This randomized clinical trial describes the second-year results of the ophthalmological device trial of epimacular brachytherapy, a proposed second-line therapy for neovascular age-related macular degeneration.
Key Points
Question
Does epimacular brachytherapy reduce the number of anti–vascular endothelial growth factor injections that patients with chronic, active neovascular age-related macular degeneration require without sacrificing their visual acuity?
Findings
In this randomized clinical trial of 363 participants with neovascular age-related macular degeneration, epimacular brachytherapy did not reduce the frequency of anti–vascular endothelial growth factor injections and was associated with worse visual acuity at month 24 compared with as-needed ranibizumab monotherapy.
Meaning
These findings do not support the addition of epimacular brachytherapy to anti–vascular endothelial growth factor treatment for neovascular age-related macular degeneration.
Abstract
Importance
Although anti–vascular endothelial growth factor (VEGF) treatment offers better outcomes than the natural history of neovascular age-related macular degeneration (ARMD), a less burdensome, less expensive, and more durable treatment is needed.
Objective
To assess the efficacy and safety of epimacular brachytherapy (EMB) for chronic, active, neovascular ARMD.
Design, Setting, and Participants
The Macular Epiretinal Brachytherapy vs Ranibizumab (Lucentis) Only Treatment (MERLOT) pivotal device trial was conducted at 24 National Health Service hospitals across the UK. Patients who had neovascular ARMD and received intravitreal ranibizumab were enrolled between November 10, 2009, and January 30, 2012. Eligible patients were randomized 2:1 and were stratified by lens status and angiographic lesion type to receive either EMB plus as-needed ranibizumab or as-needed ranibizumab monotherapy. Participants were followed up monthly for 24 months and then assessed at a final visit at month 36. Masking of participants and clinicians was not possible, but best-corrected visual acuity (BCVA) and imaging were analyzed by masked assessors. Analysis followed the intent-to-treat approach.
Interventions
Pars plana vitrectomy with 24 Gy EMB plus as-needed ranibizumab vs as-needed ranibizumab monotherapy.
Main Outcomes and Measures
Coprimary outcomes were the number of as-needed ranibizumab injections and the mean change in Early Treatment Diabetic Retinopathy Study (ETDRS) BCVA with a noninferiority margin of –5 ETDRS letters. Secondary outcomes were the percentage of participants losing fewer than 15 ETDRS letters and gaining 0 or more or 15 or more ETDRS letters and the mean change in angiographic total lesion size, choroidal neovascularization size, and foveal thickness on optical coherence tomography.
Results
Of 363 participants, 329 (90.6%) completed 24 months of follow-up (222 participants in the EMB group and 107 in the ranibizumab group). The mean (SD) age of the combined groups was 76.5 (7.4) years. The mean (SD) number of ranibizumab injections was 9.3 (6.7) in the EMB group and 8.3 (4.5) in the ranibizumab group, with a difference of 1.0 injection (95% CI, –0.3 to 2.3; P = .13). The mean (SD) BCVA change was –11.2 (15.7) ETDRS letters in the EMB group and –1.4 (10.9) ETDRS letters in the ranibizumab group, with a difference of 9.8 ETDRS letters (95% CI, –6.7 to –12.9). In the EMB group, 65.6% of participants (160 of 244) lost fewer than 15 ETDRS letters vs 86.6% (103 of 119) in the ranibizumab group, with a difference of 21% (95% CI, 12.4%-29.5%; P < .001). Microvascular abnormalities occurred in 20 of 207 eyes (9.7%) in the EMB group and 1 of 97 eyes (1.0%) in the ranibizumab group. These abnormalities occurred outside the foveal center, and there were no unexpected safety concerns.
Conclusions and Relevance
The MERLOT trial found that despite the acceptable safety of EMB, it did not reduce the number of ranibizumab injections and was associated with worse visual acuity than anti-VEGF treatment alone; these results do not support EMB use as an adjunct treatment for chronic, active neovascular ARMD.
Trial Registration
ClinicalTrials.gov Identifier: NCT01006538
Introduction
Neovascular age-related macular degeneration (ARMD) is the leading cause of blindness in European and North American nations.1,2 Since their introduction, anti–vascular endothelial growth factor (VEGF) agents have become the mainstay of treatment for neovascular ARMD. Although results from clinical trials3,4 and real-world data5 have shown that anti-VEGF agents offer much better outcomes than the natural history of the disease, the burden of treatment is substantial, requiring regular hospital visits and repeated injections. Furthermore, not all patients respond to treatment, and among those who do, many do not maintain their response. Long-term follow-up reports of pivotal anti-VEGF clinical trials showed that after initial visual acuity (VA) gains in the first 2 years, participants experienced a decline from baseline: loss of 11 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at 3.5 years after exit from the CATT (Comparison of Age-Related Macular Degeneration Treatments Trials),6 and loss of 8.6 ETDRS letters at the 7-year follow-up in the ranibizumab registration trials.7 The same trend of diminished VA (loss of 2 ETDRS letters at 3 years) has been observed in a large UK database study.8 Therefore, a need exists for a less burdensome, less expensive, and more durable treatment modality.
Radiotherapy was first used for the treatment of neovascular ARMD in 1993.9 Radiation selectively targets proliferating endothelial cells, inflammatory cells, and fibroblasts.10 Epimacular brachytherapy (EMB) is designed to deliver radiation to neovascular ARMD lesions through an endoscopic probe containing a strontium-90 source. After pars plana vitrectomy, the probe is held over the neovascular ARMD lesion to deliver 24 Gy of β radiation for 3 to 4 minutes. Because the radiation dose decreases exponentially with increasing distance from the source, the dose received by the optic nerve and lens is minimal.11
The EMB device was tested in patients with chronic, active neovascular ARMD in the MERITAGE (Macular Epiretinal Brachytherapy in Treated Age-Related Macular Degeneration) study.11,12 This uncontrolled multicenter trial found a loss of fewer than 15 ETDRS letters in 81% of participants after 12 months and in 68% after 24 months, despite participants’ receipt of fewer anti-VEGF injections than before the administration of EMB.11,12
On the basis of these results, our study group commenced the MERLOT (Macular Epiretinal Brachytherapy vs Ranibizumab [Lucentis] Only Treatment) trial to investigate the efficacy and safety of EMB for patients with active, chronic neovascular ARMD.13 The hypothesis was that EMB would reduce the number of injections that patients require without sacrificing their VA. The previously published 12-month results of the MERLOT trial did not support the use of EMB13; however, EMB typically takes several months to have an effect, and radiation damage is thought to be more likely in the second year after treatment.11,12,14,15 Thus, the month-12 results are insufficient to establish the efficacy and safety of EMB. Herein we report the month-24 results of the MERLOT trial.
Methods
The design and methodology of the MERLOT trial have been published elsewhere.13 Briefly, MERLOT was a pivotal randomized clinical trial of a surgical device that recruited participants from 24 National Health Service hospitals in the United Kingdom. Research ethics committee approval was obtained for all sites. Written informed consent was obtained from all participants. Participants did not receive any compensation or incentives. The trial adhered to the tenets of the Declaration of Helsinki,16 and investigators followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The study protocol is available in Supplement 1.
Randomization and Masking
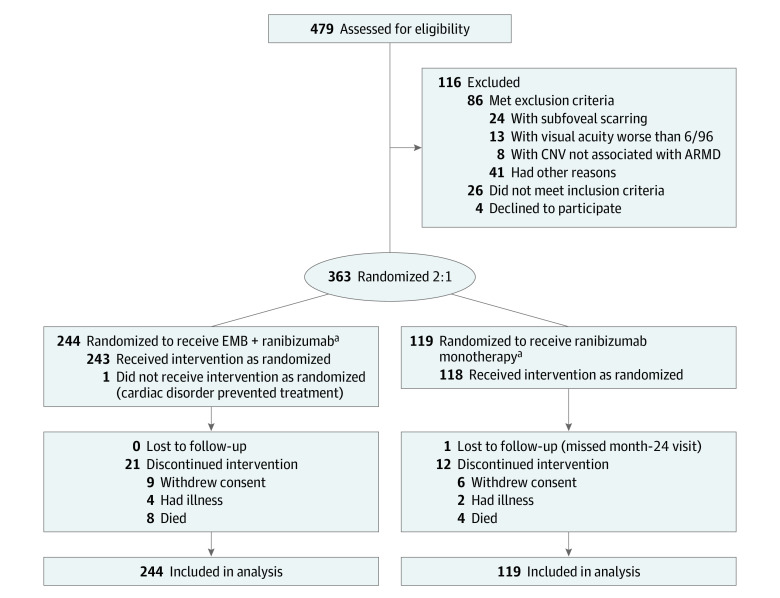
After their eligibility was confirmed, participants were randomized 2:1 and stratified by lens status and angiographic lesion type to receive either EMB plus as-needed ranibizumab (n = 244) or as-needed ranibizumab monotherapy (n = 119), which served as the control group (Figure 1). A commercially available system (MedSciNet Studies; MedSciNet AB) was used for randomization. Masking of participants and clinicians was not possible, but the assessment of best-corrected visual acuity (BCVA) and imaging were masked.
Figure 1. CONSORT Diagram .
ARMD indicates age-related macular degeneration; CNV, choroidal neovascularization; and EMB, epimacular brachytherapy.
aOne participant in the ranibizumab monotherapy group did not receive the intervention as randomized because they withdrew consent prior to baseline and were found to be ineligible because of prior photodynamic therapy. Two participants in the EMB group were enrolled in error; one had previous vitrectomy and the other had visual acuity worse than 6/96.
Participants and Treatments
The study enrolled 363 participants with chronic, active neovascular ARMD who had received at least 3 anti-VEGF injections and thereafter received ranibizumab as needed. To be classified as having active neovascular ARMD, participants must have received at least 4 ranibizumab injections in the previous 12 months or 2 ranibizumab injections in the previous 6 months. The eligibility criteria are outlined in eAppendix 1 in Supplement 2.
Participants in the EMB group underwent a 20-, 23-, or 25-gauge full pars plana vitrectomy. The EMB probe (NeoVista Inc) was positioned for 3 to 4 minutes to deliver 24 Gy over the region that the investigator determined on fundus fluorescein angiography (FFA) to have maximum disease activity. Both treatment groups received 0.5 mg of intravitreal ranibizumab (Lucentis; Novartis) at baseline and then at monthly visits if they met the predefined retreatment criteria (eAppendix 2 in Supplement 2).
Visits and Assessments
Refraction, BCVA testing using the ETDRS protocol, ocular examination, and optical coherence tomography (OCT) were performed at screening and monthly thereafter. Ophthalmoscopic photography and FFA were performed at baseline, month 12, and month 24. Color photographs, FFA images, and OCT images were graded by netWORK UK. Graders were masked to participant site and treatment group. One of our senior arbitrators (U.C.) checked the gradings and subsequently signed off on these grades.
Efficacy and Safety Outcomes
The coprimary efficacy outcomes at month 24 were mean change in BCVA from baseline and mean number of as-needed ranibizumab injections. Secondary outcomes were the percentage of participants losing fewer than 15 ETDRS letters and gaining 0 or more or 15 or more ETDRS letters, the mean change in total angiographic lesion size, choroidal neovascularization (CNV) size, and foveal thickness on OCT.
Safety measures included all adverse events (AEs) and serious adverse events (SAEs), which were coded using the MedDRA (Medical Dictionary for Regulatory Activities) Preferred Terms, version 19.0.17 AEs of particular interest were cataract and microvascular abnormalities (MVAs). The presence or absence of MVA was determined masked by the Reading Center from multimodal image grading at baseline, month 12, and month 24; and confirmed by a senior grader, checked by a senior Reading Center clinician, and approved by an expert Reading Center clinician (U.C.).
Subgroup Analysis
The prespecified statistical analysis plan detailed an exploratory subgroup analysis of the influence of baseline characteristics on the coprimary outcomes.13 Baseline variables were lens status (phakic or pseudophakic), BCVA (≤53 or >53 ETDRS letters), lesion type (predominantly classic, minimally classic, or occult), and lesion size (≤3.5 or >3.5 optic disc areas).
Statistical Analysis
Data analysis followed the intent-to-treat approach and was conducted from April 9, 2016, to June 18, 2017. The hypothesis was that EMB would reduce the frequency of ranibizumab retreatment injections without sacrificing BCVA. Therefore, the EMB group was tested against the control group (ranibizumab monotherapy) for fewer injections (superiority) and noninferior BCVA. Both coprimary outcomes had to be met to reject the null hypothesis, so the type I error rate was not adjusted. The calculation of sample size, randomization, stratification, and subgroup analyses were described previously.13
Sample-size calculation assumptions13 comprised a mean (SD) baseline VA of 65.5 (14.6) ETDRS letters in both EMB and ranibizumab groups as well as a mean (SD) number of 8 (4) injections in the ranibizumab group and 6 (3) injections in the EMB group. A sample size of 220 participants in the EMB group and 110 in the ranibizumab group gives 90% power with a 5–ETDRS letter BCVA noninferiority margin and greater than 90% power to detect a difference of 2 injections with a 2-sided significance level of 5% (an increase to 363 participants allows for 10% attrition).
The efficacy population comprised all randomized participants. Multiple imputation was performed for 36 participants with missing month-24 BCVA. The mean BCVA calculation excluded counting fingers, hand movements, and perception of light vision (predetermined; results to be described separately). No imputation was made for ranibizumab retreatment in participants who withdrew from the MERLOT trial before month 24 or for missing imaging data.
The safety population comprised all participants who received at least 1 treatment. This analysis was conducted by the actual treatment received (EMB or ranibizumab monotherapy).
Unless otherwise specified, continuous variables were summarized using mean (SD), and categorical variables were summarized using proportions. These analyses were conducted with 2-sided tests with a type I error rate of 5%. P values were calculated using the stratified Mantel-Haenszel test controlling for baseline lens status and lesion type. P < .05 indicated statistical significance.
Results
Between November 10, 2009, and January 30, 2012, 363 participants were randomized (Figure 1). Of these participants, 350 (96.4%) completed 12 months of follow-up and 329 (90.6%) completed 24 months of follow-up (222 participants in the EMB group and 107 participants in the ranibizumab group). The mean (SD) age of these participants was 76.9 (7.2) years in the EMB group and 75.8 (7.6) in the ranibizumab group, with a combined mean (SD) age of 76.5 (7.4) years. The race/ethnicity of all participants was white. More female than male participants made up the EMB group (155 of 244 [63.5%]), but sex did not influence the coprimary outcome. Baseline ocular characteristics were well balanced (eTable 1 in Supplement 2).
Ranibizumab Retreatments
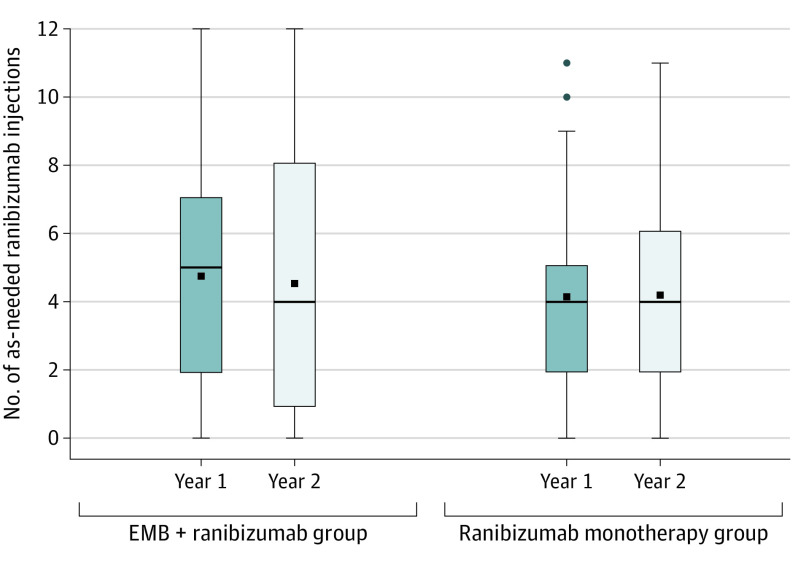
The mean (SD) number of as-needed ranibizumab injections from month 1 to month 24 inclusive was 9.3 (6.7) in the EMB group (n = 244) and 8.3 (4.5) in the ranibizumab group (n = 119), with a difference of 1.0 injection (95% CI, –0.3 to 2.3; P = .13). Figure 2, Tables 1 and 2, and eFigure 1 in Supplement 2 show the number of as-needed ranibizumab injections.
Figure 2. Number of As-Needed Ranibizumab Injections From Month 1 to Month 24 by Treatment Group.
The top of the box shows the third quartile, and the bottom shows the first quartile, with the median shown as a line across the box. The top error bar represents the observations still within 1½ times of the upper quartile, and the bottom error bar represents the observations within 1½ times the lower quartile. The mean is shown as a square inside the box, and the outliers are shown as circles. The number of injections is represented separately for year 1 and year 2. The baseline ranibizumab given for preexisting disease to participants in both arms is not included.The epimacular brachytherapy (EMB) plus as-needed ranibizumab group comprised 244 participants, whereas the as-needed ranibizumab monotherapy group consisted of 119 participants.
Table 1. Number of As-Needed Ranibizumab Injections per Year.
| Time | No. of ranibizumab injections, mean (SD) | |
|---|---|---|
| EMB + ranibizumab group (n = 244) | Ranibizumab monotherapy group (n = 119) | |
| Year 1: mo 1-12 | 4.8 (3.2) | 4.1 (2.4) |
| Year 2: mo 13-24 | 4.5 (3.8) | 4.2 (2.7) |
| Total: mo 1-24 | 9.3 (6.7) | 8.3 (4.5) |
Abbreviation: EMB, epimacular brachytherapy.
Table 2. Primary and Secondary Outcomes at Month 24.
| Variable | EMB + ranibizumab group | Ranibizumab monotherapy group | Difference (95% CI) | ||
|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | ||
| Coprimary outcomes | |||||
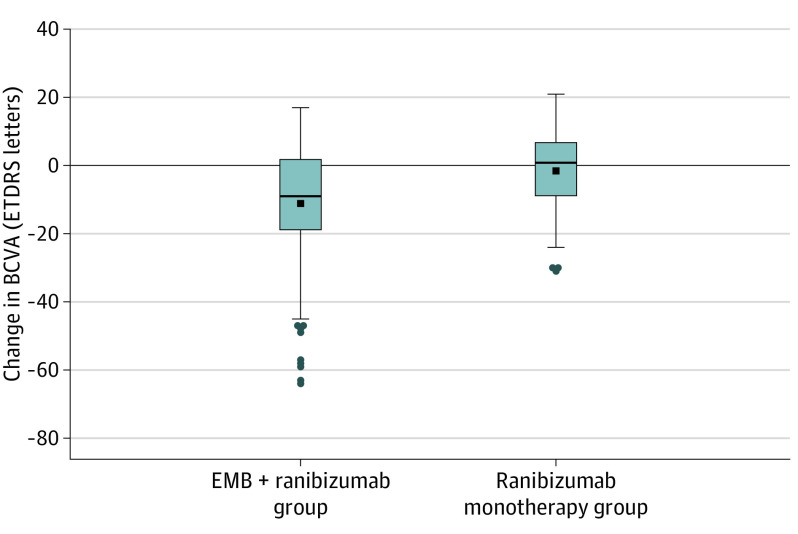
| BCVA change from baseline, ETDRS lettersa | 243 | –11.2 (15.7) | 119 | –1.4 (10.9) | –9.8 (–6.7 to –12.9) |
| Number of ranibizumab retreatments | 244 | 9.3 (6.7) | 119 | 8.3 (4.5) | 1.0 (–0.3 to 2.3) |
| Secondary outcomes: BCVA | |||||
| <15 ETDRS letter loss from baseline, No. (%) | 244 | 160 (65.6) | 119 | 103 (86.6) | 21 (12.4 to 29.5) |
| ≥0 ETDRS letter gain from baseline, No. (%) | 244 | 69 (28.3) | 119 | 61 (51.3) | 23 (12.4 to 33.6) |
| ≥15 ETDRS letter gain from baseline, No. (%) | 244 | 2 (0.8) | 119 | 4 (3.4) | 2.6 (–0.9 to 6.0) |
| Secondary outcomes: FFA and OCT | |||||
| Change in total lesion size, mm2 | 196 | 4.1 (8.7) | 96 | 2.1 (5.8) | 2.0 (0.04 to 3.9) |
| Change in total CNV size, mm2 | 196 | 2.6 (10.0) | 96 | 0.04 (6.3) | 2.6 (0.3 to 4.7) |
| Change in foveal thickness, μm | 213 | 7.0 (193) | 100 | –20.0 (130) | 27 (–14.8 to 68.8) |
Abbreviations: BCVA, best-corrected visual acuity; CNV, choroidal neovascularization; EMB, epimacular brachytherapy; ETDRS, Early Treatment Diabetic Retinopathy Study; FFA, fundus fluorescein angiography; OCT, optical coherence tomography.
Results are based on analysis of covariance model of the change in visual acuity, adjusting for baseline visual acuity, baseline lens status, and baseline lesion type.
Visual Acuity
At month 24, the mean (SD) change in BCVA from baseline was –11.2 (15.7) ETDRS letters in the EMB group (n = 243) and –1.4 (10.9) ETDRS letters in the ranibizumab group (n = 119), with a difference of 9.8 ETDRS letters (95% CI, –6.7 to –12.9) (Table 2; Figure 3). One participant in the EMB group with a BCVA of 53 ETDRS letters at baseline, 28 ETDRS letters at month 12, and perception of light at month 24 was not included in mean BCVA and CI calculations (because it was predetermined that a BCVA of counting fingers or worse would be analyzed separately). This exclusion did not alter the inference of the trial given that BCVA was significantly worse in the EMB group. The mean change in BCVA over time is shown in eFigure 2 in Supplement 2.
Figure 3. Change in Early Treatment Diabetic Retinopathy Study (ETDRS) Best-Corrected Visual Acuity (BCVA) at Month 24.
The top of the box shows the third quartile, and the bottom shows the first quartile, with the median shown as a line across the box. The top error bar represents the observations still within 1½ times of the upper quartile, and the bottom error bar represents the observations within 1½ times the lower quartile. The mean is shown as a square inside the box, and the outliers are shown as circles. The epimacular brachytherapy (EMB) plus as-needed ranibizumab group comprised 243 participants, whereas the as-needed ranibizumab monotherapy group consisted of 119 participants.
The percentage of participants losing fewer than 15 ETDRS letters was 65.6% (160 of 244) in the EMB group and 86.6% (103 of 119) in the ranibizumab group, with a difference of 21% (95% CI, 12.4%-29.5%; P < .001). The percentage of participants gaining 0 or more ETDRS letters was 28.3% (69 of 244) in the EMB group and 51.3% (61 of 119) in the ranibizumab group, with a difference of 23% (95% CI, 12.4%-33.6%; P < .001). The percentage of participants gaining 15 or more ETDRS letters was 0.8% (2 of 244) in the EMB group and 3.4% (4 of 119) in the ranibizumab group, with a difference of 2.6% (95% CI, –0.9% to 6.0%; P = .03) (Table 2).
Optical Coherence Tomography and FFA
At month 24, the mean (SD) foveal thickness increased by 7.0 (193) μm in the EMB group (n = 213) and decreased by –20.0 (130) μm in the ranibizumab group (n = 100), with a difference of 27 μm (95% CI, –14.8 μm to 68.8 μm; P = .19). The mean (SD) total lesion size increased by 4.1 (8.7) mm2 in the EMB group (n = 196) and 2.1 (5.8) mm2 in the ranibizumab group (n = 96), with a difference of 2.0 mm2 (95% CI, 0.04 mm2 to 3.9 mm2; P = .05). The mean (SD) CNV size increased by 2.6 (10.0) mm2 in the EMB group and 0.04 (6.3) mm2 in the ranibizumab group, with a difference of 2.6 mm2 (95% CI, 0.3 mm2 to 4.7 mm2; P = .02).
Subgroup Analysis
Most subgroups tended to favor the ranibizumab group with respect to the mean number of as-needed ranibizumab injections (eFigure 3 in Supplement 2), but this finding reached statistical significance only in the pseudophakic subgroup (8.9 [5.8] injections in the EMB group vs 6.7 [4.3] injections in the ranibizumab group, with a difference of 2.2 injections [95% CI, 0.1-4.3; P = .04]). Predominantly classic lesions (8.0 [5.5] injections in the EMB group vs 8.7 [5.0] injections in the ranibizumab group, with a difference of –0.7 injections [95% CI, –4.2 to 2.9; P = .69]) and small (≤3.5 disc areas) lesions (8.2 [6.2] injections in the EMB group vs 9.0 [4.5] injections in the ranibizumab group, with a difference of –0.75 injections [95% CI, –2.3 to 0.8; P = .34]) tended to favor the EMB group. With respect to VA, all subgroups favored the ranibizumab group (eFigure 4 in Supplement 2).
Safety
Study eye AEs occurring in the first 24 months are listed in eTable 2 in Supplement 2. Clinically significant cataract was the most frequent study eye AE, occurring in 66.0% of eyes (161 of 244) in the EMB group and 20.2% (24 of 119) in the ranibizumab group. Investigator-reported reduced VA also occurred more frequently in the EMB group than in the ranibizumab group (12.3% [30 of 244] vs 5.0% [6 of 119]).
eTable 3 in Supplement 2 shows all study eye SAEs and their relatedness to treatment. All other AEs and SAEs are shown in eTables 4 and 5 in Supplement 2. Nine deaths (3.7%) occurred in the EMB group and 4 deaths (3.4%) in the ranibizumab group over 24 months.
At month 24, the Reading Center identified MVAs in 20 of 207 eyes (9.7%) in the EMB group and 1 of 97 eyes (1.0%) in the ranibizumab group. The MVAs were attributed to radiation in 17 of 20 eyes (85.0%) in the EMB group, but in 3 of 20 eyes (15.0%) the cause could not be identified despite additional expert review by 2 Reading Center clinicians (U.C. and T.P.). The main features were retinal vessel staining or leakage and capillary nonperfusion. The location of the MVA, progression over 24 months, and relatedness to vision are detailed in eTable 6 in Supplement 2. The MVAs were sometimes subtle, and none involved the foveal center.
Discussion
It was hypothesized that EMB would decrease the number of ranibizumab retreatments that participants needed while enabling them to maintain noninferior VA.13 Compared with the ranibizumab group, however, the EMB group required more ranibizumab injections and had worse BCVA. The magnitude of the VA difference between groups was statistically significant and clinically meaningful, at –11.2 ETDRS letters for the EMB group vs –1.4 ETDRS letters for the ranibizumab group.
Emerging postvitrectomy cataract may have adversely affected BCVA in the EMB group, unless this emergence prompted the removal of subclinical, preexisting lens opacity, which could improve VA. However, the prespecified subgroup analysis of eyes that were pseudophakic at baseline favored the ranibizumab group. Therefore, the worse BCVA in the EMB group cannot be attributed to postvitrectomy cataract alone.
These results differ somewhat from those observed in the CABERNET (CNV Secondary to AMD Treated with Beta Radiation Epiretinal Therapy) study, which enrolled treatment-naive participants.14 Participants in the MERLOT trial had a more substantial decline in VA compared with those in the CABERNET study (–4.8 vs –0.5 ETDRS letters at month 12 and –11.2 vs –2.5 ETDRS letters at month 2413,14). A possible explanation for this difference is that the CABERNET study enrolled participants with treatment-naive disease who typically show an improvement in BCVA after the initiation of the ranibizumab treatment, whereas the MERLOT trial included participants with chronic, active disease who had already commenced anti-VEGF therapy and thus did not get the initial lift in VA at commencement of anti-VEGF therapy. The different proportion of participants with occult lesions in the EMB group between the MERLOT and CABERNET trials (74% vs 37%) may also explain the different BCVA outcomes.
The structural outcomes mirrored the VA decline, EMB being associated with an increase in retinal thickness on OCT and total lesion size and CNV size on FFA at month 24.
Because a previous responder analysis study14 found that eyes with a smaller lesion size and predominantly classic lesions had a positive response to radiation treatment, the present study prespecified a similar subgroup analysis. However, no benefit was observed for either the number of ranibizumab treatments or VA in the EMB group compared with the ranibizumab group. Instead, all subgroup analyses showed better VA in the ranibizumab monotherapy group.
Despite the results of the MERLOT trial, radiotherapy delivered by other means has produced more favorable results. The INTREPID (IRay in Conjunction With Anti-VEGF Treatment for Patients with Wet AMD) study, a large double-masked, randomized, sham-controlled clinical trial of stereotactic radiotherapy in combination with anti-VEGF therapy, met its primary outcome.18,19 The INTREPID study reported that significantly fewer ranibizumab retreatments were required in the active group and those with small (<4 mm), actively leaking lesions had significantly better BCVA compared with sham despite 55% fewer injections.20
Several possible explanations may be given for why the results with EMB (MERLOT) were so different from the results with stereotactic radiotherapy (INTREPID). A key difference was the device used to deliver radiation. The EMB probe uses a strontium source, and the energy decreases exponentially with increasing distance from the probe such that the effective tissue dose relies on precise positioning of the probe on the surface of the retina in the area of maximal disease activity. Although an FFA was used to guide positioning, the area of greatest activity can be difficult to locate, particularly for occult lesions, which represented 74% of participants in the radiation group. In addition, stereotactic radiotherapy avoids vitrectomy, which is known to cause cataract21 and to decrease the half-life of anti-VEGF drugs.22,23 To understand and explore the potential of stereotactic radiotherapy, the National Institute for Health Research and the Medical Research Council in the UK have funded a large, multicenter, double-masked, randomized, sham-controlled clinical trial targeting the best responders subgroup identified in the INTREPID study.24
In terms of safety, more AEs and SAEs were found in the EMB group, but this finding does not necessarily point to a safety concern. Cataract surgical procedure was the most common ocular AE and was most likely secondary to vitrectomy rather than to EMB.21 The proportion of arteriothrombotic events25 and neoplasia were similar between the EMB and ranibizumab groups.
Most retinal MVAs occurred in year 2, with only 1 event occurring in year 1 and worsening over year 2, indicating the importance of long-term follow-up. Participants with MVAs had worse BCVA compared with those without MVAs but not significantly so, and none of the MVAs involved the foveal center.
Strengths and Limitations
Strengths of the MERLOT trial include its size; its multicenter, randomized design; and the independent analysis of FFA and OCT images by Reading Center clinicians. The main limitation of the study is the lack of masking of participants and clinicians, which is inherent in most surgical trials. However, the key study assessments such as BCVA and OCT, which in turn drive ranibizumab retreatment, were performed by masked assessors.
Conclusions
The use of EMB as a second-line therapy for chronic neovascular ARMD was not supported by the data. More specifically, the MERLOT trial results at month 24 confirmed that EMB does not reduce the number of as-needed ranibizumab injections and that EMB is associated with worse VA than the standard of care. Despite having marketing authorization in Europe, we believe that the EMB device tested in this study should not be used as part of standard clinical care in patients with chronic, active neovascular ARMD.
Trial protocol
eAppendix 1. Eligibility Criteria
eAppendix 2. Ranibizumab Retreatment Criteria
eFigure 1. The Percentage of Participants by Number of Ranibizumab Injections From Month 1 to Month 24
eFigure 2. The Mean Change in Best-Corrected Visual Acuity (BCVA) Over Time From Baseline to Month 24 in the Epimacular Brachytherapy (EMB) and Ranibizumab Monotherapy Groups
eFigure 3. Subgroup Analysis of the Number of As-Required Ranibizumab Injections From Month 1 to Month 24 Inclusive, Comparing the Epimacular Brachtherapy Group to the Ranibizumab Monotherapy Group
eFigure 4. Subgroup Analysis of the Change in Best-Corrected Visual Acuity (BCVA) From Baseline to Month 24 Comparing the Epimacular Brachytherapy Group to the Ranibizumab Monotherapy Group
eTable 1. Baseline Ocular Characteristics
eTable 2. Study Eye Adverse Events
eTable 3. Ocular Serious Adverse Events in Study Eye, and Relatedness to
Treatment
eTable 4. Adverse Events, Excluding Those in the Study Eye
eTable 5. All Serious Adverse Events Excluding Those in the Study Eye
eTable 6. Retinal Vascular Abnormalities in the Epimacular Brachytherapy Group
Data sharing statement
References
- 1.Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012;96(5):752-756. doi: 10.1136/bjophthalmol-2011-301109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. doi: 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193-201. doi: 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 5.Holz FG, Bandello F, Gillies M, et al. ; LUMINOUS Steering Committee . Safety of ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. Br J Ophthalmol. 2013;97(9):1161-1167. doi: 10.1136/bjophthalmol-2013-303232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maguire MG, Martin DF, Ying GS, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123(8):1751-1761. doi: 10.1016/j.ophtha.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; SEVEN-UP Study Group . Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292-2299. doi: 10.1016/j.ophtha.2013.03.046 [DOI] [PubMed] [Google Scholar]
- 8.Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121(5):1092-1101. doi: 10.1016/j.ophtha.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 9.Chakravarthy U, Houston RF, Archer DB. Treatment of age-related subfoveal neovascular membranes by teletherapy: a pilot study. Br J Ophthalmol. 1993;77(5):265-273. doi: 10.1136/bjo.77.5.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirwan JF, Constable PH, Murdoch IE, Khaw PT. Beta irradiation: new uses for an old treatment: a review. Eye (Lond). 2003;17(2):207-215. doi: 10.1038/sj.eye.6700306 [DOI] [PubMed] [Google Scholar]
- 11.Dugel PU, Petrarca R, Bennett M, et al. Macular epiretinal brachytherapy in treated age-related macular degeneration: MERITAGE study: twelve-month safety and efficacy results. Ophthalmology. 2012;119(7):1425-1431. doi: 10.1016/j.ophtha.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 12.Petrarca R, Dugel PU, Bennett M, et al. Macular epiretinal brachytherapy in treated age-related macular degeneration (MERITAGE): month 24 safety and efficacy results. Retina. 2014;34(5):874-879. doi: 10.1097/IAE.0000000000000026 [DOI] [PubMed] [Google Scholar]
- 13.Jackson TL, Desai R, Simpson A, et al. ; Macular Epiretinal Brachytherapy versus Ranibizumab (Lucentis) Only Treatment (MERLOT) Study Group . Epimacular brachytherapy for previously treated neovascular age-related macular degeneration (MERLOT): a phase 3 randomized controlled trial. Ophthalmology. 2016;123(6):1287-1296. doi: 10.1016/j.ophtha.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 14.Dugel PU, Bebchuk JD, Nau J, et al. ; CABERNET Study Group . Epimacular brachytherapy for neovascular age-related macular degeneration: a randomized, controlled trial (CABERNET). Ophthalmology. 2013;120(2):317-327. doi: 10.1016/j.ophtha.2012.07.068 [DOI] [PubMed] [Google Scholar]
- 15.Jackson TL, Dugel PU, Bebchuk JD, et al. ; CABERNET Study Group . Epimacular brachytherapy for neovascular age-related macular degeneration (CABERNET): fluorescein angiography and optical coherence tomography. Ophthalmology. 2013;120(8):1597-1603. doi: 10.1016/j.ophtha.2013.01.074 [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.MedDRA Welcome to the ICH MedDRA website. Accessed June 1, 2020. https://www.meddra.org/how-to-use/support-documentation/english
- 18.Jackson TL, Chakravarthy U, Kaiser PK, et al. ; INTREPID Study Group . Stereotactic radiotherapy for neovascular age-related macular degeneration: 52-week safety and efficacy results of the INTREPID study. Ophthalmology. 2013;120(9):1893-1900. doi: 10.1016/j.ophtha.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 19.Jackson TL, Chakravarthy U, Slakter JS, et al. ; INTREPID Study Group . Stereotactic radiotherapy for neovascular age-related macular degeneration: year 2 results of the INTREPID study. Ophthalmology. 2015;122(1):138-145. doi: 10.1016/j.ophtha.2014.07.043 [DOI] [PubMed] [Google Scholar]
- 20.Jackson TL, Shusterman EM, Arnoldussen M, Chell E, Wang K, Moshfeghi DM; INTREPID Study Group . Stereotactic radiotherapy for wet age-related macular degeneration (INTREPID): influence of baseline characteristics on clinical response. Retina. 2015;35(2):194-204. doi: 10.1097/IAE.0000000000000283 [DOI] [PubMed] [Google Scholar]
- 21.Jackson TL, Donachie PH, Sparrow JM, Johnston RL. United Kingdom National Ophthalmology Database Study of Vitreoretinal Surgery: report 1; case mix, complications, and cataract. Eye (Lond). 2013;27(5):644-651. doi: 10.1038/eye.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakinoki M, Sawada O, Sawada T, Saishin Y, Kawamura H, Ohji M. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest Ophthalmol Vis Sci. 2012;53(9):5877-5880. doi: 10.1167/iovs.12-10164 [DOI] [PubMed] [Google Scholar]
- 23.Chen YY, Chen PY, Chen FT, Chen YJ, Wang JK. Comparison of efficacy of intravitreal ranibizumab between non-vitrectomized and vitrectomized eyes with diabetic macular edema. Int Ophthalmol. 2018;38(1):293-299. doi: 10.1007/s10792-017-0462-1 [DOI] [PubMed] [Google Scholar]
- 24.Neffendorf JE, Desai R, Wang Y, et al. StereoTactic radiotherapy for wet age-related macular degeneration (STAR): study protocol for a randomised controlled clinical trial. Trials. 2016;17(1):560. doi: 10.1186/s13063-016-1676-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antiplatelet Trialistsʼ Collaboration Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6921):81-106. doi: 10.1136/bmj.308.6921.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix 1. Eligibility Criteria
eAppendix 2. Ranibizumab Retreatment Criteria
eFigure 1. The Percentage of Participants by Number of Ranibizumab Injections From Month 1 to Month 24
eFigure 2. The Mean Change in Best-Corrected Visual Acuity (BCVA) Over Time From Baseline to Month 24 in the Epimacular Brachytherapy (EMB) and Ranibizumab Monotherapy Groups
eFigure 3. Subgroup Analysis of the Number of As-Required Ranibizumab Injections From Month 1 to Month 24 Inclusive, Comparing the Epimacular Brachtherapy Group to the Ranibizumab Monotherapy Group
eFigure 4. Subgroup Analysis of the Change in Best-Corrected Visual Acuity (BCVA) From Baseline to Month 24 Comparing the Epimacular Brachytherapy Group to the Ranibizumab Monotherapy Group
eTable 1. Baseline Ocular Characteristics
eTable 2. Study Eye Adverse Events
eTable 3. Ocular Serious Adverse Events in Study Eye, and Relatedness to
Treatment
eTable 4. Adverse Events, Excluding Those in the Study Eye
eTable 5. All Serious Adverse Events Excluding Those in the Study Eye
eTable 6. Retinal Vascular Abnormalities in the Epimacular Brachytherapy Group
Data sharing statement