General Considerations
The respiratory system is the primary interface for inhaled compounds and the center for gas exchange in the body. Alveoli are tiny, balloon-shaped structures that allow the rapid exchange of oxygen and carbon dioxide between the lungs and pulmonary capillaries (Fig. 155.1 ). For gas exchange to occur, the molecules must be capable of traversing three structures: the alveolar epithelium, the interstitial space, and the capillary endothelium. If ventilation is reduced because of lung disease, or perfusion is decreased through small-vessel vasculitis, lung function is impaired. In addition, inhalation of foreign particles or infection can cause inflammation and mucus production, which may act as potential barriers to gas exchange if these secretions are found on the respiratory surfaces.
Fig. 155.1.
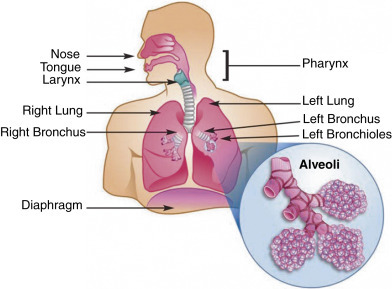
The human respiratory system.
From Quintero D, Guidot DM. Focus on the lung. Alcohol Res Clin Rev. 2010;33[3]:219–228. PubMed PMID: 23584063.
The airway distal to the larynx is normally sterile owing to several protective mechanisms, both mechanical and humoral. The mucus-covered ciliated epithelium that lines the lower respiratory tract propels sputum to the larger bronchi and trachea, evoking the cough reflex. The respiratory secretions contain substances that exert nonspecific antimicrobial actions: alpha1 antitrypsin, lysozyme, and lactoferrin. At the level of the alveoli, potent defense mechanisms are present, including alveolar macrophages, a rich vasculature capable of rapidly delivering lymphocytes and granulocytes, and an efficient lymphatic drainage network.
Bronchitis is inflammation of the mucous membranes that line the bronchi, the airways that carry air to and from the lungs. Pneumonia is inflammation of lung tissue caused by a bacterial, viral, or fungal infection in one or both lungs accompanied by infiltration and inflammation of the alveoli. Both acute bronchitis and pneumonia are characterized by the development of a cough with or without the production of sputum.
Acute bronchitis often occurs during the course of an acute viral illness such as the common cold or influenza. Viruses cause about 90% of cases of acute bronchitis. Chronic bronchitis is one type of chronic obstructive pulmonary disease (COPD) and is characterized by recurrent episodes of bronchitis for at least 3 months over 2 or more consecutive years. In chronic bronchitis, innate immune cells, including macrophages and neutrophils, increase the levels of airway inflammation through the excessive secretion of cytokines and chemokines that recruit and activate other immune cells and release tissue-destructive proteases.1 Several pulmonary toxicants have been found to be associated with chronic bronchitis, including particulate matter (i.e., air pollution), organic dusts (e.g., grain, hay, animal by-products, microorganisms), silicates, gases (e.g., nitrous oxides, methane, ozone), mycotoxins, pesticides, and metals (e.g., arsenic, cadmium, lead). Cigarette smoking is the major risk factor for COPD and continues to be one of the leading causes of cigarette smoke–related death worldwide.2
Although pneumonia may appear in healthy individuals, it is usually seen in those who are immune compromised, particularly drug and alcohol abusers. The growing population of those with chronic lung diseases and other debilitating illnesses and a history of the use of respiratory therapy, immunosuppressive drugs, and other such technologies has contributed to the further increase of nosocomial and opportunistic pneumonias, which have high mortality rates. Acute pneumonia is the seventh-leading cause of death in the United States.3 It is particularly dangerous in the elderly.
In healthy individuals, pneumonia most often follows an insult to the host defense mechanisms: viral infection (especially influenza), cigarette smoke and other noxious fumes, impairment of consciousness (which depresses the gag reflex, allowing aspiration), neoplasms, and hospitalization (Table 155.1 ). In immunocompetent, nonelderly adults, cigarette smoking is the strongest independent risk factor for invasive pneumococcal disease.4
Table 155.1.
Etiologies of Common Pneumonias
| Type | Percentage |
|---|---|
| Viral (influenza) | 20 (3) |
| Mycoplasmal | 10–20 |
| Bacterial | 12 |
| Bacterial superimposed on viral | 6 |
| Chlamydia | 10 |
| Unknown cause (Legionnaires’ disease, toxic) | 38 |
Data from Branch WT Jr. Office practice of medicine. Philadelphia: Saunders; 1982: 57–76.
Diagnostic Summary
Bronchitis
The diagnosis of acute bronchitis is usually made by ruling out other causes of an acute cough—such as pneumonia, the common cold, acute asthma, or an exacerbation of chronic obstructive pulmonary disease (Fig. 155.2 ).
Fig. 155.2.
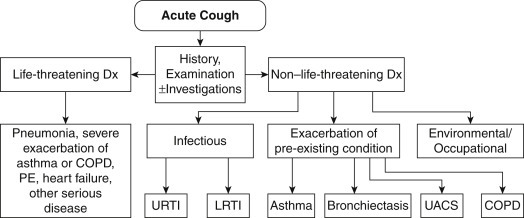
Algorithm for assessment of acute cough in patients 15 or more years of age. COPD, Chronic obstructive pulmonary disease; LRTI, lower respiratory tract infection; UACS, upper airway cough syndrome; URTI, upper respiratory tract infection.
From Dicpinigaitis PV, Colice GL, Goolsby MJ, Rogg GI, Spector SL, Winther B. Acute cough: a diagnostic and therapeutic challenge. Cough. 2009;5:11. PubMed PMID: 20015366.
In patients with the presumed diagnosis of acute bronchitis, viral cultures, serological assays, and sputum analyses should not be routinely performed because the responsible organism is rarely identified in clinical practice.
In patients with acute cough and sputum production suggestive of acute bronchitis, the absence of the following findings reduces the likelihood of pneumonia sufficiently to eliminate the need for a chest radiograph: (1) heart rate greater than 100 beats per minute; (2) respiratory rate greater than 24 breaths per minute; (3) oral body temperature above 38°C; and (4) chest examination findings of focal consolidation, egophony, or fremitus.
Pneumonia
The diagnosis of pneumonia is usually made by physical examination and confirmed by a chest x-ray. Common physical examination findings include:
-
•
Rales (a bubbling or crackling sound)—Rales on one side of the chest and rales heard while the patient is lying down are strongly suggestive of pneumonia.
-
•
Rhonchi (abnormal rumblings indicating the presence of thick fluid)
-
•Percussion—A dull thud instead of a healthy hollow, drum-like sound indicates certain conditions that suggest pneumonia, including:
-
•Consolidation (a condition in which the lung becomes firm and inelastic)
-
•Pleural effusion (fluid buildup in the space between the lungs and the surrounding lining)
-
•
Examination of the sputum suggestive of infection includes the presence of blood; a positive Gram stain; and thick, opaque, yellow-, green-, or brown-colored sputum. Sputum culture and sensitivity are not always helpful in identifying the cause of pneumonia due to contamination of the sample with throat or mouth bacteria.
A urine test (Binax NOW, Binax Inc., Scarborough, Maine) can detect Streptococcus pneumonia or Legionella pneumophila antigens within 15 minutes. It may identify up to 77% of pneumonia cases and may rule out the infection in 98% of patients who do not have S. pneumoniae. However, the test is not very useful in diagnosing S. pneumoniae as a cause of pneumonia in children because the organism is common in this population with or without pneumonia. L. pneumophila is the bacterium that causes Legionnaires’ disease, a severe form of pneumonia.
A chest x-ray is nearly always taken to confirm a diagnosis of pneumonia, but a positive result is not necessary to make the clinical diagnosis. A positive chest x-ray for pneumonia may reveal lung infiltrates or complications of pneumonia such as pleural effusions.
Special Considerations With Pneumonia
There are more than 100 types of bacteria, viruses, and fungi known to cause bronchitis or pneumonia. The three most common forms of pneumonia are viral, mycoplasmal, and pneumococcal.
Viral pneumonia
Viral pneumonia is most often caused by one of several viruses: adenovirus, influenza, parainfluenza, and respiratory syncytial virus. Viral pneumonia is responsible for about 30% of pneumonia cases and may develop as a complication of viral upper respiratory infections. Immunocompromised individuals are at risk for more serious viral pneumonia. Antibiotics are of no value in treating viral pneumonia unless a secondary bacterial infection develops.
Clinical summary for viral pneumonia
-
•
People who are at risk for more serious viral pneumonia are often immunocompromised.
-
•
Antibiotics are of no value in viral pneumonia.
-
•
Symptoms of viral pneumonia often begin slowly and may not be severe at first.
-
•The most common symptoms of viral pneumonia are as follows:
-
•Cough (some patients with pneumonia may cough up mucus or even bloody mucus)
-
•Fever, which may be mild or high
-
•Shaking chills
-
•Shortness of breath (may only occur when on merely climbing stairs)
-
•
Mycoplasmal pneumonia
Mycoplasma is a genus of bacteria that lack cell walls. Mycoplasmal pneumonia is caused by the bacterium Mycoplasma pneumoniae. Various studies suggest that M. pneumoniae is responsible for 15% to 50% of all cases of pneumonia in adults and even more than those in school-age children. It is often referred to as “walking pneumonia” because symptoms tend to be milder than pneumonia caused by other organisms. M. pneumoniae infections are one of the most common etiologies of community-acquired pneumonia (CAP). Antibiotics are usually not necessary but may speed recovery. Effective classes of antibiotics that may be effective against M. pneumoniae include macrolides, quinolones, and tetracyclines.
Clinical summary for mycoplasmal pneumonia
-
•
Most commonly occurs in children or young adults
-
•
Insidious onset over several days
-
•
Nonproductive cough, minimal physical findings, temperature generally less than 102°F
-
•
Headache and malaise are common early symptoms
-
•
White blood cell count is normal or slightly elevated
-
•
X-ray pattern is patchy or inhomogeneous
Pneumococcal pneumonia
Pneumococcal pneumonia (due to S. pneumoniae) is the most common bacterial pneumonia and the most common cause of pneumonia requiring hospitalization. Careful clinical judgment is necessary in determining the severity of the disease and the status of the patient’s immune system because it is often necessary to administer antibiotics or to refer for hospitalization, especially for elderly or immunocompromised patients.
Unfortunately, most reports show an increase in resistance rates to antibiotic therapy and an increase in the proportion of highly resistant strains.5, 6, 7 In two multinational studies, the worldwide prevalence of penicillin- and macrolide-resistant S. pneumoniae ranged from 18% to 22% and from 24% to 31%, respectively.8, 9 Given this information, it is important to consider natural treatments in cases resistant to antibiotics or as an adjunctive treatment to strengthen the immune response and increase the therapeutic effect.
Clinical summary for pneumococcal pneumonia
-
•
Pneumonia is usually preceded by upper respiratory tract infection.
-
•
There is a sudden onset of shaking, chills, fever, and chest pain.
-
•
Sputum is pinkish or blood-specked at first, then becomes rusty at the height of the infection, and finally becomes yellow and mucopurulent during resolution.
-
•
Gram-positive diplococci are present in the sputum smear.
-
•
A rapid urine test (Binax NOW) for S. pneumoniae antigens is positive.
-
•
Initially, chest excursion is diminished on the involved side, breath sounds are suppressed, and fine inspiratory rales are heard.
-
•
Later, classic signs of consolidation appear (bronchial breathing, crepitant rales, dullness).
-
•
Leukocytosis is present.
-
•
Radiograph shows lobar or segmental consolidation.
Therapeutic Considerations
The basic approach in the treatment of bronchitis and pneumonia is to use an expectorant, mucolytic, and immune-supportive nutrients. Although antibiotics are of limited value in acute bronchitis, they certainly play a role in treating pneumonia.
Expectorants
Botanical expectorants have a long history of use in bronchitis and pneumonia. Because impaired cough reflexes have been thought to play a role in recurrent bronchitis and pneumonia,10 it seems reasonable that these botanicals would be useful in helping relieve this condition and preventing recurrences. Botanical expectorants act to increase the quantity, decrease the viscosity, and promote expulsion of the secretions of the respiratory mucous membranes. Many also have antibacterial and antiviral activity. Some expectorants are also antitussives; however, Lobelia inflata, a commonly used expectorant, helps promote the cough reflex.11 Therefore Lobelia may be more effective at clearing the lungs than other expectorants when the cough is productive. Other commonly used expectorants include Glycyrrhiza glabra (licorice), Pelargonium sidoides (South African geranium), Hedera helix (ivy), and wild cherry bark.
Pelargonium sidoides (South African Geranium)
P. sidoides is a medicinal plant in the geranium family that is native to South Africa. Umckaloaba, its common name, is a close approximation of the word in the Zulu language that means “severe cough” and is a testimony to its effect in bronchitis. The plant has an intricate grouping of thick dark-red rhizomes and tubers underground that allows it to withstand the frequent grass fires in its habitat. Extracts from the rhizomes and tubers have been shown to exert a number of effects beneficial in upper respiratory tract infections, particularly bronchitis. Most of the research has been conducted using an ethanolic extract known as EPs 7630 (also marketed as Umcka), and it is an approved drug for the treatment of acute bronchitis in Germany. It is produced using 11% ethanol to yield a drug/extract ratio of 1:8 to 10. The primary active ingredients include highly oxygenated coumarins (e.g., umckalin) and polyphenolic compounds.12
Research with EPs 7630 shows that it exerts a three-pronged approach in acute bronchitis: (1) it enhances immune function; (2) it has some antimicrobial effects, including antimycobacterial13 and antiviral activity,14 and appears to inhibit the attachment of bacteria, viruses, and perhaps other organisms to mucous membranes of the respiratory tract12; and (3) it acts as an expectorant.12 These effects are mediated by the activation of macrophages (with the involvement of cytokine interferon-gamma) and the consequent increase in the production of nitric oxide (NO) (Fig. 155.3 ). Regarding its antiviral effects, EPs 7630, at concentrations up to 100 mcg/mL, interfered with the replication of seasonal influenza A virus strains (H1N1, H3N2), respiratory syncytial virus, human coronavirus, parainfluenza virus, and coxsackievirus but did not affect replication of highly pathogenic avian influenza A virus (H5N1), adenovirus, or rhinovirus.14
Fig. 155.3.
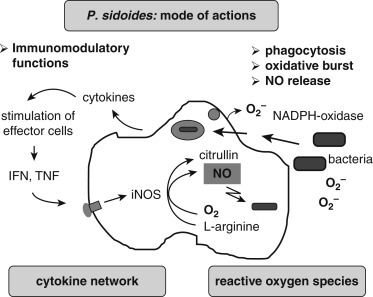
Illustration of cytotoxic defense mechanisms of activated macrophages induced by the root extract of Pelargonium sidoides.
From Kolodziej H. Antimicrobial, antiviral, and immunomodulatory activity studies of Pelargonium sidoides 9Eps 7630) in the context of health promotion. Pharmaceuticals (Basel). 2011;4[10]:1295–1314. PubMed PMID: 27721327.
A 2008 meta-analysis of four randomized clinical trials (RCTs) of EPs 7630 comprising 1647 patients with acute bronchitis supports its safety and efficacy in acute bronchitis.15 Inclusion criteria required patients to have been diagnosed with acute bronchitis within 48 hours and that they should not have received antibiotic therapy and had no obvious contraindications to therapy. The primary outcome or review of efficacy for most of the trials involved changes in the Bronchitis Severity Score (BSS), which includes the symptoms of coughing, expectoration, chest pain, dyspnea, and wheezing from baseline versus after 7 days of treatment. Included in this review was a double-blind placebo-controlled study in 468 adults with recent onset of acute bronchitis who were given either placebo or EPs 7630 for 1 week. The results showed a significantly greater improvement in symptoms in the treatment group compared with the placebo group. On average, participants who received the real treatment were able to return to work 2 days earlier than those given placebo. In another included study, 742 children with acute bronchitis showed a drop of at least 80% in the severity of component symptoms of the BSS within 2 weeks of therapy, and over 88% of the treating physicians rated the performance as “successful.”
In 2018 a review of eight RCTs found that the early administration of EPs 7630 not only reduced the severity of symptoms resulting from infection of the airways but also preponed the start of symptom improvement, with a reduction of illness duration and an earlier resumption of usual activities in the affected patients.16
Additional studies provide evidence of the safety and efficacy of EPs 7630 in acute bronchitis as well as further insight on dosage. In one study, 406 patients with acute bronchitis were randomly assigned to one of four parallel treatment groups—10-mg EPs 7630 tablets three times a day (30-mg group), 20-mg EPs 7630 tablets three times a day (60-mg group), 30-mg EPs 7630 tablets three times a day (90-mg group), or placebo three times a day (control group)—for a treatment period of 7 days.17 The primary endpoint was the change in the total BSS score from baseline to day 7. Between day 0 and day 7, the mean BSS score decreased by 2.7 (control group), 4.3 (30-mg group), 6.1 (60-mg group), and 6.3 (90-mg group), respectively. These results indicated that the 20-mg tablets of EPs 7630 taken three times daily constitute the optimal dose. Similar results were seen in a study of 400 children with acute bronchitis using the same dosage assessment.18
In another study, 200 children with acute bronchitis were randomized to receive either EPs 7630 in liquid form or placebo for 7 consecutive days.19 Dosage was based on age: from ages 1 to 6 years, 10 drops three times daily; from ages 6 to 12 years, 20 drops three times daily; and from ages 12 to 18 years, 30 drops three times daily. From baseline to day 7, the mean BSS score improved significantly more for EPs 7630 compared with placebo (3.4 vs. 1.2 points). On day 7, secondary measures of treatment outcome were significantly better, satisfaction with treatment more pronounced, onset of effect faster, and time of bed rest shorter compared with placebo.
In clinical studies with umcka in more than 2500 adults and children, adverse events occurred on par with a placebo and mainly consisted of mild gastrointestinal complaints and skin rashes. There were no known drug interactions.12
Hedera helix (Ivy)
In Europe, herbal preparations containing extracts from the leaves of ivy enjoy great popularity for the relief of cough as well as asthma. In 2007 more than 80% of herbal expectorants prescribed in Germany comprised ivy extract and amounted to nearly 2 million prescriptions nationwide. Ivy leaf contains saponins that show expectorant, mucolytic, spasmolytic, bronchodilatory, and antibacterial effects. The mucolytic and expectorant action of ivy is based on indirect β2-adrenergic effects, and this action is due to the saponins α-hederin and hederacoside C, the latter of which is metabolized to α-hederin when ingested. The indirect effect is that α-hederin inhibits the intracellular uptake of β2 receptors and leads to an increased β2-adrenergic response of the cell.20
Ivy is often used as a monopreparation, with very good safety, compliance, and efficacy ratings from postmarketing surveillance studies in both acute and chronic bronchitis.21, 22
A randomized, placebo-controlled, double-blind trial was conducted to assess the efficacy and safety of ivy leaves cough liquid in the treatment of 181 adult patients with acute cough. 23 Participants were treated with either ivy leaves cough liquid containing EA 575 or with placebo three times a day for 1 week. The evaluation of the visual analogue scale (VAS), BSS, and Verbal Category Descriptive (VCD) score revealed that subjects treated with ivy leaves cough liquid showed statistically significant and clinically relevant reductions in cough severity, severity of symptoms associated with cough, and bronchitis compared with the placebo group. In addition, an early onset of efficacy was observed: significant reductions of cough severity were detected within 48 hours after the first intake. Treatment advantage was observed at all following visits and even 7 days after the end of treatment compared with placebo.
One double-blind study in acute bronchitis used a combination of ivy and thyme (Thymus vulgaris).24 The 361 patients with acute bronchitis and 10 or more coughing fits during the day, onset of bronchial mucus production with impaired ability to cough up at a maximum of 2 days before recruitment, and a BSS score of greater than or equal to 5 points were randomly assigned to an 11-day treatment (5.4 mL three times daily) with either thyme–ivy combination syrup or placebo syrup. The mean reduction in coughing fits on days 7 to 9 relative to baseline was 68.7% under the thyme–ivy combination compared with 47.6% under the placebo. In the thyme–ivy combination group, a 50% reduction in coughing fits from baseline was reached 2 days earlier compared with the placebo group. The symptoms of acute bronchitis (BSS) improved rapidly in both groups, but the regression of symptoms was faster, and the responder rates compared with placebo were higher at visit 2 (83.0% vs. 53.9%) and visit 3 (96.2% vs. 74.7%) under the treatment of the thyme–ivy combination. Treatment was well tolerated, with no difference in the frequency or severity of side effects between the thyme–ivy combination and placebo groups.
Mucolytics
A mucolytic agent should be used to improve the quality of the mucus secretions so as to promote expectoration. Guaifenesin (also known as glycerol guiacolate) is a derivative of a compound originally isolated from beech wood. Guaifenesin is an approved over-the-counter expectorant and mucolytic. Alternatives include N-acetylcysteine and bromelain.
N-Acetylcysteine
N-acetylcysteine (NAC) has an extensive history of use as a mucolytic in the treatment of acute and chronic lung conditions. It directly splits the sulfur linkages of mucoproteins, thereby reducing the viscosity of bronchial and lung secretions. As a result, it improves bronchial and lung function, reduces cough, and improves oxygen saturation in the blood.
NAC is helpful in all lung and respiratory tract disorders, especially chronic bronchitis and COPD. A detailed analysis of 39 trials concluded that oral NAC reduces the risk of exacerbations (severe worsening) and improves symptoms in patients with chronic bronchitis compared with placebo.
In addition to its effects as a mucolytic, NAC increases the synthesis of glutathione, a major antioxidant for the entire respiratory tract and lungs. The typical dosage for NAC is 200 mg three times daily.
Bromelain
Bromelain is a useful adjunctive therapy for bronchitis and pneumonia owing to its fibrinolytic, anti-inflammatory, and mucolytic actions as well as enhancement of antibiotic absorption.25 Bromelain’s mucolytic activity is responsible for its effectiveness in respiratory tract diseases, including pneumonia, bronchitis, and sinusitis.26
Immune and Barrier Function Support
Vitamin C
In the early part of the 20th century, before the advent of effective antibiotics, many controlled and uncontrolled studies demonstrated the efficacy of large doses of vitamin C in bronchitis and pneumonia but only when they were started on the first or second day of infection.27 If administered later, vitamin C tended only to lessen the severity of the disease. Researchers also demonstrated that in pneumonia, white blood cells take up large amounts of vitamin C.
The value of vitamin C supplementation in elderly patients with pneumonia was demonstrated in a double-blind study of 57 elderly patients hospitalized for severe acute bronchitis and pneumonia.28 The patients were given either 200 mg/day of vitamin C or a placebo. Patients were assessed by clinical and laboratory methods (vitamin C levels in the plasma, white blood cells, and platelets; sedimentation rates; and white blood cell counts and differential). Patients receiving vitamin C demonstrated substantially increased vitamin C levels in all tissues, even in the presence of an acute respiratory infection. Using a clinical scoring system based on major symptoms of respiratory infections, results indicated that the patients receiving the vitamin C fared significantly better than those on placebo. The benefit of vitamin C was most obvious in patients with the most severe illness, many of whom had low plasma and white blood cell levels of vitamin C on admission.
Vitamin A
Vitamin A supplementation appears to be of value, especially in children with measles. This may be because of the increased rate of excretion of vitamin A found during severe infections such as pneumonia. One study evaluated 29 patients with pneumonia and sepsis and found that their mean excretion rate of vitamin A was 0.78 mmol/day. Subjects with fever excreted significantly more retinol than did those without fever. A remarkable 34% of the patients excreted more than 1.75 mmol/day of retinol, which is equivalent to 50% of the U.S. recommended daily allowance.29
This may be particularly important for children. A randomized, double-blind trial of 189 children with measles (average age 10 months) in South Africa evaluated the efficacy of vitamin A in reducing complications. Providing 400,000 IU (120 mg of retinyl palmitate), one-half on admission and one-half a day later, reduced the death rate by more than 50% and the duration of pneumonia, diarrhea, and hospital stay by 33%.30
However, another study did not show any benefit from vitamin A supplementation. The difference may be due to the lower dose (100,000 IU) used in the second study or that vitamin A was not limited to children with pneumonia as a complication of measles, a condition known to also decrease vitamin A levels.31
Evidence also indicates there are positive results using vitamin A concomitantly with zinc supplementation. One study of 2482 children aged 6 months to 3 years revealed that those children given initial high doses of vitamin A followed by 4 months of elemental zinc (10 mg/day for infants and 20 mg/day for children older than 1 year) brought about a reduced incidence of pneumonia, which was not seen in the group given only vitamin A.32
Vitamin E
Patients with influenza complicated by pneumonia experience a sharp rise in lipid peroxidation (LPO) products, especially those who are seriously ill. Administration of α-tocopherol promotes a significant decrease in the levels of lipid peroxidation products and a more benign clinical course.33
Garlic
Allium sativum (garlic) has exhibited a broad spectrum of antibiotic activity against both gram-positive and gram-negative bacteria.34 In vitro studies have demonstrated garlic to be an effective antibacterial agent against S. pneumoniae.35 Therefore its use should be considered in cases of antibiotic resistance or as an adjunct to antibiotic therapy. Alternatively, berberine-containing plants like Hydrastis canadensis (goldenseal) may be helpful.
Bottle Blowing and Salt Pipes
A Swedish study was carried out with 145 adults hospitalized for community-acquired pneumonia.36 These patients were divided into three groups. Group A was given early mobilization with no breathing-associated exercises, group B was instructed to sit up and take 20 deep breaths 10 times daily, and group C was instructed to sit up and blow bubbles in a bottle containing 10 mL water through a plastic tube 20 times on 10 occasions daily. In this study, length of hospitalization was significantly modified in groups B and C: group A patients were hospitalized for a mean of 5.3 days, group B for 4.6 days, and group C for only 3.9 days. The number of days with fever was lowest in the bottle-blowing group. It should be noted that early mobilization itself is known to significantly decrease hospital stays in pneumonia patients.37 Despite the positive clinical results, C-reactive protein levels, peak expiratory flow, and vital capacity were not significantly affected.
Although it is not completely understood why the patients who performed bottle blowing had shorter hospital stays, it seems that the changes in respiratory pressure associated with this exercise may be involved in providing an environment for more efficient bacterial clearance. Another study also found decreased impairment of pulmonary function and an increase in total lung capacity in patients who had undergone coronary artery bypass.38 This modality or another similar activity, like playing a wind instrument, may well prove useful as a means of decreasing the frequency and duration of respiratory events in patients who are vulnerable to respiratory infections like pneumonia.
An alternative to bubble blowing is the use of a salt pipe. These pipes are inhaler-type devices containing tiny salt particles said to ease breathing. The practice originated in central Europe, where individuals with respiratory complaints would spend time in salt caves or mines to help relieve their breathing problems.
Therapeutic Approach
As previously mentioned, the basic approach is to use an expectorant, mucolytic, and immune-supportive nutrients to help resolve the condition. Some general physical modalities and measures that may be helpful include the following:
-
•
Diathermy to chest and back: 30 min/day
-
•
Mustard poultice: once/day
-
•
Lymphatic massage: three times a day
-
•
Postural drainage: three times a day
-
•
Bottle-blowing therapy: blowing bubbles in a bottle containing 10 mL water through a plastic tube 20 times on 10 occasions a day
-
•
Getting plenty of rest
-
•
Drinking enough liquids
-
•
Using a humidifier
Expectorants
Choose one or more of the following:
Lobelia inflata
-
•
Dried herb: 50 to 200 mg three times a day
-
•
Tincture: 10 to 20 drops three times a day
-
•
Fluid extract: 8 to 10 drops three times a day
Glycyrrhiza glabra
-
•
Powdered root: 1 to 2 g three times a day
-
•
Fluid extract (1:1): 2 to 4 mL (0.5–1 tsp) three times a day
-
•
Solid (dry-powdered) extract (4:1): 250 to 500 mg three times a day
Pelargonium sidoides
Dosage recommendations for EPs 7630 or equivalent preparation are as follows:
Adults: 1.5 mL three times a day or 20-mg tablets three times a day for up to 14 days
Children: age 7 to 12 years, 20 drops (1 mL) three times a day; age 6 years or less, 10 drops (0.5 mL) three times a day
Hedera helix
Ivy leaf is available primarily in tincture and fluid extract forms and the dry powdered extract in capsules and tablets. Based on clinical studies, the daily dosages are to deliver the following equivalent to dried herbal substance: 1 to 5 years: 150 mg; 6 to 12 years: 210 mg; above 12 years: 420 mg. Therefore the typical dosage for adults and children over 12 years of age for a 4:1 dried powdered extract is 100 mg daily.
Mucolytics
Choose one or more of the following.
Guaifenesin
Adults and children 12 years of age and older: 200 to 400 mg every 4 hours. It is inadvisable to take more than 2400 mg in a 24-hour period.
The dosage for children age 6 to 11 years is 100 to 200 mg every 4 hours and no more than 1200 mg in a 24-hour period. For children age 2 to 5 years, 50 to 100 mg every 4 hours and no more than 600 mg in 24 hours. Guaifenesin is not recommended for children under 2 years of age.
N-Acetylcysteine
-
•
200 mg three times a day
Bromelain (1200–1800 Milk Clotting Units [MCU])
-
•
500 to 750 mg three times a day between meals
Supplements
-
•
Vitamin A: 50,000 IU/day for 1 week or beta-carotene 200,000 IU/day (Note: Vitamin A should not be used in menstruating women owing to possible teratogenic effects.)
-
•
Vitamin C: 500 mg every 2 hours
-
•
Vitamin E: 200 IU/day
-
•Choose one of the following:
- Bioflavonoids (mixed citrus): 1000 mg/day
- Grape seed (Vitis vinifera) extract (95% procyanidolic oligomers) 150 to 300 mg/day
- Pine bark extract (Pinus pinaster) 150 to 300 mg/day
-
•
Zinc: 30 mg/day
Additional Recommendations for Pneumococcal Pneumonia
Choose one or both of the following.
Garlic
A commercial garlic product should provide a daily dose equal to at least 4000 mg of fresh garlic, which translates to at least 10 mg alliin or a total allicin potential of 4000 mcg (see Chapter 50, Allium cepa).
Hydrastis canadensis
Given berberine’s broad-spectrum antimicrobial activity and immune-enhancing effects, berberine-containing plants are an important consideration. The dosage should be based on berberine content. Because of the wide range of quality in goldenseal preparations, standardized extracts are recommended. The following dosages are intended to be given three times daily:
-
•
Dried root or as an infusion (tea): 2 to 4 g
-
•
Tincture (1:5): 6 to 12 mL (1.5–3 tsp)
-
•
Fluid extract (1:1): 2 to 4 mL (0.5–1 tsp)
-
•
Solid (powdered dry) extract (4:1 or 8%–12% alkaloid content): 250 to 500 mg
References
See www.expertconsult.com for a complete list of references.
Footnotes
Previous edition contributor.
References
- 1.Tetley T.D. Inflammatory cells and chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy. 2005;4(6):607–618. doi: 10.2174/156801005774912824. PubMed PMID: 17305517. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses – United States, 2000-2004. MMWR. Morbidity Mortality Weekly Report. 2008;57(45):1226–1228. PubMed PMID: 19008791. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Vital Statistics Reports. 2003;52(9):9. [Google Scholar]
- 4.Nuorti J.C., Butler J.C., Farley M.M., et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 5.Bauer T., Ewig S., Marcos M.A., et al. Streptococcus pneumoniae in community-acquired pneumonia: how important is drug resistance? Med Clin North Am. 2001;85:1367–1379. doi: 10.1016/s0025-7125(05)70385-0. [DOI] [PubMed] [Google Scholar]
- 6.Cunha B.A. Clinical relevance of penicillin-resistant Streptococcus pneumoniae. Semin Respir Infect. 2002;17:204–214. doi: 10.1053/srin.2002.34686. [DOI] [PubMed] [Google Scholar]
- 7.Garau J. Treatment of drug-resistant pneumococcal pneumonia. Lancet Infect Dis. 2002;2:404–415. doi: 10.1016/s1473-3099(02)00316-x. [DOI] [PubMed] [Google Scholar]
- 8.Felmingham D. Evolving resistance patterns in community-acquired respiratory tract pathogens: first results from the PROTEKT global surveillance study. Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin. J Infect. 2002;44(suppl A):3–10. [PubMed] [Google Scholar]
- 9.Jacobs M.R., Felmingham D., Appelbaum P.C., et al. The Alexander Project 1998–2000: susceptibility of pathogens isolated from community-acquired lower respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother. 2003;52:229–246. doi: 10.1093/jac/dkg321. [DOI] [PubMed] [Google Scholar]
- 10.Niimi A., Matsumoto H., Ueda T., et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax. 2003;58:152–153. doi: 10.1136/thorax.58.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cambar P.J., Shore S.R., Aviado D.M. Bronchopulmonary and gastrointestinal effects of lobeline. Arch Int Pharmacodyn. 1969;177:1–27. [PubMed] [Google Scholar]
- 12.Brendler T., van Wyk B.E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae) J Ethnopharmacol. 2008;119(3):420–433. doi: 10.1016/j.jep.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Kim C.E., Griffiths W.J., Taylor P.W. Components derived from Pelargonium stimulate macrophage killing of Mycobacterium species. J Appl Microbiol. 2009;106(4):1184–1193. doi: 10.1111/j.1365-2672.2008.04085.x. [DOI] [PubMed] [Google Scholar]
- 14.Michaelis M., Doerr H.W., Cinatl J., Jr. Investigation of the influence of EPs([R]) 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine. 2011;18(5):384–386. doi: 10.1016/j.phymed.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agbabiaka T.B., Guo R., Ernst E. Pelargonium sidoides for acute bronchitis: a systematic review and meta-analysis. Phytomedicine. 2008;15(5):378–385. doi: 10.1016/j.phymed.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Careddu D., Pettenazzo A. Pelargonium sidoides extract EPs 7630: a review of its clinical efficacy and safety for treating acute respiratory tract infections in children. Int J General Med. 2018;11:91–98. doi: 10.2147/IJGM.S154198. PubMed PMID: 29563828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthys H., Lizogub V.G., Malek F.A., et al. Efficacy and tolerability of EPs 7630 tablets in patients with acute bronchitis: a randomised, double-blind, placebo-controlled dose-finding study with a herbal drug preparation from Pelargonium sidoides. Curr Med Res Opin. 2010;26(6):1413–1422. doi: 10.1185/03007991003798463. [DOI] [PubMed] [Google Scholar]
- 18.Kamin W., Maydannik V., Malek F.A., et al. Efficacy and tolerability of EPs 7630 in children and adolescents with acute bronchitis—a randomized, double-blind, placebo-controlled multicenter trial with a herbal drug preparation from Pelargonium sidoides roots. Int J Clin Pharmacol Ther. 2010;48(3):184–191. doi: 10.5414/cpp48184. [DOI] [PubMed] [Google Scholar]
- 19.Kamin W., Maydannik V.G., Malek F.A., et al. Efficacy and tolerability of EPs 7630 in patients (aged 6-18 years old) with acute bronchitis. Acta Paediatr. 2010;99(4):537–543. doi: 10.1111/j.1651-2227.2009.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieben A., Prenner L., Sorkalla T., et al. Alpha-hederin, but not hederacoside C and hederagenin from Hedera helix, affects the binding behavior, dynamics, and regulation of beta 2-adrenergic receptors. Biochemistry. 2009;48(15):3477–3482. doi: 10.1021/bi802036b. [DOI] [PubMed] [Google Scholar]
- 21.Stauss-Grabo M., Atiye S., Warnke A., et al. Observational study on the tolerability and safety of film-coated tablets containing ivy extract (Prospan Cough Tablets) in the treatment of colds accompanied by coughing. Phytomedicine. 2011;18(6):433–436. doi: 10.1016/j.phymed.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Hecker M., Runkel F., Voelp A. Treatment of chronic bronchitis with ivy leaf special extract–multicenter post-marketing surveillance study in 1,350 patients. Forsch Komplementarmed Klass Naturheilkd. 2002;9(2):77–84. doi: 10.1159/000057269. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer A., Kehr M.S., Giannetti B.M., Bulitta M., Staiger C. A randomized, controlled, double-blind, multi-center trial to evaluate the efficacy and safety of a liquid containing ivy leaves dry extract (EA 575) vs. placebo in the treatment of adults with acute cough. Pharmazie. 2016;71(9):504–509. doi: 10.1691/ph.2016.6712. PubMed PMID: 29441845. [DOI] [PubMed] [Google Scholar]
- 24.Kemmerich B., Eberhardt R., Stammer H. Efficacy and tolerability of a fluid extract combination of thyme herb and ivy leaves and matched placebo in adults suffering from acute bronchitis with productive cough: a prospective, double-blind, placebo-controlled clinical trial. Arzneimittelforschung. 2006;56(9):652–660. doi: 10.1055/s-0031-1296767. [DOI] [PubMed] [Google Scholar]
- 25.Kelly G.S. Bromelain: a literature review and discussion of its therapeutic applications. Altern Med Rev. 1996;1:243–257. [Google Scholar]
- 26.Rimoldi R., Ginesu F., Giura R. The use of bromelain in pneumological therapy. Drugs Exp Clin Res. 1978;4:55–66. [Google Scholar]
- 27.Klenner F.R. Virus pneumonia and its treatment with vitamin C. South Med Surg. 1948;110(2):36–38. [PubMed] [Google Scholar]
- 28.Hunt C., Chakravorty N.K., Annan G., et al. The clinical effects of vitamin C supplementation in elderly hospitalized patients with acute respiratory infections. Int J Vitam Nutr Res. 1994;64:212–219. [PubMed] [Google Scholar]
- 29.Stephensen C.B., Alvarez J.O., Kohatsec J. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr. 1994;60:88–92. doi: 10.1093/ajcn/60.3.388. [DOI] [PubMed] [Google Scholar]
- 30.Hussey G.D., Klein M. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med. 1990;323:160–164. doi: 10.1056/NEJM199007193230304. [DOI] [PubMed] [Google Scholar]
- 31.Kjolhede C.L., Chew F.J., Gadomski A.M., et al. Clinical trial of vitamin A as adjuvant treatment for lower respiratory tract infections. J Pediatr. 1995;126:807–812. doi: 10.1016/s0022-3476(95)70416-7. [DOI] [PubMed] [Google Scholar]
- 32.Bhandari N., Bahl R., Taneja S., et al. Effect of routine zinc supplementation on pneumonia in children aged 6 months to 3 years: randomised controlled trial in an urban slum. BMJ. 2002;324:1358. doi: 10.1136/bmj.324.7350.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagibina M.V., Neifakh E.A., Krylov V.F., et al. The treatment of pneumonias in influenza using antioxidants. Ter Arkh. 1996;68:33–35. [PubMed] [Google Scholar]
- 34.Sivam G.P. Protection against Helicobacter pylori and other bacterial infections by garlic. J Nutr. 2001;131:1106S–1108S. doi: 10.1093/jn/131.3.1106S. [DOI] [PubMed] [Google Scholar]
- 35.Dikasso D., Lemma H., Urga K., et al. Investigation on the antibacterial properties of garlic (Allium sativum) on pneumonia causing bacteria. Ethiop Med J. 2002;40:241–249. [PubMed] [Google Scholar]
- 36.Bjorkqvist M., Wiberg B., Bodin L., et al. Bottle-blowing in hospital-treated patients with community-acquired pneumonia. Scand J Infect Dis. 1997;29:77–82. doi: 10.3109/00365549709008669. [DOI] [PubMed] [Google Scholar]
- 37.Mundy L.M., Leet T.L., Darst K., et al. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest. 2003;124:883–889. doi: 10.1378/chest.124.3.883. [DOI] [PubMed] [Google Scholar]
- 38.Westerdahl E., Lindmark B., Almgren S.O., et al. Chest physiotherapy after coronary artery bypass graft surgery—a comparison of three different deep breathing techniques. J Rehabil Med. 2001;33:79–84. doi: 10.1080/165019701750098920. [DOI] [PubMed] [Google Scholar]


