Abstract
We estimated 10-year (2020–2030) trajectories for human immunodeficiency virus incidence in 6 US cities. Estimated incidence will only decrease in 2 of 6 cities, with the overall population-weighted incidence decreasing 3.1% (95% credible interval [CrI], ˗1.0% to 8.5%) by 2025, and 4.3% (95% CrI, ˗2.6% to 12.7%) by 2030 across cities. Targeted, context-specific combination implementation strategies will be necessary to meet the newly established national targets.
Keywords: HIV/AIDS, dynamic transmission model, epidemiological projection, “Ending the HIV epidemic” plan
(See the Editorial Commentary by Fojo and Dowdy on pages 2199–200 and Viewpoints by Eisinger et al on pages 2212–7.)
Concerted efforts and significant investments in human immunodeficiency virus (HIV) prevention and care have resulted in a 69% decline in mortality and a 48% reduction in new diagnoses since incidence peaked in the United States (US) in the mid-1990s [1, 2]. However, despite more than $20 billion of annual federal funding directed toward domestic HIV efforts, 38 000 new cases are diagnosed each year. HIV disproportionately affects African Americans (43.6% of new diagnoses in 2017), men who have sex with men (MSM) (69.9%), and those residing in the southern states (52.2%) [1]. Rather than a homogeneous national epidemic, the HIV epidemic in the US is a collection of diverse microepidemics, characterized by relatively small geographic regions with different epidemiological conditions, demographics, and healthcare infrastructure, concentrated primarily in urban centers [3] and “hotspot” counties [4, 5]. The public health response across cities has been highly heterogeneous, with fundamental differences in health systems infrastructure, funding, and local legislation, as well as racism, structural stigma, and HIV laws/policies, culminating in widely disparate rates of new HIV diagnoses [3], which are at risk of further divergence with anticipated demographic shifts [6].
On 5 February 2019 at the State of the Union Address, President Trump announced the intention to end the HIV epidemic in the US by reducing new infections by 75% within 5 years and by 90% within 10 years. To reach these goals, the Department of Health and Human Services is proposing to target 48 counties plus Washington, District of Columbia and San Juan, Puerto Rico, which together comprise 50% of new HIV diagnoses, along with 7 southern states with disproportionate HIV incidence in rural areas [7]. Using simulation modeling to account for epidemiological and structural diversity in 6 US cities accounting for 24% of HIV prevalence in the US, we project HIV incidence over 10 years (2020–2030) holding access to HIV treatment, care, and prevention services constant to assess the extent to which these targets can be reached at current resource and implementation levels.
METHODS
We adapted and calibrated a dynamic, compartmental HIV transmission model [8–10] to replicate the city-level HIV microepidemics in Atlanta, Georgia; Baltimore, Maryland; Los Angeles, California; Miami (Dade County), Florida; New York City (NYC); and Seattle, Washington (King County). The model tracked individuals susceptible to HIV through the course of infection, diagnosis, treatment with antiretroviral therapy (ART), and ART dropout. In each city, the adult population aged 15–64 years was partitioned by biological sex (male, female), HIV risk group (MSM, people who inject drugs [PWID], MSM-PWID, and heterosexual), race/ethnicity (black/African American, Hispanic/Latinx, and non-Hispanic white/others) and sexual risk behavior level (high-risk vs low-risk). The model captured heterogeneity in the risk of HIV transmission, age (via differential maturation and mortality rates for people living with HIV and the general population across cities), and disparities in access to health and prevention services, including HIV testing, ART, syringe service programs, medication for opioid use disorder, and targeted pre-exposure prophylaxis for high-risk MSM. We conducted an extensive evidence synthesis to compile the 1667 parameters needed to populate the model for each city, drawn from 11 primary database analyses, 59 peer-reviewed publications, and 24 public health and surveillance reports [9]. We calibrated the model to match new diagnoses and deaths across race/ethnic and HIV risk groups (17 targets total) and validated against external incidence estimates from 2012 to 2015 [10]. Our evidence synthesis [9] and calibration process [10] are documented in detail elsewhere.
We projected HIV incidence in the adult population accounting for external estimates of population growth and demographic shifts in each city over a 10-year time horizon (2020–2030) [9], and estimated the population-weighted average HIV incidence across these cities. In the projections, all health services were held at their 2015 levels except for pre-exposure prophylaxis, which was held at 2017 levels to account for the recent rapid growth in uptake among MSM (population projections and estimated HIV service levels are detailed in the Supplementary Appendix). Projected trends in HIV incidence accounted for the uncertainty in the model’s parameter estimates. The projections (and their 95% credible intervals [CrIs]) were estimated using 2000 calibration parameter sets in addition to probabilistic sensitivity analysis on all other uncertain model parameters determining epidemic dynamics [10].
RESULTS
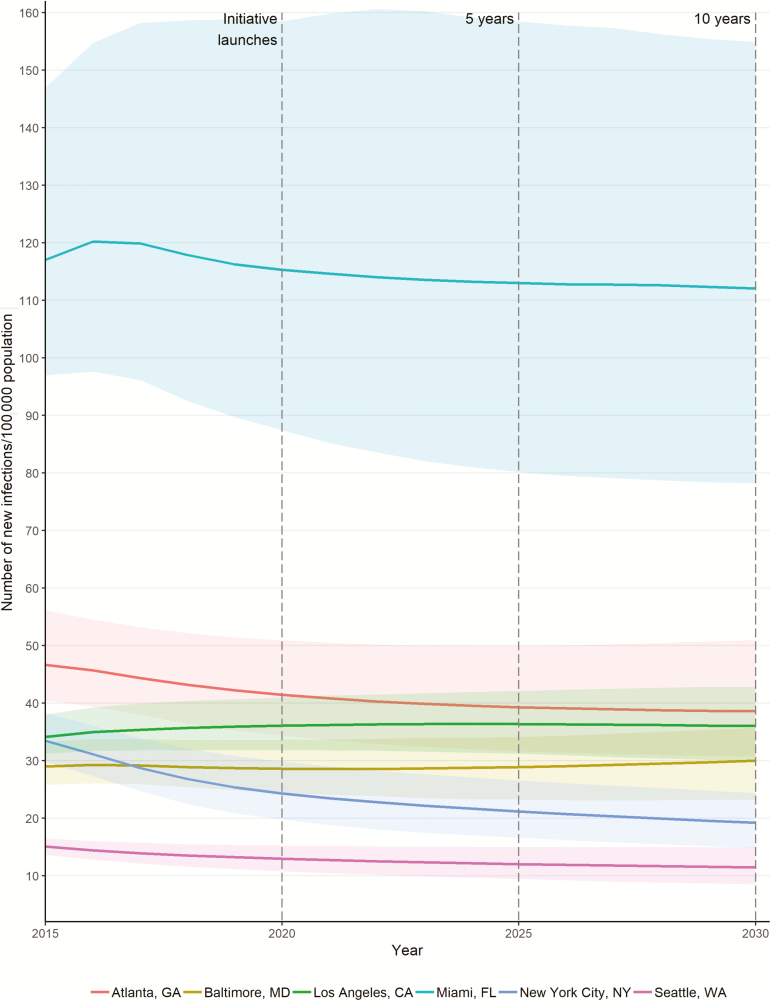
Holding service levels constant, we estimated that the HIV incidence rate (with 95% CrI) per 100 000 adults will decrease in NYC (from 24 [20–30] to 19 [15–24]) and Seattle (from 13 [11–15] to 11 [8–15]), and remain relatively constant in Atlanta at 40 (31–51), Baltimore at 30 (23–36), and Los Angeles at 36 (30–43). In contrast, Miami will remain the highest at 112 (78–155) per 100 000 (Figure 1).
Figure 1.
Model projections for human immunodeficiency virus (HIV) incidence (rate, per 100 000 adults) under current health service levels in 6 US cities, 2015–2030. We highlight 2020 (the year of initiative launch), 2025 (5-year target), and 2030 (10-year target), in accordance with the Trump Administration’s “Ending the HIV Epidemic: A Plan for America” initiative. Abbreviations: CA, California; FL, Florida; GA, Georgia; MD, Maryland; NY, New York; WA, Washington.
We estimated that the overall population-weighted HIV incidence, in comparison to the figures in 2020, will decrease 3.1% (95% CrI, ˗1.0% to 8.5%) by 2025 (ranging from a 0.6% increase in Los Angeles to a 12.2% decrease in NYC) and 4.3% (95% CrI, ˗2.6% to 12.7%) by 2030 (from a 5.2% increase in Atlanta to a 19.7% decrease in NYC) under current conditions in the selected US cities.
DISCUSSION
We estimate that current differences in HIV incidence across the selected cities will widen, leading to a 10-fold difference between Seattle and Miami by 2030 if current levels of service provision are maintained. The underlying factors driving these projections are as diverse as the cities themselves. The projected declines in HIV incidence for NYC are driven by strong political support and growing funding levels, as well as more generous public insurance and coordinated interventions, including substantial scale-up of ART. Within Seattle’s HIV epidemic, already low rates of incidence will be reinforced by the enhanced prevention efforts among younger MSM implemented in recent years.
In Los Angeles, we project a decline in HIV incidence among African American MSM that will be offset by an increase in HIV incidence among white and Hispanic MSM (Supplementary Figure 1). The projected decline among African American MSM is due in part to epidemic saturation; this group featured the highest prevalence of HIV of any population we modeled across cities. Miami is projected to see an increase in its number of Hispanic residents (3% growth, increasing the proportion from 68% to 71%, Supplementary Figure 2), which we estimate will drive HIV incidence, particularly among Hispanic MSM, which has been the case in recent years [11]. A similar shift is also estimated for Atlanta, the city with the highest projected population growth, despite African American MSM continuing to represent a large portion of incident cases. Finally, we project that Baltimore’s HIV epidemic will continue its shift from older PWID to younger African American and Hispanic MSM, resulting in limited reductions in rates of incidence by 2030.
As with any simulation modeling exercise, this study was limited by the simplifying assumptions regarding the course and characteristics of each city-level HIV microepidemic, as well as limitations in available data, including basic information on total volumes of HIV tests. In addition to the calibration and validation of our model [10], we conducted probabilistic sensitivity analysis to present uncertainty in each city’s HIV incidence projections.
As US cities grapple with competing priorities while facing stagnant or diminishing budgets, the initiative to end the HIV epidemic in the United States must provide policymakers, those at risk, and community stakeholders adequate resources to reach its stated goals [12]. Our results coincided with another recent modeling study, which found that current interventions were unlikely to reach the new targets by 2030 without a dramatic increase in scale and coverage in HIV care and prevention [13]. Despite the staggering advances in HIV/AIDS care, progress in reducing new infections in the US has plateaued and the benefits from these advances have not been shared equally among all those at risk or across microepidemics. The tools to drastically reduce the burden of HIV/AIDS are at hand, but their scale-up and implementation have been suboptimal [14–16]. This city-level analysis makes clear the need for a coordinated response consisting of targeted, context-specific combination implementation strategies to reduce the burden of HIV transmission and meet the bold goals of the current Administration’s initiative.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Localized HIV Modeling Study Group. Czarina N. Behrends, PhD, and Bruce R. Schackman, PhD, Department of Healthcare Policy and Research, Weill Cornell Medical College; Carlos Del Rio, MD, Hubert Department of Global Health, Emory Center for AIDS Research, Rollins School of Public Health, Emory University; Julia Dombrowski, MD, and Matthew Golden, MD, both primary with Department of Medicine, Division of Allergy and Infectious Diseases, adjunct in Epidemiology, University of Washington; Daniel J. Feaster, PhD, Department of Public Health Sciences, Leonard M. Miller School of Medicine, University of Miami; Kelly Gebo, MD, Gregory Kirk, PhD, and Shruti H. Mehta, PhD, Bloomberg School of Public Health, Johns Hopkins University; Reuben Granich, MD, Independent Public Health Consultant; Brandon D. L. Marshall, PhD, Department of Epidemiology, Brown School of Public Health, Rhode Island; Lisa Metsch, PhD, Department of Sociomedical Sciences, Mailman School of Public Health, Columbia University; Julio Montaner, MD, BC Centre for Excellence in HIV/AIDS; Faculty of Medicine, University of British Columbia; Bohdan Nosyk, PhD, BC Centre for Excellence in HIV/AIDS, Faculty of Health Sciences, Simon Fraser University; Steven Shoptaw, PhD, Centre for HIV Identification, Prevention and Treatment Services, School of Medicine, University of California, Los Angeles; Steffanie A. Strathdee, PhD, School of Medicine, University of California, San Diego.
Acknowledgments. The authors thank Benjamin Enns for his assistance in preparing data for model analysis. The authors also acknowledge support from their scientific advisory committee for providing inputs and expertise in goodness-of-fit weight determination and face validation.
Disclaimer. The funder had no direct role in the conduct of the analysis or the decision to submit the manuscript for publication.
Financial support. This work was supported by the National Institute on Drug Abuse, National Institutes of Health (grant number R01-DA-041747). This research was enabled in part by support provided by WestGrid (www.westgrid.ca) and Compute Canada (www.computecanada.ca).
Potential conflicts of interest. B. D. L. M. reports consulting fees from Simon Fraser University, West Virginia University, Yale University, and New York University. J. D. reports grants to the University of Washington from Hologic. S. H. M. reports consulting fees from Gilead Sciences. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. HIV surveillance report, 2017. Vol. 29 Atlanta, GA: CDC, 2018. [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC). HIV surveillance—United States, 1981–2008. MMWR Morb Mortal Wkly Rep 2011; 60:689. [PubMed] [Google Scholar]
- 3. Panagiotoglou D, Olding M, Enns B, et al. Localized HIV Modeling Study Group Building the case for localized approaches to HIV: structural conditions and health system capacity to address the HIV/AIDS epidemic in six US cities. AIDS Behav 2018; 22:3071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC). HIV in the southern United States. Atlanta, GA: CDC, 2016. [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC). Enhanced comprehensive HIV prevention planning and implementation for metropolitan statistical areas most affected by HIV/AIDS Available at: https://www.cdc.gov/hiv/research/demonstration/echpp/index.html. Accessed 5 July 2019.
- 6. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV surveillance supplemental report Atlanta, GA: CDC, 2019; 24. [Google Scholar]
- 7. Department of Health and Human Services. Ending the HIV epidemic: a plan for America Available at: https://www.hhs.gov/blog/2019/02/05/ending-the-hiv-epidemic-a-plan-for-america.html. Accessed 4 April 2019.
- 8. Nosyk B, Min JE, Lima VD, Hogg RS, Montaner JS. Cost-effectiveness of population-level expansion of highly active antiretroviral treatment for HIV in British Columbia, Canada: a modelling study. Lancet HIV 2015; 2:00127–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krebs E, Enns B, Wang L, et al. Localized HIV Modeling Study Group Developing a dynamic HIV transmission model for 6 U.S. cities: an evidence synthesis. PLoS One 2019; 14:e0217559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zang X, Krebs E, Min J, et al. Development and calibration of a dynamic HIV transmission model for 6 US cities. Med Decis Making 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forrest DW, Cardenas G, Dodson CS, Metsch LR, LaLota M, Spencer E. Trends in the HIV epidemic among men who have sex with men in Miami-Dade County, Florida, 2004–2014. Fla Public Health Rev 2018; 15:5. [Google Scholar]
- 12. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 13. Bradley H, Rosenberg ES, Holtgrave DR. Data-driven goals for curbing the US HIV epidemic by 2030. AIDS Behav 2019; 23:557–63. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Compendium of evidence-based interventions and best practices for HIV prevention Available at: https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.html. Accessed 4 April 2019.
- 15. Centers for Disease Control and Prevention. Syringe services programs Available at: https://www.cdc.gov/hiv/risk/ssps.html. Accessed 4 April 2019.
- 16. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. Atlanta, GA: CDC, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.