Abstract
Biological hydrogen production by microalgae is a potential sustainable, renewable and clean source of energy. However, many barriers limiting photohydrogen production in these microorganisms remain unsolved. In order to explore this potential and make biohydrogen industrially affordable, the unicellular microalga Chlamydomonas reinhardtii is used as a model system to solve barriers and identify new approaches that can improve hydrogen production. Recently, Chlamydomonas–bacteria consortia have opened a new window to improve biohydrogen production. In this study, we review the different consortia that have been successfully employed and analyze the factors that could be behind the improved H2 production.
Keywords: algae, bacteria, biohydrogen, Chlamydomonas reinhardtii, co-cultures, consortia, hydrogen
1. Introduction
Finding renewable, sustainable and clean energy sources has become one of the main priorities of our society. Hydrogen (H2) is a promising clean and carbon-free energy source with a high energy value (142 kJ/g) that can be easily interconverted with electricity and used for domestic and industrial applications. Currently, H2 production techniques include steam reforming natural gas/oil, coal gasification, biomass gasification/pyrolysis, and electrolysis and thermolysis of water. All these techniques are either polluting and/or demand a large amount of energy [1,2]. Under this scenario, the biological production of H2 (bioH2) has garnered considerable attention in recent decades, as it could be a cheap and renewable source of fuel. Different microorganisms such as microalgae, cyanobacteria, photosynthetic bacteria and some heterotrophic bacteria can produce H2 [3,4]. Algae and cyanobacteria are well-known photoautotrophic organisms able to convert CO2 into organic matter and release O2 during this process. Under specific conditions, H2 production is linked to photosynthetic activity. Non-oxygenic photosynthetic bacteria can also use light and organic acids (and other chemical forms) to obtain energy and produce H2, without releasing O2. Heterotrophic bacteria, on the other hand, can degrade organic matter and release CO2, with some of them also producing H2. Among them, photobiological H2 evolution by green algae and cyanobacteria has attracted considerable attention since, potentially, they do not require organic carbon sources to produce H2, only water and sunlight [4,5,6]. Moreover, microalgae and cyanobacteria are the most dominant photosynthetic organisms on Earth, which increases their biotechnological interest. However, photosynthetic H2 production is still inefficient for industrial implementation due to its low yield and rate of H2 generation. One of the most important bottlenecks of biological H2 production is its sensitivity to oxygen (O2). In all the H2-producing microorganisms, O2 is a strong repressor of H2 production.
1.1. H2 Production in Green Algae
Chlamydomonas reinhardtii (Chlamydomonas throughout) is a unicellular green microalga able to grow autotrophically and heterotrophically that has been chosen as a model system to study H2 photoproduction. There are three different pathways that can lead to H2 production in Chlamydomonas. Two of them are linked to the photosynthetic electron chain, while a third is linked to fermentative metabolism. In the photosystem II (PSII)-dependent pathway (also termed the direct pathway), the electrons generated at the level of PSII from water splitting are transferred to the photosynthetic electron chain, where they ultimately reach photosystem I (PSI) and the ferredoxins (FDXs), which are the final electron donors to the hydrogenases (HYDAs) [7,8]. Since this pathway require the activity of the PSII, both electrons and O2 are simultaneously generated. In the PSII-independent (or indirect) pathway, NAD(P)H acts as a source of electrons that can directly reduce the cytochrome b6f through type II-NADH dehydrogenase (NDA2) [9,10]. Once the electrons are in the photosynthetic electron chain, they reach the PSI and the FDXs as in the PSII-dependent pathway, but in this case O2 is not co-generated with H2 since PSII does not participate in the generation of electrons [11]. In the PSII-independent pathway, starch degradation has been identified as the most common source of reductants under sulfur (S)-depleted conditions [12]. However, under hypoxia and nutrient replete conditions, acetic acid assimilation has been suggested to play an important role as source of reductants for H2 production [13,14,15,16]. The third pathway is known as the fermentative or dark pathway. Here, the Pyruvate Ferredoxin Oxidoreductase (PFR) enzyme oxidizes pyruvate to acetyl CoA under anoxic conditions. This reaction is coupled with the generation of electrons, which are transferred to the HYDAs via FDXs [8,17,18]. In Chlamydomonas, the dark H2 production is quantitatively much more reduced than H2 photoproduction.
As mentioned before, the main drawback of photohydrogen production in algae is caused by the O2 sensitivity of the HYDAs, which show inhibitory effects at both transcriptional and posttranslational levels [19,20]. Therefore, H2 photoproduction in green algae occurs under anoxic/hypoxic conditions and, at a physiological level, H2 production is a transitory phenomenon since O2 and H2 co-evolution are incompatible. This is especially true for the PSII-dependent pathway. Furthermore, the process encounters several other bottlenecks that decrease the efficiency of H2 evolution. Among them are low light conversion efficiency, the non-dissipation of the proton gradient, the competition between electron acceptors for photosynthetic electrons, the reversibility of the HYDAs, the low level of HYDAs expression, and the pH inhibition (reviewed in [21,22,23,24,25]). Several genetic modifications have successfully palliated some of these limitations [23,25,26]. Different culturing approaches have also been developed to alleviate the identified bottlenecks. Among these approaches are the modulation of the light intensity [14,27,28,29,30,31], the optimization of the photosynthetic electrons flow towards the HYDAs [29,32,33,34], the implementation of nutrient stresses, especially sulfur (S) deprivation, influencing H2 production [35,36,37,38,39], the addition of O2 scavengers into the culture media [33,40,41], or cell immobilization [42,43,44]. Moreover, in recent years, the co-cultivation of alga and bacteria has arisen as an alternative strategy to increase algal H2 production.
1.2. H2 Production in Cyanobacteria
Cyanobacteria are prokaryotic photosynthetic microorganisms able to grow heterotrophically or photoautotrophically, some of which are nitrogen-fixing. During phototrophic growth, they perform oxygenic photosynthesis using an electron transport chain similar to algae and plants. Like in microalgae, H2 production through the HYDAs can be linked to the photosynthetic activity or to the fermentative pathways. However, unlike microalgae, H2 production can also be linked to the N2 fixation mediated by the nitrogenases. Both HYDAs and nitrogenases are O2 sensitive. Among cyanobacteria, the best H2 producers link H2 production to nitrogenase activity, since cyanobacteria HYDAs are highly reversible, and their most common physiological role is related to H2 uptake. Nitrogenases are only expressed under nitrogen-limiting conditions, and nitrogenase-based H2 production is very expensive in terms of energy expenditure (e.g., 15 photons/H2 are required by nitrogenases vs. four photons/H2 by HYDAs) [8,45].
1.3. H2 Production in Non-Oxygenic Photosynthetic Bacteria
Some non-oxygenic photosynthetic bacteria can also produce H2. In this group of microorganisms, the Purple Non-Sulfur Photosynthetic (PNSP) bacteria are among the best known H2 producers. As with cyanobacteria, H2 production by PNSP bacteria is mostly linked to nitrogenase activity. ATP generated during photosynthesis is used by the nitrogenases to produce NH3 and H2. In this case, photosynthesis is not linked to water splitting and thereby O2 is not produced. Instead, the most common source of electron donors are organic acids, and the process is known as photo-fermentation. For H2 production, formate, acetate, lactate and butyrate can act as electron donors, with butyrate being the best inducer of H2 production [8,46,47]. Like cyanobacteria, two of the main factors limiting H2 production in PNSP bacteria are the simultaneous occurrence of H2 uptake and the need to establish nitrogen-deficient conditions.
1.4. H2 Production in Heterotrophic Bacteria
Many heterotrophic bacteria can produce H2 though fermentative pathways (also known as dark H2 production). Bacterial fermentation of sugars can produce a large variety of fermentative end products, including H2. There are two distinctive groups of bacteria that have been extensively studied regarding fermentative H2 production. One group is composed of strict anaerobes (represented by, e.g., Clostridium spp.), where H2 production is linked to the oxidation of pyruvate into acetyl CoA by Pyruvate Ferredoxin Oxidoreductase (PFOR). This pathway is known as the PFOR pathway. The second group are facultative anaerobes (represented by, e.g., E. coli), which, under anaerobic conditions, perform so-called mixed acid fermentation, where pyruvate can be used by Pyruvate Formate Lyase (PFL) to produces formate and acetyl CoA. Formate is then converted to CO2 and H2 by the Formate Hydrogen Lyase (FHL), and the process is known as the PFL H2-production pathway. H2 production through dark fermentation has several limiting factors, including 1) the existence of other competitive fermentation pathways and, 2) the excessive accumulation of end products (mainly ethanol, formate, acetate, lactate, succinate, glycerol and butyrate) that block H2 production [48,49,50,51,52,53].
Although numerous efforts have been made to improve H2 production in algal and bacterial systems, the integration of these two systems to improve bioH2 production has received less attention [8,21,47]. This review outlines the past and recent achievements obtained when the green algae Chlamydomonas is co-cultivated with different bacterial strains to improve H2 production.
2. Current Achievements Obtained with Chlamydomonas-Bacteria Consortia
Several studies have proven the possibility to improve H2 production when using co-cultures of alga and bacteria [21,54,55], with some of them focusing on the use of the alga Chlamydomonas.
Table 1 provides a comparative analysis of all the previously published data about H2 production in Chlamydomonas–bacteria consortia with their respective algal monocultures in terms of yield, rate and sustainability. Studies are ranked according to the total H2 production yield. Notably, most of the publications show enhancements in H2 production parameters (yield, rate and duration) in the co-cultures relative to the monocultures, with many consortia promoting a threefold yield enhancement. Different Pseudomonas sp. and Bradyrhizobium japonicum are bacterial partners that lead to the highest H2 production yields in cultures incubated in Tris-Acetate-Phosphate (TAP) medium, devoid of S (TAP-S), and they often lead to great enhancements in H2 production (up to 22.7-fold and 32.3-fold, respectively) (Table 1). Note that these two bacterial partners are not known to be H2 producers by themselves. In general, the best condition for H2 production can be obtained in TAP-S (Table 1), confirming that, as in the case of Chlamydomonas monocultures, S deprivation is a physiological condition that greatly promotes H2 production in this alga. The light intensity does not seem to be a crucial parameter for H2 production from consortia incubated in TAP-S (Table 1). H2 photoproduction in Chlamydomonas monocultures in TAP medium is scarce, unless low light intensities (below 22 PPFD) are used [14]. However, different consortia can attain noticeable H2 production in TAP medium at higher light intensities (Table 1), which open the possibility to further explore H2 production under non-stressful conditions to avoid S removal and two-phase bioreactors. Finally, the use of H2-producing bacterial strains such as wild-type strains of E. coli in media supplemented with glucose brings up the possibility to combine H2 production from both alga and bacterium [56]. This consortium can produce up to 32.7 mL/L, which is higher than the production reported for other consortia in TAP-S medium (Table 1). Similarly, other bacteria like Pseudomonas putida and Rhizobium etli can also facilitate H2 production in Chlamydomonas when incubated with sugars as the only carbon sources (Table 1).
Table 1.
Comparison of yield, rate and sustainability of H2 generation in Chlamydomonas–bacteria co-cultures versus alga monocultures. For each report, only data from co-cultures with their corresponding control monocultures are considered (when possible). Data are ranked according to the total H2 production in co-cultures.
| Chlamydomonas Strain1 | Bacterium Strain | Medium | Light Intensity (PPFD)2 |
H2 Production in Algal Monocultures | H2 Production in Co-Cultures | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Reported | Estimated (mL/L)3,4 | Estimated (mL/L)3,4,5 | Duration5 | Estimated Average Rate (mL/L∙d)3,4,5 | |||||
| Transgenic lba (based on cc849) | Bradyrhizobium japonicum | TAP-S | 60 | 20.02 (µmol/40 mL) | ≈11.22 | ≈170.5 (× 15.2) | 14 d (× 1) | ≈11.95 (× 14.9) | [57] |
| cc503 | B. japonicum | TAP-S | 200 | 70 (µmol/mg chl) | ≈13.14 | ≈141.2 (× 10.7) | ≈16 d (× 1.8) | ≈8.82 (× 6) | [58] |
| FACHB-265 | Pseudomonas sp. strain D | TAP-S | 50 | ≈10 (mL/L) | 10 | ≈130 (× 13) | ≈12 d (× 3) | ≈10.82 (× 4.3) | [59] |
| FACHB-265 | Escherichia coli and Pseudomonas sp. strain D | TAP-S | 50 | ≈20 (mL/L) | 20 | ≈125 (× 6.2) | ≈16 d (× 2) | ≈7.81 (× 3.1) | [59] |
| FACHB-265 | Bacillus subtilis and Pseudomonas sp. strain D | TAP-S | 50 | ≈20 (mL/L) | 20 | ≈110 (× 5.5) | ≈16 d (× 2) | ≈6.87 (× 2.7) | [59] |
| Transgenic hemHc-lbac (based on cc849) | B. japonicum | TAP-S | 30 | 99 (µmol/mg chl) | ≈21.19 | ≈93.2 (× 4.4) | ≈16 d (× 2) | ≈5.82 (× 2.2) | [58] |
| cc124 | B. japonicum | TAP-S | 200 | 20 (µmol/mg chl) | ≈2.43 | ≈78.4 (× 32.3) | ≈13 d (× 1.3) | ≈6.03 (× 24.8) | [58] |
| FACHB-265 | Pseudomonas sp. strain C | TAP-S | 50 | ≈10 (mL/L) | 10 | ≈65 (× 6.5) | ≈6 d (× 1.5) | ≈10.83 (× 4.3) | [59] |
| cc124 | E. coli (ΔhypF) | TAP-S | 50 | 25 (mL/L) | 25 | ≈47.3 (× 1.9) | 7 d (× 1) | ≈6.75 (× 1.9) | [60] |
| cc849 | B. japonicum | TAP-S | 60 | 12.76 (µmol/40 mL) | ≈7.15 | ≈46.5 (× 6.5) | ≈8 d (× 2) | ≈5.82 (× 3.2) | [57] |
| FACHB-265 | Herbaspirillum sp. | TAP-S | 50 | ≈10 (mL/L) | 10 | ≈40 (× 4) | ≈8 d (× 2) | ≈5 (× 2) | [59] |
| cc849 | Pseudomonas sp. | TAP-S | 50 | 15.11 (µmol/40 mL) | ≈8.46 | ≈34.7 (× 4.1) | ≈8 d (× 2) | ≈4.3 (× 2) | [61] |
| C238 | Rhodosprillum rubrum | MBM | 200 W/m2 12:12 h L–D | 0.6 (µmol/mg dry wt) | ≈8.6 | ≈34.3 (× 4) | 12 h (× 1) | ≈68.54 (4) | [62] |
| cc849 | Stenotrophomonas sp. | TAP-S | 60 | 15.11 (µmol/40 mL) | ≈8.46 | ≈33.8 (× 4) | ≈6 d (× 1.5) | ≈5.64 (× 2.6) | [61] |
| 704 | E. coli | TAP+glu6 | 12 | 9.7 (mL/L) | 9.7 | 32.7 (× 3.4) | 9 d (× 3) | ≈3.6 (× 1.1) | [56] |
| FACHB-265 | Pseudomonas sp. strain A | TAP-S | 50 | ≈10 (mL/L) | ≈10 | ≈30 (× 3) | ≈10 d (× 4) | ≈3 (× 1.2) | [59] |
| FACHB-265 | Phyllobacterium sp. | TAP-S | 50 | ≈10 (mL/L) | ≈10 | ≈30 (× 3) | ≈12 d (× 3) | ≈2.5 (× 1) | [59] |
| FACHB-265 | E. coli | TAP-S | 50 | ≈20 (mL/L) | ≈20 | ≈30 (× 1.5) | ≈12 d (× 1.5) | ≈2.5 (× 1) | [59] |
| 704 | P. putida 12264 | TAP | 12 | 17.9 (mL/L) | 17.9 | 27.6 (× 1.5) | 4 d (× 1.3) | ≈6.86 (× 1.1) | [63] |
| 704 | E. coli (ΔhypF) | TAP+glu6 | 50 | 2.5 (mL/L) | 2.5 | 26.2 (× 10.5) | 4 d (× 2) | ≈6.5 (× 5.2) | [56] |
| FACHB-265 | Bacillus subtilis | TAP-S | 50 | ≈20 (mL/L) | ≈20 | ≈25 (× 1.2) | ≈12 d (× 1.5) | ≈2.08 (× 0.8) | [59] |
| cc849 | Microbacterium sp. | TAP-S | 60 | 15.11 (µmol/40 mL) | ≈8.46 | ≈24.5 (× 2.9) | ≈6 d (× 1.5) | ≈4.09 (× 1.9) | [61] |
| 704 | P. putida 12264 | TAP+glu6 | 50 | 2.5 (mL/L) | 2.5 | 29.2 (× 11.7) | 9 d (× 4.5) | ≈3.2 (× 2.6) | [56] |
| 704 | P. putida 291 | TAP | 12 | 17.9 (mL/L) | 17.9 | 23.1 (× 1.3) | 3 d (× 1) | ≈7.7 (× 1.3) | [63] |
| 704 | P. stutzeri | TAP | 12 | 17.9 (mL/L) | 17.9 | 23.1 (× 1.3) | 4 d (× 1.3) | ≈5.79 (× 1) | [63] |
| cc124 | E. coli (ΔhypF) | TAP | 50 | NP | -- | ≈18.7 (7) | 1 d (7) | ≈18.67 (7) | [64] |
| 704 | P. putida 12264 | TAP | 100 | 0.8 (mL/L) | 0.8 | 18.2 (× 22.7) | 2 d (× 2) | ≈9.1 (× 11.4) | [63] |
| 704 | Rhizobium etli | TAP | 12 | 17.9 (mL/L) | 17.9 | 17.7 (× 1) | 3 d (× 1) | ≈5.91 (× 1) | [63] |
| 704 | E. coli | TAP | 12 | 17.9 (mL/L) | 17.9 | 17.5 (× 1) | 3 d (× 1) | ≈5.85 (× 1) | [63] |
| 704 | P. stutzeri | TAP | 50 | 4.3 (mL/L) | 4.3 | 15.5 (× 3.6) | 2 d (× 2) | ≈7.74 (× 1.8) | [63] |
| FACHB-265 | Comamonas sp. | TAP-S | 50 | ≈10 (mL/L) | ≈10 | ≈15 (× 1.5) | ≈8 d (× 2) | ≈1.87 (× 0.7) | [59] |
| 704 | P. putida 12264 | TAP | 50 | 4.3 (mL/L) | 4.3 | 14.2 (× 3.3) | 3 d (× 3) | ≈4.73 (× 1.1) | [63] |
| cc503 | Thuomonas intermedia | TAP-S + Na2S2O3 | 200 14:10 h L–D |
43 (µmol/mg chl) | ≈0.77 | ≈12.8 (× 16.6) | 17 d (× 1.9) | ≈0.75 (× 8.7) | [65] |
| 704 | R. etli | TAP+man6 | 50 | 2.5 (mL/L) | 2.5 | 13.5 (× 5.4) | 8 d (× 4) | ≈1.7 (× 1.4) | [56] |
| 704 | P. putida 291 | TAP | 50 | 4.3 (mL/L) | 4.3 | 10.3 (× 2.4) | 3 d (× 3) | ≈3.44 (× 0.8) | [63] |
| 704 | P. stutzeri | TAP | 100 | 0.8 (mL/L) | 0.8 | 8.3 (× 10.4) | 1 d (× 1) | ≈8.3 (× 10.4) | [63] |
| 704 | E. coli | TAP | 50 | 4.3 (mL/L) | 4.3 | 6.9 (× 1.6) | 2 d (× 2) | ≈3.44 (× 0.8) | [63] |
| Chlamydomonas sp. | E. coli (ΔhypF) | TAP | 50 | NP | -- | ≈6.8 (7) | 1 d (7) | ≈6.84 (7) | [65] |
| Chlamydomonas sp. | Rhodococcus sp. | TAP | Dark | ≈5.6 (mL/L) | ≈5.6 | ≈6 (× 1.1) | 4 d (× 1) | ≈1.5 (× 1.1) | [60] |
| cc124 | E. coli (ΔhypF) | TAP | 50 | NP | -- | 5.8 (7) | ≈22 h (7) | ≈6.3 (7) | [60] |
| 704 | R. etli | TAP | 50 | 4.3 (mL/L) | 4.3 | 5.6 (× 1.3) | 1 d (× 1) | ≈5.6 (× 1.3) | [63] |
| 704 | P. putida 291 | TAP | 100 | 0.8 (mL/L) | 0.8 | 3.5 (× 4.4) | 1 d (× 1) | ≈3.5 (× 4.4) | [63] |
| cc503 | T. intermedia | TAP-S | 200 14:10 h L–D |
43 (µmol/mg chl) | ≈0.8 | ≈3.4 (× 4.4) | 17 d (× 1.9) | ≈0.2 (× 2.3) | [65] |
| cc549 | E. coli (ΔhypF) | TAP-S | 50 | 0.2 (mL/L) | 0.2 | ≈2.6 (× 13.6) | 3 d (× 1.5) | ≈0.9 (× 8.8) | [60] |
| Chlamydomonas sp. & Scenedesmus sp. | E. coli (ΔhypF) | TAP | 50 | 0 (mL/L) | 0 | 1.5 (7) | ≈10 h (7) | [66] | |
| cc549 | E. coli (ΔhypF) | TAP | 50 | 0 | 0 | 1.2 (7) | ≈22 h (7) | ≈1.3 (7) | [60] |
| Chlamydomonas sp. & Scenedesmus sp. | Bacteria flora | TAP | 50 | 0 (mL/L) | 0 | 1.1 (7) | ≈12 h (7) | ≈2.3 (7) | [66] |
| 704 | R. etli | TAP | 100 | 0.8 (mL/L) | 0.8 | 0.8 (1) | 1 d (× 1) | ≈0.8 (× 1) | [63] |
| 704 | E. coli | TAP | 100 | 0.8 (mL/L) | 0.8 | 0.8 (1) | 1 d (× 1) | ≈0.8 (× 1) | [63] |
| cc849 | Azotobacter chroococcum | TAP-S | 30 | 19 (µmol/mg chl) | --8 | (× 3.8)9 | ≈12 d (× 1.5) | -- | [67] |
| cc849 | A. chroococcum | TAP-S | 100 | 19 (µmol/mg chl) | --8 | (× 3.6)9 | ≈8 d (× 1) | -- | [67] |
| cc849 | A. chroococcum | TAP-S | 200 | 28 (µmol/mg chl) | --8 | (× 5.3)9 | ≈10 d (× 1) | -- | [67] |
| Chlamydomonas sp. | Ralstonia eutropha | TAP | NP | NP | -- | ≈1.2 (7) | ≈1 d (7) | ≈1.2 (7) | [60] |
| Chlamydomonas sp. | R. eutropha (ΔhypF1F2) | TAP | NP | NP | -- | ≈1.2 (7) | ≈1 d (7) | ≈1.2 (7) | [60] |
1Chlamydomonas reinhardtii unless otherwise stated; 2 photosynthetic photon flux density (PPFD) (µmol photons · m2−1 · s−1); 3 Avogadro’s law for ideal gas is considered to estimate H2 productivity in the unit of (mL/L culture) 1 mole H2 gas (at pressure = 101.325 kPa and temperature = 273.15 K), equal to 22.41 liters of H2; 4 the average of the lowest and the highest chlorophyll concentration was considered to estimate the H2 productivity from “per mg chlorophyll” to “per liter culture”; 5 enhancements in co-cultures compared with monocultures are presented as fold changes in parentheses; 6 sugar is added when acetic acid is depleted in the culture media; 7 folds cannot be calculated because either H2 production in alga monocultures are zero or are not reported; 8 data for chlorophyll concentration was not reported; 9 reported fold change; Modified Bristol Medium (MBM); information not provided in the original report (NP); glucose (glu); mannitol (man); light–dark cycles (L–D); “≈”: data estimated from the original study (rounded values).
To contextualize the achievements obtained using Chlamydomonas–bacteria consortia, Table 2 lists some of the most successful strategies described in Chlamydomonas for H2 production, including monocultures and co-cultures, and ranks them by the total H2 yield obtained. Monocultures using genetically modified strains and S deprivation can lead to the highest H2 yields. However, the use of Chlamydomonas wild-type strains co-cultured with different bacterial partners under S deprivation are also ranked within the top list. For example, different co-cultures incubated in TAP-S employing Pseudomonas sp. or Bradirizhobium japonicum have achieved ≈165–170 mL H2/L culture [57,58,59], and there is a published patent for H2 production using Chlamydomonas and Pseudomonas fluorescens co-cultures claiming to produce 196 mL/L [68]. These values obtained using co-cultures are a bit far from the maximal Chlamydomonas H2 production reported (850 mL/L) using a proton gradient mutant (pgrl5) affecting the cyclic electron transfer [69]. However, co-culturing techniques could have a great potential to further improve H2 production if genetically modified Chlamydomonas (or bacterial) strains are employed in co-cultures. Moreover, it should be noted that most studies exploring H2 production in Chlamydomonas co-cultures are very recent and there are much more possibilities to explore in this field.
Table 2.
Maximum H2 productivity achieved by Chlamydomonas using different approaches. Data are ranked according to the total H2 production yield. For each study, only the maximum reported values are considered.
| Strategy | Parental Alga Strain | Mutant Strain | Conditions | Reported H2 Production | Estimated H2 Production (mL/L)1,2 | Estimated Average H2 Production rate (mL/L∙d) | Reference |
|---|---|---|---|---|---|---|---|
| Monoculture/Genetic modification/S deprivation | cc124 | pgrl5 | TAP-S, 60 PPFD | 850 mL/L (9 days) | 850 | ≈94.4 | [69] |
| Monoculture/Genetic modification/S deprivation | cc1618 | stm6 | TAP-S, 100 PPFD | 540 mL/L (14 days) | 540 | ≈38.6 | [70] |
| Monoculture/Genetic modification/S deprivation | 11/32b | L159I-N230Y | TAP-S, 70 PPFD | 504 mL/L (12 days) | 504 | ≈42 | [71] |
| Monoculture/Genetic modification/S deprivation | 137c(cc124) | pgrl1 | TAP-S, 200 PPFD | ≈1.5 mmol/mg chl (≈5 days) |
≈437 | ≈87.4 | [34] |
| Monoculture/Genetic modification/S deprivation | cc1618 | Stm6Glc401 | TAP-S + 1 mM glucose, 450 PPDF | 361 mL/L (≈8 days) | 361 | ≈46 | [72] |
| Consortia/Pseudomonas sp. /S deprivation |
FACHB-265 | -- | TAP-S, 200 PPFD | 170.8 mL/L (13 days) | 170.8 | 13.1 | [59] |
| Consortia/Bradirizhobium japonicum/S deprivation | cc849 | Transgenic lba strain | TAP-S, 60 PPFD | 298.54 µmol/40 mL (14 days) | ≈170.5 | ≈11.95 | [57] |
| Consortia/Bradirizhobium japonicum/S deprivation | cc503 | -- | TAP-S, 200 PPFD | 310 µmol/mg chl (16 days) |
≈164.9 | ≈10.3 | [58] |
| Monoculture/S deprivation | 137c (cc125) | -- | TAP-S | ≈155 mL/L (≈4 days) | ≈155 | ≈38.75 | [36] |
| Monoculture/Mg deprivation | 137c (cc125) | -- | TAP-Mg, 80 PPFD | 6.3 mmol/L (≈8 days) | ≈141.1 | ≈16.9 | [73] |
| Monoculture/S deprivation/acetate free | UTEX 90 (cc1010) | -- | T(A)P-S3, 50 PPFD, N2 purging | 118 mL/L (4.5 days) | 118 | 26.2 | [74] |
| Monoculture/O2 scavenging | cc503 | -- | TAP + NaHSO3, 200 PPFD | ≈150 µmol/30mL (3 days) | ≈112.05 | ≈37.3 | [75] |
| Monoculture/Genetic modification | cc849 | hemHc-lbac | TAP-S, N2 purging, dark incubation, 50 PPFD | 3.3 mL/40 mL (≈5 days) |
82.5 | ≈16.5 | [76] |
| Monoculture/Light modulation | cc124/cc4533 | -- | TAP, 1 s light pulses (180 PPFD) + 9 s dark periods under Argon atmosphere | 3.26 mmol/L (2.25 days) | ≈73.06 | ≈32.5 | [29] |
| Monoculture/acetic acid supplementation/Light modulation | 704 | -- | TAP + acetic acid supplementation, daily aeration, 12 PPFD | 65 mL/L (9 days) | 65 | ≈10 | [14] |
| Consortia/E. coli (hypF)/S deprivation | cc124 | -- | TAP-S, 50 PPFD | 47.2 mL/L (7 days) |
47.2 | 6.75 | [60] |
1 Avogadro’s law for ideal gas is considered to estimate H2 productivity in the unit of (mL/L culture): 1 mole H2 gas (at pressure= 101.325 kPa and temperature=273.15 K) is equal to 22.41 liters of H2; 2 the average of the lowest and the highest chlorophyll concentration was considered to estimate the H2 productivity from “per mg chlorophyll” to “per liter culture”; 3 Tris–Acetate–Phosphate (TAP) without acetate and sulfur (T(A)P-S); “≈”: Data estimated from the original study (rounded values); photosynthetic photon flux density (PPFD) (µmol photons · m2−1 · s−1).
3. Characteristics of the Algae–Bacteria Association for H2 Production
In recent years, an increased interest in the study of algal–bacterial interactions has emerged not only due to their ecological significance, but also for their biotechnological potential. It is known that algae and bacteria affect one another’s physiology and metabolism. In natural ecosystems, algal–bacterial interactions cover a whole range of relationships: mutualism, commensalism and parasitism, depending on specific species and living requirements [77]. These interactions are omnipresent in all ecosystems. Moreover, microorganisms have complex and very versatile metabolisms, allowing them to grow or to simply survive in non-optimal environments. In this sense, Chlamydomonas, for example, apart from its photoautotrophic metabolism, has a fermentative metabolism that allows this alga to consume internal reserves such as starch under anaerobic conditions, releasing H2 and other end products to the medium. Moreover, Chlamydomonas can also grow heterotrophically and is able to consume acetic acid as a carbon source. Noticeably, acetic acid is the only organic carbon form that Chlamydomonas can uptake and, under hypoxic conditions, it has also been suggested that the assimilation of this compound is connected to H2 production in this alga [14,15,16].
At a physiological level, the production of H2 by microorganisms is considered as an escape valve for the electrons generated in excess during either photosynthetic or fermentative processes. The activation of hydrogenases (or nitrogenases) occurs under very specific environmental conditions, and for most microorganisms, H2 production can be considered as a transitory event. When cultivating axenic cultures of H2-producing microorganisms in the laboratory, different growth conditions are used to maximize H2 production. However, the complex interplay between the different microorganisms has not been not studied. Understanding this interplay can provide valuable information to overcome some of the bottlenecks associated with biological H2 production.
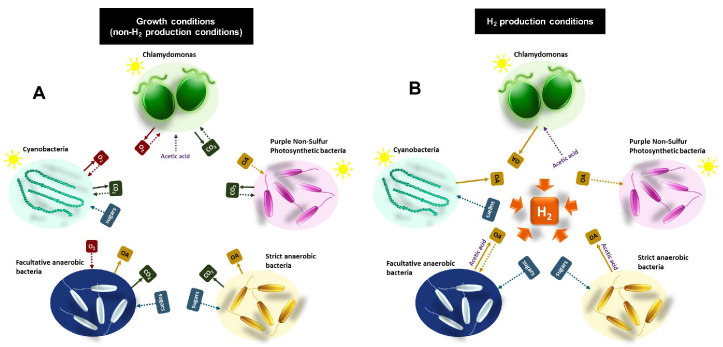
A straightforward advantage of co-culturing heterotrophic bacteria with algae is that they can efficiently remove O2 from the media, which is the most critical bottleneck associated with H2 photoproduction. At the same time, the CO2 released during bacterial fermentation can support algae and cyanobacteria growth, while the photosynthetic O2 production can support the growth of facultative anaerobic bacteria. In addition, algae and photosynthetic bacteria can theoretically combine their sunlight wavelength absorption ranges to increase the overall light-to-energy conversion efficiency for H2 production or for biomass generation. Finally, several photosynthetic and fermentative metabolites can be exchanged between microorganisms, establishing specific nutrient fluxes that can benefit H2 production and/or growth. Among these nutrient fluxes, carbon fluxes are quantitatively the most prominent, although nitrogen, phosphorous and S sources, and growth factors like Vitamin B12, have also been reported as favoring algae–bacteria interactions [78,79,80,81,82].
In the following sections, the potential mechanisms influencing H2 production in algae–bacteria cultures are discussed. They are categorized according to the impact on (1) biomass, accumulation of internal reserves, and metabolite exchange supporting H2 production, (2) net O2 evolution, and (3) the possibility to extend the solar spectrum absorption range.
3.1. Biomass, Accumulation of Internal Reserves and Metabolite Exchange Supporting H2 Production
3.1.1. Starch Accumulation could be Promoted in Co-Cultures
Starch reserves in Chlamydomonas can be connected to photobiological H2 production through the PSII-independent pathway (Figure 1). This pathway relies on the non-photochemical reduction in the PlastoQuinone (PQ) pool using the electrons derived from NAD(P)H [9,10,11,83]. The glycolytic degradation of starch is proposed to be the main source of electrons for this H2-producing pathway during S deprivation conditions [12]. Moreover, starch degradation can also feed the fermentative or dark H2 production in Chlamydomonas via the PFR pathway (Figure 1) [8,17,18]. Different nutrient stresses (mainly N and S) can promote starch accumulation in Chlamydomonas cultures under both light and dark conditions [84,85], which, in turn, can favor H2 production.
Figure 1.
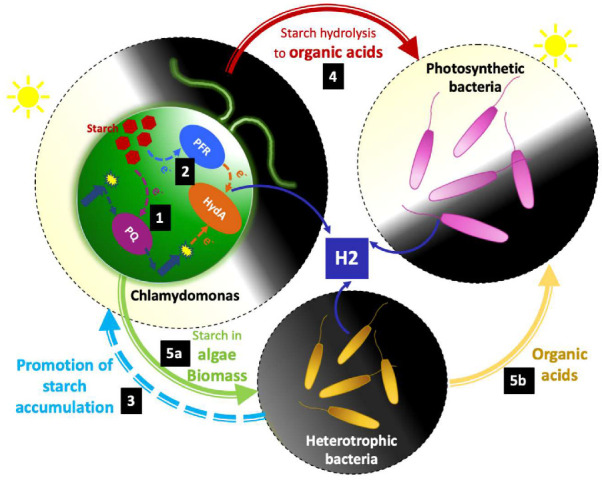
Potential starch-derived relationships between Chlamydomonas and other microorganisms during H2 production. Starch accumulated in Chlamydomonas cells can be used to feed the PII-independent (1) and fermentative (2) pathways. The accumulation of starch in Chlamydomonas can be favored when co-cultured with some bacterial strains (3). Different end products derived from Chlamydomonas starch mobilization can be excreted and used by Purple Non-Sulfur Photosynthetic (PNSP) bacteria for H2 production (4). Starch-enriched Chlamydomonas biomass can be used directly by some heterotrophic bacteria to produce H2 (5a) or in collaboration with PNSP bacteria (5b). Pyruvate Ferredoxin Reductase (PFR); PlastoQuinone (PQ); hydrogenase A (HydA).
Recently, it has been observed that co-culturing Chlamydomonas with different bacterial strains can lead to high starch accumulation in this alga. These bacterial strains include Bradyrhizobium japonicum [58], Azotobacter chroococcum [67], Pseudomonas sp. [59] and Thuomonas intermedia [65]. However, the precise reasons explaining why the starch accumulation occurs in these co-cultures have not been elucidated. In any case, co-culturing Chlamydomonas with certain bacterial strains could be used as an approach to promote starch accumulation, which potentially can enhance algal H2 production through the PSII-independent pathway or through metabolite exchange (see below sections) (Figure 1).
3.1.2. Mobilization of the Algal Starch Reserves Can Provide Organic Acids for H2 Producing Bacteria
Chlamydomonas has a very versatile fermentative metabolism and is able to quickly degrade starch reserves under anaerobic conditions to different fermentative end products including H2 [86,87,88] (Figure 1). Some end products are secreted to the medium by wild-type Chlamydomonas cultures, including acetate, ethanol and formate. Glycerol, succinate and lactate are minor end products secreted by most wild-type Chlamydomonas cells; however, the noticeable excretion of these fermentative products can be found in some Chlamydomonas mutants [86] or in some strains considered to be wild-type [89]. All these secreted end products can be theoretically used by bacteria as electron donors for H2 production (Figure 2), and some of them have been probed at an empirical level using Chlamydomonas–PNSP bacteria cultures [62,90]. Miyamoto et al. [62] reported that, when co-culturing Chlamydomonas and Rhodospirillum rubrum, they both produced H2 in dark conditions. In the case of Chlamydomonas, H2 originated from the fermentative degradation of the starch reserves, while, in the case of R. rubrum, H2 originated from the Formate Hydrogen Lyase pathway using the formate excreted by the alga as a substrate. Similarly, Miura et al. [90] reported that after incubating Chlamydomonas in the dark, the resulting medium broth was used by a marine photosynthetic bacterium, Rhodopseudomonas sp., to photoproduce H2. This Chlamydomonas medium broth was enriched with acetic acid and ethanol.
Figure 2.
Potential metabolites exchanged among different H2-producing microorganisms during growth conditions (A) and H2-producing conditions (B). The secretion and uptake of metabolites are indicated with plain and dotted arrows, respectively. Depending on the specific culture conditions the same metabolites can be secreted or accumulated. Organic Acids (OAs) mainly include ethanol, glycerol, formate, acetic acid, lactate, succinate and butyrate. When predominant, the specific OA is indicated next to the arrow.
3.1.3. Acetic Acid Exchange Can Promote H2 Production in both Algae and Bacteria
As mentioned before, the donation of fermentative metabolites from Chlamydomonas to different bacteria can promote bacterial H2 production. However, the opposite flux (from bacteria to alga) can also benefit both algal and bacterial H2 production, especially when acetic acid is produced and secreted by the bacteria [56]. In Figure 2, some of the metabolites that can be potentially exchanged between algae and other microorganisms during both growth and H2 production conditions are depicted.
Many bacteria can produce H2 though fermentative pathways (dark H2 production). In organisms using the PFOR H2-production pathway (e.g., Clostridium spp.), the highest yield is obtained when acetate is the main fermentation end product. Similarly, in organisms using the PFL H2-production pathway (e.g., E. coli), the highest yield is obtained when acetic acid and ethanol are the end products (Figure 2) [48,49]. The maximum theoretical yield of dark H2 production is assumed to be 2 to 4 mol of H2 per mol of glucose, depending on the kind of microorganisms (2 moles for facultative aerobes and 4 moles for strict anaerobes). To obtain this theoretical maximum yield, glucose must be fully converted to acetate as the terminal end product. In summary, the process in strict anaerobes, such as Clostridium sp., consists of the conversion of pyruvate to acetyl CoA and CO2 through PFOR, and electrons are donated to the hydrogenases via reduced FDX. This results in a maximum yield of 2 mol of H2 per mol of glucose. Two additional moles of H2 can be produced from the NADH produced during glycolysis via NADH:ferredoxin oxidoreductase (NFOR) which can donate electron to the FDX hydrogenase system, making an overall theoretical maximum yield of 4 mol of H2 per mol of glucose for this kind of bacteria. In facultative anaerobes, because a maximum of two molecules of formate are produced from two pyruvate molecules, the theoretical maximum yield for the PFL pathway is 2 mol of H2 per mol of glucose [48,49,50,51,91]. However, different constraints make the actual yields of dark fermentation much reduced. Two of the main drawbacks of dark H2 production are a) the existence of other fermentative competing pathways that lower the yield and b) the excessive accumulation of fermentative end products, especially acetic acid, which impairs microbial growth and H2 production [48,49,50,51,91]. Numerous studies have focused on the manipulation of Clostridium spp. and E. coli to enhance the H2 production by redirecting the fermentative pathways and reducing the accumulation of some undesired end products such as lactate, succinate or butyrate. However, the accumulation of acetic acid cannot be avoided since, in both pathways, this compound is directly linked to the production of H2, and its production is crucial to maintain an optimal energy/redox balance for the cells [48,49,50,51].
In order to solve the problematic acetic acid accumulation, integrative strategies combining dark bacteria and non-sulfur photosynthetic bacteria have been assessed. In these bacteria consortia, the organic acids generated by the dark bacteria can feed the photosynthetic bacteria for H2 production, resulting in increased H2 production yields (Figure 2) [47]. Theoretically, maximum yields in these integrative cultures can be obtained if acetic acid is the only secreted end product. Two molecules of acetate can be generated from glucose, in both facultative and strict anaerobes, which can then be converted into H2 by the PNSP bacteria, producing, theoretically, a maximum of 8 extra mol of H2, and, making the overall theoretical yield of the integrative systems 10 to 12 mol of H2 per mol of glucose. Again, these theoretical values are not reached since different limitations exist. Among others, the use of photosynthetic bacteria in these integrative systems often requires two-stage bioreactors due to the growth incompatibility and the removal of nitrogen, which strongly inhibits the H2-evolving nitrogenases [47].
The literature concerning the use of integrative systems has considered, almost exclusively, photosynthetic bacteria as the only partners able to use and remove the acetic acid resulting from dark fermentation. However, some microalgae can be used instead of (or with) photosynthetic bacteria (Figure 2). When co-culturing Chlamydomonas with different non-H2 producing bacteria in acetate-free media supplemented with sugars (glucose or mannitol), algal H2 production can be observed if acetic acid is excreted by the bacteria. The amount of acetic acid excreted by the bacteria directly correlates with the capacity of Chlamydomonas to produce H2 [56]. Moreover, as demonstrated by Fakhimi et al. [56] using E. coli and Chlamydomonas co-cultures incubated with glucose as the sole carbon source, it is possible to produce H2 in a synergetic way (60% more H2 than the sum of the respective control monocultures), with acetic acid probably being the metabolite linking dark H2 production with H2 photoproduction (Figure 2). This study entails a proof-of-concept linking dark bacteria and algae H2 production. Nonetheless, the H2 production yield obtained in E. coli–Chlamydomonas co-cultures was very low and optimizations are required.
As mentioned before, acetic acid is the only compound that Chlamydomonas can uptake as the sole carbon source for heterotrophic growth. Note that in, Chlamydomonas monocultures, no other source of organic carbon (e.g., glucose) can be used for growth or to trigger H2 production (Figure 2). Apart from growth promotion, acetic acid plays a significant role in H2 production in this alga. The presence of acetate in the medium promotes O2 consumption, represses CO2 fixation, and decreases the photosynthetic rates [92,93,94,95]; all of these factors favor H2 production. In addition, the presence of acetic acid in the culture media has been reported as a key parameter for photo-H2 production in Chlamydomonas monocultures [14] and co-cultures [56], whose role is partially independent of its capacity to promote hypoxia [14]. It has been suggested that, under light, nutrient-repleted conditions and hypoxia, the assimilation (or photoassimilation) of acetic acid, and not starch mobilization, can provide, directly or indirectly, electrons for the PSII-independent H2 production pathway [13,14]. Physiologically, the photoassimilation of acetate under hypoxia could be equivalent to the H2 photo-fermentation described in photosynthetic bacteria.
Overall, the use of microalgae such as Chlamydomonas leads to photo-H2 production, while helping to bypass the drawbacks of the acetic acid accumulation and pH acidification that prevent bacterial H2 production. The use of algae instead of photosynthetic bacteria or cyanobacteria has the advantage of avoiding the concomitant occurrence of H2 uptake and the nitrogen removal from the medium, which is required to induce nitrogenases. Moreover, compared with PNSP bacteria, algae have more compatible growth conditions with some dark bacteria. Moreover, algae, but not photosynthetic bacteria, can provide extra acetate-independent H2 production via direct H2 production (PSII-dependent pathway) or via the mobilization of the starch reserves (PSII-independent pathway). The two latter pathways can potentially surpass the theoretical maximum H2 yield of 10–12 mol H2 per mol of glucose in the solely bacterial integrative systems. However, more research is still needed to explore the potential of algae–bacteria co-cocultures for H2 production, and to better understand how the acetate metabolism is linked to H2 production in Chlamydomonas anaerobic cultures.
3.1.4. Co-Culturing Chlamydomonas with Bacteria Can Alleviate the Negative Effect of S Deprivation while Promoting H2 Production
S deprivation is a strategy widely used to enhance photobiological-H2 production in Chlamydomonas [35,36], which can lead to the highest H2 yields (Table 1). However, this strategy has several drawbacks, including growth inhibition and the loss of the cell viability (caused by the S prolonged deficiency), which reduce the potential for H2 generation. Previous studies have partially overcome the harmful effects of S deprivation using continuous or semi-continuous regimes of cultivation [96,97,98]. Recently, different studies using batch co-cultures in TAP-S [58,59,65] have obtained similar results to these previous studies, although avoiding the use of continuous or semi-continuous strategies, which can greatly simplify the overall process. For example, co-culturing Chlamydomonas with Pseudomonas sp. [59] or with Bradyrhizobium japonicum [58] in TAP-S can slow the reduction in chlorophyll, enhance starch accumulation, and maintain protein content, while favoring algal H2 production relative to algal monocultures. However, the precise reasons why these bacteria prolonged the viability of Chlamydomonas cells in TAP-S is uncertain. Interestingly, when Chlamydomonas is incubated with the sulfur-oxidizing bacterium Thuomonas intermedia [65], a considerable increase in H2 production and algal growth are observed; these effects are even more pronounced when the cultures are treated with the oxygen scavenger Na2S2O3 (Table 1). Authors propose that T. intermedia is able to oxidize S2O32− to SO42−, providing a S source for the alga to satisfy the minimum requirement for algal growth and, at the same time, maintain the S-deprived environment required for H2 photoproduction [65]. Overall, co-cultures in TAP-S require less energy inputs than continuous or semi-continuous alga monocultures and, more importantly, can support algae growth and H2 production simultaneously.
3.1.5. Starch-Enriched Alga Biomass Can Be Used as Substrate for H2 Producing Bacteria
Besides the direct supply of excreted fermentative metabolites to H2-producing bacteria by living algal cultures, algal biomass can also support H2 production by strict or facultative anaerobic bacteria. Different bacteria consortia have been probed to produce H2 from Chlamydomonas biomass. These consortia are often composed of a fermentative bacterium and a photosynthetic bacterium. The fermentative bacteria can degrade the Chlamydomonas biomass and excrete organic acids such as ethanol, formate, acetate, propionate and butyrate, which can be used by the photosynthetic bacteria to photoproduce H2 via photo-fermentation. For instance, Lactobacillus amylovorus is able to hydrolize starch from algae biomass to lactic acid, which can feed the photo-H2 production in Rhodobacter sphaeroides, Rhodobacter capsulata, Rhodospirillum rubrum and Rhodobium marinum [99,100]. Similarly, Vibrio fluvialis converted starch accumulated in Chlamydomonas to acetic acid and ethanol, which drove H2 production in Rhodobium marinum under high salt condition [101]. Likewise, Rhodobacter sphaeroides produced H2 from formate, acetate and butyrate secreted by Clostridium butyricum after anaerobic fermentation of Chlamydomonas biomass [102]. In this example, direct H2 production from Clostridium butyricum fed with Chlamydomonas biomass was also attained, which can illustrate the potential of producing H2 from algal biomass using bacteria consortia and two-step processes (Figure 1).
4. Net O2 Evolution
In Chlamydomonas, the photoproduction of H2 is unavoidably linked to the photosynthetic electron chain and thereby to O2 generation. As mentioned before, O2 is a strong inhibitor of both the expression and activity of the Chlamydomonas HYDAs [103]. The measurements of O2 in co-cultures can be indistinctly done in the liquid phase as dissolved O2 (DO2), or in the headspace. The DO2 measurements are more accurate to predict HYDAs activity in Chlamydomonas co-cultures, as demonstrated by Ban et al. [59]. Nevertheless, a good correlation between these two O2 indices and their relationship with H2 production has been observed in Chlamydomonas co-cultures [60].
In algae monocultures, the net O2 evolution is a result of the O2 inputs and outputs. The O2 inputs include initial O2 in the headspace, the DO2 in the culture media, and photosynthetic O2 generation. The light intensity directly influences the activity of the photosynthesis processes and thereby O2 generation. The O2 outputs are due to the respiratory activity. Chlamydomonas respiratory activity greatly increases when growing heterotrophically (or mixotrophically) in acetate-containing media due to the capability of this alga to use acetate as carbon source. This is the reason why most publications use either TAP or TAP-S to study H2 production in this alga.
A very simple relationship between O2 evolution and H2 production in H2-producing acetate-containing cultures is shown in Figure 3. In algal monocultures incubated in TAP medium, the O2 level quickly drops during the first 24 h. Under moderate light intensities (<50 PPFD), the photosynthetic O2 evolution is lower than the O2 consumption and the cultures remain under hypoxia for a few days, while the H2 production starts within the first 24 h. The hypoxic condition is maintained as far as acetic acid remains in the media. Once the acetic acid is fully consumed, the O2 levels rise and H2 production stops. Light intensity directly impacts the acetic acid uptake: the higher the light intensity, the faster the acetic acid uptake and the shorter the H2 production phase. At higher light intensities (>50 PPFD), there is a net positive O2 evolution and cultures do not reach hypoxia. [14] (Figure 3A). In co-cultures, the O2 outputs can be significantly increased if aerobic or facultative anaerobic bacteria are incubated in media containing organic carbon sources, which can greatly benefit H2 production. Again, most of the studies about H2 production using Chlamydomonas co-cultures are done in TAP or TAP-S media. In TAP co-cultures, the respiration rate can increase from 18% to 64% relative to Chlamydomonas monocultures, depending on the algal strain and the bacterial partners [57,61,66]. Unlike algal monocultures, no net O2 evolution is obtained in TAP co-cultures under moderate to high light intensity (50–100 PPFD), which allows H2 production at these light intensities [57,60,61,63]. A direct correlation between the presence of acetic acid in the media and the capacity to produce H2 have been observed in different Chlamydomonas–bacteria cultures [56,60,63,64]. Recently, Fakhimi et al. [63] have shown that the positive effect of the bacterial partners on H2 production can be linked to a decrease in acetate assimilation by the alga. Slower acetic acid uptake allows for a longer presence of this compound in the TAP culture medium, which, in turn, results in longer hypoxia and H2 production phases. This effect also allows for the use of higher light intensities compatible with H2 production. Distinct bacteria partners can impact the acetic acid uptake rates of Chlamydomonas differently; out of the different bacteria tested, Pseudomonas sp. showed the highest capacity to decrease the acetic acid uptake. All these data reveal that the use of co-cultures in TAP medium can help to reach hypoxia at higher light intensities than in monocultures, and they can increase the H2 yield by the means of more sustained H2 production.
Figure 3.
Typical trends of H2, O2 and acetic acid concentrations of Chlamydomonas cultures incubated in TAP (A) and TAP-S (B). In TAP cultures (A), H2 production occurs only in the presence of acetic acid, which is necessary to establish hypoxic conditions. In TAP-S cultures (B), the H2 production phase and the hypoxic phase are independent of the acetic acid concentration. Under the same light conditions, TAP cultures show faster acetic acid uptake and shorter lag phase than in TAP-S. H2 production yield and duration in TAP-S cultures is often higher than in TAP cultures. Tlag, lag phase before H2 production; tp, H2 production phase; thypoxia, hypoxia/anaerobic phase; (O2)in, initial O2 levels; (O2)hyp, minimal O2 levels compatible with H2 production.
On the other hand, S deficiency causes a decline in PSII activity and thereby in photosynthetic O2 evolution [104]. In Chlamydomonas monocultures incubated in TAP-S, at light intensities above 50 PPFD, there are 1–3 days where the cultures remain aerobic (termed as lag or oxic phase). Afterwards, an anerobic phase starts and H2 is produced; the H2 production yield in TAP-S is often higher than in TAP. In TAP-S cultures, the acetic acid is never fully consumed, and its level is neither linked to the aerobic or anaerobic phases nor to the H2 production phase [36,105] (Figure 3B). Dark incubation prior illumination or purging with noble gases are often used as strategies to quickly deplete O2 levels in the TAP-S cultures and shorten the lag phase. In co-cultures incubated in TAP-S, the respiration rate is enhanced by three to eight times during the first day compared with algal monocultures, depending on the light intensity [57,59,61,66,67]. Lakatos et al. [60] observed that after just 4h of illumination, the O2 level in the co-cultures (4–5%) were lower than in monocultures (15–16%). These observations demonstrated that, in the case of TAP-S cultures, the co-incubation with bacteria can reduce the lag phase and avoid the dark incubation or purging required to reach hypoxia and initiate H2 production.
Overall, elevating the O2 consumption rate by bacteria can improve H2 production by a) allowing the implementation of hypoxic conditions compatible with H2 production, b) decreasing the time required to establish hypoxia, c) extending the duration of the hypoxia phase, which directly influences the production phase, and d) tolerating higher light intensities without impairing the hypoxic conditions [59,60,63].
Finally, among the important aspects to be considered when setting up algae–bacteria co-cultures are the initial cell number ratios, which are one of the main concerns of many studies [57,59,61,65]. Different ratios can impact the O2 inputs and outputs and thereby the net O2 concentration in the cultures. Moreover, due to the light shading effect of the bacteria, the initial algae–bacteria ratios and light intensities should be considered and optimized at the same time [67]. According to Ban et al. [59], there is an optimum initial cell number of algae which results in the highest H2 production.
5. Extension of the Solar Spectrum Absorption Range
An important aspect of the association between microalgae and photosynthetic bacteria is the possibility to increase the range of the solar spectrum for conversion to H2. Microalgae and cyanobacteria can capture the visible portion of sunlight (400–700 nm) and generate H2, while PNSP bacteria can also capture near-infrared emissions (700–1010 nm) to produce H2. Therefore, an integrated system can lead to a better solar irradiation utilization. However, few studies have been carried in this sense using Chlamydomonas. Following this idea, Melis and Melnicki [106] studied a consortium of Chlamydomonas with Rhodospirillum rubrum to improve biomass generation. However, the light irradiance performance of this co-culture was weakly analyzed and H2 production was not reported for this co-culture. It would be interesting to perform a more thorough investigation of the light irradiance efficiency in similar co-cultures and their suitability for H2 production.
6. Final Remarks
H2 production by microalgae is being studied due for its potential to provide a clean and renewable biofuel. However, this technology is still far from industrial application due to its low rates and yields, which make it economically unviable. In the context of improving bio-H2 production, strategies based on algae–bacteria consortia are still poorly explored; however, they show great potential and could be some of the best strategies to improve H2 production. Indeed, despite the limited number of publications, the combination of Chlamydomonas with different non-H2 producing bacteria is already among the most successful strategies to attain H2 production in this alga (Table 2). However, the future of algae–bacteria consortia remains in their capacity to integrate co-cultures with other successful strategies such as physiological treatments (e.g., S or Mg deprivation), O2 scavengers, cell immobilization or light modulation. Importantly, co-cultures using genetically modified strains of both algae and bacteria could also offer great potential to further improve H2 production.
Improved H2 production in Chlamydomonas co-cultures can be explained by multiple factors, including an increase in the starch content, a decline in net O2 evolution, a decrease in the algal acetic acid uptake, metabolite exchanges, and the utilization of higher light intensities compatible with H2 production. However, there are still many questions that remain uncertain regarding how non-H2 producing bacteria promote algal H2 production.
In any case, the use of integrative systems combining different H2-producing microorganisms (alga, cyanobacteria, PNS bacteria and heterotrophic bacteria) could be the real challenge in the bio-H2 field. Combining fermentative, photofermentative and photosynthetic pathways for H2 production could be the most feasible approach to overcome the low bio-H2 production yields and make them compatible with industrial applications. In the case of microalgae, this is a very promising approach that needs to be further explored and extensively improved. A few studies have already confirmed the possibility to achieve collaborative [62,90] and even synergetic H2 production [56] when using Chlamydomonas together with different kinds of H2-producing microorganisms. This prospect can provide a new perspective on how to produce H2 from cheap raw materials or waste, taking advantage of microbial metabolic collaborations, while, at the same time, bypassing some H2 production barriers identified in both algae and bacteria (e.g., O2 withdrawal, acetic acid accumulation, pH control, or organic carbon and other nutrient supplies).
However, H2-producing microorganisms have complex and very versatile metabolisms. Unravelling the metabolic and physiological relationships that they develop in natural ecosystems is the key to creating properly designed strategies to improve H2 production when co-culturing. Finding the appropriate algal and bacterial partners, suitable raw materials, and culture conditions could be the next challenge to address efficient and sustainable H2 production.
Acknowledgments
In this section you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Author Contributions
Writing—original draft preparation, N.F., A.D. and D.G.-B.; writing—review & editing, N.F., A.D., D.G.-B. and E.F.; supervision, A.D. and D.G.-B.; project administration, A.G., E.F., A.D. and D.G.-B.; funding acquisition, A.G., E.F., A.D. and D.G.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European ERANETMED program, ERANETMED2-72-300; the Plan Propio of University of Córdoba, MOD.4.1 P.P.2016 A. DUBINI; the Spanish Government, MINECO Grant BFU2015-70649-P; the European FEDER program, Junta de Andalucía, BIO-502; the European U.E.INTERREG, 0055_ALGARED_PLUS_5_E.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- 1.Abdalla A.M., Hossain S., Nisfindy O.B., Azad A.T., Dawood M., Azad A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018;165:602–627. doi: 10.1016/j.enconman.2018.03.088. [DOI] [Google Scholar]
- 2.Züttel A., Remhof A., Borgschulte A., Friedrichs O. Hydrogen: The future energy carrier. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2010;368:3329–3342. doi: 10.1098/rsta.2010.0113. [DOI] [PubMed] [Google Scholar]
- 3.Das D., Veziroglu T.N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy. 2008;33:6046–6057. doi: 10.1016/j.ijhydene.2008.07.098. [DOI] [Google Scholar]
- 4.Chandrasekhar K., Lee Y.-J., Lee D.-W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015;16:8266–8293. doi: 10.3390/ijms16048266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oey M., Sawyer A., Ross I.L., Hankamer B. Challenges and opportunities for hydrogen production from microalgae. Plant Biotechnol. J. 2016;14:1487–1499. doi: 10.1111/pbi.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagarajan D., Lee D.-J., Kondo A., Chang J.-S. Recent insights into biohydrogen production by microalgae—From biophotolysis to dark fermentation. Bioresour. Technol. 2017;227:373–387. doi: 10.1016/j.biortech.2016.12.104. [DOI] [PubMed] [Google Scholar]
- 7.Peden E.A., Boehm M., Mulder D.W., Davis R., Old W.M., King P.W., Ghirardi M.L., Dubini A. Identification of global ferredoxin interaction networks in Chlamydomonas reinhardtii. J. Boil. Chem. 2013;288:35192–35209. doi: 10.1074/jbc.M113.483727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghirardi M.L., Dubini A., Yu J., Maness P.-C. Photobiological hydrogen-producing systems. Chem. Soc. Rev. 2009;38:52–61. doi: 10.1039/B718939G. [DOI] [PubMed] [Google Scholar]
- 9.Jans F., Mignolet E., Houyoux P.-A., Cardol P., Ghysels B., Cuiné S., Cournac L., Peltier G., Remacle C., Franck F. A type II NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc. Natl. Acad. Sci. USA. 2008;105:20546–20551. doi: 10.1073/pnas.0806896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baltz A., Dang K.-V., Beyly A., Auroy P., Richaud P., Cournac L., Peltier G. Plastidial expression of type II NAD(P)H dehydrogenase increases the reducing state of plastoquinones and hydrogen photoproduction rate by the indirect pathway in Chlamydomonas reinhardtii. Plant Physiol. 2014;165:1344–1352. doi: 10.1104/pp.114.240432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mus F., Cournac L., Cardettini V., Caruana A., Peltier G. Inhibitor studies on non-photochemical plastoquinone reduction and H2 photoproduction in Chlamydomonas reinhardtii. Biochim. Biophys. Acta (BBA) Bioenerg. 2005;1708:322–332. doi: 10.1016/j.bbabio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Chochois V., Dauvillée D., Beyly A., Tolleter D., Cuiné S., Timpano H., Ball S., Cournac L., Peltier G. Hydrogen production in Chlamydomonas: Photosystem II-dependent and -independent pathways differ in their requirement for starch metabolism. Plant Physiol. 2009;151:631–640. doi: 10.1104/pp.109.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Ballester D., Jurado-Oller J.L., Galván A., Fernández E., Dubini A. H2 production pathways in nutrient-replete mixotrophic Chlamydomonas cultures under low light. Response to the commentary article “On the pathways feeding the H2 production process in nutrient-replete, hypoxic conditions,” by Alberto Scoma and Szilvia, Z. Tóth. Biotechnol. Biofuels. 2017;10:117. doi: 10.1186/s13068-017-0801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurado-Oller J.L., Dubini A., Galván A., Fernández E., Gonzalez-Ballester D. Low oxygen levels contribute to improve photohydrogen production in mixotrophic non-stressed Chlamydomonas cultures. Biotechnol. Biofuels. 2015;8:149. doi: 10.1186/s13068-015-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbs M., Gfeller R.P., Chen C. Fermentative metabolism of Chlamydomonas reinhardtii.: III. Photoassimilation of acetate. Plant Physiol. 1986;82:160–166. doi: 10.1104/pp.82.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamberger E.S., King D., Erbes D.L., Gibbs M. H2 and CO2 evolution by anaerobically adapted Chlamydomonas reinhardtii F-60. Plant Physiol. 1982;69:1268–1273. doi: 10.1104/pp.69.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noth J., Krawietz D., Hemschemeier A., Happe T. Pyruvate:Ferredoxin Oxidoreductase is coupled to light-independent hydrogen production in Chlamydomonas reinhardtii. J. Boil. Chem. 2012;288:4368–4377. doi: 10.1074/jbc.M112.429985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Lis R., Baffert C., Couté Y., Nitschke W., Atteia A. Chlamydomonas reinhardtii chloroplasts contain a homodimeric pyruvate:ferredoxin oxidoreductase that functions with FDX1. Plant Physiol. 2012;161:57–71. doi: 10.1104/pp.112.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghirardi M.L., Togasaki R.K., Seibert M. Oxygen sensitivity of algal H2-production. Appl. Biochem. Biotechnol. 1997;63:141–151. doi: 10.1007/bf02920420. [DOI] [PubMed] [Google Scholar]
- 20.Happe T., Kaminski A. Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. JBIC J. Boil. Inorg. Chem. 2002;269:1022–1032. doi: 10.1046/j.0014-2956.2001.02743.x. [DOI] [PubMed] [Google Scholar]
- 21.Boboescu I.Z., Gherman V.D., Lakatos G., Pap B., Bíró T., Maróti G. Surpassing the current limitations of biohydrogen production systems: The case for a novel hybrid approach. Bioresour. Technol. 2016;204:192–201. doi: 10.1016/j.biortech.2015.12.083. [DOI] [PubMed] [Google Scholar]
- 22.Kruse O., Rupprecht J., Mussgnug J.H., Dismukes G.C., Hankamer B. Photosynthesis: A blueprint for solar energy capture and biohydrogen production technologies. Photochem. Photobiol. Sci. 2005;4:957. doi: 10.1039/b506923h. [DOI] [PubMed] [Google Scholar]
- 23.Dubini A., Ghirardi M.L. Engineering photosynthetic organisms for the production of biohydrogen. Photosynth. Res. 2014;123:241–253. doi: 10.1007/s11120-014-9991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubini A., Gonzalez-Ballester D. Global Warming. Springer Science and Business Media LLC; Cham, Switzerland: 2016. Biohydrogen from Microalgae; pp. 165–193. [Google Scholar]
- 25.Torzillo G., Scoma A., Faraloni C., Giannelli L. Advances in the biotechnology of hydrogen production with the microalga Chlamydomonas reinhardtii. Crit. Rev. Biotechnol. 2014;35:485–496. doi: 10.3109/07388551.2014.900734. [DOI] [PubMed] [Google Scholar]
- 26.Esquivel M.D.G., Amaro H., Pinto T., Fevereiro P., Malcata F.X. Efficient H2 production via Chlamydomonas reinhardtii. Trends Biotechnol. 2011;29:595–600. doi: 10.1016/j.tibtech.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Markov S.A., Eivazova E., Greenwood J. Photostimulation of H2 production in the green alga Chlamydomonas reinhardtii upon photoinhibition of its O2-evolving system. Int. J. Hydrogen Energy. 2006;31:1314–1317. doi: 10.1016/j.ijhydene.2005.11.017. [DOI] [Google Scholar]
- 28.Scoma A., Durante L., Bertin L., Fava F. Acclimation to hypoxia in Chlamydomonas reinhardtii: Can biophotolysis be the major trigger for long-term H2 production? New Phytol. 2014;204:890–900. doi: 10.1111/nph.12964. [DOI] [PubMed] [Google Scholar]
- 29.Kosourov S., Jokel M., Aro E.-M., Allahverdiyeva Y. A new approach for sustained and efficient H2 photoproduction by Chlamydomonas reinhardtii. Energy Environ. Sci. 2018;11:1431–1436. doi: 10.1039/C8EE00054A. [DOI] [Google Scholar]
- 30.Degrenne B., Pruvost J., Legrand J. Effect of prolonged hypoxia in autotrophic conditions in the hydrogen production by the green microalga Chlamydomonas reinhardtii in photobioreactor. Bioresour. Technol. 2011;102:1035–1043. doi: 10.1016/j.biortech.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Laurinavichene T., Tolstygina I., Tsygankov A. The effect of light intensity on hydrogen production by sulfur-deprived Chlamydomonas reinhardtii. J. Biotechnol. 2004;114:143–151. doi: 10.1016/j.jbiotec.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Milrad Y., Schweitzer S., Feldman Y., Yacoby I. Green algal hydrogenase activity is outcompeted by carbon fixation before inactivation by oxygen takes place. Plant Physiol. 2018;177:918–926. doi: 10.1104/pp.18.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagy V., Podmaniczki A., Vidal-Meireles A., Tengölics R., Kovács L., Rákhely G., Scoma A., Toth S.Z. Water-splitting-based, sustainable and efficient H2 production in green algae as achieved by substrate limitation of the Calvin-Benson-Bassham cycle. Biotechnol. Biofuels. 2018;11:69. doi: 10.1186/s13068-018-1069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolleter D., Ghysels B., Alric J., Petroutsos D., Tolstygina I., Krawietz D., Happe T., Auroy P., Adriano J.-M., Beyly A., et al. Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell. 2011;23:2619–2630. doi: 10.1105/tpc.111.086876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Ballester D., Jurado-Oller J.L., Fernández E. Relevance of nutrient media composition for hydrogen production in Chlamydomonas. Photosynth. Res. 2015;125:395–406. doi: 10.1007/s11120-015-0152-7. [DOI] [PubMed] [Google Scholar]
- 36.Melis A. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000;122:127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philipps G., Happe T., Hemschemeier A. Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta. 2011;235:729–745. doi: 10.1007/s00425-011-1537-2. [DOI] [PubMed] [Google Scholar]
- 38.Batyrova K., Gavrisheva A., Ivanova E., Liu J., Tsygankov A. Sustainable hydrogen photoproduction by phosphorus-deprived marine green microalgae Chlorella sp. Int. J. Mol. Sci. 2015;16:2705–2716. doi: 10.3390/ijms16022705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volgusheva A., Jokel M., Allahverdiyeva Y., Kukarskikh G.P., Lukashev E.P., Lambreva M.D., Krendeleva T.E., Antal T. Comparative analyses of H2 photoproduction in magnesium- and sulfur-starved Chlamydomonas reinhardtii cultures. Physiol. Plant. 2017;161:124–137. doi: 10.1111/ppl.12576. [DOI] [PubMed] [Google Scholar]
- 40.Ma W., Chen M., Wang L., Wei L., Wang Q. Treatment with NaHSO3 greatly enhances photobiological H2 production in the green alga Chlamydomonas reinhardtii. Bioresour. Technol. 2011;102:8635–8638. doi: 10.1016/j.biortech.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 41.Wei L., Yi J., Wang L., Huang T., Gao F., Wang Q., Ma W. Light intensity is important for hydrogen production in NaHSO3-treated Chlamydomonas reinhardtii. Plant Cell Physiol. 2017;58:451–457. doi: 10.1093/pcp/pcw216. [DOI] [PubMed] [Google Scholar]
- 42.Canbay E., Köse A., Oncel S.S. Photobiological hydrogen production via immobilization: Understanding the nature of the immobilization and investigation on various conventional photobioreactors. 3 Biotech. 2018;8:244. doi: 10.1007/s13205-018-1266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antal T.K., Matorin D.N., Kukarskikh G.P., Lambreva M.D., Tyystjärvi E., Krendeleva T.E., Tsygankov A., Rubin A.B. Pathways of hydrogen photoproduction by immobilized Chlamydomonas reinhardtii cells deprived of sulfur. Int. J. Hydrogen Energy. 2014;39:18194–18203. doi: 10.1016/j.ijhydene.2014.08.135. [DOI] [Google Scholar]
- 44.Kosourov S., Seibert M. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol. Bioeng. 2009;102:50–58. doi: 10.1002/bit.22050. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan A., Qian X., Ananyev G., Lun D.S., Dismukes G.C. Advances in Experimental Medicine and Biology. Springer Science and Business Media LLC; Singapore: 2018. Rewiring of cyanobacterial metabolism for hydrogen production: Synthetic biology approaches and challenges; pp. 171–213. [DOI] [PubMed] [Google Scholar]
- 46.Hu C., Choy S.-Y., Giannis A. Evaluation of lighting systems, carbon sources, and bacteria cultures on photofermentative hydrogen production. Appl. Biochem. Biotechnol. 2017;185:257–269. doi: 10.1007/s12010-017-2655-5. [DOI] [PubMed] [Google Scholar]
- 47.Hallenbeck P.C., Liu Y. Recent advances in hydrogen production by photosynthetic bacteria. Int. J. Hydrogen Energy. 2016;41:4446–4454. doi: 10.1016/j.ijhydene.2015.11.090. [DOI] [Google Scholar]
- 48.Mathews J., Wang G. Metabolic pathway engineering for enhanced biohydrogen production. Int. J. Hydrogen Energy. 2009;34:7404–7416. doi: 10.1016/j.ijhydene.2009.05.078. [DOI] [Google Scholar]
- 49.Oh Y.-K., Raj S.M., Jung G.Y., Park S. Current status of the metabolic engineering of microorganisms for biohydrogen production. Bioresour. Technol. 2011;102:8357–8367. doi: 10.1016/j.biortech.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 50.Ding C., Yang K.-L., He J. Handbook of Biofuels Production. Woodhead Publishing; Cambridge, UK: 2016. Biological and fermentative production of hydrogen; pp. 303–333. [Google Scholar]
- 51.Stephen A.J., Archer S.A., Orozco R.L., Macaskie L.E. Advances and bottlenecks in microbial hydrogen production. Microb. Biotechnol. 2017;10:1120–1127. doi: 10.1111/1751-7915.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee H.-S., Vermaas W.F., Rittmann B.E. Biological hydrogen production: Prospects and challenges. Trends Biotechnol. 2010;28:262–271. doi: 10.1016/j.tibtech.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Levin D.B., Pitt L., Love M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy. 2004;29:173–185. doi: 10.1016/s0360-3199(03)00094-6. [DOI] [Google Scholar]
- 54.Kothari R., Prasad R., Kumar V., Singh D. Production of biodiesel from microalgae Chlamydomonas polypyrenoideum grown on dairy industry wastewater. Bioresour. Technol. 2013;144:499–503. doi: 10.1016/j.biortech.2013.06.116. [DOI] [PubMed] [Google Scholar]
- 55.Chandra R., Mohan S.V. Microalgal community and their growth conditions influence biohydrogen production during integration of dark-fermentation and photo-fermentation processes. Int. J. Hydrogen Energy. 2011;36:12211–12219. doi: 10.1016/j.ijhydene.2011.07.007. [DOI] [Google Scholar]
- 56.Fakhimi N., Dubini A., Tavakoli O., Gonzalez-Ballester D. Acetic acid is key for synergetic hydrogen production in Chlamydomonas-bacteria co-cultures. Bioresour. Technol. 2019;289:121648. doi: 10.1016/j.biortech.2019.121648. [DOI] [PubMed] [Google Scholar]
- 57.Wu S., Li X., Yu J., Wang Q. Increased hydrogen production in co-culture of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Bioresour. Technol. 2012;123:184–188. doi: 10.1016/j.biortech.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 58.Xu L., Li D., Wang Q., Wu S. Improved hydrogen production and biomass through the co-cultivation of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Int. J. Hydrogen Energy. 2016;41:9276–9283. doi: 10.1016/j.ijhydene.2016.04.009. [DOI] [Google Scholar]
- 59.Ban S., Lin W., Wu F., Luo J. Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour. Technol. 2018;251:350–357. doi: 10.1016/j.biortech.2017.12.072. [DOI] [PubMed] [Google Scholar]
- 60.Lakatos G., Deák Z., Vass I., Rétfalvi T., Rozgonyi S., Rákhely G., Ördög V., Kondorosi E., Maróti G. Bacterial symbionts enhance photo-fermentative hydrogen evolution of Chlamydomonas algae. Green Chem. 2014;16:4716–4727. doi: 10.1039/c4gc00745j. [DOI] [Google Scholar]
- 61.Li X., Huang S., Yu J., Wang Q., Wu S. Improvement of hydrogen production of Chlamydomonas reinhardtii by co-cultivation with isolated bacteria. Int. J. Hydrogen Energy. 2013;38:10779–10787. doi: 10.1016/j.ijhydene.2013.02.102. [DOI] [Google Scholar]
- 62.Miyamoto K., Ohta S., Nawa Y., Mori Y., Miura Y. Hydrogen production by a mixed culture of a green alga, Chlamydomonas reinhardtii and a photosynthetic bacterium, Rhodospirillum rubrum. Agric. Boil. Chem. 1987;51:1319–1324. doi: 10.1271/bbb1961.51.1319. [DOI] [Google Scholar]
- 63.Fakhimi N., Tavakoli O., Marashi S.-A., Moghimi H., Mehrnia M., Dubini A., Gonzalez-Ballester D. Acetic acid uptake rate controls H2 production in Chlamydomonas-bacteria co-cultures. Algal Res. 2019;42:101605. doi: 10.1016/j.algal.2019.101605. [DOI] [PubMed] [Google Scholar]
- 64.Lakatos G., Balogh D., Farkas A., Ördög V., Nagy P.T., Bíró T., Maróti G. Factors influencing algal photobiohydrogen production in algal-bacterial co-cultures. Algal Res. 2017;28:161–171. doi: 10.1016/j.algal.2017.10.024. [DOI] [Google Scholar]
- 65.He J., Xi L., Sun X., Ge B., Liu D., Han Z., Pu X., Huang F. Enhanced hydrogen production through co-cultivation of Chlamydomonas reinhardtii CC-503 and a facultative autotrophic sulfide-oxidizing bacterium under sulfurated conditions. Int. J. Hydrogen Energy. 2018;43:15005–15013. doi: 10.1016/j.ijhydene.2018.06.081. [DOI] [Google Scholar]
- 66.Wirth R., Lakatos G., Maróti G., Bagi Z., Minárovics J., Nagy K., Kondorosi E., Rákhely G., Kovács K.L. Exploitation of algal-bacterial associations in a two-stage biohydrogen and biogas generation process. Biotechnol. Biofuels. 2015;8:59. doi: 10.1186/s13068-015-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L., Cheng X., Wang Q. Effect of co-cultivation of Chlamydomonas reinhardtii with Azotobacter chroococcum on hydrogen production. Int. J. Hydrogen Energy. 2017;42:22713–22719. doi: 10.1016/j.ijhydene.2017.06.223. [DOI] [Google Scholar]
- 68.Edrei J. Methods of Generating Hydrogen. US13/582,442. [(accessed on 27 December 2012)];Application. 2012 Dec 27; Available online: http://www.freepatentsonline.com/y2012/0329089.html.
- 69.Steinbeck J., Nikolova D., Weingarten R., Johnson X., Richaud P., Peltier G., Hermann M., Magneschi L., Hippler M. Deletion of Proton Gradient Regulation 5 (PGR5) and PGR5-Like 1 (PGRL1) proteins promote sustainable light-driven hydrogen production in Chlamydomonas reinhardtii due to increased PSII activity under sulfur deprivation. Front. Plant Sci. 2015;6:153. doi: 10.3389/fpls.2015.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kruse O., Rupprecht J., Bader K., Thomas-hall S., Schenk P.M., Finazzi G., Hankamer B. Improved photobiological H2 production in engineered green algal cells. J. Biol. Chem. 2005;280:34170–34177. doi: 10.1074/jbc.M503840200. [DOI] [PubMed] [Google Scholar]
- 71.Torzillo G., Scoma A., Faraloni C., Ena A., Johanningmeier U. Increased hydrogen photoproduction by means of a sulfur-deprived Chlamydomonas reinhardtii D1 protein mutant. Int. J. Hydrogen Energy. 2009;34:4529–4536. doi: 10.1016/j.ijhydene.2008.07.093. [DOI] [Google Scholar]
- 72.Oey M., Ross I.L., Stephens E., Steinbeck J., Wolf J., Radzun K.A., Kügler J., Ringsmuth A.K., Kruse O., Hankamer B. RNAi Knock-Down of LHCBM1, 2 and 3 increases photosynthetic H2 production efficiency of the green alga Chlamydomonas reinhardtii. PLoS ONE. 2013;8:e61375. doi: 10.1371/journal.pone.0061375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volgusheva A., Kukarskikh G., Krendeleva T., Rubin A., Mamedov F. Hydrogen photoproduction in green algae Chlamydomonas reinhardtii under magnesium deprivation. RSC Adv. 2015;5:5633–5637. doi: 10.1039/C4RA12710B. [DOI] [Google Scholar]
- 74.Hong M.E., Shin Y.S., Kim B.W., Sim S.J. Autotrophic hydrogen photoproduction by operation of carbon-concentrating mechanism in Chlamydomonas reinhardtii under sulfur deprivation condition. J. Biotechnol. 2016;221:55–61. doi: 10.1016/j.jbiotec.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 75.Wei L., Li X., Fan B., Ran Z., Ma W. A stepwise NaHSO3 addition mode greatly improves H2 photoproduction in Chlamydomonas reinhardtii. Front. Plant Sci. 2018;871:1532. doi: 10.3389/fpls.2018.01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu S., Huang R., Xu L., Yan G., Wang Q. Improved hydrogen production with expression of hemH and lba genes in chloroplast of Chlamydomonas reinhardtii. J. Biotechnol. 2010;146:120–125. doi: 10.1016/j.jbiotec.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 77.Ramanan R., Kim B.-H., Cho D.-H., Oh H.-M., Kim H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016;34:14–29. doi: 10.1016/j.biotechadv.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Calatrava V., Hom E., Llamas Á., Fernandez E., Galvan A. OK, thanks! A new mutualism between Chlamydomonas and methylobacteria facilitates growth on amino acids and peptides. FEMS Microbiol. Lett. 2018;365:1–9. doi: 10.1093/femsle/fny021. [DOI] [PubMed] [Google Scholar]
- 79.Xie B., Bishop S., Stessman D., Wright D., Spalding M.H., Halverson L. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J. 2013;7:1544–1555. doi: 10.1038/ismej.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hom E., Aiyar P., Schaeme D., Mittag M., Sasso S. A Chemical perspective on microalgal–microbial interactions. Trends Plant Sci. 2015;20:689–693. doi: 10.1016/j.tplants.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Kazamia E., Czesnick H., Van Nguyen T.T., Croft M.T., Sherwood E., Sasso S., Hodson S.J., Warren M.J., Smith A.G. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 2012;14:1466–1476. doi: 10.1111/j.1462-2920.2012.02733.x. [DOI] [PubMed] [Google Scholar]
- 82.Fuentes J.L., Nores I.G., Cuaresma M., Montero Z., Del Valle M.G., Vílchez C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs. 2016;14:100. doi: 10.3390/md14050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mignolet E., Lecler R., Ghysels B., Remacle C., Franck F. Function of the chloroplastic NAD(P)H dehydrogenase Nda2 for H2 photoproduction in sulphur-deprived Chlamydomonas reinhardtii. J. Biotechnol. 2012;162:81–88. doi: 10.1016/j.jbiotec.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 84.Cakmak T., Angun P., Ozkan A.D., Çakmak Z.E., Ölmez T.T., Tekinay T. Nitrogen and sulfur deprivation differentiate lipid accumulation targets of Chlamydomonas reinhardtii. Bioengineered. 2012;3:343–346. doi: 10.4161/bioe.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ball S., Dirick L., Decq A., Martiat J.-C., Matagne R. Physiology of starch storage in the monocellular alga Chlamydomonas reinhardtii. Plant Sci. 1990;66:1–9. doi: 10.1016/0168-9452(90)90162-H. [DOI] [Google Scholar]
- 86.Catalanotti C., Yang W., Posewitz M.C., Grossman A.R. Fermentation metabolism and its evolution in algae. Front. Plant Sci. 2013;4:150. doi: 10.3389/fpls.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang W., Catalanotti C., D’Adamo S., Wittkopp T.M., Ingram-Smith C.J., Mackinder L., Miller T.E., Heuberger A.L., Peers G., Smith K.S., et al. alternative acetate production pathways in Chlamydomonas reinhardtii during dark anoxia and the dominant role of chloroplasts in fermentative acetate production. Plant Cell. 2014;26:4499–4518. doi: 10.1105/tpc.114.129965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dubini A., Mus F., Seibert M., Grossman A.R., Posewitz M.C. Flexibility in anaerobic metabolism as revealed in a mutant of Chlamydomonas reinhardtii lacking hydrogenase activity. J. Boil. Chem. 2008;284:7201–7213. doi: 10.1074/jbc.M803917200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghirardi M.L., Subramanian V., Wecker M., Smolinski S., Antonio R., Xiong W., Gonzalez-Ballester D., Dubini A. Survey of the anaerobic metabolism of various laboratory wild-type Chlamydomonas reinhardtii strains. Algal Res. 2018;35:355–361. doi: 10.1016/j.algal.2018.05.002. [DOI] [Google Scholar]
- 90.Miura Y., Saitoh C., Matsuoka S., Miyamoto K. Stably sustained hydrogen production with high molar yield through a combination of a marine green alga and a photosynthetic bacterium. Biosci. Biotechnol. Biochem. 1992;56:751–754. doi: 10.1271/bbb.56.751. [DOI] [PubMed] [Google Scholar]
- 91.Hallenbeck P.C. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen Energy. 2002;27:1185–1193. doi: 10.1016/S0360-3199(02)00131-3. [DOI] [Google Scholar]
- 92.Gérin S., Mathy G., Franck F. Modeling the dependence of respiration and photosynthesis upon light, acetate, carbon dioxide, nitrate and ammonium in Chlamydomonas reinhardtii using design of experiments and multiple regression. BMC Syst. Boil. 2014;8:96. doi: 10.1186/s12918-014-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chapman S.P., Paget C.M., Johnson G., Schwartz J.-M. Flux balance analysis reveals acetate metabolism modulates cyclic electron flow and alternative glycolytic pathways in Chlamydomonas reinhardtii. Front. Plant Sci. 2015;6:474. doi: 10.3389/fpls.2015.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Endo T., Asada K. Dark Induction of the Non-Photochemical Quenching of chlorophyll fluorescence by acetate in Chlamydomonas reinhardtii. Plant Cell Physiol. 1996;37:551–555. doi: 10.1093/oxfordjournals.pcp.a028979. [DOI] [Google Scholar]
- 95.Heifetz P.B., Forster B., Osmond C.B., Giles L.J., Boynton J.E. Effects of acetate on facultative autotrophy in Chlamydomonas reinhardtii assessed by photosynthetic measurements and stable isotope analyses. Plant Physiol. 2000;122:1439–1446. doi: 10.1104/pp.122.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fedorov A.S., Kosourov S., Ghirardi M.L., Seibert M. Continuous hydrogen photoproduction by Chlamydomonas reinhardtii: Using a novel two-stage, sulfate-limited chemostat system. Appl. Biochem. Biotechnol. 2005;121:403–412. doi: 10.1385/ABAB:121:1-3:0403. [DOI] [PubMed] [Google Scholar]
- 97.Oncel S.S., Vardar-Sukan F. Photo-bioproduction of hydrogen by Chlamydomonas reinhardtii using a semi-continuous process regime. Int. J. Hydrogen Energy. 2009;34:7592–7602. doi: 10.1016/j.ijhydene.2009.07.027. [DOI] [Google Scholar]
- 98.Kosourov S., Makarova V., Fedorov A.S., Tsygankov A., Seibert M., Ghirardi M.L. The effect of sulfur re-addition on H2 photoproduction by sulfur-deprived green algae. Photosynth. Res. 2005;85:295–305. doi: 10.1007/s11120-005-5105-0. [DOI] [PubMed] [Google Scholar]
- 99.Kawaguchi H., Hashimoto K., Hirata K., Miyamoto K. H2 production from algal biomass by a mixed culture of Rhodobium marinum A-501 and Lactobacillus amylovorus. J. Biosci. Bioeng. 2001;91:277–282. doi: 10.1016/S1389-1723(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 100.Ike A., Toda N., Tsuji N., Hirata K., Miyamoto K. Hydrogen photoproduction from CO2-fixing microalgal biomass: Application of halotolerant photosynthetic bacteria. J. Ferment. Bioeng. 1997;84:606–609. doi: 10.1016/S0922-338X(97)81921-6. [DOI] [Google Scholar]
- 101.Ike A., Murakawa T., Kawaguchi H., Hirata K., Miyamoto K. Photoproduction of hydrogen from raw starch using a halophilic bacterial community. J. Biosci. Bioeng. 1999;88:72–77. doi: 10.1016/S1389-1723(99)80179-0. [DOI] [PubMed] [Google Scholar]
- 102.Kim M., Baek J., Yun Y., Junsim S., Park S., Kim S. Hydrogen production from Chlamydomonas reinhardtii biomass using a two-step conversion process: Anaerobic conversion and photosynthetic fermentation. Int. J. Hydrogen Energy. 2006;31:812–816. doi: 10.1016/j.ijhydene.2005.06.009. [DOI] [Google Scholar]
- 103.Mus F., Dubini A., Seibert M., Posewitz M.C., Grossman A.R. Anaerobic acclimation in Chlamydomonas reinhardtii. J. Boil. Chem. 2007;282:25475–25486. doi: 10.1074/jbc.M701415200. [DOI] [PubMed] [Google Scholar]
- 104.Wykoff D.D., Davies J., Melis A., Grossman A.R. The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol. 1998;117:129–139. doi: 10.1104/pp.117.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kosourov S., Seibert M., Ghirardi M.L. Effects of extracellular pH on the metabolic pathways in sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol. 2003;44:146–155. doi: 10.1093/pcp/pcg020. [DOI] [PubMed] [Google Scholar]
- 106.Melis A., Melnicki M. Integrated biological hydrogen production. Int. J. Hydrogen Energy. 2006;31:1563–1573. doi: 10.1016/j.ijhydene.2006.06.038. [DOI] [Google Scholar]