Abstract
Black ginseng (BG), which is produced by repeated steaming and drying of fresh ginseng, has various pharmacological and therapeutic properties. This study investigated the anti-hyperglycemic and hypolipidemic effects of BG ethanolic extract in type 2 diabetic db/db mice. The levels of fasting blood glucose, HbA1c, insulin levels and thiobarbituric acid reactive substances values were decreased in the groups fed BG extract (BG) (100 and 900 mg/kg BW/day), compared to the control group (CON). In the BG compared with the CON, hepatic steatosis in the liver and the size of adipocytes in muscle tissue were improved. The administration of BG regulated the glucose transporter type (GLUT) 4 and 2, and peroxisome proliferator-activated receptor (PPAR) α and γ in muscle and liver. Moreover, ginsenosides (Rh4, Rg5, and Rk1), which profiled by HPLC, regulated the markers for lipid metabolism and glucose metabolism; PPARs and GLUTs in muscle and C2C12 rather than liver cells and tissue. These findings suggested that ginsenosides (Rh4, Rg5, and Rk1) from BG extract can ameliorate type 2 diabetes through their anti-hyperglycemic and hypolipidemic effects.
Electronic supplementary material
The online version of this article (10.1007/s10068-020-00753-3) contains supplementary material, which is available to authorized users.
Keywords: Black ginseng, Anti-hyperglycemic, Hypolipidemic, Type 2 diabetic db/db mice
Introduction
Diabetes mellitus (DM) is a complex and chronic metabolic disease that caused by defects of insulin secretion or action (Herder et al., 2007). Data from the International Diabetes Federation Atlas indicated that more than 415 million people have been diagnosed with diabetes and expected that the number of diabetes patients have over 642 million by 2040 (Herder et al., 2007). There are two types of diabetes, type 1 and 2, generally. Type 2 diabetes mellitus (T2DM) accounted for 90% of diabetic patients. Diabetes is steadily increasing but the development of therapies is insufficient (Riddy et al., 2018). There is no perfect cure for insulin management, and worse yet, the wrong approach will lead to unnecessary complications and shorten the death period (Riddy et al., 2018). Previous and current diabetes therapies centered on restoring blood glucose levels to normal levels and functioning of the pancreatic β-cells (Riddy et al., 2018).
Hyperglycemia is an important factor in diagnosing diabetes and occurrence of diabetic complications. Therefore, the compounds that normalize blood glucose through the glucose uptake and disposition in the skeletal muscle, liver, and adipose have been studied extensively (Seo et al., 2016). Skeletal muscle is the main part of glucose utilization and is closely related to the improvement of hyperglycemia (Shimoda et al., 2015). The regulation of the blood glucose level is maintained by the action of various vital organs. Glucose transporters (GLUTs) regulated the glucose flux between blood and other organs such as liver and muscle (Kang et al., 2017). Glucose uptake in the muscles is mainly promoted by GLUT4, and GLUT4 released leads to increased use of glucose by stimulation of insulin (Holman and Kasuga, 1997). In comparison, GLUT2 is the main glucose transporter between the blood and liver (Thorens, 2015). Gluconeogenesis and glycogen storage in the liver are important parameters of glucose homeostasis. Previous studies have shown that activated adenosine monophosphate-activated protein kinase (AMPK) signaling pathway increases GLUT4 translocation stimulated by insulin and contraction in the muscles (Kim et al., 2013). Activated AMPK inhibited gluconeogenesis in the liver and could promote glucose uptake in peripheral tissues (Steinberg and Kemp, 2009). On the other hand, obesity is associated with fatty acid metabolic disorders in skeletal muscles. Activated peroxisome proliferator-activated receptor alpha (PPARα) promotes the activity of mitochondrial enzymes related to lipid oxidation and inhibits the enzymes activity associated with lipogenesis (Desvergne and Wahli, 1999). Also, PPARγ improve insulin sensitivity associated with lipid lowering effects in muscle (Ye et al., 2001).
Ginseng (Panax ginseng Meyer., Araliaceae) has been known as a herb of traditional physiology and function (Gillis, 1997). There are three types of ginseng on the Korean market, i.e., red, white, and black that are decided by surface colors by processes (Seo et al., 2016). Black ginseng (BG), when steamed and dried three-to-nine times undergoes the Maillard browning reaction. The steam-processed ginseng has more powerful pharmacological and biological activities than non-steamed ginseng (Sun et al., 2009). The therapeutic effects of ginseng and its ginsenosides on general health, the immune system, various types of cancer, inflammation, nervous system disorders, reproductive system and cardiovascular diseases have been well studied (Saba et al., 2016). Newly discovered ginsenosides found in BG were pharmacologically more effective than others (Sun et al., 2009).
Previous BG research focused mainly on improving both cell function and insulin resistance (Riddy et al., 2018). This study showed its effect on glucose and lipid. First, we investigated the anti-diabetic effects of BG and the mechanisms associated in type 2 diabetic mice. We also examined the mechanisms involved in the effects on lipid oxidation and glucose metabolism using skeletal muscle C2C12 cells and liver HepG2 cells. A ginsenoside, Rh4, Rg5, and Rk1 was evaluated mainly as the effective compound in BG extract.
Materials and methods
The preparation of BG extract
The 5-year old P. ginseng Mey., which is called as white ginseng (WG), was collected from the Geumsan-gun, Chungcheongnam-do province, South Korea, and were identified by Dr. Prof. Kwon-Woo Park (College of Life Science, Korea University). The voucher specimen (KUH-360) was deposited at Korea University Herbarium. All WG was cleaned with tap water to remove soil and other debris. BG was developed from raw WG by repeatedly steaming in oven (JEIO TECH, FO-600M, Daejeon, South Korea), drying five times at 95 °C for 3 h and then at 60 °C to achieve 15% water content, at which point the roots become black. To prepare the extract powder, BG was crushed and extracted three times in 10 volumes of 70% ethanol at 60 °C for 6 h using heating mantle, then was filtered and lyophilized according to the reported method (Seo et al., 2016).
HPLC
The dried BG was dissolved in 1 mL of methanol. A degassed mobile phase was prepared with acetonitrile (A) and 0.03% trifluoroacetic acid in distilled water (DW) (B). A Kinetex C18 analytical column (250 × 4.6 mm I.D., 5 μm particle size, Phenomenex Inc., CA, USA) was used. The elution conditions are shown in Additional file 1: Table S1. The flow rate was 1.0 mL/min, and the column temperature was set at 40 °C. The injection volume was 10 μL and the detection wavelength was set to 203 nm. The HPLC system consisted of an Agilent Technologies 1260 series with diode array detection (Santa Clara, CA, USA).
Animals and experimental diets
Six-week old male C57BL/KsJ (17.74 ± 0.95 g) and C57BL/KsJ-db/db (29.47 ± 1.44 g) mice were purchased from Central Lab Animal Inc. (Seoul, South Korea) and were allowed free consumption of food and water for 5 weeks. All mice were habituated for 1 week before the experiment. Four mice were allocated in each individual cage randomly and maintained at standard conditions; lighting regimen (12-h light/12-h dark), humidity (70–75%), and temperature (25 ± 2 °C). Every 2 days bedding material was changed. All in vivo experiments were performed in accordance with internationally approved guidelines for non-pathogenic organisms. The protocol was approved by the Korea University Animal Care and Use Committee (Approval No. KUIACUC-2017-2). The minimum number of animals was estimated to obtain statistical significance, and animals (six animals/group) were assigned to five experimental groups, including the control group. During the five-week study period, mice were given food and water ad libitum. Rosiglitazone, which is known to improve symptoms of type 2 diabetic patients, was used as a positive control (Investigators, 2006). C57BL/KsJ wild-type mice were administered DW (WT), and C57BL/KsJ-db/db control mice were orally administered DW (CON), rosiglitazone (0.5 mg/kg BW/day, ROG), and BG extract (100 and 900 mg/kg BW/day, BG100 and BG900) for 5 weeks (once a day). The concentration, duration, and LD50 (15 g/kg) of black ginseng in this study referred to those in the previous studies (Seo et al., 2016; Metwaly et al., 2019). After 5-weeks, mice were euthanized with CO2 inhalation in cage and sacrificed.
Blood biochemical analysis
Blood samples were collected in heparinized tubes before euthanasia. After incubation of blood samples at room temperature, centrifugation was performed at 1663×g for 10 min to obtain serum. Alkaline phosphatase (ALP) and aspartate aminotransferase (AST) were measured from the obtained serum. Creatinine, blood urea nitrogen (BUN), total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides were also measured. Whole blood collected from the five experimental groups was also used to measure hemoglobinA1c (HbA1c) levels. HbA1c was measured using the mouse HbA1c assay kit from Crystal Chem (IL, USA). Insulin concentration in serum was measured by a corresponding mouse commercial ELISA kit (Raybiotech, Inc., Norcross, GA, USA).
Lipid peroxide concentration
A sample of 0.05 g of liver tissue was weighed and homogenized by adding 0.1 M phosphate solution. The homogenized sample was mixed with TBA reagent (30% trichloroacetic acid, 1.5% thiobarbituric acid (TBA), 10 mg/mL of butylated hydroxyl toluene) and heated at 100 °C for 30 min. The supernatant obtained by centrifuging the reaction solution was measured for fluorescence at an excitation wavelength of 530 nm and an emission wavelength of 590 nm. After centrifugation, the precipitate was dissolved in 1 N NaOH and quantified. The results are expressed as the malondialdehyde (MDA) and MDA standard curves, followed by the MDA concentrations.
RNA extraction and real-time polymerase chain reaction
After sacrifice of the five experimental groups, we weighed 0.02 g of liver and muscle tissue and stored samples at − 72 °C. The cells were plated at a density of 1 × 105 cell/mL for C2C12 in six-well plates then incubated for 48 h. The cells were treated with BG extract, Rh4, Rg5, and Rk1 for 8 h. Next, 1 mL of RNAiso plus (Takara, Shiga, Japan) was added to tissues or cells. After that, chloroform and isopropanol were added to obtain mRNA from tissues and cells. DNA was synthesized using the same amount of mRNA after quantification of the separated mRNA using a NanoDrop spectrophotometer (Thermo, MA, USA). The synthesized cDNA was mixed with primers and polymerase, reacted using a real-time PCR (RT-PCR) system, and the amount of mRNA expression of the primers was observed. The quantitative RT-PCR (qRT-PCR) for analyzing mRNA gene expressions were performed using a HiPi Real-time SYBR green master mix (Elpis Biotech, Daejeon, South Korea) and an iQ5 Real-time PCR detection system (Biorad, CA, USA). All data were analyzed using the 2−ΔΔCT Method (Livak and Schmittgen, 2001) and revised with Rn18s as a housekeeping gene.
Histological analysis
To determine the pathological effects of BG extract on liver and muscle tissues, the liver and muscle tissues were fixed with 10% formalin, dehydrated for 24 h, and the tissues were embedded in paraffin. The finished block was cut into 5-μm sections, dried on a slide, and dried with xylene to remove paraffin. The tissue sections were stained with hematoxylin and eosin (H&E) and periodic-acid-Schiff (PAS). Changes in the tissues were examined with an optical microscope.
Cell culture
The mouse myoblast cell line C2C12 was obtained from the Korean Collection for Type Cultures (KCTC, Seoul, South Korea), and the human hepatoblastoma cell line HepG2 was obtained from ATCC (Manassas, VA, USA). C2C12 and HepG2 cells were maintained in Dulbecco’s Modified Eagle’s medium, DMEM supplemented with 10% fetal bovine serum at 37 °C in a humidified incubator containing 5% CO2. C2C12 and HepG2 cells were sub-cultured every 3 days, and media were changed every 3 days. The passages from 5 to 20 (C2C12) and under 30 (HepG2) were used for the experiments.
Western blots
C2C12 cells, HepG2 cells, muscle tissue, and liver tissue were washed twice with cold PBS and lysed in RIPA buffer. Equal amounts of protein from cells and tissues were using western blotting tests. GAPDH (C2C12 and HepG2) and β-actin (skeletal muscle and liver tissue) were used as a loading control. Immunoblotting was performed using monoclonal antibodies against PPARα, PPARγ, GLUT2, GLUT4 (Santacruz biotechnology), AMPK, and phospho-AMPK (Cell signaling Technology).
Statistical analysis
All the results are expressed as the mean ± SD (n = 5 or 6). Data which showed the changes of body weight, food intake, blood glucose levels and blood analysis were analyzed by one-way ANOVA followed by Duncan’s test for multiple comparisons. A difference of p < 0.05 was considered statistically significant compared with WT group was represented ‘#’, and CON group was represented ‘*’. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). The other data were analyzed by Student t test between groups. Statistically significant were represented by p values < 0.05, < 0.01, and < 0.001 compared with WT group was represented ‘#’, and CON group was represented ‘*’.
Results and discussion
Panax ginseng was classified as medicinal herb in the traditional Asian herbal pharmacopoeia (Gillis, 1997). Saponins, isolated ginsenosides which are present in ginseng have anti-hyperglycemic and anti-obesity activities in db/db and ob/ob mice (Attele et al., 2002). In the previous study, ginseng saponins were reported to reduce plasma lipids when injected intramuscularly into rats (Yamamoto et al., 1983). Crude fractions of ginsenosides have also shown an anti-obesity effect in rats fed a high-fat diet (Kim et al., 2005a). In Asia, products which used white, red, and black ginseng were sold commercially. BG went through a series of processes such as steaming and repeated drying several times. It had more biologically activities, including free radical scavenging, anti- stress, anti-cancer, and anti- inflammatory effects, than RG (Kang et al., 2008; Kim et al., 2007). Though there were studies about the effects of BG in pharmacology fields, its anti-diabetic effects have not yet been extensively investigated.
Anti-hyperglycemic and -hypolipidemic effects of black ginseng extract in type2 diabetic db/db mice
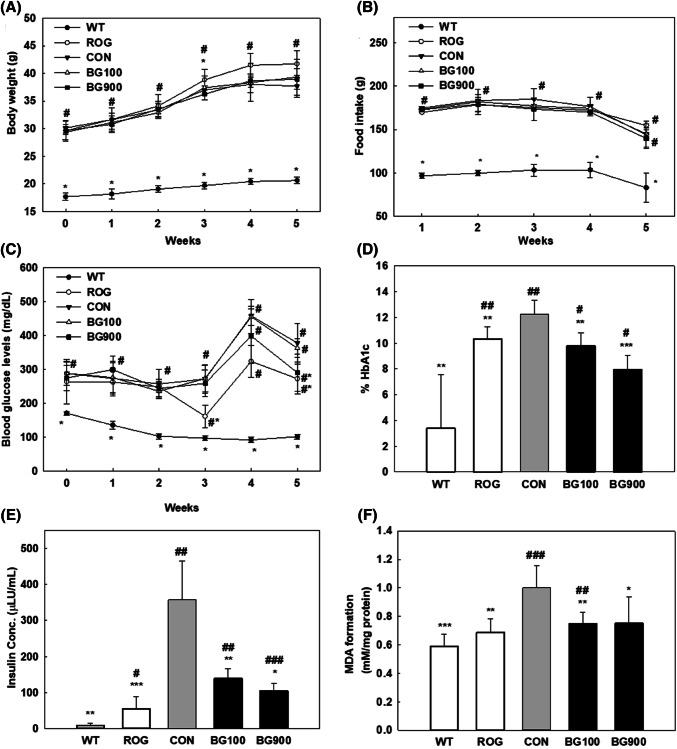
During the 5 weeks of oral administration, the body weights of the mice tended to increase in all groups (Fig. 1A). There were no significant differences in food intake between groups except the WT group (Fig. 1B). As shown in Fig. 1C, the ROG (diabetic + rosiglitazone group) and BG900 (diabetic + black ginseng 900 mg/kg BW/day group) groups after 5 weeks of administration showed significant differences in blood glucose levels from the CON group. The decrease in blood glucose levels in all treated groups after 5 weeks compared to the 4 weeks of administration is consistent with the previous study, in which the decreasing trend was found with BG and other treated groups at the same time (Seo et al., 2016). Although the levels of food intake were decrease after 5 weeks compared to 4 weeks of administration in all groups, the level of blood glucose had significant difference in ROG and B900 compared with CON. The HbA1c level was significantly lower in the BG900 (diabetic + black ginseng 900 mg/kg BW/day group) group (8.0 ± 1.072% HbA1c, p < 0.001) and BG100 (diabetic + black ginseng 100 mg/kg BW/day group) group (9.7 ± 1.0% HbA1c, p < 0.01) than in the CON group (12.2 ± 1.1% HbA1c) (Fig. 1D). As a result, it was confirmed that BG extract lowered the level of glycated hemoglobin, which is an indicator of blood glucose control. The higher serum insulin level was associated with insulin resistance and glucose intolerance (Xiong et al., 2018; Guest and Rahmoune, 2019). Although the serum insulin levels in all groups significantly increased compared with WT group (9.2 ± 6.3 μLU/mL), the ROG (55.6 ± 33.5 μLU/mL, p < 0.001), BG100 (139.2 ± 27.2 μLU/mL, p < 0.01), and BG900 (104.6 ± 22.1 μLU/mL) groups significantly decreased the serum insulin level compared with CON (357.9 ± 106.7 μLU/mL, p < 0.05) (Fig. 1E). MDA, a lipid peroxidation product, was measured as TBA reactive substances for confirmed the anti-cellular damage effect of BG extract. As a result, between the WT (0.59 ± 0.09 mM/mg protein, p < 0.001) and CON groups (1.0 ± 0.2 mM/mg protein) there was a significant difference in MDA level, and furthermore MDA level decreased in the BG fed groups (Fig. 1F). In particular, the BG100-treated group (0.75 ± 0.08 mM/mg protein, p < 0.01) showed a significant decrease in MDA levels similar to the ROG group (0.69 ± 0.10 mM/mg protein, p < 0.01) compared with the CON group. The MDA level in the BG900 group (0.75 ± 0.19 mM/mg protein, p < 0.05) also significantly decreased compared with the CON group. As shown in Table S2, there were significant differences in the values of HDL, LDL, TC, and TG in the WT group compared with the CON group. The level of ALP, an index of liver injury, LDL, and TG had significant difference in the ROG group compared with the CON group. LDL and TG level in the BG900 group remained in similar level to the ROG group, although there was no significant difference compared with the value of the CON group. AST level decreased in the BG extract-treated group compared with that in the CON group, but no significant differences were observed.
Fig. 1.
Effect of black ginseng (BG) extract on (A) body weight, (B) food intake, (C) fasting blood glucose levels, (D) hemoglobin A1c content in blood, (E) Insulin concentration in serum of mice, and (F) malondialdehyde (MDA) levels in liver tissue. WT (non-diabetic control group, wild type), ROG (diabetic + rosiglitazone group), CON (diabetic control group), BG100 (diabetic + black ginseng extract 100 mg/kg BW/day group), BG900 (diabetic + black ginseng extract 900 mg/kg BW/day group). Values are expressed as the mean ± SD (n = 6). *p values < 0.05, **p values < 0.01 and ***p values < 0.001 are considered statistically significant when compared with controls (CON), and #p values < 0.05, ##p values < 0.01 and ###p values < 0.001 are considered statistically significant when compared with wild type (WT) only by Student’s t-test
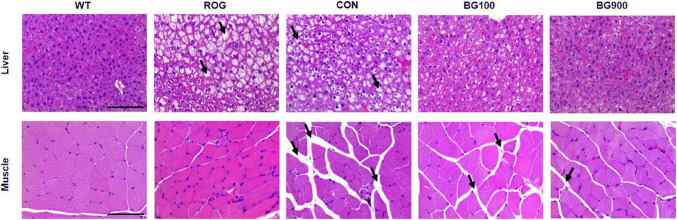
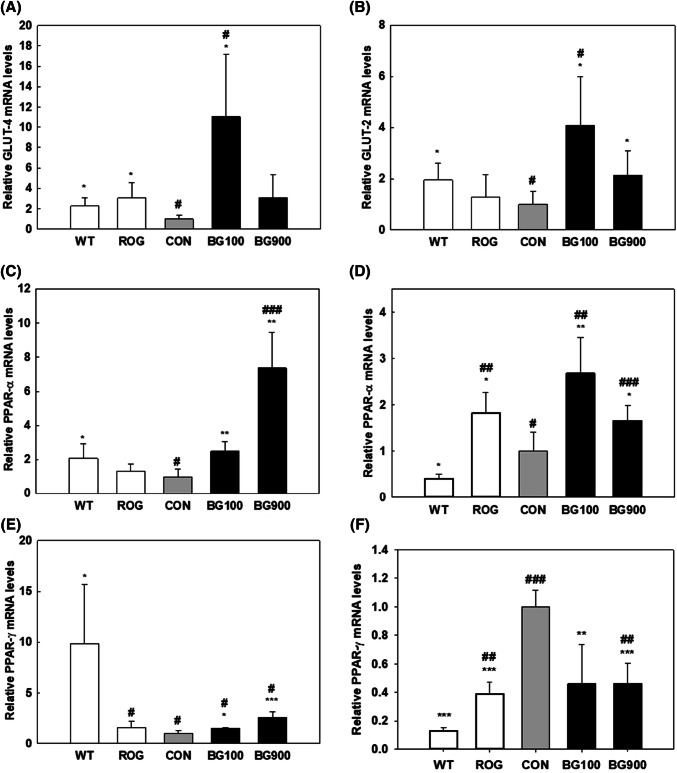
To confirm the effect of BG extract on the gastrocnemius muscle and liver, H&E staining was performed on the paraffinized muscle and liver (Fig. 2). These results show that administration of BG extract can improve muscle atrophy and hepatic steatosis in diabetic mice. The size of adipocytes in the intervals of gastrocnemius fibers was bigger in diabetic mice (CON) than in non-diabetic mice (WT), BG100, and BG900. Previous stuties reported that BG extract significantly reduced muscle damage in type 2 diabetes (Kang et al., 2017) and that muscle atrophy was result from the degree of protein degradation, and protein synthesis is associated with the mammalian target of rapamycin (mTOR) pathway, which is known as a growth-promoting enzymatic pathway (Bodine et al., 2001). And BG activated mTOR, an important regulatory protein in protein synthesis (Seo et al., 2016). These results indicate that BG can improve muscle atrophy that was due to activating mTOR and inducing protein synthesis in skeletal muscle of diabetes. In addition, the studies about nonalcoholic fatty liver disease, NAFLD with diabetes referred that the type 2 diabetes strongly related with hepatic steatosis and cirrhosis (Clark and Diehl, 2002). Our result showed that the hepatic steatosis of the liver tissue in BG900 group was improved similar as the liver tissue in WT. And the size of adipocytes among the muscle of diabetic mice administered with BG (BG100 and 900) decreased in comparison with that of diabetic mice (CON) (Fig. 2). BG extract in the db/db mice study showed significant difference in the GLUTs, and PPAR expression at liver, and more highly effective result with the extract was observed in muscle. Controlling the level of blood glucose is affected by complex actions by various organs involved the pancreas, fat, muscle, and liver (Saltiel and Kahn, 2001). Skeletal muscle is an important tissue because of its function that removing glucose in blood by insulin (Seo et al., 2016). GLUT4 is the main transporter for glucose in muscle (Kang et al., 2017), and it regulated glucose uptake (Stöckli et al., 2011). GLUT4 transfers glucose from the extracellular to intracellular milieu, and finally it increases glucose uptake (Kang et al., 2017). The GLUT4 expression in the muscle increased in the groups treated with BG100 (8.25 ± 6.43 relative quantification, p < 0.05) and 900 (2.56 ± 1.48 relative quantification, p = 0.077) compared with CON group (1.00 ± 0.18) (Fig. 3A). Up-regulation of the expression of GLUT4, effectively inhibits AMPK-regulated gluconeogenesis in peripheral tissues and improves glucose uptake (Kim et al., 2013; Steinberg and Kemp, 2009). Liver is a main organ which act a part in glucose metabolism transferring glucose between the liver and blood by regulating the expression of GLUT2 (Thorens, 2015). The mRNA level of GLUT2 in the BG100 (4.08 ± 1.92 relative quantification, p < 0.05) and 900 groups (2.13 ± 0.96 relative quantification, p < 0.05) was higher than that of the CON group (1.00 ± 0.50) in the liver (Fig. 3B). And the level of GLUT2 in BG100 group was significantly (p < 0.05) increased compared with the WT group (1.95 ± 0.65 relative quantification) in liver tissue. These results suggested that controlling glycolytic flux, BG extract regulated glucose level in liver.
Fig. 2.
Histological changes in muscle and liver following treatment with black ginseng extract. Hematoxylin and eosin-stained photomicrographs showing the muscle (×200, Bar = 50 μm) and the liver (×400, Bar = 25 μm). Effects of black ginseng extract treatment for 5 weeks on morphological changes of the gastrocnemius muscles and liver in db/db mice. Morphological changes were visualized with a light microscope after hematoxylin and eosin (H&E) staining (→: steatosis in liver; →: adipocytes in muscle). WT (non-diabetic control group, wild type), ROG (diabetic + rosiglitazone group), CON (diabetic control group), BG100 (diabetic + black ginseng extract 100 mg/kg BW/day group), BG900 (diabetic + black ginseng extract 900 mg/kg BW/day group)
Fig. 3.
The effect of BG on relative mRNA expression level. The glucose transporter 4 (GLUT4) mRNA level in the muscle (A) and the GLUT2 mRNA level in the liver (B) of db/db mice. The peroxisome proliferator-activated receptor α (PPARα) mRNA level in the muscle (C) and in liver (D) of db/db mice. The PPARγ mRNA level in the muscle (E) and in liver (F) of db/db mice. WT (non-diabetic control group, wild type), ROG (diabetic + rosiglitazone group), CON (diabetic control group), BG100 (diabetic + black ginseng extract 100 mg/kg BW/day group), BG900 (diabetic + black ginseng extract 900 mg/kg BW/day group). Bar diagram represents the mean ± standard deviation (n = 5). *p values < 0.05, **p values < 0.01 and ***p values < 0.001 are considered statistically significant when compared with controls (CON), and #p values < 0.05, ##p values < 0.01 and ###p values < 0.001 are considered statistically significant when compared with wild type (WT) only by Student’s t-test
In our study, PPARα expression increased in the gastrocnemius muscle tissue and liver of diabetic mice treated with BG extract, respectively (Fig. 3C, D). The expression level of PPARα increased in a BG dose-dependent manner (2.50 ± 0.54, and 7.36 ± 2.10), suggesting that lipid metabolism in the muscle is affected by the BG. In addition, the PPARα level in the liver increased in the BG100 (2.08 ± 0.56, p = 0.011) and BG900 (1.55 ± 0.28, p = 0.058) groups, compared with the CON group (1.00 ± 0.48). Particularly, BG had more effect on the expression of PPARα on muscle. Clinically, ginsenosides have been shown to have advantageous hypolipidemic effects (Kim and Park, 2003; Yang et al., 1999). The obese people are associated with fatty acid metabolic disorders in skeletal muscle (Houmard, 2008). Moreover, according to Lefebvre et al. activated PPARα promotes the expression of mitochondrial enzymes involved in lipid oxidation and inhibits the activation of enzymes that induce lipid synthesis (Lefebvre et al., 2006). In addition, PPARγ plays different roles in muscle and liver, respectively. In the muscle, insulin resistance is related to PPARγ, and fatty acid synthesis is linked to PPARγ in the liver (Janani and Kumari, 2015). The expression of PPARγ in muscle of BG100 group (1.56 ± 0.06, p < 0.05) and BG900 group (2.57 ± 0.59, p < 0.001) were significantly up-regulated compared with CON group (1.00 ± 0.33) (Fig. 3E). The PPARγ level in liver tissue of CON group (1.00 ± 0.12) was significantly higher compared with other groups (ROG; 0.39 ± 0.08, BG100; 0.46 ± 0.28, BG900; 0.46 ± 0.15) (Fig. 3F). The protein expression of PPARγ, PPARα, and GLUT4 in muscle of BG900 group were recovered to WT, and the protein expression of PPARγ in liver in groups except BG100 group had no difference (Fig. S1).
Profiling of BG extract
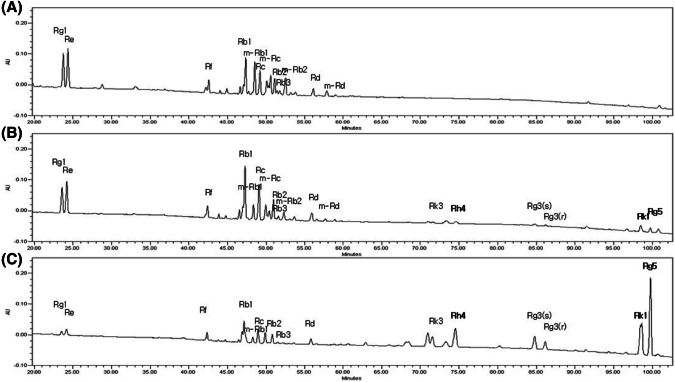
The metabolic profiles of white ginseng, WG; red ginseng, RG; black ginseng, BG and its associated function were critically affected by the repetitious steaming times. Under the optimized HPLC-UVD method, 19 major ginsenosides were clearly identified and partially assigned to both raw and steamed P. ginseng. BG having several typical ginsenoside profile in HPLC analysis to investigate key compounds that have the effect of reducing blood sugar. BG contains three distinct ginsenosides, Rh4, Rk1, and Rg5, which did not exist in WG (Fig. 4). These results are in agreement with those of recent studies (Kang et al., 2017; Metwaly et al., 2019). Specifically, RG5 (17.07 ± 0.07 mg/g), Rh4 (4.41 ± 0.08 mg/g), and Rk1 (4.21 ± 0.01 mg/g) were found in higher concentrations than in RG but were not detected in WG (Table 1). In BG, the ginsenoside content of Rg5, Rh4, and Rk1 was 10-fold, 4.7-fold, and 19-fold higher, respectively than that in RG. These changes were due to chemical modifications of ginsenosides by hydrolysis, dehydration, and decarboxylation. According to An et al. (2011) and Kang et al. (2006), major ginsenosides such as Rb1, Rb2, Rc, and Rd are transformed into Rg3 by heating, and Rg3 can be converted to Rk1 and Rg5 by heating. Particularly, deglycosylated minor ginsenosides have more potent pharmaceutical activities than the major ginsenosides (Kim et al., 2005b; Metwaly et al., 2019). This metabolic profiling approach may represent a paradigm shift for the global quality control of Korean P. ginseng products.
Fig. 4.
HPLC chromatogram of ginsenosides in (A) white ginseng (WG) and (B) red ginseng (RG) and (C) black ginseng (BG)
Table 1.
Ginsenoside content of white ginseng (WG), red ginseng (RG), and black ginseng (BG)
| Ginsenosides (mg/g) | White ginseng | Red ginseng | Black ginseng |
|---|---|---|---|
| Rg1 | 4.747 ± 0.168 | 5.126 ± 0.095 | 0.854 ± 0.066 |
| Re | 8.303 ± 0.020 | 9.485 ± 0.149 | 1.875 ± 0.193 |
| Rf | 1.301 ± 0.053 | 1.606 ± 0.067 | 1.136 ± 0.045 |
| Rb1 | 7.994 ± 0.255 | 18.702 ± 0.565 | 6.669 ± 0.275 |
| m-Rb1 | 6.978 ± 0.105 | 4.484 ± 0.220 | 1.604 ± 0.088 |
| Rc | 3.641 ± 0.044 | 8.263 ± 0.073 | 2.868 ± 0.058 |
| m-Rc | 4.124 ± 0.032 | 2.661 ± 0.071 | 0.296 ± 0.076 |
| Rb2 | 1.896 ± 0.063 | 3.980 ± 0.030 | 1.668 ± 0.067 |
| Rb3 | 0.400 ± 0.100 | 0.696 ± 0.056 | 0.290 ± 0.033 |
| m-Rb2 | 3.320 ± 0.066 | 2.239 ± 0.024 | 0.237 ± 0.082 |
| Rd | 0.677 ± 0.041 | 1.305 ± 0.064 | 0.923 ± 0.050 |
| m-Rd | 3.356 ± 0.070 | 1.914 ± 0.149 | n/d |
| Rk3 | n/d | 0.173 ± 0.044 | 2.231 ± 0.086 |
| Rh4 | n/d | 0.439 ± 0.119 | 4.410 ± 0.079 |
| F2 | n/d | n/d | n/d |
| Rg3(s) | n/d | 0.398 ± 0.149 | 4.978 ± 0.106 |
| Rg3(r) | n/d | 0.235 ± 0.062 | 2.777 ± 0.051 |
| Rk1 | n/d | 0.886 ± 0.045 | 4.195 ± 0.006 |
| Rg5 | n/d | 0.903 ± 0.085 | 17.073 ± 0.071 |
Different letters indicate signification differences at p < 0.05 by one-way ANOVA followed by Duncan’s test for multiple comparisons. The levels of Rh4, Rk1, and Rg5, used in vitro assay, were not detected in WG and considerably higher in BG compared to RG
Ginsenosides regulate expression of a gene related to lipid oxidation and insulin resistance in C2C12 and HepG2 cells
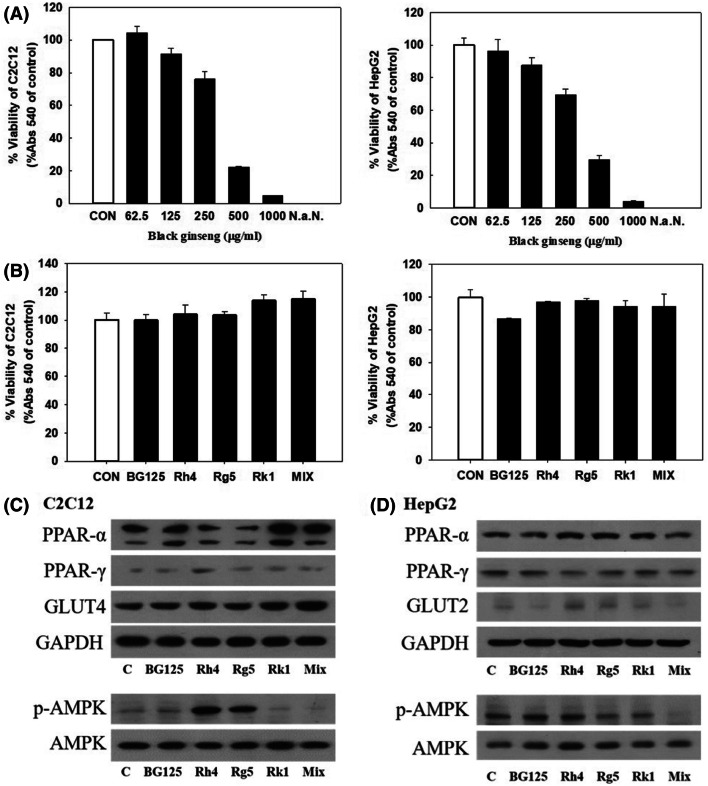
We performed the studies in C2C12 and HepG2 cells to investigate what ginsenosides had homeostasis effect on GLUTs and PPARs. The cell survival rate was found to be over 80% with 125 μg/mL of BG, and the effect of methanol percentage contained in the corresponding concentration was found not to affect viability (Fig. 5A, B). Additionally, C2C12 and HepG2 were treated with three types of ginsenosides (Rh4, 0.89 μM; Rg5, 0.69 μM; Rk1, 2.8 μM) found in 125 μg/mL of BG extract in equivalent amounts and the three combined ginsenosides (MIX: Rh4, 0.89 μM; Rg5, 0.69 μM; Rk1, 2.8 μM) (Fig. 5C, D). When treated with a single ginsenoside and mix materials on C2C12, the protein expression of PPARγ, GLUT4, and p-AMPK were up-regulated in treated with Rh4 compared with control group (‘C’) (Fig. 5C). However, PPARα protein expression was up-regulated in Rk1 and Mix groups in C2C12 (Fig. 5C). These results indicated that Rh4 is responsible for gene expression related with insulin resistance and glucose metabolism whereas, Rk1 modulates lipid oxidation in muscle. In HepG2, Rh4 and Rg5 increased PPARα expression and decreased PPARγ expression at protein level, and only Rk1 up-regulated PPARα (Fig. 5D).
Fig. 5.
(A) Viability in C2C12 Cells and HepG2 cells treated with different concentration of BG. After a 24 h exposure to BG and ginsenosides, cell viability was measured using the MTT assay. (B) Effects of BG and ginsenosides (Rh 4, Rg 5, Rk 1) on cell viability. C2C12 and HepG2 cells were treated with three types of ginsenosides (Rh4, 0.89 μM; Rg5, 0.69 μM; Rk1, 2.8 μM) found in 125 μg/mL of BG extract in equivalent amounts and the three combined ginsenosides (MIX: Rh4, 0.89 μM; Rg5, 0.69 μM; Rk1, 2.8 μM). (C) The effect of BG 125 μg/mL, ginsenoside and mixed material (Rh 4 + Rg 5 + Rk 1) on relative PPARs and GLUTs protein levels in C2C12 and HepG2 after 24 h samples treated. *p values < 0.05, **p values < 0.01 and ***p values < 0.001 are considered statistically significant when compared with the control group by Student’s t-test
In summary, BG extract has anti-diabetic activity in diabetes in vivo and in vitro. Glucose metabolism via GLUT4 in muscle and via GLUT2 in liver was affected by the BG treatment. Control of glucose metabolism and insulin resistance in muscle and liver reduces concentrations of blood glucose leading to the intake of glucose and insulin levels in the blood, and we have confirmed that this process may prevent diabetes-complicated obesity. The BG administration increased the lipid metabolism induced by PPARα in both muscle and liver. This study suggests that BG with increased content of Rh4, Rg5, and Rk1 may play as functional foods and drugs, which is distinguished from other types of ginseng. Future studies will look at the in vivo effects and further explore BG’s mechanism of action.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank School of Life Science and Biotechnology for BK21 PLUS. The authors grateful to Korea University for technical support and the Korea University CJ Food Safety Center (Seoul, South Korea) for providing the equipment and facilities. This study was supported by the business of master-work for Geumsan ginseng through the Geumsangun funded by the Geumsangun Chungnam, Republic of Korea (No. 2015-403-02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu-Jin Jeong, Email: ujinny1227@naver.com.
Moon-Jung Hwang, Email: moonca07@naver.com.
Chung-Oui Hong, Email: hco5979@hanmail.net.
Dae-Seok Yoo, Email: gator127@ginherb.re.kr.
Jin Seong Kim, Email: chiseikjs@ginherb.re.kr.
Do-Yeon Kim, Email: dykim@ginherb.re.kr.
Kwang-Won Lee, Email: kwangwon@korea.ac.kr.
References
- An Y-E, Ahn S-C, Yang D-C, Park S-J, Kim B-Y, Baik M-Y. Chemical conversion of ginsenosides in puffed red ginseng. LWT-Food Sci. Technol. 2011;44:370–374. doi: 10.1016/j.lwt.2010.09.013. [DOI] [Google Scholar]
- Attele AS, Zhou Y-P, Xie J-T, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan C-S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr. Diab. Rep. 2002;2:210–215. doi: 10.1007/s11892-002-0085-3. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem. Pharmacol. 1997;54:1–8. doi: 10.1016/S0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Guest PC, Rahmoune H. Characterization of the db/db Mouse Model of Type 2 Diabetes. pp. 195-201. In: Pre-Clinical Models. Springer (2019) [DOI] [PubMed]
- Herder C, Schneitler S, Rathmann W, Haastert B, Schneitler H, Winkler H, Bredahl R, Hahnloser E, Martin S. Low-grade inflammation, obesity, and insulin resistance in adolescents. J. Clin. Endocrinol. Metab. 2007;92:4569–4574. doi: 10.1210/jc.2007-0955. [DOI] [PubMed] [Google Scholar]
- Holman G, Kasuga M. From receptor to transporter: insulin signalling to glucose transport. Diabetologia. 1997;40:991–1003. doi: 10.1007/s001250050780. [DOI] [PubMed] [Google Scholar]
- Houmard JA. Intramuscular lipid oxidation and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1111–R1116. doi: 10.1152/ajpregu.00396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators DT. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. The Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- Janani C, Kumari BR. PPAR gamma gene–a review. Diabetes Metab. Syndr. 2015;9:46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Kang KS, Kim HY, Yamabe N, Yokozawa T. Stereospecificity in hydroxyl radical scavenging activities of four ginsenosides produced by heat processing. Bioorg. Med. Chem. Lett. 2006;16:5028–5031. doi: 10.1016/j.bmcl.2006.07.071. [DOI] [PubMed] [Google Scholar]
- Kang KS, Yamabe N, Kim HY, Park JH, Yokozawa T. Therapeutic potential of 20 (S)-ginsenoside Rg 3 against streptozotocin-induced diabetic renal damage in rats. Eur. J. Pharmacol. 2008;591:266–272. doi: 10.1016/j.ejphar.2008.06.077. [DOI] [PubMed] [Google Scholar]
- Kang O-H, Shon M-Y, Kong R, Seo Y-S, Zhou T, Kim D-Y, Kim Y-S, Kwon D-Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complement. Altern. Med. 2017;17:341. doi: 10.1186/s12906-017-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-H, Park K-S. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol. Res. 2003;48:511–513. doi: 10.1016/S1043-6618(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J. pharmacol. sci. 2005;97:124–131. doi: 10.1254/jphs.FP0040184. [DOI] [PubMed] [Google Scholar]
- Kim MK, Lee JW, Lee KY, Yang D. Microbial conversion of major ginsenoside Rb ~ 1 to pharmaceutically active minor ginsenoside Rd. J. Microbiol. 2005;43:456. [PubMed] [Google Scholar]
- Kim KT, Yoo KM, Lee JW, Eom SH, Hwang IK, Lee CY. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79-4 cells induced by oxidative stress. J. Ethnopharmacol. 111: 443-450 (2007) [DOI] [PubMed]
- Kim S-H, Hwang J-T, Park HS, Kwon DY, Kim M-S. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2013;439:66–70. doi: 10.1016/j.bbrc.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart J-C, Staels B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. Eur. J. Clin. Invest. 2006;116:571. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Metwaly AM, Lianlian Z, Luqi H, Deqiang D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules. 2019;24:1856. doi: 10.3390/molecules24101856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddy DM, Delerive P, Summers RJ, Sexton PM, Langmead CJ. G Protein–coupled receptors targeting insulin resistance, obesity, and type 2 diabetes mellitus. Pharmacol. Rev. 2018;70:39–67. doi: 10.1124/pr.117.014373. [DOI] [PubMed] [Google Scholar]
- Saba E, Jeon BR, Jeong D-H, Lee K, Goo Y-K, Kim S-H, Sung C-K, Roh S-S, Kim SD, Kim H-K. Black ginseng extract ameliorates hypercholesterolemia in rats. J. Ginseng. Res. 2016;40:160–168. doi: 10.1016/j.jgr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Seo Y-S, Shon M-Y, Kong R, Kang O-H, Zhou T, Kim D-Y, Kwon D-Y. Black ginseng extract exerts anti-hyperglycemic effect via modulation of glucose metabolism in liver and muscle. J. Ethnopharmacol. 2016;190:231–240. doi: 10.1016/j.jep.2016.05.060. [DOI] [PubMed] [Google Scholar]
- Shimoda Y, Matsuo K, Kitamura Y, Ono K, Ueyama T, Matoba S, Yamada H, Wu T, Chen J, Emoto N. Diabetes-related ankyrin repeat protein (DARP/Ankrd23) modifies glucose homeostasis by modulating AMPK activity in skeletal muscle. PloS one. 2015;10:e0138624. doi: 10.1371/journal.pone.0138624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiol. Rev. 2009;89:10251078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Stöckli J, Fazakerley DJ, James DE. GLUT4 exocytosis. J. Cell Sci. 2011;124:4147–4159. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B-S, Gu L-J, Fang Z-M, Wang C-Y, Wang Z, Lee M-R, Li Z, Li J-J, Sung C-K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC–ELSD. J. Pharm. Biomed. Anal. 2009;50:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58:221–232. doi: 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- Xiong H, Zhang S, Zhao Z, Zhao P, Chen L, Mei Z. Antidiabetic activities of entagenic acid in type 2 diabetic db/db mice and L6 myotubes via AMPK/GLUT4 pathway. J. ethnopharmacology. 2018;211:366–374. doi: 10.1016/j.jep.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kumagai A, Yamamura Y. Plasma lipid-lowering and lipogenesis-stimulating actions of ginseng saponins in tumor-bearing rats. Am. J. Chin. Med. 1983;11:88–95. doi: 10.1142/S0192415X8300015X. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu T, He K, Fu Z-G. Effect of aerobic exercise and ginsenosides on lipid metabolism in diet-induced hyperlipidemia mice. Acta Pharmacol. Sin. 1999;20:563–565. [PubMed] [Google Scholar]
- Ye J-M, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome Proliferator—Activated Receptor (PPAR)-α Activation Lowers Muscle Lipids and Improves Insulin Sensitivity in High Fat—Fed Rats: Comparison With PPAR-γ Activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.