Abstract
Mikania micrantha and Ipomoea cairica are two invasive plants widely distribute and seriously damage in Hainan island. In this study, the leaves extracts of two weeds were collected and determined for their allelopathic potentials on Chrysanthemum coronarium. The phytotoxicity bioassay showed that when the extract concentration was 50 and 100 mg/ml, the inhibited effects of M. micrantha on growth of C. coronarium were greater than by I. cairica. However, when the extract concertation at 400 mg/ml, the opposite inhibited effects were observed. We speculated this phenomenon was caused by different allelopathic compounds. Therefore, using gas chromatography–mass spectrometry, 19 and 23 compounds were identified respectively, benzoic acid and cinnamic acid were the main components in the two leaves extracts, which were selected to carry out the further bioassays. Subsequent bioassay results showed the effects of two allelochemicals on morphological index and chlorophyll content and POD activity were all negative to C. coronarium, whereas the content of MDA and activity of SOD, CAT represented adverse changes. Moreover, the inhibitions by cinnamic acid were generally greater than those by benzoic acid. Thus, the phenolic acids played the most crucial roles in the allelopathic effccts of M. micrantha and I. cairica leaves extracts.
Subject terms: Biochemistry, Plant sciences
Introduction
Exotic plant invasion is an important part of global change, and one of the most severe environmental problems facing humanity1–3. Hainan island, a tropical province of China, with light, temperature, water and soil conditions suitable for plant propagation and growth. However, its ecological environment is relatively fragile. Coupled with the increasing frequency of foreign trade and exchanges in recent years, a large number of exotic plants have settled down. Mikania micrantha is one of the most harmful invasive alien weeds, native to South and Central America, and known as mile-a-minute weeds4–6, while Ipomoea cairica is the second most important invasive plant in Hainan island in recent years7. They are perennial twining lianas with similar ecological niches, which can be associated with each other to form dominant populations in new habitats, seriously affecting agricultural and forestry production and destroying biodiversity8,9.
To clarify the invasion mechanism of M. micrantha and I. cairica, scholars have done a lot of research. On the one hand, some studies attributed their successful invasion to substantial reproduction and wide eco-physiological tolerance, which can efficiently capture and utilize environmental resources10,11. I. cairica not only has a higher photosynthetic rate and utilization rate of photosynthetic nitrogen, but also has a higher specific leaf area and lower leaf construction cost, which reflects its advantages in the utilization efficiency of carbon and nitrogen resources12. Similarly, the leaves of M. micrantha also have a relatively higher photosynthetic capacity and water using efficiency13,14. On the other hand, the allelopathic effects of M. micrantha and I. cairica are widely received as an important role in biological invasion. Allelopathy is defined as the direct or indirect inhibitory or stimulatory effects of one plant on another (including microorganisms) by producing compounds that escape from plants into the environment, and is concerned to be a powerful chemical weapon during the invasion process of alien plants15–17. It was found the extracts of M. micrantha and I. cairica can effectively inhibit the germination and seedling growth of tested plants18–21. Moreover, the soil treated with a higher concentration of M. micrantha leaves extracts was also reported to actively suppress seedling growth22. Previous studies have shown that both weeds have allelopathic effects, however, little knowledge has been reported about their effects on the same species. Whether C. chinensis will produce a stronger allelopathic effect against the invasion of M. micrantha is unknown. If so, is the growth inhibited effect of the two weeds on the colony and nearby grassland species caused by the different allelochemicals produced by the two? As early as 1964, scholars such as Muller suggested that there were intimate relationships between chemicals extracted from allelopathic plants and its distinguished competition in the new ranges23. And this competitiveness varied with the composition of the compounds24–26. Thus, we speculated that some allelochemicals play an important role in allelopathy of M. micrantha and C. chinensis.
To answer the above questions, M. micrantha and C. chinensis were used as research objects, Chrysanthemum coronarium was chosen as receiver species to measure the different effects of leaves extracts from two weeds. Then the compounds of extracts identified by gas phase and mass spectrometry (GC–MS) respectively. To investigate the critical compound in the two extracts and ascertain the dominant role in the allelopathic effect of two weeds, two components tested in the above identification procedure were carried out the next physiological and biochemical experiments on C. coronarium.
Methods and materials
Plant materials
The aerial parts of M. micrantha and I. cairica were gathered from Hainan University (Hainan province, China, 20° 3.75′ N, 110° 20.13′ E), and the leaves were cut from the vine. Then, the voucher specimen of M. micrantha and I. cairica were deposited in the Environmental Science and Engineering Laboratory of Hainan University. The numbers of the specimen were MM-20170521-001 and IC-20170521-002 respectively. Seeds of C. coronarium were purchased from Wuchang Shenniu Seeds Company (Hubei province, China). Uniform healthy seeds were collected and stored in the refrigerator at 4 °C.
Preparation of plant extracts
One hundred grams of leaves of M. micrantha and I. cairica were picked and washed with tap water, then with distilled water and dried for 2 h. Leaves were then manually chopped and placed at the bottom of an airproof glass bottle (1,000 ml), added to 700 ml distilled water into the jar and shaken well. The mixture was sealed and stored in a growth chamber at 25 °C. Every day the mixture was filtered through a Whatman no.1 filter paper and collected into a new glass bottle (2500 ml) on time, and 700 ml of distilled water was added to the glass bottle of the mixture again. Three days later, all supernatants (2,100 ml) were collected and removed to the SHB-IIIG rotary evaporator (EYELA, Japan). Then vacuum-distilled in a 60 °C water bath by N-1100 vacuum pump (EYELA, Japan) until 10 ml of brown crude resinous was obtained, that is, the concentrated plant extracts were 10 g/ml. Finally, the foliage extracts of M. micrantha and I. cairica from the above-stated were added to distilled water to diluted, with the concentration were 50 mg/ml, 100 mg/ml, 200 mg/ml, and 400 mg/ml respectively, which were optimized from prior experiments, and then stored refrigerator at 4 °C.
Phytotoxicity Bioassays of extracts of Mikania micrantha and Ipomoea cairica
An airproof glass bottle (0.3 L) was used to assess the morphological inhibitions of aqueous extracts. Thirty un-sprouted seeds of C. coronarium were selected randomly and put in a glass bottle on two sheets of filter paper moistened with 5 ml distilled water. Then 2 ml extracts of M. micrantha and I. cairica with different concentrations (50, 100, 200, 400 mg/ml) were added separately per glass bottle whereas the control bottle received 2 ml distilled water. For each species, there were five replicates. The bottles were incubated at 25 °C and 70% relative humidity with a photoperiod 12/12 h day/night. During the seed germination and seedling growth, distilled water was added to keep the filter paper moist. Seed germination was counted daily. When the radicle length was over 2 mm, the seeds were considered as germinated. The germination rate was represented by the number of seeds germinated in a day divided by the number of total seeds (30). Besides, the fresh weight, root and shoot lengths were measured after 7 days. Among them, the fresh weight was obtained by subtracting the weight of filter paper from the weight of plant seedlings + filter paper, and the length of root and shoot were measured with calipers (V5964-A150mm-1EA, Aladdin, China)27.
Preparation of organic extracts
Initially, 300 ml extracts (10 g/ml) of M. micrantha and I. cairica were filtered through a water-phase filter, then subjected to chromatography column at a flow rate of 150 ml/h for elution after the resin was activated. Then these eluants were re-filtered through 0.45 μm PTFE membrane in a Buchner funnel coupled to a vacuum that collected supernatants. After this, the supernatants were dried in a rotary evaporator until they became solid before re-dissolved by dichloromethane in a test tube (15 mm × 150 mm). Afterward, the mixture was consecutively dissolved in acetonitrile (500 μL) and derivatizing reagent (99% BSTFA + 1% TMCS, 500 μL) for 1 h at 70 °C. At the end of the process, the solutions were re-filtered through 0.45 μm PTFE membrane and saved in an airproof glass bottle after cooled, and then stored refrigerator at 4 °C28,29.
The GC–MS analysis of organic extracts
Samples of the extracts of M. micrantha and I. cairica 1 μL were injected into a 7890A gas chromatograph equipped with a splitless injector coupled with a 5975C mass spectrometer (Agilent, USA). The mass spectrometer was operated in the electron ionization (EI) mode (70 eV). Helium (99.999%) was employed as a carrier gas, and its flow rate was adjusted to 1 ml/min. The chromatographic separation was performed on an HP-5 MS column (30 m × 250 μm × 0.25 μm, Agilent). The initial temperature of the column was set at 80 °C and held for 3 min, then increased by 8 °C/min to 160 °C and maintained for 2 min, followed by a rise to 240 °C at a rate of 5 °C/min. The temperatures of the GC–MS interface, ion source and quadrupole were set to 250, 230, and 150 °C, respectively. The control samples were analyzed under the same conditions. The total ion flow chart was automatically integrated into Chemstation software (Agilent, USA). Three methods were used to identify the compounds: searching them in the NIST 2011 standard spectrum library, calculating their Kovats index values30,31, and comparing their retention times with those of authenticated standards (Sigma-Aldrich, 95–99%, USA). The relative contents of the substances were calculated by the GC peak area. The external calibration curves were also used to quantify the test concentrations of compounds extracted from the samples.
Physiological and morphological analyses of the identified compounds from extracts
According to the results of the “Identification of Compounds from The Two Extracts”, benzoic acid and cinnamic acid (Sigma-Aldrich, 95–99%) were picked for further bioassay experiments, which accounted for the highest relative contents of compounds in M. micrantha and I. cairica respectively.
Seeds of C. coronarium were used to access the morphological inhibitory effects of the extracted compounds. Thirty un-sprouted seeds of C. coronarium were selected randomly and then sown in an airtight glass bottle (0.3 L) on two sheets of filter paper moistened with 5 ml distilled water. The treatment concentrations of benzoic acid and cinnamic acid were 10, 50, 100 and 200 mg/ml, which were optimized from prior experiments. Then 2 ml of two compounds of different concentrations were added separately to the glass bottle whereas control bottles received 2 ml distilled water. The culture conditions were the same as the above phytotoxicity bioassay experiment, and the experiment had three replications. One week later, samples were collected to determine the germination and morphological indices ( shoot and root length)27.
After 15 days, samples were carried to determine the physiological indices. The malondialdehyde (MDA) content was measured using the thiobarbituric acid (TBA) reaction32. Soluble protein was determined by the Coomassie brilliant blue method33. Chlorophyll content was determined by spectrophotometrically, according to Amon34. Superoxide dismutase (SOD) activity was determined by WST-1 methods (Nanjing Jiancheng Bioengineering Institute, China)35.
Statistical analysis
The allelopathic index (RI) was used to quantify the intensity of the allelopathic effect, which described by the Eq. (1)36.
| 1 |
where C as the control data and T denoted the treatment data. RI > 0 indicated that the germination or seedling of plants was promoted at present, while RI < 0 stated the inhibition effect. The absolute value of RI represented the allelopathic intensity. The volume of sensitive effect (SE) was the sum of the corresponding RI of germination, root, and shoot lengths.
All data were presented as mean ± standard error (se) and tested by IBM SPSS Statistics 20. Significant differences (p < 0.05) were analyzed using one-way ANOVA, followed by LSD’s multiple range tests. SigmaPlot 12.5 was used to draw the graph.
Results
Effects of extracts on seed germination and seedling growth
Variations in the morphological data revealed the significant impact of extracts from two weeds on the growth of tested seeds. It can be easily seen from Table 1 that both M. micrantha and I. cairica extracts significantly inhibited the seed germination of C. coronarium, especially when the extract concentration was 400 mg/l (p < 0.05), the RI of germination rate was − 0.51 and − 0.86 respectively. Similarly, some significantly antagonistic responses were observed in the root and shoot length of C. coronarium in the treatment of the two extracts, and the antagonistic effect was enhanced with the concentration of the extracts. Besides, the root length of C. coronarium in the soaking of I. cairica extracts was always inhibited stronger than the shoot length, and the extract even slightly promoted the growth of the shoot at the concentration was 50 mg/l. In contrast, the inhibition of root length treated by the extract was stronger than the stem length was observed only when the concentration of M. micrantha extracts exceeded 200 mg/l. According to the results of SE presented in Table 1, all of them were negative. For the extract concentration of 50 and 100 mg/l, the comprehensive inhibition of the recipient plants by M. micrantha was significantly stronger than that of the I. cairica (p < 0.05). However, when the concentration of the extract was 400 mg/l, the opposite phenomenon was observed.
Table 1.
Allelopathic effects of extracts from Mikania micrantha and Ipomoea cairica on seed germination and seedling growth of Chrysanthemum coronarium.
| Plants | Concentration (mg/ml) | Response index (RI) | Synthesis effect (SE) | ||
|---|---|---|---|---|---|
| Germination rate | Root length | Shoot length | |||
| Mikania micrantha | 50 | − 0.14 a | − 0.62 a* | − 1.46 a | − 2.12 a |
| 100 | − 0.38 b | − 2.74 ab | − 2.98 ab | − 6.10 b | |
| 200 | − 0.42 b | − 4.38 b | − 2.81 a | − 7.60 b | |
| 400 | − 0.53 b | − 7.29 c | − 5.09 b | − − 12.91 c* | |
| Ipomoea cairica | 50 | − 0.11 a | − 1.10 a | 0.06 a | − 1.16 a* |
| 100 | − 0.11 a* | − 2.00 b* | − − 0.08 a* | − 2.20 a* | |
| 200 | − 0.37 a | − 3.97 ab | − 0.81 a* | − 5.18 b | |
| 400 | − 0.86 b | − 13.80 c* | − 3.36 b* | − 18.06 c | |
Lowercase letters represent that different concentrations of plant extracts have a significant difference at p < 0.05, and “*” indicates that two extracts have a significant difference at p < 0.05.
Analysis of constituents of extracts
The GC–MS data showed the extracts of M. micrantha and I. cairica contained a large number of compounds (Table 2). Nineteen compounds from the M. micrantha extracts were identified, and more than half of them were acids. The relative contents of benzoic acid and lactic acid were in the top two among them, reaching 19.36% and 15.95%. On the other hand, twenty-three compounds from the I. cairica extracts were identified. Similarly, acids were the dominant source in this mixture, the relative content of which was more than 60%. And the average relative content of cinnamic acid ranked the first among the mixture, reaching 47.56%, which were roughly triple as large as the content of 2-methyl phenyl benzoate (15.75%). Therefore, the average concentrations of benzoic acid and cinnamic acid had the maximum value gaps between two weed extracts, which were selected for further experiments.
Table 2.
Comparison among the compounds from extracts of Mikania Micrantha and Ipomoea cairica.
| Compounds | Mikania Micrantha | Compounds | Ipomoea cairica | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RI | RIlit | Identification methods | Relative content of compositions (%) | RI | RIlit | Identification methods | Relative content of compositions (%) | ||
| Phenols | Esters | ||||||||
| Phenol | 1,053 | 105151 | MS/RI/Std | 13.45 | Methyl benzoate | 1,105 | 110152 | MS/RI | 0.08 |
| o-Cresol | 1,066 | 106353 | MS/RI | 8.68 | 2-Methyl phenyl benzoate | 1900 | – | MS | 15.75 |
| p-Cresol | 1,081 | 108053 | MS/RI | 6.7 | 2-Methyl-hexadecane | 1658 | 165552 | MS/RI | 0.85 |
| 2,6-Dimethylphenol | 1,108 | 110754 | MS/RI | 3.72 | Dibutyl phthalate | 1915 | 191455 | MS/RI | 1.81 |
| 2-Tert-butyl-6-methylphenol | 2056 | – | MS | 1 | Alcohols | ||||
| Esters | Glycerol | 1,132 | 112956 | MS/RI | 0.18 | ||||
| Methyl benzoate | 1,103 | 110152 | MS/RI | 0.03 | Alkanes | ||||
| Methyl cinnamate | 1,305 | 130457 | MS/RI | 1.97 | Dodecane | 1,199 | 120058 | MS/RI | 1.89 |
| Alcohols | Tetradecane | 1,402 | 140059 | MS/RI | 1.66 | ||||
| Ethylene glycol | 1,152 | – | MS | 3.52 | Octadecane | 1801 | 180060 | MS/RI | 1.05 |
| Acids | 1-Nonadecene | 1892 | 189361 | MS/RI | 2.01 | ||||
| Hydroxyacetic acid | 1,283 | – | MS | 4.66 | Hexadecane | 1785 | 178462 | MS/RI | 0.79 |
| Lactic acid | 1,490 | 1,491.163 | MS/RI | 15.95 | Acids | ||||
| Nonanoic acid | 1587 | 1584.463 | MS/RI | 0.65 | Lactic acid | 1,495 | 1,491.163 | MS/RI | 0.18 |
| Benzoic acid | 1688 | 168764 | MS/RI/Std | 19.36 | Glycolic acid | 1,510 | 1508.663 | MS/RI | 0.47 |
| Succinic acid | 1759 | 1757.663 | MS/RI | 9.88 | Decanoic acid | 1553 | 155565 | MS/RI | 5.6 |
| Lauric acid | 1883 | 188463 | MS/RI | 1.66 | Benzoic acid | 1688 | 168764 | MS/RI/Std | 0.23 |
| Salicylic acid | 1961 | 1965.363 | MS/RI | 3.32 | Succinic acid | 1755 | 1757.663 | MS/RI | 2.12 |
| Phthalic acid | 2,135 | 2,137.263 | MS/RI | 1.69 | Cinnamic acid | 1787 | 1786.963 | MS/RI/Std | 47.56 |
| Gallic acid | 2,821 | 2,822.863 | MS/RI | 1.03 | Lauric acid | 1885 | 188463 | MS/RI | 5.16 |
| Alkanes | Hexadecanoic acid | 1937 | 193866 | MS/RI | 5.5 | ||||
| Hexadecane | 1787 | 178462 | MS/RI | 1.9 | Stearic acid | 2,489 | 2,489.163 | MS/RI | 0.69 |
| Oximes | Tetradecanoic acid | 2,578 | 257667 | MS/RI | 0.37 | ||||
| 2-Acetylthiophene-O-methyloxime | 1975 | – | MS | 0.84 | Tannic acid | 3,006 | – | MS | 1.21 |
| Phenols | |||||||||
| 2,4-di(1,1-dimethylethyl)-phenol | 1889 | – | MS/RI | 0.44 | |||||
| Ketones | |||||||||
| (4-Hydroxyphenyl)-2-pentan-2-ketone | 2,861 | – | MS | 0.42 | |||||
RI linear retention indices on the HP-5MS column (relative to n-alkanes), RIlit retention index value was consistent with that in the literature, MS by comparing the MS with that in the NIST library, Std by comparing time and MS with those of available authentic standards. “–” not found.
Morphological indices of the compounds from extracts on seedlings
Concerning the experiments on allelopathic effects of the two invasive plant extracts, the interaction between allelochemicals and concentrations was signification in the parameters evaluated (Fig. 1). The compounds from the extracts were vital inhibited seeds germination under simulating foliage extracts process (p < 0.05), which caused the germination decreased by 51.11% and 65.56% respectively at 200 mg/ml compared with control groups in 7 days. At the same time, the root length and shoot length of C. coronarium exposed to benzoic acid and cinnamic acid were also substantially suppressed compared to controls, and the inhibited effects were linear to the increasing concentrations of the compounds. In general, cinnamic acid was more inhibitory than benzoic acid (p < 0.05).
Figure 1.
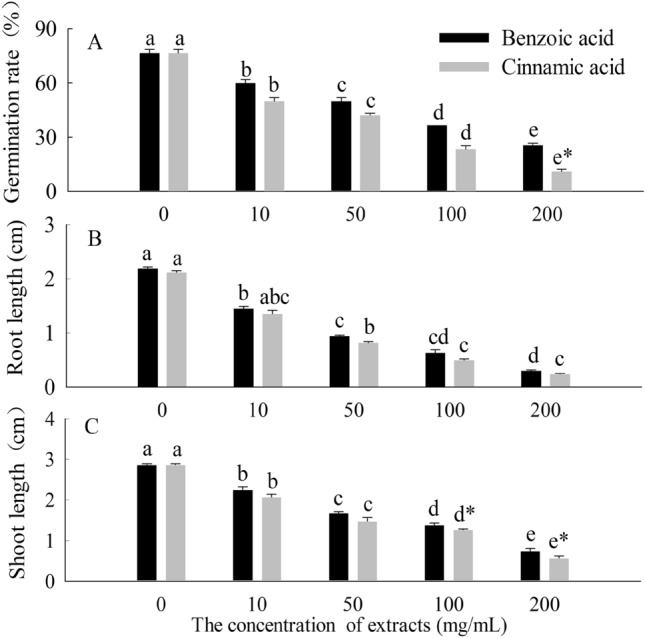
Effects of benzoic acid and cinnamic acid on germination rate (A), root length (B) and shoot length (C) of Chrysanthemum coronarium seeds and seedlings. Different lower letters indicate significant differences between the different concentrations of the same allelochemicals at the 0.05 level. The symbol of “*” indicates significant differences between the different chemicals of the same concentrations at the 0.05 level. The same as below.
Physiological indices of the compounds from extracts on seedlings
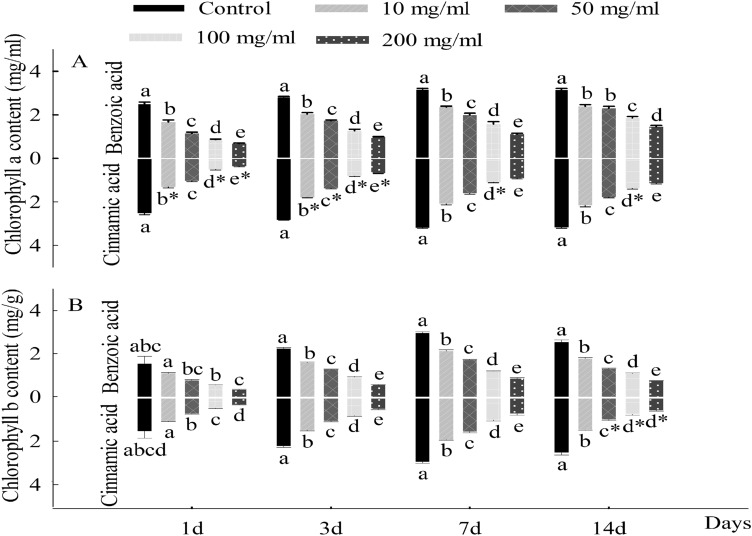
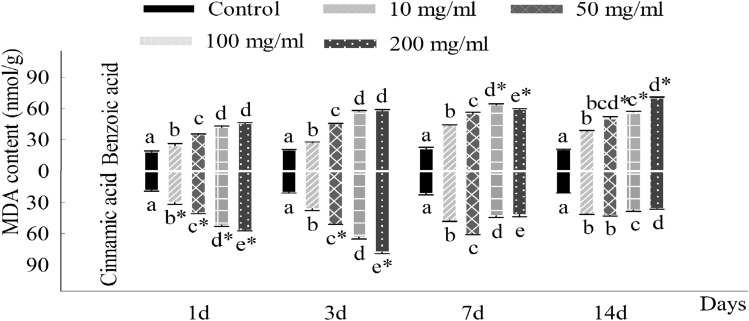
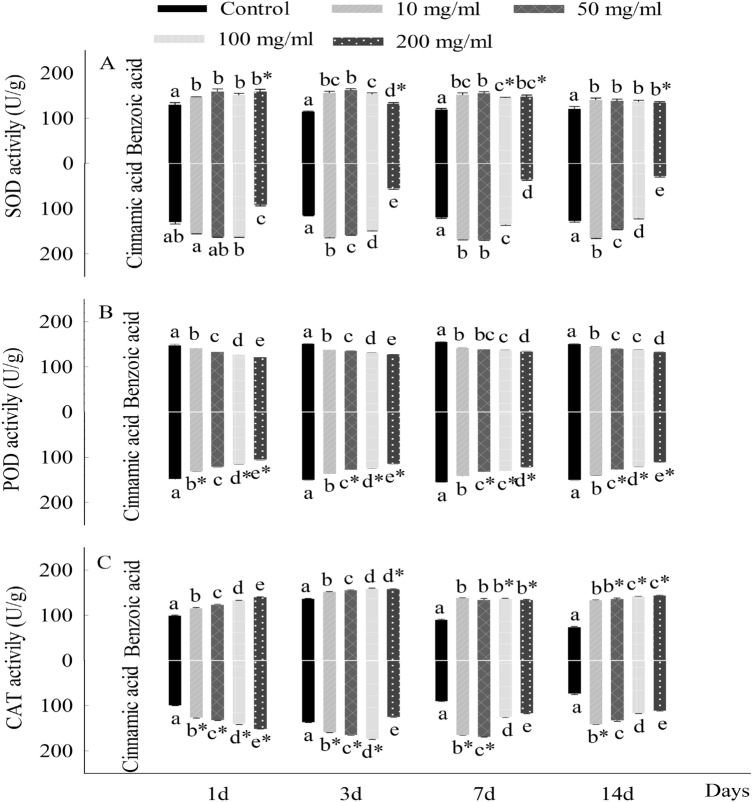
During the period of seedling growth, there existed substantial differences in the data of parameters evaluated of C. coronarium seedlings treated with different concentrations of allelochemicals. The first difference in content was at the content of chlorophyll, in which the content of chlorophyll a and chlorophyll b was positively linear to the increasing days, but negatively correlated with the concentration of the cinnamic acid and benzoic acid (Fig. 2). At the same time, the inhibited effect of cinnamic acid on chlorophyll a was stronger than that of benzoic acid during seedlings growth, but for chlorophyll b, the significant inhibition of cinnamic acid was stronger than that of benzoic acid appeared at different (50, 100 and 200 mg/l) concentrations in 14th days. Different from the previous parameter, the MDA contents of seedling in the early period (1st and 3rd day) were significantly rose (p < 0.05) with the compound concentrations increasing, compared with the control groups (Fig. 3). By contrast, on the 7th and 14th days, the MDA content of C. coronarium experienced with 100 mg/l benzoic acid was lower than that with 200 mg/l. Figure 4A, C represented the activity of SOD and CAT, which considerably increased (p < 0.05) with the benzoic acid compared to the control groups. However, the contents of SOD of treated groups were all considerably lower than contrast (p < 0.05) when seedlings exposed to cinnamic acid at 200 mg/l. Besides, the activity of POD of C. coronarium seedlings significantly (p < 0.05) declined with the chemical concentration increased, and the inhibited effect of cinnamic acid on chlorophyll a was stronger than that of benzoic acid (Fig. 4B).
Figure 2.
Effects of benzoic acid and cinnamic acid on chlorophyll a (A), chlorophyll b (B) of Chrysanthemum coronarium. Different lower letters indicate significant differences between the different concentrations of the same allelochemicals at the 0.05 level. The symbol of “*” indicates significant differences between the different chemicals of the same concentrations at the 0.05 level. The same as below.
Figure 3.
Effects of benzoic acid and cinnamic acid on malonaldehyde (MDA) content of Chrysanthemum coronarium. Different lower letters indicate significant differences between the different concentrations of the same allelochemicals at the 0.05 level. The symbol of “*” indicates significant differences between the different chemicals of the same concentrations at the 0.05 level. The same as below.
Figure 4.
Effects of benzoic acid and cinnamic acid on SOD activity (A), POD activity (B) and CAT activity (C) of Chrysanthemum coronarium seedlings. Different lower letters indicate significant differences between the different concentrations of the same allelochemicals at the 0.05 level. The symbol of “*” indicates significant differences between the different chemicals of the same concentrations at the 0.05 level. The same as below.
Discussions
Allelopathy is ubiquitous in nature, and most alien plants can make their survival a dominant position through potential allelopathic effects37. Our results showed that the extracts of M. micrantha and I. cairica significantly inhibited the seeds germination and seedling growth of C. coronarium, and the inhibitory effects increased with the concentration of extracts. This is consistent with the results of the inhibition of Brassica rapa seedling growth by the I. cairica and M. micrantha aqueous extracts reported in the previous studies38. Besides, at the concentration of 50, 100, and 200 mg/ml, the allelopathic effect of M. micrantha extracts was stronger than that of I. cairica, whereas the inhibition was reversed when concentration was increased to 400 mg/ml. Some studies pointed out that the metabolic system in plants would be disordered when the concentration of foliage extracts reaches a certain threshold39. Therefore, we infer the above different experimental phenomenon is related to the tolerance mechanism of plants, and C. coronarium is more sensitive to high concentrations of I. cairica.
According to the bioassay results of benzoic acid and cinnamic acid extracted from the extracts of M. micrantha and I. cairica respectively, we found that both allelochemicals significantly inhibited the seeds germination rate and the seedlings morphological indexes of C. coronarium. Similarly, as the concentration of allelochemicals increases, the allelopathic effect gradually increases. It was reported that benzoic acid and cinnamic acid were the most main forms of phenolic acids, and they are also important allelochemicals40. They can be extracted from the aerial parts of plants and released into the external environment, thus affecting the morphological indicators of crops above and below the ground41,42. Phenolic acids can affect seed germination by inhibiting key enzymes required for seed germination, such as phosphorylase43. It can also lead to the lack of necessary energy or inhibit the synthesis of some key enzyme intermediates during the germination process, to show a decrease in vigor, germination rate, radicle length and radicle dry weight on crop seeds44. We also found that the root growth of C. coronarium seedlings was more sensitive to phenolic acids than germination rate and stem length. It was speculated that both benzoic acid and cinnamic acid can significantly inhibit the vertical growth and cell division of the root system, to show significant changes in the root morphology, and thus affect the plant’s absorption of nutrients.
The physiological indexes of crop seedlings are affected by phenolic acid stress. The chlorophyll content of C. coronarium decreased with the increase of benzoic acid and cinnamic acid concentration, and the allelopathic effect of cinnamic acid was significantly stronger than that of benzoic acid, which was basically in agreement with Fu et al.45. Besides, in terms of seedling growth stage, the chlorophyll content of leaves showed a trend of first increasing and then decreasing, reaching the peak value on the seventh day, which was attributed to a high concentration of allelochemicals and prolonged exposure to allelochemicals causing an impaired chlorophyll synthesis, decreased stomatal opening and the stomatal opening ratio in leaves45. Besides, allelopathic stress can cause an increase in reactive oxygen species (ROS) in plants, leading to oxidative stress46. Thereby plants always enhance stress resistance by regulating antioxidant enzyme activity. In general, our study showed that SOD and CAT activities in leaves of C. coronarium were significantly higher than those in the control group during the growth under benzoic acid exposed, and only high concentration (400 mg/l) of cinnamic acid significantly inhibited SOD activity in the leaves, which is similar with Song et al.47. When the concentration of allelochemicals is high enough, the internal defense mechanism of plants collapses after reaching a threshold, and its antioxidant enzyme activity drops sharply. In addition, we observed that the POD activity of C. coronarium seedlings decreased with the increase of benzoic acid and cinnamic acid concentration, indicating that the POD activity of C. coronarium leaves was most sensitive to phenolic acids, followed by SOD activity.
Allelochemicals may destroy the balance between free radical production and scavenging system in plant tissue, thus leading to membrane lipid peroxidation. And malondialdehyde (MDA) is one of its products. The results showed that MDA content in C. coronarium leaves increased significantly during benzoic acid and cinnamic acid exposed compared with the control. In the early growth stage (1–3 days), MDA content exposed to cinnamic acid was significantly higher than that of benzoic acid and peaked on the seventh day and the third day respectively. It indicated that phenolic acids could accelerate the peroxidation of membrane lipid in chrysanthemum leaves after the tolerance threshold was reached, the plants could not resist stress through their regulation, and then MDA content began to decrease48. Therefore, we concluded that both benzoic acid and cinnamic acid could improve the antioxidant enzyme activity of C. coronarium leaves, accelerate the accumulation of reactive oxygen species in cells, and ultimately lead to the destruction of membrane structure and physiological integrity and the increase of membrane permeability.
In terms of the bioassay method used in this experiment, which has been widely used in exploring the allelopathic effect, and has the advantages of simplicity, short period, and high sensitivity25,27,49. Whereas the growing conditions of the pot experiment may be more similar to those in the natural environment than that of laboratory50. Therefore, the growth of receptor plants under allelopathic stress in the wild environment needs to be further studied. On the other hand, the germination rate was selected as the sensitivity index to evaluate plant allelopathy, whose changes would affect the abundance and competitiveness of recipient plants in the community. SOD CAT, POD, and other biological indicators directly reflect the degree of stress in seeds. However, at present, we have not studied the effect of M. micrantha and I. cairica extracts on C. coronarium seedlings at the molecular level, so the effects on cell micro-ultrastructure, cell division and elongation, growth regulation system and respiration can not be determined. These aspects will be our next research content.
Conclusions
The two typical invasive weeds in Hainan island, M. micrantha and I. cairica, their leaves extracts have negative effects on the growth of C. coronarium seeds and seedlings, and the allelopathic effect of M. micrantha extracts at low concentration was stronger than that of I. cairica extracts, while the high concentration showed an opposite effect. According to GC–MS data of leaves extracts from the two leaves, 19 and 23 chemicals were identified respectively, and each represented by benzoic acid and cinnamic acid. These two allelochemicals can lead to a sharp increase in the activity of the antioxidant enzyme, SOD and CAT and the content of MDA in the C. coronarium, thus affecting the germination rate of C. coronarium seeds and the growth of seedling roots and shoots. What’s more, the inhibitory effect of benzoic acid is stronger than that of cinnamic acid, which is one of the reasons that the allelopathic effect of I. cairica is stronger than that of M. micrantha.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Number: 31660145); the Natural Science Foundation of Hainan Province (Grant Number: 419QN174); and the Scientific Research Foundation of Hainan University (Grant Number: hdkyqd201601). The Graduate Innovation Research Program of Ordinary and Higher Universisty in Hainan Province [Grant number: Hys2018-21]
Author contributions
H.H., H.Y.M. and Y.C. designed the research, performed all analyses and wrote the first draft of the manuscript. J.H.C., Y.Q.Z., and T.Z. performed the experiment and helped to collect data. All authors read, commented on and approved the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to the data also part of an ongoing study but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mack RN, et al. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 2000;10:689–710. [Google Scholar]
- 2.Ding JQ, Mack RN, Lu P, Ren MX, Huang HW. China’s booming economy is sparking and accelerating biological invasions. Bioscience. 2008;58:317–324. [Google Scholar]
- 3.Smith-Ramesh, L. M. The aliens among us: how invasive species are transforming the planet—AND ourselves Yale University Press, New Haven Connecticut, 2017, xii + 353 pp, ISBN 978-0-300-20890-0. Biol. Invasions 1–2 (2017).
- 4.Waterhouse, D. F. Biological control of weeds: southeast Asian prospects. ACIAR Monograph (1994).
- 5.Wei CQ, Pan YM, Tang SC, Lin CH, Zhou CQ. Distribution and damage of invasive plant Mikania micrantha in Guangxi. Guihaia. 2014;34:816–820. [Google Scholar]
- 6.Peng ZB, Li JH, Jiang Y, Wang CY, Jiang JS. Damage status and invasive dynamic of an alien species Mikania micrantha in Hainan island. Subtrop. Plant Sci. 2013;42:141–145. [Google Scholar]
- 7.Liu L, et al. Survey of the species, distribution and damage situation of forest pests in the Qiongzhong County of forest pests in the Qiongzhong County of Hainan. Chin. J. Trop. Agric. 2018;38:70–74. [Google Scholar]
- 8.Zhong XQ, Huang Z, Huan SI, Zan QJ. Analysis of ecological-economic loss caused by weed Mikania micrantha on Neilingding island, Shenzhen, China. J. Trop. Subtrop. Bot. 2004;12:167–170. [Google Scholar]
- 9.Peng SL, et al. The status of noxious plants in lower subtropical region of China. Acta Ecol. Sin. 2009;29:79–83. [Google Scholar]
- 10.Mark VK, Ewald W, Markus F. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010;13:235–245. doi: 10.1111/j.1461-0248.2009.01418.x. [DOI] [PubMed] [Google Scholar]
- 11.Swamy PS, Ramakrishnan PS. Weed potential of Mikania micrantha H.B.K., and its control in fallows after shifting agriculture (Jhum) in North-East India. Agric. Ecosyst. Environ. 1987;18:195–204. [Google Scholar]
- 12.Wang RL, et al. Effects of simulated nitrogen deposition on the seeding growth, photosynthetic characteristics and allelopathic potential of invasive plant Ipomoea cairica (L.) Sweet. Allelopath. J. 2016;38:183–192. [Google Scholar]
- 13.Wang JH, Chen W, Zhu H. Ecological stoichiometry and invasive strategies of two alien species (Bidens pilosa and Mikania micrantha) in subtropical China. Ecol. Res. 2019;34:612–623. [Google Scholar]
- 14.Banerjee AK, Mukherjee A, Guo WX, Liu Y, Huang YL. Spatio-temporal patterns of climatic niche dynamics of an invasive plant Mikania micrantha kunth and its potential distribution under projected climate change. Front. Ecol. Evol. 2019;7:291. [Google Scholar]
- 15.Rice, E. L. Allelopathy. Encyclopedia of Entomology, 1, 292–293 (1984).
- 16.Callaway RM, Aschehoug ET. Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science. 2000;290:521–523. doi: 10.1126/science.290.5491.521. [DOI] [PubMed] [Google Scholar]
- 17.Callaway RM, Ridenour WM. Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004;2:436–443. [Google Scholar]
- 18.Wu JR, Chen ZQ, Peng SL. Allelopathic potential of invasive weeds: Alternanthera philoxeroide, Ipomoea cairica and Spartina alterniflora. Allelopath. J. 2006;17:279–285. [Google Scholar]
- 19.Yuan BQ, Li SS, Xiong TT, Zhang T. Cytogenetic and genotoxic effects of Ipomoea cairica (L.) Sweet leaf aqueous extract on root growth of Allium cepa var. agrogarum (L.) Allelopath. J. 2019;46:205–214. [Google Scholar]
- 20.Zuharah WF, et al. Larvicidal effectiveness of acethonilic and methanolic Ipomoea cairica extract using two extraction methods and its effects on the morphology of Culex quinquefasciatus Say mosquito. Orient. Insects. 2018;52:16–30. [Google Scholar]
- 21.Ismail BS, Chong TV. Effects of aqueous extracts and decomposition of Mikania micrantha H.B.K. debris on selected agronomic crops. Weed Biol. Manag. 2010;2:31–38. [Google Scholar]
- 22.Kaur R, Malhotra S. Effects of invasion of Mikania micrantha on germination of rice seedlings, plant richness, chemical properties and respiration of soil. Biol. Fertil. Soils. 2012;48:481–488. [Google Scholar]
- 23.Muller WH, Muller CH. Volatile growth inhibitors produced by salvia species. Bull. Torrey Bot. Club. 1964;91:327–330. [Google Scholar]
- 24.Li ZH, Wang Q, Ruan X, Pan CD, Jiang DA. Phenolics and plant allelopathy. Molecules. 2010;15:8938–8952. doi: 10.3390/molecules15128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang FJ, Guo JY, Chen FX, Liu WX, Wan FH. Identification of volatile compounds released by leaves of the invasive plant croftonweed (Ageratina adenophora, compositae), and their inhibition of rice seedling growth. Weed Sci. 2012;60:205–211. [Google Scholar]
- 26.Zhi Qun T, Zhang J, Yu JL, Wang CZ, Ju ZD. Allelopathic effects of volatile organic compounds from Eucalyptus grandis rhizosphere soil on Eisenia fetida assessed using avoidance bioassays, enzyme activity, and comet assays. Chemosphere. 2017;173:307–317. doi: 10.1016/j.chemosphere.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Shen SC, et al. Allelopathic potential of sweet potato (Ipomoea batatas) germplasm resources of Yunnan Province in southwest China. Acta Ecol. Sin. 2018;38:444–449. [Google Scholar]
- 28.Song QM, Qin FC, He H, Wang HC, Yu SX. Allelopathic potential of rain leachates from Eucalyptus urophylla on four tree species. Agrofor. Syst. 2019;93:1307–1318. [Google Scholar]
- 29.Fernandez C, et al. Potential allelopathic effect of Pinus halepensis in the secondary succession: an experimental approach. Chemoecology. 2006;16:97–105. [Google Scholar]
- 30.Smith RJ, Haken JK, Wainwright MS. Estimation of dead time and calculation of kovats indises. J. Chromatogr. A. 1985;334:95–127. [Google Scholar]
- 31.Strickler H, Kováts ES. Influence of experimental conditions on peak resolution in gas chromatography. J. Chromatogr. A. 1962;8:289–302. doi: 10.1016/s0021-9673(01)99263-5. [DOI] [PubMed] [Google Scholar]
- 32.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 33.Marshall T, Williams KM. Coomassie blue protein dye-binding assays measure formation of an insoluble protein-dye complex. Anal. Biochem. 1992;204:107–109. doi: 10.1016/0003-2697(92)90147-y. [DOI] [PubMed] [Google Scholar]
- 34.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peskin AV, Winterbourn CC. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radic. Biol. Med. 2017;103:188–191. doi: 10.1016/j.freeradbiomed.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 36.Williamson GB, Richardson D. Bioassays for allelopathy: measuring treatment responses with independent controls. J. Chem. Ecol. 1988;14:181. doi: 10.1007/BF01022540. [DOI] [PubMed] [Google Scholar]
- 37.Latif S, Chiapusio G, Weston LA. Chapter two—allelopathy and the role of allelochemicals in plant defence. Adv. Bot. Res. 2017;82:19–54. [Google Scholar]
- 38.Chen BM, Peng SL. Allelopathic potential of native invasive plants: the evidence from southern China. Allelopath. J. 2018;43:43–51. [Google Scholar]
- 39.Kato-Noguchi H, Kimura F, Ohno O, Suenaga K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017;218:66–73. doi: 10.1016/j.jplph.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Kumar N, Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019;24:e00370. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li QK, et al. Effects of two phenolic acids on root zone soil nutrients, soil enzyme activities and pod yield of peanut. J. Appl. Ecol. 2016;27:1189–1195. doi: 10.13287/j.1001-9332.201604.039. [DOI] [PubMed] [Google Scholar]
- 42.Xie DF, Zhang GC, Xia XX, Lang Y, Zhang SY. The effects of phenolic acids on the photosynthetic characteristics and growth of Populus × euramericana cv. ‘Neva’ seedlings. Photosynthetica. 2018;56:981–988. [Google Scholar]
- 43.Einhellig FA. Allelopathy: current status and future goals. Allelopathy. 1994;582:1–24. [Google Scholar]
- 44.Liu JG, Liao HX, Chen BM, Peng SL. Do the phenolic acids in forest soil resist the exotic plant invasion? Allelopath. J. 2017;41:167–175. [Google Scholar]
- 45.Fu YH, et al. Allelopathic effects of phenolic acids on seedling growth and photosynthesis in Rhododendron delavayi Franch. Photosynthetica. 2019;57:377–387. [Google Scholar]
- 46.Inderjit Plant phenolics in allelopathy. Bot. Rev. 1996;62:186–202. [Google Scholar]
- 47.Song L, Pan KW, Wang JC, Ma YH. Effects of phenolic acids on seed germination and seedling antioxidant enzyme activity of alfalfa. Acta Ecol. Sin. 2006;10:3393–3403. [Google Scholar]
- 48.Panwar R, Sharma AK, Dutt D, Pruthi V. Phenolic acids from “Parthenium hysterophorus”: evaluation of bioconversion potential as free radical scavengers and anticancer agents. Adv. Biosci. Biotechnol. 2015;6:11–17. [Google Scholar]
- 49.Zhi Qun T, Jian Z, Jun Li Y, Chun Zi W, Dan Ju Z. Allelopathic effects of volatile organic compounds from Eucalyptus grandis rhizosphere soil on Eisenia fetida assessed using avoidance bioassays, enzyme activity, and comet assays. Chemosphere. 2017;173:307–317. doi: 10.1016/j.chemosphere.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Lu XM, Siemann E, Shao X, Wei H, Ding JQ. Climate warming affects biological invasions by shifting interactions of plants and herbivores. Glob. Change Biol. 2013;19:2339–2347. doi: 10.1111/gcb.12244. [DOI] [PubMed] [Google Scholar]
- 51.Pino JA. Characterisation of volatile compounds in a smoke flavouring from rice husk. Food Chem. 2014;153:81–86. doi: 10.1016/j.foodchem.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 52.Bogusz Junior S, et al. Analysis of volatile compounds in Capsicum spp. by headspace solid-phase microextraction and GC × GC-TOFMS. Anal. Methods. 2015;7:521–529. [Google Scholar]
- 53.Niebler J, Buettner A. Pyrolysis-GC–MS–Olfactometry: a new approach to identify thermally generated odorants in frankincense. J. Anal. Appl. Pyrol. 2015;113:690–700. [Google Scholar]
- 54.Góra J. Investigation of the essential oil of Erigeron acris L. Herb. J. Essent. Oil Res. 2006;18:88–89. [Google Scholar]
- 55.Derenbach J. Phthalate esters in the Kiel Bight. Mar. Chem. 1980;8:339–346. [Google Scholar]
- 56.Leol’ko AS, Krasnykh EL, Levanova SV. Retention indices of glycerol esters. J. Anal. Chem. 2009;64:1126–1130. [Google Scholar]
- 57.Rosas JF, Zoghbi MDGB, Andrade E, Berg MEVD. Chemical composition of a methyl-(E)-cinnamate Ocimum micranthum Willd. from the Amazon. Flavour Fragr. J. 2005;20:161–163. [Google Scholar]
- 58.Hrivnak J. Open tubular column gas chromatography of the catalytic dehydrogenation products of n-dodecane. J. Chromatogr. Sci. 1972;10:701–704. [Google Scholar]
- 59.Boti JB, et al. Combined analysis of Cymbopogon giganteus Chiov. leaf oil from ivory coast by GC/RI, GC/MS and 13C-NMR. C. R. Chim. 2006;9:164–168. [Google Scholar]
- 60.Pedrol N. Volatile organic compounds of Acacia longifolia and their effects on germination and early growth of species from invaded habitats. Chem. Ecol. 2017;34:1–20. [Google Scholar]
- 61.Utsunomiya H, Kawata J, Chanoki W, Shirakawa N, Miyazawa M. Components of essential oil from woods of Prunus mume Sieb. et Zucc. J. Oleo Sci. 2005;54:609–612. [Google Scholar]
- 62.Marriott PJ. Methods for generating second dimension retention index data in comprehensive two-dimensional gas chromatography. J. Chromatogr. A. 2003;1019:3–14. doi: 10.1016/j.chroma.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Paik MJ, Lee HJ, Kim KR. Simultaneous retention index analysis of urinary amino acids and carboxylic acids for graphic recognition of abnormal state. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005;821:94–104. doi: 10.1016/j.jchromb.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Lee SM, Seo BC, Kim YS. Volatile compounds in fermented and acid-hydrolyzed soy sauces. Food Chem. Toxicol. 2006;71:C146–C156. [Google Scholar]
- 65.Fischer DCH, Limberger RP, Henriques AT, Moreno PRH. Essential oils from fruits and leaves of Siparuna guianensis (Aubl.) Tulasne from Southeastern Brazil. J. Essent. Oil Res. 2005;17:101–102. [Google Scholar]
- 66.Pombovillar E. Gas chromatography-mass spectrometry coupled with pseudo-Sadtler retention indices, for the identification of components in the essential oil of Curcuma longa L. J. Chromatogr. A. 1997;760:303–308. [Google Scholar]
- 67.Isidorov VA, Rusak M, Szczepaniak L, Witkowski S. Gas chromatographic retention indices of trimethylsilyl derivatives of mono- and diglycerides on capillary columns with non-polar stationary phases. J. Chromatogr. A. 2007;1166:207–211. doi: 10.1016/j.chroma.2007.07.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to the data also part of an ongoing study but are available from the corresponding author on reasonable request.