Abstract
Background
Since their emergence in the Americas, chikungunya (CHIKV) and Zika (ZIKV) viruses co-circulate with dengue virus (DENV), hampering clinical diagnosis. We investigated clinical and epidemiological characteristics of arboviral infections during the introduction and spread of CHIKV and ZIKV through northeastern Brazil.
Methods
Surveillance for arboviral diseases among febrile patients was performed at an emergency health unit of Salvador, Brazil, between September 2014 and July 2016. We interviewed patients to collect data on symptoms, reviewed medical records to obtain the presumptive diagnoses, and performed molecular and serological testing to confirm DENV, CHIKV, ZIKV, or nonspecific flavivirus (FLAV) diagnosis.
Results
Of 948 participants, 247 (26.1%) had an acute infection, of which 224 (23.6%) were single infections (DENV, 32 [3.4%]; CHIKV, 159 [16.7%]; ZIKV, 13 [1.4%]; and FLAV, 20 [2.1%]) and 23 (2.4%) coinfections (DENV/CHIKV, 13 [1.4%]; CHIKV/FLAV, 9 [0.9%]; and DENV/ZIKV, 1 [0.1%]). An additional 133 (14.0%) patients had serological evidence for a recent arboviral infection. Patients with ZIKV presented with rash and pruritus (69.2% each) more frequently than those with DENV (37.5% and 31.2%, respectively) and CHIKV (22.9% and 14.7%, respectively) (P < .001 for both comparisons). Conversely, arthralgia was more common in CHIKV (94.9%) and FLAV/CHIKV (100.0%) than in DENV (59.4%) and ZIKV (53.8%) (P < .001). A correct presumptive clinical diagnosis was made for 9%–23% of the confirmed patients.
Conclusions
Arboviral infections are frequent causes of febrile illness. Coinfections are not rare events during periods of intense, concomitant arboviral transmission. Given the challenge to clinically distinguish these infections, there is an urgent need for rapid, point-of-care, multiplex diagnostics.
Keywords: dengue virus, chikungunya virus, Zika virus, arbovirus, coinfection
Simultaneous transmission of dengue, chikungunya, and Zika viruses occurs in endemic regions, leading to frequent coinfections. Rash and pruritus are more common with Zika, while arthralgia is more common with chikungunya. Nevertheless, correct medical diagnosis is challenging without laboratory testing.
Dengue virus (DENV), chikungunya virus (CHIKV), and Zika virus (ZIKV) are widely distributed arboviruses, affecting tropical and subtropical areas [1]. In Brazil, the 4 DENV serotypes have co-circulated since 2010 [2], and in September 2014, the first autochthonous cases of chikungunya were reported in the country, almost simultaneously in the northern (Amapá state) and northeastern (Bahia state) regions [3, 4]. Less than 1 year later, early in 2015, autochthonous cases of Zika were first detected in the northeastern states of Bahia and Rio Grande do Norte [5, 6]. As CHIKV and ZIKV quickly spread, simultaneous co-circulation of DENV, CHIKV, and ZIKV was established in Brazil and other South and Central American countries [7].
Patients infected by DENV, CHIKV, or ZIKV may develop an acute febrile illness, with similar clinical characteristics [8]. Symptoms and signs commonly observed include fever, rash, muscle pain, arthralgia, and headache [8]. Due to the difficulty in diagnosing arboviral infections based on clinical impressions, particularly in areas with concomitant co-circulation, laboratory tests are needed for accurate diagnosis of these viral agents. Furthermore, only diagnostic methods can detect concomitant arboviral infections, which may commonly occur during concurrent epidemics and might have important implications for clinical outcomes. However, effective laboratory services are not readily available in most ambulatory and emergency units of tropical and subtropical countries [9]. Thus, few studies have systematically evaluated the frequency of arboviral infections and compared the clinical and epidemiological characteristics of single vs dual infections in settings of co-circulation.
Herein, we describe results from surveillance designed to monitor arboviral infections among acute febrile patients in Salvador, the capital of the Bahia state, northeastern Brazil, between 2014 and 2016, a period when CHIKV and ZIKV were introduced and spread throughout the country. We present clinical and epidemiological characteristics of laboratory-confirmed cases and, to determine whether human coinfections are more likely acquired through single bites of coinfected mosquitoes or multiple bites by mosquitoes carrying individual arboviruses, we also evaluate whether coinfections were more frequent than would be expected by chance based on the assumption of independent transmission.
METHODS
Study Design
From September 2014 to July 2016, we conducted enhanced surveillance to detect DENV, CHIKV, and ZIKV infections among acute febrile patients attending a public emergency health unit in Salvador, as described in the Supplementary Methods. The research ethics committees of the Gonçalo Moniz Institute (Oswaldo Cruz Foundation) and Yale University approved the study. Before enrollment, written informed consent was obtained from patients ≥18 years of age, or from guardians of patients <18 years of age, and written assent was obtained from patients 7–17 years of age.
Data and Blood Sample Collection
We interviewed the participants or their guardians using a standardized questionnaire that included demographic and clinical data. Medical records were reviewed to determine presumptive clinical diagnoses. Acute and convalescent blood samples were collected at study entry and 15 days later, respectively (Supplementary Methods). During the follow-up for convalescent blood collection, a second interview was performed to obtain data on resolution of signs and symptoms.
Arboviral Diagnosis
Acute sera, obtained as described in the Supplementary Methods, underwent RNA extraction (Maxwell Viral Total Nucleic Acid K), followed by reverse-transcription polymerase chain reaction (RT-PCR) for DENV [10], ZIKV [11], and CHIKV [12] with the AccessQuick RT-PCR system kit (Promega). In addition, we performed DENV and CHIKV immunoglobulin M (IgM) enzyme-linked immunosorbent assays (ELISAs) (Panbio Diagnostics, Brisbane, Australia and Inbios International, Seattle, Washington, respectively) on both acute and convalescent sera and tested the former with a DENV non-structural protein 1 (NS1) ELISA (Panbio Diagnostics). We did not employ a ZIKV serological assay due to the low accuracy of the available tests [13, 14].
We defined acute DENV, CHIKV, and ZIKV infections by a positive result in the DENV RT-PCR or NS1-ELISA; a positive result in the CHIKV RT-PCR, or seroconversion between acute and convalescent CHIKV IgM-ELISA; and a positive ZIKV RT-PCR, respectively (Supplementary Table). Due to potential cross-reactivity in the DENV IgM-ELISA following a ZIKV infection, we classified patients presenting DENV IgM-ELISA seroconversion between acute and convalescent samples and negative RT-PCR results for both DENV and ZIKV as acute flavivirus (FLAV) infections. Patients fulfilling the acute infection case definition for >1 arbovirus were classified as an arboviral coinfection. Finally, because a positive IgM-ELISA in the acute sample may represent a previous, recent but not acute infection in a context of intense arboviral transmission, we defined patients with a positive DENV or CHIKV IgM-ELISA in the acute sample (or in the convalescent sample when no acute sample was available) as cases of recent undetermined FLAV infection and of recent CHIKV infection, respectively.
Statistical Analyses
We calculated the overall frequency for acute and recent arboviral infections, specific frequencies of acute DENV, CHIKV, ZIKV and FLAV infections, and of coinfections, for the whole study period and monthly. Based on the detected frequencies for each virus and the assumption of independent transmission, we estimated the expected frequency of arboviral coinfections and compared them to the observed coinfection frequencies. Demographics, clinical manifestations, and presumptive clinical diagnoses were compared among patients according to their acute infection confirmation status. Medians and interquartile ranges (IQRs) or absolute and relative frequencies were used for comparisons. Two-tailed Wilcoxon–Mann-Whitney, Pearson χ2, or Fisher exact tests were used as applicable to assess statistical difference between the groups at a P < .05 significance level.
RESULTS
Laboratory Diagnosis of Arboviral Infection
We enrolled 948 acute febrile illness patients with at least 1 sample available for laboratory testing. Both acute and convalescent samples were collected from 428 (45.1%) of the patients, only acute samples from 510 (53.8%), and only convalescent samples from 10 (1.1%). Due to insufficient volumes of sera, RT-PCR for DENV and CHIKV was performed for 915 (96.5%) of the patients and RT-PCR for ZIKV was performed for 914 (96.4%). DENV serological tests were performed for 940 (99.2%) of the patients (45 tested only by IgM-ELISA, 1 tested only by NS1-ELISA, and 894 tested by both), and CHIKV IgM-ELISA was performed for 919 (96.9%).
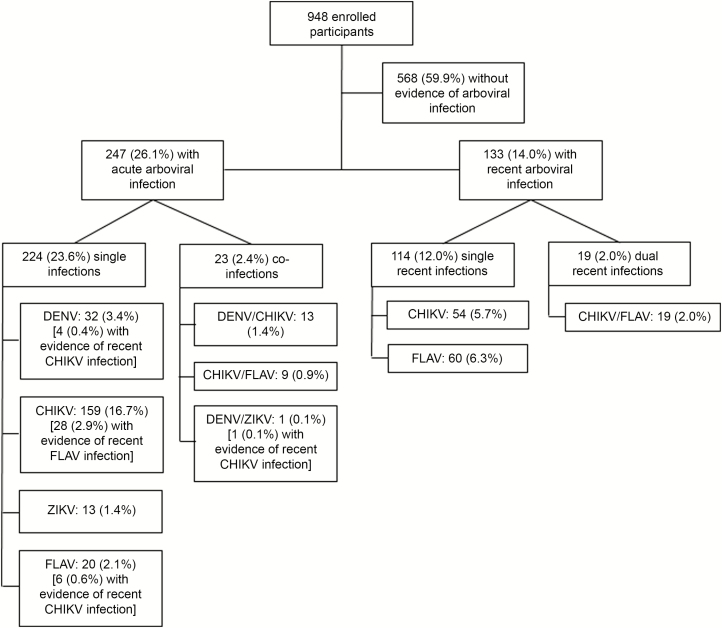
Of 948 participants, 247 (26.1%) had evidence of an acute arboviral infection, of which 224 (23.6%) were single infections and 23 (2.4%) coinfections (Figure 1). Specifically, 32 (3.4%) patients tested positive for DENV, 159 (16.7%) for CHIKV, 13 (1.4%) for ZIKV, 20 (2.1) for FLAV, 13 (1.4%) for DENV/CHIKV coinfection, 9 (0.9%) for CHIKV/FLAV coinfection, and 1 (0.1%) for DENV/ZIKV coinfection (Figure 1). Of the 45 patients with a positive DENV RT-PCR test, 5 (11.1%) were DENV-1, 17 (37.8%) were DENV-3, and 23 (51.1%) were DENV-4.
Figure 1.
Flowchart of 948 patients enrolled during an acute febrile illness surveillance study in an emergency health unit, according to the arboviral diagnosis—Salvador, Brazil, September 2014 to July 2016. Of the 247 cases of acute arboviral infection, 39 showed evidence of a recent arboviral infection. These are in addition to the other 133 recent arboviral infections shown in the figure. Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; FLAV, flavivirus; ZIKV, Zika virus.
Based on the observed frequency of DENV, CHIKV, ZIKV, and FLAV infections, the expected frequency of DENV/CHIKV coinfection, assuming that specific arboviral infections were independent events, was 0.9% (~9 cases), CHIKV/FLAV was 0.6% (~6 cases), DENV/ZIKV was 0.1% (~1 case), and CHIKV/ZIKV was 0.3% (~3 cases). The coinfection frequencies that we detected were not statistically different from expected (P > .05 for all comparisons).
Of the 247 acute arboviral infections, 39 (4.1%) had concomitant laboratory evidence for a recent infection, including 4 (0.4%) recent CHIKV among the 32 acute dengue cases, 6 (0.6%) recent CHIKV among the 20 acute FLAV cases, 28 (2.9%) recent FLAV among the 159 acute chikungunya cases, and 1 recent CHIKV in the sole acute DENV/ZIKV coinfection (Figure 1). In addition, 133 (14.0) other patients without an acute arboviral infection had laboratory evidence for a recent arboviral infection, including 54 (5.7%) with recent CHIKV infection, 60 (6.3%) with recent FLAV infection, and 19 (2.0) with dual, recent CHIKV/FLAV infections (Figure 1).
Clinical Manifestations
The median age of acute Zika patients (20 [IQR, 15–38] years) was lower than that of acute DENV (30 [IQR, 15–38] years), CHIKV (32 [IQR, 20–43] years), and FLAV patients (35 [IQR, 24–42] years), as well as of patients coinfected with DENV/CHIKV (34 [IQR, 19–34] years) and CHIKV/FLAV (47 [IQR, 37–51] years) (P < .001; Table 1). The median number of days between fever onset and study enrollment was higher for DENV (4 [IQR, 3–4] days) and lower for CHIKV (1 [IQR, 1–3] days), compared with ZIKV (2 [IQR, 2–3] days), FLAV (3 [IQR, 1.5–5] days), and DENV/CHIKV and CHIKV/FLAV (2 [IQR, 1–2] days for both) infections (P < .001; Table 1).
Table 1.
Clinical Characteristics of the Patients With Febrile Illness Enrolled in the Study According to Laboratory Diagnosis of Acute Arboviral Infection—Salvador, Bahia, Brazil, September 2014–July 2016a
| Infection | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | DENV (n = 32) |
CHIKV (n = 159) |
ZIKV (n = 13) |
FLAV (n = 20) |
DENV/CHIKV (n = 13) |
CHIKV/FLAV (n = 9) |
Negative (n = 568) |
| Demographics | |||||||
| Age,b median (IQR) | 30 (15–38) | 32 (20–43) | 20 (15–38) | 35 (24–42) | 34 (19–34) | 47 (37–51) | 26 (15–37) |
| Female sex | 15 (46.9) | 78 (49.1) | 7 (53.8) | 11 (55.0) | 10 (76.9) | 3 (33.3) | 266 (47.1) |
| Clinical manifestations | |||||||
| Days of fever,b median (IQR) | 4 (3–4) | 1 (1–3) | 2 (2–3) | 3 (1.5–5) | 2 (1–2) | 2 (1–2) | 2 (1–4) |
| Headache | 29 (93.5) | 148 (93.1) | 12 (92.3) | 20 (100.0) | 12 (92.3) | 8 (88.9) | 504 (89.2) |
| Myalgiab | 25 (80.6) | 150 (94.3) | 11 (84.6) | 17 (85.0) | 11 (84.6) | 9 (100.0) | 452 (80.4) |
| Retro-orbital pain | 20 (64.5) | 116 (73.4) | 9 (69.2) | 15 (75.0) | 7 (53.8) | 5 (55.6) | 348 (62.2) |
| Arthralgiab | 19 (59.4) | 151 (94.9) | 7 (53.8) | 15 (75.0) | 11 (84.6) | 9 (100.0) | 354 (62.3) |
| Swollen jointsb | 10 (31.2) | 63 (39.6) | 4 (30.7) | 8 (40.0) | 7 (53.8) | 4 (44.4) | 100 (17.6) |
| Vomit | 8 (25.0) | 36 (22.8) | 1 (7.7) | 5 (25.0) | 6 (46.1) | 0 | 567 (29.8) |
| Rashb | 12 (37.5) | 36 (22.9) | 9 (69.2) | 11 (55.0) | 7 (53.8) | 1 (11.1) | 186 (32.9) |
| Pruritusb | 10 (31.2) | 23 (14.7) | 9 (69.2) | 10 (50.0) | 7 (53.8) | 0 | 196 (34.5) |
| Presumptive diagnosis recorded on the medical recordc | |||||||
| DENVb | 3 (9.4) | 49 (30.8) | 1 (7.7) | 2 (10.0) | 3 (23.1) | 2 (22.2) | 60 (10.7) |
| ZIKAb | 6 (18.7) | 11 (6.9) | 3 (23.1) | 3 (15.0) | 2 (15.4) | 1 (11.1) | 34 (6.1) |
| CHIKVb | 0 | 17 (10.7) | 0 (0) | 0 | 0 | 0 | 8 (1.4) |
| UVI | 3 (9.4) | 41 (25.8) | 1 (7.7) | 4 (20.0) | 1 (7.7) | 2 (22.2) | 87 (15.6) |
| URIb,d | 2 (6.2) | 3 (1.9) | 0 (0) | 0 | 0 | 0 | 45 (8.1) |
| Gastroenteritis | 1 (3.1) | 0 (0) | 0 (0) | 0 | 0 | 0 | 13 (2.3) |
| Cystitis | 1 (3.1) | 2 (1.3) | 0 (0) | 1 (5.0) | 0 | 0 | 5 (0.9) |
| Othere | 3 (9.3) | 2 (1.3) | 1 (7.7) | 1 (5.0) | 0 | 0 | 24 (4.3) |
| None | 14 (43.7) | 61 (38.6) | 7 (53.8) | 11 (55.0) | 8 (61.5) | 4 (44.4) | 309 (55.4) |
Data are presented as no. (%) unless otherwise indicated. Of the 948 study patients, data were not shown for 1 patient with an acute DENV/ZIKV coinfection and for 133 patients with laboratory evidence of recent arboviral infection.
Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; FLAV, flavivirus; IQR, interquartile range; URI, upper respiratory infection; UVI, unspecific viral infection; ZIKV, Zika virus.
aData were not available for some variables: sex, headache, and rash (4 patients each), myalgia (7 patient), retro-orbital pain (11 patients), vomit (2 patients), medical suspicions recorded in the medical record (14 patients).
bDifferences between groups were statistically significant (P < .05).
cSum may be >100% because some patients had >1 clinical impression recorded in the medical record.
dUpper respiratory infection included pharyngitis, sinusitis, and influenza.
eOther medical suspicions were leptospirosis, pneumonia, skin infection, rotavirus, viral myositis, appendicitis, human immunodeficiency virus, and mumps.
Headache and myalgia were the most commonly reported symptoms, occurring in >80% of the arboviral patients, as well as among those with a nonarboviral febrile illness. Rash was reported more frequently by patients infected with ZIKV (69.2%), FLAV (55.0%), and DENV/CHIKV (53.8%), compared to those with DENV (37.5%), CHIKV (22.9%), CHIKV/FLAV (11.1%), and those negative for an acute or recent arboviral infection (32.9%) (P < .001; Table 1); pruritus followed a similar reporting pattern. Conversely, arthralgia was more frequently reported by patients with CHIKV (94.9%), DENV/CHIKV (84.6%), and FLAV/CHIKV (100.0%), compared to those with DENV (59.4%), ZIKV (53.8%), FLAV (75.0 %), and nonarboviral illnesses (62.3%) (P < .001). Swollen joints were more commonly reported by patients with DENV/CHIKV (53.8%), followed by CHIKV/FLAV (44.4%), FLAV (40.0%), CHIKV (39.6), DENV (31.2%), and ZIKV (30.7%) infections, and much less frequent among nonarboviral patients (17.6%) (P < .001). The sole patient with evidence for an acute DENV/ZIKV coinfection reported headache, myalgia, retro-orbital pain, rash, pruritus, arthralgia, vomiting, and swollen joints.
Nearly all (81 of 86 [94.2%]) chikungunya patients who provided a convalescent blood sample remained arthralgic (median, 18 [IQR, 13–32] days after symptom onset), as did 100% (4 of 4) of patients with DENV/CHIKV coinfection (median, 32 [IQR, 17–56] days after onset), and 100.0% (9 of 9) of the followed patients with CHIKV/FLAV coinfection (median, 15 [IQR, 13–18] days after onset). In comparison, 56.2% (9 of 16) of the followed dengue patients, 57.1% (4 of 7) of the followed Zika patients, and 65.0% (13 of 20) of the followed FLAV-infected patients maintained arthralgia (P < .001), with median follow-up of 21 (IQR, 16–44) days, 30 (IQR, 20–44) days, and 27 (IQR, 16–46) days after onset, respectively.
Presumptive Diagnoses
Despite some differences in clinical manifestations, the accuracy of presumptive diagnosis based on signs and symptoms was poor (Table 1). Among patients with acute DENV infection, only 9.4% were accurately diagnosed, while 18.7% were suspected for ZIKV and none for CHIKV. Among patients with acute CHIKV infection, the most common presumptive diagnosis was DENV (30.8%); a much smaller proportion was suspected of CHIKV (10.7%) or ZIKV (6.9%). A poor pattern of clinical diagnosis was also observed for patients with acute DENV/CHIKV coinfection, with 23.1% suspected as DENV and none suspected as CHIKV; interestingly, 15.4% were suspected of ZIKV infection. Among those with acute ZIKV infection, 23.1% were correctly diagnosed, while 7.7% were suspected as DENV and none as CHIKV.
Temporal Distribution of Arboviral Infections
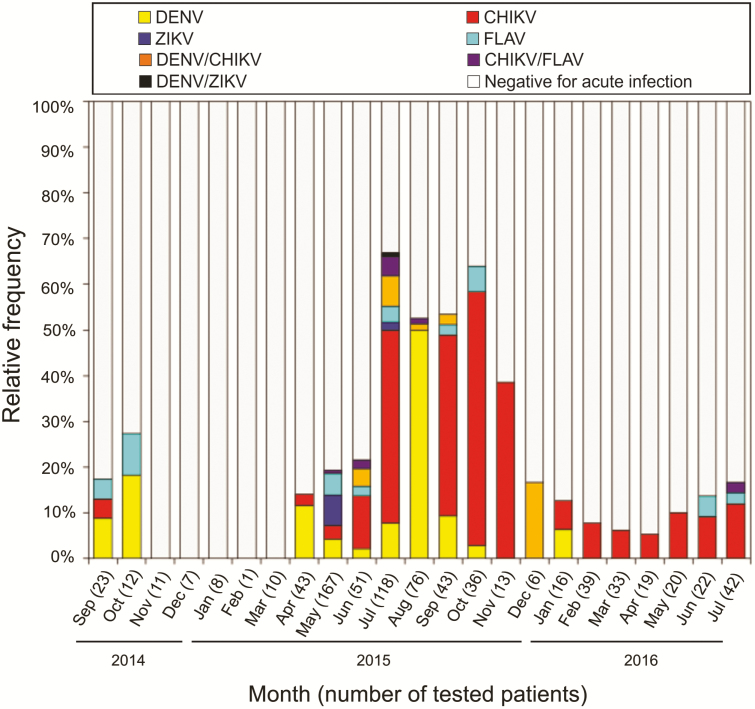
Acute arboviral infections were detected through most of the study period, except for November 2014 to March 2015 (Figure 2). Cases of acute DENV, CHIKV, and FLAV infections were confirmed from the first study month (September 2014), whereas acute ZIKV infections were only confirmed in May and July, 2015. Cases of acute DENV infection were mainly detected between April and October 2015, whereas CHIKV infections peaked between June and November 2015. Consequently, DENV/CHIKV coinfections were mainly found between June and September 2015 and DENV/ZIKV coinfections were only found in July 2015. Of note, CHIKV infections continued to be detected until the last study month, in July 2016 (Figure 2).
Figure 2.
Distribution (percentage) of 948 acute febrile illness patients according to the arboviral diagnosis by month—Salvador, Brazil, September 2014 to July 2016. Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; FLAV, flavivirus; ZIKV, Zika virus.
DISCUSSION
Our results confirmed the simultaneous transmission of DENV, CHIKV, and ZIKV in northeastern Brazil and revealed the large impact of these viruses as causes of febrile illness requiring medical care. During the study period, 26.1% of the enrolled patients were laboratory-confirmed with an acute arboviral infection. However, between July and October of 2015, when transmission of CHIKV and DENV peaked, the frequency of any arboviral infection was >50%.
Particularly noteworthy was our finding of CHIKV circulation in Salvador at the same time (September 2014) that it was first detected causing outbreaks in other Brazilian cities [3, 4], though apparently major amplification in Salvador only began in June 2015, 1 month after the ZIKV epidemic peak in May 2015 [15]. Curiously, ZIKV spread in Salvador was very rapid and the outbreak, comprising approximately 17 500 case reports, lasted only 2 months [15, 16], while the CHIKV emergence was less abrupt and lasted longer, hampering its prompt recognition, especially because public health attention was directed to the ZIKV outbreak [17]. Although our surveillance study included only 1 health unit of Salvador, our arboviral detection over time reflected previous citywide observations [15–17].
It remains unclear why ZIKV and CHIKV had different spread patterns in Salvador, both being transmitted mainly by the same Aedes (Stegomyia) aegypti mosquitoes and with the local population entirely susceptible to both. Furthermore, Salvador Ae. aegypti mosquitoes are not particularly susceptible to an American strain of ZIKV tested experimentally [18], inconsistent with the explosive amplification that was observed citywide [15]. It is possible that particularities in the interaction between viruses, vectors, and the human population produced different outcomes in terms of vectorial capacity. These may include virus strain variation, human and mosquito coinfections, human genetic diversity, variation in the sequence and timing of human arboviral infections, and even the involvement of other Aedes species, such as Aedes albopictus, in ZIKV and CHIKV transmission.
We also found that coinfections were relatively common (23 of the 247 [9.3%] acute arboviral infections detected; 2.4% of all the febrile patients studied). In addition, 38 of the 224 (17.0%) acute single arboviral infections and 133 of the 701 (20.0%) patients without an acute arboviral infection had laboratory evidence for a recent arboviral infection. The impressively high frequencies of concomitant and sequential infections were apparently due to the intense simultaneous transmission of the 3 arboviruses in Salvador during the study period.
Statistically, the likelihood of simultaneously detecting 2 independent events is estimated by multiplying their individual likelihood detection. However, 2 events could also be dependent—for example, the coinfection of people by 2 different arboviruses through the bite of a mosquito carrying >1 arbovirus, resulting in simultaneous transmission. In our study, the observed frequencies of human coinfections were not statistically different from those expected, under the assumption of independent arboviral infections. This negative finding suggests that human coinfections are nonassociated, rather than dependent events. However, as our nonassociation findings are supported merely by statistical analyses, they might not represent the true behavior of these viruses in nature. Further studies are needed to investigate potential arbovirus interactions in vectors and hosts, and to better determine whether pathogenesis and clinical outcomes of coinfections and sequential infections differ from those of single infections.
Among the arboviruses we studied, ZIKV presented the lowest frequency. This may be explained by our limited capacity for detecting ZIKV infections among the general patient population seeking medical care at the health unit because our inclusion criteria required the presence of fever, which is less common in ZIKV infections [19]. In addition, the sensitivity of ZIKV molecular diagnosis is limited [20], hampering case detection during the viremic phase of the infection, and we did not employ ZIKV serological tests due to their low accuracy [13, 14]. Finally, some patients diagnosed with an acute FLAV infection based on DENV IgM-ELISA seroconversion might actually have reflected a ZIKV infection that cross-reacted with DENV. It is important to emphasize that FLAV infections were most likely caused either by DENV or ZIKV, as there are no reports of other flaviviruses causing human infections in Salvador. Yet, we cannot completely rule out the possibility of silent circulation of other FLAV pathogens, such as yellow fever or West Nile, causing unrecognized infections.
Interestingly, Zika patients had a lower median age compared with other arboviral-infected patients. As ZIKV infections typically produce milder clinical manifestations, it is possible that ZIKV-infected children were more likely to be brought by their parents or guardians for medical care than adults. It is also possible that Zika clinical manifestations in older adults are less prominent than in children and younger adults, as previously observed in a Puerto Rico study that showed that, among individuals with laboratory-confirmed ZIKV infections, those who were symptomatic were younger than those who were asymptomatic [21]. Previous DENV exposures, which increase with age, may play an immunomodulatory role in this difference [22, 23].
As previously noticed, we also detected clinical manifestation differences between DENV, CHIKV, and ZIKV infections. Zika patients more frequently had rash and pruritus, as shown in Brazil [24] and Nicaragua [8], whereas arthralgia was more common in CHIKV patients, as reported in Trinidad [25]. However, rash and pruritus were also common among non-ZIKV patients, affecting those with DENV and CHIKV, as well as patients with nonarboviral illness. Arthralgia was also very frequent among non-CHIKV patients, occurring in >50% of DENV, ZIKV, and nonarboviral patients. As a caveat, our signs and symptoms data were based on patients’ self-reports rather than medical evaluations. Thus, imprecision for some signs, such as joint edema, may have occurred. In addition, the generalizability of our findings are limited to febrile patients and do not totally apply to ZIKV-infected patients, who frequently have no detectable fever.
Despite some clinical differences, an erroneous presumptive diagnosis was the rule. Dengue was suspected for <10% of the patients with confirmed DENV single infection, but was suspected for approximately 30% and 20% of patients with confirmed CHIKV and DENV/CHIKV infections, respectively. Chikungunya was suspected for approximately 10% of patients with CHIKV infection and for none with DENV/CHIKV coinfection. These findings may be explained by the lack of physicians’ awareness regarding high levels of CHIKV transmission in Salvador [17]. They also suggest that differences between clinical manifestations of DENV and CHIKV infection (possibly related to the severity of symptoms) made physicians suspect dengue 2–3 times more often in patients with confirmed CHIKV infections, compared to patients with confirmed DENV infections.
In summary, our study, conducted during a period of intense, simultaneous DENV, CHIKV, and ZIKV transmission, highlights the burden of arboviral diseases for febrile illness and indicates that coinfections are common in these circumstances. Given the clinical similarities among arboviral diseases and the challenge of an accurate clinical suspicion, epidemiological information on seasonality, population susceptibility, and transmission intensity is needed to improve the accuracy of presumptive clinical diagnoses. However, only with accurate diagnostic tools readily available in local health units will we be able to provide proper detection, clinical care, and surveillance of arboviral diseases.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors thank the technical staff who participated in data collection and sample processing, Renan Rosa and Perla Santana for their assistance with data management and administrative matters, the São Marcos Health Unit staff for providing health assistance to the patients, and the patients for their participation in the study.
Financial support. This work was supported by the Brazilian National Council for Scientific and Technological Development (grant numbers 400830/2013-2 and 440891/2016–7 to G. S. R.; and scholarships to I. A. D. P., L. B. T., U. K., M. G. R., and G. S. R.); the Bahia Foundation for Research Support (grant numbers PET0026/2013, APP0044/2016, and PET0022/2016 to G. S. R., and scholarship to M. M. O. S.); the Coordination for the Improvement of Higher Education Personnel, Brazilian Ministry of Education (grant number 440891/2016–7 to G. S. R. and scholarship to M. K.); the US National Institutes of Health (grant numbers R01 TW009504, R01 AI121207, and U01 AI088752 to A. I. K. and R24 AI AI120942 to S. C. W.); the Yale School of Public Health; the Federal University of Bahia; and the Oswaldo Cruz Foundation.
Potential conflicts of interest. S. C. W. owns intellectual property related to chikungunya vaccine development and has 2 patents for alphavirus vaccine development issued. A. I. K. has a patent on methods and composition for detection of flavivirus infection pending. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis 2017; 17:e101–6. [DOI] [PubMed] [Google Scholar]
- 2. Temporão JG, Penna GO, Carmo EH, et al. Dengue virus serotype 4, Roraima State, Brazil. Emerg Infect Dis 2011; 17:938–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nunes MRT, Faria NR, de Vasconcelos J, et al. Emergence and potential for spread of chikungunya virus in Brazil. BMC Med 2015; 13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teixeira MJ, Andrade AMS, Costa MC, et al. East/Central/South African genotype chikungunya virus, Brazil, 2014. Emerg Infect Dis J 2015; 21:906–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 2015; 21:1885–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aliota MT, Bassit L, Bradrick SS, et al. Zika in the Americas, year 2: what have we learned? What gaps remain? A report from the Global Virus Network. Antiviral Res 2017; 144:223–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waggoner JJ, Gresh L, Vargas MG, et al. Viremia and clinical presentation in Nicaraguan patients infected with Zika virus, chikungunya virus, and dengue virus. Clin Infect Dis 2016; 63:1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nkengasong JN, Nsubuga P, Nwanyanwu O, et al. Laboratory systems and services are critical in global health: time to end the neglect? Am J Clin Pathol 2010; 134:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lanciotti R, Calisher C, Gubler D, Chang G, Vorndam A. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 1992; 30:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balm MN, Lee CK, Lee HK, Chiu L, Koay ES, Tang JW. A diagnostic polymerase chain reaction assay for Zika virus. J Med Virol 2012; 84:1501–5. [DOI] [PubMed] [Google Scholar]
- 12. Edwards CJ, Welch SR, Chamberlain J, et al. Molecular diagnosis and analysis of chikungunya virus. J Clin Virol 2007; 39:271–5. [DOI] [PubMed] [Google Scholar]
- 13. L’Huillier AG, Hamid-Allie A, Kristjanson E, et al. Evaluation of Euroimmun anti-Zika virus IgM and IgG enzyme-linked immunosorbent assays for Zika virus serologic testing. J Clin Microbiol 2017; 55:2462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kikuti M, Tauro LB, Moreira PSS, et al. Diagnostic performance of commercial IgM and IgG enzyme-linked immunoassays (ELISAs) for diagnosis of Zika virus infection. Virol J 2018; 15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cardoso CW, Paploski IAD, Kikuti M, et al. Outbreak of exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil. Emerg Infect Dis J 2015; 21:2274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paploski IAD, Prates APPB, Cardoso CW, et al. Time lags between exanthematous illness attributed to Zika virus, Guillain-Barré syndrome, and microcephaly, Salvador, Brazil. Emerg Infect Dis J 2016; 22:1438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cardoso CW, Kikuti M, Prates AP, et al. Unrecognized emergence of chikungunya virus during a Zika virus outbreak in Salvador, Brazil. PLoS Negl Trop Dis 2017; 11:e0005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roundy CM, Azar SR, Rossi SL, et al. Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg Infect Dis 2017; 23:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 20. Fischer C, Pedroso C, Mendrone A, et al. External quality assessment for Zika virus molecular diagnostic testing, Brazil. Emerg Infect Dis 2018; 24:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lozier MJ, Burke RM, Lopez J, et al. Differences in prevalence of symptomatic Zika virus infection, by age and sex—Puerto Rico, 2016. J Infect Dis 2018; 217:1678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen J, Elong Ngono A, Angel RN, et al. Dengue virus-reactive CD8+T cells mediate cross-protection against subsequent Zika virus challenge. Nat Commun 2017; 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Priyamvada L, Quicke KM, Hudson WH, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 2016; 113:7852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azeredo EL, dos Santos FB, Barbosa LS, et al. Clinical and laboratory profile of Zika and dengue infected patients: lessons learned from the co- circulation of dengue, Zika and chikungunya in Brazil. PLoS Curr 2018; 2:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sahadeo N, Mohammed H, Allicock OM, et al. Molecular characterisation of chikungunya virus infections in Trinidad and comparison of clinical and laboratory features with dengue and other acute febrile cases. PLoS Negl Trop Dis 2015; 9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.