Abstract
The field of cell therapy and regenerative medicine can hold the promise of restoring normal tissues structure and function. Additionally, the main targets of stem cell-based therapies are chronic diseases and lifelong disabilities without definite cures such as osteoporosis. Osteoporosis as one of the important causes of morbidity in older men and post-menopausal women is characterized by reduced bone quantity or skeletal tissue atrophy that leads to an increased risk of osteoporotic fractures. The common therapeutic methods for osteoporosis only can prevent the loss of bone mass and recover the bone partially. Nevertheless, stem cell-based therapy is considered as a new approach to regenerate the bone tissue. Herein, mesenchymal stem cells as pivotal candidates for regenerative medicine purposes especially bone regeneration are the most common type of cells with anti-inflammatory, immune-privileged potential, and less ethical concerns than other types of stem cells which are investigated in osteoporosis. Based on several findings, the mesenchymal stem cells effectiveness near to a great extent depends on their secretory function. Indeed, they can be involved in the establishment of normal bone remodeling via initiation of specific molecular signaling pathways. Accordingly, the aim herein was to review the effects of stem cell-based therapies in osteoporosis.
Keywords: cell therapy, chronic diseases, mesenchymal stem cells, osteoporosis, regenerative medicine
Introduction
Osteoporosis as a chronic and long-term skeletal disorder is more common in senile people (in men after age 65 and women after age 55 years) (1–4). Accordingly, it is responsible for most of the elderly fractures through decreasing the bone mass and mineral density (BMD) (1, 5, 6). Moreover, it has been reported that osteoporosis occurs when there is an imbalance between bone cells function (7, 8). In 1993, osteoporosis is defined as “progressive systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture” by WHO (9–12). The proximal ends of the humerus and femur, the distal end of the radius, and the vertebral column are more susceptible to the osteoporotic fractures in contrast to other parts of the bone (13–15). Additionally, the hip fracture can be considered as the serious complication with high morbidity and mortality (15–17). Given the fact that the life expectancy universally is increasing and subsequently osteoporosis becomes a growing global problem with a great impact on quality of life, selecting powerful approaches for disease managing is essential. In this respect, there is no practical pharmaceutical cure (18). Recently, stem cell therapies have attained remarkable clinical consideration with a promising strategy for regenerative medicine and tissue engineering to treat various types of diseases including osteoporosis (19–26). Herein, discuss the effects of stem cell-based therapies in osteoporosis is the main objective of this review.
Bone Biology; Signaling Pathways; Bone Modeling and Remodeling
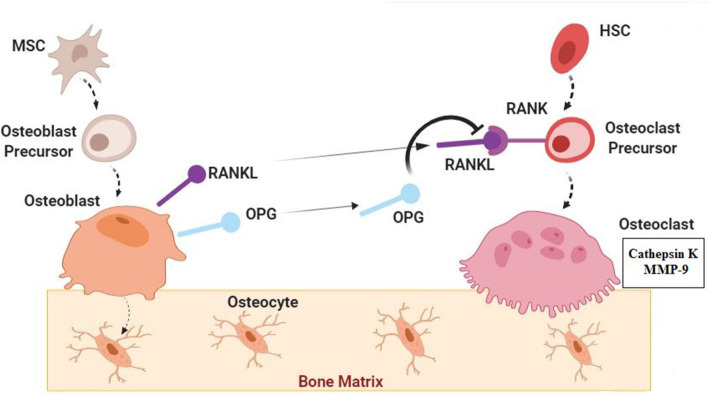
Bone as a highly dynamic tissue continuously undergoes modeling and remodeling via activation of bone cells (osteoblasts, osteoclast, and osteocytes) (Figure 1) (40–42). Herein, modeling is defined as separately happening of bone formation and resorption on the bone surface and remodeling is known as the coupling between bone formation and resorption for regeneration (43–46). The process of developing new bone material by osteoblasts is called bone formation (ossification or osteogenesis) which commences about 6 weeks after fertilization in embryos. There are two types of bone formation, including intramembranous and endochondral (27, 47). During intramembranous bone formation, mesenchymal stem cells (MSCs) are proliferated and differentiated into osteoblasts in areas of embryonic connective tissue which contain high vascularization. Additionally, the intramembranous bone formation that is involved in the formation of the flat bones of the clavicles, skull, and the mandible is known as a procedure of bone formation from fibrous membranes (48, 49). The endochondral bone formation is befallen at three sites including the physis, the epiphysis, and the cuboidal bones of the carpus and tarsus. It is a procedure in which the cartilage is commonly replaced by bone for the formation of the growing skeleton (50–52). In general, bone formation is controlled by various growth factors, cytokines, and hormones (40, 53, 54). Therein, osteoblasts can reply to these external signals through various signaling pathways and control the specific gene expression for cell fate determining (28, 29, 55). Accordingly, there are some signaling molecules with critical roles in osteoblast turnover including runt-related transcription factor 2 (Runx2), osterix (Osx), ß-Catenin, activating transcription factor 4 (Atf4), and activator protein 1(AP-1) family. Indeed, they have momentous roles in osteoblast differentiation and osteoblastogenesis to promote bone formation (27–33). Moreover, it has been demonstrated that fibroblast growth factors (FGFs), transforming growth factor β (TGF β), insulin-like growth factor 1 (IGF-1), bone morphogenetic proteins (BMPs), Notch, Wnt, and parathyroid hormone (PTH) have effective roles in the bone formation process (56–59). Bone formation and resorption must be balanced for bone mass maintenance (34, 38, 39). Bone resorption is the process of minerals dissolution and organic matrix degradation by osteoclasts, which depends on the osteoclasts secretions into the extracellular space (60–63). Some more important types of osteoclasts secretions are lysosomal enzymes (e.g., cathepsin K) and matrix metallopeptidase 9 (MMP-9) (41, 64, 65). Osteoclasts arise from the hematopoietic stem cells (HSC) via stimulation of receptor activation of NF-κB ligand (RANKL) and the monocyte/macrophage colony-stimulating factor (M-CSF) from osteoblasts membrane surface (60, 66, 67). RANKL and M-CSF are interacted with their receptors present on osteoclast precursors to stimulate osteoclast proliferation and differentiation (60, 68, 69). However, there is another signaling molecule called osteoprotegerin (OPG) which is also secreted by osteoblasts to interfere with the RANKL for inhibition of osteoclastogenesis (70–73). According to investigations, some inflammatory cytokines e.g., interleukin1 (IL-1), interlukin-6 (IL-6), and tumor necrosis factor-α (TNFα) can be involved in osteoclast differentiation and function (34–37). Several findings have indicated that imbalance between osteoclasts and osteoblasts functions can lead to some skeletal disorders including osteoporosis. In fact, these disorders are the consequence of decreased in osteoblast activity and/or increased in osteoclast activity (8, 41).
Figure 1.
Normal Bone Biology; Signaling Pathways. Bone as a dynamic tissue undergoes modeling and remodeling by activation of osteoblasts, osteoclast, and osteocytes. Mesenchymal stem cells (MSCs) are proliferated and differentiated into osteoblasts. Some signaling molecules have important roles in osteoblast turnover and function including runt-related transcription factor 2 (Runx2), osterix (Osx), ß-Catenin, activating transcription factor 4(Atf4), activator protein 1(AP-1) family, fibroblast growth factors (FGFs), transforming growth factor β (TGF β), insulin-like growth factor 1 (IGF-1), bone morphogenetic proteins (BMPs), Notch, Wnt, and parathyroid hormone (PTH) (27–33). Osteoblasts which are trapped in the bone matrix are called osteocytes. Osteoclasts are derived from the hematopoietic stem cells (HSC) through the stimulation by receptor activation of NF-κB ligand (RANKL) from osteoblasts. Osteoprotegerin (OPG) which is also secreted by osteoblasts can interfere with the RANKL and inhibit osteoclastogenesis. Osteoclasts can secrete cathepsin K and matrix metallopeptidase 9 (MMP-9) in extracellular space. Some inflammatory cytokines such as. interleukin1 (IL-1), interlukin-6 (IL-6), and tumor necrosis factor-α (TNFα) can be involved in osteoclast differentiation and function (34–37). In normal condition Bone formation (by osteoblasts) and resorption (by osteoclasts) are in balanced for bone mass maintenance (34, 38, 39).
An Overview on Osteoporosis: Imbalance Between Bone Formation and Resorption
As a result of the aging process, reduction in osteoblast number, function, and longevity, lead to bone formation decreasing However, bone resorption is exceeded due to sex hormones defection. Accordingly, individuals are predisposed to osteoporosis and osteoporotic bone fractures (74–77). In fact, osteoporotic bones due to low bone mass are fragile and brittle. The compression fractures of the vertebrae and traumatic fractures of the femoral neck and the wrist are the main issues of osteoporosis. Nevertheless, the hip fractures due to their burden are more considerable and need more attention. It is estimated that by 2050 the number of hip fractures will be more than 6 million and almost the 75% of them will be occurred in the developing countries (9). Osteoporosis can be followed by various complications and disorders. Usually, low levels of estrogen in post-menopausal women is the most well-known factor (78). In clinical diagnostic techniques of osteoporosis, dual x-ray absorptiometry [DXA] is approved as a gold standard approach to diagnose and follow the osteoporosis by calculating BMD (79). The WHO defines a set of categories to diagnose osteopenia and osteoporosis. These guidelines are based on T-score and Z-score. T-score shows the number of standard deviations above or below the mean reference value for 30 year-old healthy adults. However, Z-score measures the BMD regards to the average BMD of the same age and gender (80). According to the guidelines, a score above −1 is considered normal, a score between −1 and −2.5 indicates osteopenia, and a score below −2.5 portends the osteoporosis (79). Hereupon, for individuals with osteoporosis diagnosed, various treatments are recommended to increase the quality of life and decrease the economic burden on health care system (1).
Current Treatments and Limitations
Osteoporosis cannot be cured but some of the pharmacological and non-pharmacological treatment approaches can manage it (Table 1) through the strengthening the bones and preventing the consequent fractures. In this context, using bisphosphonates, selective estrogen receptor modulators (SERMs), teriparatide, denosumab, calcitonin, and hormone replacement therapy (HRT) are the approved methods as the pharmacological treatments for osteoporosis (94). Additionally, some of the non-pharmacological treatments are including nutritional therapy, physical exercises, vertebroplasty, and kyphoplasty. Despite the preventive and therapeutical effects of these treatments, there are some limitations and side effects around using them. Hence, it is needed to apply new and more effective approaches with fewer side effects for osteoporosis management.
Table 1.
| Treatment | Positive effects | Side effects/limitations | Type of treatment |
|---|---|---|---|
| Bisphosphonates | - Can decrease both hip and spine fracture risk through maintaining the bone mineral density | - Osteonecrosis of jaw - Gastrointestinal and renal discomfort - Atypical femoral fractures - Acute influenza-like illness |
Pharmacological |
| Teriparatide | - As a recombinant parathyroid hormone can be used to stimulate osteoblasts to reconstruct the osteoporotic bone - Can improve the bone mineral density and the bone architecture - Considered as an impressive agent to decrease the vertebral, non-vertebral, and hip fracture risks |
- Inflammation of the nose - Diarrhea - Constipation - Joint Pain |
Pharmacological |
| Hormone replacement therapy | - Safe and cost-benefit approach with positive effects on preventing the vertebral and non-vertebral fractures | - Cardiovascular, thromboembolic, and gallbladder discomforts, breast and endometrial cancers | Pharmacological |
| Selective estrogen receptor modulators | - Can be a good choice to prevent the number of hormone replacement therapy related complications - Can improve the bone mass and reduce the fracture risk |
- Have some limitations in preventing non-vertebral fractures and also have extra-skeletal side effects | Pharmacological |
| Physical exercises | - Can lead to bone loss reduction - Can conserve remain bone tissue - Can reduce the risk of bone fractures caused by falls |
- Some types of physical exercises such as abdominal sit-ups or loaded forward flexion of the spine can increase the risk of the spine compression fractures. | Non-pharmacological |
| Vertebroplasty | - Can relieve symptoms associated with vertebral compression fractures | - May lead to spinal cord or nerve root injury - May lead to infection - May lead to pulmonary embolus |
Non-pharmacological |
| Kyphoplasty | - Can relieve symptoms associated with vertebral compression fractures | - May lead to cement leaks - May lead to infection - May occur balloon rupture |
Non-pharmacological |
Cell Therapy as a Novel Approach
The clinical demand for new therapeutic methods has been led to progress in stem cell therapy and regenerative medicine (23, 95). In other words, stem cell-based therapies are becoming increasingly important in treatment of chronic and long-lasting diseases (96, 97). However, there are several parameters which need to be optimized for maximizing stem cell-based therapies potential. In this context, various basic and clinical studies related to the effects of stem cell-based therapies on diseases with no definite treatments were performed (22, 98, 99). Accordingly, some investigations were also conducted in the field of stem cell therapy for osteoporosis. Herein, the application of different types of stem cells including embryonic, induced pluripotent, and MSCs along with their secretion factors were evaluated to treat osteoporosis (100–102).
Mechanism of Stem Cells Function in Bone Remodeling and Osteoporosis
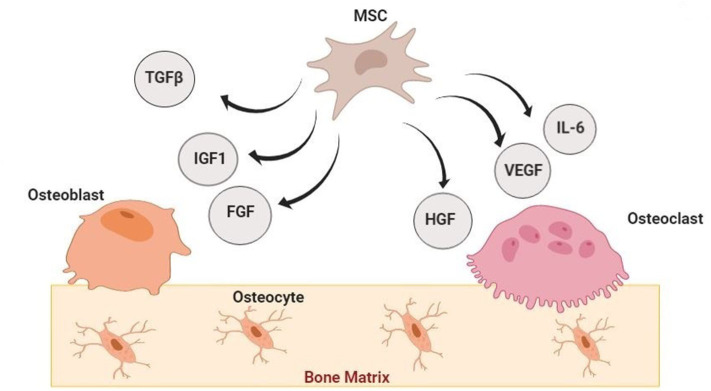
Osteoporosis is a multifactorial disorder with endogenous and exogenous components (103, 104). Cell-based regenerative medicine can be invaluable in osteoporosis treatment through bone resorption modulation, fractures susceptibility reduction, and lost mineral density enhancement. These are possible by increasing the number of progenitor stem cells and improve the function of stem cells (proliferation and differentiation into bone-forming cells) (20, 102, 105, 106). Since the bone tissue repair cascade can be controlled by local signals from various cytokines and growth factors through the inducing osteoprogenitor cells migration, differentiation, proliferation, revascularization, and extracellular matrix production (56, 107, 108), stem cells (especially MSCs) can support bone regeneration by secreting bioactive molecules such as IGF-1, TGF-β, vascular endothelial growth factor (VEGF), angiogenin, hepatocyte growth factor (HGF), IL-6, and etc. (56, 109–113). On the other hand, MSCs derived exosomes are other factors which their effects on preventing the bone loss and promoting bone remodeling processes (during osteogenesis, osteoclastogenesis, and angiogenesis) have been demonstrated in vitro and in vivo (114–116).
Embryonic, Induced Pluripotent, and Embryonic Like Stem Cells in Osteoporosis
Although particular protocols are demanded to direct differentiation of embryonic stem cells (ESCs) (from the inner cell mass of a blastocyst) and induced pluripotent stem cells (iPSCs) (embryonic–like stem cells reprogrammed from adult cells) toward the osteoblasts and osteocyte-like cells (bone-forming cells), some of investigations were shown that application of these most known pluripotent stem cells in osteoporosis treatment is limited due to ethical concerns (20, 117, 118). Recently, implementation of very small embryonic-like stem cells (VSELs) (non-hematopoietic pluripotent cells that express embryonic characteristics markers and stored during the organogenesis in organs and tissues) as the autologous treatment for decreasing the aging processes which lead to osteoporosis and other skeletal disorders is taken into consideration. However, according to some studies, VSELs population will decrease with aging (20, 119, 120).
Mesenchymal Stem Cells in Osteoporosis
In osteoporosis, there is a reduction in endogenous MSCs function (proliferation, differentiation, and consequently bones formation). Accordingly, they are the most common types of stem cells investigated in osteoporosis treatment. In this respect, examples of MSCs transplantation in osteoporotic animal models and humans were shown in Table 2. MSCs are an important example of non-hematopoietic stem cells with less ethical concerns and numerous advantages for clinical usage, containing accessibility and ease of harvesting, immunosuppressive outcomes, multi-lineal differentiation ability (especially ability to differentiate into osteoblasts), and any possibility of malignant transformation (21, 131–133). Additionally, as a subset of stromal stem cells, they can be obtained from various tissue sources. Bone marrow derive MSCs (BM-MSCs) with high osteogenic differentiation capability are the most common types of MSCs which have been used for osteoporosis (20, 24, 134–136). Herein, accumulating evidence indicates that alternation in the molecular mechanisms which modulate osteoblast differentiation in MSCs will make the MSC therapies reliable and more effective for osteoporosis (105, 137–139). While in accordance with other studies the most therapeutic impressions of MSCs are due to their supporting regenerative microenvironment ability and paracrine effects rather than their differentiation ability. In other words, MSC transplantation might open a new chapter in osteoporosis treatment specifically through paracrine effects (Figure 2) (140–143).
Table 2.
Examples of MSCs transplantation in osteoporotic animal models and humans.
| UC-MSC | ADMSC | BM-MSC | Stem cell type |
|---|---|---|---|
| −30 ovariectomized rats (2018) (121) - 30 Wistar rats (2018) (122) - 20 e Balb/c nude mice (2008) (123) |
−30 ovariectomized rats (2018) (124) - 22 SAMP6 mice (2014) (125) - 27 Balb/c nude mice (2011) (126) |
−60 estrogen deficiency-induced osteoporotic - C57/BL6 mice (2017) (127) - 22 goats (2012) (128) - 25 isogenic Wistar rats (2010) (129) - 30 number of rabbits (2006) (130) |
Animal Study |
| – | 8 participants (2012–2014) ClinicalTrials.gov Identifier: NCT01532076 (105) | 10 participants (2015–2018) ClinicalTrials.gov Identifier: NCT02566655 (105) | Clinical Trial |
Figure 2.
Paracrine Effects of Mesenchymal Stem Cells in Bone Regeneration. Mesenchymal stem cells (MSCs) can participate in bone regeneration by secreting bioactive molecules such as Insulin-like growth factor 1 (IGF-1), Transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), interleukin−6 (IL-6), and fibroblast growth factor (FGF) (140–143).
Conclusion and Future Directions
The burden of osteoporosis is raised by an increase in the proportion of older persons in societies. Routine treatments only alleviate the symptoms partially. Hence, they are not sufficient enough. Therein, regenerative medicine sheds light on the treatment of osteoporosis. Specifically, MSCs therapy is the most common technique of regenerative medicine in osteoporosis treatment. Moreover, using small molecules (e.g., PTH and oxytocin) which employ endogenous stem cells for osteoporosis treatment will be intertwined in future management (20, 144). Despite the many investigations in cell therapy for osteoporosis, further studies are still demanded to fulfill the gaps including the definite differentiation fate and biodistribution of transplanted stem cells. On the other hand, in accordance with growing advances in osteoporosis personalized medicine (the applying of specific medical treatment based on the individual characteristics of each patient), it is required to identify the important bone loss signaling pathways and genes involved in each individual (145–148). In this context, metabolomics evaluation (the principled investigation of small molecules profile in a biological system) (149, 150) also can be helpful to the osteoporosis diagnosis of individuals with a genetic capacity (151, 152). Additionally, the biomedical using of exosomal based treatments will present novel approaches in clinical practice for osteoporosis (116).
Author Contributions
BA contributed substantially to the conception and design of the study. MP conducted search strategy and data collection. MS and SA-M drafted critical revision of the article. PG and KG revised the article critically for important intellectual content. NM gave final approval of the version to be submitted and any revised version. BL agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Mohsen Khorshidi and Shokouh Salimi for their considerable assistance.
Glossary
Abbreviations
- BMD
Bone Mineral Density
- WHO
World Health Organization
- MSCs
Mesenchymal Stem Cells
- Runx2
Runt-related transcription factor 2
- OSX
Osterix
- Atf4
Activating transcription factor 4
- AP-1
Activator Protein 1
- FGFs
Fibroblast Growth Factors
- TGF-β
Transforming Growth Factor β
- IGF-1
Insulin-like Growth Factor 1
- BMP
Bone Morphogenetic Protein
- PTH
Parathyroid hormone
- MMP-9
Matrix Metallopeptidase 9
- M-CSF
Monocyte/Macrophage Colony-Stimulating Factor
- OPG
Osteoprotegerin
- HSC
Hematopoietic Stem Cells
- IL-1
Interleukin1
- Il-6
Interleukin 6
- TNFα
Tumor Necrosis Factor α
- DXA
Dual X-ray Absorptiometry
- SERMs
Selective Estrogen Receptor Modulators
- IV
Intravenous
- HRT
Hormone Replacement Therapy
- VEGF
Vascular Endothelial Growth Factor
- HGF
Hepatocyte Growth Factor
- ESCs
Embryonic Stem Cells
- iPSCs
induced Pluripotent Stem Cells
- VELs
Very small Embryonic-Like stem cells
- BM- MSCs
Bone Marrow Mesenchymal Stem Cells.
References
- 1.Sözen T, Özişik L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. (2017) 4:46. 10.5152/eurjrheum.2016.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis JR, Safford MM. Management of osteoporosis among the elderly with other chronic medical conditions. Drugs Aging. (2012) 29:549–64. 10.2165/11599620-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alswat KA. Gender disparities in osteoporosis. J Clin Med Res. (2017) 9:382. 10.14740/jocmr2970w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. (2019) 393:364–76. 10.1016/S0140-6736(18)32112-3 [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. (2002) 359:1761–7. 10.1016/S0140-6736(02)08657-9 [DOI] [PubMed] [Google Scholar]
- 6.Dobbs MB, Buckwalter J, Saltzman C. Osteoporosis: the increasing role of the orthopaedist. Iowa Orthopaed J. (1999) 19:43. [PMC free article] [PubMed] [Google Scholar]
- 7.Letarouilly J-G, Broux O, Clabaut A. New insights into the epigenetics of osteoporosis. Genomics. (2019) 111:793–8. 10.1016/j.ygeno.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Feng X, McDonald JM. Disorders of bone remodeling. Ann Rev Pathol Mech Dis. (2011) 6:121–45. 10.1146/annurev-pathol-011110-130203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization task-force for osteoporosis. Osteopor Int. (1999) 10:259. 10.1007/s001980050224 [DOI] [PubMed] [Google Scholar]
- 10.Todd J, Robinson R. Osteoporosis and exercise. Postgrad Med J. (2003) 79:320–3. 10.1136/pmj.79.932.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binkley N. Osteoporosis in men. Arquivos Brasil Endocrinol Metabol. (2006) 50:764–74. 10.1590/S0004-27302006000400021 [DOI] [PubMed] [Google Scholar]
- 12.Bawa Se. The significance of soy protein and soy bioactive compounds in the prophylaxis and treatment of osteoporosis. J Osteopor. (2010). 2010:891058. 10.4061/2010/891058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuti R, Brandi ML, Checchia G, Di Munno O, Dominguez L, Falaschi P, et al. Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med. (2019) 14:85–102. 10.1007/s11739-018-1874-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bono CM, Einhorn TA. Overview of osteoporosis: pathophysiology and determinants of bone strength. Eur Spine J. (2003) 12(Suppl. 2):S90–6. 10.1007/s00586-003-0603-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P. Bone mechanical properties and changes with osteoporosis. Injury. (2016) 47:S11–20. 10.1016/S0020-1383(16)47003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpintero P, Caeiro JR, Carpintero R, Morales A, Silva S, Mesa M. Complications of hip fractures: a review. World J Orthoped. (2014) 5:402. 10.5312/wjo.v5.i4.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y-K, Koo K-H. Osteoporotic hip fracture in the elderly patients: physicians' views. J Korean Med Sci. (2013) 28:976–7. 10.3346/jkms.2013.28.7.976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skjodt MK, Frost M, Abrahamsen B. Side effects of drugs for osteoporosis and metastatic bone disease. Br J Clin Pharmacol. (2019) 85:1063–71. 10.1111/bcp.13759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, et al. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int. (2018) 2018:2495848. 10.1155/2018/2495848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paspaliaris V, Kolios G. Stem cells in osteoporosis: from biology to new therapeutic approaches. Stem Cells Int. (2019) 2019:1730978. 10.1155/2019/1730978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aghebati-Maleki L, Dolati S, Zandi R, Fotouhi A, Ahmadi M, Aghebati A, et al. Prospect of mesenchymal stem cells in therapy of osteoporosis: a review. J Cell Physiol. (2019) 234:8570–8. 10.1002/jcp.27833 [DOI] [PubMed] [Google Scholar]
- 22.Goodarzi P, Payab M, Alavi-Moghadam S, Larijani B, Rahim F, Bana N, et al. Development and validation of Alzheimer's Disease animal model for the purpose of regenerative medicine. Cell Tissue Bank. (2019) 20:141–51. 10.1007/s10561-019-09773-8 [DOI] [PubMed] [Google Scholar]
- 23.Goodarzi P, Aghayan HR, Payab M, Larijani B, Alavi-Moghadam S, Sarvari M, et al. Human fetal skin fibroblast isolation and expansion for clinical application. Methods Mol Biol. (2019) 2109:261–73. 10.1007/7651_2019_233 [DOI] [PubMed] [Google Scholar]
- 24.Goodarzi P, Falahzadeh K, Aghayan H, Payab M, Larijani B, Alavi-Moghadam S, et al. Therapeutic abortion and ectopic pregnancy: alternative sources for fetal stem cell research and therapy in Iran as an Islamic country. Cell Tissue Bank. (2019) 20:11–24. 10.1007/s10561-018-9741-y [DOI] [PubMed] [Google Scholar]
- 25.Payab M, Goodarzi P, Heravani NF, Hadavandkhani M, Zarei Z, Falahzadeh K, et al. Stem cell and obesity: current state and future perspective. Cell Biol Transl Med. (2018) 2:1–22. 10.1007/5584_2018_227 [DOI] [PubMed] [Google Scholar]
- 26.Goodarzi P, Aghayan HR, Larijani B, Soleimani M, Dehpour A-R, Sahebjam M, et al. Stem cell-based approach for the treatment of Parkinson's disease. Med J Islamic Repub Iran. (2015) 29:168. [PMC free article] [PubMed] [Google Scholar]
- 27.Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. (2003) 19:458–66. 10.1016/S0168-9525(03)00176-8 [DOI] [PubMed] [Google Scholar]
- 28.Fung Ling Chau J, Fook Leong W, Li B. Signaling pathways governing osteoblast proliferation, differentiation and function. Histol Histopathol. (2009) 24:1593–606. 10.14670/HH-24.1593 [DOI] [PubMed] [Google Scholar]
- 29.Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. (2007) 12:3068. 10.2741/2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu S, Franceschi RT, Luo M, Fan J, Jiang D, Cao H, et al. Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone. PloS ONE. (2009) 4:e7583. 10.1371/journal.pone.0007583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer MB, Benkusky NA, Pike JW. The RUNX2 cistrome in osteoblasts characterization, down-regulation following differentiation, and relationship to gene expression. J Biol Chem. (2014) 289:16016–31. 10.1074/jbc.M114.552216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. (2012) 13:27–38. 10.1038/nrm3254 [DOI] [PubMed] [Google Scholar]
- 33.Soltanoff CS, Chen W, Yang S, Li YP. Signaling networks that control the lineage commitment and differentiation of bone cells. Crit Rev Eukaryot Gene Exp. (2009) 19:1–46. 10.1615/CritRevEukarGeneExpr.v19.i1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parra-Torres AY, Valdés-Flores M, Orozco L, Velázquez-Cruz R. Molecular aspects of bone remodeling. Topics in Osteoporosis. Rijeka: INTECH; (2013). p. 1–27. [Google Scholar]
- 35.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. J Biol Chem. (2000) 275:4858–64. 10.1074/jbc.275.7.4858 [DOI] [PubMed] [Google Scholar]
- 36.Yokota K, Sato K, Miyazaki T, Kitaura H, Kayama H, Miyoshi F, et al. Combination of tumor necrosis factor α and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthr Rheumatol. (2014) 66:121–9. 10.1002/art.38218 [DOI] [PubMed] [Google Scholar]
- 37.Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci. (2012) 9:825. 10.7150/ijms.5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorincz C, Manske SL, Zernicke R. Bone health: part 1, nutrition. Sports Health. (2009) 1:253–60. 10.1177/1941738109334213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunstan CR, Blair JM, Zhou H, Seibel MJ. Bone, mineral, connective tissue metabolism. Oxford: Elsevier; (2007). p. 495–520. [Google Scholar]
- 40.Florencio-Silva R, Sasso GRS, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. (2015) 2015:421746. 10.1155/2015/421746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilezikian JP, Raisz LG, Martin TJ. Principles of Bone Biology. New York, NY: Academic press; (2008). [Google Scholar]
- 42.Burr DB, Allen MR. Basic and Applied Bone Biology. Indiapolis: Academic Press; (2019). [Google Scholar]
- 43.Sims NA, Gooi JH (editors). Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. (2008) 19:444–51. 10.1016/j.semcdb.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 44.Allen MR, Burr DB. Bone modeling and remodeling. Basic Appl Bone Biol. (2014) 2014:75–90. 10.1016/B978-0-12-416015-6.00004-6 [DOI] [Google Scholar]
- 45.Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskel Dis. (2016) 8:225–35. 10.1177/1759720X16670154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Rep. (2014) 3:481. 10.1038/bonekey.2013.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caetano-Lopes J, Canhao H, Eurico Fonseca J. Osteoblasts and bone formation. Acta Reumatol Portuguesa. (2007) 32:103–10. [PubMed] [Google Scholar]
- 48.Lonergan SM, Topel DG, Marple DN. The Science of Animal Growth and Meat Technology. Ames, IA: Academic Press; (2018). [Google Scholar]
- 49.Netter FH. Musculoskeletal System: Anatomy, Physiology, and Metabolic Disorders. Summit, NJ: Ciba-Geigy Corp; (1987). p. 169. [Google Scholar]
- 50.Brighton CT, Sugioka Y, Hunt RM. Cytoplasmic structures of epiphyseal plate chondrocytes. Quantitative evaluation using electron micrographs of rat costochondral junctions with special reference to the fate of hypertrophic cells. J Bone Joint Surg Am. (1973) 55:771–84. 10.2106/00004623-197355040-00012 [DOI] [PubMed] [Google Scholar]
- 51.Labens R, Schramme MC, Barr AR. Orthopaedics 1: diagnosis of lameness/diseases of joints and bones. In: Mair T, Love S, Schumacher J, Smith R, Frazer G, editors. Equine Medicine, Surgery and Reproduction. Oxford: W.B. Saunders; (2013). p. 309–28. [Google Scholar]
- 52.de Crombrugghe B, Lefebvre V, Nakashima K. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr Opin Cell Biol. (2001) 13:721–8. 10.1016/S0955-0674(00)00276-3 [DOI] [PubMed] [Google Scholar]
- 53.Javed A, Chen H, Ghori FY. Genetic and transcriptional control of bone formation. Oral Maxillofacial Surg Clin. (2010) 22:283–93. 10.1016/j.coms.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SH, Kim TS, Choi Y, Lorenzo J. Osteoimmunology: cytokines and the skeletal system. BMB Rep. (2008) 41:495. 10.5483/BMBRep.2008.41.7.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayrapetyan A, Jansen JA, van den Beucken JJ. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng Part B Rev. (2015) 21:75–87. 10.1089/ten.teb.2014.0119 [DOI] [PubMed] [Google Scholar]
- 56.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGFβ and BMP. Bone. (1996) 19:S1–2. 10.1016/S8756-3282(96)00138-X [DOI] [PubMed] [Google Scholar]
- 57.Tan S, Zhang B, Zhu X, Ao P, Guo H, Yi W, et al. Deregulation of bone forming cells in bone diseases and anabolic effects of strontium-containing agents and biomaterials. Biomed Res Int. (2014) 2014:814057. 10.1155/2014/814057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marie PJ, Kassem M. Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur J Endocrinol. (2011) 165:1. 10.1530/EJE-11-0132 [DOI] [PubMed] [Google Scholar]
- 59.Yuan J, Xin F, Jiang W. Underlying signaling pathways and therapeutic applications of pulsed electromagnetic fields in bone repair. Cell Physiol Biochem. (2018) 46:1581–94. 10.1159/000489206 [DOI] [PubMed] [Google Scholar]
- 60.Xu F, Teitelbaum SL. Osteoclasts: new insights. Bone Res. (2013) 1:11 10.4248/BR201301003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res. (2015) 2015:615486. 10.1155/2015/615486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rucci N. Molecular biology of bone remodelling. Clin Cases Miner Bone Metab. (2008) 5:49–56. [PMC free article] [PubMed] [Google Scholar]
- 63.Teitelbaum SL. Bone resorption by osteoclasts. Science. (2000) 289:1504–8. 10.1126/science.289.5484.1504 [DOI] [PubMed] [Google Scholar]
- 64.Dong Z, Bonfil RD, Chinni S, Deng X, Trindade Filho JC, Bernardo M, et al. Matrix metalloproteinase activity and osteoclasts in experimental prostate cancer bone metastasis tissue. Am J Pathol. (2005) 166:1173–86. 10.1016/S0002-9440(10)62337-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henriksen K, Bollerslev J, Everts V, Karsdal MA. Osteoclast activity and subtypes as a function of physiology and pathology—implications for future treatments of osteoporosis. Endocr Rev. (2011) 32:31–63. 10.1210/er.2010-0006 [DOI] [PubMed] [Google Scholar]
- 66.Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor. Bone. (1999) 25:517–23. 10.1016/S8756-3282(99)00210-0 [DOI] [PubMed] [Google Scholar]
- 67.Yamashita T, Takahashi N, Udagawa N. New roles of osteoblasts involved in osteoclast differentiation. World J Orthoped. (2012) 3:175. 10.5312/wjo.v3.i11.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ross FP. M-CSF, c-Fms, and signaling in osteoclasts and their precursors. Ann N Y Acad Sci. (2006) 1068:110–6. 10.1196/annals.1346.014 [DOI] [PubMed] [Google Scholar]
- 69.Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. (2017) 40:706–13. 10.14348/molcells.2017.0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast–osteoclast interactions. Connect Tissue Res. (2018) 59:99–107. 10.1080/03008207.2017.1290085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wright HL, McCarthy HS, Middleton J, Marshall MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med. (2009) 2:56–64. 10.1007/s12178-009-9046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faccio R, Choi Y, Teitelbaum SL, Takayanagi H. The osteoclast: the pioneer of osteoimmunology. Osteoimmunology. (2011) 2011:141–85. 10.1016/B978-0-12-375670-1.10006-8 [DOI] [Google Scholar]
- 73.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. (2007) 9:S1. 10.1186/ar2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. (2011) 377:1276–87. 10.1016/S0140-6736(10)62349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Almeida M. Aging mechanisms in bone. BoneKEy Rep. (2012) 1:102. 10.1038/bonekey.2012.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. (2012) 4:61–76. 10.1177/1759720X11430858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai P, Song Q, Yang C, Li Z, Liu S, Liu B, et al. Loss of Rictor with aging in osteoblasts promotes age-related bone loss. Cell Death Dis. (2016) 7:e2408. 10.1038/cddis.2016.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peris P, Guanabens N, Monegal A, Suris X, Alvarez L, De Osaba MM, et al. Aetiology and presenting symptoms in male osteoporosis. Rheumatology. (1995) 34:936–41. 10.1093/rheumatology/34.10.936 [DOI] [PubMed] [Google Scholar]
- 79.El-Desouki MI. Osteoporosis in postmenopausal Saudi women using dual x-ray bone densitometry. Saudi Med J. (2003) 24:953–6. [PubMed] [Google Scholar]
- 80.Dimai HP. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T-and Z-score, and reference databases. Bone. (2017) 104:39–43. 10.1016/j.bone.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 81.Abrahamsen B. Adverse effects of bisphosphonates. Calcified Tissue Int. (2010) 86:421–35. 10.1007/s00223-010-9364-1 [DOI] [PubMed] [Google Scholar]
- 82.McClung MR. Bisphosphonates. Endocrinol Metab Clin North Am. (2003) 32:253–71. 10.1016/S0889-8529(02)00079-8 [DOI] [PubMed] [Google Scholar]
- 83.Han SL, Wan SL. Effect of teriparatide on bone mineral density and fracture in postmenopausal osteoporosis: meta-analysis of randomised controlled trials. Int J Clin Pract. (2012) 66:199–209. 10.1111/j.1742-1241.2011.02837.x [DOI] [PubMed] [Google Scholar]
- 84.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. (2010) 95:1838–45. 10.1210/jc.2009-1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.An KC. Selective estrogen receptor modulators. Asian Spine J. (2016) 10:787 10.4184/asj.2016.10.4.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kulak Júnior J, Kulak CA, Taylor HS. SERMs in the prevention and treatment of postmenopausal osteoporosis: an update. Arq Brasil Endocrinol Metab. (2010) 54:200–5. 10.1590/S0004-27302010000200016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eriksen EF, Keaveny TM, Gallagher ER, Krege JH. Literature review: the effects of teriparatide therapy at the hip in patients with osteoporosis. Bone. (2014) 67:246–56. 10.1016/j.bone.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 88.Gambacciani M, Levancini M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Przeglad menopauzalny Menopause Rev. (2014) 13:213. 10.5114/pm.2014.44996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hickey M, Elliott J, Davison SL. Hormone replacement therapy. BMJ. (2012) 344:e763. 10.1136/bmj.e763 [DOI] [PubMed] [Google Scholar]
- 90.Marcucci G, Brandi ML. Vertebroplasty and balloon kyphoplasty in osteoporosis: friends or foes? Clin Cases Min Bone Metab. (2009) 6:203. [PMC free article] [PubMed] [Google Scholar]
- 91.Moreira LD, Oliveira ML, Lirani-Galvão AP, Marin-Mio RV, Santos RN, Lazaretti-Castro M. Physical exercise and osteoporosis: effects of different types of exercises on bone and physical function of postmenopausal women. Arq Brasil Endocrinol Metab. (2014) 58:514–22. 10.1590/0004-2730000003374 [DOI] [PubMed] [Google Scholar]
- 92.Kerr C, Bottomley C, Shingler S, Giangregorio L, de Freitas HM, Patel C, et al. The importance of physical function to people with osteoporosis. Osteoporosis Int. (2017) 28:1597–607. 10.1007/s00198-017-3911-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levine JP. Pharmacologic and nonpharmacologic management of osteoporosis. Clin Cornerstone. (2006) 8:40–53. 10.1016/S1098-3597(06)80064-5 [DOI] [PubMed] [Google Scholar]
- 94.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporosis Int. (2007) 18:1023–31. 10.1007/s00198-006-0322-8 [DOI] [PubMed] [Google Scholar]
- 95.Goodarzi P, Aghayan HR, Soleimani M, Norouzi-Javidan A, Mohamadi-Jahani F, Jahangiri S, et al. Stem cell therapy for treatment of epilepsy. Acta Med Iran. (2014) 52:651–5. [PubMed] [Google Scholar]
- 96.Aghayan HR, Goodarzi P, Arjmand B. GMP-compliant human adipose tissue-derived mesenchymal stem cells for cellular therapy. In: Walker J, editor. Stem Cells and Good Manufacturing Practices. New York, NY: Humana Press; (2014). pp. 93–107. [DOI] [PubMed] [Google Scholar]
- 97.Ghodsi M, Heshmat R, Amoli M, Keshtkar AA, Arjmand B, Aghayan H, et al. The effect of fetal liver-derived cell suspension allotransplantation on patients with diabetes: first year of follow-up. Acta Med Iran. (2012) 50:541–6. [PubMed] [Google Scholar]
- 98.Yu D, Silva GA. Stem cell sources and therapeutic approaches for central nervous system and neural retinal disorders. Neurosurg Focus. (2008) 24:E11. 10.3171/FOC/2008/24/3-4/E10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuraitis D, Giordano C, Suuronen EJ, Ruel M. Cell therapy to regenerate the ischemic heart. Cardiac Regen Repair. (2014) 1:118–137. 10.1533/9780857096708.2.118 [DOI] [Google Scholar]
- 100.Li F, Zhou C, Xu L, Tao S, Zhao J, Gu Q. Effect of stem cell therapy on bone mineral density: a meta-analysis of preclinical studies in animal models of osteoporosis. PloS ONE. (2016) 11:e0149400. 10.1371/journal.pone.0149400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ilic D, Miere C, Lazic E. Umbilical cord blood stem cells: clinical trials in non-hematological disorders. Br Med Bull. (2012) 102:43–57. 10.1093/bmb/lds008 [DOI] [PubMed] [Google Scholar]
- 102.Chaparro O, Linero I. Regenerative medicine: a new paradigm in bone regeneration. Adv Techn Bone Regen. (2016) 1:253–74. 10.5772/62523 [DOI] [Google Scholar]
- 103.Marini F, Cianferotti L, Brandi ML. Epigenetic mechanisms in bone biology and osteoporosis: can they drive therapeutic choices? Int J Mol Sci. (2016) 17:1329. 10.3390/ijms17081329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alagiakrishnan K, Juby A, Hanley D, Tymchak W, Sclater A. Role of vascular factors in osteoporosis. J Gerontol Ser A Biol Sci Med Sci. (2003) 58:M362–6. 10.1093/gerona/58.4.M362 [DOI] [PubMed] [Google Scholar]
- 105.Phetfong J, Sanvoranart T, Nartprayut K, Nimsanor N, Seenprachawong K, Prachayasittikul V, et al. Osteoporosis: the current status of mesenchymal stem cell-based therapy. Cell Mol Biol Lett. (2016) 21:12. 10.1186/s11658-016-0013-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Antebi B, Pelled G, Gazit D. Stem cell therapy for osteoporosis. Curr Osteoporos Rep. (2014) 12:41–7. 10.1007/s11914-013-0184-x [DOI] [PubMed] [Google Scholar]
- 107.Martín-del-Campo M, Sampedro JG, Flores-Cedillo ML, Rosales-Ibañez R, Rojo L. Bone regeneration induced by strontium folate loaded biohybrid scaffolds. Molecules. (2019) 24:1660. 10.3390/molecules24091660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beamer B, Hettrich C, Lane J. Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS J. (2010) 6:85–94. 10.1007/s11420-009-9129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, et al. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone. (2010) 47:360–70. 10.1016/j.bone.2010.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choy L, Skillington J, Derynck R. Roles of autocrine TGF-β receptor and Smad signaling in adipocyte differentiation. J Cell Biol. (2000) 149:667–82. 10.1083/jcb.149.3.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ugarte F, Ryser M, Thieme S, Fierro FA, Navratiel K, Bornhäuser M, et al. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. (2009) 37:867–75. 10.1016/j.exphem.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 112.Byun MR, Jeong H, Bae SJ, Kim AR, Hwang ES, Hong JH. TAZ is required for the osteogenic and anti-adipogenic activities of kaempferol. Bone. (2012) 50:364–72. 10.1016/j.bone.2011.10.035 [DOI] [PubMed] [Google Scholar]
- 113.James AW. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica. (2013) 2013:684736. 10.1155/2013/684736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao M, Gao W, Papadimitriou JM, Zhang C, Gao J, Zheng M. Exosomes—the enigmatic regulators of bone homeostasis. Bone Res. (2018) 6:1–3. 10.1038/s41413-018-0039-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chu C, Wei S, Wang Y, Wang Y, Man Y, Qu Y. Extracellular vesicle and mesenchymal stem cells in bone regeneration: recent progress and perspectives. J Biomed Mater Res Part A. (2019) 107:243–50. 10.1002/jbm.a.36518 [DOI] [PubMed] [Google Scholar]
- 116.Behera J, Tyagi N. Exosomes: mediators of bone diseases, protection, and therapeutics potential. Oncoscience. (2018) 5:181. 10.18632/oncoscience.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Csobonyeiova M, Polak S, Zamborsky R, Danisovic L. iPS cell technologies and their prospect for bone regeneration and disease modeling: a mini review. J Adv Res. (2017) 8:321–7. 10.1016/j.jare.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song L, Xie XB, Peng LK, Yu SJ, Peng YT. Mechanism and treatment strategy of osteoporosis after transplantation. Int J Endocrinol. (2015) 2015:280164. 10.1155/2015/280164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kassmer SH, Krause DS. Very small embryonic-like cells: biology and function of these potential endogenous pluripotent stem cells in adult tissues. Mol Reprod Dev. (2013) 80:677–90. 10.1002/mrd.22168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ratajczak MZ, Zuba-Surma EK, Shin DM, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. (2008) 43:1009–17. 10.1016/j.exger.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hong B, Lee S, Shin N, Ko Y, Kim D, Lee J, et al. Bone regeneration with umbilical cord blood mesenchymal stem cells in femoral defects of ovariectomized rats. Osteopor Sarcop. (2018) 4:95–101. 10.1016/j.afos.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hendrijantini N, Kusumaningsih T, Rostiny R, Mulawardhana P, Danudiningrat CP, Rantam FA. A potential therapy of human umbilical cord mesenchymal stem cells for bone regeneration on osteoporotic mandibular bone. Eur J Dentist. (2018) 12:358–62. 10.4103/ejd.ejd_342_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diao Y, Ma Q, Cui F, Zhong Y. Human umbilical cord mesenchymal stem cells: osteogenesis in vivo as seed cells for bone tissue engineering. J Biomed Mater Res Part A. (2009) 91:123–31. 10.1002/jbm.a.32186 [DOI] [PubMed] [Google Scholar]
- 124.Uri O, Behrbalk E, Folman Y. Local implantation of autologous adipose-derived stem cells increases femoral strength and bone density in osteoporotic rats: a randomized controlled animal study. J Orthopaed Surg. (2018) 26:2309499018799534. 10.1177/2309499018799534 [DOI] [PubMed] [Google Scholar]
- 125.Mirsaidi A, Genelin K, Vetsch JR, Stanger S, Theiss F, Lindtner RA, et al. Therapeutic potential of adipose-derived stromal cells in age-related osteoporosis. Biomaterials. (2014) 35:7326–35. 10.1016/j.biomaterials.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 126.Cho SW, Sun HJ, Yang JY, Jung JY, Choi HJ, An JH, et al. Human adipose tissue-derived stromal cell therapy prevents bone loss in ovariectomized nude mouse. Tissue Engineering Part A. (2012) 18:1067–78. 10.1089/ten.tea.2011.0355 [DOI] [PubMed] [Google Scholar]
- 127.Qi M, Zhang L, Ma Y, Shuai Y, Li L, Luo K, et al. Autophagy maintains the function of bone marrow mesenchymal stem cells to prevent estrogen deficiency-induced osteoporosis. Theranostics. (2017) 7:4498. 10.7150/thno.17949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cao L, Liu G, Gan Y, Fan Q, Yang F, Zhang X, et al. The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials. (2012) 33:5076–84. 10.1016/j.biomaterials.2012.03.069 [DOI] [PubMed] [Google Scholar]
- 129.Ocarino ND, Boeloni JN, Jorgetti V, Gomes DA, Goes AM, Serakides R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect Tissue Res. (2010) 51:426–33. 10.3109/03008201003597049 [DOI] [PubMed] [Google Scholar]
- 130.Wang Z, Goh J, De SD, Ge Z, Ouyang H, Chong JS, et al. Efficacy of bone marrow–derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. (2006) 12:1753–61. 10.1089/ten.2006.12.1753 [DOI] [PubMed] [Google Scholar]
- 131.Hamza AA, Fikry EM, Abdallah W, Amin A. Mechanistic insights into the augmented effect of bone marrow mesenchymal stem cells and thiazolidinediones in streptozotocin-nicotinamide induced diabetic rats. Sci Rep. (2018) 8:1–8. 10.1038/s41598-018-28029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. (2013) 19:35–42. 10.1038/nm.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Su P, Tian Y, Yang C, Ma X, Wang X, Pei J, et al. Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int J Mol Sci. (2018) 19:2343. 10.3390/ijms19082343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. (2012) 16:582–92. 10.1111/j.1582-4934.2011.01335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu HY, Chiou JF, Wu AT, Tsai CY, Leu JD, Ting LL, et al. The effect of diminished osteogenic signals on reduced osteoporosis recovery in aged mice and the potential therapeutic use of adipose-derived stem cells. Biomaterials. (2012) 33:6105–12. 10.1016/j.biomaterials.2012.05.024 [DOI] [PubMed] [Google Scholar]
- 136.Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. (2012) 1:237–47. 10.5966/sctm.2011-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pino AM, Rosen CJ, Rodríguez JP. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res. (2012) 45:279–87. 10.4067/S0716-97602012000300009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Infante A, Rodríguez CI. Osteogenesis and aging: lessons from mesenchymal stem cells. Stem Cell Res Ther. (2018) 9:1–7. 10.1186/s13287-018-0995-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oryan A, Kamali A, Moshiri A, Eslaminejad MB. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs. (2017) 204:59–83. 10.1159/000469704 [DOI] [PubMed] [Google Scholar]
- 140.Nimiritsky PP, Eremichev RY, Alexandrushkina NA, Efimenko AY, Tkachuk VA, Makarevich PI. Unveiling mesenchymal stromal cells' organizing function in regeneration. Int J Mol Sci. (2019) 20:823. 10.3390/ijms20040823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PloS ONE. (2014) 9:e107001. 10.1371/journal.pone.0107001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanchooli T. Prospect of mesenchymal stem cells conditioned medium in tissue regeneration. Gene Cell Tissue. (2017) 4:965849 10.5812/gct.64645 [DOI] [Google Scholar]
- 143.Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. (2012) 26:1166–73. 10.1038/leu.2011.389 [DOI] [PubMed] [Google Scholar]
- 144.Huber BC, Grabmaier U, Brunner S. Impact of parathyroid hormone on bone marrow-derived stem cell mobilization and migration. World J Stem Cells. (2014) 6:637. 10.4252/wjsc.v6.i5.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goldshtein I, Rouach V, Shamir-Stein N, Yu J, Chodick G. Role of side effects, physician involvement, and patient perception in non-adherence with oral bisphosphonates. Adv Ther. (2016) 33:1374–84. 10.1007/s12325-016-0360-3 [DOI] [PubMed] [Google Scholar]
- 146.Arjmand B, Abdollahi M, Larijani B. Precision medicine: a new revolution in healthcare system. Iran Biomed J. (2017) 21:282–3. [PubMed] [Google Scholar]
- 147.Arjmand B, Goodarzi P, Mohamadi-Jahani F, Falahzadeh K, Larijani B. Personalized regenerative medicine. Acta Med Iran. (2017) 55:144–9. [PubMed] [Google Scholar]
- 148.Arjmand B, Larijani B. Personalized medicine: a new era in endocrinology. Acta Med Iran. (2017) 55:142–3. [PubMed] [Google Scholar]
- 149.Goodarzi P, Alavi-Moghadam S, Payab M, Larijani B, Rahim F, Gilany K, et al. Metabolomics analysis of mesenchymal stem cells. Int J Mol Cell Med. (2019) 8:30–40. 10.22088/IJMCM.BUMS.8.2.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Larijani B, Goodarzi P, Payab M, Alavi-Moghadam S, Rahim F, Bana N, et al. Metabolomics and cell therapy in Diabetes Mellitus. Int J Mol Cell Med. (2019) 8:41–48. 10.22088/IJMCM.BUMS.8.2.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao Q, Shen H, Su KJ, Zhang JG, Tian Q, Zhao LJ, et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutr Metab. (2018) 15:1–9. 10.1186/s12986-018-0296-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lv H, Jiang F, Guan D, Lu C, Guo B, Chan C, et al. Metabolomics and its application in the development of discovering biomarkers for osteoporosis research. Int J Mol Sci. (2016) 17:2018. 10.3390/ijms17122018 [DOI] [PMC free article] [PubMed] [Google Scholar]