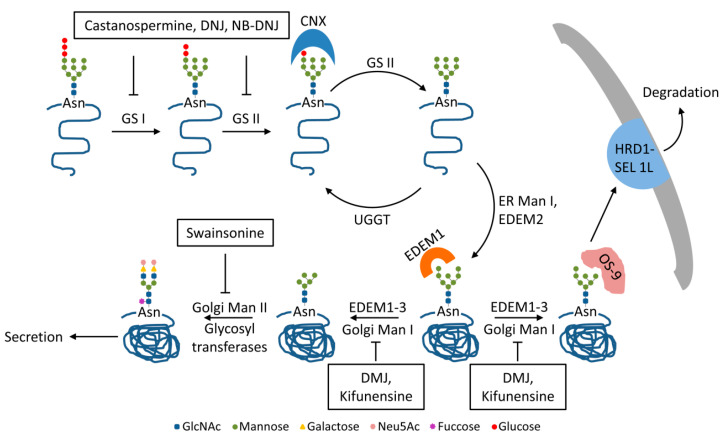
Figure 2.
N-glycan trimming of the HBV envelope glycoproteins. Following the attachment of the (GlcNAc)2Man9Glc3, precursor to nascent viral polypeptides, the ER α-glucosidase I (GS I) removes the terminal Glc, followed by ER α-glucosidase II (GS II) trimming the second Glc unit. The mono-glucosylated glycoproteins enter the protein folding cycle assisted by ER-resident chaperones [13,14]. Interaction with calnexin (CNX) was demonstrated for M and L proteins [16,17]. Subsequent to trimming of the last Glc unit by GS II, improperly folded proteins are re-glucosylated by UDP-glucose:glycoprotein glucosyltransferase (UGGT) and reenter the cycle. Correctly folded glycoproteins are subjected to further mannose (Man) trimming by ER mannosidase I (ER Man I), followed by Golgi mannosidases I and II. Finally, the N-glycan is processed by a series of glycosyl transferases, leading to complex structures on secreted glycoproteins [13,14]. The ER degradation-enhancing, mannosidase-like proteins (EDEMs )are also responsible for Man trimming, leading to Man5-7 intermediates. S and L proteins are substrates for the OS-9 lectin that delivers them to HRD1-SEL 1L complex for degradation. In contrast, EDEM trimming accelerates M protein trafficking through the secretory pathway [18,19]. Specific inhibitors for the N-glycan trimming steps are indicated [14,18].