Glycyrrhiza glabra (family: Leguminosae)
Common names: licorice, glycyrrhiza
General Description
Glycyrrhiza glabra is a perennial, temperate-zone herb or subshrub, 3 to 7 feet high, with a long, cylindrical, branched, flexible, and burrowing rootstock with runners (Fig. 85.1 ). The parts used are the dried runners and roots, which are collected in the fall.
Fig. 85.1.

Glycyrrhiza glabra.
Chemical Composition
The major active component of licorice root is the triterpenoid saponin glycyrrhizin (also known as glycyrrhizic acid or glycyrrhizinic acid), which is usually found in concentrations ranging from 6% to 10% (Fig. 85.2 ). The intestinal flora is believed to hydrolyze glycyrrhizin, yielding the aglycone molecule (glycyrrhetinic acid) and a sugar moiety, resulting in absorption of both.1
Fig. 85.2.
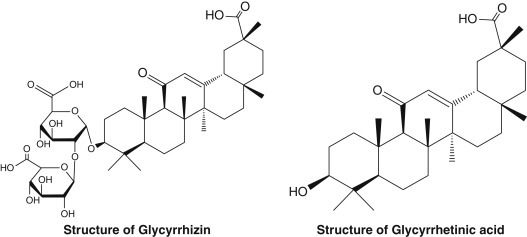
Chemical structures of glycyrrhizin and glycyrrhetinic acid.
Retrieved from https://www.sciencedirect.com/science/article/pii/S001429991730345X [accessed October 22, 2018]
A processed licorice extract, deglycyrrhizinated licorice (DGL), which is used in the treatment of peptic and aphthous ulcers, is made by removing the glycyrrhizin molecule. The active components of DGL are flavonoids. These compounds demonstrated impressive protection against chemically induced ulcer formation in animal studies.2
Other active constituents of licorice include isoflavonoids (e.g., isoflavonol, kumatakenin, licoricone, glabrol); chalcones; coumarins (e.g., umbelliferone, herniarin); triterpenoids; and sterols, lignins, amino acids, amines, gums, and volatile oils.3
History and Folk Use
The medicinal use of licorice in both Western and Eastern cultures dates back several thousand years. It was used primarily as a demulcent, expectorant, antitussive, and mild laxative. Licorice is one of the most popular components of Chinese medicines. Its traditional uses include treating peptic ulcers, asthma, pharyngitis, malaria, abdominal pain, insomnia, and infections.3
Pharmacology
Licorice is known to exhibit many pharmacological actions, including the following3:
-
•
Estrogenic
-
•
Aldosterone-like action
-
•
Anti-inflammatory (cortisol-like action)
-
•
Antiallergic
-
•
Antibacterial, antiviral, and antitrichomonas
-
•
Antihepatotoxic
-
•
Anticonvulsive
-
•
Choleretic
-
•
Anticancer
-
•
Expectorant
-
•
Antitussive activities
Although much of the pharmacology focuses on glycyrrhizin and glycyrrhetinic acid, it is worth remembering that licorice has many other components, such as flavonoids, which may have significant pharmacological effects.
Estrogenic Activity
Most herbalists generally believe that glycyrrhiza exhibits alterative action on estrogen metabolism (i.e., when estrogen levels are too high, it inhibits estrogen action, and when estrogens are too low, it potentiates estrogen action when used in greater amounts).4 Glycyrrhetinic acid has been shown to antagonize many of the effects of estrogens, particularly exogenous estrogens.5 The estrogenic action of glycyrrhiza is a result of its isoflavone content, as many isoflavone structures (e.g., daidzein and genistein from soy) are known to possess estrogenic effect. The estrogenic activity of the isoflavones appears to be more significant than the estrogen antagonism of glycyrrhetinic acid.6 Interestingly, these same components inhibit breast cancer cell growth.7
Pseudoaldosterone Activity
Long-term ingestion of glycyrrhiza in large doses leads to a well-documented pseudoaldosteronism syndrome (i.e., hypertension, hypokalemia, sodium and water retention, low plasma renin activity, and suppressed urine and serum aldosterone levels).8, 9, 10, 11, 12, 13 In normal subjects, the amount of glycyrrhizin needed to produce these side effects is between 0.7 and 1.4 g, which corresponds to approximately 10 to 14 g of the crude herb.9 Although glycyrrhiza possesses mineralocorticoid activity (about four orders of magnitude lower than aldosterone) and binds to aldosterone receptors, it is largely without effect in adrenalectomized animals or in patients with severe adrenocorticoid insufficiency. Therefore it can be concluded that its primary effects are largely a result of glycyrrhetinic acid inhibiting the breakdown of aldosterone in the liver.14 Glycyrrhizin and glycyrrhetinic acid were shown to suppress 5-β-reductase, the main enzyme in humans responsible for inactivating cortisol, aldosterone, and progesterone. These effects can be put to good use in the treatment of Addison’s disease, a severe disease of adrenal insufficiency.13
Anti-inflammatory and Antiallergic Activity
Glycyrrhiza has significant anti-inflammatory and antiallergic activity.15, 16 Although both glycyrrhizin and glycyrrhetinic acid bind to glucocorticoid receptors, and much of glycyrrhiza’s anti-inflammatory activity has been explained by its “cortisol-like effects,” many of the effects of glycyrrhiza actually antagonize or counteract cortisol.17 Antagonism to such actions of cortisol includes activation of tryptophan oxygenase, accumulation of hepatic glycogen, stimulation of hepatic cholesterol synthesis, inhibition of thymus atrophy, and inhibition of adrenocorticotropic hormone synthesis and secretion. Glycyrrhizin does, however, reinforce cortisol’s inhibition of antibody formation, stress reaction, and inflammation. Like its mineralocorticoid effect, glycyrrhiza’s major influence on glucocorticoid metabolism is probably related to its suppression of 5-β-reductase activity, thus increasing the half-life of cortisol. Glycyrrhetinic acid can also increase the conversion of cortisol to the more powerful cortisone.18
Glycyrrhiza’s major cortisol-like effect relates to its ability to inhibit phospholipase A2.19 This enzyme is responsible for cleaving lipids from biomembranes for eicosanoid metabolism. In addition to this effect, glycyrrhizin was also shown to inhibit cyclic adenosine monophosphate phosphodiesterase, thereby raising cyclic adenosine monophosphate levels and prostaglandin formation by activated peritoneal macrophages from rats.20, 21 Glycyrrhizin was shown to inhibit experimentally induced allergenic reactions, such as the Arthus phenomenon, the Shwartzman phenomenon, and Forssman anaphylaxis, and to be an antidote against many toxins, including diphtheria, tetanus, and tetrodotoxin.21, 22
Glycyrrhizin exerts antithrombotic effects but does not potentiate the inhibitory activity of antithrombin III or heparin cofactor II toward thrombin.23
Immunostimulatory and Antiviral Effects
Glycyrrhizin and glycyrrhetinic acid were shown to induce interferon.24 The induction of interferon leads to significant antiviral activity, because interferons bind to cell surfaces, where they stimulate synthesis of intracellular proteins that block the transcription of viral DNA. The induction of interferon is also followed by activation of macrophages and augmentation of natural killer cell activity.
Glycyrrhizin was shown to directly inhibit the growth of several DNA and RNA viruses in cell cultures (vaccinia, Epstein-Barr, herpes simplex, Newcastle disease, vesicular stomatitis viruses, severe acute respiratory syndrome [SARS]-associated coronavirus, and HIV) and to inactivate herpes simplex virus 1 (HSV-1) irreversibly.25, 26, 27, 28 Administration of glycyrrhizin to mice with herpetic encephalitis increased their survival rate on average about 2.5 times, whereas it reduced HSV-1 replication in the brain to 45.6% of the controls.29 Glycyrrhizin, as stated earlier, also inhibited the thymolytic and immunosuppressive action of cortisone. Other licorice components exerted immunomodulatory effects as well.30
Anticancer Effects
Licorice components exert a wide range of anticancer effects.31 The most active appear to be the flavonoids and coumarins. For example, isoliquiritigenin was shown to suppress colon cancer in mice via markedly decreasing both prostaglandin E2 and nitric oxide production in mouse macrophage cells.32 Isoliquiritigenin was also shown to significantly inhibit the proliferation of prostate and breast cancer cell lines in dose- and time-dependent manners.7, 33 Isoliquiritigenin also significantly reduced pulmonary metastasis in mouse renal cell carcinoma and prevented the leukocytopenia caused by administration of 5-fluorouracil.34 A coumarin compound, identified as licocoumarone, was shown to be the factor in licorice that induces apoptosis.35
Antibacterial Activity
Alcohol extracts of glycyrrhiza displayed antimicrobial activity in vitro against Helicobacter pylori, Staphylococcus aureus (including antibiotic resistant strains), Streptococcus mutans, Mycobacterium smegmatis, Bacillus subtilis, S. pyogenes, Haemophilus influenzae, Moraxella catarrhalis, and Candida albicans. 36, 37, 38, 39 The majority of the antimicrobial effects are due to isoflavonoid components, with the saponins having a lesser antibacterial effect.
Antihepatotoxic Activity
Glycyrrhetinic acid inhibits carbon tetrachloride and galactosamine-induced liver damage. The mechanism of action is prevention of nonenzymatic lipid peroxidation and inhibition of the production of free radicals by the enzymatic action of nicotine adenine disphosphonucleotide, reduced–cytochrome P450 reductase on CCl4.40
Memory-Enhancing Effect
Licorice may exert some memory-enhancing effects. In a study in mice, licorice was shown to enhance learning and memory in mice as determined by the elevated plus-maze and passive avoidance paradigm. Furthermore, licorice significantly reversed the amnesia induced by diazepam and scopolamine. Although anti-inflammatory and antioxidant properties may contribute favorably to the memory-enhancing effect, because scopolamine-induced amnesia was reversed as well, it is possible that the beneficial effect on learning and memory was a result of facilitation of cholinergic transmission.41
Antinephritic Activity
Glabridin, an isoflavan isolated from G. glabra, improved urinary protein excretion, total cholesterol, serum creatinine, and blood urea nitrogen levels after its oral administration to mice with glomerular disease.42
Clinical Applications
Licorice is a component of more traditional Chinese and Japanese herbal formulas than any other herb and has been commonly used in Western natural medicine and herbalism for centuries. Although extremely pharmacologically diverse, the current clinical applications of licorice can be divided into four main categories:
-
•
Use of DGL
-
•
Use of oral licorice preparations containing glycyrrhizin
-
•
Use of licorice flavonoid oil (LFO)
-
•
Use of topical preparations containing glycyrrhetinic acid
The key use of DGL is in ulcerative conditions of the gastrointestinal tract (e.g., peptic ulcers, canker sores, inflammatory bowel disease), whereas the key uses of oral licorice containing glycyrrhizin include viral infections (e.g., the common cold, HIV and AIDS, viral hepatitis); premenstrual syndrome (PMS) and menopause; acute intermittent porphyria; Addison’s disease; inflammation; syndrome X; and as a sweetening agent. Topical preparations containing glycyrrhetinic acid can be used in eczema, psoriasis, herpes, and melasma.
Deglycyrrhizinated Licorice
Although glycyrrhetinic acid was the first drug proven to promote healing of gastric and duodenal ulcers,43 most physicians using licorice in the treatment of peptic ulcers now use DGL. DGL was actually shown to be more effective than glycyrrhetinic acid, without side effects.44
DGL’s mode of action is different than that of current drugs, such as antacids and H2-receptor antagonists, which focus on reducing gastric acidity. Although effective, these treatments can be expensive, carry some risk of toxicity, disrupt normal digestive processes, and alter the structure and function of the cells that line the digestive tract. The latter factor is just one of the reasons why peptic ulcers develop again if antacids, cimetidine, ranitidine, and similar drugs are used.
Rather than inhibit the release of acid, DGL stimulates the normal defense mechanisms that prevent ulcer formation and stimulate healing of the damaged mucous membranes. Specifically, DGL increases the following45, 46:
-
•
The blood supply to the damaged mucosa
-
•
The number of cells producing the mucus that protects the mucous membranes
-
•
The amount of mucus the cells produce
-
•
The life span of the intestinal cell
In addition, several flavonoid components of G. glabra have shown significant activity against H. pylori, including antibiotic-resistant strains.38 To evaluate the effect of licorice in H. pylori eradication in 120 patients suffering from dyspepsia either with peptic ulcer disease (PUD) or nonulcer dyspepsia (NUD), licorice (380 mg twice daily) was given in addition to clarithromycin-based standard triple regimen for 2 weeks.47 H. pylori eradication was assessed 6 weeks after therapy. Response to treatment was 83.3% in the licorice group and 62.5% in the control group.
Gastric Ulcers
Numerous clinical studies over the years found DGL to be an effective antiulcer compound. DGL was shown to be extremely effective in the treatment of gastric ulcers.48, 49, 50, 51, 52 In one study, 33 gastric ulcer patients were treated with either DGL (760 mg, three times a day) or a placebo for 1 month.50 There was a significantly greater reduction in ulcer size in the DGL group (78%) than in the placebo group (34%). Complete healing occurred in 44% of those receiving DGL but only in 6% of the placebo group.
In several head-to-head comparison studies, DGL was shown to be more effective than cimetidine (Tagamet), ranitidine (Zantac), or antacids in both short-term treatment and maintenance therapy of peptic ulcers.48, 49, 52 For example, in a head-to-head comparison with Tagamet, 100 patients received either DGL (760 mg, three times a day between meals) or Tagamet (200 mg, three times a day, and 400 mg at bedtime).49 The percentage of ulcers healed after 6 and 12 weeks were similar in both groups. Although Tagamet is associated with some significant side effects, DGL is extremely safe to use.
Gastric ulcers are often a result of using alcohol, aspirin, or other nonsteroidal anti-inflammatory drugs, caffeine, and other factors that decrease the integrity of the gastric lining. Because DGL was shown in human studies to reduce the gastric bleeding caused by aspirin, DGL is strongly indicated for the prevention of gastric ulcers in patients requiring long-term treatment with ulcerogenic drugs such as aspirin, nonsteroidal anti-inflammatory agents, and corticosteroids.51
Duodenal Ulcers
DGL is also effective in duodenal ulcers. This is perhaps best illustrated by one study in patients with severe duodenal ulcers: 40 patients with chronic duodenal ulcers of 4 to 12 years’ duration and more than six relapses during the previous year were treated with DGL.53 All of the patients had been referred for surgery because of relentless pain, sometimes with frequent vomiting, despite treatment with bed rest, antacids, and anticholinergic drugs. Half of the patients received 3 g/day of DGL for 8 weeks; the other half received 4.5 g/day for 16 weeks. All 40 patients showed substantial improvement, usually within 5 to 7 days, and none required surgery during the 1-year follow-up. Although both dosages were effective, the higher dosage was significantly more effective than the lower dosage.
In another more recent study, the therapeutic effect of DGL was compared with that of antacids or cimetidine in 874 patients with confirmed chronic duodenal ulcers.52 Ninety-one percent of all ulcers healed within 12 weeks; there was no significant difference in healing rate in the groups. However, there were fewer relapses in the DGL group (8.2%) than in those receiving cimetidine (12.9%) or antacids (16.4%). These results, coupled with DGL’s protective effects and very low toxicity, suggest that DGL is a superior treatment of duodenal ulcers.
Aphthous Ulcers
Recurrent aphthous stomatitis (canker sores) is a common problem. DGL may be effective in promoting healing. In one study, 20 patients were instructed to use a solution of DGL as a mouthwash (200 mg powdered DGL dissolved in 200 mL warm water) four times daily.54 Fifteen of the 20 (75%) patients experienced 50% to 75% improvement within 1 day, followed by complete healing of the ulcers by the third day. DGL in tablet form may produce even better results.
Oral Licorice Preparations Containing Glycyrrhizin
The most popular use of oral licorice preparations containing glycyrrhizin is in the treatment of viral illnesses, particularly the common cold. Licorice has long been used in this application. This historical use is justified by its immune-enhancing and antiviral effects. In addition, licorice components were shown to exert antibacterial action against the common pathogens S. pyogenes, Haemophilus influenzae, and Moraxella catarrhalis. 55
Another popular use of licorice is in the treatment of gynecological issues, primarily PMS and menopause. Regarding PMS, because glycyrrhizin and glycyrrhetinic acid possess antiestrogenic effects and suppress the breakdown of progesterone, administration of licorice 2 weeks before the onset of menstruation (the midluteal phase) may help reduce PMS symptomatology. Clinical trials showed that taking licorice containing herbal combinations was useful in dysmenorrhea.56 Isoflavones from glycyrrhiza showed an ability to inhibit serotonin reuptake, and therefore might also exhibit some antidepressant effects in PMS.57
Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome
Glycyrrhizin-containing preparations are showing promise in the treatment of HIV-related diseases, including AIDS. Although much of the research featured intravenous administration, this route of administration might not be necessary, as glycyrrhizin and glycyrrhetinic acid are easily absorbed orally and are well tolerated. This was most evident in a recent double-blind study on the clinical effectiveness of glycyrrhizin by long-term oral administration to 16 hemophiliac patients with evidence of HIV infection.58 Patients received daily doses of 150 to 225 mg of glycyrrhizin for 3 to 7 years.
Helper and total T-lymphocyte numbers, other immune system parameters, and glycyrrhizin and glycyrrhetinic acid levels in the blood were monitored. The results indicated that orally administered glycyrrhizin was converted into glycyrrhetinic acid, which was detected in sera, without manifesting any side effects. None of the patients given the glycyrrhizin had progression of immunological abnormalities or development to AIDS. In contrast, the group not receiving glycyrrhetinic acid showed decreases in helper and total T-cell counts and antibody levels. Two of the 16 patients in the control group developed AIDS.
In another study, 10 HIV positive patients without AIDS took 150 to 225 mg/day of glycyrrhizin.59 After 1 to 2 years, none developed symptoms associated with AIDS or AIDS-related complex, whereas 1 of 10 patients of a matched control group developed AIDS-related complex, and 2 progressed to AIDS and subsequently died.
The result of glycyrrhizin in HIV-positive and AIDS patients is almost immediate improvement in immune function. In one study, 9 symptom-free HIV-positive patients received 200 to 800 mg/day of glycyrrhizin intravenously. After 8 weeks, the groups had increased T-helper cells, improved helper/suppressor ratios, and improved liver function.60
In another study, 6 AIDS patients received 400 to 1600 mg/day of glycyrrhizin intravenously.61 After 30 days, 5 of the 6 showed a reduction or disappearance of the P24 antigen, which indicates active disease. The results of these studies and others in HIV-positive and AIDS patients are encouraging.
Hepatitis
Some studies of HIV patients used an intravenous glycyrrhizin-containing product, Stronger Neo-Minophagen C (SNMC), consisting of 0.2% glycyrrhizin, 0.1% cysteine, and 2.0% glycine in physiological saline solution. This product is used in Japan primarily for the treatment of hepatitis. The other components, glycine and cysteine, appear to modulate glycyrrhizin’s actions. Glycine was shown to prevent the aldosterone effects of glycyrrhizin, whereas cysteine aids the liver in detoxification reactions.
In addition to AIDS, SNMC demonstrated beneficial results in treating chronic hepatitis B and C, often difficult infections for the body to clear.22, 62, 63, 64 Specifically, SNMC was shown to improve liver function and lower levels of liver enzymes. Glycyrrhizin therapy appears particularly helpful in patients with chronic hepatitis C who fail to respond to interferon and in those who cannot be treated with it for various reasons.
Acute Intermittent Porphyria
This disorder of heme biosynthesis is characterized by recurrent attacks of neurological and psychiatric dysfunction. The symptoms include the following:
-
•
Abdominal complaints of nausea, vomiting, and colicky pain, occasionally severe enough to present as an acute abdomen without fever or leukocytosis
-
•
Variable neurological signs and symptoms (e.g., paresthesia, hypesthesia, neuritic pain, wrist or foot drop, loss of deep tendon reflexes)
-
•
Variable mental and emotional disturbances, typically restlessness, disorientation, and visual hallucinations (seen in one third of patients)
Because estrogens are known to exacerbate or induce acute intermittent porphyria (AIP), it is quite possible that some of the so-called PMS symptoms are exacerbations of AIP caused by the midcycle estrogen surge.
A partial (50%) deficiency of uroporphyrinogen I synthase results in increased inducibility of aminolevulinic acid synthase by drugs and foreign chemicals and by 5-β-reductase steroid metabolites (potent inducers of aminolevulinic acid synthase). AIP is also associated with a marked deficiency in the activity of 5-α-reductase, resulting in increased 5-β-reductase activity.65 Glycyrrhetinic acid and glycyrrhizin were shown to significantly reduce 5-β-reductase while increasing 5-α-reductase.66 (Lead also increases 5-β-reductase activity, resulting in a presenting picture similar to AIP.66 Chronic or acute lead toxicity must be ruled out in these patients.)
Obesity and Metabolic Syndrome
Preparations containing glycyrrhetinic acid may be effective in reducing various issues related to syndrome X or metabolic syndrome. For example, in a preliminary study, 15 normal-weight subjects (7 males, 22–26 years old, and 8 females, 21–26 years old), who consumed 3.5 g/day of a commercial preparation of licorice containing glycyrrhetinic acid for 2 months, had reduced body fat mass of 1.2% in men and 2.8% in women.67 This weight loss might have been mediated not only via suppressing renin activity and aldosterone levels via inhibition of 11-β-hydroxysteroid dehydrogenase, but also via improving blood glucose control—a key goal in syndrome X.68, 69, 70
In another study, supplementation of a licorice root extract to moderately hypercholesterolemic patients for 1 month reduced plasma susceptibility to oxidation (by 19%). It also increased resistance of plasma low-density lipoprotein against three major atherogenic modifications: oxidation (by 55%), aggregation (by 28%), and retention (by 25%). It reduced plasma cholesterol levels (by 5%), which were caused by a 9% reduction in plasma low-density lipoprotein cholesterol levels, and reduced (by 14%) plasma triglyceride levels. Licorice extract supplementation also reduced systolic blood pressure by 10%.71
Addison’s Disease
As described later in “Pseudoaldosterone Activity,” licorice exerts an aldosterone-like effect that is useful in treating Addison’s disease.
Inflammation
Virtually any inflammatory or allergic condition may be reduced by licorice by the mechanisms discussed earlier in the section on “Pharmacology.” Historically, licorice was successfully used for treating asthma and other atopic conditions.3, 15
Licorice was shown to enhance the action of corticosteroids like prednisone and prednisolone, as well as the levels of the body’s own corticosteroids.72, 73 In one study, six subjects received an intravenous dose of prednisolone with or without 200 mg glycyrrhizin. Glycyrrhizin was found to significantly increase the concentration of total and free prednisolone by inhibiting its breakdown. Furthermore, the effects of prednisolone appeared to be potentiated by glycyrrhizin.73
One interesting application shown with positive clinical results in a double-blind study was reduction of postoperative sore throat.74 Forty adults who underwent elective lumbar laminectomy were randomized into two groups of 20 patients each. One group received water (Group C); the other received 0.5 g licorice in water (Group L). Both groups gargled 5 minutes before anesthesia. Postoperative sore throat incidence and severity as well as postextubation cough were reduced for all time points in the licorice group compared with the water group at rest and on swallowing. Postextubation cough was reduced in Group L compared with Group C (P <0.05). There was no difference in side effects between groups (P >0.05).
A review study evaluated the possible application of the active components of licorice, glycyrrhizin (GL) and glycyrrhetinic acid (GA), in rheumatoid arthritis (RA) treatment based on the cyclooxygenase (COX)-2/thromboxane A2 (TxA2) pathway (Fig. 85.3 ).75 The COX-2/TxA2 pathway, an auto-regulatory feedback loop, has been found to be a crucial mechanism underlying the pathogenesis of RA. Both nonsteroidal anti-inflammatory drugs (NSAIDs) and disease-modifying antirheumatic drugs (DMARDs) are typically prescribed medications for treatments of patients with RA. TxA2 is believed to be the nontarget of NSAIDs and DMARDs, and the limitations and adverse effects of those drugs may be, at least in part, caused by lack of the effects on the COX-2/TxA2 pathway. The active components of licorice, GL and GA, could not only potentiate the therapeutic effects but also decrease the adverse effects of NSAIDs or DMARDs through suppressing the COX-2/TxA2 pathway and hold the potential as a novel add-on therapy in the treatment of RA.
Fig. 85.3.
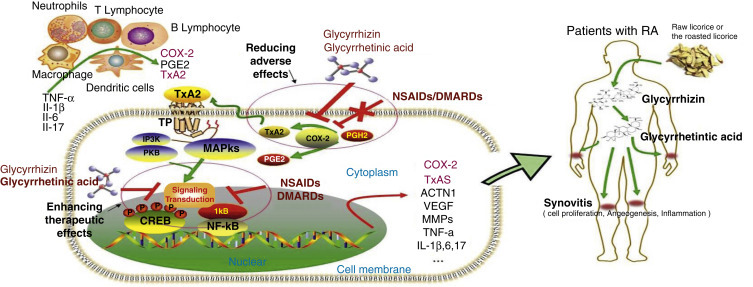
The COX-2/TxA2 pathway is a crucial mechanism underlying the toxicity reducing and efficacy enhancing effects of glycyrrhizin (GL) and glycyrrhetinic acid (GA) to NSAIDs/DMARDs. TNF, Tumor necrosis factor; IL, interleukin; RA FLS, rheumatoid arthritis fibroblast-like synoviocytes; COX, cyclooxygenase; TxA2, thromboxane A2; TP, thromboxane A2 receptor; PGH2, prostaglandin H2; PGE2, prostaglandin E2; NSAIDs, nonsteroidal anti-inflammatory drugs; DMARDs, disease modifying antirheumatic drugs; NF-κB, nuclear factor κB; CREB, cAMP response element-binding protein; MAPKs, mitogen activated protein kinases; PI3K, phosphoinositide-3-kinase; VEGF, vascular endothelial growth factor; ACTN1, α-actinin-1; MMPs, matrix metalloproteinase.
From Huang QC, Wang MJ, Chen XM, et al. Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis? Oncotarget. 2016;7[2]:1193-1202. PubMed PMID: 26498361.
Sweetening Agent
Because glycyrrhizin is 50 to 100 times sweeter than sucrose, licorice can be used as a sweetening or flavoring agent to mask the bitter taste of other medications.3
Licorice Flavonoid Oil
LFO shows promise as an antiobesity and weight loss agent. It is standardized to contain 30% polyphenols with glabridin standardized at 3%. The flavonoids are extracted with ethanol and then solubilized in medium chain triglycerides oil (hence 70% of LFO is medium chain triglycerides). The extracted licorice flavonoids are hydrophobic compounds and virtually free of the hydrophilic compounds glycyrrhizin and glycyrrhizinic acid (there is less than 0.005% glycyrrhizic acid in LFO).
LFO decreases the activity of acetyl coenzyme A carboxylase and fatty acid synthase, the rate-limiting enzymes in the fatty acid synthetic pathway, while increasing the enzymatic activity of acyl coenzyme A dehydrogenase, the rate-limiting enzyme in the fatty acid oxidative pathway. These effects are thought to be responsible for the reduction in abdominal fat in animal and human studies.76, 77, 78, 79
In a double-blind study, 56 men and 28 postmenopausal women, aged 40 to 60 years with body mass indexes of 24 to 30 kg/m2, were randomized into four groups: the placebo group took three placebo capsules per day, the low-dose group took one LFO capsule and two placebos per day (300 mg/day LFO), the middle-dose group took two LFO capsules and one placebo per day (600 mg/day LFO), and the high-dose group took three LFO capsules per day (900 mg/day LFO). The subjects in the 900 mg LFO group experienced significant decreases from baseline in body weight and body mass index compared with the placebo group (Table 85.1 ).79
TABLE 85.1.
Body Weight and Mass Compared With Baseline After Licorice Flavonoid Oil Treatment
| Group | Baseline | 4 Weeks | 8 Weeks |
|---|---|---|---|
| Body Weight (kg) | |||
| Placebo | 73.13 | 73.07 | 73.39 |
| 900 mg | 72.79 | 72.20 | 72.05 |
| Body Mass Index (kg/m2) | |||
| Placebo | 26.51 | 26.49 | 26.59 |
| 900 mg | 26.22 | 26.01 | 25.97 |
The subjects in all three LFO groups, but not the placebo group, had significantly lower body fat masses compared with baseline after 8 weeks of treatment. Computed tomographic scans showed that visceral fat mass decreased significantly compared with baseline in the 900 mg LFO group (122.37–113.02 cm2).
Another study with LFO was conducted to determine its ability to increase the muscle mass of elderly patients. Fifty subjects aged 54 to 90 years (7 men, 43 women), who underwent rehabilitation treatment for osteoarthritis of the knee, were assigned to either the LFO (300 mg per day) or placebo group. In the LFO group, muscle mass in the body trunk increased significantly after 16 weeks of LFO intake (+0.38 kg). In addition, the body fat percentage and body trunk fat percentage of the LFO group were also reduced.80
A safety study demonstrated that LFO is safe when administered once daily up to 1200 mg/day.81 There were no clinically noteworthy changes in hematologic or related biochemical parameters.
Topical Applications
Eczema and Psoriasis
Glycyrrhetinic acid exerts an effect similar to that of topical hydrocortisone in the treatment of eczema, contact and allergic dermatitis, and psoriasis.82, 83, 84, 85 In several studies, glycyrrhetinic acid was shown to be superior to topical cortisone, especially in chronic cases. For example, in one study of patients with eczema, 93% of the patients applying glycyrrhetinic acid demonstrated improvement compared with 83% using cortisone.86 In another study, a topical gel containing 2% glycyrrhetinic acid was shown to be effective for treatment of atopic dermatitis and was more effective than preparations containing 1% glycyrrhetinic acid in reducing the scores for erythema, edema, and itching over 2 weeks.82
Glycyrrhetinic acid can also be used to potentiate the effects of topically applied hydrocortisone by inhibiting 11-β-hydroxysteroid dehydrogenase, which catalyzes the conversion of hydrocortisone to an inactive form.83 It also increases the permeation of topically applied steroids. In one study, glycyrrhetinic acid in a concentration of 0.1% in gel increased diclofenac sodium flux value tenfold compared with a control gel.84
Herpes Simplex
Clinical studies showed topical glycyrrhetinic acid and derivatives to be quite helpful in reducing the healing time and pain associated with cold sores and genital herpes.87, 88 As mentioned previously, glycyrrhizin inactivates HSV-1 irreversibly and stimulates the synthesis and release of interferon.25
Melasma
Two components, glabrene and isoliquiritigenin, can inhibit tyrosinase—a key enzyme in melanin biosynthesis.89 Dermatological disorders such as melasma, age spots, and sites of actinic damage arise from the accumulation of melasma. Glabrene and isoliquiritigenin may serve as candidates for skin-lightening agents.
Dosage
The dosage of licorice for most clinical applications is based on the content of glycyrrhetinic acid. The exception is in the treatment of peptic ulcer. In this application, DGL is preferred, as it produces equally effective results compared with glycyrrhetinic acid but is free from any side effects.
For most purposes, the goal is to achieve a high level of glycyrrhetinic acid in the blood without producing side effects (discussed later in “Toxicology”). In general, the following doses three times a day are safe and effective in raising glycyrrhetinic acid levels:
-
•
Powdered root: 1 to 2 g
-
•
Fluid extract (1:1): 2 to 4 mL
-
•
Solid (dry powdered) extract (4:1): 250 to 500 mg
In the treatment of AIDS, pure glycyrrhetinic acid products or extracts standardized for glycyrrhetinic acid are recommended. Toxicity can become a problem for patients taking licorice for any period longer than 1 month (see “Toxicology” and “Drug Interactions”).
Dosage Instructions for Deglycyrrhizinated Licorice
To be effective in healing peptic ulcers, it appears that DGL must mix with saliva. DGL may promote the release of salivary compounds, which stimulate the growth and regeneration of stomach and intestinal cells. DGL in capsule form has not been shown to be effective.90, 91
The standard dosage for DGL is two to four 380-mg chewable tablets between or 20 minutes before meals. Taking DGL after meals is associated with poor results.92 DGL should be continued for 8 to 16 weeks, depending on the response.
Dosage Instructions for Licorice Flavonoid Oil
The standard dosage for LFO is 900 mg/day usually administered as 300 mg three times a day.
Toxicology
The main hazards of licorice administration are due to the aldosterone-like effects of glycyrrhetinic acid. If ingested regularly, licorice root (>3 g/day for more than 6 weeks) or glycyrrhizin (>100 mg/day) may cause sodium and water retention, hypertension, and hypokalemia.8, 9, 93, 94, 95 Individuals with existing hypertension may be more predisposed to this effect via increased sensitivity to the inhibition of 11-β-hydroxysteroid-dehydrogenase by glycyrrhetinic acid.18, 82, 96 Monitoring of blood pressure and electrolytes and increasing dietary potassium intake is suggested, as the pseudoaldosterone effects can be quite significant. The maximal effect on blood pressure with long-term ingestion is observed after 2 weeks of use.97
There is great individual variation in the susceptibility to the symptom-producing effects of glycyrrhizin, primarily due to differences in pharmacokinetics and conversion to the more potent glycyrrhetinic acid (100–200 times more active in suppressing 11-β-hydroxysteroid-dehydrogenase).98 Adverse effects are rarely observed at levels below 100 mg/day, whereas they are quite common at levels above 400 mg/day.9 However, some persons may be susceptible to long-term dosages at even lower levels, especially if the more potent glycyrrhetinic acid is available in free form. One study determined a no-effect level of glycyrrhetinic acid at 2 mg/kg, from which an acceptable daily intake of 0.2 mg/kg body weight can be extrapolated with a safety factor of 10. This translates to a consumption of 12 mg/day of glycyrrhetinic acid for a person with a body weight of 60 kg.99
Prevention of the side effects of glycyrrhizin may be possible by following a high-potassium, low-sodium diet. Although no formal trial has been performed, patients who normally consume high-potassium foods and restrict sodium intake, even those with high blood pressure and angina, have been reported to be free from the aldosterone-like side effects of glycyrrhizin.100
Licorice should probably not be used in patients with a history of hypertension or renal failure or in those who currently use digitalis preparations.
Licorice preparations containing glycyrrhizin may reduce serum and salivary testosterone levels in men. In one study, men consuming the equivalent of 500 mg of glycyrrhizin experienced a drop of 26% in serum testosterone levels.101 However, in another study, no significant effect was noted.102
Licorice intake during pregnancy is generally regarded as safe, unless hypertension becomes an issue. There was one detailed study on maternal consumption of glycyrrhizin and how it affected birth weight.103 Glycyrrhizin intake was calculated from detailed questionnaires on licorice consumption. Glycyrrhizin exposure was grouped into three levels: low (<250 mg/week), moderate (250–499 mg/week), and heavy (≥500 mg/week). Birth weight and gestational age (from ultrasound measurements) were obtained from hospital records. Babies with heavy exposure to glycyrrhizin were not significantly lighter at birth, but they were significantly more likely to be born earlier—2.52 days earlier. No other associations could be made.
Drug Interactions
No significant reports of drug interactions have appeared, although on theoretical grounds, licorice components have shown considerable interactions with various enzyme systems. Licorice root extract and purified glabridin were shown to inhibit P450 3A4, a major human drug metabolizing P450 enzyme, in time- and concentration-dependent manners, thereby potentiating the action of many drugs.104 Glycyrrhizin intake may be problematic for people on digitalis, diuretics, or antihypertensive medications. Also, individuals using oral hypoglycemic drugs or insulin need to monitor blood sugar levels closely when using glycyrrhiza.
Glycyrrhetinic acid can reduce the prevalence of side effects related to the diuretic activity of spironolactone. In a study of 32 women with polycystic ovarian syndrome, women who received 3.5 g of licorice a day with spironolactone eliminated symptoms related to volume depletion, and the activation of the renin-aldosterone system was significantly lower during spironolactone plus licorice than with spironolactone alone. The prevalence of metrorrhagia was also lower with the combined therapy.105
References
See www.expertconsult.com for a complete list of references.
References
- 1.Hattori M., Sakamoto T., Kobashi K., et al. Metabolism of glycyrrhizin by human intestinal flora. Planta Med. 1983;48:38–42. doi: 10.1055/s-2007-969875. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto K., Kakegawa H., Ueda H., et al. Gastric cytoprotective anti-ulcerogenic actions of hydroxychalcones in rats. Planta Med. 1992;58:389–393. doi: 10.1055/s-2006-961498. [DOI] [PubMed] [Google Scholar]
- 3.Chandler R.F. Licorice, more than just a flavour. Can Pharm J. 1985;118:421–424. [Google Scholar]
- 4.Kumagai A., Nishino K., Shimomura A., et al. Effect of glycyrrhizin on estrogen action. Endocrinol Jpn. 1967;14:34–38. doi: 10.1507/endocrj1954.14.34. [DOI] [PubMed] [Google Scholar]
- 5.Kraus S., Kaminskis A. The anti-estrogenic action of beta-glycyrrhetinic acid. Exp Med Surg. 1969;27:411–420. [PubMed] [Google Scholar]
- 6.Tamir S., Eizenberg M., Somjen D., et al. Estrogen-like activity of glabrene and other constituents isolated from licorice root. J Steroid Biochem Mol Biol. 2001;78:291–298. doi: 10.1016/s0960-0760(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 7.Maggiolini M., Statti G., Vivacqua A., et al. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J Steroid Biochem Mol Biol. 2002;82:315–322. doi: 10.1016/s0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- 8.Farese R.V., Jr., Biglieri E.G., Shackleton C.H., et al. Licorice-induced hypermineralocorticoidism. N Engl J Med. 1991;325:1223–1227. doi: 10.1056/NEJM199110243251706. [DOI] [PubMed] [Google Scholar]
- 9.Stormer F.C., Reistad R., Alexander J. Glycyrrhizic acid in liqourice—evaluation of health hazard. Food Chem Toxicol. 1993;31:303–312. doi: 10.1016/0278-6915(93)90080-i. [DOI] [PubMed] [Google Scholar]
- 10.Takeda R., Morimoto S., Uchida K., et al. Prolonged pseudoaldosteronism induced by glycyrrhizin. Endocrinol Jpn. 1979;26:541–547. doi: 10.1507/endocrj1954.26.541. [DOI] [PubMed] [Google Scholar]
- 11.Baron J. Side-effects of carbenoxolone. Acta Gastroenterol Belg. 1983;46:469–484. [PubMed] [Google Scholar]
- 12.Epstein M.T., Espiner E.A., Donald R.A., et al. Effect of eating liquorice on the renin-angiotensin aldosterone axis in normal subjects. BMJ. 1977;1:488–490. doi: 10.1136/bmj.1.6059.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armanini D., Karbowiak I., Funder J.W. Affinity of liquorice derivatives for mineralocorticoid and glucocorticoid receptors. Clin Endocrinol (Oxf) 1983;19:609–612. doi: 10.1111/j.1365-2265.1983.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y., Nishikawa T., Yamada K. Effects of glycyrrhetinic acid and its derivatives on delta 4-5 alpha- and 5 beta-reductase in rat liver. Arzneimittelforschung. 1979;29:647–649. [PubMed] [Google Scholar]
- 15.Kuroyanagi T., Saito M. Effect of prednisolone and glycyrrhizin on passive transfer of experimental allergic encephalomyelitis. Arerugi. 1966;15:67–74.. ([Japanese]) [PubMed] [Google Scholar]
- 16.Cyong J., Otsuka Y. A pharmacological study of the anti-inflammatory activity of Chinese herbs. A review. Acupunct Electrother Res. 1982;7:173–202. doi: 10.3727/036012982816952116. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai A., Nanaboshi M., Asanuma Y., et al. Effects of glycyrrhizin on thymolytic and immunosuppressive action of cortisone. Endocrinol Jpn. 1967;14:39–42. doi: 10.1507/endocrj1954.14.39. [DOI] [PubMed] [Google Scholar]
- 18.Van Uum S.H., Walker B.R., Hermus A.R., et al. Effect of glycyrrhetinic acid on 11 beta-hydroxysteroid dehydrogenase activity in normotensive and hypertensive subjects. Clin Sci (Colch) 2002;102:203–211. doi: 10.1042/cs20010194. [DOI] [PubMed] [Google Scholar]
- 19.Okimasa E., Moromizato Y., Watanabe S., et al. Inhibition of phospholipase A2 by glycyrrhizin, an anti-inflammatory drug. Acta Med Okayama. 1983;37:385–391. doi: 10.18926/AMO/32426. [DOI] [PubMed] [Google Scholar]
- 20.Amer M.S., Mckinney G.R., Akcasu A. Effect of glycyrrhetinic acid on the cyclic nucleotide system of the rat stomach. Biochem Pharmacol. 1974;23:3085–3092. doi: 10.1016/0006-2952(74)90593-0. [DOI] [PubMed] [Google Scholar]
- 21.Ohuchi K., Kamada Y., Levine L., et al. Glycyrrhizin inhibits prostaglandin E2 production by activated peritoneal macrophages from rats. Prostaglandins Med. 1981;7:457–463. doi: 10.1016/0161-4630(81)90033-1. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H., Ohta Y., Takino T., et al. Effects of glycyrrhizin on biochemical tests in patients with chronic hepatitis—double blind trial. Asian Med J. 1984;26:423–438. [Google Scholar]
- 23.Mendes-Silva W., Assafim M., Ruta B., et al. Antithrombotic effect of Glycyrrhizin, a plant-derived thrombin inhibitor. Thromb Res. 2003;112:93–98. doi: 10.1016/j.thromres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Abe N., Ebina T., Ishida N. Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol Immunol. 1982;26:535–539. doi: 10.1111/j.1348-0421.1982.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 25.Pompei R., Pani A., Flore O., et al. Antiviral activity of glycyrrhizic acid. Experientia. 1980;36:304. doi: 10.1007/BF01952290. [DOI] [PubMed] [Google Scholar]
- 26.Lin J.C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antiviral Res. 2003;59:41–47. doi: 10.1016/s0166-3542(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 27.Cinatl J., Morgenstern B., Bauer G., et al. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki H., Takei M., Kobayashi M., et al. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology. 2003;70:229–236. doi: 10.1159/000069334. [DOI] [PubMed] [Google Scholar]
- 29.Sekizawa T., Yanagi K., Itoyama Y. Glycyrrhizin increases survival of mice with herpes simplex encephalitis. Acta Virol. 2001;45:51–54. [PubMed] [Google Scholar]
- 30.Barfod L., Kemp K., Hansen M., et al. Chalcones from Chinese liquorice inhibit proliferation of T cells and production of cytokines. Int Immunopharmacol. 2002;2:545–555. doi: 10.1016/s1567-5769(01)00202-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z.Y., Nixon D.W. Licorice and cancer. Nutr Cancer. 2001;39:1–11. doi: 10.1207/S15327914nc391_1. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T., Takasuka N., Iigo M., et al. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004;95:448–453. doi: 10.1111/j.1349-7006.2004.tb03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanazawa M., Satomi Y., Mizutani Y., et al. Isoliquiritigenin inhibits the growth of prostate cancer. Eur Urol. 2003;43:580–586. doi: 10.1016/s0302-2838(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki S., Morita T., Endo H., et al. Isoliquiritigenin suppresses pulmonary metastasis of mouse renal cell carcinoma. Cancer Lett. 2002;183:23–30. doi: 10.1016/s0304-3835(02)00113-1. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe M., Hayakawa S., Isemura M., et al. Identification of licocoumarone as an apoptosis-inducing component in licorice. Biol Pharm Bull. 2002;25:1388–1390. doi: 10.1248/bpb.25.1388. [DOI] [PubMed] [Google Scholar]
- 36.Mitscher L., Park Y., Clark D. Antimicrobial agents from higher plants. Antimicrobial isoflavonoids and related substances from Glycyrrhiza glabra L. var. typica. J Nat Products. 1980;43:259–269. doi: 10.1021/np50008a004. [DOI] [PubMed] [Google Scholar]
- 37.Fukai T., Marumo A., Kaitou K., et al. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia. 2002;73:536–539. doi: 10.1016/s0367-326x(02)00168-5. [DOI] [PubMed] [Google Scholar]
- 38.Fukai T., Marumo A., Kaitou K., et al. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71:1449–1463. doi: 10.1016/s0024-3205(02)01864-7. [DOI] [PubMed] [Google Scholar]
- 39.Tsukiyama R., Katsura H., Tokuriki N., et al. Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob Agents Chemother. 2002;46:1226–1230. doi: 10.1128/AAC.46.5.1226-1230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiso Y., Tohkin M., Hikino H., et al. Mechanism of antihepatotoxic activity of glycyrrhizin, I: effect on free radical generation and lipid peroxidation. Planta Medica. 1984;50:298–302. doi: 10.1055/s-2007-969714. [DOI] [PubMed] [Google Scholar]
- 41.Dhingra D., Parle M., Kulkarni S.K. Memory enhancing activity of Glycyrrhiza glabra in mice. J Ethnopharmacol. 2004;91:361–365. doi: 10.1016/j.jep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Fukai T., Satoh K., Nomura T., et al. Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra. Fitoterapia. 2003;74:624–629. doi: 10.1016/s0367-326x(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 43.Doll R., Hill I., Hutton C., et al. Clinical trial of a triterpenoid liquorice compound in gastric and duodenal ulcer. Lancet. 1962;2:793–796. doi: 10.1016/s0140-6736(62)90926-1. [DOI] [PubMed] [Google Scholar]
- 44.Wilson J.A. A comparison of carbenoxolone sodium and deglycyrrhizinated liquorice in the treatment of gastric ulcer in the ambulant patient. Br J Clin Pract. 1972;26:563–566. [PubMed] [Google Scholar]
- 45.Van Marle J., Aarsen P.N., Lind A., et al. Deglycyrrhizinised liquorice (DGL) and the renewal of rat stomach epithelium. Eur J Pharmacol. 1981;72:219–225. doi: 10.1016/0014-2999(81)90276-4. [DOI] [PubMed] [Google Scholar]
- 46.Goso Y., Ogata Y., Ishihara K., et al. Effects of traditional herbal medicine on gastric mucin against ethanol-induced gastric injury in rats. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;113:17–21. doi: 10.1016/0742-8413(95)02042-x. [DOI] [PubMed] [Google Scholar]
- 47.Hajiaghamohammadi A.A., Zargar A., Oveisi S., Samimi R., Reisian S. To evaluate of the effect of adding licorice to the standard treatment regimen of Helicobacter pylori. Braz J Infect Dis. 2016;20(6):534–538. doi: 10.1016/j.bjid.2016.07.015. Epub 2016 Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan A.G., Pacsoo C., McAdam W.A. Maintenance therapy. A two year comparison between Caved-S and cimetidine treatment in the prevention of symptomatic gastric ulcer. Gut. 1985;26:599–602. doi: 10.1136/gut.26.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan A.G., McAdam W.A., Pacsoo C., et al. Comparison between cimetidine and Caved-S in the treatment of gastric ulceration, and subsequent maintenance therapy. Gut. 1982;23:545–551. doi: 10.1136/gut.23.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turpie A.G., Runcie J., Thomson T.J. Clinical trial of deglycyrrhizinised liquorice in gastric ulcer. Gut. 1969;10:299–303. doi: 10.1136/gut.10.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rees W.D., Rhodes J., Wright J.E., et al. Effect of deglycyrrhizinated liquorice on gastric mucosal damage by aspirin. Scand J Gastroent. 1979;14:605–607. doi: 10.3109/00365527909181397. [DOI] [PubMed] [Google Scholar]
- 52.Kassir Z.A. Endoscopic controlled trial of four drug regimens in the treatment of chronic duodenal ulceration. Ir Med J. 1985;78:153–156. [PubMed] [Google Scholar]
- 53.Tewari S.N., Wilson A.K. Deglycyrrhizinated liquorice in duodenal ulcer. Practitioner. 1973;210:820–823. [PubMed] [Google Scholar]
- 54.Das S.K., Das V., Gulati A.K., et al. Deglycyrrhizinated liquorice in aphthous ulcers. J Assoc Physicians India. 1989;37:647. [PubMed] [Google Scholar]
- 55.Tanaka Y., Kikuzaki H., Fukuda S., et al. Antibacterial compounds of licorice against upper airway respiratory tract pathogens. J Nutr Sci Vitaminol (Tokyo) 2001;47:270–273. doi: 10.3177/jnsv.47.270. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka T.A. Novel anti-dysmenorrhea therapy with cyclic administration of two Japanese herbal medicines. Clin Exp Obstet Gynecol. 2003;30:95–98. [PubMed] [Google Scholar]
- 57.Ofir R., Tamir S., Khatib S., et al. Inhibition of serotonin re-uptake by licorice constituents. J Mol Neurosci. 2003;20:135–140. doi: 10.1385/JMN:20:2:135. [DOI] [PubMed] [Google Scholar]
- 58.Ikegami N., Akatani K., Yamazaki S., et al. Prophylactic effect of long-term oral administration of glycyrrhizin on AIDS development of asymptomatic patients. Int Conf AIDS. 1993;9:234.. (abstract) [Google Scholar]
- 59.Ikegami N., Akatani K., Yamazaki S., et al. Abstract W.B.P; 1989. Clinical Evaluation of Glycyrrhizin on HIV-Infected Asymptomatic Hemophiliac Patients in Japan. Fifth International Conference on AIDS; p. 298. [Google Scholar]
- 60.Mori K., Sakai H., Suzuki S., et al. The present status in prophylaxis and treatment of HIV infected patients with hemophilia in Japan. Rinsho Byhori. 1989;37:1200–1208.. ([Japanese]) [PubMed] [Google Scholar]
- 61.Hattori T., Ikematsu S., Koito A., et al. Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antiviral Res. 1989;11:255–261. doi: 10.1016/0166-3542(89)90035-1. [DOI] [PubMed] [Google Scholar]
- 62.Eisenburg J. Treatment of chronic hepatitis B. Part 2. Effect of glycyrrhizinic acid on the course of illness. Fortschr Med. 1992;110:395–398. ([German]) [PubMed] [Google Scholar]
- 63.Acharya S.K., Dasarathy S., Tandon A., et al. A preliminary open trial on interferon stimulator (SNMC) derived from Glycyrrhiza glabra in the treatment of subacute hepatic failure. Indian J Med Res. 1993;98:69–74. [PubMed] [Google Scholar]
- 64.Kumada H. Long-term treatment of chronic hepatitis C with glycyrrhizin [stronger neo-minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology. 2002;62:94–100. doi: 10.1159/000048283. [DOI] [PubMed] [Google Scholar]
- 65.Anderson K., Bradlow H., Sassa S., et al. Studies in porphyria VII. Relationship of the 5 alpha-reductive metabolism of steroid hormones to clinical expression of the genetic defect in acute intermittent porphyria. Am J Med. 1979;66:644–650. doi: 10.1016/0002-9343(79)91176-8. [DOI] [PubMed] [Google Scholar]
- 66.Tomita T., Sato T., Saito K., et al. Effects of lead and arsenic on the formation of 5 beta-H steroids. Toxicol Lett. 1979;3:291–297. [Google Scholar]
- 67.Armanini D., De Palo C.B., Mattarello M.J., et al. Effect of licorice on the reduction of body fat mass in healthy subjects. J Endocrinol Invest. 2003;26:646–650. doi: 10.1007/BF03347023. [DOI] [PubMed] [Google Scholar]
- 68.Kuroda M., Mimaki Y., Sashida Y., et al. Phenolics with PPAR-gamma ligand-binding activity obtained from licorice (Glycyrrhiza uralensis roots) and ameliorative effects of glycyrin on genetically diabetic KK-A(y) mice. Bioorg Med Chem Lett. 2003;13:4267–4272. doi: 10.1016/j.bmcl.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 69.Mae T., Kishida H., Nishiyama T., et al. A licorice ethanolic extract with peroxisome proliferator-activated receptor-gamma ligand-binding activity affects diabetes in KK-Ay mice, abdominal obesity in diet-induced obese C57BL mice and hypertension in spontaneously hypertensive rats. J Nutr. 2003;133:3369–3377. doi: 10.1093/jn/133.11.3369. [DOI] [PubMed] [Google Scholar]
- 70.Armanini D., Fiore C., Mattarello M.J., et al. History of the endocrine effects of licorice. Exp Clin Endocrinol Diabetes. 2002;110:257–261. doi: 10.1055/s-2002-34587. [DOI] [PubMed] [Google Scholar]
- 71.Fuhrman B., Volkova N., Kaplan M., et al. Antiatherosclerotic effects of licorice extract supplementation on hypercholesterolemic patients: increased resistance of LDL to atherogenic modifications, reduced plasma lipid levels, and decreased systolic blood pressure. Nutrition. 2002;18:268–273. doi: 10.1016/s0899-9007(01)00753-5. [DOI] [PubMed] [Google Scholar]
- 72.MacKenzie M.A., Hoefnagels W.H., Jansen R.W., et al. The influence of glycyrrhetinic acid on plasma cortisol and cortisone in healthy young volunteers. J Clin Endocrinol Metab. 1990;70:1637–1643. doi: 10.1210/jcem-70-6-1637. [DOI] [PubMed] [Google Scholar]
- 73.Chen M.F., Shimada F., Kato H., et al. Effect of glycyrrhizin on the pharmacokinetics of prednisolone following low dosage of prednisolone hemisuccinate. Endocrinol Jpn. 1990;37:331–341. doi: 10.1507/endocrj1954.37.331. [DOI] [PubMed] [Google Scholar]
- 74.Agarwal A., Gupta D., Yadav G., et al. An evaluation of the efficacy of licorice gargle for attenuating postoperative sore throat: a prospective, randomized, single-blind study. Anesth Analg. 2009;109:77–81. doi: 10.1213/ane.0b013e3181a6ad47. [DOI] [PubMed] [Google Scholar]
- 75.Huang Q.C., Wang M.J., Chen X.M., et al. Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis? Oncotarget. 2016;7(2):1193–1202. doi: 10.18632/oncotarget.6200. PubMed PMID: 26498361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honda K., Kamisoyama H., Tominaga Y., et al. The molecular mechanism underlying the reduction in abdominal fat accumulation by licorice flavonoid oil in high fat diet-induced obese rats. Anim Sci J. 2009;80:562–569. doi: 10.1111/j.1740-0929.2009.00670.x. [DOI] [PubMed] [Google Scholar]
- 77.Kamisoyama H., Honda K., Tominaga Y., et al. Investigation of the anti-obesity action of licorice flavonoid oil in diet-induced obese rats. Biosci Biotechnol Biochem. 2008;72:3225–3231. doi: 10.1271/bbb.80469. [DOI] [PubMed] [Google Scholar]
- 78.Aoki F., Honda S., Kishida H., et al. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci Biotechnol Biochem. 2007;71:206–214. doi: 10.1271/bbb.60463. [DOI] [PubMed] [Google Scholar]
- 79.Tominaga Y., Nakagawa K., Mae T., et al. Licorice flavonoid oil reduces total body fat and visceral fat in overweight subjects: a randomized, double-blind, placebo-controlled study. Obes Res Clin Prac. 2009;3:159–178. doi: 10.1016/j.orcp.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 80.Kinoshita T., Matsumoto A., Yoshino K., Furukawa S. The effects of licorice flavonoid oil with respect to increasing muscle mass: a randomized, double-blind, placebo-controlled trial. J Sci Food Agric. 2017;97(8):2339–2345. doi: 10.1002/jsfa.8044. Epub 2016 Nov 11. [DOI] [PubMed] [Google Scholar]
- 81.Aoki F., Nakagawa K., Kitano M., et al. Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. J Am Coll Nutr. 2007;26:209–218. doi: 10.1080/07315724.2007.10719603. [DOI] [PubMed] [Google Scholar]
- 82.Saeedi M., Morteza-Semnani K., Ghoreishi M.R. The treatment of atopic dermatitis with licorice gel. J Dermatolog Treat. 2003;14:153–157. doi: 10.1080/09546630310014369. [DOI] [PubMed] [Google Scholar]
- 83.Teelucksingh S., Mackie A.D., Burt D., et al. Potentiation of hydrocortisone activity in skin by glycyrrhetinic acid. Lancet. 1990;335:1060–1063. doi: 10.1016/0140-6736(90)92633-s. [DOI] [PubMed] [Google Scholar]
- 84.Nokhodchi A., Nazemiyeh H., Ghafourian T., et al. The effect of glycyrrhizin on the release rate and skin penetration of diclofenac sodium from topical formulations. Farmaco. 2002;57:883–888. doi: 10.1016/s0014-827x(02)01298-3. [DOI] [PubMed] [Google Scholar]
- 85.Saeedi M., Morteza-Semnani K., Ghoreishi M.R. The treatment of atopic dermatitis with licorice gel. J Dermatolog Treat. 2003;14:153–157. doi: 10.1080/09546630310014369. [DOI] [PubMed] [Google Scholar]
- 86.Evans F.Q. The rational use of glycyrrhetinic acid in dermatology. Br J Clin Pract. 1958;12:269–274. [PubMed] [Google Scholar]
- 87.Partridge M., Poswillo D. Topical carbenoxolone sodium in the management of herpes simplex infection. Br J Oral Maxillofac Surg. 1984;22:138–145. doi: 10.1016/0266-4356(84)90026-3. [DOI] [PubMed] [Google Scholar]
- 88.Csonka G.W., Tyrrell D.A. Treatment of herpes genitalis with carbenoxolone and cicloxolone creams. A double blind placebo controlled trial. Br J Vener Dis. 1984;60:178–181. doi: 10.1136/sti.60.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nerya O., Vaya J., Musa R., et al. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J Agric Food Chem. 2003;51:1201–1207. doi: 10.1021/jf020935u. [DOI] [PubMed] [Google Scholar]
- 90.Bardhan K.D., Cumberland D.C., Dixon R.A., et al. Clinical trial of deglycyrrhizinised liquorice in gastric ulcer. Gut. 1978;19:779–782. doi: 10.1136/gut.19.9.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Multicentre Trial Treatment of duodenal ulcers with glycyrrhinizin acid-reduced liquorice. BMJ. 1973;3:501–503. [PMC free article] [PubMed] [Google Scholar]
- 92.Feldman H., Gilat T. A trial of deglycyrrhizinated liquorice in the treatment of duodenal ulcer. Gut. 1971;12:499–555. doi: 10.1136/gut.12.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nazari S., Rameshrad M., Hosseinzadeh H. Toxicological effects of Glycyrrhiza glabra (licorice): a review. Phytother Res. 2017;31(11):1635–1650. doi: 10.1002/ptr.5893. Epub 2017 Aug 18. [DOI] [PubMed] [Google Scholar]
- 94.Belhadj-Tahar H., Nassar B., Coulais Y., et al. Acute pseudo-aldosteronism syndrome induced by liquorice. Therapie. 2003;58:375–378. doi: 10.2515/therapie:2003058. [DOI] [PubMed] [Google Scholar]
- 95.Al-Qarawi A.A., Abdel-Rahman H.A., Ali B.H., et al. Liquorice (Glycyrrhiza glabra) and the adrenal-kidney-pituitary axis in rats. Food Chem Toxicol. 2002;40:1525–1527. doi: 10.1016/s0278-6915(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 96.Tanahashi T., Mune T., Morita H., et al. Glycyrrhizic acid suppresses type 2 11 beta-hydroxysteroid dehydrogenase expression in vivo. J Steroid Biochem Mol Biol. 2002;80:441–447. doi: 10.1016/s0960-0760(02)00033-x. [DOI] [PubMed] [Google Scholar]
- 97.Sigurjonsdottir H.A., Franzson L., Manhem K., et al. Liquorice-induced rise in blood pressure: a linear dose-response relationship. J Hum Hypertens. 2001;15:549–552. doi: 10.1038/sj.jhh.1001215. [DOI] [PubMed] [Google Scholar]
- 98.Ploeger B., Mensinga T., Sips A., et al. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab Rev. 2001;33:125–147. doi: 10.1081/dmr-100104400. [DOI] [PubMed] [Google Scholar]
- 99.Van Gelderen C.E., Bijlsma J.A., van Dokkum W., et al. Glycyrrhizic acid: the assessment of a no effect level. Hum Exp Toxicol. 2000;19:434–439. doi: 10.1191/096032700682694251. [DOI] [PubMed] [Google Scholar]
- 100.Baron J., Nabarro J., Slater J., et al. Metabolic studies, aldosterone secretion rate, and plasma renin after carbenoxolone sodium as biogastrone. BMJ. 1969;2:793–795. doi: 10.1136/bmj.2.5660.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Armanini D., Bonanni G., Mattarello M.J., et al. Licorice consumption and serum testosterone in healthy man. Exp Clin Endocrinol Diabetes. 2003;111:341–343. doi: 10.1055/s-2003-42724. [DOI] [PubMed] [Google Scholar]
- 102.Josephs R.A., Guinn J.S., Harper M.L., et al. Liquorice consumption and salivary testosterone concentrations. Lancet. 2001;358:1613–1614. doi: 10.1016/S0140-6736(01)06664-8. [DOI] [PubMed] [Google Scholar]
- 103.Strandberg T.E., Jarvenpaa A.L., Vanhanen H., et al. Birth outcome in relation to licorice consumption during pregnancy. Am J Epidemiol. 2001;153:1085–1088. doi: 10.1093/aje/153.11.1085. [DOI] [PubMed] [Google Scholar]
- 104.Kent U.M., Aviram M., Rosenblat M., et al. The licorice root derived isoflavan glabridin inhibits the activities of human cytochrome P450S 3A4, 2B6, and 2C9. Drug Metab Dispos. 2002;30:709–715. doi: 10.1124/dmd.30.6.709. [DOI] [PubMed] [Google Scholar]
- 105.Armanini D., Castello R., Scaroni C., et al. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur J Obstet Gynecol Reprod Biol. 2007;131:61–67. doi: 10.1016/j.ejogrb.2006.10.013. [DOI] [PubMed] [Google Scholar]


