This meta-analysis of randomized clinical trials evaluates the association between different central noninvasive brain stimulation therapies and efficacy and acceptability for treatment of patients with tinnitus.
Key Points
Question
Which noninvasive brain stimulation treatment was associated with the best efficacy and acceptability in tinnitus management?
Findings
In this meta-analysis of 32 unique studies including 1458 unique participants, the cathodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex combined with transcranial random noise stimulation over the bilateral auditory cortex was associated with the greatest improvement in both tinnitus severity and quality of life. Continuous theta-burst stimulation over both auditory cortices ranked more favorably than that over the left auditory cortex only.
Meaning
Regarding the efficacy and acceptability for tinnitus treatment, these findings suggest that the cathodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex combined with transcranial random noise stimulation over the bilateral auditory cortex is preferable.
Abstract
Importance
Tinnitus has a prevalence of 10% to 25% and is frequently associated with numerous complications, such as neuropsychiatric disease. Traditional treatments have failed to meet the needs of patients with tinnitus. Noninvasive brain stimulation (NIBS) can focally modify cortical functioning and has been proposed as a strategy for reducing tinnitus severity. However, the results have been inconclusive.
Objective
To evaluate the association between different central NIBS therapies and efficacy and acceptability for treatment of tinnitus.
Data Sources
ClinicalKey, Cochrane CENTRAL, Embase, ProQuest, PubMed, ScienceDirect, and Web of Science databases were searched from inception to August 4, 2019. No language restriction was applied. Manual searches were performed for potentially eligible articles selected from the reference lists of review articles and pairwise meta-analyses.
Study Selection
Randomized clinical trials (RCTs) examining the central NIBS method used in patients with unilateral or bilateral tinnitus were included in the current network meta-analysis. The central NIBS method was compared with sham, waiting list, or active controls. Studies that were not clinical trials or RCTs and did not report the outcome of interest were excluded.
Data Extraction and Synthesis
Two authors independently screened the studies, extracted the relevant information, and evaluated the risk of bias in the included studies. In cases of discrepancy, a third author became involved. If manuscript data were not available, the corresponding authors or coauthors were approached to obtain the original data. This network meta-analysis was based on the frequentist model.
Main Outcomes and Measures
The primary outcome was change in the severity of tinnitus. Secondary outcomes were changes in quality of life and the response rate related to the NIBS method in patients with tinnitus.
Results
Overall, 32 unique RCTs were included with 1458 unique participants (mean female proportion, 34.4% [range, 0%-81.2%]; mean age, 49.6 [range, 40.0-62.8] years; median age, 49.8 [interquartile range, 48.1-52.4] years). The results of the network meta-analysis revealed that cathodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex combined with transcranial random noise stimulation over the bilateral auditory cortex was associated with the greatest improvement in tinnitus severity (standardized mean difference [SMD], –1.89; 95% CI, –3.00 to –0.78) and quality of life (SMD, –1.24; 95% CI, –2.02 to –0.45) compared with the controls. Improvement in tinnitus severity ranked more favorably for continuous theta-burst stimulation (cTBS) over both auditory cortices (SMD, −0.79; 95% CI = −1.57 to −0.01) than cTBS over only the left auditory cortex (SMD, −0.30; 95% CI, −0.87 to 0.28), compared with controls. Repetitive transcranial magnetic stimulation with priming had a superior beneficial association with tinnitus severity compared with the strategies without priming. None of the investigated NIBS types had a significantly different dropout rate compared with that of the control group.
Conclusions and Relevance
This network meta-analysis suggests a potential role of NIBS interventions in tinnitus management. Future large-scale RCTs focusing on longer follow-up and different priming procedure NIBS are warranted to confirm these findings.
Introduction
In the adult population, tinnitus has a prevalence of 10% to 25%,1,2 and 6% to 25% of these persons suggest that these symptoms are severely debilitating and have an adverse effect on quality of life.3 Tinnitus is recognized as a difficult disease to identify and manage because of controversy regarding its definition and treatment. Tinnitus usually results from heterogeneous causes: (1) the auditory system (usually peripheral, rarely central), (2) the somatosensory system (head and neck), or (3) a combination of these. The condition results when the interactivity of the auditory and somatosensory systems exceeds the individual’s tinnitus threshold. Treatments to reduce tinnitus severity and tinnitus-related distress include cognitive behavioral therapy, acoustic stimulation, and educational counseling. Although somatic treatments can be effective in cases of tinnitus with a specific origin (eg, palatal myoclonus, deafferentation of the auditory system, loss of cochlear hair cells, and ototoxic drugs), no specific interventions have been proven to be effective in treating tinnitus without a specific cause.2
With the help of functional brain imaging studies, abnormal hyperactivity has been detected in the whole brain area, especially in both auditory cortices,4 the anterior cingulate cortex,5 and the insula.5 Therefore, suppression of abnormal brain hyperactivity through central noninvasive brain stimulation (NIBS) has been proposed as a tinnitus management strategy.6 The central NIBS techniques include repetitive transcranial magnetic stimulation (rTMS),7 the rTMS variant theta-burst stimulation (TBS),8,9 and transcranial electrical stimulation, such as transcranial direct current stimulation (tDCS)10 and transcranial random noise stimulation (tRNS).11 Based on the frequency applied, rTMS can induce different changes in brain activity in patients with tinnitus. For example, high-frequency rTMS induces higher brain activity, whereas low-frequency rTMS suppresses hyperactivity in the cerebral cortex.12 Transcranial DCS, in which a weak direct electrical current is passed through the brain cortex, has a similar suppressing or enhancing effect on brain activity. Similarly, tDCS has been found to have a modulating effect on the stimulated brain cortex, although the suppressing or enhancing effect due to the polarity of tDCS is still debated.8,13 The suppression of hyperactivity in the temporoparietal cortex (ie, the primary or secondary auditory cortex) through the use of rTMS or tDCS was considered a reasonable NIBS method in the management of tinnitus.14
Pairwise meta-analyses have indicated significant efficacy of tDCS15 and rTMS16 vs sham treatment in improving the severity of tinnitus. However, these meta-analyses were based on only 2 to 4 randomized clinical trials (RCTs), resulting in poor evidence of efficacy. In addition, different combination protocols of NIBS interventions have been developed for tinnitus management, including different brain stimulation targets and stimulation parameters. Specifically, additive stimulation over the prefrontal cortex (often dorsolateral prefrontal cortex [DLPFC]), also termed the priming procedure, has been reported to have an enhancing effect on tinnitus management.7,17 However, the most recent meta-analyses have not provided conclusive results relevant to clinical practice.15,16,18 In addition, traditional pairwise meta-analyses cannot provide further information about the relative efficacy of interventions that have not been directly compared in head-to-head trials, which is an essential aspect when judging the therapeutic value of an intervention. Considering these issues, we conducted a network meta-analysis of the currently published RCTs to estimate the association between different central NIBS interventions and relative efficacy and acceptability in patients with tinnitus.19
Methods
The present study did not receive any ethics committee approval or informed consent from the participants because we did not approach any specific participants or report any detailed information of specific participants. Our previous project was approved by the institutional review board of the Tri-Service General Hospital, National Defense Medical Center. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (eTable 1 in the Supplement).
Detailed information regarding the methods and materials is presented in the eMethods in the Supplement. We searched the ClinicalKey, Cochrane CENTRAL, Embase, ProQuest, PubMed, ScienceDirect, and Web of Science databases from inception to August 4, 2019. No language restriction was applied. Manual searches were performed for potentially eligible articles selected from the reference lists of review articles and pairwise meta-analyses. We included RCTs with sham-controlled, waiting list–controlled, or active-controlled design conducted in patients with tinnitus. The detailed categorization of the targets of comparison arms were listed in the node definition section of the eMethods in the Supplement.
Following the flowchart used in previous network meta-analyses,20,21,22,23,24,25,26 we extracted the relevant information from the RCTs and evaluated the risk of bias in the included studies. Two authors (J.J.C. and B.S.Z.) independently screened the studies, extracted the relevant information from the manuscripts, and evaluated the risk of bias in the included studies. In cases of discrepancy, a third author (P.T.T.) became involved. If manuscript data were not available, the corresponding authors or coauthors were approached to obtain the original data. We only extracted data on central NIBS and not peripheral stimulation.
The primary outcome was change in the severity of tinnitus after NIBS in patients with tinnitus, which could be rated using a different tinnitus questionnaire (described in the Results section) (outcomes in eMethods in the Supplement). The secondary outcomes were change in quality of life and response rate related to the NIBS method in patients with tinnitus. The detailed definition of response rate and quality of life had been presented in the outcomes in eMethods in the Supplement. Finally, the safety profile was calculated using the dropout rate, which was defined as the percentage of patients leaving the study before its conclusion for any reason.
The risk of bias was evaluated according to the Cochrane risk-of-bias tool.27 The current network meta-analysis was conducted under the frequentist model and generalized linear mixed models to make direct and indirect comparisons.28 In our analysis, the mvmeta command was applied in the STATA program, version 14.0.29 We estimated the standardized mean difference (SMD) with 95% CI for continuous variables (ie, the primary outcome of tinnitus severity and the secondary outcome of quality of life). We evaluated categorical values with the rate ratio and 95% CI (ie, the secondary outcome of response and safety of dropout) and applied a 0.5 zero-cell correction during the meta-analysis procedure. Heterogeneity among the included studies was evaluated using the τ value, which is the estimated SD of the association across the included studies.
To provide additional information for clinical applications, we calculated the surface under the cumulative ranking curve (SUCRA), which indicates the relative ranking probabilities of the treatment effects for the target outcomes.30 We conducted meta-regression to determine the associations between change in tinnitus severity and participant characteristics, such as mean age and the sex distribution. Finally, we evaluated the potential inconsistencies between the direct and indirect evidence within the network by using the loop-specific approach and identified local inconsistencies by using the node-splitting method. The design-by-treatment model was used to evaluate global inconsistencies across the entire network meta-analysis.31
Results
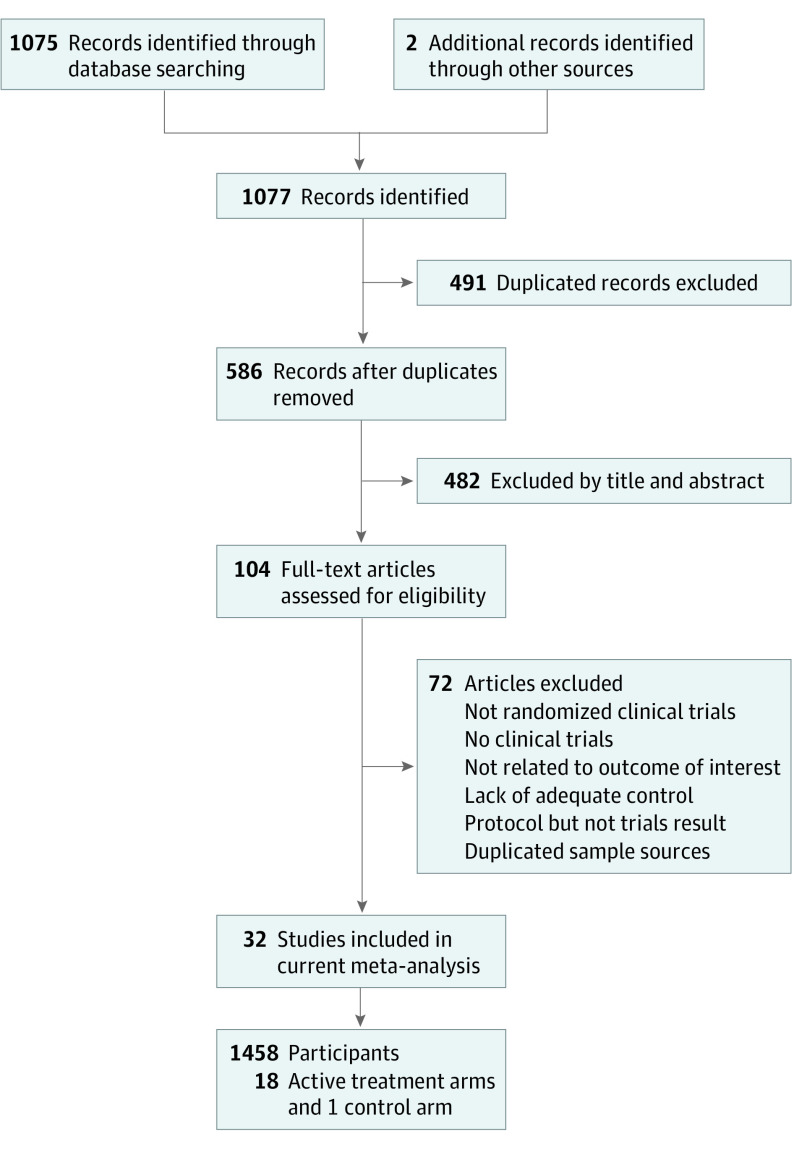
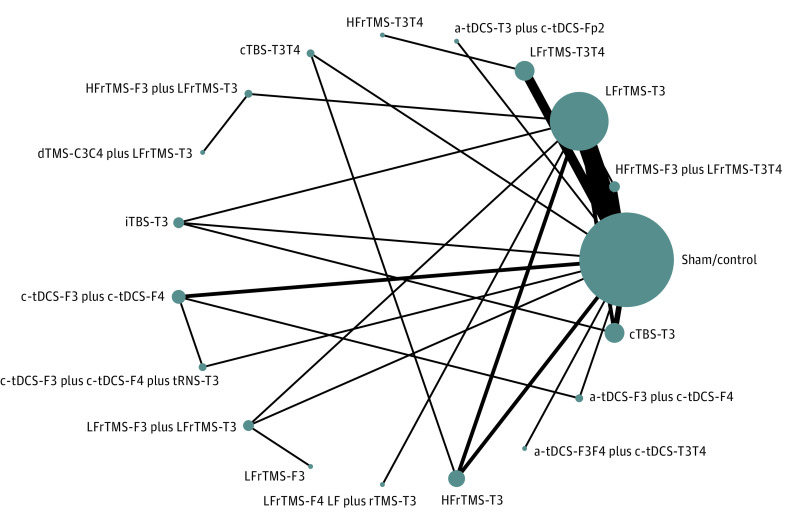
After the initial screening procedure, 104 articles were considered for full-text review (Figure 1). However, 72 were excluded for various reasons (eTable 2 in the Supplement). Finally, 32 articles7,8,9,10,11,17,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 were included in the current study (eTable 3 in the Supplement). Figure 2 depicts the entire geometric distribution of the treatment arms. The detailed categorization of the treatment arms is provided in eTable 4 in the Supplement.
Figure 1. Flowchart of the Present Network Meta-analysis.
Figure 2. The Network Structure of Changes of Severity of Tinnitus.
The lines between nodes represent direct comparisons in various trials, and the size of each circle is proportional to the size of the population involved in each specific treatment. The thickness of the lines is proportional to the number of trials connected to the network. a-tDCS indicates anodal transcranial direct current stimulation; cTBS, continuous theta-burst stimulation; c-tDCS, cathodal tDCS; dTMS, deep transcranial magnetic stimulation; F3, left dorsolateral prefrontal cortex (DLPFC); F4, right DLPFC; Fp2, right supraorbital area; HF, high-frequency; iTBS, intermittent TBS; LF, low-frequency; rTMS, repetitive TMS; T3, left auditory cortex; T4, right auditory cortex; and tRNS, transcranial random noise stimulation.
Characteristics of the Included Studies
A total of 1458 participants were included. The mean age of the participants was 49.6 years (range, 40.0-62.8 years; median, 49.8 [interquartile range (IQR), 48.1-52.4] years); the mean female proportion was 34.4% (range, 0%-81.2%; median, 30.3% [IQR, 24.7%-40.9%]), and the mean male proportion was 65.6% (range, 18.8%-100%; median, 69.7% [IQR, 59.1%-75.3%]). The mean duration of central NIBS treatment was 16.9 weeks (range, 2-54 weeks; median, 12 [IQR, 4-24] weeks). The baseline characteristics of the included participants are summarized in eTable 3 in the Supplement. The definition of response varied among the recruited studies as patient self-rated global impression, Tinnitus Handicap Inventory score reduction of greater than 7 or 10 points, Tinnitus Handicap Inventory score reduction of greater than 20% of the baseline score, and Tinnitus Questionnaire score reduction of greater than 5 or 10 points.
Primary Outcome: Change in Tinnitus Severity
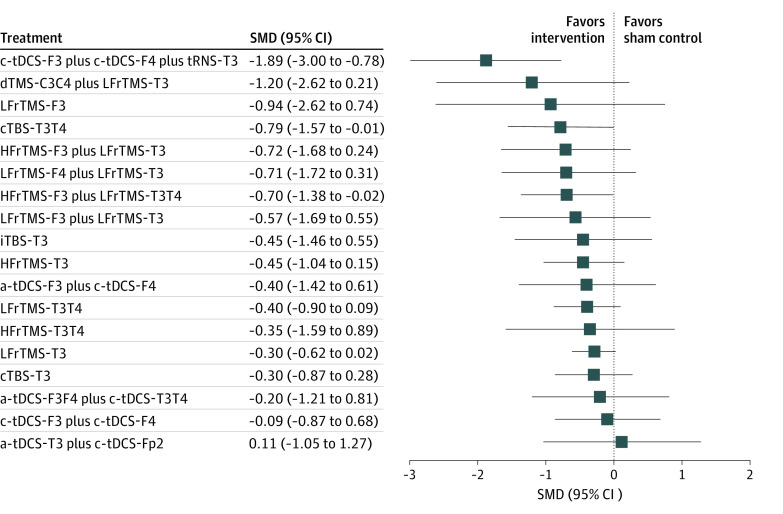
The network meta-analysis revealed that only the cathodal tDCS over the left DLPFC (F3) plus anodal tDCS over the right DLPFC (F4) plus tRNS of the left auditory cortex (T3) combination (SMD, −1.89 [95% CI, −3.00 to −0.78]), continuous TBS (cTBS) over the bilateral auditory cortices (T3T4) (SMD, −0.79 [95% CI, −1.57 to −0.01]), and high-frequency rTMS-F3 plus low-frequency rTMS-T3T4 combination (SMD, −0.70 [95% CI, −1.38 to −0.02]) were associated with significant improvement in the severity of tinnitus compared with the control (eTable 5 in the Supplement and Figure 3). The associations between an NIBS method and the change in tinnitus severity were ranked according to the SUCRA, where lower values indicate superior outcomes of tinnitus severity. In brief, the combination of cathodal tDCS-F3 plus anodal tDCS-F4 plus tRNS-T3 was associated with the largest improvement, followed by deep TMS bilateral medial frontal cortex plus low-frequency rTMS-T3 and cTBS-T3T4. In addition, rTMS with a priming procedure (ie, rTMS over the frontal lobe followed by rTMS over the auditory cortex, such as high-frequency rTMS-F3 plus low-frequency rTMS-T3T4 (SUCRA, 36.6), high-frequency rTMS-F3 plus low-frequency rTMS-T3 (SUCRA, 38.8), low-frequency rTMS-F3 plus low-frequency rTMS-T3 (SUCRA, 48.3), and low-frequency rTMS-F4 plus low-frequency rTMS-T3 (SUCRA, 38.1) was ranked more highly than rTMS without priming (ie, rTMS over the auditory cortex alone, such as low-frequency rTMS-T3 (SUCRA, 62.9), low-frequency rTMS-T3T4 (SUCRA, 54.0), and high-frequency rTMS-T3T4; SUCRA, 56.5). Finally, bilateral cTBS (ie, cTBS-T3T4; SUCRA, 32.7) was ranked more highly than unilateral cTBS (ie, cTBS-T3; SUCRA, 64.1) and intermittent TBS (iTBS) (ie, iTBS-T3; SUCRA, 53.4) (eTable 6A in the Supplement). A meta-regression using the restricted maximum likelihood estimator was performed to examine the potential association of age and sex with the change in tinnitus severity. The results reveal a nonsignificant association with the change in tinnitus severity when using the moderating variables age and sex (eTable 7A in the Supplement).
Figure 3. Forest Plot of the Changes of Severity of Tinnitus.
When the effect size is less than zero, it indicated the specified treatment was associated with higher improvement in severity of tinnitus than controls. a-tDCS indicates anodal transcranial direct current stimulation; cTBS, continuous theta-burst stimulation; c-tDCS, cathodal tDCS; dTMS, deep transcranial magnetic stimulation; F3, over the left dorsolateral prefrontal cortex (DLPFC); F4, over the right DLPFC; F3F4, over the bilateral DLPFC; Fp2, over the right supraorbital area; HF, high-frequency; iTBS, intermittent TBS; LF, low-frequency; rTMS, repetitive TMS; SMD, standardized mean difference; T3, over the left auditory cortex; T4, over the right auditory cortex; T3T4, over the bilateral auditory cortices; and tRNS, transcranial random noise stimulation.
Secondary Outcomes
Change in Quality of Life
The network meta-analysis revealed that the cathodal tDCS-F3 plus anodal tDCS-F4 plus tRNS-T3 combination (SMD, −1.24 [95% CI, −2.02 to −0.45]), low-frequency rTMS-T3T4 (SMD, −0.52 [95% CI, −0.83 to −0.20]), and high-frequency rTMS-T3 (SMD, −0.49 [95% CI, −0.93 to −0.04]) were associated with significant improvements in the quality of life of patients with tinnitus in comparison to the sham control (eTable 8A, eFigure 1A, and eFigure 2A in the Supplement). The associations between NIBS method and change in quality of life were ranked according to the SUCRA. In brief, the combination cathodal tDCS-F3 plus anodal tDCS-F4 plus tRNS-T3 was associated with the largest improvement in quality of life (SUCRA, 5.0), followed by cTBS-T3 (SUCRA, 28.8) and low-frequency rTMS-T3T4 (SUCRA, 33.4) (eTable 6B in the Supplement). The results of this meta-regression revealed a nonsignificant association with change in quality of life when using the moderating variables age and sex (eTable 7B in the Supplement).
Response Rate
The network meta-analysis revealed that none of the investigated NIBS methods were associated with significantly better response rates than the sham control (eTable 8B, eFigure 1B, and eFigure 2B in the Supplement). The associations between the NIBS methods and change in quality of life were ranked according to the SUCRA. In brief, high-frequency rTMS-T3 was associated with the highest response rate (SUCRA, 27.5) (eTable 6C in the Supplement). The results of this meta-regression revealed a nonsignificant association with the response rate when using the moderating variables age and sex (eTable 7C in the Supplement).
Safety Profile: Tolerability Reflected by Dropout Rate
In the network meta-analysis, none of the investigated NIBSs were associated with significantly different dropout rates when compared with the sham control (eTable 6D, eTable 8C, eFigure 1C, and eFigure 2C in the Supplement). The results of this meta-regression reveal a nonsignificant association with the dropout rate when using the moderating variables age and sex (eTable 7D in the Supplement).
Risk of Bias and Publication Bias
Among the included studies, we found that 134 of 224 items (59.8%) had a low risk of bias; 69 of 224 items (30.8%), an unclear risk of bias; and 21 of 224 items (9.4%), a high risk of bias. Unclear reporting of the allocation procedure or blinding of the studies further contributed to the risk of bias (eFigure 3A-B in the Supplement).
Funnel plots of the publication bias (eFigure 4A-H in the Supplement) revealed general symmetry, and the results of the Egger test indicated no significant publication bias among the articles included in the network meta-analysis. In general, the analysis did not demonstrate inconsistencies in terms of local inconsistencies, as assessed using the loop-specific approach and node-splitting method, or global inconsistencies, as determined using the design-by-treatment method except for the situation mentioned below. Overall inconsistencies were detected in the outcomes of severity of tinnitus and quality of life (eTables 9 and 10 in the Supplement).
Discussion
To our knowledge, the current study is the first comprehensive network meta-analysis performed to investigate the association between central NIBS interventions and efficacy and acceptability in patients with tinnitus. Evidence from this network meta-analysis revealed that cathodal tDCS-F3 plus anodal tDCS-F4 plus tRNS-T3 was associated with significantly greater improvement in both tinnitus severity and quality of life than the sham control and the largest improvement in both tinnitus severity and quality of life. In addition, cTBS-T3T4 was ranked more highly than cTBS-T3 or iTBS. Repetitive TMS with a priming procedure may be better at improving the severity of tinnitus. Noninvasive brain stimulation using high-frequency rTMS as the priming intervention (ie, high-frequency rTMS-F3 plus low-frequency rTMS-T3T4) was associated with significantly greater improvement in tinnitus severity than the sham control. Finally, most of the investigated NIBS methods were suggested by the dropout rate to be well tolerated.
The first main finding of this study was that the cathodal tDCS-F3 plus anodal tDCS-F4 plus tRNS-T3 combination was associated with significantly greater improvement in both tinnitus severity and quality of life than the sham control and was also associated with the greatest improvement in both the severity of tinnitus and quality of life. The cathodal tDCS-F3 plus anodal tDCS-F4 plus tRNS-T3 combination involved cathodal tDCS-F3 and tRNS-T3. Transcranial RNS is a modification of transcranial alternating current stimulation with random oscillations (ranging from 0.1-640.0 Hz).58 In 1 RCT,10 the effect of cathodal tDCS-F3 on tinnitus intensity was demonstrated. However, in a head-to-head trial,59 tRNS was proven to be superior to tDCS in suppressing tinnitus intensity and decreasing distress after a single session. Furthermore, the findings of the current network meta-analysis supported the superiority of continuous sessions of a combination of cathodal tDCS-F3, anodal tDCS-F4, and tRNS-T3 for tinnitus intensity. Based on the findings of hyperactivity detected in both auditory cortices,4 the anterior cingulate cortex,5 and the insula5 in functional brain imaging studies (ie, magnetoencephalography, functional magnetic resonance imaging, or brain positron emission tomography) for patients with tinnitus, the rationale of a combination of stimulation types over these sites is a reasonable strategy.11 Furthermore, the hypothesis of the preconditioning phenomenon can be a valid explanation of the importance of sequence of stimulation.60 According to this hypothesis, the beneficial effect of the second stimulation targeting another region (ie, the auditory cortex) of the tinnitus network is enhanced by the priming stimulation (ie, over the frontal region).61 However, only 1 RCT with waiting list controls has reported the additive effect of tRNS in patients with tinnitus recruited for this network meta-analysis.11 Although significant inconsistency was not detected within the comparison of treatment arms of cathodal tDCS-F3 plus anodal tDCS-F4, cathodal tDCS-F3 plus anodal tDCS-F4 plus tRNS-T3, and controls, which were treatment arms applied in that RCT,11 according to the side-splitting inconsistency model (side-splitting inconsistency model in eTable 7 in the Supplement), the clinicians should pay special attention when applying this result in their clinical practice. Further large-scale RCTs are required to support or refute the results of this study.
Another remarkable finding of the present network meta-analysis was that cTBS-T3T4 resulted in superior outcomes to cTBS-T3 or iTBS-T3 only. Moreover, the potential benefit of cTBS regarding severity of tinnitus has been proven in many trials, both in a single session62,63 and multiple sessions.8,52 Continuous TBS, which is reported to better suppress both pure-tone tinnitus and white noise than tonic TMS,63 had a more powerful effect on tinnitus relief than high-frequency rTMS.8 In addition, according to reports, cTBS was suggested to be able to modulate both the extralemniscal and lemniscal systems, the systems that mainly manage the sensory input to the central nervous system, whereas tonic TMS modulates only the lemniscal system.63,64 Furthermore, another RCT8 demonstrated that bilateral cTBS was more effective than unilateral stimulation. However, because bilateral cTBS (cTBS-T3T4) in patients with tinnitus was reported only in 2 RCTs included in the current network meta-analysis,8,52 future large-scale RCTs are required to support or refute this study’s results.
Finally, the present network meta-analysis identified rTMS with priming procedure as more beneficial to tinnitus severity than such stimulation without priming. Specifically, rTMS in combination with stimulation over the frontal lobe and then over the auditory cortex was superior compared with rTMS over the auditory cortex only, either in high frequency or low frequency. Furthermore, NIBS using high-frequency rTMS as the priming intervention (ie, high-frequency rTMS-F3 plus low-frequency rTMS-T3T4) was associated with significantly greater improvement in tinnitus severity than in the sham control. These findings correspond with the results of clinical brain imaging studies in which patients with tinnitus were demonstrated to have hyperactivity in multiple brain regions, including both the auditory cortex and DLPFC.4,5 In addition, the hypothesis of the preconditioning phenomenon supports multiple site interventions applied in a sequence.60 This hypothesis of the priming effect is also supported by previous clinical reports demonstrating a superior prolonged beneficial effect on tinnitus severity when a priming rTMS protocol was used in such patients for long-term follow-up compared with rTMS without priming, especially for stimulation at higher frequency.7,45,65,66 Therefore, the present network meta-analysis result can be considered as further essential evidence of the preconditioning phenomenon hypothesis. However, because of limited RCTs addressing the potential benefit of a priming procedure, future large-scale RCTs are required to support or refute the results of the current network meta-analysis.
Limitations
Several potential limitations should be considered for this network meta-analysis. First, this analysis may have been underpowered owing to the heterogeneity of the participants (eg, comorbidities, mood disorder, baseline severity of tinnitus, history of tinnitus onset, commercial machine used in each study, and follow-up duration), variety in the definition of response, and variety in tinnitus severity or quality-of-life rating scales. Although meta-regression analyses were performed to reduce the heterogeneity, some differences did exist between the included RCTs, which were attributed to other unknown factors. Second, although most of the RCTs included a sham control in their study design, the blindness of those RCTs may not have been complete because of the limitation of the commercial machine used. Third, given the relatively small number of patients and RCTs, the main results of this network meta-analysis should perhaps be conservatively applied in clinical practice. Specifically, the potential effect of additive tRNS, the priming procedure, and bilateral cTBS should be carefully interpreted because only a few RCTs reported the results of these NIBS methods (1 trial for additive tRNS,11 7 trials for priming procedure,7,17,40,41,44,45,47 and 2 trials for bilateral cTBS8,52). In addition, the relatively small number of patients and RCTs would limit the potential benefit of NIBS interventions in some outcomes. For example, although most of the NIBS interventions were associated with relatively better response than the sham or control group, the variation and CIs ranged widely, which would result in an insignificant outcome (eFigure 2B in the Supplement). Future larger-scale RCTs are warranted to support or refute the result of the present network meta-analysis. Finally, we detected significant inconsistency in some of the outcomes (ie, severity of tinnitus and quality of life). Clinicians should pay attention when applying these results in their clinical practice.
Conclusions
This study showed that the cathodal tDCS-F3 plus anodal tDCS-F4 plus tRNS-T3 combination was associated with the greatest improvement in tinnitus severity and quality of life. A specific central NIBS protocol (ie, bilateral cTBS and priming with high-frequency rTMS or tDCS) was also associated with superior improvement in tinnitus severity. All central NIBS methods had similar tolerability in terms of the dropout rate compared with the sham control. However, because some of the intervention comparisons were based on only a few RCTs, clinicians should select specific treatments with caution and avoid the one-size-fits-all treatment for all clinical conditions.
eMethods. Study Identification and Data Analysis
eTable 1. PRISMA Extension Checklist of Current Meta-analysis
eTable 2. Excluded Studies and Reasons
eTable 3. Characteristics of the Included Studies
eTable 4. Node Definition
eTable 5. League Table of the Improvement of Severity of Tinnitus
eTable 6. SUCRA of Patient Improvement, Response Rate, and Dropout Rate
eTable 7. Meta-regression Results
eTable 8. League Tables of Improvement of Quality of Life, Response Rate, and Dropout Rate
eTable 9. Inconsistency of Different Intervention
eTable 10. Estimated Between-Studies Standard Deviation of Different Outcomes
eFigure 1. Network Structure of NMA of Changes of Quality of Life, Response Rate, and Dropout Rate
eFigure 2. Forest Plots of NMA Outcomes
eFigure 3. Overview of and Detailed Risk of Bias
eFigure 4. Funnel Plots of Outcomes, Response Rate, and Dropout Rate
References
- 1.Bauer CA. Tinnitus. N Engl J Med. 2018;378(13):1224-1231. doi: 10.1056/NEJMcp1506631 [DOI] [PubMed] [Google Scholar]
- 2.Panov F, Kopell BH. Use of cortical stimulation in neuropathic pain, tinnitus, depression, and movement disorders. Neurotherapeutics. 2014;11(3):564-571. doi: 10.1007/s13311-014-0283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27(11):676-682. doi: 10.1016/j.tins.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 4.Weisz N, Müller S, Schlee W, Dohrmann K, Hartmann T, Elbert T. The neural code of auditory phantom perception. J Neurosci. 2007;27(6):1479-1484. doi: 10.1523/JNEUROSCI.3711-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanneste S, Plazier M, der Loo Ev, de Heyning PV, Congedo M, De Ridder D. The neural correlates of tinnitus-related distress. Neuroimage. 2010;52(2):470-480. doi: 10.1016/j.neuroimage.2010.04.029 [DOI] [PubMed] [Google Scholar]
- 6.De Ridder D, De Mulder G, Walsh V, Muggleton N, Sunaert S, Møller A. Magnetic and electrical stimulation of the auditory cortex for intractable tinnitus: case report. J Neurosurg. 2004;100(3):560-564. doi: 10.3171/jns.2004.100.3.0560 [DOI] [PubMed] [Google Scholar]
- 7.Formánek M, Migaľová P, Krulová P, et al. Combined transcranial magnetic stimulation in the treatment of chronic tinnitus. Ann Clin Transl Neurol. 2018;5(7):857-864. doi: 10.1002/acn3.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forogh B, Yazdi-Bahri SM, Ahadi T, Fereshtehnejad SM, Raissi GR. Comparison of two protocols of transcranial magnetic stimulation for treatment of chronic tinnitus: a randomized controlled clinical trial of burst repetitive versus high-frequency repetitive transcranial magnetic stimulation. Neurol Sci. 2014;35(2):227-232. doi: 10.1007/s10072-013-1487-5 [DOI] [PubMed] [Google Scholar]
- 9.Lorenz I, Müller N, Schlee W, Langguth B, Weisz N. Short-term effects of single repetitive TMS sessions on auditory evoked activity in patients with chronic tinnitus. J Neurophysiol. 2010;104(3):1497-1505. doi: 10.1152/jn.00370.2010 [DOI] [PubMed] [Google Scholar]
- 10.Faber M, Vanneste S, Fregni F, De Ridder D. Top down prefrontal affective modulation of tinnitus with multiple sessions of tDCS of dorsolateral prefrontal cortex. Brain Stimul. 2012;5(4):492-498. doi: 10.1016/j.brs.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 11.To WT, Ost J, Hart J Jr, De Ridder D, Vanneste S. The added value of auditory cortex transcranial random noise stimulation (tRNS) after bifrontal transcranial direct current stimulation (tDCS) for tinnitus. J Neural Transm (Vienna). 2017;124(1):79-88. doi: 10.1007/s00702-016-1634-2 [DOI] [PubMed] [Google Scholar]
- 12.Milev RV, Giacobbe P, Kennedy SH, et al. ; CANMAT Depression Work Group . Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4, neurostimulation treatments. Can J Psychiatry. 2016;61(9):561-575. doi: 10.1177/0706743716660033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath JC, Carter O, Forte JD. Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). Front Syst Neurosci. 2014;8:2. doi: 10.3389/fnsys.2014.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoare DJ, Adjamian P, Sereda M. Electrical stimulation of the ear, head, cranial nerve, or cortex for the treatment of tinnitus: a scoping review. Neural Plast. 2016;2016:5130503. doi: 10.1155/2016/5130503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song JJ, Vanneste S, Van de Heyning P, De Ridder D. Transcranial direct current stimulation in tinnitus patients: a systemic review and meta-analysis. ScientificWorldJournal. 2012;2012:427941. doi: 10.1100/2012/427941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soleimani R, Jalali MM, Hasandokht T. Therapeutic impact of repetitive transcranial magnetic stimulation (rTMS) on tinnitus: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2016;273(7):1663-1675. doi: 10.1007/s00405-015-3642-5 [DOI] [PubMed] [Google Scholar]
- 17.Kreuzer PM, Lehner A, Schlee W, et al. Combined rTMS treatment targeting the anterior cingulate and the temporal cortex for the treatment of chronic tinnitus. Sci Rep. 2015;5:18028. doi: 10.1038/srep18028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Z, Liu S, Zheng Y, Phillips JS. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database Syst Rev. 2011;(10):CD007946. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet. 2015;386(9994):628-630. doi: 10.1016/S0140-6736(15)61478-7 [DOI] [PubMed] [Google Scholar]
- 20.Hsieh MT, Tseng PT, Wu YC, et al. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: a network meta-analysis. Obes Rev. 2019;20(6):895-905. doi: 10.1111/obr.12835 [DOI] [PubMed] [Google Scholar]
- 21.Tu YK, Faggion CM Jr. A primer on network meta-analysis for dental research. ISRN Dent. 2012;2012:276520. doi: 10.5402/2012/276520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535. doi: 10.1001/jamapsychiatry.2018.4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng BS, Lin SY, Tu YK, et al. Prevention of postdental procedure bacteremia: a network meta-analysis. J Dent Res. 2019;98(11):1204-1210. doi: 10.1177/0022034519870466 [DOI] [PubMed] [Google Scholar]
- 24.Huang SW, Tsai CY, Tseng CS, et al. Comparative efficacy and safety of new surgical treatments for benign prostatic hyperplasia: systematic review and network meta-analysis. BMJ. 2019;367:5919. doi: 10.1136/bmj.l5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang CP, Tseng PT, Pei-Chen Chang J, Su H, Satyanarayanan SK, Su KP. Melatonergic agents in the prevention of delirium: a network meta-analysis of randomized controlled trials. Sleep Med Rev. 2020;50:101235. doi: 10.1016/j.smrv.2019.101235 [DOI] [PubMed] [Google Scholar]
- 26.Tseng PT, Yang CP, Su KP, et al. The association between melatonin and episodic migraine: a pilot network meta-analysis of randomized controlled trials to compare the prophylactic effects with exogenous melatonin supplementation and pharmacotherapy. J Pineal Res. Published online April 29, 2020. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JGS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2009. [Google Scholar]
- 28.Tu YK. Use of generalized linear mixed models for network meta-analysis. Med Decis Making. 2014;34(7):911-918. doi: 10.1177/0272989X14545789 [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Wang W, Zhang AB, Bai X, Zhang S. Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: A network meta-analysis. Laryngoscope. 2016;126(4):951-955. doi: 10.1002/lary.25688 [DOI] [PubMed] [Google Scholar]
- 30.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. doi: 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Del Giovane C, Chaimani A, Caldwell DM, Salanti G. Evaluating the quality of evidence from a network meta-analysis. Value Health. 2014;17(7):A324. doi: 10.1016/j.jval.2014.08.572 [DOI] [PubMed] [Google Scholar]
- 32.Anders M, Dvorakova J, Rathova L, et al. Efficacy of repetitive transcranial magnetic stimulation for the treatment of refractory chronic tinnitus: a randomized, placebo controlled study. Neuro Endocrinol Lett. 2010;31(2):238-249. [PubMed] [Google Scholar]
- 33.Bilici S, Yigit O, Taskin U, Gor AP, Yilmaz ED. Medium-term results of combined treatment with transcranial magnetic stimulation and antidepressant drug for chronic tinnitus. Eur Arch Otorhinolaryngol. 2015;272(2):337-343. doi: 10.1007/s00405-013-2851-z [DOI] [PubMed] [Google Scholar]
- 34.Chung HK, Tsai CH, Lin YC, et al. Effectiveness of theta-burst repetitive transcranial magnetic stimulation for treating chronic tinnitus. Audiol Neurootol. 2012;17(2):112-120. doi: 10.1159/000330882 [DOI] [PubMed] [Google Scholar]
- 35.Folmer RL, Theodoroff SM, Casiana L, Shi Y, Griest S, Vachhani J. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi: 10.1001/jamaoto.2015.1219 [DOI] [PubMed] [Google Scholar]
- 36.Forogh B, Mirshaki Z, Raissi GR, Shirazi A, Mansoori K, Ahadi T. Repeated sessions of transcranial direct current stimulation for treatment of chronic subjective tinnitus: a pilot randomized controlled trial. Neurol Sci. 2016;37(2):253-259. doi: 10.1007/s10072-015-2393-9 [DOI] [PubMed] [Google Scholar]
- 37.Hoekstra CE, Versnel H, Neggers SF, Niesten ME, van Zanten GA. Bilateral low-frequency repetitive transcranial magnetic stimulation of the auditory cortex in tinnitus patients is not effective: a randomised controlled trial. Audiol Neurootol. 2013;18(6):362-373. doi: 10.1159/000354977 [DOI] [PubMed] [Google Scholar]
- 38.James GA, Thostenson JD, Brown G, et al. Neural activity during attentional conflict predicts reduction in tinnitus perception following rTMS. Brain Stimul. 2017;10(5):934-943. doi: 10.1016/j.brs.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khedr EM, Rothwell JC, Ahmed MA, El-Atar A. Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: comparison of different stimulus frequencies. J Neurol Neurosurg Psychiatry. 2008;79(2):212-215. doi: 10.1136/jnnp.2007.127712 [DOI] [PubMed] [Google Scholar]
- 40.Kreuzer PM, Landgrebe M, Schecklmann M, et al. Can temporal repetitive transcranial magnetic stimulation be enhanced by targeting affective components of tinnitus with frontal rTMS? a randomized controlled pilot trial. Front Syst Neurosci. 2011;5:88. doi: 10.3389/fnsys.2011.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyong JS, Noh TS, Park MK, Oh SH, Lee JH, Suh MW. Phantom perception of sound and the abnormal cortical inhibition system: an electroencephalography (EEG) study. Ann Otol Rhinol Laryngol. 2019;128(6_suppl):84S-95S. doi: 10.1177/0003489419837990 [DOI] [PubMed]
- 42.Landgrebe M, Hajak G, Wolf S, et al. 1-Hz rTMS in the treatment of tinnitus: A sham-controlled, randomized multicenter trial. Brain Stimul. 2017;10(6):1112-1120. doi: 10.1016/j.brs.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 43.Langguth B, Kleinjung T, Frank E, et al. High-frequency priming stimulation does not enhance the effect of low-frequency rTMS in the treatment of tinnitus. Exp Brain Res. 2008;184(4):587-591. doi: 10.1007/s00221-007-1228-1 [DOI] [PubMed] [Google Scholar]
- 44.Langguth B, Landgrebe M, Frank E, et al. Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: Pooled analysis of two randomized controlled studies. World J Biol Psychiatry. 2014;15(4):276-285. doi: 10.3109/15622975.2012.708438 [DOI] [PubMed] [Google Scholar]
- 45.Lehner A, Schecklmann M, Greenlee MW, Rupprecht R, Langguth B. Triple-site rTMS for the treatment of chronic tinnitus: a randomized controlled trial. Sci Rep. 2016;6:22302. doi: 10.1038/srep22302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcondes RA, Sanchez TG, Kii MA, et al. Repetitive transcranial magnetic stimulation improve tinnitus in normal hearing patients: a double-blind controlled, clinical and neuroimaging outcome study. Eur J Neurol. 2010;17(1):38-44. doi: 10.1111/j.1468-1331.2009.02730.x [DOI] [PubMed] [Google Scholar]
- 47.Noh TS, Kyong JS, Chang MY, et al. Comparison of treatment outcomes following either prefrontal cortical-only or dual-site repetitive transcranial magnetic stimulation in chronic tinnitus patients: a double-blind randomized study. Otol Neurotol. 2017;38(2):296-303. [DOI] [PubMed] [Google Scholar]
- 48.Pal N, Maire R, Stephan MA, Herrmann FR, Benninger DH. Transcranial direct current stimulation for the treatment of chronic tinnitus: a randomized controlled study. Brain Stimul. 2015;8(6):1101-1107. doi: 10.1016/j.brs.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 49.Piccirillo JF, Garcia KS, Nicklaus J, et al. Low-frequency repetitive transcranial magnetic stimulation to the temporoparietal junction for tinnitus. Arch Otolaryngol Head Neck Surg. 2011;137(3):221-228. doi: 10.1001/archoto.2011.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piccirillo JF, Kallogjeri D, Nicklaus J, et al. Low-frequency repetitive transcranial magnetic stimulation to the temporoparietal junction for tinnitus: four-week stimulation trial. JAMA Otolaryngol Head Neck Surg. 2013;139(4):388-395. doi: 10.1001/jamaoto.2013.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plewnia C, Reimold M, Najib A, Reischl G, Plontke SK, Gerloff C. Moderate therapeutic efficacy of positron emission tomography-navigated repetitive transcranial magnetic stimulation for chronic tinnitus: a randomised, controlled pilot study. J Neurol Neurosurg Psychiatry. 2007;78(2):152-156. doi: 10.1136/jnnp.2006.095612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plewnia C, Vonthein R, Wasserka B, et al. Treatment of chronic tinnitus with θ burst stimulation: a randomized controlled trial. Neurology. 2012;78(21):1628-1634. doi: 10.1212/WNL.0b013e3182574ef9 [DOI] [PubMed] [Google Scholar]
- 53.Rossi S, De Capua A, Ulivelli M, et al. Effects of repetitive transcranial magnetic stimulation on chronic tinnitus: a randomised, crossover, double blind, placebo controlled study. J Neurol Neurosurg Psychiatry. 2007;78(8):857-863. doi: 10.1136/jnnp.2006.105007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahlsten H, Virtanen J, Joutsa J, et al. Electric field-navigated transcranial magnetic stimulation for chronic tinnitus: a randomized, placebo-controlled study. Int J Audiol. 2017;56(9):692-700. doi: 10.1080/14992027.2017.1313461 [DOI] [PubMed] [Google Scholar]
- 55.Schecklmann M, Giani A, Tupak S, et al. Neuronavigated left temporal continuous theta burst stimulation in chronic tinnitus. Restor Neurol Neurosci. 2016;34(2):165-175. doi: 10.3233/RNN-150518 [DOI] [PubMed] [Google Scholar]
- 56.Smith JA, Mennemeier M, Bartel T, et al. Repetitive transcranial magnetic stimulation for tinnitus: a pilot study. Laryngoscope. 2007;117(3):529-534. doi: 10.1097/MLG.0b013e31802f4154 [DOI] [PubMed] [Google Scholar]
- 57.Yilmaz M, Yener MH, Turgut NF, Aydin F, Altug T. Effectiveness of transcranial magnetic stimulation application in treatment of tinnitus. J Craniofac Surg. 2014;25(4):1315-1318. doi: 10.1097/SCS.0000000000000782 [DOI] [PubMed] [Google Scholar]
- 58.Van Doren J, Langguth B, Schecklmann M. Electroencephalographic effects of transcranial random noise stimulation in the auditory cortex. Brain Stimul. 2014;7(6):807-812. doi: 10.1016/j.brs.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 59.Vanneste S, Fregni F, De Ridder D. Head-to-head comparison of transcranial random noise stimulation, transcranial AC stimulation, and transcranial DC stimulation for tinnitus. Front Psychiatry. 2013;4:158. doi: 10.3389/fpsyt.2013.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang N, Siebner HR, Ernst D, et al. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56(9):634-639. doi: 10.1016/j.biopsych.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 61.Vanneste S, De Ridder D. Bifrontal transcranial direct current stimulation modulates tinnitus intensity and tinnitus-distress-related brain activity. Eur J Neurosci. 2011;34(4):605-614. doi: 10.1111/j.1460-9568.2011.07778.x [DOI] [PubMed] [Google Scholar]
- 62.Meeus O, Blaivie C, Ost J, De Ridder D, Van de Heyning P. Influence of tonic and burst transcranial magnetic stimulation characteristics on acute inhibition of subjective tinnitus. Otol Neurotol. 2009;30(6):697-703. doi: 10.1097/MAO.0b013e3181b05023 [DOI] [PubMed] [Google Scholar]
- 63.De Ridder D, van der Loo E, Van der Kelen K, Menovsky T, van de Heyning P, Moller A. Do tonic and burst TMS modulate the lemniscal and extralemniscal system differentially? Int J Med Sci. 2007;4(5):242-246. doi: 10.7150/ijms.4.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poreisz C, Paulus W, Moser T, Lang N. Does a single session of theta-burst transcranial magnetic stimulation of inferior temporal cortex affect tinnitus perception? BMC Neurosci. 2009;10:54. doi: 10.1186/1471-2202-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleinjung T, Eichhammer P, Landgrebe M, et al. Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: a pilot study. Otolaryngol Head Neck Surg. 2008;138(4):497-501. doi: 10.1016/j.otohns.2007.12.022 [DOI] [PubMed] [Google Scholar]
- 66.Lehner A, Schecklmann M, Poeppl TB, et al. Multisite rTMS for the treatment of chronic tinnitus: stimulation of the cortical tinnitus network—a pilot study. Brain Topogr. 2013;26(3):501-510. doi: 10.1007/s10548-012-0268-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Identification and Data Analysis
eTable 1. PRISMA Extension Checklist of Current Meta-analysis
eTable 2. Excluded Studies and Reasons
eTable 3. Characteristics of the Included Studies
eTable 4. Node Definition
eTable 5. League Table of the Improvement of Severity of Tinnitus
eTable 6. SUCRA of Patient Improvement, Response Rate, and Dropout Rate
eTable 7. Meta-regression Results
eTable 8. League Tables of Improvement of Quality of Life, Response Rate, and Dropout Rate
eTable 9. Inconsistency of Different Intervention
eTable 10. Estimated Between-Studies Standard Deviation of Different Outcomes
eFigure 1. Network Structure of NMA of Changes of Quality of Life, Response Rate, and Dropout Rate
eFigure 2. Forest Plots of NMA Outcomes
eFigure 3. Overview of and Detailed Risk of Bias
eFigure 4. Funnel Plots of Outcomes, Response Rate, and Dropout Rate