Abstract
The effects of extraction conditions on the acrylamide/furan content, antioxidant activity, and sensory properties of cold brew coffee were probed for samples prepared by steeping and dripping at various temperatures and for different times. Sensory properties were evaluated using a nine-point hedonic scale and an overall preference ranking test. Samples prepared by 3-h extraction featured the lowest acrylamide levels, while the lowest furan contents were observed for samples prepared by 24-h steeping and 12-h dripping. Among steeping-prepared samples, that extracted for 24 h showed the highest total phenol content, although no significant differences were observed for extraction times above 12 h, with a similar trend observed for ABTS free radical anion scavenging activity. Thus, the contents of bioactive and hazardous chemicals as well as sensory properties were found to be influenced by various extraction conditions.
Keywords: Acrylamide, Antioxidant, Cold brew coffee, Furan, Sensory test
Introduction
Since its discovery in the fifteenth century, coffee has rapidly gained global popularity to become one of the most popular beverages of modern society (Gloess et al., 2013). Currently, various types of coffee brewing (e.g., espresso, hand-drip, moka-pot, and cold brew) are implemented, depending on consumer preferences (Guenther et al., 2010).
Recently, cold brew coffee (CBC), prepared using water at either room or lower temperature to avoid prolonged exposure to heat during extraction (Siegel, 2017), has gained increasing popularity (Berry, 2018). Most commonly, CBC is prepared by steeping (also called immersion), i.e., by extraction of coffee grounds with cold water for several hours until final straining, and dripping, i.e., by gradual dripping of water through coffee grounds in a cold brew maker (Callow, 2017).
In brewed coffee, the contents of potent carcinogens (such as furan and acrylamide) produced by the Maillard reaction during coffee bean roasting are generally high and depend on brewing conditions (Altaki et al., 2011). Acrylamide, classified as probably carcinogenic to humans (Group 2A) by the International Agency for Research on Cancer (IARC, 1994), is also produced during coffee roasting, possibly via the thermally induced (Maillard) reaction of amino acids with carbonyl compounds (Mottram et al., 2002). Specifically, the main contributors to acrylamide formation via the above pathway are claimed to be asparagine and glucose (Zyzak et al., 2003). In addition to being produced by the Maillard reaction, acrylamide can also be formed from acrolein and ammonia, as commonly observed during the heating of lipid-rich foods (Krishnakumar and Visvanathan, 2014). According to the acrylamide risk assessment report of the Ministry of Food and Drug Safety (2016), the oral slope factor for acrylamide is 4.5 mg/(kg day), and the risk probabilities for 2.2 × 10−7—2.2 × 10−5 mg/(kg day) acrylamide ingestion correspond to 1 × 10−6—1 × 10−4. The European Food Safety Authority (EFSA) determined the average acrylamide level in roasted coffee and coffee beverages as 221 μg/kg (Blanch et al., 2013). Lantz et al. (2006) concluded that acrylamide present in roasted coffee is heavily extracted into brews and solubilized because of its high-water solubility (Andrzejewski et al., 2004). Furan, a volatile heterocycle with high hepatotoxic and hepatocarcinogenic activities in rodents that is also classified as possibly carcinogenic to humans (Group 2B; IARC, 1995), is present in coffee as one of the volatile aroma components generated during roasting. This compound is believed to be mainly produced by thermal degradation of sugars (alone or in the presence of amino acids) or certain amino acids and the thermal oxidation of ascorbic acid or poly-unsaturated fatty acids (Märk et al., 2006). In addition, previous studies showed that the efficiency of furan transfer from ground coffee to coffee brew is mainly determined by the beverage preparation procedure (Arisseto et al., 2011). Several studies reported that the use of some brewing methods allows the furan content of brewed coffee to be decreased to values below those of coffee beans (Arisseto et al., 2011; Kim et al., 2009).
On the other hand, coffee brews are renowned for their high antioxidant capacity (Esquivel and Jiménez, 2012), which is related to both the natural constituents of green coffee beans such as polyphenols and chlorogenic acids, and certain compounds produced during processing (Brezová et al., 2009). Brewing conditions strongly influence the antioxidant properties of brewed coffee, as the contact of water with roasted coffee grounds is a crucial extraction step (Ludwig et al., 2012). Furthermore, the total phenol content (TPC) of coffee has been correlated with its antioxidant properties (Kim et al., 2002), and CBC has been shown to have higher antioxidant activity than hot brew coffee (So et al., 2014).
The sensory characteristics of brewed coffee are affected by factors such as roasted coffee bean origin and variety, roasting degree, coffee-to-water ratio, brewing temperature, and contact time (Lane et al, 2017).
The Specialty Coffee Association of America (SCAA, 2016) suggested the best brewing practices for hot brews as contact time = 1–4 min (fine), 4–6 min (drip), 6–8 min (coarse) @ 93.0 ± 3 °C. However, the definition or suggestion of best conditions and practices for CBC has not yet been established, mostly because of the lack of related studies.
The strong influence of brewing conditions on the acrylamide/furan levels, antioxidant activity, and sensory properties of coffee calls for investigations of the chemical profiles of cold brews. Therefore, this work aimed to determine ideal CBC preparation conditions by considering the contents of potential carcinogens (acrylamide and furan), antioxidant activities (TPC and ABTS radical scavenging capacity), and sensory properties.
Materials and methods
Chemicals and reagents
Acrylamide (> 99%), d3-acrylamide (> 99%), furan (> 99%), d4-furan (> 99%), Folin & Ciocalteu phenol reagent, gallic acid, diammonium 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate), and α,α′-azodiisobutyramidine dihydrochloride were purchased from Sigma-Aldrich Corp. (St Louis, MO, USA). Anhydrous Na2CO3 was procured from Daejung Chemicals & Materials Co., Ltd. (South Korea), while NaCl was purchased from Samchun Pure Chemical Co., Ltd. (South Korea). All chemicals were of HPLC grade.
Preparation of coffee samples
Coffee beans (Arabica, Uganda) purchased from a local roasting company (Indie Coffee Roasters, Seoul, South Korea) were medium roasted in a TT20 coffee roaster (Petroncini Co., Ltd., St. Agostino FE, Italy). Roasting degree was determined based on the Agtron Roast Color Classification System established by SCAA using a commercial coffee roast degree analyzer (CM-100, Lighttells Co. Ltd., Taiwan). Roasted coffee beans were ground by a fully automatic grinder (Solis TYPE262, Supreme Electric Manufacturer Co., Ltd., China), passed through an 850-μm sieve, and stored at − 40 °C until next use.
Brewing procedures
Brewing was performed according to the golden cup standard (1:20, w/v) suggested by SCAA (2015) using steeping and dripping methods. For steeping, a glass bottle charged with ground coffee (12.5 g) and water (250 mL) was gently shaken for 20 s, brought to a temperature of 5, 10 (refrigeration temperature), or 20 °C (room temperature), and extracted for 3, 6, 12, or 24 h. The obtained extracts were filtered through coffee filter paper (FP103, Kalita Co., Japan). For dripping, a cold brew maker (Bean Plus Co., South Korea) was charged with ground coffee (12.5 g) and water (250 mL), the dripping rate was adjusted to 2 (for 3 h), 0.5 (for 6 h), or 0.25 drop/s (for 12 h), and extraction was performed at 5, 10 (refrigeration temperature), or 20 °C (room temperature) for 3, 6, or 12 h. All brew samples were stored in screw-cap vials without a headspace at 5 °C and used for further analysis on the day of preparation. The employed brewing conditions and sample notation are as follow: stepping at 5 °C for 3 h (S5-3), stepping at 5 °C for 6 h (S5-6), stepping at 5 °C for 12 h (S5-12), stepping at 5 °C for 24 h (S5-24), stepping at 10 °C for 3 h (S10-3), stepping at 10 °C for 6 h (S10-6), stepping at 10 °C for 12 h (S10-12), stepping at 10 °C for 24 h (S10-24), stepping at 20 °C for 3 h (S20-3), stepping at 20 °C for 6 h (S20-6), stepping at 20 °C for 12 h (S20-12), stepping at 20 °C for 24 h (S20-24), dripping at 10 °C for 3 h (D10-3), dripping at 10 °C for 6 h (D10-6), dripping at 10 °C for 12 h (D10-12), dripping at 20 °C for 3 h (D20-3), dripping at 20 °C for 6 h (D20-6) and dripping at 20 °C for 12 h (D20-12).
Determination of acrylamide content
Acrylamide was quantified by LC–ESI–MS/MS according to the slightly modified method of Andrzejewski et al. (2004).
Standard aqueous solutions with acrylamide levels of 1, 5, 10, 25, 50, and 100 ng/g were prepared. Typically, 1 mL of a 200 ng/mL d3-acrylamide solution was mixed with a 9-mL brewed coffee sample in a 50-mL tube. The tube was vortexed for 30 s and centrifuged on a Maxi-Spin centrifuge (LaboGene 2236R, LaboGene, South Korea) (0.45 μm PVDF filtration tube) at 9011 x g for 15 min. An Oasis HLB column was sequentially conditioned with 3.5 mL of methanol and the same amount of water. Next, a 1.5-mL sample was loaded into cartridge followed by 0.5 mL of water. After all eluent was discarded, 1.5 mL of water was added into the column, and the eluate was collected. A Bond Elut-Accucat SPE column was sequentially conditioned with 2.5 mL of methanol and the same amount of water, and the eluate collected from the HLB column was passed through. The first eight drops were discarded, and the remaining eluate was collected and analyzed by ultra-performance liquid chromatography (Nexera X2, Shimadzu, Japan) coupled with tandem mass spectrometry (MS/MS, Triple Quad 4500 System, AB SCIEX, USA). The Synergi™ Hydro-RP 80-Å column (4 µm, 250 × 2.0 mm) was maintained at 30 °C, and the flow rate of the mobile phase (0.2 vol % acetic acid and 0.5 vol % methanol in water) was set to 0.3 mL/min. The MS/MS conditions corresponded to a source temperature of 120 °C, a capillary voltage of 5.5 kV, and a curtain gas pressure of 25 psi. Multiple reaction monitoring ions for acrylamide and d3-acrylamide were 72 > 55 and 75 > 58, respectively. An injection volume of 20 µL was used.
Determination of furan content
Furan was quantified by a modification of the method reported by Goldmann et al. (2005) using aqueous standard solutions (1, 5, 10, 25, 50, 100 ng/g). Briefly, a 900-µL brew sample was mixed with 0.2 g of NaCl in a 20-mL vial, which was then charged with 100 µL of d4-furan internal standard (100 µg/L) solution and immediately sealed. Solid phase microextraction was performed using a 75-µm carboxen/polydimethylsiloxane (Supelco, USA) fiber assembly. A gas chromatograph (Agilent 7890A) equipped with an autosampler (Gerstel MPS2, Gerstel, Germany) coupled to a mass detector (5975 Series MSD, Agilent Technology, USA) was used for furan content determination. The pre-conditioned fiber (1 h at 250 °C) was heated to 40 °C for 20 min, and then probed and held at 250 °C for 5 min to desorb volatiles. Separation was conducted on an HP-Plot Q column (30 m × 0.32 mm × 20 μm; J&W Scientific, Santa Clara, CA, USA) in splitless mode. The carrier gas (He) flow rate was set to 44 cm/s, and the initial oven temperature of 50 °C was held for 5 min and then increased to 230 °C at 25 °C/min and held for 2 min. Qualitative analysis of furan was performed by comparing the responses of m/z 39 and 68 ions of furan with those of m/z 42 and 72 ions of d4-furan in selected ion monitoring mode, whereas quantitation was conducted by monitoring m/z 68 and 72 ions for furan and d4-furan, respectively.
TPC assay
TPC was determined by a modified method of So et al. (2014). Brewed coffee samples were pre-diluted 100-fold with distilled water, and standard solutions of gallic acid were used (10, 25, 50, and 100 mg/L). Briefly, a 200-µL pre-diluted sample and standard were mixed with 400 µL of 1 N Folin–Ciocalteau reagent in a microtube and incubated at room temperature for 3 min, which was followed by addition of 30 wt% Na2CO3 (600 µL). The microtube was incubated at room temperature for 1 h in the dark and subjected to absorbance measurements at 765 nm. TPC was calculated and expressed as µmol gallic acid equivalent (GAE) per mL of coffee brew.
ABTS free radical anion scavenging activity
The antioxidant activity of cold brews was probed by the ABTS free radical anion scavenging activity assay using a slightly modified method of Kim et al. (2002). The ABTS assay was chosen because of its better applicability to beverages containing hydrophilic, hydrophobic, and highly pigmented antioxidants compared to the previously reported DPPH assay (Floegel et al., 2011). All samples were pre-diluted 100-fold with distilled water, and 10, 25, 50, and 100 mg/mL L-ascorbic acid solutions were used as a standard. The ABTS+ solution was prepared by mixing a 2.5 mM solution of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt with a 1 mM solution of 2,2′-azobis(2-methylpropionamidine) dihydrochloride in 10 mM phosphate buffered saline (PBS) solution, pH 7.4 (1:1). The obtained mixture was placed in a water bath held at 68 °C for 40 min, cooled to room temperature, filtered through a 0.45-μm PVDF filter, and the filtrate was diluted with PBS to reach an absorbance of 0.65 ± 0.02 at 734 nm. Next, 20 µL of the pre-diluted sample and standard solutions were mixed with 980 µL of ABTS+ solution, and the mixture was incubated at 37 °C for 10 min in a water bath. Overall antioxidant activity was calculated and expressed as vitamin C equivalents in mg/L of coffee brew.
Sensory property evaluation
Sensory property evaluation was performed under the approval (No. 1041078-201807-HR-147-01) of the Chung-Ang University Institutional Review Board using 30 undergraduate and graduate students of the Chung-Ang University.
Specifically, sensory evaluation was performed using a slight modification of the procedure described in the coffee cupper’s handbook (Lingle, 2016), with descriptions developed by SCAA (2009) were as follow: the scent of something is the pleasant smell that it has (Aroma), taste caused by sugar-rich food such as sugar and honey (Sweetness), taste caused by citrus fruits or vinegars (Sourness) and taste caused by powdered medicines or caffeine (Bitterness). The results were expressed using a nine-point hedonic scale (nine = most preferred, one = least preferred) for each attribute, and overall preference tests were conducted using a ranking method.
Panel members evaluated 200-mL coffee samples in transparent and odorless plastic cups. The first evaluation probed coffee samples extracted at the same temperature and by the same method, while the second test re-evaluated samples that performed well in the first test.
Statistical analysis
All experiments were performed in triplicate, and experimental data were expressed as the mean ± standard deviation. Statistically significant differences in mean values were determined by one-way analysis of variance (ANOVA) using Duncan’s multiple range test and SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA).
Results and discussion
Acrylamide content
As acrylamide formed in coffee beans during roasting was partially transferred into brewed coffee (Lantz et al., 2006), the brewing procedure was expected to significantly affect the levels of this carcinogen in coffee brews.
Table 1 shows the levels of acrylamide in CBC samples, demonstrating that in the case of steeping, the highest acrylamide content was observed for S20-24, while the lowest was observed for S5-3 and S10-3. In the case of dripping, the highest and lowest acrylamide contents were observed for D20-12 and D10-3, respectively. Thus, acrylamide content was affected by both extraction procedure and time, increasing with the latter parameter because of acrylamide hydrophilicity, in line with previous reports (Alves et al, 2010).
Table 1.
Levels of acrylamide in CBC samples
| Coffee extraction conditions | Acrylamide levels | ||
|---|---|---|---|
| Methods | Temp (°C) | Time (h) | Mean ± SD (ng/mL) |
| Steeping | 5 | 3 | 4.0 ± 0.3a |
| 6 | 4.5 ± 0.2bc | ||
| 12 | 4.8 ± 0.2 cd | ||
| 24 | 5.2 ± 0.4de | ||
| 10 | 3 | 4.0 ± 0.3a | |
| 6 | 4.6 ± 0.2bc | ||
| 12 | 5.1 ± 0.3de | ||
| 24 | 5.4 ± 0.4e | ||
| 20 | 3 | 4.3 ± 0.2ab | |
| 6 | 4.9 ± 0.2 cd | ||
| 12 | 5.2 ± 0.2de | ||
| 24 | 5.5 ± 0.4e | ||
| Dripping | 10 | 3 | 3.9 ± 0.4a |
| 6 | 4.6 ± 0.2bc | ||
| 12 | 5.0 ± 0.3 cd | ||
| 20 | 3 | 4.1 ± 0.2ab | |
| 6 | 4.6 ± 0.3 cd | ||
| 12 | 5.2 ± 0.4d | ||
All experiments were performed in triplicate. Letters indicate significant differences at p < 0.05 within the same column measured by one-way ANOVA with Duncan’s test
Previous works estimated the acrylamide contents of brewed coffee prepared from locally purchased ground coffee as 0.5–4.21 μg/300 mL (Andrzejewski et al., 2004) and 38–77 μg/L (Alves et al., 2010), which is above the dilution-adjusted values obtained herein. Thus, our results showed that the level of acrylamide increased with increasing extraction time, being higher for samples prepared by steeping. Hence, steeping might not be ideal for avoiding acrylamide exposure.
Furan content
The high volatility of furan (boiling point = 31 °C) resulted in its loss upon the transition from roasted grounds to brewed coffee. Even though CBC prepared using longer extraction times may have generally decreased levels of furan, more detailed evidence on the actual quantities of furan is necessary.
Table 2 lists the furan contents of CBC samples, revealing that for steeping, the highest and lowest values were observed for S5-3 and S20-24, respectively. For dripping, the highest and lowest values were observed for D10-3 and D20-12, respectively. Thus, the levels of furan were significantly affected by temperature and extraction time. The negative correlation between furan content and extraction time was ascribed to the high volatility of furan and its loss during brewing.
Table 2.
Levels of furan in CBC samples
| Coffee extraction conditions | Furan levels | ||
|---|---|---|---|
| Methods | Temp (°C) | Time (h) | Mean ± SD (ng/mL) |
| Steeping | 5 | 3 | 23.7 ± 0.4j |
| 6 | 21.7 ± 0.5i | ||
| 12 | 18.7 ± 0.4f | ||
| 24 | 14.2 ± 0.3c | ||
| 10 | 3 | 21.0 ± 0.4 h | |
| 6 | 19.8 ± 0.3 g | ||
| 12 | 17.0 ± 0.5e | ||
| 24 | 15.0 ± 0.4d | ||
| 20 | 3 | 18.6 ± 0.3f | |
| 6 | 16.7 ± 0.2e | ||
| 12 | 13.1 ± 0.4b | ||
| 24 | 10.1 ± 0.2a | ||
| Dripping | 10 | 3 | 19.1 ± 0.4d |
| 6 | 18.9 ± 0.5d | ||
| 12 | 15.4 ± 0.4b | ||
| 20 | 3 | 17.3 ± 0.4c | |
| 6 | 15.9 ± 0.3b | ||
| 12 | 12.3 ± 0.3a | ||
All experiments were performed in triplicate. Letters indicate significant differences at p < 0.05 within the same column measured by one-way ANOVA with Duncan’s test
Previous studies have shown that furan levels are affected by coffee extraction conditions, e.g., values of 20–78, 12–20, and 117–244 ng/mL were obtained for home drip coffee maker–prepared, instant, and espresso capsule coffee, respectively (Altaki et al., 2011; Arisseto et al., 2011). Moreover, a significant decrease of furan level in canned coffee was observed after 5-min heating at 60 °C without the lid (Kim et al., 2009), and the furan content of espresso was shown to always exceed that in coffee obtained using a home drip coffee maker. These results were ascribed to the fact that the pressure applied in the espresso machine exceeds that in the home drip maker, which increases the efficiency of furan extraction from coffee powder (Altaki et al., 2011). Taken together, the furan levels obtained herein were similar to or lower than previously reported ones. Hence, the extraction of coffee at 5 °C is not recommended, as it results in increased furan content.
TPC
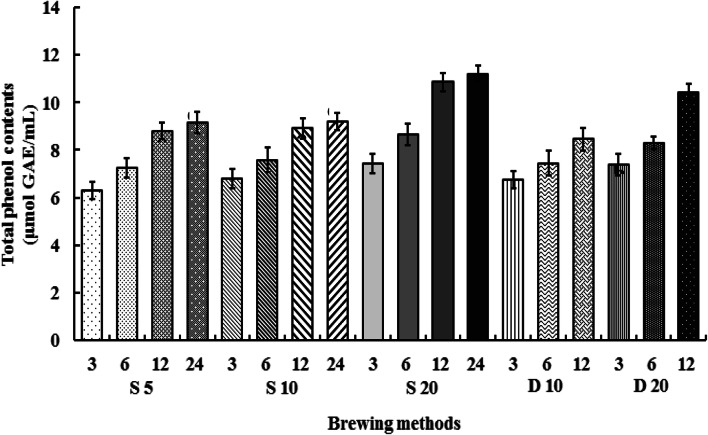
The TPC assay determines the total polyphenol content of coffee based on a redox reaction (Vignoli et al., 2011). The TPCs of CBC samples are given in Fig. 1. For steeping, the highest and lowest values were obtained for S20-24 and S5-3, respectively, whereas for dripping, the maximum and minimum values were observed for D20-12 and D10-3, respectively. The maximal value among all samples was observed for 24-h steeping at 20 °C. Thus, TPC significantly increased with increasing extraction time regardless of the brewing method.
Fig. 1.
TPCs of CBC samples obtained for n = 3 replicates. Letters indicate significantly different (p < 0.05) values according to Duncan’s test
Sacchetti et al. (2009) determined the TPC of brewed coffee prepared by solid–liquid extraction as 14.6–22.7 µmol GAE/mL for a coffee-to-water ratio of 1:10 (w/w). CBC extracted and stored at 20 °C featured a higher TPC (18.97 ± 0.33 µmol GAE/mL) than coffee extracted at 4 °C (So et al., 2014). The dilution-corrected values obtained herein are close to those reported previously. Overall, extraction at 20 °C was concluded to result in higher TPC than that at 5 and 10 °C.
ABTS free radical anion scavenging activity
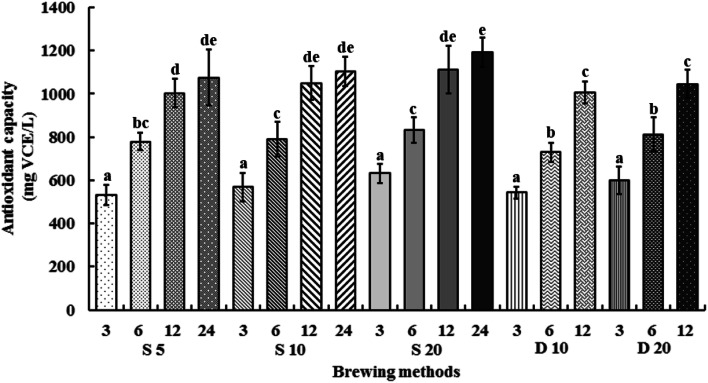
The ABTS activities of CBC samples are presented in Fig. 2. In the case of steeping, maximal and minimal values were obtained for S20-24 and S5-3, respectively, while the maxima and minima in the case of dripping were observed for D20-12 and D10-3, respectively. Thus, ABTS radical anion scavenging activity of cold brews followed a trend similar to that of TPC, significantly increasing with increasing extraction time regardless of the brewing method. Additionally, the highest activity among all samples was observed for 24-h steeping at 20 °C. Overall, extraction at 20 °C resulted in higher TPC than extraction at 5 and 10 °C.
Fig. 2.
ABTS free radical anion scavenging activities of CBC samples obtained for n = 3 replicates. Letters indicate significantly different (p < 0.05) values according to Duncan’s test
Previous works investigated how extraction rates may limit the extractable concentration of soluble coffee compounds through intragranular diffusion in hot brew coffee (as compared with cold brew coffee) because of the drastic time differences between the two processes (Fuller and Rao, 2017). This difference may explain the higher antioxidant activities of our cold brew samples. In view of the destruction of antioxidants upon exposure to high temperature, CBC featured a higher antioxidant activity than hot brew coffee.
Sensory properties
The sensory characteristics of brewed coffee depend on factors such as origin and species variety of roasted coffee beans, roasting degree, coffee-to-water ratio, brewing temperature, and contact time (Lane et al, 2017).
Table 3 shows the sensory properties of CBC prepared by steeping, demonstrating that in the case of extraction at 5 °C, the highest aroma (6.9 points) and sweetness (4.1) ranks were obtained for the sample brewed for 3 h, while the highest sourness (4.0 points) and bitterness (5.2 points) ranks were obtained for samples brewed for 6 and 24 h, respectively. The highest overall rank (3.8 points) was obtained for the sample brewed for 3 h. Cordoba et al. (2019) conducted sensory evaluation of cold brew coffees extracted for 14 h and 22 h. Higher scores were reported in the sensorial evaluation of cold brew coffee when prepared using the shortest time (14 h). For steeping at 10 °C, the 6-h sample featured the highest aroma (5.5 points) and sweetness (4.1 points) ranks. However, sourness was not significantly affected by brewing time. The 24-h sample showed the highest bitterness rank of 5.8 points, while the overall rank (3.5 points) was highest for the 6-h sample. In the case of steeping at 20 °C, the highest sweetness rank (6.0 points) was observed for the 3-h sample, while the highest sourness (4.6 points) and bitterness (5.8 points) ranks were obtained for 12-h and 24-h samples, respectively. In terms of overall preference, the highest rank of 3.2 points was observed for the 6-h sample.
Table 3.
Sensory evaluation of CBCs prepared by steeping and dripping
| Steeping | S5 | S10 | S20 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S5-3 | S5-6 | S5-12 | S5-24 | S10-3 | S10-6 | S10-12 | S10-24 | S20-3 | S20-6 | S20-12 | S20-24 | |
| Aroma | 6.9 ± 1.3b | 5.4 ± 1.0a | 6.0 ± 1.0a | 5.8 ± 1.4a | 5.3 ± 1.0ab | 5.5 ± 1.1b | 4.8 ± 1.0a | 4.8 ± 0.9a | 5.8 ± 1.3b | 6.0 ± 1.1b | 5.0 ± 1.0a | 4.9 ± 0.8a |
| Sweetness | 4.1 ± 1.5b | 3.1 ± 1.2a | 2.9 ± 0.9a | 3.4 ± 1.4ab | 3.8 ± 1.5ab | 4.1 ± 0.8b | 3.9 ± 1.4ab | 3.0 ± 1.4a | 3.4 ± 1.1b | 3.8 ± 1.5b | 2.5 ± 0.8a | 3.4 ± 1.2b |
| Sourness | 3.8 ± 1.7b | 4.0 ± 1.4b | 3.5 ± 1.1ab | 2.8 ± 1.3a | 4.5 ± 1.0a | 5.2 ± 1.6a | 4.6 ± 1.2a | 4.7 ± 0.8a | 3.4 ± 1.4a | 3.1 ± 1.6a | 4.6 ± 1.2b | 3.8 ± 1.4ab |
| Bitterness | 4.2 ± 1.6a | 3.7 ± 1.4a | 5.2 ± 1.9b | 5.4 ± 1.3b | 4.5 ± 1.2a | 4.4 ± 1.3a | 5.4 ± 1.0b | 5.8 ± 1.3b | 4.6 ± 1.4a | 5.0 ± 1.6a | 4.5 ± 1.1a | 5.8 ± 1.1b |
| Overall preference | 3.8 ± 0.4b | 2.1 ± 0.9a | 1.7 ± 0.8a | 2.1 ± 0.8a | 1.6 ± 1.0ab | 3.5 ± 0.5c | 2.1 ± 0.6b | 1.3 ± 0.6a | 2.4 ± 0.8b | 3.2 ± 0.9c | 1.7 ± 0.9a | 1.9 ± 0.7ab |
| Dripping | D10 | D20 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D10-3 | D10-6 | D10-12 | D20-3 | D20-6 | D20-12 | |||||||
| Aroma | 5.1 ± 1.2b | 5.7 ± 1.2c | 4.7 ± 1.5a | 5.8 ± 1.0b | 4.6 ± 1.2a | 4.8 ± 1.5a | ||||||
| Sweetness | 4.1 ± 1.3a | 4.5 ± 1.3a | 4.3 ± 1.3a | 4.2 ± 1.1a | 3.9 ± 0.9a | 3.9 ± 0.9a | ||||||
| Sourness | 4.6 ± 1.6a | 6.0 ± 1.3b | 4.7 ± 1.4a | 6.3 ± 0.7b | 5.5 ± 1.2a | 5.2 ± 1.3a | ||||||
| Bitterness | 5.1 ± 0.9ab | 5.1 ± 1.0a | 5.9 ± 0.9c | 4.2 ± 1.0a | 5.5 ± 0.5b | 6.2 ± 0.7c | ||||||
| Overall preference | 1.9 ± 0.8b | 2.5 ± 0.5c | 1.4 ± 0.5a | 2.8 ± 0.4b | 1.5 ± 0.5a | 1.7 ± 0.8a | ||||||
Letters indicate significant differences at p < 0.05 for the same temperature measured by one-way ANOVA with Duncan’s test
Table 3 shows the sensory properties of dripping-prepared CBC samples, showing that for 10 °C, the highest aroma (5.7 points) and sourness (4.5 points) ranks were observed for the 6-h sample, while sweetness showed no significant difference. The highest bitterness rank of 5.9 points was observed for the 12-h sample. The overall rank was highest (2.5 points) for the 6-h sample. For 20 °C, the 3-h sample showed the highest aroma (5.8 points) and sourness (6.3 points) ranks, while the 12-h sample showed the highest bitterness (6.2 points) rank. The highest overall rank (2.8 points) was observed for the 6-h sample. Table 4 shows the overall preference rankings of CBC samples, revealing that the sample brewed by steeping at 5 °C for 3 h showed the highest aroma (6.6 points), sweetness (5.9 points), and overall preference (4.2 points) ranks.
Table 4.
Sensory evaluation of selected steeping- and dripping-prepared CBCs
| Steeping | Dripping | ||||
|---|---|---|---|---|---|
| S5-3 | S10-6 | S20-6 | D10-6 | D20-3 | |
| Aroma | 6.6 ± 1.6b | 5.7 ± 1.7ab | 5.0 ± 1.3a | 5.2 ± 1.5a | 5.6 ± 1.4ab |
| Sweetness | 5.9 ± 1.1c | 4.9 ± 1.1b | 4.2 ± 1.3ab | 4.2 ± 1.2ab | 3.8 ± 1.2a |
| Sourness | 5.3 ± 1.4b | 4.4 ± 1.3a | 4.0 ± 1.4a | 3.8 ± 1.4a | 4.3 ± 1.3a |
| Bitterness | 4.2 ± 1.7a | 4.4 ± 1.6a | 5.7 ± 1.3b | 4.4 ± 1.4a | 4.1 ± 1.5a |
| Overall preference | 4.2 ± 1.4c | 3.1 ± 1.3b | 3.1 ± 1.2b | 2.2 ± 1.3a | 2.6 ± 1.4b |
Letters indicate significant differences at p < 0.05 for the same temperature measured by one-way ANOVA with Duncan’s test
Contrary to previous studies on CBC sensory properties, where extraction time was positively correlated with preference for both steeping and dripping (Kim and Kim, 2014), our study revealed an opposite trend. Additionally, the steeping method was characterized by a higher preference of sweet taste, whereas the dripping method was characterized by a higher overall intensity of bitterness, as confirmed by the fact that the caffeine contents of dripping cold brews exceeded those of dripping cold brews. The concentration of caffeine is known to influence the perceived bitterness of brewed coffee (Gloess et al., 2013). Thus, longer brewing times of CBC samples resulted in more efficient caffeine extraction, and, hence, in greater bitterness. On the other hand, our study shows that the preference for bitterness increased with increasing extraction time. Angeloni et al. (2019) reported that the flavour can vary depending on the cold brew method (dripping and immersion), but in general they found that cold brew coffees can exhibit higher intensities of sugar caramelization, sweetness, and bitterness. Overall, it was concluded that cold brewing should be performed using short extraction times because of the higher preference obtained under these conditions.
This work focused on optimizing the conditions of coffee cold brewing, considering the contents of potential carcinogens (acrylamide and furan), antioxidant activities (TPC and ABTS radical scavenging capacity), and sensory properties. The levels of acrylamide increased with increasing extraction time regardless of extraction temperature, being minimal when extraction was performed for 3 h. On the contrary, the levels of furan decreased with increasing extraction temperature and time, being minimal in cases of 24-h steeping and 12-h dripping. TPC increased with increasing extraction time, being maximal in samples prepared by 24-h steeping, but was hardly dependent on time at times above 12 h. ABTS free radical anion scavenging activities increased with increasing extraction time, following a trend similar to that observed for TPC. The sensory properties of cold brews were evaluated using a nine-point hedonic scale and an overall preference ranking test, and the most preferred sample was found to be produced by 3-h steeping at 5 °C. In summary, this study demonstrates that the levels of different chemicals and sensory properties of cold brew coffee can be controlled by modulation of corresponding extraction methods.
Acknowledgements
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2018.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ji-Won Han, Email: w_w3333@naver.com.
Hoon Boo, Email: streem3@hanmail.net.
Myung-Sub Chung, Email: chungms@cau.ac.kr.
References
- Altaki MS, Santos FJ, Galceran MT. Occurrence of furan in coffee from Spanish market: contribution of brewing and roasting. Food Chem. 2011;126:1527–1532. doi: 10.1016/j.foodchem.2010.11.134. [DOI] [PubMed] [Google Scholar]
- Alves RC, Soares C, Casal S, Fernandes JO, Oliveira MBP. Acrylamide in espresso coffee: influence of species, roast degree and brew length. Food Chem. 2010;119:929–934. doi: 10.1016/j.foodchem.2009.07.051. [DOI] [Google Scholar]
- Andrzejewski D, Roach JA, Gay ML, Musser SM. Analysis of coffee for the presence of acrylamide by LC–MS/MS. J. Agric. Food Chem. 2004;52:1996–2002. doi: 10.1021/jf0349634. [DOI] [PubMed] [Google Scholar]
- Angeloni G, Guerrini L, Masella P, Innocenti M, Bellumori M, Parenti A. Characterization and comparison of cold brew and cold drip coffee extraction methods. J. Sci. Food Agric. 2019;99:391–399. doi: 10.1002/jsfa.9200. [DOI] [PubMed] [Google Scholar]
- Arisseto A, Vicente E, Soares Ueno M, Verdiani Tfouni SA, De Figueiredo Toledo MC. Furan levels in coffee as influenced by species, roast degree, and brewing procedures. J. Agric. Food Chem. 2011;59:3118–3124. doi: 10.1021/jf104868g. [DOI] [PubMed] [Google Scholar]
- Berry D. Competition heats up in the cold brew category. Food Business News (2018) Available from: https://www.foodbusinessnews.net/articles/7365-competition-heats-up-in-the-cold-brew-category. Accessed: 14th May. 2018
- Blanch GP, Morales FJ, Moreno FDLP, Ruiz del Castillo ML. A new approach based on off-line coupling of high-performance liquid chromatography with gas chromatography–mass spectrometry to determine acrylamide in coffee brew. J. Sep. Sci. 2013;36:320–324. doi: 10.1002/jssc.201200635. [DOI] [PubMed] [Google Scholar]
- Brezová V, Šlebodová A, Staško A. Coffee as a source of antioxidants: An EPR study. Food Chem. 2009;114:859–868. doi: 10.1016/j.foodchem.2008.10.025. [DOI] [Google Scholar]
- Callow C. Cold Brew Coffee. Great Britain: Michell Beazley; 2017. [Google Scholar]
- Cordoba N, Pataquiva L, Osorio C, Moreno FLM, Ruiz RY. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel P, Jiménez VM. Functional properties of coffee and coffee by-products. Food Res. Int. 2012;46:488–495. doi: 10.1016/j.foodres.2011.05.028. [DOI] [Google Scholar]
- Floegel A, Kim DO, Chung SJ, Koo SI, Chun OK. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011;24:1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- Fuller M, Rao NZ. The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci. Rep. 2017;7:17979. doi: 10.1038/s41598-017-18247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloess AN, Schönbächler B, Klopprogge B. Comparison of nine common coffee extraction methods: instrumental and sensory analysis. Eur Food Res. Technol. 2013;236:607–627. doi: 10.1007/s00217-013-1917-x. [DOI] [Google Scholar]
- Goldmann T, Périsset A, Scanlan F, Stadler RH. Rapid determination of furan in heated foodstuffs by isotope dilution solid phase micro-extraction–gas chromatography–mass spectrometry (SPME-GC-MS) Analyst. 2005;130:878–883. doi: 10.1039/b419270b. [DOI] [PubMed] [Google Scholar]
- Guenther H, Hoenicke K, Biesterveld S, Gerhard-Rieben E, Lantz I. Furan in coffee: pilot studies on formation during roasting and losses during production steps and consumer handling. Food Addit. Contam. Part A. 2010;27:283–290. doi: 10.1080/19440040903317505. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. In: IARC monographs on the evaluation of carcinogenic risks of chemicals to humans, 63 (1994) Available from: http://monographs.iarc.fr/ENG/Classification/latest_classif.php. Accessed: 30th of SEP.2018 [PMC free article] [PubMed]
- International Agency for Research on Cancer. Furan. In: IARC monographs on the evaluation of carcinogenic risks of chemicals to humans, 63 (1995) Available from: http://monographs.iarc.fr/ENG/Classification/latest_classif.php. Accessed: 30th of SEP.2018
- Kim AR, Kim JS. Flavor contributing nonvolatile chemical and sensory characterization of cold water extraction-based coffee by different extraction methods (dripping vs steeping) and time. J. Korea Soc. Coffee Ind. 2014;3:1–9. [Google Scholar]
- Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- Kim TK, Lee YK, Park YS, Lee KG. Effect of cooking or handling conditions on the furan levels of processed foods. Food Addit. Contam. Part A. 2009;26:767–775. doi: 10.1080/02652030902774656. [DOI] [PubMed] [Google Scholar]
- Krishnakumar T, Visvanathan R. Acrylamide in food products: a review. J. Food Process Technol. 2014;5:1. [Google Scholar]
- Lane S, Palmer J, Christie B. Can cold brew coffee be convenient? A pilot study for caffeine content in cold brew coffee concentrate using high performance liquid chromatography. Arbutus Rev. 2017;8:15–23. doi: 10.18357/tar81201716816. [DOI] [Google Scholar]
- Lantz I, Ternite R, Wilkens J, Hoenicke K, Guenther H, van der Stegen GH. Studies on acrylamide levels in roasting, storage and brewing of coffee. Mol. Nutr. Food Res. 2006;50:1039–1046. doi: 10.1002/mnfr.200600069. [DOI] [PubMed] [Google Scholar]
- Lingle TR. The Coffee Cupper’s Handbook. Republic of Korea: Kwangmoonkak; 2016. [Google Scholar]
- Ludwig IA, Sanchez L, Caemmerer B, Kroh LW, De Peña MP, Cid C. Extraction of coffee antioxidants: impact of brewing time and method. Food Res. Int. 2012;48:57–64. doi: 10.1016/j.foodres.2012.02.023. [DOI] [Google Scholar]
- Märk J, Pollien P, Lindinger C. Quantitation of furan and methylfuran formed in different precursor systems by proton transfer reaction mass spectrometry. J. Agric. Food Chem. 2006;54:2786–2793. doi: 10.1021/jf052937v. [DOI] [PubMed] [Google Scholar]
- Ministry of Food and Drug Safety. Risk Assessment of Acrylamide. (2016) Available from: http://www.nifds.go.kr/brd/m_271/view.do?seq=10120 Accessed: 7th of DEC. 2018
- Mottram DS, Wedzicha BL, Dodson AT. Food chemistry: acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- Sacchetti G, Di Mattia C, Pittia P, Mastrocola D. Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J. Food Eng. 2009;90:74–80. doi: 10.1016/j.jfoodeng.2008.06.005. [DOI] [Google Scholar]
- Siegel Bari Faye. Cool, smooth and creamy WB law coffee capitalizing on the nitro cold brew trend (2017) Available from: http://www.njbiz.com/article/20171009/NJBIZ01/171009860/coolsmooth-and-creamy-wb-law-coffee-capitalizing-on-the-nitro-coldbrew-trend. Accessed: 30th of SEP. 2018
- So YJ, Lee MW, Yoo KM, Kang HJ, Hwang IK. Physicochemical characteristics and antioxidant activity of Dutch coffee depending on different extraction conditions and storage. Korean J. Food Sci. Technol. 2014;46:671–676. doi: 10.9721/KJFST.2014.46.6.671. [DOI] [Google Scholar]
- Specialty Coffee Association of America. SCAA Coffee taster’s Flavor Wheel. (2009) Available from: http://www.scaa.org/?page=resource&d=scaa-flavor-wheel. Accessed: 10th of APR. 2018
- Specialty Coffee Association of America. SCAA Standard Golden cup (2015) Available from: http://www.scaa.org/?page=resources&d=brewing-standards. Accessed: 10th of APR. 2018
- Specialty Coffee Association of America. SCAA Best practice: guidelines for using by-pass in the drip coffee brewing process (2016) Available from: http://www.scaa.org/?page=resources&d=brewing-best-practices. Accessed: 30th of SEP. 2018
- Vignoli JA, Bassoli DG, Benassi MT. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: the influence of processing conditions and raw material. Food Chem. 2011;124:863–868. doi: 10.1016/j.foodchem.2010.07.008. [DOI] [Google Scholar]
- Zyzak DV, Sanders RA, Stojanovic M. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003;51:4782–4787. doi: 10.1021/jf034180i. [DOI] [PubMed] [Google Scholar]