct of a cluster randomized controlled trial—PRoMPT (Primary care Monitoring for depressive Patient’s Trial) [ISRCTN66386086]—study protocol. BMC Public Health. 2005;5:101. doi: 10.1186/1471-2458-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Staat & Gesellschaft—Gesundheitsausgaben-Statistisches Bundesamt (Destatis) [Internet]. https://www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/Gesundheit/Gesundheitsausgaben/Gesundheitsausgaben.html. Cited 31 Jan 2019.
- 82.Kassenärztliche Bundesvereinigung [Internet]. http://www.kbv.de/html/index.php. Cited 21 Mar 2018.
- 83.KBV-Vergütung Psychotherapie [Internet]. http://www.kbv.de/html/17549.php. Cited 29 Aug 2018.
- 84.Byford S, Barrett B, Despiégel N, Wade A. Impact of treatment success on health service use and cost in depression: longitudinal database analysis. PharmacoEconomics. 2011;29:157–170. doi: 10.2165/11537360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 85.Dennehy EB, Robinson RL, Stephenson JJ, Faries D, Grabner M, Palli SR, et al. Impact of non-remission of depression on costs and resource utilization: from the COmorbidities and symptoms of DEpression (CODE) study. Curr Med Res Opin. 2015;31:1165–1177. doi: 10.1185/03007995.2015.1029893. [DOI] [PubMed] [Google Scholar]
- 86.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: OUP; 2006. [Google Scholar]
- 87.Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32:722–732. doi: 10.1177/0272989X12458348. [DOI] [PubMed] [Google Scholar]
- 88.Ahlert M, Breyer F, Schwettmann L. How you ask is what you get: framing effects in willingness-to-pay for a QALY. Soc Sci Med. 2016;150:40–48. doi: 10.1016/j.socscimed.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 89.Foreman DM, Hanna M. How long can a waiting list be? The impact of waiting time on intention to attend child and adolescent psychiatric clinics. Psychiatr Bull. 2000;24:211–213. [Google Scholar]
- 90.McGarry J, McNicholas F, Buckley H, Kelly BD, Atkin L, Ross N. The clinical effectiveness of a brief consultation and advisory approach compared to treatment as usual in child and adolescent mental health services. Clin Child Psychol Psychiatry. 2008;13:365–376. doi: 10.1177/1359104508090600. [DOI] [PubMed] [Google Scholar]
- 91.Williams ME, Latta J, Conversano P. Eliminating The wait for mental health services. J Behav Health Serv Res. 2008;35:107–114. doi: 10.1007/s11414-007-9091-1. [DOI] [PubMed] [Google Scholar]
- 92.Afzali HHA, Karnon J, Gray J. A proposed model for economic evaluations of major depressive disorder. Eur J Health Econ. 2012;13:501–510. doi: 10.1007/s10198-011-0321-3. [DOI] [PubMed] [Google Scholar]
- 93.Karnon J, Afzali HHA. When to use discrete event simulation (DES) for the economic evaluation of health technologies? A review and critique of the costs and benefits of DES. PharmacoEconomics. 2014;32:547–558. doi: 10.1007/s40273-014-0147-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 5 (TIFF 12727 kb)
Supplementary material 6 (TIFF 12727 kb)
Supplementary material 7 (TIFF 12727 kb)
Supplementary material 8 (TIFF 12727 kb)
Supplementary material 9 (TIFF 12727 kb)
Supplementary material 10 (TIFF 12727 kb)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.