Key Points
Question
For early breast cancer, is 5-year local control with delayed second-procedure targeted intraoperative radiotherapy (TARGIT-IORT) noninferior to whole-breast postoperative external beam radiotherapy (EBRT), and how do long-term outcomes compare?
Findings
In this randomized clinical trial including 1153 participants, delayed second-procedure TARGIT-IORT was not noninferior to EBRT at 5-year complete follow-up; however, long-term (median 9 years) mastectomy-free survival, distant disease-free survival, and overall survival were not different.
Meaning
For early breast cancer, delayed second-procedure single-dose TARGIT-IORT given by reopening the lumpectomy wound had similar long-term mastectomy-free and overall survival compared with EBRT despite higher local recurrence.
This secondary analysis of the TARGIT-A randomized clinical trial compares the effect of delayed second-procedure targeted intraoperative radiotherapy (TARGIT-IORT) with whole-breast external beam radiotherapy (ERBT) on local recurrence at 5 years and long-term survival outcomes among women with early breast cancer.
Abstract
Importance
Conventional adjuvant radiotherapy for breast cancer given daily for several weeks is onerous and expensive. Some patients may be obliged to choose a mastectomy instead, and some may forgo radiotherapy altogether. We proposed a clinical trial to test whether radiotherapy could be safely limited to the tumor bed.
Objective
To determine whether delayed second-procedure targeted intraoperative radiotherapy (TARGIT-IORT) is noninferior to whole-breast external beam radiotherapy (EBRT) in terms of local control.
Design, Setting, and Participants
In this prospective, randomized (1:1 ratio) noninferiority trial, 1153 patients aged 45 years or older with invasive ductal breast carcinoma smaller than 3.5 cm treated with breast conservation were enrolled from 28 centers in 9 countries. Data were locked in on July 3, 2019.
Interventions
The TARGIT-A trial was started in March 2000; patients were randomized after needle biopsy to receive TARGIT-IORT immediately after lumpectomy under the same anesthetic vs EBRT and results have been shown to be noninferior. A parallel study, described in this article, was initiated in 2004; patients who had their cancer excised were randomly allocated using separate randomization tables to receive EBRT or delayed TARGIT-IORT given as a second procedure by reopening the lumpectomy wound.
Main Outcomes and Measures
A noninferiority margin for local recurrence rate of 2.5% at 5 years, and long-term survival outcomes.
Results
Overall, 581 women (mean [SD] age, 63 [7] years) were randomized to delayed TARGIT-IORT and 572 patients (mean [SD] age, 63 [8] years) were randomized to EBRT. Sixty patients (5%) had tumors larger than 2 cm, or had positive nodes and only 32 (2.7%) were younger than 50 years. Delayed TARGIT-IORT was not noninferior to EBRT. The local recurrence rates at 5-year complete follow-up were: delayed TARGIT-IORT vs EBRT (23/581 [3.96%] vs 6/572 [1.05%], respectively; difference, 2.91%; upper 90% CI, 4.4%). With long-term follow-up (median [IQR], 9.0 [7.5-10.5] years), there was no statistically significant difference in local recurrence-free survival (HR, 0.75; 95% CI, 0.57-1.003; P = .052), mastectomy-free survival (HR, 0.88; 95% CI, 0.65-1.18; P = .38), distant disease-free survival (HR, 1.00; 95% CI, 0.72-1.39; P = .98), or overall survival (HR, 0.96; 95% CI, 0.68-1.35; P = .80).
Conclusions and Relevance
These long-term data show that despite an increase in the number of local recurrences with delayed TARGIT-IORT, there was no statistically significant decrease in mastectomy-free survival, distant disease-free survival, or overall survival.
Trial Registration
ISRCTN34086741, ClinicalTrials.gov Identifier: NCT00983684
Introduction
In 2018, there were 2 million new cases of breast cancer diagnosed worldwide and 626 000 deaths.1 Most patients are suitable for treatment with breast-conserving surgery and adjuvant radiotherapy, rather than total mastectomy. The TARGIT-A randomized clinical trial (accrual from 2000-2012) compared risk-adapted TARGeted intraoperative radiotherapy (TARGIT-IORT) during the initial surgical excision of the cancer2,3,4,5 with conventional whole-breast external beam radiotherapy (EBRT) over several weeks.2,6,7 The results of this trial demonstrated noninferiority particularly when TARGIT-IORT was delivered at the time of initial excision of cancer.
In 2004, 4 years after accrual began in the main TARGIT-A trial, and at the request of potentially high-volume centers, we sought and received additional ethics approval and opened a parallel study. This was previously referred to as “postpathology stratum” and recruited 1153 patients using a separate randomization table. Patients were randomized after their initial surgery to have either conventional fractionated whole-breast radiotherapy (n = 572), or to undergo a further operation to deliver delayed radiotherapy to the wound (n = 581) by reopening the original incision. This trial was initiated mainly because of the convenience of easier scheduling of delayed TARGIT-IORT in the operation theater. A potential benefit was that the inclusion criteria could be made more selective, choosing the patients with better prognosis based on the full histopathologic results that would be available after tumor excision. For example, the knowledge of the microscopically measured tumor size, grade, and nodal status could be used to select a much lower-risk patient population before randomization.
This delayed procedure was performed at a median (IQR) of 37 (29-51) days after the initial excision as a second surgical procedure in the operation theater, rather than immediate intraoperative radiotherapy given during the initial cancer operation. This article describes the long-term outcomes of this parallel study.
Methods
The TARGIT-A trial was a pragmatic, prospective, international, multicenter, open label, randomized, phase 3 trial that compared the policy of risk-adapted TARGIT-IORT vs the conventional policy of whole-breast EBRT. The trial protocol (https://njl-admin.nihr.ac.uk/document/download/2006598) and the details of sample size calculations, the process of random allocation, have been previously described.6,7 The trial protocol is available in Supplement 1. The study received ethics approval from the joint University College London and University College London Hospital committees of ethics of human research.
Participants
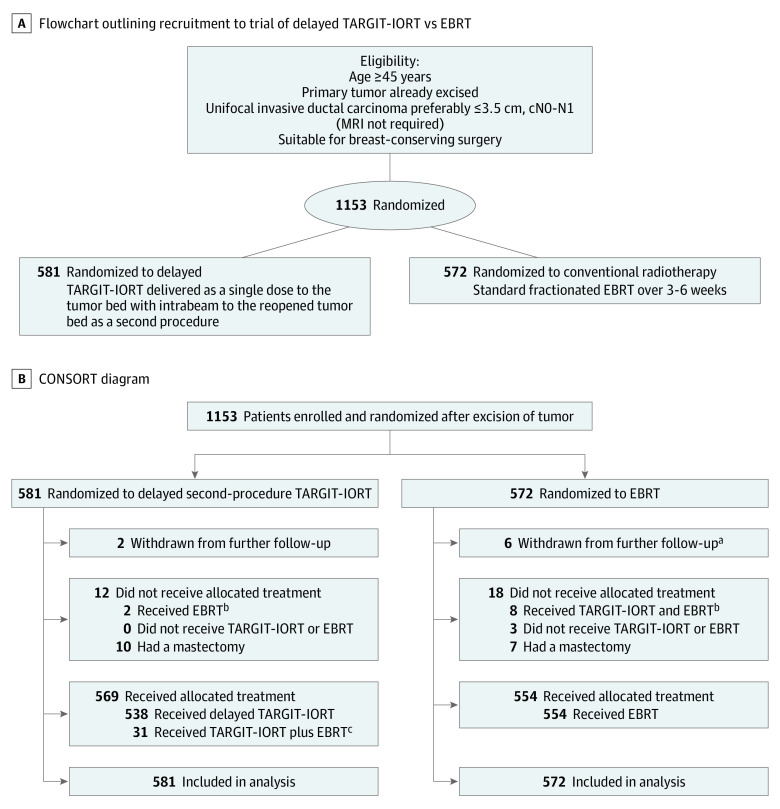
Women were eligible to participate in the delayed TARGIT-IORT trial if their breast cancer was already excised. They needed to be aged 45 years or older with unifocal breast cancer on examination and conventional imaging. Pragmatically, we permitted individual centers to prespecify the final postoperative histopathologic criteria that would make patients eligible for randomization and these were prespecified in the center’s treatment policy document. Because most centers specified criteria for eligibility: aged 50 years or older, grade 1 or 2 disease, and uninvolved nodes, only 5% of patients in the trial had any adverse prognostic criteria. All patients gave informed written consent and needed to be available for regular follow-up for at least 10 years. Follow-up clinical examination was at least every 6 months for the first 5 years and annually thereafter, including a mammogram once per year. Random allocation was in a 1:1 ratio, to receive either single-dose delayed TARGIT-IORT or EBRT as per standard schedules over several weeks, with randomization blocks stratified by center. The flow diagram and CONSORT diagram are given in Figure 1A and B.
Figure 1. Flowchart and CONSORT Diagram.
EBRT indicates whole-breast external beam radiotherapy; MRI, magnetic resonance imaging; TARGIT-IORT, targeted intraoperative radiotherapy. A, Flowchart outlining recruitment to trial of delayed TARGIT-IORT vs EBRT. B, CONSORT diagram of participant randomization.
aThe difference in number withdrawn was not statistically significant (P = .15).
bAs per protocol, 31 of 581 patients (5.3 %) allocated to delayed TARGIT-IORT received EBRT after TARGIT-IORT.
cTwo of 581 patients (0.3%) allocated to delayed TARGIT-IORT received EBRT and 8 of 572 (1.4%) allocated EBRT received TARGIT-IORT as well.
The concept and the delayed TARGIT-IORT technique have been described previously3,4,5,8,9,10,11 and enabled these patients to have their radiotherapy in 1 sitting, albeit by undergoing a second procedure, usually under a general anesthetic.12 Radiation was given over 20 to 50 minutes delivering 20 Gy to the surface of the tumor bed attenuating to 5 to 7 Gy at 1-cm depth.
The patients in the conventional arm underwent standard EBRT, which always included fractionated whole-breast radiotherapy for 3 to 6 weeks, with or without an EBRT tumor bed boost, as determined by local criteria prespecified by the collaborating center.
Statistical Analysis
The statistical analysis plan (Supplement 1) was signed off on by the chair of the independent steering committee and an independent senior statistician before the unblinded data were sent to the trial statistician for the current analysis. It specified the primary outcome as local recurrence-free survival. This outcome, consistent with the DATECAN13 and STEEP14 guidelines, estimates the chance of a patient being alive without local recurrence and therefore included local recurrence or death as events, ie, patients who had died were not censored. The other outcomes included mastectomy-free survival, distant disease-free survival, overall survival, breast cancer mortality and non–breast cancer mortality. Statistical analysis was performed using established methods, using STATA statistical software (versions 15.0 and 16.0, STATA Corp) for data compilation, validation, and analysis.13,14,15 Data analysis took place between September 11, 2019 to January 15, 2020.
In the original protocol, noninferiority was specified as being achieved if the difference in 5-year local recurrence rate did not cross a stringent margin of 2.5%. However, we have applied an even more rigorous criterion since 2013: that the upper 90% CI of the absolute difference in the binomial proportions of local recurrence rate at 5-year complete follow up should not cross 2.5% in absolute terms.
Kaplan-Meier graphs were displayed as recommended by Pocock et al,16 who recommend that the x-axis of these graphs should be extended until 10% to 20% of patients are at risk of an event. The log-rank test was used to compare the difference between survival functions and to obtain P values.
Main Outcomes and Measures
The cause of death was specified by the center. If the cause was specified as a non–breast cancer event and no distant disease was recorded, it was defined as a non–breast cancer death. If the death was recorded by the center to be related to breast cancer, or as per convention, if breast cancer was present at the time of death, or if the cause of death was recorded as unknown or uncertain, it was presumed to be a breast cancer death.
Figure 1B shows the CONSORT diagram, which describes the treatment received in each of the randomized arms. The reference date for completeness was May 2, 2018, 8 years after the first data lock. A patient was considered as having complete follow-up if they were seen for the specified duration of follow-up, had died, or had withdrawn from the trial. As the last patient was randomized in 2012, the statistical analysis plan specified that the 5-year follow-up would be considered complete if 95% of patients had complete follow-up. It also specified that 10-year follow-up would be considered complete if the patient had at least 10 years of follow-up, had been seen within 1 year of the reference date, or had died or withdrawn; the 10-year follow-up would be considered complete if this was achieved by 90% of patients. Because there was no specific trial funding for individual centers, return of follow-up relied on individual investigators and their teams’ efforts, enthused by the trial-center team. The trial statistician and the chief investigator produced reports of completeness of follow up using blinded databases on a regular basis. As recommended by the independent steering committee, the database was unblinded for analysis once the prespecified goals for completeness of follow up were achieved. The reference date for analysis was 3 July 2019, so that all events up until 2 July 2019 were included for analysis. The chief investigator/corresponding author and the trial statistician (J.S.V. and Ma.B.) had access to all data sent by the trial center for analysis; all authors were responsible for the decision to submit the article. Since the last analysis, the trial oversight has been provided by an independent steering committee, appointed by the Health Technology Assessment program of the National Institute of Health Research, Department of Health, United Kingdom.
Results
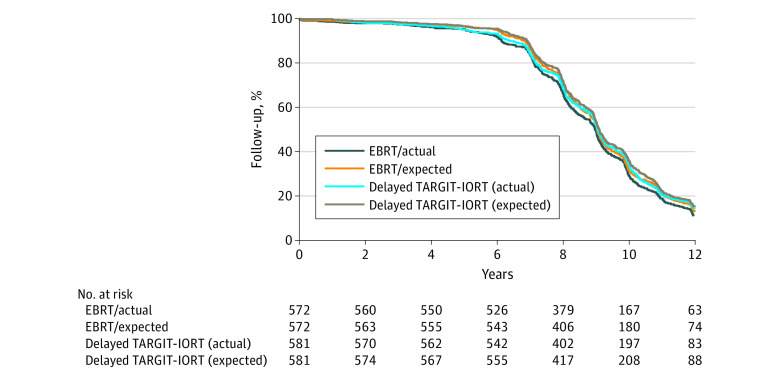
Overall, 581 women were randomized to delayed TARGIT-IORT and 572 to EBRT. The patient and tumor characteristics are given in Table 1 and were well matched between the randomization arms. Most patients were estrogen receptor positive (1119 [98%]), ERBB2 negative (1041 [94%]); 670 patients (58%) received endocrine therapy, and 40 (3.5%) received chemotherapy. The completeness of follow-up is demonstrated in Figure 2.
Table 1. Patient and Tumor Characteristics.
| Characteristic | No. (%)a | P valueb | |
|---|---|---|---|
| Delayed TARGIT-IORT (n = 581) | EBRT (n = 572) | ||
| Age, y | |||
| ≤50 | 30 (5.2) | 23 (4.02) | .54 |
| 51-60 | 166 (28.6) | 171 (29.9) | |
| 61-70 | 302 (52.0) | 284 (49.7) | |
| >70 | 83 (14.3) | 94 (16.4) | |
| Pathologic tumor size, mm | |||
| ≤10 | 294 (51.0) | 290 (51.8) | .79 |
| 11-20 | 249 (43.2) | 243 (43.4) | |
| >20 | 33 (5.7) | 27 (4.8) | |
| Unknown | 5 (0.9) | 12 (2.1) | |
| Grade | |||
| 1 | 305 (56.5) | 339 (63.8) | .06 |
| 2 | 204 (37.8) | 159 (29.9) | |
| 3 | 31 (5.7) | 33 (6.2) | |
| Unknown | 41 (7.1) | 41 (7.2) | |
| Margin | |||
| Free | 539 (92.9) | 520 (92.4) | .46 |
| DCIS only | 16 (2.8) | 18 (3.2) | |
| Invasive | 25 (4.3) | 25 (4.5) | |
| Unknown | 1 (0.2) | 9 (1.6) | |
| Lymphovascular invasion | |||
| Absent | 536 (94.7) | 533 (96.6) | .13 |
| Present | 30 (5.3) | 19 (3.4) | |
| Unknown | 15 (2.6) | 20 (3.5) | |
| Lymph nodes involved | |||
| 0 | 543 (93.6) | 537 (95.2) | .39 |
| 1-3 | 34 (5.9) | 26 (4.6) | |
| >3 | 3 (0.5) | 1 (0.2) | |
| Unknown | 1 (0.2) | 8 (1.4) | |
| ER status | |||
| Positive | 569 (98.3) | 550 (97.9) | .62 |
| Negative | 10 (1.7) | 12 (2.1) | |
| Unknown | 2 (0.3) | 10 (1.7) | |
| PgR status | |||
| Positive | 440 (81.8) | 423 (82.0) | .94 |
| Negative | 98 (18.2) | 93 (18.0) | |
| Unknown | 43 (7.4) | 56 (9.8) | |
| ERBB2 status | |||
| Positive | 30 (5.4) | 33 (6.0) | .65 |
| Negative | 526 (94.6) | 515 (94.0) | |
| Unknown | 25 (4.3) | 24 (4.2) | |
| Method of presentation | |||
| Screen detected | 420 (73.6) | 395 (70.5) | .26 |
| Symptomatic | 151 (26.4) | 165 (29.5) | |
| Unknown | 10 (1.7) | 12 (2.1) | |
| Endocrine therapy | |||
| Received | 336 (58.0) | 334 (59.4) | .63 |
| Did not receive | 243 (42.0) | 228 (40.6) | |
| Unknown | 2 (0.3) | 10 (1.8) | |
| Chemotherapy | |||
| Received | 26 (4.5) | 14 (2.5) | .07 |
| Did not receive | 553 (95.5) | 546 (97.5) | |
| Unknown | 2 (0.3) | 12 (2.1) | |
Abbreviations: DCIS, ductal carcinoma in situ; EBRT, whole-breast external beam radiotherapy; ER, estrogen receptor; PgR, progesterone receptor; TARGIT-IORT, targeted intraoperative radiotherapy.
For percentage calculation, the denominator for unknown percentages is the total number randomized (581 and 572) and the denominator for each category is the total number of known cases.
P values are given for differences between TARGIT-IORT and EBRT, calculated using a χ2 test for known values.
Figure 2. Actual Follow-up and Expected Follow-up for the Trial of Delayed Second-Procedure TARGIT-IORT vs EBRT.
EBRT indicates whole-breast external beam radiotherapy; TARGIT-IORT, targeted intraoperative radiotherapy.
At 5-year complete follow-up, the local recurrence rates were TARGIT-IORT, 23 (including 3 DCIS) of 581 (3.96%) vs EBRT, 6 (including 2 DCIS) of 572 (1.05%), giving a difference of 2.9% with its upper 90% CI of 4.4, which crossed the noninferiority margin of 2.5%.
Kaplan-Meier estimates and log-rank P values for delayed TARGIT-IORT vs EBRT are given in Table 2 and Figure 3. The median follow-up was 9 years and the differences between delayed TARGIT-IORT and EBRT were not statistically significant for local recurrence-free survival, invasive local recurrence-free survival, mastectomy-free survival, distant disease-free survival, breast cancer mortality, non–breast cancer mortality, and overall survival. No patients had uncontrolled local recurrence at the time of death.
Table 2. Twelve-Year Kaplan-Meier Estimates of Outcomes Measures for TARGIT-IORT vs EBRT.
| Outcomes | Delayed TARGIT-IORT (n = 581) | EBRT (n = 572) | Significance test for the full follow-up | |||
|---|---|---|---|---|---|---|
| Events | Kaplan-Meier estimates (95% CI) | Events | Kaplan-Meier estimates (95% CI) | HR (95% CI) | P value for log rank | |
| Local recurrence-free survivala | ||||||
| Estimate | 0.75 (0.57-1.003) | .052 | ||||
| 5-y | 41 | 92.87 (90.44-94.70) | 19 | 96.63 (94.77-97.84) | ||
| 10-y | 98 | 80.16 (76.19-83.54) | 72 | 84.36 (80.51-87.51) | ||
| 12-y | 106 | 75.30 (70.13-79.72) | 79 | 78.38 (72.32-83.27) | ||
| Invasive local recurrence-free survivala | ||||||
| Estimate | 0.75 (0.56-1.002) | .051 | ||||
| 5-y | 38 | 93.39 (91.03-95.15) | 17 | 96.99 (95.20-98.12) | ||
| 10-y | 95 | 80.68 (76.73-84.02) | 68 | 85.15 (81.35-88.23) | ||
| 12-y | 103 | 75.87 (70.72-80.24) | 75 | 79.23 (73.23-84.04) | ||
| Mastectomy-free survivala | ||||||
| Estimate | 0.88 (0.65-1.18) | .38 | ||||
| 5-y | 39 | 93.24 (90.87-95.02) | 23 | 95.93 (93.93-97.27) | ||
| 10-y | 82 | 83.79 (80.14-86.83) | 75 | 83.82 (79.94-87.01) | ||
| 12-y | 92 | 77.80 (72.57-82.16) | 79 | 80.44 (75.16-84.71) | ||
| Distant disease-free survivala | ||||||
| Estimate | 1.00 (0.72-1.39) | .98 | ||||
| 5-y | 26 | 95.49 (93.44-96.90) | 18 | 96.80 (94.97-97.97) | ||
| 10-y | 62 | 87.50 (84.13-90.19) | 62 | 86.91 (83.37 89.74) | ||
| 12-y | 71 | 81.98 (76.91-86.04) | 67 | 82.18 (76.44-86.65) | ||
| Overall survival | ||||||
| Estimate | 0.96 (0.68-1.35) | .80 | ||||
| 5-y | 19 | 96.70 (94.87-97.88) | 13 | 97.69 (96.06-98.65) | ||
| 10-y | 56 | 88.62 (85.35-91.19) | 56 | 87.77 (84.22-90.56) | ||
| 12-y | 65 | 83.13 (78.11-87.10) | 59 | 84.72 (79.52-88.70) | ||
| Breast cancer mortality | ||||||
| Estimate | 0.81 (0.43-1.52) | .50 | ||||
| 5-y | 9 | 1.58 (0.82-3.01) | 4 | 0.72 (0.27-1.90) | ||
| 10-y | 20 | 3.79 (2.45-5.83) | 16 | 3.50 (2.11-5.77) | ||
| 12-y | 21 | 4.39 (2.77-6.93) | 17 | 4.63 (2.52-8.43) | ||
| Mortality from other causes | ||||||
| Estimate | 1.02 (0.68-1.55) | .89 | ||||
| 5-y | 10 | 1.75 (0.95-3.23) | 9 | 1.60 (0.84-3.06) | ||
| 10-y | 36 | 7.90 (5.69-10.90) | 40 | 9.05 (6.62-12.31) | ||
| 12-y | 44 | 13.05 (9.35-18.05) | 42 | 11.17 (7.78-15.88) | ||
Abbreviations: EBRT, whole-breast external beam radiotherapy; HR, hazard ratio; TARGIT-IORT, targeted intraoperative radiotherapy.
Each of these survival measures include death as an event.
Figure 3. Twelve-Year Kaplan-Meier Curves Comparing Delayed Second-Procedure TARGIT-IORT vs EBRT.
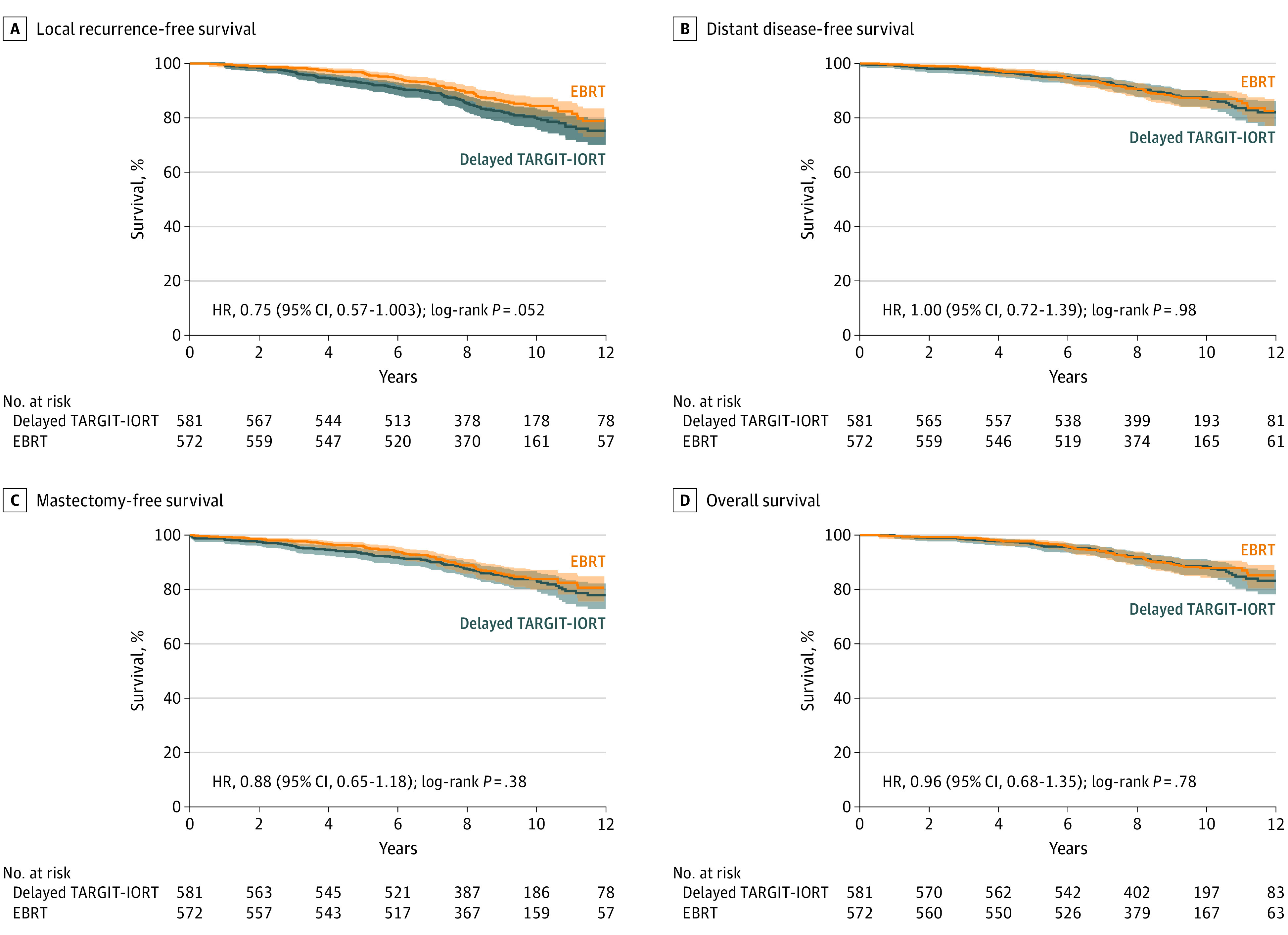
EBRT indicates whole-breast external beam radiotherapy; TARGIT-IORT, targeted intraoperative radiotherapy. In each of these Kaplan-Meier graphs, the blue lines represent delayed TARGIT-IORT with light blue shading indicating the 95% confidence intervals. The orange lines represent EBRT with light orange shading indicating the 95% confidence intervals.
Discussion
The TARGIT-A trial was originally conceived because of the clinicopathologic observation that local recurrence after breast-conserving surgery occurs predominantly in the index quadrant,17,18 despite the fact that more than 60% of patients suitable for breast conserving surgery are known to have microscopic foci of the disease outside the index quadrant.17,18,19
The delayed TARGIT-IORT approach was proposed mainly for logistical reasons. It allowed better planning of operation theaters as well as theoretically stricter selection of patients with low-risk disease based on final histopathologic analysis results. It also allowed using TARGIT-IORT in patients coming to a cancer center after having had their cancer excised in a smaller or remote hospital. Concordant with the results of our 2013 analysis, with mature follow-up (5 years complete follow-up with a median of 9 years) delayed TARGIT-IORT was found not to be noninferior to EBRT in terms of local control, with the upper 90% confidence limit of the 2.9% absolute difference in the 5-year local recurrence rate being 4.4%, which is above our stringent 2.5% noninferiority margin.
This noninferiority margin of 2.5% was decided after considerable thought,6 and is much more stringent than the 7% margin set in the in the ELIOT trial, the only other trial to our knowledge of intraoperative radiotherapy.20 We believe that it is important to consider how much the absolute differences seen in the trial matter to the patient. When considering treatments for patients with early breast cancer, local recurrence has been given great importance because of the perceived risk of consequent mastectomy, the danger of distant disease, and the potentially lower survival. The long-term data show that there was no impairment of mastectomy-free survival, distant disease-free survival, or overall survival, up to 12 years from randomization (Figure 3). Moreover, quality of life studies have shown that despite having a second procedure, the quality of life and patient-reported outcomes, such as cosmesis, breast-related quality of life, and breast pain, have been demonstrated to be superior with TARGIT-IORT,21,22 and this approach is preferred by patients even in the face of a hypothetically higher local recurrence risk.23,24 These findings may mitigate some of the patient concerns, and results of further patient preference research would help these discussions.
Limitations
The reasons for higher local recurrence with delayed second-procedure TARGIT-IORT may be multifactorial. First, the propensity of tumor recurrence in the index quadrant could be owing to a tumor promoting effect of the microenvironment of the surgical wound,25,26,27 a risk that has been shown to be beneficially manipulated by TARGIT-IORT to the fresh tumor bed,25,27,28 but perhaps not when TARGIT-IORT is given as a delayed second procedure. Second, the surgical procedure of lumpectomy has changed. Early on in the trial, the tissues around the tumor bed were often not approximated after lumpectomy, and the tumor bed remained easily identifiable as a fluid-filled cavity at the time of the second procedure, although some healing had already occurred and fibrosis was setting in by the time the delayed TARGIT-IORT was delivered (median, 37 days later). A limitation of the study was that we did not anticipate a change in surgical practice in later years, such that the tumor bed was approximated after tumor excision rather than leaving a cavity. The resultant scarring could have made it difficult to accurately locate the primary tumor bed. Given the rapid attenuation of dose, with distance from the applicator surface, adequate dose may not have reached the original tumor bed. Finally, one can also speculate that the additional surgical trauma owing to the necessary second procedure in every case of delayed TARGIT-IORT could stimulate residual cancer cells. Notwithstanding these theoretical reasons, the final judgments must be based on the long-term outcomes data.
Conclusions
Partial breast irradiation was heralded as a new standard29 at the time of the first publication of the TARGIT-A trial6 and several other supporting clinical trials have since been published: including the ELIOT trial,20 interstitial wire-brachytherapy,30 and partial breast EBRT.31,32 Based on the randomized evidence of immediate TARGIT-IORT, which has been shown to be an effective alternative to EBRT,6,7,33 it is clear that the preferred timing of using TARGIT-IORT is immediately—during the initial surgical excision of breast cancer. However, when immediate TARGIT-IORT has not been possible, the long-term data presented in this article may help inform discussions by clinicians and patients who wish to avoid a prolonged postoperative course of EBRT.
Trial Protocol
Data Sharing Statement
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953. doi: 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 2.Vaidya JS, Baum M, Tobias JS, Houghton J Targeted Intraoperative Radiothearpy (TARGIT)- trial protocol. Lancet. 1999; https://www.thelancet.com/protocol-reviews/99PRT-47.
- 3.Vaidya JS, Baum M, Tobias JS, et al. Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol. 2001;12(8):1075-1080. doi: 10.1023/A:1011609401132 [DOI] [PubMed] [Google Scholar]
- 4.Vaidya JS, Baum M, Tobias JS, Morgan S, D’Souza D. The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer. Eur J Surg Oncol. 2002;28(4):447-454. doi: 10.1053/ejso.2002.1275 [DOI] [PubMed] [Google Scholar]
- 5.Vaidya JS. A novel approach for local treatment of early breast cancer. PhD Thesis, University College London, University of London 2002. https://www.ucl.ac.uk/~rmhkjsv/papers/thesis.htm. Accessed November 9, 2019.
- 6.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376(9735):91-102. doi: 10.1016/S0140-6736(10)60837-9 [DOI] [PubMed] [Google Scholar]
- 7.Vaidya JS, Wenz F, Bulsara M, et al. ; TARGIT trialists’ group . Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383(9917):603-613. doi: 10.1016/S0140-6736(13)61950-9 [DOI] [PubMed] [Google Scholar]
- 8.Tobias JS, Vaidya JS, Keshtgar M, D’Souza DP, Baum M. Reducing radiotherapy dose in early breast cancer: the concept of conformal intraoperative brachytherapy. Br J Radiol. 2004;77(916):279-284. doi: 10.1259/bjr/17186381 [DOI] [PubMed] [Google Scholar]
- 9.Herskind C, Steil V, Kraus-Tiefenbacher U, Wenz F. Radiobiological aspects of intraoperative radiotherapy (IORT) with isotropic low-energy X rays for early-stage breast cancer. Radiat Res. 2005;163(2):208-215. doi: 10.1667/RR3292 [DOI] [PubMed] [Google Scholar]
- 10.Enderling H, Chaplain MA, Anderson AR, Vaidya JS. A mathematical model of breast cancer development, local treatment and recurrence. J Theor Biol. 2007;246(2):245-259. doi: 10.1016/j.jtbi.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 11.Herskind C, Wenz F. Radiobiological comparison of hypofractionated accelerated partial-breast irradiation (APBI) and single-dose intraoperative radiotherapy (IORT) with 50-kV X-rays. Strahlenther Onkol. 2010;186(8):444-451. et al. doi: 10.1007/s00066-010-2147-9 [DOI] [PubMed] [Google Scholar]
- 12.Vaidya JS, Walton L, Dewar J. Single dose targeted intraoperative radiotherapy (TARGIT) for breast cancer can be delivered as a second procedure under local anaesthetic. World J Surg Oncol. 2006;4:2. doi: 10.1186/1477-7819-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tunes da Silva G, Logan BR, Klein JP. Methods for equivalence and noninferiority testing. Biol Blood Marrow Transplant. 2009;15(1)(suppl):120-127. doi: 10.1016/j.bbmt.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013;381(9867):651-660. doi: 10.1016/S0140-6736(12)61852-2 [DOI] [PubMed] [Google Scholar]
- 15.Laster LL, Johnson MF, Kotler ML. Non-inferiority trials: the ‘at least as good as’ criterion with dichotomous data. Stat Med. 2006;25(7):1115-1130. doi: 10.1002/sim.2476 [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359(9318):1686-1689. doi: 10.1016/S0140-6736(02)08594-X [DOI] [PubMed] [Google Scholar]
- 17.Vaidya JS, Vyas JJ, Chinoy RF, Merchant N, Sharma OP, Mittra I. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer. 1996;74(5):820-824. doi: 10.1038/bjc.1996.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum M, Vaidya JS, Mittra I. Multicentricity and recurrence of breast cancer. [letter; comment]. Lancet. 1997;349(9046):208-208. doi: 10.1016/S0140-6736(05)60950-6 [DOI] [PubMed] [Google Scholar]
- 19.Vaidya JS, Baum M. Clinical and biological implications of the Milan breast conservation trials. Eur J Cancer. 1998;34(8):1143-1144. [DOI] [PubMed] [Google Scholar]
- 20.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14(13):1269-1277. doi: 10.1016/S1470-2045(13)70497-2 [DOI] [PubMed] [Google Scholar]
- 21.Keshtgar MR, Williams NR, Bulsara M, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat. 2013;140(3):519-525. doi: 10.1007/s10549-013-2641-8 [DOI] [PubMed] [Google Scholar]
- 22.Corica T, Nowak AK, Saunders CM, et al. Cosmesis and Breast-Related Quality of Life Outcomes After Intraoperative Radiation Therapy for Early Breast Cancer: A Substudy of the TARGIT-A Trial. Int J Radiat Oncol Biol Phys. 2016;96(1):55-64. doi: 10.1016/j.ijrobp.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 23.Alvarado MD, Conolly J, Park C, et al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res Treat. 2014;143(1):135-140. doi: 10.1007/s10549-013-2782-9 [DOI] [PubMed] [Google Scholar]
- 24.Corica T, Joseph D, Saunders C, Bulsara M, Nowak AK. Intraoperative radiotherapy for early breast cancer: do health professionals choose convenience or risk? Radiat Oncol. 2014;9:33. doi: 10.1186/1748-717X-9-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belletti B, Vaidya JS, D’Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res. 2008;14(5):1325-1332. doi: 10.1158/1078-0432.CCR-07-4453 [DOI] [PubMed] [Google Scholar]
- 26.Segatto I, Berton S, Sonego M, et al. Surgery-induced wound response promotes stem-like and tumor-initiating features of breast cancer cells, via STAT3 signaling. Oncotarget. 2014;5(15):6267-6279. doi: 10.18632/oncotarget.2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segatto I, Berton S, Sonego M, et al. p70S6 kinase mediates breast cancer cell survival in response to surgical wound fluid stimulation. Mol Oncol. 2014;8(3):766-780. doi: 10.1016/j.molonc.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabris L, Berton S, Citron F, et al. Radiotherapy-induced miR-223 prevents relapse of breast cancer by targeting the EGF pathway. Oncogene. 2016;35(37):4914-4926. doi: 10.1038/onc.2016.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azria D, Bourgier C. Partial breast irradiation: new standard for selected patients. Lancet. 2010;376(9735):71-72. doi: 10.1016/S0140-6736(10)60898-7 [DOI] [PubMed] [Google Scholar]
- 30.Strnad V, Ott OJ, Hildebrandt G, et al. ; Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO) . 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet. 2016;387(10015):229-238. doi: 10.1016/S0140-6736(15)00471-7 [DOI] [PubMed] [Google Scholar]
- 31.Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer. 2015;51(4):451-463. doi: 10.1016/j.ejca.2014.12.013 [DOI] [PubMed] [Google Scholar]
- 32.Coles CE, Griffin CL, Kirby AM, et al. ; IMPORT Trialists . Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390(10099):1048-1060. doi: 10.1016/S0140-6736(17)31145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaidya JS, Wenz F, Bulsara M, et al. An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technol Assess. 2016;20(73):1-188. doi: 10.3310/hta20730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement