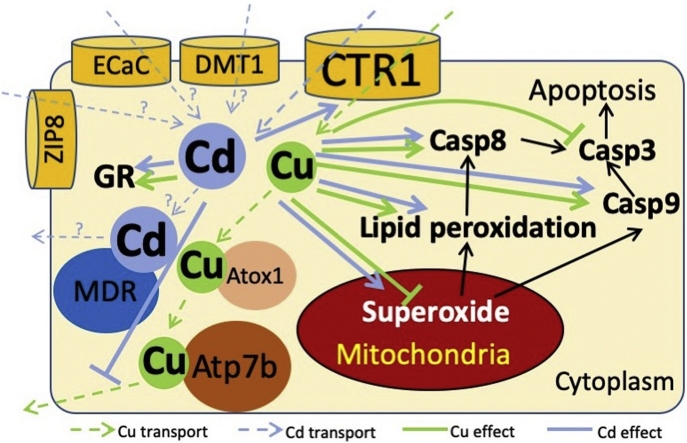
Graphical abstract
Abbreviations: z, zebrafish; BCS, bathocuproinedisulfonic acid disodium salt; Casp3, caspase 3 protein; Casp8, caspase 8 protein; Casp9, caspase 9 protein; cat, catalase gene; CAT, catalase protein; ccs, copper chaperone for superoxide dismutase gene; Cd, cadmium; Cu, copper; ef1a, elongation factor 1-alpha gene; gr, glutathione reductase gene; GR, glutathione reductase protein; gst, glutathione-S-transferase gene; GST, glutathione-S-transferase protein; LC, lethal concentration; LC50, median lethal concentration; LC20, lethal concentration of 20 % population; mtDNA, mitochondrial DNA; NAC, N-acetyl-l-cysteine; SOD, superoxide dismutase proteins; sod1, superoxide dismutase 1 gene; sod2, superoxide dismutase 2 gene; PBS, phosphate-buffered saline; VE, tocopherol (Vitamin E); ybx1, Y box-binding protein 1 gene
Keywords: Combined effects, Cytotoxicity, Mitochondrial function
Highlights
-
•
Oxidative stress and apoptosis created by Cu2+ and Cd2+ insults were studied in ZFL.
-
•
Cu2+ and Cd2+ both created lipid peroxidation, causing oxidative stress in cytoplasm.
-
•
Mitochondrial superoxide was induced by Cd2+ but supressed by Cu2+.
-
•
Cu2+ suppressed Casp3 activity, resulting in suppressed the apoptosis.
-
•
Pre-treatments of low concentration of Cu2+ protected the cell from Cd2+ insults.
Abstract
Copper (Cu) and cadmium (Cd) are widely used in industrial activities, resulting in Cu and Cd contamination in aquatic systems worldwide. Although Cu plays an essential role in many biological functions, an excessive amount of the metal causes cytotoxicity. In contrast, Cd is a non-essential metal that usually co-exists with Cu. Together, they cause oxidative stress in cells, leading to cell damage. These metal ions are also believed to cause cell apoptosis. In this study, we used a zebrafish liver cell line, ZFL, to study combined Cu and Cd cytotoxicity. Although Cd is more toxic than Cu, both were found to regulate the expression of oxidative stress related genes, and neither significantly altered the activity of oxidative stress related enzymes. Co-exposure tests with the antioxidant N-acetyl-l-cysteine and the Cu chelator bathocuproinedisulfonic acid disodium salt demonstrated that Cd toxicity was due to the oxidative stress caused by Cu, and that Cu at a low concentration could in fact exert an antioxidant effect against the oxidative stress in ZFL. Excessive Cu concentration triggered the expression of initiator caspases (caspase 8 and caspase 9) but suppressed that of an executioner caspase (caspase 3), halting apoptosis. Cd could only trigger the expression of initiator caspases; it could not halt apoptosis. However, a low concentration of Cu reduced the mitochondrial superoxide level, suppressing the Cd-induced apoptotic effects in ZFL.
1. Introduction
Cadmium (Cd) has no known function in higher organisms, although it is found in a low-molecular weight metal-binding protein, metallothionein (MT), in some animals. MTs can also bind with other divalent metal ions, such as Cu2+ and Zn 2+ [1]. Cd is found in CDCA1 in the marine diatom Thalassiosira weissflogii, which is responsible for carbonic anhydrase activity [2]. Cd usually exists in the oxidation state Cd(II) (Cd2+), but Cd(I) (Cd1+) has also been observed [3]. Cd2+ generates hydroxyl radicals by hydrogen peroxide reduction using a Cd-liberated transition metal, presumably iron [4]. In addition, exposure to sub-lethal concentrations of Cd has been shown to increase the amount of reactive oxygen species in zebrafish liver tissues [5]. Furthermore, exposure to waterborne Cd has been shown to induce oxidative stress in the brain, ovary, and liver of zebrafish [6]. Cd also caused lymphocytic infiltration and fatty degeneration in the liver of zebrafish [7].
Both Cu and Cd cause oxidative stress in biological systems. Copper (Cu) is an essential micronutrient which serves as catalytic cofactor in cytochrome c oxidase and superoxide dismutase [8]. Free Cu ions catalyse the formation of free radicals via the Fenton reaction [9]. Mitochondria are potent producers of cellular superoxide, and mitochondrial superoxide generates carbon-centred radicals that initiate lipid peroxidation [10]. Acute Cu exposure in zebrafish has been found to induce the expression of the oxidative stress related genes cytochrome c oxidase-17 and catalase [11]. Lipid peroxidation end-products, such as malondialdehyde (MDA), play a key role in oxidative stress [12].
Cells have devolved systems to deal with oxidative stress. Superoxide dismutase (SOD1 and SOD2) converts superoxide into oxygen and hydrogen peroxide (H2O2) [13,14]. The copper chaperone for superoxide dismutase (CCS) specifically restores SOD1 biosynthesis [15]. Catalase (CAT) catalyses the conversion of H2O2 to molecular oxygen and water [16]. Glutathione reductase (GR) catalyses the reduction of oxidised glutathione into reduced glutathione (GSH), an important antioxidant that scavenges free radicals and reduces oxidative stress [17]. Glutathione S-transferases (GSTs) catalyse the conjugation of glutathione to electrophilic compounds to generate less reactive and more soluble products [18].
Vitamin E is a well-known antioxidant [19] (alpha-, beta-, gamma- and delta-tocopherol). In particular, the most active form of vitamin E, alpha-tocopherol, is known to scavenge peroxyl radicals [20]. One study showed that a low dose (50 μM) of alpha-tocopherol played a protective role against oxidative stress in cell culture [21].
N-Acetyl-Cysteine (NAC) is another well-known antioxidant [22]. One study investigated the 2.0 mM NAC and 200 μM α-tocopherol improved viability, approximately 40 % and 20 % respectively, against oxidative stress created by advanced glycation end products in SH-SY5Y cells [23]. NAC was reported to enhance endogenous coenzyme Q9/10 levels, resulted in protecting against diabetes-induced cardiac injury [24]. NAC is also a well-known drug to treat patients with acute liver failure, and could protect the uterine tissue against sodium arsenite-induced oxidative stress in rats [25].
As Cu is an essential ion, a comprehensive biological system exists to balance Cu ion concentration. Notably, four proteins, namely CTR1, ATOX1, ATP7A and ATP7B, are the main regulators of Cu homeostasis [26]. CTR1 is a one way channel that imports Cu+ into the cell [8,27]. ATOX1 transports Cu2+ from the cytosol to the trans-Golgi network, where it is delivered to ATP7A or ATP7B, which are involved in the incorporation of Cu+ in copper-dependent enzymes [28,29]. ATP7A, except in the liver, and ATP7B in the liver are also involved in the excretion of excess intracellular Cu+ [30]. Notably, no such system has been found for the regulation of Cd, which enters cells via other divalent metal ion transporters, such as ECaC, DMT1 and ZIP8 [31]. One study that evaluated the role of multidrug resistance protein 1 (MDR1) in Cd transport in kidney-derived cell lines found that MDR1 could eliminate Cd from the cells [32,33]. Cd toxicity is reportedly caused by cellular Cd transport via essential metal pathways [34].
Cu in macrophages, Cd in the liver and kidneys [5,[33], [34], [35]], and lipid degradation products have been shown to induce apoptosis [36]. Apoptosis results from the activation of a series of caspases. Caspase 8 (Casp8) and caspase 9 (Casp9) are initiator caspases that propagate a lethal signal in response to the engagement of the plasma membrane death receptors or mitochondrial outer membrane permeabilization, respectively. Caspase 3 (Casp3) is an executioner caspase that, upon activation by Casp8 or Casp9, cleaves a wide panel of proteins responsible for cell integrity [37].
Zebrafish is an excellent model organism for toxicity testing and biomedical research due to some mutants display similar phenotypes of human diseases [[38], [39], [40]]. In addition, essential genes of tissues or organs are highly conserved in zebrafish when compared to human [41]. We here used a zebrafish liver cell line, ZFL, to study combined Cu and Cd cytotoxicity. The zebrafish liver cell line ZFL has been established as a model cell line for in vitro assay and cell imaging [26,[42], [43], [44]]. We examined the responses of zebrafish liver cell line (ZFL) to oxidative stress and apoptotic effects created by the administrations of Cu2+ and Cd2+. We focused on the molecular responses, such as oxidative gene expression and activities, caspase activities for apoptotic effects, by co-exposures with antioxidants and Cu ion chelator. We also have measured the lipid peroxidation (MDA level) and mitochondrial superoxide, to obtain a more comprehensive picture for oxidative stress in ZFL cells. Co-exposure and pre-treatment of Cd2+ and Cu2+ were also studied.
2. Materials and methods
2.1. Chemicals
Stock solutions of 1 M CuCl2 (CAS 10125-13-0, Sigma, St. Louis, Missouri, USA, C3279), 1 M CdCl2 (CAS 10108-64-2, Sigma, St. Louis, Missouri, USA, 20899) and 50 mM bathocuproinedisulfonic acid disodium salt (BCS) (CAS 52698-84-7, Sigma-Aldrich, St. Louis, Missouri, USA, B1125) were prepared in Nanopure water and stored at 4 °C.
Stock solution of 200 mM NAC (CAS 616-91-1, Sigma-Aldrich, St. Louis, Missouri, USA, A7250) was prepared in Nanopure water and stored at −20 °C. Stock solution of 1 M mixed tocopherol (VE) (CAS 1406-66-2, Sigma-Aldrich, St. Louis, Missouri, USA, W530066) was prepared in absolute ethanol (CAS 64-17-5, Emsure Merck, Kenilworth, New Jersey, USA, 100983) and stored at −20 °C.
2.2. Cell culture
ZFL is an adherent hepatocyte cell line [American Type Culture Collection (ATCC), CRL-2643TM] isolated from zebrafish (Danio rerio). The standard ZFL culture medium contained 50 % L-15 medium (Gibco, Waltham, Massachusetts, USA,11415064), 35 % Dulbecco’s Modified Eagle Medium (Gibco, Waltham, Massachusetts, USA,12100046) and 15 % Hans F12 (Gibco, Waltham, Massachusetts, USA, 21700075) supplemented with 0.15-g/L sodium bicarbonate, 15 mM HEPES (Gibco, Waltham, Massachusetts, USA,11344041), 10 % foetal bovine serum (Gibco, Waltham, Massachusetts, USA,10270106) and 1% antibiotic-antimycotic (Gibco, Waltham, Massachusetts, USA, 15240062) and was maintained at 28 °C as recommended by ATCC [26,[42], [43], [44]].
2.3. Chemical treatments
The cells were seeded in a serum-containing medium overnight in six-well plates, 96-well plate or confocal Petri dish according to the experiment usage which stated in particular procedure section, and the medium was then removed. All chemicals were diluted with serum-free medium to the final concentrations immediately before use. For pre-treatment, the cells seeded in the wells at suitable densities, as specified in each experiment, were treated with the chemicals diluted in serum-free medium for 24 h. The pre-treatment solution was then replaced with the exposure solution, and after exposure, the cells were used for cytotoxicity assay, real-time polymerase chain reaction (qPCR), enzymatic assay and confocal imaging. The exposure duration in each experiment was 24 h unless specified otherwise.
2.4. Cytotoxicity assay
We used alamarBlue assay (Thermo Fisher Scientific Inc., Invitrogen Corporation, Carlsbad, CA92008, USA) to determine the mortality of cell and we followed the method as reported previously [26]. The cells were exposed to the chemicals (CuCl2, CdCl2, NAC or VE) at different concentrations for 24 h. After exposure, the alamarBlue medium was added and incubated for 2 h follow to measure fluorescence signal.
2.5. Quantitative real-time PCR (qPCR)
Generally, cDNA was generated by total RNA after exposure, and all the procedure was highly similar to method as reported previously [26,44]. In brief, all qPCR amplifications and detections were performed using Takara Premix ExTaq. The DNA primers designed for use in qPCR are listed in Table 1. All primers exhibited an amplification efficiency of >1.85 (the maximum amplification efficiency was 2) and one peak in the melting curve (only one amplicon in the PCR) [45,46].
Table 1.
Nucleotide sequences of qPCR primers used in this study.
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| zef1a (NM_131263) | GCTCAAACATGGGCTGGTTC | AGGGCATCAAGAAGAGTAGTACCG |
| zybx1 (NM_131620) | CCGGCCGGTTTTGTCA | TTATTGCTCAGATGTTGGATGTTGT |
| zsod1 (NM_131294.1) | ATCAAGAGGGTGAAAAGAAGC | AAAGCATGGACGTGGAAAC |
| zsod2 (NM_199976.1) | CAGCAAGCACCATGCAACAT | CAGCTCACCCTGTGGTTCTC |
| zccs (NM_001204222.2) | TGGAGAAAGATCCAGGAGTGC | AGTCTGATCCTCCCATTCCCT |
| zcat (AF170069.1) | TGAGGCTGGGTCATCAGATA | AAAGACGGAAACAGAAGCGT |
| zgr (NM_001020554.1) | CTCCTTGGTCGCAGCATGGCT | GGCAGTGGTGGCACCGAGTTC |
| zgst (AB194127.1) | CTGAGACATCTGGGTCGAAA | AGATCTTCAACTCCGTCGTTC |
2.6. Quantification of oxidative stress enzymes
To measure catalase, superoxide dismutase, GR and glutathione S-transferase activity, 2.5 × 106 ZFL cells were seeded in a 6-cm Petri dish, exposed to the chemicals, and incubated for 24 h. The next day, the cells were harvested in 0.5-ml ice-cold buffer (100 mM potassium phosphate, 1 mM EDTA, pH 7.0) using a cell scraper. The harvested cells were then sonicated and diluted to 100 μg/mL for measurement of the total protein concentration using Pierce™ BCA Protein Assay Kit (Thermo, Waltham, Massachusetts, USA, 23225). All enzyme activities were normalised with protein contents. The cell lysate was stored at −80 °C until enzyme assays using the Catalase Assay Kit (Cayman, Ann Arbor, Michigan, USA, 707002-96WELL), Superoxide Dismutase Assay Kit (Cayman, Ann Arbor, Michigan, USA, 706002-96WELL), Glutathione Reductase Assay Kit (Cayman, Ann Arbor, Michigan, USA, 703202-96WELL) and Glutathione S-Transferase Assay Kit (Cayman, Ann Arbor, Michigan, USA, 703302-96WELL) in accordance with the manufacturer’s protocol. Absorbance was measured using a BMG CLARIOstar Microplate Reader (BMG LABTECH, Ortenberg, Germany) [26].
The catalase activity measurement is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of H2O2 [47]. The formaldehyde produced is measured colourimetrically with 4-amino-3-hydrazino- 5-mercapto-1,2,4-triazole (Purpald) as the chromogen.
Superoxide Dismutase Assay Kit utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. Tetrazolium salt react superoxide radicals produces Formazan dye which can be detected with absorbance at 440−460 nm.
Glutathione Reductase activity was determined by measuring the rate of NADPH oxidation. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm [48]. Glutathione S-Transferase activity determination is by measuring the conjugation of 1-chloro-2,4- dinitrobenzene (CDNB) with reduced glutathione. The conjugation is accompanied by an increase in absorbance at 340 nm [49].
2.7. Cellular metal content measurement
The procedure is the same as described by Kwok and Chan [26]. In brief, the exposed ZFL was trypsinized after washed by PBS, further resuspended and lysed in 0.03 M HNO3 by freeze and thaw’’ cycles. The metal concentrations were measured by using atomic absorption spectrophotometer (Hitachi Z2700 with Graphite Furnace). The metal content was nominated by cell number determined by alamarBlue assay.
2.8. Measurement of caspase activity
ZFL cells were first seeded in a 96-well black plate at a density of 1 × 104 per well and incubated overnight. The cells were exposed to the chemicals at different concentrations for 24 h. The activity of caspases was then measured using Apo-ONE® Homogeneous Caspase-3/7 Assay (Promega Madison, Wisconsin, USA, G7790), Caspase-Glo® 8 Assay (Promega, Madison, Wisconsin, USA, G8201) and Caspase-Glo® 9 Assay (Promega Madison, Wisconsin, USA, G8211) kits. The incubation time was 3 h for all of these assays, and the rest of the protocol was performed as specified in the kit user manuals. Synthetic substrates of DEVD-AFC (7-amino-4-trifluoromethyl coumarin) for Caspase3 would emit a fluorometric signal (Ex/Em = 400/505 nm) immediately after enzymatic cleavage. For the Caspase-Glo-8 and -9 assay, a substrate could be cleaved by Caspase 8 or 9 specifically to produce a glow-type luminescent signal as produced by adding luciferase in the reaction buffer. Fluorescence and luminescence were measured using a BMG CLARIOstar Microplate Reader (BMG LABTECH, Ortenberg, Germany).
2.9. Mitochondrial superoxide quantification by flow cytometry
ZFL cell were seeded in six-well plates (1 × 106 per well) and exposed to the chemicals at different concentrations for 24 h. The exposed cells were then trypsinized and washed using Hank’s balanced salt solution (HBSS) with 0.5 % bovine serum albumin. The cells were stained with 5-μg/mL MitoSOX™ Red Mitochondrial Superoxide Indicator (Invitrogen, Waltham, Massachusetts, USA, M36008) in HBSS for 30 min and then again washed with HBSS and resuspended in serum-free medium. This reagent is selectively targeted to the mitochondria and oxidized by superoxide to exhibit red fluorescence. The cells (104) were examined using a BD FACSVerse flow cytometer (BD Biosciences, Franklin Lakes, New Jersey, USA), and data were analysed using FlowJo v10 to obtain the geometric mean of the fluorescence signals emitted by the cells.
2.10. Lipid peroxidation assay
ZFL cell were seeded in six-well plates (1 × 106 per well) and exposed to the chemicals at different concentrations for 24 h. Lipid Peroxidation (MDA) Assay kit (ab118970, Abcam Cambridge, United Kingdom) was used to determine free MDA by interacting with Thiobarbituric Acid (TBA) to generate a MDA-TBA adduct, which can easily be quantified fluorometrically (Ex/Em = 532/553 nm).
2.11. Confocal microscopy imaging
ZFL cells (106) were seeded in a 34.3-mm confocal Petri dish (SPL, Gyeonggi-do, Korea, 200350) and incubated overnight, following by chemical exposure for 24 h. The exposed cells were stained by adding 50 nM MitoTracker Red CMXRos (ThermoFisher, Waltham, Massachusetts, USA, M7512), which stains mitochondria, and 5-μL Quant-iT PicoGreen dsDNA Reagent (ThermoFisher, Waltham, Massachusetts, USA, P11495), which stains DNA, in 2-ml phosphate-buffered saline (PBS) for 15 min. The cells were then washed three times with PBS, seeded in serum-free medium and imaged using the Leica TCS SP8 Confocal Microscope System.
2.12. Statistical analysis
All of the statistical analyses and graph illustrations were performed using GraphPad Prism 8.0 (* p > 0.05). The data were expressed as the mean ± standard error of the mean (SEM) of biological replicates (n = 3) unless specified otherwise. The dotted line of a linear or non-linear regression curve was considered to represent the 95 % confidence level of the curve.
3. Results
3.1. Effects of Cu2+ and Cd2+ on oxidative stress
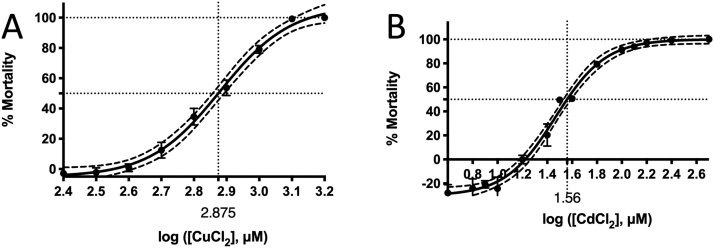
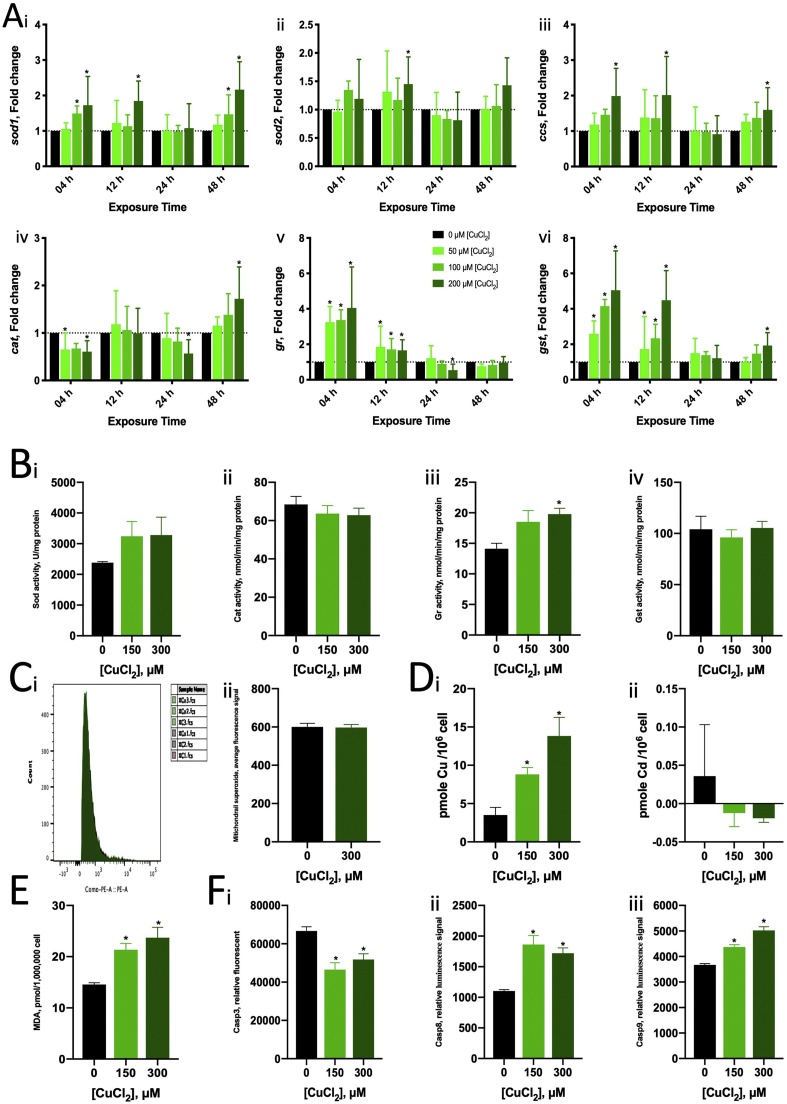
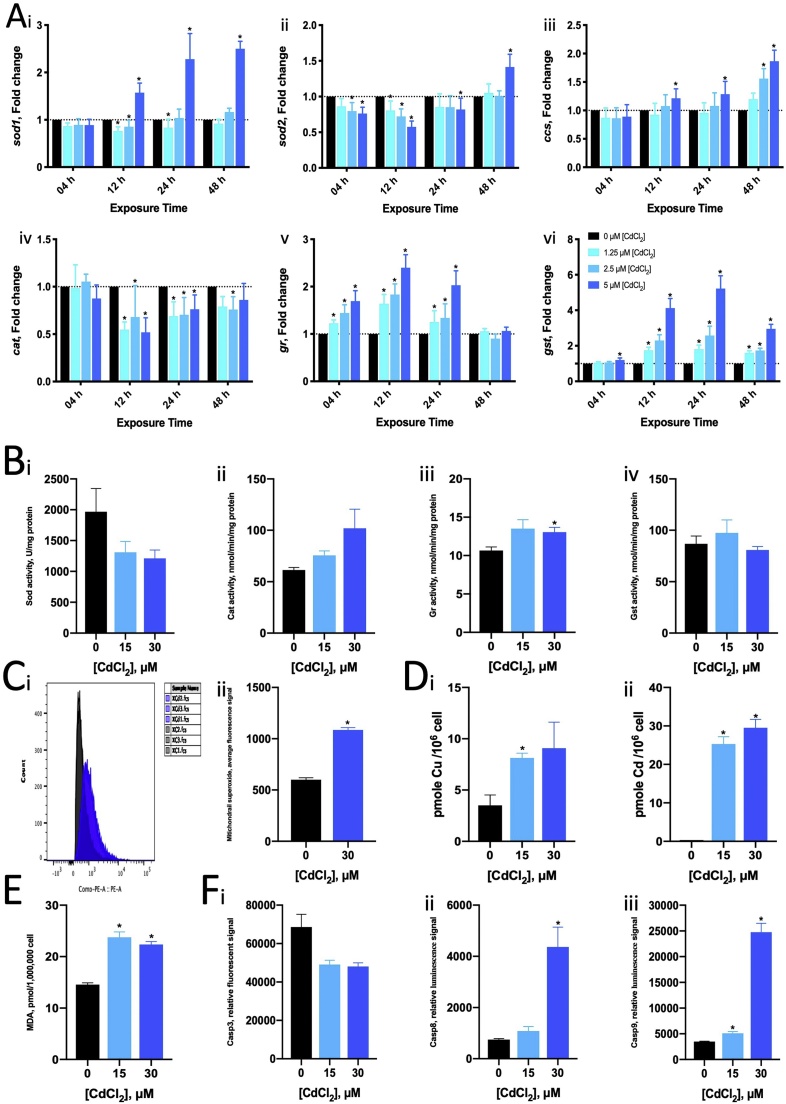
The mean LC50 values of the ZFL cells for 24-h Cu2+ and Cd2+ exposures were 750 (102.875) μM and 36.3 (101.56) μM, respectively (Fig. 1). Cu2+ (200 μM) caused a two-fold increase in zsod1 and zccs mRNA expression with 4- and 24-h exposure (Fig. 2A i, iii). Cu2+ increased zgr and zgst mRNA expression at 4 h and 12 h, but the expression returned to normal at 24 h (Fig. 5A v, vi). Cd2+ (5 μM) induced zsod1 mRNA expression at 12–24 h and 48 h (Fig. 3A i) but suppressed zsod2 (at 4, 12 and 24 h) and zcat (at 12, 24 and 48 h) mRNA expression (Fig. 3A ii, iv). Cd2+ also induced zgr (at 4, 12 and 24 h) and zgst (at 12, 24 and 48 h) mRNA expression (Fig. 3A v, vi).
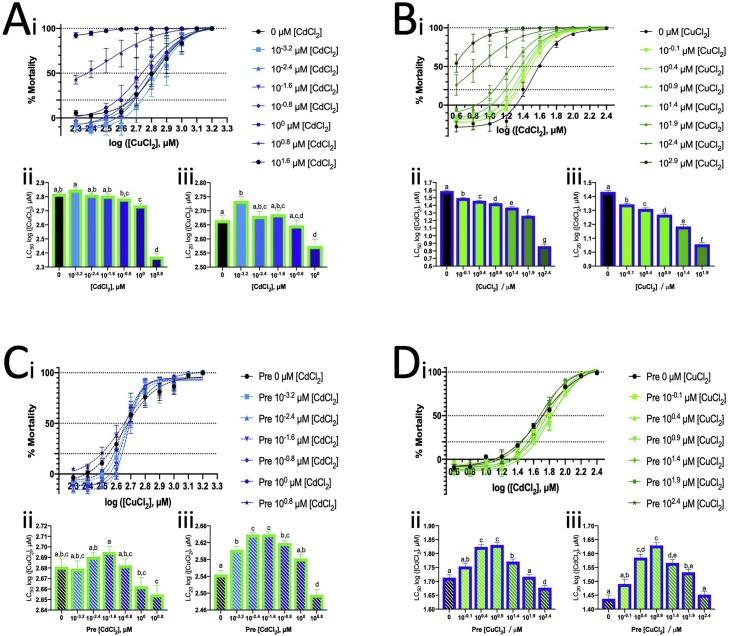
Fig. 1.
LC50 values of ZFL cells exposed to different concentrations of (A) CuCl2 and (B) CdCl2 for 24 h. The curves were generated by non-linear regression using PRISM 8.0. The dotted curve represents the 95 % confidence level of the curves.
Fig. 2.
Bioassay results of ZFL after the exposure to various concentrations of Cu2+. (A) Alterations in oxidative stress related gene expressions. The bars represent the geometric mean of fold difference derived from biological replicates (n = 3), and error bars represent the 95 % confidence level. Two-way ANOVA with Sidak’s multiple comparisons test was used to analyse and determine the statistical significance of ΔΔCt. * represents a significant difference compared with controls (without Cu) at the same time point. (i) zsod1. (ii) zsod2. (iii) zsod3. (iv) zcat. (v) zgr. (vi) zgst. (B) Alterations in the activity of oxidative stress related enzymes after 24-h exposure. * represents a significant difference compared with controls (without Cu) using the Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction). (i) SOD. (ii) CAT. (iii) GR. (iv) GST. (C) Mitochondrial superoxide in ZFL. (i) The histogram of fluorescence signal. (ii) The geometric mean of fluorescence signals in all cells. * represents a significant difference compared with controls (without Cu) using unpaired t test with Welch’s correction. (D) Cellular metal content in ZFL exposed for 24 h. * represents a significant difference compared with controls (without Cu). (i) Cu. (ii) Cd. (E) MDA in ZFL exposed for 24 h. * represents a significant difference compared with controls (without Cu) using the Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction). (F) Caspase activity in ZFL exposed for 24 h. * represents a significant difference compared with controls (without Cu) using the Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction). (i) Casp3. (ii) Casp8. (iii) Casp9.
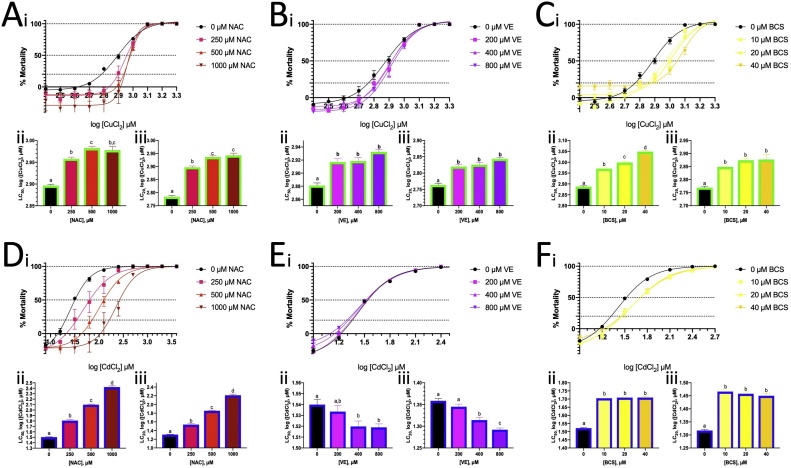
Fig. 5.
Mortality of ZFL exposed to Cu2+ or Cd2+ and co-exposure with NAC, VE or BSC for 24 h. Mortality of Cu2+-treated ZFL cells co-exposed to various concentrations of (A) NAC, (B) VE and (C) BCS. Mortality of Cd2+-treated ZFL cells co-exposed to various concentrations of (D) NAC, (E) VE and (F) BCS. (i) Mortality of ZFL. The curve was generated by non-linear regression. (ii) The LC50 values generated by (i). Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction) were used to analyse the data. Same alphabet on the bar present the same group and without significant different within the group. (iii) The LC20 values generated by (i) and the Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction) were also applied.
Fig. 3.
Bioassay results of ZFL after the exposure to various concentrations of Cd2+. (A) Alterations in oxidative stress related gene expression. The bars represent the geometric mean of fold difference derived from biological replicates (n = 3), and error bars represent the 95 % confidence level. Two-way ANOVA with Sidak’s multiple comparisons test was used to analyse and determine the statistical significance of ΔΔCt. * represents a significant difference compared with controls (without Cd) at the same time point. (i) zsod1. (ii) zsod2. (iii) zsod3. (iv) zcat. (v) zgr. (vi) zgst. (B) Alterations in the activity of oxidative stress related enzymes after 24-h exposure. * represents a significant difference compared with controls (without Cd) using the Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction). (i) SOD. (ii) CAT. (iii) GR. (iv) GST. (C) Mitochondrial superoxide in ZFL. (i) The histogram of fluorescence signal. (ii) The geometric mean of fluorescence signals in all cells. * represents a significant difference compared with the controls (without Cd) using unpaired t test with Welch’s correction. (D) Cellular metal content in ZFL exposed for 24 h. * represents a significant difference compared with controls (without Cd). (i) Cu. (ii) Cd. (E) MDA in ZFL exposed for 24 h. * represents a significant difference compared with controls (without Cd) using the Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction). (F) Caspase activity of ZFL exposed for 24 h. * represents a significant difference compared with controls (without Cd) using the Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction). (i) Casp3. (ii) Casp8. (iii) Casp9.
In terms of enzyme activity, both Cu2+ and Cd2+ upregulated GR activity but did not significantly affect SOD, CAT and GST activity (Figs. 2B, 3 B). Both Cu2+ and Cd2+ caused MDA accumulation, indicating that they induced lipid peroxidation (Figs. 2E, 3 E). Cd2+, but not Cu2+, induced mitochondrial superoxide production in ZFL cells (Figs. 2C, 3 C).
Cells contained around 4 pmole Cu and undetectable Cd per million. After exposure to 150 μM and 300 μM Cu2+, the cellular Cu content increased to 9 and 14 pmole Cu per million (Fig. 2D). For 15 μM and 30 μM Cd2+ exposure, the Cd content increased to 8 and 9 pmole Cd (Fig. 3D). Cu content increased to 8 pmole only in 15 μM Cd2+ exposure.
3.2. Cell morphology after Cu and Cd exposures
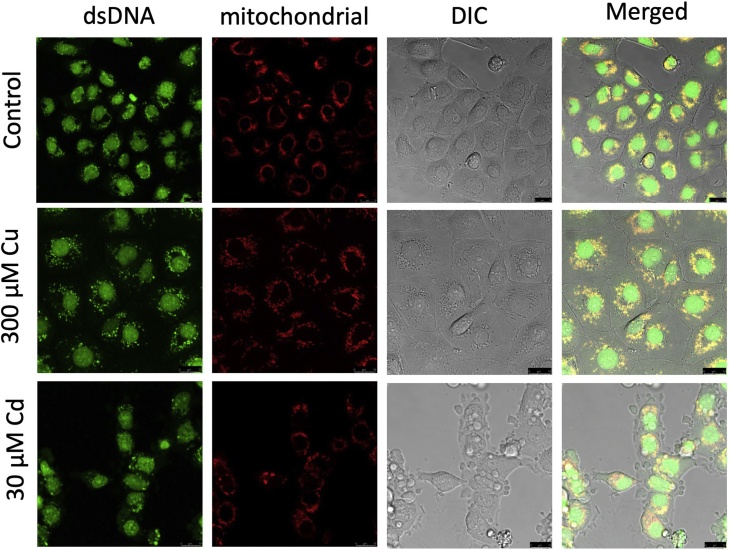
Cell behaviour was studied using fluorescent dye staining (green for dsDNA green and red for mitochondria). As the dsDNA stain was very sensitive (sensitivity of up to 25-pg/mL dsDNA, as claimed by the manufacturer), mitochondrial DNA (mtDNA) could be observed under a confocal microscope. mtDNA and genomic DNA could be distinguished by comparing the red and green fluorescence signals.
The control cells (not exposed to chemicals or metals) emitted high levels of yellow signal due to the overlapping of green and red signals, indicating intact mtDNA (Fig. 4) as well as intact cell morphology. The Cu2+-exposed cells also exhibited intact cell morphology but mainly emitted a red instead of a yellow signal, suggesting the beginning of mtDNA aggregation or depletion. The Cd2+-exposed cells had started to pack themselves into smaller pieces and exhibited widely dispersed red signal, suggesting mtDNA depletion or mitochondrial content leakage into the cytoplasm.
Fig. 4.
Confocal images of ZFL after Cu2+ and Cd2+ exposure. Green channel represents dsDNA, and red channel represents mtDNA. Differential interference contrast (DIC) was imaged using DIC microscopy. Merged images showed superimposition of red, green and DIC channels.
3.3. Cu- and Cd-induced apoptosis
Cu2+ and Cd2+ induced Casp8 and Casp9 activity, and Cu2+, but not Cd2+, suppressed Casp3 activity. Exposure to 150 μM of Cu2+ for 24 h increased Casp8 and Casp9 activity by approximately 1.7- and 1.2-fold and suppressed Casp3 activity by 0.7-fold (Fig. 2F). Exposure to 300 μM of Cu2+ showed similar effects on caspase activity. Exposure to 15 μM of Cd2+ induced Casp9 activity (by approximately 1.5-fold) but not Casp8 activity (Fig. 3F); however, an increase in Cd2+ concentration up to 30 μM increased both Casp8 and Casp9 activity by 6- and 7-fold, respectively.
3.4. Cu co-exposure with NAC, VE and BCS
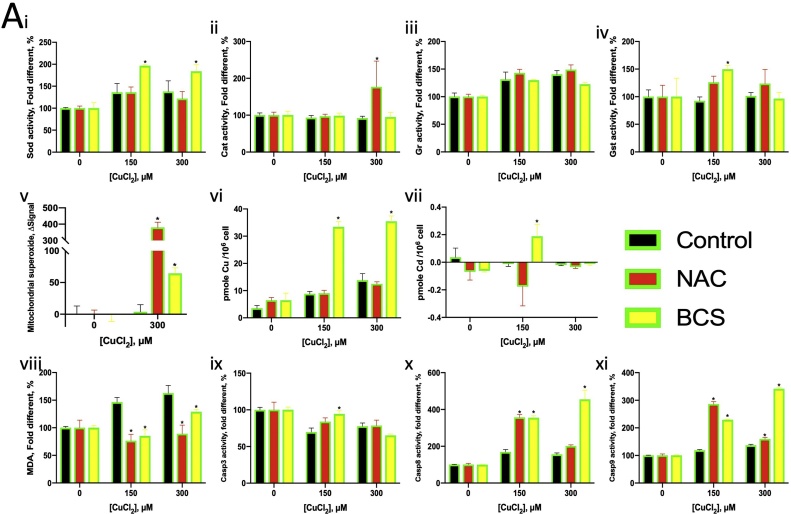
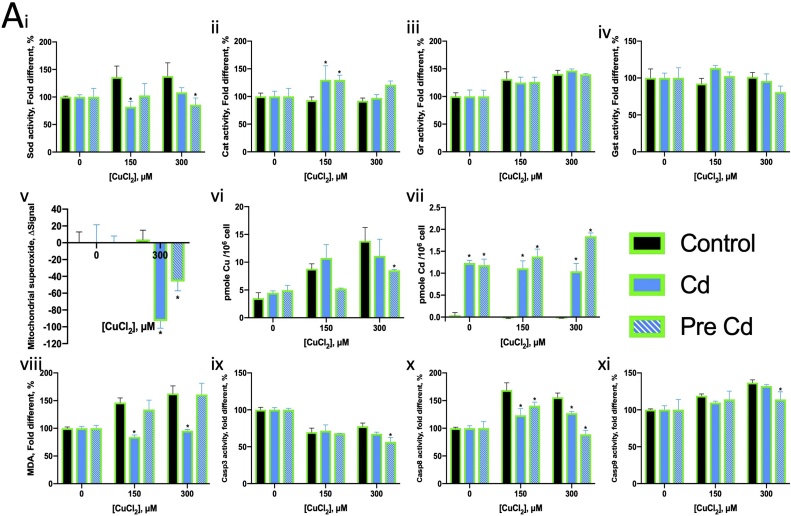
The antioxidants NAC and VE were found to increase the LC50 and LC20 values of Cu, with NAC showing a much greater effect than VE (Fig. 5A, B). Therefore, NAC (500 μM) was selected for further assays. NAC was found to upregulate CAT activity (in 150 μM Cu2+) by approximately 1.5-fold and induce mitochondrial superoxide production (in 300 μM Cu2+) (Fig. 6A ii, v). NAC suppressed lipid peroxidation, as indicated by the MDA activity induced by Cu2+ (Fig. 6A viii). NAC further enhanced Cu2+-induced Casp8 and Casp9 activity (Fig. 6A x, xi).
Fig. 6.
Normalised assay result of ZFL exposed to various concentrations of Cu2+ and Cd2+ with co-exposure to NAC or BCS compared with ZFL without co-exposure. * represents significant different compared with no co-exposure control treated with the same concentration of Cu2+ or Cd2+. (A) Cu2+ co-exposure with NAC or BCS. (B) Cd2+ co-exposure with NAC or BCS. (i) Normalised SOD activity. (ii) Normalised CAT activity. (iii) Normalised GR activity. (iv) Normalised GST activity. (v) Normalised mitochondrial superoxide level in ZFL. (vi) Cellular Cu content. (vii) Cellular Cd content. (viii) Normalised MDA concentration in ZFL. (ix) Normalised Casp3 activity. (x) Normalised Casp8 activity. (xi) Normalised Casp9 activity. Data were normalised to their own control (without metal ions but with the co-exposure substance). The controls were defined as 100 %, except for (v). The control in (v) was defined as 0.
BCS is a Cu(I) chelator and is expected to counter the effects of Cu2+. In this study, BCS increased the LC50 and LC20 values of Cu2+, thereby reducing Cu2+ toxicity (Fig. 5C). However, in addition to reducing lipid peroxidation and restoring Casp3 activity (Fig. 6A viii, ix), BCS enhanced mitochondrial superoxide production and the activity of CAT, GST (in 150 μM Cu2+ only), Casp8 and Casp9 (Fig. 6A i, iv, v, x, xi). Cu co-exposure with BCS increased the cellular Cu content (Fig. 6A vi). These findings indicate that Cu may reduce the activity of the aforementioned enzymes.
3.5. Cd co-exposure with NAC, VE and BCS
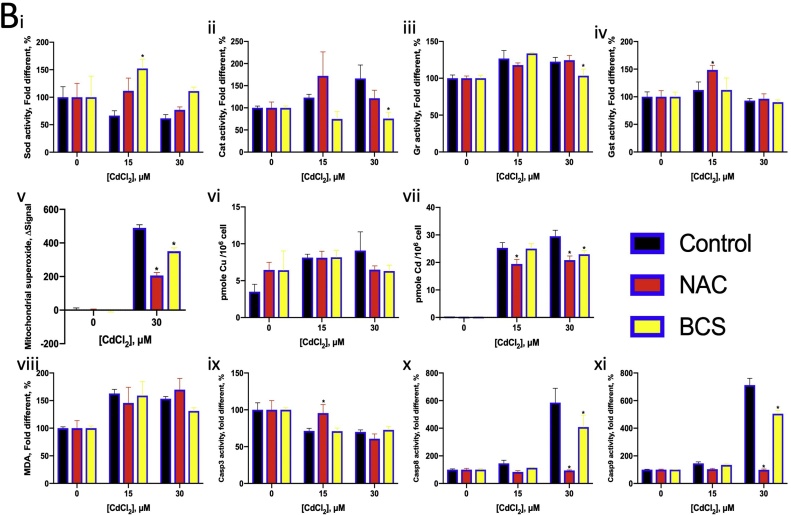
Cd2+ is well-known causative factor of oxidative stress [6]. In this study, NAC increased the LC50 and LC20 values of Cd2+, and BCS countered these effects (Fig. 5D, E). Cd2+ co-exposure with NAC (500 μM) did not significantly alter the activity of oxidative stress related enzymes, except for GST (Fig. 6B i–iv). NAC reduced mitochondrial superoxide levels and suppressed the effects of Cd2+ on Casp8 and Cas9 activity (Fig. 6B v, x, xi).
BCS essentially chelates Cu(I), but it may also be able to weakly chelate intracellular Cd2+. BCS reduced CAT activity and suppressed the effect of Cd2+ on GR activity (Fig. 6B bii, iii). Cd co-exposure with BCS and NAC decrease the cellular Cd content (Fig. 6B vii). BCS also reduced mitochondrial superoxide levels and Casp8 and Casp9 activity, which were substantially induced by Cd2+ (Fig. 6B v, x, xi).
3.6. Effects of Cd2+ pre-treatment and Cd2+ co-exposure with Cu2+
The LC50 and LC20 values of Cu2+ were determined in the presence of Cd2+ at different concentrations (Fig. 7A). High concentrations of Cd (10° and 100.8 μM) decreased the LC values of Cu2+, but less than 10−1.6 μM concentrations of Cd did not alter the LC values.
Fig. 7.
Mortalities of ZFL exposed to Cu2+ or Cd2+ with co-exposure to or pre-treatment with various concentrations of Cd2+ or Cu2+, respectively, for 24 h. (A) Mortality of ZFL due to Cu2+ co-exposure with various concentrations of Cd2+. (B) Mortality of ZFL due to Cd2+ co-exposure with various concentrations of Cu2+. (C) Mortality of Cu2+-pre-treated ZFL exposed to various concentrations of Cd2+ for 24 h. (D) Mortality of Cd2+-pre-treated ZFL exposed to various concentrations of Cu2+ for 24 h. (i) Mortality of ZFL. The curve was generated by non-linear regression. (ii) The LC50 values generated by (i). Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction) were used to analyse the data. Same alphabet on the bar represent the same group and without significant different within the group. (iii) The LC20 values generated by (i) and statistical test was same with (ii) and the Brown–Forsythe and Welch’s ANOVA tests with multiple comparison (unpaired t with Welch’s correction) were also applied.
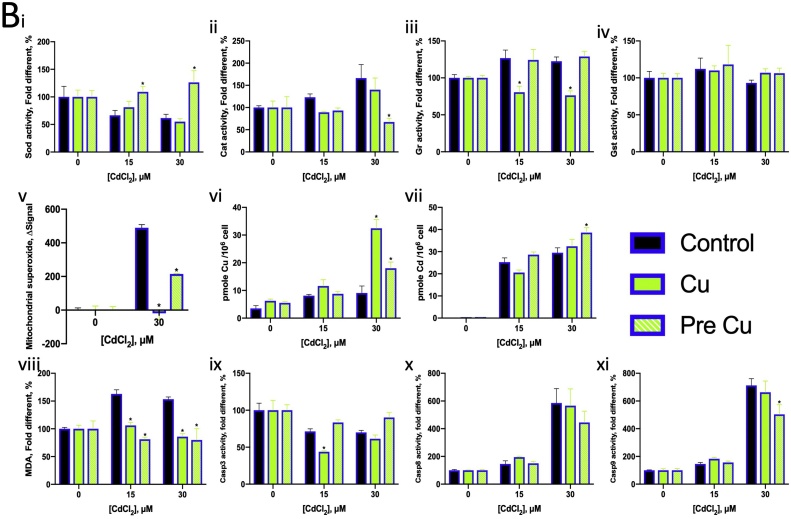
Among pre-treatments with various concentrations of Cd2+ before Cu2+ exposure, that with 10−1.6 μM Cd2+ (25 nM) increased the LC20 values of Cu, indicating that the cell became resistant to low concentrations of Cu2+ (Fig. 7C). Thus, we selected 25 nM Cd2+ concentration to evaluate the effects of Cd pre-treatment and co-exposure with 150 μM and 300 μM Cu2+. Cd2+ co-exposure with Cu2+ or Cd2+ pre-treatment did not significantly alter oxidative stress levels but slightly modified SOD and CAT activity (Fig. 8A i–iv) and reduced mitochondrial superoxide levels (Fig. 8A v). After Cd exposure or pre-treatment of Cd2+, the cells contained around 1 pmole Cd per million cells (Fig. 8A vii). The Cu content became less after exposure to 300 μM Cu2+ if the cells pre-treated with Cd2+ (Fig. 8A vi).
Fig. 8.
Normalised bioassay results of ZFL exposed to various concentrations of Cu2+ and Cd2+ and co-exposed with or pre-treated with low concentrations of Cd2+ and Cu2+, respectively. (A) Cu co-exposed with and pre-treated with low concentration of Cd. (B) Cd co-exposed with and pre-treated with low concentration of Cu. (i) Normalised SOD activity. (ii) Normalised CAT activity. (iii) Normalised GR activity. (iv) Normalised GST activity. (v) Normalised mitochondrial superoxide level in ZFL. (vi) Cellular Cu content. (vii) Cellular Cd content. (viii) Normalised MDA concentration in ZFL. (ix) Normalised Casp3 activity. (x) Normalised Casp8 activity. (xi) Normalised Casp9 activity. Data were normalised to their own control (without metal ions but with the co-exposure or pre-treatment substance). The controls were defined as 100 %, except for (v). The control in (v) was defined as 0.
Cd2+ co-exposure with Cu2+ also reduced lipid peroxidation induced by Cu2+ (Fig. 8A viii). A low concentration of Cd2+ suppressed Casp8 activity induced by Cu2+ (Fig. 8A x). Cd2+ pre-treatment also reduced Casp3 activity and suppressed Casp9 activity induced by Cu2+ (Fig. 8A ix, xi).
3.7. Effects of Cu2+ pre-treatment and Cu2+ co-exposure with Cd2+
The LC50 and LC20 values of Cd2+ were determined in the presence of Cu2+ at various concentrations (Fig. 7B). The LC values of Cd2+ decreased with the increase in Cu2+ concentration, indicating increased Cd toxicity in ZFL. However, pre-treatment with a suitable concentration of Cu2+ increased Cd2+ tolerance in ZFL. For example, 100.9 μM Cu2+ (8 μM) was found to be the most suitable concentration and was therefore selected for further assays with 15 μM and 30 μM of Cd2+. Cu2+ pre-treatment induced SOD activity and reduced CAT activity in ZFL (Fig. 8B i, ii). Cu2+ co-exposure with Cd2+ suppressed GR activity induced by Cd2+ (Fig. 8B iii). Further, Cu2+ co-exposure with Cd2+ suppressed and Cu2+ pre-treatment reduced the mitochondrial superoxide production induced by Cd2+ (Fig. 8B v). Cd co-exposure with low concentration of Cu increase the Cu cellular content. Low concentration Cu2+ promoted Cd content in 30 μM of Cd2+ exposure. Cu2+ could prevent lipid peroxidation induced by Cd2+. In addition, Cu2+ co-exposure with Cd2+ reduced Casp3 activity, and Cu2+ pre-treatment reduced Casp9 activity (Fig. 8B ix, xi).
4. Discussion
4.1. Cu2+ and Cd2+ toxicity
We used the serum-free medium for exposure instead of serum contain medium. One of the purposes is to avoid cell growth during the exposures, just like we do not feed the animal while doing acute exposures. We have experienced that the ZFL, even the other cell line we used, such as HepG2, could survive in good shape more than four days in serum-free medium based on observed under the microscope and strong signal in alamarBlue assay. There are several problems using serum containing medium for chemical exposures. Firstly, it promotes cell growth and activate metabolisms, the cells (control and non-lethal doses) in 96-well plate might saturate the surface of the well and alleviate the results in alamarBlue assay. Secondly, the composition of serum is complex and uncertain, and the proteins or chemicals may bind with or chelate the metal ions, or the hormones may stimulate the receptors if we study about the hormone related chemicals. In general, we use serum containing medium in seeding cell procedure to keep the cells in good shape, while serum free medium is used during the exposures. ZFL are well documented and known for using serum-free medium protocols for exposure [[50], [51], [52]].
The LC50 value for ZFL exposed to Cu2+ for 24 h in this study (750 μM) was similar to that in our previous study (743.3 μM) but higher than that in other studies (≤308.1 μM) [26,43,53]. However, the LC50 value of Cd2+ in this study (36.3 μM) was lower than that in other studies (59.99 μM [54] and 140.6 μM [55]). Further, the LC value of Cd2+ was 10-fold lower than that of Cu2+, indicating that Cd2+ was more toxic to ZFL than Cu2+. This finding contrasted with that in whole zebrafish and other species [56]. Previous studies have reported the following LC50 values of Cu2+ and Cd2+ for various species: adult zebrafish, 0.99 μM and 86.1 μM (96-h exposure) [57,53]; kutum (Rutilus frisii kutum) fingerlings, 11.6 μM and 211 μM (24-h exposure) [58]; Artemia urmiana, 470 μM and 703 μM (24-h exposure) [59]; Rasbora sumatrana (Cyprinidae), 0.852 μM and 12.8 μM (24-h exposure) [60]; and Poecilia reticulata (guppy) (Poeciliidae), 5.49 μM and 73.0 μM (24-h exposure) [60], respectively. The higher Cu2+ tolerance compared with Cd2+ tolerance found in ZFL was probably because ZFL is a cell line of liver origin, an organ responsible for Cu2+ elimination. Living cells have evolved cellular mechanisms to absorb, utilise and eliminate Cu2+ but not Cd2+ as Cu2+, but not Cd2+, is essential for several cellular functions [26].
Cu2+ and Cd2+ exposure promoter Cu and Cd accumulation, result in higher Cu and Cd content (Figs. 2D i, 3 D ii), matched with previous studies [26,54]. After Cd exposure, the cells retain more Cu (Fig. 3D i). That means the cells was able to absorb Cu and Cd, or in other words, Cu and Cd could go into cells [50,54].
To fully understand the effects of Cu2+ and Cd2+ on gene expression, four time points were chosen: 4, 12, 24 and 48 h. To ensure the cell viability in 48-h exposure time point, LC50 values of Cu2+ (560.6 μM) and Cd2+ (7.36 μM) for 96-h exposure, instead of 24-h exposure, were used in the oxidative stress gene profiling of ZFL exposed to Cu2+ and Cd2+ [26,50,54]. At very high metal concentrations, the cells would die, and no RNA would be available for extraction. In contrast, at very low concentrations, the cell responses would not be triggered. As the dose makes the poison, we have to use the relevant doses in all the experiments. Thus, it is very important to perform toxicity assays to determine the LC values in each experiment, we thus can pick the exposure concentration based on the LC values, usually < LC50 value and >10 % LC50 value, if we want to look at the sub-lethal effects. The doses use should hence be relevant or close to the physiological doses.
4.2. Oxidative stress related gene regulation by Cu2+ and Cd2+
The expression profiles of oxidative stress related genes were analysed at four time points (4, 12, 24 and 48 h) to determine when ZFL started showing response to Cu2+ and Cd2+ exposure (Figs. 2A, 3 A). In general, cell response to Cu started earlier at 4 h and lasted till 24 h. In contrast, cell response to Cd started later at 12 h and lasted longer till 48 h. In one study, the expression of zsod1, zsod2 and zgst showed no change, was suppressed and was induced, respectively, in zebrafish larvae after 3-h Cd exposure [61]. This result is similar to the 4-h Cd exposure result in our study.
4.3. Oxidative stress caused by Cu2+ and Cd2+
The responses of ZFL to various concentrations of Cu2+ and Cd2+ were evaluated at 24-h exposure, and as the exposure time was short, higher metal concentrations were used for testing. Both Cu2+ and Cd2+ caused oxidative stress by lipid peroxidation (Figs. 2E, 3 E). Cu2+ and Cd2+ trigger the upregulate expression of oxidative stress related gene, such as zgr and zgst. Based on central dogma, zgr and zgst expression are based on the promoter activity, not depend on the binding of Cu/Cd-glutathione complexes. The induction of gene involved a serious of transcription factor. zgr and zgst expression was induced by its own related transcription factor. In general, GR and GST are key enzyme to deal with oxidative stress, so when the cell faces oxidative stress, those related transcription factors upregulate and express more zgr and zgst. Based on this cell response, exposure to Cu2+ or Cd2+ should create oxidative stress to cells.
Although both metals regulated oxidative stress related gene expression, the related enzyme activity did not follow similar pattern or exhibit much change (Figs. 2A, B, 3 A, B). One possible explanation is that an entire family of enzymes could have contributed to the result of enzyme assays in contrast with the results of single genes analysed by qPCR, so the enzymatic assay result did not similar with qPCR, for example, the zsod gene family comprises three genes, qPCR only determined one of each gene expression, but SOD enzymatic assay measured the whole gene family activity. The facts that Cu2+ is closely monitored by Atox1 after being transported into the cell and free Cu2+ ions cannot exist in mitochondria prove that Cu2+ did not induce mitochondrial superoxide production [62].
NAC as a nutritional supplement, is a greatly applied antioxidant in vivo and in vitro [63]. It was used as antioxidant agent in many researches, for example, treating workers exposed to lead [64] and protecting SIEC02 Cells exposed by Zearalenone from oxidative stress [65]. NAC is a fast-acting antioxidant by triggering intracellular hydrogen sulfide production [66]. Besides NAC, Curcumin was reported to reduce copper-induced oxidative stress in Drosophila melanogaster [67]. The protective effect of NAC was shown to be better than Curcumin [68].
NAC reduced Cu2+ and Cd2+ toxicity by increasing their LC values, but VE did not affect Cd2+ toxicity (Fig. 5A, B, D, E). Cu and Cd is able to directly bind to the thiols group in NAC. However, If Cu binds with NAC, the complex was still not able to pass the membrane. Cu (Cu+) is imported to cell by copper transporters, Ctr1. Ions itself was not able to pass though the lipid bilayer membrane. The working principle of NAC is that NAC replenish the GSH in cell by some enzyme, such as acylase I [69]. NAC is able to react with oxidant species, but the reaction rate is much lower than GSH. we cannot totally eliminate the possibility that NAC detoxification effect of Cu and Cd is based on the chelation metal ions instead on its antioxidant activity. NAC (500 μM) was selected for further analysis, as it was found to significantly reduce the LC values of both Cu2+ and Cd2+. The effect of NAC on Cu2+ and Cd2+ co-exposure was studied to determine whether their toxicity was caused by oxidative stress. The results showed that NAC had little effect on oxidative stress related enzyme activity, as Cu2+ and Cd2+ did not directly affect them.
Since NAC contains both antioxidant and chelator properties, BCS, which is not antioxidant was chosen to chelate Cu+ and was expected to directly suppress the effects of Cu. The LC data showed that BCS could reduce both Cu2+ and Cd2+ toxicity (Fig. 5C, F). BCS chelated Cu(I) instead of Cu(II), and Ctr1 imported Cd [26,70]. The cellular Cu content increase when Cu co-exposure with BCS (Fig. 6A vi), suggesting that BCS strongly chelated Cu in the cell, leading the cells high cellular Cu tolerance. SOD activity increased in Cu co-exposure with BSC condition due to Cu cellular increased as Cu is a cofactor in SOD1 (Fig. 6A i).
We hypothesised that the Cd(I) chelated by BSC is converted from Cd2+ taken up by Ctr1. The oxidative stress caused by Cu2+ seemed to help reduce the mitochondrial superoxide levels, because of adding NAC leading to a drastic superoxide accumulation in mitochondria (Fig. 6A v). The increase in mitochondrial superoxide levels on Cu2+ chelation suggested that Cu2+ plays some role in oxidative stress regulation in mitochondria. Cu2+ induced lipid peroxidation that halted with the addition of an antioxidant or chelator, indicating that Cu2+ induced oxidative stress in the cytoplasm (Fig. 6A viii). On the other hand, Cd2+ caused oxidative stress in both the cytoplasm and mitochondria (Fig. 6B v, viii). NAC and BCS helped to reduce oxidative stress in the mitochondria but not the cytoplasm.
4.4. Cu2+ as an antioxidant in oxidative stress
One study reported that Cd does not affect Cu uptake but we found that it is not the case [71]. To further evaluate whether Cd2+ affects Cu2+ toxicity, we examined two sets of conditions, namely co-exposure with a low concentration of Cd2+ (25 nM) and the concentration of Cu2+ (150 μM and 300 μM) used previously in this study and pre-treatment of the cells with a low concentration of Cd for 24 h followed by Cu exposure. To select a Cd concentration for the experiment, the LC values of Cu with various Cd concentrations were first determined. Cd2+ made cells intolerant to Cu2+, probably because Cd2+ competed with Cu2+ for the same detoxification system (Fig. 7A). The cells pre-treated with 25 nM Cd2+ could tolerate Cu2+ (Fig. 7C). A low Cd2+ concentration decreased mitochondrial superoxide levels during Cu2+ exposure (Fig. 8A v), but raw data showed that Cu2+ co-exposure with a low concentration of Cd2+ reduced the high basal level of mitochondrial superoxide (control) (Supplementary 1e). This explained why the nominated result of mitochondrial superoxide levels were decreased sharply after Cu exposure with Cd co-espouse and pre-treatment. The same explanation could be applied to the result of lipid peroxidation (Fig. 8A viii). Next, we performed a similar experiment to determine the effect of low Cu2+ concentration on Cd2+ toxicity. The Cu concentration for further analysis was determined based on the LC values as described above, and Cu2+ co-exposure also made cells intolerant to Cd2+. The LC values of Cd2+ decreased with the increase in Cu2+ concentration (Fig. 7B).
Pre-treating cells with 8 μM Cu2+ slightly increased their tolerance to Cd2+ exposure (Fig. 7D). Co-exposure with low Cu2+ concentration could eliminate GR activity induced by Cd2+ (Fig. 8B iii). In addition, as low as 8 μM Cu2+ could terminate mitochondrial superoxide production induced by Cd2+, and Cu2+ pre-treatment showed a similar effect (Fig. 8B v). Moreover, Cu2+ could effectively reduce lipid peroxidation caused by Cd2+ (Fig. 8B viii). When the cells expose to Cd2+, together co-exposure to Cu2+ or pre-treated with Cu2+, the cells try to absorb the Cu or slow down the elimination of Cu, inferred by Cu cellular content result (Fig. 8B vi), provided more piece of evidence to suggest Cu play an antioxidant role in cells.
Cu creates oxidative stress in cell via Fenton reaction [9]. On the other hand, it serves as cofactor in SOD1 to play a role as antioxidant. These two effects sound contradictory but the doses matter. Antioxidant role is illustrious in low concentration of Cu2+ but high concentration creates oxidative stress significantly. If we consider the cell mortality mainly dependent on the oxidative stress and antioxidant effect by Cu, in theory, there is the threshold for Cu2+ to act either as prooxidant or antioxidant. It could be found in Cd2+ exposure with Cu2+ pre-treatment (Fig. 7D) and which was pre-treatment with 100.9 μM Cu2+. The antioxidant effect was maximum at this concentration, but prooxidant effect became dominant when concentration increased.
4.5. Apoptosis suppression by Cu
Numerous studies have suggested that Cu2+ and Cd2+ induce apoptosis [5,33,35,72]. In this study, caspase activity was measured to determine the induction of apoptosis as it starts with the activation of a series of caspases [37]. In general, Casp8 is activated by an external factor, namely the death receptor, and Casp9 is activated by an internal factor, namely mitochondria [73,74]. Reactive oxygen species are considered as an internal factor or part of the intrinsic pathway, and caspases are a family of end proteases that activate each other in a regulatory network. Casp3 occurs downstream of Casp8 and Casp9 in this network, and its activation leads to apoptosis.
Although Cu2+ induced Casp8 and Casp9 activation, further induction occurred only after adding NAC (Figs. 3E, 6 A ix, xi), proving that Casp8 and Casp9 induction was not due to Cu2+-induced oxidative stress. The increase in Casp3, Casp8 and Casp9 activity occurred after Cu2+ was chelated, indicating that although Cu2+ caused oxidative stress, it suppressed apoptosis (Fig. 6A xi–xi). In contrast, NAC could stop Casp8 and Casp9 induction caused by Cd2+, showing that the apoptotic effect of Cd2+ was because of oxidative stress (Fig. 6B xi, xi). Further, Cu2+ could reduce the increase in Casp3 activity caused by Cd2+ (Fig. 8A xi–xi, B xi–xi), indicating that Cu2+ reduced the apoptotic effects of Cd2+.
5. Conclusions
Cu2+ and Cd2+ caused oxidative stress in ZFL but did not have much effect on oxidative stress related enzyme activity, except for the induction of GR activity (see Graphical Abstract). NAC and BCS reduced Cu2+ and Cd2+ toxicity. Notably, Cd2+ toxicity, not Cu2+ toxicity, was due to oxidative stress. In addition, at a low concentration, Cu2+ played an antioxidant role in reducing the level of mitochondrial reactive oxygen species. High level cellular Cd level enhance Cu2+ absorption. Cu2+ could also protect ZFL from the apoptotic effect of Cd2+ exposure.
Conflict of Interest
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This project was supported by two Direct Grants for Research awarded to KMC (4053172 and 4053240) by the Biology Panel in the Chinese University of Hong Kong (CUHK). MLK received a Postgraduate Studentship from CUHK. We thank Mr LEE Chun Kit (Alan) for technical advice on flow cytometry and cell staining.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.06.012.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Klaassen C.D., Liu J., Diwan B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009;238:215–220. doi: 10.1016/j.taap.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poole J.H., Tyack P.L., Stoeger-Horwath A.S., Watwood S. A cadmium enzyme from a marine diatom. Nature. 2005;435:42. doi: 10.1038/434455a. [DOI] [PubMed] [Google Scholar]
- 3.Ho Y.P., Yang Y.C., Klippenstein S.J., Dunbar R.C. Binding energies of Ag+ and Cd+ complexes from analysis of radiative association kinetics. J. Phys. Chem. A. 1997;101:3338–3347. doi: 10.1021/jp9637284. [DOI] [Google Scholar]
- 4.Liu J., Qian S.Y., Guo Q., Jiang J.J., Waalkes M.P., Mason R.P., Kadiiska M.B. Cadmium generates reactive oxygen- and carbon-centered radical species in rats: insights from in vivo spin-trapping studies. Free Radic. Biol. Med. 2008;45:475–481. doi: 10.1016/j.freeradbiomed.2008.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu S.M., Shu L.H., Liu J.H., Chen C.H. Anti-oxidative responses on hepatic tissue of zebrafish (danio rerio) in a short duration of sub-lethal concentrations of cadmium exposure. Bull. Environ. Contam. Toxicol. 2017;98:612–618. doi: 10.1007/s00128-017-2063-0. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J.L., Yuan S.S., Wu C.W., Lv Z.M. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio) Aquat. Toxicol. 2016;180:36–44. doi: 10.1016/j.aquatox.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Renieri E.A., Sfakianakis D.G., Alegakis A.A., Safenkova I.V., Buha A., Matović V., Tzardi M., Dzantiev B.B., Divanach P., Kentouri M., Tsatsakis A.M. Nonlinear responses to waterborne cadmium exposure in zebrafish. An in vivo study . Environ. Res. 2017;157:173–181. doi: 10.1016/j.envres.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Harris E.D. Cellular copper transport and metabolism. Annu. Rev. Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- 9.Valko M., Morris H., Cronin M.T.D. Metals, toxicity and oxidative stress. Curr. Top. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 10.Brand M.D., Affourtit C., Esteves T.C., Green K., Lambert A.J., Miwa S., Pakay J.L., Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Craig P., Wood C., McClelland G. Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1882–92. doi: 10.1152/ajpregu.00383.2007. [DOI] [PubMed] [Google Scholar]
- 12.El-Aal H.A.H.M.A. Lipid peroxidation end-products as a key of oxidative stress: effect of antioxidant on their production and transfer of free radicals. In: Catala Angel., editor. Lipid Peroxidation. IntechOpen; 2012. pp. 63–88. [DOI] [Google Scholar]
- 13.Miller A.F. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 2012;586:585–595. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nojima Y., Ito K., Ono H., Nakazato T., Bono H., Yokoyama T., Sato R., Suetsugu Y., Nakamura Y., Yamamoto K., Satoh J.I., Tabunoki H., Fugo H. Superoxide dismutases, SOD1 and SOD2, play a distinct role in the fat body during pupation in silkworm bombyx mori. PLoS One. 2015;10:1–20. doi: 10.1371/journal.pone.0116007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culotta V.C., Klomp L.W.J., Strain J., Casareno R.L.B., Krems B., Gitlin J.D. The copper chaperone for superoxide dismutase. J. Biol. Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 16.Chelikani P., Fita I., Loewen P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh R.J. Glutathione: a marker and antioxidant for aging. J. Lab. Clin. Med. 2002;140:380–381. doi: 10.1067/mlc.2002.129505. [DOI] [PubMed] [Google Scholar]
- 18.Veal E.A., Mark Toone W., Jones N., Morgan B.A. Distinct roles for glutathione S-transferases in the oxidative stress response in Schizosaccharomyces pombe. J. Biol. Chem. 2002;277:35523–35531. doi: 10.1074/jbc.M111548200. [DOI] [PubMed] [Google Scholar]
- 19.Matough F.A., Budin S.B., Hamid Z.A., Alwahaibi N., Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. SQU Med. J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadoma Y., Ishihara M., Okada N., Fujisawa S. Free radical interaction between vitamin E (alpha-, beta-, gamma- and delta-tocopherol), ascorbate and flavonoids. In Vivo (Brooklyn). 2006;20:823–828. [PubMed] [Google Scholar]
- 21.Chepda T., Cadau M., Chamson A., Alexandre C., Frey J. Alpha-tocopherol as a protective agent in cell culture. Vitr. Cell. Dev. Biol. - Anim. 1999;35:491–492. doi: 10.1007/s11626-999-0058-9. [DOI] [PubMed] [Google Scholar]
- 22.Kerksick C., Willoughby D. The antioxidant role of glutathione and N-acetyl- cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2005;2:38–44. doi: 10.1186/1550-2783-2-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pazdro R., Burgess J.R. Differential effects of α-tocopherol and N-acetyl-cysteine on advanced glycation end product-induced oxidative damage and neurite degeneration in SH-SY5Y cells. Biochim. Biophys. Acta - Mol. Basis Dis. 2012;1822:550–556. doi: 10.1016/j.bbadis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Dludla P.V., Orlando P., Silvestri S., Mazibuko-mbeje S.E., Johnson R., Marcheggiani F., Cirilli I., Muller C.J.F., Louw J., Obonye N., Nyawo T., Nkambule B.B., Tiano L. N-Acetyl cysteine ameliorates hyperglycemia-induced cardiomyocyte toxicity by improving mitochondrial energetics and enhancing endogenous Coenzyme Q9/10 levels. Toxicol. Rep. 2019;6:1240–1245. doi: 10.1016/j.toxrep.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dash M., Maity M., Dey A., Perveen H., Khatun S., Jana L., Chattopadhyay S. The consequence of NAC on sodium arsenite-induced uterine oxidative stress. Toxicol. Reports. 2018;5:278–287. doi: 10.1016/j.toxrep.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok M.L., Chan K.M. Functional characterization of copper transporters zCtr1, zAtox1, zAtp7a and zAtp7b in zebrafish liver cell line ZFL. Metallomics. 2019;11:1532–1546. doi: 10.1039/C9MT00159J. [DOI] [PubMed] [Google Scholar]
- 27.Mackenzie N.C., Brito M., Reyes A.E., Allende M.L. Cloning, expression pattern and essentiality of the high-affinity copper transporter 1 (ctr1) gene in zebrafish. Gene. 2004;328:113–120. doi: 10.1016/j.gene.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Hamza I., Prohaska J., Gitlin J.D. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1215–1220. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minghetti M., Leaver M.J., George S.G. Multiple Cu-ATPase genes are differentially expressed and transcriptionally regulated by Cu exposure in sea bream, Sparus aurata. Aquat. Toxicol. 2010;97:23–33. doi: 10.1016/j.aquatox.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 30.La Fontaine S., Mercer J.F.B. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch. Biochem. Biophys. 2007;463:149–167. doi: 10.1016/j.abb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Komjarova I., Bury N.R. Evidence of common cadmium and copper uptake routes in zebrafish Danio rerio. Environ. Sci. Technol. 2014;48:12946–12951. doi: 10.1021/es5032272. [DOI] [PubMed] [Google Scholar]
- 32.Kimura O., Endo T., Hotta Y., Sakata M. Effects of P-glycoprotein inhibitors on transepithelial transport of cadmium in cultured renal epithelial cells, LLC-PK1 and LLC-GA5-COL 150. Toxicology. 2005;208:123–132. doi: 10.1016/j.tox.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Lee W.K., Torchalski B., Kohistani N., Thévenod F. ABCB1 protects kidney proximal tubule cells against cadmium-induced apoptosis: roles of cadmium and ceramide transport. Toxicol. Sci. 2011;121:343–356. doi: 10.1093/toxsci/kfr071. [DOI] [PubMed] [Google Scholar]
- 34.Arroyo V.S., Flores K.M., Ortiz L.B., Gómez-quiroz L.E., Gutiérrez-ruiz M.C. Liver and cadmium toxicity. J. Drug Metab. Toxicol. 2015;3:1–7. doi: 10.4172/2157-7609.s5-001. [DOI] [Google Scholar]
- 35.Pang J.H., Chau L.Y. Copper-induced apoptosis and immediate early gene expression in macrophages. Atherosclerosis. 1999;146:45–52. doi: 10.1016/s0021-9150(99)00126-4. [DOI] [PubMed] [Google Scholar]
- 36.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017;482:419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galluzzi L., López-Soto A., Kumar S., Kroemer G. Caspases connect cell-death signaling to organismal homeostasis. Immunity. 2016;44:221–231. doi: 10.1016/j.immuni.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 38.Ahkin Chin Tai J.K., Freeman J.L. Zebrafish as an integrative vertebrate model to identify miRNA mechanisms regulating toxicity. Toxicol. Rep. 2020;7:559–570. doi: 10.1016/j.toxrep.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craig P.M., Galus M., Wood C.M., McClelland G.B. Dietary iron alters waterborne copper-induced gene expression in soft water acclimated zebrafish (Danio rerio) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R362–R373. doi: 10.1152/ajpregu.90581.2008. [DOI] [PubMed] [Google Scholar]
- 40.Lin C.Y., Chiang C.Y., Tsai H.J. Zebrafish and Medaka: new model organisms for modern biomedical research. J. Biomed. Sci. 2016;23:1–11. doi: 10.1186/s12929-016-0236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D.S., Chan K.M. Differentially expressed proteins in zebrafish liver cells exposed to copper. Aquat. Toxicol. 2011;104:270–277. doi: 10.1016/j.aquatox.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Cheuk W.K., Chan P.C.Y., Chan K.M. Cytotoxicities and induction of metallothionein (MT) and metal regulatory element (MRE)-binding transcription factor-1 (MTF-1) messenger RNA levels in the zebrafish (Danio rerio) ZFL and SJD cell lines after exposure to various metal ions. Aquat. Toxicol. 2008;89:103–112. doi: 10.1016/j.aquatox.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Kwok M.L., Hu X.L., Meng Q., Chan K.M. Whole-transcriptome sequencing (RNA-seq) analyses of the zebrafish liver cell line, ZFL, after acute exposure to Cu2+ ions. Metallomics. 2020;12:732–751. doi: 10.1039/D0MT00005A. [DOI] [PubMed] [Google Scholar]
- 45.Dwight Z., Palais R., Wittwer C.T. uMELT: prediction of high-resolution melting curves and dynamic melting profiles of PCR products in a rich web application. Bioinformatics. 2011;27:1019–1020. doi: 10.1093/bioinformatics/btr065. [DOI] [PubMed] [Google Scholar]
- 46.Van Peer G., Mestdagh P., Vandesompele J. Accurate RT-qPCR gene expression analysis on cell culture lysates. Sci. Rep. 2012;2:1–5. doi: 10.1038/srep00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheeler C.R., Salzman J.A., Elsayed N.M., Omaye S.T., Korte D.W. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990;184:193–199. doi: 10.1016/0003-2697(90)90668-Y. [DOI] [PubMed] [Google Scholar]
- 48.Veskoukis A.S., Margaritelis N.V., Kyparos A., Paschalis V., Nikolaidis M.G. Spectrophotometric assays for measuring redox biomarkers in blood and tissues: the NADPH network. Redox Rep. 2018;23:47–56. doi: 10.1080/13510002.2017.1392695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation . J. Biol. Chem. 1974;249:7130–7149. [PubMed] [Google Scholar]
- 50.Chen Y.Y., Zhu J.Y., Chan K.M. Effects of cadmium on cell proliferation, apoptosis, and proto-oncogene expression in zebrafish liver cells. Aquat. Toxicol. 2014;157:196–206. doi: 10.1016/j.aquatox.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y.Y., Chan K.M. Transcriptional inhibition of TCDD-mediated induction of cytochrome P450 1A1 and alteration of protein expression in a zebra fi sh hepatic cell line following the administration of TCDD and Cd2+ Toxicol. Lett. 2018;282:121–135. doi: 10.1016/j.toxlet.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Z., Yang J., Chan K.M. Toxic effects of triclosan on a zebrafish (Danio rerio) liver cell line. ZFL. Aquat. Toxicol. 2017;191:175–188. doi: 10.1016/j.aquatox.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Leung K.P., Chen D., Chan K.M. Understanding copper sensitivity in zebrafish (Danio rerio) through the intracellular localization of copper transporters in a hepatocyte cell-line ZFL and the tissue expression profiles of copper transporters. Metallomics. 2014;6:1057–1067. doi: 10.1039/c3mt00366c. [DOI] [PubMed] [Google Scholar]
- 54.Zhu J.Y., Chan K.M. Mechanism of cadmium-induced cytotoxicity on the ZFL zebrafish liver cell line. Metallomics. 2012;4:1064–1076. doi: 10.1039/c2mt20134h. [DOI] [PubMed] [Google Scholar]
- 55.Ku L.L., Chan P.C.Y., Cheuk W.K., Chan K.M. Metallothionein gene expression in zebrafish embryo-larvae and ZFL cell-line exposed to heavy metal ions. Mar. Environ. Res. 2006;62:83–87. doi: 10.1016/j.marenvres.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Sarnowski P., Witeska M. The effects of copper and cadmium in single exposure or Co-exposure on growth of common carp (Cyprinus Carpio L.) Larvae. Polish J. Environ. Stud. 2008;17:791–796. [Google Scholar]
- 57.Azize Al sawafi A.G., Wang L., Yan Y. Cadmium accumulation and its histological effect on brain and skeletal muscle of zebrafish. J. Heavy Met. Toxic. Dis. 2017;2:1–6. doi: 10.21767/2473-6457.100017. [DOI] [Google Scholar]
- 58.Zahedi S., Mehrpoosh M., Vaezzade H., Dagesaracki M.Z., Hosseini S.M. LC50 determination and copper and cadmium accumulation in the gills of Kutum (Rutilus frisii Kutum) fingerlings. Terr. Aquat. Environ. Toxicol. 2012;6:71–76. [Google Scholar]
- 59.Mohiseni M., Farhangi M., Agh N., Mirvaghefi A., Talebi K. Toxicity and bioconcentration of cadmium and copper in artemia urmiana nauplii. Iran. J. Toxicol. 2017;11:33–41. doi: 10.29252/arakmu.11.1.33. [DOI] [Google Scholar]
- 60.Shuhaimi-Othman M., Nadzifah Y., Ahmad A.K. Toxicity of copper and cadmium to freshwater fishes. World Acad. Sci. Eng. Technol. 2010;65:869–871. [Google Scholar]
- 61.Mills M.G., Gallagher E.P. A targeted gene expression platform allows for rapid analysis of chemical-induced antioxidant mRNA expression in zebrafish larvae. PLoS One. 2016;12:1–20. doi: 10.1371/journal.pone.0171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hatori Y., Inouye S., Akagi R. Thiol-based copper handling by the copper chaperone Atox1. IUBMB Life. 2017;69:246–254. doi: 10.1002/iub.1620. [DOI] [PubMed] [Google Scholar]
- 63.Mokhtari V., Afsharian P., Shahhoseini M., Kalantar S.M., Moini A. A review on various uses of N-acetyl cysteine. Cell J. 2017;19:11–17. doi: 10.22074/cellj.2016.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasperczyk S., Dobrakowski M., Kasperczyk A., Zalejska-fiolka J., Pawlas N., Kapka-Skrzypczak L., Birkner E. Effect of treatment with N-acetylcysteine on non-enzymatic antioxidant reserves and lipid peroxidation in workers exposed to lead. Ann. Agric. Environ. Med. 2014;21:272–277. doi: 10.5604/1232-1966.1108590. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Li M., Zhang W., Gu A., Dong J., Li J., Shan A. Protective effect of N-acetylcysteine against oxidative stress induced by zearalenone via. Toxins (Basel) 2018;10:407. doi: 10.3390/toxins10100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ezerin D., Takano Y., Hanaoka K., Urano Y., Dick T.P. N-Acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and article N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem. Biol. 2018;25:447–459. doi: 10.1016/j.chembiol.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abolaji A.O., Fasae K.D., Iwezor C.E., Aschner M., Farombi E.O. Curcumin attenuates copper-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Toxicol. Rep. 2020;7:261–268. doi: 10.1016/j.toxrep.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bayomy N.A., Elshafey S.H., Mosaed M.M., Hegazy A.M.S. Journal of cytology & histology protective effect of curcumin versus N-acetylcystein on acetaminophen induced hepatotoxicity in adult albino rats. J. Cytol. Histol. 2015;S3:2–8. doi: 10.4172/2157-7099.S3-018. [DOI] [Google Scholar]
- 69.Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., Sergio F., Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 2018;52:751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 70.Koga T., Hirakawa C., Sakata Y., Noma H., Nonaka K., Terasaki N. Spectroscopic and electrochemical analysis of Cu(I) complex of copper sulfate electroplating solution and evaluation of plated films. ECS Trans. 2017;75:21–27. [Google Scholar]
- 71.Sampels S., Kroupova H.K., Linhartova P. Effect of cadmium on uptake of iron, zinc and copper and mRNA expression of metallothioneins in HepG2 cells in vitro. Toxicol. Vitr. 2017;44:372–376. doi: 10.1016/j.tiv.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Ganesan S., Anaimalai Thirumurthi N., Raghunath A., Vijayakumar S., Perumal E. Acute and sub-lethal exposure to copper oxide nanoparticles causes oxidative stress and teratogenicity in zebrafish embryos. J. Appl. Toxicol. 2016;36:554–567. doi: 10.1002/jat.3224. [DOI] [PubMed] [Google Scholar]
- 73.Ahmed S., Othman N.H. Honey as a potential natural anticancer agent: a review of its mechanisms. Evid.-Based Complement. Altern. Med. 2013;2013:7. doi: 10.1155/2013/829070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang N., Cao S.J., Zhou Y., He H., Tashiro S.I., Onodera S., Qiu F., Ikejima T. Inhibition of caspase-9 by oridonin, a diterpenoid isolated from Rabdosia rubescens, augments apoptosis in human laryngeal cancer cells. Int. J. Oncol. 2015;47:2045–2056. doi: 10.3892/ijo.2015.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.