Abstract
Heavy metals in groups 3–16 in periods 4 and greater. They exist naturally in the earth’s crust. People are exposed to heavy metals by the inhalation of polluted air and via the intake of contaminated food. People are exposed to lead (Pb), one of heavy metals, by consuming foods that are contaminated from the environment. Pb is ubiquitous in the environment and accumulates in plants and animals that eat contaminated plants. The Pb in foods before and after processing were analyzed via Inductively coupled plasma with mass spectrometry to determine the effects of the procedures on the Pb migration and residue. This analytical method was verified to have a limit of detection of 0.011–0.859 µg/kg, acceptable linearity with the regression coefficient of 0.999, relative recoveries of 78.1–89.9% and repeatability of 1.4–7.7%. The amount of Pb was reduced during the following processes: more than 79.6% by extracting ginseng, extracting red ginseng and balloon flower roots via alcohol, more than 47.9% by blanching Chwinamul, more than 18.2% by brewing coffee with cold and hot water, more than 22.2% by extracting juices from fruits and peeling fruits. Therefore, proper cooking and food processing can be advantageous in terms of reducing exposure to Pb.
Keywords: Lead, Migration rate, Residual rate, Food processing, Exposure
Introduction
Heavy metals are all metals in groups 3–16 in periods 4 and greater (Hawkes, 1997). As industries develop, contamination by heavy metals in the environment is becoming serious (Tian et al., 2009). Although heavy metals exist naturally in the earth’s crust, the environment can be contaminated with heavy metals by human activities, including mining, processing metals ore, and pesticide/herbicide applications (Onakpa et al., 2018; Zhuang et al., 2009). People are exposed to heavy metals by the inhalation of polluted air and via the intake of contaminated food. Plants accumulate heavy metals from the environment through soil, air and water, and animals consume heavy metals by eating contaminated plants (Peralta-Videa et al., 2009).
Heavy metals are one of the most hazardous elements to human health. Arsenic (Ar), cadmium (Cd), mercury (Hg), and Pb are included in a list of 20 hazardous substances created by the U.S. Environmental Protection Agency (EPA) in 2001 (Das et al., 2011). Especially, WHO recently withdrew the provisional tolerable weekly intake (PTWI) of Pb as health guidance value because it could no longer be considered health protective (WHO, 2010). Furthermore, WHO concluded that it was impossible to establish a new PTWI. Therefore, it is very important to characterize Pb in food among heavy metals.
Pb is classified as a 2B carcinogen by the International Agency for research on Cancer. Short-term exposure to Pb affects the nervous system, causing various symptoms, such as headache and irritability. The symptoms of long-term exposure to Pb are deterioration of memory, delay of reaction time, and diminished cognition (Järup, 2003; Memon et al., 2005). Pb has been regulated over the last decades, and contamination with Pb has been reduced by global actions, such as the phasing-out of leaded petrol (Larsen et al., 2002). Some studies have monitored the level of Pb in different food categories to assess its risk by dietary intake. The European Food Safety Authority (EFSA) determined the levels of Pb in different food categories and assessed the risk of Pb by food intake (EFSA, 2010). While most foods were contaminated with Pb at levels below 1 mg/kg, game meat (including the kidney and liver) and algae-based supplements were highly contaminated with levels over 1 mg/kg (EFSA, 2010). The Pb contents in dried vegetables, fungi, and tea were higher than those in other food categories. Foods made from the organs of plants, such as vegetables and fruits, are contaminated with Pb because plants easily take up heavy metals from the soil and air. Fish and meat are also contaminated with Pb through food chains (Peralta-Videa et al., 2009). Previous studies analyzed teas, noodles, fruits, vegetables, ginseng, edible oils, and seaweeds to determine the levels of heavy metals (Angelova et al., 2004; Jothi and Uddin, 2014; Karak and Bhagat, 2010; Khan et al., 2001; Yang et al., 2016). However, they analyzed the raw materials people do not eat. People cook and process the raw material to eat. It is necessary to analyze Pb content in food which is ready to eat. There are some studies that have examined the change in heavy metals content in food before and after cooking. The Pb content in rice was found to decrease as the milling degree of rice was increased (Kim et al., 2015). The Pb concentration was increased in tea infusion as the infusion time increased (Subbiah et al., 2017). However, there is a lack of studies examining the effects of different processing and cooking methods on the change in the Pb content. In this study, we analyzed Pb in some foods before and after processing and cooking and determined the effects of cooking methods on the change in the Pb contents.
Materials and methods
Chemicals and materials
The standards of Pb of 1000 mg/kg were obtained from Merck (Darmstadt, Germany) and were used to generate solutions of 100 µg/kg with 3% nitric acid or use as standard calibration curves. Nitric acid (purity of 60%) and hydrogen peroxide (purity of 30%) (Wako, Osaka, Japan) of electronic grade were used as the extraction and digestion solvents. Water was used after being purified by a Milli-Q System (Millipore, Bedford, MA, USA). The purity of the argon gas utilized was 99.9998%. The certified reference materials (CRMs) of different food matrixes, including edible oil, peach leaves and ginseng, were obtained from FAPAS (TET009RM, York, UK), NIST (1547, Gaithersburg, MD, USA) and KRISS (Daejun, Korea), respectively.
Samples
Dried ginsengs, dried red ginsengs and dried balloon flower roots, originating from Korea, were chosen to compare the levels of Pb in food before and after extraction with alcohol; these vegetables were purchased at Korean markets. Ginsengs, red ginsengs and balloon flower roots are the most consumed roots in Korea based on the Korea National Health and Nutrition Examination survey (KNHANES) (CDC, 2010), and they are often consumed as crude alcoholic extracts. Chwinamuls, Korean seasoned aster, was selected to compare the Pb contents in food before and after blanching. Coffee was purchased from a Korean open market to observe the change in Pb content before and after brewing. Apples and grapes grown in Korea were purchased in Korean markets. These fruits were peeled and extracted to compare the Pb content in the skin, flesh, and juices.
Cooking and processing methods
Extracting fruit juice
A screw press expeller, ANG-7700P (Angel Co. Busan, Korea), made with stainless steel, was used to extract the juice from fruits. Apples and grapes were washed before pressing at optimum screw-speed of 82 rpm.
Peeling fruits
The apples and grapes were washed, and the skins and cores were removed by hand. A knife made from stainless steel was used to peel the fruits.
Extracting root vegetables
Root vegetables, such as ginseng and red ginseng, are often consumed as extracts (Kang et al., 2009). For this study, 50% ethanol was used to extract root vegetables, such as ginsengs, red ginsengs and balloon flower roots with a solvent extractor, E-816 HE (Buchi Labortechnik AG, Flawil, Switzerland). The vegetables were extracted for 6.5 h at 83 °C followed by evaporation for 20 min at 150 °C.
Brewing coffee
There are several methods for brewing coffee. Recently, brewing coffee with cold water is used as widely as using hot water (Eun et al., 2014). For this study, 10 g of ground coffee beans were put in filter paper, made from polyethylene and polypropylene, and they were brewed with 150 mL water at 98 °C for 2.5 min and at 25 °C for 3, 9 and 12 h.
Blanching vegetables
The seasoned aster (100 g) was boiled in a pot of 1 L of water at 100 °C for 1 and 3 min. The aster was stirred with chopsticks during boiling, and the boiled water was drained from the seasoned aster.
Microwave-assisted acid digestion
Samples (0.20 g) were weighed and placed in 20 mL polytetrafluoroethylene (PTFE) flasks. The samples were digested with 10 mL of 60% nitric acid for 15 min at 80 °C and 25 min at 140 °C; the samples were digested again with 1 mL of 30% hydrogen peroxide. Then the samples were digested by a microwave oven, Easy Control-280 (Milestone, Sorisloe, Italy), for 43 min at the different aforementioned temperature conditions. The digestion temperature was increased from 0 to 80 °C at a rate of 16 °C/min and decreased to 50 °C at a rate of 10 °C/min. The temperature was maintained at 180 °C for 20 min after being increased to 180 °C for 15 min. After cooling the flasks, the flasks were washed with 25 g of distilled water.
Determination of Pb with ICP-MS
The Pb was quantified and qualified with inductively coupled plasma with mass spectrometry (ICP-MS) (Dionex ICS-5000+SP, Thermo Fisher, USA). For ICP, the rf power was 1.3 kW, and the flow rates of the argon gas for plasma, auxiliary and nebulizer were 15 mL/min, 0.9 L/min, and 1–1.1 L/min, respectively. The selected ion monitoring (SIM) mode was used to measure Pb at m/z 208 for MS.
Quality Control
The quality of the data was controlled by verifying the analytical method with performance parameters. The limit of detection (LOD) was calculated by multiplying the standard deviations of concentrations of 10 blank samples by 3. The limit of quantification (LOQ) was calculated by multiplying the standard deviations of the concentration of 10 blank samples by 10. The calibration curves for quantifying the levels of Pb were obtained by regression equations that utilized working standards in a range of 0.01–20 µg/kg. The linearity of the calibration curve was estimated by calculating the coefficient of correlation of the regression equation. The recovery and repeatability were calculated by analyzing the CRMs of edible oil with Pb of 0.332 mg/kg, ginseng powder with Pb of 10.749 mg/kg, and peach leaves with Pb of 0.869 mg/kg. Double distilled deionized water was analyzed in every sample batch in order to check the instruments for contamination. Analytical instruments, such as ICP-MS and microwaves, were calibrated and maintained every 3 months.
Statistical analysis
All the samples were cooked, processed, and analyzed 3 times; the means and standard deviations of the results were calculated. Student’s t-test was conducted to identify significant differences in the levels of Pb in food before and after cooking. Microsoft Office Excel 2007 (Microsoft Corporation, Redmond, WA, USA) was used for all the statistical analysis.
Results and discussion
Quality control
The results of performance parameters verified the analytical methods (Table 1). The LOD and LOQ for measuring Pb in different food CRMs from 0.011 to 0.859 µg/kg and from 0.036 to 2.863 µg/kg, respectively. The regression coefficient of the calibration curve was 0.999 with good linearity. The relative recoveries of Pb in the CRMs were ranged from 78.1 to 89.9%, and the relative standard deviations from 1.4% to 7.7%. These results were acceptable for precision of an analytical method by the criteria of AOAC (Taverniers et al., 2004).
Table 1.
Calibration curve, detection limits (LOD and LOQ), precision (repeatability and recovery) for analytical method
| Heavy metals | Calibration curve | Regression coefficient | Food | LOD (µg/kg) | LOQ (µg/kg) | Recovery (%) | Repeatability (%) |
|---|---|---|---|---|---|---|---|
| Pb | y = 1.0032x − 0.0465 | 0.999 | Ginseng powder | 0.859 | 2.863 | 89.919 | 2.963 |
| Edible oil | 0.011 | 0.036 | 78.071 | 1.385 | |||
| Peach leaves | 0.122 | 0.405 | 81.452 | 7.723 |
Change in the Pb contents by extracting root vegetables
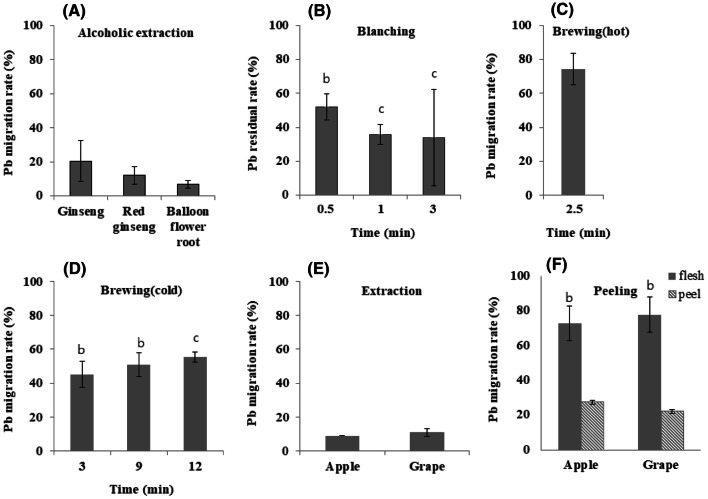
Table 2 shows the concentration of Pb and the change in the Pb amount when extracting roots with alcohol. Ginsengs, red ginsengs, and balloon flower roots contained 8.967 ± 0.278 µg (89.670 ± 2.780 µg/kg), 16.567 ± 0.173 µg (165.670 ± 1.730 µg/kg) and 22.700 ± 9.289 µg (227.000 ± 92.890 µg/kg) of Pb, respectively. Balloon flower roots contained a higher amount of Pb than the other roots, and red ginsengs were contaminated with Pb more than ginsengs. The amount of Pb in the ginseng alcoholic extractions, red ginseng alcoholic extractions, and balloon flower root alcoholic extractions were significantly reduced to 1.826 ± 0.782 µg (2.200 ± 0.942 µg/kg), 1.985 ± 0.821 µg (2.335 ± 0.966 µg/kg) and 1.505 ± 0.507 µg (2.120 ± 0.714 µg/kg), respectively (Table 2). Less than 20% of the Pb in ginseng, red ginseng and balloon flower roots was transferred to the alcoholic extractions (Fig. 1A). However, bioactive compounds, such as ginsenosides in ginsengs and red ginsengs, are easily extracted to alcohol, and alcoholic extractions of balloon flower roots showed higher hypotensive activity than water extractions (Kang et al., 2008; Kwon et al., 2003). Therefore, a beneficial way to consume ginsengs, red ginsengs, and balloon flower roots is as alcoholic extractions in order to consume less Pb and consume bioactive compounds.
Table 2.
The changes of contents of Pb during alcoholic extraction of food
| Food | Classify | Concentration of Pb (µg/kg) | Amount of Pb (µg) | Reduction rate (%) |
|---|---|---|---|---|
| Ginseng | Ctrl (100 g) | 89.670 ± 2.780 | 8.967 ± 0.278a | – |
| Extraction (830 g) | 2.200 ± 0.942 | 1.826 ± 0.782b | 79.6 | |
| Red ginseng | Ctrl (100 g) | 165.670 ± 1.730 | 16.567 ± 0.173a | – |
| Extraction (850 g) | 2.335 ± 0.966 | 1.985 ± 0.821b | 88.1 | |
| Balloon flower root | Ctrl (100 g) | 227.000 ± 92.890 | 22.700 ± 9.289a | – |
| Extraction (710 g) | 2.120 ± 0.714 | 1.505 ± 0.507b | 93.4 |
†Values with different letters in amounts of Pb are significantly different with p-value (< 0.05)
Fig. 1.
Migration and residual rates of Pb, (A) during extracting roots with alcohol, (B) during blanching Chwinamul, (C) during brewing coffee with cold water, (D) brewing coffee with hot water, (E) during extracting juice from apples and grapes, and (F) during peeling skins of apples and grapes. †Values with different letters in each column are significantly different with p-value (< 0.05)
Change in the Pb contents by blanching vegetables
Chwinamul contained 5.493 ± 0.571 µg (54.930 ± 5.710 µg/kg) of Pb, which was reduced to 2.860 ± 0.430 µg (22.292 ± 3.352 µg/kg) after blanching for 0.5 min. The Pb content was reduced to 1.968 ± 0.325 µg (15.505 ± 2.560 µg/kg) after 1 min and to 1.867 ± 1.564 µg (15.065 ± 12.620 µg/kg) after 3 min (Table 3). The Pb content was significantly reduced after blanching for 0.5 and 1 min by 47.9% and 64.2%, respectively. However, the Pb content was not significantly decreased after blanching for 3 min due to a high standard deviation (Fig. 1B). Pb can be easily transferred to boiling water even after blanching for short periods of time, such as 0.3 min. This result occurs because Pb attaches to some minerals and easily dissolves in water, allowing plants to take up Pb from the water in soil (Roussel et al., 2000). Therefore, the Pb in vegetables would be hydrophilic and easily transferred to water during blanching. To reduce the exposure to Pb by consuming vegetables, it would be better to blanch the vegetables for a short period of time.
Table 3.
The changes of contents of Pb during blanching Chwinamul
| Food | Classify | Concentration of Pb (µg/kg) | Amount of Pb (µg) | Reduction rate (%) |
|---|---|---|---|---|
| Chwinamul | Ctrl (100 g) | 54.930 ± 5.710 | 5.493 ± 0.571a | – |
| 0.5 min (128.30 g) | 22.292 ± 3.352 | 2.860 ± 0.430b | 47.9 | |
| 1 min (126.93 g) | 15.505 ± 2.560 | 1.968 ± 0.325c | 64.2 | |
| 3 min (123.93 g) | 15.065 ± 12.620 | 1.867 ± 1.564c | 66.0 |
†Values with different letters in amounts of Pb are significantly different with p-value (< 0.05)
Change in the Pb contents by brewing coffee
Coffee beans contained 0.242 ± 0.068 µg (24.200 ± 6.800 µg/kg) of Pb. Following a brew time of 2.5 min, the Pb content insignificantly decreased to 0.198 ± 0.027 µg (1.320 ± 0.180 µg/kg) . This result shows that Pb is easily transferred from coffee beans to liquid coffee after only brewing for a short time. On the other hand, the amount of Pb in cold coffee brewed for 3, 9, and 12 h was 0.109 ± 0.018 µg (0.727 ± 0.120 µg/kg) 0.124 ± 0.012 µg (0.827 ± 0.080 µg/kg), and 0.170 ± 0.040 µg (1.133 ± 0.267 µg/kg), respectively (Table 4). The amount of Pb was significantly decreased by 55% when cold brewed for 3 h and decreased as the brewing time increased. However, the migration rates of Pb by cold brewing were still lower than those by hot brewing (Fig. 1C, D). Currently, some consumers prefer to drink cold brewed coffee rather than hot brewed coffee; this preference is actually a good way to reduce the exposure to Pb when drinking coffee.
Table 4.
The changes of contents of Pb during brewing coffee with hot and cold water
| Food | Classify | Concentration of Pb (µg/kg) | Amount of Pb (µg) | Reduction rate (%) |
|---|---|---|---|---|
| Coffee (hot water) | Ctrl (10 g) | 24.200 ± 6.800 | 0.242 ± 0.068a | – |
| 2.5 min (150 g) | 1.320 ± 0.180 | 0.198 ± 0.027a | 18.2 | |
| Coffee (cold water) | 3 h (150 g) | 0.727 ± 0.120 | 0.109 ± 0.018b | 55.0 |
| 9 h (150 g) | 0.827 ± 0.080 | 0.124 ± 0.012b | 48.8 | |
| 12 h (150 g) | 1.133 ± 0.267 | 0.170 ± 0.040c | 29.8 |
†Values with different letters in amounts of Pb are significantly different with p-value (< 0.05)
Change in the Pb contents by extracting fruit juice
The juice from apples and grapes is often extracted to be consumed. Apple juice and grape juice contained 0.837 ± 0.000 µg (1.176 ± 0.000 µg/kg) and 0.702 ± 0.000 µg (1.134 ± 0.702 µg/kg) of Pb, respectively (Table 5).
Table 5.
The changes of contents of Pb during peeling and extracting fruits
| Processing | Food | Classify | Concentration of Pb (µg/kg) | Amount of Pb (µg) | Reduction rate (%) |
|---|---|---|---|---|---|
| Peeling | Apple | Ctrl (525.61 g) | 11.001 ± 1.237 | 5.782 ± 0.650a | – |
| Flesh (447.74 g) | 9.392 ± 1.322 | 4.205 ± 0.592b | 27.3 | ||
| Grape | Ctrl (818 g) | 3.066 ± 0.125 | 2.508 ± 0.102a | – | |
| Flesh (197.65 g) | 9.871 ± 2.191 | 1.951 ± 0.433b | 22.2 | ||
| Extraction | Apple | Ctrl (837 g) | 6.908 ± 0.777 | 5.782 ± 0.650a | – |
| Extraction (712 g) | 1.176 ± 0.000 | 0.837 ± 0.000b | 85.5 | ||
| Grape | Ctrl (702 g) | 3.573 ± 0.145 | 2.508 ± 0.102a | – | |
| Extraction (619 g) | 1.134 ± 0.000 | 0.702 ± 0.000b | 72.0 |
†Values with different letters in amounts of Pb are significantly different with p-value (< 0.05)
A mere 14.9% of the Pb in apples was transferred to juice, and only 28.0% of the Pb in grapes was transferred to juice (Fig. 1E). Consumers can reduce the exposure to Pb by consuming apples and grapes as extracted juice. However, apples are an important source of flavonoids, such as chlorogenic acid and catechins, and chlorogenic acid and catechins are reduced during juice extraction by 50% and 3%, respectively (Van der Sluis et al., 2002). Therefore, apple juice can not only reduce the risk of exposure to Pb but also decrease the benefits of exposure to antioxidants. It is necessary to consider risks and benefits when choosing how to cook and consume foods.
Change in the Pb contents by peeling fruits
Apples and grapes contained 5.782 ± 0.650 µg (11.001 ± 1.237 µg/kg) and 2.508 ± 0.102 µg (3.066 ± 0.125 µg/kg) of Pb, respectively. Following the peeling of the skins, apple flesh and grape flesh had 4.205 ± 0.592 µg (9.392 ± 1.322 µg/kg) and 1.951 ± 0.433 µg (9.871 ± 2.191 µg/kg) of Pb, respectively (Table 5). Pb was decreased by peeling and extracting procedure up to 85.5% (Fig. 1F). Most of the Pb uptaken by the plants were distributed to the flesh in apples and grapes, and the skins do not contain much Pb. However, the amount of skin (77.87 g) of apple was less than those of flesh (447.74 g). Therefore, the concentration of Pb in the skin was 20.252 µg/kg, and it was much higher than those in flesh. Furthermore, most of the antioxidant compounds, such as phenolics and flavonoids, were distributed to the apple skins (Lago-Vanzela et al., 2011; Wolfe et al., 2003; Xia et al., 2010). Therefore, consuming apples and grapes with their skin is better than eating them without their skin, since skin removal reduces Pb exposure very minimally while decreasing antioxidant consumption.
Acknowledgements
This research was supported by a Grant (18161MFDS020) from Ministry of Food and Drug in 2018.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joon-Goo Lee, Email: capbox@korea.kr.
Jeong-Yun Hwang, Email: dmsepf@korea.kr.
Hye-Eun Lee, Email: hyen0310@korea.kr.
Jang-Duck Choi, Email: cjd5388@korea.kr.
Gil-Jin Kang, Email: tatabox96@naver.com.
References
- Angelova V, Ivanova R, Delibaltova V, Ivanov K. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp) Ind. Crops Prod. 2004;19:197–205. doi: 10.1016/j.indcrop.2003.10.001. [DOI] [Google Scholar]
- Das SK, Grewal AS, Banerjee M. A brief review: heavy metals and their analysis. Int. J. Pharm. Sci. Rev. Res. 2011;11:13–18. [Google Scholar]
- Eun JB, Jo MY, Im JS. Physicochemical characteristics of coffee extracts using different extraction methods. Korean J. Food Sci. Technol. 2014;46:723–728. doi: 10.9721/KJFST.2014.46.6.723. [DOI] [Google Scholar]
- European Food Safety Agency (EFSA) EFSA panel on contaminants in the food chain (CONTAM); Scientific opinion of lead in food. EFSA J. 2010;8:1–151. [Google Scholar]
- Hawkes SJ. What is a “heavy metal”? J. Chem. Educ. 1997;74:1374. doi: 10.1021/ed074p1374. [DOI] [Google Scholar]
- Järup L. Hazards of heavy metal contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- Jothi JS, Uddin MB. Detection of heavy metals in some commercial brands of noodles. Eur. Acad. Res. 2014;2:10667–10679. [Google Scholar]
- Kang TH, Park HM, Kim YB, Kim H, Kim N, Do JH, Kang C, Cho Y, Kim SY. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J. Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Kang YS, Hong KP, Jung DC, Hong S, Lee JH, Nah SY, Lim Y, Bae DH. Calcium channel-blocking activity of Chinese balloon flower (Platycodon grandiflorum) for producing Blood pressure-lowering functional foods. Food Sci. Biotechnol. 2008;17:156–160. [Google Scholar]
- Karak T, Bhagat RM. Trace elements in tea leaves, made tea and tea infusion: a review. Food Res. 2010;43:2234–2252. doi: 10.1016/j.foodres.2010.08.010. [DOI] [Google Scholar]
- Khan IA, Good JA, Walker LA, Abourashed EA, Schlenk D, Benson WH. Determination of heavy metals and pesticides in Ginseng products. J. AOAC Int. 2001;84:936–939. doi: 10.1093/jaoac/84.3.936. [DOI] [PubMed] [Google Scholar]
- Kim JK, Lee JH, Kim JE, Bae IA, Kim KS, Lee ES, Kwon SD, Park JH, Lee KS. Investigation of heavy metal contents by milling degrees of rice. Korean J. Environ. 34: 303-308 (2015)
- Korea Centers for Disease Control and Prevention (CDC) Guideline for the evaluation of the fourth Korea national health and nutrition examination survey. Osong: Korea Centers for Disease Control and Prevention; 2010. [Google Scholar]
- Kwon JH, Lee GD, Bélanger JM, Paré JRJ. Effect of ethanol concentration on the efficiency of extraction of ginseng saponins when using a microwave-assisted process (MAPTM) Int. J. Food Sci. Technol. 2003;38:615–622. doi: 10.1046/j.1365-2621.2003.00688.x. [DOI] [Google Scholar]
- Lago-Vanzela ES, Da-Silva R, Gomes E, Garcia-Romero E, Hermosin-Gutiérrez I. Phenolic composition of the edible parts (flesh and skin) of Bordô Grape (Vitis labrusca) using HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2011;59:13136–13146. doi: 10.1021/jf203679n. [DOI] [PubMed] [Google Scholar]
- Larsen EH, Andersen NL, Moller A, Petersen A, Mortensen GK, Petersen J. Monitoring the content and intake of trace elements from food in Denmark. Food Addit. Contam. 2002;19:33–46. doi: 10.1080/02652030110087447. [DOI] [PubMed] [Google Scholar]
- Memon SQ, Hasany SM, Bhanger MI, Khuhawar MY. Enrichment of Pb(II) ions using phthalic acid functionalized XAD-16 resin as a sorbent. J. Colloid Interface Sci. 2005;291:84–91. doi: 10.1016/j.jcis.2005.04.112. [DOI] [PubMed] [Google Scholar]
- Onakpa MM, Njan AA, Kalu OC. A review of heavy metal contamination of food crops in Nigeria. Ann. Glob. Health. 2018;84:488–494. doi: 10.29024/aogh.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey G. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int. J. Biochem. Cell B. 2009;41:1665–1677. doi: 10.1016/j.biocel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Roussel C, Néel C, Bril H. Minerals controlling arsenic and lead solubility in an abandoned gold mineral mine tailings. Sci. Total Envion. 2000;263:209–219. doi: 10.1016/S0048-9697(00)00707-5. [DOI] [PubMed] [Google Scholar]
- Subbiah S, Oates RP, Dhanakodi K, Annamalai SK, Muraleedharan N. Impact of brewing time on heavy metal leaching in black tea from South India. Austin Environ. Sci. 2017;2:1–4. [Google Scholar]
- Taverniers L, Loose MD, Bockstaele EV. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal. Chem. 2004;23:535–552. doi: 10.1016/j.trac.2004.04.001. [DOI] [Google Scholar]
- Tian D, Zhu F, Yan W, Fang X, Xiang W, Deng X, Wang G, Peng C. Heavy metal accumulation by panicled goldenrain tree (Koelreuteria paniculata) and common elaeocarpus (Elaeocarpus decipens) in abandoned mine soils in southern China. J. Environ. Sci. 2009;21:340–345. doi: 10.1016/S1001-0742(08)62274-3. [DOI] [PubMed] [Google Scholar]
- Van der Sluis AA, Dekker M, Skrede G, Jongen WMF. Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. J. Agric. Food Chem. 2002;50:7211–7219. doi: 10.1021/jf020115h. [DOI] [PubMed] [Google Scholar]
- Wolfe K, Wu X, Liu RH. Antioxidnat activity of apple peels. J. Agric. Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). Summary report of the seventy-third meeting of JECFA. Joint FAO/WHO expert committee on food additives, Geneva (2010) http://www.who.int/foodsafety/publications/chem/summary73.pdf
- Xia EQ, Deng GF, Guo YJ, Li HB. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010;11:622–646. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Lee HK, Lee SY, Kim SG, Kim GB. Heavy metal contents and food safety assessment of processed seaweeds and cultured lavers. J. Korean Soc. Mar. Environ. Energy. 2016;19:203–210. doi: 10.7846/JKOSMEE.2016.19.3.203. [DOI] [Google Scholar]
- Zhuang P, McBride MB, Xia H, Li N, Li Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009;407:1551–1561. doi: 10.1016/j.scitotenv.2008.10.061. [DOI] [PubMed] [Google Scholar]