Abstract
目的
探讨血清DKK1水平与多发性骨髓瘤(MM)患者病程及MM骨病的关系。
方法
纳入2010–2014年诊治的145例MM患者及43名正常对照者,通过全身骨骼X线检查确定骨病情况,ELISA法检测血清dickkopf1 (DKK1)蛋白表达水平。
结果
145例患者中初治者79例,缓解者19例,复发进展者47例。初治组DKK1水平为2 155(646~35 251) ng/L,明显高于正常对照组[1487(646~2 577) ng/L,P=0.000]、缓解组[1 136(431~3 582) ng/L,P=0.001]及复发进展组[1 695(431~3 582)ng/L,P=0.037],差异均有统计学意义。复发进展组患者DKK1表达稍高于缓解患者,但差异无统计学意义(P=0.078)。合并骨病者的血清DKK1水平[2 519(646~35 251)ng/L]显著高于无骨病者[1 910(660~26 925)ng/L],差异有统计学意义(P=0.005),溶骨性病变部位0、1~3、>3处患者血清DKK1水平分别为1 910(660~26 925)、2 155(1 369~5 974)、2 547 (646~35 251)ng/L,差异有统计学意义(P=0.018)。
结论
MM患者血清DKK1表达水平不仅与MM疾病状态相关,且与溶骨性病变及其数目相关,为临床进行DKK1靶向治疗MM提供了理论依据。
Keywords: 多发性骨髓瘤, 骨病, DKK1
Abstract
Objective
To study the association between the level of serum DKK1 and disease course of multiple myeloma (MM) as well as myeloma bone disease.
Methods
This study enrolled 145 cases of MM (including 79 newly diagnosed MM, 19 responded MM, 47 relapsed or progressive MM) whose lytic bone disease were evaluated by conventional radiography, ELISA was used to detect the concentration of serum DKK1.
Results
Serum DKK-1 elevated in newly diagnosed MM compared with normal donors [2 155(646–35 251)vs 1 487(646–2 577)ng/L, P=0.000], those responded [1 136(431–3 582)ng/L, P=0.001] and relapsed/progressive MM [1 695(431–3 582)ng/L, P=0.037], and the level of relapsed/progressive MM was marginally higher than the responded ones. Moreover, MM patients without lytic lesions in conventional radiography had significantly lower DKK-1 levels than those with lytic bone disease [1 910(660–26 925)vs 2 519(646–35 251)ng/L, P=0.005]. Notably serum DKK-1 correlated with the number of bone lesions [0 vs 1–3 vs >3 lesions: 1 910(660–26 925)vs 2 155 (1 369–5 974)vs 2 547(646–35 251)ng/L, P=0.018].
Conclusion
DKK-1 serum concentration correlated with disease course of MM and myeloma bone disease, indicating that DKK-1 was an important factor for the extent of bone disease, which supporting the hypothesis of DKK-1 as a therapeutic target in myeloma bone disease.
Keywords: Multiple myeloma, Myeloma bone disease, Dickkopf1
约80%的多发性骨髓瘤(multiple myeloma,MM)患者存在MM骨病(myeloma bone disease,MBD)。研究显示MBD的主要病理机制为成骨细胞受抑、破骨细胞数量增加且活性增强所导致的骨稳态失衡[1]–[2]。近些年来,随着双膦酸盐药物的使用,显著地降低了骨相关事件的发生,但考虑其肾功能损害及下颌骨坏死等不良反应[3],因此迫切需要更安全有效的治疗策略。DKK1为Wnt信号通路抑制剂,其可与Wnt受体复合体的低密度脂蛋白受体相关蛋白5结合,通过抑制下游重要的成骨分化调控分子转录从而抑制成骨细胞前体细胞的分化[4]–[6]。DKK1由浆细胞产生并分泌,在MM患者骨髓及外周血中均可检测到,且在合并MBD的患者中高表达[7]。多个临床前研究显示DKK1在抑制成骨细胞的同时激活破骨细胞,从而导致骨稳态破坏[8]–[10]。在本研究中,我们对MM患者的血清DKK1蛋白水平进行检测,旨在探讨血清DKK1水平与MM患者病程及MBD的关系。
病例和方法
1.病例:以2010年至2014年入住我院的145例MM患者为研究对象。MM诊断及疗效评价参照国际MM工作组标准[11],145例患者中初治者79例,缓解者19例,复发进展者47例。所有患者初次入院时均进行全身骨骼X线片检查,包括颅骨、胸骨、肋骨、骨盆、四肢长骨等部位。以43名正常献血员为正常对照。患者及正常对照者均签署知情同意书。所有患者均常规进行双膦酸盐预防或治疗MBD。
2.标本采集与检测:采集患者及正常对照者外周血10 ml,室温静置待其凝固后,常规离心后收集上清储存于−80 °C备用。采用ELISA法检测血清中DKK1蛋白表达水平,试剂为R&D美国公司产品。严格按照试剂盒说明书进行操作。
3.MBD诊断及分级:按照全身骨骼X线片检查结果分为:1级:无溶骨性损坏或仅有骨质疏松;2级:1~3处溶骨性损坏;3级:3处以上溶骨性损害或存在病理性骨折[12]。
4.随访与生存分析:随访截止时间为2014年5月1日,通过电话方式进行随访。总生存(OS)时间为自诊断时至死亡或随访结束。无进展生存(PFS)时间为自诊断时至疾病进展或随访结束。
5.统计学处理:采用SPSS 17.0软件进行统计学分析。对变量常规进行正态检验,非正态分布的数值变量采用中位数及全距表示,两组样本之间比较采用Mann-Whitney U检验,多样本之间采用Kroskal-Wallis检验。生存分析采用Kaplan-Meier生存曲线,对可能影响患者预后的相关危险因素建立COX模型进行多因素分析。P≤0.05为差异有统计学意义。
结果
1.DKK1表达水平与MM患者疾病状态的相关性分析:79例初治MM患者的DKK1水平为2 155(646~35 251)ng/L,明显高于正常对照组[1 487(646~2 577)ng/L,P=0.000]、缓解组[1 136(431~3 582) ng/L,P=0.001]及复发进展组[1 695 (431~3 582)ng/L,P=0.037],差异均有统计学意义。复发进展组患者DKK1表达高于缓解患者,但差异无统计学意义(P=0.078)。根据患者诱导化疗药物不同将19例缓解期患者分为沙利度胺治疗组(7例)和硼替佐米治疗组(12例),两组患者血清DKK1水平差异无统计学意义[1 136(713~3 582)对1 098(431~2 954) ng/L,P=0.609]。
2.血清DKK1水平与MM患者MBD的相关性分析:骨骼X线检查结果显示,79例初治患者中50例存在不同程度的MBD(1~3处者7例,3处以上者43例)。合并MBD者的血清DKK1水平显著高于无骨病患者[2 519 (646~35 251)对1 910(660~26 925)ng/L,P=0.005],且DKK1水平与发生MBD的数目有关,1~3处者与>3处者分别为2 155(1 369~5 974)和2 547(646~35 251) ng/L,差异有统计学意义(P=0.018)。
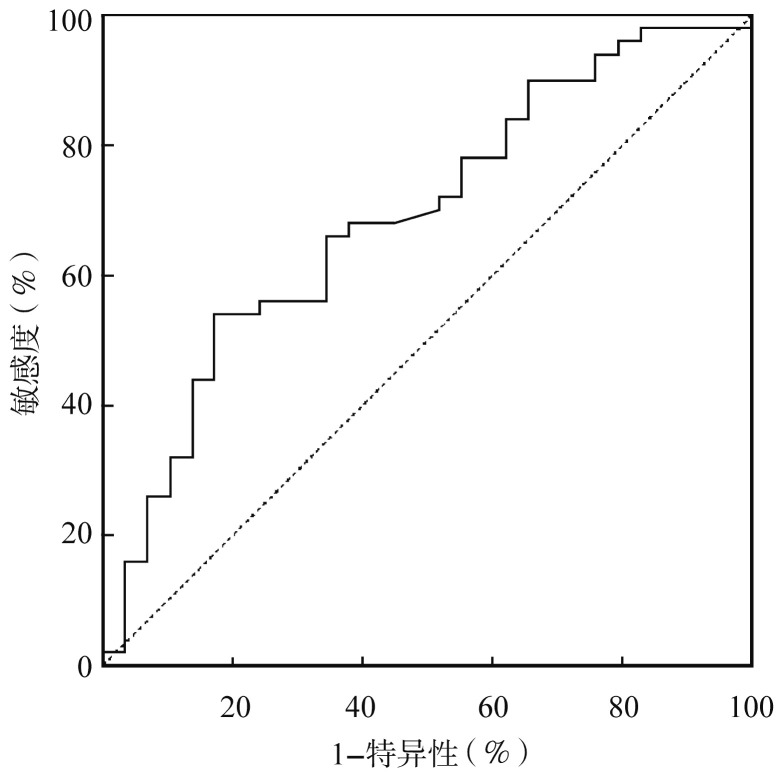
在初治患者中用血清DKK1水平诊断MBD的受试者工作特征曲线(ROC曲线)下面积为0.689(P=0.005)(图1),根据ROC曲线所得诊断阈值为2 480.5 ng/L,其诊断MBD的特异性为82.8%,敏感性为54.0%。
图1. 血清DKK1水平诊断多发性骨髓瘤骨病的受试者工作曲线.
3.血清DKK1水平与MM患者临床特征的关系:根据ROC曲线所得阈值,将初治患者分为血清DKK1水平正常(47例)和升高(32例)组。两组患者的临床特征见表1。结果显示:血清DKK1水平升高患者中ISS Ⅲ期者比例较正常组高(58.1%对35.6%,P=0.039);流式细胞术检测浆细胞比例>10%者较正常组高(55.2%对28.6%,P=0.029)。
表1. 不同血清DKK1水平的初治多发性骨髓瘤患者临床特征比较.
| 临床因素 | 正常组(47例) | 升高组(32例) | P值 |
| 性别[例数(%)] | 0.190 | ||
| 男 | 30(66.7) | 16(51.6) | |
| 女 | 15(33.3) | 15(48.4) | |
| 年龄[岁,M(范围)] | 59(36~76) | 60(34~74) | 0.936 |
| HGB[g/L,M(范围)] | 99(60~140) | 85(51~151) | 0.123 |
| PLT[×109/L,M范围)] | 167(84~718) | 200.5(41~335) | 0.537 |
| 骨髓浆细胞比例[M(范围)] | 0.240(0~0.855) | 0.320(0.020~0.915) | 0.218 |
| LDH升高a | 7/45(15.6) | 5/31(16.1) | 0.947 |
| 髓外浸润a | 6/40(15.0) | 4/28(14.3) | 0.935 |
| D-S分期a | 0.500 | ||
| Ⅰ期 | 3/42(7.1) | 0 | 0.145 |
| Ⅱ期 | 3/42(7.1) | 3/31(9.7) | 0.635 |
| Ⅲ期 | 36/42(85.7) | 28/31(90.3) | 0.288 |
| ISS分期a | 0.051 | ||
| Ⅰ期 | 6/45(13.3) | 2/31(6.5) | 0.340 |
| Ⅱ期 | 23/45(51.1) | 11/31(35.5) | 0.181 |
| Ⅲ期 | 16/45(35.6) | 18/31(58.1) | 0.039 |
| 肾功能异常发生率a | 6/42(14.3) | 9/31(29.0) | 0.126 |
| 免疫球蛋白类型a | 0.208 | ||
| IgG | 24/44(54.6) | 10/31(32.3) | |
| IgA | 11/44(25.0) | 7/31(22.6) | |
| IgD | 1/44(2.3) | 3/31(9.7) | |
| 轻链κ型 | 4/44(9.1) | 4/31(12.9) | |
| 轻链λ型 | 3/44(6.8) | 7/31(22.6) | |
| 不分泌型 | 1/44(2.3) | 0 | |
| 浆细胞比例>10%ab | 12/42(28.6) | 16/29(55.2) | 0.029 |
注:LDH升高:LDH>247 U/L;D-S:Durie-Salmon;ISS:国际分期系统;a:阳性例数/检测例数(%);b:流式细胞术检测
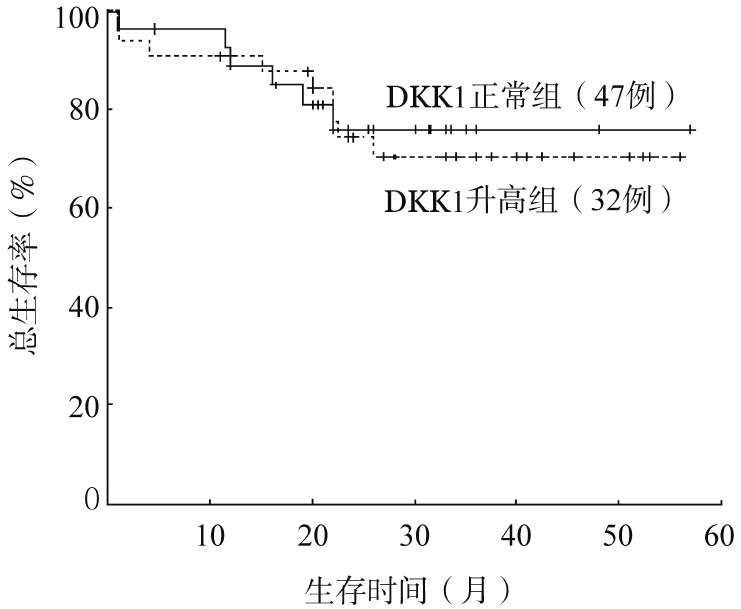
79例初治患者中位随访时间为26(1~52)个月,生存分析显示血清DKK1水平正常与升高患者的中位PFS时间(54对31个月,P=0.485)和中位OS时间(均未达到,P=0.396)比较差异均无统计学意义(图2)。将治疗方案、DKK1水平、溶骨性病变部位数等纳入COX模型进行预后多因素分析,均非影响患者生存的独立预后因素(P值均>0.05)(表2)。
图2. 血清DKK1水平对初治多发性骨髓瘤患者总生存的影响.
表2. 79例初治多发性骨髓瘤患者预后危险因素的COX模型多因素分析.
| 影响因素 | HR | 95%CI | P值 |
| 治疗方案(沙利度胺/硼替佐米) | 0.391 | 0.229~1.779 | 0.391 |
| DKK1水平(升高/正常) | 0.729 | 0.237~2.245 | 0.582 |
| 溶骨性病变部位数(1~3处/>3处) | 0.788 | 0.446~1.391 | 0.410 |
讨论
多项研究表明DKK1与MM及MBD相关。Tian等[7]研究发现MBD患者的浆细胞高表达DKK1 mRNA,而正常对照者及无MBD患者的浆细胞则不表达该分子。DKK1在MM患者的骨髓及外周血中均可检测到,且两者含量呈正相关。进一步研究证明重组DKK1蛋白或含有高水平DKK1的骨髓液上清可以抑制成骨细胞前体细胞的分化,而针对DKK1的单克隆抗体可拮抗该过程。Durie等[13]采用单核苷酸多态性芯片区分患者MBD程度,结果显示DKK1表达水平与MBD相关。Giuliani等[14]的研究表明MM患者骨髓中DKK1水平明显高于意义未明的单克隆丙球蛋白病(MGUS)患者及正常对照者,且与Durie-Salmon分期呈正相关。另外一个纳入184例初治MM患者的临床研究结果亦显示ISS分期Ⅱ/Ⅲ期者血清DKK1水平显著高于Ⅰ期者,且血清DKK1水平与有无MBD及病变的数目相关[15]。另有研究者发现MM患者在硼替佐米治疗及自体造血干细胞移植后血清DKK1水平下降,且仅在有治疗反应者中明显下降[12],[16]–[17]。
我们的研究结果亦显示初治MM患者的DKK1水平明显高于正常对照和缓解者(后两者近似),复发进展者稍高于缓解者,该结果再次提示血清DKK1可能由肿瘤性浆细胞分泌,因此与肿瘤负荷相关,有望作为疾病监测的指标,并进行靶向治疗。血清DKK1水平不仅与有无MBD相关,且与病变部位数紧密相关。采用血清DKK1水平诊断MBD的特异性为82.8%,敏感性为54.0%。尽管敏感性有待提高,但相对传统X线检查其安全性突出。我们期待更加敏感且特异的血清学分子标志出现。
考虑到DKK1与肿瘤负荷的潜在相关性,针对DKK1的靶向治疗正在进行中。Yaccoby等[10]的研究表明抗DKK1治疗可以降低移植原代肿瘤细胞的SCID-rab小鼠的肿瘤负荷,增加成骨细胞(以表达骨钙蛋白为标志)数目,同时降低破骨细胞(抗酒石酸酸性磷酸酶染色阳性细胞)数目,进一步为DKK1单克隆抗体的临床试验提供理论基础。
综上,在本研究中,我们发现血清DKK1表达水平不仅与MM疾病状态相关,且与MBD及其病变部位数相关,为DKK1靶向治疗提供了一定的理论依据。
Funding Statement
基金项目:国家自然科学基金(81101794、81172255);天津市科技支撑计划重大(抗癌专项)项目(12ZCDZSY17600);卫生公益性行业科研专项(201202017)
References
- 1.Roodman GD. Pathogenesis of myeloma bone disease[J] Leukemia. 2009;23(3):435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 2.Edwards CM, Zhuang J, Mundy GR. The pathogenesis of the bone disease of multiple myeloma[J] Bone. 2008;42(6):1007–1013. doi: 10.1016/j.bone.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jantunen E. Bisphosphonate therapy in multiple myeloma: past, present, future[J] Eur J Haematol. 2002;69(5-6):257–264. doi: 10.1034/j.1600-0609.2002.02796.x. [DOI] [PubMed] [Google Scholar]
- 4.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments[J] Nat Med. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 5.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators[J] Oncogene. 2006;25(57):7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 6.Morvan F, Boulukos K, Clement-Lacroix P, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass[J] J Bone Miner Res. 2006;21(6):934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 7.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma[J] N Engl J Med. 2003;349(26):2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 8.Qiang YW, Chen Y, Stephens O, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma[J] Blood. 2008;112(1):196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma[J] Blood. 2009;114(2):371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaccoby S, Ling W, Zhan F, et al. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo[J] Blood. 2007;109(5):2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group[J] Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 12.Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma[J] Br J Haematol. 2006;135(5):688–692. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 13.Durie BG, Van Ness B, Ramos C, et al. Genetic polymorphisms of EPHX1, Gsk3beta, TNFSF8 and myeloma cell DKK-1 expression linked to bone disease in myeloma[J] Leukemia. 2009;23(10):1913–1919. doi: 10.1038/leu.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliani N, Morandi F, Tagliaferri S, et al. Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment[J] Cancer Res. 2007;67(16):7665–7674. doi: 10.1158/0008-5472.CAN-06-4666. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser M, Mieth M, Liebisch P, et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma[J] Eur J Haematol. 2008;80(6):490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 16.Politou MC, Heath DJ, Rahemtulla A, et al. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation[J] Int J Cancer. 2006;119(7):1728–1731. doi: 10.1002/ijc.22033. [DOI] [PubMed] [Google Scholar]
- 17.Heider U, Kaiser M, Mieth M, et al. Serum concentrations of DKK-1 decrease in patients with multiple myeloma responding to anti-myeloma treatment[J] Eur J Haematol. 2009;82(1):31–38. doi: 10.1111/j.1600-0609.2008.01164.x. [DOI] [PubMed] [Google Scholar]