Abstract
Chitosan is a cationic polymer obtained by deacetylation of chitin, found abundantly in crustacean, insect, arthropod exoskeletons, and molluscs. The process of obtaining chitin by the chemical extraction method comprises the steps of deproteinization, demineralization, and discoloration. To obtain chitosan, the deacetylation of chitin is necessary. These polymers can also be extracted through the biological extraction method involving the use of microorganisms. Chitosan has biodegradable and biocompatible properties, being applied in the pharmaceutical, cosmetic, food, biomedical, chemical, and textile industries. Chitosan and its derivatives may be used in the form of gels, beads, membranes, films, and sponges, depending on their application. Polymer blending can also be performed to improve the mechanical properties of the bioproduct. This review aims to provide the latest information on existing methods for chitin and chitosan recovery from marine waste as well as their applications.
Keywords: polysaccharide, biopolymer, shrimp waste, chitosan blend, biotechnology
1. Introduction
The reuse of waste from the fishing industry is not a common practice, and large percentage of biomass of waste is discarded directly into the environment without previous treatment [1]. Any material that is not used during its production or consumption process due to technology or market limitations, which can cause damage to the environment when not properly managed, is considered waste [2,3]. However, this type of waste is a source of raw material with high benefits and can be used to produce biocompounds [4].
New processing and management techniques for these wastes are needed in order to generate quality co-products and reduce environmental impacts, therefore, we would have greater job creation and sustainable development of the fishing industry [5,6].
The seafood industry annually generates about 106 tons of waste, most of which is destined for composting or to be converted into low value-added products such as animal feed and fertilizers [7]. In this context, approximately 2000 tons of chitosan is produced annually, whose main source of extraction is from shrimp and crab shell residues [8].
Chitin is a natural polysaccharide found in fungi cell walls as well as in crustacean and insect exoskeletons and is the second most abundant biopolymer in nature after cellulose. Chitin is converted to chitosan by deacetylation that involves the removal of the acetyl group [9,10].
Chitosan is widely used as a biomaterial because it has biological properties of biocompatibility, biodegradability, and atoxicity. It can be used as a therapeutic agent because it has antibacterial and antifungal characteristics, making it interesting for applications in agriculture, medicine, the environment, and the food, cosmetic, and textile industries [11,12,13].
Therefore, the purpose of this review is to provide the latest information on existing methods for chitin and chitosan recovery from marine waste as well as their applications.
2. Chitin and Chitosan History
The research on chitin isolation and characterization began in 1811 by the studies of the French chemist Henri Braconnot, in which some fungal species were subjected to an aqueous alkali treatment, which allowed the extraction of the fungine, named by him. In 1843, Lassaigne conducted research from exoskeletons of the species Bombyx mori (silkworm), in which he demonstrated the presence of nitrogen in the structure of chitin. Chitosan was discovered in 1859 by treating chitin with heated potassium hydroxide. In 1878, Ledderhose pointed out that chitin has compounds such as glycosamine and acetic acid; however, only in 1894, Gilson confirmed the presence of glycosamine units. Still, in 1894, the German Felix Hoppe-Seyler named the compound chitosan. It was in 1950 that the chemical structure of chitosan was determined [14,15].
The first reports of chitosan production appeared in 1970 in Japan and the United States. By 1986, Japan already had fifteen industries producing chitin and chitosan commercially. Japan and the United States are world leaders in chitosan production, standing out in the research of this polysaccharide in the multitude of applications of chitosan, being economically attractive and profitable [16,17].
In 1983, the first studies with fungal chitosan were carried out in Brazil, confirming the presence of chitosan in the cell walls of fungi belonging to the class Zygomycetes and Mucorales [18]. The first records of chitosan production and marketing were done by Craveiro, Craveiro, and Queiroz (1999) [19]. Most Brazilian researches are still on a bench scale, with few patent filings being observed; however, they are extremely important because of their biotechnological importance and the potential of research groups operating in the Brazilian Northeast [20].
3. Occurrence of Chitin in Nature
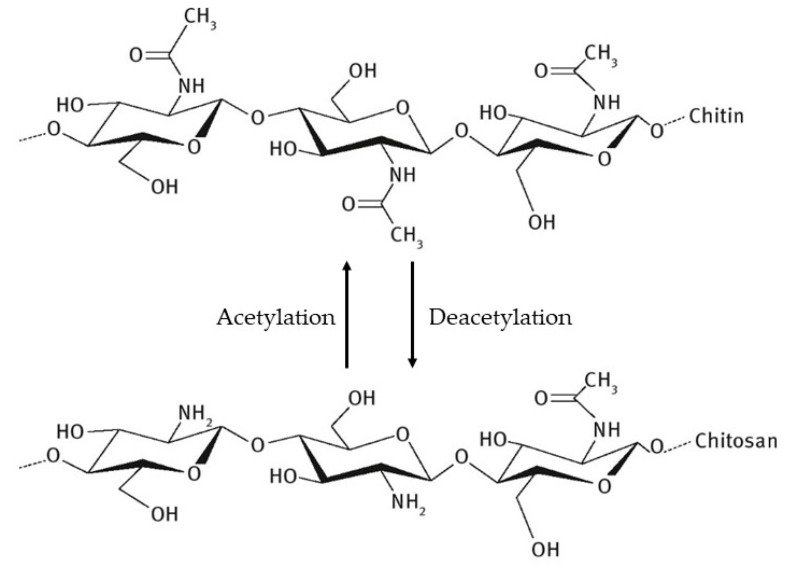
Chitin is a natural polymer that has a highly organized crystalline structure that is nitrogenous, white, and hard, having a low chemical reactivity. It is the second most abundant polysaccharide in nature, second only to cellulose. It is insoluble in water and organic solvents, presenting, after purification, as a yellowish powder. It has a high molecular weight and is chemically composed of N-acetyl-2-amino-2-deoxy-D-glucose units joined together by glycosidic bonds β (1 → 4) (Figure 1), forming a linear chain with some of the deacetylated monomer units [21,22,23].
Figure 1.
Chemical structures of chitin and chitosan.
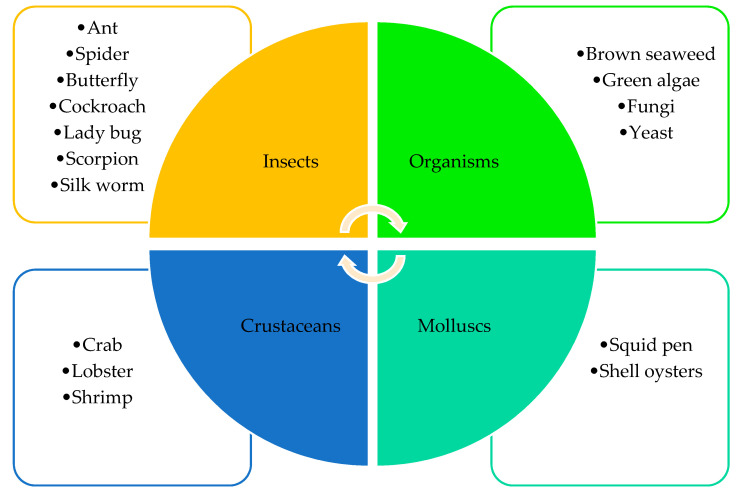
Chitin is widely distributed in nature and is the main element of the marine invertebrate exoskeleton and can be found in the structure of insects, arthropods, and molluscs [24,25,26]. Figure 2 presents the main sources of chitin production and its extraction.
Figure 2.
Sources of chitin production.
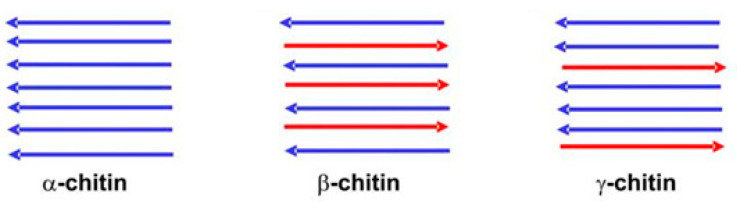
Chitin polymorphism can be visualized using X-ray diffraction, where three crystalline structures are observed, α, β, and γ, which differ by the number of chains per cell, degree of hydration, and unit size. The α-chitin is the most abundant form, being found in arthropod exoskeletons, where the dispositions of the polymeric chains are antiparallel, which favors the existence of numerous inter- and intra-chain hydrogen bonds that result in a densely packed material (Figure 3). In β-chitin, the disposition is parallel and they are found in animals that show flexibility and resistance, such as squids. The γ-chitin displays a mixture of both positions [27,28].
Figure 3.
Polymorphic structures of chitin.
Chitin derivatives have high economic value due to their biological activities and applications, being biodegradable and biocompatible polymers as well as produced by renewable natural sources. Harnessing the by-products of crustacean processing is a profitable activity because of their richness in high value-added compounds [29,30].
4. Biosynthesis of Chitin
Chitin occurs in complexes strongly linked with other substances in the cuticles of crustaceans, and some portions of polypeptides are linked to a small number of amino groups [31].
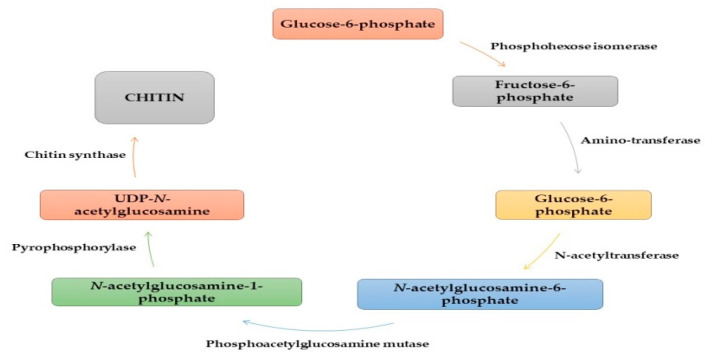
Generally, the carbon source used for chitin synthesis is glucose, starting the process of glycogen catalysis by the enzyme phosphorylase and being converted into glucose-1-P. In the presence of phosphomutase, glucose-6-P is formed and further converted to fructose-6-P by hexokinase. Intracellular fructose-6-P is converted to glucosamine-6-P via aminotransferase using L-glutamine. Then, glucosamine-6-P is converted to N-acetylglucosamine-6-P via N-acetyltransferase using acetyl co-A as a substrate. The phosphate group on it is changed from the 6-P to the 1-P position by phosphoacetylglucosamine mutase. Subsequently, N-acetylglucosamine-1-P is converted to UDP-N- acetylglucosamine via pyrophosphorylase using triphosphate as the cosubstrate. Chitin is formed from UDP-N-acetylglucosamine in the presence of the enzyme chitin synthase (Figure 4) [32,33,34]. The chitin deacetylation reaction results in chitosan [35,36].
Figure 4.
General scheme of chitin biosynthesis in biological systems.
5. Chitin Isolation from Natural Resources
Seafood is a major source of animal protein in many countries, however, besides the edible part, these raw materials have an inedible one [37]. A significant part of the environmental contamination is caused by the wastes from fishing industries, which triggers an environmental problem due to their unpleasant odor, attracting and stimulating the proliferation of insects. They can also be harmful to human health when disposed of without any kind of previous treatment [38,39].
The effluents resulting from the fishing industry, if released without previous treatment in the environment, cause physical and chemical changes in water bodies, which may cause mortality of aquatic animals and impact the local microfauna and microflora, given that this residue is characterized by its high concentrations of nitrogen, phosphorus, organic carbon, suspended solids, and oxygen [1].
Seafood waste is a potential source of raw material for chitin extraction [40]. However, due to their origin from natural resources and their chemical and physical variability, the properties of chitin and chitosan can have a direct impact on their applications [41]. Characteristics that may be related to the extraction process are molecular weight, degree of deacetylation, degree of purity, viscosity, and crystallinity [42].
5.1. Chemical Extraction
This type of extraction consists of the use of a strong alkaline solution, such as hydrolysis with sodium hydroxide at high temperatures and concentrations, causing the breakdown of polymeric chains, and establishing a high degree of chitosan deacetylation [43].
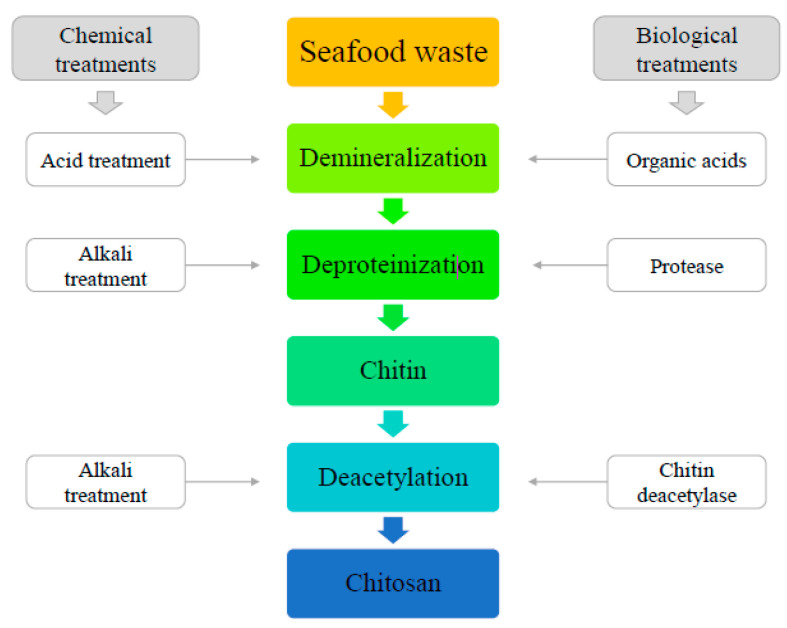
The chemical extraction method is composed of three basic steps (Figure 5), an alkaline solution deproteinization, an acid solution demineralization, and a discoloration. It is noteworthy that all these steps are directly related to the physicochemical properties of the chitin obtained [44,45]. The source for chitin extraction is subjected to washing, drying, and grinding of powder particles [46].
Figure 5.
Chitin and chitosan production by chemical and biological treatments.
This type of conventional extraction can cause problems in the disposal of waste generated, which is necessary for the neutralization and detoxification of wastewater [47].
5.1.1. Chemical Deproteinization
The deproteinization involves the disruption of chemical bonds between proteins and chitin, requiring chemicals to depolymerize the biopolymer [48], whose removal of the associated proteins is an essential step in the polysaccharide purification process [49,50].
Conventional extraction of chitin from marine waste through the deproteinization involves the use of bases and strong acids at high temperatures, which demands high energy consumption and generates effluents with high chemical concentrations, requiring appropriate treatment for their neutralization [51].
The use of strong acids and bases during the chitin extraction process leads to an increase in the cost of materials involved in the process, as well as a low-purity end product [52].
5.1.2. Chemical Demineralization
Demineralization is a process for the removal of minerals, especially calcium carbonate, using strong acids [53]. The most commonly used acids in this treatment process are sulfuric acid, hydrochloric acid, acetic acid, nitric acid, and formic acid [54,55,56].
Demineralization occurs through the decomposition of calcium carbonate in calcium chloride, with the release of carbon dioxide [57], as shown in the reaction:
| (1) |
5.1.3. Discoloration
This is an additional step during the extraction process and is performed if you wish to obtain a colorless product as it aims to eliminate astaxanthin and β-carotene pigments when they are present in the extraction source. It uses organic or inorganic solvents such as acetone, sodium hypochlorite, and hydrogen peroxide [42].
5.2. Biological Extraction
The biological extraction method involves the use of microorganisms that produce enzymes and organic acids at a relatively low cost, with a cleaner and greener process, favoring the production of quality chitin [58,59].
The biological extraction process has been made more attractive by obtaining high-quality products, with the cost of production being affordable and not generating high concentration chemical effluents, as mentioned in the chemical process [60].
Biological methods often used for chitin extraction are enzymatic deproteinization and fermentation using microorganisms [61,62].
5.2.1. Enzymatic Deproteinization
Enzymatic deproteinization of the waste from the fishing industry to obtain hydrolyzed protein is a method based on the addition of enzymes for protein fragmentation, having the advantage of not producing environmental degradation products [51,62].
Proteases are of utmost importance for protein removal during chitin extraction from fishing industry waste [63]. The proteases involved in the protein removal process from seafood residues are papain, trypsin, pepsin, alkalase, and pancreatin [64,65].
5.2.2. Fermentation
Hydrolyzed proteins can be obtained by proteolytic enzymes produced by the lactic acid bacteria activated due to a low pH in the medium. The advantage of this process is that it allows the recovery of value-added by-products such as proteins, enzymes, and pigments that can be applied, for example, in the food industry [66].
The efficiency of fermentation through microorganisms depends directly on the amount of inoculum, glucose concentration in the medium, the pH during the culture, and fermentation time. This type of extraction using microorganisms is a tendency in biotechnology and bioremediation researches [67].
Fermentation can be performed using protease-producing bacteria such as Bacillus subtilis, Pseudomonas aeruginosa, Pseudomonas maltophilia, and Serratia marcescens [68,69,70].
6. Chitin to Chitosan Conversion
Chitosan is a polysaccharide obtained by chitin deacetylation reaction through alkaline hydrolysis and subsequent treatment with acid solutions, consisting of 2-amino-2-deoxy-D-glycopyranose units joined by glycosidic bonds β (1 → 4) (Figure 5). However, the polymers differ in relative proportion and solubility of these units. They can function as an ion exchange resin for being soluble in organic acids and diluted minerals; nevertheless, their precipitation occurs with a pH value above 6.0 [71,72,73].
Chitosan are all chitin derivatives having a degree of deacetylation of 50% or more. The relative proportions of these units generate distinct structural characteristics, such as the degree of deacetylation and molecular weight, whose structural characteristics are related to the physicochemical and biological properties of the polymer [30,74,75].
Chitosan has the characteristic of solubility in acidic media because of the free amino groups being protonated (NH3 +), where the precipitation tendency increases from the moment the pH approaches 6.0, regarding the increase of -NH2 clusters in the molecular structure. Thus, amino groups make it possible for them to bind to negatively charged materials, such as other polysaccharides, enzymes, and cells, being insoluble in water, concentrated acids, acetone, and alcohol [76,77,78].
In recent years, many studies have been performed on chitosan and have shown a close dependence relationship between the structural and morphological characteristics of chitin, chitosan and their derivatives, their properties, and potential applications [79].
This polymer can be found in nature in small quantities in the cell walls of some fungi (Zygomycetes), or it can be obtained by alkaline hydrolysis of chitin from crustacean and exoskeletons of arthropods [80]. Crustacean shells contain 15% to 20% of chitin, 25% to 40% of protein, and 40% to 55% of calcium carbonate; the latter is responsible for crustacean rigidity [81].
7. Chitin and Chitosan Blend with Other Polymers
Polymer blending is defined as the homogeneous mixture of two or more different polymer species. Many blends are produced for financial reasons to reduce the costs of a technical application. However, the blend may lead to better properties of the product obtained [82].
Chitin and chitosan can be combined with poly (vinyl alcohol), alginate, collagen, cellulose acetate, among others to improve their mechanical properties [83,84,85,86]. Because chitosan is soluble in aqueous acid solutions, it can take different shapes, such as particles, films, sponges, membranes, gels, fibers, including others [87].
8. Chitin and Chitosan Applications
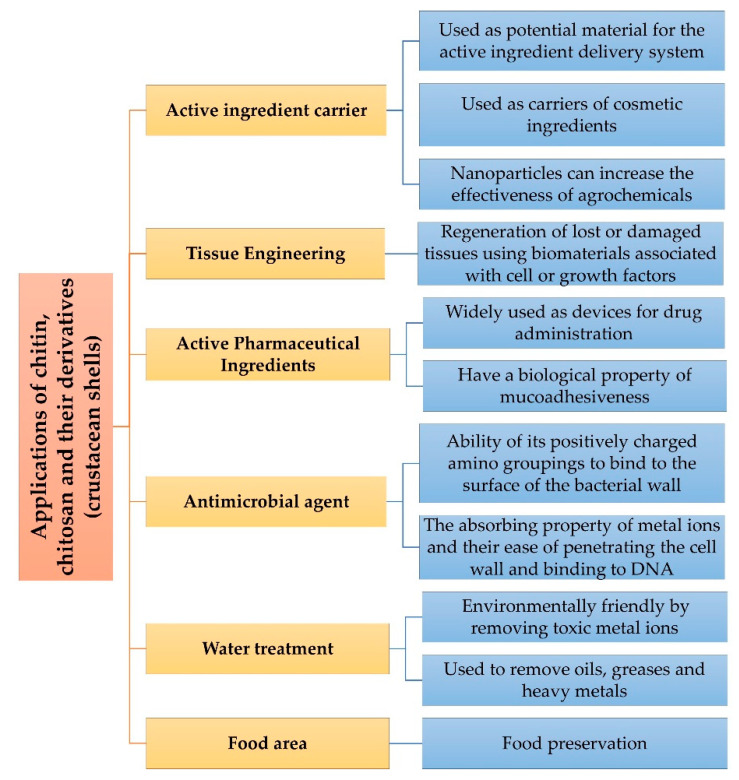
New technological approaches are needed to improve human lives and the environment. What makes chitosan a material of industrial interest are its broad spectrum of properties, such as water insolubility, cationic biopolymer behavior, positive global charge in biological pH, and easily capable of forming gels. These properties make it interesting for agricultural applications, medicine, environment, and food, as indicated in Figure 6 [88,89].
Figure 6.
Flowchart of the summary of the main applications of chitin/chitosan.
8.1. Active Ingredient Carrier
Nanotechnology has applications in many areas, such as engineering, medicine, pharmaceuticals, and agriculture, revolutionizing various processes and products [90]. Chitosan is a polymer of great industrial and biotechnological interest because of its abundant extraction source, being biocompatible and positively charged, defining it as a potential material for the active ingredient delivery system [91].
Chitin and chitosan-based nanomaterials can be used as carriers of cosmetic ingredients, such as chitin nanofibrils face masks capable of releasing active ingredients at different doses and time, and can be used as antibacterial, anti-inflammatory, sunscreen, anti-aging cosmetics depending on the active ingredient selected [92].
Nanotechnology can also be applied in agriculture, favoring agroindustry to be greener. Nanoparticles can increase the effectiveness of agrochemicals, resulting in lower doses and fewer applications, as well as reducing the risk of environmental contamination and promoting effective pest control in agriculture [93,94].
8.2. Tissue Engineering
The engineering of artificial tissues represents major advances in the biomedical field as it assists in reconstructive processes, favoring improvement in human life quality [95]. It involves the regeneration of lost or damaged tissues using biomaterials associated with cell or growth factors [96].
An important requirement for scaffolding is to have an interconnected structure with high porosity to ensure proper nutrient penetration and diffusion into cells [97].
Characteristics for choosing a biomaterial for tissue engineering are: presence of interconnected pores, controlled biodegradability, modifiable chemical surface, mechanical properties similar to the site of implantation, insignificant toxicity, and ease of obtaining desirable shapes and sizes [98].
8.3. Active Pharmaceutical Applications
Several polymers have been used in the production of mucoadhesive delivery systems; nevertheless, chitosan and its derivatives are the most broadly used due to characteristics of atoxicity, biocompatibility, antimicrobial activity, and adequate permeation [99].
Chitosan-based nanoparticles are widely used as devices for drug administration because they have useful features as a drug-loading vehicle. It has a biological property of mucoadhesiveness, implying the transient opening of epithelial junctions for drug entry [100].
Chitosan is a natural polyaminosaccharide with a non-toxic, non-allergenic, biocompatible, and biodegradable characteristic, and derivatives from chitosan are reported as anticoagulants. The literature described that the chitosan has a similar close structure like heparin, and based on this feature, many molecules of chitosan derivatives have been synthesized [101,102,103,104,105].
Anticoagulants are clinically used in different medical conditions like thrombosis and have the maximum annual growth rate among the top ten treatment areas. The developed compounds exhibited a faster onset of action and potency than nicoumalone after one hour of the drug administration. The sulphated N-alkyl derivatives of chitosan were suggested as the more potent anticoagulants than sulfated quaternary derivatives/sulfated chitosan [106,107].
In this sense, the most prominent commercial application of chitosan is its use as a hemostatic functional system. Therefore, chitosan-based wound dressings are available on the market for clinical use as products HemCon® Bandage and ChitoFlex wound dressings (HemCon Medical Technologies, UK), as well as CELOX™ (Medtrade Products, England); all products are FDA approved (http://www.hemcon.com and http://www.celoxmedical.com, respectively) [108].
8.4. Antimicrobial Agent
Many investigations reported the chitosan antimicrobial activity, but the mechanism has not yet been fully elucidated and studies are fundamental in the search for clarification of the potential of chitosan. One of the most studied properties of chitosan is its antimicrobial activity, related to the ability of its positively charged amino groupings to bind to the surface of the bacterial wall or the plasma membrane because they have negative charges. Thus, there is a change in cell permeability, favoring the flow of ions and proteins from the cytoplasm into the extracellular space and causing cell death [109]. Higher degree of acetylation, higher molecular weight of chitosan, and the antibacterial activity mediates the changes in cell permeability and blocks the transport of the bacteria [110,111,112]. Lower degree of acetylation of chitosan and lower pH favor antibacterial activity; however, the reduction of the molecular weight and the activity is toward Gram-negative bacteria, as well as the molecular weight and degree of acetylation influenced the antifungal activity with various fungi [113].
Other factors related to chitosan antimicrobial activity are the absorbing property of metal ions and their ease of penetrating the cell wall and binding to DNA, inhibiting messenger RNA synthesis, and low molecular weight of chitosan molecule induced inhibition of DNA transcription and mRNA synthesis in E. coli [110,111,112,113,114,115].
Therefore, the antimicrobial activity of chitosan and its derivatives is widely explored for the production of self-preserving materials through food protection and packaging. Chitosan films have a large application in food packaging materials, forming a protective antimicrobial barrier and preserving the nutritional quality of foods [116,117].
8.5. Water Treatment
Water and the range of services generated by this depleted natural resource contribute to poverty reduction, economic growth, as well as social and environmental sustainability, contributing to improvements in social welfare [118].
Wastewater from the food, textile, vegetable oil processing, oil production, and domestic sewage companies is a major source of pollution, given that it contains various organic compounds and is not properly treated before being discharged into the effluents [119].
Adsorption is the adhesion or fixation of molecules or electrostatic bonding of a fluid to a solid surface, enabling the elimination of compounds, metal ions, or other materials using an inactive sorbent of biological origin or natural products, by means of attractive forces between the material removed and the biosorbent [120]. The literature cites some adsorbents that are effective in removing toxic metal ions and are environmentally friendly, such as chitin, chitosan, cellulose, and guarana [121]. Chitosan is used to remove oils, greases, and heavy metals [122,123].
The increase of the degree of chitosan deacetylation is related to a greater number of amino groups, which are the main sorption centers, with being the degree of deacetylation and pH the main factors that affect the absorption capacity of chitosan [124].
8.6. Chitosan Applications in Food Technology
Chitosan biopolymer is biocompatible, nonantigenic, nontoxic, and biofunctional molecule and has attracted notable attention as a potential food preservative of natural origin [125,126,127]. Chitosan from shrimp isolation was preconized as GRAS (Generally Recognized As Safe) based on the scientific procedures for multiple technical effects in several food categories (GRAS Notice No. GRN 000443) [108,128].
Applications of chitosan were investigated for extension of shelf life of bread by retarding starch retrogradation and/or by inhibiting microbial growth have been observed. The authors evaluated chitosan molecule with 493 kDa coating on shelf life of baguette surface using 0.5%, 1.0%, or 1.5% chitosan diluted in 1.0% acetic acid. The results indicated barrier properties of chitosan to baguette-coated 1% chitosan, less weight loss, hardness, and retrogradation compared with the control during storage for 36 h at 25 °C [129,130].
Chitosan obtained by extraction from a bio-waste product using many energy-efficient methods. Chitosan is much cheaper as compared to other biopolymers. Nevertheless, the exceptional properties of chitosan make it a relatively stronger candidate for food packaging applications. The most popular and most economical way for production of chitosan is from deacetylation process of chitin. However, it is also possible to obtain chitosan directly from some fungi cell walls [131,132] and other organisms [133,134]. Chitosan film has been widely used to extend the shelf life of food, with addition of Ca2+ ions changes the permeation rate of CO2 and O2 through the chitosan membranes and increases the useful life of the fruits [17] Raw materials to prepare a series of films of chitosan and glycerol formulation showed strawberry preservation [135]. Coating with chitosan films by immersion in a 1% polysaccharide solution containing 0.1% Ca2+ prevents changes in the sensory properties of vegetables. In addition, chitosan is also useful in the production of paper for food packaging coated with it, which acts as an inhibitor of microbial growth [136]. Films formed by chitosan and polyvinyl alcohol with lignin nanoparticles are characterized by increased strength compared to films formed by individual components, antibacterial action against gram-negative microorganisms, and the synergistic antioxidant effect of chitosan and lignin [137].
9. Conclusions and Future Trends
As a major by-product of the seafood waste, a massive amount of crustacean shell waste is generated each year that can be used to produce value-added chitin, which can be converted to chitosan using a relatively simple deacetylation process. As the bio-waste product using many energy-efficient methods, chitosan is much cheaper as compared to other biopolymers. In the present review, chitin and chitosan has been presented as ideal renewable agents for native form or upgraded and incorporated with antimicrobial particles and natural compounds with multifunctional applications. Demands for alternative materials in various fields of biotechnology and industry are driven by technological advancement, favoring the increasing use of biopolymers and having chitosan as the most abundant and renewable polysaccharide, thus attracting more attention from researchers.
Chitin can be easily obtained from marine animals, crustacean residues, insects, and microorganisms. Chitosan is obtained by deacetylation of chitin, presenting antibacterial and antifungal properties, biocompatibility, mucoadhesivity, atoxicity, among others. Since previous decades, chitosan has been very important in several industrial applications, including biomedicine, textile, food, pharmaceutical, and cosmetic industries. For future applications, chitosan-based materials can be used as advanced composites or fibers, having a promising utility for accelerating tissue repair and wound healing processes for the pharmaceutical and biomedical industries. In addition, chitosan has become attractive to boost studies involving tissue engineering.
Therefore, this polymer is very attractive for application in several areas due to its characteristics, giving this polysaccharide a very promising future as a biomaterial. These include development of new smart future trends to biomaterials as promising wound healing effects and therapeutic molecules that are released at the same time as the microbial growth.
Acknowledgments
The authors would like to thank the Nucleus of Research in Environmental Sciences and Biotechnology (NPCIAMB) of the Catholic University of Pernambuco (UNICAP) for allowing them to the use of the laboratories.
Author Contributions
Conceptualization, V.P.S. and G.M.d.C.-T; methodology, V.P.S., N.S.S.M. and P.C.S.V.M.; software, V.P.S., L.d.O.F. and M.A.B.d.L.; validation, V.P.S., N.S.S.M., L.d.O.F. and G.M.d.C.-T.; formal analysis, V.P.S. and G.M.d.C.-T.; investigation, V.P.S., N.S.S.M. and G.M.d.C.-T.; data curation, V.P.S., L.d.O.F. and M.A.B.d.L.; writing—original draft preparation, V.P.S., N.S.S.M. and G.M.d.C.-T.; writing—review and editing, G.M.d.C.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Council for Scientific and Technological Development (CNPq)- under process Nr. 314422/2018-8 and Coordination for the Improvement of Higher Level Education Personnel (CAPES)- Edital Pró-Equipamentos CAPES nº 11/2014 and processes V.P.S. and Foundation for the Support of Science and Technology of the State of Pernambuco (FACEPE)- process Nr. APQ-0291-2.12/15.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Wang S.L., Nguyen V.B. Production of potent antidiabetic compounds from shrimp head powder via Paenibacillus conversion. Process. Chem. 2019;76:18–24. doi: 10.1016/j.procbio.2018.11.004. [DOI] [Google Scholar]
- 2.Liu C., Cai W., Cuixia Z., Ma M., Rao W., Li W., He K., Gao M. Developing the ecological compensation criterion of industrial solid waste based on energy for sustainable development. Energy. 2018;157:940–948. doi: 10.1016/j.energy.2018.05.207. [DOI] [Google Scholar]
- 3.Bedoić R., Ćosić B., Duić N. Technical potential and geographic distribution of agricultural residues, co-products and by-products in the European Union. Sci. Total Environ. 2019;686:568–579. doi: 10.1016/j.scitotenv.2019.05.219. [DOI] [PubMed] [Google Scholar]
- 4.Mohanty B., Mohanty U., Pattanaik S.S., Panda A., Jena A.K. Future prospects and trends for effective utilization of fish processing wastes in India. Innovat. Farm. 2018;3:1–5. [Google Scholar]
- 5.Tsakanika A., Clauzet M., May P.H. Envolvendo os pescadores artesanais no desenvolvimento sustentável urbano e periurbano no Brasil. Revibec. 2018;28:1–20. [Google Scholar]
- 6.Sivaraman I., Krishnan M., Radhakrishnan K. Better Management Practices for sustainable small-scale shrimp farming. J. Clean. Prod. 2019;214:559–572. doi: 10.1016/j.jclepro.2018.12.172. [DOI] [Google Scholar]
- 7.Schmitz C., Auza L.G., Koberidze D., Rasche S., Fischer R., Bortesi L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs. 2019;17:452. doi: 10.3390/md17080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz I., Rodríguez C., Gillet D., Moerschbacher B.M. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018;23:1151–1160. doi: 10.1007/s11367-017-1290-2. [DOI] [Google Scholar]
- 9.Arasukumar B., Prabakaran G., Gunalan B., Moovendhan M. Chemical composition, structural features, surface morphology and bioactivities of chitosan derivatives from lobster (Thenus unimaculatus) shells. Int. J. Biol. Macromol. 2019;135:1237–1245. doi: 10.1016/j.ijbiomac.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Lucas-Bautista J.A., Bautista-Baños S., Ventura-Aguilar R.I., Gómez-Ramírez M. Determinación de quitina en hongos postcosecha y de quitinasas en frutos de papaya “Maradol”. Rev. Mex. Fit. 2019;37:1–7. doi: 10.18781/R.MEX.FIT.1902-3. [DOI] [Google Scholar]
- 11.Srinivasan H., Kanayairam V., Ravichandran R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018;107:662–667. doi: 10.1016/j.ijbiomac.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Wu H., Williams G.R., Wu J., Wu J., Niu S., Li H., Wang H., Zhu L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018;180:304–313. doi: 10.1016/j.carbpol.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Medina E., Caro N., Abugoch L., Gamboa A., Díaz-Dosque M., Tapia C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019;240:191–198. doi: 10.1016/j.jfoodeng.2018.07.023. [DOI] [Google Scholar]
- 14.Synowiecki J., Al-Khateeb N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003;43:145–171. doi: 10.1080/10408690390826473. [DOI] [PubMed] [Google Scholar]
- 15.Vaz J.M., Pezzoli D., Chevallier P., Campelo C.S., Candiani G., Mantovani D. Antibacterial Coatings Based On Chitosan For Pharmaceutical And Biomedical Applications. Curr. Pharm. 2018;24:866–885. doi: 10.2174/1381612824666180219143900. [DOI] [PubMed] [Google Scholar]
- 16.Knorr D. Recovery and utilization of chitin and chitosan in food processing waste management. Food Technol. 1991;45:114–120. [Google Scholar]
- 17.Mehebub M.S., Mahmud N.U., Rahman M., Surovy M.Z., Gupta D.R., Hasanuzzaman M., Rahman M., Islam M.T. Chitosan biopolymer improves the fruit quality of litchi (Litchi chinensis Sonn) Acta Agrobot. 2019;72:1–9. doi: 10.5586/aa.1773. [DOI] [Google Scholar]
- 18.Campos-Takaki G.M., Beakes G.W., Dietrich S.M.C. Electron microscopic X-ray microprobe and cytochemical study of isolated cell walls of mucoralean fungi. T. Brit. Mycol. Soc. 1983;80:536–541. doi: 10.1016/S0007-1536(83)80053-9. [DOI] [Google Scholar]
- 19.Craveiro A.A., Craveiro A.C., Queiroz D.C. Quitosana: A fibra do futuro, 2 ed. Fortaleza; Padetec-Disal, Brazil: 2004. [Google Scholar]
- 20.Batista A.C.L., Souza Neto F.E., Paiva W.S. Review of fungal chitosan: Past, present and perspectives in Brazil. Polímeros. 2018;28:275–283. doi: 10.1590/0104-1428.08316. [DOI] [Google Scholar]
- 21.Núñez-Gómez D., Nagel-Hassemer M.E., Lapolli F.R., Lobo-Recio M.A. Potencial dos resíduos do processamento de camarão para remediação de águas contaminadas com drenagem ácida mineral. Polímeros. 2016;26:1–7. doi: 10.1590/0104-1428.1757. [DOI] [Google Scholar]
- 22.Xu R., Mao J., Penh N., Luo X., Chang C. Chitin/clay microspheres with hierarchical architecture for highly efficient removal of organic dyes. Carbohydr. Polym. 2018;188:143–150. doi: 10.1016/j.carbpol.2018.01.073. [DOI] [PubMed] [Google Scholar]
- 23.Chen C., Li D., Yano H., Abe K. Insect cuticle-mimetic hydrogels with high mechanical properties achieved via the combination of chitin nanofiber and gelatin. J. Agric. Food Chem. 2019;67:5571–5578. doi: 10.1021/acs.jafc.9b00984. [DOI] [PubMed] [Google Scholar]
- 24.Mao X., Guo N., Sun J., Xue C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017;143:814–823. doi: 10.1016/j.jclepro.2016.12.042. [DOI] [Google Scholar]
- 25.Anwar W., Javed M.A., Shahid A.A., Nawaz K., Akhter A., Rehman M.Z.U., Hameed U., Iftikhar S., Haider M.S. Chitinase genes from Metarhizium anisopliae for the control of whitefly in cotton. R. Soc. Open Sci. 2019;6:1–12. doi: 10.1098/rsos.190412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Q., Wang Y., Han Q., Ji L., Zhang H., Fei Z., Wang Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr. Polym. 2019;209:266–275. doi: 10.1016/j.carbpol.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Mehrabani M.G., Karimian R., Rakhshaei R., Pakdel F., Eslami H., Fakhrzadeh V., Rahimi M., Salehi R., Kafil H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018;116:966–976. doi: 10.1016/j.ijbiomac.2018.05.102. [DOI] [PubMed] [Google Scholar]
- 28.Ru G., Wu S., Yan X., Liu B., Gong P., Wang L., Feng J. Inverse solubility of chitin/chitosan in aqueous alkali solvents at low temperature. Carbohydr. Polym. 2019;206:487–492. doi: 10.1016/j.carbpol.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Seok H.Y., Rejinold N.S., Lekshmi K.M., Cherukula K., Park I.K., Kim Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release. 2018;280:20–30. doi: 10.1016/j.jconrel.2018.04.050. [DOI] [PubMed] [Google Scholar]
- 30.Arrouze F., Desbrieres J., Rhazi M., Essahli M., Tolaimate A. Valorization of chitins extracted from North Morocco shrimps: Comparison of chitin reactivity and characteristics. J. Appl. Polym. Sci. 2019;136:1–10. doi: 10.1002/app.47804. [DOI] [Google Scholar]
- 31.Kurita K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006;8:203–226. doi: 10.1007/s10126-005-0097-5. [DOI] [PubMed] [Google Scholar]
- 32.Elieh-Ali-Komi D., Hamblin M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016;4:411–427. [PMC free article] [PubMed] [Google Scholar]
- 33.Brigham C.J. Chitin and chitosan: Sustainable, medically relevant biomaterials. Int. J. Biotech. Well. Indus. 2017;6:41–47. doi: 10.6000/1927-3037.2017.06.02.1. [DOI] [Google Scholar]
- 34.Elsoud M.M.A., El Kady E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019;43:1–12. doi: 10.1186/s42269-019-0105-y. [DOI] [Google Scholar]
- 35.BenBettaieb N., Karbowiak T., Bornaz S. Spectroscopic analyses of the influence of electron beam irradiation doses on mechanical, transport properties and microstructure of chitosan-fish gelatin blend films. Food Hydrocoloids. 2014;48:37–51. doi: 10.1016/j.foodhyd.2014.09.038. [DOI] [Google Scholar]
- 36.Xu W., Mohan A., Pitts N.L., Udenigwe C., Mason B. Bile acid-binding capacity of lobster shell-derived chitin, chitosan and chitooligosaccharides. Food Biosci. 2020;33:100476. doi: 10.1016/j.fbio.2019.100476. [DOI] [Google Scholar]
- 37.Tolesa L.D., Gupta B.S., Lee M.J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019;130:818–826. doi: 10.1016/j.ijbiomac.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Souza F.M., Ferreira R.M.S., Barbosa R.C. Utilização da casca de camarão para produção de quitina. Rev. Scir. 2015;7:1–11. [Google Scholar]
- 39.Abreu F.L., Vasconcelos F.P., Albuquerque M.F.C. A Diversidade no Uso e Ocupação da Zona Costeira do Brasil: A Sustentabilidade como Necessidade. Conex. Cienc. Tec. 2017;11:8–16. doi: 10.21439/conexoes.v11i5.1277. [DOI] [Google Scholar]
- 40.Casadidio C., Peregrina D.V., Gigliobianco M.R., Deng S., Censi R., Di Martino P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs. 2019;17:369. doi: 10.3390/md17060369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibitoye E.B., Lokman I.H., Hezmee M.N.M., Goh Y.M., Zuki A.B.Z., Jimoh A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018;2:2–025022. doi: 10.1088/1748-605X/aa9dde. [DOI] [PubMed] [Google Scholar]
- 42.El Knidri H., Belaabed R., Addaou A., Laajed A., Lahsini A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018;120:1181–1189. doi: 10.1016/j.ijbiomac.2018.08.139. [DOI] [PubMed] [Google Scholar]
- 43.Henry García Y., Troncoso-Rojas R., Tiznado-Hernández M.E., Báez-Flores M.E., Carvajal-Millan E., Rascón-Chu A., Lizardi-Mendoza J., Martínez-Robinson K.G. Enzymatic treatments as alternative to produce chitin fragments of low molecular weight from Alternaria alternata. J. Appl. Polym. Sci. 2019;136:47339. doi: 10.1002/app.47339. [DOI] [Google Scholar]
- 44.Ali M.E.A., Aboelfadl M.M.S., Selim A.M., Khalil H.F., Elkady G.M. Chitosan nanoparticles extracted from shrimp shells, application for removal of Fe (II) and Mn (II) from aqueous phases. Sep. Sci. Technol. 2018;53:2870–2881. doi: 10.1080/01496395.2018.1489845. [DOI] [Google Scholar]
- 45.Küçükgülmez A. Extraction of Chitin from Crayfish (Astacus leptodactylus) Shell Waste. Alint. Zir. Bilim. Derg. 2018;33:99–104. doi: 10.28955/alinterizbd.432139. [DOI] [Google Scholar]
- 46.Santos V.P., Maia P., Alencar N.S., Farias L., Andrade R.F.S., Souza D., Ribeaux D.R., Franco L.O., Campos-Takaki G.M. Recovery of chitin and chitosan from shrimp waste with microwave technique and versatile application. Arq. Inst. Biol. 2019;86:1–7. doi: 10.1590/1808-1657000982018. [DOI] [Google Scholar]
- 47.Doan C.T., Tran T.N., Nguyen V.B., Vo T.P.K., Nguyen A.D., Wang S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019;131:706–715. doi: 10.1016/j.ijbiomac.2019.03.117. [DOI] [PubMed] [Google Scholar]
- 48.Yadav M., Goswami P., Paritosh K., Kumar M., Pareek N., Vivekanand V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresources. 2019;6:8–28. doi: 10.1186/s40643-019-0243-y. [DOI] [Google Scholar]
- 49.Avelelas F., Horta A., Pinto L.F.V., Marques S.C., Nunes P.M., Pedrosa R., Leandro S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs. 2019;17:239. doi: 10.3390/md17040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng X., Li P.P., Chen X., Kang Y., Xie Y., Li. X., Xie T., Zhang Y. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2019;126:867–876. doi: 10.1016/j.ijbiomac.2018.12.222. [DOI] [PubMed] [Google Scholar]
- 51.Lopes C., Antelo L.T., Franco-Uría A., Alonso A.A., Pérez-Martín R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Clean. Prod. 2018;172:4140–4151. doi: 10.1016/j.jclepro.2017.01.082. [DOI] [Google Scholar]
- 52.Broquá J., Zanin B.G., Flach A.M., Mallmann C., Taborda F.G.D., Machado L.E.L., Alves S.M.L., Silva M.M., Dias R.J.S.P., Reis O.V., et al. Different aspects of chemical and biochemical methods for chitin production a short review. Nanomed. Res. J. 2018;10:1477–2577. [Google Scholar]
- 53.Sugiyanti D., Darmadji P., Anggrahini S., Anwar C., Santoso U. Preparation and Characterization of Chitosan from Indonesian Tambak Lorok Shrimp Shell Waste and Crab Shell Waste. Pak. J. Nutr. 2018;17:446–453. doi: 10.3923/PJN.2018.446.453. [DOI] [Google Scholar]
- 54.Samrot A.V., Burman U., Philip S.A., Sobrana N., Chandrasekaran K. Synthesis of curcumin loaded polymeric nanoparticles from crab shell derived chitosan for drug delivery. Inform. Med. Unlocked. 2018;10:159–182. doi: 10.1016/j.imu.2017.12.010. [DOI] [Google Scholar]
- 55.Ali M., Shakeel M., Mehmood K. Extraction and characterization of high purity chitosan by rapid and simple techniques from mud crabs taken from Abbottabad. Pak. J. Pharm. Sci. 2019;32:171–175. [PubMed] [Google Scholar]
- 56.Tanabtabzadeh M.S., Javanbakht V., Golshirazi A.H. Extraction of Betacyanin and Betaxanthin Pigments from Red Beetroots by Chitosan Extracted from Shrimp Wastes. Waste Biomass Valor. 2019;10:641–653. doi: 10.1007/s12649-017-0086-8. [DOI] [Google Scholar]
- 57.Buanasari B., Sugiyo W., Fitriani N., Suryaningsih S. Potential of Chitosan From Local Crab (Portunus Pelagicus) to Enhance Storability of Musa Paradisiaca, L. J. Bahan. Alam. Terb. 2019;8:41–46. doi: 10.15294/jbat.v8i1.16423. [DOI] [Google Scholar]
- 58.Chakravarty J., Yang C.L., Palmer J., Brigham C.J. Chitin extraction from lobster shell waste using microbial culture-based methods. Appl. Food Biotech. 2018;5:141–154. doi: 10.22037/afb.v5i3.20787. [DOI] [Google Scholar]
- 59.Aranday-García R., Saimoto H., Shirai K., Ifuku S. Chitin biological extraction from shrimp wastes and its fibrillation for elastic nanofiber sheets preparation. Carbohydr. Polym. 2019;213:112–120. doi: 10.1016/j.carbpol.2019.02.083. [DOI] [PubMed] [Google Scholar]
- 60.Gachhi D.B., Hungund B.S. Two-phase extraction, characterization, and biological evaluation of chitin and chitosan from Rhizopus oryzae. J. Appl. Pharm. Sci. 2018;8:116–122. doi: 10.7324/JAPS.2018.81117. [DOI] [Google Scholar]
- 61.Dun Y., Li Y., Xu J., Hu Y., Zhang C., Liang Y., Zhao S. Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. Int. J. Biol. Macromol. 2019;123:420–426. doi: 10.1016/j.ijbiomac.2018.11.088. [DOI] [PubMed] [Google Scholar]
- 62.Marzieh M.N., Zahra F., Tahereh E., Sara K.N. Comparison of the physicochemical and structural characteristics of enzymatic produced chitin and commercial chitin. Int. J. Biol. Macromol. 2019;139:270–276. doi: 10.1016/j.ijbiomac.2019.07.217. [DOI] [PubMed] [Google Scholar]
- 63.Doan C., Tran T.N., Nguyen V.B., Nguyen A.D., Wang S.L. Conversion of squid pens to chitosanases and proteases via Paenibacillus sp. TKU042. Mar. Drugs. 2018;16:83. doi: 10.3390/md16030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Admassu H., Gasmalla M.A.A., Yang R., Zhao W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018;83:6–16. doi: 10.1111/1750-3841.14011. [DOI] [PubMed] [Google Scholar]
- 65.Zamora-Sillero J., Gharsallaoui A., Prentice C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018;20:118–130. doi: 10.1007/s10126-018-9799-3. [DOI] [PubMed] [Google Scholar]
- 66.Castro R., Guerrero-Legarreta I., Bórquez R. Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol. Rep. 2018;20:1–5. doi: 10.1016/j.btre.2018.e00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abirami S., Nagarajan D. Extraction of Chitin from Shrimp Shell Wastes by Using Bacillus licheniformis and Lactobacillus plantarum. Inter. J. Recent Res. Aspects. 2018:307–315. [Google Scholar]
- 68.Ghorbel-Bellaaj O., Maalej H., Nasri M., Jellouli K. Fermented Shrimp Waste Hydrolysates: Promising Source of Functional Molecules with Antioxidant Properties. J. Culin. Sci. Technol. 2018;16:357–377. doi: 10.1080/15428052.2017.1394950. [DOI] [Google Scholar]
- 69.Gong X., Tian W., Bai J., Qiao K., Zhao J., Wang L. Highly efficient deproteinization with an ammonifying bacteria Lysinibacillus fusiformis isolated from brewery spent diatomite. J. Biosci. Bioeng. 2019;127:326–332. doi: 10.1016/j.jbiosc.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Navarrete-Bolaños J.L., González-Torres I., Vargas-Bermúdez V.H., Jiménez-Islas H. A biotechnological insight to recycle waste: Analyzing the spontaneous fermentation of shrimp waste to design the hydrolysis process of chitin into n-acetylglucosamine. Rev. Mex. Ing. Quim. 2020;19:263–274. doi: 10.24275/rmiq/Bio544. [DOI] [Google Scholar]
- 71.Dilarri G., Mendes C.R., Martins A.O. Síntese de biofilmes de quitosana reticulados com tripolifosfato atuando como agente quelante na fixação de nanopartículas de prata. Cienc. Eng. 2016;25:97–103. doi: 10.14393/19834071.2016.27704. [DOI] [Google Scholar]
- 72.Kidibule P.E., Santos-Moriano P., Jiménez-Ortega E., Ramírez-Escudero M., Limón M.C., Ramavha M., Plou F.J., Sanz-Aparicio J., Fernández-Lobato M. Use of chitin and chtosan to produce new chitooligosaccharides by chitinase Chit42: Enzymatic activity and strutural basis of protein specificity. Microb. Cell. Fact. 2018;17:1–13. doi: 10.1186/s12934-018-0895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barbosa P.F.P., Cumba L.R., Andrade R.D.A., Carmo D.R. Chemical modifications of cyclodextrin and chitosan for biological and environmental applications: Metals and organic pollutants adsorption and removal. J. Polym. Environ. 2019;27:1352–1366. doi: 10.1007/s10924-019-01434-x. [DOI] [Google Scholar]
- 74.Younes I., Frachet V., Rinaudo M., Jellouli K., Nasri M. Cytotoxicity of chitosans with different acetylation degrees and molecular weights on bladder carcinoma cells. Int. J. Biol. Macromol. 2016;84:200–207. doi: 10.1016/j.ijbiomac.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 75.Baroudi A., García-Payo C., Khayet M. Structural, mechanical, and transport properties of electron beam-irradiated chitosan membranes at different doses. Polymers. 2018;10:117. doi: 10.3390/polym10020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopes S.A., Veiga I.G., Moraes A.M. Desenvolvimento de dispositivo de quitosana e xantana para a liberação tópica ou em tecidos moles de indometacina. Bluch. Chem. Eng. Proc. 2015;1:13205–13212. [Google Scholar]
- 77.Monteiro A.A.S., Richter A.R., Maciel J.S., Feitosa J.P.A., Paula H.C.B., Monteiro-Paula R.C. Effect of chemical modification on the solubility and swelling of microspheres based on carboxymethyl cashew gum and chitosan. Polímeros. 2015;25:31–39. doi: 10.1590/0104-1428.1779. [DOI] [Google Scholar]
- 78.He X., Xing R., Liu S., Qin Y., Li K., Yu H., Li P. The improved antiviral activities of amino-modified chitosan derivatives on Newcastle virus. Drug Chem. Toxicol. 2019;1:1–6. doi: 10.1080/01480545.2019.1620264. [DOI] [PubMed] [Google Scholar]
- 79.Rolim A.E.H., Carvalho F.A., Costa R.C.C., Rosa F.P. Arcabouços de quitosana-propriedades físico-químicas e biológicas para o reparo ósseo. Rev. Virtual. Quim. 2018:10. [Google Scholar]
- 80.Heidari F., Razavi M., Bahrololoom M.E., Tahriri M., Rasoulianboroujeni M., Koturi H., Tayeb L. Preparation of natural chitosan from shrimp shell with different deacetylation degree. Mater. Res. Innov. 2018;22:177–181. doi: 10.1080/14328917.2016.1271591. [DOI] [Google Scholar]
- 81.Rajoka M.S.R., Zhao L., Mehwish H.M., Wu Y., Mahmood S. Chitosan and its derivatives: Synthesis, biotechnological applications, and future challenges. Appli. Microbiol. Biotechnol. 2019;103:1557–1571. doi: 10.1007/s00253-018-9550-z. [DOI] [PubMed] [Google Scholar]
- 82.Müller K., Zollfrank C., Schmid M. Natural Polymers from Biomass Resources as Feedstocks for Thermoplastic Materials. Macromol. Mater. Eng. 2019;304:1800760–1800777. doi: 10.1002/mame.201800760. [DOI] [Google Scholar]
- 83.Castel-Molieres M., Conzatti G., Torrisani J., Rouilly A., Cavalie S., Carrere N., Tourrette A. Influence of homogenization technique and blend ratio on chitosan/alginate polyelectrolyte complex properties. J. Med. Biol. Eng. 2018;38:10–21. doi: 10.1007/s40846-017-0304-7. [DOI] [Google Scholar]
- 84.Oliveira P.N., Montembault A., Sudre G., Alcouffe P., Marcon L., Gehan H., Lux F., Albespy K., Centis V., Campos D., et al. Self-crosslinked fibrous collagen/chitosan blends: Processing, properties evaluation and monitoring of degradation by bi-fluorescence imaging. Int. J. Biol. Macromol. 2019;131:353–367. doi: 10.1016/j.ijbiomac.2019.02.134. [DOI] [PubMed] [Google Scholar]
- 85.Kasai D., Chougale R., Masti S., Chalannavar R., Malabadi R., Gani R., Gouripur G. An Investigation into the Influence of Filler Piper nigrum Leaves Extract on Physicochemical and Antimicrobial Properties of Chitosan/Poly (Vinyl Alcohol) Blend Films. J. Polym. Environ. 2019;27:472–488. doi: 10.1007/s10924-018-1353-x. [DOI] [Google Scholar]
- 86.Gopi S., Pius A., Kargl R., Kleinschek K.S., Thomas S. Fabrication of cellulose acetate/chitosan blend films as efficient adsorbent for anionic water pollutants. Polym. Bull. 2019;76:1557–1571. doi: 10.1007/s00289-018-2467-y. [DOI] [Google Scholar]
- 87.Aranaz I., Acosta N., Civera C., Elorza B., Mingo J., Castro C., Gandía M.L.L., Caballero A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers. 2018;10:213. doi: 10.3390/polym10020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thamilarasan V., Sethuraman V., Gopinath K., Balalakshmi C., Govindarajan M., Mothana R.A., Siddiqui N.A., Khaled J.M., Benelli G. Single step fabrication of chitosan nanocrystals using Penaeus semisulcatus: Potential as new insecticides, antimicrobials and plant growth promoters. J. Clust. Sci. 2018;29:375–384. doi: 10.1007/s10876-018-1342-1. [DOI] [Google Scholar]
- 89.Mirzaie A., Hasanzadeh M., Jouyban A. Cross-linked chitosan/thiolated graphene quantum dots as a biocompatible polysaccharide towards aptamer immobilization. Int. J. Biol. Macromol. 2019;123:1091–1105. doi: 10.1016/j.ijbiomac.2018.11.139. [DOI] [PubMed] [Google Scholar]
- 90.Kumaraswamy R.V., Kumari S., Choudhary R.C., Pal A., Raliya R., Biswas P., Saharan V. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018;113:494–506. doi: 10.1016/j.ijbiomac.2018.02.130. [DOI] [PubMed] [Google Scholar]
- 91.Yang J., Wang Y., Li M., Wu H., Zhen T., Xiong L., Sun Q. pH-Sensitive Chitosan–Sodium Phytate Core–Shell Hollow Beads and Nanocapsules for the Encapsulation of Active Ingredients. J. Agric. Food Chem. 2019;67:2894–2905. doi: 10.1021/acs.jafc.8b03919. [DOI] [PubMed] [Google Scholar]
- 92.Morganti P., Coltelli M.B. A New Carrier for Advanced Cosmeceuticals. Cosmetics. 2019;6:10. doi: 10.3390/cosmetics6010010. [DOI] [Google Scholar]
- 93.Pascoli M., Lopes-Oliveira P.J., Fraceto L.F., Seabra A.B., Oliveira H.C. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energ. Ecol. Environ. 2018;3:137–148. doi: 10.1007/s40974-018-0090-2. [DOI] [Google Scholar]
- 94.Campos E.V.R., Proença P.L.F., Oliveira J.L., Melville C.C., Vechia J.F.D., Andrade D.J., Fraceto L.F. Chitosan nanoparticles functionalized with β-cyclodextrin: A promising carrier for botanical pesticides. Sci. Rep. 2018;8:2067–2082. doi: 10.1038/s41598-018-20602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos K.O., Barbosa R.C., Buriti J.S., Bezerra Junior A.G., Sousa W.J.B., Barros S.M.C., Oliveira R.J., Fook M.V.L. Thermal, chemical, biological and mechanical properties of chitosan films with powder of eggshell membrane for biomedical applications. J. Therm. Anal. Calorim. 2018;136:725–735. doi: 10.1007/s10973-018-7666-0. [DOI] [Google Scholar]
- 96.Ahmed S., Annu, Ali A., Sheikh J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018;116:849–862. doi: 10.1016/j.ijbiomac.2018.04.176. [DOI] [PubMed] [Google Scholar]
- 97.Soundarya S.P., Menon A.H., Chandran S.V., Selvamurugan N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018;119:1228–1239. doi: 10.1016/j.ijbiomac.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 98.Baranwal A., Kumar A., Priyadharshini A., Oggu G.S., Bhatnagar I., Srivastava A., Chandra P. Chitosan: An undisputed bio-fabrication material for tissue engineering and bio-sensing applications. Int. J. Biol. Macromol. 2018;110:110–123. doi: 10.1016/j.ijbiomac.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 99.Ways M., Lau W.M., Khutoryanskiy V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 2018;10:267. doi: 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahsan S.M., Thomas M., Reddy K.K., Sooraparaju S.G., Asthana A., Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- 101.Heise K., Hobisch M., Sacarescu L., Maver U.J., Hobisch J., Reichelt T., Sega M., Fischer S., Spirk S. Low-molecular-weight sulfonated chitosan as template for anticoagulant nanoparticles. Int. J. Nanomed. 2018;13:4881–4894. doi: 10.2147/IJN.S172230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhong Z., Ji X., Xing R., Liu S., Guo Z., Chen X., Li P. The preparation and antioxidant activity of the sulfanilamide derivatives of chitosan and chitosan sulphates. Bioorg. Med. Chem. 2007;15:3775–3782. doi: 10.1016/j.bmc.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 103.Vikhoreva G., Bannikova G., Stolbushkina P., Panov A., Drozd N., Makarov V., Gal’braikh L. Preparation and anticoagulant activity of a low molecular weight sulfated chitosan. Carbohydr. Polym. 2005;62:327–332. doi: 10.1016/j.carbpol.2005.05.022. [DOI] [Google Scholar]
- 104.Desai U.R. New antithrombin-based anticoagulants. Med. Res. Rev. 2004;24:151–181. doi: 10.1002/med.10058. [DOI] [PubMed] [Google Scholar]
- 105.Drozd N.N., Sher A.I., Makarov V.A., Vikhoreva G.A., Gorbachiova I.N., Galbraich L.S. Comparison of antithrombin activity of the polysulphate chitosan derivatives in vitro and in vivo system. Thromb. Res. 2001;102:445–455. doi: 10.1016/S0049-3848(01)00266-3. [DOI] [PubMed] [Google Scholar]
- 106.Imran M., Sajwan M., Alsuwayt B., Asife M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm. J. 2020;28:25–32. doi: 10.1016/j.jsps.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Subhapradha N., Suman S., Ramasamy P., Saravanan R., Shanmugam V., Srinivasan A., Shanmugam A. Anticoagulant and antioxidant activity of sulfated chitosan from the shell of donacid clam Donax scortum (Linnaeus, 1758) IJNPND. 2013;3:39–45. doi: 10.4103/2231-0738.106990. [DOI] [Google Scholar]
- 108.Raafat D., Sahl H.-G. Chitosan and its antimicrobial potential–a critical literature survey. Microb. Biotechnol. 2009;33:186–201. doi: 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perinelli D.R., Fagioli L., Campana R., Lam J.K.W., Baffone W., Palmieri G.F., Casettari L., Bonacucina G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. 2018;117:8–20. doi: 10.1016/j.ejps.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 110.Divya K., Jisha M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018;16:101–112. doi: 10.1007/s10311-017-0670-y. [DOI] [Google Scholar]
- 111.Zheng L.Y., Zhu J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohyd. Polym. 2003;54:527–530. doi: 10.1016/j.carbpol.2003.07.009. [DOI] [Google Scholar]
- 112.Yang N.J., Hinner M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. Methods Mol. Biol. 2015;1266:29–53. doi: 10.1007/978-1-4939-2272-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Younes I., Rinaudo M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Marine Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sudarshan N.R., Hoover D.G., Knorr D. Antibacterial action of chitosan. Food Biotechnol. 1992;6:257–272. doi: 10.1080/08905439209549838. [DOI] [Google Scholar]
- 115.Gomes L.P., Anjo S.I., Manadas B., Coelho A.V., Paschoalin V.M.F. Proteomic analyses reveal new insights on the antimicrobial mechanisms of chitosan biopolymers and their nanosized particles against Escherichia coli. Int. J. Mol. Sci. 2020;21:225. doi: 10.3390/ijms21010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khaneghah A.M., Hashemi S.M.B., Limbo S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018;111:1–19. doi: 10.1016/j.fbp.2018.05.001. [DOI] [Google Scholar]
- 117.Mujtaba M., Morsi R.E., Kerch G., Elsabee M.Z., Kaya M., Labidi M.K.J., Khawar K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019;121:889–904. doi: 10.1016/j.ijbiomac.2018.10.109. [DOI] [PubMed] [Google Scholar]
- 118.Ferreira N., Olievira C.M., Pinhero A.S.B., Nunes J.R.S., Castrillon S.K.I. Escassez hídrica: Estudo de caso em uma comunidade rural do pantanal mato-grossense. Rev. Ibero-Am. Ciênc. Ambient. 2018;9:88–102. doi: 10.6008/CBPC2179-6858.2018.001.0007. [DOI] [Google Scholar]
- 119.Vidal R.R.L., Moraes J.S. Removal of organic pollutants from wastewater using chitosan: A literature review. Int. J. Environ. Sci. Technol. 2019;16:1741–1754. doi: 10.1007/s13762-018-2061-8. [DOI] [Google Scholar]
- 120.Richards S., Dawson J., Stutter M. The potential use of natural vs commercial biosorbent material to remediate stream waters by removing heavy metal contaminants. J. Environ. Manage. 2019;231:275–281. doi: 10.1016/j.jenvman.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 121.Al-Manhel A.J., Al-Hilphy A.R.S., Niamah A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saud. Soc. Agric. Sci. 2018;17:186–190. doi: 10.1016/j.jssas.2016.04.001. [DOI] [Google Scholar]
- 122.Jawad R.J., Ismail M.H.S., Siajam S.I. Adsorption of heavy metals and residual oil from palm oil mill effluent using novel adsorbent of alginate and mangrove composite beads coated by chitosan in a packed bed column. Engineer. J. 2018;19:1–14. doi: 10.31436/iiumej.v19i1.734. [DOI] [Google Scholar]
- 123.Lichtfouse E., Morin-Crini N., Fourmentin M., Zemmouri H., Nascimento I.O.C., Queiroz L.M., Tadza M.Y.M., Pico-Corrales L.A., Pei H., Wilsom L.D., et al. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019;17:1603–1621. doi: 10.1007/s10311-019-00900-1. [DOI] [Google Scholar]
- 124.Bugajska P., Filipkowska U., Jóźwiak T., Kuczajowska-Zadrożna M. The influence of chitosan flake deacetylation degree on orthophosphate sorption efficiency from aqueous solutions. Prog. Chem. Appl. Chitin. Deriv. 2018;23:33–44. doi: 10.15259/PCACD.23.003. [DOI] [Google Scholar]
- 125.Hirano S., Itakura C., Seino H., Akiyama Y., Nonaka I., Kanbara N., Kawakami T. Chitosan as an ingredient for domestic animal feeds. J. Agric. Food Chem. 1990;38:1214–1217. doi: 10.1021/jf00095a012. [DOI] [Google Scholar]
- 126.Li Q., Dunn E.T., Grandmaison E.W., Goosen M.F.A. Applications and properties of chitosan. J. Bioact. Comp. Polym. 1992;7:370–397. doi: 10.1177/088391159200700406. [DOI] [Google Scholar]
- 127.Sagoo S., Board R., Roller S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002;19:175–182. doi: 10.1006/fmic.2001.0474. [DOI] [Google Scholar]
- 128.Chauhan S., Nagaich U., Jain N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019;9:195–204. doi: 10.15171/apb.2019.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Butler B.L., Vergano P.J., Testin R.F., Bunn J.M., Wiles J.L. Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J. Food Sci. 1996;61:953–955, 961. [Google Scholar]
- 130.Nadarajah K., Prinyawiwatkul W., No H.K., Sathivel S., Xu Z. Sorption behavior of crawfish chitosan films as affected by chitosan extraction processes and solvent types. J. Food Sci. 2006;71:E33–E39. doi: 10.1111/j.1365-2621.2006.tb08894.x. [DOI] [Google Scholar]
- 131.Campos-Takaki G.M., Dietrich S.M.C. Characterization of cell walls from Mucoralean fungi: Biochemical composition, Electron microscopy and X--Ray microanalysis. In: Mendez-vilas A., editor. Current Research Topics in Applied Microbiology and Microbial Biotechnology. World Scientific Publishing; Toh Tuck Link, Singapore: 2009. pp. 121–125. [Google Scholar]
- 132.Berger L.R.R., Stamford T.C.M., Stamford-Arnaud T.M., de Alcântara S.R.C., da Silva A.C., da Silva A.M., Nascimento A.E., Campos-Takaki G.M. Green Conversion of Agroindustrial Wastes into Chitin and Chitosan by Rhizopus arrhizus and Cunninghamella elegans Strains. Int. J. Mol. Sci. 2014;15:9082–9102. doi: 10.3390/ijms15059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vilar Junior J.C., Ribeaux D.R., Alves da Silva C.A., Campos-Takaki G.M. Physicochemical and Antibacterial Properties of Chitosan Extracted from Waste Shrimp Shells. Inter. J. Microbiol. 2018;2016:1–7. doi: 10.1155/2016/5127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tyliszczak B., Drabczyk A., Kudłacik-Kramarczyk S., Sobczak-Kupiec A. Sustainable Production of Chitosan. In: Królczyk G., Wzorek M., Król A., Kochan O., Su J., Kacprzyk J., editors. Sustainable Production: Novel Trends in Energy, Environment and Material Systems. Studies in Systems, Decision and Control. Vol. 198. Springer; Cham, NY, USA: 2020. pp. 45–60. [Google Scholar]
- 135.Liua Y., Yuana Y., Duan S., Liu C., Hu B., Liu A., Wu D., Cui H., Lin L., He J. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020;155:249–259. doi: 10.1016/j.ijbiomac.2020.03.217. [DOI] [PubMed] [Google Scholar]
- 136.Zakaria S., Chia C.H., Wan Ahmad W.H., Kaco H., Chook S.W., Chan C.H. Mechanical and Antibacterial Properties of Paper Coated with Chitosan. Sains Malaysiana. 2015;44:905–911. doi: 10.17576/jsm-2015-4406-18. [DOI] [Google Scholar]
- 137.Yang W., Owczarek J.S., Fortunati E., Kozanecki M., Mazzaglia A., Balestra G.M., Kenny J.M., Torre L., Puglia D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016;94:800–811. doi: 10.1016/j.indcrop.2016.09.061. [DOI] [Google Scholar]