Abstract
Background: With more than 300,000 new cases reported each year in the United States of America (USA), Lyme disease is a major public health concern. Borrelia burgdorferi sensu stricto (Bbss) is considered the primary agent of Lyme disease in North America. However, multiple genetically diverse Borrelia species encompassing the Borrelia burgdorferi sensu lato (Bbsl) complex and the Relapsing Fever Borrelia (RFB) group are capable of causing tickborne disease. We report preliminary results of a serological survey of previously undetected species of Bbsl and RFB in California and Mexico using a novel immunoblot technique. Methods: Serum samples were tested for seroreactivity to specific species of Bbsl and RFB using an immunoblot method based on recombinant Borrelia membrane proteins, as previously described. A sample was recorded as seropositive if it showed immunoglobulin M (IgM) and/or IgG reactivity with at least two proteins from a specific Borrelia species. Results: The patient cohort consisted of 90 patients residing in California or Mexico who met the clinical case definition of chronic Lyme disease. Immunoblot testing revealed that 42 patients were seropositive for Bbsl (Group 1), while 56 patients were seropositive for RFB (Group 2). Eight patients were seropositive for both Bbsl and RFB species. Group 1 included patients who were seropositive for Bbss (14), B. californiensis (eight), B. spielmanii (10), B. afzelii/B. garinii (10), and mixed infections that included B. mayonii (three). Group 2 included patients who were seropositive for B. hermsii (nine), B. miyamotoi (seven), B. turicatae (nine), and B. turcica (two). In the remaining Group 1 and Group 2 patients, the exact Borrelia species could not be identified using the immunoblot technique. Conclusions: Lyme disease is associated with a diverse group of Borrelia species in California and Mexico. Current testing for Lyme disease focuses on detection of Bbss, possibly resulting in missed diagnoses and failure to administer appropriate antibiotic therapy in a timely manner. The genetic diversity of Borrelia spirochetes must be considered in future Lyme disease test development.
Keywords: Lyme disease, Borrelia burgdorferi, Relapsing Fever Borrelia, Borrelia miyamotoi, tickborne disease, immunoblot
1. Introduction
Borrelia spirochetes are a significant cause of disease worldwide. Borrelia species fall within the family Spirochaetaceae and are characterized as Gram-negative, helical bacteria moving via periplasmic axial filaments [1,2,3]. Currently, there are at least 53 known Borrelia species categorized into three groups: approximately 22 species fall within the Lyme Disease group (B. burgdorferi sensu lato, Bbsl), and approximately 29 fall within the Relapsing Fever Borrelia (RFB) group. These two groups contain agents of Lyme disease (LD) and relapsing fever (RF), respectively [4,5,6]. The remaining species fall within a third genetically distinct group, and these species remain unclassified and primarily associated with reptiles [4,5,7].
The Bbsl group comprises genetically diverse bacteria that are distributed worldwide primarily throughout the Northern hemisphere and are vectored by ixodid (hard) ticks [6,8]. In the United States of America (USA), LD is currently the largest vector-borne illness and causes an array of symptoms including musculoskeletal, neuropsychiatric, and cardiac problems and, on rare occasions, even death [9,10]. At least 11 Bbsl genospecies were identified in North America including Bbss, B. americana, B. andersoni, B. bissettii, B. californiensis, B. carolinensis, B. garinii, B. kurtenbachii, B. laneii, B. mayonii, and B. spielmanii [6,11].
The RFB complex spirochetes are likewise genetically diverse but are primarily vectored by argasid (soft) ticks and the human body louse [12,13]. They are widely distributed throughout much of the world, and they are a significant cause of illness on five out of seven continents [12,13]. RFB are endemic to the Western USA, Southwest Canada, parts of Mexico, Central and South America, the Mediterranean, much of Asia, and throughout Africa [12]. While the ecology and epidemiology of RFB in Africa is well understood, distribution of RFB outside the African continent is less well known [13]. Multiple species of RFB are reported to cause disease in humans, and two species are associated with high fatality rates: B. duttonii, which is found in East Africa, and B. recurrentis, which is widely distributed through much of the world including Africa, South America, Europe, and Asia [12]. In North America, B. hermsii and B. turicatae are the species most commonly reported [12].
RFB infection should be considered a major public health concern. Individuals infected with RFB can develop flu-like symptoms such as recurrent fevers, arthralgias, myalgias, headaches, and nausea, as well as more severe symptoms, such as central nervous system involvement [14,15]. Patients infected with certain RFB species such as B. miyamotoi are said to exhibit more significant symptoms than patients with Bbsl infection [14]. Dissemination of various RFB spirochetes into the bloodstream is said to be between 100 and 1000 times faster than Bbsl species, resulting in extensive morbidity and mortality [10,16,17]. Like many spirochetal infections, prompt antibiotic treatment of RFB infections results in a better clinical outcome, although treatment may sometimes trigger a severe Jarisch–Herxheimer reaction [18].
The symptoms caused by vector-borne pathogens including Bbsl and RFB spp. are not specific, and patients infected by either Bbsl or RFB may also be infected with other vector-borne pathogens such as Babesia, Ehrlichia, Anaplasma, Rickettsia, or Bartonella, resulting in overlapping symptoms that cannot be differentiated in terms of the individual pathogen [19]. Diagnosis and reporting of LD cases is based on insensitive serological detection of Bbss infection, and it fails to take into account the genetic diversity of spirochetes capable of causing LD and LD-like illness [1,20,21,22]. Although research into the diagnosis and treatment of RFB infection is limited in scope, a recent pilot study suggested that seroreactivity against both Bbsl and RFB could be widespread in California, thus complicating the diagnosis of LD in that state [15].
To assess the impact of testing limitations and to determine levels of exposure to Bbsl and RFB species in California and Mexico, we employed a recently developed modified Western blot procedure, termed the line immunoblot, which uses recombinant antigens from common strains and species of the Bbsl and RFB groups for the serological diagnosis of LD and RF [1,2]. Testing was conducted over an eight-month period in 2019 on patients with suspected tickborne disease. Our findings confirm that the serotype makeup of spirochetal exposure in the USA and Mexico appears to be more complex than acknowledged previously.
2. Materials and Methods
2.1. Patients and Data Collection
Our patient cohort was recruited from a medical practice located in San Francisco, CA, USA, specializing in the diagnosis and treatment of tickborne diseases. The Western Institutional Review Board (WIRB), Puyallup, WA approved the anonymous retrospective data collection protocol and consent form (IRB Approval #1148461). Patients of either sex qualified for the study provided they were at least 18 years of age, had a medical history of musculoskeletal, neuropsychiatric, and/or cardiac symptoms consistent with LD, and gave written informed consent for data collection. Subjects were included in the study if they met the case definition of untreated or previously treated chronic LD with symptoms lasting more than six months, as described in detail elsewhere [23,24]. Patients were not required to have had a documented tickbite or erythema migrans rash for participation in the study because serological testing was used to detect exposure rather than active infection. De-identified patient samples were coded according to the patient’s place of residence. Blood was drawn and serum was separated at independent laboratories including BioReference®, LabCorp®, and AnyLabTestNow®, and serum samples were transported to the reference laboratory for immunoblot testing (see below).
A total of 175 human sera expected to be negative for Bbsl and RFB were obtained from the Centers for Disease Control and Prevention (CDC), College of American Pathologists, New York State Department of Health, New York Biologics (Southampton, NY, USA) and IGeneX Reference Laboratory (Milpitas, CA, USA). The IGeneX samples were leftover sera received for routine testing for tick-borne diseases that would otherwise be discarded. Testing of control sera was performed by laboratory personnel in a blinded fashion in the same manner as testing of clinical samples from Bbsl and RFB patients. Control sera were used to evaluate immunoblot specificity/sensitivity and were not intended as population controls for the sample cohort.
2.2. Antigen Preparation
Recombinant target proteins were obtained by cloning hybrid gene constructs or portions of genes into pET vectors, expressing the gene products in Escherichia coli (GenScript, Piscataway, NJ, USA), then isolating the proteins to >90% purity, as previously described [1,2]. Bbsl recombinant proteins were derived from several US and European species of Bbsl including Bbss strains B-31 and 297 for the detection of the following targets: P18, P23 (OspC), P28, P30, P31 (OspA), P34 (OspB) P39 (BmpA), P41, P45, P58, P66, P93, the variable surface antigen of Bbss (VlsE), and C6 (a hybrid protein containing the immunodominant region of VlsE from different Bbsl species), as previously described [1]. The targeted Bbsl species were Bbss (B-31 and 297), B. californiensis, B. spielmanii, B. afzelii/B. garinii, B. mayonii, and B. valaisiana. Recombinant proteins from different RFB species were derived for the detection of four target antigens, BipA, GlpQ, fHbp, and FlaB, as previously described [2]. The targeted RFB species were B. hermsii, B. miyamotoi, B. turicatae, and B. turcica.
2.3. Preparation of Antigen Strips
Antigen strips for Bbsl and RFB immunoblots were prepared as previously described [1,2]. In brief, purified proteins and control proteins were diluted (7–19 ng protein/line) and sprayed in straight lines on nitrocellulose sheets (Amersham Protran, GE Healthcare Life Science) using a BioDot liquid dispenser (BioDot, Irvine, CA, USA). Protein L (Sigma, St. Louis, MO, USA) and mixed human immunoglobulin M (IgM) and IgG (Sigma, St. Louis, MO, USA) were used as control proteins for detecting the addition of human serum and for detecting the addition of alkaline phosphatase conjugated anti-human antibodies. The sheets were then blocked with 5% non-fat dry milk and sliced into 3-mm-wide strips.
2.4. Detection of Antibody Reactivity
Serological immunoblot testing was performed at IGeneX Reference Laboratory, a high-complexity testing facility with Clinical Laboratory Improvement Amendments (CLIA) certification.
The reactivity between Borrelia-specific antibodies from test sera and Borrelia antigens on immunoblots was detected as previously described [1,2]. In brief, strips were labeled, soaked in diluent (100 mM Tris, 0.9% NaCl, 0.1% Tween-20, and 1% non-fat dry milk) for 5 min in a trough; then, a 10-μL aliquot of the test or control serum was added to the strip. Strips with sera were incubated at room temperature for one hour, washed three times with wash buffer (KPL, Gaithersburg, MD, USA) at room temperature, and the final wash solution was then aspirated. To detect IgG and IgM reactivity, strips were incubated with alkaline phosphatase-conjugated goat anti-human IgG at 1:10,000 dilution or IgM at 1:6000 dilution, respectively (KPL, Gaithersburg, MD, USA), for one hour, and then washed three times. To visualize bands of antibody/antigen reactivity, the strips were reacted with a chromogenic substrate, 5-bromo-4-chloro-3-indolylphosphatenitro-blue tetrazolium (BCIP/NBT, KPL, Gaithersburg, MD, USA), and the reaction was terminated by washing with distilled water after the calibration control produced a visible band at 39 kDa. Bands demonstrating an intensity lower than that of the calibration control were reported as negative. Human sera from patients with confirmed Borrelia infection were used as positive controls and sera from uninfected persons were used as negative controls. All immunoblot testing of patient samples was performed with simultaneous testing of positive and negative control serum samples.
2.5. Scoring of Immunoblots
For Bbsl immunoblots, the following bands (in kDa) were scored for IgG reactivity: 18, 23 (OspC), 28, 30, 31 (OspA), 34 (OspB), 39 (BmpA), 41 (FlaB), 45, 58, 66, and 93. The following bands (in kDa) were scored for IgM reactivity: 23 (OspC), 31 (Osp A), 34 (Osp B), 39 (BmpA), 41 (FlaB), and 93. Interpretation of immunoblots was determined by two different criteria: the CDC criteria, and the in-house criteria, as previously described [1]. Based on the CDC criteria, IgM reactivity was interpreted as positive if two of the three antigen bands of 23, 39, and 41 kDa were positive, and IgG reactivity was interpreted as positive if five of the 10 antigen bands of 18, 23, 28, 30, 39, 41, 45, 58, 66, and 93 were positive. Based on the in-house criteria, IgM reactivity was considered positive if two out of the four bands of 23, 31, 39, and 41 kDa were present, and IgG reactivity was considered positive if two out of the six bands of 23, 31, 34, 39, 41, and 93 kDa were present.
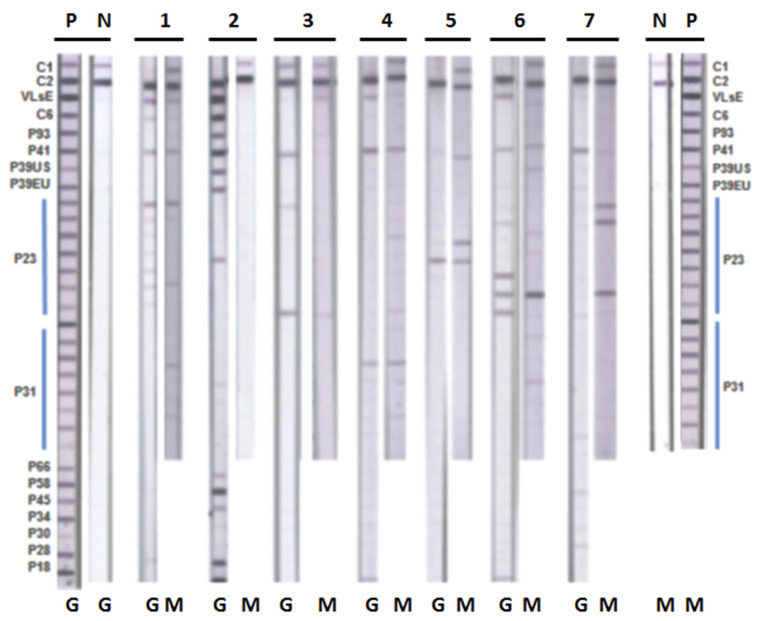
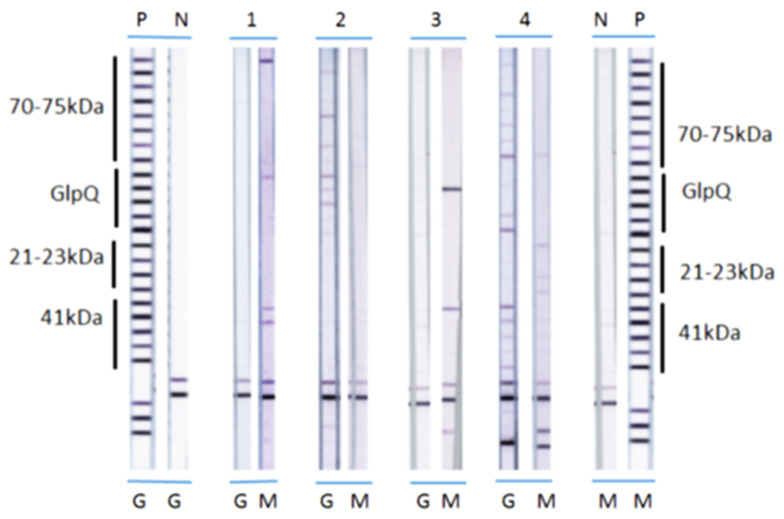
For RFB immunoblots, detection of either IgG or IgM antibodies against FlaB, as well as any two out of the three antigens BipA, GlpQ, and fHbp, gave the best specificity for RFB species. Detection of either IgG or IgM antibodies to one or more proteins within each antigen type was regarded as a positive reaction for that antigen type. Applying the same criteria for reactivity of either IgM or IgG antibodies to the four RFB antigens led to optimum sensitivity of detection, as previously described [2]. Immunoblot reactivity for Bbsl and RFB in representative patient serum samples is shown in Figure A1 and Figure A2 (Appendix A).
3. Results
Our cohort consisted of 90 patients who met the clinical case definition of chronic Lyme disease (CLD), as recently described [24]. Seventy-seven patients resided in the state of California and 13 resided in Mexico. Immunoblot testing revealed that all 90 subjects with suspected LD in our cohort were seropositive for Borrelia; a total of 42 patients (47%) were seropositive for Bbsl (Group 1), and a total of 56 patients (62%) were seropositive for RFB (Group 2). Thirty-four patients (38%) were seropositive for Bbsl alone, 48 patients (53%) were seropositive for RFB alone, and eight patients (9%) were positive for both Bbsl and RFB. The test results of these subjects in terms of place of residence, species of Borrelia seroreactivity, and IgM/IgG reactivity are summarized in Table A1 (Appendix A).
Group 1 consisted of patients seroreactive for Bbss (14), B. californiensis (eight), B. spielmanii (10), B. afzelii/B. garinii (10), and mixed infections that included B. mayonii (three). Group 2 included patients who were seroreactive for B. hermsii (nine), B. miyamotoi (seven), B. turicatae (nine), and B. turcica (two). Results for Group 1 and Group 2 seroreactivity are summarized in Table A2 (Appendix A). Sera from four patients in Group 1 were seropositive for two or more species of Bbsl, while sera from two patients in Group 2 were seropositive for two species of RFB. Seroreactivity to the exact Borrelia species in the remaining Group 1 and Group 2 patients could not be defined using the immunoblot technique.
As shown in Table A1 (Appendix A), 14/42 patients (33%) in the Bbsl group had antibodies to Bbss based on reactivity with Bbss-specific antigens derived from the B-31 and/or 297 strains. Four had antibodies to Bbss only, while the remaining 10 patients reacted with Bbss and other Bbsl species. In five samples (8, 12, 51, 86, and 88), the signal intensity with multiple species including Bbss was strong. In the remaining five samples (22, 31, 52, 63, and 64), the signal with other species was much stronger than Bbss.
The results obtained with the 175 control sera that were expected to be negative for Bbsl and RFB yielded a false positive rate of 2.3% (4/175 samples) for the Bbsl immunoblot and 2.9% (5/175 samples) for the RFB immunoblot (Table A3, Appendix A). False positive tests for Bbsl were seen with a healthy endemic serum (one control), viral infection (one control), and syphilis (two controls). False positive tests for RFB were seen with an allergy patient serum (one control), multiple sclerosis (one control), viral infection (one control), and syphilis (two controls).
4. Discussion
Using the new immunoblot test, our pilot study of patients residing in California and Mexico who met the CLD case definition revealed that all had exposure to either Bb or RFB species. Positive immunoblots were further characterized at the species level for the following Bbsl: Bbss, B. californiensis, B. spielmanii, B. afzelii/B. garinii, and B. mayonii; they were also characterized at the species level for the following RFB: B. hermsii, B. miyamotoi, B. turicatae, and B. turcica. Most sera were reactive to either Bb spp. alone (38%) or to RFB spp. alone (53%), with few seropositive to both Bb spp. and RFB spp. (9%). The lack of widespread dual reactivity suggests that cross-reactivity in our immunoblots between RFB and Bb spp. is unlikely. Immunoblot testing of control sera demonstrated excellent specificities of 97.7% for the Bbsl assay and 97.1% for the RFB assay (Table A3, Appendix A). In addition, our data indicate that, in California and Mexico, exposure to RFB spp. appears to be more common than exposure to Bb spp., and this distribution is consistent with published distribution maps [13].
Spirochetes falling into the Bbsl complex are distributed worldwide, with most cases of LD reported in the USA, Europe, and Asia [8,25]. The CDC states that approximately 30,000 cases of LD are reported in the USA each year using surveillance criteria featuring two-tier Bbss testing; however, when tracked using other methods, it is estimated that more than 300,000 people develop LD in the USA annually [26,27]. The fact that CDC surveillance criteria featuring two-tier Bbss testing captures less than one out of every ten cases shows that LD is underreported, and the results of our study suggest that some people with suspected LD who fail to meet surveillance criteria may be infected either by Bbsl or by RFB that are not cross-reactive with Bbss on two-tier testing.
The immunoblot testing used in our study enabled differentiation of Bbsl into six categories: Bbss, B. californiensis, B. spielmanii, B. mayonii, the European species B. afzelii/B. garinii, and undifferentiated Bb spp. Based on surveillance reporting in the USA, the distribution pattern of Bbss is characterized by human cases reported in all 50 states with the majority reported in the Northeast, upper Midwest, and northern California [26,27]. However, other Bbsl species are not detected by commercial testing in the USA. Until recently, Bbss, B. garinii, and B. afzelii were considered to be the only etiologic agents of LD. Currently, nine species are said to have pathogenic potential: B. afzelii, B. bavariensis, B. bissettii, Bbss, B. garinii, B. kurtenbachii, B. lusitaniae, B. spielmanii, and B. valaisiana [28]. Nine other species including B. californiensis were not isolated from humans [28]. B. afzelii, B. garinii, and B. spielmanii are considered to be Borrelia species primarily found in Eurasia, while B. californiensis is considered to be a North American species [28]. Our understanding of the pathogenicity of Borrelia species is evolving, and some species that were not isolated from humans and are not considered to be pathogenic may be capable of causing illness.
The immunoblot testing used in our study enabled differentiation of RFB into five categories: B. hermsii, B. miyamotoi, B. turicatae, B. turcica and undifferentiated RFB spp. RFB are found worldwide and are a significant cause of morbidity and mortality, particularly in impoverished African countries [13]. RF caused by B. crocidurae was recently reported to be the most common tickborne infection affecting the human population in Senegal, and, in a cohort of 206 febrile patients, 13% were determined to have detectable B. crocidurae DNA in blood samples [29]. In northwest Morocco, B. hispanica infection was found to be responsible for over 20% of unexplained fever cases [30]. Although the ecology and epidemiology of RFB in Africa is well described [13], advances in molecular testing led to widespread identification of Borrelia spp. in human specimens, thus challenging prevailing thought regarding geographic distribution and prevalence of RFB infection [15]. For example, a recent study reported that approximately 23% of a cohort of 301 patients in Russia with confirmed Borrelia infection demonstrated positive molecular testing for B. miyamotoi [31]. RFB is also a growing concern in the Western USA, Central America, and South America [13,32].
RF is not reportable nationally in the USA, and there is no standard case definition [33]. In 2011, RF was reportable in 12 Western states, yielding 504 cases, with 70% of the cases reported in three states: California (33%), Washington (25%), and Colorado (11%) [17,33]. In the USA, most human cases of RF are said to occur in the Western states, and the species causing disease include B. miyamotoi, B. hermsii, B. lonestari, B. parkeri, B. turicatae, and B. mazzotii [4,5,17,33,34,35,36,37,38]. Most cases in the USA are caused by B. hermsii, transmitted by Ornithodoros hermsi ticks that typically live in rodent nests in coniferous forests at elevations between 1500 and 8000 feet [17,33]. These soft ticks are nocturnal rapid feeders and often go unnoticed when they bite humans during sleep [16,17]. In California, B. miyamotoi, B. hermsii, and B. parkeri human infections were reported [32], and B. coriaceae was detected in ticks, although human infection was not confirmed [32,39]. B. miyamotoi was originally detected in Japan in 1995, but it is thought to have recently arrived in the Western hemisphere [38,40]; in the USA, it was detected in the North American ixodid ticks I. scapularis and I. pacificus [35,38,41,42].
The genetic diversity of Borrelia spirochetes, as well as the symptoms of infection that are as diverse as the organisms causing them, makes it challenging to diagnose Borrelia-associated disease [15,43]. It is important to recognize that classification is a human concept and the organisms encompassing the genus Borrelia fall within a continuous spectrum of organisms rather than into well-defined genetically distinct groups that are easily categorized. Some species that genetically fall into the RFB group, such as B. miyamotoi and B. lonestari, are vectored by ixodid ticks rather than argasid ticks, and there is, therefore, a proposal to classify them into a new group—hard tick-borne relapsing fever (HTBRF) [4,13,38,42,44]. B. miyamotoi demonstrates genetic differences in accord with different geographic regions and different vectors, leading to the proposal to establish grouping into a B. miyamotoi sensu lato complex [14,35,45,46,47,48,49,50].
In addition, two recently described Borrelia species of unknown pathogenicity, B. turcica and B. tachyglossi, are vectored by the same ixodid ticks as Bbsl, yet genetically they more closely resemble spirochetes of the RFB complex. The genomes of these two RFB species also contain genes orthologous to Bbsl-specific genes, such that these organisms demonstrate a phenotypic link between the Bbsl and RFB Borrelia groups [51]. To further complicate classification, moving Bbsl into its own genus, Borreliella, was recently proposed; however, because the Bbsl and RFB groups share infectious and biological similarities—both are bacterial zoonoses causing illness in humans and animals, are vectored by hematophagous arthropods, and are almost identical microscopically—a split of the well-established Borrelia genus might create confusion among medical, scientific, and lay populations and remains controversial [52,53].
Another controversial issue is the transmission time that is required for ticks to infect humans with Borrelia species. Although older animal models suggested that transmission of Bbss required 24–48 h of tick feeding, more recent reports of animal and human Borrelia infection challenged this dogma [54,55,56,57,58]. Furthermore, transmission of RFB may occur significantly faster than Bbsl, requiring seconds to a few hours in animal models and humans [59,60,61]. Thus, RFB may pose a greater risk of infection to human populations due to more rapid transmission from ticks.
A case definition for chronic LD was recently proposed that includes all members of the Borrelia spirochete complex that would encompass both the LD and the RFB groups [24]. As the genus Borrelia comprises both Bb and RFB in a continuum of genetically related organisms that overlap ecologically and cause an array of similar clinical manifestations, a logical approach would be to adopt the definition proposed by Stricker and Fesler categorizing RFB along with Bbsl as causative agents of LD. As we demonstrated in this study, since there is variable antigenic expression in Borrelia spirochetes and overlapping species distribution, clinical distinction between Bbsl and RFB may be difficult given our limited testing capability. A broader definition of LD that includes all Borrelia species, therefore, makes sense.
This study highlights the dire need for a diagnostic approach that acknowledges the complexity and genetic diversity of Borrelia spirochetes. Commercially available serological testing kits, as endorsed by the CDC, are highly specific for Bbss, and have poor sensitivity [1,20,21,22,62]. The CDC case definition for LD is narrowly drawn and the laboratory criteria needed to qualify as a positive case are rigid [63]. Commercial serological tests are based on antigens of just one Bbss strain, B-31, and this test protocol is, therefore, not capable of detecting antibody reactivity to Borrelia species and strains that lack cross-reactivity with B-31—a limitation that excludes detection of many Borrelia pathogens [1,15,43].
Bb and RFB immunoblots have unique band patterns and, therefore, serological tests based on Bb antigens do not adequately detect RFB antigens [1,15,43]. Testing for Borrelia spirochetes should ideally encompass the full spectrum of organisms, including both Bbsl and RFB. Testing for RFB is currently available in only a handful of national reference laboratories, academic laboratories, or specialty laboratories using microscopy and molecular assays, and serological testing for RFB using “in-house” tests are only available in a few laboratories [13,15,38,43]. Microscopy is nonspecific; spirochetes are only visible when there are high bacterial loads in the blood [13,15,43], and artefacts such as pseudospirochetes (filaments derived from erythrocytes) can be mistaken for spirochetes [15,64]. Ideally, a microscopic diagnosis should be confirmed by testing using other methodologies such as serological assays. Unfortunately, because commercially available test kits for RFB are not available, RFB infection is only sporadically detected, resulting in a lack of understanding of the geographical distribution of human exposure to RFB species [15]. In contrast, the immunoblot testing described in this report detected exposure to a wide variety of Bbsl and RFB species in our pilot study.
Both Bb-seropositive patients and RFB-seropositive patients tended to be more frequently IgM positive than IgG or dual IgM/IgG positive. Prolonged IgM reactivity was demonstrated in patients with late or longstanding LD [65,66,67]. Furthermore, IgM reactivity was demonstrated in human subjects with persistent Bb infection despite treatment with antibiotics [67,68]. Our cohort met the case description for CLD and had symptoms consistent with LD and other tickborne co-infections, such as musculoskeletal, neuropsychiatric, and/or cardiac manifestations. More recently, prolonged IgM seroreactivity was shown to occur in patients with late RFB infection [15]. Our study corroborates the findings of previous studies showing that prolonged IgM reactivity is associated with both Bb and RFB infections and suggests that these infections may be persistent. The fact that IgM reactivity in Borrelia infections is likely to persist long after the onset of symptoms should be recognized when designing testing protocols for diagnosis.
In summary, exposure to Bbsl and RFB is a cause for concern in California and Mexico. Bbsl and RFB infections may explain LD symptoms in patients who are seronegative for Bbss. Some patients may demonstrate dual exposure to both Bb and RFB species, further complicating diagnosis and treatment. Immunoblot testing for both Bbsl and RFB allows the detection of a diverse group of Borrelia serotypes and will promote better understanding of human exposure to pathogenic Borrelia in California and Mexico.
Appendix A
Figure A1.
Immunoblot reactivity for Borrelia burgdorferi sensu lato (Bbsl). Immunoblot strips with recombinant Borrelia antigens were incubated with representative patient serum samples, as described in Section 2. Blots were interpreted according to IGeneX criteria (see text). P—positive control; N—negative control; G—Bbsl immunoblot immunoglobulin G (IgG); M—Bbsl immunoblot IgM; Lane 1—B. burgdorferi B-31 positive IgM and IgG; Lane 2—B. mayonii positive IgG; Lane 3—B. speilmanii positive IgG; Lane 4—B. californiensis positive IgM and IgG; Lane 5—mixed infection B. mayonii and B. californiensis positive IgM; Lane 6—B. burgdorferi European species positive IgM and IgG; Lane 7—Bbss and B. burgdorferi European species positive IgM.
Figure A2.
Immunoblot reactivity for Relapsing Fever Borrelia (RFB). Immunoblot strips with recombinant Borrelia antigens were incubated with representative patient serum samples, as described in Section 2. P—positive control; N—negative control; G—RFB immunoblot IgG; M—RFB immunoblot IgM; 1—B. hermsii positive IgM; 2—B. turicatae positive IgG; 3—B. miyamotoi positive IgM; 4—B. turcica-like positive IgG.
Table A1.
Test results of study subjects showing place of residence, species of Borrelia seroreactivity, and IgM/IgG reactivity. CDC+, positive by CDC recommended surveillance criteria; +, positive by IGeneX in-house criteria; −, negative; IND, indeterminate.
| # | State/Country | Bb IgM | Bb IgG | Bb IgM and IgG | RFB IgM | RFB IgG | RFB IgM and IgG | Species | Bbss IgM or IgG |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mex | CDC+ | + | + | − | − | − | B. afzelii/B. garinii | |
| 2 | CA | + | + | + | − | − | − | Bbss | + |
| 3 | CA | − | − | − | + | − | − | B. hermsii | |
| 4 | CA | − | − | − | − | + | − | RFB sp. | |
| 5 | CA | CDC+ | − | − | − | − | − |
B. mayonii
B. spielmanii |
|
| 6 | CA | CDC+ | − | − | − | − | − | Bbss | + |
| 7 | CA | − | − | − | + | − | − | RFB sp. | |
| 8 | CA | CDC+ | − | − | − | − | − | Bbsl, Bbss | + |
| 9 | CA | − | − | − | − | + | − | B. miyamotoi | |
| 10 | CA | CDC+ | + | + | − | − | − | B. californiensis | |
| 11 | Mex | CDC+ | − | − | − | − | − | Bb sp. | |
| 12 | CA | + | − | − | − | − | − | Bbsl, Bbss | + |
| 13 | CA | − | − | − | + | − | − | RFB sp. | |
| 14 | CA | + | − | − | + | − | − |
Bb sp. B. hermsii |
|
| 15 | CA | − | − | − | + | + | + |
B. miyamotoi RFB sp. |
|
| 16 | CA | CDC+ | − | − | + | − | − |
B. spielmanii RFB sp. |
|
| 17 | CA | − | − | − | − | + | − | B. miyamotoi | |
| 18 | CA | − | − | − | + | − | − | RFB sp. | |
| 19 | CA | − | − | − | + | − | − | RFB sp. | |
| 20 | CA | − | − | − | − | + | − | RFB sp. | |
| 21 | CA | − | − | − | − | + | − | B. turicatae | |
| 22 | CA | CDC+ | CDC+ | + | − | − | − |
B. spielmanii (IgM) B. mayonii (IgG), Bbss |
+ |
| 23 | CA | − | − | − | + | + | + | RFB sp. | |
| 24 | CA | − | − | − | − | + | − | RFB sp. | |
| 25 | Mex | − | − | − | − | + | − | B. turicatae | |
| 26 | CA | − | − | − | + | − | − | RFB Sp. | |
| 27 | CA | − | − | − | − | + | − | B. miyamotoi | |
| 28 | CA | − | − | − | − | + | − | B. turicatae | |
| 29 | CA | CDC+ | + | + | − | − | − | B. spielmanii | |
| 30 | Mex | − | − | − | + | − | − | RFB sp. | |
| 31 | CA | CDC+ | CDC+ | + | + | − | − |
B. californiensis RFB sp. Bbss |
+ |
| 32 | CA | CDC+ | − | − | − | − | − | B. afzelii/B. garinii | |
| 33 | CA | − | − | − | + | − | − | RFB sp. | |
| 34 | CA | − | − | − | + | − | − | RFB sp. | |
| 35 | Mex | CDC+ | + | + | − | − | − | B. californiensis | |
| 36 | CA | − | − | − | − | + | − | RFB sp. | |
| 37 | CA | CDC+ | − | − | + | − | − |
B. afzelii/B. garinii B. turicatae |
|
| 38 | Mex | − | − | − | + | − | − | RFB sp. | |
| 39 | CA | − | − | − | − | + | − | RFB sp. | |
| 40 | CA | − | − | − | + | − | − | B. turicatae | |
| 41 | Mex | − | − | − | − | + | − | B. turicatae | |
| 42 | CA | − | − | − | + | − | − | RFB sp. | |
| 43 | CA | CDC+ | + | + | − | − | − | B. californiensis, B. mayonii, B. afzelii/B. garinii | |
| 44 | CA | CDC+ | − | − | − | − | − | B. spielmanii | |
| 45 | CA | CDC+ | − | − | − | + | − |
B. afzelii/B. garinii RFB sp. |
|
| 46 | CA | − | − | − | + | − | − | B. turicatae | |
| 47 | CA | CDC+ | CDC+ | + | − | − | − | Bbss | + |
| 48 | CA | + | − | − | − | + | − |
B. californiensis RFB sp. |
|
| 49 | CA | − | − | − | + | − | − | RFB sp. | |
| 50 | Mex | − | − | − | + | − | − | B. hermsii B. turcica bipA + |
|
| 51 | CA | CDC+ | − | − | − | − | − |
Bb sp. Bbss (Strong+ to all OspCs) |
+ |
| 52 | CA | CDC+ | − | − | − | − | − |
B. spielmanii Bbss |
+ |
| 53 | CA | − | − | − | + | − | − | B. turcica | |
| 54 | Mex | CDC+ | − | − | − | − | − | B. afzelii/B. garinii | |
| 55 | CA | − | − | − | + | − | − | RFB sp. | |
| 56 | CA | + | − | − | − | − | − | B. spielmanii | |
| 57 | CA | − | − | − | + | − | − |
RFB sp. B. miyamotoi |
|
| 58 | CA | − | − | − | + | − | − | RFB sp. | |
| 59 | CA | − | − | − | + | − | RFB sp. | ||
| 60 | CA | CDC+ | − | − | − | − | − | Bb sp | |
| 61 | CA | − | − | − | + | + | + | B. hermsii B. miyamotoi | |
| 62 | CA | − | − | − | − | + | − | B. miyamotoi | |
| 63 | CA | CDC+ | − | − | IND | − | − |
B. californiensis Bbss |
+ |
| 64 | CA | + | + | + | − | − | − |
B. afzelii/B. garinii Bbss |
+ |
| 65 | CA | CDC+ | − | − | + | − | − | Bbss RFB sp. |
+ |
| 66 | Mex | − | − | − | − | + | − | B. hermsii | |
| 67 | CA | CDC+ | − | − | IND | IND | − |
B. spielmanii B. afzelii/B. garinii |
|
| 68 | CA | − | − | − | + | − | − | RFB sp. | |
| 69 | CA | − | + | − | − | − | − | B. californiensis | |
| 70 | CA | − | − | − | − | + | − | B. turicatae | |
| 71 | CA | − | − | − | − | + | − | B. turicatae | |
| 72 | CA | − | + | − | − | − | − | B. spielmanii | |
| 73 | CA | − | − | − | − | + | − | B. hermsii | |
| 74 | CA | − | − | − | + | − | − | B. hermsii | |
| 75 | CA | − | + | − | − | − | − | Bb sp. | |
| 76 | Mex | − | − | − | + | − | − | RFB sp. | |
| 77 | CA | − | − | − | − | + | − | B. hermsii | |
| 78 | CA | − | + | − | − | − | − | B. californiensis | |
| 79 | CA | − | − | − | − | + | − | B. hermsii | |
| 80 | CA | − | − | − | − | + | − | RFB sp. | |
| 81 | Mex | − | + | − | − | − | − | B. afzelii/B. garinii | |
| 82 | Mex | − | + | − | − | − | − | B. spielmanii | |
| 83 | CA | − | + | − | − | − | − | B. afzelii/B. garinii | |
| 84 | CA | − | − | − | + | − | − | RFB sp. | |
| 85 | CA | − | − | − | + | − | − | RFB sp. | |
| 86 | CA | − | + | − | − | − | − | Bbsl, Bbss | + |
| 87 | CA | − | − | − | − | + | − | RFB sp. | |
| 88 | CA | − | + | − | − | − | − | Bbsl, Bbss | + |
| 89 | CA | − | + | − | − | − | − | Bb sp. | |
| 90 | CA | − | + | − | − | + | − |
Bb sp. RFB sp. |
|
| Total | IgM only 20 | IgG only 11 |
IgM and IgG 10 |
IgM only 29 |
IgG only 25 |
IgM and IgG 3 |
Bbss IgM or IgG 14 |
Table A2.
Summary of seroreactivity for subjects in Group 1 (Bbsl), Group 2 (RFB), or both.
| Immunoblot | Total |
|---|---|
| Group 1 Bbsl positive | 42 |
| Group 2 RFB positive | 56 |
| Dual Group 1 and 2 positive | 8 |
| Group 1 Bbsl positive samples | 34 (38%) |
| Bbsl alone | 8 |
| Bbss alone | 4 |
| B. afzelii/garinii alone | 6 |
| B. californiensis alone | 6 |
| B. spielmanii alone | 6 |
| B. mayonii + B. speilmanii | 2 |
| B. spielmanii + B. afzelii/garinii | 1 |
| B. afzelii/garinii, B. californiensis, B. mayonii | 1 |
| Group 2. RFB Positive Samples | 48 (53%) |
| RFB alone | 25 |
| B. hermsii alone | 7 |
| B. miyamotoi alone | 4 |
| B. turicatae alone | 8 |
| B. turcica alone | 2 |
| B. hermsii + B. turcica | 1 |
| B. hermsii + B. miyamotoi | 1 |
| Dual Group 1 and 2 | 8 (9%) |
| Bb + RFB | 2 |
| Bb + B. hermsii | 1 |
| B. californiensis + RFB | 2 |
| B. spielmanii + RFB | 1 |
| B. afzelii/garinii + RFB | 1 |
| B. afzelii/garinii + B. turicatae | 1 |
| Total cases | 90 |
Table A3.
Reference human sera for determining the specificity of Bbsl and RFB immunoblots.
| Source | Characteristic | Total No. of Sera | Bbsl Immunoblots (+) | RFB Immunoblots (+) |
|---|---|---|---|---|
| CDC Reference Set (n = 25) | Fibromyalgia | 2 | 0 | 0 |
| Healthy endemic | 7 | 1 | 0 | |
| Healthy non-endemic | 6 | 0 | 0 | |
| Mononucleosis | 2 | 0 | 0 | |
| Multiple sclerosis | 2 | 0 | 1 | |
| Rheumatoid arthritis | 2 | 0 | 0 | |
| Severe Periodontitis | 2 | 0 | 0 | |
| Syphilis | 2 | 0 | 1 | |
| CAP and NYSHD (n = 42) Autoimmunity and Allergy |
Autoimmune | |||
| ANA (+) | 3 | 0 | 0 | |
| ANA (−) | 2 | 0 | 0 | |
| DNA (+) | 1 | 0 | 0 | |
| Rheumatoid factor (+) | 9 | 0 | 0 | |
| Rheumatoid factor (−) | 8 | 0 | 0 | |
| Allergy (n = 19) | ||||
| IgG (+) | 13 | 0 | 1 | |
| Spec. IgE (+) | 4 | 0 | 0 | |
| Spec. IgE (−) | 2 | 0 | 0 | |
| NYB (n = 21) Viruses and RPR (+) |
Epstein–Barr virus (EBV) | 4 | 1 | 0 |
| Human immunodeficiency virus (HIV) | 4 | 0 | 0 | |
| Cytomegalovirus (CMV) | 5 | 0 | 1 | |
| Hepatitis C virus (HCV) | 0 | 0 | 0 | |
| RPR (+) | 8 | 2 | 1 | |
| IGeneX (n = 87) | Bartonella henselae infection | 7 | 0 | 0 |
| Human granulocytic anaplasmosis | 16 | 0 | 0 | |
| Babesia microti infection | 14 | 0 | 0 | |
| Babesia duncani Infection | 41 | 0 | 0 | |
| Human monocytic ehrlichiosis | 5 | 0 | 0 | |
| Negative controls | 4 | 0 | 0 | |
| False Positives | 175 | 4 | 5 | |
| Specificity | 97.7% | 97.1% | ||
ANA—anti-nuclear antibodies; CAP—College of American Pathologists; CDC—Center for Disease Control; NYB—New York Biologics, Southampton, NY; NYSH—New York State Department of Health; RF—rheumatoid factor; RPR—rapid plasma regain test for syphilis.
Author Contributions
Conceptualization, J.S.S., M.J.M., R.B.S.; methodology, J.S.S., I.D.C., J.J.B.; software, M.C.F.; validation, J.S.S., R.B.S..; formal analysis, J.S.S., M.C.F., M.J.M., J.J.B.; investigation, M.C.F., R.B.S.; resources, J.S.S., I.D.C.; data curation, J.S.S., I.D.C.; writing—original draft preparation, M.J.M.; writing—review and editing, M.C.F., M.J.M., J.S.S., J.J.B., R.B.S.; visualization, J.S.S., I.D.C.; supervision, J.S.S., R.B.S.; project administration, R.B.S.; funding acquisition, R.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Lindorf Family Foundation, Newark, OH.
Conflicts of Interest
J.S.S. is President and Laboratory Director of IGeneX Reference Laboratory, Milpitas, CA, USA. R.B.S. is the owner of Union Square Medical Associates, a medical practice that treats patients with tick-borne diseases in San Francisco, CA, USA. The other authors report no conflicts of interest in this work. An abstract describing preliminary results of this study was presented at the Western Medical Research Conference, Carmel, CA, USA on 23 January 2020.
References
- 1.Liu S., Cruz I.D., Ramos C.C., Taleon P., Ramasamy R., Shah J. Pilot study of immunoblots with recombinant Borrelia burgdorferi antigens for laboratory diagnosis of Lyme disease. Healthcare. 2018;14:99. doi: 10.3390/healthcare6030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah J.S., Liu S., Du Cruz I., Poruri A., Maynard R., Shkilna M. Line immunoblot assay for tick-borne relapsing fever and findings in patient sera from Australia, Ukraine and the USA. Healthcare. 2019;7:121. doi: 10.3390/healthcare7040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todar K. Todar’s Online Textbook of Bacteriology. University of Wisconsin-Madison, Department of Bacteriology; Madison, WI, USA: 2006. [Google Scholar]
- 4.Cutler S.J. Relapsing fever Borreliae: A global review. Clin. Lab Med. 2015;35:847–865. doi: 10.1016/j.cll.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Cutler S.J., Ruzic-Sabljic E., Potkonjak A. Emerging borreliae—Expanding beyond Lyme borreliosis. Mol. Cell Probes. 2017;31:22–27. doi: 10.1016/j.mcp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Rose I., Yoshimizu M.H., Bonilla D.L., Fedorova N., Lane R.S., Padgett K.A. Phylogeography of Borrelia spirochetes in Ixodes pacificus and Ixodes spinipalpis ticks highlights differential acarological risk of tick-borne disease transmission in northern versus southern California. PLoS ONE. 2019;14:e0214726. doi: 10.1371/journal.pone.0214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panetta J.L., Sima R., Calvani N.E.D., Hajdušek O. Reptile-associated Borrelia species in the goanna tick (Bothriocroton undatum) from Sydney, Australia. Parasit Vectors. 2017;20:616. doi: 10.1186/s13071-017-2579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Lyme Borreliosis (Lyme Disease) [(accessed on 24 October 2019)]; Available online: https://www.who.int/ith/diseases/lyme/en/
- 9.Rochlin I., Ninivaggi D.V., Benach J.L. Malaria and Lyme disease - the largest vector-borne US epidemics in the last 100 years: Success and failure of public health. BMC Public Health. 2019;19:804. doi: 10.1186/s12889-019-7069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz A.M., Hinckley A.F., Mead P.S., Hook S.A., Kugeler K.J. Surveillance for Lyme Disease—United States, 2008–2015. MMWR Surveill. Summ. 2017;66:1–12. doi: 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maraspin V., Ruzic-Sabljic E., Strle F. Lyme Borreliosis and Borrelia spielmanii. Emerg. Infect. Dis. 2006;12:1177. doi: 10.3201/eid1207.060077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin M.S., Schwan T.G., Anderson D.E., Jr., Borchardt S.M. Tick-borne relapsing fever. Infect. Dis. Clin. N. Am. 2008;22:449–468. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez J.E., Krishnavahjala A., Garcia M.N., Bermudez S. Tick-borne relapsing fever spirochetes in the Americas. Vet. Sci. 2016;3:16. doi: 10.3390/vetsci3030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telford S.R., III, Goethert H.K., Molloy P.J., Berardi V.P., Chowdri H.R., Gugliotta J.L. Borrelia miyamotoi disease: Neither Lyme disease nor relapsing fever. Clin. Lab. Med. 2015;35:867–882. doi: 10.1016/j.cll.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middelveen M.J., Shah J.S., Fesler M.C., Stricker R.B. Relapsing fever Borrelia in California: A pilot serological study. Int. J. Gen. Med. 2018;11:373–382. doi: 10.2147/IJGM.S176493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbour A.G. Relapsing Fever and Other Borrelia Diseases. In: Guerrant R.L., Walker D.H., Weller P.F., editors. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. W.B. Saunders; Edinburgh, UK: 2011. pp. 295–302. [Google Scholar]
- 17.CDC Tick-Borne Relapsing Fever (TBRF) [(accessed on 24 October 2019)];2016 Information for Clinicians. Available online: https://www.cdc.gov/relapsing-fever/clinicians/index.html.
- 18.Koton Y., Bisharat N. Tick-borne relapsing fever with severe Jarisch-Herxheimer reaction. ISR Med. Assoc. J. 2018;20:62–63. [PubMed] [Google Scholar]
- 19.Eickhoff C., Blaylock J. Tickborne diseases other than Lyme in the United States. Cleve Clin. J. Med. 2017;84:555–567. doi: 10.3949/ccjm.84a.16110. [DOI] [PubMed] [Google Scholar]
- 20.Stricker R.B., Johnson L. Lyme wars: Let’s tackle the testing. BMJ. 2007;335:1008. doi: 10.1136/bmj.39394.676227.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stricker R.B., Johnson L. Serologic tests for Lyme disease: More smoke and mirrors. Clin. Infect. Dis. 2008;47:1111–1112. doi: 10.1086/592121. [DOI] [PubMed] [Google Scholar]
- 22.Cook M.J., Puri B.K. Commercial test kits for detection of Lyme borreliosis: A meta-analysis of test accuracy. Int. J. Gen. Med. 2016;9:427–440. doi: 10.2147/IJGM.S122313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron D.J., Johnson L.B., Maloney E.L. Evidence assessments and guideline recommendations in Lyme disease: The clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti Infect. Ther. 2014;12:1103–1135. doi: 10.1586/14787210.2014.940900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stricker R.B., Fesler M.C. Chronic Lyme Disease: A Working Case Definition. Am. J. Infect. Dis. 2018;14:1–44. doi: 10.3844/ajidsp.2018.1.44. [DOI] [Google Scholar]
- 25.Golovchenko M., Vancová M., Clark K., Oliver J.H., Jr., Grubhoffer L., Rudenko N. A divergent spirochete strain isolated from a resident of the southeastern United States was identified by multilocus sequence typing as Borrelia bissettii. Parasit Vectors. 2016;9:68. doi: 10.1186/s13071-016-1353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinckley A.F., Connally N.P., Meek J.I., Johnson B.J., Kemperman M.M., Feldman K.A., White J.L., Mead P.S. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis. 2014;59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidsson M. The financial implications of a well-hidden and ignored chronic Lyme disease pandemic. Healthcare. 2018;6:16. doi: 10.3390/healthcare6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudenko N., Golovchenko M., Grubhoffer L., Oliver J.H., Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011;2:123–128. doi: 10.1016/j.ttbdis.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parola P., Diatta G., Socolovschi C., Mediannikov O., Tall A., Bassene H., Trape J.F., Raoult D. Tick-Borne Relapsing Fever Borreliosis, Rural Senegal. Emerg. Infect. Dis. 2011;17:883–885. doi: 10.3201/eid1705.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarih M., Garnier M., Boudebouch N., Bouattour A., Rihani A., Hassar M., Gern L., Postic D., Cornet M. Borrelia hispanica relapsing fever, Morocco. Emerg. Infect. Dis. 2009;15:1626–1629. doi: 10.3201/eid1510.090403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karan L., Makenov M., Kolyasnikova N., Stukolova O., Toporkova M., Olenkova O. Dynamics of spirochetemia and early PCR detection of Borrelia miyamotoi. Emerg. Infect. Dis. 2018;24:860–867. doi: 10.3201/eid2405.170829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause P.J., Carroll M., Fedorova N. Human Borrelia miyamotoi infection in California: Serodiagnosis is complicated by multiple endemic Borrelia species. PLoS ONE. 2018;13:e0191725. doi: 10.1371/journal.pone.0191725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forrester J.D., Kjemtrup A.M., Fritz C.L. Tickborne Relapsing Fever—United States, 1990–2011. MMWR. 2015;64:58–60. [PMC free article] [PubMed] [Google Scholar]
- 34.Burkot T.R., Mullen G.R., Anderson R., Schneider B.S., Happ C.M., Zeidner N.S. Borrelia lonestari DNA in adult Amblyomma americanum ticks, Alabama. Emerg. Infect. Dis. 2001;7:471–473. doi: 10.3201/eid0703.017323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bunikis J., Tsao J., Garpmo U., Berglund J., Fish D., Barbour A.G. Typing of Borrelia relapsing fever group strains. Emerg. Infect. Dis. 2004;10:1661–1664. doi: 10.3201/eid1009.040236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwan T.G., Raffel S.J., Schrumpf M.E., Schrumpf M.E., Webster L.S., Marques A.R., Spano R., Rood M., Burns J., Hu R. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, USA. Emerg. Infect. Dis. 2009;15:1026–1031. doi: 10.3201/eid1507.090223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vuyyuru R., Liu H., Manser T., Alugupalli K.R. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc. Natl. Acad. Sci. USA. 2011;108:20707–20712. doi: 10.1073/pnas.1108776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagemakers A., Staarink P.J., Sprong H., Hovius J.W.R. Borrelia miyamotoi: A widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31:260–269. doi: 10.1016/j.pt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Nieto N.C., Teglas M.B. Relapsing fever group Borrelia in Southern California rodents. J. Med. Entomol. 2014;51:1029–1034. doi: 10.1603/ME14021. [DOI] [PubMed] [Google Scholar]
- 40.Padgett K., Bonilla D., Kjemtrup A. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS ONE. 2014;9:e110853. doi: 10.1371/journal.pone.0110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook V.J., Fedorova N., Macdonald W.P. Unique strain of Borrelia miyamotoi in Ixodes pacificus ticks, California, USA. Emerg. Infect. Dis. 2016;22:2205–2207. doi: 10.3201/eid2212.152046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen N.T.T., Röttgerding F., Devraj G., Lin Y.-P., Koenigs A., Kraiczy P. The complement binding and inhibitory protein CbiA of Borrelia miyamotoi degrades extracellular matrix components by interacting with plasmin(ogen) Front. Cell Infect. Microbiol. 2018;8:23. doi: 10.3389/fcimb.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Middelveen M.J., Cruz I.D., Fesler M.C., Stricker R.B., Shah J.S. Detection of tick-borne infection in Morgellons disease patients by serological and molecular techniques. Clin. Cosmet. Investig. Dermatol. 2018;11:561–569. doi: 10.2147/CCID.S184521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krause P.J., Barbour A.G. Borrelia miyamotoi: The newest infection brought to us by deer ticks. Ann. Intern Med. 2015;163:141–142. doi: 10.7326/M15-1219. [DOI] [PubMed] [Google Scholar]
- 45.Mun J., Eisen R.J., Eisen L., Lane R.S. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J. Med. Entomol. 2006;43:120–123. doi: 10.1603/0022-2585(2006)043[0120:DOABMS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Barbour A.G. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect. Genet. Evol. 2014;27:551–558. doi: 10.1016/j.meegid.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takano A., Toyomane K., Konnai S., Ohashi K., Nakao M., Ito T. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS ONE. 2014;9:e104532. doi: 10.1371/journal.pone.0104532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krause P.J., Fish D., Narasimhan S., Barbour A.G. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukhacheva T.A., Salikhova I.I., Kovalev S.Y. Multilocus spacer analysis revealed highly homogeneous genetic background of Asian type of Borrelia miyamotoi. Infect. Genet. Evol. 2015;31:257–262. doi: 10.1016/j.meegid.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Wroblewski D., Gebhardt L., Prusinski M.A., Meehan L.J., Halse T.A., Miusser K.A. Detection of Borrelia miyamotoi and other tick-borne pathogens in human clinical specimens and Ixodes scapularis ticks in New York State, 2012–2015. Ticks Tick Borne Dis. 2017;8:407–411. doi: 10.1016/j.ttbdis.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Gofton A.W., Margos G., Fingerle V., Hepner S., Loh S.M., Ryan U., Irwin P., Oskam C.L. Genome-wide analysis of Borrelia turcica and ‘Candidatus Borrelia tachyglossi’ shows relapsing fever-like genomes with unique genomic links to Lyme disease Borrelia. Infect. Genet. Evol. 2018;66:72–81. doi: 10.1016/j.meegid.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Bergström S., Normark J. Microbiological features distinguishing Lyme disease and relapsing fever spirochetes. Wien Klin. Wochenschr. 2018;130:484–490. doi: 10.1007/s00508-018-1368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margos G., Marosevic D., Cutler S., Derdakova M., Diuk Wasser M., Emler S., Kahl O. There is inadequate evidence to support the division of the genus Borrelia. Int. J. Syst. Evol. Microbiol. 2017;67:1081–1084. doi: 10.1099/ijsem.0.001717. [DOI] [PubMed] [Google Scholar]
- 54.Hynote E.D., Mervine P.C., Stricker R.B. Clinical evidence for rapid transmission of Lyme disease following a tickbite. Diagn Microbiol. Infect. Dis. 2012;72:188–192. doi: 10.1016/j.diagmicrobio.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Cook M.J. Lyme borreliosis: A review of data on transmission time after tick attachment. Int. J. Gen. Med. 2014;8:1–8. doi: 10.2147/IJGM.S73791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofhuis A., van de Kassteele J., Sprong H., van den Wijngaard C.C., Harms M.G., Fonville M. Predicting the risk of Lyme borreliosis after a tick bite, using a structural equation model. PLoS ONE. 2017;12:e0181807. doi: 10.1371/journal.pone.0181807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sertour N., Cotté V., Garnier M., Malandrin L., Ferquel E., Choumet V. Infection kinetics and tropism of Borrelia burgdorferi sensu lato in mouse after natural (via ticks) or artificial (needle) infection depends on the bacterial strain. Front. Microbiol. 2018;9:1722. doi: 10.3389/fmicb.2018.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pospisilova T., Urbanova V., Hes O., Kopacek P., Hajdusek O., Sima R. Tracking of Borrelia afzelii transmission from infected Ixodes ricinus nymphs to mice. Infect. Immun. 2019;87:e00896-18. doi: 10.1128/IAI.00896-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwan T.G., Piesman J. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 2002;8:115–121. doi: 10.3201/eid0802.010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyle W.K., Wilder H.K., Lawrence A.M., Lopez J.E. Transmission dynamics of Borrelia turicatae from the arthropod vector. PLoS Negl. Trop. Dis. 2014;8:e2767. doi: 10.1371/journal.pntd.0002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Breuner N.E., Dolan M.C., Replogle A.J. Transmission of Borrelia miyamotoi sensu lato relapsing fever group spirochetes in relation to duration of attachment by Ixodes scapularis nymphs. Ticks Tick Borne Dis. 2017;8:677–681. doi: 10.1016/j.ttbdis.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah J.S., Cruz I.D., Narciso W., Lo W., Harris N.S. Improved sensitivity of Lyme disease Western blots prepared with a mixture of Borrelia burgdorferi strains 297 and B31. Chronic Dis. Int. 2014;1:7. [Google Scholar]
- 63.CDC Lyme Disease (Borrelia burgdorferi): 2017 Case Definition. [(accessed on 24 October 2019)];2017 Available online: https://wwwn.cdc.gov/nndss/conditions/lyme-disease/case-definition/2017/
- 64.Greene R.T., Walker R.L., Greene C.E. Pseudospirochetes in animal blood being cultured for Borrelia burgdorferi. J. Vet. Diagn. Investig. 1991;3:350–352. doi: 10.1177/104063879100300416. [DOI] [PubMed] [Google Scholar]
- 65.Craft J.E., Fischer D.K., Shimamoto G.T., Steere A.C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J. Clin. Investig. 1986;78:934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalish R.A., Leong J.M., Steere A.C. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect. Immun. 1995;63:2228–2235. doi: 10.1128/IAI.63.6.2228-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lomholt H., Lebech A.M., Hansen K., Brandrup F., Halkier-Sørenson L. Long-term serological follow-up of patients treated for chronic cutaneous borreliosis or culture-positive erythema migrans. Acta Derm. Venereol. 2000;80:362–366. doi: 10.1080/000155500459312. [DOI] [PubMed] [Google Scholar]
- 68.Middelveen M.J., Sapi E., Burke J., Filush K.R., Franco A., Fesler M.C., Stricker R.B. Persistent Borrelia infection in patients with ongoing symptoms of Lyme disease. Healthcare. 2018;6:33. doi: 10.3390/healthcare6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]