Abstract
Background and Purpose
The entire kallikrein–kinin system is present in the skin, and it is thought to exert a relevant role in cutaneous diseases, including psoriasis. The present study was designed to evaluate the relevance of kinin receptors in the development and progression of a model of psoriasis in mice.
Experimental Approach
The effects of kinin B1 and B2 receptor knockout and of kinin receptor antagonists (SSR240612C or FR173657) were assessed in a model of psoriasis induced by imiquimod in C57BL/6 mice. Severity of psoriasis was assessed by histological and immunohistochemical assays of skin, along with objective scores based on the clinical psoriasis area and severity index.
Key Results
Both kinin receptors were up‐regulated following 6 days of imiquimod treatment. Kinin B1 and B2 receptor deficiency and the use of selective antagonists show morphological and histological improvement of the psoriasis hallmarks. This protective effect was associated with a decrease in undifferentiated and proliferating keratinocytes, decreased cellularity (neutrophils, macrophages, and CD4+ T lymphocytes), reduced γδ T cells, and lower accumulation of IL‐17. The lack of B2 receptors resulted in reduced CD8+ T cells in the psoriatic skin. Relevantly, blocking kinin receptors reflected the improvement of psoriasis disease in the well‐being behaviour of the mice.
Conclusions and Implications
Kinins exerted critical roles in imiquimod‐induced psoriasis. Both B1 and B2 kinin receptors exacerbated the disease, influencing keratinocyte proliferation and immunopathology. Antagonists of one or even both kinin receptors might constitute a new strategy for the clinical treatment of psoriasis.
Abbreviations
- ACEi
ACE inhibitors
- BK
bradykinin
- DC
dendritic cells
- γδT
γδ T cells
- KO
knockout mice
- K14
keratin 14
- Ly6G
lymphocyte antigen 6 complex locus G6D
- PASI
Psoriasis Area Severity Index
- PCNA
proliferating cell nuclear antigen
- Th17
T helper 17
- TPA
12‐O‐tetradecanoylphorbol‐13‐acetate
- WT
wild‐type mice
What is already known
Kinins are inflammatory mediators involved in a wide range of physiological and pathophysiological processes.
Kinin peptides are released in the skin due to injury and/or inflammation.
What this study adds
Activation of the kinin system is associated with the severity of psoriasis disease in mice.
Treatment with kinin receptor antagonists attenuates the symptoms of psoriasis.
What is the clinical significance
This study provides new insights into the role of the kinin system in psoriasis disease.
Kinin receptors can be a potential target for the treatment of chronic skin inflammation.
1. INTRODUCTION
Psoriasis is a multifactorial disease and one of the most common immune‐mediated chronic inflammatory skin disorders (>3% population worldwide), characterized by erythematous scaly plaques, leukocyte infiltration, and altered growth and differentiation of skin‐resident cells (Eberle, Brück, Holstein, Hirahara, & Ghoreschi, 2016). There is experimental evidence associating the use of angiotensin‐converting enzyme inhibitors (ACEi) and the induction or aggravation of psoriatic manifestations (Gilleaudeau, Vallat, Carter, & Gottlieb, 1993; Wolf, Tamir, & Brenner, 1990). Furthermore, there is an association between the angiotensin‐converting enzyme (ACE) gene polymorphism and the susceptibility to psoriasis development in the Chinese population (Chang et al., 2007).
ACE is a dipeptidylcarboxypeptidase that hydrolyses the decapeptide angiotensin I to the active octapeptide, angiotensin II, and it also inactivates the vasoactive peptide bradykinin (BK) and other kinin peptides. Interactions of kinins with the constitutive bradykinin B2 receptors or with the inducible bradykinin B1 receptors have been implicated in many physiological and pathological processes, including vasodilation and release of inflammatory mediators (Calixto et al., 2004; Seliga et al., 2018). Several studies have shown that kinins are released during tissue trauma or injury and following inflammatory responses, with increased levels in diseases such as lupus, cancer, and psoriasis (Costa‐neto et al., 2008; Dutra, 2017; Poblete et al., 1991; Schremmer‐Danninger, Hermann, Fink, Fritz, & Roscher, 1999; Segawa et al., 2009). However, only a few studies support the role of kinins in cutaneous disorders (Poblete et al., 1991; Schremmer‐Danninger et al., 1999; Sharma & Al‐Sherif, 2006). In non‐inflamed human skin, both kinin receptors are constitutively expressed and are up‐regulated during disease (Schremmer‐Danninger et al., 1999). We have previously demonstrated that both kinin receptors play an important role during chronic skin inflammation and hyperproliferative processes (Pietrovski et al., 2011), and it is known that kinins act directly on keratinocytes, inducing differentiation and migration (Matus et al., 2008; Vidal et al., 2005).
Nevertheless, there are still many questions regarding the relevance and the effects of kinins in the skin, including in chronic inflammatory diseases. Thus, the present study was undertaken to investigate the role of kinin receptors in the development of psoriasiform skin inflammation caused by imiquimod in mice.
2. METHODS
2.1. Animals
All animal care and experimental procedures followed the recommendation of the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Use Ethics Committee of the Biological Sciences Section of the Federal University of Paraná (CEUA/BIO) under number 1185. All efforts were made to minimize the number of animals used and their suffering. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2011) and with the recommendations made by the British Journal of Pharmacology.
Adult male and female C57BL/6 (wild‐type, WT; RRID:MGI:5656552) and kinin receptor knockout (KOB1, KOB2, and KOB1B2) mice (8–10 weeks old, 20–25 g) were used in these experiments. The generation of mice lacking kinin B1 receptor (KOB1), kinin B2 receptor (KOB2), and both receptors (KOB1B2) was obtained and previously described by Pesquero et al. (2000), Borkowski et al. (1995), and Cayla et al. (2002), respectively. The C57BL/6 background has been used to provide better genetics than other strains for the use of the imiquimod model in the study of psoriasis (Swindell et al., 2017). Both sexes were included in the experimental design to discount any sex differences in response to disease, as recommended by the British Journal of Pharmacology (Docherty et al., 2019).
The animals were housed in the animal care facility at the Biological Sciences Section, Federal University of Paraná, under standard laboratory conditions. Food and water were supplied ad libitum, under a 12‐h light/dark cycle (lights on at 7 a.m.) in an environment with temperature (23 ± 2°C) and humidity (60 ± 10%) controlled. The mice were kept in groups of six to nine animals in solid‐bottom polypropylene cages (size: 18 cm × 34 cm × 41 cm), with autoclaved wood‐shaving bedding. All animals were allowed to acclimate at least 2 days prior to the experiment and were used only once. The experiments were conducted during the light phase. The animals were randomly divided into naive, control, and experimental groups. Some animals received i.p. injections of different doses of the non‐peptide B1 receptor antagonist SSR240612C (0.1, 0.3, or 1.0 mg·kg−1), the non‐peptide B2 receptor antagonist FR173657 (3, 10, or 30 mg·kg−1), or the vehicle (DMSO, 0.03% v/v) as indicated in figures and figure legends.
2.2. Imiquimod‐induced psoriasis‐like skin inflammation in mice
Mouse back skin was shaved 24 h prior to any treatment (Day 0). On Day 1, animals were treated topically with commercially available imiquimod cream (80 mg of 5% preparation; Aldara™ cream) on the shaved back skin, once a day for six consecutive days, as previously described by Van Der Fits et al. (2009). In addition, 30 min before imiquimod application, some WT mice were treated (i.p.) with the non‐peptide B1 receptor antagonist SSR240612C (0.1, 0.3, or 1.0 mg·kg−1) or the non‐peptide B2 receptor antagonist FR173657 (3, 10, or 30 mg·kg−1) or vehicle (0.03 % v/v DMSO) daily, during six consecutive days. The choice of the dose‐range for each drug was based on previously published data (Christianne et al., 1999; Gougat et al., 2004). On the seventh day, the animals were killed by isoflurane overdose, and skin samples were collected for analysis (Figure 1a).
FIGURE 1.
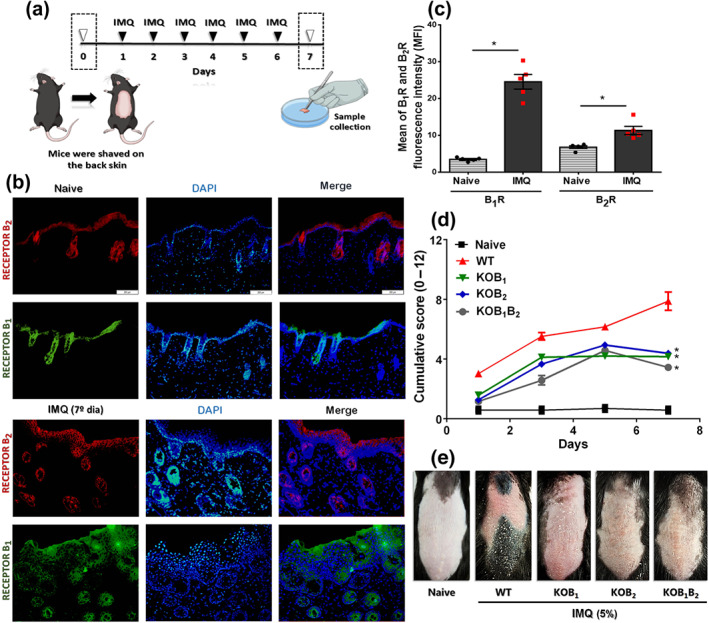
Participation of kinin receptors in the development and progression of imiquimod (IMQ)‐induced psoriasis in mice. (a) Imiquimod was applied daily on the shaved back of wild‐type (WT) and kinin receptor knockout mice (KOB1, KOB2, and KOB1B2) for a total of six applications. (b) Fluorescence microscopy images illustrate the presence of B1 (green) and B2 (red) kinin receptors under physiological skin conditions, as well as in the psoriasis‐like lesions induced by imiquimod treatment. (c) Measurement of the mean fluorescence intensity (MFI), showing the occurrence of both kinin receptors on healthy skin and the higher index of B1 and B2 receptors (B1, B2R) in psoriasiform skin. The MFI was measured from slides of five different animals per group. Data are the mean ± SEM (n = 5). (d) The PASI cumulative score (erythema plus scaling plus thickness) shows the severity of the inflammatory process established in the skin of WT and kinin receptor knockout mice treated with imiquimod for 6 days. The naive group did not receive any treatment. (e) Phenotypical representation of psoriasiform skin lesions in WT and knockout (KOB1, KOB2, and KOB1B2) mice after 6 days of treatment with imiquimod . On Day 6, representative photos of the shaved dorsal skin of the mice were taken. The PASI was blindly assessed at each time point (Days 0 to 7) by four different investigators, and then the scores of each animal was averaged and the comparison between groups was assessed. The values are presented as the mean ± SEM of 20 individual animals per group, from two independent experiments. No outliers were removed from the database. In (c), *P < .05, significantly different from the naïve group; one‐way ANOVA, with the Newmann–Keuls post hoc test. In (d), *P < .05, significantly different from WT mice treated with imiquimod; two‐way ANOVA, with the Newmann–Keuls post hoc test
The values of n in the experiments were chosen based on previous results with similar experimental protocols and considering the 3R principles, according to the BJP guidelines. The naive group did not receive any treatment. Data collection and evaluation of all experiments were performed blindly, without knowledge of the knockout or treatment group.
2.3. Psoriasis Area and Severity Index
To evaluate the imiquimod ‐induced inflammation on the shaved back skin of C57BL/6 mice, an objective scoring system was developed based on the clinical Psoriasis Area and Severity Index (PASI). Mice were evaluated daily and individually regarding erythema, scaling, and thickening. Each index was independently scored on a 0‐to‐4 scale (0, none; 1, slight; 2, moderate; 3, marked; 4, very marked; data not shown). The cumulative score (erythema plus scaling plus thickening) was used to measure the severity of skin inflammation (scale 0–12; Van Der Fits et al., 2009). The area score was not taken into account, because each mouse had the same experimental area. All evaluations were performed blindly and by three to four different investigators. The score for each mouse was averaged, and trend lines were generated to observe changes in skin lesions.
2.4. Histological and immunohistochemical staining
The skin samples collected were fixed overnight in an ALFAC solution (85 ml of 80% alcohol, 10 ml of 40% formalin, and 5 ml of glacial acetic acid). Subsequently, the samples were subjected to a serial dehydration, embedded in paraffin, sectioned at 5 μm and stained with haematoxylin and eosin (H&E). To evaluate the cellularity and the epidermis thickness, the images were captured at 200× magnification with a digital camera coupled to Olympus BX51 microscope (Olympus, Tokyo, Japan) and analysed with ImageJ® software version 1.48 (National Institute of Health, USA, RRID:SCR_003070). Five ocular fields per section were captured and subjected to evaluation. The full epidermal thickness was determined by measuring the distance between the top and the bottom of the epidermis. For each biopsy sample, the epidermis thickness was assessed blindly with a minimum of seven random measurements on the captured images, the average was calculated, and the final value was expressed in micrometres.
The antibody ‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). Paraffin‐embedded skin sections (5 μm) were deparaffinized with xylene, rehydrated with serial concentrations of ethanol, treated with 0.1‐M glycine to block aldehydes radicals, and then incubated with 3% H2O2 solution and 1% PBS/BSA to eliminate endogenous peroxidase activity and non‐specific binding sites, respectively. Subsequently, the sections were incubated for 2 h with goat polyclonal antibody to PCNA (isotype: IgG, 1:100, Santa Cruz Biotech. Cat# sc‐9857, RRID:AB_2160372) or anti‐K14 antibody (isotype: IgG, goat polyclonal antibody, 1:100, Santa Cruz Biotechnology Cat# sc‐17104, RRID:AB_10181889) at room temperature in a moist chamber. Thereafter, the slides were incubated with donkey anti‐goat IgG secondary antibody conjugated to horseradish peroxidase (HRP; 1:100, Santa Cruz Biotechnology Cat# sc‐2020, RRID:AB_631728) for 1 h at room temperature in a moist chamber. Peroxidase‐binding sites were detected by staining with a DAB Substrate Kit (BD Pharmingen, San Jose, CA, USA). The number of immunostained cells positive for PCNA and K14 in the epidermis was quantified with ImageJ® software version 1.48 (National Institute of Health, USA). The presence of brown granules in the epidermis was defined as a positive signal. For negative controls, the sections were processed as described above without primary antibody (data not shown).
2.5. Immunofluorescence detection
To assess the expression of B1 receptors, B2 receptors, Ly6G, CD11b, CD3, CD4, CD8, and IL‐17, frozen cryosections of the skin (5 μm) were fixed in ice‐cold acetone, permeabilized with Triton X‐100, and blocked in 1% BSA in PBS. Thereafter, the slides were incubated overnight at 4°C with anti‐ B1 receptor (isotype: IgG, goat polyclonal antibody, 1:300, Santa Cruz Biotech. Cat# sc‐15048, RRID:AB_2064033), anti‐ B2 receptor (isotype: IgG, rabbit polyclonal antibody, 1:300, Santa Cruz Biotech. Cat# sc‐25671, RRID:AB_2064183), anti‐Ly6G (isotype: IgG, rat monoclonal antibody, 1:300, Santa Cruz Biotech. Cat# sc‐53515, RRID:AB_783639), anti‐CD11b (isotype: IgG, rat monoclonal antibody, 1:300, BD Biosciences Cat# 557396, RRID:AB_396679), anti‐CD3 (isotype: IgG, rat monoclonal antibody,1:300, Santa Cruz Biotech. Cat# sc‐70618, RRID:AB_1120358), anti‐CD4 (isotype: IgG, goat monoclonal antibody,1:300, Santa Cruz Biotech. Cat# sc‐70670, RRID:AB_1120429), anti‐CD8 (isotype: IgG, rat monoclonal antibody,1:300, Santa Cruz Biotech. Cat# sc‐18860, RRID:AB_627184), or anti‐IL17 (isotype: IgG, rat polyclonal antibody, 1:300, Abcam. Cat# ab91649, RRID:AB_10712684). On the following day, the samples were washed twice with PBS and incubated with their respective secondary antibody (dilution 1:300) for 2 h at room temperature in a moist chamber. Finally, sections were stained with DAPI and mounted with Fluoromount‐G (Thermo Fisher Scientific, Inc., USA). The images were acquired using an Olympus BX51 microscope (Olympus, Tokyo, Japan). The omission of the primary antibody was used as a negative control, and the selectivity of the kinin receptor antibodies was tested in the respective knockout tissue (Figure S1). The mean fluorescence intensity (MFI) or the number of labelled cells was determined by direct count using ImageJ® software version 1.48 (National Institute of Health, USA).
2.6. Flow cytometry analysis
In order to evaluate the immune cell phenotypes, skin samples were collected from imiquimod‐treated WT and kinin receptor knockout mice or control untreated animals submitted to the imiquimod‐induced psoriasis model. Briefly, skin samples were cut into small pieces and placed in 500‐μl DMEM with 2.5 mg·ml−1 of collagenase II (Catalog # 17101015, Gibco®), 2.5 mg·ml−1 of collagenase IV (Catalog # C5138, Sigma‐Aldrich, EUA), 1.5 μl·ml−1 of Pierce™ Universal Nuclease for Cell Lysis (Catalog #88700, Thermo Fisher Scientific, EUA), and 1.0 mg·ml−1 of hyaluronidase (Catalog # H1136, Sigma‐Aldrich, EUA). Samples were incubated for 45 min at 37°C under constant shaking. Cell suspension was disaggregated and filtered through a 75‐μm cell strainer into 15‐ml conical tubes. Tubes were centrifuged at 1200 rpm at 4°C for 10 min and washed with DMEM plus 10% FCS. Cells were surface stained using the following antibodies: V450 Rat anti‐Mouse CD4 (BD Biosciences. Cat# 560468, RRID:AB_1645271), APC‐conjugated Anti‐F4/80 (isotype: IgG, rat monoclonal antibody, Thermo Fisher Scientific. Cat# 17‐4801‐82, RRID:AB_2784648), PE‐conjugated Anti‐CD3 (isotype: IgG, rat monoclonal antibody, BD Biosciences. Cat# 555275, RRID:AB_395699), PE‐conjugated Anti‐Ly‐6G and Ly‐6C (isotype: IgG, rat monoclonal antibody, BD Biosciences. Cat# 553128, RRID:AB_394644), and FITC‐conjugated anti‐γδ TCR antibody (isotype: IgG, Armenian Hamste monoclonal antibody, BD Biosciences. Cat# 553177, RRID:AB_394688) and stained with Fixable Viability Stain 450 (FVS450‐ Catalog #562247, BD Biosciences) or Zombie NIR™ Fixable Viability Kit (Catalog #423105, Biolegend) for 20 min at room temperature in the dark. Finally, the cells were washed with FACS buffer (PBS solution plus 1% FCS), fixed with PFA 1% for 20 min in the dark, washed with FACS buffer, resuspend in 300 μl of FACs buffer, and analysed in a FACSCanto™ flow cytometry (BD Biosciences, San Diego, CA) and FlowJo software (version 10.6.1, Becton Dickinson, Ashland, OR, RRID:SCR_008520).
2.7. Nest‐building assay
In order to evaluate the well‐being of mice with psoriasis‐like disease, the nest building assay was used (Jirkof, 2014). On Day 6 of imiquimod treatment, mice were individually housed approximately 1 h before the dark phase, and results assessed the next morning. Each cage was supplied with a 5 cm × 5 cm pressed cotton batting nest (Sussex®), and the nests were scored using a scale (0–5). Briefly, the score used was (0) untouched material; (1) material manipulated but scattered around cage; (2) material used to make flat nest; (3) material used to make a cup nest; (4) material finely shredded, nest with an incomplete dome; and (5) nest with a complete and enclosed dome (Gaskill & Pritchett‐Corning, 2016).
2.8. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
The experimental results are expressed as mean ± SEM of n observations, where n represents the number of animals. Power analyses with α = 0.05 and 1 − β = 0.90 using an estimated effect size of 1 (based on previous studies) yielded a minimum necessary sample size of six animals per treatment group. The sample size calculation was performed using the G*Power3.1.9.2 software (Heinrich‐Heine‐Universität Düsseldorf). Statistical analysis was undertaken only when each group size was at least n = 5, n being the number of independent variables (technical replicates were not treated as independent variables).
The data sets were analysed either by one‐way ANOVA or two‐way ANOVA, as indicated in figure legends. The Newman–Keuls post hoc test was conducted only if F achieved P < 0.05 and there was no significant variance inhomogeneity. Outliers were tested by the Grubbs test using GraphPad Prism. No outliers were removed from the database.
For the PASI and Nest Building data, four to five different investigators performed the score evaluation, and then the scores of each animal were averaged and the comparison between groups was assessed by two‐way or one‐way ANOVA, respectively, followed by a post hoc Newman–Keuls test. The reason for this normalization is the possibility to compare the improvement of the pathological condition over the days between different treatments or knockout animals. The number of animals in each group included for statistical tests is shown in the figure legend.
This study was designed to generate groups of equal size, using randomization and blind data analysis. The unequal group sizes of the groups were attributed to different sources, depending on the wide variety of experimental approaches or economy of resources to comply with the 3Rs' (Replacement, Reduction, and Refinement) rule in experimentation with animals. Statistical significance was set at P < 0.05. All tests were carried out using GraphPad Prism statistical software version 8.0, San Diego, California, USA (GraphPad Software, CA, USA, RRID:SCR_008520).
2.9. Materials
Imiquimod (as Aldara cream) was commercially obtained (Curitiba, Brazil); FR173657 was kindly provided by Astellas Pharma (Germany) and SSR240612C by Sanofi‐Aventis (Japan).
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019).
3. RESULTS
3.1. Evidence of kinin receptors in imiquimod‐induced psoriasis
In naive untreated skin, immunoreactivity for B1 and B2 receptors was detected mainly in the epidermis (Figure 1b,c). Six days of imiquimod treatment resulted in a marked increase in the fluorescent signal of both B1 and B2 receptors (Figure 1c), easily noted in the representative pictures (Figure 1b).
The PASI clinical score (Figure 1d) showed that imiquimod promoted a gradual increase in psoriatic‐like lesions, causing erythema, scaling, and skin thickening in the WT group when compared to naive (untreated) mice ( Figure 1d,e). The importance of kinin receptors to this response was demonstrated in the receptor‐deficient (KO) animals. Thus, KOB1, KOB2, and KOB1B2 mice treated with imiquimod demonstrated a reduction in all PASI parameters, when compared to WT mice treated with imiquimod (Figure 1d,e).
Similar results were observed when animals were treated daily with SSR240612C (selective B1 receptor antagonist) or FR173657 (selective B2R antagonist) prior to imiquimod treatment (Figure 2a). Accordingly, the SSR240612C‐ and FR173657‐treated groups presented lower PASI scores, indicating lower severity of psoriatic phenotypes at all doses evaluated when compared to the vehicle group ( Figure 2b–d). In contrast, the vehicle group did not present data that were different from those of the control group.
FIGURE 2.
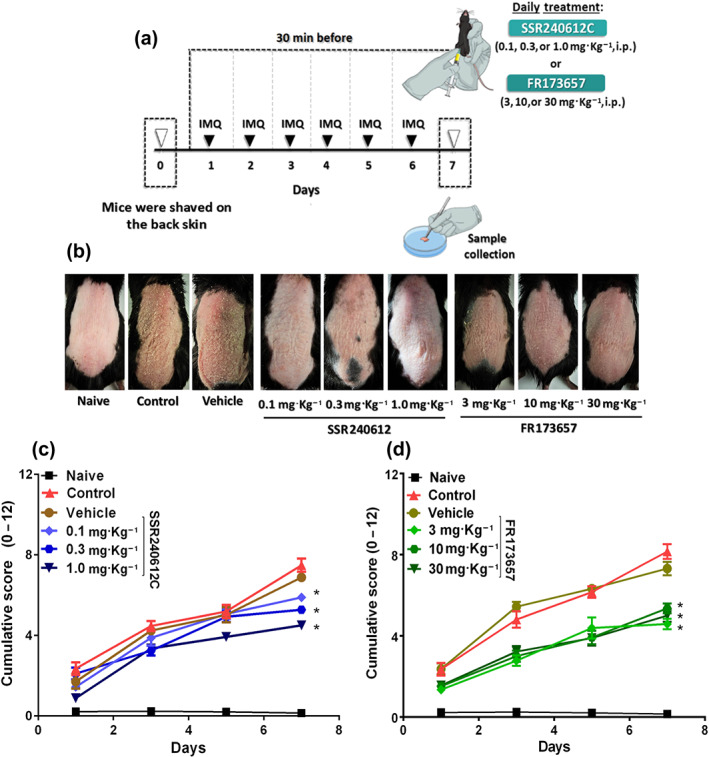
SSR240612C and FR173657 treatment protected against psoriasis‐like lesions induced by imiquimod (IMQ). In an attempt to further evaluate the participation of kinin receptors in the imiquimod ‐induced psoriasis model, (a) WT mice were treated daily with SSR240612C (0.1, 0.3, or 1.0 mg·kg−1) or FR173657 (3, 10, or 30 mg·kg−1), 30 min before imiquimod application. (b) Morphological cutaneous features of imiquimod‐induced psoriasis in mice that received SSR240612C or FR173657 on Day 7 of the experimental protocol. (c) Kinin B1 receptor participation in imiquimod‐induced psoriasis was assessed by the PASI cumulative score (erythema plus scaling plus thickening) from animals that received the SSR240612C treatment. (d) PASI cumulative score evaluation in mice that received FR173657 to evaluate kinin B2 receptor participation in the imiquimod‐induced psoriasis model. The naive group did not receive any treatment. The PASI was blindly assessed at each time point (Days 0 to 7) by five different investigators, and then the scores of each animal was averaged and the comparison between groups was assessed. The values are presented as the mean ± SEM of 18 individual animals each from two independent experiments. No outliers were removed from the database. *P < .05, significantly different from vehicle; two‐way ANOVA with the Newmann–Keuls post hoc test
3.2. A lack of kinin receptors reduces severity of psoriatic lesions in skin
Histopathological analysis of back skin by H&E staining (Figure 3a) demonstrated that WT mice treated with imiquimod showed epidermal thickening and cellular infiltrate, not seen in naïve untreated mice (Figure 3). Imiquimod ‐treated WT animals showed an increase in the number of stained nuclei, indicating increased cellular infiltrate (Figure 3a,b). In contrast, analysis of imiquimod ‐induced psoriatic lesions in mice lacking kinin receptors demonstrated a significant reduction in cellularity, when compared to WT‐treated animals (Figure 3a–d). Epidermal hyperplasia induced by imiquimod, promoting thickening, was also evident in WT animals, compared to the naive group ( Figure 3a,c). As shown in Figure 3c, the psoriatic skin from KOB1, KOB2, and KOB1B2 mice presented a reduction in this epidermal thickening compared to the WT group ( Figure 3c).
FIGURE 3.
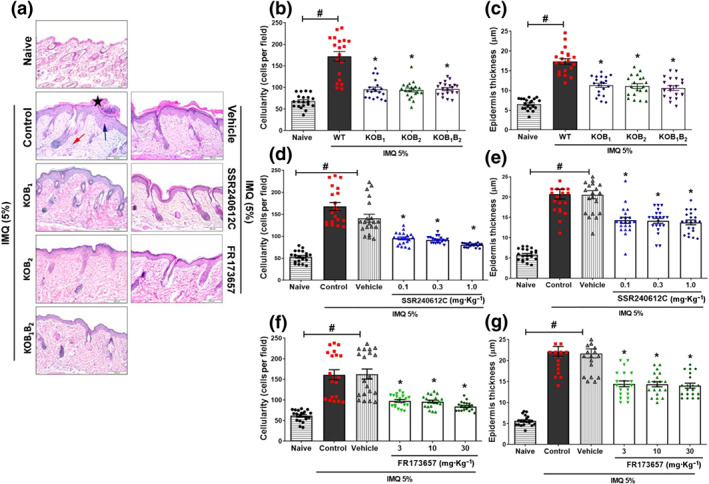
Contribution of B1 and B2 kinin receptors to the development of the histological hallmark of psoriatic skin lesions induced by imiquimod (IMQ). On Day 7 of the experimental protocol, dorsal skin samples were collected and submitted to histopathological evaluation. (a) Representative images of H&E‐stained sections of psoriatic lesions for each treatment regimen. Analyses showing more pronounced (b) cellularity and (c) epidermis thickness in WT mice than in KOB1, KOB2, and KOB1B2 mice treated with imiquimod. Treatment with SSR240612C reduced (d) the inflammatory cells and the (e) epidermal hyperplasia imiquimod ‐induced. FR173657 treatment promoted lower (f) cellular infiltration and (g) epidermal thickness in imiquimod ‐induced psoriasis‐like skin inflammation model. Skin samples of animals that did not receive treatment (naive) were collected from each of the groups and subjected to the same analysis. The black star indicates hyperkeratosis, while the blue arrow indicates epidermal hyperplasia and the red arrow demonstrates the intense cellular influx induced by treatment with imiquimod . The results are presented as the mean ± SEM of 20 individual animals each from two independent experiments. Cell count and epidermis thickness were determined in seven random fields from three non‐continuous serial sections per mouse, with each symbol representing the average value of an individual mouse. No outliers were removed from the database. *P < .05, significantly different from the WT control group (for knockouts animals) or compared to the vehicle group (for animals treated with antagonists). # P < .05, significantly different from naive group; one‐way ANOVA, followed by the Newman–Keuls post hoc test
Consistent with the results in receptor‐deficient mice, the number of infiltrating cells and the epidermal thickness were also reduced in the animals treated with SSR240612C (Figure 3a,d,e), with reductions in all doses evaluated compared to the vehicle. As reported in Figure 3f, the number of infiltrating leukocytes and epidermal hyperplasia were markedly attenuated in animals treated with FR172357, compared with the vehicle group (Figure 3a,f,g).
To further address the possible involvement of kinin receptors in the modulation of keratinocyte proliferation, we investigated the presence of immunolabelled cells for PCNA, a protein related to DNA replication (Strzalka & Ziemienowicz, 2011).
The skin of naive WT mice contained only a few PCNA‐positive cells located mostly in the basal keratinocyte layer. In psoriatic skin lesions of imiquimod ‐treated WT mice, there were significantly more PCNA‐positive cells, with positive stained keratinocytes arranged along different layers in the epidermis, reflecting unregulated proliferation (Figure 4a). In contrast, reductions in proliferating keratinocytes were observed in imiquimod ‐treated mice that did not express either kinin B1 or B2 receptors, as well as in mice deficient for both receptors (Figure 4a,c).
FIGURE 4.
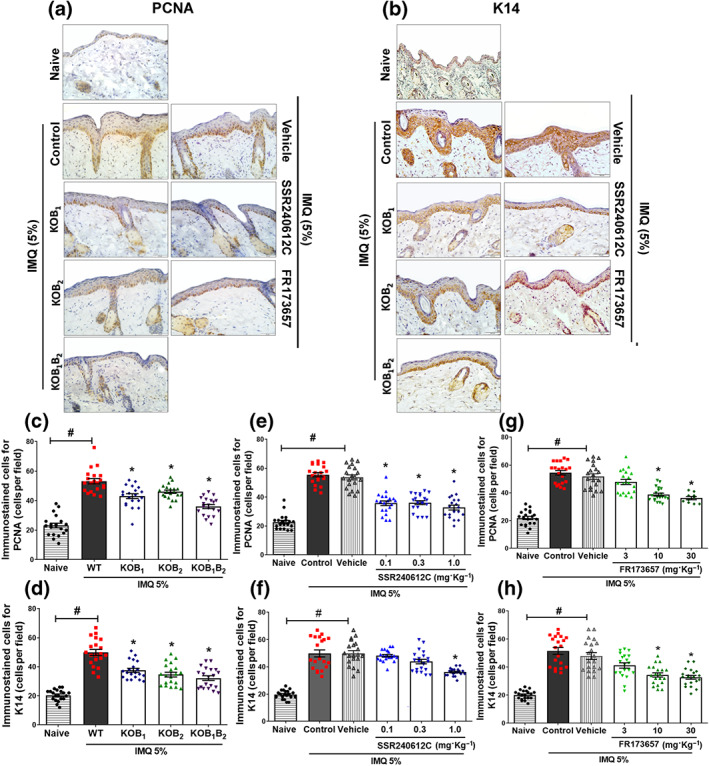
Involvement of B1 and B2 kinin receptors in keratinocyte differentiation and proliferation. Immunohistochemical analysis of mouse skin submitted to 6 days of IMQ treatment. Staining for (a) PCNA or (b) K14 representative images from each group is shown. Quantification of epidermis immuno‐positive cells for (c) PCNA and (d) K14 in WT, KOB1, KOB2, and KOB1B2 mice, as well as mice treated with different doses of (e and g) SSR240612C or (f and h) FR173657 and submitted to the imiquimod ‐induced psoriasis model. The values are presented as the mean ± SEM of 20 individual animals each from two independent experiments. The number of immunostained brown cells was quantified at the epidermis from four non‐continuous serial sections per mouse, with each symbol representing the average value from an individual mouse. No outliers were removed from the database. *P < .05, significantly different from the WT control group (for knockout animals) or compared to the vehicle group (when treated with the antagonists); # P < .05, significantly different from naive and WT imiquimod ‐treated mice or between naive and vehicle‐treated mice; one‐way ANOVA, followed by the Newman–Keuls post hoc test
Importantly, as shown in Figure 4, SSR240612C treatment decreased the number of PCNA‐positive cells at all doses, relative to the vehicle‐treated group ( Figure 4a,e). The B2 antagonist, FR 172357, at higher doses (10 and 30 mg·kg−1) also presented less PCNA‐staining keratinocytes (P < 0.05) compared to the vehicle group (Figure 4a,g). Once again, no alteration was observed in the vehicle‐treated group when compared to the imiquimod group.
K14‐positive cells were localized in the basal layer of the epidermis in the naive group (Figure 4b). In contrast, keratinocytes positive for K14 were found to be increased in imiquimod ‐treated WT mice, reflecting dedifferentiation of the epidermis (Figure 4b,d,f,h). The same effect was noted in the vehicle‐treated imiquimod group (Figure 4b,f,h). As shown in Figure 4d, the absence of B1 and B2 kinin receptors resulted in a reduction in the number of K14‐positive keratinocytes when compared to the imiquimod‐treated WT group. Similarly, even fewer K14‐positive cells were evident in psoriatic skin of mice lacking both kinin receptors (Figure 4b,d). As shown in Figure 4b,f, the psoriatic skin from B1R antagonist‐treated mice (1.0 mg·kg−1) exhibited fewer undifferentiated keratinocytes compared to the vehicle group. Likewise, FR172357 (10 and 30 mg·kg−1) administration caused a decrease in K14‐labelled keratinocytes, compared to the vehicle group (Figure 4b,h).
3.3. Kinins modulate immune cell subsets in the psoriatic skin
An analysis of the composition of the cellular infiltrate following imiquimod treatment revealed a massive infiltration of neutrophils, compared to the naïve untreated group (Figures 5a,b and 8a). Again, this was dependent on kinin receptors as all knockout mice treated with imiquimod exhibited a reduction in Ly6G+‐positive cells, compared to the WT group (Figure 5a,b). Likewise, the pretreatment with SSR240612C (1.0 mg·kg−1) or with FR 173657 (30 mg·kg−1) caused a reduction in neutrophil accumulation, when compared to the vehicle group (Figure 5a,b).
FIGURE 5.
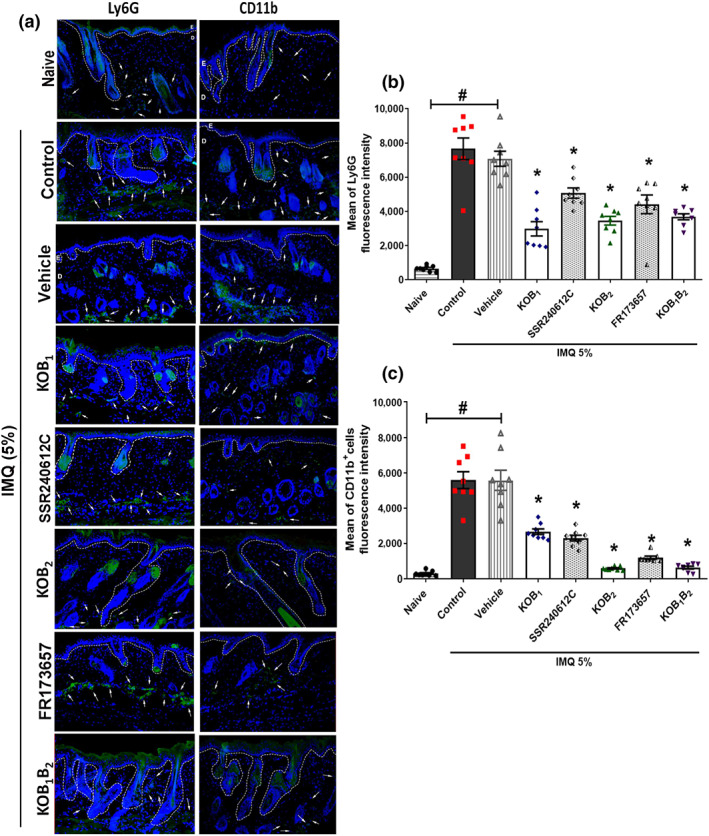
Contribution of B1 and B2 kinin receptors to the Ly6G+ and CD11b+ response induced by 6 days of imiquimod (IMQ) treatment. Mouse dorsal skin samples were collected and submitted to immunofluorescence assay on Day 7 of imiquimod ‐induced psoriasis model. Psoriatic lesions from WT, knockout (KOB1, KOB2, and KOB1B2) mice, and WT mice treated with SSR240612C (1.0 mg·kg−1, i.p.) or FR173657 (30 mg·kg−1, i.p.) were submitted to immunofluorescence analysis. (a) One representative picture is shown for each experimental group. The mean fluorescence intensity (MFI) of (b) Ly6G+ and (c) CD11b+ immunostaining cells in psoriatic skin. Naive (untreated group) samples were collected and subjected to the same analysis. The white dotted lines indicate the basal membrane. The white arrows indicate some of the positively immunolabelled cells. Data are reported as mean ± SEM of eight individual animals per group. The MFI was analysed from three non‐consecutive serial sections per mouse, each symbol representing the average value from an individual mouse. No outliers were removed from the database. *P < .05, significantly different from the WT control group (for knockout mice) or from the vehicle group (for animals treated with antagonists); # P < .05 significantly different from naive and WT imiquimod ‐treated mice or between naive and vehicle‐treated mice; one‐way ANOVA, followed by the Newman–Keuls post hoc test
FIGURE 8.
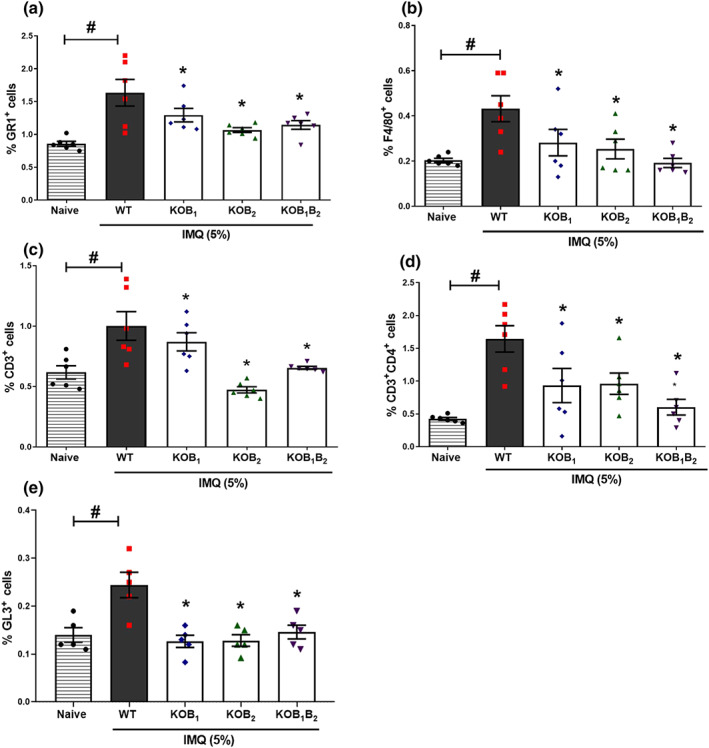
Analysis of the immune cell subset phenotype in the skin of mice lacking kinin receptors submitted to the IMQ‐induced psoriasis model. The number of (a) GR1+‐, (b) F4/80+‐, (c) CD3+‐, and (d) CD4+‐positive cells was quantified by flow cytometry in psoriatic skin lesion of WT and kinin receptor knockout mice treated daily with imiquimod for 6 days. Using FACSCanto™ flow cytometry, 500.000 events were acquired, and the results expressed in percentage. The values are presented as the mean ± SEM of six individual animals per group. (e) The percentage of GL3+‐positive cells in dorsal skin of WT and KO mice, data are means ± SEM of five individual animals per group. Symbols represent values for individual mouse and bars represent mean values for each group. No outliers were removed from the database. *P < .05, significantly different from the WT control group; # P < .05, significantly different from naive and WT imiquimod‐treated mice; one‐way ANOVA with the Newman–Keuls post hoc test
A similar increase in the number of macrophages was found in response to treatment with imiquimod Figures 5 and 8). As shown in Figure 5b, while imiquimod ‐treated WT mice showed an increase in the MFI of CD11b+ cells, blockade of kinin receptors resulted in depletion of skin macrophages (Figure 5).
Immunofluorescent staining of skin section for CD3 demonstrated that imiquimod treatment resulted in an increase in the presence of T cells compared to the naive group (Figures 6, 7a, and 8c). As was found with other cellular infiltrates, psoriatic KOB1, KOB2, and KOB1B2 mice showed reduced numbers of CD3+ T cell infiltrates, compared to the WT group (Figures 7b and 8c). Supporting these findings, SSR240612C and FR173657 administration also reduced the number of CD3+ T cells when compared to the vehicle group (Figure 7b).
FIGURE 6.
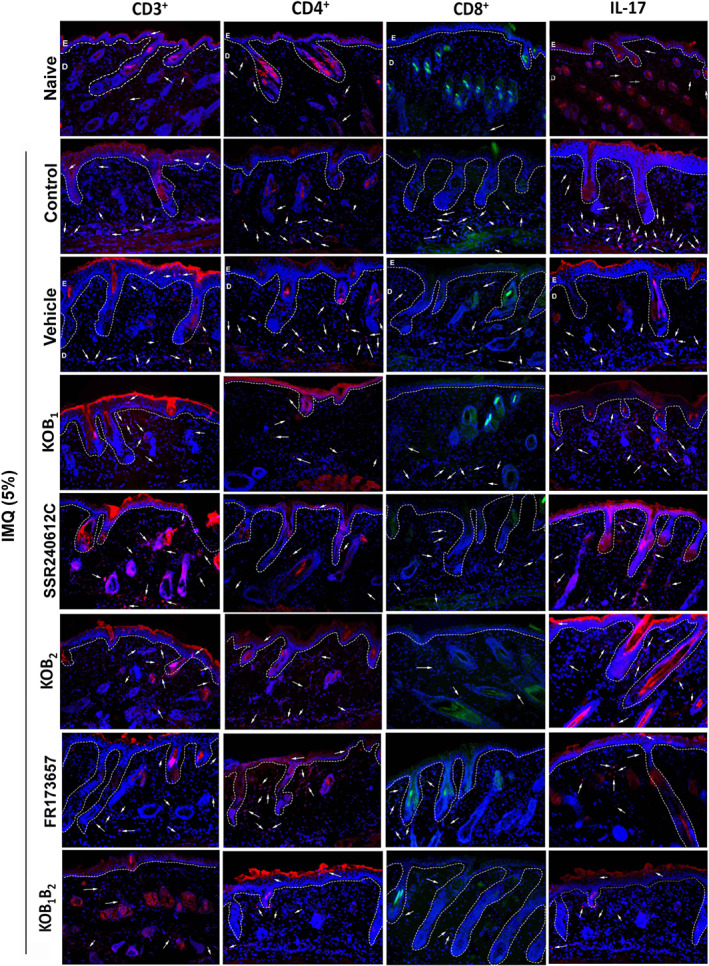
Relevance of B1 and B2 kinin receptors to the immune response induced by imiquimod (IMQ). On Day 7 of the experimental protocol, dorsal skin samples from mice were collected and examined by immunofluorescence assay. Representative images immunostained for CD3+, CD4+, CD8+, and IL‐17 in the psoriatic lesions induced by imiquimod in WT mice, knockout mice (KOB1, KOB2, and KOB1B2), and WT mice treated with vehicle (DMSO 0.03%, v/v), SSR240612C (1.0 mg·kg−1), or FR173657 (30 mg·kg−1). Naive (untreated group) samples were collected and subjected to the same analysis. Kinin‐B1 and B2 receptors were evaluated in three non‐consecutive serial sections from five mice each. The white dotted lines indicate the basal membrane. The white arrows indicate some of the positively immunolabelled cells
FIGURE 7.
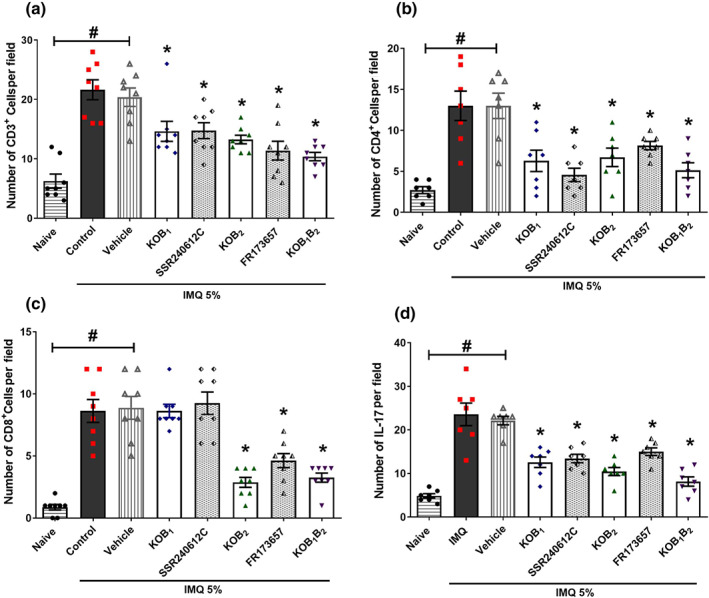
Analysis of kinin receptor participation in the immune response triggered by imiquimod (IMQ) in the skin. The number of (a) CD3+‐, (b) CD4+‐, and (c) CD8+‐positive cells was evaluated in the psoriatic lesion of imiquimod‐treated mice. (d) IL‐17 was also detected in the imiquimod‐induced, psoriatic skin of knockout and WT mice treated with the antagonists. Data are means ± SEM of eight (CD3+ T lymphocyte), seven (CD4+ T cell), eight (CD8+ T cells), and seven (IL‐17) individual animals each from two separate experiments. Positively immunolabelled cells were quantitated by counting fluorescent dots from three non‐consecutive serial sections per mouse. Symbols represent values for individual mouse and bars represent mean values for each group. No outliers were removed from the database. *P < .05, significantly different from the WT control group (for knockouts animals) or from the vehicle group (for animals treated with antagonists); # P < .05 between naive and WT imiquimod‐treated mice or between naive and vehicle‐treated mice; one‐way ANOVA with the Newman–Keuls post hoc test
The increase in CD3+ cells was due to an increase in both the CD8+ and CD4+ subsets (Figures 6, 7, and 8). Psoriatic skin from WT mice exhibited a higher number of CD4+ T lymphocytes (Figures 7b and 8d), but in skin samples from kinin receptor knockout mice, this increase was attenuated. Reinforcing these findings, SSR240612C or FR173657 administration also reduced the number of CD4+ infiltrating cells present in the imiquimod‐induced psoriatic lesions, compared to the vehicle group (13 ± 1.5 positive cells per field; Figures 6 and 7b).
Imiquimod also increased the amount of CD8+ T cells in psoriatic lesions, compared to the naive group ( Figures 6 and 7c). Whereas KOB2 mice exhibited reduced CD8+‐positive cells, an effect that was similar to the KOB1B2 group (Figure 7c), interestingly, no significant changes were observed in the KOB1 group relative to the WT group (Figure 7c). Once again, the pharmacological blockade of B2 receptors with the antagonist FR173657 diminished the number of CD8+ T cells, compared to the vehicle group. However, the B1 receptor antagonist (SSR240612C; Figure 7c) did not affect the numbers of CD8+ T cells, confirming the results seen with the receptor‐KO animals.
As shown in Figure 7d, imiquimod increased IL‐17 accumulation in psoriatic lesions compared to the naive group. Moreover, mice lacking B1, B2, or both kinin receptors exhibited suppression of this IL‐17 accumulation, compared with the WT group ( Figure 6d). Treatment with SSR240612C or FR 173657 also decreased the IL‐17 detected in the imiquimod psoriatic lesion, compared to the vehicle group (Figure 7d).
As observed with other T cell populations, mice lacking kinin receptors showed a significant reduction in the number of skin γδ T cells (gated on GL3+ cells) that accumulated in psoriatic lesions induced by imiquimod, compared to the numbers in imiquimod‐treated WT mice (Figure 8e).
3.4. Relation of kinin receptors and imiquimod‐induced increase in the weight of lymphoid organs
KOB2 and KOB1B2 animals showed attenuated imiquimod ‐induced splenomegaly, compared to the WT group ( Figure S2A). No significant differences were observed between the mean spleen weight of KOB1 and WT animals treated with imiquimod. Imiquimod also increased the weight of axillary lymph nodes, an effect that was reversed by the deletion of B2 receptors as well as in the double receptor knockout mice. No changes were observed in the weight of the axillary lymph nodes of KOB1 mice (Figure S2B). Moreover, the weight of the inguinal lymph node increased following imiquimod treatment, compared with that in the naive group (Figure S2C), but the absence of kinin receptors (KOB1, KOB2, and KOB1B2) prevented the changes in lymph node weight (Figure S2C).
Selective antagonists partly prevented the imiquimod‐induced changes in the lymphoid organs, when compared with the vehicle group. FR173657 (30 mg·kg−1) caused a reduction in the spleen weight, while SSR240612C (1.0 mg·kg−1) did not promote significant changes, relative to the vehicle control (Figure S3A). A protective effect with SSR240612C and FR173657 treatment was observed for both the axillary and inguinal lymph node weights, compared to the vehicle group (Figure S3B,C).
3.5. Effects of blocking the kinin system on the well‐being of mice with imiquimod‐induced psoriasis
WT psoriatic mice showed a reduction in the nest building score compared to the naive group (Figure 9a,b). In contrast, the lack of B1 and B2 receptors led to higher nesting scores in imiquimod ‐treated knockout mice, compared to the WT group ( Figure 9a,b). Again, mice treated with the kinin antagonists, SSR240612C or FR173657, displayed improved nest scores compared with the vehicle group ( Figure 9c,d;).
FIGURE 9.
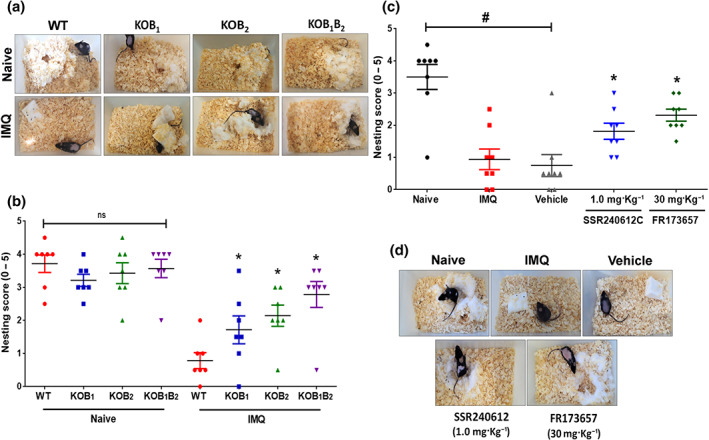
Well‐being evaluation of mice treated with imiquimod (IMQ). Mice behaviour of nest building was used as an indicator of animal well‐being in psoriasis‐like skin disease. (a) Representative images of nests built by WT and knockout (KOB1, KOB2, and KOB1B2) mice treated with imiquimod for 6 days, as well as their respective naive group (healthy mice). (b) Nest building score. Data are means ± SEM of seven individual animals per group. (c) Illustrative picture of nests built by WT mice treated with SSR240612C (0.1 mg·kg−1), FR173657 (30 mg·kg−1), or vehicle (DMSO, 0.03%, v/v) and submitted to the imiquimod‐induced psoriasis model. (d) Nesting scores. The results are presented as the mean ± SEM of eight individual animals per group. Symbols represent values for individual mouse for each group. No outliers were removed from the database. *P < .05, significantly different from (in b) imiquimod‐treated WT mice or (in c) the vehicle group; # P < .05 between naive and WT imiquimod‐treated mice or between naive and vehicle‐treated mice; one‐way ANOVA with the Newman–Keuls post hoc test
4. DISCUSSION
To understand the involvement of the kinin system in psoriasis, we used the imiquimod‐induced mouse model of psoriasis. Our previous study found that the lack of kinin action positively interfered with hyperproliferation of epidermis and leukocyte migration, stimulated by repeated topical administration of TPA on the skin of mice (Pietrovski et al., 2011). Human psoriasis presents typical histopathological hallmarks, including epidermal hyperplasia, hyperkeratosis, and a mixed inflammatory infiltrate in the dermis, which all appear after imiquimod treatment in mice (Kim, 2019; Van Der Fits et al., 2009). Here, our data showed that both B1 and B2 receptors affected chronic skin inflammation, participating in keratinocyte proliferation and immunopathology in this model of imiquimod‐induced psoriasis.
Many studies have described that B2 receptors are constitutively expressed and participate in physiological and acute pathological processes, whereas the B1 receptors are induced by inflammatory process or following tissue injury, in a similar way to the induction of COX 2 (Calixto et al., 2004; Scharfstein, Ramos, & Barral‐Netto, 2017). Accordingly, we found that in the imiquimod‐induced psoriasis model, there was also a dramatic increase in the expression of both kinin receptors in the skin, as compared to healthy mouse skin (Figure 1). Indeed, imiquimod treatment led to overexpression of B1 receptors by more than fivefold, as already observed in many inflammatory states. Our findings suggest that kinins play a major role in imiquimod‐induced psoriasis‐like inflammation, which we have confirmed with knockout mice and with selective antagonists (Figures 2 and 3), and it raises the possibility of using the appropriate receptor antagonists as targets in the treatment of psoriasis.
The accumulation of neutrophils and other inflammatory cells in the entire skin, including the epidermis is a well‐known characteristic of psoriasis histopathology. Kinin receptor knockout mice, as well as SSR240612C and FR173657 treated mice, presented lower cellular infiltration and reduced imiquimod ‐induced hyperplasia, caused by uncontrolled keratinocyte proliferation (Figures 3, 4, 5, 6, 7, 8). The lower numbers of neutrophils and macrophages in the skin of mice that received SSR240612C or FR173657 or in receptor‐deficient animals in the psoriasis model provided evidence of the importance of the two receptors, over the course of the inflammation (Figures 5 and 8). Several studies have described the effects of the kinin system in adhesion and migration of polymorphonuclear leukocytes in humans and in research animals (Guevara‐Lora, Labedz, Skrzeczynska‐Moncznik, & Kozik, 2011; Paegelow, Trzeczak, Böckmann, & Vietinghoff, 2002). As kinins are vasoactive peptides, as expected, they are able to promote an increase in vascular permeability and vasodilation, an event that is related to the recruitment of immune cells (Costa‐neto et al., 2008; Maurer et al., 2011). Besides, activation of both kinin receptors causes the release of cytokines and chemokines and induces the expression of ICAM‐I and VCAM‐I, modulating polymorphonuclear infiltrates (Dos Santos et al., 2008; Shaw & Harper, 2011). In fact, the expression of both kinin receptors in cells of the immune system, such as monocytes, neutrophils, dendritic cells, and lymphocytes, has been confirmed by other studies (Dutra, 2017) suggesting that the kallikrein–kinin system may have significant effects on many types of immune cells.
Our current study confirms and extends the notion that kinin receptor stimulation modulates keratinocyte proliferation and differentiation observed in psoriatic skin. Noticeably, mice lacking B1 and B2 receptors presented a reduction in the number of proliferating and undifferentiated keratinocytes in psoriasiform skin (Figure 4). Corroborating these results, SSR240612C and FR173657 were effective in reducing the higher proliferation rates and faulty differentiation of keratinocytes induced by imiquimod . Actually, BK elevates intracellular calcium in keratinocytes (Coutant, Wolff‐Winiski, & Ryder, 1998; Rosenbach, Liesegang, Binting, & Czarnetzki, 1993) via PLC (Talwar, Fisher, & Voorhees, 1990), and kinin mitogenic activity was associated with the generation of multiple second messengers that converge to activate MAPK (Golias, Charalabopoulos, Stagikas, Charalabopoulos, & Batistatou, 2007). Moreover, in the skin, the inflammatory process is characterized by the interference of many other factors, including cytokines such as TNF‐α, IL‐6 and IL‐17, which directly influence the proliferation of keratinocytes. In this context, kinins are known to sustain the inflammatory state, inducing the release of pro‐inflammatory cytokines, IL‐1 and TNF‐α (Cunha et al., 2007; Tiffany & Burch, 1989). Thus, in inflammatory conditions, increased expression of the kinin receptors in the epidermis may be related to keratinocyte proliferation, as well as to the release of other mediators that contribute to this phenomenon.
Additionally, both kinin receptors are involved in keratinocyte differentiation (Figure 4). Supporting this, the activation of both B1 and B2 receptors triggers signalling pathways that are involved in keratinocyte differentiation, resulting in c‐Fos expression, NF‐κB nuclear translocation, and filaggrin and cytokeratin expression (Seliga et al., 2018; Tang, Leung, & Lai, 2011). Thus, the differentiation effect of kinins can be directly on keratinocytes and/or through release of factors that induce differentiation, but this event needs to be investigated further.
It is now well characterized that in psoriasis, T cells play a dominant pathogenic role in the initiation and maintenance of the skin disorder (Romanelli, Volpe, Borsellino, Romanelli, & Chiricozzi, 2018). Notably, kinin receptor knockout mice showed a marked reduction in the CD3+ and CD4+ T cell population (Figures 6, 7, and 8), while CD8+ T cells appeared to be more strongly influenced by B2 receptors (Figures 6c and 7c). Again, the use of selective antagonists confirms that these effects of kinins are not an adaptation caused by the genomic change in knockout animals (Figures 6 and 7). This constitutes an important indication that kinins are likely to be involved in the modulation of the immune system following an inflammatory condition, as well as previously described in autoimmune diseases (Aliberti et al., 2003; Dutra, 2017). The evaluation of thymidine incorporation in kinin receptor knockout mice has previously shown a marked decrease in the cell proliferation rate in the lymph node and spleen, suggesting the participation of B1 and B2 receptors in the modulation of lymphocyte proliferation (Dutra et al., 2011). Aliberti et al. (2003) highlighted that kinins activate DC to produce IL‐12 through the activation of B2 kinin receptors, driven to a Th1 type of response, preventing eosinophil infiltration in the pleura of challenged mice. Furthermore, in the current study, we found that blockade of both receptors prevented the enlargement of inguinal and axillary lymph nodes caused by imiquimod , which may be related to the lower rate of proliferation and/or migration of immune cells (Figures S2 and S3). Moreover, B2 receptors, but not B1 receptors, are the only receptors involved in the splenomegaly induced by imiquimod.
Although the mechanism of action of imiquimod causing psoriasis is not completely understood, the involvement of the IL‐23/IL‐17 axis is undeniable (Dunussi‐Joannopoulos et al., 2011). Herein, we noted that the absence of kinin receptors promoted an intense reduction in IL‐17 accumulation in psoriatic lesions (Figures 6 and 7d). A similar effect was observed in the experimental autoimmune encephalomyelitis, where the blockade of B1 receptors also reduced IL‐17 levels (Dutra et al., 2011). Recent findings have stated that γδ T cells are major IL‐17‐producing cells in the skin following IL‐23 stimulation (Cai, Fleming, & Yan, 2012). Additionally, our data show that genetic or pharmacological blockade of kinin receptors promoted a significant reduction in the γδ T cells subset in our imiquimod‐induced mouse model of psoriasis (Figure 8e). Thus, it is possible that the kinin system is somehow modulating IL‐17 production by interfering with the migration of γδ T cells to the skin, reducing psoriasis development. Actually, the influence of kallikrein–kinin system on IL‐17‐driven immunity has already been proposed by Ramani et al., (2016).
Additionally, the improvement of the psoriatic phenotype was accompanied by an improved well‐being index when kinin receptors were blocked (Figure 9). This is an important result since patients with psoriasis present an increased risk for depression and other emotional disorders (Gooderham et al., 2017). This is a further indication that the influence of kinins in this chronic inflammatory disease is quite significant and may be an additional target in the treatment of psoriasis, whose other alternatives involve immunosuppressive treatment with many undesirable effects.
In conclusion, our findings suggest that both kinin B1 and B2 receptors play an important role in the modulation of immune response, as well as in the deregulation of keratinocyte proliferation and differentiation induced by imiquimod . The selective non‐peptide kinin antagonists SSR240612C and FR173657 could be interesting options in the treatment of psoriasis, although further studies are necessary.
AUTHOR CONTRIBUTIONS
B.S.S. performed most of the experiments. B.S.S., D.A.G.B.M., M.F.O., and D.A.C. did the data analysis. R.L.T.M., M.B., and J.B.P. created and provided the knockout mice. B.S.S., M.F.O., and D.A.C. drafted the manuscript. J.B.P., M.B., A.B., D.A.W., W.L.H., and J.B.C. helped with interpretation of results, reviewed and/or edited the manuscript, and contributed to the discussion. M.F.O., D.A.C., and J.B.C. managed the project and contributed to the fundraising.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1. Fluorescence microscopic images illustrate the absence in B1 and B2 kinin receptors expression in knockout animals by immunostaining. On day 7 of the experimental protocol, mouse dorsal skin samples were collected and submitted to immunofluorescence assay. Representative images show the presence of B1 (green) or B2 (red) kinin receptors in the psoriatic skin lesions of knockout mice (KOB1, KOB2 and KOB1B2) induced by IMQ treatment. Kinin‐B1 and B2 receptors were evaluated in 3 non‐consecutive serial sections from 5 mouse each. Scale bars represent 200 μm.
Figure S2. Estimation of (A) splenic (B) axillary lymph node and (C) inguinal lymph node weight of WT and knockout mice (KOB1, KOB2, and KOB1B2) treated for with IMQ for 6 days. Organ weight results are expressed in milligrams per kilogram body weight. (A) The values are presented as the mean ± SEM (Naive: n=6, WT: n = 7, KOB1: n = 6, KOB2: n = 6 and KOB1B2: n = 6). Symbols represent values for individual mouse and bars represent mean values for each group. (B) Data are mean ± SEM (Naive: n=10, WT: n = 10, KOB1: n = 7, KOB2: n = 6 and KOB1B2: n = 6). Symbols represent the average weight of axillary lymph node for each animal and bars represent mean values for each group. (C) Data are mean ± SEM (Naive: n=8, WT: n = 12, KOB1: n = 10, KOB2: n = 8 and KOB1B2: n = 6). Symbols represent the average weight of inguinal lymph node for each animal and bars represent mean values for each group. No outliers were removed from the database. The data are the result of the combination in a single data set of two independent experiments. The unequal group sizes of the groups were attributed to different experimental approaches. *p<0.05 according to one‐way ANOVA with the Newmann‐Keuls post‐hoc test. Values were compared to WT mice treated with IMQ. #p<0.05 between Naive and WT IMQ treated mice.
Figure S3. Effect of SSR240612C (0.1 mg/kg) and FR173657 (30 mg/kg) treatment on the (A) splenic (B) axillary lymph node and (C) inguinal lymph node weight of WT mice submitted to the IMQ model. Organ weight results bare expressed in milligrams per kilogram body weight. (A) The results are presented as the mean ± SEM (Naive: n=11, Control: n=8, Vehicle: n= 6, SSR240612C: n=6 and FR173657: n= 6). Symbols represent values for individual mouse and bars represent mean values for each group. (B) Data are means ± SEM (Naive: n=12, Control: n=12, Vehicle: n= 9, SSR240612C: n=8 and FR173657: n= 10). Symbols represent the average weight of axillary lymph node for each animal and bars represent mean values for each group. (C) Data are means ± SEM (Naive: n=6, Control: n=11, Vehicle: n= 10, SSR240612C: n=7 and FR173657: n= 8). Symbols represent the average weight of inguinal lymph node for each animal and bars represent mean values for each group. The data are the result of the combination in a single data set of two independent experiments. No outliers were removed from the database. The unequal group sizes of the groups were attributed to different experimental approaches. *p<0.05 according to one‐way ANOVA with the Newmann‐Keuls post‐hoc test. #p<0.05 between Naive and Control group (WT IMQ treated mice).
Soley BS, Silva LM, Mendes DAGB, et al. B1 and B2 kinin receptor blockade improves psoriasis‐like disease. Br J Pharmacol. 2020;177:3535–3551. 10.1111/bph.15077
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliberti, J. , Viola, J. P. B. , Vieira‐de‐Abreu, A. , Bozza, P. T. , Sher, A. , & Schartstein, J. (2003). Cutting edge: Bradykinin induces IL‐12 production by dendritic cells: A danger signal that drives Th1 polarization. The Journal of Immunology, 170(11), 5349–5353. 10.4049/jimmunol.170.11.5349 [DOI] [PubMed] [Google Scholar]
- Arora, N. , Shah, K. , & Pandey‐Rai, S. (2016). Inhibition of imiquimod‐induced psoriasis‐like dermatitis in mice by herbal extracts from some Indian medicinal plants. Protoplasma, 253(2), 503–515. 10.1007/s00709-015-0829-y [DOI] [PubMed] [Google Scholar]
- Bertram, C. , Misso, N. L. , Fogel‐Petrovic, M. , Figueroa, C. , Thompson, P. J. , & Bhoola, K. D. (2007). Comparison of kinin B1 and B2 receptor expression in neutrophils of asthmatic and non‐asthmatic subjects. International Immunopharmacology, 7(14), 1862–1868. 10.1016/j.intimp.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Böckmann, S. , & Paegelow, I. (2000). Kinins and kinin receptors: Importance for the activation of leukocytes. Journal of Leukocyte Biology, 68(5), 587–592. [PubMed] [Google Scholar]
- Borkowski, J. A. , Ransom, R. W. , Seabrook, G. R. , Trumbauer, M. , Chen, H. , Hill, R. G. , … Hess, J. F. (1995). Targeted disruption of a B2 bradykinin receptor gene in mice eliminates bradykinin action in smooth muscle and neurons. Journal of Biological Chemistry, 270(23), 13706–13710. 10.1074/jbc.270.23.13706 [DOI] [PubMed] [Google Scholar]
- Bourdet, B. , Pécher, C. , Minville, V. , Jaafar, A. , Allard, J. , Blaes, N. , … Tack, I. (2010). Distribution and expression of B2‐kinin receptor on human leukocyte subsets in young adults and elderly using flow cytometry. Neuropeptides, 44(2), 155–161. 10.1016/j.npep.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Cai, Y. , Fleming, C. , & Yan, J. (2012). New insights of T cells in the pathogenesis of psoriasis. Cellular and molecular immunology. Nature Publishing Group. 10.1038/cmi.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto, J. B. , Cabrini, D. A. , Ferreira, J. , & Campos, M. M. (2000). Kinins in pain and inflammation. Pain, 87, 1–5. 10.1016/S0304-3959(00)00335-3 [DOI] [PubMed] [Google Scholar]
- Calixto, J. B. , Medeiros, R. , Fernandes, E. S. , Ferreira, J. , Cabrini, D. A. , & Campos, M. M. (2004). Kinin B1 receptors: Key G‐protein‐coupled receptors and their role in inflammatory and painful processes. British Journal of Pharmacology, 143(7), 803–818. 10.1038/sj.bjp.0706012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, D. J. (2000). Towards understanding the kallikrein‐kinin system: Insights from measurement of kinin peptides. Brazilian Journal of Medical and Biological Research, 33(6), 665–677. 10.1590/S0100-879X2000000600008 [DOI] [PubMed] [Google Scholar]
- Cayla, C. , Merino, V. F. , Cabrini, D. A. , Silva, J. A. , Pesquero, J. B. , & Bader, M. (2002). Structure of the mammalian kinin receptor gene locus. International Immunopharmacology, 2(13–14), 1721–1727. 10.1016/S1567-5769(02)00175-3 [DOI] [PubMed] [Google Scholar]
- Chang, Y. C. , Wu, W. M. , Chen, C. H. , Lee, S. H. , Hong, H. S. , & Hsu, L. A. (2007). Association between the insertion/deletion polymorphism of the angiotensin I‐converting enzyme gene and risk for psoriasis in a Chinese population in Taiwan. British Journal of Dermatology, 156(4), 642–645. 10.1111/j.1365-2133.2006.07716.x [DOI] [PubMed] [Google Scholar]
- Christianne, B.‐M. , Calheiros, A. S. , Silva, M. R. , Cordeiro, R. S. , Teixeira, M. M. , & Martins, M. A. (1999). Suppressive effect of distinct bradykinin B2 receptor antagonist on allergen‐evoked exudation and leukocyte infiltration in sensitized rats. British Journal of Pharmacology, 127, 315–320. 10.3357/ASEM.3306.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa‐neto, C. M. , Dillenburg‐pilla, P. , Heinrich, T. A. , Parreiras‐e‐silva, L. T. , Pereira, M. G. A. G. , Reis, R. I. , & Souza, P. P. C. (2008). Participation of kallikrein–kinin system in different pathologies, 135–142. 10.1016/j.intimp.2007.08.003 [DOI] [PubMed]
- Coutant, K. D. , Wolff‐Winiski, B. , & Ryder, N. S. (1998). Fluvastatin enhances receptor‐stimulated intracellular Ca2+ release in human keratinocytes. Biochemical and Biophysical Research Communications, 245(2), 307–312. 10.1006/bbrc.1998.8429 [DOI] [PubMed] [Google Scholar]
- Cunha, T. M. , Verri, W. A. , Fukada, S. Y. , Guerrero, A. T. G. , Santodomingo‐garzón, T. , Poole, S. , … Cunha, F. Q. (2007). TNF‐α and IL‐1β mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor. European Journal of Pharmacology, 573, 221–229. 10.1016/j.ejphar.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty, J. R. , Stanford, S. C. , Panattieri, R. A. , Alexander, S. P. , Cirino, G. , George, C. H. , … Sobey, C. G. (2019). Sex: A change in our guidelines to authors to ensure that this is no longer an ignored experimental variable. British Journal of Pharmacology, 176, 1–6. 10.1111/bph.14761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos, A. C. , Roffê, E. , Arantes, R. M. E. , Juliano, L. , Pesquero, J. L. , Pesquero, J. B. , … Carvalho‐Tavares, J. (2008). Kinin B2 receptor regulates chemokines CCL2 and CCL5 expression and modulates leukocyte recruitment and pathology in experimental autoimmune encephalomyelitis (EAE) in mice. Journal of Neuroinflammation, 5(i), 1–10. 10.1186/1742-2094-5-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunussi‐Joannopoulos, K. , Renauld, J.‐C. , Dumoutier, L. , Warnier, G. , Lemaire, M. M. , Hendrickx, E. , … de Heusch, M. (2011). IL‐22 is required for imiquimod‐induced psoriasiform skin inflammation in mice. The Journal of Immunology, 188(1), 462–469. 10.4049/jimmunol.1102224 [DOI] [PubMed] [Google Scholar]
- Dutra, R. C. , Leite, D. F. P. , Bento, A. F. , Manjavachi, M. N. , Patrício, E. S. , Figueiredo, C. P. , … Calixto, J. B. (2011). The role of kinin receptors in preventing neuroinflammation and its clinical severity during experimental autoimmune encephalomyelitis in mice. PLoS ONE, 6(11). 10.1371/journal.pone.0027875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra, R. C. (2017). Kinin receptors: Key regulators of autoimmunity. Autoimmunity reviews. Elsevier B.V; 10.1016/j.autrev.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Eberle, F. C. , Brück, J. , Holstein, J. , Hirahara, K. , & Ghoreschi, K. (2016). Recent advances in understanding psoriasis. F1000Research, 5(0), 770 10.12688/f1000research.7927.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry, L. , & Baker, B. S. (2007). Triggering psoriasis: The role of infections and medications. Clinics in Dermatology, 25(6), 606–615. 10.1016/j.clindermatol.2007.08.015 [DOI] [PubMed] [Google Scholar]
- Gaskill, B. N. , & Pritchett‐Corning, K. R. (2016). Nest building as an indicator of illness in laboratory mice. Applied Animal Behaviour Science, 180, 140–146. 10.1016/j.applanim.2016.04.008 [DOI] [Google Scholar]
- Gilleaudeau, P. , Vallat, V. P. , Carter, D. M. , & Gottlieb, A. B. (1993). Angiotensin‐converting enzyme inhibitors as possible exacerbating drugs in psoriasis. Journal of the American Academy of Dermatology, 28(3), 490–492. 10.1016/S0190-9622(08)81761-6 [DOI] [PubMed] [Google Scholar]
- Golias, C. , Charalabopoulos, A. , Stagikas, D. , Charalabopoulos, K. , & Batistatou, A. (2007). The kinin system ‐ bradykinin: Biological effects and clinical implications. Multiple role of the kinin system ‐ bradykinin. Hippokratia, 11(3), 124–128. [PMC free article] [PubMed] [Google Scholar]
- Gooderham, M. , Lawson, F. , Kimball, A. B. , Srivastava, B. , de Jong, E. M. G. J. , Fakharzadeh, S. , … Langley, R. G. (2017). Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). Journal of the American Academy of Dermatology, 78(1), 70–80. 10.1016/j.jaad.2017.08.051 [DOI] [PubMed] [Google Scholar]
- Gougat, J. , Ferrari, B. , Sarran, L. , Planchenault, C. , Poncelet, M. , Maruani, J. , … Finance, O. (2004). SSR240612 [(2R)‐2‐[((3R)‐3‐(1,3‐benzodioxol‐5‐yl)‐3‐{[(6‐methoxy‐2‐naphthyl)sulfonyl]amino}propanoyl)amino]‐3‐(4‐{[2R,6S)‐2,6‐dimethylpiperidinyl]methyl}phenyl)‐N‐isopropyl‐N‐methylpropanamide hydrochloride], a new nonpeptide antagonist of the bradykinin B1 receptor: Biochemical and pharmacological characterization). The Journal of Pharmacology and Experimental Therapeutics, 309(2), 661–669. 10.1124/jpet.103.059527 [DOI] [PubMed] [Google Scholar]
- Guevara‐Lora, I. , Labedz, A. , Skrzeczynska‐Moncznik, J. , & Kozik, A. (2011). Bradykinin and des‐Arg10‐kallidin enhance the adhesion of polymorphonuclear leukocytes to extracellular matrix proteins and endothelial cells. Cell Communication & Adhesion, 18(4), 67–71. 10.3109/15419061.2011.617854 [DOI] [PubMed] [Google Scholar]
- Guevara‐Lora, I. , Stalinska, K. , Augustynek, B. , & Labedz‐Maslowska, A. (2014). Influence of kinin peptides on monocyte‐endothelial cell adhesion. Journal of Cellular Biochemistry, 115(11), 1985–1995. 10.1002/jcb.24870 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … Davies, J. A. (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirkof, P. (2014). Burrowing and nest building behavior as indicators of well‐being in mice. Journal of Neuroscience Methods, 234, 139–146. 10.1016/j.jneumeth.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2011). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Journal of Cerebral Blood Flow and Metabolism, 31(4), 991–993. 10.1038/jcbfm.2010.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. C. (2019). The imiquimod induced psoriatic animal model: Scientific implications. Biomedical Journal of Scientific & Technical Research, 13(1), 2018–2020. 10.26717/BJSTR.2019.13.002347 [DOI] [Google Scholar]
- Lowe, J. R. , Dixon, J. S. , Guthrie, J. A. , & McWhinney, P. (1986). Serum and synovial fluid levels of angiotensin converting enzyme in polyarthritis. Annals of the Rheumatic Diseases, 45(11), 921–924. 10.1136/ard.45.11.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus, C. E. , Ehrenfeld, P. , Pavicic, F. , Gonzalez, C. B. , Concha, M. , Bhoola, K. D. , … Figueroa, C. D. (2016). Activation of the human keratinocyte B1 bradykinin receptor induces expression and secretion of metalloproteases 2 and 9 by transactivation of epidermal growth factor receptor. Experimental Dermatology, 25(9), 694–700. 10.1111/exd.13038 [DOI] [PubMed] [Google Scholar]
- Matus, C. E. , Ehrenfeld, P. , Pavicic, F. , Sarmiento, J. M. , Astroza, A. , Sanchez, T. , … Figueroa, C. D. (2008). Activation of kinin B1 receptor triggers differentiation of cultured human keratinocytes. British Journal of Dermatology, 159(4), 792–803. 10.1111/j.1365-2133.2008.08784.x [DOI] [PubMed] [Google Scholar]
- Maurer, M. , Bader, M. , Bas, M. , Bossi, F. , Cicardi, M. , Cugno, M. , … Magerl, M. (2011). New topics in bradykinin research. Allergy: European Journal of Allergy and Clinical Immunology, 66, 1397–1406. 10.1111/j.1398-9995.2011.02686.x [DOI] [PubMed] [Google Scholar]
- Paegelow, I. , Trzeczak, S. , Böckmann, S. , & Vietinghoff, G. (2002). Migratory responses of polymorphonuclear leukocytes to kinin peptides. Pharmacology, 66(3), 153–161. 10.1159/000063797 [DOI] [PubMed] [Google Scholar]
- Pesquero, J. B. , Araujo, R. C. , Heppenstall, P. A. , Stucky, C. L. , Silva, J. A. , Walther, T. , … Bader, M. (2000). Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proceedings of the National Academy of Sciences, 97(14), 8140–8145. 10.1073/pnas.120035997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrovski, E. F. , Paludo, K. S. , Mendes, D. A. , Guimarães, F. D. , Veiga, S. S. , de Freitas Buchi, D. , … Ferreira, J. (2011). B1 and B2 kinin receptor participation in hyperproliferative and inflammatory skin processes in mice. Journal of Dermatological Science, 64(1), 23–30. 10.1016/j.jdermsci.2011.06.016 [DOI] [PubMed] [Google Scholar]
- Poblete, M. T. , Reynolds, N. J. , Figueroa, C. D. , Burton, J. L. , Muller‐Esterl, W. , & Bhoola, K. D. (1991). Tissue kallikrein and kininogen in human sweat glands and psoriatic skin. British Journal of Dermatology, 124(3), 236–241. 10.1111/j.1365-2133.1991.tb00567.x [DOI] [PubMed] [Google Scholar]
- Ramani, K. , Garg, A. V. , Jawale, C. V. , Conti, H. R. , Whibley, N. , Jackson, E. K. , … Biswas, P. S. (2016). The kallikrein‐kinin system: A novel mediator of IL‐17‐driven anti‐Candida immunity in the kidney. PLoS Pathogens, 12, 1–25. 10.1371/journal.ppat.1005952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli, P. , Volpe, E. , Borsellino, G. , Romanelli, M. , & Chiricozzi, A. (2018). Scanning the immunopathogenesis of psoriasis. International Journal of Molecular Sciences, 19 10.3390/ijms19010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbach, T. , Liesegang, C. , Binting, S. , & Czarnetzki, B. M. (1993). Inositol phosphate formation and release of intracellular free calcium by bradykinin in HaCaT keratinocytes. Archives of Dermatological Research, 285(7), 393–396. 10.1007/BF00372131 [DOI] [PubMed] [Google Scholar]
- Sauder, D. N. (2002). Immunomodulatory and pharmacologic properties of imiquimod. Journal of the American Academy of Dermatology, 43(1), S6–S11. 10.1067/mjd.2000.107808 [DOI] [PubMed] [Google Scholar]
- Scharfstein, J. , Ramos, P. I. P. , & Barral‐Netto, M. (2017). G protein‐coupled kinin receptors and immunity against pathogens In Advances in immunology (1st ed., Vol. 136) (pp. 29–84). Elsevier Inc. 10.1016/bs.ai.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Schremmer‐Danninger, E. , Hermann, A. , Fink, E. , Fritz, H. , & Roscher, A. A. (1999). Identification and occurrence of mRNAs for components of the kallikrein‐kinin system in human skin and in skin diseases. Immunopharmacology, 43, 287–291. 10.1016/S0162-3109(99)00100-9 [DOI] [PubMed] [Google Scholar]
- Segawa, I. , Tanji, O. , Hsu, P. S. , Izaki, S. , Hibino, T. , & Kon, S. (2009). Modulation of plasma and tissue kallikreins in psoriasis vulgaris and psoriasis pustulosa. Dermatology, 179(1), 116–117. 10.1159/000248461 [DOI] [PubMed] [Google Scholar]
- Seliga, A. , Lee, M. H. , Fernandes, N. C. , Zuluaga‐Ramirez, V. , Didukh, M. , Persidsky, Y. , … Sriram, U. (2018). Kallikrein‐kinin system suppresses type I interferon responses: A novel pathway of interferon regulation. Frontiers in Immunology, 9, 1–13. 10.3389/fimmu.2018.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, J. N. , & Al‐Sherif, G. J. (2006). Pharmacologic targets and prototype therapeutics in the kallikrein‐kinin system: Bradykinin receptor agonists or antagonists. The Scientific World JOURNAL, 6, 1247–1261. 10.1100/tsw.2006.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, O. M. , & Harper, J. L. (2011). Bradykinin receptor 2 extends inflammatory cell recruitment in a model of acute gouty arthritis. Biochemical and Biophysical Research Communications, 416(3–4), 266–269. 10.1016/j.bbrc.2011.10.137 [DOI] [PubMed] [Google Scholar]
- Soley, B. S. , Horinouchi, C. S. , Pawloski, P. L. , Otuki, M. F. , & Cabrini, D. A. (2016). Kinin receptors in skin wound healing. Journal of Dermatological Science, 82(2), 95–105. 10.1016/j.jdermsci.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Strzalka, W. , & Ziemienowicz, A. (2011). Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Annals of Botany, 107, 1127–1140. 10.1093/aob/mcq243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell, W. R. , Michaels, K. A. , Sutter, A. J. , Diaconu, D. , Fritz, Y. , Xing, X. , … Ward, N. L. (2017). Imiquimod has strain‐dependent effects in mice and does not uniquely model human psoriasis. Genome Medicine, 9(1), 1–21. 10.1186/s13073-017-0415-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar, H. S. , Fisher, G. J. , & Voorhees, J. J. (1990). Bradykinin induces phosphoinositide turnover, 1,2‐diglyceride formation, and growth in cultured adult human keratinocytes. Journal of Investigative Dermatology, 95(6), 705–710. 10.1111/1523-1747.ep12514507 [DOI] [PubMed] [Google Scholar]
- Tang, S. C. W. , Leung, J. C. K. , & Lai, K. N. (2011). The kallikrein‐kinin system. Diabetes and the Kidney, 170, 145–155. 10.1159/000325650 [DOI] [PubMed] [Google Scholar]
- Thakor, P. , Padmanabhan, M. , Johnson, A. , Pararajasingam, T. , Thakor, S. , & Jorgensen, W. (2010). Ramipril‐induced generalized pustular psoriasis: Case report and literature review. American Journal of Therapeutics, 17(1), 92–95. 10.1097/MJT.0b013e31818f9e99 [DOI] [PubMed] [Google Scholar]
- Tiffany, C. W. , & Burch, M. (1989). Bradykinin stimulates tumor necrosis factor and interleukin‐1 release from macrophages. FEBS Letters, 247(2), 189–192. 10.1016/0014-5793(89)81331-6 [DOI] [PubMed] [Google Scholar]
- Ueyama, A. , Yamamoto, M. , Tsujii, K. , Furue, Y. , Imura, C. , Shichijo, M. , & Yasui, K. (2014). Mechanism of pathogenesis of imiquimod‐induced skin inflammation in the mouse: A role for interferon‐α in dendritic cell activation by imiquimod. Journal of Dermatology, 41(2), 135–143. 10.1111/1346-8138.12367 [DOI] [PubMed] [Google Scholar]
- Van Der Fits, L. , Mourits, S. , Voerman, J. S. A. , Kant, M. , Boon, L. , Laman, J. D. , … Lubberts, E. (2009). Imiquimod‐induced psoriasis‐like skin inflammation in mice is mediated via the IL‐23/IL‐17 axis. The Journal of Immunology, 182, 5836–5845. 10.4049/jimmunol.0802999 [DOI] [PubMed] [Google Scholar]
- Vianna, R. M. J. , Ongali, B. , Regoli, D. , Calixto, J. B. , & Couture, R. (2003). Up‐regulation of kinin B1 receptor in the lung of streptozotocin‐diabetic rat: Autoradiographic and functional evidence. British Journal of Pharmacology, 138(1), 13–22. 10.1038/sj.bjp.0704999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal, M. A. , Astroza, A. , Matus, C. E. , Ehrenfeld, P. , Pavicic, F. , Sanchez, T. , … Figueroa, C. D. (2005). Kinin B2 receptor‐coupled signal transduction in human cultured keratinocytes. Journal of Investigative Dermatology, 124(1), 178–186. 10.1111/j.0022-202X.2004.23518.x [DOI] [PubMed] [Google Scholar]
- Wolf, R. , Tamir, A. , & Brenner, S. (1990). Psoriasis related to angiotensin‐converting enzyme inhibitors. Dermatologica, 181, 51–53. 10.1159/000247861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Fluorescence microscopic images illustrate the absence in B1 and B2 kinin receptors expression in knockout animals by immunostaining. On day 7 of the experimental protocol, mouse dorsal skin samples were collected and submitted to immunofluorescence assay. Representative images show the presence of B1 (green) or B2 (red) kinin receptors in the psoriatic skin lesions of knockout mice (KOB1, KOB2 and KOB1B2) induced by IMQ treatment. Kinin‐B1 and B2 receptors were evaluated in 3 non‐consecutive serial sections from 5 mouse each. Scale bars represent 200 μm.
Figure S2. Estimation of (A) splenic (B) axillary lymph node and (C) inguinal lymph node weight of WT and knockout mice (KOB1, KOB2, and KOB1B2) treated for with IMQ for 6 days. Organ weight results are expressed in milligrams per kilogram body weight. (A) The values are presented as the mean ± SEM (Naive: n=6, WT: n = 7, KOB1: n = 6, KOB2: n = 6 and KOB1B2: n = 6). Symbols represent values for individual mouse and bars represent mean values for each group. (B) Data are mean ± SEM (Naive: n=10, WT: n = 10, KOB1: n = 7, KOB2: n = 6 and KOB1B2: n = 6). Symbols represent the average weight of axillary lymph node for each animal and bars represent mean values for each group. (C) Data are mean ± SEM (Naive: n=8, WT: n = 12, KOB1: n = 10, KOB2: n = 8 and KOB1B2: n = 6). Symbols represent the average weight of inguinal lymph node for each animal and bars represent mean values for each group. No outliers were removed from the database. The data are the result of the combination in a single data set of two independent experiments. The unequal group sizes of the groups were attributed to different experimental approaches. *p<0.05 according to one‐way ANOVA with the Newmann‐Keuls post‐hoc test. Values were compared to WT mice treated with IMQ. #p<0.05 between Naive and WT IMQ treated mice.
Figure S3. Effect of SSR240612C (0.1 mg/kg) and FR173657 (30 mg/kg) treatment on the (A) splenic (B) axillary lymph node and (C) inguinal lymph node weight of WT mice submitted to the IMQ model. Organ weight results bare expressed in milligrams per kilogram body weight. (A) The results are presented as the mean ± SEM (Naive: n=11, Control: n=8, Vehicle: n= 6, SSR240612C: n=6 and FR173657: n= 6). Symbols represent values for individual mouse and bars represent mean values for each group. (B) Data are means ± SEM (Naive: n=12, Control: n=12, Vehicle: n= 9, SSR240612C: n=8 and FR173657: n= 10). Symbols represent the average weight of axillary lymph node for each animal and bars represent mean values for each group. (C) Data are means ± SEM (Naive: n=6, Control: n=11, Vehicle: n= 10, SSR240612C: n=7 and FR173657: n= 8). Symbols represent the average weight of inguinal lymph node for each animal and bars represent mean values for each group. The data are the result of the combination in a single data set of two independent experiments. No outliers were removed from the database. The unequal group sizes of the groups were attributed to different experimental approaches. *p<0.05 according to one‐way ANOVA with the Newmann‐Keuls post‐hoc test. #p<0.05 between Naive and Control group (WT IMQ treated mice).


