Abstract
Prescription drug monitoring programs (PDMPs) have become a widely embraced policy to address the US opioid crisis. Despite mixed scientific evidence on their effectiveness at improving health and reducing overdose deaths, 49 states and Washington, DC have adopted PDMPs, and they have received strong bipartisan legislative support. This article explores the history of PDMPs, tracking their evolution from paper-based administrative databases in the early 1900s to modern-day electronic systems that intervene at the point of care. We focus on two questions: how did PDMPs become so widely adopted in the United States, and how did they gain popularity as an intervention in the contemporary opioid crisis? Through this historical approach, we evaluate what PDMPs reflect about national drug policy and broader cultural understandings of substance use disorder in the United States today. (Am J Public Health. 2020;110:1191–1197. 10.2105/AJPH.2020.305696)
As public recognition of the US opioid epidemic has grown over the last decade, policymakers have suggested a wide variety of strategies to address the crisis. Many of these policies have generated controversy, as legislators debate what the end goal of addressing the crisis should be, and whether harm reduction interventions are an appropriate response. In contrast to the politically volatile nature of harm reduction strategies such as safe injection sites and sterile needle exchange programs, interventions to reform opioid-prescribing behaviors have found broad coalitions of support. Preventative prescribing efforts that restrict the supply of available opioids—both licit and illicit—are some of the most commonly proposed solutions, receiving strong support from academics, policymakers, and the popular press.
Although the root causes of the US opioid crisis are multiple and complex, there remains a set of conventional narratives that emphasize iatrogenesis—addiction induced via physicians’ prescribing behaviors—as an important early driver of the epidemic.1 Given the durability of these narratives, as well as growing discourse on the pharmaceutical industry’s culpability in spurring the opioid epidemic,2 efforts to reduce opioid-prescribing activity at the systemwide level are unsurprising and necessary. One popular policy solution has been the use of prescription drug monitoring programs (PDMPs): databases that track controlled substance prescriptions from health care providers, usually on a statewide level.3 In these systems, providers look up a patient’s prescription history for opioids and other controlled substances in a centralized database and assess patient risk for misuse or dependence before recording their new prescription in the database. In this way, PDMPs are designed to make opioid-prescribing practices safer and prevent patients from obtaining opioid prescriptions from multiple providers inappropriately—a phenomenon called “doctor shopping.”4
Although the empirical evidence on the efficacy of PDMPs is mixed, they have garnered widespread support from policymakers and the popular press over the past decade: 49 states having adopted a PDMP as of 2018.5 Recent bipartisan federal opioid legislation provides continued funding and support for PDMPs,6 and some advocates have gone as far as suggesting that PDMP use should be legally mandated each time a physician writes a prescription for an opioid.7 Today, PDMPs are an accepted and widely disseminated mechanism to prevent doctor shopping behaviors and reduce physician-induced opioid addiction.
There has been little historical analysis of how PDMPs became such a widely adopted strategy to fight an epidemic in which many public health responses remain controversial and are inconsistently implemented. Most assessments of PDMPs have come from health services research and economics, and focus on PDMPs’ effectiveness at reducing opioid prescribing and overdose deaths. This work seems to be free of the controversy surrounding research on programs like naloxone distribution, safe injection sites, and medical treatments such as buprenorphine and methadone. Implementing electronic surveillance systems that reduce physician agency in prescribing has become a “safe” policy recommendation that sees little pushback—a surprising reality considering the historical importance of physician independence and the confidential nature of the doctor–patient relationship.8 Evaluating how policymakers and politicians have arrived at support for PDMPs can reveal much about our cultural understandings of opioid use and addiction in the 21st century.
In this article, we seek to fill the gaps in our knowledge of PDMPs by placing their current popularity in the context of these programs’ longer history, as well as in the context of broader public health efforts to address the contemporary opioid epidemic. Through this historical perspective, we seek to understand how PDMPs gained popularity as a public health intervention to address the opioid epidemic, despite mixed scientific evidence of their efficacy and a wide range of potentially more effective strategies.
BUILDING A SURVEILLANCE NETWORK
Prescription drug monitoring in the United States began well before the contemporary opioid crisis. Historians such as Acker, Campbell, Courtwright, and others have shown that record-keeping and police surveillance were crucial elements of the US narcotic control system from its 19th-century origins.9 The earliest documented PDMP in the United States (although not termed as such) dates to 1914, when New York State established a short-lived system to track prescriptions of opiates under the Boylan Act.10 Unlike earlier systems that emphasized pharmacist record-keeping, the New York model was the first to require prescribing physicians to submit duplicate prescription forms to a centralized state database. This New York system emerged on the heels of the Harrison Narcotic Tax Act of 1914, during an era when the federal government was making an unprecedented effort to regulate the sale and usage of addictive drugs.11 Many providers who were deemed “over-prescribers” of opioids during this period were prosecuted, leading to a culture of “opio-phobia” that endures in the American medical profession today.12 In the New York model, physicians were required to write prescriptions for controlled substances on state-issued, numbered prescription notes, which were then sent to a state registry by the pharmacy. Pharmacists were required to verify these prescriptions with the prescribing physician by telephone or other methods before dispensing the drug. This New York prescription tracking system lasted only three years. In 1917 the Boylan Act was superseded by the more permissive Whitney Act, which permitted physicians to prescribe opioids with fewer restrictions because of concerns that supply-side restrictions were fueling the illicit opioid market.13
The next state-level PDMP was the “California Triplicate Prescription Program,” established in 1939. Administrated by the Bureau of Narcotic Enforcement, the California program set the blueprint for later PDMPs in the 20th century; it used state-issued prescription forms for controlled substances such as opiates and cocaine, to be completed in duplicate or triplicate so that the dispensing pharmacist could send a record of the prescription to a state database via mail.14 Several other states implemented PDMPs modeled on the California program in subsequent decades: Hawaii (1943), Illinois (1961), Idaho (1967), New York (1973),15 Rhode Island (1978), Texas (1981), and Michigan (1988), among others.16
PATIENT PRIVACY AND CONSTITUTIONALITY
Although physician prescribing behaviors and drug distribution practices had both been adjudicated by the US court system from the early 20th century, the legal questions around narcotic prescription surveillance took on a new valence during the 1960s and 1970s, when a series of prominent legal decisions articulated medical privacy as a constitutionally protected right.17 The 1965 decision in Griswold v Connecticut stated that a right to privacy can be inferred from several amendments in the Bill of Rights, and that this right to privacy specifically applied to married couples seeking contraception. Later decisions rendered in the cases of Roe v Wade and Doe v Bolton upheld that although states do have the right to interfere in health care, their power is limited in various ways.18 Both cases touched on the importance of the doctor–patient relationship and an implied, if not specifically enumerated, constitutional right to privacy in medical care.
In 1977, the legality of PDMPs was explicitly adjudicated in the courts. In the case Whalen v Roe, a group of patients and physicians challenged the legality of New York’s PDMP, which was established after the passage of the New York Controlled Substances Act of 1972. In New York’s PDMP system, the names and addresses of all persons who were prescribed Schedule II drugs (such as oxycodone and hydromorphone) were registered in a centralized database. The plaintiffs alleged that this practice “violated the patient’s right of privacy and interfered with the doctor’s right to prescribe treatment for his patient solely on the basis of medical considerations.”19 Whalen considered whether the records generated by the New York PDMP violated a “right to privacy” as protected by the Fourteenth Amendment. The Supreme Court ultimately ruled that disclosure of patient identifiers in the process of prescribing controlled substances was not a violation of Fourteenth Amendment freedoms.20
The Whalen opinion established a clear difference between laws that mandated PDMPs and those that criminalized abortion; the PDMP was understood as a state administrative reporting requirement, rather than a case of a state prohibiting the delivery of a medical procedure. Justice John Paul Stevens specifically highlighted that the decision to use or prescribe controlled substances is entirely left to the physician and the patient, and that the state is not intervening to prevent the use of opioids. In this way, the Whalen ruling defined the PDMP as a state law enforcement tool for preventing unlawful diversion of controlled substances, not an instrument of medicine and public health.
PRESCRIPTION DRUG MONITORING PROGRAMS IN THE ELECTRONIC ERA
The rise of the Internet in the 1990s revolutionized the use of PDMPs in the United States. During these same years, the medical community was adopting a more liberal stance toward pain management, even deeming pain the “fifth vital sign.”21 This combination of technological and professional change contributed to the widespread growth of PDMPs in the 21st century.
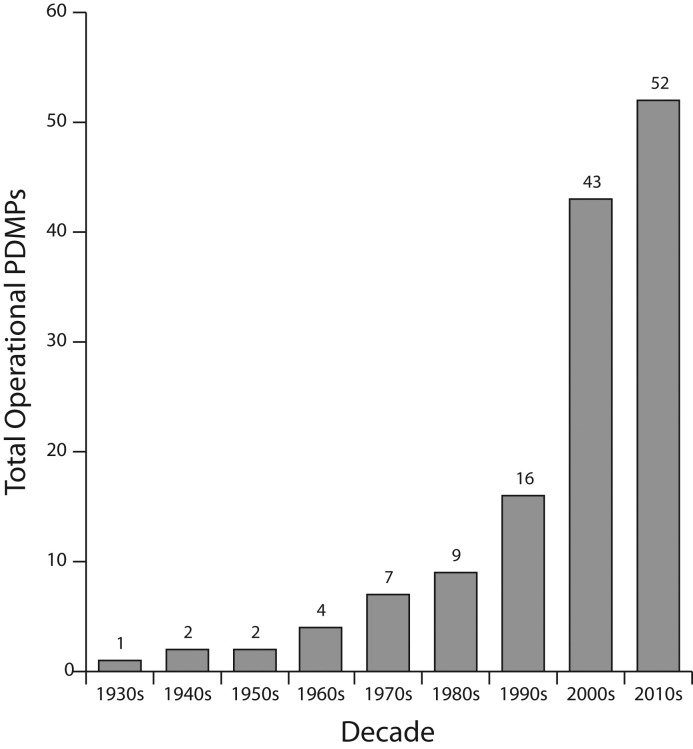
The electronic era saw a sharp increase in PDMP adoption across the United States. In 1990, Oklahoma became the first state to have a completely electronic PDMP with routine electronic data transmissions from pharmacies to a centralized database. Five more state PDMPs were established in the 1990s—in Nevada, Massachusetts, Utah, Indiana, and Kentucky—and another in the US territory of Guam. The 2000s saw another wave of expansion, with 27 PDMPs coming online between 2000 and 2009, and an additional eight between 2010 and 2019. PDMPs are now in use in 49 states, the District of Columbia, Guam, and Puerto Rico.22 Missouri is now the only state without a statewide PDMP, although the city of St. Louis operates one.23 Figure 1 illustrates this growth of operational PDMPs in the United States.
FIGURE 1—
Total Operational Prescription Drug Monitoring Programs (PDMPs) in the United States
Source. Fishman et al.24
The Internet fundamentally changed the way PDMPs were used. For the first time, prescribers could query PDMP databases directly and in real time to obtain information on a patient’s past and current controlled substance prescriptions. At the same time that these new PDMP capabilities were coming online, the medical community was experiencing a revolution in how to conceptualize and treat pain. Driven in part by the professionalization of new medical specialties such as pain medicine and palliative care, as well as Purdue Pharma’s aggressive marketing of OxyContin, medical societies and physicians began to monitor and treat pain—especially chronic, nonmalignant pain—more aggressively.25 This focus on pain management fueled demand for opioid prescriptions, and created new markets to supply them.26
In this context, electronic PDMPs became prominent tools for monitoring opioid misuse. Today, some states even mandate that prescribers query the PDMP before writing an opioid prescription, rather than simply reporting their prescription to the PDMP for record-keeping purposes.27 In many ways, there has been a seamless transition, both functional and perceived, of the purpose of PDMPs; once understood primarily as law enforcement systems to track narcotics, these databases are now seen as both clinical and public health tools, meant to help medical practitioners make informed decisions about prescribing during an active episode of care.
As their use has changed, so too has the way that PDMPs are characterized by state governments. In recent decades, many PDMPs have transitioned their administration from a law enforcement department such as a bureau of narcotics enforcement or attorney general’s office to a medical or public health department. For example, Pennsylvania, which established its PDMP within the attorney general’s office in 1972, moved program administration to its state health department in 2016.28 These administrative realignments reflect the changing nature of PDMPs, as well as a shift in perception of PDMPs from punitive law enforcement surveillance systems to preventative public health tools.
Despite this shift in perception, however, PDMPs are still used by law enforcement today. Records obtained through Freedom of Information Act requests show that local and state police access PDMPs to surveil both prescribers and patients.29 Although nearly all discussions of PDMPs in recent legislation30 and official documentation31 reference them as tools of public health, they also retain their original purpose as instruments of police power. Of course, many areas of public health, such as quarantine and vaccination, entail the threat of coercion. And in the history of narcotics control in particular, the state has consistently placed a heavy emphasis on criminalizing substance use disorders and those who suffer from them. It is important to recognize that PDMPs have the potential to fall into step with this historical legacy.
VIEWS OF SCIENTISTS AND POLICYMAKERS
A core tension in the use of PDMPs in the 21st century is their broad acceptance in combating the opioid epidemic compared with other interventions. Why are PDMPs nearly ubiquitous, whereas harm reduction programs such as clean needle exchanges and medical treatment are contentious and present in far fewer areas? In describing the history of PDMPs, it is critical to contrast the mixed scientific literature on their efficacy with the high level of support policymakers have expressed for them.
EFFICACY, OUTCOMES, AND MIXED MESSAGES
As PDMP adoption increased in the 21st century, health services researchers studied these programs’ impacts on physicians and patients in greater depth. Many studies show that PDMPs reduce opioid-prescribing rates—evidence that seems to suggest these programs’ utility in addressing the opioid epidemic. A 2018 study evaluated four state PDMPs and found a reduction in opioid-prescribing dosages attributable to these monitoring programs.32 Other studies found that state PDMP programs that require prescribers to query the system before writing a prescription reduce opioid prescribing, whereas those without mandatory checks have little effect.33 However, it remains unclear what forces are driving this reduction. In the best-case scenario, a reduction in opioid prescriptions suggests that physicians are being more diligent in their own prescribing behaviors and curbing opioid diversion behaviors such as doctor shopping. However, physicians could also be writing fewer opioid prescriptions because they feel that PDMPs pose onerous administrative burdens on their practice, or because they fear loss of licensure or even imprisonment for prescribing controlled substances. In these cases, patients with legitimate needs for opioid prescriptions may have more difficulty obtaining appropriate pain treatment—and in desperate circumstances even turn to illicit sources.
Although PDMPs may reduce inappropriate opioid prescribing, many public health advocates have argued that the true measure of their effectiveness is a reduction in the rate of opioid overdose deaths.34 Here, the scientific literature on PDMPs is less optimistic. A 2011 study found that although states with PDMPs had lower opioid-prescribing rates than those without, they did not have lower rates of opioid overdose deaths.35 A 2018 study found that although supply-side restrictions such as PDMPs decrease overdose deaths from prescription pill use, they may actually increase heroin-related deaths, as individuals who are denied medical prescriptions turn to riskier, illicit sources of nonprescription opioids.36 The few studies that do find that PDMPs reduce mortality use narrowly defined outcome measures, such as one study set in Florida that measured only the rate of oxycodone-specific overdose.37 Others have found no effect of PDMPs on drug overdose mortality,38 or a small impact, with a reduction of 1.12 deaths per 100 000 individuals.39 Although PDMPs likely reduce opioid prescribing, they may not reduce—and could potentially increase—mortality from opioid misuse.
PRESCRIPTION DRUG MONITORING PROGRAMS AND POLICY
As the extent of morbidity and mortality from the contemporary opioid epidemic became clear, PDMPs garnered significant public policy attention. A variety of recent federal policy efforts support the implementation of new PDMPs and incentivize use of existing systems. The Harold Rogers Prescription Drug Monitoring Program, established by the Department of Justice in 2003, gives states federal grant funding to establish and operate PDMPs.40 President Trump’s Commission on Combating Drug Addiction and the Opioid Crisis focused many of its recommendations on funding, expanding, and improving PDMP systems.41 The commission dedicated significant portions of its final report to supply-side restriction policies, including PDMPs. The 2018 Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act requires state Medicaid programs to order their health care providers to query state PDMPs prior to prescribing opioids.42 Given our polarized current political climate, the bipartisan support that PDMPs have enjoyed is remarkable.
SKEPTICAL SCIENCE AND EXUBERANT POLICY
When one compares the mixed evidence on the effectiveness of PDMPs with the enthusiasm they elicit from policymakers, the obvious question arises: “What explains this difference?” We suggest that this disconnect may be due, at least in part, to policymakers’ efforts to deliver expedient solutions to the opioid crisis that avoid difficult (and time-consuming) social questions about the nature of addiction and its treatment.43 Today, rhetoric about opioid use as a moral failure and a matter of personal responsibility persists, despite broad medical consensus that opioid use disorder is a chronic, relapsing disease and the development of several effective medical treatments. Reluctant to confront the continuing stigmas around substance use, and pressured to present immediate solutions, policymakers may be turning to PDMPs as a politically safe strategy to address the opioid epidemic. Reliance on supply-side restrictions such as PDMPs may have serious consequences for physicians, such as added administrative and quality measurement requirements that are associated with provider burnout.44 They may also pose medical and legal risks for patients who face unexpected interruptions in their opioid prescriptions.45 Importantly, the risk of overdose death increases after any period of abstinence, such as one that might follow a period of restricted prescription opioid access.46 Individuals who turn to illicit substances such as heroin and fentanyl when their prescriptions are interrupted face additional risks to morbidity and mortality.
Harm reduction solutions to the opioid crisis, such as medical treatment, safe injections sites, and naloxone distribution, do not enjoy the same sort of broad policy consensus that PDMPs receive today.47 One explanation for this discrepancy is that unlike supply-side restrictions, harm reduction approaches force policymakers to confront difficult questions about how we should collectively conceptualize opioid use disorder, and how we should define its treatment and care. Despite activists’ strenuous efforts to destigmatize life-saving medical treatments such as buprenorphine, methadone, and naloxone, politicians and policymakers remain deeply divided over whether these treatments should be available and encouraged.48 The morality of other life-saving treatment policies, such as clean needle exchanges and safe injection sites, also remains deeply contested.49 These historically and culturally entrenched stigmas about substance use disorders and those who suffer from them remain immense hurdles to implementing harm reduction policies and extending access to care.
Supply-side restrictions such as PDMPs, on the other hand, appeal as politically safe solutions with little moral ambiguity. These systems target “bad actors” such as “pill mill” physicians who write large numbers of inappropriate opioid prescriptions at the behest of pharmaceutical companies, or patients who divert their pills through doctor shopping tactics. Digital-age PDMPs are touted not as law enforcement tools but as public health instruments that help physicians improve their clinical decision-making. As a result, politicians who are desperate to take action in a challenging political climate may be turning to PDMPs as their policy of choice. Of course, tempering our political enthusiasm for PDMPs would not resolve the deep philosophical and political divisions over what counts as morally appropriate treatment of substance use disorders. A policy focus on supply-side restrictions such as PDMPs, however, could be one of many factors that contribute to the underprovision of more politically complicated, but more clinically effective, public health strategies such as harm reduction treatments.
CONCLUSIONS
The path to universal adoption of PDMPs in the United States was at first slow and tenuous, then sudden and widespread. Emerging as an early form of narcotics enforcement in the early 20th century, PDMPs have been transformed from tools of law enforcement surveillance to systems of public health monitoring, even as modern PDMPs continue to serve both purposes. This reframing reflects the changing nature of PDMPs in the digital age. It also illustrates the appeal of prescription monitoring as a politically expedient preventative policy in a thorny political climate. PDMPs are certainly useful tools in addressing some aspects of the opioid epidemic—such as improving opioid-prescribing practices and preventing opioid diversion through doctor shopping. But ultimately, policymakers must recognize that these programs are only one facet of a broader solution to this urgent public health crisis. Finally, there are many facets of PMDPs not discussed in detail in this article that should be areas for future research. These include concerns about their administrative burdens, their poor integration with electronic health records, and the challenges they pose for patients who have legitimate need for intensive pain management.
ACKNOWLEDGMENTS
A. Botelho was supported by award number T32GM007753 from the National Institute of General Medical Sciences.
Note. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
Footnotes
See also Fraser, p. 1117.
ENDNOTES
- 1.Beauchamp Gillian A., Winstanley Erin L, Ryan Shawn A., Lyons Michael S. Moving Beyond Misuse and Diversion: The Urgent Need to Consider the Role of Iatrogenic Addiction in the Current Opioid Epidemic. American Journal of Public Health. 2014;(11):2023–2029. doi: 10.2105/AJPH.2014.302147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Zee Art. The Promotion and Marketing of OxyContin: Commercial Triumph, Public Health Tragedy. American Journal of Public Health. 2009;(2):221–227. doi: 10.2105/AJPH.2007.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kristin Finklea K., Bagalman Erin, Sacco Lisa N. Prescription Drug Monitoring Programs. Washington, DC: Congressional Research Service; 2014. [Google Scholar]
- 4.Worley Julie. Prescription Drug Monitoring Programs, a Response to Doctor Shopping: Purpose, Effectiveness, and Directions for Future Research. Issues in Mental Health Nursing. 2012;(5):319–328. doi: 10.3109/01612840.2011.654046. [DOI] [PubMed] [Google Scholar]
- 5.Prescription Drug Monitoring Program Training and Technical Assistance Center. “Prescription Drug Monitoring Frequently Asked Questions (FAQ),” http://www.pdmpassist.org/content/prescription-drug-monitoring-frequently-asked-questions-faq (accessed October 20, 2018); Substance Abuse and Mental Health Services Administration, “Prescription Drug Monitoring Programs: A Guide for Healthcare Providers,” 2017, https://store.samhsa.gov/sites/default/files/d7/priv/sma16-4997.pdf (accessed May 7, 2020)
- 6.Miliard Mike. “Senate Passes Sweeping Opioid Response Bill With eRx, EHR, PDMP Provisions,” Healthcare IT News, September 9, 2018, https://www.healthcareitnews.com/news/senate-passes-sweeping-opioid-response-bill-erx-ehr-pdmp-provisions (accessed December 20, 2019)
- 7.Haffajee Rebecca L., Jena Anupam B., Weiner Scott G. Mandatory Use of Prescription Drug Monitoring Programs. Journal of the American Medical Association. 2015;(9):891–892. doi: 10.1001/jama.2014.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starr Paul. The Social Transformation of American Medicine: The Rise of a Sovereign Profession and the Making of a Vast Industry. New York, NY: Basic Books; 1982. [Google Scholar]
- 9. Caroline Jean Acker, Creating the American Junkie: Addiction Research in the Classic Era of Narcotic Control (Baltimore, MD: Johns Hopkins University Press, 2006); Nancy Campbell, Discovering Addiction: The Science and Politics of Substance Abuse Research (Ann Arbor, MI: University of Michigan Press; 2007); David Courtwright, Dark Paradise: A History of Opiate Addiction in America (Cambridge, MA: Harvard University Press, 2001).
- 10.Musto David F. The American Disease: Origins of Narcotic Control. Oxford, UK: Oxford University Press; 1999. p. 105. [Google Scholar]
- 11. Harrison Narcotics Act, 38 Stat 785 (1914); Joseph F. Spillane, “The Road to the Harrison Narcotics Act: Drugs and Their Control, 1875–1918,” in Federal Drug Control: The Evolution of Policy and Practice, ed. Jonathon Erlen and Joseph Spillane (Binghamton, NY: Pharmaceutical Products Press, 2004), 1–24; Harrison Narcotics Act.
- 12.Dineen Kelly K., DuBois James M. Between a Rock and a Hard Place: Can Physicians Prescribe Opioids to Treat Pain Adequately While Avoiding Legal Sanction? American Journal of Law & Medicine. 2016;(1):7–52. doi: 10.1177/0098858816644712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marion Nancy E., Oliver Willard M., editors. Drugs in American Society: An Encyclopedia of History, Politics, Culture, and the Law. Santa Barbara, CA: ABC-CLIO; 2014. [Google Scholar]
- 14. Prescription Drug Monitoring Program Training and Technical Assistance Center, “History of Prescription Drug Monitoring Programs,” March 2018, https://www.pdmpassist.org/pdf/PDMP_admin/TAG_History_PDMPs_final_20180314.pdf (accessed February 19, 2018)
- 15. New York State Office of the Attorney General, “Internet System for Tracking Over-Prescribing: A Proposal Addressing New York’s Prescription Drug Abuse and Drug Diversion Epidemic,” 2012, https://ag.ny.gov/sites/default/files/press-releases/2012/ISTOP%20REPORT%20FINAL%201.10.12.pdf (accessed February 20, 2020)
- 16.Sacco Lisa N., Duff Johnathan H., Sarata Amanda K. Prescription Drug Monitoring Programs. Washington, DC: Congressional Research Service; 2018. [Google Scholar]
- 17. For example, United States v Doremus and Webb et al. v United States. See David T. Courtwright, “A Century of American Narcotic Policy,” in Treating Drug Problems: Volume 2, ed. Dean R. Gerstein and Henrick J. Hardwood (Washington, DC: National Academies Press, 1992), 9; Direct Sales Co. v United States, 319 US 703 (1943).
- 18. Roe v Wade, 410 US 113 (1973).
- 19. Whalen v Roe, 429 US 589 (1977). [PubMed]
- 20. Ibid.
- 21.Campbell James N. APS 1995 Presidential Address. Journal of Pain. 1996;(1):85–88. [Google Scholar]
- 22.Fishman Scott M., Papazian Jennifer, Gonzalez Susana, Riches Paul S., Gilson Aaron. Regulating Opioid Prescribing Through Prescription Monitoring Programs: Balancing Drug Diversion and Treatment of Pain. Pain Medicine. 2004;(3):309–324. doi: 10.1111/j.1526-4637.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- 23.Lange Julia, Gaddis Gary, Varner Emily. Resident Access to the St. Louis County Prescription Drug Monitoring Program: Why PDMPs Matter and How to Gain Access. Missouri Medicine. 2018;(6):487–493. [PMC free article] [PubMed] [Google Scholar]
- 24. Fishman et al., “Regulating Opioid Prescribing Through Prescription Monitoring Programs.”.
- 25.McCaffery Margo, Pasero Christine L. Pain Ratings: The Fifth Vital Sign. American Journal of Nursing. 1997;(2):15–16. doi: 10.1097/00000446-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Phillips Donald M. JCAHO Pain Management Standards Are Unveiled. Journal of the American Medical Association. 2000;(4):428–429. doi: 10.1001/jama.284.4.423b. [DOI] [PubMed] [Google Scholar]
- 27. Dhaval M. Dave, Anca M. Grecu, and Henry Saffer, “Mandatory Access Prescription Drug Monitoring Programs and Prescription Drug Abuse,” National Bureau of Economic Research, June 2017, https://www.nber.org/papers/w23537 (accessed February 20, 2020)
- 28. Michael Siget, “The Prescription Drug Monitoring Program: Your Prescriber Questions Answered!” Pennsylvania Medical Society, September 6, 2016, https://www.pamedsoc.org/detail/article/Capitol-Update-Blog-Sept-6-2016 (accessed February 20, 2020)
- 29. Beth Schwartzapfel, “Guess Who’s Tracking Your Prescription Drugs?” The Marshall Project, 2017, https://www.themarshallproject.org/2017/08/02/guess-whos-tracking-your-prescription-drugs (accessed February 22, 2020)
- 30. Achieving Better Care By Monitoring All Prescriptions Program (ABC-MAP) Act, PL 2911.
- 31. Siget, “The Prescription Drug Monitoring Program: Your Prescriber Questions Answered!”.
- 32.Haffajee Rebecca L., Mello Michelle M., Zhang Fang et al. Four States With Robust Prescription Drug Monitoring Programs Reduced Opioid Dosages. Health Affairs. 2018;(6):964–974. doi: 10.1377/hlthaff.2017.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas C. Buchmueller and Colleen Carey, “The Effect of Prescription Drug Monitoring Programs on Opioid Utilization in Medicare,” American Economic Journal: Economic Policy 10, no. 1 (February 2018): 77–112.
- 34. Leo Beletsky, “Deploying Prescription Drug Monitoring to Address the Overdose Crisis: Ideology Meets Reality,” Indiana Health Law Review 15, no. 2 (2018): 139–187; Erin P. Finley, Ashley Garcia, Kristen Rosen, et al. “Evaluating the Impact of Prescription Drug Monitoring Program Implementation: A Scoping Review,” BMC Health Services Research 17, no. 1 (2017): 420. [DOI] [PMC free article] [PubMed]
- 35.Paulozzi Leonard J., Kilbourne Edwin M., Desai Hema A. Prescription Drug Monitoring Programs and Death Rates from Drug Overdose. Pain Medicine. 2011;(5):747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 36.Pitt Allison L., Humphreys Keith, Brandeau Margaret L. Modeling Health Benefits and Harms of Public Policy Responses to the US Opioid Epidemic. American Journal of Public Health. 2018;(10):1394–1400. doi: 10.2105/AJPH.2018.304590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delcher Chris, Wagenaar Alexander C., Goldberger Bruce A., Cook Robert L., Maldonado-Molina Mildred. “Abrupt Decline in Oxycodone-Caused Mortality After Implementation of Florida’s Prescription Drug Monitoring Program. Drug and Alcohol Dependence. 2015:63–68. doi: 10.1016/j.drugalcdep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Li Guohua, Brady Joanne E., Lang Barbara H., Giglio James, Hanna Wunsch, Charles DiMaggio. Prescription Drug Monitoring and Drug Overdose Mortality. Injury Epidemiology. 2014;(1):9. doi: 10.1186/2197-1714-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patrick Stephen W., Fry Carrie E., Jones Timothy F., Buntin Melinda B. Implementation of Prescription Drug Monitoring Programs Associated With Reductions in Opioid-Related Death Rates. Health Affairs. 2016;(7):1324–1332. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. “Harold Rogers Prescription Drug Monitoring Program: FY 2016 Competitive Grant Announcement,” Bureau of Justice Assistance, https://www.bja.gov/Funding/pdmp16.pdf (accessed February 20, 2020)
- 41. The President’s Commission on Combating Drug Addiction and the Opioid Crisis, Report of Commissioners, November 1, 2017, https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Final_Report_Draft_11-1-2017.pdf (accessed February 20, 2020)
- 42.Musumeci MaryBeth, Tolbert Jennifer. “Federal Legislation to Address the Opioid Crisis: Medicaid Provisions in the SUPPORT Act,” Kaiser Family Foundation, 2018, https://www.kff.org/medicaid/issue-brief/federal-legislation-to-address-the-opioid-crisis-medicaid-provisions-in-the-support-act (accessed February 20, 2020)
- 43. Corey S. Davis and Derek H. Carr, “Legal and Policy Changes Urgently Needed to Increase Access to Opioid Agonist Therapy in the United States,” International Journal of Drug Policy 73 (2019): 42–48; Rebecca L. Haffajee, Amy S.B. Bohnert, and Pooja A. Lagisetty, “Policy Pathways to Address Provider Workforce Barriers to Buprenorphine Treatment,” American Journal of Preventive Medicine 54, no. 6 (2018): S230–S242. [DOI] [PMC free article] [PubMed]
- 44. Marcus A. Bachhuber, Brendan Saloner, Marc LaRochelle, et al., “Physician Time Burden Associated With Querying Prescription Drug Monitoring Programs,” Pain Medicine 19, no. 10 (2018): 1952–1960; N. Lance Downing, David W. Bates, and Christopher A. Longhurst, “Physician Burnout in the Electronic Health Record Era: Are We Ignoring the Real Cause?” Annals of Internal Medicine 169, no. 1 (2018): 50–51; Mark A. Rothstein, “The Opioid Crisis and the Need for Compassion in Pain Management,” American Journal of Public Health 107, no. 8 (2017): 1253–1254.
- 45. Ibid.
- 46.Wilder Christine M., Miller Shannon C., Tiffany Elizabeth, Winhusen Theresa, Winstanley Erin L., Stein Michael D. Risk Factors for Opioid Overdose and Awareness of Overdose Risk Among Veterans Prescribed Chronic Opioids for Addiction or Pain. Journal of Addictive Diseases. 2016;(1):42–51. doi: 10.1080/10550887.2016.1107264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu Andrea. “Treating Opioid Addiction With a Drug Raises Hope and Controversy,” National Public Radio, May 17, 2016, https://www.npr.org/sections/health-shots/2016/05/17/478387232/treating-opioid-addiction-with-a-drug-raises-hope-and-controversy (accessed December 20, 2019)
- 48.Friedmann Peter D., Schwartz Robert P. Just Call It ‘Treatment,’. Addiction Science & Clinical Practice. 2012;(1):10. doi: 10.1186/1940-0640-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schumaker Erin. “Safe Injection Sites Might Not Solve The Opioid Crisis, But They Won’t Make It Worse,” Huffington Post, August 31, 2018, https://www.huffingtonpost.com/entry/safe-injection-sites-opioid-justice-department_us_5b87f8a7e4b0162f4720565f (accessed December 20, 2019)