Abstract
The high frequency of infection by Anisakis simplex (A. simplex) has led to an increase in IgE sensitization, turning allergy to this parasite a relevant contemporary health problem. Improving the lack of conventional diagnosis test specificity is crucial to better understand these clinical scenarios. Specific IgE (sIgE) to A. simplex extract by ImmunoCAP (Anisakis-sIgE) was determined in sera from 403 blood donors (BD) from Cantabria (North of Spain) of which 51 subjects resulted sensitized. Among these latter, 47 were asymptomatic (sABD). The values of total IgE, prick-test, Anisakis-sIgE, and sIgE to Ani s 1 (anti-rAni s 1) and Ani s 7 (anti-rAni s 7) were compared between 46 sABD and 49 A. simplex allergic patients. The IgE seroprevalence by ImmunoCAP among BD was 12.65%. Allergic patients and sABD showed significant differences in all serum biomarkers evaluated. The area under the curve was assessed for Anisakis-sIgE (0.892), sIgE-rAni s 1 (0.672) and sIgE-rAni s 7 (0.668). After a severe reaction, significantly higher levels of Anisakis-sIgE and sIgE anti-rAni s 1 were detected. Determinations of sIgE by ImmunoCAP, Ani s 1 and Ani s 7 presented different sensitization patterns between allergic and asymptomatic individuals. The Ani s 1 allergen arises as a possible biomarker to detect patients at risk of suffering severe allergic reactions.
Subject terms: Immunology, Microbiology, Biomarkers, Molecular medicine
Introduction
Anisakis simplex (A. simplex) is a nematode belonging to the Anisakidae family in whose life cycle participate fish, crustaceans and marine mammals1. Humans can become an incidental host due to the ingestion of raw or uncooked fish harboring third-stage larvae2. In the last decades the prevalence of infected fish by A. simplex has increased dramatically and in parallel, health problems associated with this parasite becoming a public health concern3–6. In the center and the northern areas of Spain, there is a tendency of consuming fresh fish either raw or prepared using techniques requiring light cooking (e.g., hake and anchovies) which favors the contact with the live larvae7–9.
It is generally accepted that an infection by Anisakis spp. larvae is required to produce symptoms in humans10,11. After a first penetration of the gastrointestinal mucosa by live Anisakis larvae, the released antigens induce the production of IgE antibodies in response to the parasite infection7,12–14. In subsequences exposures to the larvae, sensitized individuals can develop allergic IgE-mediated symptoms some minutes to hours after the intake of parasitized fish15. Clinical manifestations can range from mild to moderate, (such as urticaria, angioedema, bronchospasm) or even anaphylaxis13. The local mucosa damage produced by alive larvae may also induce gastrointestinal symptoms, such as dyspepsia, vomiting, abdominal pain12,15,16. When these type of clinical features are associated to IgE-mediated symptoms, patients suffer a gastro-allergic anisakiasis7,17,18. Anisakis simplex sensitization has been also considered as a triggering and/or worsening factor of other diseases such as urticaria/angioedema and dyspepsia13,19,20.
Due to the low specificity of traditional test such as skin prick test (SPT) and Anisakis-sIgE (ImmunoCAP)21, several authors proposed the use of component-resolved diagnosis (CRD) to investigate which allergens are responsible for a given allergic reaction. CRD is a relevant diagnostic tool that can provide relevant information on severity risks and can guide allergists on the management of allergic patients17,22–25.
Fourteen A. simplex allergens have been described and classified in three groups according to its origin: excretory/secretory, somatic and cuticular proteins7,17,26–28. The first group includes relevant major allergens as Ani s 1 and Ani s 7, which are frequently used to measure sIgE responses to A. simplex in different populations including non-symptomatic blood donors, fish processing workers and patients suffering gastroallergic anisakiasis or chronic urticaria9,17,29.
Recently, Viñas et al.30 reported that the presence of IgG and IgE antibodies to Ani s 1 and Ani s 3 allergens in serum can aid to differentiate between patients with and without urticaria in regions where Anisakis infections are frequent. Also, cross reactivity between A. simplex allergens and those from house dust mites (HDM) or shellfish among others has been proposed as the origin of positive sIgE to complete A. simplex extract (ImmunoCAP) in asymptomatic population31,32.
However, the role of Ani s 1 and Ani s 7 allergens as biomarkers of severity in Anisakis-induced acute allergic reactions were never evaluated. To address this issue, we compared the anti-Ani s 1 and anti-Ani s 7 sIgE responses in a population of sABD with that of a population of patients presenting acute allergic symptoms to this nematode.
Results
Population characteristics: demographics and consumption habits
Among 403 BD randomly selected, 381 referred to be asymptomatic when consuming fish and 47 of them presented with Anisakis-sIgE > 0.35 KUA/L (sABD) (See Supplementary Fig. S1 online). Gender and age were the only demographic variables with significant differences between allergic group and sABD (p < 0.001 and p = 0.01, respectively) (Table 1). Based on the anonymous questionnaire answers, the overall frequency of fish consumption did not show significant differences between groups (p 0.142). As expected, raw fish intake (57.4% in allergic vs. 31.1% in sABD; p = 0.011) and restaurant fish consumption (28.3% in allergic vs. 2.8% in sABD; p = 0.002) was more frequent in allergic patients (see Supplementary Table S1 online).
Table 1.
Demographics and fish consumption habits. Allergic patients and sensitized asymptomatic blood donors to Anisakis simplex (sABD).
| Allergic patients (n = 49) | sABD (n = 46) | p value | |
|---|---|---|---|
| Demographics | |||
| Females, % | 60.4 | 25 | < 0.001* |
| Agea, yearb | 54.96 (11.63) | 48.32 (12.78) | 0.010* |
| Coastal residencya | 83.7% | 81% | 0.734 |
| Occupation related to fishery | 12.9% | 5.7% | 0.311 |
| Fish consumption habits | |||
| Frequency, n (%) | No significant differences | ||
| Conservation, n (%) | No significant differences | ||
| Restaurant consumption, n (%) | 13 (28.3%) | 1 (2.8%) | 0.002* |
| Raw preparation, n (%) | 8 (16.7%) | 1 (2.3%) | 0.020* |
| Species, n (%) | No significant differences | ||
| Fish farm source, n (%) | 7 (17.5%) | 11 (44.0%) | 0.021* |
| Tolerance of frozen fish | 42 (91.3%) | 44 (100%) | 0.045* |
| Raw fish consumption | 27 (57.4%) | 14 (31.1%) | 0.011* |
| Homemade fish-preserves | No significant differences | ||
Data extracted from an anonymous questionnaire. aInformation related to age and residency was collected in all allergic patients and in 44 and 42 of sABD, respectively. Occupation was registered in 31 allergic patients and in 35 ABD. bMean (standard deviation). *Significant differences.
Thirty-one sABD accepted a follow up consultation 2 years after their recruitment. Most of them were still asymptomatic when eating fish except one of them who experienced an anaphylactic reaction after eating infected fish (undercooked see bass), 1 year after the inclusion date (see Supplementary Fig. S1 online).
Prevalence of Anisakis simplex sensitization and conventional diagnosis test results
Considering the population of 403 BD, the sIgE seroprevalence measured by ImmunoCAP (sIgE > 0.35 kUA/L) was 12.65% (n = 51). Median values of total IgE and Anisakis-sIgE in the allergic group were 366 IU/mL and 37.80 kUA/L, respectively; and 35.15 IU/mL and 1.79 kUA/L in the sABD. These differences were statistically significant between both groups (p < 0.001) (Table 2a). Anisakis-sIgE distributed by classes revealed that the majority of the allergic patients (91.9%) presented with levels greater than 3.5 kUA/L (Class 3–6), the 79.6% above 8 kUA/L, the 73.5% above 10 kUA/L and the 63.2% above 17.5 kUA/L (see Supplementary Fig. S2a online). In the sABD group all IgE values were ≤ Class 4 and the 91.3% of which were below 17.5 kUA/L (Class 1–3) the 83.4% below 8 kUA/L and 67.4% below 3.5 kUA/L (see Supplementary Fig. S2a online).
Table 2.
Results of conventional and component-resolved diagnosis test. (a) Comparison of median values (IQR) between allergic patients and sABD. (b) Comparison of positives results in component-resolved diagnosis test. (c) Sensitivity and specificity of Trisakis 170 (rAni s 1, rAni s 7, or both) considering only allergic patients as true positives.
| Allergic patients (n = 49) | sABD (n = 46) | p | |
|---|---|---|---|
| (a) | |||
| Total IgE (IU/mL) | 366 (105–692) | 35.15 (14.67–116.25) | < 0.001* |
| Anisakis-sIgE (kUA/L) | 37.80 (9.01–88.75) | 1.79 (0.79–5.50) | < 0.001* |
| rAni s 1 (OD) | 1.47 (0.22–1.69) | 0.13 (0.01–1.30) | < 0.005* |
| rAni s 7 (OD) | 1.34 (0.77–1.67) | 0.57 (0.18–1.40) | < 0.005* |
| SPT (mm) | 5.50 (4.50–9.00) | 5.00a (2.87–6.12) | 0.074 |
| (b) | |||
| Positive rAni s 1, n (%) | 38 (77.5%) | 24 (52.2%) | 0.009* |
| Positive rAni s 7, n (%) | 47 (95.9%) | 41 (89.1%) | 0.206 |
| Double positive | 38 (77.6%) | 24 (52.2%) | 0.009* |
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| (c) | ||||
| rAni s 1 | 61.3% | 66.7% | 77.5% | 47.8% |
| rAni s 7 | 95.9% | 10.9% | 53.4% | 71.4% |
| Double positive | 47.8% | 77.5% | 61.3% | 66.7% |
Cut-off values: Anisakis-sIgE > 0.35 kUA/L; SPT ≥ 3 mm; rAni s 1 = 0.09 (OD); rAni s 7 = 0.05 (OD). aSPT were performed in 21 out of 46 sABD. OD, optical density. *Significant differences.
All allergic patients had a positive SPT (5.50 mm) and 13 out of 21 sABD tested (76.2%) were also positive (median 5.00 mm) with no significant differences between groups (p 0.074) (Table 2a).
Component-resolved diagnosis test results
The sera from all allergic patients (n = 49) and 46 sABD were analyzed using the Trisakis-170 ELISA kit to detect specific anti-rAni s 1 and anti-rAni s 7 sIgE antibodies. Considering the group of allergic patients, 38/49 (77.5%) and 47/49 (95.9%) sera tested positive for rAni s 1 and rAni s 7, respectively. Two sera negative for rAni s 7 tested also negative for rAni s 1 (double negatives). Three sera (6.1%) from the rAni s 7 positive allergic population (ODs = 0.12, 0.31 and 0.83) testing negative to rAni s 1 were also negative by SPT. On the other hand, 24/46 (52.2%) and 41/46 (89.1%) sera from the sABD group tested positive for rAni s 1 and rAni s 7, respectively.
Interestingly, the statistical analysis of the allergic and sABD populations revealed significant differences (p < 0.001) between the percentages of sera testing positive for rAni s1 in the allergic (77.5%) versus the sABD population (52.2%), but not between the corresponding positive values for rAni s 7 in both populations (96% and 89%, respectively; Table 2b). Significant statistical differences (p < 0.001) were also found when comparing the percentages of sera testing positive to both recombinant allergens (double positives) in the allergic population (77.6%) versus the sABD population (52.2%) (Table 2b).
Finally, it is noteworthy that, although the OD ELISA values may be not comparable when they fall out of the linear region of the ELISA curve, the mean serum sIgE OD values in the allergic group were also significantly higher than in sABD (1.47 and 0.13 for sIgE-Ani s 1, 1.34 and 0.57 for sIgE anti-Ani s 7, respectively; p < 0.005; Table 2a).
Correlation analysis between sIgE measured by ImmunoCAP and component-resolved diagnosis and clinical symptoms
A still non-solved problem in Anisakis-induced allergy is to know which parasite allergens, among those inducing sensitization, are responsible of the allergic symptoms showed by many patients after being parasitized. Since, as showed above, the percentage of subjects having sIgE to Anisakis allergens may be different in allergic and sensitized non-allergic populations, we investigated whether these differences can be related with clinical symptoms using a given cut-off.
As all analyzed sera were selected using the ImmnoCAP method, discrimination between groups was not possible at a cut-off value of ≥ 0.35 kU/L (class 1). However, when the cut-off was increased to ≥ 3.5 kU/L, a plateau in ImmunoCAP and CRD values (sIgE anti-rAni s 1 and anti-rAni s 7) was observed, suggesting that these methods could also be used to discriminate between symptomatic and asymptomatic populations (see Supplementary Fig. S2b, S2c and Table S2 online). For a sIgE to Anisakis cut-off of 3.5 kU/L, the obtained values for sensitivity and specificity were 84.9% and 58.8%, respectively. However, these values reached 100% when only classes 5 (50–99.9 kU/L) and 6 (≥ 100 kU/L) were considered. According to previously validated cut-off values for sIgE anti-rAni s 1 and anti-rAni s 7 (ELISA) to detect Anisakis sensitization, their ability to discriminate between allergic and non-allergic subjects was poor with 61.3% and 95.9% sensitivity for rAni s 1 and Ani s 7, respectively, but only 66.7% and 10.9% specificity, respectively (Table 2c).
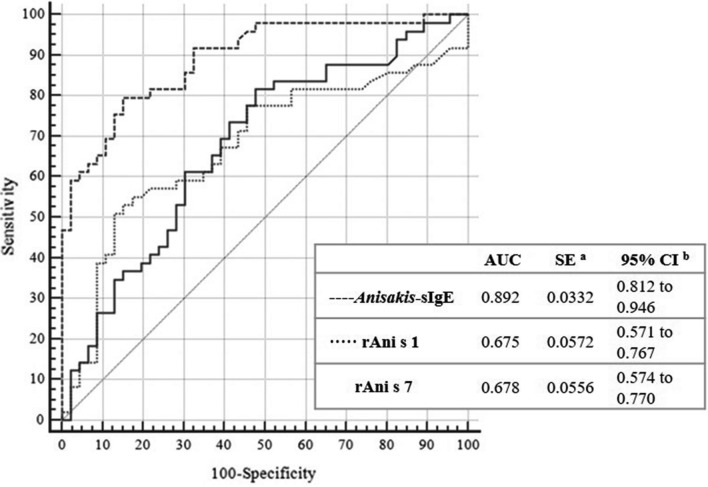
To better evaluate the ability of Trisakis-170 recombinant allergens and ImmunoCAP to predict clinical symptoms in Anisakis-sensitized patients we performed a ROC analysis to calculate the better cut-off values for each allergen (Fig. 1; Table 3a). The area under the curve (AUC) for Anisakis-sIgE values by ImmunoCAP was 0.892, while for rAni s 1 and Ani s 7 were 0.675 and 0.678, respectively. Differences between areas were statistically significant (p < 0.0001; Fig. 1).
Figure 1.
Receiver operating characteristics (ROC) curves of Anisakis-sIgE (InmunoCAP), rAni s 1 (ELISA) and rAni s 7 (ELISA). The sample was defined as positive group (n = 49; 51,58%), patients or blood donor with symptoms related to fish intake and as negative group (n = 46; 48,42%), sensitized blood donor without symptoms (sABD) after eating fish. Cut-off values: Anisakis-sIgE, 0.35 kUA/L; Ani s 1, 0.09; Ani s 7, 0.05. aDeLong et al.51, bbinomial exact. OD, optical density.
Table 3.
Receiver operating characteristics (ROC) curves and Youden Index cut-offs of Anisakis-sIgE (InmunoCAP), rAni s 1 (ELISA) and rAni s 7 (ELISA). (a) Comparison of receiver operating characteristics (ROC) curves of Anisakis-sIgE (InmunoCAP), rAni s 1 (ELISA) and rAni s 7 (ELISA). (b) Comparison of Youden index cut-offs of Anisakis-sIgE (InmunoCAP), rAni s 1 (ELISA) and rAni s 7 (ELISA).
| Anisakis-sIgE ~ rAni s 1 | Anisakis-sIgE ~ rAni s 7 | rAni s 1 ~ rAni s 7 | |
|---|---|---|---|
| (a) Comparison of ROC data | |||
| Difference between areas | 0.217 | 0.214 | 0.00355 |
| Standard Errora | 0.0440 | 0.0403 | 0.0503 |
| 95% confidence interval | 0.131 to 0.304 | 0.135 to 0.293 | − 0.0951 to 0.102 |
| z statistic | 4.946 | 5.304 | 0.0705 |
| Significance level | p < 0.0001 | p < 0.0001 | p = 0.9438 |
| Anisakis-sIgE | rAni s1 | rAni s 7 | |
|---|---|---|---|
| (b) Youden Index | |||
| Youden index J | 0.6437 | 0.3798 | 0.3381 |
| 95% Confidence intervalc | 0.4689 to 0.7471 | 0.1996 to 0.5035 | 0.1603 to 0.4725 |
| Associated criterion | > 7.9 | > 1.464 | > 0.589 |
| 95% Confidence intervalc | > 1.8 to> 26.6 | > 1.34 to > 1.576 | > 0.053 to> 1.165 |
| Sensitivity | 79.59 | 51.02 | 81.63 |
| Specificity | 84.78 | 86.96 | 52.17 |
The sample was defined as positive group (n = 49; 51,58%), patients or blood donors with symptoms related to fish intake; and as negative group (n = 46; 48,42%), sensitized blood donor without symptoms after eating fish. Cut-off values: Anisakis-sIgE > 0.35 kUA/L; SPT ≥ 3 mm; rAni s 1 = 0.09 (OD); rAni s 7 = 0.05 (OD). aDeLong et al.51, bbinomial exact. cBCa bootstrap confidence interval (1,000 iterations; random number seed: 978). OD, optical density.
When the Youden index was assessed for Anisakis-sIgE by ImmunoCAP the best reported cut-off point was ≥ 7.9 KUA/L showing a 79.59% sensitivity and 84.78% specificity. Positive likelihood ratio was 5.2 (IC 95:2.6–10.5) and negative likelihood ratio 0.24 (IC 95: 0.1–0.4). However, for rAni s 1 and Ani s 7 extremely high cut-offs were required (OD > 1.464 and OD > 0.589 for Ani s 1 and Ani s 7, respectively) to achieve moderate values of sensitivity and specificity (51.02% and 86.9% for rAni s 1 and 81.6% and 52.17% for rAni s 7) (Table 3b; see Supplementary Table S2 online).
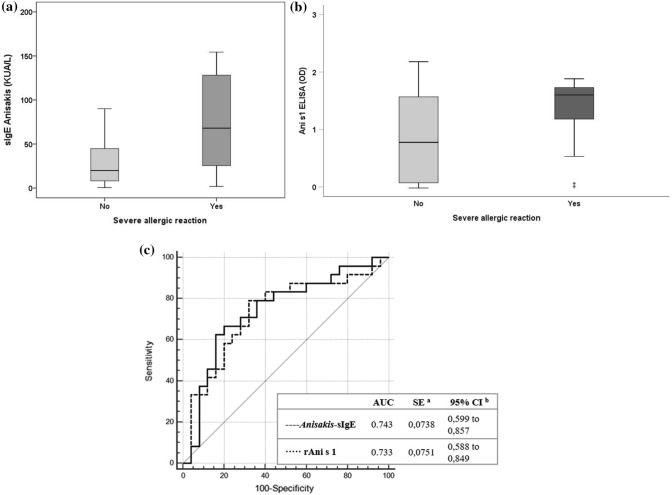
These results suggest that ImmunoCAP is better than CRD to predict the apparition of clinical symptoms of allergy after Anisakis infections. Nevertheless, the results obtained with CRD showing a better correlation of allergy symptoms with positivity to rAni s 1 versus rAni s 7 suggests that the former is more relevant in inducing allergic symptoms during Anisakis infections than rAni s 7. In this sense, after comparing the levels of sIgE anti-whole Anisakis antigens (ImmunoCAP) and anti-rAni s 1, we find significant differences in mean values between patients who have suffered a severe reaction, compared with those who experiences mild to moderate reactions (Fig. 2a, b).
Figure 2.
Comparation of Anisakis-sIgE (InmunoCAP) and rAni s 1 (ELISA) results between allergic patients who suffered a mild to moderate reaction with those who experience a severe allergic reaction. (a) Values of Anisakis-sIgE (mean of 0.65 and 1.61, respectively; p = 0.002). (b) Values of sIgE to rAni s 1 (mean of 15.90 and 72.60, respectively; p = 0.042). (c) Receiver operating characteristics (ROC) curves of Anisakis-sIgE (InmunoCAP) and rAni s 1 (ELISA) defined as positive group (n = 24; 48.98%), patients who presented a severe reaction after eating fish infected by AS and as negative group (n = 25; 51.02%), patients who presented mild to moderate reactions when eating fish infected by A. simplex. Cut-off values: Anisakis-sIgE > 0.35 kUA/L; Ani s 1 = 0.09. aDeLong et al.51, bbinomial exact.
House dust mites (Dermatophagoides pteronyssinus) and shellfish (shrimp) sensitization in asymptomatic sensitized individuals
It is well known that A. simplex shares allergen epitopes with house dust mites (HDM) such as D. pteronyssinus (DPT), and shellfish33. In this study, we observed that most of sABD (43 sera) tested also for sIgE antibodies to HDM and shrimp. However, although all these presented with variable amounts of sIgE anti-DPT (range: 2.86–978 kUA/L) only 6/43 seropositive sera to shrimp (range: 0.00–3 kUA/L). In addition, a positive correlation was observed in the sABD between sIgE to DPT and shrimp with total IgE, Anisakis-sIgE and sIgE anti-rAni s 7 (Spearman’s Rho) (Table S3 online). Specifically, the highest R values (R ≥ 0.5) were obtained comparing total IgE versus sIgE to Anisakis by ImmunoCAP (R = 0.52), total IgE versus sIgE to DPT (R = 0.88), total IgE versus sIgE to shrimp (R = 0.62), sIgE to Anisakis by ImmunoCAP versus sIgE to rAni s 7 (R = 0.66), and sIgE to shrimp versus sIgE to DPT (R = 0.63). However, significant R values were also obtained for other combinations including Ani s 7 versus sIgE to shrimp or DTP (see Supplementary Table S3 online).
Discussion
It is well known that anisakiasis is a parasitic infection which may course or not with associated allergic symptoms. Currently, the demonstration that an allergic patient has specific circulating IgE antibodies induced during a previous infection by Anisakis can be done by several in vitro techniques. However, to predict which subjects having anti-Anisakis IgE antibodies are at a risk of suffering allergic symptoms after a second contact with the parasite, and to dilucidate which Anisakis allergens are clinically relevant are questions that remain to be solved. In the last years, precision medicine applied to A. simplex allergy has tried to discriminate between allergy, cross reactivity and asymptomatic sensitization to this parasite through molecular diagnosis, detecting sIgE to different A. simplex major allergens such as Ani s 1 and Ani s 717,34,35. A possible way to investigate these problems is searching for differences in the Anisakis antigenic profiles recognized by sensitized—allergic—versus sensitized non-allergic subjects. As the number of commercially available Anisakis allergens is limited, and as a proof of concept, in this study we investigated with which frequency allergic and non-allergic Anisakis sensitized patients recognize the Ani s 1 and Ani s 7 allergens in Cantabria.
Considering the population of BD, our results show a seroprevalence of Anisakis-sIgE (tested by ImmunoCAP) of 12.65% in Cantabria, which is consistent with previous studies carried out in other Spanish geographic areas9. No seroprevalence values were obtained for rAni s 1 and Ani s 7 since only ImmunoCAP-positive sera were tested using the Trisakis-170 test. However, sABD tested also positive by Trisakis-170 (89.13%), we estimated that a seroprevalence of 12–15% is expected in Cantabria. No significant differences between allergic patients and sABD were found in frequency of fish intake, which can be explained because Cantabria is a region with a high rate of fish consumption. On the other hand, gender and age showed differences between the group of allergic and sABD, which can be due to the fact that blood donors are usually young males meanwhile Th2-driven allergic diseases are more prevalent in females36 (Table 1).
When the cut-off of Anisakis-sIgE (tested by ImmunoCAP) was analyzed based on clinical features in allergic patients and in sABD, we observed that an increase from 0.35 to 7.9 kUA/L improved its clinical significance (likelihood ratio of 5.2–0.24). Thus, a positive test result will be 5.2 times more likely in patients with Anisakis allergy than in asymptomatic population, while a negative test is 4.2 times (i.e. 1/0.24) more likely in patients without Anisakis allergy.
Regarding the population of allergic patients, only two sera out of 49 (4.1%) tested negative by rAni s 7, while 11/49 (22.4%) tested negative by rAni s 1. In our population, no serum tested positive to rAni s 1 and negative to rAni s 7. The proportions of sensitized patients against rAni s 7 and rAni s 1 in our allergic population were similar to those previously reported for patients with gastro-allergic anisakiasis or Anisakis-induced chronic urticaria17 and confirm previous reports on the immunodominant IgE response to these allergens (mainly to Ani s 7) during the infections by A. simplex28,34,37. Like for the allergic population, the percentage of sera testing positive to Ani s 7 in the sABD group was also extremely high (41/46; 89.1%), this percentage dropped to 52.2% (24/46) for rAni s 1.
The high percentage of seropositivity to rAni s 1 in the allergic population (77.5%) and a lower value in the sABD population (52.2%), compared with the responses to rAni s 7 in both populations (95.9% and 89.1%, respectively), suggests that Ani s 1 is a clinically relevant allergen, or at least, more clinically relevant than Ani s 7. Although Ani s 7 is the most immunodominant reported Anisakis allergen, it also induces high levels of IgG4 antibodies during Anisakis acute infections (e.g. gastroallergic anisakiasis), which could have a protecting role against developing some allergic symptoms (e.g., chronic urticarial) to this allergen38. Moreover, the repetitive structure variants composing this secreted antigen37 suggests it may be involved in evasion of the immune response in the definitive natural host, for example, acting as a decoy antigen. In contrast, Ani s 139 is probably of higher clinical relevance because it is structurally related with the family of Kunitz-type serin protease inhibitors largely reported as clinically-relevant allergens27,40.
Using a sample of 43 sera from the sABD population, we observed that all of them were also sensitized to DPT (IgE positive by ImmunoCAP) while only 6/43 (13.9%) tested positive for shrimp. As several Anisakis allergens (e.g. Ani s 3 and Der p 10—tropomyosin—or Ani s 2 and Der p 11—paramyosin) were reported to induce cross-reactive with mites, insects or shrimp32,41, a doubt arises about whether the positive correlations observed between IgE to DTP, Anisakis and shrimp (see Supplemental Table S3 online) are due to cross-reactions or, alternatively, that such patients were sensitized to several allergens. Although based in the present data, none of the hypothesis can be totally discarded, some data suggests that concomitant sensitizations are more probable in our cohort of patients. While cross-reacting antigens (e.g., tropomyosin), which are present in Anisakis, DTP and shrimp, may boost IgE antibody responses to each other thus provoking false positive results in ImmunoCAP (targeted with whole antigen mixtures), such cross-reactivity is improbable with Trisakis-170 analysis, mainly with Ani s 7. This hypothesis is supported by three facts: (1) the Ani s 7 allergen included in the kit is a polypeptide of 283 residues, which does not have relevant sequence identity with any know human allergen37; no cross-reactions with mites were not previously reported for this allergen, and (2) there is experimental evidence that Cantabria is a region having one of the highest allergen concentrations of D. pteronyssinus (ref https://alergiaweb.files.wordpress.com/2014/03/mapa-acarolc3b3gico-de-espac3b1a-de-l-leti.pdf).
Comparing the results obtained between determinations of sIgE by ImmunoCAP and Trisakis 170 only 5 sera were negative with the latter in the population of sABD. This raises the question of whether they are false sensitizations due to cross-reactivity that are frequently associated to the ImmunoCAP method or false negative results due to a small number of infected subjects that do not induce antibodies to Ani s 1/Ani s 7. Another possibility, that the test Trisakis 170 was less sensitive than ImmunoCAP seems less probable as this test detected patients with, as little as, 0.35 kUA/L by ImmnoCAP.
A preferential response to some other Anisakis allergens such as Ani s 4 (cystatin), Ani s 5 (SXP/RAL protein), Ani s 11 or Ani s 13 (haemoglobin)41–43, can explain that some sera test positive by ImmunoCAP and negative to Trisakis 170. Also, cross-reactions due to Ascaris infections was also reported as a possible cause of detecting sensitization to Anisakis in asymptomatic patients30,44,45, which can be related with IgE responses to pan-allergens as paramyosin (Ani s 2)31,46.
In summary, in this study we reported for the first time 12.65% prevalence values of Anisakis sensitization in Cantabria. We showed a different intensity and frequency of response to Anisakis-sIgE measured by ImmunoCAP and Ani s 1 by ELISA, respectively, between A. simplex allergic patients and asymptomatic sensitized population. Also, higher frequency of recognition of the rAni s 1 allergen was found in patients who has experienced a severe reaction compare which those who had suffered a mild to moderate one (Fig. 2).
In consequence, anti-Anisakis sIgE ImmunoCAP values ≥ 7.9 kUA/L and high OD signals to the rAni s 1 allergen could be potential biomarkers to recognize patients at risk of suffering severe allergic reaction after a re-infection by the parasite.
Materials and methods
All methods were carried out in accordance with relevant guidelines and regulations.
Study design, subjects and serum samples
A cross-sectional study with prospective data collection was performed at the Marques de Valdecilla University Hospital. The study was approved by the Clinical Research Ethics Committee of Cantabria (CEIC. Protocol number: 2016.074). All patients included in the study gave written informed consent for blood extraction, an anonymous survey about fish consumption habits and a subsequent consult if necessary.
The prevalence of IgE sensitization to A. simplex (Anisakis-sIgE > 0.35 KUA/L, ImmunoCAP) was investigated in a group of 403 BD randomly recruited at the Blood and Tissue Bank of Cantabria, Spain (control group) (see Supplementary Fig. S1 online). Among them, 51 subjects tested positive to Anisakis by ImmunoCAP, of which 47 individuals reported no previous allergic/gastrointestinal symptoms after fish consumption, chronic urticaria or dyspepsia (sABD group) (see Supplementary Fig. S1 online). One serum was lost during storage, so the remaining 46 sABD sera were used for further studies. Moreover, a follow up two years after the recruitment date was performed among this asymptomatic subgroup. The purpose was to verify if subjects belonging to the sABD group develop any clinical symptom after fish consumption during that time. In parallel, sera from 49 patients with allergy to A. simplex, were recruited for comparisons at the Allergy Department of Marques de Valdecilla University Hospital. On the inclusion day, a written survey (which included demographic data, fish consumption details and contact information) was completed by all subjects and a blood sample was collected. A Vacutainer SST II Advance (Becton Dickinson) tube was used to obtain and separate the serum sample. After a clot was formed, tubes were centrifuged at 2,000 g for 15 min at 25 °C. Serum was collected and stored at − 20 °C until use.
The group of allergic patients to A. simplex had experienced mild to severe allergic or gastrointestinal symptoms (urticaria, angioedema, dyspnea, nauseas, vomiting, abdominal pain, anaphylaxis) a few hours after eating suspicious A. simplex infected fish, presented with Anisakis-sIgE values greater than 0.35 KUA/L and sIgE to fish < 0.35 KUA/L11,47.
The sample size was determined previously assuming an A. simplex sensitization prevalence around 13%9,48 for a population of 588,656 (https://www.ine.es/jaxiT3/Datos.htm?t=2893), considering a 5% precision and a 95% confidence level, to detect a meaningful difference (20–25%) in the main variables evaluated between groups.
Skin testing and determination of Specific IgE to Anisakis simplex by ImmunoCAP
All allergic patients and 21 out of 47 of the enrolled sABD underwent SPT to A. simplex using commercial extracts (Laboratorios LETI, Spain; 165 mcg/mL). SPTs were performed on the volar side of the forearm by using disposable 1-mm-tip lancets and were also conducted with histamine (Roxall; 10 mg/ml) as positive and saline solution (0.9% NaCl) as negative controls. Readings were taken at 20 min after application. A mean wheal diameter of ≥ 3 mm was considered positive. sIgE to whole A. simplex extract (Anisakis-sIgE) was determined by ImmunoCAP system (Thermo Fisher Scientific Inc., Uppsala, Sweden) according to the manufacturer’s instructions. A positive result was considered Anisakis-sIgE > 0.35 kUA/l. The same method was used to determinate total IgE, and sIgE to fish.
Determination of specific IgE to rAni s 1 and rAni s 7 by ELISA
Fourty-six sABD and the 49 allergic patients (both groups with Anisakis-sIgE > 0.35 KUA/L) were analyzed by ELISA (Trisakis-170 ELISA. Parasitology Laboratory at Santiago de Compostela University, Spain) to detect sIgE-Ani s 1 and sIgE-rAni s 7 allergens, as previously described28,49,50. Cut off values (calculated absorbance) were considered OD = 0.09 for rAni s 1 and OD = 0.05 and rAni s 728,37.
Specific IgE to Dermatophagoides pteronyssinus and shrimp by InmunoCAP
sIgE to DPT and shrimp was determined by ImmunoCAP system (Thermo Fisher Scientific Inc., Uppsala, Sweden) in all allergic patients and in 43 sABD following the manufacture instructions.
Statistical analysis
To analyze possible associations between demographic characteristics (independent variables) and clinically relevant sensitization to A. simplex (dependent variable) a univariate and multivariate logistic regression analyses were used. Statistical analysis of diagnosis test results was carried out by logistic regression analysis and Receiver Operating Characteristics (ROC) curve. A p value of 0.05 or less was considered significant. The SPSS (version 20.0) software package was used for all data analyses. The correlation between serum levels were analyzed by MedCalc Statistical Software version 19.1 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019).
Ethics approval and consent to participate
A cross-sectional study with prospective data collection was performed at the Marques de Valdecilla University Hospital. The study was approved by the Clinical Research Ethics Committee of Cantabria (CEIC. Protocol Number: 2016.074). All patients included in the study gave written informed consent for blood extraction, an anonymous survey about fish consumption habits and a subsequent consult if necessary.
Supplementary information
Acknowledgments
We are very grateful to all medical stuff members of the Allergy Department (HUMV) for their contribution in patient recruitment and the nursing team for their help in processing the serum sample and for performing skin testing. We thank Dr. Arroyo and Dr. Amunárriz and all nursery staff of Banco de Sangre y Tejidos de Cantabria (Fundación Marqués de Valdecilla, Spain), for their help in obtaining serum samples from the donors as well as with the volunteers that granted us their valuable consent to donated sera and time to fill the questionnaire. We also have a special mention to the laboratory staff of the Immunology Department (HUMV), especially to Raquel Hondal. Dr. Florencio M. Ubeira and Dr. Victoria Martínez-Sernández received financial support from the Consellería de Cultura, Educación e Ordenación Universitaria (Xunta de Galicia), Spain, and the European Regional Development Fund. The founders had no role in the design, outcome of the experiments or interpretation of the data.
Abbreviations
- A. simplex
Anisakis simplex
- Anisakis-sIgE
Specific IgE-antibodies to Anisakis simplex
- AUC
Area under the curve
- BD
Blood donors
- CRD
Component-resolved diagnosis
- ELISA
Enzyme-linked immunosorbent assay
- HDM
House dust mites
- IgE
Immunoglobulin E
- MC
Mast cell
- sABD
Sensitized asymptomatic blood donors
- sIgE
Specific immunoglobulin E
- sIgE anti-rAni s 1
Specific IgE-antibodies to recombinant Ani s 1 allergen
- sIgE anti-rAni s 7
Specific IgE-antibodies to recombinant Ani s 7 allergen
- SPT
Skin prick test
- rAni s 1
Recombinant Ani s 1 allergen
- rAni s 7
Recombinant Ani s 1 allergen
- ROC
Receiver operating curve
Author contributions
L.V.S., M.L.H. and F.R.R. designed experiments, L.V.S., P.M.C., F.R.R. performed research, and L.V.S., M.L.H., P.M.C., and F.R.R. analyzed data. L.V.S. wrote the first draft of the manuscript. V.M.S. and F.M.U. performed ELISA experiments. V.M. assisted with patient data collection. All co-authors contributed to writing the manuscript and approved its final version.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Leticia de las Vecillas and Pedro Muñoz-Cacho.
Change history
10/28/2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Supplementary information
is available for this paper at 10.1038/s41598-020-67786-w.
References
- 1.Hochberg NS, Hamer DH. Anisakidosis: perils of the deep. Clin. Infect. Dis. 2010;51:806–812. doi: 10.1086/656238. [DOI] [PubMed] [Google Scholar]
- 2.Køie M, Berland B, Burt MDB. Development to third-stage larvae occurs in the eggs of Anisakis simplex and Pseudotetranova decipiens (Nematoda, Ascaridoidea, Anisakidae) Can. J. Fish. Aquat. Sci. 1995;52:134–139. [Google Scholar]
- 3.Bao M, et al. Assessing the risk of an emerging zoonosis of worldwide concern: anisakiasis. Sci. Rep. 2017;7:43699. doi: 10.1038/srep43699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzucco W, et al. Anisakis sensitization in different population groups and public health impact: a systematic review. PLoS ONE. 2018;13:e0203671. doi: 10.1371/journal.pone.0203671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso MAT, et al. Recurrence of anaphylaxis in a Spanish series. J. Investig. Allergol. Clin. Immunol. 2013;23:383–391. [PubMed] [Google Scholar]
- 6.Bruschi F. Helminth infections and their impact on global public health. Springer, Berlin. 2014 doi: 10.1007/978-3-7091-1782-8. [DOI] [Google Scholar]
- 7.Audicana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008;21:360–379. doi: 10.1128/CMR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuéllar C, Valls A, de Frutos C, Rodero M, Daschner A. Avidity studies in Anisakis simplex: associated allergic diseases. J. Allergy. 2013;2013:1–6. doi: 10.1155/2013/106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valiñas B, et al. Prevalence of and risk factors for IgE sensitization to Anisakis simplex in a Spanish population. Allergy. 2001;56:667–671. doi: 10.1034/j.1398-9995.2001.00987.x. [DOI] [PubMed] [Google Scholar]
- 10.Audicana MT, Ansotegui IJ, Corres LF, Kennedy MW. Anisakis simplex: dangerous—dead and alive? Trends Parasitol. 2002;18(1):20–25. doi: 10.1016/s1471-4922(01)02152-3. [DOI] [PubMed] [Google Scholar]
- 11.Sastre J, et al. A double-blind, placebo-controlled oral challenge study with lyophilized larvae and antigen of the fish parasite, Anisakis simplex. Allergy Eur. J. Allergy Clin. Immunol. 2000;55:560–564. doi: 10.1034/j.1398-9995.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Daschner A, Cuéllar C, Rodero M. The Anisakis allergy debate: does an evolutionary approach help? Trends Parasitol. 2012;28:9–15. doi: 10.1016/j.pt.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Gómez A, et al. Anisakis simplex only provokes allergic symptoms when the worm parasitises the gastrointestinal tract. Parasitol. Res. 2004;93:378–384. doi: 10.1007/s00436-004-1085-9. [DOI] [PubMed] [Google Scholar]
- 14.Del Rey Moreno A, et al. Sensitization to Anisakis simplex s.l. in a healthy population. Acta Trop. 2006;97:265–269. doi: 10.1016/j.actatropica.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 15.del Pozo V, et al. Immunopathogenesis of human gastrointestinal infection by Anisakis simplex. J. Allergy Clin. Immunol. 1999;104:637–643. doi: 10.1016/s0091-6749(99)70336-2. [DOI] [PubMed] [Google Scholar]
- 16.Esp, R., Clin, C., Intern, M. & Dic, R. Casos Clínicos. 2018, 118–121 (2018).
- 17.Cuéllar C, et al. Ani s 1 and Ani s 7 recombinant allergens are able to differentiate distinct Anisakis simplex-associated allergic clinical disorders. Arch. Dermatol. Res. 2012;304:283–288. doi: 10.1007/s00403-012-1206-8. [DOI] [PubMed] [Google Scholar]
- 18.Chung YB, Lee J. Clinical characteristics of gastroallergic anisakiasis and diagnostic implications of immunologic tests. Allergy Asthma Immunol. Res. 2014;6:228–233. doi: 10.4168/aair.2014.6.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toro C, et al. Seropositivity to a major allergen of Anisakis simplex, Ani s 1, in dyspeptic patients with Helicobacter pylori infection: histological and laboratory findings and clinical significance. Clin. Microbiol. Infect. 2006;12:453–458. doi: 10.1111/j.1469-0691.2006.01376.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan AP. Chronic urticaria: pathogenesis and treatment. J. Allergy Clin. Immunol. 2004;114:465–474. doi: 10.1016/j.jaci.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzo S, et al. Usefulness of currently available methods for the diagnosis of Anisakis simplex allergy. Allergy. 2000;55:627–633. doi: 10.1034/j.1398-9995.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- 22.Baeza ML, et al. Characterization of allergens secreted by Anisakis simplex parasite: clinical relevance in comparison with somatic allergens. Clin. Exp. Allergy. 2004;34:296–302. doi: 10.1111/j.1365-2222.2004.01883.x. [DOI] [PubMed] [Google Scholar]
- 23.Gamboa PM, et al. Diagnostic utility of components in allergy to Anisakis simplex. J. Investig. Allergol. Clin. Immunol. 2012;22:13–19. [PubMed] [Google Scholar]
- 24.Caballero ML, Moneo I. Specific IgE determination to Ani s 1, a major allergen from Anisakis simplex, is a useful tool for diagnosis. Ann. Allergy Asthma Immunol. 2002;89:74–77. doi: 10.1016/S1081-1206(10)61914-X. [DOI] [PubMed] [Google Scholar]
- 25.Moneo I, Carballeda-Sangiao N, González-Muñoz M. New perspectives on the diagnosis of allergy to Anisakis spp. Curr. Allergy Asthma Rep. 2017;17:27. doi: 10.1007/s11882-017-0698-x. [DOI] [PubMed] [Google Scholar]
- 26.Mehrdana F, Buchmann K. Excretory/secretory products of anisakid nematodes: biological and pathological roles. Acta Vet. Scand. 2017;59:42. doi: 10.1186/s13028-017-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008;121:847–852.e7. doi: 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Anadón AM, et al. Diagnosing human anisakiasis: recombinant Ani s 1 and Ani s 7 allergens versus the UniCAP 100 fluorescence enzyme immunoassay. Clin. Vaccine Immunol. 2010;17:496–502. doi: 10.1128/CVI.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerončić A, et al. Anisakis sensitization in the Croatian fish processing workers: behavioral instead of occupational risk factors? PLoS Negl. Trop. Dis. 2020;14:e0008038. doi: 10.1371/journal.pntd.0008038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viñas M, Postigo I, Suñén E, Martínez J. Urticaria and silent parasitism by ascaridoidea: component-resolved diagnosis reinforces the significance of this association. PLoS Negl. Trop. Dis. 2020;14:e0008177. doi: 10.1371/journal.pntd.0008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guarneri F, Guarneri C, Benvenga S. Cross-reactivity of Anisakis simplex: possible role of Ani s 2 and Ani s 3. Int. J. Dermatol. 2007;46:146–150. doi: 10.1111/j.1365-4632.2006.03091.x. [DOI] [PubMed] [Google Scholar]
- 32.Bernardini R, et al. Cross-reactivity between IgE-binding proteins from Anisakis simplex and Dermatophagoides pteronyssinus. Int. J. Immunopathol. Pharmacol. 2005;18:671–675. doi: 10.1177/039463200501800408. [DOI] [PubMed] [Google Scholar]
- 33.Shafique RH, Inam M, Ismail M, Chaudhary FR. Group 10 allergens (tropomyosins) from house-dust mites may cause covariation of sensitization to allergens from other invertebrates. Allergy Rhinol. (Provid.) 2012;3:e74–e90. doi: 10.2500/ar.2012.3.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anadón AM, et al. The Anisakis simplex Ani s 7 major allergen as an indicator of true Anisakis infections. Clin. Exp. Immunol. 2009;156:471–478. doi: 10.1111/j.1365-2249.2009.03919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muraro A, et al. Precision medicine in allergic disease-food allergy, drug allergy, and anaphylaxis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy Asthma and Immunology. Allergy. 2017;72:1006–1021. doi: 10.1111/all.13132. [DOI] [PubMed] [Google Scholar]
- 36.Selmi C, Gershwin ME. Sex and autoimmunity: proposed mechanisms of disease onset and severity. Expert Rev. Clin. Immunol. 2019;15:607–615. doi: 10.1080/1744666X.2019.1606714. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez E, et al. Novel sequences and epitopes of diagnostic value derived from the Anisakis simplex Ani s 7 major allergen. Allergy Eur. J. Allergy Clin. Immunol. 2008;63:219–225. doi: 10.1111/j.1398-9995.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 38.Daschner A, et al. Specific IgG 4: possible role in the pathogenesis and a new marker in the diagnosis of Anisakis-associated allergic disease. Scand. J. Immunol. 2014;79:120–126. doi: 10.1111/sji.12129. [DOI] [PubMed] [Google Scholar]
- 39.Moneo I, Caballero ML, Gómez F, Ortega E, Alonso MJ. Isolation and characterization of a major allergen from the fish parasite Anisakis simplex. J. Allergy Clin. Immunol. 2000;106:177–182. doi: 10.1067/mai.2000.106732. [DOI] [PubMed] [Google Scholar]
- 40.Aalberse RC. Structural biology of allergens. J. Allergy Clin. Immunol. 2000;106:228–238. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 41.Carballeda-Sangiao N, et al. Ani s 11-like protein is a pepsin- and heat-resistant major allergen of Anisakis spp. and a valuable tool for Anisakis allergy component-resolved diagnosis. Int. Arch. Allergy Immunol. 2016;169:108–112. doi: 10.1159/000444981. [DOI] [PubMed] [Google Scholar]
- 42.González-Fernández J, et al. Haemoglobin, a new major allergen of Anisakis simplex. Int. J. Parasitol. 2015;45:399–407. doi: 10.1016/j.ijpara.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Mahillo AI, et al. Cloning and characterisation of the Anisakis simplex allergen Ani s 4 as a cysteine-protease inhibitor. Int. J. Parasitol. 2007;37:907–917. doi: 10.1016/j.ijpara.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Carballeda-Sangiao N, et al. Anisakis/Ascaris IgE ratio improves specificity for the diagnosis of Anisakis simplex sensitization in travellers and immigrants. Acta Trop. 2014;138:1–4. doi: 10.1016/j.actatropica.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Brusca I, et al. Use of a comprehensive diagnostic algorithm for Anisakis allergy in a high seroprevalence Mediterranean setting. Eur. Ann. Allergy Clin. Immunol. 2020;52:131–141. doi: 10.23822/EurAnnACI.1764-1489.118. [DOI] [PubMed] [Google Scholar]
- 46.Caballero ML, et al. Isolation of Ani s 5, an excretory–secretory and highly heat-resistant allergen useful for the diagnosis of Anisakis larvae sensitization. Parasitol. Res. 2008;103:1231–1233. doi: 10.1007/s00436-008-1105-2. [DOI] [PubMed] [Google Scholar]
- 47.Brown SGA. Clinical features and severity grading of anaphylaxis. J. Allergy Clin. Immunol. 2004;114:371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 48.Puente P, et al. Anisakis simplex: the high prevalence in Madrid (Spain) and its relation with fish consumption. Exp. Parasitol. 2008;118:271–274. doi: 10.1016/j.exppara.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Lin AH, Nepstad I, Florvaag E, Egaas E, Van Do T. An extended study of seroprevalence of anti-anisakis simplex IgE antibodies in norwegian blood donors. Scand. J. Immunol. 2014;79:61–67. doi: 10.1111/sji.12130. [DOI] [PubMed] [Google Scholar]
- 50.Mladineo I, Poljak V, Martínez-Sernández V, Ubeira FM. Anti-Anisakis IgE seroprevalence in the healthy croatian coastal population and associated risk factors. PLoS Negl. Trop. Dis. 2014;8:e2673. doi: 10.1371/journal.pntd.0002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeLong ER, DeLong DM, Clarke-Pearson DL, Ubeira FM. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 2014;44:837–845. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.