Abstract
The last decade has witnessed a revival of the interest in the hormone melatonin, partly attributable to the discovery that variance in MTNR1B—the melatonin receptor 1b gene—is a risk factor for impaired fasting glucose and type-2-diabetes. Despite intensive investigation, there exists considerable confusion and seemingly conflicting data on the metabolic effects of melatonin and MTNR1B variation, and disagreement on whether melatonin may be metabolically beneficial or deleterious, a critical question for melatonin-agonist/antagonist drug development, and dosing time. We provide a conceptual framework—anchored in the dimensions of “time”—to reconcile paradoxical findings in the literature. We propose that the relative timing between elevated melatonin concentrations and glycemic challenges should be considered in order to better understand the mechanisms and therapeutic opportunities of melatonin signaling in glycemic health and disease.
Keywords: Circadian system, melatonin, MTNR1B, glucose metabolism, type 2 diabetes
Highlights
Melatonin has been investigated mostly for its role in sleep and circadian regulation. The recent discovery of MTNR1B as a novel type 2 diabetes (T2D) gene has sparked great interest in the role of melatonin in glucose control among diabetologists and basic researchers alike.
Despite intensive research, there exist conflicting data regarding the effects of melatonin and MTNR1B genotype on glucose control, and disagreement on whether melatonin may increase or decrease fasting glucose, glucose tolerance and T2D risk.
The concurrence of elevated melatonin concentrations with food intake decreases glucose tolerance, while high melatonin during fasting may facilitate β-cell recovery.
Shift workers, night eaters, and melatonin users are susceptible to the adverse effect brought by the concurrence of food intake and high melatonin levels.
INTRODUCTION
Despite intensive research, there exists conflicting human data regarding the effects of melatonin and MTNR1B on glucose control, and disagreement on whether melatonin may be metabolically ‘beneficial’ or ‘deleterious’. These conflicting data are not limited to one research field or methodology, but span across controlled trials and genome-wide association studies (GWAS), in vivo and in vitro approaches. As a result, currently there are opposing recommendations for the use of melatonin, melatonin agonists, and melatonin antagonists for the purposes of glycemic management. Even so, companies are already initiating programs to develop melatonin-based therapies in the fight against type 2 diabetes (T2D). We thus believe clarification is urgently needed in this fast-moving field.
Melatonin has been investigated mostly for its role in sleep and circadian regulation, including acute effects as well as circadian phase-shifting effects (Box 1). However, in recent years its role in glucose control and in T2D risk or treatment has received increasing attention. This is partly due to the discovery of T2D risk variants in MTNR1B, and partly because of the compelling evidence for the adverse impact of circadian disruption on glucose metabolism [1]. Melatonin, as a circadian hormone, peaks during the nighttime. In humans, the nighttime rise in circulating melatonin levels coincides with the trough of the endogenous circadian rhythm in glucose tolerance (Box 1), which also happens to be a naturally fasting period. This raises the question whether melatonin contributes to the circadian regulation of glucose metabolism. Despite the substantial scientific effort to understand this role of melatonin, different studies reach conflicting conclusions which have not been reconciled [2]. Laboratory rodent models are usually nocturnal and eat when their endogenous melatonin levels are high. Moreover, the widely-used mice models often have severe defects in melatonin synthesis [3]. Thus, it is not surprising that it can be very difficult to translate melatonin research from laboratory rodent models into human. Unexpectedly, even among human research, seeming contradictions exist with studies showing that elevated melatonin concentrations are associated with improved glucose control and others with impaired glucose control. While the most common hypothesis is based on the action of melatonin on impairing glucose homeostasis by inhibiting insulin secretion, an explanation is still missing for the contradictory results [2]. Some have proposed the ‘equilibrium hypothesis’ which submits that either exaggerated or dampened melatonin signaling in common and rare variant carriers, respectively, becomes detrimental for glucose homeostasis [4]. Others suggest an ‘age-related chronobiological hypothesis’ that emphasizes the importance of the circadian system and the deterioration with age [5], and others have defended the ‘loss of function hypothesis’ and the indirect effect of melatonin on glucose metabolism through its role on the central biological clock [2]. However, none of these hypotheses are currently satisfying.
Box 1.
The organization of the circadian system, melatonin, and glucose metabolism.
The circadian system is evolved to generate approximately 24-h rhythms in behavioral and physiological processes. This allows the organism to anticipate and adapt to the predictable rhythmic changes in the environment (e.g., light-dark cycle) brought about by the earth’s rotation. In mammals, endogenous circadian rhythms are produced by a multi-oscillator system composed of the central circadian pacemaker, located in the hypothalamic suprachiasmatic nucleus (SCN), as well as peripheral clocks in virtually every organ, tissue, and cell. The SCN is primarily entrained by light through direct photic inputs received from the retina and transmitted via the retino-hypothalamic tract. Then it functions to synchronize peripheral clocks via a combination of neuronal, behavioral, and endocrine outputs. One of the most well-known endocrine outputs from the SCN is melatonin.
Melatonin, or N-acetyl-5-methoxy tryptamine, is a hormone synthesized and released into the circulation at night [38]. The primary physiological function of melatonin is to convey information on the timing and length of the night to the rest of the body [73]. The main source for circulating melatonin is the pineal gland. In mammals, the production of melatonin from the pineal gland is regulated by a multi-synaptic sympathetic projection from the SCN. Circulating melatonin is regulated by two primary factors, i.e., circadian phase and light exposure. In diurnally-entrained individuals, melatonin levels start to rise a few hours prior to habitual bedtime, remain high during the night, decline in the first few hours after the habitual wake time, and remain very low during the rest of the day. Light exposure during the night causes an acute and dose-dependent reduction of melatonin (while darkness during the day does not raise melatonin production). Both the circadian rhythm and light suppression mechanisms are mediated through the SCN, which receives visual input from intrinsically photosensitive retinal ganglion cells via the retino-hypothalamic tract.
There is a diurnal rhythm in glucose tolerance in healthy humans, with a decrease in glucose tolerance across the waking day [74, 75]. Such rhythm persists when the influence of the environmental/behavioral cycles are removed, indicating circadian regulation [33]. Melatonin, with the nighttime rise and its inhibitory action on glucose tolerance, can contribute to the nighttime reduction in glucose tolerance in human [24]. Some other generally accepted regulatory pathways include autonomic nerve system [76], oscillating hormones (e.g. glucocorticoids [77]) and the cell-autonomous peripheral clocks that can influence organ function (e.g., insulin section in pancreatic islets [78], insulin sensitivity of adipocytes [79]).
Here we provide a conceptual framework—in which “time” plays a central role—with the goal of reconciling those paradoxical findings. In this framework, we consider differences in study conditions with respect to time of day, duration of melatonin exposure, circadian phase-shifting effects of melatonin, or concurrence of elevated melatonin concentrations and glycemic challenge, among others.
MELATONIN IMPACT ON GLUCOSE TOLERANCE
In the following section, we summarize the key studies that have advanced our understanding, but at the same time have fueled this controversy.
Is melatonin beneficial for T2D?
Several lines of evidence support a beneficial role of melatonin in glucose tolerance, including human epidemiologic studies, clinical trials and genetic studies (Table 1). Furthermore, prolonged melatonin treatment (>12h) enhances post-exposure glucose-stimulated insulin secretion from cultured non-diabetic human islets [6]. It has been also suggested melatonin stimulates both glucagon and insulin release from cultured human islets [7]. Low urinary levels of the primary metabolite of melatonin, 6-sulfatoxy melatonin, have been prospectively associated with increased insulin resistance and risk of T2D [8, 9]. Also, in patients with T2D and insomnia, a significant decrease in glycated hemoglobin (HbA1c) levels was found after a 5-month open label trial of repeated nighttime melatonin administration, although HbA1c levels were not affected in the preceding 3-week randomized, double blind, crossover trial [10]. Moreover, a genetic study together with a recent functional genomics study has reported that rare coding variants in the melatonin receptor gene MTNR1B, that inhibit melatonin binding or signaling, are collectively associated with increased risk of T2D [11, 12]; although recent well-powered analysis of coding variants from exome and genome sequencing studies do not support these initial findings [13, 14].
Table 1. Controversies in the literature and potential explanations by the Timing Model.
This table captures the published literature through multiple human controlled trials, genetic experimental studies, and genome wide association studies (GWAS) which support either the beneficial or deleterious roles of melatonin in glucose control and T2D risk. It includes the hypothesized mechanism that explains this apparent contradiction based on the Timing Model.
| Study design/model | Seemingly beneficial effects on glucose control | Hypothesized mechanism (circadian model) | Seemingly deleterious effects on glucose control | Hypothesized mechanism (circadian model) |
|---|---|---|---|---|
| Association study | low nighttime melatonin (urinary 6-sulphatoxy melatonin) prospectively associated with increased insulin resistance and T2D risk 6,7 | low nighttime melatonin signaling during fasting impairs beta-cell recovery and/or sleep | ||
| Open label study | long term (5mo) nighttime administration in T2D w/insomnia decreased HbA1C (however, no effect in RCT) 8 | high nighttime melatonin signaling during fasting improves beta-cell recovery and/or sleep | ||
| Genetics | rare loss-of-function MTNR1B variation associated with increased T2D risk (observation has not been replicated)9,11,81 | low nighttime melatonin signaling during fasting impairs beta-cell recovery and/or sleep | common gain-of-function MTNR1B variant impairs insulin secretion and increases T2D risk 12–17 | high melatonin signaling and longer window of melatonin production with gain-of-function variant impair glucose tolerance if eating in late evening, night, or early morning |
| Placebo-controlled study | daytime melatonin administration impairs glucose tolerance 12,20,21 | high (exogenous) melatonin impairs glucose tolerance | ||
| Placebo-controlled genetic study | daytime melatonin administration impairs glucose tolerance more in carriers of common MTNR1B variant 12,22 | high (exogenous) melatonin with gain-of function variant impairs glucose tolerance | ||
| Experimental study | late dinner timing, when endogenous melatonin concentrations elevated, impairs glucose tolerance (RCT) 23 | high (endogenous) melatonin impairs glucose tolerance | ||
| Experimental genetic study | late dinner timing, when endogenous melatonin concentrations elevated, impairs glucose tolerance more in carriers of common MTNR1B variant (RCT) 23 | high (endogenous) nighttime melatonin with gain-of function variant impairs glucose tolerance |
T2D: Type 2 Diabetes; RCT: Randomized controlled trial.
Is melatonin deleterious for T2D?
While the studies mentioned above suggest a beneficial effect of melatonin on glycemic control, multiple controlled trials, genetic experimental studies and GWAS support a deleterious role of melatonin in glucose control and T2D risk (Table 1). First, GWAS have shown that the rs10830963 risk variant of MTNR1B, which confers increased expression of the receptor in human pancreatic islets [15], is associated with impaired fasting glucose, measures of decreased insulin secretion and increased T2D risk [15–20]. Furthermore, in vitro, melatonin administration stimulates secretion of somatostatin—an insulin-inhibitory hormone—in human pancreatic islets[21, 22]. Also, placebo-controlled human experimental studies have demonstrated that acute melatonin administration worsens glucose tolerance, both in aged women [23] and in young women [24], and both in the morning and in the evening [24]. More importantly, this effect is exacerbated in carriers of the MTNR1B rs10830963 diabetes risk variant as opposed to non-carriers[25].
Consistently, using a randomized, cross-over design, we have shown that consuming a late versus an early dinner, which was associated respectively with elevated and low melatonin concentrations, impairs glucose tolerance in MTNR1B risk allele carriers but not in non-carriers [26]. The interaction of dinner timing with MTNR1B genotype supports a causal role of endogenous physiological melatonin concentrations in the impairment of glucose tolerance[24]. These results suggest that increased melatonin signaling is a risk factor for T2D.
MTNR1B may not only influence insulin release, but may also impact other aspects related to glucose homeostasis. The common MTNR1B risk allele has one of the strongest effects of any of the >243 known T2D risk single nucleotide polymorphisms (SNPs) on lowering the disposition index, which is the product of insulin secretion and insulin sensitivity and captures β-cell function in the context of concomitant insulin resistance [18, 27, 28]. The molecular mechanisms underlying genotype-specific changes in MTNR1B expression and their impact on insulin secretion—and possibly insulin sensitivity—are currently being unraveled [29]. However, it is already described that T2D risk allele for this SNP increases FOXA2-bound enhancer activity in islet- and liver-derived cells [29]. Several studies have reported that rs10830963 is associated with increased MTNR1B mRNA levels in human pancreatic islets [30]. However, the true presence of MT2 protein (the product of MTNR1B) in islets remains uncertain [31].
THE IMPORTANCE OF TIMING: A CENTRAL HYPOTHESIS
The seemingly contradictory effects of melatonin on glucose metabolism may appear difficult to reconcile at first glance, but they are consistent with the Timing Model proposed here. We hypothesize that the concurrence of a) elevated melatonin concentrations and b) food intake adversely influences glucose metabolism in humans, i.e., impairs glucose tolerance.
This concurrence can be due to eating at night, or elevated melatonin levels during the day. Under both circumstances, the high melatonin levels may suppress insulin release and/or insulin sensitivity, resulting in impaired glucose tolerance (Figure 1A, bottom right). By the same token, if we eat during the day, when melatonin levels are low, glucose tolerance is expected not to be negatively affected (Figure 1A, bottom left).
Figure 1. Effect of melatonin on glucose metabolism – importance of timing, and relevance for the general population, clinical practice, and research.
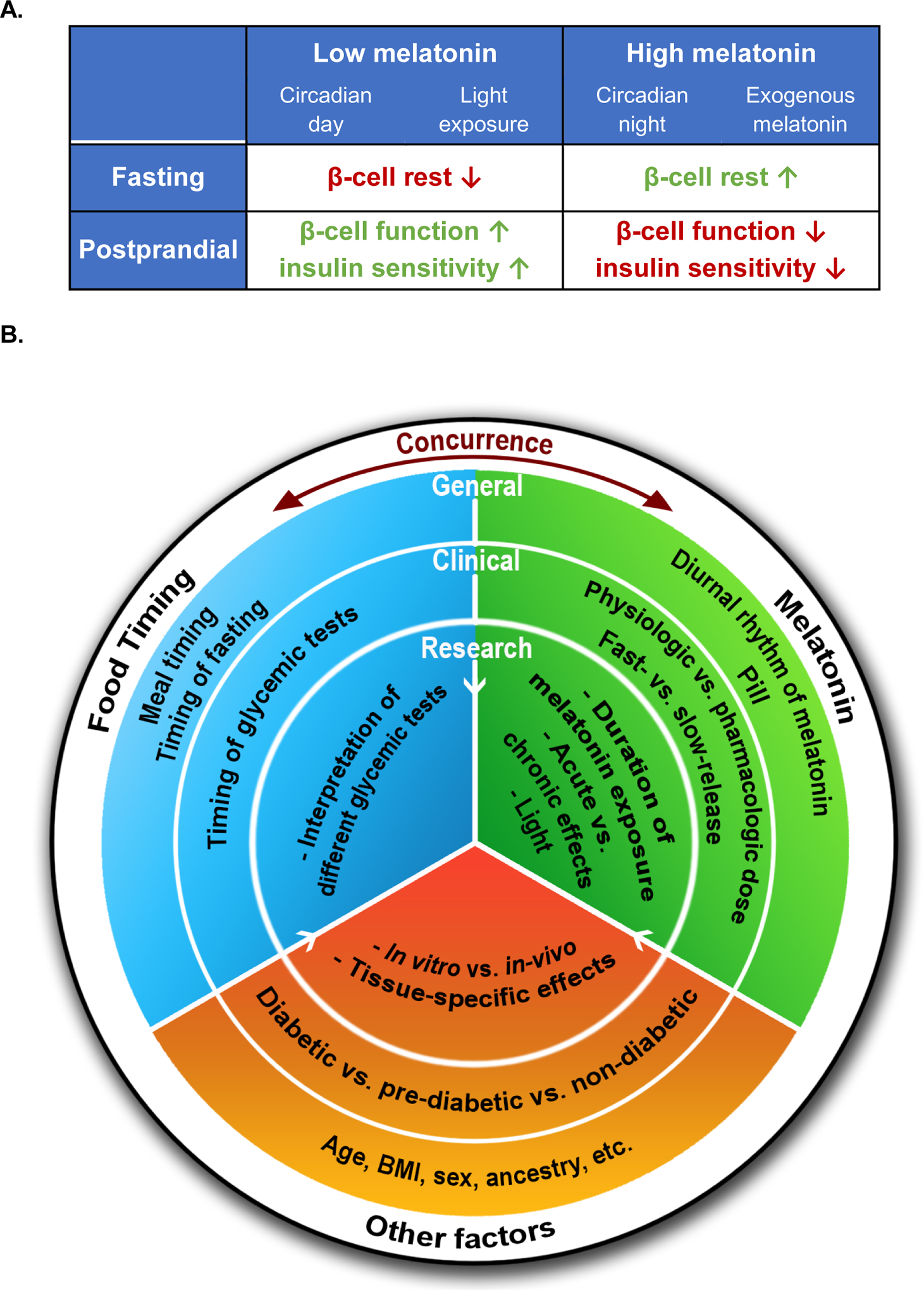
(A) The two columns represent the situations when circulating melatonin levels are low (left) or high (right). Left: the SCN inhibits the pineal gland to produce melatonin during the circadian day (Circadian day); or light inhibits endogenous melatonin production from the pineal gland (Light exposure). Right: the SCN stimulates the pineal gland to produce melatonin during the circadian night (Circadian night); or exogenous melatonin administration increases melatonin concentrations. The two rows describe whether the high/low melatonin levels concur with fasting (top) or feeding (bottom). The subsequent impacts of the four scenarios on glucose metabolism are indicated: the arrows indicate whether there is an increase (↑) or decrease (↓) of the effect under the corresponding situation. Color represents whether the effect improves (green) or impairs (red) glucose tolerance. (B) This schematic diagram summarizes the different insights provided by our current understanding on the effects of melatonin (green) on glycemic control and its interaction with food timing (blue) and other factors (orange). It also summarizes the unanswered key research questions (inner colored circle) in this field that could have further implications for clinical practice (middle colored circle) and the general population (outer colored circle). “Food Timing” in the white outer ring refers to either food timing or—more generally—other glycemic challenges. The concurrence between food timing and melatonin signaling is central to our Timing Model. The white arrows indicate that the factors in the outer circles also apply to the inner circles.
We also hypothesize that at night, during fasting, the naturally high melatonin levels may be physiologically beneficial to maintain normoglycemia as compared to that during the biological day [15, 32] (Figure 1A, top right and top left). Melatonin has also been shown to be able to restore β-cell function in human T2D islets [6].
In this Timing Model, low melatonin (during eating) supports high glucose tolerance, while high melatonin (during fasting) would facilitate β-cell recovery. Therefore, the abnormal state of high melatonin concurrent with food intake, as seen in nocturnally feeding populations, shift-workers or users of exogenous melatonin, may result in dysregulation of glucose metabolism leading to increased risk of T2D. Furthermore, abnormally low melatonin levels or decreased melatonin receptor signaling during the night (as might occur in carriers of rare loss-of-function mutations in MTNR1B), may limit β-cell recovery, and chronically raise T2D risk.
Thus, it is critical to consider the timing of the physiological melatonin cycle relative to the daily feeding-fasting cycle in order to understand the impact of melatonin on glucose metabolism.
REVISITING CONFLICTING RESULTS
With this new hypothesis of a central role of the concurrence of food intake/glycemic load with elevated melatonin levels, we can revisit the seemingly conflicting results in the literature (Table 1). We will also address other aspects to be considered (Boxes 2 & 3).
Box 2.
Potential explanations of controversies by melatonin signaling specifics
Melatonin doses and formulation.
Many cellular, model organism or human studies do not consider whether the impact on glucose metabolism of physiologic doses of melatonin (nighttime circulating melatonin concentrations are typically ~10–100 pg/ml) differs from that of much higher, pharmacologic doses (peak levels after typical OTC oral dose of 1–5 mg are ~500–10,000 pg/mL, depending on the dose, formulation, and interindividual variability), these considerations may reveal important biology of therapeutic relevance[80]. Further studies are required to test whether different melatonin formulations (i.e., slow-release or fast release forms of pharmacologic dosing) exhibit differential effects on glucose metabolism.
Tissue-specificity.
In vitro studies performed in different cell types and tissues, such as α or β cells in pancreatic islets, liver or adipose tissue may reveal different effects of melatonin on factors related to glucose control that might explain some of the conflicting results found on whole body glucose control[81]. Furthermore, melatonin’s effect on glucose control could partially be mediated through effects on the suprachiasmatic nucleus (SCN), which has an important role in glucose control[33].
Melatonin receptor subtypes.
The existence of two high-affinity receptor subtypes (MT1 and MT2) for melatonin may also explain differential effects of melatonin on glucose control. Melatonin effects on both the organellar and organismal level are likely to be well orchestrated composites of signaling through one, both (MT1 and MT2 receptors), or hetero-dimeric receptor types[82]. Furthermore, melatonin is highly lipophilic and easily crosses cell membranes, and may also affect metabolism without involving the receptors[83, 84].
Intron versus exon.
The single nucleotide polymorphisms (SNP) near the MTNR1B receptor gene may affect glucose control quite differently depending on whether the SNP is located in a regulatory (intron) or coding (exon) region. A coding loss-of-function variant would be expected to affect melatonin receptor signaling in all tissues where it is expressed, whereas a regulatory loss-of-function variant may only be operative in specific tissues, depending on the enhancer element and transcription factors involved.
Box 3.
Potential explanations of controversies by study design
In vitro vs. in vivo.
In vitro approaches allow for the testing of mechanisms under controlled conditions. However, in vitro studies are by nature reductionistic and do not give a full picture of the complex reciprocal interaction between the tissues and the whole organism through circulating and nervous system signaling present in the in vivo system.
Population characteristics.
Population characteristics such as sex, age, ancestry, BMI, T2D risk, other comorbidities, and medication use could also contribute to the controversial results. Furthermore, the common MTNR1B risk variant appears to affect the transition from a normoglycemic to prediabetic status more strongly than the transition from prediabetes to diabetes[85]. Therefore, the selection of the population may affect the results of the study.
Observational vs. experimental approaches.
Reconciling the differential effects of melatonin on glucose control may also relate to the type of study, e.g., because they address different mechanistic pathways, they are limited by the associational nature of the study, or there is limited generalizability to real life. Observational studies can relatively easily obtain large data sets, reveal associations between factors, and may point to relevant human biology. However, only subsequent rigorous experimental and interventional studies under controlled conditions can definitively test causality and direction of effect and physiologic mechanisms across multiple tissues. Therefore, results may seem contradictory, which may be due to residual or unmeasured confounding or reverse causality in observational studies. A possible exception in this regard applies to observational genetic studies, where the arrow of time is unidirectional (the genetic variant is present at conception) and there is no reverse causality of the phenotype on germline genetic variation. Currently, new analysis methods such as Mendelian Randomization are helping in testing causality.
Potential explanations by the Timing Model:
1-. Studies that have shown beneficial effects of melatonin on glucose tolerance have generally examined the role of melatonin during fasting conditions at night.
Under these conditions, in addition to the already described improved β-cell recovery, melatonin at night is known to be beneficial for sleep and thereby it may improve glucose control [33, 34]. Indeed, it has been shown that sleep restriction can affect glucose control, either indirectly through increases in hunger, in appetite, and in time availability to eat, that can lead to an increase in body weight; or more directly affecting glucose tolerance and insulin sensitivity. Both excess body weight or impaired glucose metabolism can increase the risk of developing T2D [35]. On the other hand, nighttime melatonin may improve circadian coordination and alignment among the multiple peripheral clocks [36–38].
2-. Studies that have shown deleterious effects of melatonin on glucose tolerance have generally examined the relationship between increased melatonin signaling concurrent with food intake.
Normally, melatonin levels decline over the first few hours after awakening and increase in the last hours prior to sleep, creating two windows of risk if one eats at those times with high endogenous melatonin levels [26]. A third time window is when people eat during the habitual sleep episode or biological night itself, as is true in night-eating and typically in night workers. Of interest, in carriers of the common MTNR1B T2D risk variant (Figure 2), not only is signaling enhanced because of increased receptor expression, but also the duration of elevated melatonin levels may be extended, with a delayed decline in the morning; this effect increases the probability of concurrence with food intake in the morning[39]. These findings are consistent with a detrimental effect of elevated melatonin signaling concurrent with food intake or another glycemic challenge.
Figure 2. Effect of melatonin timing on glucose metabolism; interaction with common genetic variant in MTNR1B.
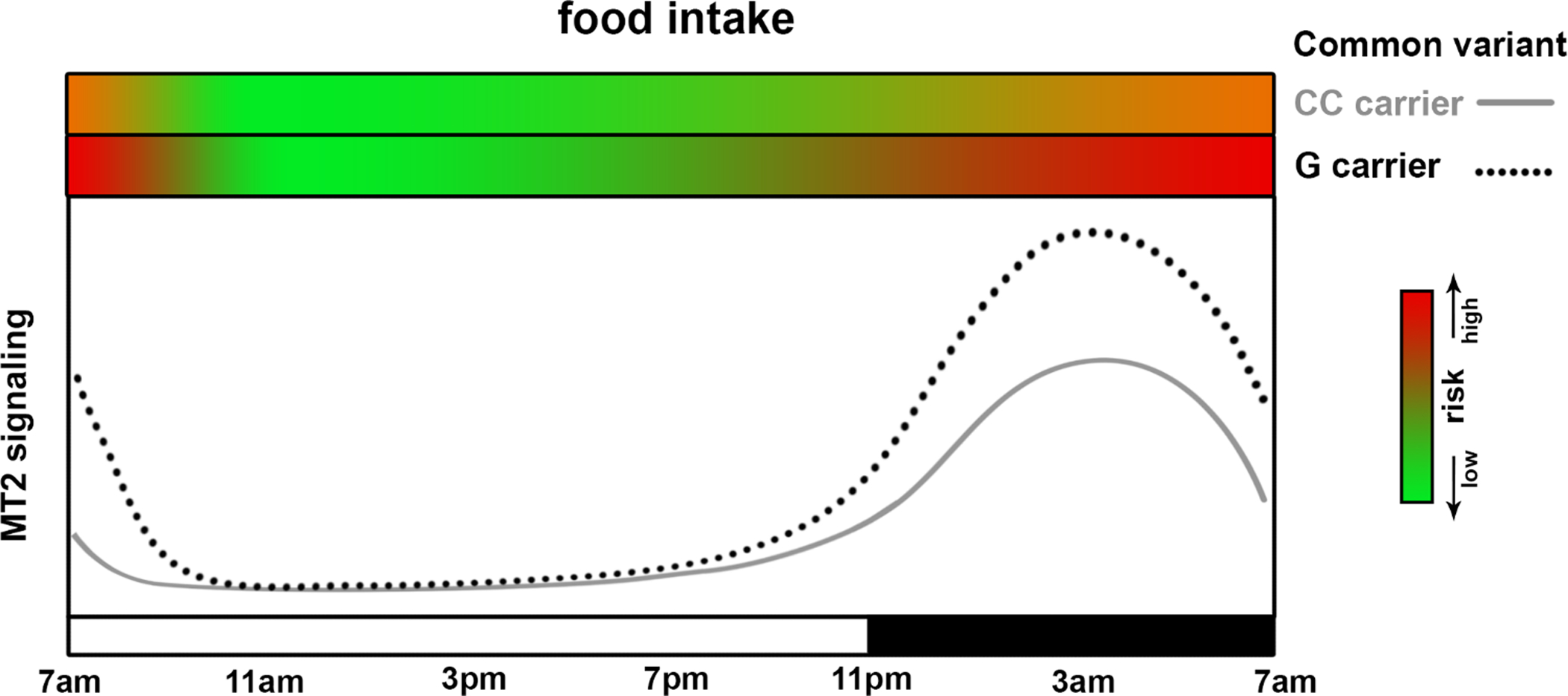
This figure shows the diurnal pattern of melatonin receptor type 2 (MT2) signaling in homozygous carriers of the C allele (grey solid line; non-risk carriers) or G allele carriers (black dotted line; risk carriers) at the common MTNR1B risk single nucleotide polymorphism rs10830963. The X-axis indicates the clock time with black box representing sleep episode in an example individual with a habitual bedtime of 11 PM. With the increased melatonin production at night, both genotypes have enhanced MT2 signaling at night as compared to the day. Since the G allele is the gain-of-function receptor variant, G carriers have higher MT2 signaling in general. It is likely that the inhibitory effect of melatonin on glucose-induced insulin secretion from pancreatic islets is enhanced in G carriers. Thus, G carriers have a higher risk to experience postprandial hyperglycemia when the endogenous melatonin level is high during the biological night. However, the inhibition of insulin secretion by melatonin activation of MT2 receptor is mostly shown in in-vitro experiments with rodent β-cell. Further studies with human islets are required to confirm such effect. The two green-red gradient bars on the top of the panel indicate the risk of glucose intolerance (red as high, green as low) when food intake happens at the given MT2 signaling level (dependent both on genotype and circadian phase).
The Timing Model proposed in this Opinion piece is hypothetical, and while it is consistent with the reported results it has not been fully tested. Future studies are needed to test the different hypotheses following from our model (see Outstanding Questions).
Outstanding Questions.
Given the central hypothesis that elevated melatonin signaling during glycemic challenges (e.g., meal, OGTT, ivGTT) inhibits insulin release and impairs glucose tolerance, does daytime melatonin administration impair glucose tolerance less in rare loss-of-function MTNR1B variants? Similarly, do glycemic challenges at night, when endogenous melatonin concentrations are elevated, impair glucose tolerance less in rare loss-of-function MTNR1B variants?
Given the central hypothesis that elevated melatonin signaling while fasting suppresses insulin release and enhances beta-cell recovery, does nighttime melatonin suppression impair beta-cell recovery, worsening next day glucose tolerance? Does the common gain-of-function MTNR1B variant enhance next day glucose tolerance if fasting overnight? Consistently, do rare loss-of-function MTNR1B variants, decrease beneficial effect of fasting overnight on next day glucose tolerance?
Given that the endogenous melatonin rhythm varies between individuals and possibly by time of year can be influenced by photoperiod, what are the potential roles of chronotype, season, and latitude in the effects of melatonin on glucose control?
Given that melatonin is widely used for its chronobiotic and soporific properties, what is the optimal dose and formulation to maximize efficacy while minimizing adverse metabolic side effects?
SOCIETAL RELEVANCE
If this hypothesis is confirmed, a large proportion of society may be adversely affected by the concurrence of food intake and high melatonin levels. Some potential examples are listed below.
Shift workers:
In the developed world a large percentage of the work force (14% in the US) perform night shifts, either permanently or in rotating shifts, or on irregular schedules [40, 41]. There is convincing epidemiological data documenting that night shift work is a risk factor for developing T2D [42–45]. Compared to nurses reporting no shift work, nurses who report rotating or permanent night shift work had increased risk of T2D dependent on duration of shift work [43]. Furthermore, a recent study has shown that an increased intensity of night work (number of nights working per month) correlates with increased T2D risk [45]. Indeed, night work is generally associated with chronic and recurrent ‘misalignment’ between the endogenous circadian timing system and the daily sleep/wake, fasting/feeding, and metabolic cycles [33, 41, 46, 47]. This chronic misalignment is thought to be a primary cause of the increased risk for diabetes in shift workers [42, 46, 48].
In early work with real shift workers, evidence for glucose intolerance (and other metabolic abnormalities) was shown following a meal during a night shift when circadian misalignment was assessed and confirmed [49]. Metabolic abnormalities were also present in simulated shift work studies, notably in fat metabolism (triglycerides) [41]. Eating a night time meal while melatonin levels are high during night shifts, was thus considered a possible mechanism for an increased risk of heart disease and diabetes.
Our recent in-laboratory studies of experimental circadian misalignment provide further direct evidence for the adverse impact of misalignment between the endogenous circadian timing system and the fasting/feeding and sleep/wake cycle on cardiometabolic functions, including glucose control [50–54]. Even in mild forms of circadian misalignment, such as with social jet lag and later chronotype, there is evidence for increased risk of metabolic alternations [55, 56].
Night eaters:
Eating at the wrong times of the 24-h day for human physiology (such as eating late at night) is associated with increased hyperglycemia and increased risk for T2D [57, 58]. While many factors may be implicated in the increased T2D risk when eating late at night, MTNR1B genetic studies show that the effect of late eating was stronger in MTNR1B T2D risk allele-carriers than in non-carriers [26], suggesting a contribution of melatonin signaling to this deleterious effect.
Melatonin users:
An estimated 3 million adult Americans have used melatonin in the last 30 days, mainly to treat sleeping problems and without medical prescription; and the rates of consumption have doubled in recent years [59]. Our finding of a significant reduction in glucose tolerance, insulin sensitivity, and disposition index following melatonin administration in the evening [24] is particularly relevant for those populations that use melatonin to address sleep complaints. Indeed, the evening is the main time for the use of melatonin administration for the improvement of sleep. In addition, melatonin is used in the evening to phase-advance the circadian system in the treatment of delayed sleep phase syndrome (DSPS) and non-24-hour sleep-wake disorder (“Non-24h”)[60]. The timing of melatonin administration is typically several hours before bedtime in these conditions [61], and thus elevated melatonin concentrations would adversely affect glucose tolerance primarily in people eating in the evening and at night. Moreover, for people taking melatonin for the treatment of DSPS, even eating dinner many hours before bedtime may adversely affect glucose control because the most effective timing of melatonin administration is approximately 5–7 h prior to habitual bedtime in this population [62].
Of further concern would be the dose of melatonin. In healthy adults, oral administration of melatonin (0.05mg) causes plasma melatonin concentration to peak close to its nocturnal physiological level. A more commonly used dose, i.e., 5 mg oral fast release, is enough to cause plasma melatonin levels to remain 10 times higher than physiological peak levels even 6 hours after administration [63], which could results in elevated melatonin levels in the following morning. Some publications have even advocated much larger than 5 mg doses for various potential treatments [64]. Such doses will lead to continuously high circulating melatonin. The range of melatonin circulating concentrations which affect glucose tolerance should be carefully delimited, although even physiologic melatonin concentrations appear sufficient to result in impaired glucose tolerance [26].
Nevertheless, these recommendations may change depending on the ethnicity, and may be applicable for Caucasian, Japanese, African American, Mexican American or Chinese, but perhaps less applicable for Asian Indian people whereas moderate effects have been reported for the genetic variant. Importantly, the effect is apparent at early ages so these recommendations should be applicable for children and adolescents [2].
USING TIMING TO IMPROVE HEALTH
In this Opinion article, we suggest that the concurrence of melatonin with food has a deleterious effect on glycemic control. If these results are confirmed in large clinical trials it would be advisable in the general population 1) to change food timing to avoid concurrence of food with elevated melatonin levels (both endogenous production at night and exogenous melatonin administration). Potential solutions could be moving dinner to an earlier time that may result in better glucose tolerance, especially in MTNR1B carriers [26]. Or, moving breakfast slightly later, to not occur immediately upon awakening, which may limit the concurrence of elevated circulating melatonin concentrations with food intake and thereby improve glycemic control, especially in MTNR1B risk allele carriers [25, 26, 65, 66]. The potential benefit of postponing breakfast may surprise some, especially given that glucose tolerance is higher in the morning [50, 54], as long as it is not so early that melatonin is still high, which would correspond to the biological night. Of interest, apart from interindividual variations in morning melatonin levels, different times of the year with different photoperiods may affect morning melatonin levels [67] especially in carriers of the common MTNR1B risk variant; thus further studies should assess the potential role of season and latitude when studying glucose control. 2) Suppression of endogenous melatonin by light - an alternative approach to avoid concurrence of food with high endogenous melatonin may be to suppress endogenous melatonin production by light during critical temporal windows. However, we should consider that the suppression of melatonin has been hypothesized to lead to other adverse health consequences, so timing, duration, dose, light history, and wavelength would need to be carefully examined [68]. Furthermore, these effects of light exposure on circadian phase should be taken into account [69], given the effect of the circadian system on glucose control [33]. 3) Timing of melatonin administration may be also considered, particularly in vulnerable populations, such as patients with T2D using exogenous melatonin [62]. For these people, it could be recommended to separate melatonin administration at least two hours from food intake to limit concurrence of melatonin with the glycemic challenge. Individual chronotype or circadian rhythm disorder may be also considered to correctly advise the timing of melatonin administration. Indeed, the effect of melatonin on glucose control depends on the timing relative to the endogenous circadian phase, not clock time [70–72].
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Several levels of society may be affected by this Timing Model (Figure 2). Once the central hypothesis is fully tested, in the general population, it may be better to avoid eating meals, especially high glycemic meals, close to exogenous melatonin intake or during the night when the endogenous melatonin levels are normally high. For clinical practice, since most glycemic assessments are performed in the morning, clinicians may find that people have better glucose tolerance in the absence of melatonin. However, if the test is done very early in the morning or if patients display late chronotypes – i.e., in both cases testing would occur especially early relative to their circadian phase – then melatonin may be still elevated and thereby impair glucose control. Therefore, this aspect may be considered when interpreting the glycemic test results. For research into glycemic control, internal circadian time - whether melatonin levels are high or low - should be considered, and perhaps even whether the melatonin signaling is influenced by genetic variants of the MT2 receptor. Up to now, the search for the optimal dose and formulation (e.g., fast/slow-release) of melatonin is mostly focused on its chronobiotic and soporific properties. However, future studies may also consider the potential adverse effects of these different dosages and formulations on glucose metabolism to maximize efficacy and minimize adverse metabolic side effects. An integrated approach to study the role of melatonin, the melatonin receptors, and their genetics on glucose control should include in vitro mechanistic studies, interventional laboratory studies and both observational and interventional studies in real-life settings, such as shift work, late-night eating and exogenous melatonin use, to successfully translate these scientific observations into targeted guidelines or therapies for at-risk individuals (see Outstanding Questions).
In this Opinion, we have proposed timing as a new factor to consider in the clinic, for the diagnosis, prevention and treatment of T2D. Moreover, timing can help unravel the confusion and apparent contradictions in the epidemiologic, clinical and basic investigations. For society, timing is also a relevant factor, especially in vulnerable populations, including late-night eaters, shift workers, and individuals using exogenous melatonin, who together constitute a large proportion of our society.
Glossary
- Circadian rhythm
term originating from the Latin words circa meaning “around” and dies meaning “day”; endogenous biological rhythm with period of approximately 24 hours that is self-sustaining and that persists independent of external environmental and behavioral influences.
- Glucose tolerance
the ability of the body to uptake glucose (sugar) from the circulation system into organs and tissues, such as muscle and adipose tissue.
- Insulin resistance
resistance to the effects of insulin on glucose uptake, metabolism, or storage; is manifested by decreased insulin-stimulated glucose transport and metabolism in adipocytes and skeletal muscle and by impaired suppression of hepatic glucose output.
- Melatonin
a natural hormone mainly synthesized and released into blood stream by the pineal gland in a circadian manner: causing high circulating concentrations at night and near-undetectable concentrations during the day in both diurnal and nocturnal mammals. It is well known as a phase marker of the timing of the central clock, for its central role in the entrainment of the circadian system and for its soporific properties.
- MTNR1B
the gene encoding the high affinity melatonin receptor 1B (also known as MT2), a member of the melatonin receptor family expressed in many tissues, including pancreatic islets. Single nucleotide polymorphisms in MTNR1B have been revealed to be associated with increased fasting blood glucose levels and type 2 diabetes incidence according to several genome-wide association studies.
- Pancreatic β-cells
a type of cells found in pancreatic islets that synthesize insulin and thereby play a dominant role in the regulation of glucose metabolism. When the blood glucose concentration is increasing, e.g., after a meal, the β-cells secrete the hormone insulin to reduce blood glucose.
- Pancreatic β-cell recovery
Recovery of β-cell function (i.e., insulin secretion), which can be achieved by temporary suppression of insulin secretion, e.g., by reducing the peripheral insulin demand. This appears to confer a certain degree of β-cell protection by replenishing insulin secretory capacity and reducing oxidative/ER stress.
- Type 2 diabetes
a metabolic disorder resulting from the interaction between a genetic predisposition and behavioral and environmental risk factors that is characterized by insulin resistance and subsequent progressive loss or dysfunction of pancreatic insulin-producing β-cells, resulting in multiple long-term complications and organ damage.
- Hyperglycemia
high blood sugar. This is a major medical concern, and affects people with both type 1 and type 2 diabetes. There are two main kinds: 1) fasting hyperglycemia (blood sugar above 130 mg/dL after 8 hours of no eating or drinking); 2) post-prandial hyperglycemia (blood sugar higher than 180 mg/dL 2 hours after eating). Chronic high blood sugar can cause damage to nerves, blood vessels and organs, which are common complications of diabetes.
- Shift work
a work schedule that differs from the traditional 9:00 am–5:00 pm day. It can involve evening or night shifts, early morning shifts, or rotating shifts.
References
- 1.Mason IC et al. (2019) Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karamitri A and Jockers R (2019) Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol 15 (2), 105–125. [DOI] [PubMed] [Google Scholar]
- 3.Kennaway DJ (2019) Melatonin research in mice: a review. Chronobiol Int 36 (9), 1167–1183. [DOI] [PubMed] [Google Scholar]
- 4.Mulder H (2017) Melatonin signalling and type 2 diabetes risk: too little, too much or just right? Diabetologia 60 (5), 826–829. [DOI] [PubMed] [Google Scholar]
- 5.Hardeland R (2017) Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J Pineal Res 62 (1). [DOI] [PubMed] [Google Scholar]
- 6.Costes S et al. (2015) Activation of Melatonin Signaling Promotes beta-Cell Survival and Function. Mol Endocrinol 29 (5), 682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramracheya RD et al. (2008) Function and expression of melatonin receptors on human pancreatic islets. J Pineal Res 44 (3), 273–9. [DOI] [PubMed] [Google Scholar]
- 8.McMullan CJ et al. (2013) Melatonin secretion and the incidence of type 2 diabetes. JAMA 309 (13), 1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMullan CJ et al. (2013) Association of nocturnal melatonin secretion with insulin resistance in nondiabetic young women. Am J Epidemiol 178 (2), 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garfinkel D et al. (2011) Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab Syndr Obes 4, 307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnefond A et al. (2012) Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet 44 (3), 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karamitri A et al. (2018) Type 2 diabetes-associated variants of the MT2 melatonin receptor affect distinct modes of signaling. Sci Signal 11 (545). [DOI] [PubMed] [Google Scholar]
- 13.Fuchsberger C et al. (2016) The genetic architecture of type 2 diabetes. Nature 536 (7614), 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flannick J et al. (2019) Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuomi T et al. (2016) Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell metabolism 23 (6), 1067–77. [DOI] [PubMed] [Google Scholar]
- 16.Chambers JC et al. (2009) Common genetic variation near melatonin receptor MTNR1B contributes to raised plasma glucose and increased risk of type 2 diabetes among Indian Asians and European Caucasians. Diabetes 58 (11), 2703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparso T et al. (2009) G-allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release: studies involving 19,605 Europeans. Diabetes 58 (6), 1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyssenko V et al. (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41 (1), 82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prokopenko I et al. (2009) Variants in MTNR1B influence fasting glucose levels. Nat Genet 41 (1), 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouatia-Naji N et al. (2009) A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 41 (1), 89–94. [DOI] [PubMed] [Google Scholar]
- 21.Picinato MC et al. (2002) Melatonin inhibits insulin secretion and decreases PKA levels without interfering with glucose metabolism in rat pancreatic islets. J Pineal Res 33 (3), 156–60. [DOI] [PubMed] [Google Scholar]
- 22.Zibolka J et al. (2018) Distribution and density of melatonin receptors in human main pancreatic islet cell types. J Pineal Res 65 (1), e12480. [DOI] [PubMed] [Google Scholar]
- 23.Cagnacci A et al. (2001) Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxf) 54 (3), 339–46. [DOI] [PubMed] [Google Scholar]
- 24.Rubio-Sastre P et al. (2014) Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 37 (10), 1715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garaulet M et al. (2015) Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism 64 (12), 1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Minguez J et al. (2017) Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin Nutr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prokopenko I et al. (2014) A central role for GRB10 in regulation of islet function in man. PLoS Genet 10 (4), e1004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahajan A et al. (2018) Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 50 (11), 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaulton KJ et al. (2015) Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet 47 (12), 1415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persaud SJ and Jones PM (2016) A Wake-up Call for Type 2 Diabetes? N Engl J Med 375 (11), 1090–2. [DOI] [PubMed] [Google Scholar]
- 31.Bonnefond A and Froguel P (2017) The case for too little melatonin signalling in increased diabetes risk. Diabetologia 60 (5), 823–825. [DOI] [PubMed] [Google Scholar]
- 32.She M et al. (2014) Melatonin receptors in diabetes: a potential new therapeutical target? Eur J Pharmacol 744, 220–3. [DOI] [PubMed] [Google Scholar]
- 33.Qian J and Scheer F (2016) Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol Metab 27 (5), 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiegel K et al. (2009) Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 5 (5), 253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reutrakul S and Van Cauter E (2018) Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 84, 56–66. [DOI] [PubMed] [Google Scholar]
- 36.Lockley SW et al. (2000) Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol 164 (1), R1–6. [DOI] [PubMed] [Google Scholar]
- 37.Hardeland R et al. (2012) Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res 52 (2), 139–66. [DOI] [PubMed] [Google Scholar]
- 38.Scheer FA and Czeisler CA (2005) Melatonin, sleep, and circadian rhythms. Sleep medicine reviews 9 (1), 5–9. [DOI] [PubMed] [Google Scholar]
- 39.Lane JM et al. (2016) Impact of Common Diabetes Risk Variant in MTNR1B on Sleep, Circadian, and Melatonin Physiology. Diabetes 65 (6), 1741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Division of Health Interview Statistics, N.C.f.H.S. (2011) 2010 National Health Interview Survey (NHIS) ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2010/samadult_freq.pdf, (accessed).
- 41.Arendt J (2010) Shift work: coping with the biological clock. Occup Med (Lond) 60 (1), 10–20. [DOI] [PubMed] [Google Scholar]
- 42.Karlsson B et al. (2001) Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occupational and environmental medicine 58 (11), 747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan A et al. (2011) Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 8 (12), e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang XS et al. (2011) Shift work and chronic disease: the epidemiological evidence. Occupational medicine 61 (2), 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vetter C et al. (2018) Night Shift Work, Genetic Risk, and Type 2 Diabetes in the UK Biobank. Diabetes Care 41 (4), 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roden M et al. (1993) The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am J Physiol 265 (1 Pt 2), R261–7. [DOI] [PubMed] [Google Scholar]
- 47.Sack RL et al. (1992) Melatonin rhythms in night shift workers. Sleep 15 (5), 434–41. [DOI] [PubMed] [Google Scholar]
- 48.Ha M and Park J (2005) Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health 47 (2), 89–95. [DOI] [PubMed] [Google Scholar]
- 49.Lund J et al. (2001) Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol 171 (3), 557–64. [DOI] [PubMed] [Google Scholar]
- 50.Morris CJ et al. (2015) Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences of the United States of America 112 (17), E2225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheer FA et al. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106 (11), 4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris CJ et al. (2016) Circadian misalignment increases cardiovascular disease risk factors in humans. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris CJ et al. (2016) Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab, jc20153924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian J et al. (2018) Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koopman ADM et al. (2017) The Association between Social Jetlag, the Metabolic Syndrome, and Type 2 Diabetes Mellitus in the General Population: The New Hoorn Study. J Biol Rhythms 32 (4), 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anothaisintawee T et al. (2017) Later chronotype is associated with higher hemoglobin A1c in prediabetes patients. Chronobiol Int 34 (3), 393–402. [DOI] [PubMed] [Google Scholar]
- 57.Mattson MP et al. (2014) Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A 111 (47), 16647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakajima K and Suwa K (2015) Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. Journal of diabetes and metabolic disorders 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clarke TC et al. (2015) Trends in the use of complementary health approaches among adults: United States, 2002–2012. National health statistics reports 79. [PMC free article] [PubMed] [Google Scholar]
- 60.Sack RL et al. (2007) Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 30 (11), 1484–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auger RR et al. (2015) Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 11 (10), 1199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burgess HJ et al. (2010) Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab 95 (7), 3325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deacon S and Arendt J (1995) Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res 688 (1–2), 77–85. [DOI] [PubMed] [Google Scholar]
- 64.Sack RL et al. (1997) Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep 20 (10), 908–15. [DOI] [PubMed] [Google Scholar]
- 65.Eckel RH et al. (2015) Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr Biol 25 (22), 3004–10. [DOI] [PubMed] [Google Scholar]
- 66.Lane JM et al. (2016) Impact of common diabetes risk variant in MTNR1B on sleep, circadian and melatonin physiology. Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arendt J (1998) Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod 3 (1), 13–22. [DOI] [PubMed] [Google Scholar]
- 68.Schernhammer ES and Schulmeister K (2004) Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer 90 (5), 941–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang AM et al. (2011) The human circadian system adapts to prior photic history. J Physiol 589 (Pt 5), 1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wittenbrink N et al. (2018) High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest 128 (9), 3826–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skene DJ et al. (2018) Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc Natl Acad Sci U S A 115 (30), 7825–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laing EE et al. (2017) Blood transcriptome based biomarkers for human circadian phase. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arendt J (1994) Melatonin and the mammalian pineal gland, Springer Science & Business Media. [Google Scholar]
- 74.Van Cauter E et al. (1991) Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 88 (3), 934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Cauter E et al. (1997) Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 18 (5), 716–38. [DOI] [PubMed] [Google Scholar]
- 76.Kalsbeek A et al. (2014) Circadian control of glucose metabolism. Mol Metab 3 (4), 372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Cauter E et al. (1992) Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol 262 (4 Pt 1), E467–75. [DOI] [PubMed] [Google Scholar]
- 78.Pulimeno P et al. (2013) Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia 56 (3), 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carrasco-Benso MP et al. (2016) Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aldhous M et al. (1985) Plasma concentrations of melatonin in man following oral absorption of different preparations. Br J Clin Pharmacol 19 (4), 517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peschke E et al. (2013) Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon. International journal of molecular sciences 14 (4), 6981–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Owino S et al. (2016) Melatonin Signaling Controls the Daily Rhythm in Blood Glucose Levels Independent of Peripheral Clocks. PLoS One 11 (1), e0148214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barrenetxe J et al. (2004) Physiological and metabolic functions of melatonin. J Physiol Biochem 60 (1), 61–72. [DOI] [PubMed] [Google Scholar]
- 84.Luchetti F et al. (2010) Melatonin signaling and cell protection function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 24 (10), 3603–24. [DOI] [PubMed] [Google Scholar]
- 85.Walford GA et al. (2012) Common genetic variants differentially influence the transition from clinically defined states of fasting glucose metabolism. Diabetologia 55 (2), 331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


