Abstract
COVID-19 can be divided into three clinical stages, and one can speculate that these stages correlate with where the infection resides. For the asymptomatic phase, the infection mostly resides in the nose, where it elicits a minimal innate immune response. For the mildly symptomatic phase, the infection is mostly in the pseudostratified epithelium of the larger airways and is accompanied by a more vigorous innate immune response. In the conducting airways, the epithelium can recover from the infection, because the keratin 5 basal cells are spared and they are the progenitor cells for the bronchial epithelium. There may be more severe disease in the bronchioles, where the club cells are likely infected. The devastating third phase is in the gas exchange units of the lung, where ACE2-expressing alveolar type II cells and perhaps type I cells are infected. The loss of type II cells results in respiratory insufficiency due to the loss of pulmonary surfactant, alveolar flooding, and possible loss of normal repair, since type II cells are the progenitors of type I cells. The loss of type I and type II cells will also block normal active resorption of alveolar fluid. Subsequent endothelial damage leads to transudation of plasma proteins, formation of hyaline membranes, and an inflammatory exudate, characteristic of ARDS. Repair might be normal, but if the type II cells are severely damaged alternative pathways for epithelial repair may be activated, which would result in some residual lung disease.
Keywords: ACE2, SARS-CoV-2, type II cells
INTRODUCTION
COVID-19 is a devastating disease, but details on the pathogenesis of this disease are not known. The respiratory epithelium is the initial site of the infection (22, 29). One can formulate a perspective of what is likely happening based on previous studies with SARS. Both SARS-CoV-1 and SARS-CoV-2, the designation for the virus that causes the disease COVID-19, use ACE2 as the receptor. By single-cell RNA profiling, ACE2 expression level in lung cells is low (33). The site of infection is thought to most likely occur in cells that express both ACE2 and the serine protease TMPRSS2, and these sites are corneal conjunctiva in the eye, ciliated and secretory cells in the nose, ciliated and secretory cells in the conducting airways, and alveolar type II cells in the gas exchange area of the lung (33). ACE2 is the docking site whereby the virus attaches and can enter the cell and start replicating. The role of the protease activity of ACE2 in the pathogenesis of the disease has been postulated but is complex and unresolved (12, 18, 23). ACE2 is protective and can block the development of acute lung injury (14, 18). Shed ACE2 or recombinant ACE2 might be useful both to decrease lung inflammation and to be a decoy to inhibit viral uptake (12). On the other hand, HCoV-NL63 is a relatively innocuous respiratory coronavirus that also uses ACE2 for viral entry (23).
PHASES OF PATHOGENESIS
The pathogenesis of COVID-19 can be divided into three phases based on the site of the respiratory epithelium infected. The first phase of the disease is in the nose. Here the virus likely infects ciliated and secretory cells, replicates, but apparently does not induce a vigorous innate immune response (33). This is the stage for asymptomatic individuals who can transmit the disease. Respiratory viruses that circulate in the community grow intracellularly and need a host to survive. It is reasonable to assume that the nasal cavity that houses these respiratory viruses has a blunted innate immune response (41). We need to learn more about the magnitude of the innate immune response to the virus located in the nasal passages compared with viral infection in the intrapulmonary conducting airways (41). It might be informative to add PCR tests for interferons, interferon response genes, and viral sensing genes to the positive nasal swabs to identify subjects who have a higher risk of disease progression (26, 48).
The second phase of COVID-19 takes place along the conducting airways, the bronchi and bronchioles. The virus infects ciliated cells as the disease progresses deeper into the lung. SARS-CoV-1 infects ciliated but not mucus cells in air-liquid interface cultures (15, 30). Similarly in nonhuman primates, SARS-CoV-2 infects ciliated cells (29). When the infection reaches this region, one can speculate that a more vigorous innate immune response is generated and the patient feels ill. Likely the infection and recruitment of inflammatory cells impairs clearance and allows the infection to spread deeper into the lung. Keratin 5-expressing basal cells are apparently spared from direct infection (29). These basal cells are the progenitor cells for the airway epithelium, and hence the epithelium of the conducting airways can readily repair and recover from the infection. Primate, hamster, and single-cell expression studies for ACE2 have all indicated that club cells in the bronchioles might be an important site of infection (3, 29, 33). Direct studies of club cells have not yet been reported, but they are located in the very distal bronchioles in humans (24).
The third phase of the disease is the lethal phase, as the infection spreads into the gas exchange portion of the lung and infects alveolar type II cells (Fig. 1). The patient is now hypoxic and has scattered subpleural ground glass densities on radiographic imaging. The gas exchange structures are an exquisite example of biologic engineering. There is close apposition of the endothelium to the type I cells to allow rapid exchange of oxygen and carbon dioxide as blood passes through the pulmonary capillaries. However, this anatomic arrangement is very delicate. Type I cells are extremely large flat cells that are easily damaged in many forms of lung injury. These cells are critical for gas exchange and transepithelial ion movement to keep the alveolus relatively free of fluid. Type I cells use CLIC5 as the chloride channel, whereas type II cells use CFTR. Both cell types express SCNN1A, SCNN1B, and SCNN1C as the sodium channel and ATP1A1 and ATP1B1 as the Na-K-ATPase. Both cell types likely contribute to the active resorption of alveolar fluid (5, 20). Rapid flooding of alveoli early in the disease suggests that type I cells are also damaged or infected (22, 26). Both type I cells and type II cells appear to be infected by SARS-CoV-2 in nonhuman primates (22, 29). SARS-CoV-1 infects highly differentiated type II cells more readily than poorly differentiated type II cells, which is also reflected in their relative ACE2 protein expression (21, 26). The protein expression of ACE2 in freshly isolated type II cells is highly variable and may indicate susceptibility to more severe disease (26). Type II cells make and secrete pulmonary surfactant, which is required for effective gas exchange. One of the early changes in surfactant in the development of ARDS is a switch in the minor anionic phospholipids from phosphatidylglycerol to phosphatidylinositol (8, 11). The composition and biophysical properties of surfactant will likely be impaired as in other forms of ARDS, lead to higher surface tension, and promote alveolar flooding in the diseased portions of the lung (2, 8, 11).
Fig. 1.
Infection of human type II cells with SARS-CoV. Human type II cells were cultured at an air-liquid interface so as to maintain their state of differentiation and infected with SARS-CoV-1 (26). The viral particles (white arrows) are seen in vesicles near normal-appearing lamellar bodies and mitochondria. There was no observed cytopathic effect under the conditions of these cultures.
PHASES OF ALVEOLAR INVOLVEMENT
There are apparently two phases for the alveolar involvement in COVID-2 (6). The early phase is characterized by profound hypoxia, normal compliance, and focal alveolar flooding, and the late phase is similar to traditional inflammatory ARDS. In the early phase, the compliance is normal because most of the lung is normal and uninvolved, and the patient is breathing close to normal functional residual capacity at the lower end of the pressure-volume curve. The physiological alterations that cause the initial hypoxia require additional studies. Focal alveolar flooding occurs, likely due to damage to both the epithelium and the endothelium. In SARS, endothelial cells are not thought to be infected directly in spite of the fact that they express some ACE2 (35). We need more detailed studies on possible infection of endothelial cells. The flooding could be due to a combination of factors, including high surface tension, death of type II cells, leak of serum proteins into the alveolar space, formation of fibrin exudates, interference with the adsorption of surfactant to the alveolar surface, and reduced active transport to pump sodium from the alveolar space into the interstitium. High alveolar surface tension would translate into increased transmural microcapillary pressure and microvascular leak (2). Alveoli tend to flood in an all-or-none manner, which would be expected in terms of the Laplace relationship and increased surface tension in the injured alveoli (31). However, the reason for the continued perfusion of these hypoxic flooded units remains to be defined. It may be due to an interference with local (9, 39) or central hypoxic vasoconstriction, due to local production of a vasodilator such as nitric oxide or PGI2, or due to transmission of the high surface tension to more negative interstitial pressures and vasodilation of the pulmonary microvasculature or a combination of these factors. Defining the physiological abnormalities of the early phase with hypoxia and normal compliance may require studies with primates. Although primates do not appear to develop a lethal infection, they have been very useful to define the cells that are infected, the effect of age, and response to therapy (22, 29, 42).
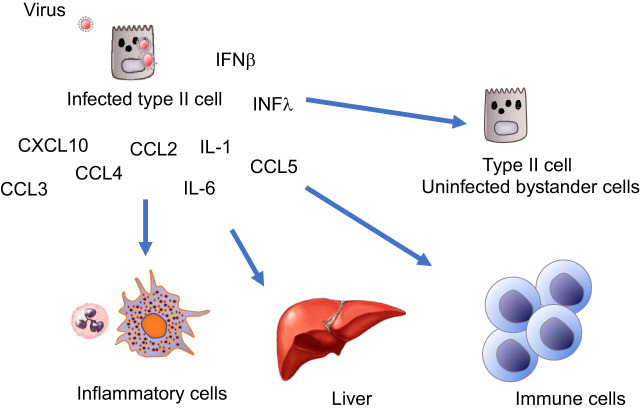
The infected type II cells will be among the first responders and can initiate the innate immune response (Fig. 2). In response to SARS-CoV-1 and influenza, type II cells express high levels of interferon lambda, interferon beta, CXCL10 (IP10), CXCL11, CCL5 (RANTES), and IL6 (26, 38). The signal can be greatly amplified by healthy bystander epithelial and inflammatory cells that will secrete additional interferon-responsive cytokines (13). CXCL10 has recently been shown to predict severe disease in COVID-19 patients. as it did with SARS (34, 44). Inflammatory cells will be recruited and enter the space. The cell-cell interactions and signaling during the apparent cytokine storm are very complex (1, 7). The infected cells will propagate the virus, and the viral particles will spill over into adjacent alveoli. This can be through pores of Kohn or due to the fact that some type II cells have apical surfaces in more than one alveolus. There may also be cell-to-cell transmission such that type II cells get infected and they infect adjacent type I cells (29). This type of injury is explosive, because the virus replicates rapidly and spreads. Individual lobules will fill with fluid, causing the characteristic radiographic images (Fig. 3). Along with the damage to type I and type II cells, there will be extensive damage to the endothelium. The result will be leakage of fibrinogen and other plasma proteins into the alveolus that can impair the ability of surfactant to absorb to the surface and lower surface tension. In addition, there will be some multinucleated giant cells, and some of these cells with be epithelial in origin (10). The next phase will include additional migration of fibroblasts and inflammatory cells into the alveolar lumen and appositional atelectasis and loss of some gas units. The loss of type II cells means loss of progenitor cells for type I cells. In most forms of lung injury there is type II cell proliferation, development of transitional type II cells, and differentiation into type I cells, which leads to restoration of the alveolar epithelium (Fig. 3C) (17, 28, 32). This might not occur in severely damaged areas of the lung in COVID-19. There are also normal pathways to limit fibrosis by the epithelium. Alveolar epithelial cells can inhibit fibroblast proliferation and the expression of extracellular matrix genes in fibroblasts (4, 25). The time course for these changes will vary in different parts of the lung of the lung as new areas get infected and in response to the innate and acquired immune response.
Fig. 2.
Bystander amplification and multiple cell-cell interactions in the cytokine response. The cytokine response is very complex. Virus-infected epithelial cells release a variety of cytokines during infection, and nearby bystander cells amplify the response, especially in response to interferon beta and lambda. Soon thereafter the recruited inflammatory cells, immune cells, and other organs such as the liver produce additional mediators and cytokines and further amplify the response.
Fig. 3.

Proposed infection sequence of alveolar epithelial cells to SARS-CoV-2. A: panel shows the virus infecting both type I and type II cells. The relative infectivity will be related to the density of ACE2 expression, which is likely higher on type II cells than type I cells. B: panel describes the early stage of alveolar flooding and hyaline membrane formation followed by inflammation and diffuse alveolar damage (ARDS). The flooding occurs because of damage to the alveolar epithelium and endothelium and high surface tension due to the impaired production and adsorption of pulmonary surfactant. C: panel shows the normal repair process where type II cells proliferate and then differentiate into transitional type II cells and finally into type I cells. Normal transitional type II cells can be identified by coexpression of markers of type II cells and type I cells as well as keratin 8. D: panel depicts the aberrant repair that occurs after severe influenza infection in which distal airway epithelial cells migrate and form a cuboidal epithelium. Some of these cells express keratin 5 and others keratin 8 and markers of club cells.
Alternative pathways for epithelial regeneration have been studied in several systems including the murine model of influenza (Fig. 3D). In influenza as in SARS, type II cells are the alveolar cells primarily infected and can be destroyed in severe infections (21, 26, 40). Airway-derived stem cell populations are activated once the type II cell population is severely depleted, as likely occurs with severe SARS-CoV-2 infection (45). In mice infected with the murine adapted strain of influenza A/PR8/1934 (H1N1), airway-derived epithelial cells proliferate and migrate into the lung parenchyma, forming clusters seen in paraffin-embedded sections (Fig. 3D) (19, 36, 43, 47). Lineage tracing has shown that these cells are heterogeneous (17, 36, 45, 47). There are nonlineage labeled airway cells that express keratin 5 and migrate into the alveolar compartment (27, 36, 43). Club cell-derived stem cells can also proliferate, migrate, express keratin 8, and migrate into the alveolar compartment (32, 46, 47). However, it is not clear that these alternatively derived epithelial cells can fully differentiate into functional type I and II cells (27, 45). During COVID-19 infection there will likely be different repair pathways present in different parts of the lung. In the normal pathway for the transition of type II cells to type I cells, resting type II cells are activated, express keratin 8, and differentiate into type I cells (16, 32). The normal pathway will likely be indicated by transitional cells that express keratin 8 and markers of both type I cells and type II cells such as RAGE and Muc1, and the areas of alternate pathways of repair will be indicated by cells expressing club cell antigens and keratin 8 or clusters of keratin 5 cells in the alveolar walls (4, 16, 32). If these alternate pathways for epithelial repair are activated in the most severe disease, there may be some scarring and residual disease. The degree of scarring and residual disease after COVID-19 is not currently known. However, both nonhuman primates and hamsters apparently recover fully and would not likely use these alternative pathways (3, 22).
SUMMARY
In summary, COVID-19 can be easily understood by considering where the infection is located. The responses in the nose, the conducting airways, and the alveoli will be different. We need to learn more about the innate immune response in these three sites. We also need to know more about the infection and response in club cells, which have a restricted location in the human lung (24, 37). The disease in the gas exchange portions of the lung can be very severe and may take a long time to fully recover. Now we just have to await the development of effective therapeutics and a vaccine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
R.J.M. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Aevermann BD, Pickett BE, Kumar S, Klem EB, Agnihothram S, Askovich PS, Bankhead A 3rd, Bolles M, Carter V, Chang J, Clauss TR, Dash P, Diercks AH, Eisfeld AJ, Ellis A, Fan S, Ferris MT, Gralinski LE, Green RR, Gritsenko MA, Hatta M, Heegel RA, Jacobs JM, Jeng S, Josset L, Kaiser SM, Kelly S, Law GL, Li C, Li J, Long C, Luna ML, Matzke M, McDermott J, Menachery V, Metz TO, Mitchell H, Monroe ME, Navarro G, Neumann G, Podyminogin RL, Purvine SO, Rosenberger CM, Sanders CJ, Schepmoes AA, Shukla AK, Sims A, Sova P, Tam VC, Tchitchek N, Thomas PG, Tilton SC, Totura A, Wang J, Webb-Robertson BJ, Wen J, Weiss JM, Yang F, Yount B, Zhang Q, McWeeney S, Smith RD, Waters KM, Kawaoka Y, Baric R, Aderem A, Katze MG, Scheuermann RH. A comprehensive collection of systems biology data characterizing the host response to viral infection. Sci Data 1: 140033, 2014. doi: 10.1038/sdata.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert RK, Lakshminarayan S, Hildebrandt J, Kirk W, Butler J. Increased surface tension favors pulmonary edema formation in anesthetized dogs’ lungs. J Clin Invest 63: 1015–1018, 1979. doi: 10.1172/JCI109369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, Liang R, Cao J, Chen Y, Tang K, Luo C, Cai JP, Kok KH, Chu H, Chan KH, Sridhar S, Chen Z, Chen H, To KK, Yuen KY. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. In press. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correll KA, Edeen KE, Zemans RL, Redente EF, Serban KA, Curran-Everett D, Edelman BL, Mikels-Vigdal A, Mason RJ. Transitional human alveolar type II epithelial cells suppress extracellular matrix and growth factor gene expression in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 317: L283–L294, 2019. doi: 10.1152/ajplung.00337.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem 25: 055–062, 2010. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 201: 1299–1300, 2020. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gralinski LE, Bankhead A 3rd, Jeng S, Menachery VD, Proll S, Belisle SE, Matzke M, Webb-Robertson BJ, Luna ML, Shukla AK, Ferris MT, Bolles M, Chang J, Aicher L, Waters KM, Smith RD, Metz TO, Law GL, Katze MG, McWeeney S, Baric RS. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio 4: e00271-13, 2013. doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregory TJ, Longmore WJ, Moxley MA, Whitsett JA, Reed CR, Fowler AA 3rd, Hudson LD, Maunder RJ, Crim C, Hyers TM. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest 88: 1976–1981, 1991. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimmer B, Kuebler WM. The endothelium in hypoxic pulmonary vasoconstriction. J Appl Physiol (1985) 123: 1635–1646, 2017. doi: 10.1152/japplphysiol.00120.2017. [DOI] [PubMed] [Google Scholar]
- 10.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol 170: 1136–1147, 2007. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Günther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 153: 176–184, 1996. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 12.Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc 9: e016219, 2020. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock AS, Stairiker CJ, Boesteanu AC, Monzón-Casanova E, Lukasiak S, Mueller YM, Stubbs AP, García-Sastre A, Turner M, Katsikis PD. Transcriptome analysis of infected and bystander type 2 alveolar epithelial cells during influenza A virus infection reveals in vivo Wnt pathway downregulation. J Virol 92: e01325-18, 2018. doi: 10.1128/JVI.01325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79: 14614–14621, 2005. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang P, Gil de Rubio R, Hrycaj SM, Gurczynski SJ, Riemondy KA, Moore BB, Omary MB, Ridge KM, Zemans RL. Ineffectual type 2–to–type 1 alveolar epithelial cell differentiation in idiopathic pulmonary fibrosis: persistence of the KRT8hi transitional state. Am J Respir Crit Care Med 201: 1443–1447, 2020. doi: 10.1164/rccm.201909-1726LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juul NH, Stockman CA, Desai TJ. Niche cells and signals that regulate lung alveolar stem cells in vivo. Cold Spring Harb Perspect Biol 2020: a035717, 2020. [Erratum in Cold Spring Harb Perspect Biol 12: a040303, 2020]. doi: 10.1101/cshperspect.a035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147: 525–538, 2011. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason RJ, Williams MC, Widdicombe JH, Sanders MJ, Misfeldt DS, Berry LC Jr. Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci USA 79: 6033–6037, 1982. doi: 10.1073/pnas.79.19.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mossel EC, Wang J, Jeffers S, Edeen KE, Wang S, Cosgrove GP, Funk CJ, Manzer R, Miura TA, Pearson LD, Holmes KV, Mason RJ. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology 372: 127–135, 2008. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munster V, Feldmann F, Williamson BN, van Doremalen N, Perez-Perez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, Avanzato VA, Rosenke R, Hanley PW, Saturday G, Scott D, Fischer ER, de Wit E. Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2 (Preprint). bioRxiv 2020.03.21.001628, 2020. doi: 10.1101/2020.03.21.001628 [DOI] [PMC free article] [PubMed]
- 23.Nicholls J, Peiris M. Good ACE, bad ACE do battle in lung injury, SARS. Nat Med 11: 821–822, 2005. doi: 10.1038/nm0805-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plopper CG, Hill LH, Mariassy AT. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp Lung Res 1: 171–180, 1980. doi: 10.3109/01902148009069646. [DOI] [PubMed] [Google Scholar]
- 25.Portnoy J, Pan T, Dinarello CA, Shannon JM, Westcott JY, Zhang L, Mason RJ. Alveolar type II cells inhibit fibroblast proliferation: role of IL-1alpha. Am J Physiol Lung Cell Mol Physiol 290: L307–L316, 2006. doi: 10.1152/ajplung.00102.2005. [DOI] [PubMed] [Google Scholar]
- 26.Qian Z, Travanty EA, Oko L, Edeen K, Berglund A, Wang J, Ito Y, Holmes KV, Mason RJ. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol 48: 742–748, 2013. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray S, Chiba N, Yao C, Guan X, McConnell AM, Brockway B, Que L, McQualter JL, Stripp BR. Rare SOX2+ airway progenitor cells generate KRT5+ cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem Cell Reports 7: 817–825, 2016. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riemondy KA, Jansing NL, Jiang P, Redente EF, Gillen AE, Fu R, Miller AJ, Spence JR, Gerber AN, Hesselberth JR, Zemans RL. Single cell RNA sequencing identifies TGFβ as a key regenerative cue following LPS-induced lung injury. JCI Insight 5: e123637, 2019. doi: 10.1172/jci.insight.123637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, de Meulder D, van Amerongen G, van den Brand J, Okba NM, Schipper D, van Run P, Leijten L, Sikkema R, Verschoor E, Verstrepen B, Bogers W, Langermans J, Drosten C, Fentener van Vlissingen M, Fouchier R, de Swart R, Koopmans M, Haagmans BL. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368: 1012–1015, 2020. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol 79: 15511–15524, 2005. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staub NC. Pulmonary edema. Physiol Rev 54: 678–811, 1974. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- 32.Strunz M, Simon LM, Ansari M, Mattner LF, Angelidis I, Mayr CH, Kathiriya J, Yee M, Ogar P, Sengupta A, Kukhtevich I, Schneider R, Zhao Z, Neumann JH, Behr J, Voss C, Stoger T, Lehmann M, Konigshoff M, Burgstaller G, O’Reilly M, Chapman HA, Theis FJ, Schiller HB. Longitudinal single cell transcriptomics reveals Krt8+ alveolar epithelial progenitors in lung regeneration (Preprint). bioRxiv 705244, 2019. doi: 10.1101/705244 [DOI]
- 33.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL; HCA Lung Biological Network . SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26: 681–687, 2020. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang NL, Chan PK, Wong CK, To KF, Wu AK, Sung YM, Hui DS, Sung JJ, Lam CW. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem 51: 2333–2340, 2005. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol 203: 740–743, 2004. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517: 621–625, 2015. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker SR, Hale S, Malkinson AM, Mason RJ. Properties of isolated nonciliated bronchiolar cells from mouse lung. Exp Lung Res 15: 553–573, 1989. doi: 10.3109/01902148909069618. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Nikrad MP, Phang T, Gao B, Alford T, Ito Y, Edeen K, Travanty EA, Kosmider B, Hartshorn K, Mason RJ. Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am J Respir Cell Mol Biol 45: 582–591, 2011. doi: 10.1165/rcmb.2010-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR, Shin HS, Wu S, Isakson BE, Witzenrath M, de Wit C, Fleming I, Kuppe H, Kuebler WM. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest 122: 4218–4230, 2012. doi: 10.1172/JCI59176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinheimer VK, Becher A, Tönnies M, Holland G, Knepper J, Bauer TT, Schneider P, Neudecker J, Rückert JC, Szymanski K, Temmesfeld-Wollbrueck B, Gruber AD, Bannert N, Suttorp N, Hippenstiel S, Wolff T, Hocke AC. Influenza A viruses target type II pneumocytes in the human lung. J Infect Dis 206: 1685–1694, 2012. doi: 10.1093/infdis/jis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wesolowska-Andersen A, Everman JL, Davidson R, Rios C, Herrin R, Eng C, Janssen WJ, Liu AH, Oh SS, Kumar R, Fingerlin TE, Rodriguez-Santana J, Burchard EG, Seibold MA. Dual RNA-seq reveals viral infections in asthmatic children without respiratory illness which are associated with changes in the airway transcriptome. Genome Biol 18: 12, 2017. [Erratum in Genome Biol 19: 49, 2018]. doi: 10.1186/s13059-016-1140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, van Doremalen N, Leighton I, Yinda CK, Perez-Perez L, Okumura A, Lovaglio J, Hanley PW, Saturday G, Bosio CM, Anzick S, Barbian K, Cihlar T, Martens C, Scott DP, Munster VJ, de Wit E. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2(Preprint). bioRxiv 2020.04.15.043166, 2020. doi: 10.1101/2020.04.15.043166 [DOI] [PMC free article] [PubMed]
- 43.Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V, Jackson JR, Xu J, Lee DK, Gotts JE, Matthay MA, Shannon JM, Chapman HA, Vaughan AE. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol 19: 904–914, 2017. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Shen C, Li J, Yuan J, Wei J, Huang F, Wang F, Li G, Li Y, Xing L, Peng L, Yang M, Cao M, Zheng H, Wu W, Zou R, Li D, Xu Z, Wang H, Zhang M, Zhang Z, Gao GF, Jiang C, Liu L, Liu Y. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. In press. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yee M, Domm W, Gelein R, Bentley KL, Kottmann RM, Sime PJ, Lawrence BP, O’Reilly MA. Alternative progenitor lineages regenerate the adult lung depleted of alveolar epithelial type 2 cells. Am J Respir Cell Mol Biol 56: 453–464, 2017. doi: 10.1165/rcmb.2016-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng D, Limmon GV, Yin L, Leung NH, Yu H, Chow VT, Chen J. Regeneration of alveolar type I and II cells from Scgb1a1-expressing cells following severe pulmonary damage induced by bleomycin and influenza. PLoS One 7: e48451, 2012. doi: 10.1371/journal.pone.0048451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng D, Yin L, Chen J. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol 50: 595–604, 2014. doi: 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J, Banovich N, Barbry P, Brazma A, Desai T, Duong TE, Eickelberg O, Falk C, Farzan M, Glass I, Haniffa M, Horvath P, Hung D, Kaminski N, Krasnow M, Kropski JA, Kuhnemund M, Lafyatis R, Lee H, Leroy S, Linnarson S, Lundeberg J, Meyer K, Misharin A, Nawijn M, Nikolic MZ, Ordovas-Montanes J, Pe’er D, Powell J, Quake S, Rajagopal J, Tata PR, Rawlins EL, Regev A, Reyfman PA, Rojas M, Rosen O, Saeb-Parsy K, Samakovlis C, Schiller H, Schultze JL, Seibold MA, Shalek AK, Shepherd D, Spence J, Spira A, Sun X, Teichmann S, Theis F, Tsankov A, van den Berge M, von Papen M, Whitsett J, Xavier R, Xu Y, Zaragosi LE, Zhang K; HCA Lung Biological Network . SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035.e19, 2020. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]