Abstract
Silkworm pupae oil (SPO) has been reported to have various biological activities in improving blood circulation. However, the protective action of SPO against vascular disorders remains unknown. A new formulation of SPO was prepared through an esterification and saponification process. The composition of unsaturated fatty acids in silkworm pupae oil sodium salt (SPOS) was then analyzed by LC/MS to show α-linolenic acid (11.0%), linoleic acid (73.2%), palmitic acid (3.1%), oleic acid (12.0%), and stearic acid (0.7%). The in vitro studies were performed to find out the efficacy of SPOS on platelet-derived growth factor (PDGF-BB) induced vascular smooth muscle cell (VSMC) migration and proliferation. PDGF-BB (10 ng/mL) induced abnormal migration and proliferation of VSMCs, whereas exposure to SPOS (30 μg/mL) significantly reduced the PDGF-BB-induced cell migration and proliferation. The extracellular signal-regulated kinase1/2 (ERK1/2) and phosphorylation of ERK1/2 were determined by immunoblot analysis and the ERK1/2 phosphorylation in PDGF-BB-stimulated VSMCs was downregulated by SPOS (30 μg/mL) treatment. These results indicate that SPOS may be a helpful and useful agent as a functional food and drug against vascular disorders.
Keywords: Silkworm pupae oil sodium salt (SPOS), α-Linolenic acid, Cholesterol, PDGF-BB, ERK1/2
Introduction
Cardiovascular disease is a major factor of mortality worldwide, and tightly involves the development of atherosclerotic lesions (Schuett et al., 2015). Atherosclerosis is defined as coronary stenosis induced by monocyte-mediated inflammation, endothelial dysfunction and hyperplasia of vascular smooth muscle cells (VSMCs) in the arterial wall, and may be life threatening by advancement to acute myocardial infarction (Minamino and Komuro, 2002; Wolf et al., 2014). In clinical therapy for acute coronary artery disease, percutaneous coronary intervention such as stent therapy is widely utilized worldwide (Marx et al., 2011). Despite successful treatment, 20–30% of patients are exposed to risk factors for restenosis. However, a comprehensive prescription has not been established (Weintraub, 2007).
Abnormal migration and hyperplasia of VSMCs are key events in vascular disorders such as atherosclerosis and restenosis (Newby and Zaltsman, 2000). Especially, platelet-derived growth factors (PDGF) stimulate the activation of mitogen-activated protein kinases (MAPKs) underlying the extracellular signal-regulated kinase (ERK) 1/2 which regulate these pathogenic phenomenon (Kavurma and Khachigian, 2003; Lee et al., 2015; Ross et al., 1986). Therefore, a possible therapy for the prevention of restenosis could be achieved by the inhibition of ERK1/2 phosphorylation.
Polyunsaturated fatty acids (PUFAs) have various biological effects such as anti-oxidant, reducing low-density lipoprotein cholesterol levels and improving cardiovascular functions (Bird et al., 2018). Diverse crop seeds and edible insects have mainly polyunsaturated fats (Bitok and Sabaté, 2018). In Asia, the silkworm pupae have been used for sustainable food production systems such as edible ingredients and natural medicine because of their high proteins, essential amino acids, and unsaturated fatty acid contents (Longvah et al., 2011). Particularly, the silkworm pupae oil (SPO) and peptides exhibit pharmaceutical effects including lowering of blood sugar, anti-thrombosis, and inhibition of fat storage in the liver; also, they contain the angiotensin I (Ang I)—converting enzyme inhibitory activity (Wei et al., 2009; Wu et al., 2015; Yeo et al., 2013; Zou et al., 2017). The composition of bioactive compounds in silkworm pupae oil such as amino acid, fatty acid, and α-linolenic acid has been recently studied (Cho et al., 1983; Kim et al., 2010; Longvah et al., 2012; Terés et al., 2008). Other reports have shown that linolenic acid activates inflammation whereas oleic acid generates nitric oxide from endothelial cells (Saraswathi et al., 2004; Young et al., 1998). However, the inhibitory effects of silkworm pupae oil (SPO) on VSMCs on migration and proliferation have not been established and it is necessary to improve the unpleasant appearance of silkworm pupa and the odor of the oil treatment process. Therefore, we investigated the stable structure from silkworm pupae oil and prepared sodium salt of silkworm pupae oil due to its high aqueous solubility and easy preparation. Here we report SPOS preparation and their biological effects on vascular disorders.
Materials and methods
Materials
Silkworm pupae oil was provided by CH Chemical Co. (Seoul, Korea). Sodium hydroxide (NaOH) and methanol were purchased from Daejung (Siheung, Korea). Cell culture materials were obtained from Gibco BRL (Gaithersburg, MD, USA). The EZ-cytox cell viability assay kit was supplied by Daeil Lab Service (Seoul, Korea). β-Actin, extracellular signal-regulated kinase1/2 (t-ERK1/2), and phosphorylation of extracellular signal-regulated kinase1/2 (p-ERK1/2) antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). All other chemicals were purchased from Sigma Corporation.
Preparation of silkworm pupae oil sodium salt (SPOS) and SPOS tablet
Synthesis of silkworm pupae oil methyl ester (SPOM)
Silkworm pupae oil (30 g) was added to a solution comprising of hydrochloric acid (1 mL) in methanol (400 mL), and the mixed solution was stirred at 60 °C for 12 h. At the end of the reaction, methanol was removed with vacuum evaporator and the remaining mixture was neutralized with 50 mL of aqueous NaHCO3 (50%, w/w). The product was extracted with hexane and dried over MgSO4 to give a pale yellow liquid.
Synthesis of silkworm pupae oil sodium salt (SPOS)
SPOM (5 g) was added to 50 mL of NaOH aqueous solution (10%, w/w) and stirred at 120 °C for 3 h. After cooling the reaction mixture, 100 mL of isopropyl ether was added to the liquid mixture and the organic layer was separated. Acetonitrile (20 mL) was added to the organic layer, and the mixture was concentrated with vacuum evaporator at 30 °C. The obtained solid was filtered and washed with cold dichloromethane. The wet solid was dried with vacuum drier at 60 °C for 12 h to give a pale white solid of SPOS.
Preparation of silkworm pupae oil sodium salt (SPOS) tablet
SPOS tablets were prepared by the direct compression method. The SPOS (1.0 g) was mixed with magnesium stearate (0.5 g), cellulose (1.5 g), lactose (7.0 g), and citric acid (1.0 g). The resultant powder mixture was directly compressed by a tablet compression machine.
Characterization of chrysalis oil sodium salt
1H NMR spectroscopy
Silkworm pupae oil, the methyl ester (SPOM) and the sodium salt (SPOS) were characterized by Bruker NMR (Billerica, MA, USA). Silkworm pupae oil and methyl ester were dissolved in methanol-d4 whereas sodium salt was dissolved in D2O for 1H NMR analysis.
FTIR analysis
The FTIR spectra of silkworm pupae oil methyl ester and sodium salt were characterized by FTIR-6300 (Easton, MD, USA) with a range from 1400 to 3000 cm−1. To obtain the spectra, KBr was added to the SPOM and SPOS and pressed into a pellet for analysis.
Differential scanning calorimetry (DSC) analysis
The thermal endothermic and exothermic behavior of SPOS was measured by Netzsch DSC (Düsseldorf, Nordrhein-Westfalen, Germany). The heating rate was fixed at 5 K/min and the flow rate of pure argon gas (Ar, 99.9999%) was maintained at 50 mL/min for DSC measurements.
Measurement of critical micelle concentration (CMC)
The critical micelle concentration (CMC) of silkworm pupae oil sodium salt was analyzed by UV/Visible spectrophotometer (Optizen POP, Seoul, Korea). The absorbance at each concentration of an aqueous solution of SPOS with benzoyl acetone (7.0 x10−5 M) was measured at 312 nm. The absorbance at each pH of aqueous solution of SPOS with benzoyl acetone was also measured at 312 nm.
Dissolution test of silkworm pupae oil sodium salt
The tablet of silkworm pupae oil sodium salt was dissolved in 900 mL of distilled water and stirred with a paddle at 37 °C for 2 h. At predetermined time points, 1 mL of the solution was collected, and absorbance was measured at 254 nm. When the absorbance reached a plateau, it was established that the solution was completely dissolved. The dissolution curve of SPOS tablet was determined by operating the dissolution tester (EDT-08Lx, ELECTROLAB, Mumbai, India).
Composition of unsaturated fatty acid in SPOS
The composition of unsaturated fatty acids in SPOS was analyzed by LC–MS (UPLC/1290 Infinity and MS Q-TOF/G6550A (Agilent Technologies Inc, Palo Alto, CA, USA)) equipped with an electrospray ionization (ESI) source. Agilent C18 extend column (150 × 2.1 mm I.D.; 1.7 μm) was used to separate fatty acids. Flow rates were maintained at 0.3 mL/min. Solvent A was 0.1% formic acid/water, and solvent B was 0.1% formic acid/acetonitrile. Gradient separations were performed from 15 to 50% of solvent B for 40 min, followed by 50 to 15% solvent B for 20 min. The injection volume was 2 μL. The flow of liquid chromatography was introduced into the mass spectrometry source. The MS capillary voltage was 3.5 kV.
Cell culture and proliferation assay
The cell culture and proliferation assay was performed as previously reported (Lee et al., 2014). Cell culture materials were obtained from Gibco BRL (Gaithersburg, MD, USA). Rat VSMCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin, and maintained at 37 °C in a 5% CO2 atmosphere. Cells were seeded at a density of 2 × 103 cells/mL per well in a 96-well microplate containing FBS-supplemented DMEM and incubated for 24 h, after which they were incubated in a serum deficient media for a further 24 h. Cells were exposed to varying concentrations of SPOS (3, 10, and 30 µg/mL) in either PDGF-BB-containing or PDGF-BB-deficient DMEM, and incubated for 24 h. The cell viability was calculated by comparing to SPOS untreated control cells at 450 nm. Cell proliferation was determined using an EZ-cytox cell viability assay kit.
Scratch wound healing assay
Scratch wound healing assay was performed as previously reported (Lee et al., 2014). VSMCs were seeded at a density of 4 × 104 cells/mL per well in 6-well plates, and incubated in FBS-supplemented medium for 24 h. The medium was substituted with a serum-free medium. After serum starvation for 24 h, a scratch wound was made using a sterile 200-µL pipette tip, across the monolayer of cells in each well. This was followed by incubation with or without the SPOS (3, 10, and 30 µg/mL) in either PDGF-BB or PDGF-BB-deficient DMEM for an additional 24 h. Images of the cells were obtained by an inverted microscope (IX71; Olympus, Tokyo, Japan) equipped with a charge-coupled device (CCD) camera. The migration distance was determined using the Image J software package. The scratch wound areas were measured by comparing to the SPOS untreated control cells in PDGF-BB-deficient media.
Western blot analysis
Western blot assay was performed as previously reported (Lee et al., 2016). To analyze protein expression, we performed Western blotting using specific antibodies. Briefly, 20 µg of protein was used for electrophoresis. After boiling, the separated proteins on 12% polyacrylamide gels were transferred onto polyvinylidene difluoride membranes in transfer buffer at 4 °C for 2 h. 5% of bovine serum albumin (BSA) in tris-buffered saline (TBS) was used to prevent non-specific protein binding at room temperature for 1 h, following which it was washed in TBS with 0.1% Tween 20 (TBS/T). The membranes were incubated overnight at 4 °C with either t-ERK1/2 antibody, or p-ERK1/2 antibody, both at a 1:2000 dilution. The membranes were washed with TBS/T, followed by incubation with 1:5000 diluted IgG secondary antibody conjugated to horseradish peroxidase. The expression levels of proteins were analyzed using chemiluminescence (ECL plus kit; Amersham Pharmacia Biotech). The developed protein bands were visualized and quantified using the Image J Software.
Sprout-out growing test
The sprout-out growing tests were carried out as previously described (Lee et al., 2016). Briefly, the endothelium and adventitium of the aorta from SD rats (5-weeks-old, n = 3) were removed mechanically and enzymatically, and the internal vessels were cut into 1-mm2 strips. The strips were embedded in 48-well plates coated with matrigel, after which they were subjected to PDGF-BB (10 ng/mL) and SPOS (30 μg/mL) treatment in FBS-free DMEM for 5 days. The strips were stained with Diff-Quik (Baxter Healthcare) and photographed, and the length of sprout was measured using the Image J software.
Statistical analysis
The results are expressed as the mean ± standard error (SE) of at least three independent experiments. Student’s t test compared differences between the two groups, whereas one-way analysis of variance (ANOVA) with Tukey’s test was used for multiple comparisons (Prism 4.00 for Windows, GraphPad, San Diego, CA, USA). A P-value of < 0.05 was considered as statistically significant.
Results and discussion
Establishment of powder formation from silkworm pupae oil
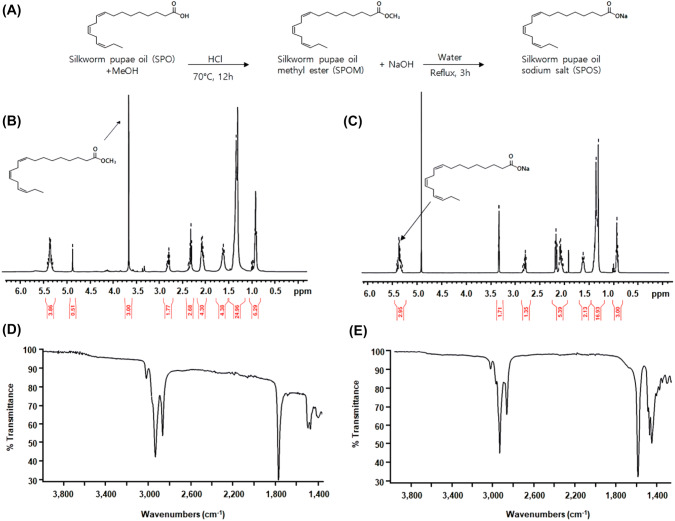
The SPOS were synthesized as shown in Fig. 1(A). The procedure of SPO modification has two steps: (1) esterification and (2) saponification. For esterification from SPO, the first chemical reaction of SPO involved treatment with methanol and HCl at 60 °C for 12 h. Modification of the pupae oil into silkworm pupae oil methyl ester (SPOM) was determined by 1H NMR (Fig. 1(B)). The signals centered at 0.91, 1.35, and 1.65 ppm were attributable to the methyl protons of the stearic acid chain, and to the protons of the methyl group in the linolenyl chain, respectively. The multiplet centered at ca. 2.10 and 2.35 ppm were assigned to the methylene allylic protons of the linoleic and linolenyl chain, while the multiplet centered at 2.84 ppm was assigned to the methylene bis-allylic protons of the linolenyl chain. The spectra showed a well-distinguishable peak as singlet at 3.65 ppm, attributable to the protons of the methyl group in SPOM. The resonance ca.5.36 ppm was assigned to the olefinic protons of the unsaturated fatty acid component. Next, for the saponification of SPOM, the solution was refluxed with NaOH and water for 3 h. After cooling the mixture, organic solvents such as isopropyl ether and acetonitrile were used as the aqueous methanolic solution in order to solidify SPOS. After filtration and washing with aqueous volatile solvent, the wet solid was dried under reduced pressure to give a pale white solid of SPOS. As shown in Fig. 1(C), SPOS was identified by 1H NMR. There was no peak at 3.65 ppm, which was observed in the counterpart 1H-NMR spectra of SPOM meaning hydrolysis of methyl ester. Other peaks of SPOS from 0.91 ppm to 2.84 ppm were similar to those of SPOM. In all the NMR spectra recorded, we identified that SPOS was synthesized from SPOM by saponification. SPOM was obtained in liquid form, while SPOS was obtained in solid form. This means that SPOS can be formulated with tablets. Here we first applied SPOS to make tablet.
Fig. 1.
The characterization of silkworm pupae oil. (A) The reaction scheme of silkworm pupae oil. (B) 1H-NMR of silkworm pupae oil methyl ester (SPOM). (C) 1H-NMR of silkworm pupae oil sodium salt (SPOS). (D) FTIR spectra of silkworm pupae oil methyl ester (SPOM). (E) FTIR spectra of silkworm pupae oil sodium salt (SPOS)
Characterization of SPOS by FTIR
The FTIR spectra of SPOM and SPOS are shown in Fig. 1(D) and 1(E). The methyl ester peaks were detected at 1760 cm−1 (Fig. 1(D)). However, no peak was observed at the same position in Fig. 1(E), indicating the disappearance of methyl ester. The peak at 1595 cm−1 in Fig. 1(E) was identified as the strong C=O stretching of carboxylic sodium salt. Therefore, we suggest that the process of SPOS generation were successfully established.
Fatty acid composition of SPOS
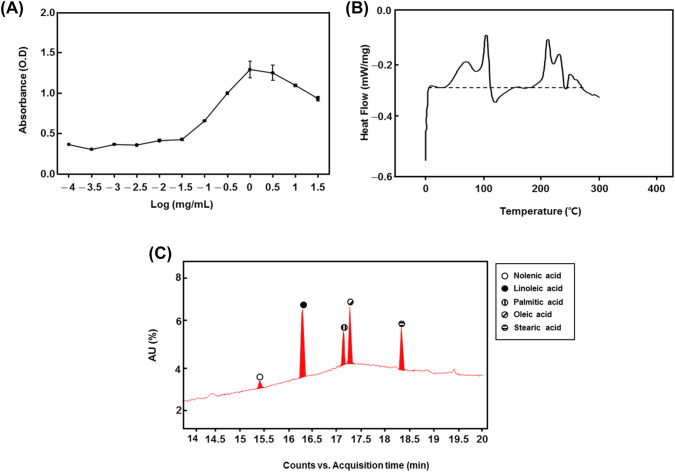
DSC and LC–MS were performed to analyze the fatty acid composition of SPOS (Fig. 2(B) and 2(C)). A total of 5 constituent fatty acids were detected, which are listed in Table 1: α-linolenic acid (11.0%), linoleic acid (73.2%), palmitic acid (3.1%), oleic acid (12.0%), and stearic acid (0.7%).
Fig. 2.
Characterization of SPOS. (A) The measurement of critical micelle concentration (CMC) of silkworm pupae oil sodium salt (SPOS); (B) The analysis of differential scanning calorimetry (DSC) of SPOS; (C) LC–MS analysis of SPOS. The circle indicates the major peaks within 15.01–18.5 min, respectively
Table 1.
LC/MS data of silkworm pupae oil sodium salt (SPOS)
| Sample E (SPOS) | [M-H]-(m/z) | Area of EIC peak | Area % |
|---|---|---|---|
| α-Linolenic acid | 277.2173 | 3,373,125 | 11 |
| Linoleic acid | 279.233 | 22,345,656 | 73.2 |
| Palmitic acid | 255.233 | 958,094 | 3.1 |
| Oleic acid | 281.2486 | 3,650,890 | 12 |
| Stearic acid | 283.2643 | 209,816 | 0.7 |
| Total | 30,537,581 | 100 | |
From the LC–MS study, we identified 5 active fatty acids such as α -linolenic acid, linoleic acid, palmitic acid, oleic acid, and stearic acid. The fatty acid can reduce the levels of ROS-induced oxidative stress (Duval et al., 2002). In particular, Nowak et al. (2017) demonstrated that elevated ROS and oxidative stress may induce atherosclerosis through inducing abnormal migration and proliferation of VSMC. Therefore, we suggest that SPOS can contribute to cardiovascular disease prevention by reducing oxidative stress.
SPOS attenuates the migration and proliferation of PDGF-BB-stimulated VSMCs
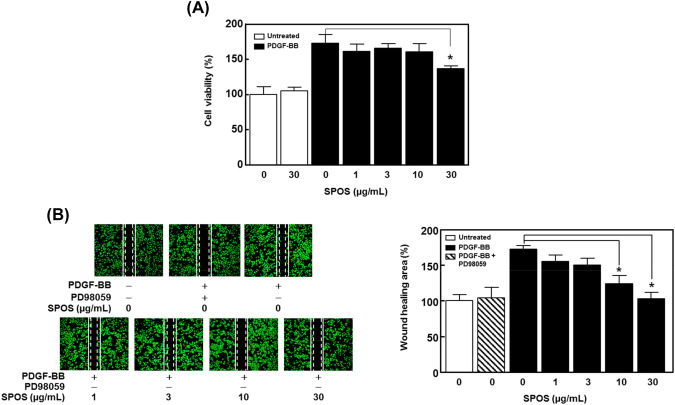
To explore the effect of SPOS on PDGF-induced abnormal migration and proliferation of VSMCS, we first evaluated for cell proliferation by performing the XTT reaction. As shown in Fig. 3(A), PDGF-BB (10 ng/mL) significantly increases the cell growth, whereas SPOS (30 μg/mL) significantly regulates the PDGF-BB-induced cell proliferation (n = 4). Next, we carried out the scratch wound healing assay to assess whether SPOS regulates the PDGF-BB-induced migration of VSMCs. The PD98059, used to prevent the SMC migration through the ERK 1/2, was used as a positive control. As shown in Fig. 3(B), PDGF-BB (10 ng/mL) increased VSMC proliferation by 72.69 ± 1.98% compared with the untreated group. In the presence of SPOS, PDGF-BB-induced VSMC proliferation was reduced to 48.89 ± 3.69% for the 10 μg/mL treatment and to 69.95 ± 2.08% with 30 μg/mL of SPOS. Abnormal proliferation and migration of VSMCs are major causes of atherosclerosis and restenosis (Shah, 2003). In our results, SPOS significantly reduced PDGF-BB-induced migration and proliferation suggesting that SPOS may be a good candidate for the treatment of vascular disorders.
Fig. 3.
Effect of SPOS on migration and proliferation of PDGF-BB stimulated VSMCs. VSMCs were treated with 30 µg/mL of SPOS and cultured in the presence or absence of PDGF-BB (10 ng/mL). (A) The graph shows the representative cell proliferation from four independent experiments. (B) The figures show the wound healing assay. Dotted white lines indicate the initial scratches and solid white lines indicated migrated cell (n = 3). Results are presented as mean ± standard error. Values are not significantly different based on Tukey’s multiple range test (P < 0.05)
SPOS regulates the PDGF-BB-induced phosphorylation of ERK 1/2 in VSMCs
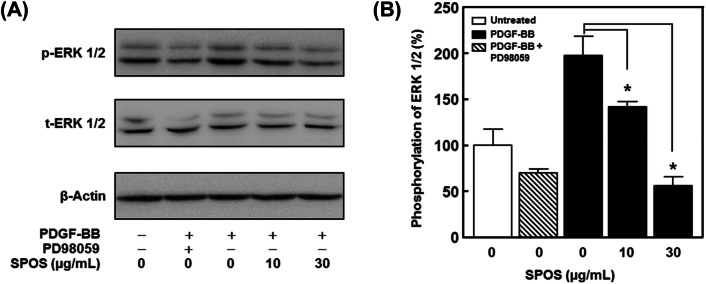
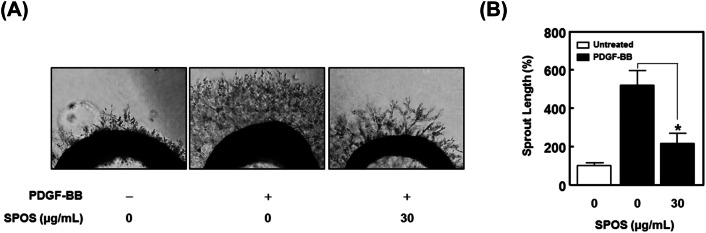
We further determined the effect of SPOS on ERK1/2 phosphorylation in PDGF-BB-stimulated VSMCs. As shown in Fig. 4(B), the PDGF-BB-induced ERK1/2 phosphorylation was reduced in a dose dependent manner to 55.38 ± 8.90% and 141.72 ± 6.29% after treatment with 10 μg/mL and 30 μg/mL of SPOS. Furthermore, the treatment of aortic rings with SPOS (30 μg/mL) inhibited PDGF-BB-induced sprout outgrowth (Fig. 5).
Fig. 4.
The effect of SPOS on migration and proliferation of PDGF-BB stimulated VSMCs. (A) Cell lysates were analyzed by immunoblotting with specific antibodies. (B) Statistical results obtained from panel A. The band intensities were evaluated and indicated the effect of SPOS on PDGF-BB-activated ERK1/2 phosphorylation (p-ERK). These values were normalized to total ERK1/2 (t-ERK 1/2). The basal levels of phosphorylation are deemed to be 100% (n = 3). Results are presented as mean ± standard error. Values are not significantly different based on Tukey’s multiple range test (P < 0.05)
Fig. 5.
Effect of SPOS on PDGF-BB-induced aortic sprout growth. (A) The results were observed on day 5. (B) Statistical results obtained from panel A. The level of the untreated group was expressed as 100% (n = 3). Results are presented as mean ± standard error. Values are not significantly different based on Tukey’s multiple range test (P < 0.05)
Regulating the ERK1/2 phosphorylation is of particular importance, since it is involved in the migration and proliferation of VSMC in response to PDGF-BB (Ross et al., 1986). Although much research has focused on the energy sources of unsaturated fatty acids, there is increasing evidence that unsaturated fatty acid directly regulates cell signaling in vascular diseases. Lee et al. (2007). reported that activated- ERK1/2 regulates the pathogenesis of VSMCs. As described above, our data indicates that SPOS significantly down regulated ERK1/2 phosphorylation. Therefore, we suggest that SPOS has the potential to inhibit abnormal migration and proliferation of VSMCs.
Through this study, we demonstrate that the modified sodium salt of silkworm pupae oil (SPOS) containing active compounds such as linolenic acid, linoleic acid, palmitic acid, oleic acid, and stearic acid attenuates the PDGF-BB-induced migration and proliferation of SMCs. The anti-proliferation and anti-migration effects of SPOS are associated with the downregulation of phosphorylation of ERK 1/2 expression. Taken together, our results indicate that application of SPOS may be a promising approach to realize a more effective outcome in patients with atherosclerosis.
Acknowledgements
This research was supported by the academic research fund of Hoseo University in 2016 (2016-0305).
Compliance with ethical standards
Conflict of interest
None of the authors of this study has any financial interest or conflict with industries or parties.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Young Jin Kim, Email: kyg2991kr@naver.com.
Kang Pa Lee, Email: gostop48@konkuk.ac.kr.
Do Young Lee, Email: leedoyeong@naver.com.
Yun Tae Kim, Email: ssarima2@naver.com.
Suji Baek, Email: baeksooji@konkuk.ac.kr.
Myeong Sik Yoon, Email: msyoon@hoseo.edu.
References
- Bird JK, Calder P, Eggersdorfer M. The role of n-3 long chain polyunsaturated fatty acids in cardiovascular disease prevention, and interactions with statins. Nutrients. 2018;10:E775. doi: 10.3390/nu10060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitok E, Sabaté J. Nuts and cardiovascular disease. Prog. Cardiovasc. Dis. 2018;61:33–37. doi: 10.1016/j.pcad.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Cho S, Yang S, Cha W. Studies on the amino acid and fatty acid composition of white grub and Chrysalis of silkworm. Ind. Tech. Res. 1983;83:105–116. [Google Scholar]
- Duval C, Augé N, Frisach MF, Casteilla L, Salvayre R, Nègre-Salvayre A. Mitochondrial oxidative stress is modulated by oleic acid via an epidermal growth factor receptor-dependent activation of glutathione peroxidase. Biochem. 2002;367:889–894. doi: 10.1042/bj20020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavurma MM, Khachigian LM. ERK, JNK, and p38 MAP kinases differentially regulate proliferation and migration of phenotypically distinct smooth muscle cell subtypes. J. Cell. Biochem. 2003;89:289–300. doi: 10.1002/jcb.10497. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park J, Zhoh C, Hong S, Ryu K. The component analysis of male silkworm’s extract. J. Kor. Soc. Esthe. Cosm. 2010;5:37–45. [Google Scholar]
- Lee CK, Lee HM, Kim HJ, Park HJ, Won KJ, Roh HY, Choi WS, Jeon BH, Park TK, Kim B. Syk contributes to PDGF-BB-mediated migration of rat aortic smooth muscle cells via MAPK pathways. Cardiovascular. Research. 2007;74:159–168. doi: 10.1016/j.cardiores.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Lee KP, Park ES, Kim DE, Park IS, Kim JT, Hong H. Artemisinin attenuates platelet-derived growth factor BB-induced migration of vascular smooth muscle cells. Nutr. Res. Pract. 2014;8:521–525. doi: 10.4162/nrp.2014.8.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Sudjarwo GW, Jung SH, Lee D, Lee DY, Lee GB, Baek S, Kim DY, Lee HM, Kim B. Carvacrol inhibits atherosclerotic neointima formation by downregulating reactive oxygen species production in vascular smooth muscle cells. Atherosclerosis. 2015;240:367–373. doi: 10.1016/j.atherosclerosis.2015.03.038. [DOI] [PubMed] [Google Scholar]
- Lee KP, Kim JE, Kim H, Chang HR, Lee DW, Park WH. Bo-Gan-Whan regulates proliferation and migration of vascular smooth muscle cells. BMC. Complement. Altern. Med. 2016;16:306–314. doi: 10.1186/s12906-016-1292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longvah T, Mangthya K, Ramulu P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food. Chem. 2011;128:400–403. doi: 10.1016/j.foodchem.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Longvah T, Manghtya K, Qadri SS. Eri silkworm: a source of edible oil with a high content of α-linolenic acid and of significant nutritional value. J. Sci. Food. Agric. 2012;92:1988–1993. doi: 10.1002/jsfa.5572. [DOI] [PubMed] [Google Scholar]
- Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ. Cardiovasc. Interv. 2011;4:104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Komuro I. Role of telomere in endothelial dysfunction in atherosclerosis. Curr. Opin. Lipidol. 2002;13:537–543. doi: 10.1097/00041433-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J. Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Nowak WN, Deng J, Ruan XZ, Xu Q. Reactive Oxygen Species Generation and Atherosclerosis. Arterioscler Thromb Vasc. Biol. 2017;37(5):e41–e52. doi: 10.1161/ATVBAHA.117.309228. [DOI] [PubMed] [Google Scholar]
- Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Saraswathi V, Wu G, Toborek M, Hennig B. Linoleic acid-induced endothelial activation role of calcium and peroxynitrite signaling. J. Lipid. Res. 2004;45:794–804. doi: 10.1194/jlr.M300497-JLR200. [DOI] [PubMed] [Google Scholar]
- Schuett KA, Lehrke M, Marx N, Burgmaier M. High-risk cardiovascular patients: clinical features, comorbidities, and interconnecting mechanisms. Front. Immunol. 2015;6:591–600. doi: 10.3389/fimmu.2015.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK. Inflammation, neointimal hyperplasia, and restenosis: as the leukocytes roll, the arteries thicken. Circulation. 2003;10:2175–2177. doi: 10.1161/01.CIR.0000069943.41206.BD. [DOI] [PubMed] [Google Scholar]
- Terés S, Barceló-Coblijn G, Benet M, Alvarez R, Bressani R, Halver J, Escriba P. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13811–13817. doi: 10.1073/pnas.0807500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZJ, Liao AM, Zhang HX, Liu J, Jiang ST. Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology. Bioresour. Technol. 2009;100:4214–4219. doi: 10.1016/j.biortech.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Weintraub WS. The pathophysiology and burden of restenosis. Am. J. Cardiol. 2007;100:S3–S9. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Wolf D, Stachon P, Bode C, Zirlik A. Inflammatory mechanisms in atherosclerosis. Hämostaseologie. 2014;34:63–71. doi: 10.5482/HAMO-13-09-0050. [DOI] [PubMed] [Google Scholar]
- Wu Q, Jia J, Yan H, Du J, Gui Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: biochemical characterization and molecular docking study. Peptides. 2015;68:17–24. doi: 10.1016/j.peptides.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Yeo YH, Cho MH, Jeon BD, Seo HB, Kwon TD, Ryu SP. Changes of pupa powder ingestion on inflammatory cytokine in rats. J. Exerc. Nutr. Biochem. 2013;17:71–80. doi: 10.5717/jenb.2013.17.3.71. [DOI] [Google Scholar]
- Young VM, Toborek M, Yang F, Mcclain CJ, Hennig B. Effect of linoleic acid on endothelial cell inflammatory mediators. Metabolism. 1998;47:566–572. doi: 10.1016/S0026-0495(98)90241-4. [DOI] [PubMed] [Google Scholar]
- Zou Y, Hu T, Shi Y, Liao S, Liu J, Mu L, Chen C. Silkworm pupae oil exerts hypercholesterolemic and antioxidant effects in high-cholesterol diet-fed rats. J. Sci. Food. Agr. 2017;97:2050–2056. doi: 10.1002/jsfa.8009. [DOI] [PubMed] [Google Scholar]