To the Editor,
Since angiotensin converting enzyme 2 (ACE2) was identified as the host receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), speculation has arisen over the impact on the Renin-Angiotensin-Aldosterone System (RAAS).1 ACE2 metabolizes angiotensin II (AngII) to angiotensin 1,7, which counterbalances the vasoconstrictive and pro-inflammatory properties of AngII.2 Animal models of SARS suggested that high AngII, due to attenuation of ACE2 activity by the virus, directly drives lung injury.3 In a study of 12 patients with SARS-CoV-2, Liu et al. reported significantly higher plasma levels of AngII compared to controls. No healthy control had an AngII level >200 pg/ml, while all but one in the infected group had levels >200 pg/ml, with values reaching as high as 500 pg/ml.4 However, methodologic concerns were raised over these measurements.5 As multiple clinical trials have begun to ameliorate the effects of AngII in patients with coronavirus disease-2019 (COVID-19), we aimed to replicate the finding by Liu et al. in a larger sample.
Adults who presented to the University of Cincinnati Medical Center (UCMC) Emergency Department (ED) with suspected COVID-19 and had a clinically indicated blood draw were prospectively enrolled via an institutional review board-approved waiver of informed consent. Samples were obtained in the presence of a protease inhibitor in pre-chilled tubes to prevent degradation of AngII peptides. Following collection, samples were centrifuged at 2000g for 15 min at 4 °C within 3 h of collection and frozen at −80 °C until analysis. Inclusion in this analysis was dependent on a positive reverse transcription polymerase chain reaction (RT-PCR) test for COVID-19 via a standard-of-care nasopharyngeal swab. Patients taking medications targeting the RAAS, such as ACE inhibitors and angiotensin receptor blockers, were excluded. Deidentified samples from healthy, normotensive outpatient adults were used as controls. Plasma concentration of AngII was measured using ELISA (Enzo Life Sciences, Farmingdale, NY, USA). Plasma concentration of aldosterone was measured using Diasorin Liaison XL (DiaSorin S.p.A. Saluggia, Italy).
COVID-19 positive patients were stratified into subgroups based on ED disposition (hospitalized vs. discharged) and need for intensive care unit admission (ICU vs. no ICU). Comparisons of plasma concentrations of AngII and aldosterone were performed using the Mann-Whitney U test. The relationship between AngII and aldosterone was evaluated using the Spearman's rho.
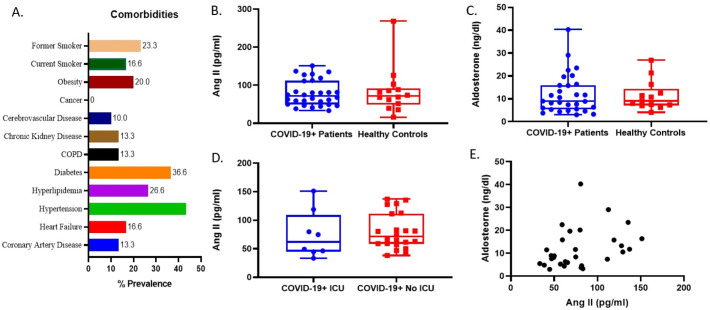
Forty-three COVID-19+ patients were enrolled in the study. Following exclusion of those on RAAS medications, a total of 30 patients were included. Fourteen healthy controls were included as a comparison group. Median age in the control group was 42 (IQR: 37–46) vs. 46.5 (IQR: 37–66) years in the COVID-19 group (p = 0.200). Males made up 60% of COVID-19+ patients and 85% of controls (p = 0.088). Comorbidities of the COVID patients are presented in Fig. 1A. Median blood pressure in the ED was 134 (IQR: 113–141)/72 (IQR: 68–78) mmHg. Eighteen (60%) patients were hospitalized with eight (16.6%) requiring intensive care.
Fig. 1.
Comorbidities of included COVID-19 patients (A). Plasma angiotensin 2 (AngII) and aldosterone levels were comparable in patients with COVID-19 versus healthy controls (B,C). AngII concentrations were similar between COVID-19 patients requiring ICU admission vs. those not requiring ICU admission (D). AngII levels significantly correlated with aldosterone levels in the COVID-19 cohort (p = 0.03) (E).
Median plasma AngII concentrations were nearly equal between patients with COVID-19 and healthy controls (71.4 (IQR: 49.7–97.8) vs. 71.5 (IQR: 65.1–95.8) pg/ml, p = 0.990) (Fig. 1B). Similarly, aldosterone concentrations were comparable in patients with and without COVID-19 (8.9 (IQR: 5.8–16.2) vs. 9.0 (IQR: 7.4–12.2) ng/dL, p = 0.865) (Fig. 1C). When comparing hospitalized vs. discharged COVID-19 patients, there were no significant differences in AngII (80.2 (IQR: 65.4–119.1) vs. 61.0 (IQR: 45.7–71.4) pg/ml, p = 0.185) Likewise, no differences were observed between patients requiring ICU support vs. no ICU support (77.3 (IQR: 62.3–99.5) vs. 71.4 (IQR: 59.5–112.6) pg/ml, p = 0.440) (Fig. 1D). The concentrations of AngII and aldosterone were found to be significantly correlated (rho = 0.39 (95%CI: 0.23–0.66); p = 0.033) (Fig. 1E).
Unlike the observation of Liu et al.,4 we did not observe elevated plasma AngII in patients with COVID-19. There are several important caveats when considering these findings. First, circulating AngII may not necessarily reflect the lung milieu. Thus, our findings do not exclude the potential benefits of RAAS medications. Nonetheless, the lack of detectable AngII systemic spillover raises doubts as to role of this peptide in driving multi-organ disease. Second, measurement of AngII is challenging due to protein degradation. We employed an ELISA-based method to compare our findings to Liu et al. Other biochemical approaches should be applied in future studies. However, as would be expected physiologically, we found consistency in AngII and aldosterone levels, with aldosterone concentration measured on a clinically validated platform. Third, circulating AngII may be reflective of several changes in the RAAS and human physiology.5 The limited sample size prohibits detailed analysis by patient baseline characteristics.
We did not observe any differences in AngII with respect to COVID-19 severity, and importantly, did not observe the drastically elevated AngII levels as reported by Liu et al. The AngII measurements in this study do not necessarily provide insight into the interaction between SARS-CoV-2 and ACE2, as AngII can be metabolized in pathways outside ACE2. Measurements of other RAAS parameters, including ACE and ACE2 are required to fully understand these observations. We encourage replication of our findings in future studies.
Funding
This study was funded by the University of Cincinnati College of Medicine Special Coronavirus (COVID-19) Research Pilot Grant Program. Further material support was provided by CinCor Pharma.
Declaration of competing interest
The authors declare no conflicts of interests.
References
- 1.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-converting enzyme 2 and anti-hypertensives (angiotensin receptor blockers and angiotensin converting enzyme inhibitors) in coronavirus disease 2019 (COVID-19) Mayo Clin Proc. 2020;19 doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry B.M., Vikse J. Clinical characteristics of Covid-19 in China. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 3.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]