Summary
We present here PhotoGal4, a phytochrome B-based optogenetic switch for fine-tuned spatiotemporal control of gene expression in Drosophila explants. This switch integrates the light-dependent interaction between phytochrome B and PIF6 from plants with regulatory elements from the yeast Gal4/UAS system. We found that PhotoGal4 efficiently activates and deactivates gene expression upon red- or far-red-light irradiation, respectively. In addition, this optogenetic tool reacts to different illumination conditions, allowing for fine modulation of the light-dependent response. Importantly, by simply focusing a laser beam, PhotoGal4 induces intricate patterns of expression in a customized manner. For instance, we successfully sketched personalized patterns of GFP fluorescence such as emoji-like shapes or letterform logos in Drosophila explants, which illustrates the exquisite precision and versatility of this tool. Hence, we anticipate that PhotoGal4 will expand the powerful Drosophila toolbox and will provide a new avenue to investigate intricate and complex problems in biomedical research.
Subject Areas: Optical Imaging, Genetics, Cell Biology, Technical Aspects of Cell Biology
Graphical Abstract

Highlights
-
•
PhotoGal4 activates gene expression in response to red light in Drosophila explants
-
•
Exposure to far-red light allows for PhotoGal4 deactivation
-
•
PhotoGal4 can activate gene expression in subgroups of cells within a single tissue
Optical Imaging; Genetics; Cell Biology; Technical Aspects of Cell Biology
Introduction
As a powerful genetic model organism, Drosophila melanogaster continues to be a source of innovation in the development of gene expression systems, from the more traditional UAS/Gal4 approach to the more complex heat shock-, hormone- and chemical-related systems (Fischer et al., 1988; Osterwalder et al., 2001; Sun et al., 2007; Tanguay, 1988). Despite their functionality, these classical tools still have few caveats that impinge accurate spatiotemporal control of transgene expression. For example, with heat shock-dependent systems, full activation of transcription occurs only at high temperatures, which may trigger unwanted cellular stress. On the other hand, hormonal- and chemical-dependent promoters lack precise temporal transcriptional activation or deactivation and can be accompanied by complex pharmacokinetics and pleiotropic effects (Landis et al., 2015). Therefore a rapid and reversible inducible system that could offer fine spatiotemporal resolution without toxicity is urgently needed. In this regard, optogenetic approaches seem to check all the boxes. Optogenetics exploits photo-sensing proteins that change conformation in the presence of light to manipulate critical physiological processes in plants, fungi, and cyanobacteria. As these photo-sensing molecules use light as the activating force, inducible systems based on this principle are fast, accurate, and can be quickly reversed by simply removing the stimulus (de Mena et al., 2018; Pathak et al., 2013, 2014).
Among the vast optogenetic repertoire, phytochrome B (PhyB)-based tools have special interest owing to their high stability and unique light-dependent reversibility. In this case, a two-hybrid-like system capitalizes on the light-dependent heterodimerization between the photoreceptor PhyB from Arabidopsis thaliana and its interacting partner Pif6 (Hughes et al., 2012; Shimizu-Sato et al., 2002). Briefly, in the absence of light, PhyB exists in a red-absorbing form, known as Pr, in association with the endogenous chromophore phycocyanobilin (PCB) (Levskaya et al., 2009; Muller et al., 2013a). Then, upon red light irradiation (∼630 nm), the Pr inactive form undergoes a conformational change switching into the active form (Pfr), which interacts with its cofactor Pif6 to exert a variety of functions (Auldridge and Forest, 2011; Davis et al., 1999). Conversely, when illuminated with far-red light (>730 nm), PhyB reverts to the inactive Pr form and dissociates from Pif6. Thus, based on the original design of Shimizu-Sato and co-workers (Shimizu-Sato et al., 2002), we combined the PhyB photosensory N-terminal domain with the Gal4-DNA binding domain (Gal4-DBD) and PIF6 with the VP16 activator domain (Pif6-VP16 AD) to engineer a bicistronic system (Figure 1). This resulted in a tunable light-dependent regulator of gene expression with rapid ON/OFF kinetics and reduced background.
Figure 1.
Schematic Representation of the PhotoGal4 Light-Dependent Gene Expression System
In the dark, phytochrome B (PhyB) remains in its inactive form. Upon incorporation of the chromophore PCB and red light (∼630nm) stimulation, PhyB undergoes a conformational change, turning into the active form. Then, PhyB heterodimerizes with phytochrome-interacting factor 6 (Pif6) to bring together the Gal4 DNA binding domain (DBD) and VP16 activation domain (AD) at the UAS binding sequence, which triggers transcription of the gene of interest. Conversely, upon far-red (>730 nm) illumination, PhyB and Pif6 dissociate, halting transcription immediately.
Here, we introduce PhotoGal4 as a highly sensitive and flexible PhyB-based light-dependent system for spatiotemporal control of gene expression in fruit fly tissues. As a proof of concept, we used Drosophila ex vivo tissue cultures to show how gene expression can be successfully adjusted in a light- and chromophore-dependent manner at different stages of development in the eye. Importantly, we found that manipulation of light frequency and intensity allows for fine-tuned control over the expression levels of the target gene. Moreover, targeting illumination at confined areas within the eye disc triggered PhotoGal4-mediated transcription in selected subset of cells, resulting in personalized sub-patterns of gene expression. Therefore, we anticipate that PhotoGal4 will be a valuable resource to investigate complex biological, developmental, and pathological processes in ex vivo paradigms with remarkable resolution.
Results
Engineering and Validation of PhotoGal4 in Drosophila Cultured Cells
Our PhyB-based inducible system, PhotoGal4, consists of a single bicistronic vector encoding for PhyB fused to the Gal4-DNA binding domain (PhyB-Gal4 DBD) and Pif6 fused to the VP16 transcriptional activator domain (Pif6-VP16 AD) (Figure 2A). In our design, we opted for a previously reported form of PhyB that contains N-PAS2-GAF-PHY motifs, but lacks PAS and histidine kinase-related domains (Beyer et al., 2015). This truncated PhyB (1–650 amino acid [aa]) was previously reported to have a more robust and consistent expression than its full-length counterpart (Buckley et al., 2016). We also chose a shorter form of Pif6 consisting only of its activated phytochrome binding domain. This truncated form of Pif6 (1–100 aa) can still associate with PhyB while maintaining its photo-reversible capabilities (Levskaya et al., 2009). Thus, the chimeric PhyB-Gal4 DBD and Pif6-VP16 AD sequences tagged, respectively, with HA and V5 were subcloned into an actin expression vector separated by the ribosomal skipping sequence T2A (Figure 2A), which allows expression of both proteins under a single promoter. Therefore, the resulting construct includes all the required components to trigger light-dependent activation of any UAS transgene. We first confirmed that both hybrid proteins (PhyB-Gal4DBD-HA and Pif6-VP16AD-V5) are produced in PhotoGal4-transfected S2R+ cells through western blot and immunohistochemistry (Figures 2B and 2C). Next, to test the light-dependent activity of PhotoGal4, we co-transfected Drosophila S2R+ cells with the PhotoGal4 vector and a reporter plasmid carrying UAS-Luciferase. Due to the absence of the chromophore PCB in animal cells, this compound was provided exogenously to allow PhyB-Pif6 interactions (Shimizu-Sato et al., 2002). Thus, 20 h after transient transfection, we supplemented the S2R+ cell culture medium with 10 μM PCB. As expected, transfected cells supplemented with PCB and irradiated with pulses of red light (10 s ON/10 s OFF, 0.75 mW cm−2) for an extra 24 h showed an ∼20-fold increase in Luciferase expression (p > 0.01) compared with cells kept in total darkness or lacking PCB. Moreover, transfected cells cultured in dark conditions or without PCB showed similar Luciferase levels to cells transfected with the Luciferase vector alone (Figure 2D). This result suggests that PhotoGal4 activity relies entirely on light and PCB for transcriptional activation.
Figure 2.
Light-Dependent Activation of PhotoGal4 in S2R+ Cells
(A) Schematic diagram of the Actin-PhotoGal4 construct. The Actin promoter regulates PhotoGal4, which consists of PhyB-650 amino acid fused to Gal4 DBD and the hemagglutinin (HA) tag on one side and the transcriptional activation domain of VP16 fused to Pif6 and the V5 tag on the other side, separated by the T2A sequence.
(B) Western blot analysis of PhotoGal4 components upon transient transfection in S2R+ cells. Untransfected cells were used as a negative control, and α-tubulin was used as internal control.
(C) Immunodetection of PhyB-Gal4DBD-HA and Pif6-VP16AD-V5 in S2R+ cells with anti-HA (left panel, Alexa 564) and anti-V5 (right panel, Alexa 564) antibodies. Scale bar, 10 μm.
(D) Luciferase activity levels in cells transfected only with PhotoGal4, UAS-Luciferase, or both. The plasmid pAC-renilla was used as control for normalization. After transfection, cells were kept in the dark or illuminated with red light pulses (630 nm; 10 s ON 10 s OFF) and cultured in media supplemented with PCB or DMSO for 24 h. Average levels of triplicates for luciferase activity are shown. ∗p < 0.01 by two-tailed Student's t test. All data are represented as mean ± S.D.
Characterization of PhotoGal4 Expression in Drosophila Ex Vivo Cultures
Drosophila ex vivo cultures are powerful experimental paradigms that facilitate long-term imaging and real-time analysis of a wide variety of processes such as cell migration, cell competition, circadian oscillations, developmental remodeling, and wound healing to name a few (Mezan et al., 2016; Prithviraj et al., 2012; Stramer and Wood, 2009; Tsao et al., 2016, 2017). Thus, we reasoned that implementation of PhotoGal4 in Drosophila ex vivo cultures would provide a new opportunity to address key questions in complex multicellular organs and tissues. Given the relatively flat structure of the eye imaginal disc in Drosophila, we tested our prototype system in this experimental context. As a first step, we created transgenic flies bearing the PhotoGal4 cassette under the control of three copies of the eye-specific GMR promoter (Figure S1A). Then, we proved that both PhyB-Gal4DBD and Pif6-VP16 AD were successfully expressed in these flies through western blots (Figure S1B). Moreover, protein distribution was restricted to cells posterior to the morphogenetic furrow, consistent with the expression domain of the GMR promoter in the developing larval eye disc (Hay et al., 1994) (Figure S1C). Of note, none of the seven GMR-PhotoGal4 lines (Figure S1D) created display any apparent changes in morphology, structure, and organization of the adult eye even in homozygosis (Figure S1E), suggesting that PhotoGal4 is innocuous in flies.
PhotoGal4 Drives Light-Dependent Transcription Ex Vivo
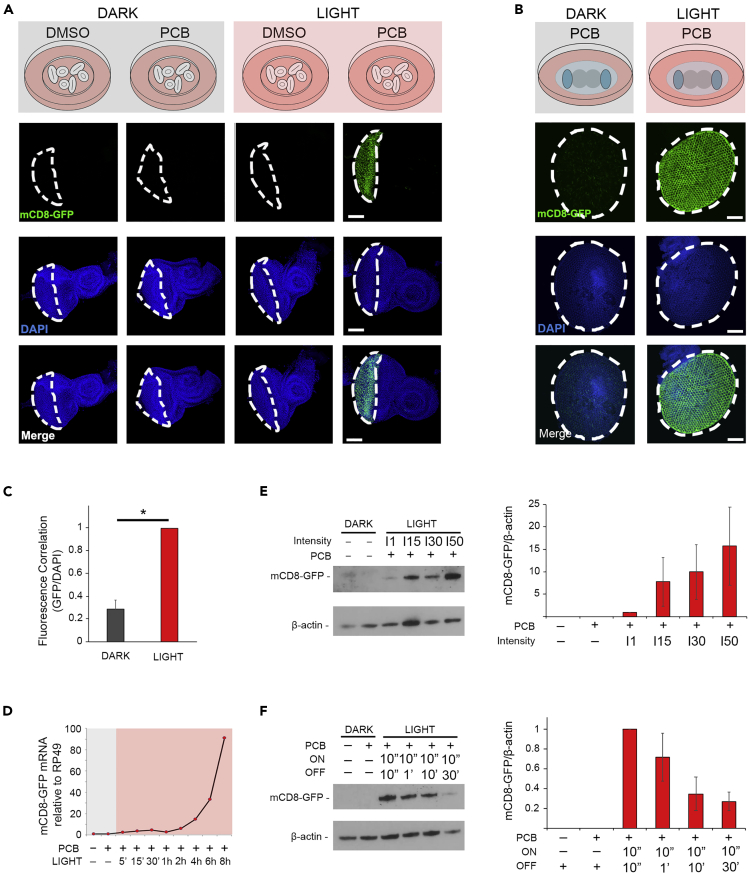
To demonstrate the light-dependent activity of PhotoGal4 in ex vivo cultures, we first crossed GMR-PhotoGal4 flies with flies carrying a UAS-CD8-GFP reporter. Then, from the progeny, we dissected and cultured four sets of 10 eye-antennal discs from third-instar larvae, and incubated them in four different conditions: light, darkness, and absence or presence of the chromophore PCB (100 μM) (Figure 3). The experiment was performed in a closed incubator with a 225 LED lamp placed 3 inches from the top of the ex vivo chambers. Fifteen hours after dissection, we observed highly significant GFP expression in samples exposed to continuous red-light pulses (630 nm, 10 s ON/10 s OFF, 0.75mW cm−2) and PCB (Figure 3A, column 4). In contrast, tissues grown in the dark with or without PCB as well as tissues grown under a red light but in the absence of PCB do not have detectable accumulation of GFP protein (Figure 3A, columns 1–3). Next, we tested the ability of PhotoGal4 to activate gene expression in a later stage of development. Thus, we dissected and cultured prepupal retinas throughout 15 h in similar conditions to those described earlier. As expected, prepupal retinas cultured in PCB-supplemented media and exposed to continuous red-light pulses (630 nm, 10 s ON/10 s OFF) showed significantly higher expression of GFP compared with controls (p < 0.01) (Figures 3B, 3C, and S2). Overall, these results support the robust PCB- and light-dependent activation of PhotoGal4 in the developing fly eye.
Figure 3.
PhotoGal4 Activates Gene Expression in the Developing Eye of Drosophila in a Light-Dependent Manner
(A and B) Imaginal discs (A) or prepupal retinas (B) from flies expressing PhotoGal4 and UAS-mCD8-GFP constructs were dissected and grown in ex vivo culture media supplemented with 100 μM PCB or DMSO, either in the dark or under constant pulses of red light for 15 h (10 s ON, 10 s OFF) (n = 6 for each condition). Each image is the result of a projection of a z stack capture. White dotted lines mark the area where the GMR promoter drives PhotoGal4 expression. Scale bar, 100 μm.
(C) Relative fluorescence correlation for GFP protein levels of prepupal retinas cultured in dark or illuminated with red light in the presence of PCB. ∗p < 0.01 by two-tailed Student's t test. All data are represented as mean ± S.D.
(D) Light response curve of CD8-GFP transcription at different periods of illumination. Groups of 10 imaginal eye discs were cultured for a total of 8 h and exposed to continuous pulses of red light (∼630nm 10s ON, 10s OFF) for the indicated amount of time (5′, 15′, 30′, 1 h, 2 h, 4 h, 6 h or 8 h). Controls were kept in dark for the entire duration of the experiment. mRNA levels were quantified by qPCR. Fold changes in GFP mRNA relative to the internal control RP49 are shown.
(E) Expression and quantification of CD8-GFP in response to increasing light intensities (I1, I15, I30, and I50). Groups of 10 eye discs were illuminated for 8 h with continuous light pulses (∼630 nm 10 s ON, 50 s OFF) at the indicated intensities. Control samples were kept in dark during the whole experiment, and β-actin was used as internal control for normalization. All data are represented as mean ± S.D.
(F) Expression and quantification of CD8-GFP in response to the frequency of light pulses. Groups of 10 eye discs were illuminated for 8 h with red light (∼630 nm) at decreasing light pulse frequencies where a 10-s light pulse was provided every 10 s, 1 min, 10 min, or 30 min. β-Actin was used as internal control for normalization. Control samples were kept in dark for the entire duration of the experiment. All data are represented as mean ± S.D.
See also Figures S1, S2, and S5.
Next, to further determine the versatility of PhotoGal4, we performed experiments at different periods of light irradiation (Figure 3D), under increasing light intensities (Figure 3E), or with different frequencies of light pulses (Figure 3F). For this, we used a customized programmable light-emitting diode (LED) lamp placed half an inch over the plate containing the eye disc explants. To analyze the effect of the duration of stimuli over time, we incubated groups of 10 eye-antennal discs under continuous pulses of light for periods ranging from 0 to 480 min. We opted for long illumination pulses (10 s ON/10 s OFF, 1.2 mW cm−2) to maximize light-induced expression levels (Muller et al., 2014). In our experimental conditions, global red-light irradiation is innocuous to cells when working in ranges of intensities between 0 and 2 mW cm−2 and cycles equal or inferior to 10 s. Thus, 8 h after the initial light pulse, we harvested all samples and processed them for mRNA. As anticipated, CD8-GFP mRNA levels correlated with the duration of light exposure showing a 2-fold expression level only after 2 h, reaching ∼100-fold after 8 h (Figure 3D). In contrast, control groups expressing PhotoGal4 in the presence of PCB but without light failed to activate the CD8-GFP reporter transgene. Then, to assess the effect of light potency, we tested four different intensities including I50, I30, I15, and I1, correlating with ∼100% (1.9 mW cm−2), ∼60% (1.2 mW cm−2), ∼30% (0.6 mW cm−2), and ∼2% (0.05 mW cm−2) of maximum LED intensity, respectively. We found that high GFP expression levels correlate directly with high light intensity values (Figure 3E). Last, we performed experiments at different frequencies of light/dark pulses including 10 s/10 s, 10 s/1 min, 10 s/10 min, and 10 s/30 min (630 nm 1.2 mW cm−2). All groups were cultured under the same conditions of light intensity and PCB concentration (100 μM) for 8 h before harvesting. As expected, GFP protein levels decreased as the light pulses became sparser in time (Figure 3F). Altogether, these data demonstrate that PhotoGal4 can regulate gene expression levels in a customized manner by simply adjusting conditions of the light stimuli such as intensity, duration of exposure, or frequency of light cycles.
PhotoGal4 Activity Is Fully Reversible
One of the most interesting and unique features of the PhyB-based optogenetic system is its capability to be switched off under far-red irradiation (>720 nm). To test whether the light-dependent activity of PhotoGal4 could be reversed, we exposed ex vivo cultures of developing eye discs to pulses of 10 s of 630 nm light (1.2 mW cm−2) and 10 s of >750 nm (1.9 mW cm−2) filtered bright-field light followed by 1 min of darkness. As far-red pulses are provided immediately after red pulses, the PhyB/Pif6 interaction induced by red light is expected to dissociate immediately after far-red light exposure. Accordingly, tissues exposed to far-red light showed a significant decrease in GFP accumulation compared with those tissues exposed exclusively to red light (Figures 4A and 4B), suggesting that PhotoGal4 was effectively inactivated. Quantification of GFP mRNA confirmed the significant differences between activated and inactivated PhotoGal4-expressing tissues (p < 0.01) (Figure 4C). Besides, to confirm that PhotoGal4 inactivation was due specifically to far-red irradiation and not to other unforeseen factors, we reproduced the protocol in tissues expressing a mutant version of our system, mutPhotoGal4, whose activation depends solely on PCB administration and functions independently of light (Figures S3A–S3D). Thus, illumination of mutPhotoGal4-expressing tissues with red and/or far-red light should bear no effect on the final levels of gene expression. As predicted, mutPhotoGal4 activation was irreversible after far-red light irradiation and both experimental groups showed similar expression levels of GFP after 8 h of red or red/far-red irradiation (Figure S3E).
Figure 4.
Far-Red Light Reverses PhotoGal4 Function
(A) Reversibility of PhotoGal4-dependent expression of CD8-GFP in eye imaginal discs cultured ex vivo with PCB. Samples were subjected to 10-s pulses of red light alone or followed by 10 s of filtered far-red light (>720 nm) and 40 s of darkness for a total period of 8 h. White-dotted lines mark the expected area of PhotoGal4 expression under the GMR promoter. Scale bar, 100 μm.
(B) Fluorescence correlation between samples grown under pulses of red light alone and those exposed to red and far-red light treatments (averaged values of n = 3). ∗p < 0.01 by two-tailed Student's t test. The data are represented as mean ± S.D.
(C) Quantification of GFP mRNA levels from samples treated with red or red and far-red light. Values were normalized to RP49 internal transcripts. ∗p < 0.01 by two-tailed Student's t test. The data are represented as mean ± S.D.
See also Figure S3.
PhotoGal4 Allows Optical Sculpting of Gene Expression Patterns
A highly desirable characteristic of any inducible expression system is the ability to easily and selectively activate target genes with fine spatiotemporal resolution. To assess this feature in PhotoGal4, we capitalized on light beams to control the direction of light to specific regions of interest (ROIs). We first demonstrated that a brief irradiation (250 ms every 50 min for 12 h) with the red-light beam (∼630 nm, 2.8 mW cm−2) of an inverted widefield microscope at 5 mm distance from the specimen produces comparable GFP fluorescence values to those obtained using LED. Following this protocol, we directed the light beam specifically to one of the two eye discs in a single third-instar larval culture (Figure S4A). As a result, only the eye disc confined within the ROI showed significant levels of GFP expression, whereas its counterpart grown in the dark displayed undetectable levels of GFP (Figure 5A). This suggests that activation of gene expression in particular sub-regions of a tissue carrying PhotoGal4 could be achieved by simply limiting the area of illumination.
Figure 5.
Personalized Activation of PhotoGal4 in the Developing Eye of Drosophila
For a Figure360 author presentation of Figure 5, see https://doi.org/10.1016/j.isci.2020.101308.
(A) Specific activation of CD8-GFP expression in only one of the two eye discs connected to the brain of a single third-instar Drosophila larva. A widefield microscope was used to direct 250-ms pulses of 630 nm every 50 min for a total period of 15 h to only one of the two eye discs cultured ex vivo with 100 μM PCB. Note that GFP is detected within the expression domain of the GMR promoter (white dotted lines) only in the presence of light. Red dotted lines delimit the areas kept in darkness or exposed to red light. Scale bar, 200 μm.
(B) Regions of interest were created to activate CD8-GFP expression in only one-half of the GMR territory (white dotted lines) within the eye imaginal disc. Specimens were exposed to 150-ms pulses of 561 nm at 0.5% laser power from a confocal fluorescent microscope every 30 min for 8 h and cultured for a total period of 15h (7 h in dark). Scale bar, 100 μm.
(C) Manipulation of PhotoGal4 to imprint a happy face-like pattern of GFP expression within the pupal retina. Scale bar, 100 μm.
(D) A PhotoGal4-induced “GFP tattoo” showing the initials of the University of Florida (UF) in the pupal retina. Scale bar, 100 μm. All final images are projections of z stack captures for DAPI (351 nm) and GFP (488 nm) channels.
See also Figures S4 and S5.
To test this hypothesis, we created a more restricted ROI within one single developing eye disc by using a confocal microscope. We first confirmed that exposure to short pulses (150 ms every 50 min for 9 h) of 631-nm laser at low levels (0.5% of maximum laser power) induces similar GFP expression to that obtained with the LED light source. Then, we manipulated the laser beam to specifically illuminate only one-half of the GMR territory while keeping the other half in the dark (Figure S4B). Although all the eye primordium express PhotoGal4 through the GMR promoter (Figure S1), only the half exposed to the red-light beam displayed GFP accumulation within the ROI (Figure 4B). Next, we decided to go one step further and explored the possibility of generating more complex patterns of expression. Given that prepupal retinas offer a bigger canvas to operate, we used this paradigm to imprint “GFP tattoos” in distinct configurations within the GMR territory. Thus, we designed ROIs to specifically engrave an “emoji-like” face as well as the initials of the University of Florida (UF). Strikingly, we successfully engraved the expected “GFP tattoos” upon red-light illumination (Figures 5C and 5D). These extraordinary results indicate that even intricate sub-patterns of gene expression can be created within the same tissue (Figure 5D), highlighting the versatility and specificity of the PhotoGal4 system.
Discussion
Despite the tremendous contribution of classic Drosophila gene expression systems, there is still a need for more time-restrictive and spatial-accurate tools to improve our understanding of many biological processes. The emergence of a new generation of tools based on light-sensitive proteins aimed to fill this gap (de Mena et al., 2018). As light acts as the stimuli, these optogenetic systems promised a faster and finer way of selectively activating gene expression with minimal side effects. Thus, motivated by this technology, we implemented PhotoGal4, a multipurpose PhyB-based optogenetic switch, to regulate gene expression in Drosophila tissues.
Over the years, different photo-sensing proteins have been adapted to manipulate gene expression. For instance, within the blue-light spectrum, systems such as EL222 or CRY-CIB effectively trigger gene expression in different animal cell models (Motta-Mena et al., 2014; Pathak et al., 2013; Reade et al., 2017; Taslimi et al., 2016). However, these systems require constant light stimulation for activation because removal of the stimuli results in deactivation (de Mena et al., 2018). In contrast, PhyB-based systems are uniquely attractive because they can be turned ON and OFF by switching between red or far-red light for an unlimited period of time. Moreover, activated PhyB remains stable during its half-life, allowing for shorter periods of red-light pulses at longer intervals, which reduces the risk of phototoxicity compared with blue-light systems (Buckley et al., 2016; de Mena et al., 2018; Levskaya et al., 2009; Pathak et al., 2013). Unfortunately, no PhyB-based optogenetic system is yet available for manipulation of gene expression in Drosophila.
The first attempt to bring optogenetics to the Drosophila community for transgene control proposed a blue-light expression system. The authors created a UAS cassette carrying the light-responsive CRY2/CIBN heterodimer components hooked to the split LexA transcriptional activator (Chan et al., 2015). In this concept, a fully functional LexA is reconstituted upon activation through any Gal4 driver and blue-light illumination, resulting in expression of a target gene fused to the LexAop promoter. This platform worked well in Drosophila cultured cells and showed up to 10-fold activation of a GFP reporter; however, it displayed elevated basal transcription levels in vivo even in dark conditions. As a solution, the authors introduced a feedback mechanism through the transcriptional repressor Gal80, which reduces the strength of the Gal4-dependent UAS-CRY2/CIBN-LexA construct, but at the same time complicates genetic manipulation. Besides, this system relies on the LexAop promoter for gene activation, whose application is limited compared with thousands of available UAS strains. To bypass these limitations, we opted for a PhyB-based system that could be exploited to manipulate expression of any available UAS transgene with high versatility and low background.
When tested in Drosophila cultured cells, PhotoGal4 consistently induced more than 20-fold increase in reporter gene expression under standard light conditions with negligible background (Figures 2B–2D). We then capitalized on the relatively flat structure of the eye imaginal disc to fully assess the transcriptional attributes of PhotoGal4 in Drosophila tissues ex vivo (Figures S5A and S5B). We demonstrated that PhotoGal4 allows for a high level of spatiotemporal regulation (Figures 3A and 3B) with low background (Figures 3D–3F) and rapid photo-reversibility (Figure 4). Of note, we found that the system facilitates fine-tune regulation of gene induction through modification of the red-light conditions. For instance, a higher frequency and duration of red-light pulses correlated with an increment in the expression levels of the reporter gene (Figures 3D and 3F). A boost in the intensity of the light source also led to higher expression levels (Figure 3E). Besides, we observed that reporter gene expression was already perceptible by qRT-PCR within 2 h after PhotoGal4 activation (Figure 3D). These results suggest that manipulation of the red-light conditions such as duration of stimuli, number of cycles, and intensity of light could potentially allow for user-defined levels of final transcriptional products.
On the other hand, the bicistronic nature of PhotoGal4 provides simplicity and flexibility, making it easier to operate in Drosophila (Figures 2A and S1A). For instance, the engineering of PhotoGal4 allows for easy exchange of tissue-specific driver sequences, facilitating its use in a broad range of tissues and organs. However, the most interesting feature of PhotoGal4 is its unique ability to switch it off. Most optogenetic transcriptional regulators rely on the spontaneous return to the dark state of the photo-sensing protein to cease gene expression (Di Ventura and Kuhlman, 2016; Pathak et al., 2014), whereas far-red light permits switching off PhyB-based tools almost instantly at any desirable moment (Buckley et al., 2016; de Mena et al., 2018; Levskaya et al., 2009; Pathak et al., 2013). Our results demonstrated that providing pulses of far-red light immediately after activating red light pulses reversed PhotoGal4 to its inactive form, resulting in a lack of reporter gene expression (Figure 4). We believe that this reversibility will be instrumental to selectively switch ON/OFF gene expression at will at different experimental points and/or developmental stages.
Unlike classical chemically inducible systems, the use of targeted light to activate PhotoGal4 allows sculpting of complex spatial patterns in a particular group of cells. Here, we demonstrated that PhotoGal4 has the ability to restrict gene expression to a specific organ (Figure 5A), defined areas within that organ (Figure 5B), and even sophisticated patterns of expression in different shapes (Figures 5C and 5D). This is achieved by simply creating ROIs with a confocal microscope. However, in theory, specialized microscopy involving digital micromirror device or two-photon technology could provide more restricted patterns of expression, potentially even to a single-cell resolution.
In conclusion, this work demonstrates that PhotoGal4 can be used as a high-resolution device to sculpt gene expression in response to light quantity, duration of exposure, and trajectory of the beam. We anticipate that the ability to control gene expression “a la carte” will provide Drosophila researchers with customizable platforms to study gene function at specific times and sites, with switching options, and possibly in a dose-dependent manner. Thus, further implementation of PhotoGal4 in intact flies may play a transformative role in promoting widespread applications of optogenetics to achieve ultrasensitive and reversible manipulation of any gene in Drosophila with remarkable precision.
Limitations of the Study
Some limitations of our study must be acknowledged. Our system requires exogenous chromophore (PCB) for proper PhotoGal4 function. However, we believe that the addition of PCB exogenously acts as an extra safeguard for fly manipulation and experimentation. Most photo-sensing inducible systems require working under safelight conditions and total avoidance of environmental white light to remain inactive (Hernandez-Candia et al., 2019). In contrast, the PCB-dependent function of PhotoGal4 permits safe handling of specimens under standard light conditions until PCB is incorporated, reducing the risk of undesired photo-activation and leakiness. Another potential issue is that the supplemented PCB may not reach proper concentrations in certain tissues in vivo, which may affect the potency of PhotoGal4. However, it has been shown that mammalian cultured cells expressing cyanobacterial enzymes PcyA and HO1 can successfully transform endogenous heme groups into PCB (Kyriakakis et al., 2018; Muller et al., 2013b; Uda et al., 2017; Youichi Uda et al., 2020). We strongly believe that genetic synthesis of PCB will pave the way for more practical applications of PhotoGal4.
Another potential drawback is that PhyB-based systems can be activated by wavelengths below the 630-nm spectrum under certain conditions. In our hands, long exposures of high intensity blue or green light led to significant expression of reporter gene (data not shown), coinciding with previous reports (Adrian et al., 2017). However, this could be bypassed by immediately providing cycles of deactivating far-red light pulses. In this regard, when far-red light irradiates the tissue, PhyB becomes inactive and dissociates from Pif6, which precludes further target gene activation. It is worth to mention, however, that all previously activated proteins will remain in the cell for the duration of their half-life. This should be taken into consideration when performing gene expression experiments that require narrow periods of activation and deactivation.
Last, PhotoGal4 functions specifically in ex vivo paradigms at present. Therefore, one final concern refers to its future implementation in adult intact flies due to cuticle and tissue penetration issues. Previous studies indicate that red and far-red light systems provide higher penetration than those associated with blue and UV wavelengths, while showing less phototoxicity (Ash et al., 2017; Kwon et al., 2009). However, accurate targeting of deep tissues within live organisms remains a challenge due to dispersion and diffusion of light through multiple cell layers. Fortunately, this scenario is changing with the development of new live imaging approaches. For instance, surgical windows in head and thorax of living fruit flies are becoming common practice for central nervous system imaging in freely walking flies (Chen et al., 2018; Huang et al., 2018; Simpson and Looger, 2018). Similarly, adaptation of multiphoton microscopy to live flies is facilitating high-definition imaging of deep structures such as the brain mushroom bodies (Aragon et al., 2019). Thus, we believe that the combination of these technologies with PhotoGal4 will capture the imagination of the community and stimulate exciting new ideas to expand the horizons and applications of our system in the future.
Resource Availability
Lead Contact
Requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Diego E. Rincon-Limas (diego.rincon@neurology.ufl.edu).
Materials Availability
All constructs and Drosophila lines generated in this study will be made available on request to the Lead Contact without restriction; however, requestor will cover shipping costs. This study did not generate new unique reagents.
Data and Code Availability
Requests for raw data can be directed to the Lead Contact. All the DNA sequences for constructs designed in this study are located within Transparent Methods. This study did not generate large datasets, computer codes, or algorithms.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Jared Toettcher for providing the blueprints and software to create and manipulate the RED LED programmable lamp. We also thank all Rincon-Limas lab members for valuable discussions. This work was supported by NIH grants R21NS088866 and R01AG059871 to D.E.R.-L., by an HHMI-sponsored postdoctoral fellowship from the Life Sciences Research Foundation to L.D.M, and by NIH Grant 1S10OD020026 to the University of Florida CTAC core for the Nikon A1RMP system.
Author Contributions
L.D.M. and D.E.R.-L designed the study; L.D.M. performed the experiments; L.D.M. and D.E.R.-L. analyzed data; L.D.M. and D.E.R.-L. wrote and edited the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101308.
Contributor Information
Lorena de Mena, Email: lorena.demenaalvarez@neurology.ufl.edu.
Diego E. Rincon-Limas, Email: diego.rincon@neurology.ufl.edu.
Supplemental Information
References
- Adrian M., Nijenhuis W., Hoogstraaten R.I., Willems J., Kapitein L.C. A phytochrome-derived photoswitch for intracellular transport. ACS Synth. Biol. 2017;6:1248–1256. doi: 10.1021/acssynbio.6b00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon M.J., Wang M., Shea J., Mok A.T., Kim H., Lett K.M., Barkdull N., Schaffer C.B., Xu C., Yapici N. Non-invasive multiphoton imaging of neural structure and activity in Drosophila. bioRxiv. 2019 doi: 10.1101/798686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash C., Dubec M., Donne K., Bashford T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017;32:1909–1918. doi: 10.1007/s10103-017-2317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auldridge M.E., Forest K.T. Bacterial phytochromes: more than meets the light. Crit. Rev. Biochem. Mol. Biol. 2011;46:67–88. doi: 10.3109/10409238.2010.546389. [DOI] [PubMed] [Google Scholar]
- Beyer H.M., Naumann S., Weber W., Radziwill G. Optogenetic control of signaling in mammalian cells. Biotechnol. J. 2015;10:273–283. doi: 10.1002/biot.201400077. [DOI] [PubMed] [Google Scholar]
- Buckley C.E., Moore R.E., Reade A., Goldberg A.R., Weiner O.D., Clarke J.D. Reversible optogenetic control of subcellular protein localization in a live vertebrate embryo. Dev. Cell. 2016;36:117–126. doi: 10.1016/j.devcel.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y.B., Alekseyenko O.V., Kravitz E.A. Optogenetic control of gene expression in Drosophila. PLoS One. 2015;10:e0138181. doi: 10.1371/journal.pone.0138181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.L., Hermans L., Viswanathan M.C., Fortun D., Aymanns F., Unser M., Cammarato A., Dickinson M.H., Ramdya P. Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nat. Commun. 2018;9:4390. doi: 10.1038/s41467-018-06857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.J., Vener A.V., Vierstra R.D. Bacteriophytochromes: phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science. 1999;286:2517–2520. doi: 10.1126/science.286.5449.2517. [DOI] [PubMed] [Google Scholar]
- de Mena L., Rizk P., Rincon-Limas D.E. Bringing light to transcription: the optogenetics repertoire. Front. Genet. 2018;9:518. doi: 10.3389/fgene.2018.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ventura B., Kuhlman B. Go in! Go out! Inducible control of nuclear localization. Curr. Opin. Chem. Biol. 2016;34:62–71. doi: 10.1016/j.cbpa.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J.A., Giniger E., Maniatis T., Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988;332:853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- Hay B.A., Wolff T., Rubin G.M. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Hernandez-Candia C.N., Wysoczynski C.L., Tucker C.L. Advances in optogenetic regulation of gene expression in mammalian cells using cryptochrome 2 (CRY2) Methods. 2019;164-165:81–90. doi: 10.1016/j.ymeth.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Maxey J.R., Sinha S., Savall J., Gong Y., Schnitzer M.J. Long-term optical brain imaging in live adult fruit flies. Nat. Commun. 2018;9:872. doi: 10.1038/s41467-018-02873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R.M., Bolger S., Tapadia H., Tucker C.L. Light-mediated control of DNA transcription in yeast. Methods. 2012;58:385–391. doi: 10.1016/j.ymeth.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon K., Son T., Lee K.J., Jung B. Enhancement of light propagation depth in skin: cross-validation of mathematical modeling methods. Lasers Med. Sci. 2009;24:605–615. doi: 10.1007/s10103-008-0625-4. [DOI] [PubMed] [Google Scholar]
- Kyriakakis P., Catanho M., Hoffner N., Thavarajah W., Hu V.J., Chao S.S., Hsu A., Pham V., Naghavian L., Dozier L.E. Biosynthesis of orthogonal molecules using ferredoxin and ferredoxin-NADP(+) reductase systems enables genetically encoded PhyB optogenetics. ACS Synth. Biol. 2018;7:706–717. doi: 10.1021/acssynbio.7b00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis G.N., Salomon M.P., Keroles D., Brookes N., Sekimura T., Tower J. The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila. Aging (Albany NY) 2015;7:53–69. doi: 10.18632/aging.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levskaya A., Weiner O.D., Lim W.A., Voigt C.A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezan S., Feuz J.D., Deplancke B., Kadener S. PDF signaling is an integral part of the drosophila circadian molecular oscillator. Cell Rep. 2016;17:708–719. doi: 10.1016/j.celrep.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta-Mena L.B., Reade A., Mallory M.J., Glantz S., Weiner O.D., Lynch K.W., Gardner K.H. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat. Chem. Biol. 2014;10:196–202. doi: 10.1038/nchembio.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K., Engesser R., Metzger S., Schulz S., Kampf M.M., Busacker M., Steinberg T., Tomakidi P., Ehrbar M., Nagy F. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res. 2013;41:e77. doi: 10.1093/nar/gkt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K., Engesser R., Timmer J., Nagy F., Zurbriggen M.D., Weber W. Synthesis of phycocyanobilin in mammalian cells. Chem. Commun. (Camb) 2013;49:8970–8972. doi: 10.1039/c3cc45065a. [DOI] [PubMed] [Google Scholar]
- Muller K., Engesser R., Timmer J., Zurbriggen M.D., Weber W. Orthogonal optogenetic triple-gene control in Mammalian cells. ACS Synth. Biol. 2014;3:796–801. doi: 10.1021/sb500305v. [DOI] [PubMed] [Google Scholar]
- Osterwalder T., Yoon K.S., White B.H., Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak G.P., Strickland D., Vrana J.D., Tucker C.L. Benchmarking of optical dimerizer systems. ACS Synth. Biol. 2014;3:832–838. doi: 10.1021/sb500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak G.P., Vrana J.D., Tucker C.L. Optogenetic control of cell function using engineered photoreceptors. Biol. Cell. 2013;105:59–72. doi: 10.1111/boc.201200056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prithviraj R., Trunova S., Giniger E. Ex vivo culturing of whole, developing Drosophila brains. J. Vis. Exp. 2012:4270. doi: 10.3791/4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reade A., Motta-Mena L.B., Gardner K.H., Stainier D.Y., Weiner O.D., Woo S. TAEL: a zebrafish-optimized optogenetic gene expression system with fine spatial and temporal control. Development. 2017;144:345–355. doi: 10.1242/dev.139238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Sato S., Huq E., Tepperman J.M., Quail P.H. A light-switchable gene promoter system. Nat. Biotechnol. 2002;20:1041–1044. doi: 10.1038/nbt734. [DOI] [PubMed] [Google Scholar]
- Simpson J.H., Looger L.L. Functional imaging and optogenetics in Drosophila. Genetics. 2018;208:1291–1309. doi: 10.1534/genetics.117.300228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B., Wood W. Inflammation and wound healing in Drosophila. Methods Mol. Biol. 2009;571:137–149. doi: 10.1007/978-1-60761-198-1_9. [DOI] [PubMed] [Google Scholar]
- Sun Y., Chen X., Xiao D. Tetracycline-inducible expression systems: new strategies and practices in the transgenic mouse modeling. Acta Biochim. Biophys. Sin (Shanghai) 2007;39:235–246. doi: 10.1111/j.1745-7270.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- Tanguay R.M. Transcriptional activation of heat-shock genes in eukaryotes. Biochem. Cell Biol. 1988;66:584–593. doi: 10.1139/o88-069. [DOI] [PubMed] [Google Scholar]
- Taslimi A., Zoltowski B., Miranda J.G., Pathak G.P., Hughes R.M., Tucker C.L. Optimized second-generation CRY2-CIB dimerizers and photoactivatable Cre recombinase. Nat. Chem. Biol. 2016;12:425–430. doi: 10.1038/nchembio.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C.K., Ku H.Y., Lee Y.M., Huang Y.F., Sun Y.H. Long term ex vivo culture and live imaging of Drosophila larval imaginal discs. PLoS One. 2016;11:e0163744. doi: 10.1371/journal.pone.0163744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C.K., Ku H.Y., Sun Y.H. Long-term live imaging of Drosophila eye disc. J. Vis. Exp. 2017:55748. doi: 10.3791/55748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda Y., Goto Y., Oda S., Kohchi T., Matsuda M., Aoki K. Efficient synthesis of phycocyanobilin in mammalian cells for optogenetic control of cell signaling. Proc. Natl. Acad. Sci. U S A. 2017;114:11962–11967. doi: 10.1073/pnas.1707190114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youichi Uda H.M., Goto Yuhei, Aoki Kazuhiro. Improvement of phycocyanobilin synthesis for genetically encoded phytochrome-based optogenetics. bioRxiv. 2020 doi: 10.1101/2020.1101.1115.908343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for raw data can be directed to the Lead Contact. All the DNA sequences for constructs designed in this study are located within Transparent Methods. This study did not generate large datasets, computer codes, or algorithms.