Abstract.
Newborn screening (NBS) can detect 21-hydroxylase deficiency (21-OHD), allowing for early treatment initiation. However, many patients present with adrenal crises or hyponatremia at their first visit. Age (in days) of hyponatremia development in infants with salt-wasting (SW)-type 21-OHD remains unclear. Therefore, we determined the earliest age of hyponatremia diagnosis in this retrospective observational study using medical records of 40 patients with classic 21-OHD in Niigata Prefecture, Japan, from April 1989 to March 2019. We determined the earliest diagnosis of hyponatremia (serum sodium levels < 130 mEq/L) and created a sodium decrease rate model to estimate hyponatremia development age. Of 23 patients with SW-type 21-OHD, 10 (43.5%) were identified during NBS; the earliest case to present with hyponatremia was at day 7. Serum sodium levels were significantly and negatively correlated with age in days, and hyponatremia was estimated to develop at 6.6 d after birth. Genotype or serum 17-hydroxyprogesterone levels were not associated with sodium decrease rate. Thus, hyponatremia development age is earlier (within 7 d) than the previously described time-point (10–14 d) in infants with SW-type 21-OHD. Efforts to reduce the time lag from obtaining results to consultation may be required in patients with high 17-hydroxyprogesterone levels on NBS.
Keywords: 21-hydroxylase deficiency, hyponatremia, congenital adrenal hyperplasia, salt wasting, newborn screening
Introduction
21-hydroxylase deficiency (21-OHD) is an autosomal recessive inherited disease caused by genetic abnormalities in the CYP21A2 gene. According to the residual enzyme activity, 21-OHD is classified into classic salt wasting (SW), classic simple virilizing (SV), or non-classic forms. Patients with the SW type (the most severe presentation) develop hyponatremia, hypovolemic shock, and adrenal crises, because of lack of mineralocorticoids and glucocorticoids (1). In Japan, since 1989, newborn mass screening (NBS) has been used to measure 17-hydroxyprogesterone (17-OHP) in filter paper blood and 21-OHD has been detected in approximately 1 in 18,000–19,000 births (2, 3). Although 21-OHD can be detected and treated early owing to the NBS program, we frequently encounter patients with hyponatremia and adrenal crises at their first visit. According to the Nelson Textbook of Pediatrics, hyponatremia in neonates with the SW type occurs at 10–14 d after birth (4), but no studies about the actual timing of this hyponatremia development exist. In our clinical setting, we have encountered cases of hyponatremia occurring earlier than reported in the textbook. Therefore, a clinical question was presented regarding the earliest age, in days, at which SW-type 21-OHD reduced serum sodium levels. However, it is very difficult to examine the natural course of serum sodium changes in patients with 21-OHD, because they should be treated promptly regardless of whether hyponatremia has occurred, as recommended in the Japanese guidelines for 21-OHD (5). Therefore, in the present study, we determined the earliest age (in days) at which hyponatremia was diagnosed and estimated age of hyponatremia development by creating a sodium decrease rate model. The purpose of this study was to elucidate the precise timing of hyponatremia development in patients with SW-type 21-OHD.
Patients and Methods
We extracted data from the records of 40 patients diagnosed with classic 21-OHD in Niigata Prefecture, Japan, from April 1989 to March 2019. 21-OHD was diagnosed based on the criteria in the Guidelines for Diagnosis and Treatment of 21-Hydroxylase Deficiency (5). These include clinical manifestations, endocrinological findings (e.g., elevated serum 17-OHP), and exclusions of P450 oxidoreductase, 3β-hydroxysteroid dehydrogenase, and 11β-hydroxylase deficiencies. Exclusion criteria were lack of serum sodium levels data before hydrocortisone replacement and presence of severe complications other than 21-OHD. Moreover, cases treated with intravenous crystalloid glucose solutions before hydrocortisone replacement were excluded.
We defined hyponatremia as serum sodium < 130 mEq/L, as described in another 21-OHD-related study (6). We defined the SW-type deficiency as the presence of symptoms and signs of circulatory shock, dehydration, and hyponatremia or CYP21A2 mutations with the estimated enzyme activity of 0–1% for both alleles (7) and the SV type based on other classical 21-OHD characteristics.
This was a multicenter retrospective observational study. From the medical records of the patients, we extracted data on serum or whole blood sodium levels, serum 17-OHP, serum potassium levels, plasma renin activity, body weight before the start of treatment, and gene mutation types. We included the serum sodium levels taken during the first visit and the whole blood serum taken during the examination on the transition date.
The primary endpoint was to determine the earliest age, in days, at which hyponatremia was diagnosed based on the medical record date. The secondary endpoint was to investigate the factors associated with serum sodium or sodium decrease rate in SW-type 21-OHD and estimate the age, in days, of hyponatremia development by creating a sodium decrease rate model.
For gene analysis, we used multiplex ligation-dependent probe amplification (SALSA MLPA P050 CAH Probemix, MRC Holland, Amsterdam, Netherlands) and direct sequencing to identify CYP21A2 mutations (8). We classified genotypes with estimated enzymatic activity of both alleles at 0% into group null and the remaining genotypes into group A. We plotted the serum sodium data in the SW type against age (in days) at the first visit or hyponatremia development, and created a sodium decrease rate model with liner regression.
Statistical analyses were performed using EZR Version 3.5.2. (9) and Stata Version 16.1. Background data of patients with 21-OHD are presented as mean ± standard deviation. Differences in birth weight, gestational age, age (in days) at the first visit, weight change rate at the first visit, and 17-OHP levels between SW and SV types were assessed using t-tests. Univariate analysis was performed between serum sodium levels and age (in days) as well as between serum sodium level and weight change rate at the first visit using a linear regression model. In addition, analysis of covariance was performed for serum sodium level and age (in days) between factors* considered to be associated with sodium decrease rate. Statistical significance was set at 5%.
* The factors associated with sodium decrease rate including birth weight (BW), gestational age (GA), 17-OHP levels in NBS, plasma renin activity (PRA) and genotype, which were classified as follows:

**17-hydroxyprogesterone (17-OHP) levels in newborn screening (NBS) were measured by direct method.
The Niigata University Ethics Committee approved this study (Ethics Committee Approval Number: 2019-0095). Information related to the content of research is posted on the hospital homepage. Patients and/or their family were given the opportunity to refuse having their medical record data used for this study.
Results
Of the 40 cases diagnosed with classical 21-OHD, 15 (37.5%) were SV type and 25 (62.5%) were SW type. Two patients in the SW type were excluded because of unknown serum sodium level before treatment or history of infusion treatment before hydrocortisone replacement. Table 1 shows the clinical features of both types. Of the 23 cases in the SW type (9 girls and 14 boys), 10 (43.5%) were detected during NBS and 13 (56.5%) by other causes including ambiguous genitalia, family history of 21-OHD, vomiting, body weight loss, and hyponatremia. The mean age (in days) at the first visit in the SW type was 12.5 ± 4.7 d in those detected by NBS and 6.9 ± 6.5 d in the other cases (p = 0.02). In addition, weight loss was apparent in almost all patients diagnosed with the SW type and the mean weight change rate was −5.5 ± 4.6%. We found no significant weight loss in the patients of the SV type (6.4 ± 13.8%), and the patients in the SW type had significant weight loss compared with those in the SV type (p < 0.001).
Table 1. Background of patients with 21-hydroxylase deficiency (21-OHD).
Age, in days, at change in serum sodium level
Of the 23 patients in the SW type, we observed hyponatremia in 17 (73.9%), with day 7 being the earliest development day (patient number 4, Table 2), and all patients whose first visit was later than 10 d after birth presented with hyponatremia. In only three cases (patient numbers 1, 2, and 7, Table 2) of SW type 21-OHD, the natural course of blood sodium levels was confirmed. One patient had sodium levels monitored everyday (patient number 1, Table 2) and presented hyponatremia at 9 d, whereas other patients presented at 7 and 10 d.
Table 2. Hyponatremia development day.
Associations of serum sodium level with age (in days) and weight change rate at the first visit
There was a strong negative correlation between serum sodium and age (in days) [r = −0.89; 95% confidence interval (CI): −0.95 to −0.74, p < 0.001]. In contrast, there was no correlation between serum sodium and weight change rate at the first visit (r = 0.33; 95% CI: −0.12 to 0.66).
Analysis of covariance between birth weight, weight change rate, serum 17-OHP level, and genotype
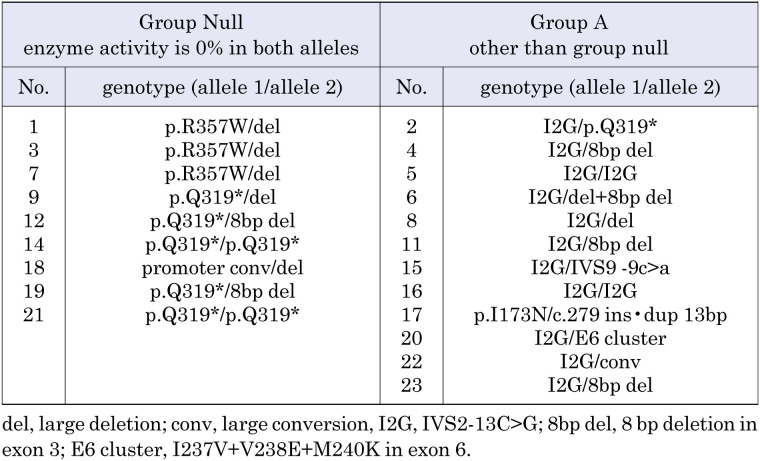
We did not find any significant factors associated with sodium decrease rate (Fig. 1). Analysis of the CYP21A2 mutation was performed in 21 of 23 cases. We classified 9 cases into group null and 12 into group A (Table 3). There were no significant differences between the two groups (p = 0.812, Fig. 1C).
Fig. 1.
Analysis of covariance. We performed analysis of covariance for serum sodium level and age (in days) among four factors. No factor was associated with sodium decrease rate. A: Birth weight (p = 0.586). B: 17-OHP levels in NBS (p = 0.749). C: Genotype (p = 0.812).
Table 3. Genotype in group null and group A.
Gestational age was excluded from the analysis because 22 of 23 cases were classified as group 2. Plasma renin activity was also excluded from the analysis because all cases exceeded 20 ng/mL/h and it often exceeded the measurement sensitivity. One patient (patient number 17, Table 2) was excluded from all analyses with this model because of diagnosis on repeated NBS and genotype (I173N) corresponding to the SV type.
Sodium decrease rate model
Since a strong negative correlation was confirmed between serum sodium levels and age (in days) and there were no factors associated with sodium decrease rate, single regression analysis between serum sodium level and age (in days) was performed. The calculated regression equation was: y = −1.34x + 138.9 (95% CI: slope −1.7 to −1.0; intercept 135.4–142.3) and the age when serum sodium level was < 130 mEq/L was estimated to be 6.6 d (95% CI: 5.3–8.7) after birth (Fig. 2). One case (patient number 17, Table 2) was excluded from this model as described above.
Fig. 2.
Sodium decrease rate model. We plotted serum sodium data in the SW type and age at first visit or hyponatremia development, and created a sodium decrease rate model. The age when serum sodium was 130 mEq/L was estimated to be 6.6 d (95% CI: 5.3–8.7) after birth.
Discussion
Our study of 23 patients with SW-type 21-OHD found that the earliest age of hyponatremia development was 7 d, and all of the patients with first visits later than 10 d presented with hyponatremia. In addition, development of hyponatremia in patients with the SW type was estimated to occur at approximately 7 d after birth using a sodium rate decrease model.
However, hyponatremia in patients with the SW type is thought to develop only later in life (i.e. not during the first day after birth). The time required for presentation of hyponatremia is 10–14 d after birth according to the Nelson Textbook of Pediatrics (4), about 2 wk according to the Williams Textbook of Endocrinology (10) and 7–14 d according to a published reference (11). However, we were not able to find any reference citations for the information presented in any of these resources.
Before NBS was implemented, 21-OHD was presumably discovered based on symptoms such as poor feeding, vomiting, and shock. Therefore, descriptions in the above textbooks likely represent the age whereby such symptoms were observed. In addition, not all US states currently perform NBS for 21-OHD, which may also affect the timing of hyponatremia. In contrast, we calculated the age at which serum sodium level was < 130 mEq/L, regardless of the symptoms, in this study. Our results suggest that this age is much earlier than the previous report.
The Japanese guidelines for 21-OHD suggest that prompt treatment should be started regardless of whether hyponatremia has occurred (5). Some cases were diagnosed immediately after birth because of ambiguous genitalia or a family history of 21-OHD and were started on glucocorticoid replacement before the development of hyponatremia. For this reason, it is difficult to examine the natural course of serum sodium changes in patients with SW-type 21-OHD. In our study, 7 out of 10 patients with SW-type 21-OHD without hyponatremia at the first visit were started on hydrocortisone replacement immediately, and we were able to follow the natural course of sodium levels in only three patients, who presented hyponatremia at 9, 7, and 10 d.
For this study, for this study, a sodium decrease rate model was created to predict the age at which hyponatremia would occur. As a result, we found a strong negative correlation between the serum sodium and the age (in days), and no other factors were associated with sodium decrease rate. The age (in days) at serum sodium < 130 mEq/L was estimated to be 6 to 9 d. Indeed, the earliest age at which hyponatremia was diagnosed was 7 d of age, consistent with this model. Our results show that hyponatremia may occur within 7d in patients with the severe SW-type 21-OHD.
Genotype–phenotype correlations in congenital adrenal hyperplasia due to 21-hydroxylase deficiency are based on in vitro CYP21A2 activity (12). Since the degree of aldosterone secretion failure is defined by the degree of residual activity, we hypothesized that the difference in the sodium decrease rate models depends on the genotype. However, we could not find any significant differences between the null and A genotype groups. This may be because some genotypes cannot accurately predict the phenotype, although most match the phenotype severity (13). In addition, sodium levels are regulated by a balance of sodium intake, sodium excretion, and body water content. Sodium intake of the patients, which depends on the nutritional status before the first visit, was unknown and this also may have affected our sodium decrease rate model. Since the number of cases was small, further studies will be necessary to assess the association between development of hyponatremia and genotype.
Similarly, we found no significant correlations between serum sodium and weight change rate. This is probably because of two factors. First, the age at first visit varied from case to case although the body weight physiologically decreased several days after birth; thus, comparing it with the weight change rate is difficult. Second, factors other than 21-OHD affect body gain such as the amount of milk ingested.
This study has some limitations. First, we investigated only the age of hyponatremia “diagnosis” and not “development”. The actual onset of hyponatremia is considered to be earlier as there are many cases in which hyponatremia has already developed before the first visit. Second, we estimated the age (in days) of hyponatremia development using a linear model; however, whether this model is consistent with the natural course of serum sodium changes in patients with 21-OHD remains unclear. Further studies are warranted to validate this consistency. Third, we did not find information on the nutritional status or general condition of each newborn before the visit, and other bias effects may have affected our analysis. Finally, this study covers only patients in the Niigata Prefecture; whether the sodium decrease rate model can be applied in other regions requires a larger number of studies.
Conclusion
The age at which SW-type 21-OHD develops hyponatremia, within 7 d of age, is earlier than the previously described 10–14 d of age. This suggests that we need to make efforts to reduce the time lag from receiving results of NBS to consultation for those with high 17-hydroxyprogesterone by NBS.
Conflict of interests
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgments
We would like to thank Dr. Kazuo Takeuchi, Department of Pediatrics, Nagaoka Chuo General Hospital, for cooperation in providing patient data.
References
- 1.White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 2000;21: 245–91. [DOI] [PubMed] [Google Scholar]
- 2.Suwa S. Nationwide survey of neonatal mass-screening for congenital adrenal hyperplasia in Japan. Screening 1994;3: 141–51. doi: 10.1016/0925-6164(94)90022-1 [DOI] [Google Scholar]
- 3.Nagasaki K, Asami T, Nomura M, Hokari K, Otabe N. Neonatal screening for 21-hydroxylase deficiency in Niigata, Japan: 20 years experience and results. J Jpn Soc for Mass-screening 2010;20: 223–7. [Google Scholar]
- 4.Kilegman RM,, St Geme III JW, Blum NJ, et al. Nelson Textbook of Pediatrics,21st edition. Philadelphia: Elsevier; 2019. p. 2973–6. [Google Scholar]
- 5.Ishii T, Anzo M, Adachi M, Onigata K, Kusuda S, Nagasaki K, et al. Mass Screening CommitteeJapanese Society for Pediatric EndocrinologyJapanese Society for Mass Screening. Guidelines for diagnosis and treatment of 21-hydroxylase deficiency (2014 revision). Clin Pediatr Endocrinol 2015;24: 77–105. doi: 10.1297/cpe.24.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koyama S, Toyoura T, Saisho S, Shimozawa K, Yata J. Genetic analysis of Japanese patients with 21-hydroxylase deficiency: identification of a patient with a new mutation of a homozygous deletion of adenine at codon 246 and patients without demonstrable mutations within the structural gene for CYP21. J Clin Endocrinol Metab 2002;87: 2668–73. doi: 10.1210/jcem.87.6.8522 [DOI] [PubMed] [Google Scholar]
- 7.Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab 2009;23: 181–92. doi: 10.1016/j.beem.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usui T, Nishisho K, Kaji M, Ikuno N, Yorifuji T, Yasuda T, et al. Three novel mutations in Japanese patients with 21-hydroxylase deficiency. Horm Res 2004;61: 126–32. [DOI] [PubMed] [Google Scholar]
- 9.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48: 452–8. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melmed S, Auchus RJ, Goldfine AB, et al. Williams Textbook of Endocrinology, 13th edition. Philadelphia: Elsevier; 2015. p. 535–6. [Google Scholar]
- 11.Merke DP. Genetics and clinical presentation of classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on July 08, 2019). [Google Scholar]
- 12.Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest 1992;90: 584–95. doi: 10.1172/JCI115897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RC, Mercado AB, Cheng KC, New MI. Steroid 21-hydroxylase deficiency: genotype may not predict phenotype. J Clin Endocrinol Metab 1995;80: 2322–9. [DOI] [PubMed] [Google Scholar]