Abstract
The current appearance of the new SARS coronavirus 2 (SARS-CoV-2) and it quickly spreading across the world poses a global health emergency. The serious outbreak position is affecting people worldwide and requires rapid measures to be taken by healthcare systems and governments. Vaccinations represent the most effective strategy to prevent the epidemic of the virus and to further reduce morbidity and mortality with long-lasting effects. Nevertheless, currently there are no licensed vaccines for the novel coronaviruses. Researchers and clinicians from all over the world are advancing the development of a vaccine against novel human SARS-CoV-2 using various approaches. Herein, we aim to present and discuss the progress and prospects in the field of vaccine research towards SARS-CoV-2 using adenovirus (AdV) replication deficient-based strategies, with a comprehension that may support research and combat this recent world health emergency.
Keywords: SARS-CoV-2, vaccine, adjuvant, adenovirus, COVID-19
1. Introduction
In December 2019, the World Health Organization (WHO) reported a cluster of pneumonia cases of unknown origin registered in Wuhan, Hubei Province, China [1]. Most of these cases were epidemiologically connected to the Huanan Seafood Wholesale Market, probably related to animal contact. Subsequently, the first incidence of human-to-human transmission occurred [2] and the disease, now named coronavirus disease 19 (COVID-19), had a rapid and global dissemination. The emerging pathogen was detected and rapidly characterized as a new member of the beta-coronavirus genus, nearly associated to several bat coronaviruses and to severe acute respiratory syndrome coronavirus (SARS-CoV) [3]. The whole genome sequencing highlighted that SARS coronavirus 2 (SARS-CoV-2) has in common a sequence homology with SARS-CoV, within the receptor-binding motif that precisely interacts with the mankind receptor Angiotensin Converting Enzyme 2 (ACE2). This aspect provides valuable indications for vaccine development. The human-to-human spread of SARS-CoV-2 has now been confirmed, and the World Health Organization (WHO) has declared COVID-19 a health emergency of global concern. Furthermore, the new SARS-coronavirus was found in patients and is considered to be the causative agent of the new emerging lung disease [1], acute kidney injury (AKI) [4,5], ischemia stroke and blood clots [5,6], and affects the nervous, vascular, digestive, urinary and haematological systems [7]. Therefore, this recent issue poses a global health emergency.
Vaccines are an important and effective public health tool, able to contribute to the prevention and control of infectious diseases that can cause serious, even fatal, complications [8] representing a safe strategy compared to therapeutic medicines [9]. Despite tremendous global efforts to contain SARS-CoV-2, the virus continues to spread around the world. Therefore, the development of novel and effective vaccines for SARS-CoV-2 are urgently warranted, since some natural infections may not result in long-lasting protective immunity. The disease-induced immunity is often induced by a single natural infection, while an immunity caused by vaccine administration usually occurs after few administrations. Nevertheless, immunization with vaccines, in contrast to some natural infections, induces long-lasting protecting immunity [10].
Herein, we will focus on the use of adenoviruses as attractive vaccine adjuvants and vectors, highlighting the clinical prospects on vaccine generation against SARS-CoV-2. We hope that this brief review will support the global community and help in designing the appropriate immune intervention for the prophylactic vaccines against SARS-CoV-2.
2. Vaccines as a Tool to Prevent Infectious Diseases
Vaccination is one of the most meaningful achievements of medicine that has enormously limited the spread of infectious diseases over the last 20 decades [11,12]. Discovered in the 1700s, vaccination is considered a useful economic strategy able to preserve human health by preventing or eradicating infectious diseases, thus helping to maintain a high quality of life [13]. During the 20th century, mass vaccination led to a huge decrease in the incidence and morbidity of many infectious diseases and, interestingly, in various countries vaccination became a routine strategy that led to the reduction and elimination of a number of infectious diseases [12]. Nevertheless, while the smallpox vaccine represents a successful vaccine achievement which lead to the disease being eradicated globally, there is still the urgency to develop safe and cost-effective vaccines against recent diseases such as HIV and SARS-CoV-2, which are currently severe issues for public health worldwide.
In addition to directly preventing diseases, vaccinations can also reduce disease transmission. In fact, due to the herd immunity, persons who do not receive a vaccination have a reduced risk of being infected once a high percentage of the population has been immunized [14]. Moreover, vaccination can also limit the use of antibiotic based therapy [15,16], thus affecting the antimicrobial resistance, as proved [12]. Interestingly, being effective in reducing disease currency and associated mortality, vaccination also avoids additional costs of medical care.
Despite this, there is still a need for long-lasting vaccine platforms. Therefore, developing better ways to prevent and manage pandemic threats will be critical to control infectious diseases.
3. SARS-CoV-2 Structure and Protein Composition
SARS-CoV-2 is close enough to both Middle East respiratory syndrome-related coronavirus (MERS-CoV) and SARS-CoV, and it displays features typical of betacoronaviruses as follows: envelope and positive single-stranded ss-RNA (29,903 bp) [16,17,18,19,20]. The RNA organisational genome structure, starting from 5′ to 3′, uncovers the following: 5′-untranslated-region (UTR), replicase complex (ORF1a and ORF1b), four structural proteins (S,E,M,N)-3′, and non-structural ORFs [21,22]. ORF1a and ORF1b encompass about two-thirds of the genome [2]. According to the authors [23,24], ORF1a and ORF1b undergo frameshift mutations resulting in both pp1a (440–500 kDa) and pp1ab (740–810 kDa) non-structural polypeptides (nsps). These nsps are operated by viral proteases: chymotrypsin (3CLpro), main Mpro, and two papain producing sixteen nsps [23,25,26]. The SARS-CoV nsps are dispensable for virus replication (nsp12 harbouring RNA-dependent RNA polymerase RdRP) and blocking the host innate immune response [14]. The varial folding and RNA synthesis depend on the specific proteins M, E and N, respectively [27,28]. Interestingly, SARS-2-S and SARS-S show the following similarities: (1) amino acids (~76% identity) [15,21,27,29]; (2) entry into the host cells using two receptors (angiotensin-converting enzyme 2-ACE2 and cellular serine protease-TMPRSS2) and (3) priming spike-protein (S protein) [27,30]. There were found [17,23] to be at least 41 modifications in SARS-CoV-2’s transcript [5], with the most frequent motive of AAGAA [25]. The SARS-CoV-2’s genomic and proteomic sequences are published in GISAID, NCBI, NMDC and CNCB/NGDC. To date, it was shown that SARS-CoV-2 differs from other CoVs in features, such as: (1) S protein short anchor, (2) small ORFs’ distinctive amount and position, and (3) papain-like protease PLPpro’s one copy [2].
SARS-CoV-2 Antigen Selection
SARS-CoV-2’s complete sequences have an impact on developing vaccines and antiviral agents [6]. Vaccine candidates target spike (S) and nucleocapsid proteins, including subunit and recombinant vector vaccines, as well as DNA vaccines [7]. Lucchese [18] proposed unique pentapeptides of SARS-CoV-2 that are considered as preventive measures against novel coronavirus (2019-nCoV). Pentapeptides are oligopeptidic sequences (n = 37) of the spike protein that are absent in humans [18]. Thus, both the S and nucleocapsid (N) proteins of SARS-CoV-2 are attractive immunogens for vaccine development [16]. The viral pentapeptides (n = 933) [18] are unfamiliar to the human immune system. Among these, the S glycoproteins (n = 107) utilize the receptor-binding domain (RBD) to bind to a human ACE2 receptor [18]. The ~1200 aa long S protein with the S1/S2 processing site exhibits different motifs among coronaviruses [19]. In 2019-nCoV, the RBD includes a receptor-binding motif (RBM), implicating eight conserved residues to contact ACE2 [30]. The S2-protein consists of the fusion amino acids (FP) and a second S2 cleavage site for virus entry [19,30]. The S2 subunit is highly conserved, thus it is a target for antiviral agents [23]. Interestingly, it is important for an ideal vaccine to induce highly potent neutralizing antibodies without inducing any disease-enhancing antibodies [31]. In fact, the antibody-dependent enhancement (ADE) of some viral infections can increase the virus cell entry and replication due to the presence of virus-specific antibodies [32]. However, the mechanisms of ADE still remain to be better studied and understood. Therefore, the identification of potent and effective viral epitopes and their safety assessment is an important aspect to be taken into consideration.
SARS-CoV-2’s RBD contains the neutralizing antigens, but does not have any linear immunodominant (ID) sites [27]. The RBD of SARS-CoV-2 showed a contrasting ID scene compared with its correlative in SARS-CoV. Vaccines against SARS-CoV-2 using the S linear antigenic epitopes instead of the entire S protein guarantee better safety [27]. Interestingly, ORF3b and ORF8 have no homology with SARS-CoV, thus will help with the construction of an adequate vaccine. Furthermore, Zhang et al. [27] reported that some protein-specific epitope titers outreached the microneutralization titers by several orders of magnitude. Additionally, because of the low level of neutralizing antibody measured in in the recovered patients, it is supposed to observe a compelling difference between specific and neutralizing antibodies. Authors also reported comparable antiviral activity followed by the immunization program, with epitopes compared to vaccination with the complete RBD fragment. This observation gives evidence that epitope-based vaccines can generate equivalent protection compared to subunit vaccines. Alternative vaccines based on DNA, expressing full-length S proteins or its fragments as well as nucleocapsids, have advantages (safety; neutralizing antibodies) and disadvantages (immune responses; TH2 cell-distortive immune response; delayed-type hypersensitivity) [18]. Thus, to increase the efficiency, these vaccines are administered with adjuvants [26]. Moreover, the inactivated whole-virus vaccines, containing CoV genetically attenuated in replication [33], are under study. The antiviral discovery processes includes the mRNA-based vaccines consisting of mRNAs encoding full-size S formulated on cationic lipid nanoparticles [33]. As far as it is known, vaccine development focuses on mRNA encoding S, E, M and N protein, all of which are potent in inducing the antigen-specific immune reactions [33].
4. Adenoviruses as a Promising Vaccine Adjuvant
A vaccine, in order to be effective and long-lasting, should induce protective immunity in a population towards a specific epitope/antigen. Such immunity is generated by the immunization process containing an element of the disease inducing agent (e.g., a killed or live attenuated form of the pathogen). Currently, many of the most successful and safe vaccines used attenuated variants of a target pathogen resulting in a mild infection able to produce long-lasting immunity [12]. Protein-based vaccines depend on the presence of adjuvants in order to induce both an innate and adaptive immune response and subsequently to generate protective immunological memory to the vaccine antigen, since proteins alone are often not very immunogenic [34]. Therefore, adjuvants are considered as additional key components of the vaccine for a successful immunization process within an individual by increasing the adaptive response to a vaccine [34].
One of such potent adjuvants can be the adenovirus vector [35,36,37,38,39,40,41,42,43,44,45,46,47]. In an effort to design vaccines rationally, different studies have tested replication-defective adenovirus (Ad) vectors as a vaccine platform [20,48]. Adenovirus vectors are promising as: (1) a vaccine vector because of their properties to stimulate the immune responses, and (2) an adjuvant because of their ability to stimulate the immune reaction [49]. On the top of adenovirus immunogenicity, adenoviral vectors have been extensively characterized showing: (1) a genome that can be easily modified by inserting exogenous transgenes of an interest, and (2) a relatively stable viral capsid. Moreover, adenovirus vectors can either encode for various exogenous antigens or can present those epitopes on the surface of the capsid, modulating a strong immune response to the specific antigens [50]. Furthermore, adenoviruses are able to activate various innate immune signalling pathways resulting in the secretion of a number of proinflammatory cytokines, paving the route for potent and adequate immune cell stimulation by inducing a strong adaptive humoral and cellular immune reaction which are translated into an immunological memory to the vaccine antigen.
Adenovirus-based vaccines can utilize replication-competent or replication-defective vectors, constructed by removing or replacing the E1A and E1B genes (early transcript 1A and 1B), thus erasing the replication features of vectors [51]. The viral E3 and E4 genes are often removed in order to diminish the virus clearance from the host [51]. Adenovirus itself works as a strong immune activator, enhancing the immunogenicity of the vaccine antigens, attracting antigen presenting cells to the side of infection, boosting T cell priming and inducing development of proinflammatory responses. Therefore, adenovirus properties of activating innate and adaptive immune responses helps develop immunity to the exogenous antigens and develop a protective immunity [52,53,54,55].
Adenoviruses exhibit several advantages over other viruses, such as: vaccinia virus (VV), lentivirus, retrovirus, adeno-associated virus (AAV) and herpesvirus. They are highly immunogenic and have an ability to induce potent innate and adaptive immune responses in a host. In contrast to a lentivirus or retrovirus, adenoviruses do not integrate the viral genomic DNA into the hosts’ chromosome, thus reducing the risk of mutagenesis [2,3]. In turn, AAVs are less pathogenic than adenoviruses, but on other hand big scale manufacturing of AAV is more complexed and challenging [4].
However, there are also some drawbacks of using adenoviruses (AdVs) as an adjuvant. This includes pre-existing immunity in humans leading to the production of neutralizing antibodies. Nevertheless, this limitation can be easily overcome by replacing the adenovirus hexon sequence or fiber knob domain (chimeric adenovirus) from a different serotype. Importantly, due to the versatility and diversity of adenovirus serotypes, they are valuable and preferable vectors for vaccine productions against a broad spectrum of pathogens [5,6]. AdVs have been classified into seven subtypes: A–G into 67 various serotypes [56]. This classification relies on similarities in genome homology, tropism and analyses of neutralizing antibodies against capsid antigen, phylogenetic assessment encoding protease, the hexon structure, and the viral DNA polymerase [57]. Because of the broad versatility and diversity of adenovirus subtypes, they are valuable and preferable vectors for vaccine productions against a broad spectrum of pathogens [57,58]. Many adenoviruses exhibit very low seroprevalence: AdV2, AdV26 and AdV35 are considered as promising candidates for a vaccine development [59]. Both adenoviruses AdV26 and AdV35 have been tested in clinical studies, where they have been proven to be well tolerated and safe. However, their efficacy and immunogenic potency is lower in comparison to the most well studied and used, AdV5. Since the neutralizing antibodies against various serotypes and T cells do not cross react with different subtypes, it gives broad possibilities for vaccine development, utilizing various vectors or their combinations [60,61].
In addition to their immune-stimulatory properties (both innate and adaptive), adenoviral-based vaccine vectors have been well characterized. Interestingly, their safety profile is well known and proven in many clinical studies [24,62,63]. Taking all this into account, using genetic engineering tools, it is doable to construct and produce Ad-based vectors to induce the strong and effective immune response towards the target vaccine antigen. This scientific rationale for the adenovirus-based vaccine construct strategy may contribute to a more effective vaccine, able to generate a specific immune response depending on the vaccine antigen.
5. Clinical Prospects on COVID-19 Vaccine Development
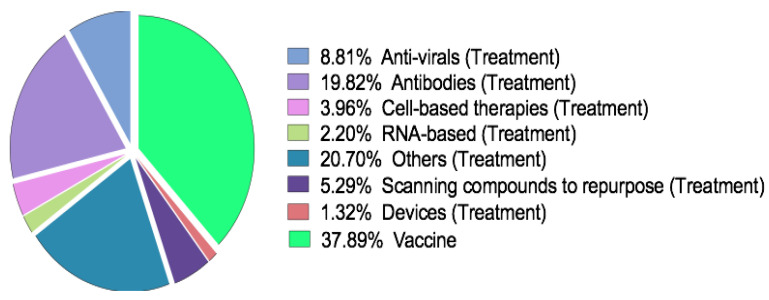
Up to date, there is no available preventive vaccine against SARS-CoV-2 and no drug agent with proven clinical potency. However, there are multiple candidates that might be effective in prevention or treatment. Over 900 clinical trials have been registered at clinicaltrials.gov (27 April 2020) and over 1100 worldwide, including 123 trials with a EudraCT (27 April 2020) on COVID-19 covering both treatment and preventive approaches (Figure 1).
Figure 1.
Coronavirus disease 19 (COVID-19) treatments and vaccines in a research pipeline with a percentage distribution.
Although no vaccines are available on the market for both SARS and Middle East respiratory syndrome (MERS), considerable effort has been put into the vaccine development against these diseases, thus representing a valuable example to produce an effective vaccine for COVID-19. After the SARS epidemic in 2002–2003, the majority of vaccines [64] targeted the spike (S) glycoprotein of the virus [65]. Different vaccine strategies have been proposed based on a live-attenuated, inactivated virus, recombinant viral vectors, DNA, virus-like particles (VLPs) and soluble proteins [66]. Strategies based on recombinant viral vectors used genetically engineered viruses, which differ from the SARS-CoV and are able to express components of the SARS-CoV [67]. Currently, only vaccines based on an inactivated SARS virus, DNA and soluble proteins based on the SARS S glycoprotein, reached a clinical stage (phase I). As for SARS vaccines, most of them are based on the S glycoprotein as well. Vaccines based on inactivated and live attenuated viruses, recombinant viral vectors, nanoparticle DNA and soluble proteins have been developed and tested in animal models. Interestingly, so far only one DNA-based vaccine has been tested in phase I [68].
Currently there are at least two promising adenovirus-based vaccines against COVID-19 undergoing testing in clinical studies. The first one is based on the human replication-defective AdV5 vector (Ad5-nCoV) coding for the full-length S protein currently being tested in China (mid. April 2020, Phase I/II of the clinical study, NCT04313127, NCT04324606). Preclinical data on Ad5-nCoV showed the safety profile and the ability of the tested vaccine to generate strong immune responses in tested animal models. Another promising vaccine is based on Chimpanzee adenovirus (ChAdOx1) engineered in UK and is being tested in the phase I/II clinical trial (April 2020, NCT04324606) on 510 volunteers. The adenovirus vector is also replication-deficient and has been armed with genes encoding antigens in order to stimulate humoral and cytotoxic T-cells.
As reported by CEPI in April 2020, 115 total vaccine contestants are in early stages of development [69]. Selected clinical studies on a COVID-19 vaccine have been shortlisted and are presented in Table 1.
Table 1.
Selected clinical studies on vaccine research against SARS coronavirus 2 (SARS-CoV-2).
| Name | Description | Phase of the Trial | Number of Participants | Location | References |
|---|---|---|---|---|---|
| INO-4800 | DNA plasmid | Phase I | 40 | USA | NCT04336410 |
| Ad5-nCoV | Recombinant AdV 5 | Phase I | 108 | China | NCT04313127 |
| Ad5-nCoV | Recombinant AdV 5 | Phase II | 500 | China | NCT04324606 |
| ChAdOx1 nCoV-19 | Adenovirus vector | Phase I, II | 510 | UK | NCT04276896 |
| LV-SMENP-DC | Lentiviral vaccine, DCs modified with a lentiviral vector | Phase I, II | 100 | China |
NCT04276896 [70] |
| Covid-19/aAPC | Lentiviral vector, pathogen specific artificial antigen presenting DCs | Phase I | 100 | China |
NCT04299724 [70] |
| mRNA-1273 | Lipid nanoparticle containing mRNA | Phase I | 45 | USA | NCT04283461 |
| rhACE2 | Recombinant ACE2 (angiotensin-converting enzyme 2) |
- | 24 | China | NCT04287686 |
|
Washed microbiota
transplantation |
Washed microbiota transplantation |
- | - | China | NCT04251767 |
| BCG Vaccination to Protect Healthcare Workers Against COVID-19 (BRACE) | Bacillus Calmette–Guérin (BSG) vaccine | Phase 3 | 4170 | Australia | NCT04327206 |
6. Conclusions
In the face of a current unprecedented world pandemic, urgent and rapid research focused on the production of clinically proven vaccines is in high demand.
The SARS-CoV-2 vaccination approach and technology should be supported and guided by a better understanding of the SARS pathogenesis in mankind, including the route of virus dissemination within the body. These aspects can support the generation of vaccines able to prevent and inhibit SARS-CoV-2 spread and, above all, infection of the vital and target organs. So far, unfortunately, we still have limited knowledge about SARS-CoV-2 and there are still a lot of open questions, in particular the immune reaction in response to the virus invasion, that is crucial for vaccine generation, remains unclear. All these issues need to be addressed in the near future for a successful vaccine development.
The potential of adenoviruses as vaccine vectors is attributed to their ability to drive a robust humoral and cellular adaptive immune response. Over recent years, there has been significant progress made in the field of adenovirus vector vaccine development. Currently we are entering an exciting stage, focused on the development of adenovirus-based vaccines, and the results from on-going clinical trials may contribute to improve our knowledge and develop an effective vaccine against COVID-19.
Persistent and constant international cooperation along with joint attempts are crucial to unravel the outstanding unanswered queries about the new SARS-CoV-2. Importantly, the cooperation on national and international levels encompasses: academics, governments and industries, which are imperative to limit dissemination of COVID-19 and to prevent future outbreaks.
Author Contributions
Conceptualization: M.G., L.K., M.S.; writing original draft: M.G., L.K., M.S.; writing review and editing: M.G., M.S., S.S., P.C., K.W.P., M.W. and L.K. All authors have read and agreed to the published version of the manuscript.
Funding
M.S. was supported by Warsaw University of Technology; EU COST action CA17140 (M.G. & L.K.); SONATINA (2019/32/C/NZ7/00156), National Science Centre, Poland (L.K.); MINIATURA (2018/02/X/ NZ7/00727), National Science Centre, Poland (L.K.); by PRID-J (Grant Number: GARO_SID19_02) funded by University of Padua (M.G.).
Conflicts of Interest
L.K. is an employee in Targovax Oy in Finland. All the other authors declare no potential conflicts of interest.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F., Fung K.S., Tang H.L., Yan W.W., Chan H.W., et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin H., Hong C., Chen S., Zhou Y., Wang Y., Mao L., Li Y., He Q., Li M., Su Y., et al. Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke Vasc. Neurol. 2020 doi: 10.1136/svn-2020-000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remy V., Largeron N., Quilici S., Carroll S. The Economic Value of Vaccination: Why Prevention Is Wealth. Value Health. 2014;17:A450. doi: 10.1016/j.jval.2014.08.1211. [DOI] [PubMed] [Google Scholar]
- 9.Zhou W., Pool V., Iskander J., English-Bullard R., Ball R., Wise R., Haber P., Pless R., Mootrey G., Ellenberg S., et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)--United States, 1991-2001. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2003;52:1–24. [PubMed] [Google Scholar]
- 10.Clerici M., Tacket C.O., Via C.S., Lucey D.R., Muluk S.C., Zajac R.A., Boswell R.N., Berzofsky J.A., Shearer G.M. Immunization with subunit human immunodeficiency virus vaccine generates stronger T helper cell immunity than natural infection. Eur. J. Immunol. 1991;21:1345–1349. doi: 10.1002/eji.1830210603. [DOI] [PubMed] [Google Scholar]
- 11.Andre F.E., Booy R., Bock H.L., Clemens J., Datta S.K., John T.J., Lee B.W., Lolekha S., Peltola H., Ruff T.A., et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008;86:140–146. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badur S., Ota M., Ozturk S., Adegbola R., Dutta A. Vaccine confidence: The keys to restoring trust. Hum. Vaccines Immunother. 2020;16:1007–1017. doi: 10.1080/21645515.2020.1740559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre F. Vaccinology: Past achievements, present roadblocks and future promises. Vaccine. 2003;21:593–595. doi: 10.1016/S0264-410X(02)00702-8. [DOI] [PubMed] [Google Scholar]
- 14.Plotkin’s Vaccines. [(accessed on 10 June 2020)]; Available online: https://www.sciencedirect.com/book/9780323357616/plotkins-vaccines#book-info.
- 15.Palmu A.A., Jokinen J., Nieminen H., Rinta-Kokko H., Ruokokoski E., Puumalainen T., Borys D., Lommel P., Traskine M., Moreira M., et al. Effect of pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) on outpatient antimicrobial purchases: A double-blind, cluster randomised phase 3–4 trial. Lancet Infect. Dis. 2014;14:205–212. doi: 10.1016/S1473-3099(13)70338-4. [DOI] [PubMed] [Google Scholar]
- 16.Klugman K.P., Black S. Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects. Proc. Natl. Acad. Sci. USA. 2018;115:12896–12901. doi: 10.1073/pnas.1721095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 18.Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses. 2019;11 doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C., Zhou D. Adenoviral vector-based strategies against infectious disease and cancer. Hum. Vaccines Immunother. 2016;12:2064–2074. doi: 10.1080/21645515.2016.1165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 22.Bezstarosti K., Lamers M.M., Haagmans B.L., Demmers J.A.A. Targeted proteomics for the detection of Sars-COV-2 proteins. bioRxiv. 2020 doi: 10.1101/2020.04.23.057810. [DOI] [Google Scholar]
- 23.Features, Evaluation and Treatment Coronavirus (COVID-19) [(accessed on 10 June 2020)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/
- 24.Ranki T., Joensuu T., Jager E., Karbach J., Wahle C., Kairemo K., Alanko T., Partanen K., Turkki R., Linder N., et al. Local treatment of a pleural mesothelioma tumor with ONCOS-102 induces a systemic antitumor CD8(+) T-cell response, prominent infiltration of CD8(+) lymphocytes and Th1 type polarization. Oncoimmunology. 2014;3:e958937. doi: 10.4161/21624011.2014.958937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The Architecture of SARS-CoV-2 Transcriptome. Cell. 2020 doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enjuanes L., Zuniga S., Castano-Rodriguez C., Gutierrez-Alvarez J., Canton J., Sola I. Molecular Basis of Coronavirus Virulence and Vaccine Development. Adv. Virus Res. 2016;96:245–286. doi: 10.1016/bs.aivir.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J.-D., Zhang B.-Z., Hu Y.-F. Mapping the Immunodominance Landscape of SARS-CoV-2 Spike Protein for the Design of Vaccines against COVID-19. bioRxiv. 2020 doi: 10.1101/2020.04.23.056853. [DOI] [Google Scholar]
- 28.Lucchese G. Epitopes for a 2019-nCoV vaccine. Cell. Mol. Immunol. 2020;17:539–540. doi: 10.1038/s41423-020-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med. Wkly. 2020;150:w20249. doi: 10.4414/smw.2020.20249. [DOI] [PubMed] [Google Scholar]
- 32.Tetro J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22:72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z., Xiao X., Wei X., Li J., Yang J., Tan H., Zhu J., Zhang Q., Wu J., Liu L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020 doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuryk L., Moller A.W., Jaderberg M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology. 2019;8:e1532763. doi: 10.1080/2162402X.2018.1532763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuryk L., Moller A.W., Vuolanto A., Pesonen S., Garofalo M., Cerullo V., Jaderberg M. Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuryk L., Moller A.S.W., Garofalo M., Cerullo V., Pesonen S., Alemany R., Jaderberg M. Antitumor-specific T-cell responses induced by oncolytic adenovirus ONCOS-102 (AdV5/3-D24-GM-CSF) in peritoneal mesothelioma mouse model. J. Med. Virol. 2018;90:1669–1673. doi: 10.1002/jmv.25229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capasso C., Magarkar A., Cervera-Carrascon V., Fusciello M., Feola S., Muller M., Garofalo M., Kuryk L., Tähtinen S., Pastore L., et al. A novel in silico framework to improve MHC-I epitopes and break the tolerance to melanoma. OncoImmunology. 2017;6:e1319028. doi: 10.1080/2162402X.2017.1319028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirvinen M., Capasso C., Guse K., Garofalo M., Vitale A., Ahonen M., Kuryk L., Vähä-Koskela M., Hemminki A., Greco D., et al. Boosting the Immunogenicity of an Oncolytic Vaccinia Virus By Expression of DAI Can Enhance Anti-Tumor Immunity in Humanized Mice. Mol. Ther. 2015;23:S31. doi: 10.1016/S1525-0016(16)33676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuryk L., Moller A.W. Chimeric oncolytic Ad5/3 virus replicates and lyses ovarian cancer cells through desmoglein-2 cell entry receptor. J. Med. Virol. 2020 doi: 10.1002/jmv.25677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuryk L., Moller A.W., Jaderberg M. Abscopal effect when combining oncolytic adenovirus and checkpoint inhibitor in a humanized NOG mouse model of melanoma. J. Med. Virol. 2019 doi: 10.1002/jmv.25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuryk L., Haavisto E., Garofalo M., Capasso C., Hirvinen M., Pesonen S., Ranki T., Vassilev L., Cerullo V. Synergistic anti-tumor efficacy of immunogenic adenovirus ONCOS-102 (Ad5/3-D24-GM-CSF) and standard of care chemotherapy in preclinical mesothelioma model. Int. J. Cancer. 2016;139:1883–1893. doi: 10.1002/ijc.30228. [DOI] [PubMed] [Google Scholar]
- 43.Garofalo M., Villa A., Rizzi N., Kuryk L., Rinner B., Cerullo V., Yliperttula M., Mazzaferro V., Ciana P. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J. Control. Release Off. J. Control. Release Soc. 2019;294:165–175. doi: 10.1016/j.jconrel.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Garofalo M., Villa A., Crescenti D., Marzagalli M., Kuryk L., Limonta P., Mazzaferro V., Ciana P. Heterologous and cross-species tropism of cancer-derived extracellular vesicles. Theranostics. 2019;9:5681–5693. doi: 10.7150/thno.34824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garofalo M., Villa A., Rizzi N., Kuryk L., Mazzaferro V., Ciana P. Systemic Administration and Targeted Delivery of Immunogenic Oncolytic Adenovirus Encapsulated in Extracellular Vesicles for Cancer Therapies. Viruses. 2018;10:558. doi: 10.3390/v10100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuryk L., Møller A.-S.W., Jaderberg M. Quantification and functional evaluation of CD40L production from the adenovirus vector ONCOS-401. Cancer Gene Ther. 2018 doi: 10.1038/s41417-018-0038-x. [DOI] [PubMed] [Google Scholar]
- 47.Lipiec A., Kuryk L. Onkolityczne wektory wirusowe w immunoterapii nowotworów. Immunoterapia PZWL. 2018;1:31–44. [Google Scholar]
- 48.Sharma P.K., Dmitriev I.P., Kashentseva E.A., Raes G., Li L., Kim S.W., Lu Z.H., Arbeit J.M., Fleming T.P., Kaliberov S.A., et al. Development of an adenovirus vector vaccine platform for targeting dendritic cells. Cancer Gene. 2018;25:27–38. doi: 10.1038/s41417-017-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartman Z.C., Appledorn D.M., Amalfitano A. Adenovirus vector induced innate immune responses: Impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Small J.C., Ertl H.C. Viruses—From pathogens to vaccine carriers. Curr. Opin. Virol. 2011;1:241–245. doi: 10.1016/j.coviro.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wold W.S., Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hendrickx R., Stichling N., Koelen J., Kuryk L., Lipiec A., Greber U.F. Innate Immunity to Adenovirus. Hum. Gene. 2014;25:265–284. doi: 10.1089/hum.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lowenstein P.R., Castro M.G. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: Peculiarities, mechanisms, and consequences. Gene. 2003;10:946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- 54.Sharma A., Krause A., Xu Y., Sung B., Wu W., Worgall S. Adenovirus-based vaccine with epitopes incorporated in novel fiber sites to induce protective immunity against Pseudomonas aeruginosa. PLoS ONE. 2013;8:e56996. doi: 10.1371/journal.pone.0056996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia W., Channappanavar R., Zhang C., Li M., Zhou H., Zhang S., Zhou P., Xu J., Shan S., Shi X., et al. Single intranasal immunization with chimpanzee adenovirus-based vaccine induces sustained and protective immunity against MERS-CoV infection. Emerg. Microbes Infect. 2019;8:760–772. doi: 10.1080/22221751.2019.1620083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crenshaw B.J., Jones L.B., Bell C.R., Kumar S., Matthews Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines. 2019;7 doi: 10.3390/biomedicines7030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh S., Kumar R., Agrawal B. Adenoviral Vector-Based Vaccines and Gene Therapies: Current Status and Future Prospects. Adenoviruses. 2019 doi: 10.5772/intechopen.79697. [DOI] [Google Scholar]
- 58.Tatsis N., Ertl H.C. Adenoviruses as vaccine vectors. Mol. Ther. J. Am. Soc. Gene Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen H., Xiang Z.Q., Li Y., Kurupati R.K., Jia B., Bian A., Zhou D.M., Hutnick N., Yuan S., Gray C., et al. Adenovirus-based vaccines: Comparison of vectors from three species of adenoviridae. J. Virol. 2010;84:10522–10532. doi: 10.1128/JVI.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holterman L., Vogels R., van der Vlugt R., Sieuwerts M., Grimbergen J., Kaspers J., Geelen E., van der Helm E., Lemckert A., Gillissen G., et al. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: Low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 2004;78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barouch D.H., Pau M.G., Custers J.H., Koudstaal W., Kostense S., Havenga M.J., Truitt D.M., Sumida S.M., Kishko M.G., Arthur J.C., et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 62.Vassilev L., Ranki T., Joensuu T., Jager E., Karbach J., Wahle C., Partanen K., Kairemo K., Alanko T., Turkki R., et al. Repeated intratumoral administration of ONCOS-102 leads to systemic antitumor CD8(+) T-cell response and robust cellular and transcriptional immune activation at tumor site in a patient with ovarian cancer. Oncoimmunology. 2015;4:e1017702. doi: 10.1080/2162402X.2015.1017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ranki T., Pesonen S., Hemminki A., Partanen K., Kairemo K., Alanko T., Lundin J., Linder N., Turkki R., Ristimaki A., et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer. 2016;4:17. doi: 10.1186/s40425-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spiering M. Primer on the Immune System. Alcohol Res. Curr. Rev. 2015;37:171. [PMC free article] [PubMed] [Google Scholar]
- 65.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrovsky N., Aguilar J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 67.Lauer K.B., Borrow R., Blanchard T.J. Multivalent and Multipathogen Viral Vector Vaccines. Clin. Vaccine Immunol. CVI. 2017;24 doi: 10.1128/CVI.00298-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Modjarrad K., Roberts C.C., Mills K.T., Castellano A.R., Paolino K., Muthumani K., Reuschel E.L., Robb M.L., Racine T., Oh M.-d., et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019;19:1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Institute T.M. COVID-19 Treatment and Vaccine Tracker. [(accessed on 29 May 2020)]; Available online: https://milkeninstitute.org/covid-19-tracker.
- 70.Thanh Le T., Andreadakis Z., Kumar A., Gomez Roman R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020 doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]