Abstract
Background and aims
Atherosclerosis is an important contributing factor to cardiovascular mortality. The role of Helicobacter pylori (H. pylori) infection in atherosclerosis is inconsistent and sometimes controversial. The present study aimed to determine if H. pylori infection is associated with carotid atherosclerosis.
Methods
17,613 males and females with both carotid ultrasonic examination and 13C-urea breath test for H. pylori infection were screened by a major Chinese university hospital from March 2012 to March 2017 for the study. Baseline demographics, cardiac risk factors, and laboratory studies were obtained. After exclusion for pre- specified conditions, 12,836 individuals were included in the analysis, including 8157 men (63.5%) and 4679 women (36.5%). Analysis was also made for 5-year follow-up data of 1216 subjects (869 males and 347 females) with and without H. pylori infection for development and progression of carotid atherosclerosis.
Results
After adjusting for age, sex, body mass index, lipid profile, hypertension, renal function, diabetes mellitus, and smoking, H. pylori infection was found as an independent risk factor for carotid atherosclerosis in males under 50 years, but not in older males or females (odds ratio 1.229, 95% CI 1.054–1.434, p =0.009). Follow-up data analysis showed that the incidence of carotid atherosclerosis from no atherosclerosis to detectable lesions was significantly higher in young males with persistent H. pylori infection than those without H. pylori infection (p = 0.028) after 3 years.
Conclusions
These data suggest that H. pylori infection might be an important risk factor for carotid atherosclerosis in young Chinese males under 50.
Keywords: Helicobacter pylori, Carotid atherosclerosis, Gender difference, Cardiovascular risk factor
1. Introduction
Atherosclerosis is among the principal contributors to cardiovascular diseases (CVDs), especially coronary artery diseases (CAD) and stroke [1]. The incidence of stroke in China has increased dramatically in the last thirty years [2], and is a leading cause of death [3]. Although traditional CVD risk factors in the Chinese population are common [4–6], other novel risk factor for the development of atherosclerosis in the carotid artery are being explored as additional reasons for the increased rate of stroke.
Gut microorganisms have been shown to significantly contribute to the development of atherosclerosis and related CVD [7]. Helicobacter pylori (H. pylori) colonizes the human gastric epithelium in a significant portion of the general population worldwide, from 30% to 50% in developed countries up to 80% in developing countries [8]. Increasing data indicated that H. pylori infection is associated with extra gastrointestinal diseases including CVDs [9,10]. However, the relationship between H. pylori infection and atherosclerosis in both coronary artery and carotid artery has been inconsistent and sometimes controversial, with the findings ranging from a strong positive association to a mild association, to no association [11–13]. The association of H. pylori infection and carotid atherosclerosis in Chinese patients has not been defined in an adequate sample size. The present study aimed to determine if H. pylori infection could be associated with increased risk for carotid atherosclerosis.
2. Patients and methods
2.1. Study population
Patients who underwent a carotid ultrasonic examination and a 13C-urea breath test [13C-UBT]) at the Third Xiangya Hospital of Central South University in Changsha, Hunan, China, during their annual health evaluation were screened from March 2012 to March 2017 for the study. Based on the study methods, the population was divided into two groups: a cross-sectional study for the single measurement group, and a retrospective cohort study for patients with follow up measurements up to 5 years (Fig. 1A). Patients were excluded from the study if any of the following conditions was present: 1) history of H. pylori eradication, 2) use of any antibiotics, proton pump inhibitors, or H2-receptor blockers 3 months before the tests, 3) age < 20 or > 70 years, 4) connective tissue diseases or immunological diseases,5) mental disorders, 6) asthma or COPD, 7) hematological disorders, 8) thyroid diseases, 9) malignancies, 10) recent (within 3 months) or chronic infection (over 3 months) except H. pylori infection, 11) congestive heart failure, and 12) abnormal liver function. Patients with CAD were not excluded from the study since carotid atherosclerosis and CAD share similar risk factors, and it was felt that exclusion of the subjects with CAD could remove the subgroup population who might be at increased risk for carotid atherosclerosis with H. pylori infection, leading to potential selection bias. Of note, the patients with CAD accounted only for about 3% of all participating subjects for the present study, and there was no stroke patient in the database. The study was conducted according to the principles of the Declaration of Helsinki, and approved by the Clinical Research Ethics Committee of the Third Xiangya Hospital of Central South University, Changsha, Hunan, China. Written informed consent was obtained from all patients prior to their participation.
Fig. 1.
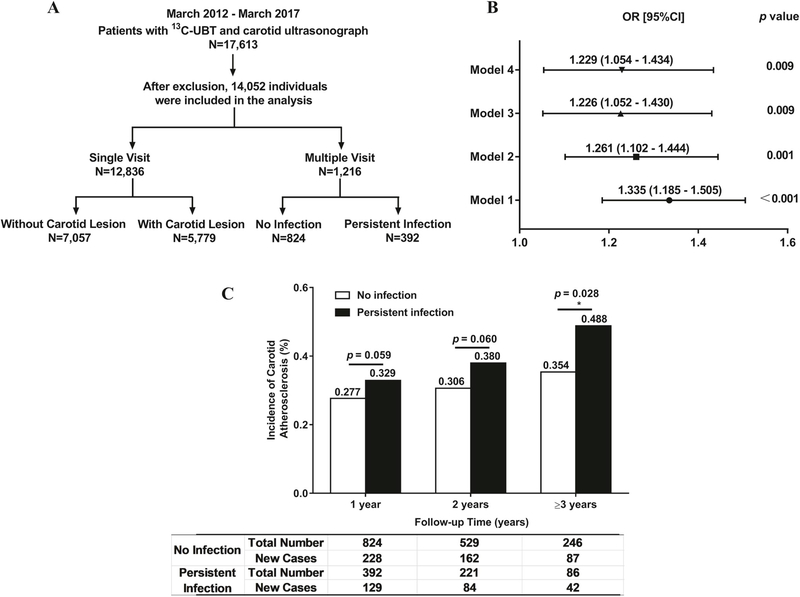
Study design and relationship between H. pylori infection and carotid atherosclerosis.
(A) The subjects were screened and divided into different groups based on the methods of analysis for the study. (B) After adjusting for age, sex, BMI, lipid profile, HTN, DM, smoking, and alcohol use, H. pylori infection was found to be an independent risk factor for carotid atherosclerosis for male patients ≤50 years. Model 1: not adjusted; Model 2: adjusted for age, sex, smoking, alcohol use, and BMI; Model 3: adjusted for all the factors in Model 2 plus blood pressure, HDL, LDL, and total cholesterol; Model 4: adjusted for all the factors in Model 3 plus diabetes mellitus. BMI: body mass index; DM: diabetes mellitus; HDL: high-density lipoprotein; LDL: low-density lipoprotein; HTN: hypertension. (C) Analysis on the follow-up patients showed that patients with persistent H. pylori infection had significantly increased risk for carotid atherosclerosis after 3 years of follow up only in males under 50 years, not in females (p = 0.028).
2.2. Carotid ultrasonography and atherosclerosis detection
Carotid intima-media thickness (CIMT) was defined as the distance between the aortic intima and the media-adventitia interface. The presence of increased CIMT and carotid atherosclerosis was determined with a Siemens Acuson™ Sequoia 512 Doppler ultrasound (Siemens, German) at 1 cm proximal to the common carotid artery bifurcation of the left and right common carotid arteries. The average CIMT of three separate measurements was used for analysis for each subject. Carotid atherosclerotic plaque was defined as CIMT > 1.4 mm, or the presence of focal wall thickening at least 50% greater than that of the surrounding vessel wall as described [14].
2.3. Detection of H. pylori infection
On the same day as the carotid ultrasound examination, a two-stage 13C-UBT test was performed for each subject to determine the presence of H. pylori infection after fasting for at least 6 h. The status of H. pylori infection was determined by analyzing the exhaled breath samples using 13C infrared spectrometry for each patient. A receiver-operating characteristic curve analysis was performed to define the cut-off delta-over-baseline (DOB) values. DOB≥4 [15] was considered as a positive reaction, and DOB < 4 as a negative reaction.
2.4. Other clinical data collection
Other clinical data including anthropometry and laboratory tests were collected for the study as described [16]. The height and weight were taken without shoes and with light clothing. Body-mass-index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Waist circumference was taken at the minimum circumference between the iliac crest and the rib cage using a non-stretchable standard tape. Seated blood pressures were obtained by skilled medical personnel after subjects rested for 15 min using an electronic sphygmomanometer, and the average of three readings was recorded for analysis. The individual was considered to be hypertensive when the systolic blood pressure was over 130 mmHg or diastolic blood pressure over 80 mmHg or on anti-hypertensive drugs.
An overnight fasting blood sample was obtained from an antecubital vein for each subject for measurements of serum lipids, glucose, creatinine, blood urea nitrogen, and uric acid with a Hitachi 7170S autoanalyzer (Hitachi, Tokyo, Japan). The subject was considered to have hyperlipidemia when the individual’s triglyceride (TG) was ≥2.26 mmol/L, or total cholesterol (TC) ≥6.22 mmol/L, or HDL-C < 1.04 mmol/L. The patient was classified as diabetic when the fasting plasma glucose concentration was over 6.11 mmol/L or on antidiabetic drugs like insulin.
2.5. Statistical analysis
Categorical variables were expressed as percentage (%), and analyzed using chi-square (x2) test. Quantitative variables were expressed as mean ± standard deviation (SD) (X ± SD), and analyzed using oneway ANOVA. Adjusted odds ratios (ORs) were estimated with logistic regression analysis models. A cross-sectional study was performed for the single measurement group to determine the association between H. pylori infection and carotid atherosclerosis. A retrospective cohort study was performed for the incidence of carotid atherosclerosis for the patients with follow up measurements. Based on the distribution of patients with carotid atherosclerosis in different age groups, the cut-off age of 50 years old was used to identify the specific population with increased risk for carotid atherosclerosis associated with H. pylori infection. All statistical analyses were performed using the SPSS software (Windows Version 25.0, Chicago, IL, USA). The difference was considered statistically significant when a p value was < 0.05.
3. Results
3.1. Factors associated with carotid atherosclerosis
A total of 17,613 patients were screened with both carotid ultrasonography and a13C-urea breath test. The study population included 12,836 individuals of whom 8157 men (65.5%) and 4679 women (36.5%) (Table 1). Based on the presence of carotid atherosclerosis on carotid artery color ultrasonography, the patients were divided into two groups: 5779 patients with carotid atherosclerosis, and 7057 individuals without carotid atherosclerosis.
Table 1.
Baseline characteristics for the participants with and without carotid atherosclerosis.
| Withoutcarotid atherosclerosis (N = 7057) | With carotid atherosclerosis (N = 5779) | p value | |
|---|---|---|---|
| H. pylori infection, n (%) | 2348 (33.3%) | 2079 (36.0%) | 0.001 |
| Age (years) | 41.7 ± 7.7 | 54.0 ± 9.5 | 0.000 |
| Male, n (%) | 4347 (61.6%) | 3810 (65.9%) | 0.000 |
| BMI (kg/m2) | 24.4 ± 3.5 | 25.1 ± 3.2 | 0.000 |
| Waist-hip ratio | 0.891 ± 0.073 | 0.915 ± 0.062 | 0.000 |
| SBP (mmHg) | 121.4 ± 15.5 | 133.1 ± 18.6 | 0.000 |
| DBP (mmHg) | 78.3 ± 12.0 | 83.1 ± 12.1 | 0.000 |
| Hypertension, n (%) | 1482 (21.0%) | 2699 (46.7%) | 0.000 |
| Diabetes mellitus, n (%) | 1369 (19.4%) | 2092 (36.2%) | 0.000 |
| Hyperlipidaemia, n (%) | 1750 (24.8%) | 2231 (38.6%) | 0.000 |
| Smoking, n (%) | 2865 (40.6%) | 2456 (42.5%) | 0.031 |
| Alcohol, n (%) | 3057 (43.3%) | 3230 (55.9%) | 0.000 |
| Coronary heart disease, n (%) | 176 (2.5%) | 220 (3.8%) | 0.000 |
| Family history of CHD, n (%) | 816 (11.6%) | 701 (12.1%) | 0.067 |
| ALT (U/L) | 30.0 ± 23.0 | 28.8 ± 20.0 | 0.051 |
| AST (U/L) | 24.0 ± 11.0 | 24.5 ± 10.4 | 1.000 |
| Serum total bilirubin (umol/L) | 15.4 ± 5.6 | 15.6 ± 5.5 | 0.697 |
| Serum total protein (g/L) | 72.8 ± 4.4 | 72.4 ± 4.4 | 0.370 |
| Serum albumin (g/L) | 46.7 ± 2.8 | 45.7 ± 2.9 | 0.476 |
| A/G | 1.8 ± 0.3 | 1.8 ± 0.3 | 0.969 |
| Seroglobulin (g/L) | 26.1 ± 3.6 | 26.7 ± 3.9 | 0.890 |
| Blood urea nitrogen (mmol/L) | 4.6 ± 1.2 | 5.1 ± 1.4 | 1.000 |
| Creatinine (umol/L) | 68.8 ± 15.5 | 72.0 ± 29.5 | 0.500 |
| Uric acid (mg/dL) | 317.7 ± 96.0 | 331.6 ± 92.2 | 0.346 |
| Total cholesterol (mmol/L) | 5.01 ± 0.97 | 5.31 ± 1.05 | 0.000 |
| Triglycerides (mmol/L) | 2.00 ± 2.07 | 2.12 ± 1.95 | 0.014 |
| HDL-cholesterol (mmol/L) | 1.46 ± 0.38 | 1.42 ± 0.37 | 0.001 |
| LDL-cholesterol (mmol/L) | 2.66 ± 0.83 | 2.95 ± 0.89 | 0.000 |
| Fibrinogen (g/L) | 2.7 ± 0.6 | 2.9 ± 0.7 | 0.200 |
Data were expressed as mean ± standard deviation or n (%), where appropriate. A/G: serum albumin/seroglobulin; ALT: alanine aminotransferase; AST: aspartate transaminase; DBP: diastolic blood pressure; HDL: high-density lipoprotein; LDL: Low-density lipoprotein; SBP: systolic blood pressure.
Patients with atherosclerosis had significantly higher rate of H. pylori infection, and had higher BMI, higher incidence of HTN, DM, smoking, alcohol use, higher levels of total cholesterol and low-density lipoprotein cholesterol (LDL), and lower level of high-density lipoprotein cholesterol (HDL) than those without carotid atherosclerosis (p < 0.05) (Table 1). Male patients had a significantly higher prevalence of carotid atherosclerosis than females (46.7% vs. 42.1%, p < 0.001).
3.2. H. pylori infection and carotid atherosclerosis: differences in age and gender
H. pylori-positive male patients had a significantly higher incidence of carotid atherosclerosis (48.54% vs. 45.67%, p = 0.006) and thicker CIMT (0.713 ± 0.098 vs. 0.700 ± 0.100, p = 0.001) compared with H. pylori-negative male subjects. However, no difference in the incidence of carotid atherosclerosis or CIMT was observed in females with and without H. pylori infection (Table 2). Further analysis showed that there was an age difference in male patients in the association between H. pylori infection and atherosclerosis. Fig. 1B showed that H. pylori infection significantly increased the risk for carotid atherosclerosis (OR = 1.335, 95% CI = 1.185–1.505, p < 0.001) and CIMT (0.689 ± 0.084 vs. 0.672 ± 0.087, p < 0.001) (Table 2) for males younger than 50 years, but not in males over 50 years.
Table 2.
Incidence of carotid atherosclerosis and carotid intima-media thickness in subiects with and without H. pylori infection.
| Incidence of carotid atherosclerosis |
p value | Carotid intima-media thickness (mm) |
p value | |||
|---|---|---|---|---|---|---|
| WithoutH. pylori infection | With H. pylori infection | Without H. pylori infection | With H. pylori infection | |||
| Male | 2381 (45.67%) | 1429 (48.54%) | 0.006 | 0.700 ± 0.100 | 0.713 ± 0.098 | 0.001 |
| ≤50 | 945 (27.09%) | 659(33.17%) | 0.004 | 0.672 ± 0.087 | 0.689 ± 0.084 | < 0.001 |
| > 50 | 1436(83.25%) | 770 (80.46%) | 0.514 | 0.756 ± 0.106 | 0.763 ± 0.120 | 0.607 |
| Female | 1301 (42.13%) | 668 (41.99%) | 0.700 | 0.665 ± 0.106 | 0.662 ± 0.093 | 0.052 |
Data were expressed as mean ± standard deviation or n (%), where appropriate.
Binary logistic regression analysis was performed to determine the impact of various factors on the likelihood for carotid atherosclerosis for male patients under 50 years old. Model 4 included all predictors that were statistically significant with single factor analysis (Fig. 1B). The p value of Omnibus test for the model coefficients was < 0.001, indicating that the model was able to identify the factors for male patients under 50 years of age with carotid atherosclerosis. As shown in Table 3, H. pylori infection, alcohol use, age, systolic blood pressure, HTN, CAD, and LDL-cholesterol were independent factors for increased risk of carotid atherosclerosis for males under 50 years. H. pylori infection was associated with a significant increase in CIMT for the patients without carotid atherosclerosis (0.664 ± 0.084 vs. 0.637 ± 0.088, p = 0.002). However, there was no difference in CIMT between subjects with and without H. pylori infection in patients with carotid atherosclerosis (0.732 ± 0.091 vs. 0.732 ± 0.094, p = 0.989) (Supplementary Table I), suggesting that the existing atherosclerotic burden might overshadow the effect of H. pylori infection on CIMT.
Table 3.
Risk factors for carotid atherosclerosis in male patients under 50 years. Binary logistic regression analysis was performed to assess the impact of a number of factors on the likelihood for carotid atherosclerosis.
| Risk factor | Odds ratio (95%CI) | p value |
|---|---|---|
| H. pylori infection, n (%) | 1.229(1.054–1.434) | 0.009* |
| Age (years) | 1.144 (1.126–1.163) | < 0.001* |
| Alcohol, n (%) | 1.445 (1.204–1.734) | < 0.001* |
| BMI (kg/m2) | 0.997 (0.97–1.025) | 0.849 |
| SBP (mmHg) | 1.014 (1.007–1.021) | < 0.001* |
| Blood glucose, (mmol/L) | 1.057 (0.965–1.157) | 0.235 |
| Total cholesterol, (mmol/L) | 1.033(0.813–1.313) | 0.790 |
| LDL-cholesterol, (mmol/L) | 1.243 (1.106–1.397) | < 0.001* |
| HDL-cholesterol, (mmol/L) | 0.825 (0.318–2.141) | 0.692 |
| HDL/TC | 0.132 (0.001–1.721) | 0.412 |
| Hypertension, n (%) | 1.476(1.198–1.820) | < 0.001* |
| Diabetes mellitus, n (%) | 1.225 (0.925–1.621) | 0.157 |
| Hyperlipidaemia, n (%) | 1.040 (0.822–1.314) | 0.745 |
| Coronaryheartdisease, n(%) | 2.995 (1.418–6.324) | 0.004* |
| Serum total bilirubin, (umol/L) | 0.924 (0.852–1.002) | 0.057 |
| Serum albumin, (g/L) | 1.080 (0.946–1.23) | 0.243 |
| Uricacid, (mg/dL) | 1.001 (1.000–1.002) | 0.092 |
| A/G | 0.390 (0.12–1.262) | 0.116 |
| Blood urea nitrogen, (mmol/L) | 1.057 (0.991–1.127) | 0.094 |
A/G: serum albumin/seroglobulin; BMI: body mass index; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SBP: systolic blood pressure; TC: total cholesterol.
3.3. Persistent H. pylori infection increased the incidence of carotid atherosclerosis in males
A total of 1216 patients had follow-up carotid ultrasonography for up to 5 years. All study patients received an annual carotid ultrasound and 13C-UBT test. Patient with a continuous positive breath test every year was considered persistent H. pylori positive while patients who never had a positive breath test during the annual breath test were considered persistent negative for H. pylori infection. The incidence of carotid atherosclerosis was significantly higher in subjects with persistent H. pylori infection than those without infection after three years (Fig. 1C). A total of 332 individuals without carotid atherosclerosis at the initial ultrasound study were followed for 3–5 years and had follow-up carotid ultrasonography (Supplementary Table II). Of these, 246 patients were persistently negative for H. pylori infection, and 86 patients were persistently positive for H. pylori infection. A total of 129 patients developed carotid atherosclerosis during follow up from 2012 to 2017. Among them, 94 cases (72.87%) were males, and 35 cases (27.13%) were females. Males with persistent H. pylori infection had a significantly higher incidence of carotid atherosclerosis than those never infected after 3 years (50.91% vs. 35.29%, p = 0.040), especially in males under 50 years (48.00% vs. 30.72%, p = 0.028) (Table 4), but not in older males or female patients.
Table 4.
Incidence of carotid atherosclerosis in men and women after follow up for over 3 years.
| No infection | Persistent infection | p value | |||||
|---|---|---|---|---|---|---|---|
| Total number | New cases | Incidence | Total number | New cases | Incidence | ||
| Male | 187 | 66 | 35.29% | 55 | 28 | 50.91% | 0.040* |
| ≤5 | 166 | 51 | 30.72% | 50 | 24 | 48.00% | 0.028* |
| > 50 | 21 | 15 | 71.43% | 5 | 4 | 80.00% | 1.000 |
| Female | 59 | 21 | 35.59% | 31 | 14 | 45.16% | 0.495 |
p < 0.05.
4. Discussion
The present study demonstrated that (1) H. pylori infection is common in China, with an infection rate of 36.1% for males and 31.6% for females; (2) after adjusting for age, gender, BMI, and other risk factors for atherosclerosis, H. pylori infection is an independent risk factor for carotid atherosclerosis, especially in Chinese males under 50 years of age, but not in older males nor in females; (3) progression of atherosclerosis can be detected in young Chinese males who have persistent H. pylori infection, when followed over at least 3 years.
The relationship between H. pylori infection and atherosclerosis has been inconsistent and sometime controversial. The prevalence of serologically confirmed H. pylori infection was significantly higher in patients with angiographically documented CAD, supporting a positive association between H. pylori infection and CAD [17–19]. However, a meta-analysis with inclusion of 18 epidemiological studies and over 10,000 patients showed no positive relationship between H. pylori infection and CAD [20]. In contrast, data supporting a positive relationship between H. pylori infection and carotid atherosclerosis with increased CIMT were consistent in most of the studies [21–25]. The reason(s) for the significant difference in consistency on the relationship between H. pylori infection and CAD vs. carotid atherosclerosis is unclear. It could be very likely due to the different imaging modalities used for detection of CAD (coronary angiogram) and carotid atherosclerosis (carotid ultrasound). Carotid ultrasound could easily detect early atherosclerotic lesions, while coronary angiogram could not. In a recent study, investigators used cardiac multidetector computed tomography to identify subclinical coronary atherosclerotic lesions in healthy subjects without clinical CVD, and found that patients with current H. pylori infection were 3-fold more likely to have subclinical and yet significant coronary atherosclerosis than patients without H. pylori infection [26]. One of the major features of atherosclerosis is thickening of the intima-media in the arteries that could not be detected with angiogram. Carotid artery is considered an early site of atherosclerosis and superficial. Thus, carotid ultrasound examination is an ideal and sensitive non-invasive image modality to diagnose and monitor the progression of atherosclerosis [27], although it has not been widely used clinically for atherosclerosis screening at this point.
It is very concerning that cardiovascular mortality has been increasing since 2010, especially in males, for unknown reasons [28]. It is also reported that patients with ST elevation myocardial infarction over the past 20 years are getting younger [29]. The reasons for this reverse trend in cardiovascular mortality and mobility have yet to be defined. In the present study, we found that H. pylori infection selectively increased the risk for carotid atherosclerosis in young male patients (≤50 years), not in older males or female patients. A recent study [30] that analyzed a large database with a study population of 208,196 showed that mortality rate was significantly lower in patients with early eradication of H. pylori infection. The cumulative CAD rate was significantly decreased in younger patients (< 65 years old) with H. pylori eradication therapy within 1 year compared to those patients without eradication at all. Interestingly, the treatment of H. pylori eradication did not have a benefit in older patients (> 65 years old). These data strongly suggested that H. pylori infection could be a significant risk factor for atherosclerosis and CAD in young patients, and could provide a potential explanation for young patients who develop CAD without a clear etiology. It is unclear why H. pylori infection dose not increase the risk for atherosclerosis in patients older than 50 years. It is possible that other significant risk factors like diabetes mellitus, hypertension, and hyperlipidemia play a dominant role in the development of atherosclerosis in this age group of patients. Further studies are needed to investigate the mechanism(s) on the selective effect of H. pylori infection on atherosclerosis in the young population.
There are substantial gender differences in many CVDs including (but not limited to) myocardial infarctions, heart failure, hypertension, and cardiac hypertrophy [31]. It is well known that premenopausal women are relatively protected from CVDs when compared to men. Typically, women are almost 10 years older than men when they have their first myocardial infarction [32]. It was believed that the decreased cardiovascular morbidity and mortality in young females was due to possible cardio-protective effects of estrogen [33]. However, several large clinical studies, including the HERS trials and the Women’s Health Initiative study [34,35], showed that hormone replacement therapies (HRT) had no cardiovascular benefit in post-menopausal women. In contrast, there might have been an increased risk of CAD during the first year of HRT, and there was an increased risk of nonfatal ventricular arrhythmias among women on HRT [34]. Thus, the mechanism(s) for decreased CVD risk in premenopausal women is still unclear. Data from the present study showed that the prevalence of H. pylori infection was the same in males and females, and yet, H. pylori infection only increased the risk for carotid atherosclerosis in male patients ≤50 years, but not in older males or female patients. It is possible that the significant gender and age difference in the development of atherosclerosis associated with H. pylori infection may be one of the reasons for the decreased risk of CAD in young females. Further studies are needed to confirm these findings with both patients and experimental models.
It is not surprising that H. pylori infection increases the risk for atherosclerosis. It has been reported that H. pylori infection promotes the release of IL-1, IL-6, TNF-ɑ, and other cytokines, and activates local and systemic inflammatory response, thus accelerating the development of atherosclerosis [36–38]. H. pylori infection could also lead to malabsorption of vitamin B12, which could increase serum level of homocysteine, and promote the development of atherosclerosis [39]. In addition, H. pylori could promote the oxidation of low-density lipoproteins (LDL) and increase atherosclerotic plaque formation with decreased plaque stability [40,41]. In the present study, we observed that the blood lipid profiles were different between H. pylori-infected patients and those without H. pylori infection. The levels of LDL-cholesterol in patients with H. pylori infection were significantly higher than those without H. pylori infection, while the level of high-density lipoprotein cholesterol (HDL-C) was significantly decreased in patients with H. pylori infection than those without H. pylori infection (Supplementary Table III). Patients with H. pylori seropositivity were shown to have increased brachial-ankle pulse wave velocity (a marker of atherosclerosis), and impaired glucose metabolism [42]. It is believed that H. pylori could interact with gastric epithelial cells to upregulate the expression of adhesion molecules, and secrete cytokines, which could activate leukocytes, damage the vascular endothelium, aggravate local and systematic inflammatory responses, and thus promote the development and progression of atherosclerosis.
There were a few limitations in the present studies, including (1) the retrospective nature of the study and the fact that it involved only one center with one ethnic population, (2) there was no detailed information on medications for the subjects in the database, and (3) there might be potential intra- and inter-observer variation in the assessment of CIMT. The database did not provide adequate information for the evaluation of potential intra- and inter-observer variation in the assessment of CIMT. However, the possibility for potential intra- and inter-observer variation could be the same for each subject in the study.
4.1. Conclusions
The data from the present study suggested that H. pylori infection was an independent risk factor for carotid atherosclerosis only in male patients under 50 years, not in older males or female patients.
Supplementary Material
HIGHLIGHTS.
H. pylori infection might be a novel risk factor for carotid atherosclerosis for young males.
The findings might provide an explanation for the observation that males develop atherosclerosis earlier than females.
The data could justify screening and treatment for H. pylori infection in young males with premature atherosclerosis.
Acknowledgments
The authors wish to thank the staff in the Department of Health Management, the Third Xiangya Hospital of Central South University, Changsha, Hunan, China, for their assistance on database maintenance and protection.
Financial support
This work was supported by grants from the National Natural Science Foundation of China (No. 81570509), Changsha Science and Technology Project, Changsha, China (No. kq1701091), New Xiangya Talent Project of the Third Xiangya Hospital of Central South University, Changsha, China (No. 20150310), and China Scholarship Council, China (201806370189 to LZ), and partially supported by Hunan Provincial Natural Science Foundation, China (2018JJ6136 to CX) and NIH, United States (ES026200 and HL148196 to ZL).
Footnotes
Declaration of competing interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2019.10.005.
References
- [1].Bhatt DL, Steg PG, Ohman EM, et al. , International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis, JAMA 295 (2006) 180–189. [DOI] [PubMed] [Google Scholar]
- [2].Wang W, Jiang B, Sun H, et al. , Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults, Circulation 135 (2017) 759–771. [DOI] [PubMed] [Google Scholar]
- [3].Yang G, Wang Y, Zeng Y, et al. , Rapid health transition in China, 1990–2010: findings from the global burden of disease study 2010, Lancet (London, Engl) 381 (2013) 1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xu Y, Wang L, He J, et al. , Prevalence and control of diabetes in Chinese adults, JAMA 310 (2013) 948–959. [DOI] [PubMed] [Google Scholar]
- [5].Li W, Gu H, Teo KK, et al. , Hypertension prevalence, awareness, treatment, and control in 115 rural and urban communities involving 47 000 people from China, J. Hypertens. 34 (2016) 39–46. [DOI] [PubMed] [Google Scholar]
- [6].O’Donnell MJ, Xavier D, Liu L, et al. , Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study, Lancet (London, Engl) 376 (2010) 112–123. [DOI] [PubMed] [Google Scholar]
- [7].Chistiakov DA, Bobryshev YV, Kozarov E, et al. , Role of gut microbiota in the modulation of atherosclerosis-associated immune response, Front. Microbiol 6 (2015) 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goh KL, Chan WK, Shiota S, et al. , Epidemiology of Helicobacter pylori infection and public health implications, Helicobacter 16 (Suppl 1) (2011) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pietroiusti A, Diomedi M, Silvestrini M, et al. , Cytotoxin-associated gene-A–positive Helicobacter pylori strains are associated with atherosclerotic stroke, Circulation 106 (2002) 580–584. [DOI] [PubMed] [Google Scholar]
- [10].Franceschi F, Di Simone N, D’Ippolito S, et al. , Antibodies anti-CagA cross-react with trophoblast cells: a risk factor for pre-eclampsia? Helicobacter 17 (2012) 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oshima T, Ozono R, Yano Y, et al. , Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects, J. Am. Coll. Cardiol 45 (2005) 1219–1222. [DOI] [PubMed] [Google Scholar]
- [12].Franceschi F, Sepulveda AR, Gasbarrini A, et al. , Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis, Circulation 106 (2002) 430–434. [DOI] [PubMed] [Google Scholar]
- [13].Mach F, Sukhova GK, Michetti M, et al. , Influence of Helicobacter pylori infection during atherogenesis in vivo in mice, Circ. Res 90 (2002) E1–E4. [DOI] [PubMed] [Google Scholar]
- [14].Makita S, Nakamura M, Hiramori K, The association of C-reactive protein levels with carotid intima-media complex thickness and plaque formation in the general population, Stroke 36 (2005) 2138–2142. [DOI] [PubMed] [Google Scholar]
- [15].Gu L, Li S, He Y, et al. , Bismuth, rabeprazole, amoxicillin, and doxycycline as firstline Helicobacter pylori therapy in clinical practice: a pilot study, Helicobacter (2019) e12594. [DOI] [PubMed] [Google Scholar]
- [16].Dai H, Wang W, Chen R, et al. , Lipid accumulation product is a powerful tool to predict non-alcoholic fatty liver disease in Chinese adults, Nutr. Metab 14 (2017) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schottker B, Adamu MA, Weck MN, et al. , Helicobacter pylori infection, chronic atrophic gastritis and major cardiovascular events: a population-based cohort study, Atherosclerosis 220 (2012) 569–574. [DOI] [PubMed] [Google Scholar]
- [18].Yu XJ, Yang X, Feng L, et al. , Association between Helicobacter pylori infection and angiographically demonstrated coronary artery disease: a meta-analysis, Exp Ther Med 13 (2017) 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tabata N, Sueta D, Akasaka T, et al. , Helicobacter pylori seropositivity in patients with interleukin-1 polymorphisms is significantly associated with ST-segment elevation myocardial infarction, PLoS One 11 (2016) e0166240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Danesh J, Peto R, Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies, Br. Med. J 316 (1998) 1130–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gabrielli M, Santoliquido A, Cremonini F, et al. , CagA-positive cytotoxic H. pylori strains as a link between plaque instability and atherosclerotic stroke, Eur. Heart J 25 (2004) 64–68. [DOI] [PubMed] [Google Scholar]
- [22].Markus HS, Risley P, Mendall MA, et al. , Helicobacter pylori infection, the cytotoxin gene A strain, and carotid artery intima-media thickness, J. Cardiovasc. Risk 9 (2002) 1–6. [DOI] [PubMed] [Google Scholar]
- [23].Rozankovic PB, Huzjan AL, Cupic H, et al. , Influence of CagA-positive Helicobacter pylori strains on atherosclerotic carotid disease, J. Neurol 258 (2011) 753–761. [DOI] [PubMed] [Google Scholar]
- [24].Sawayama Y, Ariyama I, Hamada M, et al. , Association between chronic Helicobacter pylori infection and acute ischemic stroke: fukuoka Harasanshin Atherosclerosis Trial (FHAT), Atherosclerosis 178 (2005) 303–309. [DOI] [PubMed] [Google Scholar]
- [25].Shan J, Bai X, Han L, et al. , Association between atherosclerosis and gastric biomarkers concerning Helicobacter pylori infection in a Chinese healthy population, Exp. Gerontol 112 (2018) 97–102. [DOI] [PubMed] [Google Scholar]
- [26].Lee M, Baek H, Park JS, et al. , Current Helicobacter pylori infection is significantly associated with subclinical coronary atherosclerosis in healthy subjects: a cross-sectional study, PLoS One 13 (2018) e0193646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nagai Y, Matsumoto M, Metter EJ, The carotid artery as a noninvasive window for cardiovascular risk in apparently healthy individuals, Ultrasound Med. Biol 28 (2002) 1231–1238. [DOI] [PubMed] [Google Scholar]
- [28].Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, et al. , Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities, Circulation 133 (2016) 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mentias A, Hill E, Barakat AF, et al. , An alarming trend: change in the risk profile of patients with ST elevation myocardial infarction over the last two decades, Int. J. Cardiol 248 (2017) 69–72. [DOI] [PubMed] [Google Scholar]
- [30].Wang JW, Tseng KL, Hsu CN, et al. , Association between Helicobacter pylori eradication and the risk of coronary heart diseases, PLoS One 13 (2018) e0190219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Czubryt MP, Espira L, Lamoureux L, et al. , The role of sex in cardiac function and disease, Can. J. Physiol. Pharmacol 84 (2006) 93–109. [DOI] [PubMed] [Google Scholar]
- [32].Radovanovic D, Nallamothu BK, Seifert B, et al. , Temporal trends in treatment of ST-elevation myocardial infarction among men and women in Switzerland between 1997 and 2011, Eur Heart J Acute Cardiovasc Care 1 (2012) 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bell JR, Bernasochi GB, Varma U, et al. , Sex and sex hormones in cardiac stress–mechanistic insights, J. Steroid Biochem. Mol. Biol 137 (2013) 124–135. [DOI] [PubMed] [Google Scholar]
- [34].Grady D, Herrington D, Bittner V, et al. , Cardiovascular disease outcomes during 6.8 years of hormone therapy: heart and Estrogen/progestin Replacement Study follow-up (HERS II), JAMA 288 (2002) 49–57. [DOI] [PubMed] [Google Scholar]
- [35].Rossouw JE, Anderson GL, Prentice RL, et al. , Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial, JAMA 288 (2002) 321–333. [DOI] [PubMed] [Google Scholar]
- [36].Russo F, Jirillo E, Clemente C, et al. , Circulating cytokines and gastrin levels in asymptomatic subjects infected by Helicobacter pylori (H. pylori), Immunopharmacol. Immunotoxicol 23 (2001) 13–24. [DOI] [PubMed] [Google Scholar]
- [37].Consolazio A, Borgia MC, Ferro D, et al. , Increased thrombin generation and circulating levels of tumour necrosis factor-alpha in patients with chronic Helicobacter pylori-positive gastritis, Aliment. Pharmacol. Ther 20 (2004) 289–294. [DOI] [PubMed] [Google Scholar]
- [38].Maciorkowska E, Kaczmarski M, Panasiuk A, et al. , Soluble adhesion molecules ICAM-1, VCAM-1, P-selectin in children with Helicobacter pylori infection, World J. Gastroenterol 11 (2005) 6745–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sipponen P, Laxen F, Huotari K, et al. , Prevalence of low vitamin B12 and high homocysteine in serum in an elderly male population: association with atrophic gastritis and Helicobacter pylori infection, Scand. J. Gastroenterol 38 (2003) 1209–1216. [DOI] [PubMed] [Google Scholar]
- [40].Hoffmeister A, Rothenbacher D, Bode G, et al. , Current infection with Helicobacter pylori, but not seropositivity to Chlamydia pneumoniae or cytomegalovirus, is associated with an atherogenic, modified lipid profile, Arterioscler. Thromb. Vasc. Biol 21 (2001) 427–432. [DOI] [PubMed] [Google Scholar]
- [41].Laurila A, Bloigu A, Nayha S, et al. , Association of Helicobacter pylori infection with elevated serum lipids, Atherosclerosis 142 (1999) 207–210. [DOI] [PubMed] [Google Scholar]
- [42].Yoshikawa H, Aida K, Mori A, et al. , Involvement of Helicobacter pylori infection and impaired glucose metabolism in the increase of brachial-ankle pulse wave velocity, Helicobacter 12 (2007) 559–566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.