Abstract
Ionic liquids (ILs) are a special category of molten salts solely composed of ions with varied molecular symmetry and charge delocalization. The versatility in combining varied cation–anion moieties and in functionalizing ions with different atoms and molecular groups contributes to their peculiar interactions ranging from weak isotropic associations to strong, specific, and anisotropic forces. A delicate interplay among intra- and intermolecular interactions facilitates the formation of heterogeneous microstructures and liquid morphologies, which further contributes to their striking dynamical properties. Microstructural and dynamical heterogeneities of ILs lead to their multifaceted properties described by an inherent designer feature, which makes ILs important candidates for novel solvents, electrolytes, and functional materials in academia and industrial applications. Due to a massive number of combinations of ion pairs with ion species having distinct molecular structures and IL mixtures containing varied molecular solvents, a comprehensive understanding of their hierarchical structural and dynamical quantities is of great significance for a rational selection of ILs with appropriate properties and thereafter advancing their macroscopic functionalities in applications. In this review, we comprehensively trace recent advances in understanding delicate interplay of strong and weak interactions that underpin their complex phase behaviors with a particular emphasis on understanding heterogeneous microstructures and dynamics of ILs in bulk liquids, in mixtures with cosolvents, and in interfacial regions.
1. Introduction
Ionic liquids (ILs) are liquid molten salts, typically composed of bulky and asymmetrical organic cations and organic or inorganic anions with their melting points below 100 °C. The history of ILs generally credits the German chemist Paul Walden with the first documented salt material at ambient temperature in 1914.1 He synthesized an ionic salt, ethylammonium nitrate (EAN), which displays a melting point of 12 °C and a rather low viscosity. Unfortunately, apart from a brief mention of this work in a study of parachor and chemical constitutions of some fused metals and salts in 1929,2 this early report did not receive much consideration from various scientific communities, and it was not anticipated that such salt materials would become of widespread interest in the future.
Nearly 40 years later, Hurley and Wier recognized the potential benefit of decreasing melting points of molten salt materials via synthesis of organic chloroaluminates by mixing aluminum compounds with alkylpyridinium chloride salts. The obtained organic chloroaluminates are now considered as the first generation of ILs.3 However, these haloaluminate ILs suffer from their high sensitivity to atmospheric moisture and thus require handling under strict anhydrous conditions to avoid their hydrolysis. In addition, it is not feasible to regulate their acidity and basicity.3 More specific investigations of these haloaluminate compounds started in the 1970s.4,5 In the 1980s, ILs were proposed as solvents for organic synthesis, and scientific interest in ILs began to spread and the range of investigations began to broaden. A significant contribution from Wilkes and Zaworotko on “Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids” in 1992 is seen by most researchers as ushering a new stage for the development of ILs,5,6 even though some ILs had been predicted previously7 and other air- and moisture-stable ILs have been used in laboratory settings.8 Unlike haloaluminate salts, this new generation of ILs can be obtained, handled, and stored outside a glovebox. By a careful selection of cation–anion combinations, it is possible to prepare a large variety of ILs. These pioneering works and significant breakthroughs in IL communities opened up avenues for a surge of research on ILs and initiated paramount research activities across areas of physics, chemistry, biology, materials science and engineering, and environmental science, which are subjects of numerous reviews,9−34 special themed issues in prestigious journals,35−42 and book chapters.43−49
ILs have multifaceted and remarkable physicochemical characteristics, such as negligible volatilities, reasonable conductivity–viscosity properties, extended liquid-state temperature ranges, wide electrochemical windows, high thermal- and chemical-oxidative stabilities, as well as excellent capabilities to dissolve liquid and solid solute molecules having distinct polarities.25,50 An additional fascinating character of ILs is that these physicochemical quantities related to hydrophobicity, polarity, and solvent power as well as their microstructural organization can be widely tuned by combinations of different cations and anions, by introducing a controllable amount of solutes in IL matrixes, and by mutating specific atoms in constituent ions.25,50 Therefore, ILs are always referred to tunable, tailorable, task-specific, and designer solvents. These striking features render ILs as dependable candidates and benign alternatives to conventional molecular solvents in material synthesis to control precise structures and patterns of nanomaterials;23,24 valuable reaction media in catalytic chemistry to provide optimized chemical enantioselectivity;24,51 promising working fluids in separation technology via absorption of specific gas molecules;29,52 unique tunable platforms to design task-specific advanced materials to dissolve celluloses and proteins;23,29 reliable solvent electrolytes in electrochemical devices with tunable electrochemical windows and ion conductivities;14,19,53−57 and useful lubricants and lubricant additives in tribology to reduce frictions between solid sliding contacts under harsh conditions.22,30,58 As a result of enormous number of promising applications, the playing field for additional applications and related investigations for physicists, chemists, biologists, materials scientists, and engineers is vast and has yet to see its limitation.
The rapid upswing and wide applications of ILs in academia and industrial communities stem from a direct consequence of peculiar intra- and intermolecular interactions among constituent ions. These molecular interactions range from weak, isotropic, and nonspecific forces (e.g., solvophobic, van der Waals (vdW), dispersion forces, etc.) to strong (Coulombic), anisotropic, and specific forces (e.g., charge, dipole, and multiple interactions as well as hydrogen bonding (HB) interactions, etc.). Favorable vdW and dispersion associations among apolar moieties and decisive Coulombic interactions among polar moieties in constituent ions are key driving forces to construct remarkable liquid structures in IL matrixes.18,30 In addition, Coulombic interactions among ion species are isotropic, which enables a substantial assortment of secondary directional interactions, such as dipole–dipole, dipole–induced dipole, multipoles, and possible π–π stacking interactions as well as HB coordinations between ion species having heteroaromatic rings with delocalized charges.17,59−61 These delicate interactions have considerable entropic contribution, facilitate additional stabilization, and direct the formation of remarkable ion clusters, paving the road for complex, higher order self-assembled liquid structures of ILs in bulk liquids and in confined environments.15,18,30,62,63
Moreover, not all but a vast number of ILs can be categorized as having polar and apolar components, and therefore can be regarded as nanosegregated fluids with polar (apolar) networks permeated by apolar (polar) domains. A subtle balance of intermolecular interactions between constituent ions and associations between polar and apolar components defines their peculiar transport properties including thermal conductivities (for heat transfer), liquid viscosities (for momentum transfer), and diffusion coefficients (for mass transfer). In addition, ion (electrical) conductivities of ILs are significant64,65 as ILs are solely composed of ions. These properties play central roles in electrochemical applications.
ILs are generally much more viscous than their neutral binary mixture counterparts,66−68 and some ILs become less viscous when ion charges are more homogeneously distributed over molecular frameworks or with addition of molecular solutes to IL matrixes. These aspects are expected to weaken Coulombic interactions among constituent ions and thereafter influence their dynamic properties. ILs exhibit slow dynamics that are typically characterized by subdiffusivities and nonexponential relaxations. The overall nonexponential dynamics of ILs are ascribed to a superposition of exponential relaxations of constituent ions with different relaxation times,66,69 which is in accordance with relaxations of glass forming liquids,70 colloids, and polymer melts71 that exhibit distinct structural and dynamical heterogeneities at extended spatiotemporal scales.
Microstructural and dynamical heterogeneities are hallmark features of striking properties that ILs can possess. To date, almost all known physical chemistry techniques have been adopted to study these heterogeneities of ILs. Spectroscopic experiments (two-dimensional infrared (2D IR) spectroscopy,72−75 dielectric spectroscopy,76,77 direct recoil spectroscopy (DRS),78,79 Fourier transform-infrared (FT-IR) spectroscopy,80−82 light scattering spectroscopy,83,84 nuclear magnetic resonance (NMR) spectroscopy,85−88 optical-heterodyne-detected optical Kerr effect (OHD-OKE) spectroscopy,89−95 sum frequency generation (SFG) vibrational spectroscopy,96−101 ultrafast infrared spectroscopy,91−93,102 neutron diffraction,103,104 reflectivity105−107 and scattering spectroscopies,108−113 X-ray photoelectron spectroscopy (XPS),114−116 X-ray diffraction (XRD),117,118 X-ray reflectivity (XRR),119−122 and X-ray scattering spectroscopies,123−126etc.), atomic force microscopy (AFM),127−130 scanning tunneling microscopy (STM),131−134 surface force techniques (surface force apparatus (SFA),135,136 and surface force balance (SFB)137−140), polarization sensitive pump–probe (PSPP) measurements,75,141 and computer simulations (density functional theory (DFT) calculations,142−151ab initio,152−154 atomistic,75,150,155−181 and coarse-grained (CG) molecular dynamics (MD) simulations55,66−68,133,182−185) have provided tremendous insights into heterogeneous microstructures and dynamics of ILs in bulk liquids, in mixtures with cosolvents, and in interfacial regions.
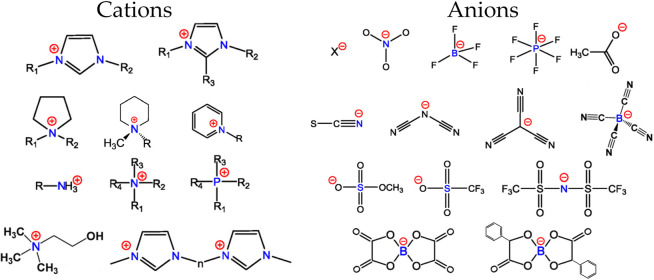
The purpose of this review is to trace recent advances in understanding striking and complex phase behaviors of ILs and to describe how delicate interplay of strong interactions and weak associations underpins their complex physicochemical properties with a particular emphasis on microstructural and dynamical heterogeneities of ILs at varied conditions. The versatility in combining different cations and anions with varied charge delocalization, and the flexibility in mutating specific atoms in constituent ions indicate that a huge number of ILs with distinct physicochemical and structural properties is accessible for applications. It is predicted that there are ∼106 pure ILs that can be easily prepared in laboratory, leading to a possibility of 1012 binary combinations and 1018 ternary IL mixtures potentially available.9 These numbers are still growing exponentially due to advanced synthetic procedures and technologies. It is difficult to coordinate microstructural and dynamical heterogeneities of all possible ILs. Therefore, in this contribution, we mainly focus on four IL families consisting of imidazolium, pyrrolidinium, (tetra)alkylammonium, and tetraalkylphosphonium cations due to their extensive usage in industrial applications. The anions of interest are the most popular ones, which can be either inorganic or organic entities including halides, nitrate ([NO3]), tetrafluoroborate ([BF4]), hexafluorophosphate ([PF6]), acetate ([OAc]), alkylphosphate, alkylsulfonate, alkylsulfate ([CnSO4]), trifluoromethylsulfonate ([TFO]), bis(trifluoromethanesulfonyl)imide ([NTF2]), and orthoborates. A nonexhaustive list of ion species discussed in this review is provided in Figure 1.
Figure 1.
Chemical structures of typical cations and anions. Cations: dialkylimidazolium ([CnCmIM]), trialkylimidazolium, dialkylpyrrolidinium ([CnCmPYRR]), 1-alkyl-methylpiperidinium ([CnMPIP]), N-alkylpyridinium ([CnPYRI]), alkylammonium, tetraalkylammonium ([Ni,j,k,l]), tetraalkylphosphonium ([Pi,j,k,l]), 2-hydroxyethyl-trimethylammonium (cholinium, [CH]), and di-imidazolium ([Cn(MIM)2]). Anions: halides, nitrate, tetrafluoroborate, hexafluorophosphate, acetate, thiocyanate, dicyanamide, tricyanomethanide, tetracyanoborate, methylsulfate, trifluoromethanesulfonates, bis(trifluoromethylsulfonyl)imide, bis(oxalato)borate, and bis(mandelato)borate. Adapted with permission from ref (13). Copyright 2017 American Chemical Society.
2. Pure Ionic Liquids
In IL community, ILs were originally assumed to fall within a conventional scheme of molecular liquids as coherent, irregular, and essentially homogeneous systems.4,17,18 Bulk ILs were widely treated as similar to high-temperature molten salts (like NaCl) or highly concentrated salt solutions. However, more recently it has been determined that ILs present diverse ordering structures compared to conventional molecular liquids driven by a combination of short-range HB, vdW, and solvophobic interactions and long-range Coulombic associations among constituent ions. Generally, ion interactions impose a degree of short-range ordering structures (ion pairs, ion clusters, etc.) and result in distinct mesoscopic organization (HB networks, amphiphilic combinations of polar and apolar components, micelle-like and bicontinuous liquid morphologies) in IL matrixes. ILs exhibit structural heterogeneities at multiple length scales, which is one of the most distinctive properties of ILs, and therefore it is feasible to fine-tune ILs’ physicochemical and structural properties with desirable macroscopic functionalities for promising applications.
2.1. IL Crystal Structures
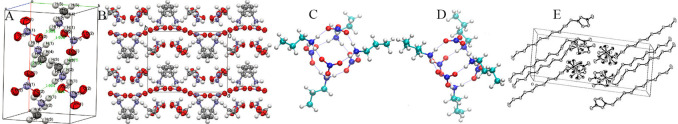
IL crystal structures provide clues to their liquid structures especially for local intermolecular interactions. Alkylammonium nitrate ILs are the most studied ILs since they are the first ILs ever synthesized in laboratory.1 Bodo et al. reported crystal structure of methylammonium nitrate (MAN) using experimental techniques and DFT calculations.186 A solid phase existing at high temperatures is a polymorph and has a high degree of disorder, corresponding to an ionic plastic phase where both cations and anions retain a more or less fixed reticular position. [NO3] anions are asymmetrically coordinated with MA cations via HB interactions, that is, a given [NO3] anion is not engaged in three equally strong HBs; instead, one HB is significantly weaker than the other two (Figure 2A). Such an asymmetric HB structure was also observed in theoretical calculations of MAN ion clusters.187,188 It should be noted that there is no nanoscale apolar segregation in MAN as methyl groups are too small. For alkylammonium cations with alkyl chains longer than C1, the corresponding nitrate ILs present some long-range ordering structures over a nanometer length scale, which stem from self-assembly of hydrophobic alkyl chains within polar networks.108,189−195
Figure 2.
Molecular packing of (A) MAN and (B) EtAN crystal structures. Reproduced with permission from ref (186). Copyright 2011 American Chemical Society. Reproduced with permission from ref (196). Copyright 2012 Royal Society of Chemistry. Minimum energy structures of (C) PAN and (D) BAN ILs determined from atomistic simulations. Reproduced with permission from ref (188). Copyright 2012 American Chemical Society. (E) Unit cell of [C12MIM][PF6] crystal structure. Reproduced with permission from ref (197). Copyright 1998 Royal Society of Chemistry.
The crystal structure of ethanolammonium nitrate (EtAN) consists of two lamellar-like layers composed of EtA cations taking vertical configurations (Figure 2B).196 Half of [NO3] anions are located between neighboring ammonium moieties forming polar domains, and the other [NO3] anions are interspersed between ethyl chains. Lengthening alkyl chains in alkylammonium cations leads to distinct structures as observed in propylammonium nitrate (PAN) (Figure 2C) and butylammonium nitrate (BAN) ILs (Figure 2D).188,198 Raman spectra revealed that PA cations exhibit trans conformations in low temperature crystalline phase and undertake a crystal polymorphism transition with increasing temperatures to a phase in which PA cations are characterized by gauche conformations. Such a structural rearrangement takes place both in polar domains, in which there is a dominance of Coulombic and HB interactions between ammonium groups and [NO3] anions and in apolar domains, where there are strong vdW interactions among alkyl groups. The distorted cation–anion structures remain in the liquid phase at high temperatures but can be arrested in isles with distinct microscopic heterogeneities at high pressures.
The temperature dependence of ethylammonium chloride (EAC) and propylammonium chloride (PAC) crystal structures was investigated by in situ X-ray powder diffraction spectroscopy.199−201 A polymorphic transition, with a reconstructive character, was observed for PAC from a monoclinic phase formed at low temperatures to a tetragonal phase formed at high temperatures. For EAC polymorphs, the thermal expansion is small and anisotropic, which is attributed to EAC’s liquid organization characterized by an anisotropic framework consisting of apolar and polar domains, whereas an isotropic thermal expansion was observed for PAC attributing to striking intermolecular interactions between nitrogen atoms in PA cations and Cl anions. In addition, microscopic isostructurality was reported for other PA halides (Br and I) at room temperature.200
In contrast to alkylammonium ILs, imidazolium ILs show different crystal structures depending on anion structures and cation alkyl chain lengths. [C2MIM] ILs were extensively studied because they exhibit low melting points and high ion conductivities. When [C2MIM] cations are coordinated with halides, [C2MIM]F is unstable and has never been isolated under ambient conditions.202 [C2MIM]Cl exhibits an orthorhombic crystal structure containing four ion pairs in an asymmetric unit cell,203 in which Cl anions are situated in positions with characteristic C–H···Cl HB interactions. Similar HB structures were also observed in [C2MIM]Br and [C2MIM]I crystals.204 [C2MIM][BF4] exhibits a peculiar monoclinic crystal structure in which [C2MIM] cations exhibit one-dimensional (1D) pillar structures with one imidazolium ring facing the next one, and they are linked by H(methylene)···π interactions,205 whereas in ILs consisting of [C2MIM] cations and hexafluorocomplex anions, [C2MIM] cations form a similar 1D pillar structure with anions positioned in a zigzag arrangement along the same direction.195,202
XRD spectra showed that multiple polymorphs with rotational cation isomers are obtained for ILs consisting of [C4MIM] cations coupled with Cl, Br, I, [BF4], and [PF6] anions at crystalline and liquid states.206 Both monoclinic crystal structures with trans–trans configurations and orthorhombic crystal structures having gauche–trans configurations are available depending on conformations of butyl chains in cations and their delicate associations with anions. In monoclinic [C4MIM]Cl crystal structure, butyl chains take a trans–trans conformation, while in orthorhombic [C4MIM]Cl and [C4MIM]Br crystal structures, butyl chains exhibit gauche–trans conformations. Complementary Raman spectra207 revealed that [C4MIM] cations in these ILs form mesostructures in liquid regions that are similar to column structures found in crystals.206 Two different mesoscopic rotational isomers coexist in IL matrixes and are crucial in hindering crystallization of [C4MIM] ILs.208,209
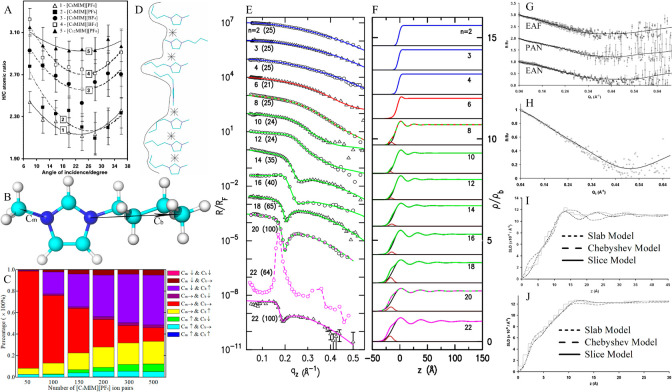
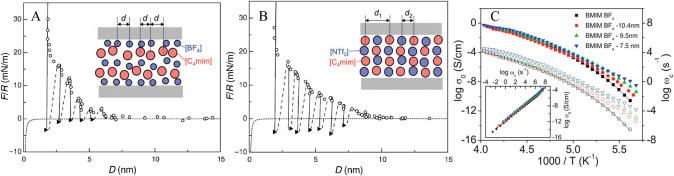
Imidazolium cations with alkyl chains longer than C14 can form a thermotropic smectic liquid crystal phase between liquid and solid states, which is similar to that observed in alkylammonium ILs with cations having long alkyl chains and some other protic ILs with cations having intermediate alkyl chains.10,210 ILs consisting of halide anions and 1-alkyl-3-methylimidazolium ([CnMIM]) cations with n = 12–18 exhibit bilayer crystal lattices,197 in which anion species and imidazolium rings form polar sheets that are separated by apolar domains consisting of interdigitated cation alkyl chains. The interlayer distance between polar sheets ranges from 2.4 to 3.3 nm depending on cation alkyl chain length (Figure 2E). The thermal behavior of a series of ILs consisting of [CnMIM] cations (n = 12–18) and anions having different coordinating abilities and sizes from Cl to [NTF2] was studied using small-angle X-ray scattering (SAXS) experiments and differential scanning calorimetry (DSC).211 These ILs form lamellar, sheetlike arrays in crystalline phase and enantiomeric structures in the smectic liquid crystal phase at higher temperatures, except for ILs containing [NTF2] anions which directly melt and form isotropic liquids. Layer spacing in crystal mesophase determined from SAXS spectra increases with cation alkyl chain length and with coordination ability of anions, following an order of Cl > Br > [BF4] > [TFO] > [NTF2].
Among many different anion species, [NTF2] anions exhibit striking behavior due to their diffuse charge distribution. The delocalized negative charge along S–N–S core in [NTF2] anion reduces ion–ion interactions and results in a suppression of liquid-crystallinity and fluidizes imidazolium ILs with low melting points.212 XRD, vibrational spectra, and DFT calculations213,214 showed that [NTF2] anions adopt a higher energy, less stable cis geometry in [C1MIM][NTF2] constrained by bifurcated C–H···O and C–H···N HBs resulting in the formation of fluorous layers in solid state structures.
2.2. Hydrogen Bonding and π–π Stacking Structures
2.2.1. Hydrogen Bonding Structures and Dynamics
When cations contain hydrogen atoms and anions have lone electron pairs, it is possible to form cation–anion HBs with cations being dominant HB donors and anions being dominant HB acceptors, respectively. It should be noted that HB in ILs is not a binary on–off phenomenon but occurs on a graduated scale, which makes demarking HB difficult. Because of a wide range of cations and anions constituting ILs, the characteristics and features of HB interactions are quite system dependent.149,153,215−218 In protic cations, hydrogen atoms in HB donors are often covalently bonded to heavy atoms carrying formal charges, and aprotic cations tend to have C–H groups as primary HB donor units. A large range of potential HB acceptors exist in anions ranging from strong HB acceptors like halides to weak HB acceptors with minimal HB interactions. In many cases, alkyl chains in ions can be functionalized with, but not limited to, alcohol, amine, carboxylic acid, and ether groups.219−228 These functional groups add further opportunities to fine-tune HB interactions in IL matrixes.
Evans and co-workers made the first suggestion that there are well-defined HB structures in alkylammonium ILs.229 They found that transferring hydrocarbons and rare gases from cyclohexane to EAN has negative enthalpies and thus speculated that proton donors and acceptors in constituent ions form a three-dimensional (3D) HB network resembling water. While this speculation was never seriously disputed, HB interactions in EAN and its derivative IL systems were convincingly established by Ludwig and co-workers in 2009.230 In combination with DFT calculations, the deconvoluted vibrational bands in FT-IR spectra for EAN, PAN, and dimethylammonium nitrate ILs are assigned to intermolecular stretching and bending modes of N–H···NO3 HBs. In these ILs, the characteristic symmetric and asymmetric stretching modes, as well as bending modes in low-frequency region of FT-IR spectra, are consistent with those of pure liquid water. These observations are rationalized by the formation of similar HB structures in these protic ILs, while unlikely to be tetrahedral, are structurally reminiscent of water, owing to different ion structures and donor–acceptor capabilities of alkylammonium nitrate ILs.
By choosing specific cation–anion moieties and changing the number of alkyl chains in alkylammonium cations, it is feasible to tune dominant forces from HB to Coulombic interactions by switching from protic ILs consisting of primary, secondary, and tertiary ammonium cations to aprotic tetraalkylammonium ILs.231−233 FT-IR spectrum of [N1,1,1,1][NO3] shows a broad vibrational band attributing to librational contributions of interacting ions, whereas FT-IR spectrum of [N0,1,1,1][NO3] exhibits a distinct vibrational band at approximately 170 cm–1, which is associated with N–H···NO3 HB interactions since no other intramolecular vibrational motion of alkylammonium or nitrate is observed in this frequency range. This interpretation was further supported by DFT calculated frequencies of [N1,1,1,1][NO3] and [N0,1,1,1][NO3] ion clusters. The energy per ion pair for [N0,1,1,1][NO3] (one HB donor) is ∼49 kJ/mol, higher than that for [N1,1,1,1][NO3] (no HB donor), demonstrating the formation of a single HB between [N0,1,1,1] cation and [NO3] anion. In addition, this value is 2 times larger than that of HBs in water (∼22 kJ/mol), indicating that [N0,1,1,1][NO3] possesses stronger HBs than water.234
Solid-state NMR spectra in combination with atomistic simulation results revealed that N-D deuterons in [N0,2,2,2] cations have the lowest deuteron quadrupole coupling constants in all reported tetraalkylammonium cations and have strong HB interactions with anion species.235 For hydroxyl-functionalized tetraalkylammonium ILs,236 N-D deuterons have two types of HB interactions: a regular type Coulomb-enhanced cation–anion HBs and an unusual type cation–cation HBs. The formation of cation clusters prevents these ILs from crystallizing, and HBs between cation species persist at low temperatures, resulting in supercooling liquids and glass formation. Both neutron diffraction experiments and atomistic simulation results demonstrated that the elusive like-charge attraction is almost competitive with conventional ion-pair formation104 and thus leads to enhanced ion pairing structures in comparison with dispersion forces between alkyl groups.237 These findings revealed a new era of controlling IL nanostructures via HB interactions between like-charged ions, which impact diverse areas including electrochemical charge storage (batteries and catalysis), electrodeposition, and lubrication.
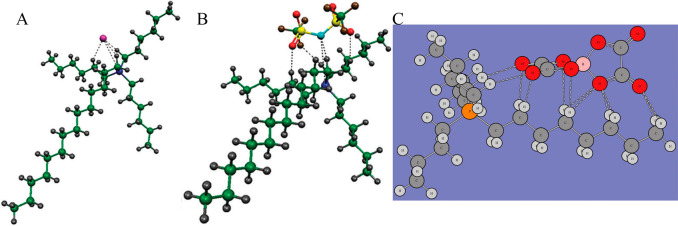
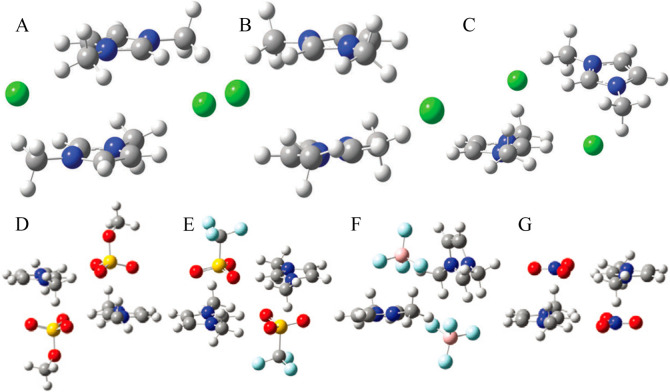
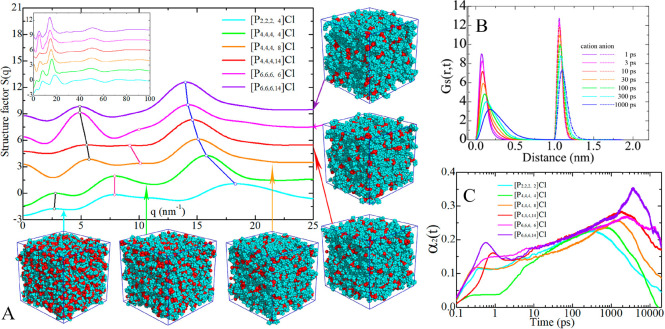
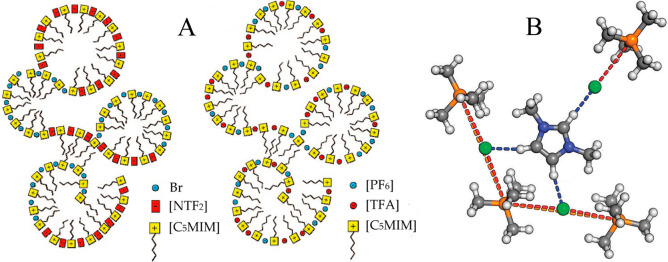
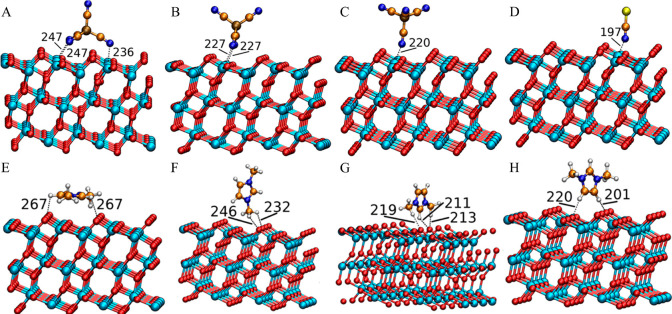
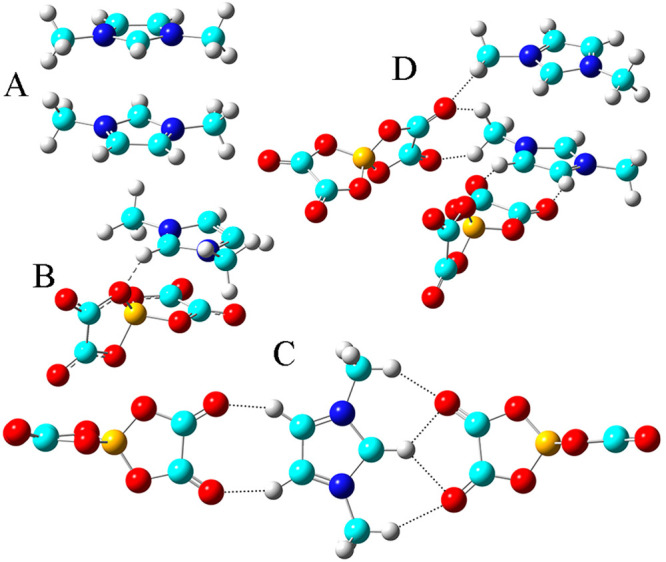
In addition, a number of DFT calculations and atomistic simulations indicated that hydrogen atoms in methylene units that are covalently bonded to central atoms in tetraalkylphosphonium cations assume preferential orientations toward electronegative atoms in anions via intermediate HB interactions.142−144,238 In a representative [P6,6,6,14]Cl, Cl anions interact with hydrogen atoms in central P(CH2)4 groups in cations, resulting in a cradlelike structure with Cl anions sitting in regions formed by three alkyl chains in [P6,6,6,14] cations (Figure 3A).142−144,239,240 For large anions, like [NTF2] and orthoborates, the most negatively charged atoms are always coordinated with polar moieties in cations via strong electrostatic interactions and preferential HB coordinations (Figure 3B). A synergistic effect of these intermolecular interactions promotes a constrained orientation of large anion species around tetraalkylphosphonium cations, like the piggy-back structure of bis(oxalato)borate ([BOB]) anion on [P4,4,4,8] cation (Figure 3C).164
Figure 3.
Optimized ion pair structures of (A) [P6,6,6,14]Cl and (B) [P6,6,6,14][NTF2] ion pairs determined from quantum chemical calculations. Reproduced with permission from ref (144). Copyright 2011 Royal Society of Chemistry. (C) Optimized [P4,4,4,8][BOB] ion pair structure obtained from DFT calculations. Reproduced with permission from ref (164). Copyright 2014 American Chemical Society.
When tetraalkylammonium cations are functionalized with hydroxyl groups, a subtle energy balance between Coulombic, HB interactions, and dispersion forces governs unique properties of ILs.80,219 FT-IR spectra of [CH][NTF2] display a well-defined vibrational feature, which is assigned to a jumping-and-pecking motion of cations attributing to intermolecular vibrational modes between hydroxyl groups in [CH] cations and oxygen atoms in [NTF2] anions.80,241 Repulsive Coulombic interactions between [CH] cations are replaced by cooperative HB interactions between cations, which are, in principle, similar to those of alcohol dimers.241 Addition of alcohols to pure [CH] clusters results in enhanced “kinetic stability”,241 leading to the formation of ring structures with [CH] cations separated by neutral alcohols. The enhanced cooperative HB interactions and reduced Coulombic repulsions contribute to distinct thermodynamic stabilities of these ion clusters with increased melting temperatures and viscosities as well as decreased ion conductivities in comparison with those for [N1,1,1,3][NTF2]. Rotational dynamics and HB lifetimes for [CH][NTF2] consist of two time scales.219 The short time contribution to rotational correlation times and HB lifetimes is around picoseconds, whereas the long time contribution decays with relaxation correlation times in nanosecond range, demonstrating importance and longevity of ion pairs stabilized by HBs.
HB interactions between imidazolium cations and paired anions were fairly debatable in early investigations since their contributions to ion arrangements are indistinguishable from strong electrostatic interactions. In addition, similar to other IL systems, the principle adopted to define a HB has a significant effect on its interpretation.17,242 Nowadays, there is clear evidence of HB interactions in imidazolium ILs, and it is widely accepted that HB interactions between constituent ions in aprotic and protic imidazolium ILs behave as usual cation–anion interactions although sometimes HB interactions occur between ion species carrying the same charge.243,244 Evidences of HB interactions in imidazolium ILs were reported via a variety of experimental characterizations (FT-IR,81,245 NMR,245 Raman spectroscopies,244etc.), DFT calculations,81,245,246 and atomistic simulations.153,215,216,247 Local and directional HB interactions in imidazolium ILs are indicated by short C–H···anion distances, downfield shifted C–H proton chemical shifts, red-shifted C–H frequencies, and by DFT calculated frequencies of IL clusters consisting of [C2MIM] cations paired with thiocyanate ([SCN]), dicyanamide ([N(CN)2]), [HSO4], [C2SO4], and [NTF2].59,244,245 The observed differences in low frequency region of vibrational spectra of these ILs stem from specific cation–anion interactions, e.g., stretching and bending modes of C–H···anion HB interactions.
Hunt and co-workers performed intensive DFT calculations to elucidate HB structures in imidazolium ILs.17,149,248 HB donor sites in aprotic imidazolium cations are C–H units, which can be a C–H on ring moiety or a C–H from methylene or methyl groups of alkyl chains (Figure 4). DFT calculations revealed an array of different HB types between [C4MIM] cations and Cl anions.249,250 The primary HB interaction is C(2)–H···Cl with a HB distance of ∼2 Å, which is very short considering the sum of vdW radii for Cl and H atoms.250 It is noteworthy that this HB is not linear, as Cl sits slightly displaced, forming another weak HB with neighboring alkyl C–H unit. Large anions prefer to position over imidazolium rings.251 These remarkable cation–anion configurations with anions sitting on top of imidazolium rings result in a slight blue-shift of computed C(2)–H vibration due to distinct anion-π donor–acceptor interactions.
Figure 4.
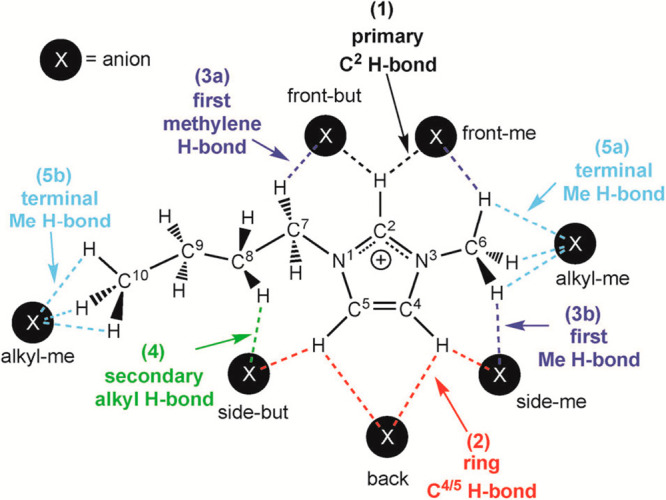
All possible HB donor sites in [C4MIM] cation to coordinate anions denoted as filled black circles with a white X. Different HB types are color coded and numbered as primary (1) with C(2) of imidazolium ring (black), ring (2) with C(4) and C(5) at rear part of imidazolium ring (red), first methylene (3a) or first methyl (3b) with C(6) or C(7) groups in alkyl chain (dark blue), secondary (4) with lateral methylene groups in alkyl chain (green), and terminal methyl (5) with terminal C(6) or C(10) methyl groups in alkyl chain (light blue). Reproduced with permission from ref (17). Copyright 2015 Royal Society of Chemistry.
HB interactions between imidazolium cations and their paired anions are affected by electronic characteristics and steric hindrance effects of alkyl chains. Alkyl chains can rotate to stabilize distributions of anions, and long alkyl chains can form secondary supporting HBs with anions. For all alkyl substituents in imidazolium cations, methylene groups bonded to nitrogen atoms on imidazolium rings have a distinctive electronic character and tend to form intermediate HBs, and the other methylene/methyl groups have varied weak HB capabilities depending on local conformations of alkyl chains.17,165,248,250,252 Generally, multiple HBs are formed between imidazolium cations and anions via different HB donor and acceptor sites with a wide range of HB strengths (Figure 4). Secondary HBs of anions with cation alkyl chains are viewed as influencing primary ones via displacing anions from an ideal linear arrangement.250 A key feature of these HB structures is that alkyl chains are able to reorient to maintain a more linear C–H HB interaction, while ring C–Hs cannot.149 A chelating ability of these HBs is relevant; if one HB is broken, another can keep the anion in the local vicinity allowing the broken HB to reform.61,217,248,253 These features are important for the formation of cation–anion HB networks in imidazolium ILs.248
Most imidazolium cations can be functionalized with various moieties, among which protonation of N(3) position and methylation of C(2) position hold specific significance.220 The former can tune the cation’s hydrophilicity from aprotic to protic. The hydrogen atom at N(3) position serves as a strong HB donor site and has significant and preferential HB interactions with anions and polar solutes in ILs.75,94,163,254,255 A combination of OHD-OKE, NMR, Raman, and FT-IR spectra and atomistic simulations demonstrated that strong competitive HB interactions among cations, anions, and water molecules can significantly affect molecular mobilities and rotational dynamics of ion species as well as hydrodynamic behavior of ILs and IL–water mixtures.75,94,254
Methylation of C(2) position in imidazolium rings disrupts predominant cation–anion HB interactions, leading to surprising changes in physicochemical properties of ILs via adjusting respective contributions of Coulombic forces, vdW associations, and HB interactions to yield distinct cation–anion coordination patterns.75,94,255,256 Elimination of C(2)-H···anion HBs by alkyl substitution tends to increase phase transition temperatures and liquid viscosities,59,218 as manifested in experimental studies of [C4C1MIM]X (1-butyl-2,3-dimethylimidazolium) (X = Cl, Br, I, [BF4], and [PF6]) ILs in comparison with [C4MIM]X ILs.257 Based on computational results, it was argued that an increase in rotational barrier of butyl chains facilitates alkyl chain association, and an overcompensation of phase transition entropy decreases with increasing transition enthalpy. A so-called “‘entropy theory”’ was proposed, but it is still presumed that HBs stabilize imidazolium ILs.221 Ludwig et al. suggested another explanation that directional cation–anion HBs destroy charge symmetry resulting in fluidized ILs.230,233,258,259 HBs can be regarded as “defects” in Coulombic networks of ILs. These defects increase ion dynamics, leading to decreased melting points and viscosities, as observed in FT-IR spectra and DFT calculations of [C2MIM][NTF2] and [C2C1MIM][NTF2] (1-ethyl-2,3-dimethylimidazolium) ion clusters. Additional studies showed that cation–anion interactions in imidazolium ILs are enhanced by HB interactions as indicated by frequency shifts to higher wavenumbers in FT-IR and terahertz spectra.59,222,223,260
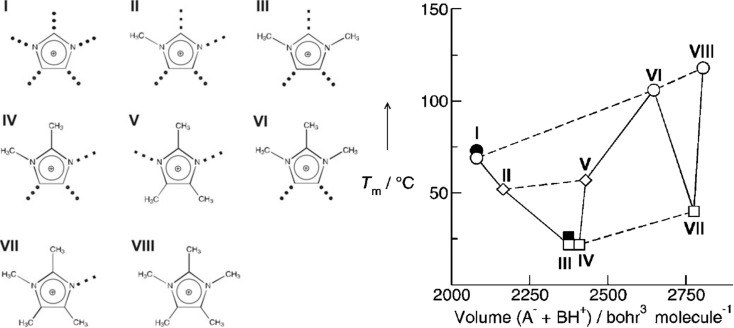
A combination of these two effects, namely, protonation of N(3) position and methylation of C(2) position in imidazolium rings, leads to complex phase behaviors of imidazolium ILs. Ludwig and co-workers designed a series of polymethylated imidazolium cations with varied methyl groups and hydrogen atoms on imidazolium rings to reduce conformational flexibility of imidazolium cations (Figure 5).222,223,261 By varying cation structures in a systematic way, it was shown that increasing interaction strength leads to a frequency shift to higher wavenumbers.261 Supported by DFT calculations of IL clusters, a nearly linear relationship was observed between calculated binding energies per ion pair and the observed frequency shifts. These results are referred to enhanced cation–anion interactions. For protic imidazolium ILs, it was shown that the vibrational bands assigned to cation–anion interactions can be well separated from other low-frequency vibrational modes, which arise from librational and rattling motions of ions. Stronger HBs further shift vibrational energies to higher frequency and result in distinct vibrational bands which can be used for studying phase transitions of ILs and cation–anion interaction strengths as a function of temperature. HBs have significant influence on ILs’ physicochemical properties, such as liquid viscosities, melting points, and enthalpies of vaporization (Figure 5).59,218,262 In addition, Noack et al. performed experimental studies on similar ILs and found that changes in electron density can adjust locations and strengths of interionic interactions, leading to reduced configurational variations.260 They suggested that neither “entropy theory”221 nor “defect hypothesis”59,222 alone is capable of explaining changes in physicochemical properties of ILs but complements each other.
Figure 5.
Left: Protonation and methylation of imidazolium rings at varied positions. Right: Plot of melting points (Tm) versus ion pair volumes for representative imidazolium [NTF2] ILs. As indicated by dashed lines, there is an increase in Tm with increasing ion pair volumes for ILs with no specific interaction site (●), with one interaction site (□), and with two interaction sites (◊). Reproduced with permission from ref (261). Copyright 2011 Wiley-VCH Verlag GmbH & Co. KGaA.
There are other ideas discussing the relevance of inter- and intramolecular HB interactions on physicochemical properties of ILs.263,264 Zahn et al. showed that the absence of HBs at C(2) position in imidazolium rings results in a reduced free movement of anions and an increased melting point of ILs, which is referred to flat energy landscapes of ion pairs.264 Izgorodina et al. investigated two possible structural and energetic sources for decreased ion conductivities of imidazolium ILs due to methylation of C(2) position; first, ion associations, as suggested by the Walden rule, and second, variations of potential energy surface profiles that favor ion transport in non-C(2)-methylated imidazolium ILs.263 It was shown that the increased liquid viscosities of C(2)-methylated-imidazolium ILs, attributing to a high potential energy barrier between energetically preferred conformations on potential energy surface, inhibit an overall ion transport.
It should be noted that methylation of C(2) position on imidazolium rings is also essential to improve chemical stabilities and tribological properties of ILs.221,263 Trialkylimidazolium ILs are considered as promising electrolytes for electrochemical applications265 due to the lack of an acidic proton at C(2) position and enhanced thermal and chemical stabilities221 in comparison with dialkylimidazolium ILs. Thermal stability is an important property when it is necessary to select appropriate ILs for applications at high temperatures, such as thermal fluids and lubricants and solvents for organic reactions at elevated temperatures. ILs with low thermal and chemical stabilities have reduced efficiencies in some physicochemical processes, which may lead to hazardous byproducts.266 Therefore, accurate data and knowledge of physicochemical properties of ILs are essential for engineering liquid flows for industrial applications and warrant extensive investigations combining experimental characterizations and computational studies.
Despite a wide range of anions, to date, HBs are formed with anions having a limited number of atom types. Anions, and their HB characteristics, can be classified by increasing structural complexity. The simplest anions are monatomic halides and small diatomics.250,267 Then follows highly symmetric multiatomic anions which exhibit similar structures but weaker HB interactions compared with monatomic anions.268 The next level of complexity includes small, less symmetric anions with an increasing number of strong HB acceptor sites, such as [OAc], [TFO], and [HSO4]. The most complex anions have more than one type of HB acceptors. Nitrogen centered anion species such as [NTF2] and [N(CN)2] have both an electron rich central nitrogen atom and pendant groups containing oxygen and nitrogen atoms, respectively. In most cases, oxygen atoms in anions have preferential HB interactions, but in the liquid phase all electronegative atoms can form HBs with cation species.269
In typical ILs, ions assume configurations to maximize HB interactions, and there can be a fine balance between a small number of shorter stronger directional HB interactions and a large number of weaker looser HB interactions.252,268,270 The density of HBs within ILs is very high, which facilitates networking. HB networking is expected to be maximized when the number of HB donor sites in cations is equal to the number of HB acceptor sites in anions. Perfectly matched HB donor and acceptor sites lead to the formation of “closed” rings and clusters or a rigid HB network in which all ions are held in place with well-defined configurations. A loss of HB sites or a restriction of alkyl chain rotation due to HB interactions can reduce entropy, which can be balanced by enhanced enthalpies of multiple HBs. A mismatch creates “‘loose’” HB acceptors or HB donors, i.e., “defects” within a HB network, which tend to facilitate fluidity and enhance dynamic properties of HB networks.187
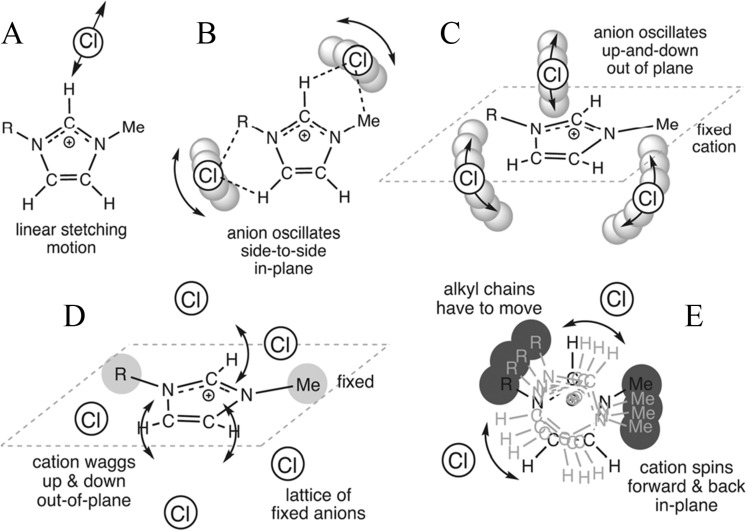
Recent investigations have featured the significance of time scales of HB interactions on ILs’ physicochemical properties.153,215,216,247,253 Both continuous and intermittent HB dynamics were examined for representative imidazolium ILs. For [C4MIM][PF6], continuous HB lifetimes are related to rotations of anions leading to a rapid breaking and forming of HBs, and intermittent HB lifetimes are associated with caging and librational motions of ions in local heterogeneous environments,271 respectively. Skarmoutsos et al. studied temperature dependence of HB dynamics in “hot” [C2MIM]Cl and “cold” [C4MIM]Cl ILs.253 It was shown that HB dynamics change dramatically for an ∼100 °C temperature variation even though the average number of HBs remains constant. In “hot” [C2MIM]Cl IL, C(2)–H forms the strongest HB having the slowest intermittent HB dynamics with a time constant of ∼100–120 ps, which is similar to rotational dynamics of N–N vectors in imidazolium rings. These computational results indicate that in the time it takes the entire imidazolium ring to rotate, adjacent anions have moved away, breaking HB structures (Figure 6). In “cold” [C4MIM]Cl IL, ring C–H sites dominate HB dynamics and intermittent HBs last for approximately 5 ns, whereas rotations of imidazolium ring planes occur on a much longer time scale. These HB dynamics can be intrinsically rationalized by underlying HB structures in these two ILs. In “hot” [C2MIM]Cl IL, a dynamic HB network exists, which contains a large number of single HBs. Once one HB breaks, the respective ions forming this HB are less likely to remain colocated, and they will form new HBs with different ions. A high density of ions in IL matrix is likely to be important as ions should be sufficiently close together that new HB arrangements will be readily formed. Thus, in “hot” [C2MIM]Cl IL, HBs are rapidly breaking and forming between different cations and anions. As this IL cools, a higher proportion of bifurcated HBs occurs. Ions are colocated for longer times, and lifetimes for individual HBs are extended (particularly for ring HBs). Even in “cold” [C4MIM]Cl IL, HBs are still breaking and reforming, but it is much more likely that these are happened between the same ion pairs, and intermittent HB lifetimes are dramatically extended.
Figure 6.
Cartoons indicating (A–C) movements of cations and anions for breaking and reforming cation–anion HBs, (D) out-of-plane wagging movements of anions around cations, and (E) cation in-plane spinning movements. Reproduced with permission from ref (253). Copyright 2014 Royal Society of Chemistry.
For imidazolium cations coupled with large anions, like [BOB], it was shown that the decay of continuous HB dynamics of C(2)–H in coordinating [BOB] anions is much faster than that of intermittent HB dynamics in a given IL matrix, which is consistent with computational results for [CnMIM]Cl ILs at elevated temperatures.253,272 It is noteworthy that residence lifetimes for HB dynamics, either ring C–H or alkyl C–H units, are much longer than those for water and alcohols under ambient conditions and are somewhat comparable with those of [C2MIM]Cl, [C2MIM][BF4], and [C2MIM][NTF2] ILs at high temperatures and with those of [C4MIM]Cl and [C4MIM][BF4] ILs over a wide temperature range.156 The continuous and intermittent C(2)–H···O ([BOB]) HB dynamics are described by a stretched biexponential decay function, which are distinct to those of C(2)–H···Cl HB dynamics. Diffusive properties of Cl anions around cation species in [C2MIM]Cl and [C4MIM]Cl ILs are described by a correlation function with three decay components.253 This observation is attributed to remarkable [BOB] anion structures compared with monatomic Cl anions in coordinating imidazolium cations.156,253,272 [BOB] anions have multiple HB acceptor sites, promoting their constrained distributions in polar domains and the formation of HB networks in heterogeneous IL matrixes. Assuming that imidazolium cations are fixed on regular lattices, the rotational dynamics of [BOB] anions around imidazolium ring planes, either up-and-down or side-to-side angular motions, desire a large energy to break ion structures and HB networks and therefore are not favored in [CnMIM][BOB] ILs. The fast decay of C(2)–H···O HB dynamics in [CnMIM][BOB] ILs can be rationalized by a linear in-and-out stretching mode along C–H bonds in cations. The librational motions of Cl anions in IL matrixes, such as up-and-down and side-to-side angular motions relative to imidazolium ring planes and linear stretching vibration along the C–H bonds, lead to a fast decay of continuous C(2)–H···Cl HB dynamics (Figure 6).253 Furthermore, lengthening cation alkyl chains leads to a substantial increase in residence lifetimes for both continuous and intermittent HB dynamics.217
2.2.2. π–π Stacking Structures
In addition to HB coupling, π-type interactions are correlated with preferential electrostatic interactions and favorable dispersion associations among heteroaromatic rings, such as imidazolium, triazolium, thiazolium, pyrazolium, pyridinium cations, orthoborate anions, and their derivatives, despite the strong repulsive electrostatic forces between ring moieties having like charges.6,60,273−278
Matthews and Hunt performed DFT calculations to explore microstructural and energetic landscapes of π–π stacking structures in [C1MIM]Cl ion pair dimers.149,252,279 Imidazolium ring stacking structures are described as electron deficient π–π stacking interactions, and a competitive on-top ion pair structure is identified as a peculiar anion-donor π-acceptor coordination pattern between imidazolium ring planes and anions. Ion pair dimers display a subtle balance of varied microstructural features. The low-energy middle π–π stacking conformer (Figure 7A) exhibits a front and side arrangement where imidazolium rings exhibit parallel-displaced and stacking structures, analogous to benzene dimers,149,279 whereas in a low-energy diagonal conformer (Figure 7C) front and top arrangements dominate.279 An energy barrier of ∼6.1 kJ/mol exists for conversion of a π–π stacking structure (Figure 7B) to a diagonal conformer with no π–π stacking (Figure 7C), indicating that such a structural conversion is a facile process in the liquid phase.149,252 In addition, rotation of methyl groups within π–π stacking structures facilitates the formation of linear secondary alkyl C–H···Cl HBs, which decay faster with distance than primary ring C–H···Cl HBs and C(2)···Cl anion−π interactions. Theoretical analysis showed that a subtle structural difference of these stacking structures of [C1MIM]Cl ion pair dimers is mainly attributed to an array of weak and strong HBs, anion−π, and π–π stacking interactions. The energy differences between cation–anion HB interactions, anion−π associations, and cation–cation π–π stacking interactions are all very small (<10 kJ/mol), and their competition creates a very delicate balance of forces within liquid environments. These interactions fluctuate in strength, forming a small number of strong interactions and a larger number of moderate interactions with very little cost in energy. This is striking and in contrast to biological systems and some crystal structures where π–π stacking, anion−π, and HB interactions impart remarkable secondary structures.279
Figure 7.
Optimized [C1MIM]Cl ion pair dimer structures determined from quantum chemistry calculations ((A) and (B) are middle configurations, and (C) is the diagonal configuration). Reproduced with permission from ref (149). Copyright 2014 Royal Society of Chemistry. Ion pair dimer structures for (D) [C1MIM][C1SO4], (E) [C1MIM][TFO], (F) [C1MIM][BF4], and (G) [C1MIM][NO3] obtained from DFT calculations. Reproduced with permission from ref (252). Copyright 2014 IOP Publishing.
Furthermore, the impact of anion electronic structures on disruption of π–π stacking interactions was identified by a substitution of Cl with a range of large anions with diffuse charge (i.e., [NO3], [C1SO4], [TFO], and [BF4]).252 A diagonal ion pair dimer structure is the most stable configuration in energy for large anions, reflecting a propensity of large multidentate [C1SO4] (Figure 7D), [TFO] (Figure 7E) and [BF4] anions (Figure 7F) to favor top interactions. Based on analysis of molecular orbitals and electronic structures, it is evident that there is a subtle interplay between traditional in-plane HB coordinations and peculiar interplanar anion−π interactions, the latter of which is particularly prominent for [C1MIM][NO3] ion pair dimers (Figure 7G). All these interactions have a significant impact on structural arrangements in ILs and highlight the influence of dispersion forces and the importance of HB interactions on the formation of π–π stacking structures in imidazolium ILs.
Subsequent first-principles calculations also revealed preferential π–π stacking interactions in [C2MIM]Cl and [C2MIM][SCN] ILs and their mixtures.277 A weak π–π ordering structure was observed in [C2MIM][SCN] in comparison with that in [C2MIM]Cl, and an intermediate π–π stacking structure was observed in their mixture with an equimolar fraction. In addition, π–π stacking dimer structure was also formed between imidazolium ring planes in [C2MIM][NO3],6 [C2MIM]2[SO4],6 and [C2MIM][NTF2]275 crystal structures owing to a substantial screening of charge–charge repulsive forces among cation species mediated by anions. XRD data, complemented by atomistic simulations, revealed that Br anions in [C2MIM]Br liquid phase are symmetrically distributed around [C2MIM] cations and are more closer to ring moieties than those in crystal structures.276 Thus, π-type interactions are recognized as a key component for local structuring of imidazolium ILs.
For imidazolium cations with intermediate alkyl chains, dispersion interactions between alkyl chains play a dominant role.280 Nuclear Overhauser effect experiments suggested a local short-ranged cation–cation stacking structure in [C4MIM][BF4] and in its C(2)-methylated analogue.281 A further lengthening cation alkyl chains results in the formation of ionic liquid crystals, in which interdigitation of alkyl chains facilitates alignment of imidazolium rings such that π–π stacking interaction becomes more significant. Various lyotropic liquid crystalline phases were obtained in mixtures of [CnMIM]Br ILs (n = 12, 14, and 16) with p-xylene and water.282 Strong π–π stacking interactions of imidazolium rings and cation−π interactions with p-xylene have unique influences in determining structural properties, especially the thickness of water channels in mixtures. In addition, both rheological steady and dynamic moduli of these liquid crystalline phases increase with lengthening cation alkyl chains, leading to their promising applications in fabrication of nanomaterials.
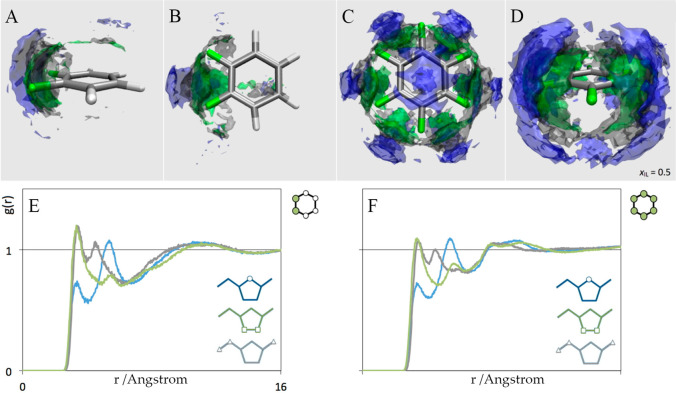
Moreover, an incorporation of benzene (and its fluorinated derivatives) in imidazolium ILs can significantly alter microstructures and, in particular, cation–cation interactions.152,283,284 Benzene molecules tend to displace anions by intercalation into high-charge density domains. This intercalation is attributed to π–π stacking interactions between imidazolium ring planes and benzene molecules rationalized by attractive arrangements of quadrupole moments.283,285−287Ab initio and atomistic MD simulations showed that quadrupole moments of aromatics are an almost linear function of the number of fluorine substitutions,89,152 which is fully reflected in spatial arrangements of imidazolium rings around aromatics. Cations are mainly located above/below benzene plane (Figure 8) because of strong diamagnetic influence of aromatic electrons. As benzene is progressively fluorinated, cations migrate to equatorial plane of aromatics, experiencing a milder paramagnetic effect. Lengthening alkyl chains in imidazolium cations contributes to a finite probability for benzene to be found both in apolar domains and in polar networks, indicating that benzene experiences different local environments in heterogeneous IL matrixes.288 Concerning dynamic features, atomistic simulations showed that rotation of benzene in [C4MIM]Cl is controlled by vdW and π–π interactions on short time scales (picoseconds) and by solvent charge associations on long time scales (hundreds of picoseconds).289 OHD-OKE experiments on [C1MIM][NTF2]-benzene89 and [C8MIM][BF4]-benzene290 mixtures showed different intensities in comparison with their ideal mixing spectra, which is attributed to suppressed translational motions of benzene in mixtures.
Figure 8.
Spatial distributions of C(2) (blue), C(5) (green), and C(6) (gray) atoms around (A, B) 1,2-difluorobenzene and (C, D) hexafluorobenzene molecules in equimolar IL–aromatic mixtures. Radial distribution functions between carbon atoms in imidazolium cations (C(2), blue; C(5), green; C(7), gray) and fluorine atoms in (E) 1,2-difluorobenzene and (F) hexafluorobenzene in equimolar IL–aromatic mixtures. Reproduced with permission from ref (152). Copyright 2014 American Chemical Society.
2.2.3. Hydrogen Bonding vs π–π Stacking Interactions
HB coordinations and π–π stacking interactions have distinct effects on stabilization of liquid structures of ILs having heteroaromatic rings and multiple HB donor and acceptor sites.252,279 For imidazolium cations paired with small anion species, such as Cl,277,279 [NO3],252 and [SCN],277 π–π stacking structures coexist with HB coupling between ion species, promoting the formation of decent microscopic ion structures in bulk ILs.252,277,279Ab initio molecular dynamics (AIMD) simulations revealed distinct π–π stacking conformations in [C2MIM][SCN], which decrease dramatically in [C2MIM][N(CN)2] and [C2MIM][B(CN)4] (tetracyanoborate) ILs.153 HB interactions are very pronounced in [C2MIM][N(CN)2] and [C2MIM][SCN] ILs with anions taking in-plane configurations of imidazolium rings, while HB interactions are almost absent in [C2MIM][B(CN)4] with [B(CN)4] anions taking on-top conformations above/below imidazolium rings.291 The small size of [SCN] anion together with its strong HB capability stabilize local ion arrangements as was pointed out for [CnMIM]Cl ion pair dimers.149,279 Cyano ILs are generally viscous and their liquid dynamics are well correlated with rotational dynamics of cyano groups.292 Both microstructural and dynamical quantities of these ILs exhibit similar sequence as their viscosities, inferring that high viscosity of [C2MIM][SCN] might be related to enhanced π–π stacking interactions between imidazolium rings.
For ILs composed of imidazolium cations paired with large anion species, like [C1SO4]252 and [NTF2],59,275,278 both π–π stacking interactions and HB coordinations get weakened. These anions have multiple HB acceptors and have preferential HB coordinations with imidazolium cation hydrogen atoms, promoting the formation of HB networks with distinct HB strength and directionality. Additionally, these large anions prefer configurations above and below imidazolium rings or exhibit tilted orientations in equatorial region of imidazolium rings, leading to cation–cation π-type coordination being partially weakened or totally screened due to anion size effect. Therefore, HB networks overtake π-type interactions and have a significant effect on local ionic structures and complex liquid morphologies of ILs.
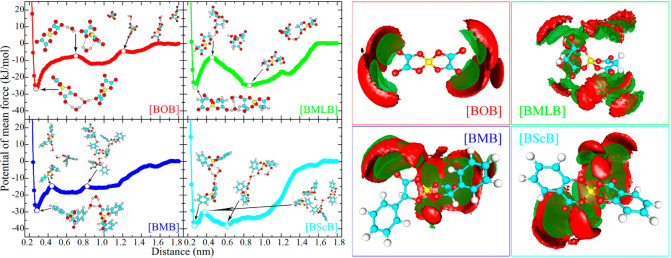
The subtle balance of HB and π–π stacking interactions among ion species, either competitive or cooperative, will be more sophisticated if anions have planar rings, such as orthoborate anions.61,217,293−296 For [CnMIM][BOB] ILs, AIMD simulations revealed that preferential HB interactions and remarkable π–π coordination among neighboring imidazolium and oxalato ring planes coexist in ILs (Figure 9).61,217 HBs are formed between imidazolium cation hydrogen atoms and [BOB] anion oxygen atoms but with different HB features. At short radial distances, imidazolium rings exhibit π–π stacking associations (Figure 9A) and present complicated orientational distributions at intermediate and large radial distances due to intervention of other ions. Spatial associations between imidazolium and oxalato ring planes are characterized by short-range π–π stacking structures (Figure 9B) and displaced offset stacking conformations mediated by peculiar in-plane HB interactions (Figure 9C) and by distinct perpendicular distributions at intermediate radial distances because of attractive electrostatic interactions. The intermolecular interactions between oxalato ring planes are mediated by repulsive Coulombic interactions and steric hindrance effect, contributing to tilted orientations of oxalato ring planes to neighboring ones in local ionic environments (Figure 9D). A gradual lengthening of cation alkyl chains results in a substantial increase in interaction strength for all HBs. However, the strengthened HB interactions result in weakened π–π stacking coordinations between imidazolium and oxalato ring planes, demonstrating significant competitive characteristics for HB and π–π stacking interactions in [CnMIM][BOB] ILs. In addition, intermittent and continuous HB dynamics exhibit a decent cooperative correlation feature with rotational and translational dynamics of ring moieties with increasing alkyl chains in imidazolium cations. The competitive structural trade-off and cooperative dynamical interplay of HB and π–π stacking interactions in [CnMIM][BOB] ILs are essentially correlated with preferential and collective associations among cation alkyl units and decisive Coulombic interactions among imidazolium and oxalate ring moieties in heterogeneous IL matrixes. These computational data may provide important physical insights for a thorough understanding of striking microstructures and dynamical quantities, and mesoscopic liquid morphologies of [CnMIM][BOB] ILs as well as their macroscopic functionalities in industrial applications, for example, as promising solvent electrolytes in electrochemical devices or as alternative lubricants or lubricant additives in tribology.
Figure 9.
(A) Imidazolium ring pairs are featured with π–π stacking orientation. Imidazolium and oxalate ring pairs are described by (B) π–π stacking and (C) parallel displaced offset stacking configurations as well as hydrogen bonding interactions. (D) Intermolecular oxalato ring pairs are characterized by tilted distributions promoting their HB coordinations with cation hydrogen atoms. Reproduced with permission from ref (61). Copyright 2017 American Chemical Society.
2.3. Free Ions, Ion Pairs, and Ion Clusters
Since ILs are concentrated and solventless ion solutions, intimate ion pairs would be a natural expectation and many endeavors have been focused on describing bulk ILs as a large population of neutral ion pairs plus a small concentration of “free” ions or ion clusters.18,135,271,297−303 Both mass spectrometric data and theoretical calculations suggested that distillation of ILs mainly occurs via neutral ion pair clusters of composition, followed by dissociation of large ion aggregates to lower order ion pairs and thereafter to small charged ion clusters in gas phase depending on the amount of internal energy for depositing charged clusters into neutral ion pairs upon evaporation.304,305 These findings indicate that ion pairs might be available in bulk liquid phase, similar to that for a description of aqueous electrolyte solutions and Coulomb fluids. In addition, there are some postulations addressing that ILs form clustered supramolecular structures to maintain HB networks,306 whereas other studies focused on ion cluster models including possible formation mechanism of IL ion clusters and effect of IL ion clusters on interfacial structures, liquid morphologies, and self-assembly processes of ILs in bulk liquids and in interfacial regions.297 It should be noted that the concept of the ion cluster mainly comes from theoretical calculations and atomistic simulations. These studies should be carefully interpreted as there is no rigorous criteria to define an ion cluster and the distinction between different ion cluster models is arbitrary.307
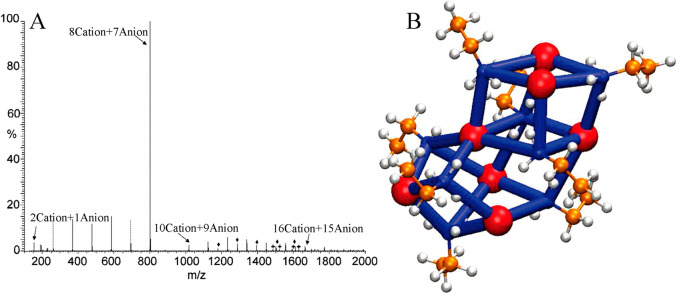
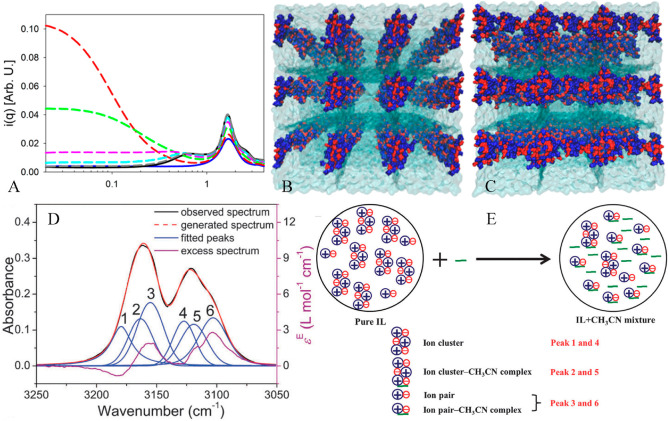
Weingartner et al. suggested the formation of EAN ion pairs via measurement of critical behavior of EAN–octanol mixtures.308 The obtained constant value describing ion pair associations from ion conductivity data is an order of magnitude larger than theoretical prediction of ion pairing behaviors in mixtures, suggesting that EAN may exist in a chemical equilibrium condition between “free” ions and ion pairs in the liquid region.298 Ion pair lifetimes are close to those of ion coupling phenomena in alkali metal nitrate molten salts but are much larger than those for dilute aqueous electrolytes. Kennedy and Drummond proposed that protic ILs are composed of net charged ion clusters as they observed distinct ion aggregates with varied ion sizes from positive ion spectra of pure protic ILs, such as a dominant ion cluster consisting of eight cations and seven anions in EAN and PAN ILs (Figure 10A).299 Therefore, it was suggested that EAN and PAN ILs are polydispersed mixtures consisting of aggregated ions and charged ion clusters. DFT calculations supported this hypothesis and showed that this particular ion cluster model is thermodynamically favorable for EAN in the gas phase and is the most stable species for entropic and enthalpic considerations because this ion cluster forms the most compact HB network in which all proton donors in EA cations and HB acceptors in [NO3] anions are involved in an optimal way (Figure 10B).309,310
Figure 10.
(A) Electrospray ionization mass spectrometry positive ion mode of EAN. Reproduced with permission from ref (299). Copyright 2009 American Chemical Society. (B) The most stable ″8Cation+7Anion” model for EAN obtained from thermochemistry calculations. Hydrogen, carbon, and nitrogen atoms in EA cations are represented by white, orange, and blue spheres. [NO3] anions are represented as red spheres. Reproduced with permission from ref (309). Copyright 2009 American Chemical Society.
Dielectric spectra, theoretical calculations,301,311 and atomistic simulations271,300 on a range of aprotic imidazolium,76,312 pyrrolidinium,313 pyridinium,313 and tetraalkylammonium313 ILs did not display signatures of ion pair formation in bulk liquids. In an interesting work, Gebbie and co-workers provided a distinct view of bulk IL structures from DLVO (Derjaguin–Landau–Vervey–Overbeek) fits of SFA data.135,136 For [C4MIM[NTF2] confined between two charged solid surfaces, a weak attractive force spanning from 3 to 30 nm was obtained, which is independent of applied electric potentials. The fitting of such a long-range force with DLVO theory predicts a negligible concentration of free ions in bulk liquids. Therefore, it was speculated that [C4MIM][NTF2] is a dilute electrolyte solution consisting of a large proportion of neutral ion pairs and a small fraction of dissociated free ion species, which is akin to the description of water consisting of an overwhelming majority of neutral water molecules plus some H3O+ and OH– ions. It should be noted that while long-ranged forces might be real in IL matrixes,314 the main conclusion of this work is conflict with many experimental and computational studies. An additional description indicated that DLVO theory is unsuitable to characterize phase behaviors of ILs because (1) ILs show complex association and dissociation equilibria,315,316 (2) ion species change allegiances among neighboring counterions but not to single ion pairs or ion clusters in bulk liquids,300,301 and (3) a long-range repulsive force was missing which should be accompanied by the long-ranged attractive force.
In another case, ion and electrical conductivities of [C4MIM][PF6] deviate from the Nernst–Einstein relationship,271 which is attributed to correlated motion of ions having opposite charge and anticorrelated motion of ion species having the same charge over multiple timescales up to nanoseconds.157,262,317,318 This scenario is distinct to that in electrolyte solutions, where positively correlated motion of ion species has a substantial contribution to decreased impedance conductivity.318 On the basis of microstructural and dynamical analysis, this observation indicates that cation–anion interactions can be described using ion association instead of ion pair as each ion group is not solely paired to a single counterion nearby but to multiple counterions in ionic atmosphere. Additional atomistic simulations demonstrated that the formation of different ion associations is not so important to describe bulk IL structures as these ion units are weakly maintained together due to a small separation of ion species in bulk IL matrixes.300 Therefore, it was suggested that the origin of destabilization of ion associations is caused by an overscreening of electrostatic charges in the first solvation shell.301
Modeling of bulk ILs as a continuum consisting of ion couples migrating together is not appropriate, which cannot be reconciled with their intrinsic character of low vapor pressure. It is known that the vapor pressure of liquid materials is correlated with ionicity, which is well represented in the Walden plot of molar conductivity against fluidity.319 A separated neutral ion pairing unit does not have any contribution to ion conductivity and charge transferability. Therefore, ILs having a high proportion of neutral ion pairs or ion pairing aggregates should be “poor” liquids as ion conductivity and charge transferability will be less than expected from liquid viscosity. However, to some extent, most ILs are “good” liquids and have high ion conductivity, charge transferability, and low vapor pressure.320 This might be attributed to the fact that spatial distributions of ion species in bulk ILs are highly heterogeneous with each ion surrounded by a shell of counterions exhibiting different configurations due to a delicate intermolecular interaction of central ion with neighboring counterions.
In contrast to pure ILs, some other investigations indicated that ILs may form ion pairing structures in IL–molecular solvent mixtures. The direct-contacted and solute-separated ion pairing structures were suggested in [C4MIM][BF4]–water and [C4MIM][BF4]–dimethyl sulfoxide mixtures.321 In addition, [C4MIM][PF6]–naphthalene322 and 1-methyl-4-cyanopyridinium [NTF2]–methylnaphthalene323 mixtures also exhibit cation–anion ion pairing and cation–aromatic pairing groups across a wide range of solute concentrations. Therefore, even the ion pairing concept is helpful to understand liquid structures in electrolyte solutions, it is not feasible to describe bulk IL structures. Transient ion pairing structures might exist in IL matrixes with their lifetimes shorter than picoseconds; however, it should be addressed that the overall liquid structures of bulk ILs are more complicated than a continuum of ion pairs, ion couples, and ion clusters in ion solutions.
2.4. Microstructures and Mesoscopic Liquid Morphologies
2.4.1. Alkylammonium ILs
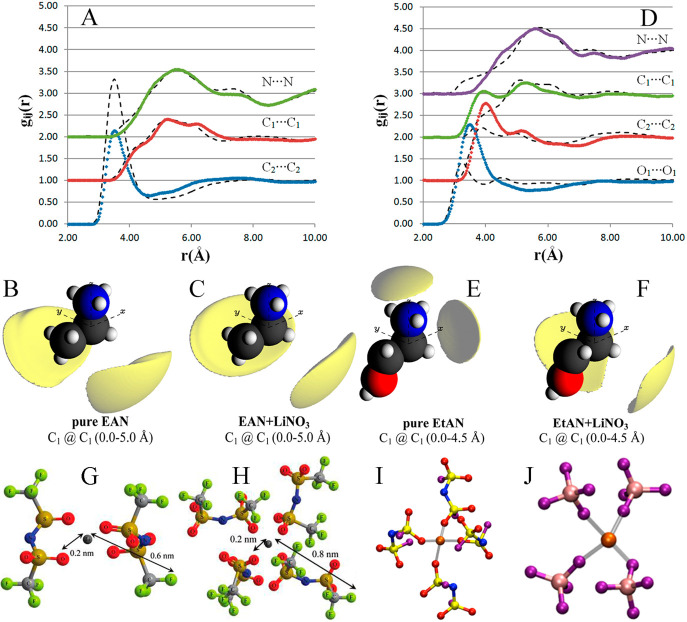
Atkin and Warr108 and Umebayashi et al.189 investigated microstructures of EAN using complementary wide-angle X-ray scattering (WAXS) and small-angle neutron scattering (SANS) techniques, respectively. Both studies indicated that nanoscale heterogeneity exists in EAN with polar and apolar domains throughout the bulk liquid matrix, suggesting a disordered, locally smectic, or bicontinuous liquid structure. The solvophobic interactions among alkyl units are essentially responsible for production of nanostructural heterogeneity. In addition, both electrostatic and HB interactions between amine and [NO3] groups play a significant role in stabilizing microstructures in the EAN matrix.
Lengthening alkyl chains in alkylammonium cations leads to pronounced nanoscopic liquid structures in which apolar and polar domains are segregated and partially interdigitated, with ions in more precisely defined positions relative to one another.108,192,195,324,325 HB networks are more stable and get stronger in ILs having longer cation alkyl chains owing to a distinct hydrophobic effect stemming from alkyl chain contacts. Equilibration within apolar domains, as evident from stretching dynamics of C–H bonds, is faster than that in polar domains and shows a remarkably low thermal activation, indicating that ILs are not only structurally heterogeneous but also dynamics vary considerably among different substructures. Both ends of alkylammonium cations, namely, charged N–H head groups and hydrophobic tail C–H groups, exhibit rotational dynamics on different time scales. These rotational dynamics are heterogeneous and are governed by a local propellerlike motion of cations, which is intrinsically attributed to structural heterogeneity in IL matrixes.187,193 The N–H head groups exhibit slow dynamics because of strong Coulombic interactions and preferential HB interactions with [NO3] anions, whereas the tail C–H groups exhibit fast dynamics due to their weaker vdW interactions with surrounding atoms. In particular, rotational dynamics of tail C–H groups show marginal dependence on cation alkyl chain length, while rotations of head N–H groups slow down with lengthening cation alkyl chains, demonstrating that dynamical heterogeneities are enhanced in ILs with longer alkyl chains in alkylammonium cations. Such a slowdown is mainly correlated with a decreased number of [NO3] anions near N–H groups, which presumably leads to an increase in energy barrier for their rotations and thus gives rise to a broad distribution of N–H rotation times. Furthermore, the relative free energy landscapes of cation–anion interactions exhibit a progressively deeper well as cation alkyl chains get longer.192,326,327 These observations indicate that IL nanostructures are analogous to a surfactant mesophase, and thus the area ratio of polar and apolar domains will be useful to predict self-assembled liquid morphologies in alkylammonium ILs.190
Addition of hydroxyl groups to terminal methyl groups in EA cations leads to a disruption of solvophobic associations between alkyl chains in EtAN, resulting in small ion aggregates, rather than a spongelike liquid morphology with extended networks.191,224 EtAN has a less ordered liquid arrangement than that of EAN since [NO3] anions are competitively coordinated with both ammonium and hydroxyl groups in EtA cations via strong HB interactions.328,329 Generally, inclusion of additional HBs represents a source of defects in polar networks, leading to more disordered microstructures in ILs.94,223,255,330 In addition, both translational and rotational dynamics of constituent ions slow down due to the formation of HB networks in IL matrixes. When an external electric field is applied, EtAN requires a lower electric field strength than that for EAN to emit ion pairs.331 The applied electric field can effectively reduce the number of hydroxyl–[NO3] HBs but is less effective in disrupting HBs between N–H head groups and [NO3] anions.
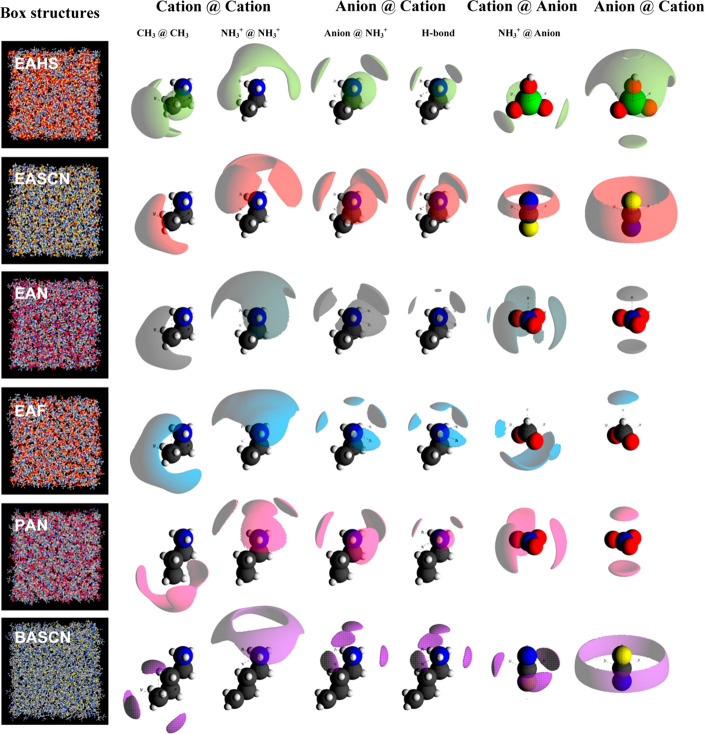
Besides variations in cation structures, anions also have a significant effect on microstructures in a range of ILs consisting of primary alkylammonium cations coupled with Br, [NO3], [OAc], [HSO4], [SCN], formate, triflate ([CF3SO3]), and alkylsulfonate anions.190,324,325,329,332−334 Representative spatial distributions of HB acceptors around donor sites are shown in Figure 11. Ion arrangements in these ILs are consequently attributed to a delicate balance of ion dimensions and varied intermolecular forces among constituent ions. While similar nanostructures characterized by bicontinuous liquid morphologies were formed in all these protic ILs, there is a substantial difference in HB features. More cation–anion HBs are formed when anions have multiple HB acceptors and build up a dense, cooperative HB network.332 HB geometries are intrinsically related to capability of each ion to accommodate HBs in bicontinuous networks rather than creating a different structure in bulk liquids. When liquid structures are such that there is a high proportion of linear HBs, attractions between ion species increase and ILs exhibit solidlike phase behavior. In contrast, nanostructures with bifurcated or trifurcated HBs produce weak and bent HBs, leading to decreased cation–anion attractions, and therefore ILs are more fluidlike materials. These differences in HB interactions are reflected in macroscopic physicochemical properties, like melting points, glass transition temperatures, ion conductivities, and liquid viscosities of alkylammonium ILs.18
Figure 11.
Nanostructures in primary alkylammonium protic ILs. Column 1 (left), box structures; column 2, uncharged groups in cations around uncharged groups in cations; column 3, charged groups in cations around charged groups in cations; column 4, charged groups in anions around charged groups in cations; column 5, HB acceptors in anions around HB donor sites in cations; column 6, charged groups in cations around charged groups in anions; and column 7, charged groups in anions around charged groups in anions. Reproduced with permission from ref (332). Copyright 2013 Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission from ref (190). Copyright 2014 American Chemical Society. Reproduced with permission from ref (18). Copyright 2015 American Chemical Society.
2.4.2. Imidazolium ILs
The first speculation of the existence of mesoscopic liquid structures in imidazolium ILs was suggested on the basis of experimental measurement of diffusive properties of electroactive solute molecules dissolved in imidazolium IL–water mixtures.335 Neutral and charged solutes exhibit distinct diffusion coefficients, indicating that binary imidazolium IL–water mixtures should not be considered as homogeneous liquids but have to be treated as nanostructured solvents consisting of polar and apolar domains. Later systematic measurements of physicochemical properties (densities, viscosities, diffusion coefficients, ion conductivities, etc.) of [CnMIM][NTF2] ILs with cations having varied alkyl chains262 and a suggestion of structural heterogeneity being the underlying microscopic origin of the “red-edge effect” observed in fluorescence spectroscopy64 showed that experimental and computational studies are consistent with the hypothesis of microstructural and dynamical heterogeneities in imidazolium ILs.
Subsequent molecular simulations further suggested that aprotic imidazolium ILs can self-assemble and form solvent nanostructures. CG simulations of [CnMIM][NO3] ILs demonstrated that imidazolium rings and [NO3] anions are relatively homogeneously distributed in bulk liquids. However, alkyl groups aggregate into spatially heterogeneous apolar domains, which is attributed to a competitive coordination between long-range electrostatic interactions among charged groups and short-range collective solvophobic associations between hydrophobic alkyl chains.336,337 This observation is more significant for cations having long alkyl chains, contributing to the formation of liquid crystal-like structures.184,338,339 The application of external electric fields can substantially affect spatial heterogeneities of ILs, which are first disordered from spatially heterogeneous to spatially homogeneous structures, and thereafter get reordered to nematic-like structures with a gradual increase in external electric field strength.340,341 Translational diffusion of ions increases in the homogeneous regime and decreases in the nematic-like region, attributing to a competition between electrostatic interactions among ion species and the formation of ion cage structures under external electric fields.
ILs composed of imidazolium cations paired with varied spherical anions (F, Cl, Br, [BF4], and [PF6]) present a similar picture of heterogeneous microstructures in IL matrixes but with a few important differences.342,343 Polar domains in imidazolium ILs have heterogeneous distributions and form continuous ion channels in IL matrixes. Polar domains coexist with apolar domains consisting of hydrophobic alkyl groups. Simulation snapshots showed in Figure 12A–F, particularly those rendered under green (apolar domain) and red (polar domain) convention, provide a clear visualization on the evolution of heterogeneous liquid structures as cation alkyl chain length increases. For imidazolium cations with short alkyl chains, like C1 and C2, small and globular apolar “islands” are formed within interpenetrating polar networks. Lengthening cation alkyl chains enables apolar “islands” to aggregate into spongelike liquid nanostructures. [C4MIM] cation marks a microstructural transition between these two solvent morphologies.
Figure 12.
Snapshots of simulation systems containing 700 [CnMIM][PF6] ion pairs. [C2MIM][PF6] with (A) CPK color coding and (B) red (polar domain)-green (apolar domain) color coding, (C) [C4MIM][PF6], (D) [C6MIM][PF6], (E) [C8MIM][PF6], and (F) [C12MIM][PF6]. Reproduced with permission from ref (343). Copyright 2006 American Chemical Society. (G) X-ray diffraction patterns for supercooled [CnMIM]Cl IL. Reproduced with permission from ref.280 Copyright 2007 American Chemical Society. (H) X-ray diffraction patterns for [CnMIM][PF6] ILs at 25 °C. Reproduced with permission from ref (345). Copyright 2008 Elsevier. (I) SWAXS data for [CnMIM][NTF2] ILs at room temperature. Reproduced with permission from ref (346). Copyright 2009 IOP Publishing.
Whether these computational results are interpreted as indications of micelle-like structures or bicontinuous liquid morphologies, X-ray scattering data are compelling evidence of self-assembled solvent nanostructures. S/WAXS spectra exhibit a well-defined peak at ∼0.3 Å–1 for [CnMIM]Cl (Figure 12G) and [CnMIM][PF6] (Figure 12H) ILs with cation alkyl chains longer than C4.280,344,345 Not only peak amplitudes increase but also peak positions shift toward low q values with lengthening cation alkyl chains. Enlarging anions from small Cl (Figure 12G) to intermediate [PF6] (Figure 12H) and then to large and asymmetric [NTF2] (Figure 12I) has no discernible effect on the dependence of characteristic size of nanoscale heterogeneity on cation alkyl chain length,280 highlighting the importance of apolar aggregation in self-assembly of imidazolium ILs in bulk liquids.280,346
While these experimental studies provide valuable results, the model used to rationalize scattering data at low q values is minimalistic. It does not take into consideration privileged correlations between similar polar–polar, apolar–apolar, and different polar–apolar moieties. Hardacre et al. conducted SANS experiments on imidazolium ILs and elucidated local ion–ion distributions via an empirical potential structural refinement (EPSR) fitting of SANS spectra.347,348 Microstructural arrangements in imidazolium ILs exhibit a remarkable charge ordering feature which, to some extent, resembles ion structures in crystalline state following an onionlike alternating cation–anion shells. [C1MIM] cation is not amphiphilic because of the very short methyl groups, and thus its bulk liquid structures are principally determined by electrostatic interactions.348 This cation serves as a reference for description of bulk correlation peak changes with lengthening cation alkyl chains. SANS spectra showed that enhanced structural heterogeneities are mainly originated from decreased symmetry of imidazolium cations as alkyl chain length increases.347 For [CnMIM][PF6] ILs with cations having intermediate alkyl chains that are long enough to produce apolar segregations, an unambiguous scattering peak was observed in the low q region (Figure 12H).345 This peak moves to low q values (large spatial distances), sharpens, and increases in intensity with lengthening cation alkyl chains. These results indicate that lattice expansion and correlation are entirely related to apolar domains with an increase of ∼2 Å per methylene unit.344,347
Margulis and co-workers155 came to a similar conclusion to that of Hardacre et al.347 via performing atomistic simulations of bulk [C6MIM]Cl, [C8MIM][PF6], and [C10MIM][PF6] ILs. The first sharp diffraction peak (FSDP) at low q values in the scattering spectra is regarded as an indicator of the mesoscopic liquid structure, attributing to segregation of polar and apolar domains in IL matrixes. While cation anisotropy may be important for the formation of FSDPs, it appears that FSDPs can be described by a much simpler consideration of solvation shell asymmetry.347 In addition, neutron scattering data for protic190 and aprotic347 ILs are generally consistent with the spatial distance of a spongelike liquid phase, and thus the scattering peak for a sponge phase formed in these ILs can be considered as a gauge of bulk self-assembly due to an amphiphilic feature of constituent ions.
For ILs composed of imidazolium cations paired with [NTF2] anions, neutron scattering data and theoretical calculations showed that [C1MIM][NTF2] has a negligible long-range alternating counterion ordering.275 Russina et al. conducted S/WAXS characterizations on a series of [CnMIM][NTF2] (1 < n < 10) ILs and highlighted their microstructural heterogeneities.346 Three decent diffraction peaks are observed with two peaks at high q values displaying marginal dependence on the cation alkyl chain length and one peak at a low q value showing strong dependence of amplitude and position on cation alkyl chain length (Figure 12I). The latter is a signature of microstructural heterogeneity with its size related to alkyl chain segregation in apolar domains,349 similar to that observed in imidazolium ILs consisting of Cl, [BF4], and [PF6] anions.344,346,348
Mutating a methylene (methyl) group with specific atoms, like hydrogen,94,254 oxygen,225,350 fluorine,278,351 silicon,85,352 selenium,353 and even phenyl groups,354−356 leads to distinct microstructural arrangements in heterogeneous IL matrixes.
Functionalization of alkyl chains using ether and hydroxyl groups disturbs a delicate balance of Coulombic, HB, and dispersion interactions, contributing to enhanced intermolecular interactions between constituent ions.227,360,361 This has an overall effect on disrupting mesoscopic ordering structures, as revealed from peaks at low q values, which either have decreased scattering intensities or are totally disappeared. These changes in low q scattering are attributed to flexibility and increased polarity of alkoxy chains and favorable associations of ether and hydroxyl moieties with imidazolium rings via HB interactions.225 Russina and Triolo compared SAXS data for [C6MIM][NTF2] with its ether-substituted counterpart [(C1OC1)2MIM][NTF2] (1-methoxyethoxymethyl-3methylimidazolium) and presented experimental evidence for mesoscopic liquid morphologies in these imidazolium ILs.225 Later Shimizu et al. carried out a systematic study on [CnMIM][NTF2] ILs (n = 3, 6, 9) and their ether-substituted [(C1OC1)n/3MIM][NTF2] analogues.350 A clear bulk structural peak is observed in the low q region for [CnMIM][NTF2] ILs, but this does not occur when ether groups are present even though all ILs are isoelectronic (Figure 13A). SAXS data and atomistic simulations demonstrated that a suppression of nanostructures and the corresponding prepeaks in scattering structural functions for ether-substituted imidazolium cations occurs along the entire IL series, and this suppression of nanostructures is not due to any modification of ILs’ polar networks but rather due to different morphologies of the surrounding apolar domains.362 The microstructures in the apolar domains in [CnMIM][NTF2] ILs are described by bulky segregated structures, which are transitioned to thin enveloping ones in [(C1OC1)n/3MIM][NTF2] ILs. Such a microstructural transition is attributed to an inability of alkoxy chains for their effective side-by-side packing and the kinks along ether-substituted alkyl chains, which lead to scorpionlike intramolecular interactions among imidazolium rings and ether-substituted alkyl chains. As polyether chains are relatively polar, inter- and intramolecular driving forces are weak for spatial segregation of apolar moieties. This induces kinks along ether-substituted chains to form a clustered liquid morphology, which is similar to that in EtAN224,363,364 and in ILs with imidazolium cations bearing hydroxyl and carboxyl terminal groups.226,227,365,366
Figure 13.
(A) SWAXS spectra for [C6MIM][NTF2] and [C1OC2OC1MIM][NTF2] ILs. Reproduced with permission from ref (225). Copyright 2012 Royal Society of Chemistry. (B) Experimental (red) and computational (blue) structure factors for four silicon-substituted imidazolium [NTF2] ILs. Reproduced with permission from ref (357). Copyright 2016 AIP Publishing LLC. (C) Comparison of SWAXS data for [C5C5IM][NTF2] and [C9MIM][NTF2] ILs. Reproduced with permission from ref (358). Copyright 2011 American Chemical Society. (D) SWAX scattering intensities for double-headed [C12(MIM)2][NTF2]2 at varied temperatures. Reproduced with permission from ref (359). Copyright 2014 Royal Society of Chemistry.
The silicon-substituted imidazolium cations have advantages in some applications in comparison with carbon based imidazolium cations, and thus they are frequently used as solvent electrolytes and gas absorbents.352 [SiMIM] (1-methyl-3-trimethylsilylmethylimidazolium) cation is very similar to [CnMIM] cations, in which the Si–C bond is slightly longer and more polar than the C–C bond, and therefore the [SiMIM] cation has a larger cation size and the Si atom has more excess charge than the corresponding carbon atom in [CnMIM] cation. Both electronic and size effects make intermolecular correlations in [SiMIM] ILs weaker than in [CnMIM] ILs,357 leading to a low viscosity of [SiMIM][NTF2].367−369 OHD-OKE spectroscopy revealed a generic correlation of low intermolecular vibrational frequencies for [SiMIM][NTF2] with its decreased liquid viscosity, which is distinct from that of [CnMIM][NTF2] ILs.367,368 An alkylsiloxy-substituted [SiOSiMIM][NTF2] (1-methyl-3-pentamethyldisiloxymethylimidazolium) has a decreased glass transition temperature and a low shear viscosity.368 Despite its polar nature, pentamethyl-disiloxymethyl chains in [SiOSiMIM] cations are long enough to display a distinct FSDP in X-ray scattering structural function (Figure 13B).357 A dimethylphenylsilylmethyl substitution on imidazolium cations causes a substantial increase in liquid viscosities and glass transition temperatures.368 It should be noted that [PhSiMIM][NTF2] (1-dimethylphenylsilylmethyl-3-methylimidazolium) has higher shear viscosity and glass transition temperature than other silicon-substituted ILs, likely due to stronger intermolecular interactions. The diffusivities of small solutes in silicon-substituted ILs are faster than those in alkyl-substituted ILs with similar viscosities due to flexibilities of silicon-substituted alkyl chains and weak cation–solute interactions.370
Functionalizing imidazolium cations with aromatic moieties (benzyl groups) leads to significant and systematic changes in thermophysical properties of ILs. They have increased glass transition and melting temperatures arising from additional π–π interactions.371 Motivated by striking phase behaviors and distinct molecular dynamics of [CnMIM][NTF2]–benzene mixtures,89,372 Xue et al. performed femtosecond optical-heterodyne-detected Raman-induced Kerr effect (OHD-RIKE) experiments and atomistic simulations on [BzMIM][NTF2] (1-benzyl-3-methylimidazolium) and compared results with those for an equimolar [C1MIM][NTF2]–benzene mixture.354 Kerr spectra showed marginal differences in spectral densities for phenyl and imidazolium rings, indicating a very similar local environment for these two ring moieties. Furthermore, intermolecular part of Kerr spectrum for [BzMIM][NTF2] has lower intensity and higher frequency and is broader than that for the 1:1 [C1MIM][NTF2]–benzene mixture.373 These results are rationalized as being a consequence of local liquid structures, which have π–π stacking complexes involving a benzene molecule sandwiched between two imidazolium rings in 1:1 [C1MIM][NTF2]–benzene mixture. It should be noted that [BzMIM][NTF2] is a glass-forming liquid,355,371 whereas 1:1 [C1MIM][NTF2]–benzene mixture forms an inclusion crystal with a congruent melting temperature,374 which is mainly maintained by π–π stacking interactions between benzene and imidazolium ring planes and anion−π interactions of [NTF2] with benzene π electrons.
For imidazolium ILs, most cations are characterized by an asymmetric feature with one nitrogen atom attached to either a hydrogen atom or a methyl group, and the other nitrogen is covalently bonded to an alkyl chain with varied chain length. It is known that cation symmetry provides distinct structural organizations that allow fine-tuning IL’s physicochemical properties. There are two ways in tuning imidazolium cation symmetry. One is to bond two alkyl chains having the same number of carbon atoms to two nitrogen atoms in imidazolium rings.358,375−378 The other is to functionalize terminal methyl group in long alkyl chain with an imidazolium ring.379,380 The obtained symmetric imidazolium cations are described by double-tailed and double-headed structures, respectively.
For double-tailed imidazolium ILs, S/WAXS spectra showed that microstructural heterogeneity for an IL consisting of asymmetric imidazolium cations is larger than that for an IL consisting of double-tailed symmetric imidazolium cations.358,375,376 Local liquid structures of double-tailed symmetric imidazolium ILs are more tightly packed and exhibit more solidlike behavior than ILs with asymmetric cations, as indicated by a high q peak being narrower for [C5C5IM][NTF2] than that for [C9MIM][NTF2] (Figure 13C). Despite there are some controversies for the trend shift on enthalpies of vaporization, a nanostructuration effect in symmetric and asymmetric ILs is observed in their physicochemical properties.262,358,375 [Cn/2Cn/2IM][NTF2] ILs have higher volatilities and lower entropies of vaporization than [Cn–1MIM][NTF2] ILs having the same number of carbon atoms in their alkyl substituents.358,381 Moreover, [Cn/2Cn/2IM][NTF2] ILs exhibit a striking odd–even feature in their enthalpies and entropies of vaporization.381,382 With respect to intermolecular dynamics, OKE spectra for ILs having symmetric imidazolium cations are higher in frequency and broader than those for ILs having asymmetric imidazolium cations. In addition, an explicit difference was observed in the dependence of spectral parameters of the intermolecular part of the OKE spectrum of [CnCnIM][NTF2] ILs on cation alkyl chains from ethyl to propyl groups,358,375,376 indicating that alkyl chain segregation occurs at n = 3.
Besides distinct microstructural and dynamical heterogeneities in double-tailed imidazlium ILs, double-headed (divalent or dicationic) imidazolium ILs exhibit different microstructures,359,383−385 dynamics,385−387 and solvent properties.380,386,387 S/WAXS experiments and atomistic simulations showed enhanced spatial heterogeneities in [Cn(MIM)2][NTF2]2 ILs characterized by changes in scattering intensities and heterogeneity order parameters as the alkyl chain length increases from n = 3 to 6, and 12.359 The bulk liquid structures and ion diffusivities of double-headed imidazolium ILs are substantially depend on the length of alkyl chain linkage separating two imidazolium rings.383,385,388 Double-headed imidazolium ILs with short alkyl linkages exhibit almost identical mesostructural features as that for monovalent imidazolium ILs, regardless of anion types, whereas double-headed imidazolium ILs with long alkyl spacers between imidazolium rings display a very small prepeak and a low microstructural heterogeneity.359,385,389 Moreover, anions have a weak effect on bulk liquid structures, but they are well organized around imidazolium rings with similar spatial distributions as in monovalent ILs,389,390 leaving a low-density region around the alkyl linkage between two imidazolium rings in double-headed imidazolium ILs.385,390 Variations in temperatures have a slight influence on locally assembled polar and apolar nanostructures for double-headed imidazolium ILs in comparison with monovalent ones. The scattering peaks at 0.9 Å–1 and 1.4 Å–1 are shifted toward lower q values as temperature increases (Figure 13D), which are reflected in variations in heterogeneity order parameters determined from atomistic simulations.359,383−385,388 In addition, double-headed ILs are “subionic”, relatively “superfragile”, and moderately non-Newtonian fluids with positive Gibbs free energies of activation.391
In general, microstructural heterogeneity of ILs is accompanied by distinct dynamic heterogeneity of constituent ions.11 Dynamic heterogeneity implies a distribution of spatial regions with varied relaxation rates, which are determined by a competition between short-range vdW interactions arising from hydrophobic alkyl chains and long-range Coulombic interactions from polar groups. This picture provides an appealing insight into microscopic origin of nonexponentiality that arises from ensembles of ions having fast and slow dynamics.392 Indeed the diffusion mechanism of ions in IL matrixes is more complicated than originally expected,393 which may have additional hints for rationalizing striking dynamical quantities in self-assembled IL matrixes.
The experimental data from ultrafast infrared spectroscopy and OHD-OKE spectroscopy are used as evidence to describe heterogeneous dynamics of ions in inhomogeneous ILs, which occur on different time scales. Fayer and co-workers performed ultrafast spectroscopy to study rotations and local structural fluctuation dynamics of CO2 in [CnMIM][NTF2] ILs, which are promising solvents for CO2 capture.91,92,394,395 The rotational dynamics of CO2 occur on three time scales, corresponding to two different time scales of restricted wobbling-in-a-cone motions and a long-time complete diffusive rotational randomization. In addition, the complete rotational randomization of CO2 and the structural fluctuation of ILs in supported IL membranes ([C2MIM][NTF2] in poly(ether sulfone)) are slower than those in bulk liquids by approximately 2–3-fold in spite of large pore size (350 nm) in supported membranes. Experimental results indicated that variations of IL structures induced by polymer interface span more than hundred nanometers from the interfacial region, influencing dynamics of ion species and rotational dynamics of CO2.395,396 Furthermore, rotation of CO2 slows down with lengthening cation alkyl chains but less than that in liquid viscosities of imidazolium ILs (Figure 14A).394 These experimental results demonstrated that once there are substantial apolar regions in IL matrixes, making these regions larger does not change long-time-scale spectral diffusion dynamics experienced by CO2. Therefore, if a process is to be optimized for carbon capture, liquid dynamics that actually influence CO2 absorption should be systematically considered. OHD-OKE experiments showed that rotational relaxations of ILs have a complex time dependence that span from a few hundred femtoseconds to hundreds of nanoseconds with several power laws and a final exponential decay (Figure 14B).90,91,93,397 The power laws reflect dynamics on time scales during which a molecule is “caged” by surrounding molecules, and the final exponential decay is the diffusive complete rotational randomization of ion species.
Figure 14.
(A) Isotropic spectral diffusion of CO2’s asymmetric stretching in [CnMIM][NTF2] ILs. Reproduced with permission from ref (394). Copyright 2016 American Chemical Society. (B) Representative OHD-OKE data for [C4MIM][BF4]. Reproduced with permission from ref (91). Copyright 2014 Elsevier.
Related information on dynamical heterogeneities of ILs were obtained from NMR experiments,87,262,398 atomistic156−158 and CG66−68,133,184,336 simulations on single (van Hove correlation function, incoherent intermediate scattering function, non-Gaussian parameter, diffusional anisotropy, etc.) and collective (self-diffusivity, thermal and ion conductivity, shear viscosity, rotation, etc.) dynamical quantities. Despite some discrepancies between NMR measurements and simulation results, the dynamical heterogeneities of ILs and changes in single and collective dynamical quantities with temperatures are qualitatively captured. In general, imidazolium cations have enhanced heterogeneous dynamical quantities in comparison with their paired anion species, and a small fraction of highly mobile cations contribute to their distinct self-diffusivities in IL matrixes.66−68,185 Imidazolium cations structurally relax faster but rotationally relax slower than their coupled anion species at all temperatures. In addition, there is a distinct temperature dependence of rotational anisotropy for imidazolium cations but only a weak temperature dependence for anion species. Ion conductivities of ILs are significantly influenced not only by ion sizes but also by shapes of constituent ions. Variations in self-diffusion coefficients, viscosities, and ion conductivities with temperatures follow a Vogel–Fulcher–Tammann (VFT) equation.262 All in all, the dynamical behaviors of ILs are extremely complex and consist of many different relaxation modes spanning multiple time scales. The sizes and shapes of IL ions and delicate interplay of interactions among constituent ions contribute to distinct dynamical heterogeneities of ILs in heterogeneous ionic environments.156
In addition to temperature changes,399 effects of other external constraints, such as pressure,400−405 shear flow,406 and electric field,407,408 on microstructural and dynamical heterogeneities of bulk ILs have been investigated via numerous experimental and computational studies. Imidazolium ILs consisting of either small halides (Cl,409,410 Br409), [BF4],401,411 [PF6],404,412 or [NTF2] anions400,405 exhibit complicated phase behaviors due to conformational flexibilities of alkyl chains. The external constraints can significantly affect conformational equilibrium of imidazolium cations and anions, like [NTF2],400,405,409 which directly contribute to distinct IL structural transitions. For [CnMIM][NTF2] ILs, the presence of sandwiched structures of [NTF2] anions between neighboring imidazolium rings leads to a substantial hindrance for alkyl chains to curl and consequently to dissolve polar–apolar alternations at high pressures.400 This behavior is distinct from that of [CnMIM][BF4] ILs where locations of [BF4] anions favor curling of alkyl chains and consequent changes in liquid structural organization.411 In addition, [CnMIM][NTF2] ILs with n = 3–10 form a glassy state at high pressures. Intriguingly, the glass transition pressure slightly increases up to n = 5, reaches a plateau at n = 8, and increases again at n = 10.405 This is completely different from high-pressure glass formation of [CnMIM][BF4] ILs.403 These findings are intrinsically correlated with the fact that [CnMIM][NTF2] ILs are resistant to external pressures and prefer to retain their local liquid structures by essential conformational adjustments of [CnMIM] cations and [NTF2] anions at high pressures.82
2.4.3. Pyrrolidinium ILs
Pyrrolidinium ILs are widely used in electrochemical applications because of their wider electrochemical windows and higher electrochemical stabilities than imidazolium analogues.413,414 SAXS and atomistic simulations showed that effects of alkyl chain lengths and temperatures on nanoscale organization of [CnMPYRR][NTF2] ILs415−418 are similar to those reported for [CnMIM][NTF2] IL series even though imidazolium cations are planar and aromatic and pyrrolidinium cations are nonplanar and nonaromatic.280,346,347 However, the FSDPs for [CnMPYRR][NTF2] ILs with long cation alkyl chains display a remarkable shift to high q values with increasing temperatures,417 which is rationalized by a competition between strong charge ordering associations of ions and vdW interactions between alkyl chains in [CnMPYRR] cations. This observation highlights a charge ordering pattern of polar moieties resulting from electrostatic ion ordering at short distances and a complex association among cation alkyl chain at long distances. The self-aggregation of alkyl chains and the dependence of polar group ordering on alkyl chain length provide a clear view of heterogeneous microstructures in [CnMPYRR][NTF2] ILs. Rotation dynamics of [CnMPYRR] cations and [NTF2] anions are anisotropic, and the degree of anisotropy increases with decreasing temperatures. Electrostatic interactions between constituent ions are mainly responsible for decreased ion conductivities and increased viscosities of [CnMPYRR][NTF2] ILs with larger effects at lower temperatures.419
In addition, constituent species (head and tail groups in cations and anions) of [CnMPYRR][NTF2] ILs are structurally complex and can be conformationally modified by applying pressures.419−422 The Margulis and Castner Jr. groups did comprehensive studies on microstructures of ILs consisting of a common [NTF2] anion coupled with varied pyrrolidinium cations (1-(cyclohexylmethyl)-1-methylpyrrolidinium, 1-(2-ethylhexyl)-1-methylpyrrolidinium, and 1-alkyl-1-methylpyrrolidinium) via SAXS experiments and atomistic simulations.415,418,423 Both polarity and charge orderings decrease with increasing pressures as correlations of polar and apolar moieties are susceptible to applied pressures.422 Alkyl tails in cations possess an increased number of gauche defects at higher pressures, leading to their increased bending and curling in heterogeneous ILs.424 In addition, [CnMPYRR][NTF2] ILs exhibit distinct changes in polarity and charge alternations upon pressuring these ILs with cations having branched and cyclic tails.415,423
Besides variations of pyrrolidinium cation alkyl chains, the inclusion of an additional methylene unit in pyrrolidinium ring leads to piperidinium and pyridinium ILs characterized by distinct microstructures and complex phase behaviors, as revealed from S/WAXS experiments.126 Lengthening cation alkyl chains in [CnMPIP][NTF2] ILs (n = 1–8) leads to a decrease in thermal stabilities and ion conductivities of ILs.425 Although prominent peaks are observed in low q region in S/WAXS plots for [CnMPIP][NTF2] ILs with alkyl chains ranging from C2 to C7, their peak positions are distinctly higher than those for imidazolium ILs, indicating a smaller characteristic length of microstructural heterogeneity in nonaromatic ILs than that in aromatic ILs.
Variations of cation structures from [C4MPYRR] to [C4HPYRI] and [C4MIM] lead to significant changes in low-frequency Kerr spectra when these cations are coupled with [NTF2] anions.426 For ILs containing aromatic cations, such as [C4MIM][NTF2] and [C4HPYRI][NTF2], spectral intensities in the low-frequency region below 20 cm–1 increase and representative spectral peaks in the high-frequency region at around 80 cm–1 shift to lower frequencies with increasing temperatures. These shifts are attributed to distinct activation of translational and vibrational motions of ion species at high thermal energies and fast librational motions of aromatic rings due to large free volumes and weak intermolecular interactions at high temperatures. Small differences between imidazolium and pyridinium ILs are correlated with a delicate interplay of intermolecular interactions (vdW, HB, π–π stacking, and Coulombic interactions) among constituent ions and, in particular, electronic structures of cations.427 In contrast, ILs containing nonaromatic cations only exhibit an increase in spectral intensities at the low-frequency region, while spectra at the high-frequency region show little change with increasing temperatures, indicating that the presence of aromatic rings influences temperature-dependent spectral features, especially at the high-frequency region.
The presence of static microstructural heterogeneity of pyrrolidinium ILs leads to distinct solvation dynamics of (dye) solute molecules, either neutral or charged, in IL matrixes.428,429 Solvatochromic measurements indicated that the extent of energetic heterogeneity of solutes in IL matrixes is significantly correlated not only with microstructural but also with dynamical heterogeneities arising from viscous glassy nature of ionic environments.69,428,429 Small neutral and charged dye molecules explore locally “soft” (mostly apolar with low electrostriction) domains and locally “stiff” (mostly charged with high electrostriction) domains, respectively. These domains of low and high frictions are associated with jump and cage regimes. The enhanced diffusivities of neutral tracers in low friction domains, associated with apolar groups in constituent ions, have a substantial bearing on large positive deviations from the Stokes–Einstein hydrodynamics of ILs compared to conventional molecular solvents.430 In contrast, the diminished mobilities of charged tracers involve lengthy caging dynamics separated by jump events, which are often caused by a recovery or loss of counterions. Charged solutes are strongly coupled with polar moieties of solvent ions and are not an innocent spectator of stiff and soft solvent regions but instead participate in creating electrostriction they experience.428 As cations in less viscous ILs are often asymmetric, it is expected that a small positively charged probe will have stronger interactions with anions than with cations.431,432 Therefore, small ion tracers will become an intrinsic component of polar networks and their diffusions are strongly correlated with that of ion groups comprising polar networks in IL matrixes.
2.4.4. Tetraalkylammonium and Tetraalkylphosphonium ILs
In protic alkylammonium ILs consisting of either primary, secondary, or tertiary ammonium cations, anions prefer to coordinate with N–H groups via preferential HB interactions, which are absent in aprotic tetraalkylammonium ILs.433,434 SAXS435,436 and atomistic simulations437 of [N1,n,n,n][NTF2] ILs (n = 4, 6, and 8) showed that low q peaks in scattering structural functions depend on alkyl chain length in [N1,n,n,n] cations, while intermediate and high q peaks do not. These conjugated studies delineate the nature of marked nanoscale segregation of polar and apolar domains in ILs. Polar domains consist of 3D networks of ion channels, and apolar domains are arranged as a continuous microphase permeating polar networks. These results agree well with previous studies of [CnMIM][PF6]438 and [CnMIM][NTF2] ILs,439 and provide another confirmation that not only ILs are nanostructured solvent media consisting of polar and apolar domains but also that their liquid structures can be further decomposed according to different categories of liquid morphologies.440 In addition, [N1,n,n,n][NTF2] ILs are not very viscous, but they exhibit a (low-frequency) macroscopic solidlike response to a low shear strain even at 100 °C above the glass transition temperature,441 indicating collective elastic intermolecular interactions and long relaxation time scales. Therefore, the role of shear elasticity has to be considered for a better understanding of their odd properties, such as a non-Arrhenius temperature dependence of viscosities and ion conductivities as well as deviations of these dynamical properties from the Nernst–Einstein equation and their variations in the Walden plot, which are intrinsically ascribed to changes in high-frequency shear modulus with lengthening alkyl chains in tetraalkylammonium cations.442
Functionalization of the [N1,1,1,2] cation with a terminal hydroxyl group leads a good capability of [CH] cation in coordinating amino acid anions leading to a new type of ILs. These ILs are wholly composed of renewable biomaterials with inherent biocompatibility and low toxicity and hence can be used in enzymatic extraction and biocatalysis.443 XRD and IR spectra as well as AIMD and atomistic MD simulations444,445 revealed that amine groups in anions do not show strong interactions with [CH] cations, that is, amino acid anions are “more acid than amino”, contributing to their similarities with alkylcarboxylate anions.325 The liquid structures of [CH] amino acid ILs are substantially composed of two main docking interactions acting between different charge moieties: a strong HB feature connecting carboxyl terminals in amino acid anions to hydroxyl groups in [CH] cations, which determines short-range structural behavior and a substantial ion coupling as basic building blocks of IL matrixes.446 A remarkable change was observed in translational and rotational responses of [CH] amino acid ILs to external electric fields.447 Effective dipolar alignments of constituent ions with electric fields exhibit striking rotational mobilities of ions in the direction of electric field, which decrease with electric field frequency and increase with electric field strength.
SAXS experiments and atomistic simulations of ILs consisting of [N2,2,2,8], [N2,2,2,(2O2O2)] (2-ethoxyethoxy-ethyltriethylammonium), and their phosphonium analogues showed that ILs with cations having four alkyl substituents exhibit FSDPs in their scattering structure functions, which are less intense or totally absent in diether-substituted analogues.420,448,449 In ILs with cations having four alkyl substituents, anions are excluded from locations in which they are found in other types of ILs, eliminating long-range alternating polar–apolar patterns.450 In addition, the inclusion of ether functionalities in tetraalkylammonium and tetraalkylphosphonium cations disturbs both long-ranged charge ordering and intermediate-ranged ordering in a rather subtle manner.451 Ether groups have stronger interionic interactions with cation polar groups, resulting in larger surface tensions than ILs with hydrophobic alkyl groups and exhibiting a more flexible and less segregated polar domains in apolar networks in ILs.452−454 These two structural features contribute to distinct transport properties of ether-substituted cations in comparison with their alkyl-substituted counterparts.
The specific effect of changing cation core from nitrogen to phosphorus atoms on cation–anion interactions, thermodynamics, microstructures, dynamical, and transport properties was investigated combining XPS,114 femtosecond OHD-RIKES,452 quasi-elastic light scattering spectroscopy, and broadband dielectric spectroscopy83 as well as atomistic simulations.453,455 Tetraalkylphosphonium ILs exhibit lower shear viscosities and glass transition temperatures and higher ion conductivities than the corresponding tetraalkylammonium ILs.83,452,454,455 These properties are attributed to flexible molecular structures and weaker liquid structuring of tetraalkylphosphonium cations in comparison with tetraalkylammonium homologues.453 DFT calculations showed that phosphorus atoms in tetraalkylphosphonium cations carry more positive charge than nitrogen atoms in tetraalkylammonium cations, and a noticeable charge delocalization occurs in tetraalkylammonium cations in comparison with tetraalkylphosphonium congener.455 These features contribute to a less ordered nanoscale aggregation of hydrophobic alkyl moieties and, consequently, inhibit cooperative relaxation dynamics in apolar networks in tetraalkylphosphonium ILs. Relative to α structural relaxation, these ILs present pronounced secondary structural relaxations that are strongly dependent on atomic identity of charged cation centers. [P2,2,2,8][NTF2] exhibits faster secondary relaxations than [N2,2,2,8][NTF2] at all measured temperatures, of which the former is characterized by an Arrhenius temperature dependence characteristic of local β relaxation, whereas the latter exhibits a mild non-Arrhenius thermal activation, which is indicative of molecular cooperativity.
Compared with imidazolium and pyrrolidinium ILs, the dependence of nanoscopic liquid morphologies of tetraalkylphosphonium ILs on alkyl chain length of cations is complicated as cations are composed of four alkyl chains and each one can be tuned by varying the chain length.238,239,294,456 Tetraalkylphosphonium cations with short alkyl chains exhibit both liquid and solid states,457,458 such as [P1,2,2,4][PF6], in which the [P1,2,2,4] cation exhibits a distinct transport mechanism involving a crankshaft motion around the isobutyl group.457,458 The microstructural liquid morphology of [P2,2,2,4]Cl is described by bicontinuous interpenetration of the polar and apolar networks (Figure 15A). Lengthening the cation alkyl chains results in a polar network composed of central polar moieties in cations and anions being partially fractured or totally partitioned within the apolar framework.238 The variations of liquid morphologies and heterogeneous microstructures in the [Pn,n,n,m]Cl IL matrixes are qualitatively manifested in decent adjacency correlation peaks and polarity alternation peaks observed at high and low q values in scattering structural functions, and their peak positions gradually shift to lower q values with lengthening alkyl chains in cations (Figure 15A). The peaks for charge alternations located in the intermediate q range present a complicated dependence on cation alkyl chain length due to a complete cancellation of positive contributions from ions having the same charge and negative contributions from ions having an opposite charge. These liquid structure changes are obviously manifested in dynamical properties. The terminal carbon atoms of alkyl chains exhibit an overall higher translational diffusivity than central phosphorus atoms in cations, which exhibit comparable translational diffusivities as Cl anions due to their decisive Coulombic associations in polar domains in IL matrixes. Lengthening cation alkyl chains leads to a concomitant shift of van Hove correlation functions to large radial distances (Figure 15B) and of non-Gaussian parameters to long time scales (Figure 15C), respectively, corresponding to increased translational diffusion heterogeneities of constituent ions in constrained ionic environments.
Figure 15.
(A) Computational X-ray scattering structural functions and representative liquid morphologies of tetraalkylphosphonium Cl ILs. Polar domains (red) are composed of P(CH2)4 moieties in cations and anions, and the apolar entity (cyan) consists of the remaining alkyl groups in cations. (B) Self-part of van Hove correlation functions Gs(r,t) for ion species in [P6,6,6,14]Cl at seven different times. (C) Non-Gaussian parameters α2(t) for Cl anions in six tetraalkylphosphonium Cl IL matrixes. Reproduced with permission from ref (238). Copyright 2017 AIP Publishing LLC.
The [P6,6,6,14] ILs have been extensively investigated in experimental and computational studies due to a versatility of [P6,6,6,14] cations in coordinating various anions, from small spherical ones (halides, [BF4], [PF6], etc.)459−462 to bulky asymmetrical ones with diffuse charges,462,463 like [NTF2].464,465 The FSDPs in scattering structural functions for [P6,6,6,14] ILs are independent of anion types and increase in intensity with decreasing temperatures.462 In addition, charge and polarity orderings in [P6,6,6,14] ILs display appreciable sensitivity to external pressures.460 Polarity ordering diminishes as pressure increases from ambient to a transition pressure. Upon further pressuring ILs, an additional peak corresponding to crystalline order emerges on a relatively short length scale. This observation is attributed to enhanced polar–polar and apolar–apolar correlations and decreased polar–apolar correlations. With increasing pressures, the probability of finding more gauche kinks along C6 chains is systematically increased, whereas a mixed effect is observed for C14 chains in [P6,6,6,14] cations.
ILs consisting of [P6,6,6,14] cations coupled with chelated orthoborate anions have striking tribological features.58,294,466 Atomistic simulations indicated that there are mainly four high probability regions for orthoborate anions to coordinate [P6,6,6,14] cations. Boron atoms in [BOB] anions exhibit dispersed tetrahedral distributions around [P6,6,6,14] cations. An increase in anion sizes from [BOB] to bis(malonato)borate ([BMLB]), bis(mandelato)borate ([BMB]) and bis(salicylato)borate ([BScB]) leads to an expansion of spatial distributions of boron atoms in these orthoborate anions around [P6,6,6,14] cations. Spatial distributions of oxygen atoms in orthoborate anions are characterized by a trefoil-like structure and follow a similar tendency as those of boron atoms as anion size increases. These distinct ion structures lead to significant changes in their liquid viscosities and rheological properties.294,467,468
3. Ionic Liquid–Molecular Solvent Mixtures
3.1. Solvation Thermodynamics and Structures of Water in ILs
For IL related research in laboratory and industrial applications, an inevitable issue is the presence of residual impurities in ILs. As an omnipresent compound, water is one of the most common contaminants found in IL samples. On one side, this is caused by ILs’ intrinsic hygroscopic feature, even those described by hydrophobic characteristics can absorb a considerable amount of water from atmosphere, and on the other side many processes including chemical synthesis and liquid extractions and purifications involve water. It has been well recorded that trace amounts of water can dramatically alter microstructures in ILs, resulting in significant changes in physicochemical and rheological properties of ILs as well as reactivities and selectivities of reactions taking place in IL matrixes.469
Water–ion interaction strength qualitatively determines the trend of excess chemical potentials and therefore miscibility of water in ILs.470 Anthony et al. found that excess chemical potentials of water in [C4MIM][PF6], [C8MIM][BF4], and [C8MIM][PF6] ILs lie between −15 kJ/mol and −20 kJ/mol and increase slowly with temperatures and cation alkyl chains.471 Lynden-Bell et al. calculated excess chemical potentials of a series of solutes (water, methanol, dimethyl ether, acetone, and propane) dissolved in [C1MIM]Cl IL matrix using a thermodynamic integration method.472 Water and methanol have large excess chemical potentials (−29 kJ/mol and −14 kJ/mol, respectively), which are attributed to specific HB interactions in stabilizing water and methanol in [C1MIM]Cl. Apolar molecules like dimethyl ether and acetone have excess chemical potentials of ∼7 kJ/mol while the value for propane is of approximately 26 kJ/mol.
Wang et al. showed that free energies of solvating one water molecule from gas phase into bulk [P6,6,6,14] orthoborate IL matrixes are positive and exhibit linear dependence on temperatures.473 At a given temperature, these solvation free energies are intrinsically related to orthoborate anion structures with an order of [BMLB] > [BScB] > [BMB] > [BOB], which is qualitatively consistent with experimentally determined residual water contents in the corresponding ILs. In addition, less solvation free energies are required to dissolve the second one within the first solvation shell of the first solvated water molecule, attributing to preferential pairwise intermolecular interactions between two water molecules and favorable multibody interactions of solvated water molecules with neighboring anion species at short and intermediate distances.
Seddon and co-workers provided guidelines on mutual miscibilities of ILs with water.474 In general, ILs containing highly fluorinated and charge-delocalized anions, such as [NTF2], tricyanomethanide and [PF6], are hydrophobic and are immiscible with water.475−477 ILs having hydrophilic ions, such as halides, ethanoate, [NO3], trifluoroacetate, and carboxylate, are totally miscible with water, and those consisting of [BF4], [CF3SO3], and phosphate anions are either miscible or immiscible with water depending on cation substituents. In addition, cations also affect miscibilities of ILs in water with hydrophobicity of cations following an order of [CnMIM] < [CnMPYRI] < [CnMPYRR] < [CnMPIP] < tetraalkylammonium < tetraalkylphosphonium. Furthermore, hydrophobicity of cations increases with lengthening cation alkyl chains, indicating a decreased polarity of cations.478
It is understood that solvation structures of water in ILs depend primarily on strength of electrostatic ion–water interactions, which, in turn, are essentially determined by ion sizes and the amount of charge on the ion surface.470 Interactions of water with small ions are favorable while those with large ions are much weak due to their delocalized charges.470,475,477,479 In addition, HB interactions contribute to significant coordination of water with constituent ions due to a dual feature of water having HB interactions with both cations and anions, which further complicates solvation structures of multiple water molecules in ILs. The water–water potential of mean force profiles for [P6,6,6,14] orthoborate ILs indicate substantial interactions of solvated water molecules with neighboring ions depending on local ionic environments (Figure 16).473 A characteristic deep potential minimum registered at ∼0.28 nm corresponds to the formation of a water dimer complex, which has strong coordination with neighboring anions and forms stable ring structures via HB interactions. As the separation distance between solvated water molecules increases, there is not enough space between solvated water molecules for an ion to squeeze in, leading to the formation of unstable intermediates. These intermediates contribute to gradually enhanced water–water interactions until the formation of metastable ion-separated water association structures at intermediate separation distance. The association patterns for solvated water molecules at large separations are described by remarkable characteristics depending on specificities of anions and mild interactions of solvated water with surrounding ions, leading to remarkable spatial association patterns of solvated water molecules around orthoborate anions (Figure 16).473
Figure 16.
Left, potential of mean force curves of two solvated water molecules in IL matrixes, and representative configurations of solvated water molecules in coordinating neighboring anion species in [P6,6,6,14] orthoborate ILs. Right, spatial distributions of hydrogen (green contour) and oxygen (red contour) atoms in solvated water molecules around neighboring orthoborate anions. Reproduced with permission from ref (473). Copyright 2016 AIP Publishing LLC.
3.2. IL–Water Mixtures
Extensive experimental characterizations (NMR,161,254,321,480−483 infrared and Raman spectroscopies,151,230,482−488 X-ray and neutron scattering spectroscopies,109,111,159,487,489etc.), theoretical studies,31,477 DFT calculations,172,483,490 and atomistic simulations72,160,162,167−169,171−174,178,180,491−493 have been performed to characterize phase behavior changes when mixing ILs with water. Variations in liquid structures, translational and rotational dynamics of ion species, and evolution of microscopic liquid morphologies of IL–water mixtures with a gradual increase of water concentration in ILs can be roughly classified into several compositional regimes. Representative changes of microstructural and dynamical quantities in [P6,6,6,14][BOB]–water mixtures are illustrated in Figure 17.164,293,491
Figure 17.
Representative snapshots of [P6,6,6,14][BOB]–water mixtures containing varied water mole fractions of Xwater = (A) 0.33, (B) 0.5, (C) 0.8, and (D) 0.95. Cations, anions, and water molecules are represented by green, red, and blue spheres, respectively. Reproduced with permission from ref (491). Copyright 2015 American Chemical Society. (E) Translational diffusion coefficients of all ion species in mixtures having different water concentrations at 333 K. Reproduced with permission from ref (293). Copyright 2016 American Chemical Society.
In IL–water mixtures with a small water mole fraction (generally Xwater ≤ 0.2), cation groups and anion species are strongly coupled together via decisive Coulombic interactions and preferential HB interactions and possibly π–π stacking interactions in ILs containing heteroaromatic rings. Microscopic liquid organization is characterized by isolated polar domains dispersed in a connected apolar network or by interpenetrating polar and apolar networks. Most water molecules are scattered in IL matrixes and are preferentially coordinated with ion species nearby, leading to the local ionic structures described by solute-shared ion pairs486 (Figure 17A). The restricted spatial configuration of water molecules in IL–water mixtures results in their constrained rotational relaxation within a cone of angles due to their dual feature in coordinating both cations and anions via HB interactions and a significant increase in translational diffusions of ion species in mixtures as water concentration increases (Figure 17E).110,159,165,480,494
For IL–water mixtures having intermediate water concentrations (generally 0.2 < Xwater ≤ 0.9), small clusters, such as water dimers, trimers, and water channels, appear and dominate distribution of water aggregates in IL–water mixtures (Figure 17B,C). These water clusters serve as bridges connecting anions between isolated polar domains and mediating their distributions in mixtures.485,495 The local ion structures in these IL–water mixtures are described by solute-mediated ion pairs, leading to increased spatial associations between ions and, thus, have a strong effect on translational and rotational dynamics of ion species in mixtures (Figure 17E).111,151,166,488,496,497
The further addition of water into IL–water mixtures results in a dynamic percolation of water molecules throughout the entire simulation box (Figure 17D). The local ionic environment is described by interpenetrating polar and apolar networks or loose micelle-like aggregates in a highly branched water network.168,494 The formation of water networks promotes a rapid increase in ion dynamics in these water-concentrated mixtures. These IL–water mixtures are characterized by substantial spatiotemporal heterogeneities attributing to a competition between strong electrostatic and preferential HB interactions between polar moieties in ion species, and persistent dispersion associations between hydrophobic moieties in constituent ions.482,484−486,490,496
In addition to variations in microstructures and mesoscopic liquid morphologies determined from atomistic simulations, a combination of 2D IR spectroscopy,72−75,488 OHD-OKE spectroscopy,74,75,141,498 time-resolved ultrafast infrared spectroscopy,93,102 and PSPP measurements74,75,90 provides comprehensive characterizations on multi-time scale dynamics of ILs ranging from ultrafast vibrational motions of well-defined functional groups to rotational relaxation of constituent ions in IL–water mixtures at ground and excited states.91,102,499 Take [CnMIM][BF4]–water mixtures for example, rotational dynamics of water in [CnMIM][BF4] ILs (n = 4, 6, 8, and 10) characterized using 2D IR spectroscopy and PSPP experiments showed that water’s hydroxyl absorption spectra are independent of cation alkyl chain length, indicating that dispersed water molecules feel fairly similar local ionic environments in all studied mixtures.74 However, rotational relaxation (PSPP experiments) and spectral diffusion (structural dynamics using 2D IR experiments) measurements of hydroxyl groups display alkyl chain length dependence. These dynamics slow when cation alkyl chains become long enough to form apolar segregations in IL-water mixtures. At low water concentrations, all hydroxyls interact mainly with anions because of their dispersed distributions in heterogeneous IL matrixes. As water concentration increases, a water-associated water population forms, absorbing in a new spectral region red-shifted from absorption of isolated or anion-associated water populations. At sufficiently high water concentrations, water clusters grow and water molecules experience dynamics approach those of bulk water, and IL structures are fluidized by addition of water. Both rotations of hydroxyls and their spectral diffusions, tracking fluctuations in local structures and interactions, speed up noticeably with increasing water content in certain concentration ranges. The water concentration at which dilute water dynamics change to fluidized dynamics depends on cation alkyl chain length, which determines the extent and ordering of apolar domains. Increasing water concentrations and lengthening cation alkyl chains tend to modify IL structural orderings but with opposite and competing effects on dissolved water dynamics.
It should be noted that water exhibits different solubilities in [CnMIM][BF4] ILs depending on alkyl chain length in cations. Both [C2MIM][BF4] and [C4MIM][BF4] ILs are infinitely miscible with water, whereas in [C6MIM][BF4] IL water saturates at Xwater = 0.75.94 The Debye–Stokes–Einstein (DSE) plot for [C2MIM][BF4]–water mixtures has two hydrodynamic regimes of Xwater < 0.67 and Xwater > 0.80 with a crossover region in between (Figure 18A).94 Atomistic simulations showed that there is no connected apolar domains in [C2MIM] ILs. The local ionic environments in [C2MIM][BF4] can restructure without paying a significant penalty for disrupting local ion structures in polar domains to accommodate more water molecules in the IL matrix. The DSE curve for [C6MIM][BF4]–water mixtures is linear, indicating that [C6MIM][BF4]–water mixtures do not substantially change local ionic environments as water concentration increases (Figure 18C).90,94 [C6MIM][BF4]–water mixtures are hydrodynamic, likely due to favorable dispersion associations among cation alkyl chains, making it unfavorable for ion species to change local ionic structures to accommodate additional water molecules. Instead of disrupting the apolar framework in the IL matrix, [C6MIM][BF4] saturates with water. [C4MIM][BF4] is an intermediate item, in which apolar domains are interconnected and present to a limited extent. Similarly, two linear regimes, a low water content regime with a moderate slope and a high water content regime with a steep slope, are observed in the DSE curve for [C4MIM][BF4]–water mixtures (Figure 18B).90,94 However, the transition point for [C4MIM][BF4]–water mixtures is between Xwater = 0.25 and Xwater = 0.50, which is lower than that for [C2MIM][BF4]–water mixtures. [C4MIM] cations are able to restructure local ionic environments to accommodate more water molecules, leading to two linear regimes in the DSE curve because apolar domains in [C4MIM][BF4]–water mixtures are less extensive than those in [C6MIM][BF4]–water mixtures.
Figure 18.
Debye–Stokes–Einstein plots for IL–water mixtures consisting of (A) [C2MIM][BF4], (B) [C4MIM][BF4], and (C) [C6MIM][BF4] ILs with water at varied ratios of ion pairs:water. Reproduced with permission from ref (94). Copyright 2018 AIP Publishing LLC.
It is known that protonation of N(3) position in imidazolium rings changes imidazolium cations from aprotic to protic. The hydrogen atoms in N(3)–H groups serve as strong HB donor sites and have preferential HB interactions with water molecules and anions.75,94,163,254 OHD-OKE spectra showed that [C2MIM][NTF2]–water mixtures exhibit hydrodynamic behavior at all water concentrations up to saturation, whereas [C2HIM][NTF2]–water (1-ethyllimidazolium) mixtures display distinct phase behaviors at specific water concentrations.75,94 Atomistic simulations demonstrated a substantial jump in the formation of N(3)–H···(H2O)n clusters in [C2HIM][NTF2]–water mixtures upon increasing water concentrations. Cluster formation contributes to significant deviations of rotational relaxations of [C2HIM][NTF2]–water mixtures from hydrodynamic behavior due to dominant HB interactions of water with N(3)–H groups over other HB donor sites in [C2HIM] and [C2MIM] cations. This structural variation is confirmed by FT-IR spectra showing an asymmetric O–D stretching that is indicative of water clusters in [C2HIM][NTF2]–water mixtures.
In addition to aprotic and protic imidazolium ILs, water exhibits distinct microstructures and dynamics in protic alkylammonium ILs.154,230,500−503 AIMD simulations showed that HBs formed between MAN and water are characterized by distinct HB structures and dynamics, resulting in a significant incorporation of water into HB networks of MAN.154,500 Water slows down rotational dynamics of cations and anions in MAN and, to some extent, changes polarization of IL ions. However, in EAN–water mixtures, water changes EAN nanostructures mainly due to its HB interactions with EA cations and [NO3] anions.503 In addition to bicontinuous polar and apolar domains in the EAN IL matrix, separate EAN aggregations and water domains are also evident. Water neither dilutes molecularly dispersed IL ions nor does it swell polar networks of existing IL nanostructures. Instead, water is principally associated with ion polar groups, and its net effect is to increase effective cation headgroup size and to create an interfacial curvature around apolar domains. This changes EAN nanostructures in EAN–water mixtures from a near-zero mean curvature to a branched (locally cylindrical) network.224,500,503
S/WAXS and atomistic simulations were combined to explore liquid structures and dynamics of IL–water mixtures consisting of primary, secondary, and tertiary alkylammonium cations in conjunction with Cl, formate, and alkanoates anions.199,502,504 For IL–water mixtures consisting of primary alkylammonium cations coupled with small anions, like EAC–water and PAC–water mixtures, there exists a complex liquid structure where cation groups and anion species do not possess a completely closed hydration shell of their own. In particular, a dominant percentage of solute-shared ion pair structures are formed in mixtures with low water concentrations, in which dispersed water molecules act as linkers between cations and anions via HB interactions. This percentage decreases with increasing water concentrations in mixtures, but in conditions of very high dilution, solvent-shared ion pair structures continue to survive.199 Lengthening cation alkyl chains leads to distinct thermal behaviors in hexyl-, octyl-, and decylammonium Cl IL–water mixtures.501 For ILs having large anions, liquid viscosities of IL–water mixtures exhibit characteristic behaviors of concentrated salt solutions, with water diluting protic ILs.502,504 Ion conductivities of IL–water mixtures display complicated changes with a gradual increase of water concentrations due to a subtle interplay of available “free” ions in mixtures and changes in liquid viscosities of mixtures.
3.3. IL–Alcohol Mixtures
ILs are polar liquids, and polarities of pure ILs are higher than acetone, dimethyl sulfoxide, and dichloromethane but lower than water and close to short chain alcohols.505 Mixtures of ILs with alcohols showed reduced viscosities and enhanced functionalities in chemical engineering applications.9,18 In these processes, it is especially significant to understand how microstructures and molecular arrangements of ILs change upon mixing and in which way miscibility and physicochemical properties of IL–alcohol mixtures are related to their characteristics in applications.
3.3.1. Alkylammonium IL–Alcohol Mixtures
Both EAN and methanol are amphiphilic and their bulk liquid structures are characterized by extended HB networks. Hence, they are expected to exhibit ideal mixing characters.506,507 However, though macroscopically homogeneous, EAN–methanol mixtures are heterogeneous fluids at the mesoscopic level.194,508,509 Fluorescence anisotropy measurements, XRD spectra, and atomistic simulations revealed that even in methanol enriched mixtures, most EAN ion species are clustered and preserve their spongelike liquid organization, and methanol molecules cannot fully dissociate EAN clusters.510 Such heterogeneous liquid structures contribute to distinct dynamic heterogeneities of EAN–methanol mixtures as revealed from fluorescence anisotropy measurements.510 Additional analysis of time-resolved anisotropy data together with DSE hydrodynamic theory predicts that reorientation times for EAN and methanol are comparable and close to the stick hydrodynamic line in mixtures with low EAN concentrations. EAN–methanol mixtures with intermediate EAN concentrations exhibit both Newtonian and Arrhenius behaviors, and their liquid viscosities are independent of temperatures.510 Furthermore, SAXS spectra showed that strong cohesive forces in EAN and PAN ILs can induce intermediate chain length alcohols to self-organize into microemulsion-like and micelle-like structures in ILs, which is in contrast to their immiscibilities with EtA ILs and water.191,194,511 This indicates that EA and PA cations act as cosurfactants and enable alcohols to aggregate, whereas EtA cations cannot serve this function because their terminating hydroxyl groups render EtA cations nonamphiphilic.
Shrivastav et al. performed additional atomistic simulations to explore the effect of alcohol alkyl chain length on liquid organization and HB interactions in equimolar mixtures of BAN with primary alcohols.512 All BAN–alcohol mixtures exhibit two decent peaks in the low q region in structural functions, indicating the presence of bicontinuous heterogeneous ordering structures, which depend considerably on alcohol alkyl chain length.191 Alcohols prefer to reside with their hydrophobic tails in apolar domains and their polar heads in polar domains in the IL matrixes.194,511,513 This is distinct from distributions of polar solutes, like water, which reside in polar regions,230,503,504 while apolar solutes prefer apolar domains.191,513 Lengthening alcohol alkyl chains produces significant variations in microstructural ordering of polar and apolar domains in BAN–alcohol mixtures and exhibits enhanced HB interactions of hydroxyls in alcohols with anions and other alcohols.326
Both miscibility and liquid organization of binary alkylammonium IL–alcohol mixtures are structured on two distinct length scales: one is associated with self-assembled alcohol aggregates and the other is associated with the underlying IL nanostructures, both of which are primarily sensitive to the ratio of alcohol alkyl chain length to alkylammonium cation alkyl chain length.191,509 Alcohols with alkyl chains shorter than twice that of alkylammonium cations disrupt intrinsic amphiphilic IL nanostructures and reduce periodic orders but do not generate a qualitatively different solution structures. Long chain alcohols cannot be accommodated within intrinsic IL nanostructures to any significant extent and instead are expelled into large amphiphilic aggregates. The self-assembled liquid structures depend on detailed chemical compositions of IL–alcohol mixtures. Globular micelles are formed at low alcohol concentrations, but micelles tend to percolate into bicontinuous structures at high alcohol concentrations, which are alcohol-rich but contain some cation groups acting as cosurfactants. EtA cations cannot act as cosurfactants to induce self-assembly of alcohols and therefore lead to immiscibility once alcohol alkyl chains exceed twice the EtA cation alkyl chains.191,514 It should be noted that these liquid structural transitions are affected by anion structures and anion HB capacities.326 Ethylammonium formate (EAF) has a less pronounced bulk nanostructure than EAN and PAN ILs,190 and its miscibility with octanol results from a weak solvophobic driving force. Therefore, fewer and smaller aggregates are formed in EAF–octanol mixtures.
These observations provide valuable physical insights into microstructural features and general stabilization mechanisms of weakly structured mixtures and reveal new pathways for identifying molecules and ILs that are likely to generate weakly structured fluids for facilitating aggregations of nontraditional amphiphiles and for supporting self-assembly of nontraditional surfactants. Understanding these systems is an important step toward applications of functional ILs as solvent electrolytes for electrochemical devices and their effective utilization to assemble new forms of soft matter.191,194,515
3.3.2. Imidazolium and Pyrrolidinium IL–Alcohol Mixtures
The mutual solubility of imidazolium ILs and alcohols are intrinsically related to significant interactions of ILs with alcohols.516,517 These interactions are important characters affecting physicochemical behaviors of IL–alcohol mixtures.518,519 A general feature is that a gradual lengthening of alcohol alkyl chains leads to a systematic decrease in solubility of ILs in alcohols, which is deduced from solubility of [C2MIM][NTF2] in propanol, butanol, and pentanol,520 and of [C8MIM][BF4] in butanol and pentanol,521 as well as solubility of other ILs containing different cation–anion moieties in various alcohols.516,522,523 Lengthening alcohol alkyl chains leads to a decrease in excess molar enthalpies and increases in molar excess volumes and in upper critical solution temperature (UCST) of IL–alcohol mixtures. These properties indicate that packing structures and interactions between unlike molecules become less important in mixtures containing long chain alcohols.519,524 By contrast, an increase in alkyl chain length in imidazolium cations leads to enhanced hydrophobicity of ILs and therefore results in a decrease in UCST of IL–alcohol mixtures.518,525,526 Decreasing HB opportunities of alcohols with C(2)-methylated imidazolium cations results in an increase in UCST, while the coupled anions have a significant impact on this property,525,527 which diminishes with increasing alkyl chain length in cations.522,523,528
Calorimetric data together with computational results showed evidence of effects of anion’s basicity on IL–alcohol interactions and of cation structures on the solvation process of alcohols in ILs.16,529 The effect of anion basicity on molar enthalpies of solvation at infinite dilution of propanol in [C4MIM] ILs is described by a sequence of anion enthalpy values: [PF6] > tri(pentafluoroethyl)trifluorophosphate ([FAP]) > [NTF2] > [N(CN)2] > trifluoroacetic acid ([TFA]). This trend of increasing exothermic enthalpies from [C4MIM][PF6] to [C4MIM][TFA] is rationalized by a decrease in cavitation energy (endothermic contribution) and an increase in IL–alcohol interaction strength (exothermic contribution). In addition, variations in molar enthalpies of solvation of propanol in [C4MIM] ILs are linearly correlated with HB interactions of anions with propanol in IL–propanol mixtures.519,530 These experimental and computational results are generally consistent with previous findings on delicate interactions of different solutes with anions in protic and aprotic ILs.513,531,532
The aggregation process of alcohols in ILs and dynamical quantities of ion species in IL–alcohol mixtures are highly dependent on the nature of anions and size of alcohols as alcohols have predominant interactions with anions, particularly in halogenated IL–alcohol mixtures.533,534 Atomistic simulations suggested that the formation of apolar networks, as has been reported for IL–water mixtures, does not take place in IL–alcohol mixtures when halides are present,533,534 as observed from a significant presence of locally ordered structures in [CnMIM]Cl–alcohol mixtures.535 However, in solvophobic IL–alcohol mixtures with fluorinated anions, the formation of apolar aggregates and polar networks depends on anion sizes.533,536 Diffusivities of constituent ions in IL–alcohol mixtures increase with increasing alcohol concentrations. Two different regimes are observed: (i) at low alcohol concentrations, diffusion coefficients of alcohols increase very slowly, which is associated with viscosities of mixtures, and (ii) at high alcohol concentrations, diffusion coefficients of alcohols show a rapid increase, which is related to percolation of alcohol domains breaking IL polar networks.536,537
Concerning liquid dynamics of IL–alcohol mixtures, particularly those involving HB fluctuations, Kramer et al. conducted a series of 2D IR measurements of spectral diffusions and PSPP measurements of vibrational population dynamics and rotation relaxation of hydroxyl stretching mode of methanol and ethanol molecules in [C2MIM][NTF2].397 Long time scale spectral diffusion (structural evolution) observed in 2D IR spectra showed a clear but not dramatic slowing as alcohol alkyl chain increases: 28 ps for methanol and 34 ps for ethanol, both of which are larger than that for water (23 ps) in the same IL. Frequency–frequency correlation functions characterizing structural evolution in IL–alcohol mixtures reported a variety of time scales for HB dynamics and water rotation relaxation ranging from a few hundred femtoseconds to tens of picoseconds. Rotational correlation functions for these IL–alcohol mixtures exhibit several periods of restricted angular wobbling-in-a-cone motions before a complete rotational randomization. These experimental results suggest a weakening of angular potential from methanol to ethanol with decreased HB strength.538
Similar microstructural and dynamical changes were also observed in mixtures of alcohols with pyrrolidinium, pyridinium, and piperidinium ILs.529,538 Molar enthalpies of solvation at infinite dilution of propanol in ILs consisting of [NTF2] and [FAP] anions coupled with [C4MPYRR], [C4PYRI], and [C4MPIP] cations were studied to examine the influence of cation structural factors (size, aromaticity, charge distribution, and acidity).538 For [NTF2] ILs, there is no significant dependence of solvation enthalpies on cation structures, which is an indirect evidence of substantial interactions of alcohols with [NTF2] anions. Regarding [FAP] ILs, there is a significant difference in solvation enthalpies of propanol in [C4MPYRR][FAP] in comparison with that in [C4MIM][FAP].539 This is attributed to an nonaromaticity of [C4MPYRR] cations and a decrease in their acidic character, leading to decreased cation–alcohol dispersive and HB interactions.538
In general, solvation of alcohols in ILs can be described as a two-step process: creation of a cavity in ILs and relaxation of ions in cavities to establish preferential IL–alcohol interactions.519 Experimental and computational results of solvation enthalpies of alcohols in ILs are interpreted as a balance between an endothermic contribution of cavitation effect in IL solvents and an exothermic contribution of solute–solvent interactions. These results provide a detailed effect of cations and anions on solvation of alcohols in ILs.
3.3.3. Tetraalkylammonium and Tetraalkylphosphonium IL–Alcohol Mixtures
Mixing [N1,1,1,4][NTF2] with ethanol and propanol is expected to result in a competition between ion–ion and ion–alcohol interactions.540 Solubility data of ethanol and propanol in [N1,1,1,4][NTF2] indicate that mixing enthalpy is positive for these two alcohols because they must overcome strong Coulombic ion pair interactions to solvate in IL matrix. In addition, structural and energetic properties of [N1,1,1,4][NTF2]–alcohol mixtures provide important molecular-level information that can be used to understand and predict solubility trends for other alcohols in ILs. Furthermore, small variations in alcohol structures lead to a wealth of microstructures and liquid morphologies in IL–alcohol mixtures, highlighting a resilience of IL polar networks to addition of alcohols with different polarities.531 Charge ordering peaks for all IL–alcohol mixtures exhibit higher intensities than those for pure ILs and are all shifted to lower q values due to expansions of apolar networks in IL–alcohol mixtures. It is noteworthy that the expansion of apolar networks and the partial breakdown of polar networks (domains) in [CH] ILs are totally different from those in [CnMIM] ILs. The modified polar networks are more filamentous in the former case, whereas in the latter case they are more globular in shape (some portions are expanded or broken while others still retain a fairly degree of 3D connectivity). Microstructures and liquid morphologies of these IL–alcohol mixtures are, in fact, determined by an intricate balance between different types of interactions (electrostatic, solvophobic, vdW, HB, etc.) that are privileged among intervening species.
In another work, atomistic simulations revealed that [P6,6,6,14]Cl–methanol mixtures exhibit a microstructural evolution from IL-like to methanol-like with a gradual addition of methanol into [P6,6,6,14]Cl.459 In mixtures with high methanol concentrations, cation–anion pairs are widely separated by methanol molecules. The central polar units in [P6,6,6,14] are overwhelmingly surrounded by Cl anions and subsequently by methanol molecules, and methanol hydroxyl groups are preferentially solvated by anion species. In addition, liquid viscosities of [P6,6,6,14]Cl–methanol mixtures show a nonlinear dependence on mole fractions of ILs with XIL < 0.1, which is in contrast to a linear data variation with mole fractions of ILs with XIL > 0.1.541
For molecules interacting mainly via permanent dipole moments (1,2-difluorobenzene, ethers) or permanent electric quadrupole moments (benzene, hexafluorobenzene), molecules tend to swell existing IL apolar domains and prefer to locate near polar networks with orientations to maximize favorable multipole–charge interactions. Such a charge-template arrangement also works in the other sense: IL polar networks are generally flexible and can be rearranged to accommodate solute molecules, even if such rearrangement implies expanding polar networks. In the case of alcohol solutes, IL polar networks have to simultaneously accommodate alkyl moieties and hydroxyl groups in alcohols. This impact leads to a general loss of ion–ion interactions in close solvation shells and a ramification in polar networks. Nonetheless, IL polar networks can form well-organized string-like arrangements and exist up to fairly high solute concentrations. In IL–alcohol mixtures, a second network composed of alcohols and anions forms at intermediate alcohol concentrations. Such a secondary network causes a sheathing of original IL polar networks, and is probably responsible for neutron diffraction and X-ray scattering patterns at low q values.
3.4. IL–Acetonitrile Mixtures
Acetonitrile (ACN) is an important organic solvent, and its mixtures with ILs have been utilized as reaction media for organic synthesis and thermally stable electrolytes in batteries and solar cells with low toxicity and high efficiency.62,542 In addition, ACN is structurally similar to methanol but with different HB donation capability, which contributes to its intrinsic macroscopic functionalities in electrochemical applications.62,543
A large inhomogeneity was observed in EAN–ACN mixtures with EAN being a minority component, as identified from combined SAXS and atomistic simulations (Figure 19A).544 It is EAN’s wormlike liquid structure that is responsible for density fluctuations in EAN–ACN mixtures, which are even denser than pure EAN due to preferential HB interactions.545 EAN and ACN have significant reciprocal effects: EAN ions orient toward dipolar ACN molecules and ACN effectively interacts with EA cations preventing self-association of EAN, which promotes mutual solubility of these two components (Figure 19B,C). Microstructural heterogeneities in EAN–ACN mixtures are manifested in their transport properties at micro- and mesoscopic levels.546,547 Diffusion coefficients, ion conductivities of constituent ions, and shear viscosities of EAN–ACN mixtures exhibit strong deviations from ideal mixing, which is attributed to the formation of large ion clusters that behave similarly to colloidal aggregates. A similar inhomogeneity was also observed in PAN–ACN mixtures but with smaller magnitude than in EAN–ACN mixtures.118
Figure 19.
(A) SAXS patterns for EAN–ACN mixtures with EAN concentrations of 0.0 (blue), 0.1 (red), 0.3 (green), 0.5 (magenta), 0.7 (cyan), 0.9 (gray), and 1.0 (black). (B,C) Representative snapshots of EAN–ACN mixture with XEAN = 0.1. Reproduced with permission from ref (544). Copyright 2017 American Chemical Society. (D) Deconvoluted IR spectrum in imidazolium ring C–H stretching region of a [C4MIM][BF4]–ACN mixture with XACN = 0.5. (E) Schematic model of a transformation of ion species in dilution process. Reproduced with permission from ref (245) Copyright 2013 Royal Society of Chemistry.
Microstructrues and dynamics of [C4MIM]X–ACN mixtures were extensively studied using S/WAXS,220,245 IR,82,548 NMR,88,548 OHD-RIKE,95 dielectric77,549 and terahertz spectroscopies,550 as well as theoretical245,548 and computational95,551−553 approaches. Ion species are nanostructurally organized into polar networks and apolar domains, and ACN molecules are localized in interfacial regions with nitrogen atoms pointing toward imidazolium rings because of HB interactions.95 A gradual increase of IL concentration in mixtures leads to a structural transition of mixtures from “IL dissolved in ACN” to “ACN dissolved in IL”, and a phase behavior transition of hydrodynamic boundary conditions from a “stick” regime to a “slip” regime. In IL–ACN mixtures with high IL concentrations, ACN diffuses much slower than expected due to strong ion–ACN interactions and the presence of ACN clusters in charge-enriched domains.77,553 Another series of studies showed that dilution of [C4MIM][BF4] by ACN results in a local structural transition from large ion clusters to small ion pairing structures (Figure 19D,E).245,548 ACN cannot break apart strong electrostatic interactions among constituent ions, but they can separate large ion aggregates into small ion pairing clusters with a gradual increase of ACN concentration.88,245,548,552 For [C4MIM]X–ACN mixtures used as solvent electrolytes in electrochemical devices, microstructures and differential capacitances of pure ILs and IL–ACN mixtures can be described by a theoretical concept termed “counter-charge layer in generalized solvent”.551 An electrical double layer (EDL) structure is composed of a counter-charge layer consisting of polarized IL ions for balancing electrode charge, and the differential capacitances of EDLs are related to detailed interfacial ion structures that will be discussed comprehensively in section 5.
The microstructural and dynamical variations in IL–ACN mixtures further influence their solvent properties, especially in determining solvent–solute interactions. It was found that nitrogen-containing solutes (hexan-1-amine, dipropylamine) diffuse more slowly than oxygen-containing solutes (1-hexanol and dipropylether) in [C4MIM][NTF2]–ACN mixtures, indicating enhanced IL–nitrogen interactions compared to IL–oxygen interactions in heterogeneous IL–ACN mixtures.220 It should be noted that not only ACN but also other molecular components (water, dichloromethane, methanol, and t-butanol) with varied polarity, size, and isomerism can affect solvent power (dipolarity and polarizability) of ILs.554 At low concentrations of molecular compounds, liquid properties of mixtures are dominated by properties of ILs. Conversely, when the quantity of molecular compound is increased, ILs become solute ions in a molecular medium, that is, an electrolyte solution. Between these two extremes, it is expected that there will be remarkable microstructural changes in mixtures with a gradual addition of molecular solutes into ILs, such as the swelling of IL networks, the formation of impetrating polar and apolar networks, and the disruption of large IL cluster into small ion pairs and then possibly solvated isolated ions in mixtures.
4. Ionic Liquid–Ionic Liquid Mixtures
As ILs are solely consisting of cations and anion species, blending two or more ILs represents an important extension of ILs because IL mixtures have interesting physicochemical properties and may outperform pure IL components for a given process. A binary IL–IL mixture consists of either a common cation coupled with two different anions or a common anion coupled with two cations belonging to the same or different cation families. Molecular associations and liquid structures in IL–IL mixtures will be more complicated than those in respective pure IL matrixes and will have a significant effect on their macroscopic functional performance in specific applications.
4.1. IL–IL Mixtures Containing the Same Cation
Neutron and X-ray scattering spectra327,329 showed that even small protic ILs can be nanostructured in the bulk liquid phase and have distinct rheological properties. Viscosities of pure protic alkylammonium ILs are strongly correlated with strength of HB networks and solvophobic nanostructures in ILs.363 Pure alkylammonium ILs behave as Newtonian fluids at low shear rates but exhibit striking shear thinning feature at high shear rates. However, rheological properties of binary protic alkylammonium IL–IL mixtures demonstrated fundamental differences in cation–anion interactions due to an offsetting effect of HB interactions and the formation of solvophobic nanostructures in mixtures.363,555 Mixtures containing the same protic alkylammonium cation coupled with formate and [NO3] anions can resist heavier flow fields than respective ILs due to different HB capabilities of two anions.259,333 These protic alkylammonium IL mixtures are a representative demonstration of double-salt ILs in which constituent ions do not retain their individual nature.556 Although pure alkylammonium ILs and their mixtures exhibit great similarities with respect to overall ion-alternating structures, each type of the statistically distributed ions experiences a completely different local ionic environments in heterogeneous IL matrixes.
For imidazolium IL–IL mixtures, it was found that [C4MIM][NTF2]x[C1C1PO4]1–x (dimethylphosphate) mixtures exhibit significant positive excess molar volumes, whereas [C4MIM][NTF2]x[TFO]1–x mixtures show small deviations from ideality,557,558 which is attributed to greater HB capability of [C1C1PO4] anions compared to that of [TFO] anions in coordinating imidazolium cations. In addition, thermal and ion conductivities of imidazolium IL–IL mixtures exhibit either expected behavior upon mixing559,560 or minor deviations from anticipated values561,562 depending on a delicate interplay of interactions among constituent ions. Generally, deviations in phase behaviors of mixtures are related to variations in short-range interactions and ion asymmetries, the latter of which are crucial for ion associations and dissociations in mixtures.315,563,564
There are some experimental attempts to understand phase behaviors of imidazolium IL–IL mixtures.558,561,565,566 OKE spectra for [C5MIM]Brx[NTF2]1–x mixtures are well described by weighted sums of spectra for pure ILs, whereas OKE spectra for [C5MIM][PF6]x[TFA]1–x mixtures are nonadditive.561 Either additive or nonadditive of OKE spectra depends on relative sizes of anions in mixtures, assuming that mixtures are assembled into polar and apolar networks at the nanoscopic level.336,564 For anions with similar sizes (i.e., [PF6] and [TFA]), polar networks in mixtures are described by random co-networks, whereas for anions that differ greatly in sizes (i.e., [NTF2] and Br), polar networks are characterized by “block co-networks”, a nanostructural organization resembling that of block copolymer (Figure 20A). Anion sizes and shapes have significant effects on spatial arrangements of ions in IL–IL mixtures in terms of interion distances and Coulombic attractions between oppositely charged ions.563,567 NMR results showed that both static and dynamic free volumes are strongly correlated for imidazolium IL–IL mixtures.558 Given a relationship between free volumes and transport properties such as liquid viscosities and ion conductivities, these results provide an intrinsic link between thermodynamics of mixing and liquid structures of IL–IL mixtures.
Figure 20.
(A) Schematics of nanostructural organization in binary [C5MIM]Brx[NTF2]1–x (left) and [C5MIM][PF6]x[TFA]1–x (right) mixtures. Reproduced with permission from ref (561). Copyright 2008 American Chemical Society. (B) Ion cluster cage formed by one [C1MIM] cation, three Cl anions, and four [P1,1,1,1] cations obtained from DFT calculations. Reproduced with permission from ref (142). Copyright 2015 Royal Society of Chemistry.
Focusing on electrochemical applications of pyrrolidinium and pyridinium IL–IL mixtures, the beneficial synergic effect on ion conductivities and electrochemical stabilities of mixtures was investigated combining experimental and computational studies.568−570 Binary [C4MPYRI][BF4]x[N(CN)2]1–x mixtures exhibit two well-defined regions in which physicochemical properties resemble those of pure ILs, separated by a critical concentration of [BF4].568 Liquid structures do not change remarkably once this critical concentration is reached, leading to an almost ideal mixing from a thermodynamic point of view. This points to a simple anion dilution effect leading to liquid regions dominated by interactions between [C4MPYRI] cations and [N(CN)2] anions with [BF4] anions being dispersed or liquid regions dominated by interactions between [C4MPYRI] cations and [BF4] anions with [N(CN)2] anions being dispersed.568 Atomistic simulations indicated a weak structural change with an increase of [C4MPYRI][BF4] concentration in mixtures, arising from decreased sizes of apolar domains. This structural change leads to positive excess molar volumes but without significant changes in energetic properties or in the manner in which ions interact.
4.2. IL–IL Mixtures Containing a Same Anion
IL–IL mixtures containing two substantially different cations and a common anion have a rich structural chemistry, even if these two cations belong to the same cation family.571−573 Canongia Lopes et al. found very small positive excess volumes for binary imidazolium IL mixtures composed of cations with varied alkyl chains coupled with [NTF2] anions.574 In addition, several thermophysical properties, such as liquid densities, enthalpies of mixing, conductivities, and viscosity data, also exhibit practically “ideal” (linear) mixing behavior with small excess deviations arising from structural differences between imidazolium cations.559,575 For a [C2MIM]0.5[C6MIM]0.5[NTF2] mixture, microphase separation was observed between polar and apolar domains whose sizes are intermediate to those of two pure IL components.572 SANS experiments and atomistic simulations of [C2MIM]x[C12MIM]1–x[NTF2] mixtures demonstrated that both physicochemical properties and liquid structures change substantially as a function of chemical composition of mixtures.113,573 Liquid structures of mixtures with molar fractions of [C12MIM][NTF2] less than 0.3 are described as aggregates of amphiphilic [C12MIM] cations in a relatively polar [C2MIM][NTF2] solvent, whereas liquid structures of mixtures with molar fractions of [C12MIM][NTF2] more than 0.3 are characterized by bicontinuous polar and apolar networks, where C12 chains percolate throughout mixtures forming a continuous apolar subphase. With a gradual increase of [C12MIM][NTF2] concentration in mixtures, the length scale of apolar subphase increases and phase behaviors of mixtures become more reminiscent of pure [C12MIM][NTF2]. These [C2MIM]x[C12MIM]1–x[NTF2] mixtures become more disordered with increasing temperatures, but the fundamental liquid structures do not change substantially. In addition, these mixtures exhibit different response to the addition of water. For mixtures with less [C12MIM][NTF2], water is incorporated into polar networks, whereas mixtures with more [C12MIM][NTF2] are too hydrophobic to incorporate sufficient water and to change liquid structures.
In addition to aprotic IL–IL mixtures consisting of a common anion coupled with two imidazolium cations with varied alkyl chains, different mixing behaviors were observed in thermo-dynamical quantities of protic–aprotic mixtures with cations belonging to different cation families.259,567,570,576,577 Atomistic simulations and experimental measurements showed that binary mixtures composed of alkylammonium protic ILs and imidazolium aprotic ILs exhibit almost ideal mixing behavior with a gradual microstructural transition between two pure IL components,576,578 which is rationalized by a structural similarity of two ILs.557,579 In particular, a noteworthy association was observed upon mixing PAN/BAN and [C2MIM][BF4] ILs. In these mixtures, [C2MIM] cations are integrated into protic networks and [BF4] anions occupy previously empty regions near protic cation tails. It is significant that a subtle microstructural change in these mixtures leads to complicated variations in transport properties of constituent ions.578 For EAN–[C2MIM][BF4] mixtures, a novel conductivity curve exhibits pronounced deviations from the simple ideal mixing rule, with three different regions defined by a local maximum, reflecting enhanced translational dynamics relative to ideal mixing behavior, and a global minimum at intermediate concentrations. These regions are defined by the onset of the formation of EAN HB networks (XEAN = 0.2) and the virtual disappearance of aprotic IL structures (XEAN = 0.7), where long-range ordering for [C2MIM][BF4] breaks down.
In addition to these mixing behaviors observed in protic–aprotic IL–IL mixtures where ions differ considerably in sizes and in HB forming abilities,580 there are some mutually immiscible binary IL–IL mixtures,570,581,582 which are in disagreement to the intuitive rule of thumb similia similibus solvuntur. Two phases were obtained for [C2MIM]x[P6,6,6,14]1–x[NTF2] mixtures over a wide temperature range.581,583 In addition, mutually immiscible binary mixtures were also obtained if [NTF2] anions in [CnMIM]x[P6,6,6,14]1–x[NTF2] mixtures are replaced by Cl anions.581,583 A large negative entropy of dissolving [CnMIM]Cl ILs in [P6,6,6,14]Cl was observed when imidazolium cation alkyl chains are shorter than C5.581 DFT calculations revealed that solvation of [C1MIM]Cl in [P6,6,6,14]Cl not only exhibits a large negative entropy but also leads to enhanced diffusion of [P6,6,6,14] cations compared to [C1MIM] cations in mixtures.142 Both unexpected features are attributed to the formation of large symmetric ion cluster cages in which a [C1MIM] cation is surrounded by three Cl anions in the first solvation shell and four [P6,6,6,14] cations in the second solvation shell (Figure 20B). This cage structural motif illustrated that atoms involved in HB interactions in mixtures remain their positions similar to those in pure ILs because polar domains in [C1MIM]Cl and [P6,6,6,14]Cl ILs fit well with each other. Lengthening imidazolium cation alkyl chains disturbs cage structures and enhances π–π stacking of imidazolium rings in [CnMIM]x[P6,6,6,14]1–xCl mixtures. Both aspects decrease liquid structural ordering in mixtures.
Even though there are some variations for IL–IL mixtures consisting of a common anion and two cations, it is clear that these mixtures are distinct systems in comparison with pure IL components. Both experimental and computational studies showed that a range of microstructural and physicochemical properties can be controlled simply by fine-tuning chemical compositions of mixtures rather than by synthesizing a large number of pure ILs. These IL–IL mixtures provide a promising path for rational selection of appropriate ILs for particular applications.
4.3. Imidazolium IL–Imidazole Mixtures
For imidazolium ILs, there is a set of extraordinary binary mixtures consisting of imidazolium ILs and neutral amphoteric imidazole molecules.163,584,585 Imidazole molecules provide both proton donor and acceptor sites to establish extended HB networks and facilitate proton transfer in ionic materials. AIMD simulations revealed that proton migration in imidazole involves two steps with a strong HB between two adjacent imidazole molecules (∼0.3 ps) and thereafter a rotation of imidazole molecules because of HB cleavage (∼30 ps). Imidazole molecules tend to form strong HBs with anions in mixtures, like selenocyanate ([SeCN]),586 leading to a restricted short time angular wobbling-in-a-cone sampling of [SeCN] anions in heterogeneous solvent environments.
Mixing a protic [C2HIM][NTF2] with imidazole perturbs the native HB networks in the IL matrix. Due to a structural similarity, imidazolium cations and imidazole molecules are interchangeable and exhibit competing features in coordinating [NTF2] anions.163,585 These two species form HBs with [NTF2] anions with varied HB strengths depending on the detailed composition of IL–imidazole mixtures, leading to significant changes in microstructures and dynamics in binary mixtures. At low imidazole concentrations, HB network configurations promote proton transfer (Grotthuss mechanism) in mixtures where imidazole molecules act as base molecules pulling protons from neighboring imidazolium cations. In intermediate imidazole concentrations, mixtures display a nonideal mixing behavior rationalized by competitive ion–ion and ion–imidazole interactions and preferential HB interactions. For a [C2HIM][NTF2]–imidazole mixture with an equimolar fraction, a microstructural transition occurs from an ion network mainly stabilized by electrostatic forces among constituent ions to a mixed phase held together by site specific HB interactions. This composition marks a steep increase in ion conductivities resulting from the formation of HBs between neighboring imidazole molecules. Liquid structures of [C2HIM][NTF2]–imidazole mixtures with high imidazole concentrations are characterized by highly dissociated and rapidly diffusing ions in a neutral solvent. Charge transport in these imidazole-concentrated mixtures is characterized by a vehicular mechanism. These observations provide important physical insights into a potential usage of protic ILs as solvent electrolytes in electrochemical devices and demonstrate that manipulation of HB networks is a valuable approach to fine-tune charge transport mechanisms in IL mixtures.
4.4. IL–Li Salt Mixtures
IL mixtures containing Li salts are useful systems for Li ion batteries in terms of safety, manufacturing cost, and performance.560,587−590 Usually, Li salts containing IL anions are preferable because of a higher solubility with respect to mixtures with dissimilar anions. Therefore, IL–Li salt mixtures can be considered as a ternary component system consisting of IL cations, Li ions, and common anions.
Focusing on ILs as solvent electrolytes in batteries, representative studies were devoted to analysis of LiNO3 in alkylammonium nitrate ILs. At ambient conditions, EAN and LiNO3 are miscible up to ∼0.2 molar fraction of LiNO3 in EAN.591,592 An overall structural scenario in EAN–LiNO3 mixtures, as determined from joint SAXS and atomistic simulations,591,593 can be described as (1) EAN can essentially retain its nanostructure and liquid morphology upon addition of LiNO3 and only interactions among ammonium groups and [NO3] anions being slightly perturbed and (2) Li ions are solvated in polar domains in IL matrixes, where they coordinate with [NO3] anions either in a monodentate or in a bidentate manner, leading to a solidlike short-range pseudolattice ordering structure.
However, it is noteworthy that solvated Li ions have distinct capabilities in coordinating ions in IL matrixes and can be described using a similar idea of “structure-making” and “structure-breaking”, which are well-established concepts for description of dilute aqueous electrolytes.594,595 LiNO3 is a “structure-breaking” salt for EAN. Li ions are incorporated into polar domains of spongelike nanostructures. They disrupt alignments of ethyl chains in apolar domains and weaken cation–anion HB interactions, resulting in fewer linear HBs and a higher proportion of bifurcated HBs (Figure 21A–C).596 Conversely, LiNO3 is a “structure-making” salt for EtAN. Li ions induce a long-range rearrangement of EtAN ion species, such that a bicontinuous instead of a clustered nanostructure is formed, which is attributed to favorable trans conformations of EtA cations (Figure 21D–F).596 In addition, heterogeneous structures in IL–Li salt mixtures are further corroborated by experimental characterizations of microstructural and rheological properties of EAN–LiNO3 and EtAN–LiNO3 mixtures in confined environments.595 EAN is weakly dependent, with only small changes in structural forces, frictions, and liquid viscosities at low concentrations of Li ions,592 whereas EtAN’s behavior is strongly subjected to Li ion concentrations. At low Li ion concentrations, liquid viscosities of EtAN–LiNO3 mixtures increase. Structural forces and the associated friction profiles deviate from those for pure EtAN, which has a direct consequence on boundary layers, as demonstrated from a large reduction in friction forces at high loads. A high Li ion concentration in EtAN leads to enhanced changes in liquid viscosities and structural forces. Lengthening alkyl chains in primary alkylammonium cations from EA to PA and BA and changes in cation structures from primary to secondary and tertiary ammonium cations have a significant impact on solvation structures of Li ions in mixtures as Li ions are preferentially accommodated into polar networks to coordinate anions.597 With an increase of Li ion concentrations in mixtures, Li ions progressively erode HB networks, decreasing the extent of HB networks and inducing orientation disorders in polar domains. These effects are more pronounced for ILs having long cation alkyl chains due to a lower degree of cation–anion HB interactions. Large secondary and tertiary ammonium cations have sparsely packed 3D ion structures in IL matrixes, which are easily perturbed by Li salts.
Figure 21.
Local structures in apolar domains for IL–LiNO3 mixtures. Comparison of radial distribution functions for (A) EAN–LiNO3 and (D) EtAN–LiNO3 mixtures (solid lines) with those for pure ILs (dashed lines). Spatial distribution function plots of carbon atoms bonded to nitrogen atoms in EA and EtA cations as a function of distance and angular position from a central cation for (B) EAN, (C) EAN–LiNO3 mixture, (E) EtAN, and (F) EtAN–LiNO3 mixture. Reproduced with permission from ref (596). Copyright 2014 American Chemical Society. Representative ion structures of (G) [Li(NTF2)2]− and (H) [Li(NTF2)4]3–. Reproduced with permission from ref (598). Copyright 2008 American Chemical Society. Typical Li+ coordination complexes of (I) [Li(FSI)4]3– found in [C3MPYRR][FSI] and of (J) [Li(BF4)4]3– found in [C2MIM][BF4] at 298 K and with XLi+ = 0.05. Reproduced with permission from ref (599). Copyright 2014 American Chemical Society.
OHD-OKE spectra demonstrated that addition of LiNTF2 salt to [C4MIM][NTF2] leads to an increase in liquid viscosity, a decrease in ion mobility, and distinct rotation relaxation dynamics of ions in IL–Li salt mixtures.431,600,601 These changes are essentially correlated with distinct variations of microstructures in mixtures with a gradual addition of LiNTF2 salt. Competitive binding of large cations to [NTF2] anions acts as an enhancer of Li ion diffusivities in mixtures by reducing LiNTF2 aggregate sizes and keeping a tetra-coordination feature of Li ions.598 IR and DFT calculations provided direct evidence supporting the presence of strongly coordinated [Li(NTF2)2]− structure being a major adduct in [CnMIM][NTF2]–LiNTF2 mixtures (Figure 21G).598,602,603 In general, [Li(NTF2)n+1]n− clusters are highly stable species due to strong Coulombic interactions between Li ions and oxygen atoms in [NTF2] anions (Figure 21H).598,603−605
A series of interesting works demonstrated that hybrid electrolytes consisting of imidazolium ILs and Li salts having different anions are promising electrolytes for Li ion batteries. For [C2MIM][NTF2]–LiNTF2 mixtures, replacing [NTF2] with bis(fluorosulfonyl)imide ([FSI]) or combining both anions leads to enhanced ion conductivities in mixtures.589 The [C2MIM][FSI]0.8[LiNTF2]0.2 electrolyte holds peculiar significance due to its high thermal stability, arising from a specific solvation structure of Li ions in coordinating [NTF2] and [FSI] anions.606 In such a way, a remarkable EDL structure is formed on the electrode surface protecting [C2MIM] cations from decomposition and thereby improving cathodic stability. A nontrivial nature of these EDL structures together with their dependence on electrolytes and electrode materials607 are on par with organic electrolytes in terms of viscosities, ion and electrical conductivities when taking into account their anticipated operating temperatures.608
The IL ion-Li coordination pattern in protic imidazolium ILs is very different to that in aprotic imidazolium ILs.609,610 The coordination number of Li ions in protics is always smaller than that in aprotics, which makes Li ions more “free” to move in protic imidazolium ILs.611,612 Furthermore, the charge valence of inorganic salt cations like Mg2+ and Ca2+ has a significant effect on HB networks in IL–salt mixtures than those in their monovalent counterparts.590,613 Vibrational spectra and AIMD simulation results indicated that there are three different potential energy environments for [NTF2] anions in IL–Mg salt mixtures in contrast to the fact that there are only two coordination patterns in IL–Na and IL–Li salt mixtures.614 Additional investigations revealed noteworthy coordination patterns of strontium, uranyl, and perrhenate salts with imidazolium ILs in IL–salt mixtures, which provide valuable clues for extraction of these salts in context of nuclear waste processing.615
In challenging electrochemical applications, pyrrolidinium ILs have prominence for their outstanding capabilities in dissolving Li salts, giving rise to IL–Li electrolytes for innovative uses in Li ion devices.616,617 In comparison with those observed in imidazolium ILs, doping Li salts into pyrrolidinium analogues results in distinct microstructures.599,618−620 For example, at low levels of Li salt doping, both [C4MPYRR][NTF2] and [C3MPYRR][FSI] ILs exhibit stable Li solvation structures containing either two anions, both having bidentate ligand bonds, or three anions, one being bidentate and the other two being monodentate (Figure 21I), whereas [BF4] ILs exhibit a tendency toward a solvation shell having four monodentate anions (Figure 21J).599 A high level of Li doping leads to the formation of highly coordinated Li ion network structures where a single anion participates in multiple Li ion solvation shells. A similar [Li(NTF2)n+1]n− adduct structure is observed in [CnMPYRR][NTF2]–LiNTF2 mixtures.603,604,621 SAXS experiments showed a nonmonotonic variation of charge alternation distances with and without addition of Li salts into pyrrolidinium and imidazolium ILs as a function of cation alkyl chain length, highlighting a competitive feature of steric hindrance and vdW interactions of alkyl tails in cations and a reconfiguration of [NTF2] anions in coordinating Li ions.599,620,622
It should be noted that transport and conductivities of Li ions in IL–Li salt mixtures are governed by their binding strength with anions and intrinsic liquid viscosities of ILs.599,619,623,624 In a [C4MPYRR]0.9Li0.1[NTF2] mixture, diffusivities of ion species are significantly smaller than those in pure IL matrix due to a larger viscosity of this IL–Li salt mixture and the formation of peculiar Li–anion adducts in this mixture.604,625 These findings are rationalized by a hopping diffusion process of Li–anion adducts in mixture because of a disruption of adduct coordination shells by [NTF2] anions (structure–diffusion mechanism) rather than a Brownian motion of the whole Li–anion adducts (vehicular mechanism).593,618,625 Ion conductivities of IL–Li salt mixtures increase with increasing concentrations of Li ions, with this effect being greater at elevated temperatures. However, the contribution of Li ions to ion conductivities does not proportionally increase with Li ion concentrations but saturates at a high doping level.604
5. Ionic Liquids in Interfacial Regions
Applications of ILs in gas separations, biphasic extractions, and in electrochemical devices involve their contact with gas, liquid, and solid materials. Microstructural and dynamical properties of ILs on surfaces and in interfacial regions determine their functionalities in physical and chemical processes occurring across phase boundaries. Given that interfacial regions are highly inhomogeneous, ILs exhibit fundamentally different physicochemical and structural quantities as that in bulk liquids. In the following subsections, we mainly focus on a description of heterogeneous microstructures and dynamics of ILs in varied interfacial regions and their effects on macroscopic functional performance of ILs in electrochemical and tribological applications.
5.1. IL–Vapor (Gas) Interface
The IL–vapor (gas) interface holds a special significance for particular applications of ILs involving adsorption and desorption of gas molecules.626 In these applications, chemical compositions and molecular arrangements of ions in interfacial regions are distinct to those in bulk liquids because ions experience unbalanced forces. A wide range of experimental techniques including DRS,78,79,627 neutron reflectometry,106,628−630 SFG,96,98,100 XRR,105,119,628,629 XPS,115,626 and molecular simulations631−633 have been employed to provide detailed information on surface compositions, interfacial structures, and dynamics of ions at the IL–vapor interface.
Watson et al. first reported experimental investigations of interfacial compositions and molecular orientations of imidazolium ILs using DRS spectroscopy.79,627 Both imidazolium cations and anions in [CnMIM][PF6] (n = 4, 8, and 12) and [CnMIM][BF4] ILs (n = 4 and 8) are present at the IL–vapor interface with no significant segregation. Imidazolium rings in these two ILs exhibit similar orientations being perpendicular to the IL–vapor interface. However, butyl chains are positioned differently with those in [C4MIM][PF6] being parallel to the IL–vapor interface and those in [C4MIM][BF4] projecting into bulk liquids with a shift of N–CH3 groups closer to the IL–vapor interface. Lengthening cation alkyl chains from C4 to C8 has little effect on the H/C ratio for all ILs at the IL–vapor interface, but a further increase to C12 results in a substantial increase in the H/C ratio for [C12MIM][PF6] with methyl groups close to the IL–vapor interface (Figure 22A). In another series of studies, SFG spectra for hydrophobic [C4MIM][PF6] and [C4MIM][NTF2] ILs and hydrophilic [C4MIM][BF4] IL suggested that imidazolium rings are most likely lying parallel to the IL–vapor interface with butyl chains projecting into the vapor phase with a tilt angle from the surface normal direction.438,634 Moreover, SFG spectra illustrated that anions have a negligible effect on preferred orientations of [C4MIM] cations in the IL–vapor interfacial region.97,635
Figure 22.
(A) Experimentally determined atomic H/C ratios for representative ILs at the IL–vapor interface. Reproduced with permission from ref (79). Copyright 2001 Royal Society of Chemistry. (B) Definition of Cm and Cb vectors in [C4MIM] cation and (C) preferential distributions of [C4MIM] cations, characterized with bivariate orientations of Cm and Cb vectors, at the IL–vapor interface for simulation systems containing varied numbers of [C4MIM][PF6] ion pairs on the graphene surface. The notations →, ↑, and ↓ correspond to Cm and Cb vectors being parallel and perpendicular to the IL–vapor interface with terminal carbon atoms protruded into the vapor phase and projected into bulk liquids, respectively. Reproduced with permission from ref (645). Copyright 2013 Royal Society of Chemistry. (D) Schematic conformation of [C4MIM] in the IL–vapor interface. Reproduced with permission from ref (646). Copyright 2012 Royal Society of Chemistry. (E) Fresnel-normalized (symbols) and model-fitted (lines) XRR curves of the IL–vapor interface of [CnMIM][NTF2] ILs at specific temperatures. Different colors indicate different surface phases. Curves in two colors denote a transition between surface phases. (F) Electron density profiles (normalized to bulk electron density) obtained from model fits in part E. Reproduced with permission from ref (647). Copyright 2018 National Academy of Sciences. (G) XRR profiles for EAN (Δ), PAN (○), and EAF (□) IL–vapor interface, and for (H) EtAN–vapor interface. Reproduced with permission from ref (629). Copyright 2011 Royal Society of Chemistry. Scattering length density profiles for (I) EAN–vapor and (J) EtAN–vapor interfaces from the slab fit (dotted line), Chebyshev fit (dashed line), and slice fit (solid line) models. Reproduced with permission from ref (364). Copyright 2012 Royal Society of Chemistry.
XRR and neutron reflectivity are complementary techniques in providing electron density profiles across the IL–vapor interface.105,106,628,630 Bowers, Vergara-Gutierrez, and Webster carried out neutron reflectivity on [C4MIM][BF4] and [C8MIM][PF6] ILs.636 A surface layering structural model was suggested with imidazolium rings and alkyl tail moieties being segregated at the IL–vapor interface forming a lamellar structure extending to two layers of the alkyl chains. On the other hand, XRR studies of [C4MIM][PF6] and [C4MIM][BF4] ILs reported by Deutsch et al. indicated the presence of surface layering structures at the IL–vapor interface.630 Two types of molecular arrangements in the IL–vapor interfacial region were proposed, one with alkyl chains parallel to the IL–vapor interface and the other one with alkyl chains normal to the IL–vapor interface. In addition, XRR spectra revealed an increase in electron density (∼10–12%) at the IL–vapor interface, which is higher than that in bulk ILs and is mainly attributed to adsorption of anions at the IL–vapor interface. These experimental results indicate the presence of both cations and anions in the IL–vapor interfacial region, which is consistent with experimental data obtained from DRS spectra.78,627
Applications of external fields can significantly change interfacial ion structures.637,638 An intriguing work conducted by Jurado et al. demonstrated that an extended SFA repeatedly imposing and releasing confinement on [C6MIM][C2SO4] IL film induced a phase transition that was not relieved by the final removal of such an external constraint.639 Atomistic simulations of the [C8MIM][C8SO4] IL film indicated that a lamellar structure is not restricted to the IL–vapor interfacial region but instead extends across a slab of ∼9 nm.640 A systematic variation of alkyl chain lengths in [CnMIM][CmSO4] ILs showed similar layering structures at the IL–vapor interface,99,100,641,642 which is also supported by surface tension measurements with a decreased trend with increasing alkyl chain lengths of cations and anions if the corresponding counterion is fixed.643 When [CnMIM] cations and [CmSO4] anions are functionalized with ether groups in the alkyl chains, both of them still favor tail-outward orientations at the IL–vapor interface, and bulk liquid phase preserves an alternation of polar and apolar regions.644 These experimental and computational observations indicate that different polymorphic phases of ILs can be “engineered” on the nanoscopic level via confinement perturbations or other external stimuli, and the observed liquid morphological transitions are very attractive for energy applications particularly for electrochemical devices.
It should be noted that not only IL ion structures but also IL film thickness can have a significant effect on distributions of cations in the IL–vapor interfacial region if the IL film is confined on a solid substrate.645 With a gradual increase of the IL film thickness, preferential orientations of cations in [C4MIM][PF6] at the IL–vapor interface exhibit a progressive transition from a dominant flat distribution in an IL monolayer to that described by multiple favorable orientations having varied proportions (Figure 22B,C).645,648 In these favorable orientations, the main orientation of imidazolium rings is essentially perpendicular to the IL–vapor interface with some angular tilt (Figure 22D).646 The formation of compact IL–vapor interfacial layering structures further complicates dynamical quantities of ILs in comparison with those in bulk liquids. In the IL–vapor interfacial region, translational mobilities of terminal carbon atoms in [C4MIM] butyl chains648 and rotational dynamics of a short molecular axis in [C4MIM] cations271 are faster than those in other layers of confined IL films and in bulk liquids of simulation systems without confinement. Rotational dynamics of [C4MIM] cations generally follow a Kohlrausch–Williams–Watts behavior in the IL–vapor interfacial region, whereas in bulk liquids of confined IL films, the temperature dependence of translational diffusions and rotational relaxations of [C4MIM] cations is characterized by a VFT feature over a wide temperature range.271
Systematic lengthening alkyl chains in imidazolium cations and enlarging anions from halides to [BF4], [PF6], [TFO], and complex anions containing perfluoroalkyl groups such as [NTF2], [FAP], and bis(perfluoroethylsulfonyl)imide lead to significant changes in interfacial structures and molecular arrangements in the IL–vapor interfacial region.115,626,646,649 In general, lengthening the alkyl chains in the imidazolium cations and changing asymmetric [CnMIM] to symmetric [CnCnIM] cations tend to increase the probability for alkyl chains to point into the vapor phase and the coverage of the IL–vapor interface by alkyl groups with an expense of imidazolium rings and anions.633 However, this interfacial structural enhancement decreases with enlarging anion sizes, which promotes a disruption of interfacial alkyl layer structures, leading to cations with enhanced orientation freedom and more alkyl chains being able to point into liquid phase. In addition, no significant surface segregation of anions relative to imidazolium rings occurs in the IL–vapor interfacial layers, indicating that imidazolium rings and anions are located at similar distances from the interface, forming a densely packed polar layer due to strong Coulombic interactions. Temperature has a negligible influence on the overall density profiles and a relatively small effect on molecular orientations in the IL–vapor interfacial region. Ions stay considerably longer in the IL–vapor interfacial layers than they do in subinterfacial layers and ion exchange dynamics between consecutive layers are associated with distinct ion diffusions and rotational dynamics of ions within layers.
The evolution of IL–vapor interfacial structures with lengthening alkyl chains in imidazolium cations from Coulombic to vdW interaction domination was clarified in a systematic Angstrom-resolution synchrotron XRR study of [CnMIM][NTF2] ILs.647 A progressive change in electron density profiles was observed from a typical “simple liquid” for n < 6 (akin to that of simple liquids like water and organic solvents), through a nonmonotonic layered solvent for 8 < n < 20 (single high-density surface-segregated monolayer), to a liquid crystal surface phase for n = 22 which reverts to a “conventional” layered surface phase at high temperatures (Figure 22E,F). The layered surface phase consists of alternating polar and apolar parallel slabs, and layer spacing is larger than extended alkyl chains in imidazolium cations but smaller than twice that length, indicating that alkyl chains in lateral packing slabs are flexible, kinked, and partially interdigitated.375,650,651
Compared to bulk liquid structures of protic ILs, XRR spectra showed that EAN, PAN, and EAF ILs exhibit extended IL–vapor interfacial structures spanning at least five ion pair diameters (Figure 22G,I).119,629 All three ILs exhibit similar X-ray scattering length density profiles consisting of two parts. The top interfacial layer is a diffuse layer composed of multiple cations and anions arranged in such a way that polar groups are surrounded by cation alkyl chains, protecting hydrophilic species from the hydrophobic gaseous phase. Below this are layers enriched with apolar alkyl groups and polar domains consisting of cation ammonium moieties and anions, which gradually decay to bulk liquid structures. Lengthening cation alkyl chains from EA to PA results in pronounced interfacial structures. Alkyl chains in PA cations impart larger solvophobicity than those in EA cations, leading to well-defined segregation of polar and apolar domains in a heterogeneous PAN matrix. Conversely, replacing [NO3] with formate anions reduces HB interactions, and thus the interfacial region does not extend as far into bulk liquids. This suggests that solvophobicity determines the sharpness of segregation of interfacial structures while HB determines the extent of interfacial ordering.
The IL–vapor interfacial structures of EtAN (Figure 22H) are distinct from those of EAN, PAN, and EAF ILs.364 A simple model used to fit XRR spectra revealed that electron densities increase from zero to bulk values over a distance of ∼11 Å (Figure 22J), a distance of approximately twice the EtAN ion pair dimension. Polar head and tail moieties in EtA cations are internalized to form, along with [NO3] anions, the interior of the surface aggregates, which are coated by methyl moieties in contact with the vapor phase. However, surface orientations of alkyl groups in EtA cations are quite different from those of EA cations due to a less amphiphilic character of EtA than EA.119 This is a clear illustration of how designer properties of ILs can be exploited to control interfacial nanostructures. If an (relatively) unstructured interface is desired, it is better to incorporate polar functional groups into alkyl chains to disrupt solvophobic interactions. This prevents the formation of a layered subsurface and facilitates a rapid adsorption and transport of species from the vapor phase through the interfacial region into bulk liquids, desirable for applications such as CO2 absorption.
For ILs consisting of pyrrolidinium cations coupled with [NTF2] anions, experimental features observed in SFG and XPS spectra resemble those for imidazolium ILs with alkyl chains projecting into the vapor phase.652,653 Pyrrolidinium rings appear to be parallel to the surface plane with alkyl chains taking tilted configurations relative to the surface normal direction. Atomistic simulations showed that [C4MPYRR][CF3SO3], [C4MPYRR][NTF2], and [C4MPYRR][FAP] ILs exhibit distinct segregation of polar and apolar domains in the IL–vapor interfacial region, where liquid morphologies of apolar domains are independent of anion types.654 The presence of a discontinuity in local density leads to a small charge segregation at the IL–vapor interface, which is less substantial for [C4MPYRR][FAP] as [FAP] anions are voluminous and motivate a lack of interfacial ordering at the IL–vapor interface.
5.2. IL–Carbon Interface
Carbon materials span a wide range of active porous carbons with various ordering structures including C60,655 onionlike carbons (OLCs),181,656,657 carbon nanotubes (CNTs),658−660 graphene/graphite,661−663 and 3D ordered mesoporous carbon matrixes.664−666 Carbon materials have distinct benefits, such as high hydrophobic surface areas, large pore volumes, and good thermal and mechanical stabilities and thus exhibit remarkable potential as promising materials for confining ILs. Interest in the IL–carbon interface has been fueled by the desire to understand the solvation structures of C60, CNTs, graphene/graphite, and their derivatives in ILs659,667,668 as well as applications of ILs in supercapacitors, where ILs and carbon materials are used as solvent electrolytes and electrodes, respectively.669,670
5.2.1. IL–OLC Interface
Formed by vacuum annealing of nanodiamond powder, OLCs can be viewed as concentric graphene shells, and their surface is fully accessible for ion accumulation.671 Therefore, OLCs are novel electrode materials for supercapacitors.672 It was found that supercapacitors with OLC as an electrode and [N2,2,2,2][BF4]-PC (propylene carbonate) as an electrolyte have high energy density and ultrahigh power density.672 Furthermore, EDL capacitance increases as OLC becomes smaller.656 To understand the underlying physical origin of these striking phenomena, Cummings and co-workers performed intensive atomistic simulations to study interfacial structures of ILs near idealized spherical OLCs.181,673 An almost linear relationship of surface charge density versus electrode potential applied over a large voltage range was found for EDLs of ILs near OLC electrodes,181 leading to a differential capacitance versus electrode potential curve that is nearly flat (Figure 23A). These results are in contrast to the observed U-, bell-, and camel-shaped curves observed in many theoretical, computational, and experimental studies of ILs on planar electrodes. The flat capacitance–potential curve is ascribed to an almost constant charge overscreening behavior, and the normalized capacitance of EDLs on OLCs increases with curvatures of OLCs and decreases with sizes of OLCs, which is in good agreement with available experimental measurements.672
Figure 23.
(A) Effect of electrode curvature on differential capacitance of ILs on OLCs and planar electrodes. R is the radius of an OLC, and it is infinity for a planar electrode. Reproduced with permission from ref (181). Copyright 2012 American Chemical Society. (B) Differential capacitance as a function of applied electric potential for pure [C6(MIM)2][NTF2]2, [C6(MIM)2](NTF2)2–ACN, and [C6(MIM)2](NTF2)2–PC mixtures with an IL concentration of 5%. Reproduced with permission from ref (674). Copyright 2014 IOP Publishing.
In addition, ILs consisting of double-headed imidazolium cations show distinct interfacial structures and enhanced capacitive performance over monovalent counterparts near OLC electrodes.181,673 A high concentration of anions is accumulated near the OLC electrodes to neutralize the charge of the double-headed cations. For [Cn(MIM)2][BF4]2 ILs, the average capacitance at the OLC electrode characterized by positive charges is higher than that at the OLC electrode characterized by negative charges, which is mainly attributed to the smaller size of [BF4] anions than [Cn(MIM)2] cations. In the latter case, differential capacitance decreases with increasing cation alkyl chain length. In order to facilitate the usage of double-headed imidazolium IL electrolytes in supercapacitors without compromising their power density, organic solvents such as PC and ACN are always used to increase ion conductivities, charging, and discharging rates (Figure 23B).674 However, the reason for the underlying enhanced functionalities of the IL–solvent mixtures and the charging/discharging mechanisms are yet to be understood. This is an important direction of research for future electrochemical applications.
5.2.2. IL–CNT Interface
The confinement of ILs within CNT materials is of particular significance due to their fascinating surface structures. The initially empty CNTs can be filled spontaneously with ILs to reach a saturated state despite of their hydrophobic feature. The spontaneous diffusion of ILs into CNTs has been described with atomistic simulations.658,675−677 The cations of [C4MIM][PF6] are always faster than anions to enter CNTs in the filling process owing to favorable dispersion interactions of alkyl groups with CNT surface and preferential electronic interactions of imidazolium rings with CNT π-electrons.658 Atomistic simulations showed that a single [C4MIM] cation can enter a CNT (9,9) from the bulk liquid region driven by a favorable free energy of −27 kJ/mol, whereas the corresponding value for a single [PF6] anion to enter this CNT is approximately 32 kJ/mol, indicating that it is very difficult for [PF6] anions to spontaneously enter this CNT.658 However, for a [C4MIM][PF6] ion pair, the obtained free energy value is around −27.6 kJ/mol, indicating that it is energetically favorable for ion pairs to enter CNTs. A hypothesis that a cation “pulls” an anion into CNT channels from bulk IL matrix gives a reasonable explanation for the insertion of [C4MIM][PF6] into CNTs. Furthermore, CNTs with larger diameters can accommodate more ions and provide faster filling speed for ions entering CNTs.658 It is noteworthy that the smallest diameter of CNTs capable of being filled by common ILs is around 0.95 nm, as revealed from atomistic simulations677 and experiments.678,679 This value is consistent with that determined from theoretical calculations considering the vdW radii of atoms constituting ions and sp2 carbon atoms of CNTs.679,680 Below this threshold, ILs do not spontaneously enter CNTs under normal conditions.
In addition to solvation of ILs inside CNTs, ILs are also good solvents to maintain dispersion of CNTs.659,667,668 Weak vdW interactions between ILs and CNTs guide enthalpy alteration upon CNT solvation and increase monotonically with CNT diameters. Therefore, solvation of CNTs in ILs is strongly prohibited entropically. Functionalization of CNTs with hydrophilic groups is definitely helpful to enhance interactions of CNTs with ILs. At the macroscopic level, IL–CNT mixtures exhibit varied rheological and viscoelastic features depending on temperatures, concentrations, and types of CNTs dissolved in ILs.681 Addition of multiwalled CNTs in the dilute regime of mixtures provokes a decrease in liquid viscosity at high flow fields, but concentrated dispersions of CNTs in ILs always have a high viscosity in comparison with that for pure ILs. Aligned multiwalled CNTs have a more pronounced effect on liquid viscosities than nonaligned CNTs in ILs.
The confinement of ILs in CNTs leads to anomalous variations of the ILs’ phase behavior from the liquid phase to high-melting-point crystallites, which is attributed to ordered arrangements of ions inside CNTs.678,682,683 The freezing behavior of ILs in CNTs is a nonequilibrium process that is fully suppressed in bulk liquids and only occurs in nanoconfinement or at very low temperatures.682 Conversely, the presence of electrical charges at the IL–solid interface and changes in the local cation–anion ratio have a significant effect on a thawing thermal behavior.684 For example, a downshift of melting point of confined [C3MPYRR][NTF2] is attributed to high ion concentrations and nonstoichiometric local ion arrangements in the interfacial region impeding the formation of extended ionic liquid crystal structures. Both freezing and thawing phase transitions of ILs in CNTs are strongly influenced by geometric constraints and strong interactions of ILs with CNT walls.685 The interior radius and chiralities of CNTs and doping atoms in CNTs686 can affect the melting temperatures of confined ILs.687,688 ILs inside smaller CNTs are more stable than those in larger CNTs, which is attributed to enhanced interactions of ILs with CNT walls. In addition, ILs inside zigzag CNTs have lower energy and are more stable than those in armchair configurations and therefore have higher melting temperatures. Furthermore, the glass transition temperature of [C2MIM][PF6] encapsulated in a zigzag CNT is further increased when nitrogen atoms are doped into CNTs.686
ILs confined in CNTs are expected to display distinct microstructural characteristics that are not present in the bulk liquid phase. ILs exhibit diverse solvation structures in CNTs, resulting in heterogeneous density distribution along the radial55,182,183,675,677,688−692 and axial directions of CNTs.658,688−690 Atomistic simulations showed that internal solvation structures of [C2MIM][BF4] in CNTs are described by a single file distribution of ion species in CNT (7,7) (Figure 24A), zigzag distributions of cation–anion structures in CNT (8,8) (Figure 24B), chiral ion distributions in CNT (10,10) (Figure 24C), disordered ion structures in CNT (12,12) (Figure 24D), staggered pentagonal ion structures with alternating cation and anion layers in CNT (15,15) (Figure 24E), and staggered octagonal ion structures with disordered ion configurations in CNT (20,20) (Figure 24F).677 Additionally, for [C4MIM][PF6] confined in CNTs, the number of ion layers increases from two to four with an increase of CNT diameters from 2.0 to 3.7 nm (Figure 24G–J).689 The preferential ion structures inside CNTs are strongly associated with pore loading of [C4MIM][PF6] inside CNTs.675,689,693 An increase in pore loading results in a concomitant increase in mass density distributions near the central regions of CNTs. Imidazolium cations prefer to align along CNTs and exhibit dense packing structures in the IL–CNT interfacial region compared to other layers farther from CNTs.686 This behavior is attributed to hydrophobic interactions of CNTs with cation alkyl chains and π–π stacking interactions between CNTs and imidazolium rings, whereas anions prefer to locate in the vicinity of imidazolium rings due to preferential intermolecular interactions.675,677,689,693
Figure 24.
Snapshots of IL species in the first internal solvation shell of a CNT. Red and green spheres represent locations of center-of-mass of [C2MIM] and [BF4], respectively. (A) CNT (7,7), (B) CNT (8,8), (C) CNT (10,10), (D) CNT (12,12), (E) CNT (15,15), and (F) CNT (20,20). Reproduced with permission from ref (677). Copyright 2009 American Chemical Society. Snapshots of [C4MIM][PF6] confined inside multiwalled CNTs with diameters of (G) 2.0 nm, (H) 2.5 nm, (I) 3.0 nm, and (J) 3.7 nm. Reproduced with permission from ref (689). Copyright 2010 American Chemical Society.
CNT diameters and pore loading of ILs inside CNTs have a remarkable influence on dynamic quantities of ILs, such as diffusion and ion conductivity.676,680,686,688,694−697 An increase in diffusion coefficients, as observed for [C8MIM][BF4] in membranes consisting of vertically aligned CNTs (CNT forests consisting of multiwalled CNTs with 2 or 3 walls and with a 4 nm internal diameter),697 is likely due to a frustration of self-organization of [C8MIM][BF4] on the nanoscopic scale. Ohba et al.680,693 specified that a destruction of HB and electrostatically driven polar networks in [C2MIM]Cl and frictionless movement of ions along CNT walls contribute to their enhanced mobilities inside CNTs. The hydrophobicity and HB structures of confined ILs in CNTs are assumed to play major roles, because some hydrophobic ILs, such as [C4MIM][PF6]689 and [C4MIM][NTF2] ILs,694 characterized by hydrophobic features exhibit slow dynamics when they are confined within CNTs. Translational dynamics of confined IL ions are highly heterogeneous and depend on their relative distance from CNT walls.678,698 Additionally, ion and electric conductivities of ILs increase with increasing temperatures87 and decrease with lengthening imidazolium cation alkyl chains.675
Besides imidazolium ILs that are widely studied via experimental and computational characterizations, other ILs consisting of nonaromatic cations, such as EAN and its derivatives, can also form varied ion pair structures inside CNTs.660,699 EAN exhibits peculiar, well-defined ion pair structures inside CNTs having various diameters660 and has cylindrical double-shell solvation structures around the CNTs regardless of the CNT diameters.699 In the first solvation shell, methyl groups in EA cations are closer to CNT walls than the amine groups, while [NO3] anions tend to be in contact with CNT walls with three oxygen atoms facing bulk liquids. In addition, asymmetric stretching intensities of C–H in EA cations and N–O in [NO3] anions at the EAN–CNT interface are slightly higher than those in bulk liquids owing to enhanced accumulation and significant orientation of cations and anions in interfacial regions.
5.2.3. IL–Graphene (Graphite) Interface
Besides CNTs, graphene and graphite provide another attractive confinement environments for ILs. Functional performance of ILs in IL–graphene systems, either in electrochemical devices or in lubrication, is strongly correlated with their wetting states on graphene surface.700,701 Experimental characterizations showed that diverse wetting states of liquid droplets on graphene can be achieved by changing the thickness of graphene layers on substrates and by careful manipulation of IL–substrate interactions via a judicious selection of cations and anion species.702,703
SFG spectra for [C4MIM][C1SO4] on a graphene surface showed that methyl groups of [C1SO4] anions align greater than 40° from the surface normal direction, while [C4MIM] cations exhibit a weak parallel alignment along the graphene surface, leading to a wetting state with a contact angle of 58 ± 2°.101,704 Another SFG measurement of [C4MIM][N(CN)2] demonstrated that only [N(CN)2] anions were aggregated on the bare barium fluoride substrate, whereas both [C4MIM] cations and [N(CN)2] anions were detected on barium fluoride substrate coated with graphene, which is accompanied by an increase of the contact angle from 57° for IL on bare barium fluoride substrate to 69° for IL on substrate covered by a single graphene layer. In addition, [C4MIM][N(CN)2] forms ordered interfacial layers at different potentials, but concentrations and orientations of butyl chains exhibit negligible changes as the electrode potential varies.705 More physical insights obtained from various experimental studies lay a solid foundation for a comprehensive understanding of interfacial IL structures on the electrified graphene surface and their utilities in specific applications.130,663,706−711
DFT calculations provided detailed microstructures of ILs at varied wetting states on the graphene surface.146,147,274,712,713 Imidazolium cations interact more strongly with neutral graphene than anions due to π–π stacking interactions between imidazolium ring planes and solvophobic associations of alkyl chains with graphene.274,709,714,715 Imidazolium cations have a small band gap in comparison with pyrrolidinium analogues when they are paired with Br and [BF4] anions and a larger band gap when they are coupled with [PF6] and [NTF2] anions. These anions interact with graphene via different π type interactions.145,714 Binding energies of representative ILs on neutral graphene surface follow an order of [C4MIM][PF6] (−15.01 kcal/mol) > [C4MIM][N(CN)2] (−14.38 kcal/mol) > [N1,1,1,4][N(CN)2] (−13.53 kcal/mol) > [C4MIM][BF4] (−13.04 kcal/mol) > [N1,1,1,4][PF6] (−12.88 kcal/mol) > [N1,1,1,4][NTF2] (−11.69 kcal/mol) > [C4MIM][NTF2] (−10.53 kcal/mol) > [N1,1,1,4][BF4] (−9.61 kcal/mol).712,714,715 In addition, anions have stronger coordinations than cations with positively charged graphene surface. Interactions between halides and graphene are even stronger than those for molecular anions due to preferential and cooperative anion···π interactions.715 However, at negatively charged graphene surface, cation alkyl chains prefer to align “epitaxially” along graphene lattice, which induces quasi-crystallization of (imidazolium) cations on graphene. Besides DFT calculations, atomistic and CG simulations showed that wetting graphene with ILs is essentially related to IL droplet size,703 interaction strength between graphene and ILs,716 coating materials,703 temperatures,710,716 and external electric fields.717,718 The interfacial energy of solid–liquid interface is a good indicator to describe affinity of ILs to graphene.719 Imidazolium cations functionalized with benzyl groups have low interfacial energies due to favorable interactions between ring moieties and graphene and thus can easily wet the graphene surface. Imidazolium cations having long alkyl chains are characterized by low interfacial energies in comparison with those having short alkyl chains, but their affinities to graphene surface are weaker than that of pyrrolidinium analogues.
ILs confined in neutral graphene slits possess symmetric layering structures between graphene walls and exhibit distinct microstructural720−725 and dynamical heterogeneities.588,645,648,695,710,720,722,723,726,727 Ions in interfacial layers close to graphene walls exhibit slower dynamics than those in the central region of confined IL films. If graphene walls have a large slit width, ions in the central region of confined IL films usually have similar dynamical quantities and comparable relaxation times to those in bulk liquids without confinement. In addition, diffusions of ions in directions perpendicular to the pore walls are significantly slower than those along the pore walls as ions must traverse dense ion layers in the perpendicular direction.695,722,723,728
Heterogeneous microstructures and dynamical quantities of ILs in the IL–graphene interfacial region are essentially correlated with their functionalities in applications, such as in electrochemical devices20,133,729−735 and in lubrication.736−738 Yan and co-workers performed intensive atomistic simulations studying effects of anion structures,739,740 temperatures,741 and specific interactions of ions with graphene742,743 on EDL structures and capacitive functionalities of imidazolium ILs confined between graphene electrodes. On one side, specific adsorption of imidazolium cations on the graphene electrode causes a positive potential of zero charge (PZC) on a positively charged electrode and a depression of capacitance at positive polarization.742 On the other side, adsorption of imidazolium cations effectively lowers the surface charge and electrode potential on the negatively charged graphene electrode and thus raises capacitance at negative polarization.742 For specifically absorbed ions, such as [C1MIM], variations in the electrode potentials near PZC do not affect the cation absorption structures to a significant extent but alter the anion layer next to the absorbed cation interfacial layer.742 Consequently, the local minimum of camel-shaped differential capacitance curve, though commonly observed in experiments and simulations, may not be related to PZC, whereas interfacial co-ion structures next to absorbed counterions may contribute to a striking relationship between differential capacitances and applied potentials by altering an effective EDL thickness.
For [C3MPYRR] ILs near a graphite electrode, cations exhibit perpendicular orientations at potentials near the PZC, and anion species in the first interfacial layer exhibit increased parallel orientation to the electrode with a gradual increase in applied electrode potentials.744,745 The asymmetry of the capacitance–electrode polarity curve for [C3MPYRR][NTF2] is attributed to strong interactions of fluorine atoms in [NTF2] anions with a graphite electrode, with the relatively large footprint of [NTF2] anions in comparison with [C3MPYRR] cations and the tendency of C3 chains in [C3MPYRR] cations to reside in the IL–graphite interfacial region even at high positive potentials (Figure 25A). Replacing [NTF2] with [FSI] does not lead to a significant increase in differential capacitance near a positively charged graphite electrode,746 whereas a 30% higher differential capacitance is observed on a negatively charged graphite electrode for [C3MPYRR][FSI] in comparison with [C3MPYRR][NTF2] (Figure 25A).744 This behavior is correlated with two microstructural characteristics of EDL: (a) a closer approach of [FSI] to the electrode surface than [NTF2] (Figure 25B) and (b) a faster anion desorption rate (vs potential decrease) for [FSI] from the electrode surface than [NTF2]. An observed decrease in capacitance and a disappearance of the minimum in the capacitance–potential curve near PZC with increasing temperatures are most probably attributed to an enhanced crowding effect of ions in IL–graphite interfacial layers.
Figure 25.
(A) Differential capacitance versus electrode potential for [C3MPYRR][NTF2] and [C3MPYRR][FSI] ILs. (B) Representative snapshots of interfacial ion orientations for [C3MPYRR][NTF2] and [C3MPYRR][FSI] ILs within 8 Å from the electrode surface (green vertical lines). Reproduced with permission from ref (746). Copyright 2011 American Chemical Society. (C) Differential capacitance versus EDL potential for varied electrode surfaces. Inserted image snapshots indicate electrolyte structures near flat (left) and rough (right) surfaces at ∼2.5 V. Reproduced with permission from ref (747). Copyright 2011 American Chemical Society. (D) Comparison of absolute values of capacitances obtained from simulations in atomically flat and rough slit pores as a function of pore dimensions for [C2MIM][NTF2] IL. Reproduced with permission from ref (745). Copyright 2015 American Chemical Society.
Further atomistic simulations indicated that the atomic level roughness of the graphene electrode can qualitatively change the dependence of capacitance on the applied potentials.747,748 ILs on an atomically flat basal plane of the graphite surface exhibit camel-shaped differential capacitance curves, whereas those on an atomically corrugated prismatic face of the graphite surface have large differential capacitances characterized by bell shapes at low double-layer potentials (Figure 25C,D). Both bell-shaped and camel-shaped capacitance–potential curves are correlated with variations of the EDL structures as a function of the applied potential due to a strong dependence of electrolyte packing structures on the surface and a large difference in the intermolecular potential energies of electrolyte ions near the flat and rough graphene surfaces. In addition, ILs on an atomically flat graphene electrode exhibit a slight decrease in capacitance with increasing temperatures due to thicker EDL structures,739,749 whereas the influence of temperatures on differential capacitance is more pronounced for ILs confined on atomically corrugated graphene surface with ion dimension comparable to the size of corrugation pattern on graphene surface. Therefore, interpretation of experimental capacitance data should take detailed knowledge of the electrode topography and roughness into consideration, and it should go beyond the typical route of considering electrolyte behavior near a flat surface. These computational studies further indicate that controlling the electrode surface roughness might be an effective route to improve energy densities of EDL capacitors.
Addition of ACN into imidazolium and tetraalkylphosphonium ILs improves ion conductivities and thus enhances power and energy densities of supercapacitors as ACN facilitates dissociation of cation–anion pairs in EDL.674,750,751 However, addition of PC into imidazolium ILs results in distinct changes in interfacial ion structures and capacitances of supercapacitors. PC molecules exhibit outstanding co-ion expulsion capabilities due to their strong absorption onto the graphite surface in contrast to ACN. In addition, doping inorganic salts (Li+,752−754 K+,753 Mg2+,752 Ca2+,755 and Al3+755) and molecular solvents (water,754,756 methanol,757 ethanol757) in ILs and generating vacancy sites on the graphene surface757,758 leads to significant variations in interfacial structures and dynamics of confined ions in comparison with those in bulk liquids and thereafter variations in capacitance–potential curves.
A significant experimental observation showed that the energy density of supercapacitors can be significantly increased by confining ILs in the graphene slits with pore widths at the subnanometer scale, but this reduces their power density and compromises the key advantage of supercapacitors.728,745,759−766 Molecular simulations using a phenomenological model revealed that charging ionophilic pores with pore widths comparable to ion diameters is a complex process (Figure 26A).759 It is adsorption-driven at high potentials but depend on the ion concentrations, and it is dominated either by co-ion desorption or by adsorption of both types of ions at low potentials (Figure 26B). Diffusivities of ions in subnanometer pores exhibit a strong dependence on ion densities and local chemical compositions (Figure 26C) during charging over a few orders of magnitude and can exceed a few times the bulk liquid ion diffusion under similar conditions.759,767 A fast demixing transition inside narrow pores due to an instant expulsion of co-ions from the pores above a certain electrode potential threshold and the ability of conductive pores to maintain elevated densities of counterions after demixing lead to distinct capacitance enhancement.728,760 During cyclic charging and discharging, a nanopore system is driven far from the equilibrium state. Indeed, the internal state of nanopores, in particular, total ion densities, not only deviates from those under quasi-static charging and discharging conditions but also varies greatly as the scan rate changes.
Figure 26.
(A) Side-view snapshot of simulation system for charging narrow electrode pores with ILs, and top-view perspectives of ion species inside a negatively charged electrode pore when a voltage of 3 V is applied between electrodes. Blue and orange spheres represent cations and anions, respectively. (B) A plot showing averaged total and charge densities during instant charging (blue solid line) and in equilibrium conditions (black dash-dot-dot line). Green dash-dot line corresponds to an ionophobic pore. (C) Normalized diffusion coefficients of cations along an equilibrium path and along charge densities corresponding to instant charging at 3 V. Reproduced with permission from ref (759). Copyright 2014 Nature Publishing Group. (D) EDLC simulation cell consists of CG [C4MIM][PF6] electrolyte confined between two porous electrodes. The same configuration at each potential is shown twice: ion distribution (left) and degree of charging of electrode atoms (right), in which carbon atoms are color coded according to partial charge q they carry (green, q < 0; yellow, q ≈ 0; and red, q > 0). Reproduced with permission from ref (765). Copyright 2012 Nature Publishing Group. Adsorption of ion species in a nanoporous carbon electrode at null potential, and distribution of degree of confinement experienced by ion species in (E) pure [C4MIM][PF6] and in (F) [C4MIM][PF6]–ACN mixtures. (G) Representative configurations of ion species for their four adsorption modes (gray rods for C–C bonds, gray spheres for carbon atoms that are in a coordination sphere of central molecules, red for [C4MIM] cations, green for [PF6] anions, and blue for ACN molecules, respectively.). Reproduced with permission from ref (766). Copyright 2013 Nature Publishing Group.
For supercapacitors, the charge storage mechanism is a complicated process intrinsically correlated with multiple factors such as relative ratios of pore sizes over ion dimensions669 and desolvation effects of ILs in confined environments.670 For negative polarization, the adsorption of cations onto solid substrates dominates, whereas for positive polarization, charging proceeds by an ion exchange effect with anions replacing cations in the interfacial regions. Experimental measurements using NMR and in situ electrochemical quartz crystal microbalance indicated that adsorbed ions are partially solvated.768 Atomistic simulations demonstrated that charge screening,20,761,769 ion rearrangement and confinement (Figure 26D),765,766 and pore surface properties21,733,759,770−773 have significant effects on charging dynamics and differential capacitances. ILs absorbed onto solid electrodes exhibit varied configurations and have striking coordinations with different adsorption sites like edge sites (concave curvature), plane sites (graphene sheetlike structure), hollow sites (convex curvature), and pocket sites (inside a subnanometer carbon pore) (Figure 26E–G).766 These different adsorption sites have distinct effects on power performance of electrochemical devices.774
It should be noted that molecular dynamics of ions near electrified nanoporous electrode are described by multiple time scales arising from solvation, electrosorption, and ion confinement effects.775 Diffusivities of confined ions are always slower than those of bulk electrolytes due to the confinement effect and strong electrostatic attractions with pore walls when a potential is applied, which significantly hinder their utilizations as electrolytes for ensuring fast charging supercapacitors.776 Therefore, molecular simulations with effective prescreening methods will be an economical procedure for selection and design appropriate IL candidates with enhanced properties to meet specific application requirements.777,778
5.2.4. IL–3D Mesoporous Carbon Interface
In addition to cylindrical CNTs and graphene slits, confinement of ILs within 3D carbon materials having complicated pore sizes and pore geometries, such as CMK-3664 and CMK-5,665,666 was also investigated via atomistic simulations. These CMKs are amorphous carbon materials characterized as interconnected mesoporous but with different pore size, shape, and surface roughness, which have a significant effect on microstructural and dynamical quantities of confined ILs. Large spatial structural heterogeneities of confined ILs are observed in the axial direction of CMK-3 at a pore loading below bulk IL density, suggesting that ions tend to cluster together to create domains with similar local densities as those of bulk liquids (Figure 27A,C).664 For the same ILs confined within CMK-5, ILs adsorbed at the outer surface of carbon nanopipes have a dramatic effect on density distributions of ILs confined inside carbon nanopipes (Figure 27B,C).665,666 Ions are more closer to carbon walls in CMK-5 than those in the CMK-3 matrix due to preferential interactions of IL ions inside CMK-5 nanopipes with those outside CMK-5 nanopipes. The surface curvature and mesoporous arrangement in CMK-3 and CMK-5 result in different confinement effects on dynamical properties of ILs. ILs adsorbed outside CMK-5 nanopipes have faster dynamics than those outside CMK-3 having a similar pore size.664 Exterior ion absorption can affect dynamics of ions inside nanopipes, but this effect is IL-specific. It should be noted that all confined ions inside CMK carbon materials exhibit slower translational and rotational dynamics compared to those in bulk IL matrixes.664,666
Figure 27.
Density profiles of [C2MIM][NTF2] IL inside (A) CMK-3 and (B) CMK-5 carbon materials. Areas with high cation densities are depicted in dark shades of gray. (C) Density distributions of [C2MIM] cations and [NTF2] anions outside CMK-3 nanorods and inside/outside CMK-5 nanopipes along the direction indicated by solid arrows up to distances indicated by dashed circles. Reproduced with permission from ref (666). Copyright 2016 Taylor & Francis. Representative snapshots of [C2MIM][NTF2] IL inside a coconut shell carbon material with pore sizes of (D) 0.75 nm and (E) 1.23 nm. Reproduced with permission from ref (727). Copyright 2014 American Chemical Society.
Compared with constrained microstructures of ILs within carbon matrixes having regular nanopores, it was shown that ILs exhibit bulk liquidlike microstructures inside porous carbon materials having varied pore size and pore geometry.727 [C2MIM][NTF2] prefers to form ion layers on a coconut shell carbon surface with ions parallel to surface walls.727 Different to well-defined ion layering structures in slit pores, confined ion species exhibit significant spatiotemporal heterogeneities in coconut shell carbon materials due to complex pore geometries of carbon materials (Figure 27D,E), wherein ions near pore walls move more slowly than those in central regions of the pores, similar to observations in other slit pores.722,725 Furthermore, a gradual increase in averaged pore size in carbon materials leads to small and nonmonotonic variations in ion transport properties which, in turn, leads to a nonuniform and weakened confinement effect of irregular carbon materials compared with those with regular pore geometries.
5.3. IL–Metal Interface
5.3.1. IL–Hg Interface
Mercury provides a very smooth liquid surface which enables one to carry out XRR experiments at sub-Angstrom resolution, as demonstrated by investigations of a mercury-supported [C4MPYRR][FAP] IL Langmuir film in both the lateral and longitudinal directions.122 At a low surface coverage (90 Å2/molecule), a monolayer ion pair structure is formed at the IL–Hg interface, whereas a bilayer surface-parallel ion pair structure is formed at a high surface coverage (50 Å2/molecule) (Figure 28A). Surface-parallel ion pairs self-organize into stripes, exhibiting 1D, smecticlike order, implying a checkerboard-like tilted ion surface structure. This checkerboard pattern at the IL–Hg interface has similarities with interfacial structures of Hg-supported Langmuir films of alcohols, thiols, and fatty acids.779 In these Langmuir films, full 2D lateral ordering structures eventually emerge upon increasing cation alkyl chain lengths, irrespective of irregular ion shapes and strong Coulombic interactions between constituent ions. However, XRR spectra for [CnMIM][NTF2] ILs demonstrated that IL–Hg interfacial structures are highly disordered, and diffuse surface-normal electron density profiles exhibit gradual Hg penetration into IL films and surface-normal structural evolution over a period of hours.780 Unlike localized charges in pyrrolidinium cations, charge delocalizations in imidazolium cations and [NTF2] anions increase their affinities to Hg atoms, leading to a lateral segregation of ions into polar and apolar domains at the IL–Hg interface with residual Hg in apolar domains.
Figure 28.
(A) Measured (symbols) and Lorentzian-plus-linear background fitted (line) diffraction peak for [C4MPYRR][FAP] on the Hg surface at a surface coverage of 50 Å2/molecule. Reproduced with permission from ref (122). Copyright 2011 American Physical Society. STM images of [C4MPYRR][NTF2] on Ag(111) surface recorded at 100 K. (B) Image at submonolayer coverage exhibits four Ag terraces. (C) At monolayer coverage, large Ag terrace is covered by an ordered 2D crystalline phase, while small Ag terraces are occupied by a disordered 2D glass phase. Reproduced with permission from ref (781). Copyright 2013 American Chemical Society.
In electrochemical applications of the IL–Hg interface, a thorough understanding of the dependence of differential capacitance and charging curves of the IL EDL structures on constituent ions is important.782,783 Costa et al. found that interactions of positively charged imidazolium rings with the Hg surface gradually diminish with lengthening cation alkyl chains due to preferential hydrophobic interactions of alkyl chains with the Hg surface. Variations in cation alkyl chains lead to remarkable changes in capacitance–potential curves for [CnMIM][NTF2] ILs (n = 2, 4, and 6) on Hg electrode.430,782,783 In addition, differential capacitance of ILs increases with increasing temperatures.784 Both noncoincidence and positive temperature coefficients of PZC are distinct to predictions of classical Gouy–Chapman theory, which is suitable for description of phase behaviors of dilute aqueous electrolytes.785,786
5.3.2. IL–Au Interface
Potential-induced surface reconstruction of single-crystalline Au electrode is always an important issue in electrochemical surface science. Concentrated solvent electrolytes (ILs) interact strongly with Au atoms and destroy the long-range order of a single-crystal Au surface at certain potentials.787−790 STM characterizations of [C4MPYRR][NTF2] and [CnMIM] ILs on Au(hkl) substrates demonstrated that Au(111) and Au(100) can be reconstructed into the usual surface structures at sufficiently negative potentials,134,791 indicating that solvent adsorption of ILs has a remarkable influence on electrode processes compared to aqueous electrolytes.
A comprehensive investigation of adsorption of ILs on an unreconstructed Au(100) surface in a wide potential range revealed that [C4MIM][PF6] exhibits a 2D phase transition upon cathodic excursion.792 For [C4MIM][BF4], ordered adsorption of [BF4] anions occurs at the anodic side of the bell-shaped capacitance maximum, while adsorption of [C4MIM] cations is on the cathodic side of the capacitance maximum and follows a potential-promoted disorder–order transition.134 However, [C2MIM][NTF2] and [C8MIM][NTF2] ILs absorb onto the Au(111) surface and form a 2D liquid phase at ambient temperature, whereas they condense into 2D islands with short-range ordering glasslike structures at lower temperatures.793 These findings indicate that (1) the formation of IL–Au interfacial structures is dominated by a tendency to optimize anion adsorption geometries and (2) cation alkyl chains prefer standing upright rather than lying flat along the Au surface.134,793−801 A close similarity was found in the adlayer structures of [C4MPYRR][NTF2] on the Au(111) surface.795,796 At room temperature, the growth of 2D IL film occurs up to one monolayer coverage, with both anions and cations in direct contact with the Au substrate. At lower temperatures, ion mobilities in the IL–Au interfacial region are frozen. Both a 2D crystalline phase with long-range order and a disordered 2D glass state are formed. Annealing experiments revealed that the 2D crystalline phase is thermally more stable than the 2D glass state against melting, and its stability is strongly influenced by coverage of adsorbates on the underlying Au(111) surface.795,796
Atkin and co-workers elucidated interfacial structures of various ILs on charged Au electrodes via AFM experiments.802,803 At null potential, interfacial behaviors of ILs are consistent with a discontinuous sliding process. This effect is less pronounced when a positive or negative potential is applied. ILs consisting of imidazolium cations coupled with [FAP] anions exhibit similar lubrication behavior: a high friction at positive potentials and a low friction at negative potentials, suggesting that imidazolium cations are more lubricating than [FAP] anions.802,804−807 The lateral force varies as a function of applied potential and ion structures because of changes in interfacial compositions of confined ion layers from anion-enriched (at positive potentials) to mixed (at null potential) and then to cation-enriched (at negative potentials) structures (Figure 29A). The length of alkyl chains in imidazolium cations has a significant influence on lubricity at similar negative potentials. C6 chains in [C6MIM] cations produce a well-formed interfacial layer that provides a lubricating sliding plane (left column in Figure 29D), whereas C4 chains in [C4MIM] cations lead to a less defined interfacial layer and reduced lubricity (left column in Figure 29C). When the cation alkyl chain length is further reduced to C2, imidazolium rings in [C2MIM] cations orientate parallel to the Au surface, and lubricity is increased relative to [C4MIM] cations (left column in Figure 29B). However, when interfacial layers are [FAP] enriched, imidazolium cations become irrelevant and a same friction coefficient is obtained at positive potentials independent of cation alkyl chain length (right columns in Figure 29B–D).
Figure 29.
(A) Lateral force against normal load for different surface potentials for [C4MPYRR][FAP] confined between the Au(111) electrode surface and a silica colloid probe. Reproduced with permission from ref (805). Copyright 2012 American Physical Society. Typical force versus apparent separation distance for the silica colloid probe approaching the Au (111) surface in (B) [C2MIM][FAP], (C) [C4MIM][FAP], and (D) [C6MIM][FAP]. Left column, ILs at negative potentials (−2.0 V for [C2MIM][FAP], −1.0 V for [C4MIM][FAP], and −2.0 V for [C6MIM][FAP]). Middle column, ILs at null potential. Right column, ILs at positive potentials (1.5 V for [C2MIM][FAP], 1.0 V for [C4MIM][FAP], and 1.5 V for [C6MIM][FAP]). Reproduced with permission from ref (803). Copyright 2013 Royal Society of Chemistry.
A combined AFM-STM investigation provides a comprehensive understanding of EDL structures in the IL–Au interfacial region.133,808 Both [C2MIM][NTF2] and [C4MPYRR][NTF2] ILs form multiple ion pair layers (3–5 layers) on the Au(111) surface at an open circuit potential.809 The [C4MPYRR][NTF2]–Au interface appears highly structured (in part wormlike), while the [C2MIM][NTF2]–Au interface exhibits weak interfacial structuring. Therefore, it requires a large force to rupture the innermost IL solvation layer for [C4MPYRR][NTF2] in comparison with that for [C2MIM][NTF2]. This remarkable difference is ascribed to stronger interactions of [C4MPYRR] cations than [C2MIM] cations with the Au(111) surface. Similar experimental features were also observed for ILs consisting of [FAP] anions coupled with either imidazolium or pyrrolidinium cations.810−812
Addition of inorganic salt precursors810,813−818 or water819−821 can significantly affect the IL–Au interfacial structures. AFM investigations revealed that typical multilayered IL interfacial structures are retained only at quite low salt concentrations. IL EDL structures will be disturbed when a large amount of salt is introduced into the IL–Au interfacial region, where new EDL structures consisting of salt ions will be formed. The width of the innermost layer is dependent on salt concentrations and applied electrode potentials. For IL–water mixtures, a clear transition from “water in IL” to “IL in water” was observed with a gradual increase of water concentration in ILs.819 Above a certain water concentration, cation–anion intermolecular interactions are drastically weakened, and a transition from a multilayered interfacial structure to a classic double-layer structure occurs at specific electrode potentials. As more water molecules adsorb onto the Au surface, the thickness of the interior EDL structures increases and the thickness of the neutral exterior layers (beyond the first two layers in the IL–Au interfacial region) remain constant.820
In addition to heterogeneous microstructures in the IL–Au interfacial region, the dynamic evolution of these interfacial structures upon charging Au electrodes was studied using various experimental techniques including surface-enhanced infrared absorption spectroscopy (SEIRAS),822−824 surface plasmon resonance,825 STM,131,132 and AFM measurements.826,827 Distinct stepwise transitions were observed in the IL–Au interfacial region with increasing electrode charge density. SEIRAS measurements revealed hysteretic cation–anion exchanges and rotations of ions in the first IL–Au interfacial layer.822,823 During potential scans for ILs consisting of sufficiently large ion groups, a fast and barrierless change in the local ionic environment occurs first in the overlayers, and thereafter a slow replacement of ions with their counterions occurs in the IL–Au interfacial layers to compensate the surface charge when an overpotential exceeding a critical value is applied. These results highlight the significance of a steric hindrance effect of ion species on their replacement in the interfacial layers.826,828,829
Extensive experimental and computational investigations were performed to address effects of IL ion structures and EDL interfacial structures on differential capacitance of ILs upon charging Au electrodes.748,830−834 It was revealed that IL capacities follow an order of [C4MIM][PF6] < [C4MIM][NTF2] < [C4MIM][BF4]. Lengthening the cation alkyl chains does not obviously affect the general shape of capacitance–potential curves but influences capacitances in a systematic way due to constrained distributions of alkyl chains in the IL–Au interfacial region. In addition, enlarging anions from small spherical ones (Cl, [BF4], and [PF6] etc.) to large nonspherical and multidendate species ([NTF2], [FAP] etc.) further complicates the dependence of differential capacitances of ILs on cation alkyl chain lengths and potentials required for a transition from overscreening to overcrowding IL–Au interfacial layers due to distinct interactions between confined ions and Au atoms. It is noteworthy that an IL composed of tetramer imidazolium cations and [NTF2] anions exhibits outstanding electrochemical properties in EDL devices.835 Furthermore, an introduction of ether spacers to the imidazolium ring moieties makes these oligomers fluidic, and multivalent electrostatic interactions ensured by their oligomeric structures play a vital role in their functional performance in electrochemical devices.
5.3.3. IL–Ag Interface
Metallic silver is a catalyst in oxygen-assisted coupling reactions.836 Potential applications of Ag alloys in heterogeneous catalysis raise additional interest in understanding interactions of Ag with ILs. Research on ultrathin IL layers on the Ag substrate showed that both [CnMIM] cations and [NTF2] anions adsorb in a checkerboard arrangement with both ions in contact with the Ag(111) surface,837 similar to that observed in the IL–Au(111) interfacial region.838 Deposition of a large amount of [C1MIM][NTF2] on Ag(111) revealed an initial 3D IL film morphology.839,840 In contrast, a quasi 2D film morphology was found from the beginning of depositing [C8MIM][NTF2] on the Ag(111) substrate, indicating a remarkable effect of imidazolium cation alkyl chain length on IL film growth kinetics. In addition, STM investigations reported that [C2MIM][NTF2] and [C8MIM][NTF2] ILs exhibit similar ion mobilities at submonolayer coverage on the Ag(111) surface as that on the Au(111) substrate.793 When [C8MIM][NTF2] and [C8MIM][PF6] ILs are sequentially deposited onto the Ag(111) surface, a pronounced enrichment of [NTF2] anions was observed at the IL–vapor interface due to a rapid anion exchange at the IL–Ag interface.841 It is the larger adsorption energy and surface tension of [C8MIM][PF6] than those of [C8MIM][NTF2] that contribute to exchange of [NTF2] with [PF6] anions in the IL–Ag interfacial region.
For deposition of [C4MPYRR][NTF2] on the Ag(111) surface at room temperature followed by a gradual cool-down to ∼100 K, a coexistence of a 2D liquid phase, a disordered 2D glass phase, and an ordered 2D crystalline phase was observed (Figure 28B).781 Dynamic STM measurements at low temperatures resolved exchanges of adspecies at crystalline–liquid and disordered glass–liquid phase boundaries (Figure 28C). In addition, a dynamic equilibrium between the liquid phase and the crystalline phase is attributed to weak adsorbate–adsorbate associations and a low surface diffusion barrier. DFT calculations revealed an equal adsorption of [C4MPYRR] cations and [NTF2] anions laterally placed side by side with [NTF2] anions exhibiting cis configurations, SO2 groups binding to the Ag surface, and CF3 groups pointing toward the IL–vapor interface. ILs interact with the Ag surface through weak electrostatic (dipole induced dipole) interactions and preferential dispersion interactions, leading to a distinct electron solvation behavior of [C4MPYRR][NTF2] at the IL–Ag(111) interface.842
Besides these extensive studies of ILs confined on Hg, Au, and Ag surfaces, there are some other metals that are used as substrates to support ILs for specific applications, such as Li,843 Cu,116,799,844,845 Ti,466,846 Fe,847,848 Ni,847 Ru,849 Pd,850 Cd,851 In,852 Pt,853,854 and Bi.855 ILs exhibit varied interfacial structures and dynamical quantities in IL–metal interfacial regions depending on the delicate interplay of interactions among constituent ions and metal atoms that are in direct contact with confined ILs. These IL–metal interfacial systems have distinct electrochemical applications.
5.4. IL–Al2O3 Interface
Mezger et al. reported temperature-dependent microstructures of [FAP] ILs on a charged Al2O3 substrate using XRR spectroscopy.121 A pronounced interfacial layering structure with the innermost layer spacing of ∼8 Å was observed for [C4MPYRR][FAP] at the IL–Al2O3 interface, indicating a double-layer interfacial structure with [C4MPYRR] cations being in contact with the Al2O3 surface (Figure 30A). [FAP] anions are repelled from the negatively charged Al2O3 surface and form a second anion layer on the interfacial cation layer. Such an EDL structure is totally distinct from those of dilute aqueous electrolytes near charged substrates, owing to strong correlations between oppositely charged ions. This EDL structure leads to some apparently counterintuitive observation like charge inversion and attraction between like-charged objects.856,857 A gradual addition of PC solutes in [C4MPYRR][FAP] leads to reduced ion–ion correlations and a decreased correlation length of interfacial layer structures. At high concentrations, PC molecules accumulate laterally within the IL–Al2O3 interfacial layers and reduce Coulombic repulsions between like-charged ions.
Figure 30.
Total (black) and individual (red for cations and blue for anions) electron densities for (A) [C4MPYRR][FAP], (B) [C6MIM][FAP], and (C) [N4,4,4,4][FAP] at the IL–Al2O3 interface. Red and blue lines indicate Gaussian distributions for cations and anions contributing to their respective partial electron density profiles. The gray bar corresponds to the electron density of the Al2O3 substrate without roughness. Reproduced with permission from ref (121). Copyright 2008 American Association for the Advancement of Science.
Replacing [C4MPYRR] with [C6MIM] and [N4,4,4,4] cations leads to negligible variations in molecular layer structures at the IL–Al2O3 interface, but there are distinct changes in the interfacial electron densities (Figure 30B,C).121 However, additional experimental characterizations showed that [C4MIM][PF6] and [C4MIM][BF4] ILs exhibit different IL–Al2O3 interfacial behaviors.858 The former exhibits strong, exponentially decaying, and alternate-charge layering structures at the IL–Al2O3 interface, whereas the latter does not show interfacial layer structures but only a single dense layer at the IL–Al2O3 interface. This interfacial structural discrepancy is attributed to different correlations between constituent ions of these two ILs. In additional, preferential HB interactions between Al2O3 interfacial groups and ILs can also induce lateral ordering structures characterized by an excess of cations in the IL–Al2O3 interfacial region.121,858
Saramago and co-workers characterized the wetting properties of [C8MIM][BF4] IL films on representative solid substrates (silicon, boron-silicate glass, and aluminum) and found that only aluminum substrate is wetted and the corresponding interfacial structures are strongly dependent on time and IL ion concentrations in ethanol solution.859,860 IL films deposited from highly diluted IL in ethanol exhibit liquidlike lamellar structures, whereas IL films deposited from concentrated IL in ethanol present solidlike or solid–liquid coexistence structures.128 These interfacial structures are mainly determined by London dispersion forces between confined ion species and substrates. For [C8MIM][BF4], its reduced surface tension is well described by Guggenheim’s universal curve for simple, purely dispersive fluids. However, for [C2OH-MIM][BF4] (1-(2-hydroxyethyl)-3-methylimidazolium), there is a distinct deviation of reduced surface tension toward lower values, which is a typical behavior of molecular fluids having strong HB interactions.
SEIRAS spectra showed that adsorption of [C4MIM][NTF2] on a well-ordered Al2O3 surface is molecular and reversible.861 Strong changes in relative intensities of [NTF2] anion related vibrational bands were observed in the submonolayer region due to distinct accumulation of [NTF2] anions in the IL–Al2O3 interfacial region, indicating a pronounced interfacial orientation of [NTF2] anions. DFT calculations demonstrated that [NTF2] anions predominately adopt a cis conformation with slightly tilted orientation with respect to the IL–Al2O3 interface, preferentially interacting with interfacial atoms via SO2 groups. In addition, characteristic differences were observed in monolayer adsorption spectra of [C2MIM][TFO] on a bare Al2O3/NiAl(110) surface and that covered by Pd nanoparticles.862 [TFO] anions are less oriented on the Al2O3 surface, while they appear to stand up on the Pd nanoparticles with CF3 groups directed toward the vapor phase due to stronger intermolecular interactions of SO3 groups with Pd atoms.
5.5. IL–Silica Interface
A microporous silica matrix is a promising framework for confining ILs because of its simple synthesis procedure and nontoxicity. When ILs are confined in silica matrixes, an obvious difference is observed in melting points of ILs in comparison with those in bulk liquids. The effect of silica confinement on melting points of ILs, either increases863 or decreases,864,865 is contradictory from different reports. Most studies stated that melting endotherms of confined ILs are detected at relatively lower temperatures and sometimes disappear compared to bulk ILs, resulting in liquidlike phase behavior of ILs below their solidification temperatures.865−868 ILs consisting of [C2MIM] cations coupled with [N(CN)2], [C2SO4], [SCN], and [TFO] anions have decreased melting temperatures when they are confined within mesoporous silica monoliths, in which melting temperatures of [C2MIM][N(CN)2] and [C2MIM][TFO] ILs are depressed by approximately 14 and 8 °C, respectively.868 [C4MIM][C8SO4] shows significant changes in melting, crystallization, and glass transition temperatures in a nanoporous silica matrix.864 In addition, melting temperature depression for ILs confined within a porous silica matrix shows a linear variation with the inverse of the mean pore diameter of the microporous silica matrix.864,868 Furthermore, immobilization of imidazolium cations on the silica surface can also lead to a melting point depression for confined ILs depending on the weight proportion of immobilized ions and the loading amount of ILs on the silica surface.869 In contrast to melting temperature depression, it was shown that compressed gases play an important role in increasing melting temperatures of imidazolium ILs entrapped within mesoporous silica matrixes.863,870
These nonbulk thermodynamic phase behaviors of ILs confined in silica matrixes are essentially correlated with delicate interactions of silica surface atoms with confined ion species. The silica surface contains Si atoms, Si–O, silanol SiOH, and silane SiH2 groups.63,127,871,872 Depending on the specificity of the ion species and their relative positions in confined environments, ILs exhibit varied coordination features with these interfacial groups, as revealed from combined SFG, FT-IR spectra, and theoretical calculations.127,864,866,873,874 In general, strong charge-balancing and electrostatic interactions and preferential HB interactions between confined ion species and silica interfacial groups contribute to remarkable microstructures and distinct orientations of ILs on the silica surface.63,125,871,873,875−877 For ILs consisting of [C4MIM] cations coupled with [BF4], [PF6], [TFO], and [NTF2] anions, atomistic simulations showed that [C4MIM] cations attach exclusively onto the negatively charged silica surface covered by Si(OH)2 surface groups, with imidazolium ring planes perpendicular to the interfacial Si(OH)2 groups and butyl chains elongated above the Si(OH)2 groups along the silica surface (Figure 31).63 Anions exhibit random orientation distributions in subsequent anion layers because interactions between the adsorbed anion species and interfacial Si(OH)2 groups are partially screened.63,864,875,878,879 However, anions are particularly absorbed onto positively charged silica surface covered by silane SiH2 groups. The main axes of asymmetric [NTF2] and [TFO] anions are parallel and perpendicular to interfacial SiH2 groups, respectively (Figure 31).63 Additional simulations demonstrated that silica surface with irregularly distributed SiOH groups is less efficient in trapping anions such that confined ILs are less localized on the amorphous SiO2 surface.878 In addition, there are significant positive dipole determined short-range SiOH-anion interactions in the IL–SiO2 interfacial region, which can be perturbed by strong external fields. Therefore, an effective way to control interfacial structures of ILs on the silica surface is to regulate the concentration of interfacial hydroxyl groups via appropriate physicochemical treatments of the SiO2 surface.
Figure 31.
Representative configurations of ILs consisting of [C4MIM] cations coupled with [BF4], [PF6], [TFO], and [NTF2] anions confined on silica surfaces covered by positively charged SiH2 (top) and negatively charged Si(OH)2 (bottom) interfacial groups. Reproduced with permission from ref (63). Copyright 2014 Royal Society of Chemistry.
Owing to substantial interactions of ILs with silica surface groups, confined ions with flexible structures usually have distinct conformations near the silica surface.867,878,880−882 Even though the trans conformer is dominant for [NTF2] anions in bulk ILs, both Raman spectroscopy330,881 and atomistic simulations880 indicated that [NTF2] anions preferably adopt a cis conformation, allowing efficient packing of [NTF2] anions in the IL–SiO2 interfacial region.881 An increase in degree of confinement880 or loading amount of ILs330 into silica matrix leads to a gradual increase in the cis/trans ratio, and the cis conformer becomes dominant. In addition, conformational changes of [NTF2] anions resulting from the confinement effect lead to prominent variations in spectroscopic properties, especially fluorescence,865 FT-IR,864,867,873 and Raman vibrational spectra of anion species.330,873,881,883
Because of the distinct confinement effect and preferential distributions of ions on the silica surface, ILs exhibit remarkable interfacial layering structures.869,875,878,884 [C4MIM][NTF2] and [C4MIM][BF4] ILs confined between two silica surfaces display considerable oscillatory solvation force profiles with varied features depending on ion pair dimensions and structure-forming abilities of ILs (Figure 32A,B).884 [C4MIM][NTF2] in a confined silica matrix shows two XRD peaks, indicating that both cations and anions coexist in the interfacial layer with a checkerboard ion arrangement.125,878,885 Force–distance profiles revealed that interfacial structures of [C4MPYRR][NTF2] are much weaker than those of [C4MIM][NTF2], and addition of LiNTF2 salt can further abate interfacial IL nanostructures. Similar interfacial layering structures of ILs confined within porous silica materials were also observed in extensive atomistic simulations.125,875,877,882,886−888
Figure 32.
Normal force rescaled with the radius of the surface curvature (F/R) as a function of surface separation distance between two silica surfaces in (A) [C4MIM][BF4] and (B) [C4MIM][NTF2] ILs. Open circles and closed triangles correspond to data on approach and on retraction, respectively. Dotted lines represent vdW attractions between silica surfaces calculated from the Lifshitz theory. Solid and dashed lines correspond to stable and unstable regions, respectively, in the force profiles. Reproduced with permission from ref (884). Copyright 2010 Royal Society of Chemistry. Schematic spatial distributions of ion species between silica surfaces are shown in the insets. Reproduced with permission from ref (125). Copyright 2018 Royal Society of Chemistry. (C) Temperature dependence of ion conductivity σ0(T) (open symbols) and characteristic rate of charge transport ωc (filled symbols) for [C4MIM][BF4] in the bulk liquid region and in confined silica nanopores. Lines represent VFT fitting of the bulk data. Inset: ion conductivity σ0 versus charge transport ωc for bulk and confined [C4MIM][BF4] IL. Reproduced with permission from ref (889). Copyright 2012 Royal Society of Chemistry.
Besides distinct interfacial layering structures, dynamical quantities of confined ILs and the intrinsic response of interfacial structures to external variables, such as shear and electric fields, are important for their usage in lubrication and electrochemical applications. Studies have been mainly focused on addressing translational873,875,878,879,883,890,891 and rotational dynamics,876,890 shear viscosities,880,892 ion and thermal conductivities,877,889 and tribological884,892 and dielectric relaxations869 of ILs in confined environments. Unlike ILs in bulk liquids, dynamics of confined ILs, either increase882,893 or decrease873,877,880,894 with different trends and extents, depend on the delicate interplay of interactions among constituent ions and interfacial groups and the loading fraction of ILs in confined environments.330,877,880,886 Jacob et al. measured self-diffusion coefficients of [C6MIM][PF6] and [C4MIM][BF4] ILs confined within micro- and mesoporous silica membranes.889,895 NMR data exhibit a distinct temperature dependence of translational diffusion data of hydrophilic [C4MIM][BF4] on pore sizes in silica matrixes.889 Ion conductivities of [C4MIM][BF4] in mesopores silica matrixes present a peculiar thermal activation described by VTF character, exhibiting a stepwise increase in ion conductivities with decreasing pore diameters of silica matrixes at low temperatures (Figure 32C).889
A silica surface functionalized with various chemical groups, such as tributylsilyls,893 metal carbonyls,896 and even IL groups,897,898 offers additional pathways for tuning heterogeneous dynamics of confined ions in silica matrixes. Diffusion coefficients of hydrophobic [C6MIM][PF6] IL in untreated silica membranes (hydrophilic) decrease by approximately 1 order of magnitude compared to that in bulk liquids. However, a remarkable increase in diffusion coefficients is observed upon decorating silica membranes with hexamethyldisilazane groups.889,895 In another case, both [C6MIM][NTF2] and diethylmethylammonium methanesulfonate ILs display higher ion conductivities in silica matrixes functionalized with tributylsilyl groups as compared with those in untreated silica nanopores.893 A similar feature with enhanced proton conductivity is observed in [C8HIM][NTF2]–imidazole mixtures confined in nanopores of silica particles.899 Proton conductivity occurs due to an establishment of new N···H–N HBs and fast proton exchange events in polar domains, which are decoupled from molecular diffusions of constituent ions in heterogeneous IL matrixes.
For ILs used as lubricants or lubricant additives, their tribological and antiwear properties are intrinsically correlated with a balance of molecular shapes of ILs and atomic structures of solid surfaces and possible residual impurities (such as water) in ILs.892,900−905 Spencer and co-workers studied representative tribological behaviors of silica/silicon tribopairs lubricated with fluorinated ILs.901,902,906 XPS and Raman spectra showed that a mechanical form of wear dominates within a wide speed range for [C2MIM][NTF2] and [C6MIM][NTF2] ILs.906 In contrast, the corresponding [FAP] ILs exhibit significantly different XPS and Raman spectra, suggesting a different boundary lubrication mechanism depending on contact pressure.901,902 Furthermore, effect of water on antiwear properties of IL lubricants depends on hydrophilicities of anions and surface types.903,904 Addition of water into hydrophilic [C4MIM][BF4] results in a disruption of solvation IL layers and thereafter the formation of an interfacial water phase on silica via HB interactions of water with IL ions and silica interfacial groups.904
5.6. IL–Mica Interface
Mica is a layered alumina silicate with two layers of silica tetrahedra sandwiching a layer of alumina octahedra. Mica has been extensively used as a model surface to gain insights into microstructures and dynamical properties of liquids and solutions in confined environments.107 Horn and co-workers measured oscillatory forces between atomically smooth mica surfaces immersed in EAN and EAN–water mixtures.907 Four to five oscillations are obtained for EAN before strong repulsion is observed, which prevents a closer approach of mica surfaces. The step period of 0.5–0.6 nm is consistent with the EAN ion pair diameter, indicating that EA cations and [NO3] anions are present in approximately equal numbers at the IL–mica interface. For EAN–water mixtures with low EAN concentrations, EAN behaves as a simple electrolyte and EDL force between mica surfaces decreases with a gradual increase of EAN concentration, consistent with DLVO theoretical prediction.856 As EAN concentration increases, EA cations adsorb to mica surfaces in a manner that is described by an ion-exchange model, and the EDL force becomes weak and is completely replaced by a short-range solvation force extending up to several nanometers.
Atkin and co-workers studied interfacial structural quantities of alkylammonoium ILs confined between a Si3N4 AFM tip and a mica surface.129,130,908,909 Two significant features, a series of repeating “push-through” at discrete separations on the AFM tip approach and retraction and a significant increase in the rupture force closer to the mica surface, were consistently obtained from extensive AFM measurements (Figure 33). In addition, oscillatory force profiles for EAN–mica systems exhibit a significant temperature dependence. An increase in temperature leads to a decrease in the number of solvation layers in the EAN–mica interfacial region and a decreased force that is required to rupture the innermost solvation layer due to reduced liquid viscosity.910 However, the force barriers associated with interfacial ordering structures are largely unaffected by temperatures, indicating that boundary IL layers remain in the interfacial region even at high temperatures.
Figure 33.
Interfacial data for EAN–, PAN–, EtAN–, and dimethylethylammonium formate (DMEAF)–mica systems. Column 1 (left) shows forces versus separation distances for an AFM tip to approach and to retract from the mica surface. Reproduced with permission from ref (129). Copyright 2007 American Chemical Society. Reproduced with permission from ref (908). Copyright 2009 American Chemical Society. Column 2 shows typical amplitude (dotted) and phase (black) data documented when an oscillating AFM tip approaching a mica surface dispersed in ILs. Column 3 shows topographic images of the innermost ion layer adsorbed to a mica surface. Column 4 (right) shows topographic images of the first near-surface layer of IL–mica systems. Insets present section analysis of interfacial IL structures near mica surfaces indicated by a blue line. Reproduced with permission from ref (912). Copyright 2015 Royal Society of Chemistry.
Lengthening the alkyl chains in alkylammonium cations from C2 to C3 leads to notable changes in the solvation forces for alkylammonium nitrate ILs confined on a mica surface.129,908,911,912 C3 chains in PA cations pack efficiently without layering as they confer more orientation freedom than C2 chains in EA cations, and thus fewer and more compressible interfacial layers are detected in the PAN–mica interfacial region (Figure 33). Additional AFM characterization of the PAN–alcohol mixtures on the mica surface showed that butanol can pack into the native PAN nanostructural region and causes swelling of polar and apolar networks with minimal structural variation.913 Alkyl chains in octanol and dodecanol are too long to simply accommodate themselves alongside PA cations. Even if hydroxyl groups in octanol and dodecanol are solvated in polar domains, alkyl chains in these two alcohols can transverse apolar domains in PAN. In addition, dissolved inorganic ions (Li+, Na+, Mg2+, and Al3+) compete effectively with PA cations in coordinating negatively charged sites on mica surface even at low ion concentrations, leading to distinct interfacial structures at the IL–mica interface.914
Covalently tethering a hydroxyl group to the terminal methyl unit in the EA cation leads to a dramatic change in the oscillatory force profile for EtAN on the mica surface.908,911,912 EtAN exhibits less ordered interfacial structures and fewer interfacial layers than EAN and PAN near the mica surface (Figure 33). In addition, substitution of primary alkylammonium cations with secondary and tertiary ones reduces the number of solvation layers at the IL–mica interface and weakens adsorption of cations onto the mica surface (Figure 33).908,912 These two variations are intrinsically related to intermolecular cohesive forces between ions and preferential coordinations of ions with the mica surface.
In comparison with EAN, [C2MIM][NTF2] exhibits distinct interfacial structures as revealed by AFM experiments, which are correlated with intrinsic molecular structures of constituent ions and their abilities to self-assemble at the mica surface.909,915−917 AFM imaging revealed that EAN self-assembles in a wormlike liquid morphology at the mica surface, whereas [C2MIM] cations adsorb in a more isolated fashion but still in rows templated by the mica surface. In addition, EA cations remain adsorbed to mica surface at high forces, whereas [C2MIM] cations desorb at a relatively low pressure, which is attributed to electrostatic attractions of charged atoms on the mica surface with localized charges in the EA cations being stronger than that with delocalized charges in [C2MIM] cations. This indicates that for applications where strong surface adsorption is desirable, such as in tribology, ions with localized charges are preferred; however, for applications where access to the solid interfacial region is required, such as in electrochemical devices like dye-sensitized solar cells (DSSCs), ions with delocalized charges should be employed as the solutes are more readily diffused from the solution to solid surfaces.
In another series of systematic studies, interfacial microstructures of [CnMIM][NTF2] ILs confined between negatively charged mica sheets were studied using the SFB technique,107,137−140 neutron reflectivity,107 X-ray scattering spectroscopy,124,125 and atomistic simulations.124,125,918,919 Both [C4MIM[NTF2] and [C6MIM][NTF2] ILs exhibit clear oscillatory forces with alternating repulsive and attractive regions and increased amplitude with decreasing relative distance between the two mica surfaces.137,920 The oscillation period for [C4MIM][NTF2] is close to one ion pair dimension, indicating that ions are arranged in alternating cation–anion monolayers between mica surfaces (Figure 34A). [C6MIM][NTF2] is more structured between mica surfaces with a larger repeat distance although alkyl chain length is merely and incrementally increased relative to [C4MIM][NTF2], indicating a structural transition from alternating cation–anion monolayers for [C4MIM][NTF2] to tail-to-tail cation bilayers for [C6MIM][NTF2] driven by solvophobic self-assembly of [C6MIM] cations in confined environments (Figure 34B). Furthermore, a combination of SFB and neutron reflectivity experiments demonstrated that [C10MIM][NTF2] exhibits clear evidence of interfacial layering structures between mica surfaces. These results were later reproduced by atomistic simulations138,919 and are rationalized by delicate electrostatics and chemical interactions controlling interfacial structures of ILs in confined environments.139,639,921
Figure 34.
Normal force (FN) renormalized with curvature radius (R) between mica surfaces across (A) [C4MIM][NTF2], (B) [C6MIM][NTF2], (C) [C4MPYRR][NTF2], (D) [C8MPYRR][NTF2], (E) [C10MPYRR][NTF2], and (F) [C4MPYRR]0.5[C10MPYRR]0.5[NTF2] mixture as a function of separation distance between mica surfaces. Open diamond points were measured on approach and open circles on retraction of mica surfaces. Schematics indicate possible layering structures for pure ILs and IL–IL mixtures. Reproduced with permission from ref (920). Copyright 2013 Royal Society of Chemistry.
One thing should be mentioned is that the mica surface is very reactive and readily adsorbs hydrocarbons and water species, leading to the formation of a few Angstrom thick contamination layer on the mica surface.904,915,922,923 XPS data revealed that [C1MIM][NTF2] and [C4MIM][NTF2] ILs exhibit complete dewetting behavior on a clean mica surface, but they form thin IL films on a fully carbon-covered mica surface.924 A considerable surface enhancement of [NTF2] anions is detected at submonolayer coverage in the IL–mica interfacial region, where [NTF2] anions are located above imidazolium rings that are parallel to the mica surface. [NTF2] anions adopt a cis conformation with CF3 groups pointing away from the mica surface. These film growth and interfacial structural features strongly differ from that found for submonolayer coverage of ILs on the Au surface, where confined cations and anions in [CnMIM][NTF2] ILs adsorb next to each other.838,925 The presence of water in [CnMIM][NTF2] ILs alters not only interfacial layering structures but also lateral and orientation ordering and aggregation of cation hydrophobic tails in the interfacial region. For [C4MIM][NTF2] confined between mica surfaces, water weakens adsorption of [C4MIM] cations onto mica surfaces and displaces ions from mica surfaces, and therefore interfacial layering structures nearly diminish. A general feature concerning the effect of water on the IL interfacial structures can be understood based on available experimental and computational studies of IL–water–mica systems.915,922,923 In the presence of water, interfacial structures near the mica surface are electrifiable via surface atom dissociation. Electrification of the mica surface is correlated with self-organization of interfacial ions and adsorption of water at the IL–mica interface. Water often, but not always, weakens interfacial layering structures, which can be traced back to the fact that water is both a dielectric solvent and a molecular liquid.918,926 For water-stable ILs, water may be leveraged to improve functional performance of ILs in applications. Therefore, adopting interfacial water is promising to manipulate IL interfacial structures (and dynamics) and potentially allows more flexibility in specific applications. In this regard, encouraging results have already been reported for using interfacial water to improve capacitive energy storage and lubrication.756,927,928
Novel interfacial behaviors were also observed in a homologous series of [CnMPYRR][NTF2] ILs.421,920,926,929,930 [CnMPYRR] cations (n = 4, 6, and 8) exhibit consistent stable alternating cation–anion layers, akin to [CnMIM][NTF2] ILs with very similar film thickness.920,931 This implies a tendency for alkyl chains to lie more or less parallel along the mica surface, leading to a decrease in the number density of cations within the interfacial layers as the alkyl chain length increases, a gradually increased mismatch in maximum possible ion concentration of anion and cation layers, and a frustration of overscreened alternating cation–anion interfacial layering structures. These mismatches contribute to a substantial lowering of oscillatory forces for [C8MPYRR][NTF2] (Figure 34D) compared to [C4MPYRR][NTF2] (Figure 34C) confined between mica surfaces. A substantially different oscillatory force is observed in [C10MPYRR][NTF2], which is attributed to a flip from monolayer to bilayer interfacial structures (Figure 34E). This change is different from that for [C6MIM][NTF2] as C10 chains of [C10MPYRR] cations interdigitate and [NTF2] anions reside between neighboring pyrrolidinium rings.926,929
It is noteworthy that the monolayer-to-bilayer transition and the resulting bilayer architectures for [CnMIM][NTF2] and [CnMPYRR][NTF2] ILs are intrinsically different in alkyl chain length transition and interfacial bilayer thickness.920 Crossover from the anion–cation monolayer to cation bilayer structures occurs between C4 and C6 for [CnMIM][NTF2] ILs and between C8 and C10 for [CnMPYRR][NTF2] ILs. This difference is attributed to distinct cation–cation interactions. Planar imidazolium rings with delocalized charges can interact favorably via π–π stacking interactions, allowing a close approach of alkyl chains and favorable dispersion interactions in apolar domains. Pyrrolidinium cations, on the other hand, cannot stack at such close distances and, therefore, require long alkyl chains to drive bilayer formation.932 In addition, [CnMIM][NTF2] and [CnMPYRR][NTF2] ILs exhibit qualitatively different interfacial bilayer architectures in the IL–mica interfacial region. A toe-to-toe bilayer structure is proposed for confined [C6MIM][NTF2] with [NTF2] anions sitting on top of imidazolium rings; however, C10 chains of [C10PYRR] cations are significantly interdigitated with [NTF2] anions positioned with pyrrolidinium polar groups in the same plane in the bilayer structures.920
Furthermore, oscillatory structural forces for a [C4MPYRR]0.5[C10MPYRR]0.5[NTF2] mixture confined between mica surfaces are distinct to those for pure IL components with both [C4MPYRR] and [C10MPYRR] cations being present in the confined IL film, rather than one component being more substantially surface active than the other and thus segregating from the bulk mixture in the thin IL film (Figure 34F).920 The interfacial layer thickness of this mixture differs appreciably from that of alternating ion layers (monolayers) and is more similar to that of a bilayer structural motif for [C10MPYRR][NTF2], indicating the formation of bilayer-like interfacial structures in the [C4MPYRR]0.5[C10MPYRR]0.5[NTF2] mixture. However, this bilayer-like interfacial structure is slightly thinner than that for pure [C10MPYRR][NTF2] and is substantially more compressible and requires a low force to rupture or squeeze-out. In this mixture, [C10MPYRR] cations dictate interfacial structures, which are reminiscent of the effect of mixtures on bulk nanostructures, where cations with long alkyl chains are predominant in determining apolar domain sizes.225
It should be addressed that both anion sizes and shapes (halides, [BF4], [PF6], [N(CN)2], [FSI], [NTF2], [FAP], etc.) have a significant influence on oscillatory force profiles for ILs confined between mica surfaces.140,237,916,917,933−935 [PF6] anions appear to be more conducive to nanostructure formation than [NTF2], [NO3], and formate anions, underlining the importance of anion features related to their symmetries and charge distributions. This points to a new avenue for molecularly designing IL architectures and, thereafter, tuning interfacial phenomena for particular applications, such as adhesion, lubrication, and electrokinetic flows.917 Where mobility and transfer of ion species to and from solid interface is desirable (heterogeneous catalysis, batteries, supercapacitors, DSSCs, etc.), IL containing multiple sterically hindered allylic functional groups can be considered to maximize compressibility of IL solvation layers and minimize IL–substrate associations in interfacial regions. Conversely, in situations where adsorption of ILs to solid surface is required (e.g., electrode surface restructuring and lubrication), ions with symmetric shapes having localized charge centers are preferable.934
5.7. IL–TiO2 Interface
TiO2 is a representative photoanode material in DSSCs,936 and the IL–TiO2 interface is of particular interest in DSSC research as detailed knowledge of interfacial structures may shed light on device differences in regard to function and durability.936 A combination of XPS and extended X-ray absorption fine structure spectra showed that [C4MIM][BF4] absorbs onto the anatase(101) surface, the most thermodynamically stable and dominant surface exposed in the TiO2 substrate,937 in an ordering manner via electrostatic interactions at a low coverage with imidizolium rings oriented at 32 ± 4° from the anatase surface.938,939 With an increase in coverage of [C4MIM][BF4] on the anatase(101) surface, the influence of the TiO2 surface on interfacial orientations of confined ions at the uppermost IL–vapor layers is reduced and interfacial ordering structures are partially or totally lost. Above specific temperatures, both cations and anions undergo surface-induced degradation on the anatase(101) surface, leading to a production of varied ion species detected by XPS.938,940 The decomposition mechanism of ILs on the anatase(101) surface is distinct depending on ion structures and temperatures. [BF4] anions most likely react with interfacial atoms at oxygen-vacancy sites resulting in an incorporation of fluorine atoms into oxygen vacancies at the anatase surface.938
Atomistic simulations provided complementary results for a thorough understanding of interfacial structures at the IL–TiO2 interface.941−944 It was found that layering effect for [C2MIM][NTF2] confined inside a rutile(110) slit is more pronounced compared to its confinement in a graphitic slit having the same slit width.725 Both [C2MIM] cations and [NTF2] anions adopt multiple orientations and exhibit significantly slower diffusion rates near rutile walls than near graphite walls,725 which is attributed to specific interactions between confined ion species and interfacial atoms of the rutile walls. Strong electrostatic and dispersion interactions are present between individual atoms in [C2MIM][NTF2] and Ti and oxygen atoms on rutile walls, whereas only vdW interactions are present between confined ions and carbon atoms at graphitic walls.
Barbara and co-workers performed AIMD simulations to study interfacial structures of [C2MIM][SCN] and [C2MIM][B(CN)4] ILs on the anatase(101) surface.942−944 These two ILs exhibit dense interfacial layers at the IL–TiO2 interface with [C2MIM] cations being perpendicular to the anatase surface because of HB interactions of C(2)–H atoms with interfacial oxygen atoms on the anatase surface. Most [B(CN)4] anions exhibit face conformation (Figure 35A), where three CN groups have preferential interactions with the anatase(101) surface. The less populated edge (Figure 35B) and vertex (Figure 35C) conformations with two and one CN groups in contact with the anatase(101) surface are also observed. Favorable N–Ti interactions of [SCN] anions with the anatase(101) surface result primarily in perpendicular distributions of [SCN] anions with nitrogen atoms closest to the anatase(101) surface (Figure 35D). In addition, absorbed imidazolium cations exhibit varied adsorption geometries on the anatase(101) surface (Figure 35E–H) and tend to cause an energetic downward shift of TiO2 band levels by accepting electron density from the anatase surface. Anions are observed to raise energy levels by donating electron density to the anatase surface.942 Both effects take place simultaneously and counteract each other, leaving a complicated charge transfer phenomenon at the IL–TiO2 interface. In general, if it aims to achieve a maximum energetic upward shift, the adopted ILs should consist of cations with highly delocalized or screened positive charges, and their interactions with the TiO2 surface should be minimal. Additionally, ILs can be changed systematically to increase or decrease the band edge position to match the band levels appropriately. If alignment of the band levels is satisfactory, different ILs that yield similar band shifts can be employed to improve other aspects for peculiar applications, such as compatibilities of electrolytes with dye molecules, redox mediators, and solar cell sealing materials.945
Figure 35.
Adsorption geometries of ion species on the anatase(101) surface determined from DFT calculations. [B(CN)4] with (A) face conformation, (B) edge conformation, and (C) vertex conformation. (D) [SCN] with “N coordination” conformation. [C1MIM] with (E) “flat” conformation, (F) “vertical” conformation, (G) “H5 coordination” conformation, and (H) “H4 coordination” conformation. Labeled distances are in picometers. Reproduced with permission from ref (943). Copyright 2015 American Chemical Society.
Furthermore, there are some sporadic experimental and computational investigations on absorption of ILs on various metal oxide surfaces including CeO2,946,947 ZnO,948,949 VO2,950,951 SrTiO3,952,953 CoO/Co3O4,954 FeO,955,956 ZrO2,957 and even on some metal sulfide surfaces, like GeS2958 and MoS2.959,960 These studies illustrated that delicate intermolecular interactions between IL ions and metal oxide surfaces and acidity of the metal oxide surfaces have a significant effect on short- and long-term thermal stabilities of supported ILs. A detailed elucidation of these interactions offers new opportunities for rational design of materials, such as solid catalysts on supported IL layers for heterogeneous catalysis and supported IL membranes for gas separation.
6. Conclusions and Outlook
Over the last 2 decades, ILs have attracted increasing attention in academia and industrial communities owing to their numerous useful physicochemical and structural properties. Due to a countless number of combinations of cation–anion moieties and mixtures with cosolvents, a thorough understanding of their hierarchical structures and dynamics is highly significant for rational selection and design of ILs with desired properties and thereafter maximizing their functionalities in applications including catalysis, gas capture and separation, energy storage and harvesting, and lubrication. This is a uniting theme across different IL types including alkylammonium, imidazolium, pyrrolidinium, pyridinium, piperidinium, and tetraalkylammonium and tetraalkylphosphonium cations with either organic or inorganic anions. An important feature of these ILs is that they are more complex than molecular solvents and show rich diversities in various aspects ranging from molecular structures of constituent ions, intra- and intermolecular interactions, dynamical quantities, and self-assembled liquid morphologies of ILs in bulk liquids. Therefore, ILs have been extensively investigated in different ways including free ions, ion pairs, ion clusters, ion continuum, HB networks, and bicontinuous sponge structures with interpenetrating polar and apolar networks via advanced experimental techniques and molecular simulations. In addition, nanoconfined ILs, owing to distinct spatial confinement and dominant surface forces at short length scale, offer new and attractive features, such as distinct phase transitions and depressed transport properties. These microstructural, dynamical, and transport properties are attributed to a complex interplay of constituent ions with surrounding ions in bulk liquids, with cosolvent molecules and inorganic salts in IL mixtures, and with solid surfaces in confined environments. A specific feature of ILs, either in bulk liquids or in interfacial regions, is microstructural and dynamical heterogeneities, which are hallmark characteristics of their unique properties. These heterogeneities of ILs arise because ILs are composed of cations and anions or, alternatively, polar and apolar groups, leading to repeating and correlated microstructures in bulk liquids and in interfacial regions on nanometer dimensions. The diversified solvent structures of ILs are the origin of much past, current, and future interest in ILs, which are implicated in almost all aspects of their chemistry.
At the current stage, the IL research field has reached an astonishing level, enriched with an unexpected diversity of ions with distinct capacities for self-assembly in bulk liquids and in confined environments. This diversity has catalyzed IL research, and their chemical and self-assembled structures can be used to unlock their potential to impact many areas of scientific research and technological applications. As this field matures, there needs to be increasing economic cost/benefit analysis of ILs, how to select and how to design appropriate ions to achieve peculiar microstructures, distinctive mesoscopic liquid morphologies, and specific macroscopic functions, which will be critical for ILs to compete with established liquids and materials. Multiscale modeling approaches, including first principle calculations and ab initio, atomistic, and CG MD simulations, provide not only complementary results but also critical physical insights for understanding spectacular phenomena taking place in bulk liquids and in interfacial regions under harsh conditions. In future work, molecular simulations will be generally adopted in two modes within the IL community: accurate prediction of physicochemical and structural quantities of IL systems and providing qualitative insights into the structure–property relationship for maximizing their utilization in applications. It is anticipated that in a long period of time in the future, multiscale modeling simulations will be on an equal footing with experimental investigations to explore important properties of ILs in a wide range of applications. An integration of multiscale simulation results and experimental characterizations is expected to unveil fundamental mechanisms governing distinct microstructural and dynamical heterogeneities of ILs in a wide range of physical and chemical environments and to fine-tune these properties in an intelligent fashion. A comprehensive understanding of fundamental properties of ILs can provide unprecedented guidance for preselection and design of appropriate IL candidates and advancing their functionalities in industrial applications while minimizing environmental effects for a sustainable future.
Acknowledgments
Y.-L. Wang gratefully acknowledges financial support from the Knut and Alice Wallenberg Foundation (Grant KAW 2018.0380). F. Mocci acknowledges financial support from Progetto Fondazione di Sardegna (Grant CUP F71I17000170002). Z.-Y. Lu acknowledges financial support from the National Science Foundation of China (Grants 21534004 and 21833008). J. Yuan is grateful for financial support from ERC Starting Grant NAPOLI-639720 from the European Research Council, Swedish Research Council Grant 2018-05351, Dozentenpreis 15126 from Verband der Chemischen Industrie e.V. (VCI) in Germany, and the Wallenberg Academy Fellow program (Grant KAW 2017.0166) in Sweden. A. Laaksonen acknowledges the Swedish Science Council for financial support and partial support from a grant from the Ministry of Research and Innovation of Romania (CNCS–UEFISCDI, Project Number PN-III-P4-ID-PCCF-2016-0050, within PNCDI III). M. D. Fayer acknowledges the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant No. DEFG03-84ER13251 and Air Force Office of Scientific Research under AFOSR Award No. FA9550-16-1-0104 for support of this research.
Glossary
Abbreviations
- 1D
one-dimensional
- 2D
two-dimensional
- 3D
three-dimensional
- AIMD
ab initio molecular dynamics
- ACN
acetonitrile
- AFM
atomic force microscopy
- BAN
butylammonium nitrate
- B(CN)4
tetracyanoborate
- BF4
tetrafluoroborate
- BMB
bis(mandelato)borate
- BMLB
bis(malonato)borate
- BOB
bis(oxalato)borate
- BScB
bis(salicylato)borate
- BzMIM
1-benzyl-3-methylimidazolium
- C1C1PO4
dimethylphosphate
- (C1OC1)2MIM
1-methoxyethoxymethyl-3-methylimidazolium
- C2C1MIM
1-ethyl-2,3-dimethylimidazolium
- C2HIM
1-ethyllimidazolium
- C2OH-MIM
1-(2-hydroxyethyl)-3-methylimidazolium
- C4C1MIM
1-butyl-2,3-dimethylimidazolium
- CF3SO3
triflate
- CG
coarse-grained
- CH
2-hydroxyethyl-trimethylammonium (cholinium)
- CnCmIM
dialkylimidazolium
- CnCmPYRR
dialkylpyrrolidinium
- CnMIM
1-alkyl-3-methylimidazolium
- Cn(MIM)2
di-imidazolium
- CnMPIP
1-alkyl-methylpiperidinium
- CnPYRI
N-alkylpyridinium
- CnSO4
alkylsulfate
- CNT
carbon nanotube
- DFT
density functional theory
- DLVO
Derjaguin-Landau-Vervey-Overbeek
- DMEAF
dimethylethylammonium formate
- DRS
direct recoil spectroscopy
- DSC
differential scanning calorimetry
- DSE
Debye–Stokes–Einstein
- DSSCs
dye-sensitized solar cells
- EAC
ethylammonium chloride
- EAF
ethylammonium formate
- EAN
ethylammonium nitrate
- EDL
electrical double layer
- EPSR
empirical potential structure refinement
- EtAN
ethanolammonium nitrate
- FAP
tris(pentafluoroethyl)trifluorophosphate
- FSDP
first sharp diffraction peak
- FSI
bis(fluorosulfonyl)imide
- FT
Fourier transform
- HB
hydrogen bonding
- IL
ionic liquid
- IR
infrared
- MAN
methylammonium nitrate
- MD
molecular dynamics
- N2,2,2,(2O2O2)
2-ethoxyethoxy-ethyltriethylammonium
- N(CN)2
dicyanamide
- Ni,j,k,l
tetraalkylammonium
- NMR
nuclear magnetic resonance
- NO3
nitrate
- NTF2
bis(trifluoromethanesulfonyl)imide
- OAc
acetate
- OHD-OKE
optical-heterodyne-detected optical Kerr effect
- OHD-RIKE
optical-heterodyne-detected Raman-induced Kerr effect
- OLC
onion like carbon
- PAC
propylammonium chloride
- PAN
propylammonium nitrate
- PC
propylene carbonate
- PF6
hexafluorophosphate
- PhSiMIM
1-dimethylphenylsilylmethyl-3-methylimidazolium
- Pi,j,k,l
tetraalkylphosphonium
- PSPP
polarization sensitive pump–probe
- PZC
potential of zero charge
- SANS
small-angle neutron scattering
- SAXS
small-angle X-ray scattering
- SCN
thiocyanate
- SeCN
selenocyanate
- SEIRAS
surface-enhanced infrared absorption spectroscopy
- SFA
surface force apparatus
- SFB
surface force balance
- SFG
sum frequency generation
- SiMIM
1-methyl-3-trimethylsilylmethylimidazolium
- SiOSiMIM
1-methyl-3-pentamethyldisiloxymethylimidazolium
- STM
scanning tunneling microscopy
- TFA
trifluoroacetic acid
- TFO
trifluoromethylsulfonate
- UCST
upper critical solution temperature
- vdW
van der Waals
- VFT
Vogel–Fulcher–Tammann
- WAXS
wide-angle X-ray scattering
- XPS
X-ray photoelectron spectroscopy
- XRD
X-ray diffraction
- XRR
X-ray reflectivity
Biographies
Yong-Lei Wang received his Ph.D. in 2012 from Jilin University, China, and in 2013 from Stockholm University, Sweden, under the joint supervision of Prof. Zhong-Yuan Lu and Prof. Aatto Laaksonen. From 2013 to 2016, he worked as a postdoctoral fellow with Prof. Aatto Laaksonen at Stockholm University and with Prof. Lars Kloo at KTH Royal Institute of Technology. In 2016, he received financial support from the Knut and Alice Wallenberg Foundation and worked as a Wallenberg Fellow with Prof. Michael D. Fayer at Stanford University and with Prof. Jiayin Yuan at Stockholm University. His research mainly focuses on numerical algorithm development for efficient handling of long-range Coulombic interactions, multiscale modelling of ionic liquids and polymeric fluids (charged soft matter systems), and computational studies of luminescent properties of transition-metal complexes in biological systems.
Bin Li was born in Siping, Jilin, China. He received his Ph.D. in 2013 from the Institute of Theoretical Chemistry, Jilin University, under the supervision of Prof. Zhong-Yuan Lu. Then he performed research as a postdoc at Technical University of Darmstadt, Germany, in the group of Prof. Nico van der Vegt (2013–2014), and Lund University, Sweden, in the group of Prof. Jan Forsman (2015–2017). After that, he moved to the National Center for Nanoscience and Technology, China, as an Assistant Professor. Currently he is an Assistant Professor at the School of Chemical Engineering and Technology, Sun Yat-sen University, China (2019–present). He mainly focuses on molecular simulations of soft matter systems, including ionic liquids properties and applications on supercapacitors as well as self-assembly of polymers and colloids.
Sten Sarman received his Ph.D. degree in physical chemistry at KTH Royal Institute of Technology, Sweden. Thereafter, he has worked at Australian National University in Canberra, Australia and Göteborg University and Stockholm University in Sweden. His main interest is various aspects of statistical thermodynamics and transport properties of liquids, including flow properties of ionic liquids, liquid crystals, and integral equation theory of inhomogeneous liquids close to walls or in narrow slits.
Francesca Mocci graduated in Chemistry in 1999 at Cagliari University, where she obtained her Ph.D. in Chemistry in 2002, under the supervision of Prof. Giuseppe Saba. She has been Aggr. Prof. in Physical Methods in Organic Chemistry at the Faculty of Pharmacy of Cagliari University since 2007. She has been a visiting professor for several years at Stockholm University (2011–2016). Her scientific activity is mainly directed at the study of conformational preferences and structural organization of organic and bio-organic systems, with particular attention to the interactions in highly charged systems.
Zhong-Yuan Lu received his Ph.D. from Jilin University, China, in 1999, with Professor Ze-Sheng Li as the advisor. After receiving a Ph.D., he stayed in the group of Professor Reinhard Hentschke as a postdoc at Bergische Universitaet-Gesamthochshule Wuppertal, Germany, until 2003. He then moved back to Jilin University and started his own research group as an Associate Professor. In 2010, he was awarded with the National Nature Science Foundation for Outstanding Youth Scholars of China. Now he is a Full Professor at Jilin University. His research interest is multiscale simulation of polymers.
Jiayin Yuan studied chemistry at Shanghai Jiao Tong University (1998–2002), China. He received a M.Sc. degree from University of Siegen (Germany) in 2004 and a Ph.D. from Bayreuth University (Germany) in 2009. He joined the Max Planck Institute of Colloids and Interfaces in Potsdam as a postdoc and then became a group leader. He received the European Research Council Starting Grant in 2014, Dr. Hermann-Schnell Award in 2015, the Dozentenpreis from the Fund of Chemical Industry in 2016, and a Wallenberg Academy Fellow in Sweden in 2017. From December 2018, he is a Full Professor at Stockholm University (Sweden). He is interested in functional polymers and carbon materials.
Aatto Laaksonen is a Professor of Physical Chemistry at Stockholm University. He received a Ph.D. in 1981 from Stockholm University and was a postdoctoral fellow at Daresbury Laboratory (U.K.) in 1982 with Vic Saunders and at IBM Poughkeepsie/Kingston (U.S.) from 1983 to 1985 with Enrico Clementi. He had a sabbatical at Dalhousie University 1993–1995 (Canada) with Rod Wasylishen. He has been a visiting professor at JAERI 2002, 2005 (Japan), at Cagliari University 2008, 2009, 2011, 2015 (Sardinia), at Nanjing Tech University 2018–present (China), and at Petru Poni Institute for Macromolecular Chemistry 2018–present (Romania). Research areas include multiscale modelling/simulations including method and model development in bio and materials sciences and green chemical engineering.
Michael D. Fayer received a B.S. degree in Chemistry from University of California at Berkeley where he was Phi Beta Kapa. He also received his Ph.D. from University of California at Berkeley in 1974, working with Professor Charles B. Harris. After graduate school, Fayer went directly to Stanford University as an Assistant Professor of Chemistry in 1974. He has been a Full Professor since 1984 and became the David Mulvane Ehrsam and Edward Curtis Franklin Professor of Chemistry in 2000. Fayer pioneered the development and use of ultrafast nonlinear optical spectroscopy for studying dynamics in complex condensed matter molecular systems. Over the last 45 years, he has advanced the field, expanding from visible to infrared and to multidimensional techniques as he pursued a wide array of fundamentally important problems ranging from water to proteins. Fayer also developed detailed theoretical descriptions of nonlinear experiments and molecular systems. In 1979, Fayer performed the first picosecond (ultrafast at the time) photon echoes on molecular crystals. In 1993, the Fayer group conducted the first ultrafast infrared echo experiments, dubbed vibrational echoes, using an infrared free electron laser. These initial experiments set the stage for the current widespread field of ultrafast 2D IR spectroscopy. By the early 2000s, Fayer was using 2D IR spectroscopy to study hydrogen bonding, leading to a detailed understanding of water hydrogen bond dynamics. Fayer opened a new era of spectroscopic research on the dynamics of complex molecular system through his ground-breaking development and application of ultrafast nonlinear optical experiments. His work in both visible and IR has spread widely and stimulated a vast amount of research around the world that has amplified and extended the fields. Fayer has received many honors for his scientific work. He was elected to the National Academy of Sciences of U.S.A. in 2007 and the American Academy of Arts and Sciences in 1999. He received the Pittsburgh Spectroscopy Award from SSP in 2018, the Ahmed Zewail Award in Ultrafast Science and Technology from ACS in 2014, the Arthur L. Schawlow Prize in Laser Science from APS in 2012, the Ellis R. Lippincott Award from OSA in 2009, the E. Bright Wilson Award for Spectroscopy from ACS in 2007, and the Earl K. Plyler Prize for Molecular Spectroscopy from APS in 2000.
The authors declare no competing financial interest.
References
- Walden P. Molecular Weights and Electrical Conductivity of Several Fused Salts. Bull. Acad. Imper. Sci. (St. Petersburg) 1914, 8, 405–422. [Google Scholar]
- Sugden S.; Wilkins H. CLXVII.—The Parachor and Chemical Constitution. Part XII. Fused Metals and Salts. J. Chem. Soc. 1929, 0, 1291–1298. 10.1039/JR9290001291. [DOI] [Google Scholar]
- Hurley F. H.; Wier T. P. The Electrodeposition of Aluminum from Nonaqueous Solutions at Room Temperature. J. Electrochem. Soc. 1951, 98, 207–212. 10.1149/1.2778133. [DOI] [Google Scholar]
- Welton T. Ionic liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. 10.1007/s12551-018-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes J. S. A. Short History of Ionic Liquids—From Molten Salts to Neoteric Solvents. Green Chem. 2002, 4, 73–80. 10.1039/b110838g. [DOI] [Google Scholar]
- Wilkes J. S.; Zaworotko M. J. Air and Water Stable 1-Ethyl-3-methylimidazolium Based Ionic Liquids. J. Chem. Soc., Chem. Commun. 1992, 13, 965–967. 10.1039/c39920000965. [DOI] [Google Scholar]
- Cooper E. I.; Angell C. A. Versatile Organic Iodide Melts and Glasses with High Mole Fractions of LiI: Glass Transition Temperatures and Electrical Conductivities. Solid State Ionics 1983, 9, 617–622. 10.1016/0167-2738(83)90304-1. [DOI] [Google Scholar]
- Poole S. K.; Shetty P. H.; Poole C. F. Chromatographic and Spectroscopic Studies of Solvent Properties of A New Series of Room-temperature Liquid Tetraalkylammonium Sulfonates. Anal. Chim. Acta 1989, 218, 241–264. 10.1016/S0003-2670(00)80302-5. [DOI] [Google Scholar]
- Plechkova N. V.; Seddon K. R. Applications of Ionic Liquids in the Chemical Industry. Chem. Soc. Rev. 2008, 37, 123–150. 10.1039/B006677J. [DOI] [PubMed] [Google Scholar]
- Binnemans K. Ionic Liquid Crystals. Chem. Rev. 2005, 105, 4148–4204. 10.1021/cr0400919. [DOI] [PubMed] [Google Scholar]
- Ediger M. D. Spatially Heterogeneous Dynamics in Supercooled Liquids. Annu. Rev. Phys. Chem. 2000, 51, 99–128. 10.1146/annurev.physchem.51.1.99. [DOI] [PubMed] [Google Scholar]
- Welton T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- Dong K.; Liu X.; Dong H.; Zhang X.; Zhang S. Multiscale Studies on Ionic Liquids. Chem. Rev. 2017, 117, 6636–6695. 10.1021/acs.chemrev.6b00776. [DOI] [PubMed] [Google Scholar]
- MacFarlane D. R.; Forsyth M.; Howlett P. C.; Kar M.; Passerini S.; Pringle J. M.; Ohno H.; Watanabe M.; Yan F.; Zheng W.; Zhang S.; Zhang J. Ionic Liquids and Their Solid-State Analogues as Materials for Energy Generation and Storage. Nat. Rev. Mater. 2016, 1, 15005. 10.1038/natrevmats.2015.5. [DOI] [Google Scholar]
- Zhang S.; Zhang J.; Zhang Y.; Deng Y. Nanoconfined Ionic Liquids. Chem. Rev. 2017, 117, 6755–6833. 10.1021/acs.chemrev.6b00509. [DOI] [PubMed] [Google Scholar]
- Podgorsek A.; Jacquemin J.; Pádua A. A.; Costa Gomes M. F. Mixing Enthalpy for Binary Mixtures Containing Ionic Liquids. Chem. Rev. 2016, 116, 6075–6106. 10.1021/acs.chemrev.5b00379. [DOI] [PubMed] [Google Scholar]
- Hunt P. A.; Ashworth C. R.; Matthews R. P. Hydrogen Bonding in Ionic Liquids. Chem. Soc. Rev. 2015, 44, 1257–1288. 10.1039/C4CS00278D. [DOI] [PubMed] [Google Scholar]
- Hayes R.; Warr G. G.; Atkin R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. 10.1021/cr500411q. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Sun J.; Zhang X.; Xin J.; Miao Q.; Wang J. Ionic Liquid-Based Green Processes for Energy Production. Chem. Soc. Rev. 2014, 43, 7838–7869. 10.1039/C3CS60409H. [DOI] [PubMed] [Google Scholar]
- Burt R.; Birkett G.; Zhao X. S. A Review of Molecular Modelling of Electric Double Layer Capacitors. Phys. Chem. Chem. Phys. 2014, 16, 6519–6538. 10.1039/c3cp55186e. [DOI] [PubMed] [Google Scholar]
- Fedorov M. V.; Kornyshev A. A. Ionic Liquids at Electrified Interfaces. Chem. Rev. 2014, 114, 2978–3036. 10.1021/cr400374x. [DOI] [PubMed] [Google Scholar]
- Somers A. E.; Howlett P. C.; MacFarlane D. R.; Forsyth M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. 10.3390/lubricants1010003. [DOI] [Google Scholar]
- Niedermeyer H.; Hallett J. P.; Villar-Garcia I. J.; Hunt P. A.; Welton T. Mixtures of Ionic Liquids. Chem. Soc. Rev. 2012, 41, 7780–7802. 10.1039/c2cs35177c. [DOI] [PubMed] [Google Scholar]
- Hallett J. P.; Welton T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. 10.1021/cr1003248. [DOI] [PubMed] [Google Scholar]
- Castner E. W. Jr; Margulis C. J.; Maroncelli M.; Wishart J. F. Ionic Liquids: Structure and Photochemical Reactions. Annu. Rev. Phys. Chem. 2011, 62, 85–105. 10.1146/annurev-physchem-032210-103421. [DOI] [PubMed] [Google Scholar]
- Armand M.; Endres F.; MacFarlane D. R.; Ohno H.; Scrosati B. Ionic-Liquid Materials for the Electrochemical Challenges of the Future. Nat. Mater. 2009, 8, 621–629. 10.1038/nmat2448. [DOI] [PubMed] [Google Scholar]
- Wang Y. L.; Hedman F.; Porcu M.; Mocci F.; Laaksonen A. Non-Uniform FFT and Its Applications in Particle Simulations. Appl. Mathematics 2014, 5, 520–541. 10.4236/am.2014.53051. [DOI] [Google Scholar]
- Santos C. S.; Baldelli S. Gas-Liquid Interface of Room-Temperature Ionic Liquids. Chem. Soc. Rev. 2010, 39, 2136–2145. 10.1039/b921580h. [DOI] [PubMed] [Google Scholar]
- Soukup-Hein R. J.; Warnke M. M.; Armstrong D. W. Ionic Liquids in Analytical Chemistry. Annu. Rev. Anal. Chem. 2009, 2, 145–168. 10.1146/annurev-anchem-060908-155150. [DOI] [PubMed] [Google Scholar]
- Zhou F.; Liang Y.; Liu W. Ionic Liquid Lubricants: Designed Chemistry for Engineering Applications. Chem. Soc. Rev. 2009, 38, 2590–2599. 10.1039/b817899m. [DOI] [PubMed] [Google Scholar]
- Ma C.; Laaksonen A.; Liu C.; Lu X.; Ji X. The Peculiar Effect of Water on Ionic Liquids and Deep Eutectic Solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. 10.1039/C8CS00325D. [DOI] [PubMed] [Google Scholar]
- Yuan J.; Antonietti M. Poly(ionic liquid)s: Polymers Expanding Classical Property Profiles. Polymer 2011, 52, 1469–1482. 10.1016/j.polymer.2011.01.043. [DOI] [Google Scholar]
- Fellinger T. P.; Thomas A.; Yuan J.; Antonietti M. 25th Anniversary Article:“Cooking Carbon with Salt”: Carbon Materials and Carbonaceous Frameworks From Ionic Liquids and Poly(Ionic Liquid)s. Adv. Mater. 2013, 25, 5838–5855. 10.1002/adma.201301975. [DOI] [PubMed] [Google Scholar]
- Men Y.; Kuzmicz D.; Yuan J. Poly(Ionic Liquid) Colloidal Particles. Curr. Opin. Colloid Interface Sci. 2014, 19, 76–83. 10.1016/j.cocis.2014.03.012. [DOI] [Google Scholar]
- Endres F. Interfaces of Ionic Liquids. Phys. Chem. Chem. Phys. 2012, 14, 5008–5009. 10.1039/c2cp90031a. [DOI] [PubMed] [Google Scholar]
- Endres F. Physical Chemistry of Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 1648. 10.1039/c001176m. [DOI] [PubMed] [Google Scholar]
- Muldoon M. J.; Nockemann P.; Lagunas M. C. Crystal Engineering with Ionic Liquids. CrystEngComm 2012, 14, 4873. 10.1039/c2ce90050e. [DOI] [Google Scholar]
- Austen Angell C.; Ansari Y.; Zhao Z. Ionic Liquids: Past, Present and Future. Faraday Discuss. 2012, 154, 9–27. 10.1039/C1FD00112D. [DOI] [PubMed] [Google Scholar]
- Perkin S.; Salanne M. Interfaces of Ionic Liquids. J. Phys.: Condens. Matter 2014, 26, 280301. 10.1088/0953-8984/26/28/280301. [DOI] [PubMed] [Google Scholar]
- Rogers R. D.; Voth G. A. Ionic Liquids. Acc. Chem. Res. 2007, 40, 1077–1078. 10.1021/ar700221n. [DOI] [PubMed] [Google Scholar]
- Perkin S.; Kirchner B.; Fayer M. D. Preface: Special Topic on Chemical Physics of Ionic Liquids. J. Chem. Phys. 2018, 148, 193501. 10.1063/1.5039492. [DOI] [PubMed] [Google Scholar]
- Castner E. W. Jr; Wishart J. F. Spotlight on Ionic Liquids. J. Chem. Phys. 2010, 132, 120901. 10.1063/1.3373178. [DOI] [PubMed] [Google Scholar]
- Marcus Y.Ionic Liquid Properties: From Molten Salts to RTILs; Springer-Verlag: Berlin-Heidelberg, 2016. [Google Scholar]
- Electrodeposition From Ionic Liquids; Endres F., Abbott A., MacFarlane D. R., Eds.; John Wiley & Sons, 2017. [Google Scholar]
- The Structure of Ionic Liquids; Caminiti R., Gontrani L., Eds.; Springer-Verlag: Berlin-Heidelberg, 2014. [Google Scholar]
- Electrochemistry in Ionic Liquids. Vol. 1: Fundamentals; Torriero A. A. J., Eds.; Springer-Verlag: Berlin-Heidelberg, 2015. [Google Scholar]
- Structures and Interactions of Ionic Liquids; Zhang S., Wang J., Lu X., Zhou Q., Eds.; Springer-Verlag: Berlin-Heidelberg, 2013. [Google Scholar]
- Wang Y.-L.; Sarman S.; Golets M.; Mocci F.; Lu Z.-Y.; Laaksonen A.. Multigranular Modeling of Ionic Liquids. In Ionic Liquids: Synthesis, Properties, Technologies and Applications; Fehrmann R., Santini C., Eds.; De Gruyter, 2019. [Google Scholar]
- Mocci F.; Laaksonen A.; Wang Y.-L.; Saba G.; Lai A.; Marincola F. C.. CompChem and NMR Probing Ionic Liquids. In The Structure of Ionic Liquids; Caminiti R., Gontrani L., Eds.; Springer-Verlag: Berlin-Heidelberg, 2014. [Google Scholar]
- Wood N.; Stephens G. Accelerating the Discovery of Biocompatible Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 1670–1674. 10.1039/b923429b. [DOI] [PubMed] [Google Scholar]
- Chauvin Y.; Mussmann L.; Olivier H. A Novel Class of Versatile Solvents for Two-Phase Catalysis: Hydrogenation, Isomerization, and Hydroformylation of Alkenes Catalyzed by Rhodium Complexes in Liquid 1,3-Dialkylimidazolium Salts. Angew. Chem., Int. Ed. Engl. 1996, 34, 2698–2700. 10.1002/anie.199526981. [DOI] [Google Scholar]
- Lei Z.; Dai C.; Chen B. Gas Solubility in Ionic Liquids. Chem. Rev. 2014, 114, 1289–1326. 10.1021/cr300497a. [DOI] [PubMed] [Google Scholar]
- Watanabe M.; Thomas M. L.; Zhang S.; Ueno K.; Yasuda T.; Dokko K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. 10.1021/acs.chemrev.6b00504. [DOI] [PubMed] [Google Scholar]
- MacFarlane D. R.; Tachikawa N.; Forsyth M.; Pringle J. M.; Howlett P. C.; Elliott G. D.; Davis J. H.; Watanabe M.; Simon P.; Angell C. A. Energy Applications of Ionic Liquids. Energy Environ. Sci. 2014, 7, 232–250. 10.1039/C3EE42099J. [DOI] [Google Scholar]
- Li B.; Ma K.; Wang Y.-L.; Turesson M.; Woodward C. E.; Forsman J. Fused Coarse-Grained Model of Aromatic Ionic Liquids and Their Behaviour at Electrodes. Phys. Chem. Chem. Phys. 2016, 18, 8165–8173. 10.1039/C6CP00202A. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Wei S.; Wu Y.; Wang Y.-L.; Zhang M.; Roy D.; Wang H.; Yuan J.; Zhao Q. Poly(Ionic Liquid)-Derived Graphitic Nanoporous Carbon Membrane Enables Superior Supercapacitive Energy Storage. ACS Nano 2019, 13, 10261–10271. 10.1021/acsnano.9b03514. [DOI] [PubMed] [Google Scholar]
- Bi S.; Banda H.; Chen M.; Niu L.; Chen M.; Wu T.; Wang J.; Wang R.; Feng J.; Chen T.; Dinca M.; Kornyshev A. A.; Feng G. Molecular Understanding of Charge Storage and Charging Dynamics in Supercapacitors with MOF Electrodes and Ionic Liquid Electrolytes. Nat. Mater. 2020, 10.1038/s41563-019-0598-7. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson N.; Bergendal E.; Wang Y.-L.; Munavirov B.; Wallinder D.; Glavatskih S.; Aastrup T.; Atkin R.; Furó I.; Rutland M. W. Electro-Responsive Surface Composition and Kinetics of an Ionic Liquid in a Polar Oil. Langmuir 2019, 35, 15692–15700. 10.1021/acs.langmuir.9b02119. [DOI] [PubMed] [Google Scholar]
- Fumino K.; Wulf A.; Ludwig R. Strong, Localized, and Directional Hydrogen Bonds Fluidize Ionic Liquids. Angew. Chem., Int. Ed. 2008, 47, 8731–8734. 10.1002/anie.200803446. [DOI] [PubMed] [Google Scholar]
- Geronimo I.; Jiten Singh N.; Kim K. S. Nature of Anion-Templated π+–π+ Interactions. Phys. Chem. Chem. Phys. 2011, 13, 11841–11845. 10.1039/c1cp20348g. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Laaksonen A.; Fayer M. D. Hydrogen Bonding versus π-π Stacking Interactions in Imidazolium-Oxalatoborate Ionic Liquid. J. Phys. Chem. B 2017, 121, 7173–7179. 10.1021/acs.jpcb.7b05564. [DOI] [PubMed] [Google Scholar]
- Gao N.; He Y.; Tao X.; Xu X.-Q.; Wu X.; Wang Y. Crystal-Confined Freestanding Ionic Liquids for Reconfigurable and Repairable Electronics. Nat. Commun. 2019, 10, 547. 10.1038/s41467-019-08433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-L.; Laaksonen A. Interfacial Structure and Orientation of Confined Ionic Liquids on Charged Quartz Surfaces. Phys. Chem. Chem. Phys. 2014, 16, 23329–23339. 10.1039/C4CP03077J. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Margulis C. J. Heterogeneity in a Room-Temperature Ionic Liquid: Persistent Local Environments and the Red-Edge Effect. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 831–836. 10.1073/pnas.0507364103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habasaki J.; Ngai K. Heterogeneous Dynamics of Ionic Liquids from Molecular Dynamics Simulations. J. Chem. Phys. 2008, 129, 194501. 10.1063/1.3005372. [DOI] [PubMed] [Google Scholar]
- Roy D.; Patel N.; Conte S.; Maroncelli M. Dynamics in an Idealized Ionic Liquid Model. J. Phys. Chem. B 2010, 114, 8410–8424. 10.1021/jp1004709. [DOI] [PubMed] [Google Scholar]
- Karimi-Varzaneh H. A.; Müller-Plathe F.; Balasubramanian S.; Carbone P. Studying Long-Time Dynamics of Imidazolium-Based Ionic Liquids with a Systematically Coarse-Grained Model. Phys. Chem. Chem. Phys. 2010, 12, 4714–4724. 10.1039/b925780b. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Zhu Y.-L.; Lu Z.-Y.; Laaksonen A. Electrostatic Interactions in Soft Particle Systems: Mesoscale Simulations of Ionic Liquids. Soft Matter 2018, 14, 4252–4267. 10.1039/C8SM00387D. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Margulis C. J. A Study of the Time-Resolved Fluorescence Spectrum and Red Edge Effect of ANF in a Room-Temperature Ionic Liquid. J. Phys. Chem. B 2006, 110, 11025–11028. 10.1021/jp061652y. [DOI] [PubMed] [Google Scholar]
- Karmakar S.; Dasgupta C.; Sastry S. Growing Length and Time Scales in Glass-Forming Liquids. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 3675–3679. 10.1073/pnas.0811082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Kim C.; Sung B. J. Simulation Study of Seemingly Fickian but Heterogeneous Dynamics of Two Dimensional Colloids. Phys. Rev. Lett. 2013, 110, 047801. 10.1103/PhysRevLett.110.047801. [DOI] [PubMed] [Google Scholar]
- Terranova Z.; Corcelli S. Molecular Dynamics Investigation of the Vibrational Spectroscopy of Isolated Water in an Ionic Liquid. J. Phys. Chem. B 2014, 118, 8264–8272. 10.1021/jp501631m. [DOI] [PubMed] [Google Scholar]
- Ren Z.; Brinzer T.; Dutta S.; Garrett-Roe S. Thiocyanate as a Local Probe of Ultrafast Structure and Dynamics in Imidazolium-Based Ionic Liquids: Water-Induced Heterogeneity and Cation-Induced Ion Pairing. J. Phys. Chem. B 2015, 119, 4699–4712. 10.1021/jp512851v. [DOI] [PubMed] [Google Scholar]
- Giammanco C. H.; Kramer P. L.; Wong D. B.; Fayer M. D. Water Dynamics in 1-Alkyl-3-methylimidazolium Tetrafluoroborate Ionic Liquids. J. Phys. Chem. B 2016, 120, 11523–11538. 10.1021/acs.jpcb.6b08410. [DOI] [PubMed] [Google Scholar]
- Bailey H. E.; Wang Y.-L.; Fayer M. D. Impact of Hydrogen Bonding on the Dynamics and Structure of Protic Ionic Liquid/Water Binary Mixtures. J. Phys. Chem. B 2017, 121, 8564–8576. 10.1021/acs.jpcb.7b06376. [DOI] [PubMed] [Google Scholar]
- Ridings C.; Lockett V.; Andersson G. Effect of the Aliphatic Chain Length on Electrical Double Layer Formation at the Liquid/Vacuum Interface in the [Cnmim][BF4] Ionic Liquid Series. Phys. Chem. Chem. Phys. 2011, 13, 17177–17184. 10.1039/c1cp20910h. [DOI] [PubMed] [Google Scholar]
- Stoppa A.; Hunger J.; Hefter G.; Buchner R. Structure and Dynamics of 1-N-alkyl-3-N-methylimidazolium Tetrafluoroborate+Acetonitrile Mixtures. J. Phys. Chem. B 2012, 116, 7509–7521. 10.1021/jp3020673. [DOI] [PubMed] [Google Scholar]
- Gannon T. J.; Law G.; Watson P. R.; Carmichael A. J.; Seddon K. R. First Observation of Molecular Composition and Orientation at the Surface of a Room-Temperature Ionic Liquid. Langmuir 1999, 15, 8429–8434. 10.1021/la990589j. [DOI] [Google Scholar]
- Law G.; Watson P. R.; Carmichael A. J.; Seddon K. R. Molecular Composition and Orientation at Surface of Room-Temperature Ionic Liquids: Effect of Molecular Structure. Phys. Chem. Chem. Phys. 2001, 3, 2879–2885. 10.1039/b101952j. [DOI] [Google Scholar]
- Knorr A.; Fumino K.; Bonsa A.-M.; Ludwig R. Spectroscopic Evidence of ‘Jumping and Pecking’of Cholinium and H-bond Enhanced Cation-Cation Interaction in Ionic Liquids. Phys. Chem. Chem. Phys. 2015, 17, 30978–30982. 10.1039/C5CP03412D. [DOI] [PubMed] [Google Scholar]
- Zaitsau D. H.; Emel’yanenko V. N.; Stange P.; Schick C.; Verevkin S. P.; Ludwig R. Dispersion and Hydrogen Bonding Rule: Why the Vaporization Enthalpies of Aprotic Ionic Liquids are Significantly Larger Than Those of Protic Ionic Liquids. Angew. Chem., Int. Ed. 2016, 55, 11682–11686. 10.1002/anie.201605633. [DOI] [PubMed] [Google Scholar]
- Umebayashi Y.; Jiang J.-C.; Lin K.-H.; Shan Y.-L.; Fujii K.; Seki S.; Ishiguro S.-I.; Lin S. H.; Chang H.-C. Solvation and Microscopic Properties of Ionic Liquid/Acetonitrile Mixtures Probed by High-Pressure Infrared Spectroscopy. J. Chem. Phys. 2009, 131, 234502. 10.1063/1.3273206. [DOI] [PubMed] [Google Scholar]
- Griffin P. J.; Holt A. P.; Tsunashima K.; Sangoro J. R.; Kremer F.; Sokolov A. P. Ion Transport and Structural Dynamics in Homologous Ammonium and Phosphonium-Based Room Temperature Ionic Liquids. J. Chem. Phys. 2015, 142, 084501. 10.1063/1.4913239. [DOI] [PubMed] [Google Scholar]
- Aliotta F.; Ponterio R. C.; Saija F.; Salvato G.; Triolo A. Excess Ehermodynamic Properties in Mixtures of a Representative Room-Temperature Ionic Liquid and Acetonitrile. J. Phys. Chem. B 2007, 111, 10202–10207. 10.1021/jp072836v. [DOI] [PubMed] [Google Scholar]
- Chung S. H.; Lopato R.; Greenbaum S. G.; Shirota H.; Castner E. W. Jr.; Wishart J. F. Nuclear Magnetic Resonance Study of the Dynamics of Imidazolium Ionic Liquids with CH2Si(CH3)3 vs CH2C(CH3)3 Substituents. J. Phys. Chem. B 2007, 111, 4885–4893. 10.1021/jp071755w. [DOI] [PubMed] [Google Scholar]
- Borodin O.; Gorecki W.; Smith G. D.; Armand M. Molecular Dynamics Simulation and Pulsed-Field Gradient NMR Studies of Bis(fluorosulfonyl)imide (FSI) and Bis[(trifluoromethyl)sulfonyl]imide (TFSI)-Based Ionic Liquids. J. Phys. Chem. B 2010, 114, 6786–6798. 10.1021/jp911950q. [DOI] [PubMed] [Google Scholar]
- Mbondo Tsamba B. E.; Sarraute S.; Traïkia M.; Husson P. Transport Properties and Ionic Association in Pure Imidazolium-Based Ionic Liquids as a Function of Temperature. J. Chem. Eng. Data 2014, 59, 1747–1754. 10.1021/je400841s. [DOI] [Google Scholar]
- Marekha B. A.; Kalugin O. N.; Bria M.; Idrissi A. Probing Structural Patterns of Ion Association and Solvation in Mixtures of Imidazolium Ionic Liquids with Acetonitrile by Means of Relative 1H and 13C NMR Chemical Shifts. Phys. Chem. Chem. Phys. 2015, 17, 23183–23194. 10.1039/C5CP02748A. [DOI] [PubMed] [Google Scholar]
- Lynden-Bell R. M.; Xue L.; Tamas G.; Quitevis E. L. Local Structure and Intermolecular Dynamics of an Equimolar Benzene and 1,3-Dimethylimidazolium Bis[(trifluoromethane)sulfonyl]amide Mixture: Molecular Dynamics Simulations and OKE Spectroscopic Measurements. J. Chem. Phys. 2014, 141, 044506. 10.1063/1.4890529. [DOI] [PubMed] [Google Scholar]
- Sturlaugson A. L.; Fruchey K. S.; Fayer M. D. Orientational Dynamics of Room Temperature Ionic Liquid/Water Mixtures: Water-Induced Structure. J. Phys. Chem. B 2012, 116, 1777–1787. 10.1021/jp209942r. [DOI] [PubMed] [Google Scholar]
- Fayer M. D. Dynamics and Structure of Room Temperature Ionic Liquids. Chem. Phys. Lett. 2014, 616, 259–274. 10.1016/j.cplett.2014.09.062. [DOI] [Google Scholar]
- Giammanco C. H.; Kramer P. L.; Yamada S. A.; Nishida J.; Tamimi A.; Fayer M. D. Carbon Dioxide in an Ionic Liquid: Structural and Rotational Dynamics. J. Chem. Phys. 2016, 144, 104506. 10.1063/1.4943390. [DOI] [PubMed] [Google Scholar]
- Shin J. Y.; Yamada S. A.; Fayer M. D. Influence of Water on Carbon Dioxide and Room Temperature Ionic Liquid Dynamics: Supported Ionic Liquid Membrane vs the Bulk Liquid. J. Phys. Chem. B 2018, 122, 2389–2395. 10.1021/acs.jpcb.8b01163. [DOI] [PubMed] [Google Scholar]
- Bailey H. E.; Wang Y.-L.; Fayer M. D. The Influence of Hydrophilicity on the Orientational Dynamics and Structures of Imidazolium-Based Ionic Liquid/Water Binary Mixtures. J. Chem. Phys. 2018, 149, 044501. 10.1063/1.5038563. [DOI] [PubMed] [Google Scholar]
- Bardak F.; Xiao D.; Hines L. G. Jr; Son P.; Bartsch R. A.; Quitevis E. L.; Yang P.; Voth G. A. Nanostructural Organization in Acetonitrile/Ionic Liquid Mixtures: Molecular Dynamics Simulations and Optical Kerr Effect Spectroscopy. ChemPhysChem 2012, 13, 1687–1700. 10.1002/cphc.201200026. [DOI] [PubMed] [Google Scholar]
- Shultz M. J.; Baldelli S.; Schnitzer C.; Simonelli D. Aqueous Solution/Air Interfaces Probed with Sum Frequency Generation Spectroscopy. J. Phys. Chem. B 2002, 106, 5313–5324. 10.1021/jp014466v. [DOI] [PubMed] [Google Scholar]
- Rivera-Rubero S.; Baldelli S. Surface Characterization of 1-Butyl-3-methylimidazolium Br–, I–, PF6–, BF4–, (CF3SO2)2N–, SCN–, CH3SO3–, CH3SO4–, and (CN)2N– Ionic Liquids by Sum Frequency Generation. J. Phys. Chem. B 2006, 110, 4756–4765. 10.1021/jp0563989. [DOI] [PubMed] [Google Scholar]
- Peñalber-Johnstone C.; Adamová G.; Plechkova N. V.; Bahrami M.; Ghaed-Sharaf T.; Ghatee M. H.; Seddon K. R.; Baldelli S. Sum Frequency Generation Spectroscopy of Tetraalkylphosphonium Ionic Liquids at the Air-Liquid Interface. J. Chem. Phys. 2018, 148, 193841. 10.1063/1.5009674. [DOI] [PubMed] [Google Scholar]
- Santos C. S.; Baldelli S. Alkyl Chain Interaction at the Surface of Room Temperature Ionic Liquids: Systematic Variation of Alkyl Chain Length (R = C1–C4, C8) in Both Cation and Anion of [RMIM][ROSO3] by Sum Frequency Generation and Surface Tension. J. Phys. Chem. B 2009, 113, 923–933. 10.1021/jp807924g. [DOI] [PubMed] [Google Scholar]
- Martinez I. S.; Santos C.; Baldelli S. Structural Study at the Gas-Liquid Interface of 1-Alkyl-3-methylimidazolium Alkylsulfates Using Surface Potential Measurements. ChemPhysChem 2012, 13, 1818–1824. 10.1002/cphc.201100985. [DOI] [PubMed] [Google Scholar]
- Baldelli S. Interfacial Structure of Room-Temperature Ionic Liquids at the Solid–Liquid Interface as Probed by Sum Frequency Generation Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 244–252. 10.1021/jz301835j. [DOI] [PubMed] [Google Scholar]
- Yan C.; Thomaz J. E.; Wang Y.-L.; Nishida J.; Yuan R.; Breen J. P.; Fayer M. D. Ultrafast to Ultraslow Dynamics of a Langmuir Monolayer at the Air/Water Interface Observed with Reflection Enhanced 2D IR Spectroscopy. J. Am. Chem. Soc. 2017, 139, 16518–16527. 10.1021/jacs.7b06602. [DOI] [PubMed] [Google Scholar]
- Humphreys E. K.; Allan P. K.; Welbourn R. J. L.; Youngs T. G. A.; Soper A. K.; Grey C. P.; Clarke S. M. A Neutron Diffraction Study of the Electrochemical Double Layer Capacitor Electrolyte Tetrapropylammonium Bromide in Acetonitrile. J. Phys. Chem. B 2015, 119, 15320–15333. 10.1021/acs.jpcb.5b08248. [DOI] [PubMed] [Google Scholar]
- Niemann T.; Neumann J.; Stange P.; Gärtner S.; Youngs T. G.; Paschek D.; Warr G. G.; Atkin R.; Ludwig R. The Double-Faced Nature of Hydrogen Bonding in Hydroxy-Functionalized Ionic Liquids Shown by Neutron Diffraction and Molecular Dynamics Simulations. Angew. Chem., Int. Ed. 2019, 58, 12887–12892. 10.1002/anie.201904712. [DOI] [PubMed] [Google Scholar]
- Daillant J.; Gibaud A.. X-ray and Neutron Reflectivity: Principles and Applications; Springer-Verlag: Berlin-Heidelberg, 2008. [Google Scholar]
- Mars J.; Hou B.; Weiss H.; Li H.; Konovalov O.; Festersen S.; Murphy B. M.; Rütt U.; Bier M.; Mezger M. Surface Induced Smectic Order in Ionic Liquids – An X-ray Reflectivity Study of [C22C1im][NTf2]. Phys. Chem. Chem. Phys. 2017, 19, 26651–26661. 10.1039/C7CP04852A. [DOI] [PubMed] [Google Scholar]
- Griffin L. R.; Browning K. L.; Clarke S. M.; Smith A. M.; Perkin S.; Skoda M.; Norman S. E. Direct Measurements of Ionic Liquid Layering at a Single Mica-Liquid Interface and in Nano-Films Between Two Mica-Liquid Interfaces. Phys. Chem. Chem. Phys. 2017, 19, 297–304. 10.1039/C6CP05757H. [DOI] [PubMed] [Google Scholar]
- Atkin R.; Warr G. G. The Smallest Amphiphiles: Nanostructure in Protic Room-Temperature Ionic Liquids with Short Alkyl Groups. J. Phys. Chem. B 2008, 112, 4164–4166. 10.1021/jp801190u. [DOI] [PubMed] [Google Scholar]
- Abe H.; Takekiyo T.; Shigemi M.; Yoshimura Y.; Tsuge S.; Hanasaki T.; Ohishi K.; Takata S.; Suzuki J.-I. Direct Evidence of Confined Water in Room-Temperature Ionic Liquids by Complementary Use of Small-Angle X-ray and Neutron Scattering. J. Phys. Chem. Lett. 2014, 5, 1175–1180. 10.1021/jz500299z. [DOI] [PubMed] [Google Scholar]
- Migliorati V.; Zitolo A.; D’Angelo P. Using a Combined Theoretical and Experimental Approach to Understand the Structure and Dynamics of Imidazolium-Based Ionic Liquids/Water Mixtures. 1. MD Simulations. J. Phys. Chem. B 2013, 117, 12505–12515. 10.1021/jp4048677. [DOI] [PubMed] [Google Scholar]
- Gao J.; Wagner N. J. Water Nanocluster Formation in the Ionic Liquid 1-Butyl-3-methylimidazolium Tetrafluoroborate ([C4mim][BF4])–D2O Mixtures. Langmuir 2016, 32, 5078–5084. 10.1021/acs.langmuir.6b00494. [DOI] [PubMed] [Google Scholar]
- Bier M.; Mars J.; Li H.; Mezger M. Salt-Induced Microheterogeneities in Binary Liquid Mixtures. Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top. 2017, 96, 022603. 10.1103/PhysRevE.96.022603. [DOI] [PubMed] [Google Scholar]
- Cabry C. P.; D’Andrea L.; Shimizu K.; Grillo I.; Li P.; Rogers S.; Bruce D. W.; Canongia Lopes J. N.; Slattery J. M. Exploring the Bulk-Phase Structure of Ionic Liquid Mixtures Using Small-Angle Neutron Scattering. Faraday Discuss. 2018, 206, 265–289. 10.1039/C7FD00167C. [DOI] [PubMed] [Google Scholar]
- Blundell R. K.; Licence P. Quaternary Ammonium and Phosphonium Based Ionic Liquids: A Comparison of Common Anions. Phys. Chem. Chem. Phys. 2014, 16, 15278–15288. 10.1039/C4CP01901F. [DOI] [PubMed] [Google Scholar]
- Lovelock K.; Kolbeck C.; Cremer T.; Paape N.; Schulz P.; Wasserscheid P.; Maier F.; Steinruck H.-P. Influence of Different Substituents on the Surface Composition of Ionic Liquids Studied Using ARXPS. J. Phys. Chem. B 2009, 113, 2854–2864. 10.1021/jp810637d. [DOI] [PubMed] [Google Scholar]
- Olschewski M.; Gustus R.; Höfft O.; Lahiri A.; Endres F. Monochromatic X-ray Photoelectron Spectroscopy Study of Three Different Ionic Liquids in Interaction with Lithium-Decorated Copper Surfaces. J. Phys. Chem. C 2017, 121, 2675–2682. 10.1021/acs.jpcc.6b10139. [DOI] [Google Scholar]
- Jeon Y.; Vaknin D.; Bu W.; Sung J.; Ouchi Y.; Sung W.; Kim D. Surface Nanocrystallization of an Ionic Liquid. Phys. Rev. Lett. 2012, 108, 055502. 10.1103/PhysRevLett.108.055502. [DOI] [PubMed] [Google Scholar]
- Campetella M.; Mariani A.; Sadun C.; Wu B.; Castner E. W. Jr; Gontrani L. Structure and Dynamics of Propylammonium Nitrate-Acetonitrile Mixtures: An Intricate Multi-Scale System Probed with Experimental and Theoretical Techniques. J. Chem. Phys. 2018, 148, 134507. 10.1063/1.5021868. [DOI] [PubMed] [Google Scholar]
- Niga P.; Wakeham D.; Nelson A.; Warr G. G.; Rutland M.; Atkin R. Structure of Ethylammonium Nitrate Surface: An X-ray Reflectivity and Vibrational Sum Frequency Spectroscopy Study. Langmuir 2010, 26, 8282–8288. 10.1021/la904697g. [DOI] [PubMed] [Google Scholar]
- Uysal A.; Zhou H.; Feng G.; Lee S. S.; Li S.; Fenter P.; Cummings P. T.; Fulvio P. F.; Dai S.; McDonough J. K.; Gogotsi Y. Structural Origins of Potential Dependent Hysteresis at the Electrified Graphene/Ionic Liquid Interface. J. Phys. Chem. C 2014, 118, 569–574. 10.1021/jp4111025. [DOI] [Google Scholar]
- Mezger M.; Schroder H.; Reichert H.; Schramm S.; Okasinski J. S.; Schoder S.; Honkimaki V.; Deutsch M.; Ocko B. M.; Ralston J.; Rohwerder M.; Stratmann M.; Dosch H. Molecular Layering of Fluorinated Ionic Liquids at a Charged Sapphire(0001) Surface. Science 2008, 322, 424–428. 10.1126/science.1164502. [DOI] [PubMed] [Google Scholar]
- Tamam L.; Ocko B.; Reichert H.; Deutsch M. Checkerboard Self-Patterning of an Ionic Liquid Film on Mercury. Phys. Rev. Lett. 2011, 106, 197801. 10.1103/PhysRevLett.106.197801. [DOI] [PubMed] [Google Scholar]
- Nemoto F.; Kofu M.; Nagao M.; Ohishi K.; Takata S.-i.; Suzuki J.-i.; Yamada T.; Shibata K.; Ueki T.; Kitazawa Y.; Watanabe M.; Yamamuro O. Neutron Scattering Studies on Short- and Long-Range Layer Structures and Related Dynamics in Imidazolium-Based Ionic Liquids. J. Chem. Phys. 2018, 149, 054502. 10.1063/1.5037217. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Rouha M.; Feng G.; Lee S. S.; Docherty H.; Fenter P.; Cummings P. T.; Fulvio P. F.; Dai S.; McDonough J.; Presser V.; Gogotsi Y. Nanoscale Perturbations of Room Temperature Ionic Liquid Structure at Charged and Uncharged Interfaces. ACS Nano 2012, 6, 9818–9827. 10.1021/nn303355b. [DOI] [PubMed] [Google Scholar]
- Tomita K.; Mizukami M.; Nakano S.; Ohta N.; Yagi N.; Kurihara K. X-ray Diffraction and Resonance Shear Measurement of Nano-Confined Ionic Liquids. Phys. Chem. Chem. Phys. 2018, 20, 13714–13721. 10.1039/C7CP08611C. [DOI] [PubMed] [Google Scholar]
- Triolo A.; Russina O.; Fazio B.; Appetecchi G. B.; Carewska M.; Passerini S. Nanoscale Organization in Piperidinium-Based Room Temperature Ionic Liquids. J. Chem. Phys. 2009, 130, 164521. 10.1063/1.3119977. [DOI] [PubMed] [Google Scholar]
- Hoffmann V.; Lahiri A.; Borisenko N.; Carstens T.; Pulletikurthi G.; Borodin A.; Atkin R.; Endres F. Nanostructure of the H-Terminated p-Si(111)/Ionic Liquid Interface and the Effect of Added Lithium Salt. Phys. Chem. Chem. Phys. 2017, 19, 54–58. 10.1039/C6CP06306C. [DOI] [PubMed] [Google Scholar]
- Bovio S.; Podesta A.; Lenardi C.; Milani P. Evidence of Extended Solidlike Layering in [Bmim][NTf2] Ionic Liquid Thin Films at Room-Temperature. J. Phys. Chem. B 2009, 113, 6600–6603. 10.1021/jp9022234. [DOI] [PubMed] [Google Scholar]
- Atkin R.; Warr G. G. Structure in Confined Room-Temperature Ionic Liquids. J. Phys. Chem. C 2007, 111, 5162–5168. 10.1021/jp067420g. [DOI] [Google Scholar]
- Werzer O.; Cranston E. D.; Warr G. G.; Atkin R.; Rutland M. W. Ionic Liquid Nanotribology: Mica-Silica Interactions in Ethylammonium Nitrate. Phys. Chem. Chem. Phys. 2012, 14, 5147–5152. 10.1039/C1CP23134K. [DOI] [PubMed] [Google Scholar]
- Müller C.; Németh K.; Vesztergom S.; Pajkossy T.; Jacob T. The Interface Between HOPG and 1-Butyl-3-methyl-imidazolium Hexafluorophosphate. Phys. Chem. Chem. Phys. 2016, 18, 916–925. 10.1039/C5CP05406K. [DOI] [PubMed] [Google Scholar]
- Wen R.; Rahn B.; Magnussen O. M. Potential-Dependent Adlayer Structure and Dynamics at the Ionic Liquid/Au (111) Interface: A Molecular-Scale in situ Video-STM Study. Angew. Chem., Int. Ed. 2015, 54, 6062–6066. 10.1002/anie.201501715. [DOI] [PubMed] [Google Scholar]
- Merlet C.; Limmer D. T.; Salanne M.; van Roij R.; Madden P. A.; Chandler D.; Rotenberg B. The Electric Double Layer Has a Life of Its Own. J. Phys. Chem. C 2014, 118, 18291–18298. 10.1021/jp503224w. [DOI] [Google Scholar]
- Su Y. Z.; Fu Y. C.; Yan J. W.; Chen Z. B.; Mao B. W. Double Layer of Au(100)/Ionic Liquid Interface and Its Stability in Imidazolium-Based Ionic Liquids. Angew. Chem., Int. Ed. 2009, 48, 5148–5151. 10.1002/anie.200900300. [DOI] [PubMed] [Google Scholar]
- Gebbie M. A.; Valtiner M.; Banquy X.; Fox E. T.; Henderson W. A.; Israelachvili J. N. Ionic Liquids Behave as Dilute Electrolyte Solutions. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 9674–9679. 10.1073/pnas.1307871110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbie M. A.; Valtiner M.; Banquy X.; Henderson W. A.; Israelachvili J. N. Experimental Observations Demonstrate that Ionic Liquids Form Both Bound (Stern) and Diffuse Electric Double Layers. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, E4122. 10.1073/pnas.1315608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkin S.; Crowhurst L.; Niedermeyer H.; Welton T.; Smith A. M.; Gosvami N. N. Self-Assembly in the Electrical Double Layer of Ionic Liquids. Chem. Commun. 2011, 47, 6572–6574. 10.1039/c1cc11322d. [DOI] [PubMed] [Google Scholar]
- Dragoni D.; Manini N.; Ballone P. Interfacial Layering of a Room-Temperature Ionic Liquid Thin Film on Mica: A Computational Investigation. ChemPhysChem 2012, 13, 1772–1780. 10.1002/cphc.201100947. [DOI] [PubMed] [Google Scholar]
- Lhermerout R.; Perkin S. Nanoconfined Ionic Liquids: Disentangling Electrostatic and Viscous Forces. Phys. Rev. Fluids 2018, 3, 014201. 10.1103/PhysRevFluids.3.014201. [DOI] [Google Scholar]
- Smith A. M.; Perkin S. Influence of Lithium Solutes on Double-Layer Structure of Ionic Liquids. J. Phys. Chem. Lett. 2015, 6, 4857–4861. 10.1021/acs.jpclett.5b02166. [DOI] [PubMed] [Google Scholar]
- Sturlaugson A. L.; Arima A. Y.; Bailey H. E.; Fayer M. D. Orientational Dynamics in a Lyotropic Room Temperature Ionic Liquid. J. Phys. Chem. B 2013, 117, 14775–14784. 10.1021/jp407325b. [DOI] [PubMed] [Google Scholar]
- Zahn S.; Stark A. Order in the Chaos: The Secret of the Large Negative Entropy of Dissolving 1-Alkyl-3-methylimidazolium Chloride in Trihexyltetradecylphosphonium Chloride. Phys. Chem. Chem. Phys. 2015, 17, 4034–4037. 10.1039/C4CP05074F. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Sarman S.; Li B.; Laaksonen A. Multiscale Modeling of the Trihexyltetradecylphosphonium Chloride Ionic Liquid. Phys. Chem. Chem. Phys. 2015, 17, 22125–22135. 10.1039/C5CP02586A. [DOI] [PubMed] [Google Scholar]
- Thompson D.; Coleman S.; Diamond D.; Byrne R. Electronic Structure Calculations and Physicochemical Experiments Quantify the Competitive Liquid Ion Association and Probe Stabilisation Effects for Nitrobenzospiropyran in Phosphonium-Based Ionic Liquids. Phys. Chem. Chem. Phys. 2011, 13, 6156–6168. 10.1039/c0cp02717k. [DOI] [PubMed] [Google Scholar]
- Yu C.-J.; Ri U.-S.; Ri G.-C.; Kim J.-S. Revealing the Formation and Electrochemical Properties of Bis(trifluoromethanesulfonyl)imide Intercalated Graphite with First-Principles Calculations. Phys. Chem. Chem. Phys. 2018, 20, 14124–14132. 10.1039/C8CP01468J. [DOI] [PubMed] [Google Scholar]
- Atilhan M.; Aparicio S. Theoretical Study of Low Viscous Ionic Liquids at the Graphene Interface. J. Phys. Chem. C 2018, 122, 1645–1656. 10.1021/acs.jpcc.7b10434. [DOI] [Google Scholar]
- Pensado A. S.; Malberg F.; Gomes M. F. C.; Pádua A. A. H.; Fernández J.; Kirchner B. Interactions and Structure of Ionic Liquids on Graphene and Carbon Nanotubes Surfaces. RSC Adv. 2014, 4, 18017–18024. 10.1039/C4RA02059F. [DOI] [Google Scholar]
- Li H.; Atkin R.; Page A. J. Combined Friction Force Microscopy and Quantum Chemical Investigation of Tribotronic Response at Propylammonium Nitrate-Graphite Interface. Phys. Chem. Chem. Phys. 2015, 17, 16047–16052. 10.1039/C5CP01952D. [DOI] [PubMed] [Google Scholar]
- Matthews R. P.; Welton T.; Hunt P. A. Competitive π Interactions and Hydrogen Bonding within Imidazolium Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 3238–3253. 10.1039/c3cp54672a. [DOI] [PubMed] [Google Scholar]
- Dong D.; Hooper J. B.; Bedrov D. Structural and Dynamical Properties of Tetraalkylammonium Bromide Aqueous Solutions: A Molecular Dynamics Simulation Study Using a Polarizable Force Field. J. Phys. Chem. B 2017, 121, 4853–4863. 10.1021/acs.jpcb.7b01032. [DOI] [PubMed] [Google Scholar]
- Voss J. M.; Marsh B. M.; Zhou J.; Garand E. Interaction Between Ionic Liquid Cation and Water: Infrared Predissociation Study of [bmim]+(H2O)n Clusters. Phys. Chem. Chem. Phys. 2016, 18, 18905–18913. 10.1039/C6CP02730J. [DOI] [PubMed] [Google Scholar]
- Dias N.; Shimizu K.; Morgado P.; Filipe E. J.; Canongia Lopes J. N.; Vaca Chávez F. N. Charge Templates in Aromatic Plus Ionic Liquid Systems Revisited: NMR Experiments and Molecular Dynamics Simulations. J. Phys. Chem. B 2014, 118, 5772–5780. 10.1021/jp503130y. [DOI] [PubMed] [Google Scholar]
- Weber H.; Kirchner B. Complex Structural and Dynamical Interplay of Cyano-Based Ionic Liquids. J. Phys. Chem. B 2016, 120, 2471–2483. 10.1021/acs.jpcb.6b00098. [DOI] [PubMed] [Google Scholar]
- Zahn S.; Wendler K.; Delle Site L.; Kirchner B. Depolarization of Water in Protic Ionic Liquids. Phys. Chem. Chem. Phys. 2011, 13, 15083–15093. 10.1039/c1cp20288j. [DOI] [PubMed] [Google Scholar]
- Annapureddy H. V.; Kashyap H. K.; De Biase P. M.; Margulis C. J. What is the Origin of the Prepeak in the X-ray Scattering of Imidazolium-Based Room-Temperature Ionic Liquids?. J. Phys. Chem. B 2010, 114, 16838–16846. 10.1021/jp108545z. [DOI] [PubMed] [Google Scholar]
- Sha M.; Liu Y.; Dong H.; Luo F.; Jiang F.; Tang Z.; Zhu G.; Wu G. Origin of Heterogeneous Dynamics in Local Molecular Structures of Ionic Liquids. Soft Matter 2016, 12, 8942–8949. 10.1039/C6SM01797E. [DOI] [PubMed] [Google Scholar]
- Liu H.; Maginn E. A Molecular Dynamics Investigation of the Structural and Dynamic Properties of the Ionic Liquid 1-n-Butyl-3-methylimidazolium Bis(trifluoromethanesulfonyl)imide. J. Chem. Phys. 2011, 135, 124507. 10.1063/1.3643124. [DOI] [PubMed] [Google Scholar]
- Kowsari M.; Alavi S.; Ashrafizaadeh M.; Najafi B. Molecular Dynamics Simulation of Imidazolium-Based Ionic Liquids. II. Transport Coefficients. J. Chem. Phys. 2009, 130, 014703. 10.1063/1.3042279. [DOI] [PubMed] [Google Scholar]
- Price D. L.; Borodin O.; González M. A.; Kofu M.; Shibata K.; Yamada T.; Yamamuro O.; Saboungi M.-L. Relaxation in a Prototype Ionic Liquid: Influence of Water on Dynamics. J. Phys. Chem. Lett. 2017, 8, 715–719. 10.1021/acs.jpclett.6b02871. [DOI] [PubMed] [Google Scholar]
- D’Angelo P.; Zitolo A.; Aquilanti G.; Migliorati V. Using a Combined Theoretical and Experimental Approach to Understand the Structure and Dynamics of Imidazolium-Based Ionic Liquids/Water Mixtures. 2. EXAFS Spectroscopy. J. Phys. Chem. B 2013, 117, 12516–12524. 10.1021/jp404868a. [DOI] [PubMed] [Google Scholar]
- Moreno M.; Castiglione F.; Mele A.; Pasqui C.; Raos G. Interaction of Water with the Model Ionic Liquid [Bmim][BF4]: Molecular Dynamics Simulations and Comparison with NMR Data. J. Phys. Chem. B 2008, 112, 7826–7836. 10.1021/jp800383g. [DOI] [PubMed] [Google Scholar]
- Mariani A.; Caminiti R.; Gontrani L. Water and Hexane in an Ionic Liquid: Computational Evidence of Association Under High Pressure. Phys. Chem. Chem. Phys. 2017, 19, 8661–8666. 10.1039/C6CP08450H. [DOI] [PubMed] [Google Scholar]
- Yaghini N.; Gómez-González V.; Varela L. M.; Martinelli A. Structural Origin of Proton Mobility in a Protic Ionic Liquid/Imidazole Mixture: Insights From Computational and Experimental Results. Phys. Chem. Chem. Phys. 2016, 18, 23195–23206. 10.1039/C6CP03058K. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Shah F. U.; Glavatskih S.; Antzutkin O. N.; Laaksonen A. Atomistic Insight into Orthoborate-Based Ionic Liquids: Force Field Development and Evaluation. J. Phys. Chem. B 2014, 118, 8711–8723. 10.1021/jp503029d. [DOI] [PubMed] [Google Scholar]
- Spickermann C.; Thar J.; Lehmann S.; Zahn S.; Hunger J.; Buchner R.; Hunt P.; Welton T.; Kirchner B. Why Are Ionic Liquid Ions Mainly Associated in Water? A Car-Parrinello Study of 1-Ethyl-3-methyl-imidazolium Chloride Water Mixture. J. Chem. Phys. 2008, 129, 104505. 10.1063/1.2974098. [DOI] [PubMed] [Google Scholar]
- Brehm M.; Weber H.; Pensado A. S.; Stark A.; Kirchner B. Proton Transfer and Polarity Changes in Ionic Liquid-Water Mixtures: A Perspective on Hydrogen Bonds from ab Initio Molecular Dynamics at the Example of 1-Ethyl-3-methylimidazolium Acetate–Water Mixtures—Part 1. Phys. Chem. Chem. Phys. 2012, 14, 5030–5044. 10.1039/c2cp23983c. [DOI] [PubMed] [Google Scholar]
- Bhargava B.; Klein M. L. Formation of Interconnected Aggregates in Aqueous Dicationic Ionic Liquid Solutions. J. Chem. Theory Comput. 2010, 6, 873–879. 10.1021/ct900674t. [DOI] [PubMed] [Google Scholar]
- Liu X.; Zhou G.; Huo F.; Wang J.; Zhang S. Unilamellar Vesicle Formation and Microscopic Structure of Ionic Liquids in Aqueous Solutions. J. Phys. Chem. C 2016, 120, 659–667. 10.1021/acs.jpcc.5b08977. [DOI] [Google Scholar]
- Heid E.; Docampo-Álvarez B.; Varela L. M.; Prosenz K.; Steinhauser O.; Schröder C. Langevin Behavior of the Dielectric Decrement in Ionic Liquid Water Mixtures. Phys. Chem. Chem. Phys. 2018, 20, 15106–15117. 10.1039/C8CP02111B. [DOI] [PubMed] [Google Scholar]
- Kobayashi T.; Reid J. E.; Shimizu S.; Fyta M.; Smiatek J. The Properties of Residual Water Molecules in Ionic Liquids: A Comparison Between Direct and Inverse Kirkwood–Buff Approaches. Phys. Chem. Chem. Phys. 2017, 19, 18924–18937. 10.1039/C7CP03717A. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Wang X.; Liu Q.; Ma X.; Fang D.; Jiang X.; Guan W. Dynamic Phase Change and Local Structures in IL-Containing Mixtures: Classical MD Simulations and Experiments. Phys. Chem. Chem. Phys. 2017, 19, 3028–3038. 10.1039/C6CP06300D. [DOI] [PubMed] [Google Scholar]
- Borodin O.; Price D. L.; Aoun B.; González M. A.; Hooper J. B.; Kofu M.; Kohara S.; Yamamuro O.; Saboungi M.-L. Effect of Water on the Structure of a Prototype Ionic Liquid. Phys. Chem. Chem. Phys. 2016, 18, 23474–23481. 10.1039/C6CP02191C. [DOI] [PubMed] [Google Scholar]
- Sha M.; Dong H.; Luo F.; Tang Z.; Zhu G.; Wu G. Dilute or Concentrated Electrolyte Solutions? Insight From Ionic Liquid/Water Electrolytes. J. Phys. Chem. Lett. 2015, 6, 3713–3720. 10.1021/acs.jpclett.5b01513. [DOI] [PubMed] [Google Scholar]
- Méndez-Morales T.; Carrete J.; Cabeza O.; Gallego L. J.; Varela L. M. Molecular Dynamics Simulation of the Structure and Dynamics of Water-1-Alkyl-3-methylimidazolium Ionic Liquid Mixtures. J. Phys. Chem. B 2011, 115, 6995–7008. 10.1021/jp202692g. [DOI] [PubMed] [Google Scholar]
- Annapureddy H. V.; Dang L. X. Pairing Mechanism Among Ionic Liquid Ions in Aqueous Solutions: A Molecular Dynamics Study. J. Phys. Chem. B 2013, 117, 8555–8560. 10.1021/jp404839w. [DOI] [PubMed] [Google Scholar]
- Hegde G. A.; Bharadwaj V. S.; Kinsinger C. L.; Schutt T. C.; Pisierra N. R.; Maupin C. M. Impact of Water Dilution and Cation Tail Length on Ionic Liquid Characteristics: Interplay Between Polar and Non-polar Interactions. J. Chem. Phys. 2016, 145, 064504. 10.1063/1.4960511. [DOI] [Google Scholar]
- Bernardes C. E.; Minas da Piedade M. E.; Canongia Lopes J. N. The Structure of Aqueous Solutions of a Hydrophilic Ionic Liquid: The Full Concentration Range of 1-Ethyl-3-methylimidazolium Ethylsulfate and Water. J. Phys. Chem. B 2011, 115, 2067–2074. 10.1021/jp1113202. [DOI] [PubMed] [Google Scholar]
- Martins V. L.; Nicolau B. G.; Urahata S. M.; Ribeiro M. C.; Torresi R. M. Influence of the Water Content on the Structure and Physicochemical Properties of an Ionic Liquid and Its Li+ Mixture. J. Phys. Chem. B 2013, 117, 8782–8792. 10.1021/jp312839z. [DOI] [PubMed] [Google Scholar]
- Pal T.; Biswas R. Composition Dependence of Dynamic Heterogeneity Time- and Length Scales in [Omim][BF4]/Water Binary Mixtures: Molecular Dynamics Simulation Study. J. Phys. Chem. B 2015, 119, 15683–15695. 10.1021/acs.jpcb.5b08763. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Ghorai P. K. Effect of Water on Structure and Dynamics of [BMIM][PF6] Ionic Liquid: An All-Atom Molecular Dynamics Simulation Investigation. J. Chem. Phys. 2016, 144, 114505. 10.1063/1.4944083. [DOI] [PubMed] [Google Scholar]
- Feng G.; Jiang D.-E.; Cummings P. T. Curvature Effect on the Capacitance of Electric Double Layers at Ionic Liquid/Onion-Like Carbon Interfaces. J. Chem. Theory Comput. 2012, 8, 1058–1063. 10.1021/ct200914j. [DOI] [PubMed] [Google Scholar]
- Richey F. W.; Dyatkin B.; Gogotsi Y.; Elabd Y. A. Ion Dynamics in Porous Carbon Electrodes in Supercapacitors Using in situ Infrared Spectroelectrochemistry. J. Am. Chem. Soc. 2013, 135, 12818–12826. 10.1021/ja406120e. [DOI] [PubMed] [Google Scholar]
- Deschamps M.; Gilbert E.; Azais P.; Raymundo-Piñero E.; Ammar M. R.; Simon P.; Massiot D.; Béguin F. Exploring Electrolyte Organization in Supercapacitor Electrodes with Solid-State NMR. Nat. Mater. 2013, 12, 351–358. 10.1038/nmat3567. [DOI] [PubMed] [Google Scholar]
- Saielli G.; Bagno A.; Wang Y. Insights on the Isotropic-to-Smectic a Transition in Ionic Liquid Crystals from Coarse-Grained Molecular Dynamics Simulations: The Role of Microphase Segregation. J. Phys. Chem. B 2015, 119, 3829–3836. 10.1021/jp5104565. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Lyubartsev A.; Lu Z.-Y.; Laaksonen A. Multiscale Coarse-Grained Simulations of Ionic Liquids: Comparison of Three Approaches to Derive Effective Potentials. Phys. Chem. Chem. Phys. 2013, 15, 7701–7712. 10.1039/c3cp44108c. [DOI] [PubMed] [Google Scholar]
- Bodo E.; Postorino P.; Mangialardo S.; Piacente G.; Ramondo F.; Bosi F.; Ballirano P.; Caminiti R. Structure of the Molten Salt Methylammonium Nitrate Explored by Experiments and Theory. J. Phys. Chem. B 2011, 115, 13149–13161. 10.1021/jp2070002. [DOI] [PubMed] [Google Scholar]
- Zahn S.; Thar J.; Kirchner B. Structure and Dynamics of the Protic Ionic Liquid Monomethylammonium Nitrate ([CH3NH3][NO3]) from ab Initio Molecular Dynamics Simulations. J. Chem. Phys. 2010, 132, 124506. 10.1063/1.3354108. [DOI] [PubMed] [Google Scholar]
- Bodo E.; Mangialardo S.; Ramondo F.; Ceccacci F.; Postorino P. Unravelling the Structure of Protic Ionic Liquids with Theoretical and Experimental Methods: Ethyl-, Propyl- and Butylammonium Nitrate Explored by Raman Spectroscopy and DFT Calculations. J. Phys. Chem. B 2012, 116, 13878–13888. 10.1021/jp3052714. [DOI] [PubMed] [Google Scholar]
- Umebayashi Y.; Chung W.-L.; Mitsugi T.; Fukuda S.; Takeuchi M.; Fujii K.; Takamuku T.; Kanzaki R.; Ishiguro S.-I. Liquid Structure and the Ion-Ion Interactions of Ethylammonium Nitrate Ionic Liquid Studied by Large Angle X-ray Scattering and Molecular Dynamics Simulations. J. Comput. Chem., Jpn. 2008, 7, 125–134. 10.2477/jccj.H2013. [DOI] [Google Scholar]
- Hayes R.; Imberti S.; Warr G. G.; Atkin R. Effect of Cation Alkyl Chain Length and Anion Type on Protic Ionic Liquid Nanostructure. J. Phys. Chem. C 2014, 118, 13998–14008. 10.1021/jp503429k. [DOI] [Google Scholar]
- Greaves T. L.; Kennedy D. F.; Kirby N.; Drummond C. J. Nanostructure Changes in Protic Ionic Liquids (PILs) Through Adding Solutes and Mixing PILs. Phys. Chem. Chem. Phys. 2011, 13, 13501–13509. 10.1039/c1cp20496c. [DOI] [PubMed] [Google Scholar]
- Campetella M.; Macchiagodena M.; Gontrani L.; Kirchner B. Effect of Alkyl Chain Length in Protic Ionic Liquids: An AIMD Perspective. Mol. Phys. 2017, 115, 1582–1589. 10.1080/00268976.2017.1308027. [DOI] [Google Scholar]
- Usui K.; Hunger J.; Bonn M.; Sulpizi M. Dynamical Heterogeneities of Rotational Motion in Room Temperature Ionic Liquids Evidenced by Molecular Dynamics Simulations. J. Chem. Phys. 2018, 148, 193811. 10.1063/1.5005143. [DOI] [PubMed] [Google Scholar]
- Jiang H. J.; FitzGerald P. A.; Dolan A.; Atkin R.; Warr G. G. Amphiphilic Self-Assembly of Alkanols in Protic Ionic Liquids. J. Phys. Chem. B 2014, 118, 9983–9990. 10.1021/jp504998t. [DOI] [PubMed] [Google Scholar]
- Mariani A.; Caminiti R.; Campetella M.; Gontrani L. Pressure-Induced Mesoscopic Disorder in Protic Ionic Liquids: First Computational Study. Phys. Chem. Chem. Phys. 2016, 18, 2297–2302. 10.1039/C5CP06800B. [DOI] [PubMed] [Google Scholar]
- Henderson W. A.; Fylstra P.; De Long H. C.; Trulove P. C.; Parsons S. Crystal Structure of the Ionic Liquid EtNH3NO3—Insights into the Thermal Phase Behavior of Protic Ionic Liquids. Phys. Chem. Chem. Phys. 2012, 14, 16041–16046. 10.1039/c2cp43079g. [DOI] [PubMed] [Google Scholar]
- Gordon C. M.; Holbrey J. D.; Kennedy A. R.; Seddon K. R. Ionic Liquid Crystals: Hexafluorophosphate Salts. J. Mater. Chem. 1998, 8, 2627–2636. 10.1039/a806169f. [DOI] [Google Scholar]
- Faria L. F.; Penna T. C.; Ribeiro M. C. Raman Spectroscopic Study of Temperature and Pressure Effects on the Ionic Liquid Propylammonium Nitrate. J. Phys. Chem. B 2013, 117, 10905–10912. 10.1021/jp4066778. [DOI] [PubMed] [Google Scholar]
- Migliorati V.; Ballirano P.; Gontrani L.; Triolo A.; Caminiti R. Thermal and Structural Properties of Ethylammonium Chloride and Its Mixture with Water. J. Phys. Chem. B 2011, 115, 4887–4899. 10.1021/jp2010138. [DOI] [PubMed] [Google Scholar]
- King M. V.; Lipscomb W. The Low-Temperature Modification of n-Propyl-ammonium Chloride. Acta Crystallogr. 1950, 3, 227–230. 10.1107/S0365110X50000562. [DOI] [Google Scholar]
- Migliorati V.; Ballirano P.; Gontrani L.; Russina O.; Caminiti R. Crystal Polymorphism of Propylammonium Chloride and Structural Properties of Its Mixture with Water. J. Phys. Chem. B 2011, 115, 11805–11815. 10.1021/jp206831d. [DOI] [PubMed] [Google Scholar]
- Matsumoto K.; Hagiwara R.; Yoshida R.; Ito Y.; Mazej Z.; Benkič P.; Žemva B.; Tamada O.; Yoshino H.; Matsubara S. Syntheses, Structures and Properties of 1-Ethyl-3-methylimidazolium Salts of Fluorocomplex Anions. Dalton Trans 2004, 144–149. 10.1039/B310162B. [DOI] [PubMed] [Google Scholar]
- Dymek C.J.; Grossie D. A.; Fratini A. V.; Wade Adams W. Evidence for the Presence of Hydrogen-Bonded Ion-Ion Interactions in the Molten Salt Precursor, 1-Methyl-3-ethylimidazolium Chloride. J. Mol. Struct. 1989, 213, 25–34. 10.1016/0022-2860(89)85103-8. [DOI] [Google Scholar]
- Elaiwi A.; Hitchcock P. B.; Seddon K. R.; Srinivasan N.; Tan Y.-M.; Welton T.; Zora J. A. Hydrogen Bonding in Imidazolium Salts and Its Implications for Ambient-Temperature Halogenoaluminate(III) Ionic Liquids. J. Chem. Soc., Dalton Trans. 1995, 3467–3472. 10.1039/dt9950003467. [DOI] [Google Scholar]
- Matsumoto K.; Hagiwara R.; Mazej Z.; Benkič P.; Žemva B. Crystal Structures of Frozen Room Temperature Ionic Liquids, 1-Ethyl-3-methylimidazolium Tetrafluoroborate (EMImBF4), Hexafluoroniobate (EMImNbF6) and Hexafluorotantalate (EMImTaF6), Determined by Low-Temperature X-ray Diffraction. Solid State Sci. 2006, 8, 1250–1257. 10.1016/j.solidstatesciences.2005.12.018. [DOI] [Google Scholar]
- Hamaguchi H.-O.; Saha S.; Ozawa R.; Hayashi S.. Raman and X-ray Studies on the Structure of [Bmim]X (X = Cl, Br, I, [BF4], [PF6]): Rotational Isomerism of the [Bmim]+ Cation. ACS Symp. Ser. 2005, Chapter 5, 90168–78. 10.1021/bk-2005-0901.ch005 [DOI] [Google Scholar]
- Berg R. W.; Deetlefs M.; Seddon K. R.; Shim I.; Thompson J. M. Raman and ab Initio Studies of Simple and Binary 1-Alkyl-3-methylimidazolium Ionic Liquids. J. Phys. Chem. B 2005, 109, 19018–19025. 10.1021/jp050691r. [DOI] [PubMed] [Google Scholar]
- Ozawa R.; Hayashi S.; Saha S.; Kobayashi A.; Hamaguchi H.-O. Rotational Isomerism and Structure of the 1-Butyl-3-methylimidazolium Cation in the Ionic Liquid State. Chem. Lett. 2003, 32, 948–949. 10.1246/cl.2003.948. [DOI] [Google Scholar]
- Hayashi S.; Ozawa R.; Hamaguchi H.-O. Raman Spectra, Crystal Polymorphism, and Structure of a Prototype Ionic-Liquid [BMIM]Cl. Chem. Lett. 2003, 32, 498–499. 10.1246/cl.2003.498. [DOI] [Google Scholar]
- Tschierske C. Molecular Self-Organization of Amphotropic Liquid Crystals. Prog. Polym. Sci. 1996, 21, 775–852. 10.1016/S0079-6700(96)00014-7. [DOI] [Google Scholar]
- Bradley A.; Hardacre C.; Holbrey J.; Johnston S.; McMath S.; Nieuwenhuyzen M. Small-Angle X-ray Scattering Studies of Liquid Crystalline 1-Alkyl-3-methylimidazolium Salts. Chem. Mater. 2002, 14, 629–635. 10.1021/cm010542v. [DOI] [Google Scholar]
- Faria L. F.; Matos J. R.; Ribeiro M. C. Thermal Analysis and Raman Spectra of Different Phases of the Ionic Liquid Butyltrimethylammonium Bis(trifluoromethylsulfonyl)imide. J. Phys. Chem. B 2012, 116, 9238–9245. 10.1021/jp3051824. [DOI] [PubMed] [Google Scholar]
- Holbrey J. D.; Reichert W. M.; Rogers R. D. Crystal Structures of Imidazolium Bis(trifluoromethanesulfonyl)imide Ionic Liquid Salts: The First Organic Salt with a cis-TFSI Anion Conformation. Dalton Trans 2004, 2267–2271. 10.1039/B405901H. [DOI] [PubMed] [Google Scholar]
- Dhumal N. R.; Noack K.; Kiefer J.; Kim H. J. Molecular Structure and Interactions in the Ionic Liquid 1-Ethyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide. J. Phys. Chem. A 2014, 118, 2547–2557. 10.1021/jp502124y. [DOI] [PubMed] [Google Scholar]
- Kohagen M.; Brehm M.; Lingscheid Y.; Giernoth R.; Sangoro J.; Kremer F.; Naumov S.; Iacob C.; Karger J.; Valiullin R.; Kirchner B. How Hydrogen Bonds Influence the Mobility of Imidazolium-Based Ionic Liquids. A Combined Theoretical and Experimental Study of 1-n-Butyl-3-methylimidazolium Bromide. J. Phys. Chem. B 2011, 115, 15280–15288. 10.1021/jp206974h. [DOI] [PubMed] [Google Scholar]
- Sun Q.; Harvey J. A.; Greco K. V.; Auerbach S. M. Molecular Simulations of Hydrogen Bond Cluster Size and Reorientation Dynamics in Liquid and Glassy Azole Systems. J. Phys. Chem. B 2016, 120, 10411–10419. 10.1021/acs.jpcb.6b07148. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L. Competitive Microstructures vs. Cooperative Dynamics of Hydrogen Bonding and π Type Stacking Interactions in Imidazolium Bis(oxalato)borate Ionic Liquids. J. Phys. Chem. B 2018, 122, 6570–6585. 10.1021/acs.jpcb.8b02899. [DOI] [PubMed] [Google Scholar]
- Fumino K.; Reimann S.; Ludwig R. Probing Molecular Interaction in Ionic Liquids by Low Frequency Spectroscopy: Coulomb Energy, Hydrogen Bonding and Dispersion Forces. Phys. Chem. Chem. Phys. 2014, 16, 21903–21929. 10.1039/C4CP01476F. [DOI] [PubMed] [Google Scholar]
- Strate A.; Neumann J.; Overbeck V.; Bonsa A.-M.; Michalik D.; Paschek D.; Ludwig R. Rotational and Translational Dynamics and Their Relation to Hydrogen Bond Lifetimes in an Ionic Liquid by Means of NMR Relaxation Time Experiments and Molecular Dynamics Simulation. J. Chem. Phys. 2018, 148, 193843. 10.1063/1.5011804. [DOI] [PubMed] [Google Scholar]
- Keaveney S. T.; Greaves T. L.; Kennedy D. F.; Harper J. B. Understanding the Effect of Solvent Structure on Organic Reaction Outcomes When Using Ionic Liquid/Acetonitrile Mixtures. J. Phys. Chem. B 2016, 120, 12687–12699. 10.1021/acs.jpcb.6b11090. [DOI] [PubMed] [Google Scholar]
- Hunt P. A. Why Does a Reduction in Hydrogen Bonding Lead to an Increase in Viscosity for the 1-Butyl-2,3-dimethyl-imidazolium Based Ionic Liquids?. J. Phys. Chem. B 2007, 111, 4844–4853. 10.1021/jp067182p. [DOI] [PubMed] [Google Scholar]
- Wulf A.; Fumino K.; Ludwig R. Spectroscopic Evidence for an Enhanced Anion-Cation Interaction From Hydrogen Bonding in Pure Imidazolium Ionic Liquids. Angew. Chem., Int. Ed. 2010, 49, 449–453. 10.1002/anie.200905437. [DOI] [PubMed] [Google Scholar]
- Fumino K.; Peppel T.; Geppert-Rybczyńska M.; Zaitsau D. H.; Lehmann J. K.; Verevkin S. P.; Köckerling M.; Ludwig R. The Influence of Hydrogen Bonding on the Physical Properties of Ionic Liquids. Phys. Chem. Chem. Phys. 2011, 13, 14064–14075. 10.1039/c1cp20732f. [DOI] [PubMed] [Google Scholar]
- Hayes R.; Imberti S.; Warr G. G.; Atkin R. Amphiphilicity Determines Nanostructure in Protic Ionic Liquids. Phys. Chem. Chem. Phys. 2011, 13, 3237–3247. 10.1039/C0CP01137A. [DOI] [PubMed] [Google Scholar]
- Russina O.; Triolo A. New Experimental Evidence Supporting the Mesoscopic Segregation Model in Room Temperature Ionic Liquids. Faraday Discuss. 2012, 154, 97–109. 10.1039/C1FD00073J. [DOI] [PubMed] [Google Scholar]
- Katsyuba S. A.; Vener M. V.; Zvereva E. E.; Fei Z.; Scopelliti R.; Laurenczy G.; Yan N.; Paunescu E.; Dyson P. J. How Strong is Hydrogen Bonding in Ionic Liquids? Combined X-ray Crystallographic, Infrared/Raman Spectroscopic, and Density Functional Theory Study. J. Phys. Chem. B 2013, 117, 9094–9105. 10.1021/jp405255w. [DOI] [PubMed] [Google Scholar]
- Fakhraee M.; Zandkarimi B.; Salari H.; Gholami M. R. Hydroxyl-Functionalized 1-(2-Hydroxyethyl)-3-methylimidazolium Ionic Liquids: Thermodynamic and Structural Properties Using Molecular Dynamics Simulations and ab Initio Calculations. J. Phys. Chem. B 2014, 118, 14410–14428. 10.1021/jp5083714. [DOI] [PubMed] [Google Scholar]
- Knorr A.; Stange P.; Fumino K.; Weinhold F.; Ludwig R. Spectroscopic Evidence for Clusters of Like-Charged Ions in Ionic Liquids Stabilized by Cooperative Hydrogen Bonding. ChemPhysChem 2016, 17, 458–462. 10.1002/cphc.201501134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. F.; Chen S.-H.; Schriver G. W.; Arnett E. M. Thermodynamics of Solution of Nonpolar Gases in a Fused Salt. Hydrophobic Bonding Behavior in a Nonaqueous System. J. Am. Chem. Soc. 1981, 103, 481–482. 10.1021/ja00392a049. [DOI] [Google Scholar]
- Fumino K.; Wulf A.; Ludwig R. Hydrogen Bonding in Protic Ionic Liquids: Reminiscent of Water. Angew. Chem., Int. Ed. 2009, 48, 3184–3186. 10.1002/anie.200806224. [DOI] [PubMed] [Google Scholar]
- Fumino K.; Reichert E.; Wittler K.; Hempelmann R.; Ludwig R. Low-Frequency Vibrational Modes of Protic Molten Salts and Ionic Liquids: Detecting and Quantifying Hydrogen Bonds. Angew. Chem., Int. Ed. 2012, 51, 6236–6240. 10.1002/anie.201200508. [DOI] [PubMed] [Google Scholar]
- Fumino K.; Ludwig R. Analyzing the Interaction Energies Between Cation and Anion in Ionic Liquids: The Subtle Balance Between Coulomb Forces and Hydrogen Bonding. J. Mol. Liq. 2014, 192, 94–102. 10.1016/j.molliq.2013.07.009. [DOI] [Google Scholar]
- Stange P.; Fumino K.; Ludwig R. Ion Speciation of Protic Ionic Liquids in Water: Transition from Contact to Solvent-Separated Ion Pairs. Angew. Chem., Int. Ed. 2013, 52, 2990–2994. 10.1002/anie.201209609. [DOI] [PubMed] [Google Scholar]
- Steiner T. The Hydrogen Bond in the Solid State. Angew. Chem., Int. Ed. 2002, 41, 48–76. . [DOI] [PubMed] [Google Scholar]
- Khudozhitkov A. E.; Stange P.; Golub B.; Paschek D.; Stepanov A. G.; Kolokolov D. I.; Ludwig R. Characterization of Doubly Ionic Hydrogen Bonds in Protic Ionic Liquids by NMR Deuteron Quadrupole Coupling Constants: Differences to H-bonds in Amides, Peptides, and Proteins. Angew. Chem., Int. Ed. 2017, 56, 14310–14314. 10.1002/anie.201708340. [DOI] [PubMed] [Google Scholar]
- Khudozhitkov A.; Neumann J.; Niemann T.; Zaitsau D.; Stange P.; Paschek D.; Stepanov A.; Kolokolov D.; Ludwig R. Hydrogen Bonding Between Ions of Like Charge in Ionic Liquids Characterized by NMR Deuteron Quadrupole Coupling Constants-Comparison with Salt Bridges and Molecular Systems. Angew. Chem., Int. Ed. 2019, 58, 17863–17871. 10.1002/anie.201912476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann T.; Li H.; Warr G. G.; Ludwig R.; Atkin R. Influence of Hydrogen Bonding Between Ions of Like Charge on the Ionic Liquid Interfacial Structure at a Mica Surface. J. Phys. Chem. Lett. 2019, 10, 7368–7373. 10.1021/acs.jpclett.9b03007. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Li B.; Sarman S.; Laaksonen A. Microstructures and Dynamics of Tetraalkylphosphonium Chloride Ionic Liquids. J. Chem. Phys. 2017, 147, 224502. 10.1063/1.4995003. [DOI] [PubMed] [Google Scholar]
- Liu X.; Zhao Y.; Zhang X.; Zhou G.; Zhang S. Microstructures and Interaction Analyses of Phosphonium-Based Ionic Liquids: A Simulation Study. J. Phys. Chem. B 2012, 116, 4934–4942. 10.1021/jp210696r. [DOI] [PubMed] [Google Scholar]
- Zhou G.; Liu X.; Zhang S.; Yu G.; He H. A Force Field for Molecular Simulation of Tetrabutylphosphonium Amino Acid Ionic Liquids. J. Phys. Chem. B 2007, 111, 7078–7084. 10.1021/jp068365e. [DOI] [PubMed] [Google Scholar]
- Strate A.; Niemann T.; Ludwig R. Controlling the Kinetic and Thermodynamic Stability of Cationic Clusters by the Addition of Molecules or Counterions. Phys. Chem. Chem. Phys. 2017, 19, 18854–18862. 10.1039/C7CP02227A. [DOI] [PubMed] [Google Scholar]
- Desiraju G. R. A Bond by Any Other Name. Angew. Chem., Int. Ed. 2011, 50, 52–59. 10.1002/anie.201002960. [DOI] [PubMed] [Google Scholar]
- Ribeiro M. C. High Viscosity of Imidazolium Ionic Liquids with the Hydrogen Sulfate Anion: A Raman Spectroscopy Study. J. Phys. Chem. B 2012, 116, 7281–7290. 10.1021/jp302091d. [DOI] [PubMed] [Google Scholar]
- Faria L. F.; Lima T. A.; Ferreira F. F.; Ribeiro M. C. Ultraslow Phase Transitions in an Anion-Anion Hydrogen-Bonded Ionic Liquid. J. Phys. Chem. B 2018, 122, 1972–1980. 10.1021/acs.jpcb.7b09497. [DOI] [PubMed] [Google Scholar]
- Zheng Y.-Z.; Wang N.-N.; Luo J.-J.; Zhou Y.; Yu Z.-W. Hydrogen-Bonding Interactions Between [BMIM][BF4] and Acetonitrile. Phys. Chem. Chem. Phys. 2013, 15, 18055–18064. 10.1039/c3cp53356e. [DOI] [PubMed] [Google Scholar]
- Dong K.; Song Y.; Liu X.; Cheng W.; Yao X.; Zhang S. Understanding Structures and Hydrogen Bonds of Ionic Liquids at the Electronic Level. J. Phys. Chem. B 2012, 116, 1007–1017. 10.1021/jp205435u. [DOI] [PubMed] [Google Scholar]
- Ramya K.; Kumar P.; Venkatnathan A. Molecular Simulations of Anion and Temperature Dependence on Structure and Dynamics of 1-Hexyl-3-methylimidazolium Ionic Liquids. J. Phys. Chem. B 2015, 119, 14800–14806. 10.1021/acs.jpcb.5b09456. [DOI] [PubMed] [Google Scholar]
- Skarmoutsos I.; Dellis D.; Matthews R. P.; Welton T.; Hunt P. A. Hydrogen Bonding in 1-Butyl- and 1-Ethyl-3-methylimidazolium Chloride Ionic Liquids. J. Phys. Chem. B 2012, 116, 4921–4933. 10.1021/jp209485y. [DOI] [PubMed] [Google Scholar]
- Dong K.; Zhang S.; Wang D.; Yao X. Hydrogen Bonds in Imidazolium Ionic Liquids. J. Phys. Chem. A 2006, 110, 9775–9782. 10.1021/jp054054c. [DOI] [PubMed] [Google Scholar]
- Hunt P. A.; Gould I. R. Structural Characterization of the 1-Butyl-3-methylimidazolium Chloride Ion Pair Using ab Initio Methods. J. Phys. Chem. A 2006, 110, 2269–2282. 10.1021/jp0547865. [DOI] [PubMed] [Google Scholar]
- Malberg F.; Pensado A. S.; Kirchner B. The Bulk and the Gas Phase of 1-Ethyl-3-methylimidazolium Ethylsulfate: Dispersion Interaction Makes the Difference. Phys. Chem. Chem. Phys. 2012, 14, 12079–12082. 10.1039/c2cp41878a. [DOI] [PubMed] [Google Scholar]
- Matthews R. P.; Ashworth C.; Welton T.; Hunt P. A. The Impact of Anion Electronic Structure: Similarities and Differences in Imidazolium Based Ionic Liquids. J. Phys.: Condens. Matter 2014, 26, 284112. 10.1088/0953-8984/26/28/284112. [DOI] [PubMed] [Google Scholar]
- Skarmoutsos I.; Welton T.; Hunt P. A. The Importance of Timescale for Hydrogen Bonding in Imidazolium Chloride Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 3675–3685. 10.1039/c3cp54551b. [DOI] [PubMed] [Google Scholar]
- Yaghini N.; Nordstierna L.; Martinelli A. Effect of Water on the Transport Properties of Protic and Aprotic Imidazolium Ionic Liquids–An Analysis of Self-Diffusivity, Conductivity, and Proton Exchange Mechanism. Phys. Chem. Chem. Phys. 2014, 16, 9266–9275. 10.1039/C4CP00527A. [DOI] [PubMed] [Google Scholar]
- Yaghini N.; Pitawala J.; Matic A.; Martinelli A. Effect of Water on the Local Structure and Phase Behavior of Imidazolium-Based Protic Ionic Liquids. J. Phys. Chem. B 2015, 119, 1611–1622. 10.1021/jp510691e. [DOI] [PubMed] [Google Scholar]
- Martinelli A.; Matic A.; Johansson P.; Jacobsson P.; Börjesson L.; Fernicola A.; Panero S.; Scrosati B.; Ohno H. Conformational Evolution of TFSI– in Protic and Aprotic Ionic Liquids. J. Raman Spectrosc. 2011, 42, 522–528. 10.1002/jrs.2713. [DOI] [Google Scholar]
- Endo T.; Kato T.; Nishikawa K. Effects of Methylation at the 2 Position of the Cation Ring on Phase Behaviors and Conformational Structures of Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2010, 114, 9201–9208. 10.1021/jp104123v. [DOI] [PubMed] [Google Scholar]
- Fumino K.; Wulf A.; Ludwig R. The Potential Role of Hydrogen Bonding in Aprotic and Protic Ionic Liquids. Phys. Chem. Chem. Phys. 2009, 11, 8790–8794. 10.1039/b905634c. [DOI] [PubMed] [Google Scholar]
- Fumino K.; Bonsa A. M.; Golub B.; Paschek D.; Ludwig R. Non-Ideal Mixing Behaviour of Hydrogen Bonding in Mixtures of Protic Ionic Liquids. ChemPhysChem 2015, 16, 299–304. 10.1002/cphc.201402760. [DOI] [PubMed] [Google Scholar]
- Noack K.; Schulz P. S.; Paape N.; Kiefer J.; Wasserscheid P.; Leipertz A. The Role of the C2 Position in Interionic Interactions of Imidazolium Based Ionic Liquids: A Vibrational and NMR Spectroscopic Study. Phys. Chem. Chem. Phys. 2010, 12, 14153–14161. 10.1039/c0cp00486c. [DOI] [PubMed] [Google Scholar]
- Peppel T.; Roth C.; Fumino K.; Paschek D.; Köckerling M.; Ludwig R. The Influence of Hydrogen-Bond Defects on the Properties of Ionic Liquids. Angew. Chem., Int. Ed. 2011, 50, 6661–6665. 10.1002/anie.201100199. [DOI] [PubMed] [Google Scholar]
- Tokuda H.; Hayamizu K.; Ishii K.; Susan M. A. B. H.; Watanabe M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 2. Variation of Alkyl Chain Length in Imidazolium Cation. J. Phys. Chem. B 2005, 109, 6103–6110. 10.1021/jp044626d. [DOI] [PubMed] [Google Scholar]
- Izgorodina E. I.; Maganti R.; Armel V.; Dean P. M.; Pringle J. M.; Seddon K. R.; MacFarlane D. R. Understanding the Effect of the C2 Proton in Promoting Low Viscosities and High Conductivities in Imidazolium-Based Ionic Liquids: Part I. Weakly Coordinating Anions. J. Phys. Chem. B 2011, 115, 14688–14697. 10.1021/jp208573y. [DOI] [PubMed] [Google Scholar]
- Zahn S.; Bruns G.; Thar J.; Kirchner B. What Keeps Ionic Liquids in Flow?. Phys. Chem. Chem. Phys. 2008, 10, 6921–6924. 10.1039/b814962n. [DOI] [PubMed] [Google Scholar]
- Liao C.; Shao N.; Han K. S.; Sun X.-G.; Jiang D.-E.; Hagaman E. W.; Dai S. Physicochemical Properties of Imidazolium-Derived Ionic Liquids with Different C-2 Substitutions. Phys. Chem. Chem. Phys. 2011, 13, 21503–21510. 10.1039/c1cp22375e. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Mu T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. 10.1021/ie5009597. [DOI] [Google Scholar]
- Hunt P. A.; Kirchner B.; Welton T. Characterising the Electronic Structure of Ionic Liquids: An Examination of the 1-Butyl-3-methylimidazolium Chloride Ion Pair. Chem. - Eur. J. 2006, 12, 6762–6775. 10.1002/chem.200600103. [DOI] [PubMed] [Google Scholar]
- Dong K.; Zhang S. Hydrogen Bonds: A Structural Insight into Ionic Liquids. Chem. - Eur. J. 2012, 18, 2748–2761. 10.1002/chem.201101645. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S.; Tokuda H.; Hayamizu K.; Watanabe M. Magnitude and Directionality of Interaction in Ion Pairs of Ionic Liquids: Relationship with Ionic Conductivity. J. Phys. Chem. B 2005, 109, 16474–16481. 10.1021/jp0533628. [DOI] [PubMed] [Google Scholar]
- Niedermeyer H.; Ashworth C.; Brandt A.; Welton T.; Hunt P. A. A Step Towards the a Priori Design of Ionic Liquids. Phys. Chem. Chem. Phys. 2013, 15, 11566–11578. 10.1039/c3cp50521a. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Leroy F.; Heggen B.; Zahn S.; Kirchner B.; Balasubramanian S.; Müller-Plathe F. Are There Stable Ion-Pairs in Room-Temperature Ionic Liquids? Molecular Dynamics Simulations of 1-n-Butyl-3-methylimidazolium Hexafluorophosphate. J. Am. Chem. Soc. 2009, 131, 15825–15833. 10.1021/ja906337p. [DOI] [PubMed] [Google Scholar]
- Qiao B.; Krekeler C.; Berger R.; Delle Site L.; Holm C. Effect of Anions on Static Orientational Correlations, Hydrogen Bonds, and Dynamics in Ionic Liquids: A Simulational Study. J. Phys. Chem. B 2008, 112, 1743–1751. 10.1021/jp0759067. [DOI] [PubMed] [Google Scholar]
- Ramírez-González P. E.; Ren G.; Saielli G.; Wang Y. Effect of Ion Rigidity on Physical Properties of Ionic Liquids Studied by Molecular Dynamics Simulation. J. Phys. Chem. B 2016, 120, 5678–5690. 10.1021/acs.jpcb.6b03379. [DOI] [PubMed] [Google Scholar]
- Tang F.; Ohto T.; Hasegawa T.; Bonn M.; Nagata Y. π+–π+ Stacking of Imidazolium Cations Enhances Molecular Layering of Room Temperature Ionic Liquids at Their Interfaces. Phys. Chem. Chem. Phys. 2017, 19, 2850–2856. 10.1039/C6CP07034E. [DOI] [PubMed] [Google Scholar]
- Deetlefs M.; Hardacre C.; Nieuwenhuyzen M.; Padua A. A.; Sheppard O.; Soper A. K. Liquid Structure of the Ionic Liquid 1,3-Dimethylimidazolium Bis{(trifluoromethyl)sulfonyl}amide. J. Phys. Chem. B 2006, 110, 12055–12061. 10.1021/jp060924u. [DOI] [PubMed] [Google Scholar]
- Aoun B.; Goldbach A.; Kohara S.; Wax J.-F.; González M. A.; Saboungi M.-L. Structure of a Prototypic Ionic Liquid: Ethyl-methylimidazolium Bromide. J. Phys. Chem. B 2010, 114, 12623–12628. 10.1021/jp1070715. [DOI] [PubMed] [Google Scholar]
- Brüssel M.; Brehm M.; Pensado A. S.; Malberg F.; Ramzan M.; Stark A.; Kirchner B. On the Ideality of Binary Mixtures of Ionic Liquids. Phys. Chem. Chem. Phys. 2012, 14, 13204–13215. 10.1039/c2cp41926b. [DOI] [PubMed] [Google Scholar]
- Weber H.; Hollóczki O.; Pensado A. S.; Kirchner B. Side Chain Fluorination and Anion Effect on the Structure of 1-Butyl-3-methylimidazolium Ionic Liquids. J. Chem. Phys. 2013, 139, 084502. 10.1063/1.4818540. [DOI] [PubMed] [Google Scholar]
- Matthews R. P.; Welton T.; Hunt P. A. Hydrogen Bonding and π–π Interactions in Imidazolium-Chloride Ionic Liquid Clusters. Phys. Chem. Chem. Phys. 2015, 17, 14437–14453. 10.1039/C5CP00459D. [DOI] [PubMed] [Google Scholar]
- Triolo A.; Russina O.; Bleif H.-J.; Di Cola E. Nanoscale Segregation in Room Temperature Ionic Liquids. J. Phys. Chem. B 2007, 111, 4641–4644. 10.1021/jp067705t. [DOI] [PubMed] [Google Scholar]
- Mele A.; Romanò G.; Giannone M.; Ragg E.; Fronza G.; Raos G.; Marcon V. The Local Structure of Ionic Liquids: Cation-Cation NOE Interactions and Internuclear Distances in Neat [BMIM][BF4] and [BDMIM][BF4]. Angew. Chem., Int. Ed. 2006, 45, 1123–1126. 10.1002/anie.200503745. [DOI] [PubMed] [Google Scholar]
- Li X.-W.; Zhang J.; Dong B.; Zheng L.-Q.; Tung C.-H. Characterization of Lyotropic Liquid Crystals Formed in the Mixtures of 1-Alkyl-3-methylimidazolium Bromide/p-Xylene/Water. Colloids Surf., A 2009, 335, 80–87. 10.1016/j.colsurfa.2008.10.031. [DOI] [Google Scholar]
- Deetlefs M.; Hardacre C.; Nieuwenhuyzen M.; Sheppard O.; Soper A. K. Structure of Ionic Liquid-Benzene Mixtures. J. Phys. Chem. B 2005, 109, 1593–1598. 10.1021/jp047742p. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Costa Gomes M. F.; Pádua A. L. A.; Rebelo L. S. P. N.; Canongia Lopes J. N. On the Role of the Dipole and Quadrupole Moments of Aromatic Compounds in the Solvation by Ionic Liquids. J. Phys. Chem. B 2009, 113, 9894–9900. 10.1021/jp903556q. [DOI] [PubMed] [Google Scholar]
- Harper J. B.; Lynden-Bell R. Macroscopic and Microscopic Properties of Solutions of Aromatic Compounds in an Ionic Liquid. Mol. Phys. 2004, 102, 85–94. 10.1080/00268970410001668570. [DOI] [Google Scholar]
- Blesic M.; Lopes J. N. C.; Pádua A. L. A.; Shimizu K.; Gomes M. F. C.; Rebelo L. S. P. N. Phase Equilibria in Ionic Liquid-Aromatic Compound Mixtures, Including Benzene Fluorination Effects. J. Phys. Chem. B 2009, 113, 7631–7636. 10.1021/jp902178g. [DOI] [PubMed] [Google Scholar]
- Holbrey J. D.; Reichert W. M.; Nieuwenhuyzen M.; Sheppard O.; Hardacre C.; Rogers R. D. Liquid Clathrate Formation in Ionic Liquid-Aromatic Mixtures. Chem. Commun. 2003, 476–477. 10.1039/b212726a. [DOI] [PubMed] [Google Scholar]
- Shimomura T.; Takamuku T.; Yamaguchi T. Clusters of Imidazolium-Based Ionic Liquid in Benzene Solutions. J. Phys. Chem. B 2011, 115, 8518–8527. 10.1021/jp203422z. [DOI] [PubMed] [Google Scholar]
- Yasaka Y.; Klein M. L.; Nakahara M.; Matubayasi N. Exploring the Reorientation of Benzene in an Ionic Liquid via Molecular Dynamics: Effect of Temperature and Solvent Effective Charge on the Slow Dynamics. J. Chem. Phys. 2011, 134, 191101. 10.1063/1.3592530. [DOI] [PubMed] [Google Scholar]
- Shirota H. Intermolecular/Interionic Vibrations of 1-Methyl-3-n-octylimidazolium Tetrafluoroborate Ionic Liquid and Benzene Mixtures. J. Phys. Chem. B 2013, 117, 7985–7995. 10.1021/jp402456g. [DOI] [PubMed] [Google Scholar]
- Fumino K.; Wittler K.; Ludwig R. The Anion Dependence of the Interaction Strength Between Ions in Imidazolium-Based Ionic Liquids Probed by Far-Infrared Spectroscopy. J. Phys. Chem. B 2012, 116, 9507–9511. 10.1021/jp306173t. [DOI] [PubMed] [Google Scholar]
- Penna T. C.; Faria L. F.; Ribeiro M. C. Raman Band Shape Analysis of Cyanate-Anion Ionic Liquids. J. Mol. Liq. 2015, 209, 676–682. 10.1016/j.molliq.2015.06.038. [DOI] [Google Scholar]
- Wang Y.-L.; Shimpi M. R.; Sarman S.; Antzutkin O. N.; Glavatskih S.; Kloo L.; Laaksonen A. Atomistic Insight into Tetraalkylphosphonium Bis(oxalato)borate Ionic Liquid/Water Mixtures. 2. Volumetric and Dynamic Properties. J. Phys. Chem. B 2016, 120, 7446–7455. 10.1021/acs.jpcb.6b02921. [DOI] [PubMed] [Google Scholar]
- Shah F. U.; Glavatskih S.; MacFarlane D. R.; Somers A.; Forsyth M.; Antzutkin O. N. Novel Halogen-Free Chelated Orthoborate-Phosphonium Ionic Liquids: Synthesis and Tribophysical Properties. Phys. Chem. Chem. Phys. 2011, 13, 12865–12873. 10.1039/c1cp21139k. [DOI] [PubMed] [Google Scholar]
- Golets M.; Shimpi M. R.; Wang Y.-L.; Antzutkin O. N.; Glavatskih S.; Laaksonen A. Understanding the Thermal Decomposition Mechanism of a Halogen-Free Chelated Orthoborate-Based Ionic Liquid: A Combined Computational and Experimental Study. Phys. Chem. Chem. Phys. 2016, 18, 22458–22466. 10.1039/C6CP03191A. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Golets M.; Li B.; Sarman S.; Laaksonen A. Interfacial Structures of Trihexyltetradecylphosphonium-Bis(mandelato)borate Ionic Liquid Confined Between Gold Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 4976–4987. 10.1021/acsami.6b14429. [DOI] [PubMed] [Google Scholar]
- Chen S.; Zhang S.; Liu X.; Wang J.; Wang J.; Dong K.; Sun J.; Xu B. Ionic Liquid Clusters: Structure, Formation Mechanism, and Effect on the Behavior of Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 5893–5906. 10.1039/C3CP53116C. [DOI] [PubMed] [Google Scholar]
- Weingärtner H.; Knocks A.; Schrader W.; Kaatze U. Dielectric Spectroscopy of the Room Temperature Molten Salt Ethylammonium Nitrate. J. Phys. Chem. A 2001, 105, 8646–8650. 10.1021/jp0114586. [DOI] [Google Scholar]
- Kennedy D. F.; Drummond C. J. Large Aggregated Ions Found in Some Protic Ionic Liquids. J. Phys. Chem. B 2009, 113, 5690–5693. 10.1021/jp900814y. [DOI] [PubMed] [Google Scholar]
- Lynden-Bell R. Screening of Pairs of Ions Dissolved in Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 1733–1740. 10.1039/B916987C. [DOI] [PubMed] [Google Scholar]
- Lee A. A.; Vella D.; Perkin S.; Goriely A. Are Room-Temperature Ionic Liquids Dilute Electrolytes?. J. Phys. Chem. Lett. 2015, 6, 159–163. 10.1021/jz502250z. [DOI] [PubMed] [Google Scholar]
- Lu H.; Li B.; Nordholm S.; Woodward C. E.; Forsman J. Ion Pairing and Phase Behaviour of an Asymmetric Restricted Primitive Model of Ionic Liquids. J. Chem. Phys. 2016, 145, 234510. 10.1063/1.4972214. [DOI] [PubMed] [Google Scholar]
- Feng G.; Chen M.; Bi S.; Goodwin Z. A. H.; Postnikov E. B.; Brilliantov N.; Urbakh M.; Kornyshev A. A. Free and Bound States of Ions in Ionic Liquids, Conductivity, and Underscreening Paradox. Phys. Rev. X 2019, 9, 021024. 10.1103/PhysRevX.9.021024. [DOI] [Google Scholar]
- Armstrong J. P.; Hurst C.; Jones R. G.; Licence P.; Lovelock K. R. J.; Satterley C. J.; Villar-Garcia I. J. Vapourisation of Ionic Liquids. Phys. Chem. Chem. Phys. 2007, 9, 982–990. 10.1039/b615137j. [DOI] [PubMed] [Google Scholar]
- Neto B. A. D.; Meurer E. C.; Galaverna R.; Bythell B. J.; Dupont J.; Cooks R. G.; Eberlin M. N. Vapors From Ionic Liquids: Reconciling Simulations with Mass Spectrometric Data. J. Phys. Chem. Lett. 2012, 3, 3435–3441. 10.1021/jz301608c. [DOI] [PubMed] [Google Scholar]
- Dupont J. On the Solid, Liquid and Solution Structural Organization of Imidazolium Ionic Liquids. J. Braz. Chem. Soc. 2004, 15, 341–350. 10.1590/S0103-50532004000300002. [DOI] [Google Scholar]
- Weingaertner H. NMR Studies of Ionic Liquids: Structure and Dynamics. Curr. Opin. Colloid Interface Sci. 2013, 18, 183–189. 10.1016/j.cocis.2013.04.001. [DOI] [Google Scholar]
- Weingärtner H.; Merkel T.; Käshammer S.; Schröer W.; Wiegand S. The Effect of Short-Range Hydrogen-Bonded Interactions on the Nature of the Critical Point of Ionic Fluids. Part I: General Properties of the New System Ethylammonium Nitrate + n-Octanol with an Upper Consolute Point Near Room Temperature. Ber. Bunsenges. Phys. Chem. 1993, 97, 970–975. 10.1002/bbpc.19930970804. [DOI] [Google Scholar]
- Ludwig R. A Simple Geometrical Explanation for the Occurrence of Specific Large Aggregated Ions in Some Protic Ionic Liquids. J. Phys. Chem. B 2009, 113, 15419–15422. 10.1021/jp907204x. [DOI] [PubMed] [Google Scholar]
- Addicoat M. A.; Stefanovic R.; Webber G. B.; Atkin R.; Page A. J. Assessment of the Density Functional Tight Binding Method for Protic Ionic Liquids. J. Chem. Theory Comput. 2014, 10, 4633–4643. 10.1021/ct500394t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner B.; Malberg F.; Firaha D. S.; Hollóczki O. Ion Pairing in Ionic Liquids. J. Phys.: Condens. Matter 2015, 27, 463002. 10.1088/0953-8984/27/46/463002. [DOI] [PubMed] [Google Scholar]
- Bren U.; Martínek V.; Florián J. Decomposition of the Solvation Free Energies of Deoxyribonucleoside Triphosphates Using the Free Energy Perturbation Method. J. Phys. Chem. B 2006, 110, 12782–12788. 10.1021/jp056623m. [DOI] [PubMed] [Google Scholar]
- Weingärtner H.; Sasisanker P.; Daguenet C.; Dyson P. J.; Krossing I.; Slattery J. M.; Schubert T. The Dielectric Response of Room-Temperature Ionic Liquids: Effect of Cation Variation. J. Phys. Chem. B 2007, 111, 4775–4780. 10.1021/jp0671188. [DOI] [PubMed] [Google Scholar]
- Perkin S.; Salanne M.; Madden P.; Lynden-Bell R. M. Is a Stern and Diffuse Layer Model Appropriate to Ionic Liquids at Surfaces?. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, E4121. 10.1073/pnas.1314188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui M. Y.; Crowhurst L.; Hallett J. P.; Hunt P. A.; Niedermeyer H.; Welton T. Salts Dissolved in Salts: Ionic Liquid Mixtures. Chem. Sci. 2011, 2, 1491–1496. 10.1039/c1sc00227a. [DOI] [Google Scholar]
- Bodo E.; Gontrani L.; Triolo A.; Caminiti R. Structural Determination of Ionic Liquids With Theoretical Methods: C8mimBr and C8mimCl. Strength and Weakness of Current Force Fields. J. Phys. Chem. Lett. 2010, 1, 1095–1100. 10.1021/jz100146r. [DOI] [Google Scholar]
- Afandak A.; Eslami H. Ion-Pairing and Electrical Conductivity in the Ionic Liquid 1-n-Butyl-3-methylimidazolium Methylsulfate [Bmim][MeSO4]: Molecular Dynamics Simulation Study. J. Phys. Chem. B 2017, 121, 7699–7708. 10.1021/acs.jpcb.7b06039. [DOI] [PubMed] [Google Scholar]
- Kashyap H. K.; Annapureddy H. V. R.; Raineri F. O.; Margulis C. J. How is Charge Transport Different in Ionic Liquids and Electrolyte Solutions?. J. Phys. Chem. B 2011, 115, 13212–13221. 10.1021/jp204182c. [DOI] [PubMed] [Google Scholar]
- Earle M. J.; Esperanca J. M.S.S.; Gilea M. A.; Canongia Lopes J. N.; Rebelo L. P.N.; Magee J. W.; Seddon K. R.; Widegren J. A. The Distillation and Volatility of Ionic Liquids. Nature 2006, 439, 831–834. 10.1038/nature04451. [DOI] [PubMed] [Google Scholar]
- Ueno K.; Tokuda H.; Watanabe M. Ionicity in Ionic Liquids: Correlation With Ionic Structure and Physicochemical Properties. Phys. Chem. Chem. Phys. 2010, 12, 1649–1658. 10.1039/b921462n. [DOI] [PubMed] [Google Scholar]
- Mele A.; Tran C. D.; De Paoli Lacerda S. H. The Structure of A Room-Temperature Ionic Liquid With and Without Trace Amounts of Water: The Role of C-H-O and C-H-F Interactions in 1-n-Butyl-3-methylimidazolium Tetrafluoroborate. Angew. Chem. 2003, 115, 4500–4502. 10.1002/ange.200351783. [DOI] [PubMed] [Google Scholar]
- Del Popolo M. G.; Mullan C. L.; Holbrey J. D.; Hardacre C.; Ballone P. Ion Association in [Bmim][PF6]/Naphthalene Mixtures: An Experimental and Computational Study. J. Am. Chem. Soc. 2008, 130, 7032–7041. 10.1021/ja710841n. [DOI] [PubMed] [Google Scholar]
- Hardacre C.; Holbrey J. D.; Mullan C. L.; Nieuwenhuyzen M.; Youngs T. G. A.; Bowron D. T.; Teat S. J. Solid and Liquid Charge-Transfer Complex Formation Between 1-Methylnaphthalene and 1-Alkyl-cyanopyridinium Bis{(trifluoromethyl)sulfonyl}imide Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 1842–1853. 10.1039/b921160h. [DOI] [PubMed] [Google Scholar]
- McDonald S.; Murphy T.; Imberti S.; Warr G. G.; Atkin R. Amphiphilically Nanostructured Deep Eutectic Solvents. J. Phys. Chem. Lett. 2018, 9, 3922–3927. 10.1021/acs.jpclett.8b01720. [DOI] [PubMed] [Google Scholar]
- Reddy T. D. N.; Mallik B. S. Protic Ammonium Carboxylate Ionic Liquids: Insight into Structure, Dynamics and Thermophysical Properties by Alkyl Group Functionalization. Phys. Chem. Chem. Phys. 2017, 19, 10358–10370. 10.1039/C6CP08884H. [DOI] [PubMed] [Google Scholar]
- Song X.; Hamano H.; Minofar B.; Kanzaki R.; Fujii K.; Kameda Y.; Kohara S.; Watanabe M.; Ishiguro S.-I.; Umebayashi Y. Structural Heterogeneity and Unique Distorted Hydrogen Bonding in Primary Ammonium Nitrate Ionic Liquids Studied by High-Energy X-ray Diffraction Experiments and MD Simulations. J. Phys. Chem. B 2012, 116, 2801–2813. 10.1021/jp209561t. [DOI] [PubMed] [Google Scholar]
- Hayes R.; Imberti S.; Warr G. G.; Atkin R. Pronounced Sponge-Like Nanostructure in Propylammonium Nitrate. Phys. Chem. Chem. Phys. 2011, 13, 13544–13551. 10.1039/c1cp21080g. [DOI] [PubMed] [Google Scholar]
- Thummuru D. N. R.; Mallik B. S. Structure and Dynamics of Hydroxyl-Functionalized Protic Ammonium Carboxylate Ionic Liquids. J. Phys. Chem. A 2017, 121, 8097–8107. 10.1021/acs.jpca.7b05995. [DOI] [PubMed] [Google Scholar]
- Greaves T. L.; Kennedy D. F.; Mudie S. T.; Drummond C. J. Diversity Observed in the Nanostructure of Protic Ionic Liquids. J. Phys. Chem. B 2010, 114, 10022–10031. 10.1021/jp103863z. [DOI] [PubMed] [Google Scholar]
- Nayeri M.; Aronson M. T.; Bernin D.; Chmelka B. F.; Martinelli A. Surface Effects on the Structure and Mobility of the Ionic Liquid C6C1ImTFSI in Silica Gels. Soft Matter 2014, 10, 5618–5627. 10.1039/C4SM00642A. [DOI] [PubMed] [Google Scholar]
- Mehta N. A.; Levin D. A. Comparison of Two Protic Ionic Liquid Behaviors in the Presence of an Electric Field Using Molecular Dynamics. J. Chem. Phys. 2017, 147, 234505. 10.1063/1.5001827. [DOI] [PubMed] [Google Scholar]
- Hayes R.; Imberti S.; Warr G. G.; Atkin R. The Nature of Hydrogen Bonding in Protic Ionic Liquids. Angew. Chem. 2013, 125, 4721–4725. 10.1002/ange.201209273. [DOI] [PubMed] [Google Scholar]
- Paschek D.; Golub B.; Ludwig R. Hydrogen Bonding in a Mixture of Protic Ionic Liquids: A Molecular Dynamics Simulation Study. Phys. Chem. Chem. Phys. 2015, 17, 8431–8440. 10.1039/C4CP05432F. [DOI] [PubMed] [Google Scholar]
- Zheng Z. P.; Fan W. H.; Roy S.; Mazur K.; Nazet A.; Buchner R.; Bonn M.; Hunger J. Ionic Liquids: Not Only Structurally But Also Dynamically Heterogeneous. Angew. Chem., Int. Ed. 2014, 54, 687–690. 10.1002/anie.201409136. [DOI] [PubMed] [Google Scholar]
- Schröder U.; Wadhawan J. D.; Compton R. G.; Marken F.; Suarez P. A. Z.; Consorti C. S.; de Souza R. F.; Dupont J. Water-Induced Accelerated Ion Diffusion: Voltammetric Studies in 1-Methyl-3-[2,6-(S)-dimethylocten-2-yl]imidazolium Tetrafluoroborate, 1-Butyl-3-methylimidazolium Tetrafluoroborate and Hexafluorophosphate Ionic Liquids. New J. Chem. 2000, 24, 1009–1015. 10.1039/b007172m. [DOI] [Google Scholar]
- Wang Y.; Voth G. A. Unique Spatial Heterogeneity in Ionic Liquids. J. Am. Chem. Soc. 2005, 127, 12192–12193. 10.1021/ja053796g. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Voth G. A. Tail Aggregation and Domain Diffusion in Ionic Liquids. J. Phys. Chem. B 2006, 110, 18601–18608. 10.1021/jp063199w. [DOI] [PubMed] [Google Scholar]
- Cao W.; Wang Y.; Saielli G. Metastable State During Melting and Solid-Solid Phase Transition of [CnMim][NO3](n = 4–12) Ionic Liquids by Molecular Dynamics Simulation. J. Phys. Chem. B 2018, 122, 229–239. 10.1021/acs.jpcb.7b09073. [DOI] [PubMed] [Google Scholar]
- Saielli G.; Wang Y. Role of the Electrostatic Interactions in the Stabilization of Ionic Liquid Crystals: Insights From Coarse-Grained MD Simulations of an Imidazolium Model. J. Phys. Chem. B 2016, 120, 9152–9160. 10.1021/acs.jpcb.6b04717. [DOI] [PubMed] [Google Scholar]
- Shi R.; Wang Y. Ion-Cage Interpretation for the Structural and Dynamic Changes of Ionic Liquids Under an External Electric Field. J. Phys. Chem. B 2013, 117, 5102–5112. 10.1021/jp311017r. [DOI] [PubMed] [Google Scholar]
- Saielli G.; Voth G. A.; Wang Y. Diffusion Mechanisms in Smectic Ionic Liquid Crystals: Insights From Coarse-Grained MD Simulations. Soft Matter 2013, 9, 5716–5725. 10.1039/c3sm50375e. [DOI] [Google Scholar]
- Urahata S. M.; Ribeiro M. C. C. Unraveling Dynamical Heterogeneity in the Ionic Liquid 1-Butyl-3-methylimidazolium Chloride. J. Phys. Chem. Lett. 2010, 1, 1738–1742. 10.1021/jz100411w. [DOI] [Google Scholar]
- Canongia Lopes J. N. A.; Pádua A. A. H. Nanostructural Organization in Ionic Liquids. J. Phys. Chem. B 2006, 110, 3330–3335. 10.1021/jp056006y. [DOI] [PubMed] [Google Scholar]
- Nemoto F.; Kofu M.; Yamamuro O. Thermal and Structural Studies of Imidazolium-Based Ionic Liquids With and Without Liquid-Crystalline Phases: The Origin of Nanostructure. J. Phys. Chem. B 2015, 119, 5028–5034. 10.1021/acs.jpcb.5b01080. [DOI] [PubMed] [Google Scholar]
- Triolo A.; Russina O.; Fazio B.; Triolo R.; Di Cola E. Morphology of 1-Alkyl-3-methylimidazolium Hexafluorophosphate Room Temperature Ionic Liquids. Chem. Phys. Lett. 2008, 457, 362–365. 10.1016/j.cplett.2008.04.027. [DOI] [Google Scholar]
- Russina O.; Triolo A.; Gontrani L.; Caminiti R.; Xiao D.; Hines Jr L. G; Bartsch R. A; Quitevis E. L; Pleckhova N.; Seddon K. R Morphology and Intermolecular Dynamics of 1-Alkyl-3-methylimidazolium Bis(trifluoromethane)sulfonylimide Ionic Liquids: Structural and Dynamic Evidence of Nanoscale Segregation. J. Phys.: Condens. Matter 2009, 21, 424121. 10.1088/0953-8984/21/42/424121. [DOI] [Google Scholar]
- Hardacre C.; Holbrey J. D.; Mullan C. L.; Youngs T. G. A.; Bowron D. T. Small Angle Neutron Scattering From 1-Alkyl-3-methylimidazolium Hexafluorophosphate Ionic Liquids ([Cnmim][PF6], n= 4, 6, and 8). J. Chem. Phys. 2010, 133, 074510. 10.1063/1.3473825. [DOI] [PubMed] [Google Scholar]
- Hardacre C.; Holbrey J. D.; Nieuwenhuyzen M.; Youngs T. G. A. Structure and Solvation in Ionic Liquids. Acc. Chem. Res. 2007, 40, 1146–1155. 10.1021/ar700068x. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Bernardes C. E. S.; Canongia Lopes J. N. Structure and Aggregation in the 1-Alkyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide Ionic Liquid Homologous Series. J. Phys. Chem. B 2014, 118, 567–576. 10.1021/jp409987d. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Bernardes C. E. S.; Triolo A.; Canongia Lopes J. N. Nano-Segregation in Ionic Liquids: Scorpions and Vanishing Chains. Phys. Chem. Chem. Phys. 2013, 15, 16256–16262. 10.1039/c3cp52357h. [DOI] [PubMed] [Google Scholar]
- Lo Celso F.; Appetecchi G. B.; Jafta C. J.; Gontrani L.; Canongia Lopes J. N.; Triolo A.; Russina O. Nanoscale Organization in the Fluorinated Room Temperature Ionic Liquid: TetraethylAmmonium (Trifluoromethanesulfonyl) (Nonafluorobutylsulfonyl)imide. J. Chem. Phys. 2018, 148, 193816. 10.1063/1.5016236. [DOI] [PubMed] [Google Scholar]
- Bulut S.; Ab Rani M. A.; Welton T.; Lickiss P. D.; Krossing I. Preparation of [Al(hfip)4]-Based Ionic Liquids with Siloxane-Functionalized Cations and Their Physical Properties in Comparison with Their [Tf2N] Analogues. ChemPhysChem 2012, 13, 1802–1805. 10.1002/cphc.201200028. [DOI] [PubMed] [Google Scholar]
- Yamada S. A.; Bailey H. E.; Tamimi A.; Li C.; Fayer M. D. Dynamics in a Room-Temperature Ionic Liquid From the Cation Perspective: 2D IR Vibrational Echo Spectroscopy. J. Am. Chem. Soc. 2017, 139, 2408–2420. 10.1021/jacs.6b12011. [DOI] [PubMed] [Google Scholar]
- Xue L.; Tamas G.; Matthews R. P.; Stone A. J.; Hunt P. A.; Quitevis E. L.; Lynden-Bell R. M. An OHD-RIKES and Simulation Study Comparing a Benzylmethylimidazolium Ionic Liquid with an Equimolar Mixture of Dimethylimidazolium and Benzene. Phys. Chem. Chem. Phys. 2015, 17, 9973–9983. 10.1039/C5CP00550G. [DOI] [PubMed] [Google Scholar]
- Tao R.; Gurung E.; Cetin M. M.; Mayer M. F.; Quitevis E. L.; Simon S. L. Fragility of Ionic Liquids Measured by Flash Differential Scanning Calorimetry. Thermochim. Acta 2017, 654, 121–129. 10.1016/j.tca.2017.05.008. [DOI] [Google Scholar]
- Shirota H.; Matsuzaki H.; Ramati S.; Wishart J. F. Effects of Aromaticity in Cations and Their Functional Groups on the Low-Frequency Spectra and Physical Properties of Ionic Liquids. J. Phys. Chem. B 2015, 119, 9173–9187. 10.1021/jp509412z. [DOI] [PubMed] [Google Scholar]
- Wu B.; Shirota H.; Lall-Ramnarine S.; Castner E. W. Jr Structure of Ionic Liquids with Cationic Silicon-Substitutions. J. Chem. Phys. 2016, 145, 114501. 10.1063/1.4962257. [DOI] [Google Scholar]
- Zheng W.; Mohammed A.; Hines L. G. Jr; Xiao D.; Martinez O. J.; Bartsch R. A.; Simon S. L.; Russina O.; Triolo A.; Quitevis E. L. Effect of Cation Symmetry on the Morphology and Physicochemical Properties of Imidazolium Ionic Liquids. J. Phys. Chem. B 2011, 115, 6572–6584. 10.1021/jp1115614. [DOI] [PubMed] [Google Scholar]
- Li S.; Bañuelos J. L.; Zhang P.; Feng G.; Dai S.; Rother G.; Cummings P. T. Toward Understanding the Structural Heterogeneity and Ion Pair Stability in Dicationic Ionic Liquids. Soft Matter 2014, 10, 9193–9200. 10.1039/C4SM01742K. [DOI] [PubMed] [Google Scholar]
- Menges F. S.; Zeng H. J.; Kelleher P. J.; Gorlova O.; Johnson M. A.; Niemann T.; Strate A.; Ludwig R. Structural Motifs in Cold Ternary Ion Complexes of Hydroxyl-Functionalized Ionic Liquids: Isolating the Role of Cation-Cation Interactions. J. Phys. Chem. Lett. 2018, 9, 2979–2984. 10.1021/acs.jpclett.8b01130. [DOI] [PubMed] [Google Scholar]
- Strate A.; Niemann T.; Michalik D.; Ludwig R. When Like Charged Ions Attract in Ionic Liquids: Controlling the Formation of Cationic Clusters by the Interaction Strength of the Counterions. Angew. Chem., Int. Ed. 2017, 56, 496–500. 10.1002/anie.201609799. [DOI] [PubMed] [Google Scholar]
- Dhungana K. B.; Margulis C. J. Comparison of the Structural Response to Pressure of Ionic Liquids with Ether and Alkyl Functionalities. J. Phys. Chem. B 2017, 121, 6890–6897. 10.1021/acs.jpcb.7b04038. [DOI] [PubMed] [Google Scholar]
- Smith J. A.; Webber G. B.; Warr G. G.; Atkin R. Rheology of Protic Ionic Liquids and Their Mixtures. J. Phys. Chem. B 2013, 117, 13930–13935. 10.1021/jp407715e. [DOI] [PubMed] [Google Scholar]
- Wakeham D.; Niga P.; Ridings C.; Andersson G.; Nelson A.; Warr G. G.; Baldelli S.; Rutland M. W.; Atkin R. Surface Structure of a “Non-Amphiphilic” Protic Ionic Liquid. Phys. Chem. Chem. Phys. 2012, 14, 5106–5114. 10.1039/c2cp23694j. [DOI] [PubMed] [Google Scholar]
- Wu B.; Kuroda K.; Takahashi K.; Castner E. W. Jr Structural Analysis of Zwitterionic Liquids vs. Homologous Ionic Liquids. J. Chem. Phys. 2018, 148, 193807. 10.1063/1.5010983. [DOI] [PubMed] [Google Scholar]
- Fakhraee M.; Gholami M. R. Effect of Anion and Alkyl Side Chain on Structural and Dynamic Features of Ester Functionalized Ionic Liquids: Confirming Nanoscale Organization. J. Phys. Chem. B 2016, 120, 11539–11555. 10.1021/acs.jpcb.6b08874. [DOI] [PubMed] [Google Scholar]
- Shirota H.; Castner E. W. Jr. Why Are Viscosities Lower for Ionic Liquids with – CH2Si(CH3)3 vs – CH2C(CH3)3 Substitutions on the Imidazolium Cations?. J. Phys. Chem. B 2005, 109, 21576–21585. 10.1021/jp053930j. [DOI] [PubMed] [Google Scholar]
- Shirota H.; Wishart J. F.; Castner E. W. Jr. Intermolecular Interactions and Dynamics of Room Temperature Ionic Liquids that Have Silyl- and Siloxy-Substituted Imidazolium Cations. J. Phys. Chem. B 2007, 111, 4819–4829. 10.1021/jp067126o. [DOI] [PubMed] [Google Scholar]
- Niedermeyer H.; Ab Rani M. A.; Lickiss P. D.; Hallett J. P.; Welton T.; White A. J. P.; Hunt P. A. Understanding Siloxane Functionalised Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 2018–2029. 10.1039/b922011a. [DOI] [PubMed] [Google Scholar]
- Endo T.; Nemugaki S.; Matsushita Y.; Sakai Y.; Ozaki H.; Hiejima Y.; Kimura Y.; Takahashi K. Fast Solute Diffusivity in Ionic Liquids with Silyl or Siloxane Groups Studied by the Transient Grating Method. Chem. Phys. 2016, 472, 128–134. 10.1016/j.chemphys.2016.03.016. [DOI] [Google Scholar]
- Serra P. B. P.; Ribeiro F. M. S.; Rocha M. A. A.; Fulem M.; Růžička K.; Santos L. M. N. B. F. Phase Behavior and Heat Capacities of the 1-Benzyl-3-methylimidazolium Ionic Liquids. J. Chem. Thermodyn. 2016, 100, 124–130. 10.1016/j.jct.2016.04.009. [DOI] [Google Scholar]
- Shirota H.; Kakinuma S.; Itoyama Y.; Umecky T.; Takamuku T. Effects of Tetrafluoroborate and Bis(trifluoromethylsulfonyl)amide Anions on the Microscopic Structures of 1-Methyl-3-octylimidazolium-Based Ionic Liquids and Benzene Mixtures: A Multiple Approach by ATR-IR, NMR, and Femtosecond Raman-Induced Kerr Effect Spectroscopy. J. Phys. Chem. B 2016, 120, 513–526. 10.1021/acs.jpcb.5b10917. [DOI] [PubMed] [Google Scholar]
- Xue L.; Tamas G.; Quitevis E. L. Comparative OHD-RIKES Study of the Low-Frequency (0–250 cm–1) Vibrational Dynamics of Dibenzyl- and Monobenzyl-Substituted Imidazolium Ionic Liquids and Benzene/Dimethylimidazolium Mixtures. ACS Sustainable Chem. Eng. 2016, 4, 514–524. 10.1021/acssuschemeng.5b01222. [DOI] [Google Scholar]
- Łachwa J.; Bento I.; Duarte M. T.; Lopes J. N. C.; Rebelo L. P. N. Condensed Phase Behaviour of Ionic Liquid-Benzene Mixtures: Congruent Melting of a [EMIM][NTf2]-C6H6 Inclusion Crystal. Chem. Commun. 2006, 2445–2447. 10.1039/B602675C. [DOI] [PubMed] [Google Scholar]
- Rocha M. A.; Neves C. M.; Freire M. G.; Russina O.; Triolo A.; Coutinho J.; Santos L. M. N. B. F. Alkylimidazolium Based Ionic Liquids: Impact of Cation Symmetry on Their Nanoscale Structural Organization. J. Phys. Chem. B 2013, 117, 10889–10897. 10.1021/jp406374a. [DOI] [PubMed] [Google Scholar]
- Xiao D.; Hines L. G. Jr; Li S.; Bartsch R. A.; Quitevis E. L.; Russina O.; Triolo A. Effect of Cation Symmetry and Alkyl Chain Length on the Structure and Intermolecular Dynamics of 1,3-Dialkylimidazolium Bis(trifluoromethanesulfonyl)amide Ionic Liquids. J. Phys. Chem. B 2009, 113, 6426–6433. 10.1021/jp8102595. [DOI] [PubMed] [Google Scholar]
- Bernardes C. E. S.; Shimizu K.; Lobo Ferreira A. I. M. C.; Santos L. M. N. B. F.; Canongia Lopes J. N. Structure and Aggregation in the 1,3-Dialkyl-imidazolium Bis(trifluoromethylsulfonyl)imide Ionic Liquid Family: 2. From Single to Double Long Alkyl Side Chains. J. Phys. Chem. B 2014, 118, 6885–6895. 10.1021/jp502968u. [DOI] [PubMed] [Google Scholar]
- Saielli G.; Margola T.; Satoh K. Tuning Coulombic Interactions to Stabilize Nematic and Smectic Ionic Liquid Crystal Phases in Mixtures of Charged Soft Ellipsoids and Spheres. Soft Matter 2017, 13, 5204–5213. 10.1039/C7SM00612H. [DOI] [PubMed] [Google Scholar]
- Pitawala J.; Matic A.; Martinelli A.; Jacobsson P.; Koch V.; Croce F. Thermal Properties and Ionic Conductivity of Imidazolium Bis(trifluoromethanesulfonyl)imide Dicationic Ionic Liquids. J. Phys. Chem. B 2009, 113, 10607–10610. 10.1021/jp904989s. [DOI] [PubMed] [Google Scholar]
- Lynden-Bell R. M.; Quitevis E. L. A Simulation Study of CS2 Solutions in Two Related Ionic Liquids with Dications and Monocations. J. Chem. Phys. 2018, 148, 193844. 10.1063/1.5008801. [DOI] [PubMed] [Google Scholar]
- Rocha M. A. A.; Coutinho J. A. P.; Santos L. M. N. B. F. Vapor Pressures of 1,3-Dialkylimidazolium Bis(trifluoromethylsulfonyl)imide Ionic Liquids with Long Alkyl Chains. J. Chem. Phys. 2014, 141, 134502. 10.1063/1.4896704. [DOI] [PubMed] [Google Scholar]
- Rocha M. A. A.; Coutinho J. A. P.; Santos L. M. N. B. F. Cation Symmetry Effect on the Volatility of Ionic Liquids. J. Phys. Chem. B 2012, 116, 10922–10927. 10.1021/jp306937f. [DOI] [PubMed] [Google Scholar]
- Ishida T.; Shirota H. Dicationic versus Monocationic Ionic Liquids: Distinctive Ionic Dynamics and Dynamical Heterogeneity. J. Phys. Chem. B 2013, 117, 1136–1150. 10.1021/jp3110425. [DOI] [PubMed] [Google Scholar]
- Bodo E.; Chiricotto M.; Caminiti R. Structure of Geminal Imidazolium Bis(trifluoromethylsulfonyl)imide Dicationic Ionic Liquids: A Theoretical Study of the Liquid Phase. J. Phys. Chem. B 2011, 115, 14341–14347. 10.1021/jp205514w. [DOI] [PubMed] [Google Scholar]
- Yeganegi S.; Soltanabadi A.; Farmanzadeh D. Molecular Dynamic Simulation of Dicationic Ionic Liquids: Effects of Anions and Alkyl Chain Length on Liquid Structure and Diffusion. J. Phys. Chem. B 2012, 116, 11517–11526. 10.1021/jp3059933. [DOI] [PubMed] [Google Scholar]
- Sahu P. K.; Das S. K.; Sarkar M. Toward Understanding Solute-Solvent Interaction in Room-Temperature Mono- and Dicationic Ionic Liquids: A Combined Fluorescence Spectroscopy and Mass Spectrometry Analysis. J. Phys. Chem. B 2014, 118, 1907–1915. 10.1021/jp500218r. [DOI] [PubMed] [Google Scholar]
- Majhi D.; Seth S.; Sarkar M. Differences in the Behavior of Dicationic and Monocationic Ionic Liquids as Revealed by Time Resolved-Fluorescence, NMR and Fluorescence Correlation Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 7844–7856. 10.1039/C7CP08630J. [DOI] [PubMed] [Google Scholar]
- Li S.; Feng G.; Bañuelos J. L.; Rother G.; Fulvio P. F.; Dai S.; Cummings P. T. Distinctive Nanoscale Organization of Dicationic Versus Monocationic Ionic Liquids. J. Phys. Chem. C 2013, 117, 18251–18257. 10.1021/jp406381g. [DOI] [Google Scholar]
- Moosavi M.; Khashei F.; Sedghamiz E. Molecular Dynamics Simulation of Geminal Dicationic Ionic Liquids [Cn(mim)2][NTf2]2-Structural and Dynamical Properties. Phys. Chem. Chem. Phys. 2018, 20, 435–448. 10.1039/C7CP05681H. [DOI] [PubMed] [Google Scholar]
- Alavi S. M.; Yeganegi S. DFT Study of Structures and Hydrogen Bonds of Imidazolium Based Halogen-Free Boron Containing Dicationic Ionic Liquids. J. Mol. Liq. 2018, 256, 330–343. 10.1016/j.molliq.2018.02.066. [DOI] [Google Scholar]
- Moosavi M.; Khashei F.; Sharifi A.; Mirzaei M. Transport Properties of Short Alkyl Chain Length Dicationic Ionic Liquids. The Effects of Alkyl Chain Length and Temperature. Ind. Eng. Chem. Res. 2016, 55, 9087–9099. 10.1021/acs.iecr.6b02881. [DOI] [Google Scholar]
- Donati C.; Glotzer S. C.; Poole P. H.; Kob W.; Plimpton S. J. Spatial Correlations of Mobility and Immobility in a Glass-Forming Lennard-Jones Liquid. Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top. 1999, 60, 3107–3119. 10.1103/PhysRevE.60.3107. [DOI] [PubMed] [Google Scholar]
- Wang B.; Kuo J.; Bae S. C.; Granick S. When Brownian Diffusion is Not Gaussian. Nat. Mater. 2012, 11, 481–485. 10.1038/nmat3308. [DOI] [PubMed] [Google Scholar]
- Giammanco C. H.; Yamada S. A.; Kramer P. L.; Tamimi A.; Fayer M. D. Structural and Rotational Dynamics of Carbon Dioxide in 1-Alkyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide Ionic Liquids: The Effect of Chain Length. J. Phys. Chem. B 2016, 120, 6698–6711. 10.1021/acs.jpcb.6b03971. [DOI] [PubMed] [Google Scholar]
- Shin J. Y.; Yamada S. A.; Fayer M. D. Carbon Dioxide in A Supported Ionic Liquid Membrane: Structural and Rotational Dynamics Measured With 2D IR and Pump–Probe Experiments. J. Am. Chem. Soc. 2017, 139, 11222–11232. 10.1021/jacs.7b05759. [DOI] [PubMed] [Google Scholar]
- Shin J. Y.; Yamada S. A.; Fayer M. D. Dynamics of a Room Temperature Ionic Liquid in Supported Ionic Liquid Membranes vs the Bulk Liquid: 2D IR and Polarized IR Pump–Probe Experiments. J. Am. Chem. Soc. 2017, 139, 311–323. 10.1021/jacs.6b10695. [DOI] [PubMed] [Google Scholar]
- Kramer P. L.; Giammanco C. H.; Fayer M. D. Dynamics of Water, Methanol, and Ethanol in a Room Temperature Ionic Liquid. J. Chem. Phys. 2015, 142, 212408. 10.1063/1.4914156. [DOI] [PubMed] [Google Scholar]
- Driver G.; Huang Y.; Laaksonen A.; Sparrman T.; Wang Y.-L.; Westlund P.-O. Correlated/Non-Correlated Ion Dynamics of Charge-Neutral Ion Couples: The Origin of Ionicity in Ionic Liquids. Phys. Chem. Chem. Phys. 2017, 19, 4975–4988. 10.1039/C6CP05801A. [DOI] [PubMed] [Google Scholar]
- Ivanov M. Y.; Prikhod’ko S. A.; Adonin N. Y.; Kirilyuk I. A.; Adichtchev S. V.; Surovtsev N. V.; Dzuba S. A.; Fedin M. V. Structural Anomalies in Ionic Liquids Near the Glass Transition Revealed by Pulse EPR. J. Phys. Chem. Lett. 2018, 9, 4607–4612. 10.1021/acs.jpclett.8b02097. [DOI] [PubMed] [Google Scholar]
- Lo Celso F.; Triolo A.; Gontrani L.; Russina O. Anion-Specific Response of Mesoscopic Organization in Ionic Liquids Upon Pressurization. J. Chem. Phys. 2018, 148, 211102. 10.1063/1.5036588. [DOI] [PubMed] [Google Scholar]
- Russina O.; Celso F. L.; Triolo A. Pressure-Responsive Mesoscopic Structures in Room Temperature Ionic Liquids. Phys. Chem. Chem. Phys. 2015, 17, 29496–29500. 10.1039/C5CP04682C. [DOI] [PubMed] [Google Scholar]
- Chen F.; You T.; Yuan Y.; Pei C.; Ren X.; Huang Y.; Yu Z.; Li X.; Zheng H.; Pan Y.; Yang K.; Wang L. Pressure-Induced Structural Transitions of a Room Temperature Ionic Liquid—1-Ethyl-3-methylimidazolium Chloride. J. Chem. Phys. 2017, 146, 094502. 10.1063/1.4977044. [DOI] [Google Scholar]
- Yoshimura Y.; Shigemi M.; Takaku M.; Yamamura M.; Takekiyo T.; Abe H.; Hamaya N.; Wakabayashi D.; Nishida K.; Funamori N.; Sato T.; Kikegawa T. Stability of the Liquid State of Imidazolium-Based Ionic Liquids Under High Pressure at Room Temperature. J. Phys. Chem. B 2015, 119, 8146–8153. 10.1021/acs.jpcb.5b03476. [DOI] [PubMed] [Google Scholar]
- Russina O.; Fazio B.; Schmidt C.; Triolo A. Structural Organization and Phase Behaviour of 1-Butyl-3-methylimidazolium Hexafluorophosphate: An High Pressure Raman Spectroscopy Study. Phys. Chem. Chem. Phys. 2011, 13, 12067–12074. 10.1039/c0cp02684k. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y.; Takekiyo T.; Koyama Y.; Takaku M.; Yamamura M.; Kikuchi N.; Wakabayashi D.; Funamori N.; Matsuishi K.; Abe H.; Hamaya N. High-Pressure Glass Formation of a Series of 1-Alkyl-3-methylimidazolium Bis(trifluoromethanesulfonyl)imide Homologues. Phys. Chem. Chem. Phys. 2018, 20, 199–205. 10.1039/C7CP06594A. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. Coupling Between the Mesoscopic Dynamics and Shear Stress of a Room-Temperature Ionic Liquid. Phys. Chem. Chem. Phys. 2018, 20, 17809–17817. 10.1039/C8CP02814A. [DOI] [PubMed] [Google Scholar]
- Wang Y. Disordering and Reordering of Ionic Liquids Under an External Electric Field. J. Phys. Chem. B 2009, 113, 11058–11060. 10.1021/jp906228d. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Dong K.; Liu X.; Zhang S.; Zhu J.; Wang J. Structure of Ionic Liquids Under External Electric Field: A Molecular Dynamics Simulation. Mol. Simul. 2012, 38, 172–178. 10.1080/08927022.2011.610894. [DOI] [Google Scholar]
- Chen F.; Fang G.; Yan Z.; Pan Y.; Liu J.; Wang L. Cation Conformational Changes of 1-Butyl-3-methylimidazolium Halides at High Pressures. J. Phys. Chem. C 2018, 122, 9320–9331. 10.1021/acs.jpcc.8b01469. [DOI] [Google Scholar]
- Abe H.; Hamaya N.; Koyama Y.; Kishimura H.; Takekiyo T.; Yoshimura Y.; Wakabayashi D.; Funamori N.; Matsuishi K. Long Periodic Structure of a Room-Temperature Ionic Liquid by High-Pressure Small-Angle X-ray Scattering and Wide-Angle X-ray Scattering: 1-Decyl-3-methylimidazolium Chloride. ChemPhysChem 2018, 19, 1441–1447. 10.1002/cphc.201701273. [DOI] [PubMed] [Google Scholar]
- Takekiyo T.; Koyama Y.; Shigemi M.; Matsuishi K.; Abe H.; Hamaya N.; Yoshimura Y. Conformational Adjustment for High-Pressure Glass Formation of 1-Alkyl-3-methylimidazolium Tetrafluoroborate. Phys. Chem. Chem. Phys. 2017, 19, 863–870. 10.1039/C6CP06212A. [DOI] [PubMed] [Google Scholar]
- Endo T.; Kato T.; Tozaki K.-i.; Nishikawa K. Phase Behaviors of Room Temperature Ionic Liquid Linked with Cation Conformational Changes: 1-Butyl-3-methylimidazolium Hexafluorophosphate. J. Phys. Chem. B 2010, 114, 407–411. 10.1021/jp909256j. [DOI] [PubMed] [Google Scholar]
- Jiang H. J.; Imberti S.; Atkin R.; Warr G. G. Dichotomous Well-Defined Nanostructure with Weakly Arranged Ion Packing Explains the Solvency of Pyrrolidinium Acetate. J. Phys. Chem. B 2017, 121, 6610–6617. 10.1021/acs.jpcb.7b03045. [DOI] [PubMed] [Google Scholar]
- Das L.; Kumar R.; Maity D. K.; Adhikari S.; Dhiman S. B.; Wishart J. F. Pulse Radiolysis and Computational Studies on a Pyrrolidinium Dicyanamide Ionic Liquid: Detection of the Dimer Radical Anion. J. Phys. Chem. A 2018, 122, 3148–3155. 10.1021/acs.jpca.8b00978. [DOI] [PubMed] [Google Scholar]
- Araque J. C.; Hettige J. J.; Margulis C. J. Ionic Liquids—Conventional Solvent Mixtures, Structurally Different but Dynamically Similar. J. Chem. Phys. 2015, 143, 134505. 10.1063/1.4932331. [DOI] [PubMed] [Google Scholar]
- Santos C. S.; Murthy N. S.; Baker G. A.; Castner E. W. Jr X-ray Scattering From Ionic Liquids with Pyrrolidinium Cations. J. Chem. Phys. 2011, 134, 121101. 10.1063/1.3569131. [DOI] [PubMed] [Google Scholar]
- Li S.; Bañuelos J. L.; Guo J.; Anovitz L.; Rother G.; Shaw R. W.; Hillesheim P. C.; Dai S.; Baker G. A.; Cummings P. T. Alkyl Chain Length and Temperature Effects on Structural Properties of Pyrrolidinium-Based Ionic Liquids: A Combined Atomistic Simulation and Small-Angle X-ray Scattering Study. J. Phys. Chem. Lett. 2012, 3, 125–130. 10.1021/jz2013209. [DOI] [Google Scholar]
- Kashyap H. K.; Hettige J. J.; Annapureddy H. V. R.; Margulis C. J. SAXS Anti-Peaks Reveal the Length-Scales of Dual Positive-Negative and Polar-Apolar Ordering in Room-Temperature Ionic Liquids. Chem. Commun. 2012, 48, 5103–5105. 10.1039/c2cc30609c. [DOI] [PubMed] [Google Scholar]
- Borodin O.; Smith G. D. Structure and Dynamics of N-methyl-N-propylpyrrolidinium Bis(trifluoromethanesulfonyl)imide Ionic Liquid from Molecular Dynamics Simulations. J. Phys. Chem. B 2006, 110, 11481–11490. 10.1021/jp061593o. [DOI] [PubMed] [Google Scholar]
- Kashyap H. K.; Santos C. S.; Daly R. P.; Hettige J. J.; Murthy N. S.; Shirota H.; Castner E. W. Jr; Margulis C. J. How Does the Ionic Liquid Organizational Landscape Change When Nonpolar Cationic Alkyl Groups Are Replaced by Polar Isoelectronic Diethers?. J. Phys. Chem. B 2013, 117, 1130–1135. 10.1021/jp311032p. [DOI] [PubMed] [Google Scholar]
- Smith A. M.; Lovelock K. R. J.; Gosvami N. N.; Licence P.; Dolan A.; Welton T.; Perkin S. Monolayer to Bilayer Structural Transition in Confined Pyrrolidinium-Based Ionic Liquids. J. Phys. Chem. Lett. 2013, 4, 378–382. 10.1021/jz301965d. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Gupta A.; Kashyap H. K. How the Structure of Pyrrolidinium Ionic Liquids is Susceptible to High Pressure. J. Phys. Chem. B 2016, 120, 3206–3214. 10.1021/acs.jpcb.6b01133. [DOI] [PubMed] [Google Scholar]
- Kashyap H. K.; Santos C. S.; Murthy N. S.; Hettige J. J.; Kerr K.; Ramati S.; Gwon J.; Gohdo M.; Lall-Ramnarine S. I.; Wishart J. F.; Margulis C. J.; Castner E. W. Structure of 1-Alkyl-1-methylpyrrolidinium Bis(trifluoromethylsulfonyl)amide Ionic Liquids with Linear, Branched, and Cyclic Alkyl Groups. J. Phys. Chem. B 2013, 117, 15328–15337. 10.1021/jp403518j. [DOI] [PubMed] [Google Scholar]
- Lima T. A.; Faria L. F.; Paschoal V. H.; Ribeiro M. C. Glass Transition and Melting Lines of an Ionic Liquid. J. Chem. Phys. 2018, 148, 171101. 10.1063/1.5030083. [DOI] [PubMed] [Google Scholar]
- Montanino M.; Carewska M.; Alessandrini F.; Passerini S.; Appetecchi G. B. The Role of the Cation Aliphatic Side Chain Length in Piperidinium Bis(trifluoromethansulfonyl)imide Ionic Liquids. Electrochim. Acta 2011, 57, 153–159. 10.1016/j.electacta.2011.03.089. [DOI] [Google Scholar]
- Kakinuma S.; Shirota H. Femtosecond Raman-Induced Kerr Effect Study of Temperature-Dependent Intermolecular Dynamics in Molten Bis(trifluoromethylsulfonyl)amide Salts: Effects of Cation Species. J. Phys. Chem. B 2018, 122, 6033–6047. 10.1021/acs.jpcb.8b03302. [DOI] [PubMed] [Google Scholar]
- Wu C.; De Visscher A.; Gates I. D. Comparison of Electronic and Physicochemical Properties Between Imidazolium-Based and Pyridinium-Based Ionic Liquids. J. Phys. Chem. B 2018, 122, 6771–6780. 10.1021/acs.jpcb.8b00764. [DOI] [PubMed] [Google Scholar]
- Araque J. C.; Yadav S. K.; Shadeck M.; Maroncelli M.; Margulis C. J. How is Diffusion of Neutral and Charged Tracers Related to the Structure and Dynamics of a Room-Temperature Ionic Liquid? Large Deviations from Stokes-Einstein Behavior Explained. J. Phys. Chem. B 2015, 119, 7015–7029. 10.1021/acs.jpcb.5b01093. [DOI] [PubMed] [Google Scholar]
- Wu E. C.; Kim H. J.; Peteanu L. A. Spectroscopic and MD Study of Dynamic and Structural Heterogeneities in Ionic Liquids. J. Phys. Chem. B 2017, 121, 1100–1107. 10.1021/acs.jpcb.6b10678. [DOI] [PubMed] [Google Scholar]
- Costa R.; Pereira C. M.; Silva F. Electric Double Layer Studies at the Interface of Mercury-Binary Ionic Liquid Mixtures with a Common Anion. RSC Adv. 2013, 3, 11697–11706. 10.1039/c3ra40584b. [DOI] [Google Scholar]
- Lawler C.; Fayer M. D. The Influence of Lithium Cations on Dynamics and Structure of Room Temperature Ionic Liquids. J. Phys. Chem. B 2013, 117, 9768–9774. 10.1021/jp405752q. [DOI] [PubMed] [Google Scholar]
- Liu H.; Maginn E. Effect of Ion Structure on Conductivity in Lithium-Doped Ionic Liquid Electrolytes: A Molecular Dynamics Study. J. Chem. Phys. 2013, 139, 114508. 10.1063/1.4821155. [DOI] [PubMed] [Google Scholar]
- Nasrabadi A. T.; Gelb L. D. Structural and Transport Properties of Tertiary Ammonium Triflate Ionic Liquids: A Molecular Dynamics Study. J. Phys. Chem. B 2017, 121, 1908–1921. 10.1021/acs.jpcb.6b12418. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S.; Shinoda W.; Miran M. S.; Kinoshita H.; Yasuda T.; Watanabe M. Interactions in Ion Pairs of Protic Ionic Liquids: Comparison with Aprotic Ionic Liquids. J. Chem. Phys. 2013, 139, 174504. 10.1063/1.4827519. [DOI] [PubMed] [Google Scholar]
- Pott T.; Méléard P. New Insight into the Nanostructure of Ionic Liquids: A Small Angle X-ray Scattering (SAXS) Study on Liquid Trialkylmethylammonium Bis(trifluoromethanesulfonyl)amides and Their Mixtures. Phys. Chem. Chem. Phys. 2009, 11, 5469–5475. 10.1039/b901582e. [DOI] [PubMed] [Google Scholar]
- Santos C. S.; Annapureddy H. V.; Murthy N. S.; Kashyap H. K.; Castner E. W. Jr; Margulis C. J. Temperature-Dependent Structure of Methyltributylammonium Bis(trifluoromethylsulfonyl)amide: X-ray Scattering and Simulations. J. Chem. Phys. 2011, 134, 064501. 10.1063/1.3526958. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Pádua A. l. A.; Canongia Lopes J. N. Nanostructure of Trialkylmethylammonium Bistriflamide Ionic Liquids Studied by Molecular Dynamics. J. Phys. Chem. B 2010, 114, 15635–15641. 10.1021/jp108420x. [DOI] [PubMed] [Google Scholar]
- Iimori T.; Iwahashi T.; Ishii H.; Seki K.; Ouchi Y.; Ozawa R.; Hamaguchi H.-O.; Kim D. Orientational Ordering of Alkyl Chain at the Air/Liquid Interface of Ionic Liquids Studied by Sum Frequency Vibrational Spectroscopy. Chem. Phys. Lett. 2004, 389, 321–326. 10.1016/j.cplett.2004.03.099. [DOI] [Google Scholar]
- Yasui Y.; Kitazumi Y.; Ishimatsu R.; Nishi N.; Kakiuchi T. Ultraslow Response of Interfacial Tension to the Change in the Phase-Boundary Potential at the Interface Between Water and a Room-Temperature Ionic Liquid, Trioctylmethylammonium Bis(nonafluorobutanesulfonyl)amide. J. Phys. Chem. B 2009, 113, 3273–3276. 10.1021/jp9006312. [DOI] [PubMed] [Google Scholar]
- Hanke K.; Kaufmann M.; Schwaab G.; Havenith M.; Wolke C. T.; Gorlova O.; Johnson M. A.; Kar B. P.; Sander W.; Sanchez-Garcia E. Understanding the Ionic Liquid [N4111][NTf2] From Individual Building Blocks: An IR-Spectroscopic Study. Phys. Chem. Chem. Phys. 2015, 17, 8518–8529. 10.1039/C5CP00116A. [DOI] [PubMed] [Google Scholar]
- Mendil-Jakani H.; Baroni P.; Noirez L.; Chancelier L.; Gebel G. Highlighting a Solid-Like Behavior in RTILs: Trioctylmethylammonium Bis(trifluoromethanesulfonyl)imide TOMA-TFSI. J. Phys. Chem. Lett. 2013, 4, 3775–3778. 10.1021/jz401938y. [DOI] [Google Scholar]
- Yamaguchi T.; Nakahara E.; Sueda K.; Koda S. Interpretation of the Variation of the Walden Product of Ionic Liquids with Different Alkyl Chain Lengths in Terms of Relaxation Spectra. J. Phys. Chem. B 2013, 117, 4121–4126. 10.1021/jp401280a. [DOI] [PubMed] [Google Scholar]
- Ke Y.; Jin W.; Yang Q.; Suo X.; Yang Y.; Ren Q.; Xing H. Nanostructured Branched-Chain Carboxylate Ionic Liquids: Synthesis, Characterization and Extraordinary Solubility for Bioactive Molecules. ACS Sustainable Chem. Eng. 2018, 6, 8983–8991. 10.1021/acssuschemeng.8b01353. [DOI] [Google Scholar]
- del Olmo L.; Lage-Estebanez I.; López R.; Garcia de la Vega J. M. Understanding the Structure and Properties of Cholinium Amino Acid Based Ionic Liquids. J. Phys. Chem. B 2016, 120, 10327–10335. 10.1021/acs.jpcb.6b06969. [DOI] [PubMed] [Google Scholar]
- Campetella M.; Le Donne A.; Daniele M.; Gontrani L.; Lupi S.; Bodo E.; Leonelli F. Hydrogen Bonding Features in Cholinium-Based Protic Ionic Liquids From Molecular Dynamics Simulations. J. Phys. Chem. B 2018, 122, 2635–2645. 10.1021/acs.jpcb.7b12455. [DOI] [PubMed] [Google Scholar]
- Campetella M.; Bodo E.; Montagna M.; De Santis S.; Gontrani L. Theoretical Study of Ionic Liquids Based on the Cholinium Cation. Ab Initio Simulations of Their Condensed Phases. J. Chem. Phys. 2016, 144, 104504. 10.1063/1.4943197. [DOI] [PubMed] [Google Scholar]
- Aparicio S.; Atilhan M.; Pala N. Insights on Cholinium- and Piperazinium-Based Ionic Liquids Under External Electric Fields: A Molecular Dynamics Study. J. Chem. Phys. 2013, 139, 224502. 10.1063/1.4839635. [DOI] [PubMed] [Google Scholar]
- Triolo A.; Russina O.; Caminiti R.; Shirota H.; Lee H. Y.; Santos C. S.; Murthy N. S.; Castner E. W. Jr. Comparing Intermediate Range Order for Alkyl- vs. Ether-Substituted Cations in Ionic Liquids. Chem. Commun. 2012, 48, 4959–4961. 10.1039/c2cc31550e. [DOI] [PubMed] [Google Scholar]
- Siqueira L. J.; Ribeiro M. C. Charge Ordering and Intermediate Range Order in Ammonium Ionic Liquids. J. Chem. Phys. 2011, 135, 204506. 10.1063/1.3662062. [DOI] [PubMed] [Google Scholar]
- Siqueira L. J.; Ribeiro M. C. Molecular Dynamics Simulation of the Ionic Liquid N-ethyl-N,N-dimethyl-N-(2-methoxyethyl)ammonium Bis(trifluoromethanesulfonyl)imide. J. Phys. Chem. B 2007, 111, 11776–11785. 10.1021/jp074840c. [DOI] [PubMed] [Google Scholar]
- Lee H. Y.; Shirota H.; Castner E. W. Jr Differences in Ion Interactions for Isoelectronic Ionic Liquid Homologs. J. Phys. Chem. Lett. 2013, 4, 1477–1483. 10.1021/jz400465x. [DOI] [PubMed] [Google Scholar]
- Shirota H.; Fukazawa H.; Fujisawa T.; Wishart J. F. Heavy Atom Substitution Effects in non-Aromatic Ionic Liquids: Ultrafast Dynamics and Physical Properties. J. Phys. Chem. B 2010, 114, 9400–9412. 10.1021/jp1021104. [DOI] [PubMed] [Google Scholar]
- Scarbath-Evers L. K.; Hunt P. A.; Kirchner B.; MacFarlane D. R.; Zahn S. Molecular Features Contributing to the Lower Viscosity of Phosphonium Ionic Liquids Compared to Their Ammonium Analogues. Phys. Chem. Chem. Phys. 2015, 17, 20205–20216. 10.1039/C5CP00340G. [DOI] [PubMed] [Google Scholar]
- Philippi F.; Rauber D.; Zapp J.; Hempelmann R. Transport Properties and Ionicity of Phosphonium Ionic Liquids. Phys. Chem. Chem. Phys. 2017, 19, 23015–23023. 10.1039/C7CP04552B. [DOI] [PubMed] [Google Scholar]
- Carvalho P. J.; Ventura S. P.; Batista M. L.; Schröder B.; Gonçalves F.; Esperança J.; Mutelet F.; Coutinho J. A. Understanding the Impact of the Central Atom on the Ionic Liquid Behavior: Phosphonium vs Ammonium Cations. J. Chem. Phys. 2014, 140, 064505. 10.1063/1.4864182. [DOI] [PubMed] [Google Scholar]
- Marták J.; Schlosser Š. New Mechanism and Model of Butyric Acid Extraction by Phosphonium Ionic Liquid. J. Chem. Eng. Data 2016, 61, 2979–2996. 10.1021/acs.jced.5b01082. [DOI] [Google Scholar]
- Chen F.; Jin L.; De Leeuw S.; Pringle J.; Forsyth M. Atomistic Simulation of Structure and Dynamics of the Plastic Crystal Diethyl(methyl)(isobutyl)phosphonium Hexafluorophosphate. J. Chem. Phys. 2013, 138, 244503. 10.1063/1.4811179. [DOI] [PubMed] [Google Scholar]
- Carignano M. A. Structure and Dynamics of [PF6][P1,2,2,4] from Molecular Dynamics Simulations. J. Phys. Chem. B 2013, 117, 15176–15183. 10.1021/jp407648b. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Sharma S.; Kashyap H. K. Composition Dependent Structural Organization in Trihexyl(tetradecyl)phosphonium Chloride Ionic Liquid-Methanol Mixtures. J. Chem. Phys. 2015, 142, 134503. 10.1063/1.4916308. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Gupta A.; Dhabal D.; Kashyap H. K. Pressure-Dependent Morphology of Trihexyl(tetradecyl)phosphonium Ionic Liquids: A Molecular Dynamics Study. J. Chem. Phys. 2016, 145, 134506. 10.1063/1.4963271. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.; Koda S. Dielectric and Shear Relaxations of Ionic Liquid Composed of Symmetric Ions. J. Chem. Phys. 2014, 141, 144503. 10.1063/1.4897988. [DOI] [PubMed] [Google Scholar]
- Hettige J. J.; Araque J. C.; Kashyap H. K.; Margulis C. J. Nanoscale Structure of Tetradecyltrihexylphosphonium Based Ionic Liquids. J. Chem. Phys. 2016, 144, 121102. 10.1063/1.4944678. [DOI] [PubMed] [Google Scholar]
- Tanner E. E. L.; Barnes E. O.; Tickell C. B.; Goodrich P.; Hardacre C.; Compton R. G. Application of Asymmetric Marcus-Hush Theory to Voltammetry in Room-Temperature Ionic Liquids. J. Phys. Chem. C 2015, 119, 7360–7370. 10.1021/acs.jpcc.5b01174. [DOI] [Google Scholar]
- Liu H.; Paddison S. J. Direct Calculation of the X-ray Structure Factor of Ionic Liquids. Phys. Chem. Chem. Phys. 2016, 18, 11000–11007. 10.1039/C5CP06199G. [DOI] [PubMed] [Google Scholar]
- Hettige J. J.; Kashyap H. K.; Margulis C. J. Anomalous Temperature Dependence of the Intermediate Range Order in Phosphonium Ionic Liquids. J. Chem. Phys. 2014, 140, 111102. 10.1063/1.4867900. [DOI] [PubMed] [Google Scholar]
- An R.; Zhou G.; Zhu Y.; Zhu W.; Huang L.; Shah F. U. Friction of Ionic Liquid–Glycol Ether Mixtures at Titanium Interfaces: Negative Load Dependence. Adv. Mater. Interfaces 2018, 5, 1800263. 10.1002/admi.201800263. [DOI] [Google Scholar]
- Sarman S.; Wang Y.-L.; Rohlmann P.; Glavatskih S.; Laaksonen A. Rheology of Phosphonium Ionic Liquids: A Molecular Dynamics and Experimental Study. Phys. Chem. Chem. Phys. 2018, 20, 10193–10203. 10.1039/C7CP08349A. [DOI] [PubMed] [Google Scholar]
- Pei H.-W.; Li B.; Laaksonen A.; Wang Y.-L. How Molecular Chiralities of Bis(mandelato)borate Anions Affect Their Binding Structures with Alkali Metal Ions and Microstructural Properties in Tetraalkylphosphonium Ionic Liquids. Front. Chem. 2020, 8, 65. 10.3389/fchem.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke C. G.; Lynden-Bell R. M. A Simulation Study of Water-Dialkylimidazolium Ionic Liquid Mixtures. J. Phys. Chem. B 2003, 107, 10873–10878. 10.1021/jp034221d. [DOI] [Google Scholar]
- Klähn M.; Stüber C.; Seduraman A.; Wu P. What Determines the Miscibility of Ionic Liquids with Water? Identification of the Underlying Factors to Enable a Straightforward Prediction. J. Phys. Chem. B 2010, 114, 2856–2868. 10.1021/jp1000557. [DOI] [PubMed] [Google Scholar]
- Anthony J. L.; Maginn E. J.; Brennecke J. F. Solution Thermodynamics of Imidazolium-Based Ionic Liquids and Water. J. Phys. Chem. B 2001, 105, 10942–10949. 10.1021/jp0112368. [DOI] [Google Scholar]
- Lynden-Bell R.; Atamas N.; Vasilyuk A.; Hanke C. Chemical Potentials of Water and Organic Solutes in Imidazolium Ionic Liquids: A Simulation Study. Mol. Phys. 2002, 100, 3225–3229. 10.1080/00268970210159488. [DOI] [Google Scholar]
- Wang Y.-L.; Sarman S.; Kloo L.; Antzutkin O. N.; Glavatskih S.; Laaksonen A. Solvation Structures of Water in Trihexyltetradecylphosphonium-Orthoborate Ionic Liquids. J. Chem. Phys. 2016, 145, 064507. 10.1063/1.4960506. [DOI] [Google Scholar]
- Seddon K. R.; Stark A.; Torres M.-J. Influence of Chloride, Water, and Organic Solvents on the Physical Properties of Ionic Liquids. Pure Appl. Chem. 2000, 72, 2275–2287. 10.1351/pac200072122275. [DOI] [Google Scholar]
- Freire M. G.; Carvalho P. J.; Gardas R. L.; Marrucho I. M.; Santos L. M.; Coutinho J. A. Mutual Solubilities of Water and the [Cnmim][Tf2N] Hydrophobic Ionic Liquids. J. Phys. Chem. B 2008, 112, 1604–1610. 10.1021/jp7097203. [DOI] [PubMed] [Google Scholar]
- Alfassi Z.; Huie R. E.; Milman B.; Neta P. Electrospray Ionization Mass Spectrometry of Ionic Liquids and Determination of Their Solubility in Water. Anal. Bioanal. Chem. 2003, 377, 159–164. 10.1007/s00216-003-2033-8. [DOI] [PubMed] [Google Scholar]
- Maiti A.; Kumar A.; Rogers R. D. Water-Clustering in Hygroscopic Ionic Liquids—An Implicit Solvent Analysis. Phys. Chem. Chem. Phys. 2012, 14, 5139–5146. 10.1039/c2cp00010e. [DOI] [PubMed] [Google Scholar]
- Aki S. N.; Brennecke J. F.; Samanta A. How Polar Are Room-Temperature Ionic Liquids?. Chem. Commun. 2001, 413–414. 10.1039/b008039j. [DOI] [Google Scholar]
- Freire M. G.; Neves C. M.; Carvalho P. J.; Gardas R. L.; Fernandes A. M.; Marrucho I. M.; Santos L. M.; Coutinho J. A. Mutual Solubilities of Water and Hydrophobic Ionic Liquids. J. Phys. Chem. B 2007, 111, 13082–13089. 10.1021/jp076271e. [DOI] [PubMed] [Google Scholar]
- Kaneko K.; Saihara K.; Masuda Y.; Yoshimura Y.; Shimizu A. Dynamic Properties of Water Molecules in Ionic Liquid/Water Mixture with Various Alkyl Chain Length. J. Mol. Liq. 2018, 264, 337–342. 10.1016/j.molliq.2018.05.043. [DOI] [Google Scholar]
- Saihara K.; Yoshimura Y.; Ohta S.; Shimizu A. Properties of Water Confined in Ionic Liquids. Sci. Rep. 2015, 5, 10619. 10.1038/srep10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeeva T. A.; Husson P.; DeVine J. A.; Costa Gomes M. F.; Greenbaum S. G.; Castner E. W. Jr Interactions Between Water and 1-Butyl-1-methylpyrrolidinium Ionic Liquids. J. Chem. Phys. 2015, 143, 064503. 10.1063/1.4928065. [DOI] [PubMed] [Google Scholar]
- Cha S.; Ao M.; Sung W.; Moon B.; Ahlström B.; Johansson P.; Ouchi Y.; Kim D. Structures of Ionic Liquid-Water Mixtures Investigated by IR and NMR Spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 9591–9601. 10.1039/C4CP00589A. [DOI] [PubMed] [Google Scholar]
- Jeon Y.; Sung J.; Kim D.; Seo C.; Cheong H.; Ouchi Y.; Ozawa R.; Hamaguchi H.-O. Structural Change of 1-Butyl-3-methylimidazolium Tetrafluoroborate+Water Mixtures Studied by Infrared Vibrational Spectroscopy. J. Phys. Chem. B 2008, 112, 923–928. 10.1021/jp0746650. [DOI] [PubMed] [Google Scholar]
- Sun B.; Jin Q.; Tan L.; Wu P.; Yan F. Trace of the Interesting “V”-Shaped Dynamic Mechanism of Interactions Between Water and Ionic Liquids. J. Phys. Chem. B 2008, 112, 14251–14259. 10.1021/jp806805r. [DOI] [PubMed] [Google Scholar]
- Cammarata L.; Kazarian S.; Salter P.; Welton T. Molecular States of Water in Room Temperature Ionic Liquids. Phys. Chem. Chem. Phys. 2001, 3, 5192–5200. 10.1039/b106900d. [DOI] [Google Scholar]
- Takamuku T.; Kyoshoin Y.; Shimomura T.; Kittaka S.; Yamaguchi T. Effect of Water on Structure of Hydrophilic Imidazolium-Based Ionic Liquid. J. Phys. Chem. B 2009, 113, 10817–10824. 10.1021/jp9042667. [DOI] [PubMed] [Google Scholar]
- van der Post S. T.; Scheidelaar S.; Bakker H. J. Water Dynamics in Aqueous Solutions of Tetra-n-alkylammonium Salts: Hydrophobic and Coulomb Interactions Disentangled. J. Phys. Chem. B 2013, 117, 15101–15110. 10.1021/jp4085734. [DOI] [PubMed] [Google Scholar]
- D’Angelo P.; Zitolo A.; Migliorati V.; Bodo E.; Aquilanti G.; Hazemann J. L.; Testemale D.; Mancini G.; Caminiti R. X-Ray Absorption Spectroscopy Investigation of 1-Alkyl-3-methylimidazolium Bromide Salts. J. Chem. Phys. 2011, 135, 074505. 10.1063/1.3625920. [DOI] [PubMed] [Google Scholar]
- Danten Y.; Cabaco M.; Besnard M. Interaction of Water Highly Diluted in 1-Alkyl-3-methylimidazolium Ionic Liquids with the PF6 and BF4 Anions. J. Phys. Chem. A 2009, 113, 2873–2889. 10.1021/jp8108368. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Sarman S.; Glavatskih S.; Antzutkin O. N.; Rutland M. W.; Laaksonen A. Atomistic Insight into Tetraalkylphosphonium-Bis(oxalato)borate Ionic Liquid/Water Mixtures. I. Local Microscopic Structure. J. Phys. Chem. B 2015, 119, 5251–5264. 10.1021/acs.jpcb.5b00667. [DOI] [PubMed] [Google Scholar]
- Jiang W.; Wang Y.; Voth G. A. Molecular Dynamics Simulation of Nanostructural Organization in Ionic Liquid/Water Mixtures. J. Phys. Chem. B 2007, 111, 4812–4818. 10.1021/jp067142l. [DOI] [PubMed] [Google Scholar]
- Serva A.; Migliorati V.; Lapi A.; Aquilanti G.; Arcovito A.; D’Angelo P. Structural Properties of Geminal Dicationic Ionic Liquid/Water Mixtures: A Theoretical and Experimental Insight. Phys. Chem. Chem. Phys. 2016, 18, 16544–16554. 10.1039/C6CP01557C. [DOI] [PubMed] [Google Scholar]
- Abe H.; Imai Y.; Takekiyo T.; Yoshimura Y. Deuterated Water Effect in a Room Temperature Ionic Liquid: N, N-diethyl-N-methyl-N-2-methoxyethyl Ammonium Tetrafluoroborate. J. Phys. Chem. B 2010, 114, 2834–2839. 10.1021/jp910947z. [DOI] [PubMed] [Google Scholar]
- López-Pastor M.; Ayora-Cañada M. J.; Valcárcel M.; Lendl B. Association of Methanol and Water in Ionic Liquids Elucidated by Infrared Spectroscopy Using Two-Dimensional Correlation and Multivariate Curve Resolution. J. Phys. Chem. B 2006, 110, 10896–10902. 10.1021/jp057398b. [DOI] [PubMed] [Google Scholar]
- Abe H.; Takekiyo T.; Yoshimura Y.; Saihara K.; Shimizu A. Anomalous Freezing of Nano-Confined Water in Room-Temperature Ionic Liquid 1-Butyl-3-methylimidazolium Nitrate. ChemPhysChem 2016, 17, 1136–1142. 10.1002/cphc.201501199. [DOI] [PubMed] [Google Scholar]
- Usula M.; Mocci F.; Marincola F. C.; Porcedda S.; Gontrani L.; Caminiti R. The Structural Organization of N-methyl-2-pyrrolidone+Water Mixtures: A Densitometry, X-ray Diffraction, and Molecular Dynamics Study. J. Chem. Phys. 2014, 140, 124503. 10.1063/1.4869235. [DOI] [PubMed] [Google Scholar]
- Bailey H. E.; Wang Y.-L.; Lynch S. R.; Fayer M. D. Dynamics and Microstructures of Nicotine/Water Binary Mixtures Near the Lower Critical Solution Temperature. J. Phys. Chem. B 2018, 122, 9538–9548. 10.1021/acs.jpcb.8b06205. [DOI] [PubMed] [Google Scholar]
- Kraack J. P.; Hamm P. Surface-Sensitive and Surface-Specific Ultrafast Two-Dimensional Vibrational Spectroscopy. Chem. Rev. 2017, 117, 10623–10664. 10.1021/acs.chemrev.6b00437. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Wan Z.; Yang Z.; Ji Y.; Li L.; Yang D.; Zhu M.; Chen X. Concentration-Dependent Hydrogen Bond Behavior of Ethylammonium Nitrate Protic Ionic Liquid-Water Mixtures Explored by Molecular Dynamics Simulations. J. Chem. Eng. Data 2017, 62, 2340–2349. 10.1021/acs.jced.7b00205. [DOI] [Google Scholar]
- Migliorati V.; Ballirano P.; Gontrani L.; Materazzi S.; Ceccacci F.; Caminiti R. A Combined Theoretical and Experimental Study of Solid Octyl and Decylammonium Chlorides and of Their Aqueous Solutions. J. Phys. Chem. B 2013, 117, 7806–7818. 10.1021/jp403103w. [DOI] [PubMed] [Google Scholar]
- Salma U.; Usula M.; Caminiti R.; Gontrani L.; Plechkova N. V.; Seddon K. R. X-ray and Molecular Dynamics Studies of Butylammonium Butanoate-Water Binary Mixtures. Phys. Chem. Chem. Phys. 2017, 19, 1975–1981. 10.1039/C6CP06860J. [DOI] [PubMed] [Google Scholar]
- Hayes R.; Imberti S.; Warr G. G.; Atkin R. How Water Dissolves in Protic Ionic Liquids. Angew. Chem., Int. Ed. 2012, 51, 7468–7471. 10.1002/anie.201201973. [DOI] [PubMed] [Google Scholar]
- Greaves T. L.; Kennedy D. F.; Weerawardena A.; Tse N. M. K.; Kirby N.; Drummond C. J. Nanostructured Protic Ionic Liquids Retain Nanoscale Features in Aqueous Solution While Precursor Brønsted Acids and Bases Exhibit Different Behavior. J. Phys. Chem. B 2011, 115, 2055–2066. 10.1021/jp1112203. [DOI] [PubMed] [Google Scholar]
- Reichardt C. Polarity of Ionic Liquids Determined Empirically by Means of Solvatochromic Pyridinium N-phenolate Betaine Dyes. Green Chem. 2005, 7, 339–351. 10.1039/b500106b. [DOI] [Google Scholar]
- Hadded M.; Mayaffre A.; Letellier P. Surface-Tension of Ideal Solutions-Application to Binary-Mixtures of Methanol and Molten Ethylammonium Nitrate at 298 K. J. Chim. Phys. Phys.-Chim. Biol. 1989, 86, 525–537. 10.1051/jcp/1989860525. [DOI] [Google Scholar]
- Heimburg T.; Mirzaev S.; Kaatze U. Heat Capacity Behavior in the Critical Region of the Ionic Binary Mixture Ethylammonium Nitrate-n-Octanol. Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top. 2000, 62, 4963–4967. 10.1103/PhysRevE.62.4963. [DOI] [PubMed] [Google Scholar]
- Russina O.; Sferrazza A.; Caminiti R.; Triolo A. Amphiphile Meets Amphiphile: Beyond the Polar-Apolar Dualism in Ionic Liquid/Alcohol Mixtures. J. Phys. Chem. Lett. 2014, 5, 1738–1742. 10.1021/jz500743v. [DOI] [PubMed] [Google Scholar]
- Varela L.; Méndez-Morales T.; Carrete J.; Gómez-González V.; Docampo-Álvarez B.; Gallego L.; Cabeza O.; Russina O. Solvation of Molecular Cosolvents and Inorganic Salts in Ionic Liquids: A Review of Molecular Dynamics Simulations. J. Mol. Liq. 2015, 210, 178–188. 10.1016/j.molliq.2015.06.036. [DOI] [Google Scholar]
- Kundu N.; Roy A.; Dutta R.; Sarkar N. Translational and Rotational Diffusion of Two Differently Charged Solutes in Ethylammonium Nitrate-Methanol Mixture: Does the Nanostructure of the Amphiphiles Influence the Motion of the Solute?. J. Phys. Chem. B 2016, 120, 5481–5490. 10.1021/acs.jpcb.6b02251. [DOI] [PubMed] [Google Scholar]
- Murphy T.; Hayes R.; Imberti S.; Warr G. G.; Atkin R. Ionic Liquid Nanostructure Enables Alcohol Self Assembly. Phys. Chem. Chem. Phys. 2016, 18, 12797–12809. 10.1039/C6CP01739H. [DOI] [PubMed] [Google Scholar]
- Shrivastav G.; Gupta A.; Rastogi A.; Dhabal D.; Kashyap H. K. Molecular Dynamics Study of Nanoscale Organization and Hydrogen Bonding in Binary Mixtures of Butylammonium Nitrate Ionic Liquid and Primary Alcohols. J. Chem. Phys. 2017, 146, 064503. 10.1063/1.4975172. [DOI] [PubMed] [Google Scholar]
- Montes-Campos H.; Otero-Mato J. M.; Méndez-Morales T.; López-Lago E.; Russina O.; Cabeza O.; Gallego L. J.; Varela L. M. Nanostructured Solvation in Mixtures of Protic Ionic Liquids and Long-Chained Alcohols. J. Chem. Phys. 2017, 146, 124503. 10.1063/1.4978943. [DOI] [PubMed] [Google Scholar]
- Chakraborty T.; Ghosh S.; Moulik S. P. Micellization and Related Behavior of Binary and Ternary Surfactant Mixtures in Aqueous Medium: Cetyl Pyridinium Chloride (CPC), Cetyl Trimethyl Ammonium Bromide (CTAB), and Polyoxyethylene (10) Cetyl Ether (Brij-56) Derived System. J. Phys. Chem. B 2005, 109, 14813–14823. 10.1021/jp044580o. [DOI] [PubMed] [Google Scholar]
- Schroer W.; Triolo A.; Russina O. Nature of Mesoscopic Organization in Protic Ionic Liquid-Alcohol Mixtures. J. Phys. Chem. B 2016, 120, 2638–2643. 10.1021/acs.jpcb.6b01422. [DOI] [PubMed] [Google Scholar]
- Domańska U.; Marciniak A. Solubility of Ionic Liquid [Emim][PF6] in Alcohols. J. Phys. Chem. B 2004, 108, 2376–2382. 10.1021/jp030582h. [DOI] [Google Scholar]
- Domanska U.; Rekawek A.; Marciniak A. Solubility of 1-Alkyl-3-ethylimidazolium-Based Ionic Liquids in Water and 1-Octanol. J. Chem. Eng. Data 2008, 53, 1126–1132. 10.1021/je700693z. [DOI] [Google Scholar]
- Vale V. R.; Rathke B.; Will S.; Schröer W. Liquid-Liquid Phase Behavior of Solutions of 1-Octyl- and 1-Decyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide (C8,10mimNTf2) in n-Alkyl Alcohols. J. Chem. Eng. Data 2010, 55, 2030–2038. 10.1021/je900988a. [DOI] [Google Scholar]
- Vaz I. C.; Bhattacharjee A.; Rocha M. A.; Coutinho J. A.; Bastos M.; Santos L. M. Alcohols as Molecular Probes in Ionic Liquids: Evidence for Nanostructuration. Phys. Chem. Chem. Phys. 2016, 18, 19267–19275. 10.1039/C6CP03616C. [DOI] [PubMed] [Google Scholar]
- Heintz A.; Lehmann J. K.; Wertz C. Thermodynamic Properties of Mixtures Containing Ionic Liquids. 3. Liquid–Liquid Equilibria of Binary Mixtures of 1-Ethyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide with Propan-1-ol, Butan-1-ol, and Pentan-1-ol. J. Chem. Eng. Data 2003, 48, 472–474. 10.1021/je0201931. [DOI] [Google Scholar]
- Heintz A.; Klasen D.; Lehmann J. K.; Wertz C. Excess Molar Volumes and Liquid-Liquid Equilibria of the Ionic Liquid 1-Methyl-3-octyl-imidazolium Tetrafluoroborate Mixed with Butan-1-ol and Pentan-1-ol. J. Solution Chem. 2005, 34, 1135–1144. 10.1007/s10953-005-7692-y. [DOI] [Google Scholar]
- Crosthwaite J. M.; Aki S. N.; Maginn E. J.; Brennecke J. F. Liquid Phase Behavior of Imidazolium-Based Ionic Liquids with Alcohols: Effect of Hydrogen Bonding and non-Polar Interactions. Fluid Phase Equilib. 2005, 228, 303–309. 10.1016/j.fluid.2004.07.014. [DOI] [Google Scholar]
- Łachwa J.; Morgado P.; Esperanca J. M.; Guedes H. J.; Canongia Lopes J. N.; Rebelo L. P. N. Fluid-Phase Behavior of {1-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide, [C6mim][NTf2], + C2–C8 n-Alcohol} Mixtures: Liquid-Liquid Equilibrium and Excess Volumes. J. Chem. Eng. Data 2006, 51, 2215–2221. 10.1021/je060307z. [DOI] [Google Scholar]
- Vale V. R.; Will S.; Schröer W.; Rathke B. The General Phase Behavior of Mixtures of 1-Alkyl-3-methylimidazolium Bis[(trifluoromethyl)sulfonyl]amide Ionic Liquids with n-Alkyl Alcohols. ChemPhysChem 2012, 13, 1860–1867. 10.1002/cphc.201100911. [DOI] [PubMed] [Google Scholar]
- Pereiro A.; Rodriguez A. Study on the Phase Behaviour and Thermodynamic Properties of Ionic Liquids Containing Imidazolium Cation with Ethanol at Several Temperatures. J. Chem. Thermodyn. 2007, 39, 978–989. 10.1016/j.jct.2006.10.017. [DOI] [Google Scholar]
- Abe H.; Fukushima R.; Onji M.; Hirayama K.; Kishimura H.; Yoshimura Y.; Ozawa S. Two-Length Scale Description of Hydrophobic Room-Temperature Ionic Liquid-Alcohol Systems. J. Mol. Liq. 2016, 215, 417–422. 10.1016/j.molliq.2015.12.011. [DOI] [Google Scholar]
- Rodrigues A. S.; Rocha M. A.; Almeida H. F.; Neves C. M.; Lopes-da-Silva J. A.; Freire M. G.; Coutinho J. o. A.; Santos L. M. Effect of the Methylation and N-H Acidic Group on the Physicochemical Properties of Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2015, 119, 8781–8792. 10.1021/acs.jpcb.5b05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosthwaite J. M.; Aki S. N.; Maginn E. J.; Brennecke J. F. Liquid Phase Behavior of Imidazolium-Based Ionic Liquids with Alcohols. J. Phys. Chem. B 2004, 108, 5113–5119. 10.1021/jp037774x. [DOI] [PubMed] [Google Scholar]
- Paduszyński K.; Królikowski M.; Domańska U. Excess Enthalpies of Mixing of Piperidinium Ionic Liquids with Short-Chain Alcohols: Measurements and PC-SAFT Modeling. J. Phys. Chem. B 2013, 117, 3884–3891. 10.1021/jp401253r. [DOI] [PubMed] [Google Scholar]
- Cláudio A. F. M.; Swift L.; Hallett J. P.; Welton T.; Coutinho J. A.; Freire M. G. Extended Scale for the Hydrogen-Bond Basicity of Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 6593–6601. 10.1039/c3cp55285c. [DOI] [PubMed] [Google Scholar]
- Bernardes C. E S; Shimizu K.; Canongia Lopes J. N Solvent Effects on the Polar Network of Ionic Liquid Solutions. J. Phys.: Condens. Matter 2015, 27, 194116. 10.1088/0953-8984/27/19/194116. [DOI] [PubMed] [Google Scholar]
- Jahangiri S.; Taghikhani M.; Behnejad H.; Ahmadi S. Theoretical Investigation of Imidazolium Based Ionic Liquid/Alcohol Mixture: A Molecular Dynamic Simulation. Mol. Phys. 2008, 106, 1015–1023. 10.1080/00268970802068495. [DOI] [Google Scholar]
- Mendez-Morales T.; Carrete J.; Cabeza O.; Gallego L.; Varela L. Molecular Dynamics Simulations of the Structural and Thermodynamic Properties of Imidazolium-Based Ionic Liquid Mixtures. J. Phys. Chem. B 2011, 115, 11170–11182. 10.1021/jp206341z. [DOI] [PubMed] [Google Scholar]
- Agrawal S.; Kashyap H. K. Structures of Binary Mixtures of Ionic Liquid 1-Butyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide with Primary Alcohols: The Role of Hydrogen-Bonding. J. Mol. Liq. 2018, 261, 337–349. 10.1016/j.molliq.2018.03.124. [DOI] [Google Scholar]
- Chen M.; Pendrill R.; Widmalm G. r.; Brady J. W.; Wohlert J. Molecular Dynamics Simulations of the Ionic Liquid 1-n-Butyl-3-methylimidazolium Chloride and Its Binary Mixtures with Ethanol. J. Chem. Theory Comput. 2014, 10, 4465–4479. 10.1021/ct500271z. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Ghorai P. K. Effect of Alcohols on the Structure and Dynamics of [BMIM][PF6] Ionic Liquid: A Combined Molecular Dynamics Simulation and Voronoi Tessellation Investigation. J. Chem. Phys. 2018, 148, 204514. 10.1063/1.5008439. [DOI] [PubMed] [Google Scholar]
- Méndez-Morales T.; Carrete J.; García M.; Cabeza O.; Gallego L. J.; Varela L. M. Dynamical Properties of Alcohol+1-Hexyl-3-methylimidazolium Ionic Liquid Mixtures: A Computer Simulation Study. J. Phys. Chem. B 2011, 115, 15313–15322. 10.1021/jp209563b. [DOI] [PubMed] [Google Scholar]
- Vaz I. C. M.; Bastos M.; Bernardes C. E. S.; Canongia Lopes J. N.; Santos L. M. N. B. F. Solvation of Alcohols in Ionic Liquids-Understanding the Effect of the Anion and Cation. Phys. Chem. Chem. Phys. 2018, 20, 2536–2548. 10.1039/C7CP07525A. [DOI] [PubMed] [Google Scholar]
- Kurnia K. A.; Lima F.; Cláudio A. F. M.; Coutinho J. A.; Freire M. G. Hydrogen-Bond Acidity of Ionic Liquids: An Extended Scale. Phys. Chem. Chem. Phys. 2015, 17, 18980–18990. 10.1039/C5CP03094C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahid A.; Maginn E. J. Monte Carlo Simulation and SAFT Modeling Study of the Solvation Thermodynamics of Dimethylformamide, Dimethylsulfoxide, Ethanol and 1-Propanol in the Ionic Liquid Trimethylbutylammonium Bis(trifluoromethylsulfonyl)imide. Phys. Chem. Chem. Phys. 2015, 17, 7449–7462. 10.1039/C4CP05961A. [DOI] [PubMed] [Google Scholar]
- Barra K. M.; Sabatini R. P.; McAtee Z. P.; Heitz M. P. Solvation and Rotation Dynamics in the Trihexyl(tetradecyl)phosphonium Chloride Ionic Liquid/Methanol Cosolvent System. J. Phys. Chem. B 2014, 118, 12979–12992. 10.1021/jp5092784. [DOI] [PubMed] [Google Scholar]
- Forse A. C.; Griffin J. M.; Merlet C.; Bayley P. M.; Wang H.; Simon P.; Grey C. P. NMR Study of Ion Dynamics and Charge Storage in Ionic Liquid Supercapacitors. J. Am. Chem. Soc. 2015, 137, 7231–7242. 10.1021/jacs.5b03958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Zhang Y.; Ma X.; Lu L.; He Y.; Deng Y. Benzonitrile as a Probe of Local Environment in Ionic Liquids. J. Phys. Chem. B 2013, 117, 2764–2772. 10.1021/jp312251q. [DOI] [PubMed] [Google Scholar]
- Mariani A.; Caminiti R.; Ramondo F.; Salvitti G.; Mocci F.; Gontrani L. Inhomogeneity in Ethylammonium Nitrate-Acetonitrile Binary Mixtures: The Highest “Low q Excess” Reported to Date. J. Phys. Chem. Lett. 2017, 8, 3512–3522. 10.1021/acs.jpclett.7b01244. [DOI] [PubMed] [Google Scholar]
- Mancini P.; Fortunato G.; Vottero L. Molecular Solvent/Ionic Liquid Binary Mixtures: Designing Solvents Based on the Determination of Their Microscopic Properties. Phys. Chem. Liq. 2004, 42, 625–632. 10.1080/00319100412331320476. [DOI] [Google Scholar]
- Mariani A.; Bonomo M.; Wu B.; Centrella B.; Dini D.; Castner E. W. Jr.; Gontrani L. Intriguing Transport Dynamics of Ethylammonium Nitrate–Acetonitrile Binary Mixtures Arising from Nano-Inhomogeneity. Phys. Chem. Chem. Phys. 2017, 19, 27212–27220. 10.1039/C7CP04592A. [DOI] [PubMed] [Google Scholar]
- Sonnleitner T.; Nikitina V.; Nazet A.; Buchner R. Do H-bonds Explain Strong Ion Aggregation in Ethylammonium Nitrate + Acetonitrile Mixtures?. Phys. Chem. Chem. Phys. 2013, 15, 18445–18452. 10.1039/c3cp51773j. [DOI] [PubMed] [Google Scholar]
- Xu J.; Deng G.; Zhou Y.; Ashraf H.; Yu Z.-W. Hydroxyl Group as IR Probe to Detect the Structure of Ionic Liquid-Acetonitrile Mixtures. J. Mol. Struct. 2018, 1161, 424–432. 10.1016/j.molstruc.2018.02.051. [DOI] [Google Scholar]
- Bešter-Rogač M.; Stoppa A.; Buchner R. Ion Association of Imidazolium Ionic Liquids in Acetonitrile. J. Phys. Chem. B 2014, 118, 1426–1435. 10.1021/jp412344a. [DOI] [PubMed] [Google Scholar]
- Asaki M.; Redondo A.; Zawodzinski T.; Taylor A. Dielectric Relaxation and Underlying Dynamics of Acetonitrile and 1-Ethyl-3-methylimidazolium Triflate Mixtures Using THz Transmission Spectroscopy. J. Chem. Phys. 2002, 116, 10377–10385. 10.1063/1.1451054. [DOI] [Google Scholar]
- Feng G.; Huang J.; Sumpter B. G.; Meunier V.; Qiao R. A “Counter-Charge Layer in Generalized Solvents” Framework for Electrical Double Layers in Neat and Hybrid Ionic Liquid Electrolytes. Phys. Chem. Chem. Phys. 2011, 13, 14723–14734. 10.1039/c1cp21428d. [DOI] [PubMed] [Google Scholar]
- Conway B.; Uitvlugt C.; Maroncelli M. Simulations of 1-Butyl-3-methylimidazolium Tetrafluoroborate + Acetonitrile Mixtures: Force-Field Validation and Frictional Characteristics. J. Phys. Chem. B 2018, 122, 7385–7393. 10.1021/acs.jpcb.8b04341. [DOI] [PubMed] [Google Scholar]
- Koverga V.; Kalugin O. N.; Miannay F.-A.; Smortsova Y.; Goloviznina K.; Marekha B.; Jedlovszky P.; Idrissi A. The Local Structure in BmimPF6/Acetonitrile Mixture: The Charge Distribution Effect. Phys. Chem. Chem. Phys. 2018, 20, 21890–21902. 10.1039/C8CP03546F. [DOI] [PubMed] [Google Scholar]
- Canongia Lopes J. N.; Costa Gomes M. F.; Husson P.; Pádua A. A. H.; Rebelo L. P. N.; Sarraute S.; Tariq M. Polarity, Viscosity, and Ionic Conductivity of Liquid Mixtures Containing [C4C1im][Ntf2] and a Molecular Component. J. Phys. Chem. B 2011, 115, 6088–6099. 10.1021/jp2012254. [DOI] [PubMed] [Google Scholar]
- Palumbo O.; Trequattrini F.; Navarra M.; Brubach J.-B.; Roy P.; Paolone A. Tailoring the Physical Properties of the Mixtures of Ionic Liquids: A Microscopic Point of View. Phys. Chem. Chem. Phys. 2017, 19, 8322–8329. 10.1039/C7CP00850C. [DOI] [PubMed] [Google Scholar]
- Chatel G.; Pereira J. F.; Debbeti V.; Wang H.; Rogers R. D. Mixing Ionic Liquids–“Simple Mixtures” or “Double Salts”?. Green Chem. 2014, 16, 2051–2083. 10.1039/c3gc41389f. [DOI] [Google Scholar]
- Clough M. T.; Crick C. R.; Gräsvik J.; Hunt P. A.; Niedermeyer H.; Welton T.; Whitaker O. P. A Physicochemical Investigation of Ionic Liquid Mixtures. Chem. Sci. 2015, 6, 1101–1114. 10.1039/C4SC02931C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks N. J.; Castiglione F.; Doherty C. M.; Dolan A.; Hill A. J.; Hunt P. A.; Matthews R. P.; Mauri M.; Mele A.; Simonutti R.; Villar-Garcia I. J.; Weber C. C.; Welton T. Linking the Structures, Free Volumes, and Properties of Ionic Liquid Mixtures. Chem. Sci. 2017, 8, 6359–6374. 10.1039/C7SC01407D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor U.; Shah J. K. Preferential Ionic Interactions and Microscopic Structural Changes Drive Nonideality in Binary Ionic Liquid Mixtures as Revealed From Molecular Simulations. Ind. Eng. Chem. Res. 2016, 55, 13132–13146. 10.1021/acs.iecr.6b03314. [DOI] [Google Scholar]
- Bayley P. M.; Best A. S.; MacFarlane D. R.; Forsyth M. Transport Properties and Phase Behaviour in Binary and Ternary Ionic Liquid Electrolyte Systems of Interest in Lithium Batteries. ChemPhysChem 2011, 12, 823–827. 10.1002/cphc.201000909. [DOI] [PubMed] [Google Scholar]
- Xiao D.; Rajian J. R.; Hines L. G. Jr; Li S.; Bartsch R. A.; Quitevis E. L. Nanostructural Organization and Anion Effects in the Optical Kerr Effect Spectra of Binary Ionic Liquid Mixtures. J. Phys. Chem. B 2008, 112, 13316–13325. 10.1021/jp804417t. [DOI] [PubMed] [Google Scholar]
- Jarosik A.; Krajewski S. R.; Lewandowski A.; Radzimski P. Conductivity of Ionic Liquids in Mixtures. J. Mol. Liq. 2006, 123, 43–50. 10.1016/j.molliq.2005.06.001. [DOI] [Google Scholar]
- Voroshylova I. V.; Ferreira E. S.; Malček M.; Costa R.; Pereira C. M.; Cordeiro M. N. D. Influence of the Anion on the Properties of Ionic Liquid Mixtures: A Molecular Dynamics Study. Phys. Chem. Chem. Phys. 2018, 20, 14899–14918. 10.1039/C8CP01541D. [DOI] [PubMed] [Google Scholar]
- Payal R. S.; Balasubramanian S. Homogenous Mixing of Ionic Liquids: Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2013, 15, 21077–21083. 10.1039/c3cp53492h. [DOI] [PubMed] [Google Scholar]
- Avula N. V.; Mondal A.; Balasubramanian S. Charge Environment and Hydrogen Bond Dynamics in Binary Ionic Liquid Mixtures: A Computational Study. J. Phys. Chem. Lett. 2018, 9, 3511–3516. 10.1021/acs.jpclett.8b01481. [DOI] [PubMed] [Google Scholar]
- Andanson J.-M.; Beier M. J.; Baiker A. Binary Ionic Liquids with a Common Cation: Insight into Nanoscopic Mixing by Infrared Spectroscopy. J. Phys. Chem. Lett. 2011, 2, 2959–2964. 10.1021/jz201323a. [DOI] [Google Scholar]
- Matthews R. P.; Villar-Garcia I. J.; Weber C. C.; Griffith J.; Cameron F.; Hallett J. P.; Hunt P. A.; Welton T. A Structural Investigation of Ionic Liquid Mixtures. Phys. Chem. Chem. Phys. 2016, 18, 8608–8624. 10.1039/C6CP00156D. [DOI] [PubMed] [Google Scholar]
- Aparicio S.; Atilhan M. Mixed Ionic Liquids: The Case of Pyridinium-Based Fluids. J. Phys. Chem. B 2012, 116, 2526–2537. 10.1021/jp212258b. [DOI] [PubMed] [Google Scholar]
- Castiglione F.; Raos G.; Battista Appetecchi G.; Montanino M.; Passerini S.; Moreno M.; Famulari A.; Mele A. Blending Ionic Liquids: How Physico-Chemical Properties Change. Phys. Chem. Chem. Phys. 2010, 12, 1784–1792. 10.1039/b921816e. [DOI] [PubMed] [Google Scholar]
- Annat G.; Forsyth M.; MacFarlane D. R. Ionic Liquid Mixtures: Variations in Physical Properties and Their Origins in Molecular Structure. J. Phys. Chem. B 2012, 116, 8251–8258. 10.1021/jp3012602. [DOI] [PubMed] [Google Scholar]
- Cha S.; Kim D. Change of Hydrogen Bonding Structure in Ionic Liquid Mixtures by Anion Type. J. Chem. Phys. 2018, 148, 193827. 10.1063/1.5010067. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Tariq M.; Rebelo L. P.; Lopes J. N. C. Binary Mixtures of Ionic Liquids with a Common Ion Revisited: A Molecular Dynamics Simulation Study. J. Mol. Liq. 2010, 153, 52–56. 10.1016/j.molliq.2009.07.012. [DOI] [Google Scholar]
- Bruce D. W.; Cabry C. P.; Canongia Lopes J. N.; Costen M. L.; D’Andrea L.; Grillo I.; Marshall B. C.; McKendrick K. G.; Minton T. K.; Purcell S. M.; Rogers S.; Slattery J. M.; Shimizu K.; Smoll E.; Tesa-Serrate M. A. Nanosegregation and Structuring in the Bulk and at the Surface of Ionic-Liquid Mixtures. J. Phys. Chem. B 2017, 121, 6002–6020. 10.1021/acs.jpcb.7b01654. [DOI] [PubMed] [Google Scholar]
- Canongia Lopes J. N.; Cordeiro T. C.; Esperança J. M.; Guedes H. J.; Huq S.; Rebelo L. P.; Seddon K. R. Deviations from Ideality in Mixtures of Two Ionic Liquids Containing a Common Ion. J. Phys. Chem. B 2005, 109, 3519–3525. 10.1021/jp0458699. [DOI] [PubMed] [Google Scholar]
- Stoppa A.; Buchner R.; Hefter G. How Ideal are Binary Mixtures of Room-Temperature Ionic Liquids?. J. Mol. Liq. 2010, 153, 46–51. 10.1016/j.molliq.2009.05.001. [DOI] [Google Scholar]
- Docampo-Alvarez B.; Gómez-González V.; Méndez-Morales T.; Rodríguez J. R.; Cabeza O.; Turmine M.; Gallego L. J.; Varela L. M. The Effect of Alkyl Chain Length on the Structure and Thermodynamics of Protic-Aprotic Ionic Liquid Mixtures: A Molecular Dynamics Study. Phys. Chem. Chem. Phys. 2018, 20, 9938–9949. 10.1039/C8CP00575C. [DOI] [PubMed] [Google Scholar]
- Lepre L. F.; Costa Gomes M.; Pádua A. l. A.; Ando R. M. A.; Ribeiro M. C. On the Regular Behavior of a Binary Mixture of Ionic Liquids. J. Phys. Chem. B 2019, 123, 6579–6587. 10.1021/acs.jpcb.9b04724. [DOI] [PubMed] [Google Scholar]
- Docampo-Álvarez B.; Gómez-González V.; Méndez-Morales T.; Rodríguez J. R.; López-Lago E.; Cabeza O.; Gallego L. J.; Varela L. M. Molecular Dynamics Simulations of Mixtures of Protic and Aprotic Ionic Liquids. Phys. Chem. Chem. Phys. 2016, 18, 23932–23943. 10.1039/C6CP03700C. [DOI] [PubMed] [Google Scholar]
- Hollóczki O.; Malberg F.; Welton T.; Kirchner B. On the Origin of Ionicity in Ionic Liquids. Ion Pairing versus Charge Transfer. Phys. Chem. Chem. Phys. 2014, 16, 16880–16890. 10.1039/C4CP01177E. [DOI] [PubMed] [Google Scholar]
- Stark A.; Brehm M.; Brüssel M.; Lehmann S. B.; Pensado A. S.; Schöppke M.; Kirchner B. A Theoretical and Experimental Chemist’s Joint View on Hydrogen Bonding in Ionic Liquids and Their Binary Mixtures. Top. Curr. Chem. 2013, 351, 149–187. 10.1007/128_2013_485. [DOI] [PubMed] [Google Scholar]
- Arce A.; Earle M. J.; Katdare S. P.; Rodríguez H.; Seddon K. R. Mutually Immiscible Ionic Liquids. Chem. Commun. 2006, 2548–2550. 10.1039/B604595B. [DOI] [PubMed] [Google Scholar]
- Omar S.; Lemus J.; Ruiz E.; Ferro V. R.; Ortega J.; Palomar J. Ionic Liquid Mixtures - An Analysis of Their Mutual Miscibility. J. Phys. Chem. B 2014, 118, 2442–2450. 10.1021/jp411527b. [DOI] [PubMed] [Google Scholar]
- Arce A.; Earle M. J.; Katdare S. P.; Rodríguez H.; Seddon K. R. Phase Equilibria of Mixtures of Mutually Immiscible Ionic Liquids. Fluid Phase Equilib. 2007, 261, 427–433. 10.1016/j.fluid.2007.06.017. [DOI] [Google Scholar]
- Hori Y.; Chikai T.; Ida T.; Mizuno M. Local Structure and Hydrogen Bond Characteristics of Imidazole Molecules for Proton Conduction in Acid and Base Proton-Conducting Composite Materials. Phys. Chem. Chem. Phys. 2018, 20, 10311–10318. 10.1039/C7CP08396C. [DOI] [PubMed] [Google Scholar]
- Turner A. H.; Imberti S.; Swadźba-Kwaśny M.; Holbrey J. D. Applying Neutron Diffraction with Isotopic Substitution to the Structure and Proton-Transport Pathways in Protic Imidazolium Bis{(trifluoromethyl)sulfonyl}imide Ionic Liquids. Faraday Discuss. 2018, 206, 247–263. 10.1039/C7FD00143F. [DOI] [PubMed] [Google Scholar]
- Shin J. Y.; Wang Y.-L.; Yamada S. A.; Hung S. T.; Fayer M. D. Imidazole and 1-Methylimidazole Hydrogen Bonding and Nonhydrogen Bonding Liquid Dynamics: Ultrafast IR Experiments. J. Phys. Chem. B 2019, 123, 2094–2105. 10.1021/acs.jpcb.8b11299. [DOI] [PubMed] [Google Scholar]
- Leclère M.; Bernard L.; Livi S.; Bardet M.; Guillermo A.; Picard L.; Duchet-Rumeau J. Gelled Electrolyte Containing Phosphonium Ionic Liquids for Lithium-Ion Batteries. Nanomaterials 2018, 8, 435. 10.3390/nano8060435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H.; Yokota Y.; Imanishi A.; Inagaki K.; Morikawa Y.; Fukui K.-I. Potential Dependent Changes in the Structural and Dynamical Properties of 1-Butyl-3-methylimidazolium Bis(trifluoromethanesulfonyl)imide on Graphite Electrodes Revealed by Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2018, 20, 19408–19415. 10.1039/C8CP02733A. [DOI] [PubMed] [Google Scholar]
- Lahiri A.; Schubert T. J.; Iliev B.; Endres F. LiTFSI in 1-Butyl-1-methylpyrrolidinium Bis(fluorosulfonyl)amide: A Possible Electrolyte for Ionic Liquid Based Lithium Ion Batteries. Phys. Chem. Chem. Phys. 2015, 17, 11161–11164. 10.1039/C5CP01337B. [DOI] [PubMed] [Google Scholar]
- Vardar G.; Sleightholme A. E.; Naruse J.; Hiramatsu H.; Siegel D. J.; Monroe C. W. Electrochemistry of Magnesium Electrolytes in Ionic Liquids for Secondary Batteries. ACS Appl. Mater. Interfaces 2014, 6, 18033–18039. 10.1021/am5049064. [DOI] [PubMed] [Google Scholar]
- Russina O.; Caminiti R.; Méndez-Morales T.; Carrete J.; Cabeza O.; Gallego L.; Varela L.; Triolo A. How Does Lithium Nitrate Dissolve in a Protic Ionic Liquid?. J. Mol. Liq. 2015, 205, 16–21. 10.1016/j.molliq.2014.08.007. [DOI] [Google Scholar]
- Prabhu S. R.; Dutt G. Does Addition of an Electrolyte Influence the Rotational Diffusion of Nondipolar Solutes in a Protic Ionic Liquid?. J. Phys. Chem. B 2015, 119, 6311–6316. 10.1021/acs.jpcb.5b02853. [DOI] [PubMed] [Google Scholar]
- Méndez-Morales T.; Carrete J. S.; Cabeza O. S.; Russina O.; Triolo A.; Gallego L. J.; Varela L. M. Solvation of Lithium Salts in Protic Ionic Liquids: A Molecular Dynamics Study. J. Phys. Chem. B 2014, 118, 761–770. 10.1021/jp410090f. [DOI] [PubMed] [Google Scholar]
- Lo Nostro P.; Ninham B. W. Hofmeister Phenomena: An Update on Ion Specificity in Biology. Chem. Rev. 2012, 112, 2286–2322. 10.1021/cr200271j. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson N.; Atkin R.; Rutland M. W. Effect of Lithium Ions on Rheology and Interfacial Forces in Ethylammonium Nitrate and Ethanolammonium Nitrate. J. Phys. Chem. C 2016, 120, 26960–26967. 10.1021/acs.jpcc.6b10626. [DOI] [Google Scholar]
- Hayes R.; Bernard S. A.; Imberti S.; Warr G. G.; Atkin R. Solvation of Inorganic Nitrate Salts in Protic Ionic Liquids. J. Phys. Chem. C 2014, 118, 21215–21225. 10.1021/jp506192d. [DOI] [Google Scholar]
- Méndez-Morales T.; Carrete J.; Rodríguez J. R.; Cabeza Ó.; Gallego L. J.; Russina O.; Varela L. M. Nanostructure of Mixtures of Protic Ionic Liquids and Lithium Salts: Effect of Alkyl Chain Length. Phys. Chem. Chem. Phys. 2015, 17, 5298–5307. 10.1039/C4CP04668D. [DOI] [PubMed] [Google Scholar]
- Umecky T.; Saito Y.; Okumura Y.; Maeda S.; Sakai T. Ionization Condition of Lithium Ionic Liquid Electrolytes Under the Solvation Effect of Liquid and Solid Solvents. J. Phys. Chem. B 2008, 112, 3357–3364. 10.1021/jp711625r. [DOI] [PubMed] [Google Scholar]
- Haskins J. B.; Bennett W. R.; Wu J. J.; Hernández D. M.; Borodin O.; Monk J. D.; Bauschlicher C. W. Jr; Lawson J. W. Computational and Experimental Investigation of Li-Doped Ionic Liquid Electrolytes:[Pyr14][TFSI], [Pyr13][FSI], and [EMIM][BF4]. J. Phys. Chem. B 2014, 118, 11295–11309. 10.1021/jp5061705. [DOI] [PubMed] [Google Scholar]
- Kadyan A.; Pandey S. Florescence Quenching within Lithium Salt-Added Ionic Liquid. J. Phys. Chem. B 2018, 122, 5106–5113. 10.1021/acs.jpcb.8b02723. [DOI] [PubMed] [Google Scholar]
- Niu S.; Cao Z.; Li S.; Yan T. Structure and Transport Properties of the LiPF6 Doped 1-Ethyl-2,3-dimethyl-imidazolium Hexafluorophosphate Ionic Liquids: A Molecular Dynamics Study. J. Phys. Chem. B 2010, 114, 877–881. 10.1021/jp909486z. [DOI] [PubMed] [Google Scholar]
- Lassègues J.-C.; Grondin J.; Aupetit C.; Johansson P. Spectroscopic Identification of the Lithium Ion Transporting Species in LiTFSI-Doped Ionic Liquids. J. Phys. Chem. A 2009, 113, 305–314. 10.1021/jp806124w. [DOI] [PubMed] [Google Scholar]
- Dubnikova F.; Zeiri Y. Structure, Energies, and Vibrational Frequencies of Solvated Li+ in Ionic Liquids: Role of Cation Type. J. Phys. Chem. A 2016, 120, 3079–3087. 10.1021/acs.jpca.5b10588. [DOI] [PubMed] [Google Scholar]
- Li Z.; Smith G. D.; Bedrov D. Li+ Solvation and Transport Properties in Ionic Liquid/Lithium Salt Mixtures: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2012, 116, 12801–12809. 10.1021/jp3052246. [DOI] [PubMed] [Google Scholar]
- Brinkkötter M.; Giffin G. A.; Moretti A.; Jeong S.; Passerini S.; Schönhoff M. Relevance of Ion Clusters for Li Transport at Elevated Salt Concentrations in [Pyr12O1][FTFSI] Ionic Liquid-Based Electrolytes. Chem. Commun. 2018, 54, 4278–4281. 10.1039/C8CC01416G. [DOI] [PubMed] [Google Scholar]
- Kerner M.; Plylahan N.; Scheers J.; Johansson P. Ionic Liquid Based Lithium Battery Electrolytes: Fundamental Benefits of Utilising Both TFSI and FSI Anions?. Phys. Chem. Chem. Phys. 2015, 17, 19569–19581. 10.1039/C5CP01891A. [DOI] [PubMed] [Google Scholar]
- Costa R.; Pereira C. M.; Silva A. F. Charge Storage on Ionic Liquid Electric Double Layer: The Role of the Electrode Material. Electrochim. Acta 2015, 167, 421–428. 10.1016/j.electacta.2015.02.180. [DOI] [Google Scholar]
- Flachard D.; Rolland J.; Obadia M.; Serghei A.; Bouchet R.; Drockenmuller E. A 1,2,3-Triazolate Lithium Salt with Ionic Liquid Properties at Room Temperature. Chem. Commun. 2018, 54, 9035–9038. 10.1039/C8CC04463E. [DOI] [PubMed] [Google Scholar]
- Menne S.; Vogl T.; Balducci A. Lithium Coordination in Protic Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 5485–5489. 10.1039/c3cp55183k. [DOI] [PubMed] [Google Scholar]
- Menne S.; Pires J.; Anouti M.; Balducci A. Protic Ionic Liquids as Electrolytes for Lithium-Ion Batteries. Electrochem. Commun. 2013, 31, 39–41. 10.1016/j.elecom.2013.02.026. [DOI] [Google Scholar]
- Kunze M.; Jeong S.; Paillard E.; Schönhoff M.; Winter M.; Passerini S. New Insights to Self-Aggregation in Ionic Liquid Electrolytes for High-Energy Electrochemical Devices. Adv. Energy Mater. 2011, 1, 274–281. 10.1002/aenm.201000052. [DOI] [Google Scholar]
- Eftekhari A.; Liu Y.; Chen P. Different Roles of Ionic Liquids in Lithium Batteries. J. Power Sources 2016, 334, 221–239. 10.1016/j.jpowsour.2016.10.025. [DOI] [Google Scholar]
- Gómez-González V.; Docampo-Álvarez B.; Cabeza O.; Fedorov M.; Lynden-Bell R. M.; Gallego L. J.; Varela L. M. Molecular Dynamics Simulations of the Structure and Single-Particle Dynamics of Mixtures of Divalent Salts and Ionic Liquids. J. Chem. Phys. 2015, 143, 124507. 10.1063/1.4931656. [DOI] [PubMed] [Google Scholar]
- Giffin G. A.; Moretti A.; Jeong S.; Passerini S. Complex Nature of Ionic Coordination in Magnesium Ionic Liquid-Based Electrolytes: Solvates with Mobile Mg2+ Cations. J. Phys. Chem. C 2014, 118, 9966–9973. 10.1021/jp502354h. [DOI] [Google Scholar]
- Chaumont A.; Klimchuk O.; Gaillard C.; Billard I.; Ouadi A.; Hennig C.; Wipff G. Perrhenate Complexation by Uranyl in Traditional Solvents and in Ionic Liquids: A Joint Molecular Dynamics/Spectroscopic Study. J. Phys. Chem. B 2012, 116, 3205–3219. 10.1021/jp209476h. [DOI] [PubMed] [Google Scholar]
- Pringle J. M. Recent Progress in the Development and Use of Organic Ionic Plastic Crystal Electrolytes. Phys. Chem. Chem. Phys. 2013, 15, 1339–1351. 10.1039/C2CP43267F. [DOI] [PubMed] [Google Scholar]
- Makhlooghiazad F.; Howlett P. C.; Wang X.; Hilder M.; MacFarlane D. R.; Armand M.; Forsyth M. Phosphonium Plastic Crystal Salt Alloyed with a Sodium Salt as a Solid-State Electrolyte for Sodium Devices: Phase Behaviour and Electrochemical Performance. J. Mater. Chem. A 2017, 5, 5770–5780. 10.1039/C6TA10340E. [DOI] [Google Scholar]
- Palumbo O.; Trequattrini F.; Vitucci F.; Paolone A. Relaxation Dynamics and Phase Transitions in Ionic Liquids: Viscoelastic Properties From the Liquid to the Solid State. J. Phys. Chem. B 2015, 119, 12905–12911. 10.1021/acs.jpcb.5b06039. [DOI] [PubMed] [Google Scholar]
- Ray P.; Vogl T.; Balducci A.; Kirchner B. Structural Investigations on Lithium-Doped Protic and Aprotic Ionic Liquids. J. Phys. Chem. B 2017, 121, 5279–5292. 10.1021/acs.jpcb.7b02636. [DOI] [PubMed] [Google Scholar]
- Pilar K.; Balédent V.; Zeghal M.; Judeinstein P.; Jeong S.; Passerini S.; Greenbaum S. Investigation of Ion Aggregation in Ionic Liquids and Their Solutions with Lithium Salt Under High Pressure. J. Chem. Phys. 2018, 148, 031102. 10.1063/1.5016049. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.; Mikawa K.-I.; Koda S.; Serizawa N.; Seki S.; Fujii K.; Umebayashi Y. Effects of Lithium Salts on Shear Relaxation Spectra of Pyrrolidinium-Based Ionic Liquids. J. Phys. Chem. B 2012, 116, 7322–7327. 10.1021/jp3032308. [DOI] [PubMed] [Google Scholar]
- Aguilera L.; Völkner J.; Labrador A.; Matic A. The Effect of Lithium Salt Doping on the Nanostructure of Ionic Liquids. Phys. Chem. Chem. Phys. 2015, 17, 27082–27087. 10.1039/C5CP03825A. [DOI] [PubMed] [Google Scholar]
- Lesch V.; Li Z.; Bedrov D.; Borodin O.; Heuer A. The Influence of Cations on Lithium Ion Coordination and Transport in Ionic Liquid Electrolytes: A MD Simulation Study. Phys. Chem. Chem. Phys. 2016, 18, 382–392. 10.1039/C5CP05111H. [DOI] [PubMed] [Google Scholar]
- Al-Masri D.; Yunis R.; Hollenkamp A. F.; Pringle J. M. A Symmetrical Ionic Liquid/Li Salt System for Rapid Ion Transport and Stable Lithium Electrochemistry. Chem. Commun. 2018, 54, 3660–3663. 10.1039/C8CC00531A. [DOI] [PubMed] [Google Scholar]
- Castiglione F.; Ragg E.; Mele A.; Appetecchi G. B.; Montanino M.; Passerini S. Molecular Environment and Enhanced Diffusivity of Li+ ions in Lithium-Salt-Doped Ionic Liquid Electrolytes. J. Phys. Chem. Lett. 2011, 2, 153–157. 10.1021/jz101516c. [DOI] [Google Scholar]
- Maier F.; Niedermaier I.; Steinrück H.-P. Perspective: Chemical Reactions in Ionic Liquids Monitored Through the Gas (Vacuum)/Liquid Interface. J. Chem. Phys. 2017, 146, 170901. 10.1063/1.4982355. [DOI] [PubMed] [Google Scholar]
- Law G.; Watson P. R. Surface Orientation in Ionic Liquids. Chem. Phys. Lett. 2001, 345, 1–4. 10.1016/S0009-2614(01)00857-0. [DOI] [Google Scholar]
- Nishi N.; Yasui Y.; Uruga T.; Tanida H.; Yamada T.; Nakayama S.-I.; Matsuoka H.; Kakiuchi T. Ionic Multilayers at Free Surface of an Ionic Liquid, Trioctylmethylammonium Bis(nonafluorobutanesulfonyl)amide, Probed by X-ray Reflectivity Measurements. J. Chem. Phys. 2010, 132, 164705. 10.1063/1.3398029. [DOI] [PubMed] [Google Scholar]
- Wakeham D.; Nelson A.; Warr G. G.; Atkin R. Probing the Protic Ionic Liquid Surface Using X-ray Reflectivity. Phys. Chem. Chem. Phys. 2011, 13, 20828–20835. 10.1039/c1cp22351h. [DOI] [PubMed] [Google Scholar]
- Sloutskin E.; Ocko B. M.; Tamam L.; Kuzmenko I.; Gog T.; Deutsch M. Surface Layering in Ionic Liquids: An X-ray Reflectivity Study. J. Am. Chem. Soc. 2005, 127, 7796–7804. 10.1021/ja0509679. [DOI] [PubMed] [Google Scholar]
- Lynden-Bell R.; Del Popolo M. Simulation of the Surface Structure of Butylmethylimidazolium Ionic Liquids. Phys. Chem. Chem. Phys. 2006, 8, 949–954. 10.1039/B514848K. [DOI] [PubMed] [Google Scholar]
- Bhargava B.; Balasubramanian S. Layering at an Ionic Liquid-Vapor Interface: A Molecular Dynamics Simulation Study of [Bmim][Pf6]. J. Am. Chem. Soc. 2006, 128, 10073–10078. 10.1021/ja060035k. [DOI] [PubMed] [Google Scholar]
- Sarangi S. S.; Raju S. G.; Balasubramanian S. Molecular Dynamics Simulations of Ionic Liquid-Vapour Interfaces: Effect of Cation Symmetry on Structure at Interface. Phys. Chem. Chem. Phys. 2011, 13, 2714–2722. 10.1039/C0CP01272F. [DOI] [PubMed] [Google Scholar]
- Rivera-Rubero S.; Baldelli S. Influence of Water on the Surface of Hydrophilic and Hydrophobic Room-Temperature Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 11788–11789. 10.1021/ja0464894. [DOI] [PubMed] [Google Scholar]
- Iwahashi T.; Miyamae T.; Kanai K.; Seki K.; Kim D.; Ouchi Y. Anion Configuration at Air/Liquid Interface of Ionic Liquid [Bmim]OTf studied by Sum-Frequency Generation Spectroscopy. J. Phys. Chem. B 2008, 112, 11936–11941. 10.1021/jp8021908. [DOI] [PubMed] [Google Scholar]
- Bowers J.; Vergara-Gutierrez M. C.; Webster J. R. Surface Ordering of Amphiphilic Ionic Liquids. Langmuir 2004, 20, 309–312. 10.1021/la035495v. [DOI] [PubMed] [Google Scholar]
- Shi R.; Wang Y. Surface Structure of Ionic Liquids Under an External Electric Field. Mol. Simul. 2017, 43, 1295–1299. 10.1080/08927022.2017.1332412. [DOI] [Google Scholar]
- Anaredy R. S.; Shaw S. K. Long-Range Ordering of Ionic Liquid Fluid Films. Langmuir 2016, 32, 5147–5154. 10.1021/acs.langmuir.6b00304. [DOI] [PubMed] [Google Scholar]
- Jurado L. A.; Kim H.; Arcifa A.; Rossi A.; Leal C.; Spencer N. D.; Espinosa-Marzal R. M. Irreversible Structural Change of a Dry Ionic Liquid Under Nanoconfinement. Phys. Chem. Chem. Phys. 2015, 17, 13613–13624. 10.1039/C4CP05592F. [DOI] [PubMed] [Google Scholar]
- Nagata Y.; Ohto T.; Backus E. H. G.; Bonn M. Molecular Modeling of Water Interfaces: From Molecular Spectroscopy to Thermodynamics. J. Phys. Chem. B 2016, 120, 3785–3796. 10.1021/acs.jpcb.6b01012. [DOI] [PubMed] [Google Scholar]
- Sloutskin E.; Lynden-Bell R.; Balasubramanian S.; Deutsch M. The Surface Structure of Ionic Liquids: Comparing Simulations with X-ray Measurements. J. Chem. Phys. 2006, 125, 174715. 10.1063/1.2361289. [DOI] [PubMed] [Google Scholar]
- Lynden-Bell R. Gas-Liquid Interfaces of Room Temperature Ionic Liquids. Mol. Phys. 2003, 101, 2625–2633. 10.1080/00268970310001592700. [DOI] [Google Scholar]
- Shimizu K.; Heller B. S.; Maier F.; Steinrück H.-P.; Canongia Lopes J. N. Probing the Surface Tension of Ionic Liquids Using the Langmuir Principle. Langmuir 2018, 34, 4408–4416. 10.1021/acs.langmuir.7b04237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettige J. J.; Amith W. D.; Castner E. W. Jr; Margulis C. J. Ionic Liquids with Symmetric Diether Tails: Bulk and Vacuum-Liquid Interfacial Structures. J. Phys. Chem. B 2017, 121, 174–179. 10.1021/acs.jpcb.6b09148. [DOI] [PubMed] [Google Scholar]
- Wang Y.-L.; Laaksonen A.; Lu Z.-Y. Influence of Ionic Liquid Film Thickness on Ion Pair Distributions and Orientations at Graphene and Vacuum Interfaces. Phys. Chem. Chem. Phys. 2013, 15, 13559–13569. 10.1039/c3cp51226f. [DOI] [PubMed] [Google Scholar]
- Hantal G.; Voroshylova I.; Cordeiro M. N. D.; Jorge M. A Systematic Molecular Simulation Study of Ionic Liquid Surfaces Using Intrinsic Analysis Methods. Phys. Chem. Chem. Phys. 2012, 14, 5200–5213. 10.1039/c2cp23967a. [DOI] [PubMed] [Google Scholar]
- Haddad J.; Pontoni D.; Murphy B. M.; Festersen S.; Runge B.; Magnussen O. M.; Steinrück H.-G.; Reichert H.; Ocko B. M.; Deutsch M. Surface Structure Evolution in a Homologous Series of Ionic Liquids. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E1100–E1107. 10.1073/pnas.1716418115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-L.; Lu Z.-Y.; Laaksonen A. Heterogeneous Dynamics of Ionic Liquids in Confined Films with Varied Film Thickness. Phys. Chem. Chem. Phys. 2014, 16, 20731–20740. 10.1039/C4CP02843K. [DOI] [PubMed] [Google Scholar]
- Lísal M.; Posel Z.; Izák P. Air–Liquid Interfaces of Imidazolium-Based [TF2N–] Ionic Liquids: Insight From Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2012, 14, 5164–5177. 10.1039/c2cp23572b. [DOI] [PubMed] [Google Scholar]
- Mezger M.; Ocko B. M.; Reichert H.; Deutsch M. Surface Layering and Melting in an Ionic Liquid Studied by Resonant Soft X-ray Reflectivity. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 3733–3737. 10.1073/pnas.1211749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli A.; Maréchal M.; Östlund Å.; Cambedouzou J. Insights into the Interplay Between Molecular Structure and Diffusional Motion in 1-Alkyl-3-methylimidazolium Ionic Liquids: A Combined PFG NMR and X-ray Scattering Study. Phys. Chem. Chem. Phys. 2013, 15, 5510–5517. 10.1039/c3cp00097d. [DOI] [PubMed] [Google Scholar]
- Aliaga C.; Baker G. A.; Baldelli S. Sum Frequency Generation Studies of Ammonium and Pyrrolidinium Ionic Liquids Based on the Bistrifluoromethanesulfonimide Anion. J. Phys. Chem. B 2008, 112, 1676–1684. 10.1021/jp077008g. [DOI] [PubMed] [Google Scholar]
- Men S.; Hurisso B. B.; Lovelock K. R. J.; Licence P. Does the Influence of Substituents Impact upon the Surface Composition of Pyrrolidinium-Based Ionic Liquids? An Angle Resolved XPS Study. Phys. Chem. Chem. Phys. 2012, 14, 5229–5238. 10.1039/c2cp40262a. [DOI] [PubMed] [Google Scholar]
- Paredes X.; Fernández J.; Pádua A. A. H.; Malfreyt P.; Malberg F.; Kirchner B.; Pensado A. S. Bulk and Liquid-Vapor Interface of Pyrrolidinium-Based Ionic Liquids: A Molecular Simulation Study. J. Phys. Chem. B 2014, 118, 731–742. 10.1021/jp406651f. [DOI] [PubMed] [Google Scholar]
- García G.; Atilhan M.; Aparicio S. Theoretical Study on the Solvation of C60 Fullerene by Ionic Liquids. J. Phys. Chem. B 2014, 118, 11330–11340. 10.1021/jp507146r. [DOI] [PubMed] [Google Scholar]
- Huang J.; Sumpter B. G.; Meunier V.; Yushin G.; Portet C.; Gogotsi Y. Curvature Effects in Carbon Nanomaterials: Exohedral versus Endohedral Supercapacitors. J. Mater. Res. 2010, 25, 1525–1531. 10.1557/JMR.2010.0195. [DOI] [Google Scholar]
- Li S.; Van Aken K. L.; McDonough J. K.; Feng G.; Gogotsi Y.; Cummings P. T. The Electrical Double Layer of Dicationic Ionic Liquids at Onion-like Carbon Surface. J. Phys. Chem. C 2014, 118, 3901–3909. 10.1021/jp409888f. [DOI] [Google Scholar]
- Dong K.; Zhou G.; Liu X.; Yao X.; Zhang S.; Lyubartsev A. Structural Evidence for the Ordered Crystallites of Ionic Liquid in Confined Carbon Nanotubes. J. Phys. Chem. C 2009, 113, 10013–10020. 10.1021/jp900533k. [DOI] [Google Scholar]
- Yu Z.; He Y.; Wang Y.; Madsen L. A.; Qiao R. Molecular Structure and Dynamics of Ionic Liquids in a Rigid-Rod Polyanion-Based Ion Gel. Langmuir 2017, 33, 322–331. 10.1021/acs.langmuir.6b03798. [DOI] [PubMed] [Google Scholar]
- Zhou G.; Li Y.; Yang Z.; Fu F.; Huang Y.; Wan Z.; Li L.; Chen X.; Hu N.; Huang L. Structural Properties and Vibrational Spectra of Ethylammonium Nitrate Ionic Liquid Confined in Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2016, 120, 5033–5041. 10.1021/acs.jpcc.6b00307. [DOI] [Google Scholar]
- Han T.; Park M.-S.; Kim J.; Kim J. H.; Kim K. The Smallest Quaternary Ammonium Salts with Ether Groups for High-Performance Electrochemical Double Layer Capacitors. Chem. Sci. 2016, 7, 1791–1796. 10.1039/C5SC02755A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov M. V.; Lynden-Bell R. M. Probing the Neutral Graphene-Ionic Liquid Interface: Insights from Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2012, 14, 2552–2556. 10.1039/c2cp22730d. [DOI] [PubMed] [Google Scholar]
- Ebeling D.; Bradler S.; Roling B.; Schirmeisen A. 3-Dimensional Structure of a Prototypical Ionic Liquid-Solid Interface: Ionic Crystal-like Behavior Induced by Molecule-Substrate Interactions. J. Phys. Chem. C 2016, 120, 11947–11955. 10.1021/acs.jpcc.6b02232. [DOI] [Google Scholar]
- Monk J.; Singh R.; Hung F. R. Effects of Pore Size and Pore Loading on the Properties of Ionic Liquids Confined Inside Nanoporous CMK-3 Carbon Materials. J. Phys. Chem. C 2011, 115, 3034–3042. 10.1021/jp1089189. [DOI] [Google Scholar]
- Lei Z.; Liu Z.; Wang H.; Sun X.; Lu L.; Zhao X. S. A High-Energy-Density Supercapacitor with Graphene-CMK-5 as the Electrode and Ionic Liquid as the Electrolyte. J. Mater. Chem. A 2013, 1, 2313–2321. 10.1039/c2ta01040b. [DOI] [Google Scholar]
- He X.; Monk J.; Singh R.; Hung F. R. Molecular Modelling of Ionic Liquids in the Ordered Mesoporous Carbon CMK-5. Mol. Simul. 2016, 42, 753–763. 10.1080/08927022.2015.1089992. [DOI] [Google Scholar]
- Fukushima T.; Kosaka A.; Ishimura Y.; Yamamoto T.; Takigawa T.; Ishii N.; Aida T. Molecular Ordering of Organic Molten Salts Triggered by Single-Walled Carbon Nanotubes. Science 2003, 300, 2072–2074. 10.1126/science.1082289. [DOI] [PubMed] [Google Scholar]
- Chaban V. V.; Fileti E. E. Free Energy of Solvation of Carbon Nanotubes in Pyridinium-Based Ionic Liquids. Phys. Chem. Chem. Phys. 2016, 18, 20357–20362. 10.1039/C6CP03497G. [DOI] [PubMed] [Google Scholar]
- Largeot C.; Portet C.; Chmiola J.; Taberna P.-L.; Gogotsi Y.; Simon P. Relation Between the Ion Size and Pore Size for an Electric Double-Layer Capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. 10.1021/ja7106178. [DOI] [PubMed] [Google Scholar]
- Chmiola J.; Largeot C.; Taberna P. L.; Simon P.; Gogotsi Y. Desolvation of Ions in Subnanometer Pores and its Effect on Capacitance and Double-Layer Theory. Angew. Chem., Int. Ed. 2008, 47, 3392–3395. 10.1002/anie.200704894. [DOI] [PubMed] [Google Scholar]
- Zhang L. L.; Zhou R.; Zhao X. Graphene-Based Materials as Supercapacitor Electrodes. J. Mater. Chem. 2010, 20, 5983–5992. 10.1039/c000417k. [DOI] [Google Scholar]
- Pech D.; Brunet M.; Durou H.; Huang P.; Mochalin V.; Gogotsi Y.; Taberna P.-L.; Simon P. Ultrahigh-Power Micrometre-Sized Supercapacitors Based on Onion-Like Carbon. Nat. Nanotechnol. 2010, 5, 651–654. 10.1038/nnano.2010.162. [DOI] [PubMed] [Google Scholar]
- Li S.; Feng G.; Fulvio P. F.; Hillesheim P. C.; Liao C.; Dai S.; Cummings P. T. Molecular Dynamics Simulation Study of the Capacitive Performance of a Binary Mixture of Ionic Liquids near an Onion-Like Carbon Electrode. J. Phys. Chem. Lett. 2012, 3, 2465–2469. 10.1021/jz3009387. [DOI] [PubMed] [Google Scholar]
- Li S.; Zhang P.; Fulvio Pasquale F; Hillesheim Patrick C; Feng G.; Dai S.; Cummings Peter T Enhanced Performance of Dicationic Ionic Liquid Electrolytes by Organic Solvents. J. Phys.: Condens. Matter 2014, 26, 284105. 10.1088/0953-8984/26/28/284105. [DOI] [PubMed] [Google Scholar]
- Shi W.; Sorescu D. C. Molecular Simulations of CO2 and H2 Sorption into Ionic Liquid 1-n-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)amide ([Hmim][Tf2N]) Confined in Carbon Nanotubes. J. Phys. Chem. B 2010, 114, 15029–15041. 10.1021/jp106500p. [DOI] [PubMed] [Google Scholar]
- Chaban V. V.; Prezhdo O. V. Nanoscale Carbon Greatly Enhances Mobility of a Highly Viscous Ionic Liquid. ACS Nano 2014, 8, 8190–8197. 10.1021/nn502475j. [DOI] [PubMed] [Google Scholar]
- Shim Y.; Kim H. J. Solvation of Carbon Nanotubes in a Room-Temperature Ionic Liquid. ACS Nano 2009, 3, 1693–1702. 10.1021/nn900195b. [DOI] [PubMed] [Google Scholar]
- Chen S.; Kobayashi K.; Miyata Y.; Imazu N.; Saito T.; Kitaura R.; Shinohara H. Morphology and Melting Behavior of Ionic Liquids Inside Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2009, 131, 14850–14856. 10.1021/ja904283d. [DOI] [PubMed] [Google Scholar]
- Li L.-J.; Khlobystov A.; Wiltshire J.; Briggs G.; Nicholas R. Diameter-Selective Encapsulation of Metallocenes in Single-Walled Carbon Nanotubes. Nat. Mater. 2005, 4, 481–485. 10.1038/nmat1396. [DOI] [PubMed] [Google Scholar]
- Ohba T.; Chaban V. V. A Highly Viscous Imidazolium Ionic Liquid Inside Carbon Nanotubes. J. Phys. Chem. B 2014, 118, 6234–6240. 10.1021/jp502798e. [DOI] [PubMed] [Google Scholar]
- Pamies R.; Espejo C.; Carrión F.; Morina A.; Neville A.; Bermúdez M. Rheological Behavior of Multiwalled Carbon Nanotube-Imidazolium Tosylate Ionic Liquid Dispersions. J. Rheol. 2017, 61, 279–289. 10.1122/1.4975108. [DOI] [Google Scholar]
- Weingarth D.; Drumm R.; Foelske-Schmitz A.; Kötz R.; Presser V. An Electrochemical in situ Study of Freezing and Thawing of Ionic Liquids in Carbon Nanopores. Phys. Chem. Chem. Phys. 2014, 16, 21219–21224. 10.1039/C4CP02727B. [DOI] [PubMed] [Google Scholar]
- Chen S.; Wu G.; Sha M.; Huang S. Transition of Ionic Liquid [Bmim][PF6] from Liquid to High-Melting-Point Crystal when Confined in Multiwalled Carbon Nanotubes. J. Am. Chem. Soc. 2007, 129, 2416–2417. 10.1021/ja067972c. [DOI] [PubMed] [Google Scholar]
- Ehre D.; Lavert E.; Lahav M.; Lubomirsky I. Water Freezes Differently on Positively and Negatively Charged Surfaces of Pyroelectric Materials. Science 2010, 327, 672–675. 10.1126/science.1178085. [DOI] [PubMed] [Google Scholar]
- Christenson H. K. Confinement Effects on Freezing and Melting. J. Phys.: Condens. Matter 2001, 13, R95–R133. 10.1088/0953-8984/13/11/201. [DOI] [Google Scholar]
- Dou Q.; Sha M.; Fu H.; Wu G. Melting Transition of Ionic Liquid [Bmim][PF6] Crystal Confined in Nanopores: A Molecular Dynamics Simulation. J. Phys. Chem. C 2011, 115, 18946–18951. 10.1021/jp201447g. [DOI] [Google Scholar]
- Taherkhani F.; Minofar B. Effect of Nitrogen Doping on Glass Transition and Electrical Conductivity of [EMIM][PF6] Ionic Liquid Encapsulated in a Zigzag Carbon Nanotube. J. Phys. Chem. C 2017, 121, 15493–15508. 10.1021/acs.jpcc.7b00911. [DOI] [Google Scholar]
- Akbarzadeh H.; Abbaspour M.; Salemi S.; Abdollahzadeh S. Investigation of the Melting of Ionic Liquid [EMIM][PF6] Confined Inside Carbon Nanotubes Using Molecular Dynamics Simulations. RSC Adv. 2015, 5, 3868–3874. 10.1039/C4RA14747B. [DOI] [Google Scholar]
- Singh R.; Monk J.; Hung F. R. A Computational Study of the Behavior of the Ionic Liquid [BMIM][PF6] Confined Inside Multiwalled Carbon Nanotubes. J. Phys. Chem. C 2010, 114, 15478–15485. 10.1021/jp1058534. [DOI] [Google Scholar]
- Shim Y.; Kim H. J. Nanoporous Carbon Supercapacitors in an Ionic Liquid: A Computer Simulation Study. ACS Nano 2010, 4, 2345–2355. 10.1021/nn901916m. [DOI] [PubMed] [Google Scholar]
- Ma K.; Wang X.; Forsman J.; Woodward C. E. Molecular Dynamic Simulations of Ionic Liquid’s Structural Variations from Three to One Layers Inside a Series of Slit and Cylindrical Nanopores. J. Phys. Chem. C 2017, 121, 13539–13548. 10.1021/acs.jpcc.7b03319. [DOI] [Google Scholar]
- Frolov A. I.; Kirchner K.; Kirchner T.; Fedorov M. V. Molecular-Scale Insights into the Mechanisms of Ionic Liquids Interactions with Carbon Nanotubes. Faraday Discuss. 2012, 154, 235–247. 10.1039/C1FD00080B. [DOI] [PubMed] [Google Scholar]
- Ohba T.; Hata K.; Chaban V. V. Nanocrystallization of Imidazolium Ionic Liquid in Carbon Nanotubes. J. Phys. Chem. C 2015, 119, 28424–28429. 10.1021/acs.jpcc.5b09423. [DOI] [Google Scholar]
- Li S.; Han K. S.; Feng G.; Hagaman E. W.; Vlcek L.; Cummings P. T. Dynamic and Structural Properties of Room-Temperature Ionic Liquids Near Silica and Carbon Surfaces. Langmuir 2013, 29, 9744–9749. 10.1021/la401172z. [DOI] [PubMed] [Google Scholar]
- Sha M.; Wu G.; Liu Y.; Tang Z.; Fang H. Drastic Phase Transition in Ionic Liquid [Dmim][Cl] Confined Between Graphite Walls: New Phase Formation. J. Phys. Chem. C 2009, 113, 4618–4622. 10.1021/jp810980v. [DOI] [Google Scholar]
- Ghoufi A.; Szymczyk A.; Malfreyt P. Ultrafast Diffusion of Ionic Liquids Confined in Carbon Nanotubes. Sci. Rep. 2016, 6, 28518. 10.1038/srep28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrod Q.; Ferdeghini F.; Judeinstein P.; Genevaz N.; Ramos R.; Fournier A.; Dijon J.; Ollivier J.; Rols S.; Yu D.; Mole R. A.; Zanotti J.-M. Enhanced Ionic Liquid Mobility Induced by Confinement in 1D CNT Membranes. Nanoscale 2016, 8, 7845–7848. 10.1039/C6NR01445C. [DOI] [PubMed] [Google Scholar]
- Kanakubo M.; Hiejima Y.; Minami K.; Aizawa T.; Nanjo H. Melting Point Depression of Ionic Liquids Confined in Nanospaces. Chem. Commun. 2006, 1828–1830. 10.1039/b600074f. [DOI] [PubMed] [Google Scholar]
- Zhou G.; Yang Z.; Fu F.; Huang Y.; Chen X.; Lu Z.; Hu N. Molecular-Level Understanding of Solvation Structures and Vibrational Spectra of an Ethylammonium Nitrate Ionic Liquid Around Single-Walled Carbon Nanotubes. Ind. Eng. Chem. Res. 2015, 54, 8166–8174. 10.1021/acs.iecr.5b01624. [DOI] [Google Scholar]
- Yin J.; Li X.; Yu J.; Zhang Z.; Zhou J.; Guo W. Generating Electricity by Moving a Droplet of Ionic Liquid Along Graphene. Nat. Nanotechnol. 2014, 9, 378–383. 10.1038/nnano.2014.56. [DOI] [PubMed] [Google Scholar]
- Kim H.-Y.; dos Santos M. C.; Cole M. W. Wetting Transitions of Water on Graphite and Graphene. J. Phys. Chem. A 2014, 118, 8237–8241. 10.1021/jp501046r. [DOI] [PubMed] [Google Scholar]
- Rafiee J.; Mi X.; Gullapalli H.; Thomas A. V.; Yavari F.; Shi Y.; Ajayan P. M.; Koratkar N. A. Wetting Transparency of Graphene. Nat. Mater. 2012, 11, 217–222. 10.1038/nmat3228. [DOI] [PubMed] [Google Scholar]
- Herrera C.; García G.; Atilhan M.; Aparicio S. Nanowetting of Graphene by Ionic Liquid Droplets. J. Phys. Chem. C 2015, 119, 24529–24537. 10.1021/acs.jpcc.5b08713. [DOI] [Google Scholar]
- Baldelli S.; Bao J.; Wu W.; Pei S.-S. Sum Frequency Generation Study on the Orientation of Room-Temperature Ionic Liquid at the Graphene-Ionic Liquid Interface. Chem. Phys. Lett. 2011, 516, 171–173. 10.1016/j.cplett.2011.09.084. [DOI] [Google Scholar]
- Xu S.; Xing S.; Pei S.-S.; Ivaništšev V.; Lynden-Bell R.; Baldelli S. Molecular Response of 1-Butyl-3-Methylimidazolium Dicyanamide Ionic Liquid at Graphene Electrode Interface Investigated by Sum Frequency Generation Spectroscopy and Molecular Dynamics Simulations. J. Phys. Chem. C 2015, 119, 26009–26019. 10.1021/acs.jpcc.5b08736. [DOI] [Google Scholar]
- Maruyama S.; Takeyama Y.; Taniguchi H.; Fukumoto H.; Itoh M.; Kumigashira H.; Oshima M.; Yamamoto T.; Matsumoto Y. Molecular Beam Deposition of Nanoscale Ionic Liquids in Ultrahigh Vacuum. ACS Nano 2010, 4, 5946–5952. 10.1021/nn101036v. [DOI] [PubMed] [Google Scholar]
- Buchner F.; Forster-Tonigold K.; Bozorgchenani M.; Gross A.; Behm R. J. Interaction of a Self-Assembled Ionic Liquid Layer with Graphite(0001): A Combined Experimental and Theoretical Study. J. Phys. Chem. Lett. 2016, 7, 226–233. 10.1021/acs.jpclett.5b02449. [DOI] [PubMed] [Google Scholar]
- Page A. J.; Elbourne A.; Stefanovic R.; Addicoat M. A.; Warr G. G.; Voïtchovsky K.; Atkin R. 3-Dimensional Atomic Scale Structure of the Ionic Liquid-Graphite Interface Elucidated by AM-AFM and Quantum Chemical Simulations. Nanoscale 2014, 6, 8100–8106. 10.1039/C4NR01219D. [DOI] [PubMed] [Google Scholar]
- Carstens T.; Gustus R.; Höfft O.; Borisenko N.; Endres F.; Li H.; Wood R. J.; Page A. J.; Atkin R. Combined STM, AFM, and DFT Study of the Highly Ordered Pyrolytic Graphite/1-Octyl-3-methyl-imidazolium Bis(trifluoromethylsulfonyl)imide Interface. J. Phys. Chem. C 2014, 118, 10833–10843. 10.1021/jp501260t. [DOI] [Google Scholar]
- Dou Q.; Sha M.; Fu H.; Wu G. Molecular Dynamics Simulation of the Interfacial Structure of [Cnmim][PF6] Adsorbed on a Graphite Surface: Effects of Temperature and Alkyl Chain Length. J. Phys.: Condens. Matter 2011, 23, 175001. 10.1088/0953-8984/23/17/175001. [DOI] [PubMed] [Google Scholar]
- Bordes E.; Douce L.; Quitevis E. L.; Pádua A. A.; Costa Gomes M. Ionic Liquids at the Surface of Graphite: Wettability and Structure. J. Chem. Phys. 2018, 148, 193840. 10.1063/1.5010604. [DOI] [PubMed] [Google Scholar]
- Herrera C.; Alcalde R.; Atilhan M.; Aparicio S. Theoretical Study on Amino Acid-Based Ionic Pairs and Their Interaction with Carbon Nanostructures. J. Phys. Chem. C 2014, 118, 9741–9757. 10.1021/jp500165t. [DOI] [Google Scholar]
- Gómez-González V.; García-Fuente A.; Vega A.; Carrete J.; Cabeza O.; Gallego L. J.; Varela L. M. Density Functional Study of Charge Transfer at the Graphene/Ionic Liquid Interface. J. Phys. Chem. C 2018, 122, 15070–15077. 10.1021/acs.jpcc.8b02795. [DOI] [Google Scholar]
- Shakourian-Fard M.; Jamshidi Z.; Bayat A.; Kamath G. Meta-Hybrid Density Functional Theory Study of Adsorption of Imidazolium- and Ammonium-Based Ionic Liquids on Graphene sheet. J. Phys. Chem. C 2015, 119, 7095–7108. 10.1021/jp512020q. [DOI] [Google Scholar]
- Ruzanov A.; Lembinen M.; Ers H.; García de la Vega J. M.; Lage-Estebanez I.; Lust E.; Ivaništšev V. B. Density Functional Theory Study of Ionic Liquid Adsorption on Circumcoronene Shaped Graphene. J. Phys. Chem. C 2018, 122, 2624–2631. 10.1021/acs.jpcc.7b12156. [DOI] [Google Scholar]
- Burt R.; Birkett G.; Salanne M.; Zhao X. S. Molecular Dynamics Simulations of the Influence of Drop Size and Surface Potential on the Contact Angle of Ionic-Liquid Droplets. J. Phys. Chem. C 2016, 120, 15244–15250. 10.1021/acs.jpcc.6b04696. [DOI] [Google Scholar]
- Dong D.; Vatamanu J. P.; Wei X.; Bedrov D. The 1-Ethyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide Ionic Liquid Nanodroplets on Solid Surfaces and in Electric Field: A Molecular Dynamics Simulation Study. J. Chem. Phys. 2018, 148, 193833. 10.1063/1.5016309. [DOI] [PubMed] [Google Scholar]
- Taherian F.; Leroy F.; Heim L.-O.; Bonaccurso E.; van der Vegt N. F. A. Mechanism for Asymmetric Nanoscale Electrowetting of an Ionic Liquid on Graphene. Langmuir 2016, 32, 140–150. 10.1021/acs.langmuir.5b04161. [DOI] [PubMed] [Google Scholar]
- Wang S.; Zhang Y.; Abidi N.; Cabrales L. Wettability and Surface Free Energy of Graphene Films. Langmuir 2009, 25, 11078–11081. 10.1021/la901402f. [DOI] [PubMed] [Google Scholar]
- Salemi S.; Akbarzadeh H.; Abdollahzadeh S. Nano-Confined Ionic Liquid [Emim][PF6] Between Graphite Sheets: A Molecular Dynamics Study. J. Mol. Liq. 2016, 215, 512–519. 10.1016/j.molliq.2016.01.035. [DOI] [Google Scholar]
- Alibalazadeh M.; Foroutan M. Specific Distributions of Anions and Cations of an Ionic Liquid Through Confinement Between Graphene Sheets. J. Mol. Model. 2015, 21, 168. 10.1007/s00894-015-2703-4. [DOI] [PubMed] [Google Scholar]
- Rajput N. N.; Monk J.; Hung F. R. Structure and Dynamics of an Ionic Liquid Confined Inside a Charged Slit Graphitic Nanopore. J. Phys. Chem. C 2012, 116, 14504–14513. 10.1021/jp3041617. [DOI] [Google Scholar]
- Singh R.; Monk J.; Hung F. R. Heterogeneity in the Dynamics of the Ionic Liquid [BMIM][PF6] Confined in a Slit Nanopore. J. Phys. Chem. C 2011, 115, 16544–16554. 10.1021/jp2046118. [DOI] [Google Scholar]
- Shen Y.; He X.; Hung F. R. Structural and Dynamical Properties of a Deep Eutectic Solvent Confined inside a Slit Pore. J. Phys. Chem. C 2015, 119, 24489–24500. 10.1021/acs.jpcc.5b08172. [DOI] [Google Scholar]
- Rajput N. N.; Monk J.; Singh R.; Hung F. R. On the Influence of Pore Size and Pore Loading on Structural and Dynamical Heterogeneities of an Ionic Liquid Confined in a Slit Nanopore. J. Phys. Chem. C 2012, 116, 5169–5181. 10.1021/jp212440f. [DOI] [Google Scholar]
- Yokota Y.; Miyamoto H.; Imanishi A.; Inagaki K.; Morikawa Y.; Fukui K.-I. Structural and Dynamic Properties of 1-Butyl-3-methylimidazolium Bis(trifluoromethanesulfonyl)imide/Mica and Graphite Interfaces Revealed by Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2018, 20, 6668–6676. 10.1039/C7CP07313E. [DOI] [PubMed] [Google Scholar]
- Rajput N. N.; Monk J.; Hung F. R. Ionic Liquids Confined in a Realistic Activated Carbon Model: A Molecular Simulation Study. J. Phys. Chem. C 2014, 118, 1540–1553. 10.1021/jp408617j. [DOI] [Google Scholar]
- He Y.; Qiao R.; Vatamanu J.; Borodin O.; Bedrov D.; Huang J.; Sumpter B. G. Importance of Ion Packing on the Dynamics of Ionic Liquids During Micropore Charging. J. Phys. Chem. Lett. 2016, 7, 36–42. 10.1021/acs.jpclett.5b02378. [DOI] [PubMed] [Google Scholar]
- Merlet C.; Salanne M.; Rotenberg B.; Madden P. A. Imidazolium Ionic Liquid Interfaces with Vapor and Graphite: Interfacial Tension and Capacitance from Coarse-Grained Molecular Simulations. J. Phys. Chem. C 2011, 115, 16613–16618. 10.1021/jp205461g. [DOI] [Google Scholar]
- Méndez-Morales T.; Burbano M.; Haefele M.; Rotenberg B.; Salanne M. Ion-Ion Correlations Across and Between Electrified Graphene Layers. J. Chem. Phys. 2018, 148, 193812. 10.1063/1.5012761. [DOI] [PubMed] [Google Scholar]
- Le Ma J.; Meng Q.; Fan J. Charge Driven Lateral Structural Evolution of Ions in Electric Double Layer Capacitors Strongly Correlates with Differential Capacitance. Phys. Chem. Chem. Phys. 2018, 20, 8054–8063. 10.1039/C7CP08075A. [DOI] [PubMed] [Google Scholar]
- Limmer D. T. Interfacial Ordering and Accompanying Divergent Capacitance at Ionic Liquid-Metal Interfaces. Phys. Rev. Lett. 2015, 115, 256102. 10.1103/PhysRevLett.115.256102. [DOI] [PubMed] [Google Scholar]
- Feng G.; Li S.; Presser V.; Cummings P. T. Molecular Insights into Carbon Supercapacitors Based on Room-Temperature Ionic Liquids. J. Phys. Chem. Lett. 2013, 4, 3367–3376. 10.1021/jz4014163. [DOI] [Google Scholar]
- Salanne M.; Rotenberg B.; Naoi K.; Kaneko K.; Taberna P.-L.; Grey C. P.; Dunn B.; Simon P. Efficient Storage Mechanisms for Building Better Supercapacitors. Nat. Energy 2016, 1, 16070. 10.1038/nenergy.2016.70. [DOI] [Google Scholar]
- Hu G.; Pandey G. P.; Liu Q.; Anaredy R. S.; Ma C.; Liu M.; Li J.; Shaw S. K.; Wu J. Self-Organization of Ions at the Interface Between Graphene and Ionic Liquid DEME-TFSI. ACS Appl. Mater. Interfaces 2017, 9, 35437–35443. 10.1021/acsami.7b10912. [DOI] [PubMed] [Google Scholar]
- Capozza R.; Benassi A.; Vanossi A.; Tosatti E. Electrical Charging Effects on the Sliding Friction of a Model Nano-Confined Ionic Liquid. J. Chem. Phys. 2015, 143, 144703. 10.1063/1.4933010. [DOI] [PubMed] [Google Scholar]
- Li H.; Wood R. J.; Rutland M. W.; Atkin R. An Ionic Liquid Lubricant Enables Superlubricity to be “Switched On” in situ Using an Electrical Potential. Chem. Commun. 2014, 50, 4368–4370. 10.1039/c4cc00979g. [DOI] [PubMed] [Google Scholar]
- Pivnic K.; Fajardo O. Y.; Bresme F.; Kornyshev A. A.; Urbakh M. Mechanisms of Electrotunable Friction in Friction Force Microscopy Experiments with Ionic Liquids. J. Phys. Chem. C 2018, 122, 5004–5012. 10.1021/acs.jpcc.8b00516. [DOI] [Google Scholar]
- Liu X.; Wang Y.; Li S.; Yan T. Effects of Anion on the Electric Double Layer of Imidazolium-Based Ionic Liquids on Graphite Electrode by Molecular Dynamics Simulation. Electrochim. Acta 2015, 184, 164–170. 10.1016/j.electacta.2015.10.064. [DOI] [Google Scholar]
- Jin W.; Liu X.; Han Y.; Li S.; Yan T. Effects of Repulsive Interaction on the Electric Double Layer of an Imidazolium-Based Ionic Liquid by Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2015, 17, 2628–2633. 10.1039/C4CP04853A. [DOI] [PubMed] [Google Scholar]
- Liu X.; Han Y.; Yan T. Temperature Effects on the Capacitance of an Imidazolium-Based Ionic Liquid on a Graphite Electrode: A Molecular Dynamics Simulation. ChemPhysChem 2014, 15, 2503–2509. 10.1002/cphc.201402220. [DOI] [PubMed] [Google Scholar]
- Si X.; Li S.; Wang Y.; Ye S.; Yan T. Effects of Specific Adsorption on the Differential Capacitance of Imidazolium-Based Ionic Liquid Electrolytes. ChemPhysChem 2012, 13, 1671–1676. 10.1002/cphc.201200013. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Han Y.; Wang Y.; Ye S.; Yan T. Comparing the Differential Capacitance of Two Ionic Liquid Electrolytes: Effects of Specific Adsorption. Electrochem. Commun. 2014, 38, 44–46. 10.1016/j.elecom.2013.10.027. [DOI] [Google Scholar]
- Vatamanu J.; Borodin O.; Smith G. D. Molecular Insights into the Potential and Temperature Dependences of the Differential Capacitance of a Room-Temperature Ionic Liquid at Graphite Electrodes. J. Am. Chem. Soc. 2010, 132, 14825–14833. 10.1021/ja104273r. [DOI] [PubMed] [Google Scholar]
- Vatamanu J.; Vatamanu M.; Bedrov D. Non-Faradaic Energy Storage by Room Temperature Ionic Liquids in Nanoporous Electrodes. ACS Nano 2015, 9, 5999–6017. 10.1021/acsnano.5b00945. [DOI] [PubMed] [Google Scholar]
- Vatamanu J.; Borodin O.; Smith G. D. Molecular Simulations of the Electric Double Layer Structure, Differential Capacitance, and Charging Kinetics for N-methyl-N-propylpyrrolidinium Bis(fluorosulfonyl)imide at Graphite Electrodes. J. Phys. Chem. B 2011, 115, 3073–3084. 10.1021/jp2001207. [DOI] [PubMed] [Google Scholar]
- Vatamanu J.; Cao L.; Borodin O.; Bedrov D.; Smith G. D. On the Influence of Surface Topography on the Electric Double Layer Structure and Differential Capacitance of Graphite/Ionic Liquid Interfaces. J. Phys. Chem. Lett. 2011, 2, 2267–2272. 10.1021/jz200879a. [DOI] [Google Scholar]
- Hu Z.; Vatamanu J.; Borodin O.; Bedrov D. A Molecular Dynamics Simulation Study of the Electric Double Layer and Capacitance of [BMIM][PF6] and [BMIM][BF4] Room Temperature Ionic Liquids Near Charged Surfaces. Phys. Chem. Chem. Phys. 2013, 15, 14234–14247. 10.1039/c3cp51218e. [DOI] [PubMed] [Google Scholar]
- Vatamanu J.; Xing L.; Li W.; Bedrov D. Influence of Temperature on the Capacitance of Ionic Liquid Electrolytes on Charged Surfaces. Phys. Chem. Chem. Phys. 2014, 16, 5174–5182. 10.1039/c3cp54705a. [DOI] [PubMed] [Google Scholar]
- Feng G.; Huang J.; Sumpter B. G.; Meunier V.; Qiao R. Structure and Dynamics of Electrical Double Layers in Organic Electrolytes. Phys. Chem. Chem. Phys. 2010, 12, 5468–5479. 10.1039/c000451k. [DOI] [PubMed] [Google Scholar]
- Vatamanu J.; Vatamanu M.; Borodin O.; Bedrov D. A Comparative Study of Room Temperature Ionic Liquids and Their Organic Solvent Mixtures Near Charged Electrodes. J. Phys.: Condens. Matter 2016, 28, 464002. 10.1088/0953-8984/28/46/464002. [DOI] [PubMed] [Google Scholar]
- Gómez-González V.; Docampo-Álvarez B.; Méndez-Morales T.; Cabeza O.; Ivaništšev V. B.; Fedorov M. V.; Gallego L. J.; Varela L. M. Molecular Dynamics Simulation of Structure and Interfacial Free Energy Barriers of Mixtures of Ionic Liquids and Divalent Salts Near a Graphene Wall. Phys. Chem. Chem. Phys. 2017, 19, 846–853. 10.1039/C6CP07002G. [DOI] [PubMed] [Google Scholar]
- Ivaništšev V.; Méndez-Morales T.; Lynden-Bell R. M.; Cabeza O.; Gallego L. J.; Varela L. M.; Fedorov M. V. Molecular Origin of High Free Energy Barriers for Alkali Metal Ion Transfer Through Ionic Liquid-Graphene Electrode Interfaces. Phys. Chem. Chem. Phys. 2016, 18, 1302–1310. 10.1039/C5CP05973A. [DOI] [PubMed] [Google Scholar]
- Vatamanu J.; Borodin O. Ramifications of Water-in-Salt Interfacial Structure at Charged Electrodes for Electrolyte Electrochemical Stability. J. Phys. Chem. Lett. 2017, 8, 4362–4367. 10.1021/acs.jpclett.7b01879. [DOI] [PubMed] [Google Scholar]
- Gómez-González V.; Docampo-Álvarez B.; Montes-Campos H.; Otero J. C.; Lago E. L.; Cabeza O.; Gallego L. J.; Varela L. M. Solvation of Al3+ Cations in Bulk and Confined Protic Ionic Liquids: A Computational Study. Phys. Chem. Chem. Phys. 2018, 20, 19071–19081. 10.1039/C8CP02933D. [DOI] [PubMed] [Google Scholar]
- Osti N. C.; Dyatkin B.; Thompson M. W.; Tiet F.; Zhang P.; Dai S.; Tyagi M.; Cummings P. T.; Gogotsi Y.; Wesolowski D. J.; Mamontov E. Influence of Humidity on Performance and Microscopic Dynamics of an Ionic Liquid in Supercapacitor. Phys. Rev. Mater. 2017, 1, 035402. 10.1103/PhysRevMaterials.1.035402. [DOI] [Google Scholar]
- Montes-Campos H.; Otero-Mato J. M.; Méndez-Morales T.; Cabeza O.; Gallego L. J.; Ciach A.; Varela L. M. Two-Dimensional Pattern Formation in Ionic Liquids Confined Between Graphene Walls. Phys. Chem. Chem. Phys. 2017, 19, 24505–24512. 10.1039/C7CP04649A. [DOI] [PubMed] [Google Scholar]
- Paek E.; Pak A. J.; Hwang G. S. Large Capacitance Enhancement Induced by Metal-Doping in Graphene-Based Supercapacitors: A First-Principles-Based Assessment. ACS Appl. Mater. Interfaces 2014, 6, 12168–12176. 10.1021/am501395j. [DOI] [PubMed] [Google Scholar]
- Kondrat S.; Wu P.; Qiao R.; Kornyshev A. A. Accelerating Charging Dynamics in Subnanometre Pores. Nat. Mater. 2014, 13, 387–393. 10.1038/nmat3916. [DOI] [PubMed] [Google Scholar]
- Xing L.; Vatamanu J.; Borodin O.; Bedrov D. On the Atomistic Nature of Capacitance Enhancement Generated by Ionic Liquid Electrolyte Confined in Subnanometer Pores. J. Phys. Chem. Lett. 2013, 4, 132–140. 10.1021/jz301782f. [DOI] [PubMed] [Google Scholar]
- Wu P.; Huang J.; Meunier V.; Sumpter B. G.; Qiao R. Complex Capacitance Scaling in Ionic Liquids-Filled Nanopores. ACS Nano 2011, 5, 9044–9051. 10.1021/nn203260w. [DOI] [PubMed] [Google Scholar]
- Guan Y.; Shao Q.; Chen W.; Zhang J.; Zhang X.; Deng Y. Flow-Induced Voltage Generation by Driving Imidazolium-Based Ionic Liquids Over a Graphene Nano-Channel. J. Mater. Chem. A 2018, 6, 11941–11950. 10.1039/C8TA02629G. [DOI] [Google Scholar]
- Futamura R.; Iiyama T.; Takasaki Y.; Gogotsi Y.; Biggs M. J.; Salanne M.; Ségalini J.; Simon P.; Kaneko K. Partial Breaking of the Coulombic Ordering of Ionic Liquids Confined in Carbon Nanopores. Nat. Mater. 2017, 16, 1225–1232. 10.1038/nmat4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher K.; Abele M.; Kondrat S.; Holm C. The Effect of Finite Pore Length on Ion Structure and Charging. J. Chem. Phys. 2017, 147, 104708. 10.1063/1.4986346. [DOI] [PubMed] [Google Scholar]
- Merlet C.; Rotenberg B.; Madden P. A.; Taberna P.-L.; Simon P.; Gogotsi Y.; Salanne M. On the Molecular Origin of Supercapacitance in Nanoporous Carbon Electrodes. Nat. Mater. 2012, 11, 306–310. 10.1038/nmat3260. [DOI] [PubMed] [Google Scholar]
- Merlet C.; Péan C.; Rotenberg B.; Madden P. A.; Daffos B.; Taberna P.-L.; Simon P.; Salanne M. Highly Confined Ions Store Charge More Efficiently in Supercapacitors. Nat. Commun. 2013, 4, 2701. 10.1038/ncomms3701. [DOI] [PubMed] [Google Scholar]
- Wu P.; Huang J.; Meunier V.; Sumpter B. G.; Qiao R. Voltage Dependent Charge Storage Modes and Capacity in Subnanometer Pores. J. Phys. Chem. Lett. 2012, 3, 1732–1737. 10.1021/jz300506j. [DOI] [PubMed] [Google Scholar]
- Black J. M.; Feng G.; Fulvio P. F.; Hillesheim P. C.; Dai S.; Gogotsi Y.; Cummings P. T.; Kalinin S. V.; Balke N. Strain-Based in situ Study of Anion and Cation Insertion into Porous Carbon Electrodes with Different Pore Sizes. Adv. Energy Mater. 2014, 4, 1300683. 10.1002/aenm.201300683. [DOI] [Google Scholar]
- Feng G.; Cummings P. T. Supercapacitor Capacitance Exhibits Oscillatory Behavior as a Function of Nanopore Size. J. Phys. Chem. Lett. 2011, 2, 2859–2864. 10.1021/jz201312e. [DOI] [Google Scholar]
- Lucio A. J.; Shaw S. K.; Zhang J.; Bond A. M. Double-Layer Capacitance at Ionic Liquid-Boron-Doped Diamond Electrode Interfaces Studied by Fourier Transformed Alternating Current Voltammetry. J. Phys. Chem. C 2018, 122, 11777–11788. 10.1021/acs.jpcc.8b00272. [DOI] [Google Scholar]
- Vatamanu J.; Hu Z.; Bedrov D.; Perez C.; Gogotsi Y. Increasing Energy Storage in Electrochemical Capacitors with Ionic Liquid Electrolytes and Nanostructured Carbon Electrodes. J. Phys. Chem. Lett. 2013, 4, 2829–2837. 10.1021/jz401472c. [DOI] [Google Scholar]
- Yokota Y.; Miyamoto H.; Imanishi A.; Takeya J.; Inagaki K.; Morikawa Y.; Fukui K.-I. Microscopic Properties of Ionic Liquid/Organic Semiconductor Interfaces Revealed by Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2018, 20, 13075–13083. 10.1039/C8CP01043A. [DOI] [PubMed] [Google Scholar]
- Park S.-W.; DeYoung A. D.; Dhumal N. R.; Shim Y.; Kim H. J.; Jung Y. Computer Simulation Study of Graphene Oxide Supercapacitors: Charge Screening Mechanism. J. Phys. Chem. Lett. 2016, 7, 1180. 10.1021/acs.jpclett.6b00202. [DOI] [PubMed] [Google Scholar]
- Kondrat S.; Kornyshev A. Charging Dynamics and Optimization of Nanoporous Supercapacitors. J. Phys. Chem. C 2013, 117, 12399–12406. 10.1021/jp400558y. [DOI] [Google Scholar]
- Pean C.; Daffos B.; Rotenberg B.; Levitz P.; Haefele M.; Taberna P.-L.; Simon P.; Salanne M. Confinement, Desolvation, and Electrosorption Effects on the Diffusion of Ions in Nanoporous Carbon Electrodes. J. Am. Chem. Soc. 2015, 137, 12627–12632. 10.1021/jacs.5b07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péan C.; Merlet C.; Rotenberg B.; Madden P. A.; Taberna P.-L.; Daffos B.; Salanne M.; Simon P. On the Dynamics of Charging in Nanoporous Carbon-Based Supercapacitors. ACS Nano 2014, 8, 1576–1583. 10.1021/nn4058243. [DOI] [PubMed] [Google Scholar]
- Schütter C.; Husch T.; Korth M.; Balducci A. Toward New Solvents for EDLCs: From Computational Screening to Electrochemical Validation. J. Phys. Chem. C 2015, 119, 13413–13424. 10.1021/acs.jpcc.5b02113. [DOI] [Google Scholar]
- Burt R.; Breitsprecher K.; Daffos B.; Taberna P.-L.; Simon P.; Birkett G.; Zhao X. S.; Holm C.; Salanne M. Capacitance of Nanoporous Carbon-Based Supercapacitors is a Trade-off Between Concentration and Separability of Ions. J. Phys. Chem. Lett. 2016, 7, 4015–4021. 10.1021/acs.jpclett.6b01787. [DOI] [PubMed] [Google Scholar]
- Ocko B.; Kraack H.; Pershan P. S.; Sloutskin E.; Tamam L.; Deutsch M. Crystalline Phases of Alkyl-Thiol Monolayers on Liquid Mercury. Phys. Rev. Lett. 2005, 94, 017802. 10.1103/PhysRevLett.94.017802. [DOI] [PubMed] [Google Scholar]
- Elfassy E.; Mastai Y.; Pontoni D.; Deutsch M. Liquid-Mercury-Supported Langmuir Films of Ionic Liquids: Isotherms, Structure, and Time Evolution. Langmuir 2016, 32, 3164–3173. 10.1021/acs.langmuir.6b00196. [DOI] [PubMed] [Google Scholar]
- Buchner F.; Forster-Tonigold K.; Uhl B.; Alwast D.; Wagner N.; Farkhondeh H.; Groß A.; Behm R. J. Toward the Microscopic Identification of Anions and Cations at the Ionic Liquid-Ag(111) Interface: A Combined Experimental and Theoretical Investigation. ACS Nano 2013, 7, 7773–7784. 10.1021/nn4026417. [DOI] [PubMed] [Google Scholar]
- Costa R.; Pereira C. M.; Silva F. Double Layer in Room Temperature Ionic Liquids: Influence of Temperature and Ionic Size on the Differential Capacitance and Electrocapillary Curves. Phys. Chem. Chem. Phys. 2010, 12, 11125–11132. 10.1039/c003920a. [DOI] [PubMed] [Google Scholar]
- Nishi N.; Hashimoto A.; Minami E.; Sakka T. Electrocapillarity and Zero-Frequency Differential Capacitance at the Interface Between Mercury and Ionic Liquids Measured Using the Pendant Drop Method. Phys. Chem. Chem. Phys. 2015, 17, 5219–5226. 10.1039/C4CP05818F. [DOI] [PubMed] [Google Scholar]
- Alam M. T.; Islam M. M.; Okajima T.; Ohsaka T. Measurements of Differential Capacitance at Mercury/Room-Temperature Ionic Liquids Interfaces. J. Phys. Chem. C 2007, 111, 18326–18333. 10.1021/jp075808l. [DOI] [Google Scholar]
- Kornyshev A. A. Double-Layer in Ionic Liquids: Paradigm Change?. J. Phys. Chem. B 2007, 111, 5545–5557. 10.1021/jp067857o. [DOI] [PubMed] [Google Scholar]
- Fedorov M. V.; Kornyshev A. A. Ionic Liquid Near a Charged Wall: Structure and Capacitance of Electrical Double Layer. J. Phys. Chem. B 2008, 112, 11868–11872. 10.1021/jp803440q. [DOI] [PubMed] [Google Scholar]
- Pilkington G. A.; Harris K.; Bergendal E.; Reddy A. B.; Palsson G. K.; Vorobiev A.; Antzutkin O. N.; Glavatskih S.; Rutland M. W. Electro-Responsivity of Ionic Liquid Boundary Layers in a Polar Solvent Revealed by Neutron Reflectance. J. Chem. Phys. 2018, 148, 193806. 10.1063/1.5001551. [DOI] [PubMed] [Google Scholar]
- Rotenberg B.; Salanne M. Structural Transitions at Ionic Liquid Interfaces. J. Phys. Chem. Lett. 2015, 6, 4978–4985. 10.1021/acs.jpclett.5b01889. [DOI] [PubMed] [Google Scholar]
- Rietzler F.; Piermaier M.; Deyko A.; Steinrück H.-P.; Maier F. Electrospray Ionization Deposition of Ultrathin Ionic Liquid Films: [C8C1Im]Cl and [C8C1Im][Tf2N] on Au(111). Langmuir 2014, 30, 1063–1071. 10.1021/la404429q. [DOI] [PubMed] [Google Scholar]
- Foulston R.; Gangopadhyay S.; Chiutu C.; Moriarty P.; Jones R. G. Mono- and Multi-Layer Adsorption of an Ionic Liquid on Au(110). Phys. Chem. Chem. Phys. 2012, 14, 6054–6066. 10.1039/c2cp23901a. [DOI] [PubMed] [Google Scholar]
- Zein El Abedin S.; Saad A.Y.; Farag H.K.; Borisenko N.; Liu Q.X.; Endres F. Electrodeposition of Selenium, Indium and Copper in an Air- and Water-Stable Ionic Liquid at Variable Temperatures. Electrochim. Acta 2007, 52, 2746–2754. 10.1016/j.electacta.2006.08.064. [DOI] [Google Scholar]
- Gnahm M.; Pajkossy T.; Kolb D. The Interface Between Au (111) and an Ionic Liquid. Electrochim. Acta 2010, 55, 6212–6217. 10.1016/j.electacta.2009.08.031. [DOI] [Google Scholar]
- Uhl B.; Huang H.; Alwast D.; Buchner F.; Behm R. J. Interaction of Ionic Liquids with Noble Metal Surfaces: Structure Formation and Stability of [OMIM][TFSA] and [EMIM][TFSA] on Au (111) and Ag (111). Phys. Chem. Chem. Phys. 2015, 17, 23816–23832. 10.1039/C5CP03787E. [DOI] [PubMed] [Google Scholar]
- Pajkossy T.; Müller C.; Jacob T. The Metal-Ionic Liquid Interface as Characterized by Impedance Spectroscopy and in situ Scanning Tunneling Microscopy. Phys. Chem. Chem. Phys. 2018, 20, 21241–21250. 10.1039/C8CP02074D. [DOI] [PubMed] [Google Scholar]
- Uhl B.; Cremer T.; Roos M.; Maier F.; Steinrück H.-P.; Behm R. J. At the Ionic Liquid|Metal Interface: Structure Formation and Temperature Dependent Behavior of an Ionic Liquid Adlayer on Au(111). Phys. Chem. Chem. Phys. 2013, 15, 17295–17302. 10.1039/c3cp52184b. [DOI] [PubMed] [Google Scholar]
- Waldmann T.; Huang H. H.; Hoster H. E.; Höfft O.; Endres F.; Behm R. J. Imaging an Ionic Liquid Adlayer by Scanning Tunneling Microscopy at the Solid-Vacuum Interface. ChemPhysChem 2011, 12, 2565–2567. 10.1002/cphc.201100413. [DOI] [PubMed] [Google Scholar]
- Su Y.; Yan J.; Li M.; Zhang M.; Mao B. Electric Double Layer of Au(100)/Imidazolium-Based Ionic Liquids Interface: Effect of Cation Size. J. Phys. Chem. C 2013, 117, 205–212. 10.1021/jp3079919. [DOI] [Google Scholar]
- Borisenko N.; Zein El Abedin S.; Endres F. An in situ STM and DTS Study of the Extremely Pure [EMIM]FAP/Au(111) Interface. ChemPhysChem 2012, 13, 1736–1742. 10.1002/cphc.201100873. [DOI] [PubMed] [Google Scholar]
- Biedron A. B.; Garfunkel E. L.; Castner E. W. Jr; Rangan S. Ionic Liquid Ultrathin Films at the Surface of Cu(100) and Au(111). J. Chem. Phys. 2017, 146, 054704. 10.1063/1.4975101. [DOI] [PubMed] [Google Scholar]
- Wang F.-X.; Pan G.-B.; Liu Y.-D.; Xiao Y. Pb Deposition onto Au(111) From Acidic Chloroaluminate Ionic Liquid. Chem. Phys. Lett. 2010, 488, 112–115. 10.1016/j.cplett.2010.02.019. [DOI] [Google Scholar]
- Zhong Y.-X.; Yan J.-W.; Li M.-G.; Zhang X.; He D.-W.; Mao B.-W. Resolving Fine Structures of the Electric Double Layer of Electrochemical Interfaces in Ionic Liquids with an AFM Tip Modification Strategy. J. Am. Chem. Soc. 2014, 136, 14682–14685. 10.1021/ja508222m. [DOI] [PubMed] [Google Scholar]
- Hayes R.; Borisenko N.; Tam M. K.; Howlett P. C.; Endres F.; Atkin R. Double Layer Structure of Ionic Liquids at the Au(111) Electrode Interface: An Atomic Force Microscopy Investigation. J. Phys. Chem. C 2011, 115, 6855–6863. 10.1021/jp200544b. [DOI] [Google Scholar]
- Li H.; Endres F.; Atkin R. Effect of Alkyl Chain Length and Anion Species on Interfacial Nanostructure of Ionic Liquids at Au(111)-Ionic Liquid Interface as a Function of Potential. Phys. Chem. Chem. Phys. 2013, 15, 14624–14633. 10.1039/c3cp52421c. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson N.; Wallinder D.; Glavatskih S.; Atkin R.; Aastrup T.; Rutland M. W. Weighing the Surface Charge of an Ionic Liquid. Nanoscale 2015, 7, 16039–16045. 10.1039/C5NR03965G. [DOI] [PubMed] [Google Scholar]
- Sweeney J.; Hausen F.; Hayes R.; Webber G. B.; Endres F.; Rutland M. W.; Bennewitz R.; Atkin R. Control of Nanoscale Friction on Gold in an Ionic Liquid by a Potential-Dependent Ionic Lubricant Layer. Phys. Rev. Lett. 2012, 109, 155502. 10.1103/PhysRevLett.109.155502. [DOI] [PubMed] [Google Scholar]
- Li H.; Rutland M. W.; Atkin R. Ionic Liquid Lubrication: Influence of Ion Structure, Surface Potential and Sliding Velocity. Phys. Chem. Chem. Phys. 2013, 15, 14616–14623. 10.1039/c3cp52638k. [DOI] [PubMed] [Google Scholar]
- Krämer G.; Hausen F.; Bennewitz R. Dynamic Shear Force Microscopy of Confined Liquids at a Gold Electrode. Faraday Discuss. 2017, 199, 299–309. 10.1039/C6FD00237D. [DOI] [PubMed] [Google Scholar]
- Kornyshev A. A.; Qiao R. Three-Dimensional Double Layers. J. Phys. Chem. C 2014, 118, 18285–18290. 10.1021/jp5047062. [DOI] [Google Scholar]
- Atkin R.; El Abedin S. Z.; Hayes R.; Gasparotto L. H. S.; Borisenko N.; Endres F. AFM and STM Studies on the Surface Interaction of [BMP]TFSA and [EMIm]TFSA Ionic Liquids with Au(111). J. Phys. Chem. C 2009, 113, 13266–13272. 10.1021/jp9026755. [DOI] [Google Scholar]
- Endres F.; Borisenko N.; El Abedin S. Z.; Hayes R.; Atkin R. The Interface Ionic Liquid(s)/Electrode(s): In situ STM and AFM Measurements. Faraday Discuss. 2012, 154, 221–233. 10.1039/C1FD00050K. [DOI] [PubMed] [Google Scholar]
- Carstens T.; Hayes R.; Abedin S. Z. E.; Corr B.; Webber G. B.; Borisenko N.; Atkin R.; Endres F. In situ STM, AFM and DTS Study of Interface 1-Hexyl-3-methylimidazolium Tris(pentafluoroethyl)-trifluorophosphate/Au (111). Electrochim. Acta 2012, 82, 48–59. 10.1016/j.electacta.2012.01.111. [DOI] [Google Scholar]
- Atkin R.; Borisenko N.; Drüschler M.; El Abedin S. Z.; Endres F.; Hayes R.; Huber B.; Roling B. An in situ STM/AFM and Impedance Spectroscopy Study of the Extremely Pure 1-Butyl-1-methylpyrrolidinium Tris(pentafluoroethyl)trifluorophosphate/Au(111) Interface: Potential Dependent Solvation Layers and the Herringbone Reconstruction. Phys. Chem. Chem. Phys. 2011, 13, 6849–6857. 10.1039/c0cp02846k. [DOI] [PubMed] [Google Scholar]
- Borisenko N.; Lahiri A.; Pulletikurthi G.; Cui T.; Carstens T.; Zahlbach J.; Atkin R.; Endres F. The Au(111)/IL Interfacial Nanostructure in the Presence of Precursors and Its Influence on the Electrodeposition Process. Faraday Discuss. 2018, 206, 459–473. 10.1039/C7FD00165G. [DOI] [PubMed] [Google Scholar]
- Hoffmann V.; Pulletikurthi G.; Carstens T.; Lahiri A.; Borodin A.; Schammer M.; Horstmann B.; Latz A.; Endres F. Influence of a Silver Salt on the Nanostructure of a Au(111)/Ionic Liquid Interface: An Atomic Force Microscopy Study and Theoretical Concepts. Phys. Chem. Chem. Phys. 2018, 20, 4760–4771. 10.1039/C7CP08243F. [DOI] [PubMed] [Google Scholar]
- McLean B.; Li H.; Stefanovic R.; Wood R. J.; Webber G. B.; Ueno K.; Watanabe M.; Warr G. G.; Page A.; Atkin R. Nanostructure of [Li(G4)]TFSI and [Li(G4)]NO3 Solvate Ionic Liquids at HOPG and Au(111) Electrode Interfaces as a Function of Potential. Phys. Chem. Chem. Phys. 2015, 17, 325–333. 10.1039/C4CP04522J. [DOI] [PubMed] [Google Scholar]
- Carstens T.; Lahiri A.; Borisenko N.; Endres F. [Py1,4]FSI-NaFSI-Based Ionic Liquid Electrolyte for Sodium Batteries: Na+ Solvation and Interfacial Nanostructure on Au(111). J. Phys. Chem. C 2016, 120, 14736–14741. 10.1021/acs.jpcc.6b04729. [DOI] [Google Scholar]
- Lahiri A.; Carstens T.; Atkin R.; Borisenko N.; Endres F. In situ Atomic Force Microscopic Studies of the Interfacial Multilayer Nanostructure of LiTFSI-[Py1,4]TFSI on Au(111): Influence of Li+ Ion Concentration on the Au(111)/IL Interface. J. Phys. Chem. C 2015, 119, 16734–16742. 10.1021/acs.jpcc.5b04562. [DOI] [Google Scholar]
- Hayes R.; Borisenko N.; Corr B.; Webber G. B.; Endres F.; Atkin R. Effect of Dissolved LiCl on the Ionic Liquid-Au(111) Electrical Double Layer Structure. Chem. Commun. 2012, 48, 10246–10248. 10.1039/c2cc35737b. [DOI] [PubMed] [Google Scholar]
- Cui T.; Lahiri A.; Carstens T.; Borisenko N.; Pulletikurthi G.; Kuhl C.; Endres F. Influence of Water on the Electrified Ionic Liquid/Solid Interface: A Direct Observation of the Transition From a Multilayered Structure to a Double-Layer Structure. J. Phys. Chem. C 2016, 120, 9341–9349. 10.1021/acs.jpcc.6b02549. [DOI] [Google Scholar]
- Zhong Y.; Yan J.; Li M.; Chen L.; Mao B. The Electric Double Layer in an Ionic Liquid Incorporated with Water Molecules: Atomic Force Microscopy Force Curve Study. ChemElectroChem 2016, 3, 2221–2226. 10.1002/celc.201600177. [DOI] [Google Scholar]
- Friedl J.; Markovits I. I.; Herpich M.; Feng G.; Kornyshev A. A.; Stimming U. Interface Between an Au(111) Surface and an Ionic Liquid: The Influence of Water on Double-Layer Capacitance. ChemElectroChem 2017, 4, 216–220. 10.1002/celc.201600557. [DOI] [Google Scholar]
- Motobayashi K.; Minami K.; Nishi N.; Sakka T.; Osawa M. Hysteresis of Potential-Dependent Changes in Ion Density and Structure of an Ionic Liquid on a Gold Electrode: In situ Observation by Surface-Enhanced Infrared Absorption Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3110–3114. 10.1021/jz401645c. [DOI] [Google Scholar]
- Motobayashi K.; Nishi N.; Inoue Y.; Minami K.; Sakka T.; Osawa M. Potential-Induced Restructuring Dynamics of Ionic Liquids on a Gold Electrode: Steric Effect of Constituent Ions Studied by Surface-Enhanced Infrared Absorption Spectroscopy. J. Electroanal. Chem. 2017, 800, 126–133. 10.1016/j.jelechem.2017.02.049. [DOI] [Google Scholar]
- Wen R.; Rahn B.; Magnussen O. M. In situ Video-STM Study of Adlayer Structure and Surface Dynamics at the Ionic Liquid/Au (111) Interface. J. Phys. Chem. C 2016, 120, 15765–15771. 10.1021/acs.jpcc.5b11590. [DOI] [PubMed] [Google Scholar]
- Nishi N.; Hirano Y.; Motokawa T.; Kakiuchi T. Ultraslow Relaxation of the Structure at Ionic Liquid Gold Electrode Interface to a Potential Step Probed by Electrochemical Surface Plasmon Resonance Measurements: Asymmetry of Relaxation Time to the Potential-Step Direction. Phys. Chem. Chem. Phys. 2013, 15, 11615–11619. 10.1039/c3cp51463c. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Cui T.; Li G.; Endres F. Interfacial Nanostructure and Asymmetric Electrowetting of Ionic Liquids. Langmuir 2017, 33, 9539–9547. 10.1021/acs.langmuir.7b00082. [DOI] [PubMed] [Google Scholar]
- Labuda A.; Grütter P. Atomic Force Microscopy in Viscous Ionic Liquids. Langmuir 2012, 28, 5319–5322. 10.1021/la300557u. [DOI] [PubMed] [Google Scholar]
- Schmidt E.; Shi S.; Ruden P. P.; Frisbie C. D. Characterization of the Electric Double Layer Formation Dynamics of a Metal/Ionic Liquid/Metal Structure. ACS Appl. Mater. Interfaces 2016, 8, 14879–14884. 10.1021/acsami.6b04065. [DOI] [PubMed] [Google Scholar]
- Delcheva I.; Beattie D. A.; Ralston J.; Krasowska M. Dynamic Wetting of Imidazolium-Based Ionic Liquids on Gold and Glass. Phys. Chem. Chem. Phys. 2018, 20, 2084–2093. 10.1039/C7CP06404G. [DOI] [PubMed] [Google Scholar]
- Jha K. C.; Liu H.; Bockstaller M. R.; Heinz H. Facet Recognition and Molecular Ordering of Ionic Liquids on Metal Surfaces. J. Phys. Chem. C 2013, 117, 25969–25981. 10.1021/jp4032404. [DOI] [Google Scholar]
- Ferreira E. S. C.; Pereira C. M.; Cordeiro M. N. D. S.; dos Santos D. J. V. A. Molecular Dynamics Study of the Gold/Ionic Liquids Interface. J. Phys. Chem. B 2015, 119, 9883–9892. 10.1021/acs.jpcb.5b04505. [DOI] [PubMed] [Google Scholar]
- Nikitina V. A.; Kislenko S. A.; Nazmutdinov R. R.; Bronshtein M. D.; Tsirlina G. A. Ferrocene/Ferrocenium Redox Couple at Au(111)/Ionic Liquid and Au(111)/Acetonitrile Interfaces: A Molecular-Level View at the Elementary Act. J. Phys. Chem. C 2014, 118, 6151–6164. 10.1021/jp4072108. [DOI] [Google Scholar]
- Ruzanov A.; Lembinen M.; Jakovits P.; Srirama S. N.; Voroshylova I. V.; Cordeiro M. N. D. S.; Pereira C. M.; Rossmeisl J.; Ivaništšev V. B. On the Thickness of the Double Layer in Ionic Liquids. Phys. Chem. Chem. Phys. 2018, 20, 10275–10285. 10.1039/C7CP07939G. [DOI] [PubMed] [Google Scholar]
- Sha M.; Dou Q.; Luo F.; Zhu G.; Wu G. Molecular Insights into the Electric Double Layers of Ionic Liquids on Au(100) Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 12556–12565. 10.1021/am502413m. [DOI] [PubMed] [Google Scholar]
- Matsumoto M.; Shimizu S.; Sotoike R.; Watanabe M.; Iwasa Y.; Itoh Y.; Aida T. Exceptionally High Electric Double Layer Capacitances of Oligomeric Ionic Liquids. J. Am. Chem. Soc. 2017, 139, 16072–16075. 10.1021/jacs.7b09156. [DOI] [PubMed] [Google Scholar]
- Xu B.; Siler C. G.; Madix R. J.; Friend C. M. Ag/Au Mixed Sites Promote Oxidative Coupling of Methanol on the Alloy Surface. Chem. - Eur. J. 2014, 20, 4646–4652. 10.1002/chem.201304837. [DOI] [PubMed] [Google Scholar]
- Lexow M.; Talwar T.; Heller B. S.; May B.; Bhuin R. G.; Maier F.; Steinrück H.-P. Time-Dependent Changes in the Growth of Ultrathin Ionic Liquid Films on Ag(111). Phys. Chem. Chem. Phys. 2018, 20, 12929–12938. 10.1039/C8CP01411F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T.; Stark M.; Deyko A.; Steinrück H.-P.; Maier F. Liquid/Solid Interface of Ultrathin Ionic Liquid Films:[C1C1Im][Tf2N] and [C8C1Im][Tf2N] on Au(111). Langmuir 2011, 27, 3662–3671. 10.1021/la105007c. [DOI] [PubMed] [Google Scholar]
- Cremer T.; Wibmer L.; Calderón S. K.; Deyko A.; Maier F.; Steinrück H.-P. Interfaces of Ionic Liquids and Transition Metal Surfaces—Adsorption, Growth, and Thermal Reactions of Ultrathin [C1C1Im][Tf2N] Films on Metallic and Oxidised Ni(111) Surfaces. Phys. Chem. Chem. Phys. 2012, 14, 5153–5163. 10.1039/c2cp40278e. [DOI] [PubMed] [Google Scholar]
- Rietzler F.; Nagengast J.; Steinrück H.-P.; Maier F. Interface of Ionic Liquids and Carbon: Ultrathin [C1C1Im][Tf2N] Films on Graphite and Graphene. J. Phys. Chem. C 2015, 119, 28068–28076. 10.1021/acs.jpcc.5b09649. [DOI] [Google Scholar]
- Lexow M.; Heller B. S.; Maier F.; Steinrück H. P. Anion Exchange at the Liquid/Solid Interface of Ultrathin Ionic Liquid Films on Ag(111). ChemPhysChem 2018, 19, 2978–2984. 10.1002/cphc.201800773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E. A.; Strader M. L.; Johns J. E.; Yang A.; Caplins B. W.; Shearer A. J.; Suich D. E.; Harris C. B. Femtosecond Electron Solvation at Ionic Liquid/Metal Electrode Interface. J. Am. Chem. Soc. 2013, 135, 10646–10653. 10.1021/ja3108593. [DOI] [PubMed] [Google Scholar]
- Yildirim H.; Haskins J. B.; Bauschlicher C. W.; Lawson J. W. Decomposition of Ionic Liquids at Lithium Interfaces. 1. Ab Initio Molecular Dynamics Simulations. J. Phys. Chem. C 2017, 121, 28214–28234. 10.1021/acs.jpcc.7b09657. [DOI] [Google Scholar]
- Buchner F.; Bozorgchenani M.; Uhl B.; Farkhondeh H.; Bansmann J.; Behm R. J. Reactive Interaction of (Sub-)Monolayers and Multilayers of the Ionic Liquid 1-Butyl-1-methylpyrrolidinium Bis(trifluoro-methylsulfonyl)imide with Coadsorbed Lithium on Cu(111). J. Phys. Chem. C 2015, 119, 16649–16659. 10.1021/acs.jpcc.5b03765. [DOI] [Google Scholar]
- Syres K. L.; Jones R. G. Adsorption, Desorption, and Reaction of 1-Octyl-3-methylimidazolium Tetrafluoroborate, [C8C1Im][BF4], Ionic Liquid Multilayers on Cu(111). Langmuir 2015, 31, 9799–9808. 10.1021/acs.langmuir.5b02932. [DOI] [PubMed] [Google Scholar]
- Gindri I. M.; Siddiqui D. A.; Frizzo C. P.; Martins M. A. P.; Rodrigues D. C. Ionic Liquid Coatings for Titanium Surfaces: Effect of IL Structure on Coating Profile. ACS Appl. Mater. Interfaces 2015, 7, 27421–27431. 10.1021/acsami.5b09309. [DOI] [PubMed] [Google Scholar]
- Lebedeva O.; Kudryavtsev I.; Kultin D.; Dzhungurova G.; Kalmykov K.; Kustov L. Self-Organized Hexagonal Nanostructures on Nickel and Steel Formed by Anodization in 1-Butyl-3-methylimidazolium Bis(triflate)imide Ionic Liquid. J. Phys. Chem. C 2014, 118, 21293–21298. 10.1021/jp507319r. [DOI] [Google Scholar]
- Dold C.; Amann T.; Kailer A. Influence of Electric Potentials on Friction of Sliding Contacts Lubricated by an Ionic Liquid. Phys. Chem. Chem. Phys. 2015, 17, 10339–10342. 10.1039/C4CP05965D. [DOI] [PubMed] [Google Scholar]
- Pensado A. S.; Pádua A. A. Solvation and Stabilization of Metallic Nanoparticles in Ionic Liquids. Angew. Chem., Int. Ed. 2011, 50, 8683–8687. 10.1002/anie.201103096. [DOI] [PubMed] [Google Scholar]
- Schernich S.; Wagner V.; Taccardi N.; Wasserscheid P.; Laurin M.; Libuda J. Interface Controls Spontaneous Crystallization in Thin Films of the Ionic Liquid [C2C1Im][OTf] on Atomically Clean Pd(111). Langmuir 2014, 30, 6846–6851. 10.1021/la500842c. [DOI] [PubMed] [Google Scholar]
- Pikma P.; Siinor L.; Selberg S.; Lust E. In situ STM Studies of Electrochemically Polished Cd (0001) Electrode in 1-Ethyl-3-methylimidazolium Tetrafluoroborate. ECS Trans. 2015, 66, 41–47. 10.1149/06627.0041ecst. [DOI] [Google Scholar]
- Liu Y.-C.; Chen Y.-C.; Hsieh Y.-T.; Sun I. W. Anomalous Voltammetric Behavior Observed for Electrodeposition of Indium in the 1-Butyl-1-methylpyrrolidinium Dicyanamide Ionic Liquid. A Result of the Ionic Liquid Cation Adsorption. J. Phys. Chem. C 2017, 121, 8907–8913. 10.1021/acs.jpcc.7b01375. [DOI] [Google Scholar]
- Drüschler M.; Huber B.; Passerini S.; Roling B. Hysteresis Effects in Potential-Dependent Double Layer Capacitance of Room Temperature Ionic Liquids at a Polycrystalline Platinum Interface. J. Phys. Chem. C 2010, 114, 3614–3617. 10.1021/jp911513k. [DOI] [Google Scholar]
- Jitvisate M.; Seddon J. R. T. Direct Measurement of the Differential Capacitance of Solvent-Free and Dilute Ionic Liquids. J. Phys. Chem. Lett. 2018, 9, 126–131. 10.1021/acs.jpclett.7b02946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.; Grozovski V.; Siinor L.; Siimenson C.; Ivaništšev V.; Lust K.; Kallip S.; Lust E. Influence of Electrode Potential and in situ STM Scanning Conditions on the Phase Boundary Structure of the Single Crystal Bi(111)|1-Butyl-4-methylpyridinium Tetrafluoroborate Interface. J. Electroanal. Chem. 2013, 709, 46–56. 10.1016/j.jelechem.2013.10.004. [DOI] [Google Scholar]
- Grosberg A. Y.; Nguyen T.; Shklovskii B. Colloquium: The Physics of Charge Inversion in Chemical and Biological Systems. Rev. Mod. Phys. 2002, 74, 329–345. 10.1103/RevModPhys.74.329. [DOI] [Google Scholar]
- Chen Y.-G.; Weeks J. D. Local Molecular Field Theory for Effective Attractions Between Like Charged Objects in Systems with Strong Coulomb Interactions. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 7560–7565. 10.1073/pnas.0600282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezger M.; Schramm S.; Schröder H.; Reichert H.; Deutsch M.; De Souza E. J.; Okasinski J. S.; Ocko B. M.; Honkimäki V.; Dosch H. Layering of [BMIM]-Based Ionic Liquids at a Charged Sapphire Interface. J. Chem. Phys. 2009, 131, 094701. 10.1063/1.3212613. [DOI] [PubMed] [Google Scholar]
- Köhler R.; Restolho J.; Krastev R.; Shimizu K.; Canongia Lopes J. N.; Saramago B. Liquid- or Solid-Like Behavior of [Omim][BF4] at a Solid Interface?. J. Phys. Chem. Lett. 2011, 2, 1551–1555. 10.1021/jz2005682. [DOI] [Google Scholar]
- Restolho J.; Mata J. L.; Shimizu K.; Canongia Lopes J. N.; Saramago B. Wetting Films of Two Ionic Liquids: [C8mim][BF4] and [C2OHmim][BF4]. J. Phys. Chem. C 2011, 115, 16116–16123. 10.1021/jp204911h. [DOI] [Google Scholar]
- Sobota M.; Nikiforidis I.; Hieringer W.; Paape N.; Happel M.; Steinrück H.-P.; Görling A.; Wasserscheid P.; Laurin M.; Libuda J. Toward Ionic-Liquid-Based Model Catalysis: Growth, Orientation, Conformation, and Interaction Mechanism of the [Tf2N] Anion in [BMIM][Tf2N] Thin Films on a Well-Ordered Alumina Surface. Langmuir 2010, 26, 7199–7207. 10.1021/la904319h. [DOI] [PubMed] [Google Scholar]
- Schernich S.; Kostyshyn D.; Wagner V.; Taccardi N.; Laurin M.; Wasserscheid P.; Libuda J. Interactions Between the Room-Temperature Ionic Liquid [C2C1Im][OTf] and Pd (111), Well-Ordered Al2O3, and Supported Pd Model Catalysts From IR Spectroscopy. J. Phys. Chem. C 2014, 118, 3188–3193. 10.1021/jp5006692. [DOI] [Google Scholar]
- Chen S.; Liu Y.; Fu H.; He Y.; Li C.; Huang W.; Jiang Z.; Wu G. Unravelling the Role of the Compressed Gas on Melting Point of Liquid Confined in Nanospace. J. Phys. Chem. Lett. 2012, 3, 1052–1055. 10.1021/jz300225n. [DOI] [PubMed] [Google Scholar]
- Singh M. P.; Singh R. K.; Chandra S. Studies on Imidazolium-Based Ionic Liquids Having a Large Anion Confined in a Nanoporous Silica Gel Matrix. J. Phys. Chem. B 2011, 115, 7505–7514. 10.1021/jp2003358. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhang Q.; Li X.; Liu S.; Ma Y.; Shi F.; Deng Y. Nanocomposites of Ionic Liquids Confined in Mesoporous Silica Gels: Preparation, Characterization and Performance. Phys. Chem. Chem. Phys. 2010, 12, 1971–1981. 10.1039/b920556j. [DOI] [PubMed] [Google Scholar]
- Tripathi A. K.; Verma Y. L.; Singh R. K. Thermal, Electrical and Structural Studies on Ionic Liquid Confined in Ordered Mesoporous MCM-41. J. Mater. Chem. A 2015, 3, 23809–23820. 10.1039/C5TA05090A. [DOI] [Google Scholar]
- Verma Y. L.; Singh R. K. Conformational States of Ionic Liquid 1-Ethyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide in Bulk and Confined Silica Nanopores Probed by Crystallization Kinetics Study. J. Phys. Chem. C 2015, 119, 24381–24392. 10.1021/acs.jpcc.5b06672. [DOI] [Google Scholar]
- Göbel R.; Hesemann P.; Weber J.; Möller E.; Friedrich A.; Beuermann S.; Taubert A. Surprisingly High, Bulk Liquid-like Mobility of Silica-Confined Ionic Liquids. Phys. Chem. Chem. Phys. 2009, 11, 3653–3662. 10.1039/b821833a. [DOI] [PubMed] [Google Scholar]
- Singh M. P.; Verma Y. L.; Gupta A. K.; Singh R. K.; Chandra S. Changes in Dynamical Behavior of Ionic Liquid in Silica Nano-Pores. Ionics 2014, 20, 507–516. 10.1007/s11581-013-1008-9. [DOI] [Google Scholar]
- Li C.; Guo X.; He Y.; Jiang Z.; Wang Y.; Chen S.; Fu H.; Zou Y.; Dai S.; Wu G.; Xu H. Compression of Ionic Liquid When Confined in Porous Silica Nanoparticles. RSC Adv. 2013, 3, 9618–9621. 10.1039/c3ra40245b. [DOI] [Google Scholar]
- Sieffert N.; Wipff G. Ordering of Imidazolium-Based Ionic Liquids at the α-Quartz(001) Surface: A Molecular Dynamics Study. J. Phys. Chem. C 2008, 112, 19590–19603. 10.1021/jp806882e. [DOI] [Google Scholar]
- Ballone P.; Del Pópolo M. G.; Bovio S.; Podestà A.; Milani P.; Manini N. Nano-Indentation of a Room-Temperature Ionic Liquid Film on Silica: A Computational Experiment. Phys. Chem. Chem. Phys. 2012, 14, 2475–2482. 10.1039/c2cp23459a. [DOI] [PubMed] [Google Scholar]
- Shah F. U.; Holmgren A.; Rutland M. W.; Glavatskih S.; Antzutkin O. N. Interfacial Behavior of Orthoborate Ionic Liquids at Inorganic Oxide Surfaces Probed by NMR, IR, and Raman Spectroscopy. J. Phys. Chem. C 2018, 122, 19687–19698. 10.1021/acs.jpcc.8b06049. [DOI] [Google Scholar]
- Gupta A. K.; Verma Y. L.; Singh R. K.; Chandra S. Studies on an Ionic Liquid Confined in Silica Nanopores: Change in Tg and Evidence of Organic-Inorganic Linkage at Pore Wall Surface. J. Phys. Chem. C 2014, 118, 1530–1539. 10.1021/jp408142a. [DOI] [Google Scholar]
- Pal T.; Beck C.; Lessnich D.; Vogel M. Effects of Silica Surfaces on the Structure and Dynamics of Room-Temperature Ionic Liquids: A Molecular Dynamics Simulation Study. J. Phys. Chem. C 2018, 122, 624–634. 10.1021/acs.jpcc.7b10567. [DOI] [Google Scholar]
- Kruk D.; Wojciechowski M.; Brym S.; Singh R. K. Dynamics of Ionic Liquids in Bulk and in Confinement by Means of 1H NMR Relaxometry – BMIMOcSO4 in an SiO2 Matrix as an Example. Phys. Chem. Chem. Phys. 2016, 18, 23184–23194. 10.1039/C6CP02377K. [DOI] [PubMed] [Google Scholar]
- Coasne B.; Viau L.; Vioux A. Loading-Controlled Stiffening in Nanoconfined Ionic Liquids. J. Phys. Chem. Lett. 2011, 2, 1150–1154. 10.1021/jz200411a. [DOI] [PubMed] [Google Scholar]
- Federici Canova F.; Mizukami M.; Imamura T.; Kurihara K.; Shluger A. L. Structural Stability and Polarisation of Ionic Liquid Films on Silica Surfaces. Phys. Chem. Chem. Phys. 2015, 17, 17661–17669. 10.1039/C5CP02299A. [DOI] [PubMed] [Google Scholar]
- Castillo M. R.; Fraile J. M.; Mayoral J. A. Structure and Dynamics of 1-Butyl-3-methylimidazolium Hexafluorophosphate Phases on Silica and Laponite Clay: From Liquid to Solid Behavior. Langmuir 2012, 28, 11364–11375. 10.1021/la300976p. [DOI] [PubMed] [Google Scholar]
- Ori G.; Villemot F.; Viau L.; Vioux A.; Coasne B. Ionic Liquid Confined in Silica Nanopores: Molecular Dynamics in the Isobaric-Isothermal Ensemble. Mol. Phys. 2014, 112, 1350–1361. 10.1080/00268976.2014.902138. [DOI] [Google Scholar]
- Martinelli A.; Nordstierna L. An Investigation of the Sol-Gel Process in Ionic Liquid-Silica Gels by Time Resolved Raman and 1H NMR Spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 13216–13223. 10.1039/c2cp41914a. [DOI] [PubMed] [Google Scholar]
- Shi W.; Luebke D. R. Enhanced Gas Absorption in the Ionic Liquid 1-n-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)amide ([Hmim][Tf2N]) Confined in Silica Slit Pores: A Molecular Simulation Study. Langmuir 2013, 29, 5563–5572. 10.1021/la400226g. [DOI] [PubMed] [Google Scholar]
- Garaga M. N.; Persson M.; Yaghini N.; Martinelli A. Local Coordination and Dynamics of a Protic Ammonium Based Ionic Liquid Immobilized in Nano-Porous Silica Micro-Particles Probed by Raman and NMR Spectroscopy. Soft Matter 2016, 12, 2583–2592. 10.1039/C5SM02736E. [DOI] [PubMed] [Google Scholar]
- Ueno K.; Kasuya M.; Watanabe M.; Mizukami M.; Kurihara K. Resonance Shear Measurement of Nanoconfined Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 4066–4071. 10.1039/b923571j. [DOI] [PubMed] [Google Scholar]
- Federici Canova F.; Matsubara H.; Mizukami M.; Kurihara K.; Shluger A. L. Shear Dynamics of Nanoconfined Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 8247–8256. 10.1039/C4CP00005F. [DOI] [PubMed] [Google Scholar]
- Kritikos G.; Vergadou N.; Economou I. G. Molecular Dynamics Simulation of Highly Confined Glassy Ionic Liquids. J. Phys. Chem. C 2016, 120, 1013–1024. 10.1021/acs.jpcc.5b09947. [DOI] [Google Scholar]
- Wu C.-M.; Lin S.-Y. Close Packing Existence of Short-Chain Ionic Liquid Confined in the Nanopore of Silica Ionogel. J. Phys. Chem. C 2015, 119, 12335–12344. 10.1021/acs.jpcc.5b01461. [DOI] [Google Scholar]
- Yan Z.; Meng D.; Wu X.; Zhang X.; Liu W.; He K. Two-Dimensional Ordering of Ionic Liquids Confined by Layered Silicate Lates via Molecular Dynamics Simulation. J. Phys. Chem. C 2015, 119, 19244–19252. 10.1021/acs.jpcc.5b05776. [DOI] [Google Scholar]
- Iacob C.; Sangoro J. R.; Kipnusu W. K.; Valiullin R.; Kärger J.; Kremer F. Enhanced Charge Transport in Nano-Confined Ionic Liquids. Soft Matter 2012, 8, 289–293. 10.1039/C1SM06581E. [DOI] [Google Scholar]
- Kruk D.; Wojciechowski M.; Verma Y. L.; Chaurasia S. K.; Singh R. K. Dynamical Properties of EMIM-SCN Confined in a SiO2 Matrix by Means of 1H NMR Relaxometry. Phys. Chem. Chem. Phys. 2017, 19, 32605–32616. 10.1039/C7CP06174A. [DOI] [PubMed] [Google Scholar]
- Cerclier C. V.; Zanotti J.-M.; Bideau J. L. Ionogel Based on Biopolymer–Silica Interpenetrated Networks: Dynamics of Confined Ionic Liquid with Lithium Salt. Phys. Chem. Chem. Phys. 2015, 17, 29707–29713. 10.1039/C5CP04889C. [DOI] [PubMed] [Google Scholar]
- Castejón H. J.; Wynn T. J.; Marcin Z. M. Wetting and Tribological Properties of Ionic Liquids. J. Phys. Chem. B 2014, 118, 3661–3668. 10.1021/jp411765f. [DOI] [PubMed] [Google Scholar]
- Garaga M. N.; Aguilera L.; Yaghini N.; Matic A.; Persson M.; Martinelli A. Achieving Enhanced Ionic Mobility in Nanoporous Silica by Controlled Surface Interactions. Phys. Chem. Chem. Phys. 2017, 19, 5727–5736. 10.1039/C6CP07351D. [DOI] [PubMed] [Google Scholar]
- Chu M.; Miller M.; Douglas T.; Dutta P. Ultraslow Dynamics at a Charged Silicon-Ionic Liquid Interface Revealed by X-ray Reflectivity. J. Phys. Chem. C 2017, 121, 3841–3845. 10.1021/acs.jpcc.6b10443. [DOI] [Google Scholar]
- Iacob C.; Sangoro J. R.; Papadopoulos P.; Schubert T.; Naumov S.; Valiullin R.; Kärger J.; Kremer F. Charge Transport and Diffusion of Ionic Liquids in Nanoporous Silica Membranes. Phys. Chem. Chem. Phys. 2010, 12, 13798–13803. 10.1039/c004546b. [DOI] [PubMed] [Google Scholar]
- Nishida J.; Yan C.; Fayer M. D. Orientational Dynamics of a Functionalized Alkyl Planar Monolayer Probed by Polarization-Selective Angle-Resolved Infrared Pump-Probe Spectroscopy. J. Am. Chem. Soc. 2016, 138, 14057–14065. 10.1021/jacs.6b08672. [DOI] [PubMed] [Google Scholar]
- Wang H. B.; Yao N.; Wang L.; Hu Y. L. Brønsted-Lewis Dual Acidic Ionic Liquid Immobilized on Mesoporous Silica Materials as an Efficient Cooperative Catalyst for Mannich Reactions. New J. Chem. 2017, 41, 10528–10531. 10.1039/C7NJ02541F. [DOI] [Google Scholar]
- Vangeli O. C.; Romanos G. E.; Beltsios K. G.; Fokas D.; Kouvelos E. P.; Stefanopoulos K. L.; Kanellopoulos N. K. Grafting of Imidazolium Based Ionic Liquid on the Pore Surface of Nanoporous Materials—Study of Physicochemical and Thermodynamic Properties. J. Phys. Chem. B 2010, 114, 6480–6491. 10.1021/jp912205y. [DOI] [PubMed] [Google Scholar]
- Garaga M. N.; Dracopoulos V.; Werner-Zwanziger U.; Zwanziger J. W.; Maréchal M.; Persson M.; Nordstierna L.; Martinelli A. A Long-Chain Protic Ionic Liquid Inside Silica Nanopores: Enhanced Proton Mobility due to Efficient Self-Assembly and Decoupled Proton Transport. Nanoscale 2018, 10, 12337–12348. 10.1039/C8NR02031K. [DOI] [PubMed] [Google Scholar]
- Arcifa A.; Rossi A.; Espinosa-Marzal R. M.; Spencer N. D. Influence of Environmental Humidity on the Wear and Friction of a Silica/Silicon Tribopair Lubricated with a Hydrophilic Ionic Liquid. ACS Appl. Mater. Interfaces 2016, 8, 2961–2973. 10.1021/acsami.5b09370. [DOI] [PubMed] [Google Scholar]
- Arcifa A.; Rossi A.; Ramakrishna S. N.; Espinosa-Marzal R.; Sheehan A.; Spencer N. D. Lubrication of Si-Based Tribopairs with a Hydrophobic Ionic Liquid: The Multiscale Influence of Water. J. Phys. Chem. C 2018, 122, 7331–7343. 10.1021/acs.jpcc.8b01671. [DOI] [Google Scholar]
- Arcifa A.; Rossi A.; Espinosa-Marzal R. M.; Spencer N. D. Environmental Influence on the Surface Chemistry of Ionic-Liquid-Mediated Lubrication in a Silica/Silicon Tribopair. J. Phys. Chem. C 2014, 118, 29389–29400. 10.1021/jp505998k. [DOI] [Google Scholar]
- Kamijo T.; Arafune H.; Morinaga T.; Honma S.; Sato T.; Hino M.; Mizukami M.; Kurihara K. Lubrication Properties of Ammonium-Based Ionic Liquids Confined Between Silica Surfaces Using Resonance Shear Measurements. Langmuir 2015, 31, 13265–13270. 10.1021/acs.langmuir.5b03354. [DOI] [PubMed] [Google Scholar]
- Sakai K.; Okada K.; Uka A.; Misono T.; Endo T.; Sasaki S.; Abe M.; Sakai H. Effects of Water on Solvation Layers of Imidazolium-Type Room Temperature Ionic Liquids on Silica and Mica. Langmuir 2015, 31, 6085–6091. 10.1021/acs.langmuir.5b01184. [DOI] [PubMed] [Google Scholar]
- Lertola A. C.; Wang B.; Li L. Understanding the Friction of Nanometer-Thick Fluorinated Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 11681–11685. 10.1021/acs.iecr.8b03044. [DOI] [Google Scholar]
- Arcifa A.; Rossi A.; Spencer N. D. Adsorption and Tribochemical Factors Affecting the Lubrication of Silicon-Based Materials by (Fluorinated) Ionic Liquids. J. Phys. Chem. C 2017, 121, 7259–7275. 10.1021/acs.jpcc.6b13028. [DOI] [Google Scholar]
- Horn R.; Evans D.; Ninham B. Double-Layer and Solvation Forces Measured in a Molten Salt and Its Mixtures with Water. J. Phys. Chem. 1988, 92, 3531–3537. 10.1021/j100323a042. [DOI] [Google Scholar]
- Wakeham D.; Hayes R.; Warr G. G.; Atkin R. Influence of Temperature and Molecular Structure on Ionic Liquid Solvation Layers. J. Phys. Chem. B 2009, 113, 5961–5966. 10.1021/jp900815q. [DOI] [PubMed] [Google Scholar]
- Segura J. J.; Elbourne A.; Wanless E. J.; Warr G. G.; Voïtchovsky K.; Atkin R. Adsorbed and Near Surface Structure of Ionic Liquids at a Solid Interface. Phys. Chem. Chem. Phys. 2013, 15, 3320–3328. 10.1039/c3cp44163f. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson N.; Atkin R.; Rutland M. W. Is the Boundary Layer of an Ionic Liquid Equally Lubricating at Higher Temperature?. Phys. Chem. Chem. Phys. 2016, 18, 9232–9239. 10.1039/C5CP05837F. [DOI] [PubMed] [Google Scholar]
- Sweeney J.; Webber G. B.; Rutland M. W.; Atkin R. Effect of Ion Structure on Nanoscale Friction in Protic Ionic Liquids. Phys. Chem. Chem. Phys. 2014, 16, 16651–16658. 10.1039/C4CP02320J. [DOI] [PubMed] [Google Scholar]
- Elbourne A.; Voïtchovsky K.; Warr G. G.; Atkin R. Ion Structure Controls Ionic Liquid Near-Surface and Interfacial Nanostructure. Chem. Sci. 2015, 6, 527–536. 10.1039/C4SC02727B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne A.; Cronshaw S.; Voïtchovsky K.; Warr G. G.; Atkin R. Near Surface Properties of Mixtures of Propylammonium Nitrate with n-Alkanols 1. Nanostructure. Phys. Chem. Chem. Phys. 2015, 17, 26621–26628. 10.1039/C5CP04786B. [DOI] [PubMed] [Google Scholar]
- McDonald S.; Elbourne A.; Warr G. G.; Atkin R. Metal Ion Adsorption at the Ionic Liquid-Mica Interface. Nanoscale 2016, 8, 906–914. 10.1039/C5NR05833C. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Li H.; Atkin R.; Priest C. Influence of Water on the Interfacial Nanostructure and Wetting of [Rmim][NTf2] Ionic Liquids at Mica Surfaces. Langmuir 2016, 32, 8818–8825. 10.1021/acs.langmuir.6b01790. [DOI] [PubMed] [Google Scholar]
- Black J. M.; Zhu M.; Zhang P.; Unocic R. R.; Guo D.; Okatan M. B.; Dai S.; Cummings P. T.; Kalinin S. V.; Feng G.; Balke N. Fundamental Aspects of Electric Double Layer Force-Distance Measurements at Liquid-Solid Interfaces Using Atomic Force Microscopy. Sci. Rep. 2016, 6, 32389. 10.1038/srep32389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitvisate M.; Seddon J. R. Near-Wall Molecular Ordering of Dilute Ionic Liquids. J. Phys. Chem. C 2017, 121, 18593–18597. 10.1021/acs.jpcc.7b04843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Fang C.; Qiao R. Effects of Water on Mica-Ionic Liquid Interfaces. J. Phys. Chem. C 2018, 122, 9035–9045. 10.1021/acs.jpcc.8b01405. [DOI] [Google Scholar]
- Payal R. S.; Balasubramanian S. Effect of Cation Symmetry on the Organization of Ionic Liquids near a Charged Mica Surface. J. Phys.: Condens. Matter 2014, 26, 284101. 10.1088/0953-8984/26/28/284101. [DOI] [PubMed] [Google Scholar]
- Smith A. M.; Lovelock K. R.; Perkin S. Monolayer and Bilayer Structures in Ionic Liquids and Their Mixtures Confined to Nano-Films. Faraday Discuss. 2014, 167, 279–292. 10.1039/c3fd00075c. [DOI] [PubMed] [Google Scholar]
- Jurado L. A.; Kim H.; Rossi A.; Arcifa A.; Schuh J. K.; Spencer N. D.; Leal C.; Ewoldt R. H.; Espinosa-Marzal R. M. Effect of the Environmental Humidity on the Bulk, Interfacial and Nanoconfined Properties of an Ionic Liquid. Phys. Chem. Chem. Phys. 2016, 18, 22719–22730. 10.1039/C6CP03777A. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Priest C. Impact of Nanoscale Surface Heterogeneity on Precursor Film Growth and Macroscopic Spreading of [Rmim][NTf2] Ionic Liquids on Mica. Langmuir 2013, 29, 11344–11353. 10.1021/la402668v. [DOI] [PubMed] [Google Scholar]
- Gong X.; Kozbial A.; Li L. What Causes Extended Layering of Ionic Liquids on the Mica Surface?. Chem. Sci. 2015, 6, 3478–3482. 10.1039/C5SC00832H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyko A.; Cremer T.; Rietzler F.; Perkin S.; Crowhurst L.; Welton T.; Steinrück H.-P.; Maier F. Interfacial Behavior of Thin Ionic Liquid Films on Mica. J. Phys. Chem. C 2013, 117, 5101–5111. 10.1021/jp3115397. [DOI] [Google Scholar]
- Hayes R.; Warr G. G.; Atkin R. At the Interface: Solvation and Designing Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 1709–1723. 10.1039/b920393a. [DOI] [PubMed] [Google Scholar]
- Smith A. M.; Parkes M. A.; Perkin S. Molecular Friction Mechanisms Across Nanofilms of a Bilayer-Forming Ionic Liquid. J. Phys. Chem. Lett. 2014, 5, 4032–4037. 10.1021/jz502188g. [DOI] [PubMed] [Google Scholar]
- Fajardo O. Y.; Bresme F.; Kornyshev A. A.; Urbakh M. Electrotunable Friction with Ionic Liquid Lubricants: How Important is the Molecular Structure of the Ions?. J. Phys. Chem. Lett. 2015, 6, 3998–4004. 10.1021/acs.jpclett.5b01802. [DOI] [PubMed] [Google Scholar]
- Lian C.; Liu K.; Liu H.; Wu J. Impurity Effects on Charging Mechanism and Energy Storage of Nanoporous Supercapacitors. J. Phys. Chem. C 2017, 121, 14066–14072. 10.1021/acs.jpcc.7b04869. [DOI] [Google Scholar]
- Freitas A. A. D.; Shimizu K.; Smith A. M.; Perkin S.; Canongia Lopes J. N. Structure and Dynamics of Mica-Confined Films of [C10C1pyrr][ntf2] Ionic Liquid. J. Chem. Phys. 2018, 148, 193808. 10.1063/1.5007809. [DOI] [PubMed] [Google Scholar]
- Smith A. M.; Lovelock K. R.; Gosvami N. N.; Welton T.; Perkin S. Quantized Friction Across Ionic Liquid Thin Films. Phys. Chem. Chem. Phys. 2013, 15, 15317–15320. 10.1039/c3cp52779d. [DOI] [PubMed] [Google Scholar]
- Smith A. M.; Lee A. A.; Perkin S. Switching the Structural Force in Ionic Liquid-Solvent Mixtures by Varying Composition. Phys. Rev. Lett. 2017, 118, 096002. 10.1103/PhysRevLett.118.096002. [DOI] [PubMed] [Google Scholar]
- Singh Payal R.; Balasubramanian S. Orientational Ordering of Ionic Liquids near a Charged Mica Surface. ChemPhysChem 2012, 13, 1764–1771. 10.1002/cphc.201100871. [DOI] [PubMed] [Google Scholar]
- Seddon J. R. T. Conservative and Dissipative Interactions of Ionic Liquids in Nanoconfinement. J. Phys. Chem. C 2014, 118, 22197–22201. 10.1021/jp508336e. [DOI] [Google Scholar]
- Bou-Malham I.; Bureau L. Nanoconfined Ionic Liquids: Effect of Surface Charges on Flow and Molecular Layering. Soft Matter 2010, 6, 4062–4065. 10.1039/c0sm00377h. [DOI] [Google Scholar]
- Hayes R.; El Abedin S. Z.; Atkin R. Pronounced Structure in Confined Aprotic Room-Temperature Ionic Liquids. J. Phys. Chem. B 2009, 113, 7049–7052. 10.1021/jp902837s. [DOI] [PubMed] [Google Scholar]
- Gorlov M.; Kloo L. Ionic Liquid Electrolytes for Dye-Sensitized Solar Cells. Dalton Trans 2008, 2655–2666. 10.1039/b716419j. [DOI] [PubMed] [Google Scholar]
- Agosta L.; Brandt E. G.; Lyubartsev A. P. Diffusion and Reaction Pathways of Water near Fully Hydrated TiO2 Surfaces from ab Initio Molecular Dynamics. J. Chem. Phys. 2017, 147, 024704. 10.1063/1.4991381. [DOI] [PubMed] [Google Scholar]
- Wagstaffe M.; Jackman M. J.; Syres K. L.; Generalov A.; Thomas A. G. Ionic Liquid Ordering at an Oxide Surface. ChemPhysChem 2016, 17, 3430–3434. 10.1002/cphc.201600774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson Z.; Walton A.; Thomas A. G.; Syres K. L. Water-Induced Reordering in Ultrathin Ionic Liquid Films. J. Phys.: Condens. Matter 2018, 30, 334003. 10.1088/1361-648X/aad24f. [DOI] [PubMed] [Google Scholar]
- Uhl B.; Hekmatfar M.; Buchner F.; Behm R. J. Interaction of the Ionic Liquid [BMP][TFSA] with Rutile TiO2 (110) and Coadsorbed Lithium. Phys. Chem. Chem. Phys. 2016, 18, 6618–6636. 10.1039/C5CP07433A. [DOI] [PubMed] [Google Scholar]
- Singh R.; Rajput N. N.; He X.; Monk J.; Hung F. R. Molecular Dynamics Simulations of the Ionic Liquid [EMIM][TFMSI] Confined Inside Rutile (110) Slit Nanopores. Phys. Chem. Chem. Phys. 2013, 15, 16090–16103. 10.1039/c3cp51266e. [DOI] [PubMed] [Google Scholar]
- Weber H.; Kirchner B. Ionic Liquid Induced Band Shift of Titanium Dioxide. ChemSusChem 2016, 9, 2505–2514. 10.1002/cssc.201600844. [DOI] [PubMed] [Google Scholar]
- Weber H.; Bredow T.; Kirchner B. Adsorption Behavior of the 1,3-Dimethylimidazolium Thiocyanate and Tetracyanoborate Ionic Liquids at Anatase (101) Surface. J. Phys. Chem. C 2015, 119, 15137–15149. 10.1021/acs.jpcc.5b02347. [DOI] [Google Scholar]
- Weber H.; Salanne M.; Kirchner B. Toward an Accurate Modeling of Ionic Liquid-TiO2 Interfaces. J. Phys. Chem. C 2015, 119, 25260–25267. 10.1021/acs.jpcc.5b08538. [DOI] [Google Scholar]
- Pinilla C.; Del Pópolo M.; Kohanoff J.; Lynden-Bell R. Polarization Relaxation in an Ionic Liquid Confined Between Electrified Walls. J. Phys. Chem. B 2007, 111, 4877–4884. 10.1021/jp067184+. [DOI] [PubMed] [Google Scholar]
- Babucci M.; Balci V.; Akçay A.; Uzun A. Interactions of [BMIM][BF4] with Metal Oxides and Their Consequences on Stability Limits. J. Phys. Chem. C 2016, 120, 20089–20102. 10.1021/acs.jpcc.6b03975. [DOI] [Google Scholar]
- Schernich S.; Laurin M.; Lykhach Y.; Steinruck H.-P.; Tsud N.; Skala T.; Prince K. C.; Taccardi N.; Matolin V.; Wasserscheid P.; Libuda J. Functionalization of Oxide Surfaces Through Reaction with 1,3-Dialkylimidazolium Ionic Liquids. J. Phys. Chem. Lett. 2013, 4, 30–35. 10.1021/jz301856a. [DOI] [PubMed] [Google Scholar]
- Yuan H.; Shimotani H.; Ye J.; Yoon S.; Aliah H.; Tsukazaki A.; Kawasaki M.; Iwasa Y. Electrostatic and Electrochemical Nature of Liquid-Gated Electric-Double-Layer Transistors Based on Oxide Semiconductors. J. Am. Chem. Soc. 2010, 132, 18402–18407. 10.1021/ja108912x. [DOI] [PubMed] [Google Scholar]
- Black J. M.; Come J.; Bi S.; Zhu M.; Zhao W.; Wong A. T.; Noh J. H.; Pudasaini P. R.; Zhang P.; Okatan M. B.; Dai S.; Kalinin S. V.; Rack P. D.; Ward T. Z.; Feng G.; Balke N. Role of Electrical Double Layer Structure in Ionic Liquid Gated Devices. ACS Appl. Mater. Interfaces 2017, 9, 40949–40958. 10.1021/acsami.7b11044. [DOI] [PubMed] [Google Scholar]
- Jeong J.; Aetukuri N.; Graf T.; Schladt T. D.; Samant M. G.; Parkin S. S. Suppression of Metal-Insulator Transition in VO2 by Electric Field-Induced Oxygen Vacancy Formation. Science 2013, 339, 1402–1405. 10.1126/science.1230512. [DOI] [PubMed] [Google Scholar]
- Nakano M.; Shibuya K.; Okuyama D.; Hatano T.; Ono S.; Kawasaki M.; Iwasa Y.; Tokura Y. Collective Bulk Carrier Delocalization Driven by Electrostatic Surface Charge Accumulation. Nature 2012, 487, 459–462. 10.1038/nature11296. [DOI] [PubMed] [Google Scholar]
- Li M.; Han W.; Jiang X.; Jeong J.; Samant M. G.; Parkin S. S. Suppression of Ionic Liquid Gate-Induced Metallization of SrTiO3(001) by Oxygen. Nano Lett. 2013, 13, 4675–4678. 10.1021/nl402088f. [DOI] [PubMed] [Google Scholar]
- Petach T. A.; Mehta A.; Marks R.; Johnson B.; Toney M. F.; Goldhaber-Gordon D. Voltage-Controlled Interfacial Layering in an Ionic Liquid on SrTiO3. ACS Nano 2016, 10, 4565–4569. 10.1021/acsnano.6b00645. [DOI] [PubMed] [Google Scholar]
- Xu T.; Waehler T.; Vecchietti J.; Bonivardi A.; Bauer T.; Schwegler J.; Schulz P. S.; Wasserscheid P.; Libuda J. Interaction of Ester-Functionalized Ionic Liquids with Atomically-Defined Cobalt Oxides Surfaces: Adsorption, Reaction and Thermal Stability. ChemPhysChem 2017, 18, 3443–3453. 10.1002/cphc.201700843. [DOI] [PubMed] [Google Scholar]
- Voeltzel N.; Giuliani A.; Fillot N.; Vergne P.; Joly L. Nanolubrication by Ionic Liquids: Molecular Dynamics Simulations Reveal an Anomalous Effective Rheology. Phys. Chem. Chem. Phys. 2015, 17, 23226–23235. 10.1039/C5CP03134F. [DOI] [PubMed] [Google Scholar]
- Cooper P. K.; Wear C. J.; Li H.; Atkin R. Ionic Liquid Lubrication of Stainless Steel: Friction is Inversely Correlated with Interfacial Liquid Nanostructure. ACS Sustainable Chem. Eng. 2017, 5, 11737–11743. 10.1021/acssuschemeng.7b03262. [DOI] [Google Scholar]
- Sandhu P.; Gindri I.; Siddiqui D.; Rodrigues D. Dicationic Imidazolium-Based Ionic Liquid Coatings on Zirconia Surfaces: Physico-Chemical and Biological Characterization. J. Funct. Biomater. 2017, 8, 50. 10.3390/jfb8040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori G.; Massobrio C.; Pradel A.; Ribes M.; Coasne B. Structure and Dynamics of Ionic Liquids Confined in Amorphous Porous Chalcogenides. Langmuir 2015, 31, 6742–6751. 10.1021/acs.langmuir.5b00982. [DOI] [PubMed] [Google Scholar]
- Perera M. M.; Lin M.-W.; Chuang H.-J.; Chamlagain B. P.; Wang C.; Tan X.; Cheng M. M.-C.; Tománek D.; Zhou Z. Improved Carrier Mobility in Few-Layer MoS2 Field-Effect Transistors with Ionic-Liquid Gating. ACS Nano 2013, 7, 4449–4458. 10.1021/nn401053g. [DOI] [PubMed] [Google Scholar]
- Chen D.; Ying W.; Guo Y.; Ying Y.; Peng X. Enhanced Gas Separation Through Nanoconfined Ionic Liquid in Laminated MoS2 Membrane. ACS Appl. Mater. Interfaces 2017, 9, 44251–44257. 10.1021/acsami.7b15762. [DOI] [PubMed] [Google Scholar]