Abstract
目的
评价高三尖杉酯碱、阿糖胞苷、柔红霉素或去甲氧柔红霉素(HAD/HAI)方案诱导治疗初治急性髓系白血病(AML)患者的长期疗效并探讨其影响因素。
方法
观察HAD/HAI方案治疗1个疗程后初治143例AML患者的完全缓解(CR)、总生存(OS)及无复发生存(RFS)率,分析WHO 2008标准诊断分型、遗传学预后分组及初始WBC等因素对患者OS、RFS率的影响,评估中剂量阿糖胞苷(MD-Ara-C)巩固治疗在提高AML患者长期生存中的作用。
结果
143例患者中112例(78.3%)1个疗程获CR。仅1例患者在诱导化疗期早期死亡。高白细胞与非高白细胞组、FLT3-ITD突变型与野生型组患者CR率差异均无统计学意义(P值分别为0.266和0.528)。遗传学预后良好、中等、不良组患者CR率分别为93.7%、71.4%和61.3%,组间差异有统计学意义(P=0.001)。中位随访24(1~104)个月,中位生存时间为30(95%CI 12~48)个月。所有患者的5年OS率为40.0%,5年RFS率为37.0%。1个疗程CR后接受巩固治疗的患者为96例,5年OS率为47.0%,5年RFS率为38.0%;其中序贯含MD-Ara-C方案(71例)和序贯标准剂量Ara-C(25例)巩固治疗组患者的5年OS率差异有统计学意义(58.0%对19.0%,P=0.004)。在序贯含MD-Ara-C方案巩固治疗的患者中,高白细胞与非高白细胞组、FLT3-ITD突变型和野生型组患者的5年OS率差异均无统计学意义(P值分别为0.419和0.482),遗传学预后良好、中等、不良组患者5年OS率差异无统计学意义(P=0.332)。
结论
HAD/HAI方案诱导化疗缓解后序贯含MD-Ara-C方案巩固治疗的策略对初治AML患者,特别是高白细胞患者,可望获得满意的长期生存率。
Keywords: 白血病,髓样,急性, 三尖杉酯碱, 抗肿瘤联合化疗方案, 治疗结果
Abstract
Objective
To estimate the long-term outcomes and the prognostic factors of homoharringtonine, cytarabine, daunorubicin or idarubicin (HAD/HAI) as induction chemotherapy in de novo acute myeloid leukemia (AML).
Methods
The CR rate, overall survival (OS) rate, relapse free survival (RFS) rate were retrospectively assayed in 143 de novo AML patients who received the HAD/HAI induction chemotherapy. The outcomes were compared among prognostic groups according to world health organization (WHO) classification, genetic prognosis and initial white blood cell (WBC) count. The role of consolidation chemotherapy consisting of middle-dosage Ara-C (MD-Ara-C) on long term survival was evaluated.
Results
Of 143 patients, 112 (78.3%) achieved CR after the first course of HAD/HAI induction treatment, and early death occurred in only one case. Notably, the CR rate of patients with an initial WBC count ≥100×109/L was not significantly different from those with an initial WBC count<100× 109/L (70.4% vs 80.2%, P=0.266). The CR rate for the patients with favorable, intermediate and unfavorable integrated genetics risk factors was 93.7%, 71.4% and 61.3%, respectively, the difference between groups was statistically significant (P=0.001). Patients with FLT3-ITD mutation obtained similar CR rate (70.6%) to that of patients with FLT3 wild type (79.3%, P=0.528).The estimated 5-year OS rate and 5-year RFS rate for all patients was 40.0% and 37.0%, respectively, with a median follow-up of 24 (range 1–104) months. The median survival time was 30 [95%CI (12, 48)] months. 5-year OS and 5-year RFS of the 96 patients who achieved CR after first course chemotherapy without undergoing allo-HSCT in complete remission was 47.0% and 38.0%, respectively. 5-year OS was significantly higher in MD-Ara-C consolidation group than in no MD-Ara-C consolidation group among CR patients without allo-HSCT (58.0%, 19.0%, respectively, P=0.004). In patients who obtained CR after first course and received MD-Ara-C consolidation without allo-HSCT, the 5-year OS of patients with hyperleukocytosis was not significantly lower than that of patients without hyperleukocytosis (55.5%, 58.8%, respectively,P=0.419). FLT3-ITD mutation patients showed similar 5-year OS to that of wild type FLT3 patients (51.4%, 60.2%, respectively, P=0.482). And furthermore, 5-year OS of favorable, intermediate and unfavorable integrated genetics groups were 59.1%, 62.5%, 51.9%, respectively (P=0.332) in this subgroup.
Conclusion
HAD/HAI induction chemotherapy with sequential consolidation of MD-Ara-C could obtain satisfactory CR rate and long-term survival rate in de novo AML, especially for patients with hyperleukocytosis or FLT3-ITD mutation. It yet remains to be verified by large sample, prospective studies.
Keywords: Leukemia, myeloid, acute; Harringtonine; Antineoplastic combined chemotherapy protocols; Treatment outcome
以柔红霉素(DNR)和去甲氧柔红霉素(IDA)为代表的蒽环类药物联合阿糖胞苷(Ara-C)(DA或IA)组成的“3+7方案”是现今国际公认的急性髓系白血病(AML)标准诱导化疗方案[1]–[2]。高三尖杉酯碱(HHT)联合DA或AA(阿克拉霉素+ Ara-C)组成HAD和HAA方案已被推荐为AML的一线诱导化疗方案[3]。我们对接受HAD (HHT+Ara-C+DNR)/HAI (HHT+Ara-C+IDA)方案诱导治疗的AML患者资料进行分析,旨在评价HAD/HAI方案对初治AML患者的长期疗效并探讨其影响因素。
病例与方法
1.病例:以2006年5月至2013年1月间在我院接受HAD或HAI方案诱导化疗的143例初治非早幼粒细胞AML患者为研究对象。所有病例根据MICM(细胞形态学、免疫表型分析、细胞遗传学和分子生物学)及WHO 2008标准[4]进行确诊和分型。
2.细胞遗传学和分子生物学检查:采集患者骨髓,用R显带方法进行染色体核型分析,用实时定量PCR或PCR联合测序检测PML-RARA、RUNX1-RUNX1T1和CBFB-MYH11融合基因以及FLT3-ITD、CEBPA和NPM1突变。遗传学预后分组标准[5]–[6]:①预后良好组:包括t(8;21)、inv(16)或t(16; 16)(p13;q22),正常核型伴NPM1阳性且FLT3-ITD阴性,或正常核型伴CEBPA阳性;②预后不良组:−5或5q−,−7或7q−,累及3q、9q、11q、21q、17p异常核型,t(6;9), t(9;22),复杂核型(≥3非关联的核型异常),FLT3-ITD阳性的正常核型;③预后中等组:正常核型及其他异常核型。
3.治疗方案:对高白细胞(WBC≥100×109/L)患者,诱导化疗前采用羟基脲、小剂量Ara-C和(或)白细胞单采术降低白血病细胞负荷,在WBC达50× 109/L左右时进行诱导化疗。HAD/HAI方案为:HHT 2.5 mg·m−2·d−1,静脉滴注,第1~7天;Ara-C 150 mg·m−2 ·d−1,静脉滴注,第1~7天;DNR 45 mg·m−2·d−1,静脉滴注,第1~3天或IDA 9 mg·m−2·d−1,静脉滴注,第1~3天。骨髓抑制期HGB<80 g/L或出现明显贫血症状时输注悬浮红细胞,PLT<20×109/L或出现出血倾向时输注血小板。中性粒细胞绝对计数(ANC)<0.5×109/L时常规应用氟康唑(200 mg/d)预防真菌感染。
缓解后治疗方案:依据患者意愿及患者一般状况、有无严重并发症、预后分组等情况,进行标准剂量Ara-C(100~200 mg·m−2·d−1,静脉滴注,第1~7天)或中剂量(MD)Ara-C(1.0 g/m2,每12 h1次,第1~4天)为主的联合诱导缓解后化疗3~6个疗程,具备HLA全相合干细胞来源患者建议行异基因造血干细胞移植(allo-HSCT)。对未缓解患者进行再诱导化疗或姑息治疗。
4.主要观察指标:1个疗程后的完全缓解(CR)、总生存(OS)及无复发生存(RFS)率,CR判断参照2001年AML国际工作组修订标准[7]。所有患者均按照WHO抗癌药物不良反应评估标准[8]进行安全性评价。观察CR患者ANC减少持续时间(化疗开始至ANC<0.5×109/L最后1天),抑制期ANC最低值及达最低值的时间。诱导化疗开始至化疗后30 d内发生的死亡事件为早期死亡。OS时间为确诊至患者死亡或末次随访时间(失访时间)。RFS期为从获CR起至患者复发、死亡或末次随访时间止。通过门诊、住院病历查阅及电话进行随访。随访截止日期为2015年4月1日。
5.统计学处理:应用SPSS18.0软件进行统计学分析。CR率采用χ2检验或Fisher's确切概率检验进行差异性检验。用Kaplan-Meier方法描述生存状态分布,采用Log-rank检验进行单因素预后生存分析,用Cox回归模型进行多因素生存分析。采用R软件竞争风险分析法计算5年累计复发率。所有的统计检验均采用双侧检验,P≤0.05为差异有统计学意义。
结果
1.患者基本状况:143例患者中,男89例,女54例,中位年龄39(14~58)岁。WHO 2008标准诊断分型:伴有重现性遗传学异常者34例,伴有多系发育异常者19例、不另作分类者90例。依照美国东部肿瘤协作组(ECOG)评分标准进行一般状况评分,2分者48例,3分者53例,4分者42例。诱导化疗前中位HGB 79(30~150)g/L,中位WBC 20.1 (0.6~409.0)×109/L,中位PLT 38(2~831)×109/L。高白细胞者27例。
染色体核型分布如下:正常核型者78例,复杂核型者6例,无分裂象者8例,存在t(8;21)者28例(其中合并−Y 17例),−7、−18各2例,del (7) (q32)、−7/2q+、del(9)(q11;q22)、i(8)(q10)、14q+/(i21q)、13q−、+ 8/del (9)(q11;q22)、del(9)(q11; q22)/+ 11、−Y、t(10;11)(p13;q23)+ mar、del(1) (p32)、1q−、t(5;11)(q33;q13)、del (19)(p12)、der(1;7)(q10;p10)、der(10)、+ 22、+ 8、+ 19各1例。FLT3-ITD突变率为13.3%(17/128),CEBPA突变率为18.1% (23/127),NPM1突变率为19.1% (22/115)。遗传学预后良好组63例、预后中等组42例、预后不良组31例、预后不明组7例。
2.诱导化疗疗效及影响疗效因素分析:80例患者接受HAD方案,63例患者接受HAI方案。143例患者中112例(78.3%)1个疗程获得CR。HAI、HAD组患者1个疗程CR率分别为79.4%、77.5% (P= 0.788)。31例未缓解患者后续治疗:1例在诱导化疗期早期死亡,11例放弃治疗,19例接受再诱导化疗(其中11例获CR)。2个疗程CR率为86.0%(123/143)。
预后相关因素对患者1个疗程CR率的影响:治疗前WBC≥100 × 109/L (P=0.266)、FLT3-ITD突变(P=0.528)、NPM1突变(P=0.396)对患者1个疗程CR率的影响差异无统计学意义;CEBPA (TAD/bZIP)突变患者CR率优于野生型者(P=0.021);遗传学预后分组间差异有统计学意义(P=0.001) (表1)。
表1. 预后相关因素对急性髓系白血病(AML)患者1个疗程完全缓解(CR)率的影响.
| 预后因素 | CR率[%,(CR例数/入组例数)] | P值 |
| WHO诊断分型 | 0.000 | |
| 伴有重现性遗传学异常AML | 94.1(32/34) | |
| 伴有多系发育异常AML | 36.8(7/19) | |
| 不另作分类AML | 81.1(73/90) | |
| 初诊WBC | 0.266 | |
| ≥100×109/L | 70.4(19/27) | |
| <100×109/L | 80.2(93/116) | |
| FLT3-ITD | 0.528 | |
| 突变型 | 70.6(12/17) | |
| 野生型 | 79.3(88/111) | |
| CEBPA(TAD/bZIP) | 0.021 | |
| 双突变型 | 100.0(16/16) | |
| 野生型 | 74.0(77/104) | |
| NPM1 | 0.396 | |
| 突变型 | 86.4(19/22) | |
| 野生型 | 75.3(70/93) | |
| 遗传学预后分组 | 0.001 | |
| 预后良好 | 93.7(59/63) | |
| 预后中等 | 71.4(30/42)a | |
| 预后不良 | 61.3(19/31)b | |
| 预后不明 | 57.1(4/7) |
注:与预后良好组比较,aP=0.002,bP=0.000;预后中等与预后不良组比较,P=0.362
3.诱导化疗期间不良反应:ANC<0.5×109/L持续中位时间为18(8~37)d,ANC<0.2×109/L持续中位时间为15 (2~35) d。中位浓缩红细胞输注量为11.75 (0~32)U,中位浓缩血小板输注量为60(5~220)U。患者常见感染为肺炎、齿龈炎、肛周感染、腹泻,中位发热持续时间为8(0~36) d(表2)。
表2. 143例急性髓系白血病患者常见的非血液学不良反应.
| 非血液学不良反应 | Ⅰ~Ⅱ级(%) | Ⅲ~Ⅳ级(%) |
| 恶心 | 48.6 | 4.2 |
| 呕吐 | 24.6 | 2.1 |
| 腹泻 | 16.3 | 2.8 |
| 便秘 | 7.0 | 1.4 |
| 口腔黏膜炎 | 17.6 | 1.4 |
| 丙氨酸转氨酶升高 | 15.5 | 1.4 |
| 发热 | 31.9 | 2.7 |
| 胆红素升高 | 6.7 | 0.7 |
| 周围神经炎 | 0 | 3.3 |
| 心脏毒性反应 | 1.4 | 0 |
| 败血症 | 19.9 | |
| 侵袭性真菌病 | 40.4 |
4.长期疗效:123例CR患者中,7例放弃后续治疗,20例行allo-HSCT, 71例接受含MD-Ara-C方案巩固治疗,25例患者接受标准剂量Ara-C巩固治疗。123例患者中失访11例(8.9%)。中位随访时间为24(1~104)个月。143例患者的中位OS时间为30(95%CI 12~48)个月(图1),3年OS率为45.0%,5年OS率为40.0%;3年RFS率为39.0%,5年RFS率为37.0%;5年累计复发率为56.9%。HAI、HAD组患者5年OS率分别为41.0%、40.0%(P=0.601)。112例1个疗程CR患者的3年RFS率为43.0%,5年RFS率为41.0%(图2)。20例allo-HSCT患者5年OS率为60.0%。
图1. 143例初治急性髓系白血病患者总生存曲线.
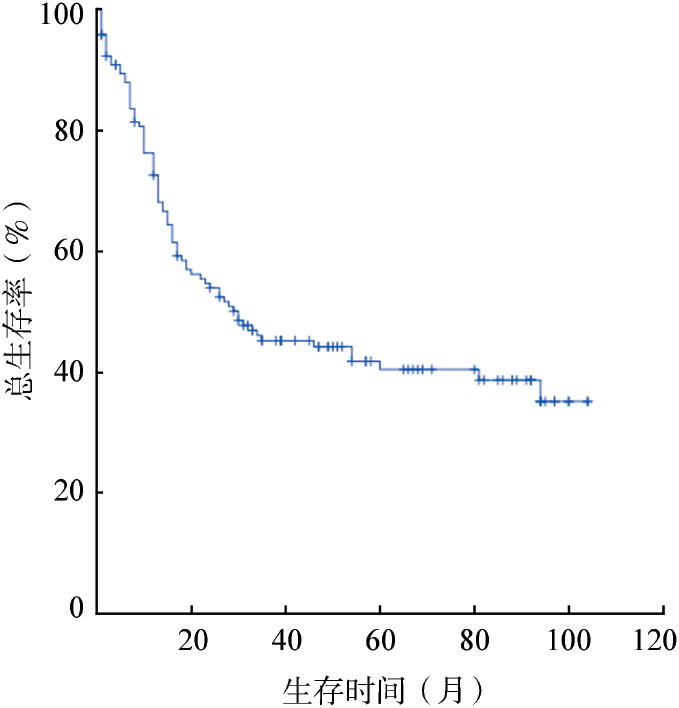
图2. 112例1个疗程获完全缓解的初治急性髓系白血病患者无复发生存曲线.

96例1个疗程CR后接受巩固治疗患者的中位OS时间为54 (95%CI 9~99)个月。3年OS率为55.0%,5年OS率为47.0%;3年RFS率为41.0%,5年RFS率为38.0%。接受含MD-Ara-C方案巩固治疗组患者的5年OS率为58.0%,明显高于接受标准剂量Ara-C巩固治疗组的19.0%(P=0.004)(图3)。影响患者长期生存的单因素分析结果见表3,结果显示WHO 2008标准诊断分型、初诊WBC、FLT3-ITD突变、CEBPA (TAD/bZIP)突变、NPM1突变及遗传学预后分组对患者的5年OS、RFS率的影响均无统计学意义(P值均>0.05),故未再将结果纳入Cox模型进行多因素分析。
图3. 不同剂量阿糖胞苷巩固治疗对1个疗程获完全缓解非移植患者总生存的影响.
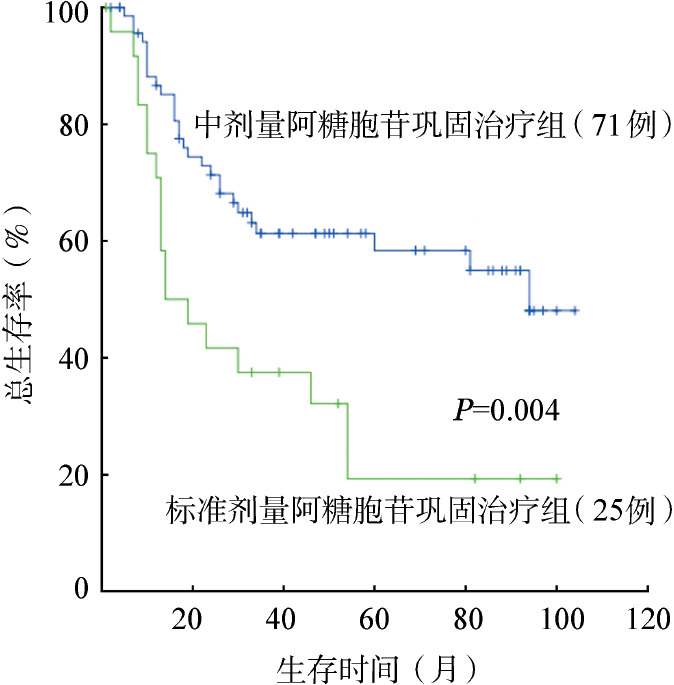
表3. 影响1个疗程完全缓解后接受含中剂量阿糖胞苷方案巩固化疗患者预后的单因素分析.
| 影响因素 | 例数 | 5年总生存 |
5年无复发生存 |
||
| 率(%) | P值 | 率(%) | P值 | ||
| WHO诊断分型 | 0.509 | 0.172 | |||
| 伴有重现性遗传学异常AML | 24 | 64.7 | 57.7 | ||
| 伴有多系发育异常AML | 7 | 50.0 | 42.9 | ||
| 不另作分类AML | 40 | 54.5 | 40.9 | ||
| 初诊WBC | 0.419 | 0.889 | |||
| ≥100×109/L | 9 | 55.5 | 55.6 | ||
| <100×109/L | 62 | 58.8 | 45.8 | ||
| FLT3-ITD | 0.482 | 0.552 | |||
| 突变型 | 7 | 51.4 | 42.9 | ||
| 野生型 | 58 | 60.2 | 48.7 | ||
| CEBPA(TAD/bZIP) | 0.702 | 0.931 | |||
| 双突变型 | 7 | 51.4 | 42.9 | ||
| 野生型 | 56 | 58.2 | 48.8 | ||
| NPM1 | 0.578 | 0.339 | |||
| 突变型 | 14 | 73.3 | 41.7 | ||
| 野生型 | 44 | 55.1 | 51.3 | ||
| 遗传学预后分组 | 0.332 | 0.619 | |||
| 预后良好 | 41 | 59.1 | 47.2 | ||
| 预后中等 | 17 | 62.5 | 52.9 | ||
| 预后不良 | 11 | 51.9 | 45.5 | ||
注:AML:急性髓系白血病
讨论
在经典的“3+7方案”基础上,采用高剂量DNR(90 mg·m−2·d−1)、中/大剂量Ara-C或三药联合等策略,尽管可提高AML患者1个疗程的CR率,但其早期病死率也偏高[9]–[12]。
HHT是从具有抗肿瘤作用的传统中药粗榧属植物中分离出的一种生物碱。我院从20世纪80年代开始应用HHT治疗AML,证实HHT与Ara-C联合(HA)方案治疗AML疗效与DA (DNR+Ara-C)方案相似,CR率分别为54%、64%,提出HHT可作为一线药物替代蒽环类药物。此外,约半数HA或DA方案诱导治疗无效的患者在互换方案后仍可获得CR,表明HHT与蒽环类药物不存在交叉耐药,HHT和DNR交替使用可提高AML患者的CR率[13]。在此基础上,HAD方案治疗初治AML患者,CR率达86.1%,疗效比HA方案有进一步提高[14]。我们的结果显示HAD/HAI方案治疗后患者的1、2个疗程CR率分别为78.3%和86.0%,与文献[15]的HAA(HHT、Ara-C、阿克拉霉素)方案疗效(78.0%)相似。
诱导化疗前高白细胞计数(WBC≥100×109/L)是AML的一个重要预后不良因素[16]–[17]。本研究中一个非常有意思的发现是采用HAD/HAI方案进行诱导化疗,高白细胞与非高白细胞患者CR率相当(分别为70.4%与80.2%,P=0.266),这可能与HHT对G1和G2期白血病细胞有较强的杀伤作用,以及本组患者的诱导治疗早期病死率较低有关。
遗传学是影响AML患者疗效的重要因素[18]–[21]。本组患者遗传学预后良好者1个疗程CR率(93.7%)明显高于预后中等(71.4%,P=0.002)及预后不良者(61.3%,P=0.000),但预后不良与预后中等组患者间CR率差异却无统计学意义(P=0.362)。FLT3-ITD突变是染色体核型正常AML患者独立的预后不良因素[22]–[23],我们的结果表明FLT3-ITD突变型患者CR率与FLT3-ITD野生型患者CR率相当(P= 0.528)。上述初步结果提示HAD/HAI方案有可能改善遗传学预后不良患者的CR率。
采用含中、大剂量Ara-C的方案进行诱导缓解后治疗对AML患者长期生存非常重要[24]。本研究中96例CR患者仅接受诱导缓解后巩固治疗,接受含MD-Ara-C方案巩固治疗组患者的5年OS率明显高于接受标准剂量MD-Ara-C治疗组(58.0%对19.0%,P=0.004)。值得注意的是,接受含MD-Ara-C方案巩固治疗的患者中,高白细胞与非高白细胞组(P=0.419)、FLT3-ITD突变型和野生型组(P= 0.482)、遗传学预后良好、中等、不良组(P=0.332)患者的5年OS率差异均无统计学意义。
总之,我们的研究结果再次证实HAD/HAI方案诱导化疗可使AML患者迅速缓解且降低早期病死率,CR后序贯含MD-Ara-C方案进行诱导缓解治疗,可使AML患者,特别是高白细胞患者获得较好的长期生存率。但由于本研究样本量较小,且为回顾性研究,这些发现尚待大样本、前瞻性研究加以验证。
Funding Statement
基金项目:卫生公益性行业科研专项(201202017)
Fund program: Health Public Welfare Industry Scientific Research Special(201202017)
References
- 1.Mandelli F, Vignetti M, Suciu S, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10[J] J Clin Oncol. 2009;27(32):5397–5403. doi: 10.1200/JCO.2008.20.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Joo YD, Kim H, et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia[J] Blood. 2011;118(14):3832–3841. doi: 10.1182/blood-2011-06-361410. [DOI] [PubMed] [Google Scholar]
- 3.成人急性髓系白血病(非急性早幼粒细胞白血病)中国诊疗指南(2011年版)[J] 中华血液学杂志. 2011;32(11):804–807. doi: 10.3760/cma.j.issn.0253-2727.2011.11.021. [DOI] [Google Scholar]
- 4.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues[M] 4th, ed. Lyon, France: IARC Press; 2008. pp. 109–139. [Google Scholar]
- 5.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study[J] Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 6.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet[J] Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia[J] J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment[J] Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Weick JK, Kopecky KJ, Appelbaum FR, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study[J] Blood. 1996;88(8):2841–2851. [PubMed] [Google Scholar]
- 10.Bishop JF, Matthews JP, Young GA, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia[J] Blood. 1996;87(5):1710–1717. [PubMed] [Google Scholar]
- 11.Willemze R, Suciu S, Meloni G, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial[J] J Clin Oncol. 2014;32(3):219–228. doi: 10.1200/JCO.2013.51.8571. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia[J] N Engl J Med. 2009;361(13):1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.卞 寿庚, 郝 玉书. 成人急性非淋巴细胞白血病化疗的研究[J] 中华血液学杂志. 1993;14(2):59–62. [Google Scholar]
- 14.Xiao Z, Xue H, Li R, et al. The prognostic significance of leukemic cells clearance kinetics evaluation during the initial course of induction therapy with HAD (homoharringtonine, cytosine arabinoside, daunorubicin) in patients with de novo acute myeloid leukemia[J] Am J Hematol. 2008;83(3):203–205. doi: 10.1002/ajh.21068. [DOI] [PubMed] [Google Scholar]
- 15.叶 佩佩, 牧 启田, 陈 菲菲, et al. HAA方案诱导治疗成人初发急性髓系白血病236例疗效观察[J] 中华血液学杂志. 2013;34(10):825–829. doi: 10.3760/cma.j.issn.0253-2727.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Dutcher JP, Schiffer CA, Wiernik PH. Hyperleukocytosis in adult acute nonlymphocytic leukemia: impact on remission rate and duration, and survival[J] J Clin Oncol. 1987;5(9):1364–1372. doi: 10.1200/JCO.1987.5.9.1364. [DOI] [PubMed] [Google Scholar]
- 17.Marbello L, Ricci F, Nosari AM, et al. Outcome of hyperleukocytic adult acute myeloid leukaemia: a single-center retrospective study and review of literature[J] Leuk Res. 2008;32(8):1221–1227. doi: 10.1016/j.leukres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia[J] J Clin Oncol. 2012;30(36):4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaidzik V, Dohner K. Prognostic implications of gene mutations in acute myeloid leukemia with normal cytogenetics[J] Semin Oncol. 2008;35(4):346–355. doi: 10.1053/j.seminoncol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Smith ML, Hills RK, Grimwade D. Independent prognostic variables in acute myeloid leukaemia[J] Blood Rev. 2011;25(1):39–51. doi: 10.1016/j.blre.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Mrozek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia[J] Blood Rev. 2004;18(2):115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 22.Rombouts WJ, Blokland I, Lowenberg B, et al. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the Flt3 gene[J] Leukemia. 2000;14(4):675–683. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- 23.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials[J] Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 24.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B[J] N Engl J Med. 1994;331(14):896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]


