Abstract
Clinical applicability of rapid diagnostic test kit for SARS-CoV-2 antibodies was evaluated. The kit detected antibodies from day 9–56 of illness. IgG bands were observed up to 1: 1000 dilutions. The kit could detect 90.5% of IgG and 61.9% of IgM antibodies of mild febrile patients without pneumonia.
Keywords: Rapid diagnostic test, Antibody, SARS-CoV-2, Titration, Point-of-care test
Introduction
Global spread of coronavirus disease 2019 (COVID-19) outbreak is rapidly progressive.1 Although detection of viral RNA by reverse transcription-polymerase chain reaction (RT-PCR) is the standard for the diagnosis of COVID-19,2 need for serologic test is increasing for an alternative diagnostic method and/or seroprevalence studies. Rapid diagnostic test (RDT) kits for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies are easy to use and require 15 min for the test. To date, many RDT kits detecting SARS-CoV-2 IgM and/or IgG antibodies have been developed, but validation data with various clinical aspects are limited.3 , 4 For clinical application of RDT kits in the field of COVID-19 management, we evaluated performance according to the day of illness, type of specimens, feasibility as a point-of-care test (POCT), severity of illness, and with ten-fold titrations.
Methods
We performed SARS-CoV-2 IgM and IgG antibody tests using a RDT kit that had been used in recent report,4 in eight pneumonic COVID-19 patients and 21 mild febrile COVID-19 patients without pneumonia. SARS-CoV-2 infections of the evaluated patients were confirmed by RT-PCR. Pneumonic patients were admitted at tertiary care hospitals. Pneumonia was documented by chest X-ray and/or computed tomography. Onset of illness and clinical course were clearly documented. Day of illness was counted from the symptom onset (symptom onset day as D1), and the first week of illness denoted illness days from D1 to D7. Mild patients were cared at a life treatment center (LTC),5 meeting all of the following conditions: 1) mentally alert, 2) body-temperature below 37.5 °C at admission, 3) under 60 year of age, 4) no underlying disease, 5) non-smoker, and 6) no radiologic evidence of pneumonia. Among these mild patients, patients who experienced febrile sense, chilling, and/or myalgia were selected. Written informed consents were obtained from the patients. This study was approved by the Institutional Review Boards of the Samsung Medical Center (IRB No. 2020-03-113 and 2020-03-120).
The RDT kits using lateral flow immunoassay principle were assembled at Korea (Wells Bio Inc., Seoul, Korea) using the materials manufactured by Jiangsu Medomics Medical Technology (Nanjing, China).4 It is able to detect both IgM and IgG separately, targeting SARS-CoV-2 spike protein and supposed to use serum, plasma, and whole blood (WB) specimen and the manufacturer reported sensitivity of the kit was 88.66% and specificity was 90.63%. Test was performed according to the manufacturer's instructions.4 Interpretation of the test results is described in the supplementary material in detail.
Results
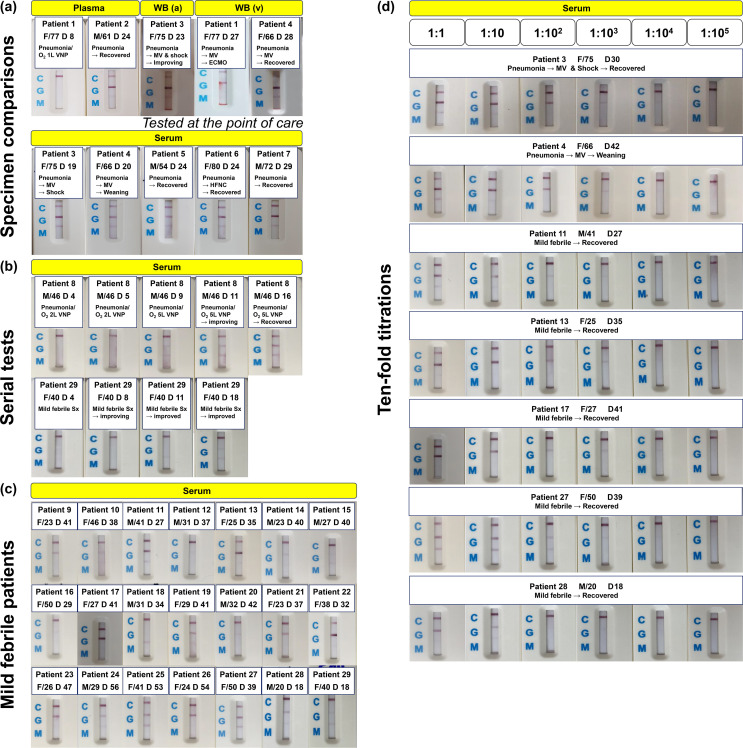
In overall, 52 specimens from 21 mild febrile and 8 pneumonic COVID-19 patients were evaluated (Supplementary Table 1). Among 43 specimens collected after the second week of illness (after D14), 41 specimens showed positive IgG bands (95.3%) and 34 showed positive IgM bands (79.1%), including very weakly positive bands. Pictures of test results are presented in Fig. 1 and supplementary material (large size of pictures as a PDF file).
Figure 1.
Tests for application of RDT kit for SARS-CoV-2 antibody into the field of COVID-19 patient management. (a) Tests using specimens from pneumonic COVID-19 patients. (b) Tests for serial specimens from a patient. (c) Tests using convalescent sera from mild febrile COVID-19 patients without pneumonia. (d) Titration tests with ten-fold dilutions of serum specimens. Pictures with larger size are presented as a supplementary material. Abbreviations: RDT, rapid diagnostic kit; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; WB, whole blood.
Specimens from pneumonic COVID-19 patients and test for various clinical aspects
A total of 28 blood specimens from eight pneumonic COVID-19 patients were tested (Fig. 1 and Supplementary Figure 1 and 2). Among 22 specimens collected from pneumonic patients after the 2nd week of illness (after D14), 22 specimens (100%) were positive for IgG bands and 21 (95.5%) were positive for IgM bands. IgG bands were clear and intense, while IgM bands were relatively fainter than IgG bands.
To assess the feasibility of RDT kit as a POCT, we tested three WB specimens at the bed-side of patients (Fig. 1-(a)). WB from arterial (taken from arterial line) and venous blood (taken from routine peripheral blood sampling) showed strong bands on IgG bands within two minutes after dropping two to three drops of blood on the test wells without adding additional dilution buffers, which were different from manufacturer's instruction as applying 15 μL WB and two to three drops of dilution buffer (Supplementary figure 1; Exact titration of specimen amount could not be done for the bed-side tests, and dilution buffers were intentionally omitted to see if the kit can exhibit test results without adding buffer.).
For the evaluation of test performance according to the types of blood specimens, we compared WB, plasma, and serum specimens taken at the same day from patient 3 (Supplementary Figure 2-(a)). The intensity of IgG band was slightly stronger while IgM band was fainter with WB specimen, compared to plasma and serum specimens. Results using plasma and serum specimens did not show noticeable differences. This difference was also noted with the samples from Patient 4, taken by four-day interval (Supplementary Figure 2-(b)).
To see performance according to the day of illness, we tested serial serum specimens collected from a pneumonic patient (Patient 8) with moderate illness and a mild febrile patient (Patient 29, Fig. 1-(b)). For Patient 8, tests were negative by D5, and IgG and IgM bands were simultaneously positive on D9 with weak intensities for pneumonic patient. IgG bands became more intense with following specimens. As we did not collect specimen between D5 and D9, whether the kit could detect IgM earlier than IgG was not be evaluated for this pneumonic patient. Meanwhile, for Patient 29, the RDT kit could detect earlier response of IgM on D11. IgG band was noticed on D18 specimen for this mild febrile patient.
Diagnostic performance for mild febrile COVID-19 patients without pneumonia
To evaluate feasibility for seroprevalence study, convalescent sera from 21 mild febrile COVID-19 patients without pneumonia were evaluated (Fig. 1-(c)). Eight patients (38.1%) were male and the mean age was 32.2 years old. The sampling day ranged from D18 to D56. Nineteen specimens (90.5%) were positive for IgG bands, including four weakly positive (Patient 10, 14, 20, and 29) and two very weakly positive bands (Patient 15 and 16). Thirteen specimens (61.9%) showed positive IgM bands, including three weakly positive (Patient 11 and 15) and seven very weakly positive bands (Patient 10, 13, 14, 19, 22, 24, and 28). One specimen was negative for both IgG and IgM, and overall intensity of bands was weaker than specimens from pneumonic patients.
Ten-fold titrations using convalescent sera of seven COVID-19 patients
Feasibility of RDT kit as a semi-quantitative method was also evaluated with ten-fold titrations. Convalescent sera collected from two pneumonic patients (Patient 3 and 4) and five mild febrile patients (Patient 11, 13, 17, 27, and 28) were diluted with PBS buffer from 1:10 to 1:100,000 (Fig. 1-(d)). Sera from two pneumonic patients showed positive IgG intensity by 1:100 dilutions, and the bands became weakly positive at 1:1000 dilutions. Meanwhile, among five mild febrile patients, none of specimens showed positive IgG intensity at 1:100 dilutions (compared to pneumonic patients by the Chi-square test, P = 0.048); four showed weakly positive intensity and one was negative.
Discussion
Based on the initial performance data of serologic test kits for emerging diseases,3 clinical feasibility should be evaluated according to the purpose of serologic test. For the diagnostic purpose, we noticed the kit can detect IgG and IgM antibodies from D9 of illness. A published serologic study using enzyme linked immunosorbent assay (ELISA) suggested that the median time of seroconversion was D12 for IgM and D14 for IgG.6 Another report also presented presence of IgM and IgG antibodies from the 3rd week of illness.7 In the present test, it was noticed that the RDT kit detected IgG antibodies in 95.3% of specimens collected after the second week of illness (after D14), suggesting that the performance of the kit would be comparable to previous reports.
Of note, we also tested feasibility of RDT kit as a semi-quantitative method with ten-fold dilution of serum specimen, and noticed that RDT kit could detect IgG antibodies of 1:1000 diluted sera. As of the current COVID-19 pandemic, convalescent plasma infusion therapy is one of potential treatment options for severe COVID-19 patients.8 , 9 For the selection of donor plasma, measurement of functional anti-SARS-CoV-2 antibodies by neutralization test is necessary. However, facilities for neutralization tests are not readily available, non-culture based methods can be used for titrations of antibodies.10 Our finding provided that RDT kit can be used for a semi-quantitative method for assessing amount antibodies, which would be useful in resource limited settings.
Lastly, performance of detecting antibodies in convalescent serum of mild illness was evaluated. The main purpose of seroprevalence study in the current pandemic would be to detect potential undiagnosed COVID-19 patients, who are likely to be mild cases. As antibody response in mild illness would be weak, whether an antibody test kit can detect serologic response in these patients is an important issue. It was noticed that the kit can detect IgG antibodies with high detection rate in mild febrile patients without pneumonia. However, since bands in these patients were relatively faint and serologic response in non-febrile patients can be weaker, more sensitive method to detect weak serologic response should be further studied.
In conclusion, in the test for RDT kit for SARS-CoV-2 antibodies, it detected IgG response from D9 with 95.3% detection rate after the second week of illness (after D14). It could be used as POCT, a semi-quantitative method, and for seroprevalence studies.
Funding
None received.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to express our sincerest condolences to the patients and families who suffered from the COVID-19 outbreak. We also greatly appreciate the health care personnel and staff members who worked together to overcome the COVID-19 outbreak. Finally, we would like to thank Ms. Jin Yang Baek and Dr. Seongman Bae for testing kits, and Wells Bio Inc., Seoul, Korea for donation of the pilot kits and.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2020.07.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Korean Society of Infectious Diseases KSoPID, Korean Society of Epidemiology , Korean Society for Antimicrobial therapy , Korean Society for Healthcare-Associated Infection Control and Prevention and Korea Centers for Disease Control and Prevention Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Kor Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong K.H., Lee S.W., Kim T.S., Huh H.J., Lee J., Kim S.Y. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020;40:351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S. Development and clinical application of A rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiang J., Yan M., Li H., Liu T., Lin C., Huang S. Evaluation of enzyme-linked immunoassay and colloidal gold- immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19) medRxiv. 2020;2020 02.27.20028787. [Google Scholar]
- 5.Peck K.R. Early diagnosis and rapid isolation: response to COVID-19 outbreak in Korea. Clin Microbiol Infect. 2020;26:805–807. doi: 10.1016/j.cmi.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344/5812996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao D.A.T., Gao D.C., Zhang D.S. Profile of specific antibodies to SARS-CoV-2: the first report. J Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Kor Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko J.H., Seok H., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 10.Ko J.H., Muller M.A., Seok H., Park G.E., Lee J.Y., Cho S.Y. Suggested new breakpoints of anti-MERS-CoV antibody ELISA titers: performance analysis of serologic tests. Eur J Clin Microbiol Infect Dis. 2017;36:2179–2186. doi: 10.1007/s10096-017-3043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.