Abstract
Mayetiola destructor (Hessian fly) is a destructive pest of wheat in several parts of the world. Here, we investigated the presence of reproductive symbionts and the effect of the geographical location on the bacterial community associated to adult Hessian flies derived from four major wheat producing areas in Morocco. Using specific 16S rDNA PCR assay, Wolbachia infection was observed in 3% of the natural populations and 10% of the laboratory population. High throughput sequencing of V3-V4 region of the bacterial 16S rRNA gene revealed that the microbiota of adult Hessian flies was significantly influenced by their native regions. A total of 6 phyla, 10 classes and 79 genera were obtained from all the samples. Confirming the screening results, Wolbachia was identified as well in the natural Hessian flies. Phylogenetic analysis using the sequences obtained in this study indicated that there is one Wolbachia strain belonging to supergroup A. To our knowledge, this is the first report of Wolbachia in Hessian fly populations. The observed low abundance of Wolbachia most likely does not indicate induction of reproductive incompatibility. Yet, this infection may give a new insight into the use of Wolbachia for the fight against Hessian fly populations.
Keywords: biological control, Hessian fly, high throughput sequencing, HTS, 16S rRNA gene
1. Introduction
Hessian fly, Mayetiola destructor (Say) (Diptera: Cecidomyiidae), is one of the most destructive insect pests of wheat in North Africa, North America and southern Europe [1]. In Morocco, damage caused by the Hessian fly can result in total crop loss if fall infestations are high and coincide with young stages of the crop [2]. The life cycle of the Hessian fly consists of eggs, three larval instars, pupae and adults. Adult flies resemble to mosquitos and have a short life span of 2 days for males and from 2 to 5 days for females with reduced flying capability [3,4,5]. Adults do not feed but mate and lay eggs on the upper surfaces of young wheat leaves. After hatching, first instar larvae crawl to the base of seedlings, where they establish a feeding site. The Hessian fly larvae and pupae develop in the same position and feed only during the first and second larval instars [6]. Feeding on the base of the seedlings causes stunting of the infested plants, which eventually become dark green and stop growing. This type of damage is observed mainly on young plants, while plants attacked at a more developed stage are not completely killed, but undergo a shortening of the internodes, and their yields in grain and straw are reduced [6,7,8]. Currently, several control methods, such as the use of classical chemical control and resistant cultivars are applied for managing Hessian fly infestations [2,9,10,11]. With the high demand of environmentally friendly alternatives, we address hereby, a biological and innovative pest management approach based on microorganisms associated with the insects. It is known that a diverse array of bacterial species is widespread in insects and has a tremendous variety of impacts which engage in obligatory or facultative symbioses, ranging from parasitism to mutualism. Bacterial symbionts have been reported to affect host biology on aspects of development, nutrition, reproduction, and fitness [12,13,14,15,16,17,18].
Recently, reproductive endosymbionts have attracted attention for their potential as new biocontrol agents, with the most studied symbionts in this area belonging to Wolbachia [19,20,21]. Wolbachia are obligatory intracellular and maternally inherited bacteria infecting a number of invertebrates especially insects [19]. Wolbachia affect the reproduction of their host by several strategies allowing them to persist and spread rapidly in host population. Several studies show that Wolbachia are able to induce thelytokous parthenogenesis, male killing, feminization of genetic males and cytoplasmic incompatibility (CI) which is the most frequent and best studied effect that Wolbachia have on their hosts [22,23]. Wolbachia was also reported to provide fitness benefit to their hosts by influencing fecundity, nutrition, development and providing resistance to pathogens [24,25,26,27,28,29,30,31,32].
Apart from Wolbachia, other inherited symbionts that can alter the biology of their insect hosts exist. Among them, Spiroplasma are mainly extracellular bacteria belonging to the phylum Tenericutes, known to cause selective death of male offspring in Drosophila flies and in a nymphalid butterfly [33,34]. Furthermore, Spiroplasma has been found to protect the infected host against natural enemies [34,35,36]. The endosymbiont Cardinium belonging to the Bacteroidetes phylum, also causes various reproductive phenotypes in numerous hosts, including cytoplasmic incompatibility, feminization and parthenogenesis [37,38,39,40,41]. Arsenophonus, belonging to the phylum Proteobacteria, establish diverse symbiotic interactions with approximately 5% of insect species, and mainly induce male killing phenomena [42,43].
To date, there are very few studies on the characterization of the bacterial community associated to Hessian fly populations. Bansal et al. 2011 based on classical sequencing, showed that for both culture-dependent and -independent methods, laboratory Hessian fly adults harbor four phyla: Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes with difference in proportion between both methods [44]. Using culture-dependent methods, Hessian fly adults were mainly dominated by Bacillus genus (62.5%). Very low levels of proteobacterial genera were detected (less than 3.1%). However, using culture-independent method Hessian fly adults were dominated mainly by members of Proteobacteria with Ochrobactrum as the most abundant genus (55%). These differences were explained by the inability of some bacterial genera belonging to Proteobacteria phylum to grow on laboratory growth media. In regards to the Wolbachia-infection, Johnson et al. 2004 [45] tested by Long PCR technique the presence of Wolbachia in Hessian fly adults from 30 different geographic origins within the North America, the Mediterranean basin, Southwest Asia, and New Zealand obtained between 2000 and 2003 in addition to six laboratory populations. The results were negative for all the natural and laboratory populations.
In the present study, we aimed to screen laboratory and natural populations of Hessian fly for reproductive symbionts. The screening results were positive only for Wolbachia infections. The characterization of these Wolbachia strains was based on the use of 16S rRNA gene. In addition, we report on the bacterial symbionts associated to natural populations of adult Hessian flies using a high throughput sequencing (HTS) approach based on the Illumina-MiSeq platform.
2. Materials and Methods
2.1. Sample Collection and DNA Isolation
Four different Hessian fly populations were obtained from bread wheat from the major wheat producing areas in Morocco: Doukkala, Fes, Chaouia, and Safi (Table 1). The plants containing Hessian fly pupae were collected in winter 2017 and 2018 and they were reared in a growth room under constant conditions (Temperature 25 ± 1 °C, Relative Humidity 51.7%) from which adults were allowed to emerge. The first generation of adults were collected. A laboratory population kept at the International Center of Agricultural Research in Dry Areas (ICARDA) in Rabat, Morocco, was also included in the screening analysis (Table 1). All samples collected were placed separately (one adult per tube) in 100% ethanol and stored at room temperature until use. Before the DNA extraction, samples were rinsed with sterile water then the DNA of the whole fly was isolated using a modified CTAB (Cetyl Trimethyl Ammonium Bromide) method [46]. The quality and quantity of DNA samples was tested using a Q5000 micro-volume UV Vis spectrophotometer (Quawell Technology, San Jose, CA, USA). DNA samples were stored in Eppendorf tubes at −20 °C until PCR amplification and amplicon sequencing analysis.
Table 1.
Number of collected Hessian fly adults from different locations.
| Region | Location | Coordinates | Number of Insects | |||
|---|---|---|---|---|---|---|
| Altitude | Latitude | Longitude | Female | Male | ||
| Safi | Jamaat Shaim | 173 | 32.24076 | −8.46976 | 32 | 27 |
| Fes | Fes | 584 | 34.01436 | −5.34543 | 23 | 30 |
| Doukkala | Khemis Zemamra | 162 | 32.614952 | −8.664802 | 19 | 8 |
| Chaouia | Sidi El Aidi | 247 | 33.07341 | −7.37935 | 16 | 9 |
| - | Laboratory colony | - | - | - | 40 | 40 |
2.2. Screening of Reproductive Symbionts and Sanger Sequencing
In total, 164 samples from wild populations and 80 samples from the laboratory colony were assayed for the presence of Wolbachia, Spiroplasma, Cardinium and Arsenophonus. The detection was performed using bacterial species-specific 16S rRNA gene-based PCR (Polymerase Chain Reaction). The mitochondrial gene 12S rRNA was used as positive control for amplification (Table S1). The amplification was performed in 25 µL reaction mixtures containing 2.5 µL KAPA Taq buffer 10×, 0.25 µL dNTPs (25 mM), 0.25 µL of KAPA Taq, 0.5 µL of the forward primer (25 µM), 0.5 µL of the reverse primer (25 µM), 1 µL of template DNA solution and was finalized with 20 µL sterile deionized water. The PCR temperature profile was 95 °C for 5 min followed by 35 cycles of 95 °C for 30 s, 30 s at the optimum annealing temperature for each pair of primers, 1 min at 72 °C and a final extension step of 72 °C for 5 min. PCR products were electrophoresed on a 1.5% agarose gel in order to examine the presence and size of the amplified fragments. The primer sequences used in this study along with the product size and annealing temperature are summarized in Table S1. The PCR-positive products were purified using polyethylene glycol (20% PEG, 2.5 M NaCl) [47] and resuspended in 15 μL water. Amplicon sequencing was performed using Sanger method.
2.3. PCR Amplification of V3-V4 Region, PCR Indexing and Illumina Sequencing
For the amplicon sequencing analysis, five individuals from each gender were randomly selected from the four natural populations of Hessian fly samples. The hypervariable V3-V4 region of the bacterial 16S rRNA gene was amplified from the total number of 40 adults using MiSeq universal primers 341F and 805R (Table S1). The first PCR reaction was performed and purified as previously described. In order to include the indexes as well as the Illumina adaptors, a second PCR were performed in 50 µl volume containing 5 µL KAPA Taq buffer 10×, 0.4 µL dNTPs (25 mM), 0.2 µL of KAPA Taq, 5 µL of the forward index primer (10 µM), 5 µL of the reverse index primer (10 µM), 2 µL of the cleaned PCR product diluted up to 10 ng.µL−1 and 32.4 µL sterile deionized water. The temperature profile used PCR was: 95 °C for 3 min followed by 8 cycles of 95 °C for 30 s, 30 s at 55 °C, 30 s at 72 °C and a final extension step of 72 °C for 3 min. The resulting amplicons were cleaned using the NucleoMag NGS (Next Generation Sequencing) Clean-up and Size Selection kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s instructions. Indexed amplicons from all samples examined were mixed in equimolar ratio (8 nM) and sequencing was performed by Macrogen using a 2 × 300 bp pair-end kit on a MiSeq platform. The datasets have been deposited to NCBI under BioProject PRJNA613835.
2.4. Bioinformatic Analysis of Amplicon Sequencing Data
After sequencing, bioinformatic analysis was performed using USEARCH v.11 [48] and QIIME2 distribution 2019.1 [49]. Briefly, paired-end reads were assembled, trimmed by length using the usearch -fastq_mergepairs option, then, the quality of assembled sequences was improved using -fastq_filter, followed by finding unique read sequences and abundances using -fastx_uniques option. Sequences were clustered into operational taxonomic units (OTUs) with -cluster_otus command based on 97% OTU clustering using UPARSE algorithm [50]. Cross-talk errors were identified and filtered with -uncross option based on UNCROSS2 algorithm [51]. Taxonomy was assigned with Qiime2 based on BLAST+ algorithm [52] against SILVA 128 release database [53].
Richness, Simpson, Shannon and Evenness indices of alpha diversity, which reflect the diversity of individual samples were calculated based on “diversity” function from “vegan” R package and plotted using “ggplot” function from “ggplot2” package. Pair wise ANOVA was used to identify significant differences of alpha diversity indices between the different locations. Beta diversity was analyzed to evaluate the similarity of bacterial communities from different locations using Generalized UniFrac distance [54] and visualized via non-metric multidimensional scaling (NMDS) plot. A permutational multivariate analysis of variance using distance matrices was calculated using “adonis” function from “vegan” R package to determine significance differences between the separated groups. Linear discriminant analysis (LDA) effect size (LEfSe) method was applied on OTUs table to identify the discriminant taxa characterizing the four different regions using the galaxy web application (http://huttenhower.sph.harvard.edu/galaxy/) [55]. A p-value < 0.05 was considered indicative of statistical significance.
2.5. Phylogenetic Analysis
The Wolbachia phylogenetic analyses were carried out based on the partial 16S rRNA gene sequences obtained from the Wolbachia-infected samples and the sequences representing the Wolbachia related OTUs obtained by HTS sequencing. First, the sequences were aligned using MUSCLE [56], with the default algorithm parameters, as implemented in MEGA 7.0 software [57] and manually adjusted. Smart Model Selection [58] was used to estimate the best model of nucleotide acid evolution for constructing phylogenies of our data based on Akaike Information Criterion [59]. For the alignment of Wolbachia sequences obtained by Sanger sequencing, the model (GTR+G+I) was selected, while the model (TN+G+I) was selected for the alignment of Wolbachia related sequence obtained by the HTS sequencing. The robustness was assessed with 1000 bootstrap replicates. Maximum-Likelihood trees were constructed using MEGA 7.0 software [57]. All 16S rRNA gene sequences generated in this study have been deposited in the GenBank database under accession numbers MT231732-MT231744 and MT229221.
3. Results
3.1. Reproductive Infection Status Assessed by PCR Screening of Natural and Laboratory Hessian Fly Populations
3.1.1. Reproduction Infection Prevalence in Natural and Laboratory Hessian Fly Populations
PCR screening methods were used to assay the presence of four reproductive symbionts: Wolbachia, Spiroplasma, Cardinium and Arsenophonus, in a laboratory colony and four natural Hessian fly populations. The screening results revealed that these flies were infected only by Wolbachia with around 3% in natural populations, and 10% in the laboratory population. Noteworthy that, the Wolbachia percentage infection in natural populations did not reveal an even distribution among the different locations. Only, Hessian flies from Doukkala (5 out of 27 screened individuals) were determined to be infected with Wolbachia. In total, 13 flies were found infected: 3 females and 2 males out of 27 individuals from Doukkala population and 8 males out of 80 individuals from the laboratory population (Table 2, Figure S1). Whereas, none of the Hessian fly populations examined were infected with Spiroplasma, Cardinium and Arsenophonus.
Table 2.
Prevalence of bacterial endosymbionts screened in natural and laboratory populations of Hessian fly.
| Population | Gender | Sample Size | Wolbachia | Spiroplasma | Cardinium | Arsenophonus |
|---|---|---|---|---|---|---|
| Doukkala | Female | 19 | + (3) | − | − | − |
| Male | 8 | + (2) | − | − | − | |
| Safi | Female | 32 | − | − | − | − |
| Male | 27 | − | − | − | − | |
| Fes | Female | 23 | − | − | − | − |
| Male | 30 | − | − | − | − | |
| Chaouia | Female | 16 | − | − | − | − |
| Male | 9 | − | − | − | − | |
| Laboratory | Female | 40 | − | − | − | − |
| Male | 40 | + (8) | ||||
| Total | 244 | + (13) | − | − | − |
+ infected samples (with number of infected individuals per population), − uninfected samples.
3.1.2. Phylogenetic Analysis of Wolbachia Sequences Obtained by Sanger Sequencing
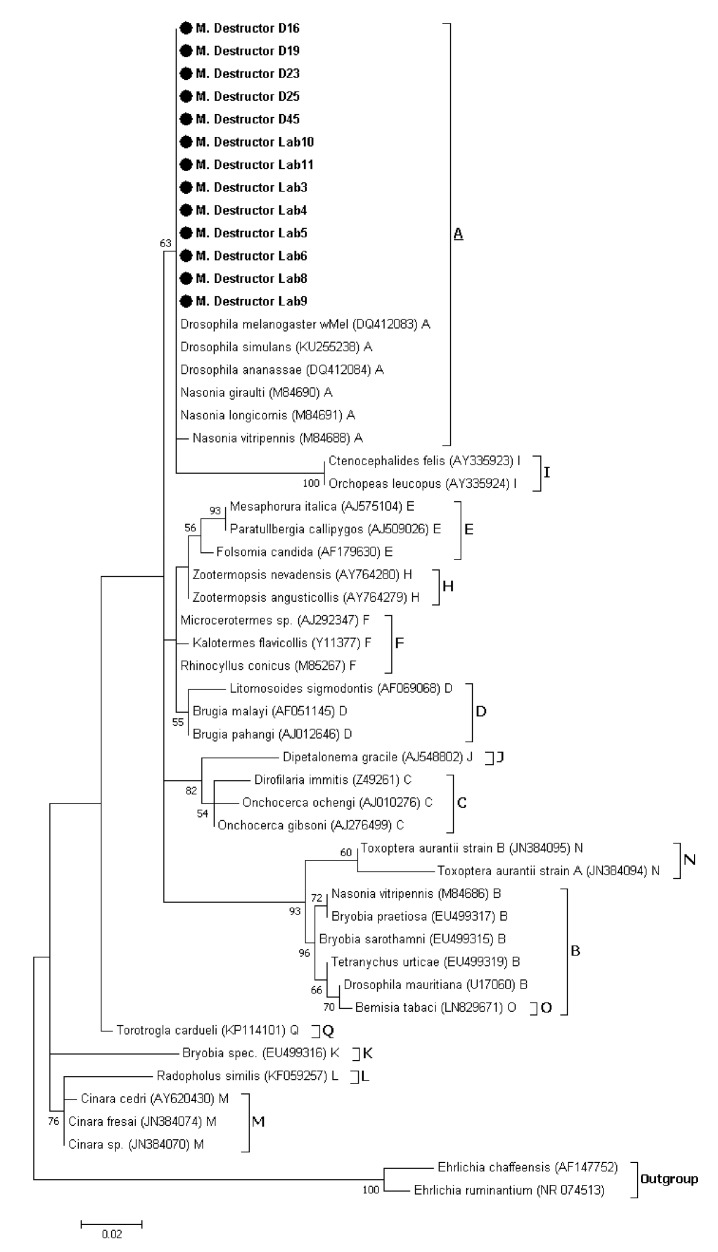
After removing manually the low-quality bases, 393 bp remained in thirteen Wolbachia sequences. The Wolbachia phylogenetic analysis was carried out on the thirteen Wolbachia-infected samples based on the partial 16S rRNA gene sequences. The results revealed that the Wolbachia strains infecting both natural and laboratory Hessian fly populations belonged to supergroup A (Figure 1). Interestingly, the Wolbachia strain sequences detected in the Hessian fly populations match perfectly with all the reference sequences of supergroup A except for Nasonia vitripennis.
Figure 1.
Maximum likelihood phylogenetic tree based on Wolbachia 16S rRNA sequences (257 bp indel-free alignment): The thirteen Wolbachia sequences present in Hessian fly positive samples are indicated in bold letters (D: samples from Doukkala region, Lab: samples from the laboratory colony) along with the other sequences represent the known supergroups from A to Q (except supergroup P). Wolbachia sequences are characterized by the names of their host species and their GenBank accession number. The number in each node represent bootstrap proportions based on 1000 replication (only values > 50% are indicated).
3.2. 16S rRNA Gene Amplicon Sequencing Reveals the Presence of Wolbachia in Natural Populations of Hessian Fly and Dynamics Indicate an Origin Effect
The bacterial community composition and diversity of 40 natural Hessian fly samples from Chaouia, Doukkala, Fes and Safi regions were investigated using Illumina high throughput sequencing of 16S rRNA gene amplicons. A total of 1,203,664 qualified paired-end reads with an average count per samples of 30,091 reads were obtained after sequencing and quality filtering. On the basis of a 97% species similarity, 101 operational taxonomic units (OTUs) classified in six phyla, 10 classes and 79 genera were obtained across all the samples (Table S2).
3.2.1. Bacterial Diversity within Hessian Flies’ Natural Populations
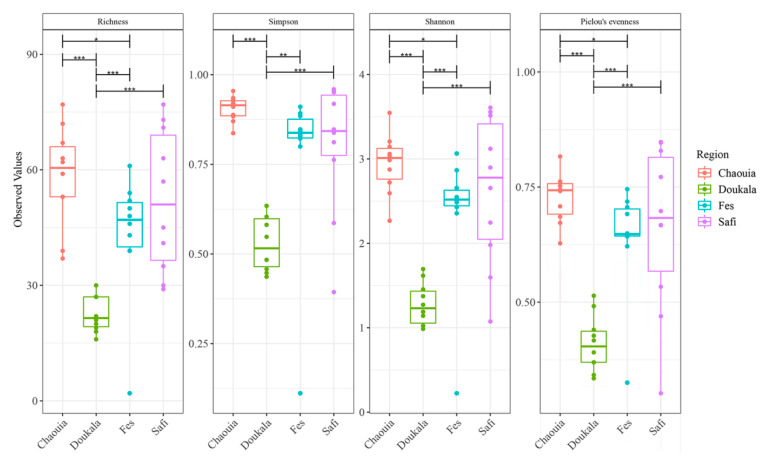
The four examined natural populations of Hessian flies exhibited different species richness and diversity indices based on the number and relative abundance of OTUs, Simpson and Shannon indices (Figure 2). In detail, the most bacterial species rich samples were those derived from Chaouia, Fes, and Safi, while samples from Doukkala exhibited statistically lower species richness and diversity than the samples from the other regions (pairwise ANOVA: p < 0.001). Additionally, samples from Chaouia region exhibited statistically higher diversity, based on the Shannon index, compared to samples from Fes region (pairwise ANOVA: p < 0.05).
Figure 2.
Species richness and diversity indices with significance differences of Hessian fly samples collected from Chaouia, Doukkala, Fes and Safi regions. Boxes represent inter-quartile range (IQR), the line within the boxes is the median, and the dots represent samples. * p < 0.05, ** p < 0.01 and *** p < 0.001.
3.2.2. Bacterial Composition of Hessian Flies’ Natural Populations
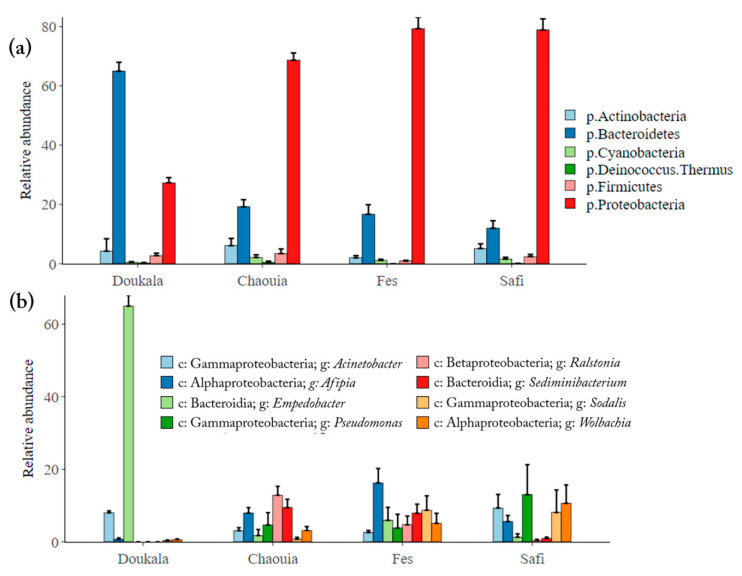
In total, most of the bacteria were identified as Proteobacteria (63.5 ± 2.9%) and Bacteroidetes (28 ± 2.7%), in addition to Actinobacteria, Cyanobacteria, Deinococcus Thermus and Firmicutes (less than 5%) (Table S2). In more detail, the Hessian flies collected from Chaouia, Fes and Safi regions shared a similar phylum distribution dominated by Proteobacteria (68.6 ± 2.4%, 79.2 ± 3.8% and 78.8 ± 3.7% respectively), Bacteroidetes (19.2 ± 2.3%, 16.6 ± 3.2% and 11.9 ± 2.4%, respectively), Actinobacteria (6.1 ± 2.4%, 1.9 ± 0.7% and 5.1 ± 1.6%, respectively) and Firmicutes (3.4 ± 1.5%, 0.9 ± 0.2% and 2.3 ± 0.8% respectively) (Figure 3a). By contrast, the Hessian flies from Doukkala region had a high relative abundance of Bacteroidetes (64.9 ± 2.9%) followed by Proteobacteria (27.2 ± 1.7%), Actinobacteria (4.2 ± 4.1%) and Firmicutes (2.7 ± 0.8%). At the class level, the most abundant taxa in the Hessian flies from Safi, Fes and Chaouia were Gammaproteobacteria, Alphaproteobacteria and Betaproteobacteria respectively. However, in Doukkala region, the Hessian flies’ microbiota was dominated mainly by Bacteroidia (Figure 3b). These results were confirmed by the LEfSe analysis and LDA scores (Figure S2A), where similar bacterial distribution patterns have been observed in the natural Hessian flies collected, suggesting that the flies’ microbiota was affected significantly by their native regions. In total, 47 OTUs were identified with LDA scores > 4.0 and p < 0.05 (Figure S2B). Eleven, 16, 8 and 12 OTUs were identified in Hessian flies from Doukkala, Chaouia, Fes and Safi respectively. LEfSe indicated that the most discriminant OTUs in Hessian flies were Bacteroidetes (p < 0.001, Kruskal-Wallis test) in Doukkala, Actinobacteria in Chaouia region, Betaproteobacteria in Fes and Gammaproteobacteria in Safi (p < 0.01, Kruskal Wallis test). Different frequencies of genera were observed between the collection regions. The most frequent genus in the Hessian flies was Empedobacter which exhibited a higher relative abundance (64.9 ± 2.9%) in Doukkala, Ralstonia (12.8 ± 2.4%) in Chaouia, Afipia (16.2 ± 3.9%) in Fes, and Pseudomonas (13 ± 8.2%) in Safi (Figure 3b). Interestingly, the genus Wolbachia was detected across all regions with a higher relative abundance in Hessian flies derived from Safi (10.6 ± 5.0%) and Fes (5.0 ± 2.7%) compared to those derived from Chaouia (3.1 ± 1.0%) and Doukkala (0.6 ± 0.1%) regions. Additionally, HTS results confirmed the absence of Arsenophonus, Cardinium and Spiroplasma genera in Hessian flies’ microbiota.
Figure 3.
Composition of Hessian flies’ microbiota at the phylum (a) and genus (b) levels. ‘p’ corresponds to phylum, ‘c’ to class and ‘g’ to genus.
3.2.3. Phylogenetic Analysis of Wolbachia Related Sequence Obtained by HTS
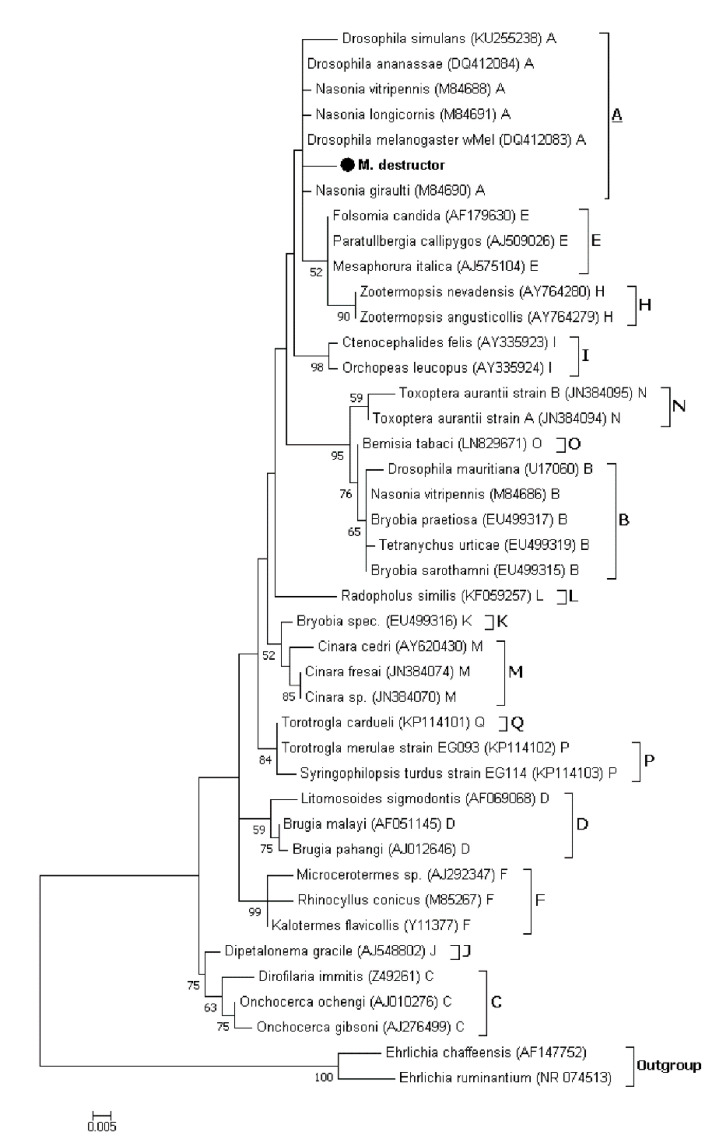
The Wolbachia infections status was assessed from the normalized libraries, using the conventional clustering of sequences into OTUs (97% sequence identity). Wolbachia related reads clustered into one OTU only, with differences in prevalence across the four studied regions. Among ten individuals per region, eight individuals were infected in Safi and Fes, nine individuals in Chaouia and the lower prevalence was detected in Doukkala with six individuals. The phylogenetic analyses based on the 16S rRNA gene placed the sequence representing the Wolbachia related OTU (443 bp) within the supergroup A sequences (Figure 4). These results indicate that the Hessian flies derived from different regions most likely carried the same Wolbachia strain.
Figure 4.
Maximum likelihood phylogenetic tree based on 16S rRNA gene of Wolbachia related OTU (443 bp full size alignment): The Wolbachia related sequence obtained from Hessian fly positive samples is indicated in bold letters along with the other sequences represent the known supergroups from A to Q. Wolbachia sequences are characterized by the names of their host species and their GenBank accession number. The number in each node represent bootstrap proportions based on 1000 replication (only values > 50% are indicated).
3.2.4. Bacterial Diversity between Hessian Flies’ Natural Populations
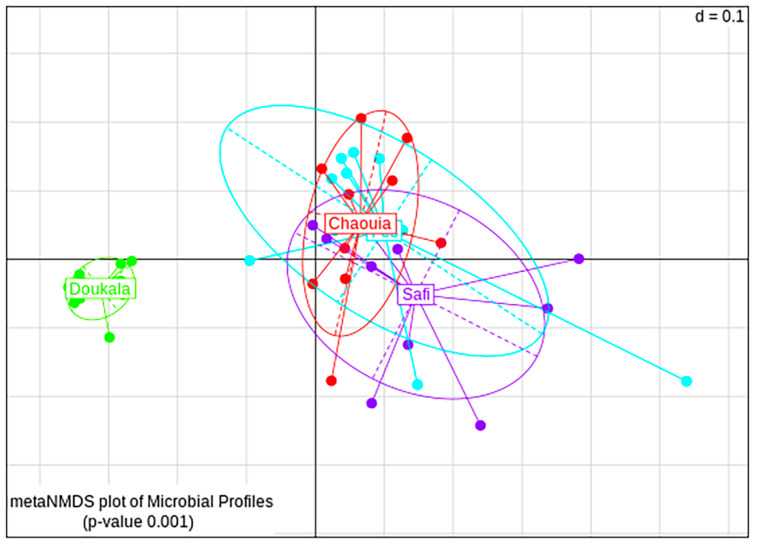
Based on beta-diversity analysis, the bacterial communities seemed to be affected statistically by the origin of the insects. The NMDS plots based on generalized UniFrac distance showed that samples derived from Chaouia, Fes and Safi overlapped, whereas samples from Doukkala formed a different cluster (PERMANOVA, p < 0.001, Figure 5). The pairwise comparison showed that the samples from the four regions were significantly separated (PERMANOVA, p < 0.05, Figure S3). The distant separation of the samples derived from the region of Doukkala resulted from the significantly high abundance of Bacteroidetes, which decreased the overall diversity of the bacterial community in this region. In contrast, no differences were observed between the bacterial communities of males and females (PERMANOVA, p = 0.217, Figure S4).
Figure 5.
Non-metric multidimensional scaling (NMDS) plot of bacterial communities for Hessian flies samples collected from Chaouia (red), Doukkala (green), Fes (cyan) and Safi (purple) (p < 0.001). ‘d’ indicates dissimilarity scale of the grid (d = 0.1 mean that the distance between two grid lines represent approximately 10% dissimilarity between the samples).
4. Discussion
In the present study, we investigated the natural and laboratory populations of Hessian flies for the presence of maternally transmitted symbionts, as well as we identified the bacterial communities and diversity present in the natural Hessian fly adults collected from four different wheat producing areas in Morocco using HTS sequencing of V3-V4 region of the 16S rRNA gene.
PCR with primers specific to the studied symbionts revealed that all the screened Hessian flies collected from Moroccan fields and a laboratory population maintained in ICARDA did not harbor any Spiroplasma, Arsenophonus or Cardinium infections. However, five out of 28 flies from Doukkala region and eight males out of 80 flies from the laboratory colony harbor Wolbachia. Wolbachia infections were also detected in HTS DNA sequence datasets from 31 out of 40 individuals of the four studied natural populations. The level of detection was significantly higher in two individuals from Safi (42.84% and 38.13%) and one individual from Fes (28.96%). However, the Wolbachia related reads represents less than 11% in the other individuals (Table S3). The difference of prevalence between the two approaches may be related to the low Wolbachia infection density and the increased sensitivity of HTS compared to the standard PCR screening [60,61]. It should be noted that Wolbachia had never been detected in natural populations of the Hessian fly [44,45,62]. To our knowledge, among the members of gall midges family, Wolbachia was reported only in two species, Asian rice gall midge (Orseolia oryzae) [63] and pine needle gall midge (Thecodiplosis japonensis) [64]. However, wide range of agricultural pests known to harbor one or multiple strains of Wolbachia including Aphids [20,46,65,66], fruit flies members of Tephritidae family [67,68,69,70,71,72] as well as members of Drosophilidae family [73,74,75,76]. Currently, there are 16 identified major supergroups of Wolbachia strains named from A to Q with exception of supergroup G which has been considered as combination of A and B supergroups [46,77,78,79,80,81]. The characterization of Wolbachia strains is primarily based on the 16S rRNA gene, multi locus sequence typing systems (MLST) using five conserved genes as molecular markers (gatB, coxA, hcpA, ftsZ and fbpA) as well as the Wolbachia surface protein (wsp) gene [82,83]. Our attempts to characterize Wolbachia strains detected in Hessian fly laboratory and natural populations using MLST and wsp genes, were constrained by amplification issues possibly due to the low infection density which complicate the detection and strain characterization of Wolbachia infections [84,85,86,87,88]. Thus, our phylogenetic analyses were based only on 16S rRNA sequences amplified using Wolbachia specific primers and the Wolbachia related sequence obtained using HTS. All the Wolbachia sequences obtained in this study appeared to be most homologous to the strains belonging to supergroup A (Figure 1 and Figure 4), suggesting that the origin of Hessian fly samples did not conduct to Wolbachia strain divergence. It has been previously observed that the density of Wolbachia in a host affects the level of CI that occurs [89]. Therefore, the low infection rate observed in our study suggests that Wolbachia most likely does not result in reproductive incompatibility between Hessian flies. Moreover, PCR detection of Wolbachia in both males and females, may suggest that no female-biased sex ratio effects should be found in response to infection. Although Wolbachia are usually transmitted vertically, the possibility that the infected population from Doukkala could be the result of horizontal transmission cannot be excluded since this mode of transmission is common among and within insect species [90,91] and could be mediated through host plants [92] or parasitoids [93]. Meanwhile, our finding sheds a new light on the ability of Hessian flies to carry a Wolbachia-infection, which may lead us to produce a stable Wolbachia-infected line using the transinfection technique. This technique aims to transfer symbiont strains to new hosts within the same species or between different species. It has already been applied for Wolbachia in Aedes albopictus [94,95], Anopheles stephensi [96], Aedes polynesiensis [97], Aedes aegypti [98,99], Ceratitis capitata [100], and Bactrocera oleae [101].
In addition to Wolbachia infection status, we assessed whether geographical origin affect the bacterial composition of Hessian flies. Based on HTS sequencing, we identified several distinct OTUs from four different Hessian fly populations (Table S2 and Figure S5). Our results revealed that the microbiota of adult Hessian flies was significantly influenced by their native regions. Samples from Chaouia, Fes and Safi exhibited a higher number of OTUs and higher species richness indicating that these samples contained more diverse microbiota compared to the samples from Doukkala region. Members of Proteobacteria were the predominant bacterial taxa in samples derived from Chaouia, Fes and Safi regions, while samples from Doukkala were dominated mainly by Bacteroidetes due to the abnormally high relative abundance of Empedobacter taxon, and Proteobacteria as a second most abundant phylum. The presence of Proteobacteria in all samples could be due to the fact that maggots recruit Proteobacteria from the wheat hosts during the feeding stages of Hessian flies (1st and 2nd instar) and these are subsequently transferred to adults across developmental stages [102,103,104,105]. At the genus level, an earlier study using culture-independent and standard sequencing approaches, identified Ochrobactrum member of Alphaproteobacteria as the dominant taxa in Hessian fly adults, followed by Alcaligenes member of Betaproteobacteria, Arthrobacter and Microbacterium members of Actinobacteria and Sphingobacterium member of Bacteroidia [44]. However, in our study, diverse bacterial genera were detected in Hessian fly across the different regions. The most abundant taxa were Ralstonia member of Betaproteobacteria in Chaouia, Pseudomonas member of Gammaproteobacteria in Safi, Afipia a member of Alphaproteobacteria in Fes and Empedobacter a member of Bacteroidetes, in Doukkala. Low abundance of the previously mentioned genera [44] (<5%) were identified as well in Hessian flies from Chaouia, Fes and Safi. The differences of the bacterial composition in Hessian flies could be influenced by the wheat cultivars used in the fields. Indeed, the effects of wheat cultivars on the Hessian fly larvae and adults’ microbiota need to be elucidated. Additional to the host plant, other factors could also contribute to shaping the bacterial composition including climatic conditions, soil microorganisms and infection with symbionts like Wolbachia [106,107,108,109,110]. Further works on the Hessian flies and their environment are therefore required to understand the origin and the role of different symbiont associated to Hessian fly populations.
5. Conclusions
The geographical location was found to affect significantly the bacterial composition in Hessian fly adults. Higher number of OTUs dominated by members of Proteobacteria were observed in the flies derived from Chaouia, Fes and Safi. However, those derived from Doukkala region were abnormally dominated by Empedobacter, a member of the Bacteroidetes/Chlorobi group, which may be due to the local environments and/or the wheat cultivar used. To the best of our knowledge, this study documents the first cases of Wolbachia infection in Hessian fly populations. Phylogenetic analyses based on 16S rRNA gene revealed the presence of only supergroup A Wolbachia strain in all the infected flies. Since the Wolbachia infection occurs in low titer, reproductive incompatibility may not be induced in the studied populations. However, the presence of this infection may give a new insight into the use of Wolbachia for the fight against Hessian fly populations. This could be tested using an experimental transfer of a Wolbachia strain known to induce a high level of cytoplasmic incompatibility into Hessian flies for the suppression of natural populations.
Acknowledgments
The authors are grateful to the ICARDA entomology staff (Abdelhadi Sabraoui and Karim El Fakhouri) for the collection and rearing of Hessian fly populations from the different wheat regions of Morocco.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/6/340/s1, Table S1: Primer pairs used in PCR amplifications, Table S2: Relative abundance (%) of different bacteria detected in Hessian fly Samples derived from Safi, Fes, Chaouia and Doukkala regions, Table S3: Hessian flies positive for Wolbachia screened using PCR and the number of Wolbachia related reads and their frequencies per samples, Figure S1: Detection of Wolbachia in Hessian flies using PCR and electrophoretic separation on 1.5% agarose gel., Figure S2: LEfSe results of Hessian flies’ microbiota, Figure S3: Pairwise non-metric multidimensional scaling (NMDS) plot of bacterial communities for Hessian fly samples collected from Chaouia (red), Doukkala (green), Fes (cyan) and Safi (purple), Figure S4: Non-metric multidimensional scaling (NMDS) plot of bacterial communities for males (cyan) and females (red) of Hessian fly samples, Figure S5: Heat map showing relative distribution and relative abundance of OTUs detected in Hessian fly samples at genus level.
Author Contributions
Conceptualization, A.M., M.E.B. and G.T.; methodology, P.S., E.A. and G.T.; analysis, N.B.M., E.A. and G.T.; resources, G.T.; writing—original draft preparation, N.B.M.; writing—review and editing, N.B.M., A.M., M.R.B., M.E.B., P.S., E.A., C.B. and G.T.; supervision, A.M. and G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been conducted with the support of the Erasmus+ Programme of the European Union. Also, this work was co-funded by Greece and the European Union in the framework of the action titled “Innovation in Aquaculture” managed by the Special Management Service for Fisheries and Sea.
Conflicts of Interest
The authors declare that there is no conflict of interest in this work.
References
- 1.El Bouhssini M., Ogbonnaya F.C., Chen M., Lhaloui S., Rihawi F., Dabbous A. Sources of resistance in primary synthetic hexaploid wheat (Triticum aestivum L.) to insect pests: Hessian fly, Russian wheat aphid and Sunn pest in the fertile crescent. Genet. Resour. Crop Evol. 2013;60:621–627. doi: 10.1007/s10722-012-9861-3. [DOI] [Google Scholar]
- 2.Lhaloui S., Buschman L., El Bouhssini M., Starks K., Keith D.L., El Houssaini K. Control of Mayetiola species (Diptera: Cecidomyiidae) with carbofuran in bread wheat, durum wheat and barley with yield loss assessment and its economic analysis. Al Awamia. 1992;77:55–73. [Google Scholar]
- 3.Lhaloui S. Biology, Host Preference, Host Suitability, and Plant. Resistance Studies of the Barley Stem Gall. Midge and Hessian Fly (Diptera: Cecidomyiidae) in Morocco. Kansas State University; Manhattan, KS, USA: 1995. [Google Scholar]
- 4.Harris M.O., Rose S. Factors influencing the onset of egglaying in a cecidomyiid fly. Physiol. Entomol. 1991;16:183–190. doi: 10.1111/j.1365-3032.1991.tb00555.x. [DOI] [Google Scholar]
- 5.Bergh J.C., Harris M.O., Rose S. Temporal Patterns of Emergence and Reproductive Behavior of the Hessian Fly (Diptera: Cecidomyiidae) Ann. Entomol. Soc. Am. 1990;83:998–1004. doi: 10.1093/aesa/83.5.998. [DOI] [Google Scholar]
- 6.Stuart J.J., Chen M.-S., Shukle R., Harris M.O. Gall Midges (Hessian Flies) as Plant Pathogens. Annu. Rev. Phytopathol. 2012;50:339–357. doi: 10.1146/annurev-phyto-072910-095255. [DOI] [PubMed] [Google Scholar]
- 7.Shukle R.H. Hessian Fly, Mayetiola destructor (Say) (Diptera: Cecidomyiidae) In: Capinera J.L., editor. Encyclopedia of Entomology. Springer; Dordrecht, The Netherlands: 2008. pp. 1794–1797. [Google Scholar]
- 8.Gagné R.J., Hatchett J.H. Instars of the Hessian Fly (Diptera: Cecidomyiidae) Ann. Entomol. Soc. Am. 1989;82:73–79. doi: 10.1093/aesa/82.1.73. [DOI] [Google Scholar]
- 9.Schmid R.B., Knutson A., Giles K.L., McCornack B.P. Hessian Fly (Diptera: Cecidomyiidae) Biology and Management in Wheat. J. Integr. Pest. Manag. 2018;9 doi: 10.1093/jipm/pmy008. [DOI] [Google Scholar]
- 10.Naber N., El Bouhssini M., Lhaloui S. Biotypes of Hessian fly (Dipt., Cecidomyiidae) in Morocco. J. Appl. Entomol. 2003;127:174–176. doi: 10.1046/j.1439-0418.2003.00738.x. [DOI] [Google Scholar]
- 11.El Bouhssini M., Lhaloui S., Amri A., Jlibene M., Hatchett J.H., Nssarellah N., Nachitt M. Wheat genetic control of Hessian fly (Diptera: Cecidomyiidae) in Morocco. Field Crops Res. 1996;45:111–114. doi: 10.1016/0378-4290(95)00063-1. [DOI] [Google Scholar]
- 12.Ami E.B., Yuval B., Jurkevitch E. Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J. 2010;4:28–37. doi: 10.1038/ismej.2009.82. [DOI] [PubMed] [Google Scholar]
- 13.Augustinos A.A., Kyritsis G.A., Papadopoulos N.T., Abd-Alla A.M.M., Cáceres C., Bourtzis K. Exploitation of the Medfly Gut Microbiota for the Enhancement of Sterile Insect Technique: Use of Enterobacter sp. in Larval Diet-Based Probiotic Applications. PLoS ONE. 2015;10:e0136459. doi: 10.1371/journal.pone.0136459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Yosef M., Pasternak Z., Jurkevitch E., Yuval B. Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. J. Evol. Biol. 2014;27:2695–2705. doi: 10.1111/jeb.12527. [DOI] [PubMed] [Google Scholar]
- 15.Gavriel S., Jurkevitch E., Gazit Y., Yuval B. Bacterially enriched diet improves sexual performance of sterile male Mediterranean fruit flies. J. Appl. Entomol. 2011;135:564–573. doi: 10.1111/j.1439-0418.2010.01605.x. [DOI] [Google Scholar]
- 16.Gavriel S., Gazit Y., Yuval B. Effect of diet on survival, in the laboratory and the field, of sterile male Mediterranean fruit flies. Entomol. Exp. Appl. 2010;135:96–104. doi: 10.1111/j.1570-7458.2010.00972.x. [DOI] [Google Scholar]
- 17.Kyritsis G.A., Augustinos A.A., Cáceres C., Bourtzis K. Medfly Gut Microbiota and Enhancement of the Sterile Insect Technique: Similarities and Differences of Klebsiella oxytoca and Enterobacter sp. AA26 Probiotics during the Larval and Adult Stages of the VIENNA 8D53+ Genetic Sexing Strain. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Contreras M., Vlisidou I. The Diversity of Insect-bacteria Interactions and its Applications for Disease Control. Biotechnol. Genet. Eng. Rev. 2008;25:203–244. doi: 10.5661/bger-25-203. [DOI] [PubMed] [Google Scholar]
- 19.Bourtzis K. Wolbachia-Based Technologies for Insect Pest Population Control. In: Aksoy S., editor. Transgenesis and the Management of Vector-Borne Disease. Volume 627. Springer; New York, NY, USA: 2008. pp. 104–113. [DOI] [PubMed] [Google Scholar]
- 20.Jeyaprakash A., Hoy M.A. Long PCR improves Wolbachia DNA amplification: Wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 21.Saridaki A., Bourtzis K. Wolbachia: More than just a bug in insects genitals. Curr. Opin. Microbiol. 2010;13:67–72. doi: 10.1016/j.mib.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Bourtzis K. Wolbachia-Induced Cytoplasmic Incompatibility to Control Insect Pests? In: Vreysen M.J.B., Robinson A.S., Hendrichs J., editors. Area-Wide Control of Insect Pests. Springer; Dordrecht, The Netherlands: 2007. pp. 125–135. [Google Scholar]
- 23.Bourtzis K., Braig H.R., Karr T.L. Cytoplasmic Incompatibility. In: Bourtzis K., Miller T.A., editors. Insect Symbiosis. Volume 1. CRC Press; Boca Raton, FL, USA: 2003. pp. 217–246. [Google Scholar]
- 24.Correa C.C., Ballard J.W.O. Wolbachia Associations with Insects: Winning or Losing Against a Master Manipulator. Front. Ecol. Evol. 2016;3 doi: 10.3389/fevo.2015.00153. [DOI] [Google Scholar]
- 25.Braquart-Varnier C., Altinli M., Pigeault R., Chevalier F.D., Grève P., Bouchon D., Sicard M. The Mutualistic Side of Wolbachia-Isopod Interactions: Wolbachia Mediated Protection against Pathogenic Intracellular Bacteria. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikoh N., Hosokawa T., Moriyama M., Oshima K., Hattori M., Fukatsu T. Evolutionary origin of insect–Wolbachia nutritional mutualism. Proc. Natl. Acad. Sci. USA. 2014;111:10257–10262. doi: 10.1073/pnas.1409284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bian G., Xu Y., Lu P., Xie Y., Xi Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes aegypti. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaser R.L., Meola M.A. The Native Wolbachia Endosymbionts of Drosophila melanogaster and Culex quinquefasciatus Increase Host Resistance to West Nile Virus Infection. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne S.E., Leong Y.S., O’Neill S.L., Johnson K.N. Variation in Antiviral Protection Mediated by Different Wolbachia Strains in Drosophila simulans. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedges L.M., Brownlie J.C., O’Neill S.L., Johnson K.N. Wolbachia and Virus Protection in Insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 31.Dobson S.L., Rattanadechakul W., Marsland E.J. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity. 2004;93:135–142. doi: 10.1038/sj.hdy.6800458. [DOI] [PubMed] [Google Scholar]
- 32.Brownlie J.C., Cass B.N., Riegler M., Witsenburg J.J., Iturbe-Ormaetxe I., McGraw E.A., O’Neill S.L. Evidence for Metabolic Provisioning by a Common Invertebrate Endosymbiont, Wolbachia pipientis, during Periods of Nutritional Stress. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiggins F.M., Hurst G.D., Jiggins C.D., vd Schulenburg J.H., Majerus M.E. The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology. 2000;120:439–446. doi: 10.1017/S0031182099005867. [DOI] [PubMed] [Google Scholar]
- 34.Xie J., Butler S., Sanchez G., Mateos M. Male killing Spiroplasma protects Drosophila melanogaster against two parasitoid wasps. Heredity. 2014;112:399. doi: 10.1038/hdy.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Łukasik P., Guo H., van Asch M., Ferrari J., Godfray H.C.J. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J. Evol. Biol. 2013;26:2654–2661. doi: 10.1111/jeb.12260. [DOI] [PubMed] [Google Scholar]
- 36.Xie J., Vilchez I., Mateos M. Spiroplasma Bacteria Enhance Survival of Drosophila hydei Attacked by the Parasitic Wasp Leptopilina heterotoma. PLoS ONE. 2010;5:e12149. doi: 10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y.-K., Chen Y.-T., Yang K., Hong X.-Y. A review of prevalence and phylogeny of the bacterial symbiont Cardinium in mites (subclass: Acari) Syst. Appl. Acarol. 2016;21:978–990. doi: 10.11158/saa.21.7.11. [DOI] [Google Scholar]
- 38.Kageyama D., Narita S., Watanabe M. Insect Sex Determination Manipulated by Their Endosymbionts: Incidences, Mechanisms and Implications. Insects. 2012;3:161–199. doi: 10.3390/insects3010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giorgini M., Monti M.M., Caprio E., Stouthamer R., Hunter M.S. Feminization and the collapse of haplodiploidy in an asexual parasitoid wasp harboring the bacterial symbiont Cardinium. Heredity. 2009;102:365–371. doi: 10.1038/hdy.2008.135. [DOI] [PubMed] [Google Scholar]
- 40.Provencher L.M., Morse G.E., Weeks A.R., Normark B.B. Parthenogenesis in the Aspidiotus nerii Complex (Hemiptera: Diaspididae): A Single Origin of a Worldwide, Polyphagous Lineage Associated with Cardinium Bacteria. Ann. Entomol. Soc. Am. 2005;98:629–635. doi: 10.1603/0013-8746(2005)098[0629:PITANC]2.0.CO;2. [DOI] [Google Scholar]
- 41.Zchori-Fein E., Perlman S.J., Kelly S.E., Katzir N., Hunter M.S. Characterization of a ‘Bacteroidetes’ symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): Proposal of ‘Candidatus Cardinium hertigii’. Int. J. Syst. Evol. Microbiol. 2004;54:961–968. doi: 10.1099/ijs.0.02957-0. [DOI] [PubMed] [Google Scholar]
- 42.Duron O., Bouchon D., Boutin S., Bellamy L., Zhou L., Engelstädter J., Hurst G.D. The diversity of reproductive parasites among arthropods: Wolbachiado not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferree P.M., Avery A., Azpurua J., Wilkes T., Werren J.H. A Bacterium Targets Maternally Inherited Centrosomes to Kill Males in Nasonia. Curr. Biol. 2008;18:1409–1414. doi: 10.1016/j.cub.2008.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal R., Hulbert S., Schemerhorn B., Reese J.C., Whitworth R.J., Stuart J.J., Chen M.-S. Hessian Fly-Associated Bacteria: Transmission, Essentiality, and Composition. PLoS ONE. 2011;6:e23170. doi: 10.1371/journal.pone.0023170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson A.J., Schemerhorn B.J., Shukle R.H. A First Assessment of Mitochondrial DNA Variation and Geographic Distribution of Haplotypes in Hessian fly (Diptera: Cecidomyiidae) Ann. Entomol. Soc. Am. 2004;97:940–948. doi: 10.1603/0013-8746(2004)097[0940:AFAOMD]2.0.CO;2. [DOI] [Google Scholar]
- 46.Augustinos A.A., Santos-Garcia D., Dionyssopoulou E., Moreira M., Papapanagiotou A., Scarvelakis M., Doudoumis V., Ramos S., Aguiar A.F., Borges P.A.V., et al. Detection and Characterization of Wolbachia Infections in Natural Populations of Aphids: Is the Hidden Diversity Fully Unraveled? PLoS ONE. 2011;6:e28695. doi: 10.1371/journal.pone.0028695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartley L.J., Bowen H. PEG precipitation for selective removal of small DNA fragments. Focus. 1996;18:27. [Google Scholar]
- 48.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 49.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, Interactive, Scalable, and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgar R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 51.Edgar R.C. UNCROSS2: Identification of cross-talk in 16S rRNA OTU tables. BioRxiv. 2018:400762. doi: 10.1101/400762. [DOI] [Google Scholar]
- 52.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J., Bittinger K., Charlson E.S., Hoffmann C., Lewis J., Wu G.D., Collman R.G., Bushman F.D., Li H. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics. 2012;28:2106–2113. doi: 10.1093/bioinformatics/bts342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lefort V., Longueville J.-E., Gascuel O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017;34:2422–2424. doi: 10.1093/molbev/msx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akaike H. Autoregressive model fitting for control. Ann. Inst. Stat. Math. 1971;23:163–180. doi: 10.1007/BF02479221. [DOI] [Google Scholar]
- 60.Comar M., D’Accolti M., Cason C., Soffritti I., Campisciano G., Lanzoni L., Bisi M., Volta A., Mazzacane S., Caselli E. Introduction of NGS in Environmental Surveillance for Healthcare-Associated Infection Control. Microorganisms. 2019;7:708. doi: 10.3390/microorganisms7120708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao Y., Fanning S., Proos S., Jordan K., Srikumar S. A Review on the Applications of Next Generation Sequencing Technologies as Applied to Food-Related Microbiome Studies. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson A.J., Morton P.K., Schemerhorn B.J., Shukle R.H. Use of a Nuclear Marker to Assess Population Structure in Hessian Fly (Diptera: Cecidomyiidae) Ann. Entomol. Soc. Am. 2011;104:666–674. doi: 10.1603/AN10154. [DOI] [Google Scholar]
- 63.Behura S.K., Sahu S.C., Mohan M., Nair S. Wolbachia in the Asian rice gall midge, Orseolia oryzae (Wood-Mason): Correlation between host mitotypes and infection status. Insect Mol. Biol. 2001;10:163–171. doi: 10.1046/j.1365-2583.2001.00251.x. [DOI] [PubMed] [Google Scholar]
- 64.KimMi G., HyunWoo O., HeeMoon P., HoYong P. Molecular identification of Wolbachia naturally infected in Thecodiplosis japonensis (Diptera: Cecidominideii) Korean J. Entomol. 2000;30:139–146. [Google Scholar]
- 65.Zytynska S.E., Weisser W.W. The natural occurrence of secondary bacterial symbionts in aphids. Ecol. Entomol. 2016;41:13–26. doi: 10.1111/een.12281. [DOI] [Google Scholar]
- 66.Wang Z., Shen Z.-R., Song Y., Liu H.-Y., Li Z.-X. Distribution and diversity of Wolbachia in different populations of the wheat aphid Sitobion miscanthi (Hemiptera: Aphididae) in China. EJE. 2009;106:49–55. doi: 10.14411/eje.2009.007. [DOI] [Google Scholar]
- 67.Asimakis E.D., Doudoumis V., Hadapad A.B., Hire R.S., Batargias C., Niu C., Khan M., Bourtzis K., Tsiamis G. Detection and characterization of bacterial endosymbionts in Southeast Asian tephritid fruit fly populations. BMC Microbiol. 2019;19:290. doi: 10.1186/s12866-019-1653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mateos M., Martinez H., Lanzavecchia S.B., Conte C., Guillén K., Morán-Aceves B.M., Toledo J., Liedo P., Asimakis E.D., Doudoumis V., et al. Wolbachia pipientis associated to tephritid fruit fly pests: From basic research to applications. BioRxiv. 2018:358333. doi: 10.1101/358333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yong H.-S., Song S.-L., Chua K.-O., Lim P.-E. Predominance of Wolbachia endosymbiont in the microbiota across life stages of Bactrocera latifrons (Insecta: Tephritidae) Meta Gene. 2017;14:6–11. doi: 10.1016/j.mgene.2017.07.007. [DOI] [Google Scholar]
- 70.Schuler H., Arthofer W., Riegler M., Bertheau C., Krumböck S., Köppler K., Vogt H., Teixeira L.A., Stauffer C. Multiple Wolbachia infections in Rhagoletis pomonella. Entomol. Exp. Appl. 2011;139:138–144. doi: 10.1111/j.1570-7458.2011.01115.x. [DOI] [Google Scholar]
- 71.Sarakatsanou A., Diamantidis A.D., Papanastasiou S.A., Bourtzis K., Papadopoulos N.T. Effects of Wolbachia on fitness of the Mediterranean fruit fly (Diptera: Tephritidae) J. Appl. Entomol. 2011;135:554–563. doi: 10.1111/j.1439-0418.2011.01610.x. [DOI] [Google Scholar]
- 72.Sun X., Cui L., Li Z. Diversity and phylogeny of Wolbachia infecting Bactrocera dorsalis (Diptera: Tephritidae) populations from China. Environ. Entomol. 2007;36:1283–1289. doi: 10.1603/0046-225X(2007)36[1283:DAPOWI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 73.Richardson K.M., Schiffer M., Griffin P.C., Lee S.F., Hoffmann A.A. Tropical Drosophila pandora carry Wolbachia infections causing cytoplasmic incompatibility or male killing. Evolution. 2016;70:1791–1802. doi: 10.1111/evo.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamm C.A., Begun D.J., Vo A., Smith C.C.R., Saelao P., Shaver A.O., Jaenike J., Turelli M. Wolbachia do not live by reproductive manipulation alone: Infection polymorphism in Drosophila suzukii and D. subpulchrella. Mol. Ecol. 2014;23:4871–4885. doi: 10.1111/mec.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mateos M., Castrezana S.J., Nankivell B.J., Estes A.M., Markow T.A., Moran N.A. Heritable Endosymbionts of Drosophila. Genetics. 2006;174:363–376. doi: 10.1534/genetics.106.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bourtzis K., Nirgianaki A., Markakis G., Savakis C. Wolbachia Infection and Cytoplasmic Incompatibility in Drosophila Species. Genetics. 1996;144:1063–1073. doi: 10.1093/genetics/144.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moreira M., Aguiar A.M.F., Bourtzis K., Latorre A., Khadem M. Wolbachia (Alphaproteobacteria: Rickettsiales) Infections in Isolated Aphid Populations from Oceanic Islands of the Azores Archipelago: Revisiting the Supergroups M and N. Environ. Entomol. 2019;48:326–334. doi: 10.1093/ee/nvy189. [DOI] [PubMed] [Google Scholar]
- 78.Gerth M. Classification of Wolbachia (Alphaproteobacteria, Rickettsiales): No evidence for a distinct supergroup in cave spiders. Infect. Genet. Evol. 2016;43:378–380. doi: 10.1016/j.meegid.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 79.Wang G.-H., Jia L.-Y., Xiao J.-H., Huang D.-W. Discovery of a new Wolbachia supergroup in cave spider species and the lateral transfer of phage WO among distant hosts. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016;41:1–7. doi: 10.1016/j.meegid.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 80.Glowska E., Dragun-Damian A., Dabert M., Gerth M. New Wolbachia supergroups detected in quill mites (Acari: Syringophilidae) Infect. Genet. Evol. 2015;30:140–146. doi: 10.1016/j.meegid.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 81.Ros V.I.D., Fleming V.M., Feil E.J., Breeuwer J.A.J. How Diverse Is the Genus Wolbachia? Multiple-Gene Sequencing Reveals a Putatively New Wolbachia Supergroup Recovered from Spider Mites (Acari: Tetranychidae) Appl. Environ. Microbiol. 2009;75:1036–1043. doi: 10.1128/AEM.01109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baldo L., Hotopp J.C.D., Jolley K.A., Bordenstein S.R., Biber S.A., Choudhury R.R., Hayashi C., Maiden M.C.J., Tettelin H., Werren J.H. Multilocus Sequence Typing System for the Endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou W., Rousset F., O’Neil S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. B Biol. Sci. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Miguel T., Zhu O., Villa T.G. Horizontal Gene Transfer Between Wolbachia and Animals. In: Villa T.G., Viñas M., editors. Horizontal Gene Transfer: Breaking Borders Between Living Kingdoms. Springer International Publishing; Cham, Switzerland: 2019. pp. 227–234. [Google Scholar]
- 85.Husnik F., McCutcheon J.P. Functional horizontal gene transfer from bacteria to eukaryotes. Nat. Rev. Microbiol. 2018;16:67. doi: 10.1038/nrmicro.2017.137. [DOI] [PubMed] [Google Scholar]
- 86.Doudoumis V., Tsiamis G., Wamwiri F., Brelsfoard C., Alam U., Aksoy E., Dalaperas S., Abd-Alla A., Ouma J., Takac P., et al. Detection and characterization of Wolbachia infections in laboratory and natural populations of different species of tsetse flies (genus Glossina) BMC Microbiol. 2012;12:S3. doi: 10.1186/1471-2180-12-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hotopp J.C.D. Horizontal gene transfer between bacteria and animals. Trends Genet. 2011;27:157–163. doi: 10.1016/j.tig.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kondo N., Nikoh N., Ijichi N., Shimada M., Fukatsu T. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc. Natl. Acad. Sci. USA. 2002;99:14280–14285. doi: 10.1073/pnas.222228199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bordenstein S.R., Bordenstein S.R. Temperature Affects the Tripartite Interactions between Bacteriophage WO, Wolbachia, and Cytoplasmic Incompatibility. PLoS ONE. 2011;6:e29106. doi: 10.1371/journal.pone.0029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmed M.Z., Barro P.J.D., Ren S.-X., Greeff J.M., Qiu B.-L. Evidence for Horizontal Transmission of Secondary Endosymbionts in the Bemisia tabaci Cryptic Species Complex. PLoS ONE. 2013;8:e53084. doi: 10.1371/journal.pone.0053084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huigens M.E., de Almeida R.P., Boons P.A.H., Luck R.F., Stouthamer R. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. Biol. Sci. 2004;271:509–515. doi: 10.1098/rspb.2003.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li S.-J., Ahmed M.Z., Lv N., Shi P.-Q., Wang X.-M., Huang J.-L., Qiu B.-L. Plantmediated horizontal transmission of Wolbachia between whiteflies. ISME J. 2017;11:1019–1028. doi: 10.1038/ismej.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmed M.Z., Li S.-J., Xue X., Yin X.-J., Ren S.-X., Jiggins F.M., Greeff J.M., Qiu B.-L. The Intracellular Bacterium Wolbachia Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission. PLoS Pathog. 2015;11:e1004672. doi: 10.1371/journal.ppat.1004672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kubota M., Morii T., Miura K. In vitro cultivation of parthenogenesis-inducing Wolbachia in an Aedes albopictus cell line. Entomol. Exp. Appl. 2005;117:83–87. doi: 10.1111/j.1570-7458.2005.00335.x. [DOI] [Google Scholar]
- 95.Calvitti M., Moretti R., Lampazzi E., Bellini R., Dobson S.L. Characterization of a New Aedes albopictus (Diptera: Culicidae)—Wolbachia pipientis (Rickettsiales: Rickettsiaceae) Symbiotic Association Generated by Artificial Transfer of the w Pip Strain From Culex pipiens (Diptera: Culicidae) J. Med. Entomol. 2010;47:179–187. doi: 10.1603/ME09140. [DOI] [PubMed] [Google Scholar]
- 96.Bian G., Joshi D., Dong Y., Lu P., Zhou G., Pan X., Xu Y., Dimopoulos G., Xi Z. Wolbachia Invades Anopheles stephensi Populations and Induces Refractoriness to Plasmodium Infection. Science. 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 97.Andrews E.S., Crain P.R., Fu Y., Howe D.K., Dobson S.L. Reactive Oxygen Species Production and Brugia pahangi Survivorship in Aedes polynesiensis with Artificial Wolbachia Infection Types. PLoS Pathog. 2012;8:e1003075. doi: 10.1371/journal.ppat.1003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McMeniman C.J., Lane R.V., Cass B.N., Fong A.W.C., Sidhu M., Wang Y.-F., O’Neill S.L. Stable Introduction of a Life-Shortening Wolbachia Infection into the Mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 99.Hoffmann A.A., Montgomery B.L., Popovici J., Iturbe-Ormaetxe I., Johnson P.H., Muzzi F., Greenfield M., Durkan M., Leong Y.S., Dong Y., et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 100.Zabalou S., Riegler M., Theodorakopoulou M., Stauffer C., Savakis C., Bourtzis K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA. 2004;101:15042–15045. doi: 10.1073/pnas.0403853101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Apostolaki A., Livadaras I., Saridaki A., Chrysargyris A., Savakis C., Bourtzis K. Transinfection of the olive fruit fly Bactrocera oleae with Wolbachia: Towards a symbiont-based population control strategy. J. Appl. Entomol. 2011;135:546–553. doi: 10.1111/j.1439-0418.2011.01614.x. [DOI] [Google Scholar]
- 102.Douglas A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Ann. Rev. Entomol. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bansal R., Hulbert S.H., Reese J.C., Whitworth R.J., Stuart J.J., Chen M.-S. Pyrosequencing Reveals the Predominance of Pseudomonadaceae in Gut Microbiome of a Gall Midge. Pathogens. 2014;3:459–472. doi: 10.3390/pathogens3020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jones R.T., Sanchez L.G., Fierer N. A Cross-Taxon Analysis of Insect-Associated Bacterial Diversity. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kikuchi Y. Endosymbiotic Bacteria in Insects: Their Diversity and Culturability. Microbes Environ. 2009;24:195–204. doi: 10.1264/jsme2.ME09140S. [DOI] [PubMed] [Google Scholar]
- 106.Toju H., Fukatsu T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: Relevance of local climate and host plants. Mol. Ecol. 2011;20:853–868. doi: 10.1111/j.1365-294X.2010.04980.x. [DOI] [PubMed] [Google Scholar]
- 107.Heinen R., Biere A., Harvey J.A., Bezemer T.M. Effects of Soil Organisms on Aboveground Plant-Insect Interactions in the Field: Patterns, Mechanisms and the Role of Methodology. Front. Ecol. Evol. 2018;6 doi: 10.3389/fevo.2018.00106. [DOI] [Google Scholar]
- 108.Pineda A., Zheng S.-J., Van Loon J.J., Pieterse C.M., Dicke M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010;15:507–514. doi: 10.1016/j.tplants.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Dittmer J., Bouchon D. Feminizing Wolbachia influence microbiota composition in the terrestrial isopod Armadillidium vulgare. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-25450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simhadri R.K., Fast E.M., Guo R., Schultz M.J., Vaisman N., Ortiz L., Bybee J., Slatko B.E., Frydman H.M. The Gut Commensal Microbiome of Drosophila melanogaster Is Modified by the Endosymbiont Wolbachia. mSphere. 2017;2 doi: 10.1128/mSphere.00287-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.