Key Points
Question
Is treatment adherence to neoadjuvant chemoradiotherapy and/or adjuvant chemotherapy associated with disease-free survival among patients with locally advanced rectal cancer?
Findings
This post hoc analysis of a phase 3 clinical trial examined 1232 patients who were treated with neoadjuvant fluorouracil-based chemoradiotherapy and adjuvant chemotherapy, both with and without the addition of oxaliplatin, in the CAO/ARO/AIO-04 phase 3 trial. Adherence to neoadjuvant chemoradiotherapy, but not adjuvant chemotherapy, was significantly associated with disease-free survival.
Meaning
Close monitoring and supportive measures to increase adherence to neoadjuvant chemoradiotherapy should be implemented as an intergral part of study designs.
Abstract
Importance
Despite numerous published phase 3 trials, the association of treatment adherence with outcomes for patients with rectal cancer remains largely unexplored.
Objective
To analyze the association of treatment adherence with disease-free survival (DFS) among patients with rectal cancer in the CAO/ARO/AIO-04 trial.
Design, Setting, and Participants
This post hoc analysis of a phase 3 randomized clinical trial was conducted from July 25, 2006, to February 26, 2010, among 1232 patients from 80 centers with T3 to T4 or node-positive rectal adenocarcinoma. Statistical analysis was performed from May 5, 2019, to February 2, 2020.
Interventions
A total of 625 patients received neoadjuvant fluorouracil-based chemoradiotherapy (nCRT), and a total of 607 patients received fluorouracil-based nCRT with addition of oxaliplatin. Of the 1126 patients who underwent curative surgery, 439 started fluorouracil-based adjuvant chemotherapy and 419 started fluorouracil-based adjuvant chemotherapy with oxaliplatin.
Main Outcomes and Measures
The association of adherence with nCRT and adjuvant chemotherapy with DFS was assessed in both groups in the as-treated population.
Results
Among the 625 patients (442 men; mean age, 63.0 years) who received fluorouracil nCRT and the 607 patients (430 men; mean age, 63.0 years) who received fluorouracil-based nCRT with addition of oxaliplatin, after a median follow-up of 50 months (interquartile range, 38-61 months), 3-year DFS in the as-treated population was 71.1% in the fluorouracil group and 75.8% in the fluorouracil-oxaliplatin group (hazard ratio [HR], 0.803; 95% CI, 0.651-0.990; P = .04). Overall, 419 patients in the fluorouracil nCRT group (67.0%) and 434 patients in the fluorouracil-oxaliplatin nCRT group (71.5%) received full doses of preoperative nCRT. Likewise, 253 of 439 patients in the fluorouracil group (57.6%) and 134 of 419 patients in the fluorouracil-oxaliplatin group (32.0%) received full doses of adjuvant chemotherapy. Adherence to nCRT was associated with 3-year DFS in both the fluorouracil group (complete vs near complete: HR, 1.325; 95% CI, 0.959-1.832; P = .09; complete vs reduced: HR, 1.877; 95% CI, 1.147-3.072; P = .01) and the fluorouracil-oxaliplatin group (complete vs near complete: HR, 1.501; 95% CI, 0.980-2.299; P = .06; complete vs reduced: HR, 1.724; 95% CI, 1.144-2.596; P = .009) in multivariable analyses. In contrast, adjuvant chemotherapy was not associated with DFS in both the fluorouracil group (complete vs near complete: HR, 0.900; 95% CI, 0.559-1.448; P = .66; complete vs incomplete: HR, 1.057; 95% CI, 0.807-1.386; P = .69) and the fluorouracil-oxaliplatin group (complete vs near complete: HR, 1.155; 95% CI, 0.716-1.866; P = .56; complete vs incomplete: HR, 1.073; 95% CI, 0.790-1,457; P = .65).
Conclusions and Relevance
To our knowledge, this is the first analysis of a phase 3 trial to assess the association of treatment adherence with some clinical outcomes for patients with rectal cancer. The findings emphasize the need for appropriate trial design with optimized nCRT dose and schedule and supportive strategies to facilitate good adherence and precision delivery, especially for intensified nCRT.
Trial Registration
ClinicalTrials.gov Identifier: NCT00349076
This post hoc analysis of a randomized phase 3 trial analyzes the association of treatment adherence with disease-free survival among patients with rectal cancer in the CAO/ARO/AIO-04 trial.
Introduction
Neoadjuvant fluorouracil-based chemoradiotherapy (fluorouracil nCRT) followed by total mesorectal excision has reduced locoregional recurrence in patients with rectal cancer but failed to improve disease-free survival (DFS) or overall survival.1,2 A meta-analysis of 10 randomized trials demonstrated that the addition of oxaliplatin to fluorouracil nCRT was not associated with improved DFS or overall survival but was associated with higher grade 3 and 4 toxic effects.3 Increased acute toxic effects may be associated with treatment adherence, which can in turn be associated with oncologic outcomes.4 However, in contrast to the strong interest in “precision oncology” during the last decade, the importance of adherence to facilitate “precision delivery” and maximize the potential benefit of treatments appears to be less emphasized. We examined the association of adherence to fluorouracil nCRT and adjuvant chemotherapy (aCTh), both with and without the addition of oxaliplatin, with DFS among 1232 patients with rectal cancer treated within the CAO/ARO/AIO-04 trial.5
Methods
The CAO/ARO/AIO-04 was an open-label, prospective, 2-group randomized phase 3 trial conducted from July 25, 2006, to February 26, 2010. The design and oncologic outcomes have been previously published5 (trial protocol in Supplement 1). All sites obtained medical ethics committee approval and written patient informed consent.
Patients were grouped into 3 nCRT adherence groups: those who received full doses of radiotherapy (50.4 Gy) and concurrent chemotherapy (complete nCRT), those who received 45 Gy or more of radiotherapy and 80% of concurrent chemotherapy (near-complete nCRT), and those who received less than 45 Gy of radiotherapy or less than 80% of concurrent chemotherapy (reduced nCRT). Likewise, adherence to aCTh was grouped as complete (those who received all cycles without dose reduction), near complete (those who received all cycles with dose reductions), and incomplete (those who discontinued aCTh).
Statistical analysis was performed from May 5, 2019, to February 2, 2020. Correlation of treatment adherence with baseline characteristics were assessed using Pearson χ2 tests. All P values were from 2-sided tests, and results were deemed statistically significant at P < .05. The association of adherence to nCRT or aCTh with DFS was examined with log-rank tests, including all baseline characteristics of clinical relevance, and plotted using the Kaplan-Meier method. Univariable and multivariable Cox proportional hazards regression modeling was used to assess the association of baseline characteristics with DFS in each of the 2 treatment groups. For all Cox proportional hazards regression models, the proportionality of hazards assumption was first assessed via Schoenfeld goodness-of-fit testing; all such tests revealed no proportional hazards violations (P > .05). Only factors that were significant (α = .05) on univariable analysis were included in the multivariable model.
Results
eFigure 1 in Supplement 2 shows the CONSORT diagram in the as-treated population. A total of 625 patients (442 men; mean age, 63.0 years) received fluorouracil nCRT, and a total of 607 patients (430 men; mean age, 63.0 years) received fluorouracil-based nCRT with the addition of oxaliplatin. Full doses of nRT and concurrent chemotherapy were tolerated in 419 patients in the fluorouracil nCRT group (67.0%) and in 434 patients in the fluorouracil-oxaliplatin group (71.5%), whereas 47 patients in the fluorouracil nCRT group (7.5%) and 88 patients in the fluorouracil-oxaliplatin group (14.5%) showed reduced nCRT adherence (eTable 1 in Supplement 2). The association of baseline characteristics with adherence to nCRT is shown in eTable 1 in Supplement 2. A total of 1126 patients underwent resection with curative intent and were eligible for aCTh. Of those, 132 did not start protocol-specified adjuvant treatment with fluorouracil, and 136 did not start protocol-specified adjuvant treatment with fluorouracil-oxaliplatin (eFigure 1 in Supplement 2), whereas 439 patients started aCTh with fluorouracil, and 419 patients started aCTh with fluorouracil-oxaliplatin. Among the group receiving aCTh with fluorouracil, 253 (57.6%) had full completion, 117 (26.7%) had dose reduction, and 69 (15.7%) discontinued treatment; among those receiving aCTh with fluorouracil-oxaliplatin, 134 (32.0%) had full completion, 205 (48.9%) had dose reduction, and 80 (19.1%) discontinued treatment.
After a median follow-up of 50 months (interquartile range, 38-61 months), 3-year DFS was 71.1% in the fluorouracil group and 75.8% in the fluorouracil-oxaliplatin group (hazard ratio [HR], 0.803; 95% CI, 0.651-0.990; P = .04). Consistent with the univariable analyses (eTable 2 in Supplement 2; Figure, A and B), treatment adherence to nCRT was significantly associated with 3-year DFS in both the fluorouracil group (complete vs near complete: HR, 1.325; 95% CI, 0.959-1.832; P = .09; complete vs reduced: HR, 1.877 95% CI, 1.147-3.072; P = .01) and the fluorouracil-oxaliplatin group (complete vs near complete: HR, 1.501; 95% CI, 0.980-2.299; P = .06; complete vs reduced: HR, 1.724; 95% CI, 1.144-2.596; P = .009) on multivariable analyses (Table 1). Other baseline characteristics that remained factors independently associated with DFS included Eastern Cooperative Oncology Group performance status (both groups), clinical tumor category and tumor localization (fluorouracil group only), and age (fluorouracil-oxaliplatin group only). In contrast, aCTh adherence was not associated with DFS in either group (Figure, C and D). Similarly, aCTh adherence was not associated with DFS in the subgroup of patients who had received complete nCRT (eFigure 2 in Supplement 2).
Figure. Disease-Free Survival of the Study Patients Based on Univariable Analysis According to Treatment Group.
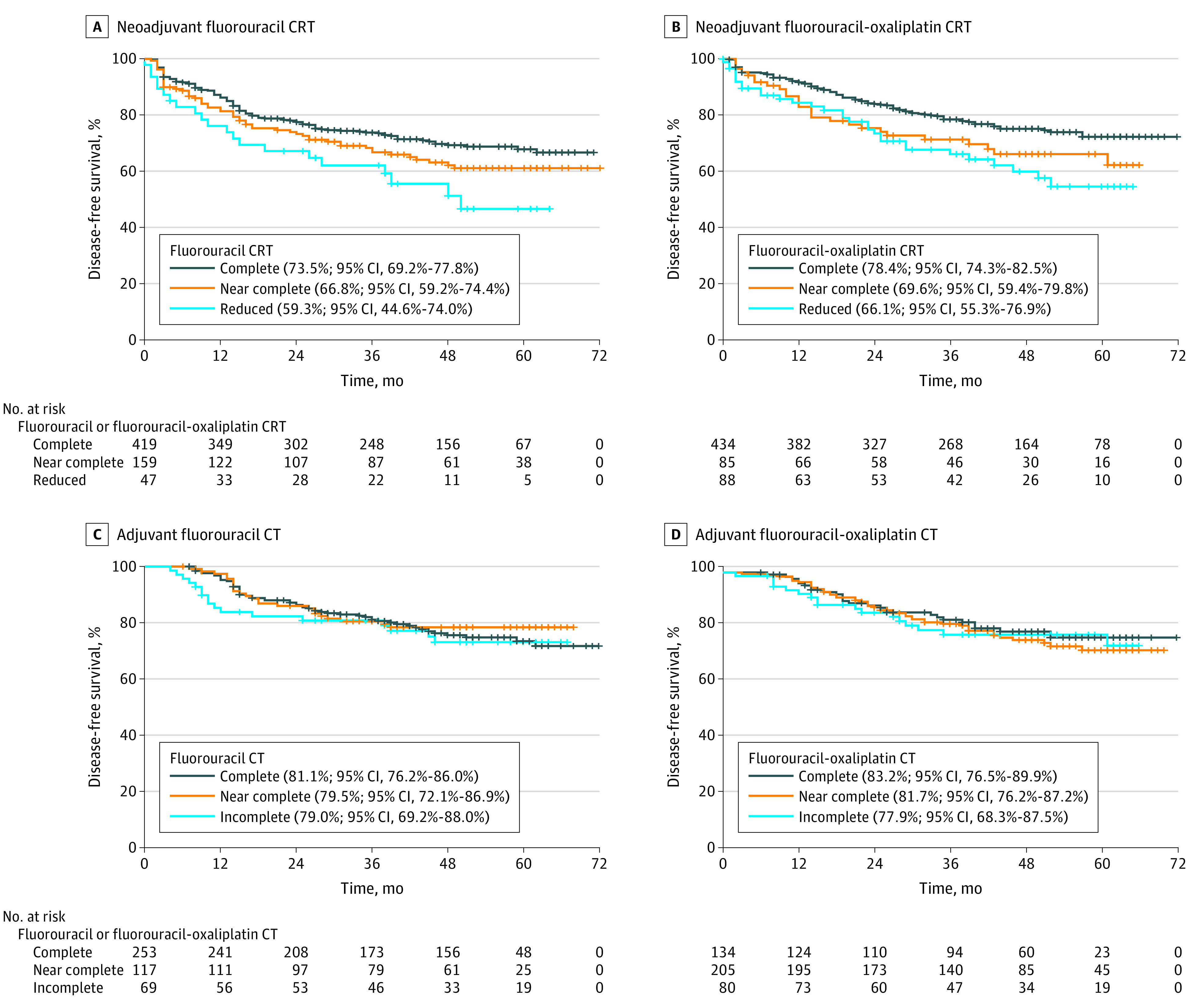
A, Neoadjuvant fluorouracil chemoradiotherapy (CRT). B, Neoadjuvant fluorouracil-oxaliplatin CRT, as shown in eTable 2 in Supplement 2. C, Adjuvant fluorouracil chemotherapy (CT) (complete vs near complete, P = .66; complete vs incomplete, P = .69). D, Adjuvant fluorouracil-oxaliplatin CT (complete vs near complete, P = .55; complete vs incomplete P = .65). Percentages refer to 3-year disease-free survival.
Table 1. Multivariable Analyses of Association of nCRT Adherence and Pretreatment Characteristics With Disease-Free Survivala.
| Multivariable analysis | 3-y Disease-free survival | |||
|---|---|---|---|---|
| Fluorouracil nCRT group | Fluorouracil-oxaliplatin nCRT group | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| nCRT adherence | ||||
| Complete vs near complete | 1.325 (0.959-1.832) | .09 | 1.501 (0.980-2.299) | .06 |
| Complete vs reduced | 1.877 (1.147-3.072) | .01 | 1.724 (1.144-2.596) | .009 |
| Age, y | ||||
| <70 vs ≥70 | NA | NA | 1.419 (0.995-2.024) | .05 |
| ECOG | ||||
| Grade 0 vs 1 | 1.416 (1.024-1.959) | .04 | 1.646 (1.138-2.380) | .008 |
| Grade 0 vs 2 | 1.442 (0.574-3.626) | .44 | 0.956 (0.132-6.898) | .96 |
| cT category | ||||
| cT2 vs cT3 | 2.153 (0.880-5.268) | .09 | NA | NA |
| cT2 vs cT4 | 3.894 (1.477-10.265) | .006 | NA | NA |
| Tumor localization | ||||
| Low vs middle | 0.607 (0.445-0.827) | .002 | NA | NA |
| Low vs high | 1.006 (0.635-1.593) | .98 | NA | NA |
Abbreviations: cT, clinical tumor stage; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; NA, not applicable; nCRT, neoadjuvant chemoradiotherapy.
Multivariable analyses were performed using the Cox proportional hazards regression model.
Discussion
Several randomized phase 3 trials have assessed tumor response and oncologic outcomes of intensified nCRT after the addition of oxaliplatin to fluorouracil-based CRT.5,6,7,8,9,10 Adherence to fluorouracil and fluorouracil-oxaliplatin nCRT was heterogeneously reported across these trials, making comparison challenging. In the STAR-01 (Studio Terapia Adiuvante Retto),7 ACCORD-12 (Actions Concertées dans les Cancers Colorectaux et Digestifs),6 NSABP R-04 (National Surgical Adjuvant Breast and Bowel Project R-04),8 and PETACC-6 (Preoperative Chemoradiotherapy and Postoperative Chemotherapy With Capecitabine and Oxaplatin vs Capecitabine Alone in Locally Advanced Rectal Cancer)11 trials, radiotherapy dose and/or concurrent chemotherapy were markedly reduced in the experimental groups (Table 2), and no improvement was reported for early effectiveness or oncologic outcomes. Conversely, in the FOWARC (Neoadjuvant FOLFOX6 Chemotherapy With or Without Radiation in Rectal Cancer),10 Chinese,9 and CAO/ARO/AIO-045 trials, adherence to nCRT was largely comparable in both groups (Table 2). In the CAO/ARO/AIO-04 trial,5 pathologic complete response and DFS were significantly improved in the experimental group. The FOWARC trial showed increased pathologic complete response in favor of oxaliplatin,10 whereas the Chinese trial revealed decreased distant metastasis,9 but neither demonstrated a DFS benefit.
Table 2. Adherence to Neoadjuvant Fluorouracil-Based CRT With or Without Oxaliplatin in Phase 3 Trials.
| Treatment component and adherence | CRT, No. (%) of patients | |
|---|---|---|
| Fluorouracil-capecitabine | Fluorouracil-capecitabine-oxaliplatin | |
| Radiotherapy for STAR-01 trial7 | ||
| Full dose | 348 (92) | 295 (84) |
| ≥90% Planned dose | 368 (97) | 319 (91) |
| ≥80% Planned dose | 375 (99) | 331 (94) |
| Chemotherapy for STAR-01 trial7 | ||
| ≥80% Planned dose of fluorouracil | 340 (90) | 282 (80) |
| Oxaliplatin | NA | 264 (75) |
| Radiotherapy for ACCORD-12 trial6 | ||
| Full dose | NR (100) | NR (87) |
| Chemotherapy for ACCORD-12 trial6 | ||
| Discontinuation | NR (2.8) | NR (8.8) |
| Dose reduction | NR (50) | NR (59) |
| Radiotherapy for NSABP R-04 trial8 | ||
| ≥80% Planned dose | NR (98) | NR (96) |
| Chemotherapy for NSABP R-04 trial8 | ||
| ≥80% Planned dose | NR (90) | NR (84) |
| Radiotherapy for PETACC-06 trial11 | ||
| >45 Gy | NR (98) | NR (92) |
| Chemotherapy for PETACC-06 trial11 | ||
| ≥90% Planned dose | NR (91) | NR (63) |
| Radiotherapy for FOWARC trial10 | ||
| ≥90% Planned dose | 134 (86) | 143 (91) |
| Chemotherapy for FOWARC trial10 | ||
| ≥90% Planned dose | 137 (88) | 150 (95) |
| Radiotherapy for Chinese trial9 | ||
| Full dose | 98 (95.1) | 96 (93.2) |
| ≥90% Planned dose | 103 (100) | 102 (99.0) |
| Chemotherapy for Chinese trial9 | ||
| Capecitabine full dose | 90 (87.4) | 88 (85.44) |
| ≥90% Planned dose | 98 (95.2) | 97 (94.17) |
| ≥80% Planned dose | 100 (97.2) | 100 (97.09) |
| Oxaliplatin full dose | NA | 84 (81.6) |
| ≥90% Planned dose | NA | 91 (88.4) |
| ≥80% Planned dose | NA | 102 (99.0) |
| Radiotherapy | ||
| Full dose | 576 (92.2) | 549 (90.4) |
| ≥45 Gy | 595 (95.2) | 564 (92.9) |
| <45 Gy | 30 (4.8) | 43 (7.1) |
| Chemotherapy for CAO/ARO/AIO-04 trial5 | ||
| Full dose of planned fluorouracil | 492 (78.7) | 517 (85.2) |
| ≥80% Planned fluorouracil | 602 (96.3) | 553 (91.1) |
| <80% Planned fluorouracil | 23 (3.7) | 54 (8.8) |
| Full dose of planned oxaliplatin | NA | 566 (93.1) |
| ≥80% Planned oxaliplatin | NA | 579 (95.4) |
| <80% Planned oxaliplatin | NA | 28 (4.6) |
Abbreviations: ACCORD, Actions Concertées dans les Cancers Colorectaux et Digestifs; CRT, chemoradiotherapy; FOWARC, Neoadjuvant FOLFOX6 Chemotherapy With or Without Radiation in Rectal Cancer; NA, not applicable; NR, not reported; NSABP R-04, National Surgical Adjuvant Breast and Bowel Project R-04; PETACC, Preoperative Chemoradiotherapy and Postoperative Chemotherapy With Capecitabine and Oxaplatin vs Capecitabine Alone in Locally Advanced Rectal Cancer; STAR, Studio Terapia Adiuvante Retto.
To date, the association of adherence to treatment with oncologic outcomes has not been reported in phase 3 trials of rectal cancer, to our knowledge. We show a significant association between complete vs reduced adherence to nCRT and DFS. Thus, the negative results reported in the studies with oxaliplatin may be explained, at least in part, by impaired adherence to nCRT in the experimental groups. These data reflect the importance of optimal dosing and supportive strategies to facilitate adherence to nCRT, especially among older patients and those with poor performance status. In the CAO/ARO/AIO-04 trial,5 60% of patients 70 years or older and 62% of patients with an Eastern Cooperative Oncology Group performance status of 1 or 2 received complete fluorouracil nCRT, whereas 67% of patients 70 years or older and 66% of patients with an Eastern Cooperative Oncology Group performance status of 1 or 2 received complete fluorouracil-oxaliplatin nCRT. Thus, age and performance status did not prohibit administration of oxaliplatin. The nCRT regimen used in the CAO/ARO/AIO-04 trial was well tolerated because it had been previously tested in phase 1/2 trials.12
In contrast to nCRT, adherence to aCTh was not associated with DFS in our study. The role of aCTh for patients with rectal cancer remains opaque, as demonstrated in 5 randomized trials; these studies were limited by low accrual, suboptimal regimens, poor treatment adherence, and early randomization before nCRT or surgery.13 All phase 2/3 trials failed to demonstrate a benefit of aCTh for DFS or overall survival except the ADORE (Adjuvant Oxaliplatin in Rectal Cancer)14 phase 2 trial, which was characterized by postsurgical randomization of patients with stage II or III disease, inclusion of young patients (median age, 54 years), and excellent adherence to fluorouracil-oxaliplatin aCTh (range, 87%-95%).
Limitations
Our study has limitations. First, this work constitutes a post hoc secondary analysis. Second, categorical rather than continuous variable definitions of treatment adherence were used to improve clinical utility; more important, these categorical definitions reflect those of previous reports (Table 2).5,6,7,8,9,10,11 Third, this analysis does not definitively demonstrate a causal association of radiotherapy and concurrent chemotherapy dose levels with DFS because poor adherence to nCRT has been associated with other factors, such as age and performance status. Fourth, other potential confounders, such as psychological and socioeconomic factors, were not documented in this trial and, hence, have not been taken into account in the mutivariable analyses.
Conclusions
To our knowledge, this is the first study to demonstrate the association of nCRT adherence with DFS among patients with rectal cancer treated within a large, randomized phase 3 trial. Phase 1/2 trials are essential to establish optimal nCRT dosing, while close monitoring and supportive measures to increase adherence to nCRT should be implemented as an integral part of study design. A shift toward total neoadjuvant treatment, rather than aCTh, may increase adherence to systemic treatment and potentially increase DFS.15
Trial Protocol
eFigure 1. Study Flow Diagram of the Present Post Hoc Analysis
eFigure 2. Prognostic Significance of Adjuvant Chemotherapy Compliance for Disease-Free Survival Based on Univariable Analysis in Patients That Received the Full Dose of nCRT for (A) Adjuvant 5-FU CT and (B) Adjuvant 5-FU/Ox CT
eTable 1. Association of Patient Characteristics With the Compliance to nCRT in the Two Treatment Groups
eTable 2. Univariable Analyses of nCRT Compliance and Pretreatment Characteristics on Disease-Free Survival in 5-FU and 5-FU/Ox Group
References
- 1.Glynne-Jones R, Wyrwicz L, Tiret E, et al. ; ESMO Guidelines Committee . Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv263. doi: 10.1093/annonc/mdy161 [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926-1933. doi: 10.1200/JCO.2011.40.1836 [DOI] [PubMed] [Google Scholar]
- 3.Hüttner FJ, Probst P, Kalkum E, et al. Addition of platinum derivatives to fluoropyrimidine-based neoadjuvant chemoradiotherapy for stage II/III rectal cancer: systematic review and meta-analysis. J Natl Cancer Inst. 2019;111(9):887-902. doi: 10.1093/jnci/djz081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleeland CS, Allen JD, Roberts SA, et al. Reducing the toxicity of cancer therapy: recognizing needs, taking action. Nat Rev Clin Oncol. 2012;9(8):471-478. doi: 10.1038/nrclinonc.2012.99 [DOI] [PubMed] [Google Scholar]
- 5.Rödel C, Graeven U, Fietkau R, et al. ; German Rectal Cancer Study Group . Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979-989. doi: 10.1016/S1470-2045(15)00159-X [DOI] [PubMed] [Google Scholar]
- 6.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30(36):4558-4565. doi: 10.1200/JCO.2012.42.8771 [DOI] [PubMed] [Google Scholar]
- 7.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773-2780. doi: 10.1200/JCO.2010.34.4911 [DOI] [PubMed] [Google Scholar]
- 8.Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11):djv248. doi: 10.1093/jnci/djv248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao D, Zhang R, Gong Z, et al. Fluorouracil-based preoperative chemoradiotherapy with or without oxaliplatin for stage II/III rectal cancer: a 3-year follow-up study. Chin J Cancer Res. 2015;27(6):588-596. doi: 10.3978/j.issn.1000-9604.2015.12.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Y, Chi P, Lan P, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol. 2019;37(34):3223-3233. doi: 10.1200/JCO.18.02309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haustermans K, Schmoll HJ, Price T, et al. FC-0250: first results of the PETACC-6 randomized phase III trial in locally advanced rectal cancer. Radiother Oncol. 2014;111(suppl 1):S96. doi: 10.1016/S0167-8140(15)30355-8 [DOI] [Google Scholar]
- 12.Rödel C, Liersch T, Hermann RM, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol. 2007;25(1):110-117. doi: 10.1200/JCO.2006.08.3675 [DOI] [PubMed] [Google Scholar]
- 13.Carvalho C, Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. 2017;18(6):e354-e363. doi: 10.1016/S1470-2045(17)30346-7 [DOI] [PubMed] [Google Scholar]
- 14.Hong YS, Kim SY, Lee JS, et al. Oxaliplatin-based adjuvant chemotherapy for rectal cancer after preoperative chemoradiotherapy (ADORE): long-term results of a randomized controlled trial. J Clin Oncol. 2019;37(33):3111-3123. doi: 10.1200/JCO.19.00016 [DOI] [PubMed] [Google Scholar]
- 15.Fokas E, Allgäuer M, Polat B, et al. ; German Rectal Cancer Study Group . Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37(34):3212-3222. doi: 10.1200/JCO.19.00308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Study Flow Diagram of the Present Post Hoc Analysis
eFigure 2. Prognostic Significance of Adjuvant Chemotherapy Compliance for Disease-Free Survival Based on Univariable Analysis in Patients That Received the Full Dose of nCRT for (A) Adjuvant 5-FU CT and (B) Adjuvant 5-FU/Ox CT
eTable 1. Association of Patient Characteristics With the Compliance to nCRT in the Two Treatment Groups
eTable 2. Univariable Analyses of nCRT Compliance and Pretreatment Characteristics on Disease-Free Survival in 5-FU and 5-FU/Ox Group


