Abstract
Commercial enological tannins from various origins have been widely used in modern wine making. In order to investigate the diverse quality of tannin products, a quick, accurate and simple UPLC method was developed, which could simultaneously determine 11 principle characteristic components of hydrolysable tannins and condensed tannins. The optimum resolution of the tannins was achieved on a Waters Acquity UPLC-BEH C18 column (2.1 mm × 50 mm, 1.7 μm) at 280 nm with gradient elution. The method was validated to achieve desired specificity, linearity, precision, accuracy and stability. The developed method was successfully applied to 30 commercial enological tannins from different origins for their quality evaluation. The 30 tannin samples were qualitatively distinguished into hydrolysable, condensed or mixture tannins, and quantificationally classified into four levels of excellent, good, fair and poor products. The method could be used for quick evaluating the quality of enological tannins in practical use.
Keywords: Thirty enological tannins, Quality evaluation, UPLC, Wines, Linearity
Introduction
Recently years, commercial tannins (TAN) have been commonly used in leather production (Romer et al., 2011), nutrient addition of animal feed (Martínez et al., 2004) and food industry, especially in wine making (Neves et al., 2010; Parker et al., 2007). Based on the different origins, they are classified into two groups: hydrolysable tannins and condensed tannins (Mämmelä et al., 2000). Hydrolysable tannins (gallotannins and ellagitannins) are glucose esters of gallic and ellagic acids that extracted from chestnut wood, oak galls or other species. Condensed tannins (proanthocyanidins) with a flavonoid core as a basic structure are from grape seed, grape skin or grape stem (Vivas et al., 1996; Vivas et al., 2000).
The use of enological tannins to improve wine quality has a long history. These tannins could, in most cases, stabilize color stability, improve astringency intensity and enrich polyphenols to red wines (Bautista-Ortín et al., 2005; Liu et al., 2013). Now a wide range of enological tannins from botanical origin are available on the market (Vivas et al., 1996; Viriot et al., 1994). Enological tannins from diverse plant species have different effects, which are highly dependent on the different chemical compositions, varying amounts and diverse qualities in practical use. Information of tannin products about the origins, recommended addition quantity, extraction solvent and an estimation of the total phenols contained are always listed on the labels. However, sometimes they are not always adequately and accurately defined, it would be necessary to verify the label information before using (Vivas et al., 2000). Correspondingly, various methods have been developed to analyze tannins from plant extracts. HPLC combined with UV detection was the principle separation method used in tannins and fluorescence detection ranked the second (Kelm et al., 2006; Schofield et al., 2001; Viriot et al., 1994; Yanagida et al., 2002). With the development of analytical method, tannins could be determined extensively by techniques such as LSIMS (Vivas et al., 1996), HPLC-DAD-MS (Comandini et al., 2014), MALDI-TOF-MS (Giovando et al., 2009; Pizzi et al., 2009), GC-MS (Sanz et al., 2008) and LC-NMR (De Rijke et al., 2006; Wolfender et al., 2001) to obtain molar mass and structure information. For identifying the tannin nature, Niola et al. (1993) performed a HPLC method of chitin-chitosan samples by acid hydrolysis. And the main components in various commercial tannin extracts were identified with LSIMS (Vivas et al., 1996). For quantifying tannin products, an HPLC-DAD-MS method reported by Comandini et al. (2014) can simultaneously determine seven compounds of hydrolysable tannin extracts from chestnut bark. Neves et al. (2010) firstly gave the detailed principle composition of grape seed tannins with HPLC. The number of studies gave us lots of qualitative or quantitative information available for tannin analysis. While the method for the simultaneous separation and quantitative determination of both hydrolysable tannins and condensed tannins for quality control are not available to date.
The objective of this study was performed to develop a new method for quick distinguishing enological tannins from different origins and evaluating the quality to ensure the correct tannin used in wine production. The 11 compounds of castalin, galic acid, vescalagin, castalagin, (+)-catechin, (–)-epicatechin, proanthocyanidin B1, proanthocyanidin B2, procyanidin dimers B2-3′-O-gallatewere, proanthocyanidin C1 and ellagic acid, which were abundant in tannin products and could be taken as species-markers, were simultaneously determined (Neves et al., 2010; Vivas et al., 1996). The method was fast and accurate for tannin quality assessment. It is of great significance to establish the method for quick distinguishing and evaluating the quality of tannins in practical use.
Materials and methods
Chemicals and samples
Castalin, vescalagin, castalagin were obtained from Shanghai ZZBIO CO., Ltd (Shanghai, China). Galic acid, ellagic acid, (+)-catechin, (–)-epicatechin were purchased from Chendu Must Bio Technology Co., Ltd. (Chengdu, China). Proanthocyanidin B1, Proanthocyanidin B2, procyanidin dimers B2-3′-O-gallatewere (B2-3′-O-G) and proanthocyanidin C1 were extracted and purified to > 95% according to a standard operation procedure and the chemical structure was confirmed by nuclear magnetic resonance spectroscopy as described previously (Zhang et al., 2015). Formic acid (assay 98–100%) was from Chemical Branch of Shandong Yuwang industrial Co., Ltd. (Shandong, China). Methanol was obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents were of the highest analytical grade available.
A total of 30 commercial enological tannin samples (TAN 1 to TAN 30) were got from the market. TAN1 to TAN6 were provided from Proenol Industria Biotecnologica Lda., TAN7 to TAN19 were purchased from Enartis Unipessoal Lda. (Portugal), TAN20 to TAN24 were from institut obert de catalunya Lda. (France), TANA25 to TAN28 were from Ahorro Energético y Biomasa LDA. (Spain), TAN29 and TAN30 were provided from Biocra´tico Lda (Portugal).
Preparation of commercial tannin samples
20 mg of tannin samples were weighted and dissolved with water in a 10 mL glass stoppered conical flask. The resulting solution was filtered using a 0.22 μm filter membrane before injection. An aliquot of 2 μL of the treated samples was injected into the UPLC system for analysis.
Instrumentation
The measurements were performed using a Waters Acquity UPLC system (Singapore, Massachusetts, USA). The chromatographic separation was achieved on a Waters Acquity UPLC-BEH C18 column (2.1 mm × 50 mm, 1.7 μm) (Waters, Massachusetts, USA) at 30 °C. The flow rate was fixed at 0.3 mL/min at 280 nm. A binary mobile phase system consisted of 0.2% formic acid in water (mobile phase A) and 0.2% formic acid in methanol (mobile phase B). The gradient elution was used as follows: 0 min (A98% : B2%), 4 min (A98% : B2%), 7 min (A92%: B18%), 12 min (A90%: B10%), 17 min (A88%: B18%), 21 min (A86%: B14%), 24 min (A70%: B30%), 28 min (A68%: B32%), 28.1 min (A0%: B100%), 30 min (A0%: B100%), 31 min (A98%: B2%).
Preparation of standard solutions and calibration solutions
The stock solutions of castalin, vescalagin, castalagin (Shanghai, China) were prepared in water at the concentration of approximately 1.00 mg/mL. Galic acid, (+)-catechin, (–)-epicatechin (Chengdu, China), proanthocyanidin B1, proanthocyanidin B2, proanthocyanidin C1 and procyanidin dimers B2-3′-O-gallate were (B2-3′-O-G) were prepared in methanol (Mo, USA) at the concentration of approximately 2.00 mg/mL. Ellagic acid (Chengdu, China) were prepared in methanol (Mo, USA) at the concentration of approximately 0.5 mg/mL. The mixed standard stock solutions were further diluted with ultra-pure water to obtain working solutions at different concentration levels as working solution. The calibration standards at six levels were prepared by appropriately mixing and further diluting the stock standard solutions. All stock solutions were stored in a refrigerator at 4 °C.
Method validation
The method was fully validated according to ICH guidelines for validation of analytical procedures.
Specificity
The specificity was shown by comparing the consistency of the retention time of each analyte between a sample and the corresponding reference standard.
Linearity, limits of detection (LOD) and quantitation (LOQ)
Appropriate amounts of the standard stock solutions were diluted with water to give six concentrations covered from 0.0102 to 0.0612 mg/mL for castalin (Shanghai, China), 0.0018 to 0.0804 mg/mL for gallic acid (Chengdu, China), 0.0080 to 0.1325 mg/mL for vescalagin (Shanghai, China), 0.0098 to 0.1225 mg/mL for castalagin (Shanghai, China), 0.0040 to 0.2020 mg/mL for (+)-catechin (Chengdu, China), 0.0048 to 0.1800 mg/mL for proanthocyanidin B1, 0.0048 to 0.2400 mg/mL for proanthocyanidin B2, 0.0064 to 0.1030 mg/mL for (–)-epicatechin (Chengdu, China), 0.0080 to 0.096 mg/mL for Proanthocyanidin C1, 0.0015 to 0.1080 mg/mL for procyanidin dimers B2-3′-O-gallate and 0.0020 to 0.0406 mg/mL for ellagic acid (Chengdu, China) respectively. The limits of detection values (LOD) were calculated as the amount of the lowest concentration producing a signal to noise ratio of 3 (S/N = 3). The limits of quantifications (LOQ) were calculated as the lowest concentration producing a signal to noise ratio of 10 (S/N = 10).
Precision and repeatability
The precision was determined by the intra- and inter-day variations. Precision was measured with mixed standard solutions consists of castalin (0.0612 mg/mL), gallic acid (0.0804 mg/mL), vescalagin (0.1325 mg/mL), castalagin (0.1225 mg/mL), proanthocyanidin B1 (0.1800 mg/mL), catechin (0.2020 mg/mL), procyanidin B2 (0.2400 mg/mL), epicatechin (0.1030 mg/mL), procyanidin dimers B2-3′-O-G (0.1080 mg/mL), procyanidin C1 (0.096 mg/mL), and ellagic acid (0.0406 mg/mL), and the repeatability was measured with commercial tannin samples prepared with TAN4, TAN23, and TAN30, respectively. The intra-day precision was analyzed for six times within the same day, while for inter-day test, the samples were examined in triplicate for three successive days. To determine the repeatability, six replicates of the same samples were prepared and analyzed. Precision and repeatability were expressed by the RSDs.
Stability
The commercial tannin samples were used to study storage stability with three parallel. The sample solutions were stored away from light at room temperature of 25 °C before analyzed using the established method at 0, 2, 4, 6, 8, 10, and 12 h. The RSDs of peak areas at different times were calculated.
Recovery
Recovery was evaluated by spiking known amounts of the analyte solutions to a certain amount of sample at three concentrations (low, middle and high) with three parallel analyses at each level. Finally, the recovery was evaluated by comparing peak area ratios of the amount detected subtracted the original amount with the amount spiked.
Data analysis
All data was obtained with triplicate analyses. Quantitative analysis was calculated by standard curve method with Empower@3 (Singapore, Massachusetts, USA) of the Waters Acquity UPLC system workstation, and methodological verification was carried out in strict accordance with the national pharmacopoeia. The RSD value and bar chart were calculated and plotted by Excel 2019. The chromatogram was fitted by Original 9.0.
Results and discussion
Optimization of chromatography conditions
To optimize the separation of analytes, varying mobile phases of methanol–water, acetonitrile–water system with some modifiers including formic acid with different proportions were investigated under different gradient elution modes. By comparison with acetonitrile–water, methanol–water provided better results in terms of separation and retention of the components, whereas acetonitrile–water system resulted in a decrease of separation of hydrolysable tannins. The addition of a small amount of formic acid to the mobile phase was proved to improve the peak symmetry and shape of analytes. With the increase of the amount of formic acid, the peak shape of proanthocyanidins was greatly improved, but the separations of caslin, galic acid and vescalagin of heavier polar species, would be decreased. Finally, a mobile phase composed of methanol–water containing 0.2% formic acid was selected for good peak symmetry and proper retention time for the analytes. Different wavelengths were tested for analysis, 280 nm was chosen for the detection of analytes.
Method validation
Specificity
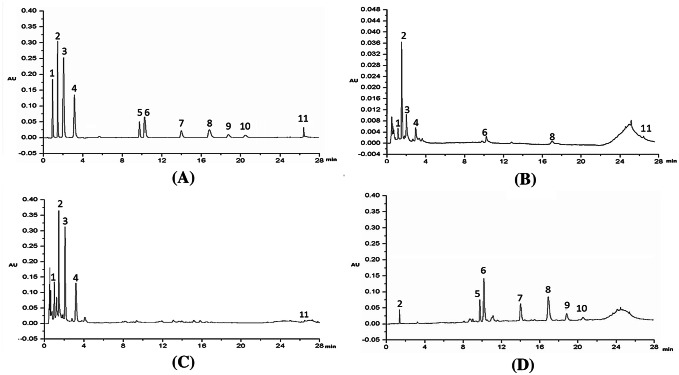
The chromatogram of the standard solution and the sample solutions were shown in Fig. 1. Typical chromatograms of standard solution, hydrolysable, condensed tannins and mixture tannins (the mixture of hydrolysable and condensed tannins) were shown in (A), (B), (C) and (D) of Fig. 1 respectively. The integration peak in the chromatogram of the sample solution was corresponding in time to the peak in the chromatogram of the standard solution. Baseline separation of the 11 analytes was achieved under the developed condition.
Fig. 1.
Chromatogram of reference standard solutiion (A), TAN23 (B), TAN4 (C), TAN30 (D). 1-castalin, 2-galic acid, 3-vescalagin, 4-castalagin, 5-proanthocyanidin B1, 6-catechin, 7-proanthocyanidin B2, 8-epicatechin, 9-procyanidin dimers B2-3′-O-gallate, 10-proanthocyanidin C1, 11-ellagic acid
Linearity, limits of detection (LOD) and quantitation (LOQ)
The stock solutions containing 11 analytes were prepared and diluted into six appropriate concentrations for the construction of calibration curves. The calibration equations were calculated by the peak areas obtained via UPLC (Massachusetts, China). The linear regression correlation values (r2) were all above 0.9990 for all the analytes as shown in Table 1. The LOD and LOQ of the 11 analytes were also listed in Table 1.
Table 1.
The linearity data of 11 compounds in tannins
| Compounds | Linear Equation | r2 | Linear range (mg/mL) | LOQ (μg/mL) | LOD (μg/mL) |
|---|---|---|---|---|---|
| Castalin | Y = 6.020 × 106X − 4.970 × 104 | 0.9997 | 0.0102–0.0612 | 10.20 | 3.26 |
| Gallic acid | Y = 1.689 × 107X − 2.634 × 104 | 0.9999 | 0.0018–0.0804 | 1.81 | 0.63 |
| Vescalagin | Y = 1.685 × 107X − 1.599 × 105 | 0.9990 | 0.0098–0.1225 | 9.80 | 3.43 |
| Castalagin | Y = 8.164 × 106X − 1.357 × 104 | 0.9996 | 0.0080–0.1325 | 7.95 | 3.64 |
| Procyanidin B1 | Y = 5.122 × 106X − 1.922 × 104 | 0.9997 | 0.0048–0.1800 | 4.80 | 2.00 |
| Catechin | Y = 4.881 × 106X − 9.436 × 103 | 0.9995 | 0.0040–0.2020 | 4.04 | 1.21 |
| Procyanidin B2 | Y = 5.086 × 106X − 1.396 × 104 | 0.9994 | 0.0048–0.2400 | 4.80 | 1.5 |
| Epicatechin | Y = 6.657 × 106X − 3.419 × 104 | 0.9998 | 0.0064–0.1030 | 6.43 | 2.19 |
| Procyanidin dimers B2-3′-O-gallatewere | Y = 5.780 × 106X − 9.046 × 102 | 0.9995 | 0.0015–0.1080 | 1.51 | 0.65 |
| Procyanidin C1 | Y = 2.5129 × 106X − 8.342 × 103 | 0.9996 | 0.0080–0.0960 | 8.00 | 2.50 |
| Ellagic acid | Y = 1.377 × 107X − 5.539 × 103 | 0.9995 | 0.0020–0.0406 | 2.03 | 0.81 |
Precision and repeatability
The inter-day and intra-day RSD for 11 standard analytes were less than 1.7 and 1.9%, indicating that the developed method was highly precise for the quantification of these components. In terms of repeatability, the RSDs of the 11 peak areas were < 2.0%. The values listed in Table 2 were all acceptable.
Table 2.
Precision, stability and repeatability data of 11 compounds
| Compounds | Intra-day | Inter-day | Stability | Repeatability |
|---|---|---|---|---|
| RSD/% | RSD/% | RSD/% | RSD/% | |
| Castalin | 1.4 | 0.9 | 2.1 | 1.3 |
| Gallic acid | 1.9 | 1.7 | 1.9 | 1.1 |
| Vescalagin | 1.0 | 1.3 | 2.5 | 1.0 |
| Castalagin | 0.2 | 0.5 | 1.9 | 1.1 |
| Procyanidin B1 | 1.2 | 0.7 | 1.1 | 1.8 |
| Catechin | 1.3 | 0.5 | 1.0 | 1.3 |
| Procyanidin B2 | 0.3 | 0.8 | 1.2 | 1.8 |
| Epicatechin | 0.6 | 1.0 | 1.1 | 0.9 |
| Procyanidin C1 | 0.8 | 1.0 | 2.2 | 1.0 |
| Procyanidin dimers B2-3′-O-gallatewere | 0.7 | 0.8 | 1.0 | 1.4 |
| Ellagic acid | 0.1 | 0.1 | 1.5 | 2.0 |
Stability
The RSD values of the tannin sample solutions were all less than 2.2% within 12 h, and the analytes were found to be stable within 12 h in the sample solution.
Recovery
The overall recoveries of the 11 analytes were 97.7% to 103.7% with RSD < 3.2%, which were shown in Table 3.
Table 3.
Recovery results of 11 analytes
| Compounds | Original/μg | Added/μg | Found/μg | Average recovery/% | RSD/% |
|---|---|---|---|---|---|
| Castalin | 114.7 | 51.00 | 164.6 | 97.7 | 1.4 |
| 102.0 | 216.5 | 99.8 | |||
| 153.0 | 266.8 | 99.5 | |||
| Gallic acid | 125.0 | 62.81 | 189.0 | 101.5 | 1.3 |
| 125.6 | 247.6 | 97.9 | |||
| 188.4 | 313.7 | 100.1 | |||
| Vescalagin | 149.0 | 78.40 | 227.1 | 99.4 | 0.9 |
| 156.8 | 305.6 | 100.0 | |||
| 235.2 | 385.7 | 100.6 | |||
| Castalagin | 90.70 | 44.52 | 136.4 | 102.7 | 1.9 |
| 89.0 | 180.0 | 100.1 | |||
| 133.6 | 225.3 | 100.8 | |||
| Procyanidin B1 | 557.4 | 500 | 1055 | 99.6 | 2.1 |
| 1000 | 1554 | 99.7 | |||
| 1500 | 2066 | 100.6 | |||
| Catechin | 83.81 | 40.4 | 125.0 | 103.2 | 2.1 |
| 80.8 | 165.1 | 99.8 | |||
| 121.2 | 206.4 | 101.2 | |||
| Procyanidin B2 | 833.3 | 500 | 1342 | 101.7 | 3.2 |
| 1000 | 1839 | 100.6 | |||
| 1500 | 2345 | 100.8 | |||
| Epicatechin | 97.30 | 51.5 | 147.9 | 98.1 | 2.1 |
| 103.0 | 199.3 | 99.1 | |||
| 154.5 | 250.9 | 99.2 | |||
| Procyanidin dimers B2-3′-O-gallatewer | 394.8 | 194.4 | 586.1 | 98.5 | 1.8 |
| 388.8 | 786.8 | 100.8 | |||
| 583.2 | 971.7 | 98.9 | |||
| Procyanidin C1 | 333.7 | 160 | 492.0 | 99.2 | 1.7 |
| 320 | 661.3 | 102.1 | |||
| 480 | 815.9 | 100.5 | |||
| Ellagic acid | 25.42 | 12.96 | 38.19 | 98.6 | 2.9 |
| 25.92 | 51.37 | 99.6 | |||
| 38.88 | 65.61 | 103.7 |
The developed UPLC method was validated to be accurate and efficient for simultaneous determination of 11 principle compounds in enological tannins. It took just 28 min for each run, which was much faster than other used HPLC methods (Kelm et al., 2006; Viriot et al., 1994). The method is specific for all the analytes, since they were well separated from other similar polyphenols and the endogenous interference from the sample substrate (Schofield et al., 2001). It was a good method for the analysis of enological tannins.
Sample analysis
The new developed UPLC method was successfully applied to simultaneous quantification of 11 analytes in 30 commercial tannin samples, including hydrolysable tannins, condensed tannins and mixture tannins. The contents of individual analyte differed from sample to sample. It was coincident with the results of other researches with the commonly used method of HPLC (Ghanem et al., 2017; Viriot et al., 1994). Castalin, vescalagin, castalagin and ellagic acid occurred in high contents in hydrolysable tannin samples. While the contents of (+)-catechin (Chengdu, China), proanthocyanidin B1, proanthocyanidin B2, (–)-epicatechin (Chengdu, China), proanthocyanidin C1 and procyanidin dimers B2-3′-O-gallate were abundant in condensed tannin samples. For the mixture tannins, both of these compounds existed. Gallic acid exited in all tannin samples and the content was relatively high.
Consequently, as the most abundant characteristic components, castalin, vescalagin, castalagin and ellagic acid were defined as the species markers of hydrolysable tannins. For condensed tannins, (+)-catechin, proanthocyanidin B1, proanthocyanidin B2, (–)-epicatechin, proanthocyanidin C1 and procyanidin dimers B2-3′-O-gallate were taken as the species-markers. With these species markers, the 30 samples were divided into hydrolysable, condensed or mixture tannins which were consistent with the labels.
To some extent, the total content phenolic compounds reflected an important parameter for the quality of tannin products in wine industry. As an important phenolic compound in wine, gallic acid was also quantified as an evaluating indicator for quality assessment. So in this study, the total content of all these species markers of hydrolysable tannins, condensed tannins and mixture tannins were evaluated for the quality assessment.
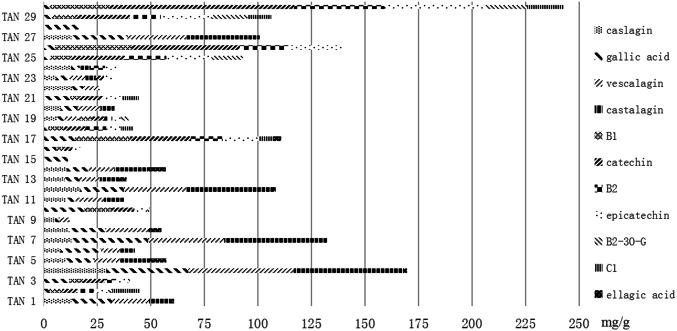
Basing on the total contents of specie-marker demonstrated in Fig. 2, all tannin samples were divided into four levels. TAN4 and TAN30 were defined as excellent products with the content higher than 150 mg/g. TAN7, 12, 17, 26, 27 and 29 samples with concentration levels between 150 and 100 mg/g were divided as good products. TAN1, 2, 3, 5, 6, 8, 10, 11, 13, 14, 18, 19, 20, 21, 22, 23, 24, 25 and 28 in normal concentration range of the total contents of 11 components were divided as fair products. TAN9, 15 and 16 with total contents lower than 25 mg/g were divided as poor products.
Fig. 2.
Total contents of 11 compounds in 30 different origins of tannins. The forms of bar represent the kinds of analytes and the lengths of the bar represent the contents of corresponding analytes in the tannin samples
This was the first attempt to establish a reliable method to differentiate the categories of tannins origin and evaluate the classification of tannins quality basing on the species constituents. It would be practical to the market to get rid of the excessive reliance on the label from the manufacturer. On the other hand, wine organizations, such as O.I.V., encourage the establishment of standardized and easy technical methods adapted to the markets, cater and large market to guarantee the authenticity of commercial tannins in order to respond satisfactorily to winemakers’ needs (Sanz et al., 2008). The developed UPLC method was approved to be a good choice. Nevertheless, more samples were still needed to improve and perfect the rule we had got.
Acknowledgements
This work was supported by Program for Liaoning Excellent Talents in University (LJQ2015106), and Scientific Research Foundation for the Returned Overseas Scholars of Shenyang Pharmaceutical University (GGJJ201606).
Compliance with ethical standards
Conflict of interest
The authors have declared that they do not have any conflict of interest.
Ethical review
This study does not involve any human or animal testing.
Informed consent
Written informed consent was obtained from all study participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Chao Yu, Email: 571196504@qq.com.
Zhe Li, Email: 949857066@qq.com.
Bao Shan Sun, Email: sun.baoshan@iniav.pt.
Yan Cui, Email: Cuiyan_13@126.com.
References
- Bautista-Ortín AB, Martínez-Cutillas A, Ros-García JM, López-Roca JM, Gómez-Plaza E. Improving colour extraction and stability in red wines: the use of maceration enzymes and enological tannins. Int. J. Food Sci. Tech. 2005;40:867–878. doi: 10.1111/j.1365-2621.2005.01014.x. [DOI] [Google Scholar]
- Comandini P, Lerma-García MJ, Simó-Alfonso EF, Toschi TG. Tannin analysis of chestnut bark samples (Castanea sativa Mill.) by HPLC-DAD–MS. Food Chem. 157: 290–295 (2014) [DOI] [PubMed]
- De Rijke E, Out P, Niessen WM, Ariese F, Gooijer C, Udo AT. Analytical separation and detection methods for flavonoids. J. Chromatogr. A. 2006;1112(1–2):31–63. doi: 10.1016/j.chroma.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Ghanem C, Taillandier P, Rizk M, Rizk Z, Nehme N, Souchard JP, El Rayess Y. Analysis of the impact of fining agents types, oenological tannins and mannoproteins and their concentrations on the phenolic composition of red wine. LWT Food Sci. Technol. 2017;83:101–109. doi: 10.1016/j.lwt.2017.05.009. [DOI] [Google Scholar]
- Giovando S, Pizzi A, Pasch H, Rode K. Synthetic tannins structure by MALDI-TOF mass spectroscopy. J. Appl. Polym. Sci. 2009;114:1339–1347. doi: 10.1002/app.30667. [DOI] [Google Scholar]
- Kelm MA, Johnson JC, Robbins RJ, Hammerstone JF, Schmitz HH. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J. Agric. Food Chem. 54: 1571–1576 (2006) [DOI] [PubMed]
- Liu YX, Liang NN, Wang J, Pan QH, Duan CQ. Effect of the prefermentative addition of five enological tannins on anthocyanins and color in red wines. J. Food Sci. 2013;78(1):C25–C30. doi: 10.1111/j.1750-3841.2012.02993.x. [DOI] [PubMed] [Google Scholar]
- Martínez TF, Moyano FJ, Diaz M, Barroso FG, Alarcon FJ. Ruminal degradation of tannin-treated legume meals. J. Sci. Food Agric. 2004;84:1979–1987. doi: 10.1002/jsfa.1907. [DOI] [Google Scholar]
- Mämmelä P, Savolainen H, Lindroos L, Kangas J, Vartiainen T. Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry. J. Chromatogra. A. 2000;891(1):75–83. doi: 10.1016/S0021-9673(00)00624-5. [DOI] [PubMed] [Google Scholar]
- Neves AC, Spranger MI, Zhao Y, Leandro MC, Sun B. Effect of addition of commercial grape seed tannins on phenolic composition, chromatic characteristics, and antioxidant activity of red wine. J. Agric. Food Chem. 2010;58:11775–11782. doi: 10.1021/jf1038037. [DOI] [PubMed] [Google Scholar]
- Niola F, Basora N, Chornet E, Vidal PF. A rapid method for the determination of the degree of N-acetylation of chitin-chitosan samples by acid hydrolysis and HPLC. Carbohyd. Res. 1993;238:1–9. doi: 10.1016/0008-6215(93)87001-9. [DOI] [Google Scholar]
- Pizzi A, Pasch H, Rode K, Giovando S. Polymer structure of commercial hydrolyzable tannins by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. J. Appl. Polym. Sci. 2009;113:3847–3859. doi: 10.1002/app.30377. [DOI] [Google Scholar]
- Parker M, Smith PA, Birse M, Francis IL, Kwiatkowski MJ, Lattey KA, Liebich B, Herderich MJ. The effect of pre- and post-ferment additions of grape derived tannin on Shiraz wine sensory properties and phenolic composition. Aust. J. Grape Wine Res. 13(1): 30–37 (2007)
- Romer FH, Underwood AP, Senekal ND, Bonnet SL, Duer MJ, Reid DG, Jan HVDW. Tannin fingerprinting in vegetable tanned leather by solid state NMR spectroscopy and comparison with leathers tanned by other processes. Molecules. 2011;16:1240–1252. doi: 10.3390/molecules16021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz ML, Martínez-Castro I, Moreno-Arribas MV. Identification of the origin of commercial enological tannins by the analysis of monosaccharides and polyalcohols. Food Chem. 2008;111:778–783. doi: 10.1016/j.foodchem.2008.04.050. [DOI] [Google Scholar]
- Schofield P, Mbugua DM, Pell AN. Analysis of condensed tannins: a review. Anim. Feed Sci. Tech. 2001;91(1):21–40. doi: 10.1016/S0377-8401(01)00228-0. [DOI] [Google Scholar]
- Vivas N, Augustin M, Lonvaud-Funel A. Influence of oak wood and grape tannins on the lactic acid bacterium Oenococcus oeni (Leuconostoc oenos, 8413) J. Sci. Food Agr. 2000;80:1675–1678. doi: 10.1002/1097-0010(20000901)80:11<1675::AID-JSFA695>3.0.CO;2-Z. [DOI] [Google Scholar]
- Vivas N, Bourgeois G, Vitry C, Glories Y, Freitas VD. Determination of the composition of commercial tannin extracts by liquid secondary ion mass spectrometry (LSIMS) J. Sci. Food Agr. 1996;72:309–317. doi: 10.1002/(SICI)1097-0010(199611)72:3<309::AID-JSFA658>3.0.CO;2-U. [DOI] [Google Scholar]
- Viriot C, Scalbert A, Du Penhoat CLH, Moutounet M. Ellagitannins in woods of sessile oak and sweet chestnut dimerization and hydrolysis during wood ageing. Phytochemistry. 1994;36:1253–1260. doi: 10.1016/S0031-9422(00)89647-8. [DOI] [Google Scholar]
- Wolfender JL, Ndjoko K, Hostettmann K. The potential of LC-NMR in phytochemical analysis. Phytochem. Analysis. 2001;12(1):2–22. doi: 10.1002/1099-1565(200101/02)12:1<2::AID-PCA552>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yanagida A, Shoji T, Kanda T. Characterization of polymerized polyphenols by size-exclusion HPLC. Biosci. Biotech. Bioch. 2002;66:1972–1975. doi: 10.1271/bbb.66.1972. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cui Y, Li L, Li Y, Zhou P, Luo L, Sun B. Preparative HSCCC isolation of phloroglucinolysis products from grape seed polymeric proanthocyanidins as new powerful antioxidants. Food Chem. 2015;188:422–429. doi: 10.1016/j.foodchem.2015.05.030. [DOI] [PubMed] [Google Scholar]