Abstract
A 72-year-old Japanese man treated with omeprazole for 11 years was admitted due to loss of consciousness and muscle weakness. Wolff-Parkinson-White syndrome-induced tachycardia was considered as the cause of syncope. His blood examination revealed rhabdomyolysis, hypokalaemia, hypomagnesaemia, hypocalcaemia, hyperlactacidaemia, hyperammonaemia and high-anion-gap metabolic acidosis. Hypomagnesaemia could be caused by magnesium malabsorption due to omeprazole use. Hypocalcaemia might be caused by the inhibitory effect of hypomagnesemia on the parathyroid gland hormone secretion. Hyperammonaemia might be caused by two reasons: (1) renal ammonium production induced by hypokalaemia; (2) inhibition of ammonium secretion by omeprazole. Both hypocalcaemia and hypokalaemia might cause chronic elevation of serum creatinine phosphokinase which ended up with rhabdomyolysis. Correction of serum electrolytes rapidly improved his muscle weakness. Discontinuation of omeprazole no longer caused these abnormalities. A physician should be aware of unexplained signs and symptoms of patients using proton-pump inhibitors to avoid life-threatening electrolyte and physiologic disturbances.
Keywords: renal system, metabolic disorders, unwanted effects / adverse reactions, fluid electrolyte and acid-base disturbances
Background
Proton-pump inhibitors (PPIs) have been widely used for patients with peptic ulcers and gastro-oesophageal reflux disease. They have been particularly used as long-term antiplatelet therapy to prevent gastric injury. With the expansion of PPI use worldwide, various kinds of adverse effects have been reported.1 Hypomagnesaemia was reported as one of the adverse effects of PPI use because of their inhibitory effects on the absorption of magnesium from the intestine.2 However, PPI-induced hypomagnesaemia has not been recognised among physicians, partly because of the latent time that varies from a few days to 13 years.3 We present a case of omeprazole-induced hypomagnesaemia after 11 years of intake. This case particularly showed a novel combination of physiologic derangements complicated by renal tubular acidosis (RTA) with high-anion-gap metabolic acidosis. Note that RTA is usually diagnosed in patients with non-anion gap, hyperchloremic metabolic acidosis.4
Case presentation
A 72-year-old Japanese man was admitted to the hospital after losing consciousness for several seconds at home. He presented with disorientation in time and place but could talk about himself in detail. His weakness has been deteriorating over the last month. He gradually lost his appetite. He has been feeling dizzy for the last 2 weeks. He has not been able to walk smoothly by himself for the last 2 days. In the emergency room, his vital signs were as follows: blood pressure 112/78 mm Hg, heart rate 117 beats/min with a regular rhythm, body temperature 36.5°C, respiratory rate 18 breaths/min and SpO2 97% in room air.
His prior medical history included duodenal ulcers about 20 years ago. Since then, he was treated with antacid medications in a different outpatient clinic. His medication was changed from famotidine to omeprazole 10 mg one time a day 11 years ago. His unexplained hypokalaemia was pointed out 3 years ago during a regular check-up and was treated with potassium chloride supplementation since then. A slightly abnormal elevation of serum creatinine phosphokinase (CPK 226 U/mL; reference range 38–213) was also pointed out just around the same time but no further investigations were done. There were no data on his previous serum magnesium level. Two years ago, he changed his primary physician to a new physician who practised near his home because of gradual weakness in his legs, which he thought was an ageing symptom. His daily medication at the new clinic was the same as at the former clinic. His daily medication included omeprazole 10 mg, potassium chloride 1200 mg, telmisartan 20 mg, allopurinol 100 mg, loxoprofen sodium hydrate 180 mg, eperisone 150 mg, teprenone 150 mg and azulene sodium sulfonate 2 g. His serum CPK elevated up to 2878 U/L (reference range 38–196) 6 days before admission. Apart from the previously mentioned medical histories, the Wolff-Parkinson-White (WPW) syndrome was detected on his ECG when he underwent a regular check-up 16 years ago. Since then, he has only been managed conservatively with yearly cardiology follow-ups. He has been pointed out his occult blood in the urine since 14 years ago. He suffered from urinary tract stone about 10 years ago, but the composition of the stone was not analysed. He had a thorough examination at the urology clinic without a renal biopsy. The cause of his microscopic haematuria was not elucidated. As for his social history, he has been an active judge. He never worked using heavy metal, battery or gasoline. His family history did not include any collagen diseases or renal diseases. He has no known allergies to either food or drug.
Investigations
Further investigations were needed to determine whether his loss of consciousness was a result of tachycardia induced by the WPW syndrome. However, the initial blood test showed several abnormal values (table 1). His serum potassium level decreased to 2.7 mEq/L. He also showed clinical evidence of extreme hypomagnesaemia (0.4 mg/dL), hypocalcaemia (6.4 mg/dL, albumin corrected 6.8 mg/dL), hyperammonaemia (176 µg/dL), hyperlactacidaemia (14 mmol/L) and high-anion-gap metabolic acidosis. Furthermore, his serum CPK was severely elevated (4622 U/L). His ECG showed delta-waves, which is a typical finding of WPW syndrome. CT imaging of the brain, chest and abdomen revealed normal findings without any renal calcification.
Table 1.
Laboratory results of the patient
| Blood chemistry | Urinalysis | Serum | |||
| ALB (g/dL) | 3.6 | pH | 5.5 | CRP (mg/dL) | 0.17 |
| AST (U/L) | 72 | Protein | 2+ | SS-A (U/mL) | <1.0 |
| ALT (U/L) | 31 | Sugar | – | SS-B (U/mL) | <1.0 |
| ALP (U/L) | 133 | Ketones | – | Hormones | |
| GTP (U/L) | 18 | Occult blood | 3+ | TSH (μIU/mL) | 2.02 |
| LDH (U/L) | 436 | Urobilinogen | normal | fT4 (ng/dL) | 1.02 |
| CPK (U/L) | 4622 | Osmolality | 445 | PRA (ng/mL/hour) | 0.7 |
| CK-MB (IU/L) | 21 | Na (mEq/L) | 106 | PAC (pg/mL) | 98.5 |
| BUN (mg/dL) | 8.4 | K (mEq/L) | 44.5 | ACTH (pg/mL) | 25.5 |
| Cre (mg/dL) | 0.73 | Cl (mEq/L) | 51 | Cortisol (μg/dL) | 12.5 |
| Na (mEq/L) | 142 | Ca (mg/dL) | 1.3 | PTH-int (pg/mL) | 62 |
| K (mEq/L) | 2.7 | Cre (mg/dL) | 87.6 | 1,25-(OH)2 vitaminD | 49.9 |
| Cl (mEq/L) | 99 | AG (mEq/L) | 99.5 | ABGA | |
| Ca (mg/dL) | 6.4 | β2MG (μg/L) | 266 | pH | 7.331 |
| P (mg/dL) | 4.2 | NAG (IU/L) | 0.9 | pCO2 (mm Hg) | 30.6 |
| Mg (mg/dL) | 0.4 | CBC | pO2 (mm Hg) | 70.7 | |
| Pb (μg/dL) | <1.0 | WBC (×109/L) | 9.2 | HCO3− (mmol/L) | 15.8 |
| NH3 (μg/dL) | 176 | RBC (×1012/L) | 3.81 | BE (mmol/L) | −8.9 |
| Lactate (mmol/L) | 14 | Hb (g/L) | 124 | AG (mEq/L) | 27.2 |
| Osmolality | 286 | Ht (%) | 36.4 | ||
| Plt (×109/L) | 233 | ||||
ABGA, arterial blood gas analysis; ACTH, adrenocorticotropic hormone; AG, anion gap; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BE, base excess; BUN, blood urea nitrogen; Ca, calcium; CBC, complete blood count; CK-MB, creatine kinase isoenzyme MB fraction; Cl, chlorine; CPK, creatinine phosphokinase; Cre, creatinine; CRP, C-reactive protein; fT4, free thyroxine; GTP, gamma-glutamyl transferase; Hb, haemoglobin; Ht, haematocrit; K, potassium; LDH, lactate dehydrogenase; Mg, magnesium; β2MG, beta-2-microglobulin; Na, sodium; NAG, N-acetyl-β-D-glucosaminidase; NH3, ammonia; P, phosphorus; PAC, plasma aldosterone concentration; Pb, lead; Plt, platelet; PRA, plasma renin activity; PTH-int, intact parathyroid hormone; RBC, red blood cell; SS-A, anti-SSA/Ro antibody; SS-B, anti-SSB/Rh antibody; TSH, thyroid-stimulating hormone; WBC, white blood cell.
His chronic use of omeprazole was suspected as the cause of hypomagnesaemia because PPI-induced hypomagnesaemia has been well-documented.1 2 A decrease in intracellular magnesium releases the magnesium-mediated inhibition of ROMK (renal outer medullary potassium) channels on the apical membrane, which increases potassium secretion into the urine.5 His refractory hypokalaemia for 3 years suggested concomitant hypomagnesaemia, which has remained undiagnosed since he was never examined for blood magnesium levels at any medical institutions.
The finding of acidaemia with hypokalaemia suggested RTA. Urine anion gap (UAG) appeared slightly high at 99.5 mEq/L (reference range 20–90), but it could have been overestimated due to lactacidaemia. Positive UAG indicated low or normal NH4 excretion.6 The urine osmolality gap was also roughly estimated from urine osmolality, urinary sodium and urinary potassium. A value below 144 mosmol/kg at best would indicate NH4 excretion of approximately below 72 mEq/L. This value, coupled with chronic metabolic acidosis, suggests impaired excretion of NH4.6 Thus, distal RTA, rather than proximal RTA, would be a more probable aetiology in this case. However, his urine pH was 5.5, which was rather lower than that seen in typical distal RTA. The occurrence of both metabolic acidosis and hypokalaemia-induced renal ammoniagenesis causes excretion of hydrogen ion as NH4 into the urine.6 His elevated blood NH3 levels could be due to both enhanced renal ammoniagenesis and reduced NH4 secretion into the urine. His electrolyte disturbances needed prompt correction and were prioritised over a bicarbonate loading test or ammonium loading test to differentiate any types of RTA, considering that progressive rhabdomyolysis and insidious tachycardia attack were at risk.
Severe hypomagnesaemia can suppress the secretion of intact parathyroid hormone (iPTH) from parathyroid glands.7 In this case, secretions of both iPTH and 1,25-dihydroxyvitamin D were relatively suppressed in spite of severe hypocalcaemia (table 1). The deficit of either iPTH or 1,25-dihydroxyvitamin D by hypomagnesaemia could be the cause of secondary hypocalcaemia.7 8
His blood concentrations of calcium, potassium and CPK were on the borderline of each of their reference ranges 8 months before admission: 7.8 mg/dL (reference range, 8.4–10.2), 3.5 mEq/L (3.6–5.0) and 211 U/L (38-213), respectively. Since hypokalaemia might also contribute to rhabdomyolysis,9 these abnormalities in addition to hypomagnesaemia could have altogether worsened muscle weakness just before admission.
Differential diagnosis
In general, relatively low urinary pH associated with metabolic acidaemia suggested proximal RTA rather than distal RTA because the absolute amount of HCO3− excretion into urine decreases in proximal RTA. In contrast, urine pH increases in distal RTA because of reduced H+ excretion in collecting duct. Additionally, hypocalcaemia could cause proximal RTA.4 We could not determine whether this was a case of concomitant proximal RTA due to the lack of a bicarbonate loading test.
The cause of high-anion-gap metabolic acidosis was considered as the result of hyperlactacidaemia. His high UAG of 99.5 mEq/L suggested persistent potassium loss in urine, which was further supported by the inappropriately high urine potassium/creatinine ratio of 0.51 mEq/mg. Usually, high UAG estimates urinary ammonium excretion except in certain conditions of high anions such as hippurate or ketoacid anions.10 Lactacidaemia could have contributed to an extremely high value of UAG in our case. We were uncertain of the aetiology of the persistent, but the gradual improvement of lactacidaemia lasting 7 days presented in this case.
In summary, our tentative diagnosis was PPI-induced hypomagnesemia with possible distal RTA.
Treatment
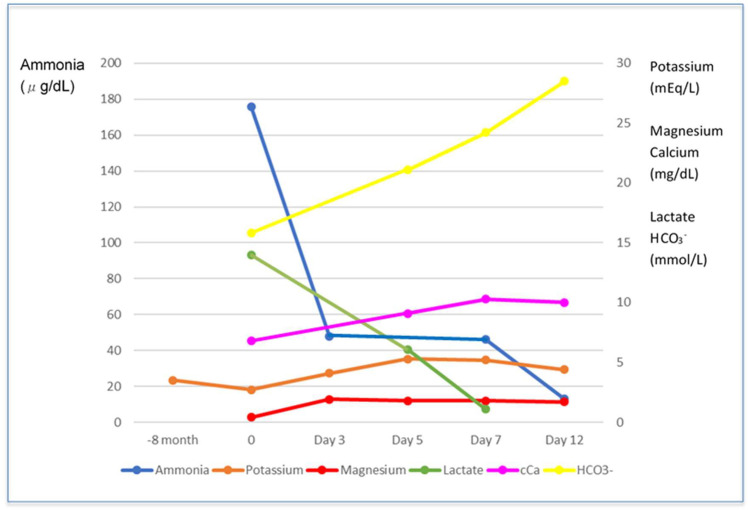
Electrolyte disturbance was first corrected with the venous infusion of magnesium and calcium supplementation, resulting in normal blood ammonia after 3 days (figure 1). It was notable that urinary pH elevated up to 7.0, correlating with blood pH, which could be normalised from above 7.35 without HCO3− supplementation. In proximal RTA, a large amount of HCO3− supplementation was usually needed in addition to potassium supplementation to correct acidaemia. At this point, we excluded proximal RTA involved in autoimmune diseases, light chain deposition disease as differential diagnoses. The indicator for proximal tubule injury was shown in the almost normal values of his spot urine specimens: urinary beta 2-microglobulin 266 µg/L (reference range 0–230) and urinary N-acetyl-beta-d-glucosaminidase 0.9 IU/L (reference range 0.7–11.2).
Figure 1.
Trajectories of blood ammonia, lactate, and bicarbonate, and serum potassium, magnesium, and corrected calcium.
On the third day of admission, potassium supplementation was changed from potassium chloride to potassium citrate because urine alkalisation was generally recommended in hyperuricaemia. Magnesium and calcium supplementation also changed from venous infusion to oral intake on the same day. His acidaemia, hypokalaemia, hypocalcaemia, hypomagnesaemia, hyperammonaemia and hyperlactacidaemia did not relapse after taking magnesium and calcium supplementation.
On the seventh day of admission, omeprazole intake was discontinued after the upper gastrointestinal endoscopy confirmed no other lesions except for previous duodenal ulcer scars. He was discharged with normalised CPK. His medications prescribed on discharge were potassium citrate 1 g, magnesium oxide 0.5 g and allopurinol 100 mg. His leg weakness recovered enough to fulfil daily livings.
Outcome and follow-up
His weakness and lethargy improved, and he was fully ambulant during his follow-up. Magnesium oxide was stopped, and only allopurinol 100 mg was continued to be prescribed. It took 47 days to achieve almost normal serum magnesium concentration (1.5 mg/dL) without magnesium oxide supplementation and omeprazole. He also underwent electrophysiologic examination specifically for his WPW syndrome and received ablation therapy for atrioventricular reciprocating tachycardia by a cardiologist after discharge.
Discussion
In a case series of hypomagnesaemia induced by PPI intake, concomitant hypokalaemia and hypocalcaemia were reported, but did not show any other metabolic abnormalities such as hyperammonaemia and hyperlactacidaemia.3 In the case of distal RTA, hyperammonaemia was caused by compensatory enhanced renal ammonia production, resulting in increased renal vein ammonia delivery.11 Hyperlactacidaemia was also reported in the case of distal RTA.11 To the best of our research on previous reports, our case presented a novel combination of metabolic derangements caused by long-term PPI use.
The latent period of hypomagnesemia caused by PPI was reported to be from 14 days to 13 years.7 The recovery period of hypomagnesemia after discontinuation of PPIs was reported to be only 4 days.7 In our case, it was estimated that hypomagnesaemia developed after at least 8 years of omeprazole ingestion, considering obvious hypokalaemia development and occasional elevation of serum CPK. However, his recovery from hypomagnesemia needed 47 days after discontinuation of omeprazole. The mechanism of magnesium absorption in the intestine after long-term use of PPI has yet been elucidated.
Patient’s perspective.
I was informed regarding lack of potassium in my body by my primary physician. I was given potassium supplementation ever since. The prescription was not switched when I started to see the present physician. Sometimes, my doctor pointed out the abnormality in muscle value observed in the blood examination, but I was asked to follow-up. Last month, I felt weakness in my legs that gradually worsened. I also visited an ear, nose and throat doctor near my house 2 weeks ago because of dizziness. He prescribed herbal medicine but it had no effect, so I stopped taking it. I was disturbed by my low appetite and weakness. I lost consciousness at home and my grandson called an ambulance. I was very surprised that I began to recover my strength to walk by myself after admission. I was quite disgusted by so many prescribed medicines because there was no improvement despite adding new medicine one after another. I never imagined that my stomach medicine was the cause of several disorders. I am very happy that I need only a few kinds of medicine after discontinuation of stomach medicine.
Learning points.
Omeprazole-induced hypomagnesaemia caused renal tubular acidosis with hypokalaemia and hypocalcaemia.
Omeprazole-induced hypomagnesaemia caused high-anion-gap metabolic acidosis with hyperlactacidaemia.
Omeprazole-induced hypomagnesemia caused hyperammonaemia which might contribute to altered mental status.
It took 47 days to achieve full recovery from hypomagnesemia without magnesium supplementation after discontinuing omeprazole.
Footnotes
Contributors: MH was involved in the clinical case, collection of data, and drafted the manuscript. NI was involved in the clinical case and edited the manuscript. Both authors have approved the final manuscript for submission and are accountable for the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kinoshita Y, Ishimura N, Ishihara S. Advantages and disadvantages of long-term proton pump inhibitor use. J Neurogastroenterol Motil 2018;24:182–96. 10.5056/jnm18001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.William JH, Danziger J. Proton-Pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol 2016;5:152. 10.5527/wjn.v5.i2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoorn EJ, van der Hoek J, de Man RA, et al. . A case series of proton pump inhibitor-induced hypomagnesemia. Am J Kidney Dis 2010;56:112–6. 10.1053/j.ajkd.2009.11.019 [DOI] [PubMed] [Google Scholar]
- 4.Yaxley J, Pirrone C. Review of the diagnostic evaluation of renal tubular acidosis. Ochsner J 2016;16:525–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C-L, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007;18:2649–52. 10.1681/ASN.2007070792 [DOI] [PubMed] [Google Scholar]
- 6.Emmett M, Palmer BF. Urine anion and osmolal gaps in metabolic acidosis - UpToDate. Topic 2348 Version 23.0. Available: https://www.uptodate.com/contents/urine-anion-and-osmolal-gaps-in-metabolic-acidosis?sectionName=LimitationsoftheUAG&search=Etiologyanddiagnosisofdistal(type1)andproximal(type2)renaltubularacidosis&topicRef= [Accessed 10 Mar 2020].
- 7.Hess MW, Hoenderop JGJ, Bindels RJM, et al. . Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012;36:405–13. 10.1111/j.1365-2036.2012.05201.x [DOI] [PubMed] [Google Scholar]
- 8.Rude RK, Adams JS, Ryzen E, et al. . Low serum concentrations of 1,25-dihydroxyvitamin D in human magnesium deficiency. J Clin Endocrinol Metab 1985;61:933–40. 10.1210/jcem-61-5-933 [DOI] [PubMed] [Google Scholar]
- 9.Lane R, Phillips M. Rhabdomyolysis. BMJ 2003;327:115–6. 10.1136/bmj.327.7407.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emmett M, Palmer BF. Etiology and diagnosis of distal (type 1) and proximal (type 2) renal tubular acidosis - UpToDate. Topic 2328 Version 24.0. Available: https://www.uptodate.com/contents/etiology-and-diagnosis-of-distal-type-1-and-proximal-type-2-renal-tubular-acidosis?source=history_widget#H7 [Accessed 10 Mar 2020].
- 11.Kurtz I. Renal Tubular Acidosis: H+/Base and Ammonia Transport Abnormalities and Clinical Syndromes. Adv Chronic Kidney Dis 2018;25:334–50. 10.1053/j.ackd.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]